The effects of phenolic acid supplementation on intestinal barrier function: a review
Li
Xia†
,
Xiulian
Lin†
,
Yuanjiao
Zhou
,
Yamei
Li
,
Yingyan
Liao
,
Yan
Lin
,
Limei
Lin
*,
Ping
Wu
* and
Jingchen
Xie
 *
*
Key Laboratory for Quality Evaluation of Bulk Herbs of Hunan Province, School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, Hunan, China. E-mail: limei_lin@hnucm.edu.cn; 13607437848@163.com; 004108@hnucm.edu.cn
First published on 8th December 2025
Abstract
The intestinal barrier is critically implicated in the pathogenesis of diverse diseases, and its impairment constitutes a common pathological hallmark across numerous conditions. Therefore, restoring intestinal barrier function is a key therapeutic strategy for mitigating or treating associated diseases. Phenolic acid compounds, secondary metabolites derived from plants, exhibit diverse pharmacological properties, including anti-inflammatory, antioxidant, and antibacterial activities. This review summarizes the sources, absorption, and metabolic pathways of phenolic acids, delving into their mechanisms for maintaining intestinal barrier integrity. Key mechanisms encompass the upregulation of tight junction protein expression, modulation of mucus secretion, inhibition of oxidative stress and inflammatory responses, and regulation of gut microbiota composition. Furthermore, we summarize the therapeutic potential of phenolic acid compounds in treating diseases associated with intestinal barrier dysfunction, discuss their clinical applications and safety profiles, and critically evaluate challenges in clinical translation. This review aims to provide a scientific foundation for the potential therapeutic application of phenolic acid compounds. However, their definitive clinical value necessitates further validation through rigorous clinical trials.
1. Introduction
Phenolic acids are widely distributed plant secondary metabolites. As dietary supplements, phenolic acids provide a broad spectrum of health benefits, for example, antioxidant, antimicrobial and anti-inflammatory effects.1–3 In recent years, public research on phenolic acids has increased, especially in intestinal diseases. Currently, millions of people around the world suffer from intestinal diseases, including IBS,4 IBD5 and intestinal infections.6 The treatment of these intestinal diseases is limited to managing symptoms and they cannot be completely eradicated. Therefore, identifying the key pathogenic mechanisms of intestinal diseases is critical to overcoming this problem.The intestinal barrier, the first line of defense between the gut and the external environment, plays a vital role in regulating the transport of nutrients, water, and waste while resisting pathogen invasion. Its integrity is closely associated with the development and progression of various intestinal diseases.7,8 Therefore, preventing or repairing intestinal barrier damage may represent a promising approach for treating these conditions.9 Several phenolic acids, such as chlorogenic acid,10 gallic acid,11 caffeic acid,12 and ferulic acid,13 have been shown to repair damaged intestinal barriers by upregulating the expression of tight junction proteins. Protocatechuic acid,14 sinapic acid,15 and vanillic acid16 can also exert protective effects on the intestinal barrier through their anti-inflammatory and antioxidant properties. Similarly, studies have demonstrated the beneficial effects of phenolic acid rich foods on intestinal barrier integrity.17,18
This review systematically synthesizes the molecular mechanisms through which phenolic acids protect the intestinal barrier, including increasing tight junction protein expression, regulating mucus secretion, reducing oxidative stress, modulating intestinal microbiota composition, and normalizing immune function. Furthermore, it evaluates their therapeutic potential and clinical translation barriers, providing a scientific foundation and future research directions for developing phenolic acid-based interventions targeting barrier repair.
2. Sources, dietary forms, and absorption and metabolism of phenolic acids
2.1 Sources of phenolic acid
Phenolic acids are ubiquitous in foods, including fruits (such as apples, lemons, grapes, pomegranates and strawberries), vegetables (such as peppers, onions, eggplants and coriander), beans (such as peas, mung beans and kidney beans), grains (such as barley, wheat, rye and millet), and oilseeds (such as flax, rapeseed and mustard seeds).19 Additionally, the gut microbiota can metabolize precursors such as cinnamic acid into a variety of phenolic acids via the shikimate and phenylpropanoid pathways, including salicylic acid,20 chlorogenic acid,21 protocatechuic acid,22 gallic acid,23 coumaric acid,24 caffeic acid,25 ferulic acid, and sinapic acid.26 Dietary anthocyanins, procyanidins, and quercetin undergo biotransformation into chlorogenic acid,27–29 while anthocyanin-3-glucoside (C3G) yields metabolites including protocatechuic acid, vanillic acid, ferulic acid and derivatives.30 Similarly, flavanol compounds are metabolized by the gut microbiota to 15 phenolic acids, including caffeic acid and ferulic acid,31 all of which exhibit significant antioxidant and anti-inflammatory activities. Additionally, during the processes of absorption and metabolism, phenolic acids can undergo interconversion: hepatic metabolism converts ferulic acid to vanillic acid;32 the gut microbiota metabolizes protocatechuic acid to vanillic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, and 3-methoxybenzoic acid;33 and colonic microorganisms degrade chlorogenic acid to caffeic acid.342.2 Dietary forms of phenolic acid
In food matrices, hydroxycinnamic acids are commonly present as glycerides. For instance, ethyl p-coumarate has been identified in certain varieties of radish and tomato.35 Chlorogenic acid exists as ethyl chlorogenic acid in unripe sunflower seeds,36 while caffeic acid and monoglycerides of p-coumaric acid have been isolated from pineapple stems.37 Hydroxybenzoic acids are predominantly present in foods as glycosides. Caffeic acid, protocatechuic acid, and chlorogenic acid are found in currants, blueberries, and gooseberries, respectively, in the form of 4-O-glycosidic bonds.38 Para-hydroxybenzoic acid occurs as glycosides in star anise,39 and glycoside forms of various phenolic acids, including salicylic acid, vanillic acid, and eugenic acid, have been detected in a wide range of fruits and vegetables such as onions, potatoes, and cherries.40 In general, phenolic acids are predominantly found in foods as esters or glycosides, typically conjugated with natural compounds such as sterols, triterpenes, and lignans.2.3 Absorption and metabolism of phenolic acids
Phenolic acids undergo dynamic processes of absorption, metabolic processing and excretion (Fig. 1), which determine their bioavailability. Free phenolic acids (such as caffeic acid) are partially absorbed in the stomach through passive diffusion, whereas conjugated phenolic acids require hydrolysis mediated by the colonic microbiota for biological activation. The metabolic processing of phenolic acids occurs in the colon and liver. Conjugated phenolic acids and macromolecular phenolic acids are initially metabolized in the colon and then reach the liver through the hepatic portal vein for further metabolism, where they undergo phase I metabolic reactions in the liver through structural modifications (hydroxylation, decarboxylation, and thiolation) followed by phase II binding reactions (glucuronidation, methylation, and sulfation). Structural modifications in phase I metabolism can activate or enhance the pharmacological activities of certain phenolic acids. Phase II binding reactions reduce the potential toxicity of phenolic acids and increase their hydrophilicity, promoting the excretion of water-soluble metabolites in bile or urine.41 Bulky conjugated metabolites are eliminated in bile, whereas smaller conjugated metabolites, such as monosulfate derivatives, are excreted in urine.42Evidence indicates significant inter-compound variation in the absorption, metabolism, and excretion profiles of different phenolic acids. Following jejunal perfusion, ferulic acid demonstrates an absorption rate of approximately 56%.43 Subsequent metabolism within colonic epithelial cells yields feruloyl glucuronide, sulfate conjugates, and dihydroferulic acid.44 Post-absorption, the majority of ferulic acid undergoes urinary excretion as glucuronide conjugates, while the free acid fraction enters the liver via the portal circulation for further biotransformation.45 Conjugated derivatives, specifically sulfoglucuronide forms, constitute up to 84% of the total urinary ferulic acid metabolites.46 Notably, fecal excretion of ferulic acid remains negligible irrespective of the administered dose.45 Protocatechuic acid is predominantly absorbed in the intestine47 and undergoes metabolism in both the liver and colon;48 its absorption across the gastric mucosa remains poorly characterized. Post-metabolism, protocatechuic acid and its metabolites—including the free acid, glucuronide, sulfate, and methylated conjugates—are excreted via both urinary and fecal routes in rodent and human models.49–51 Caffeic acid undergoes rapid systemic absorption. Detection of free caffeic acid in gastric effluent within 5 minutes following intraportal administration, reaching levels equivalent to 89.7% of the administered dose, indicates efficient absorption via this route.52 Conversely, perfusion studies revealed substantially lower intestinal absorption rates: 12.4% in the duodenum and 19.5% in the jejunoileum, respectively.53,54 Caffeic acid is subject to extensive hepatic metabolism.55–57 Within 72 hours post-administration, urinary excretion accounts for up to 68% of ingested caffeic acid derivatives, markedly exceeding the 4.6% recovered in feces.56 Human data indicate that only approximately 11% of the ingested dose is excreted renally as the unmetabolized parent compound.58 This suggests that the remainder undergoes elimination as conjugated or oxidized metabolites, potentially over a prolonged elimination phase.
3. Protective effects of phenolic acids on intestinal barrier function
The four major functional units of the intestinal mucosal barrier are biological barrier, chemical barriers, epithelial barrier and immune barriers. Studies have shown that phenolic acids and their derivatives play a protective role in intestinal barrier function. Specifically, phenolic acids increase the expression of tight junction proteins, thereby reducing intestinal permeability, promoting the secretion of intestinal mucus and serving as a “physical barrier” to prevent the invasion of pathogens. In addition, the antioxidant properties of phenolic acids help prevent further deterioration of the damaged intestinal barrier; at the same time, phenolic acids regulate the intestinal microbiota and immune response, jointly maintaining the integrity of the intestinal barrier. Their multifaceted effects highlight the potential of phenolic acids as therapeutic agents to maintain intestinal health (Table 1).| Phenolic acid | Structural formula | Model | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Chlorogenic acid |
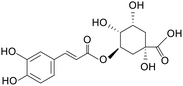
|
ICR mice treated with 3% DSS for 7 days | 250, 500 mg kg−1 | Increased expression of tight junction protein mRNA; reduced oxidative stress; inhibited release of inflammatory factors | 10 |
| BALB/c mice treated with 3% DSS for 7 days | 20, 40 mg kg−1 | Relieved colitis and inhibited IL-1β | 59 | ||
| C57BL/6 mice treated with 3% DSS for 5 days | 50 mg kg−1 | Reduced the polarization of M1 macrophages; reduced colon inflammation | 60 | ||
| C57BL/6 mice treated with 3% DSS for 8 days | 1000 μM | Significantly inhibited mRNA expression IL-1β | 61 | ||
| C57BL/6 mice treated with 2.5% DSS for 7 days | 1000 μM | Improved infiltration of T cells, neutrophils, and macrophages by inhibiting NF-κB signaling | 62 | ||
| C57BL/6 mice treated with 5% DSS for 7 days | 30, 60 and 120 mg/kg | Relieved oxidative stress; reduced the release of pro-inflammatory factors IL-1 and IL-6 | 63 | ||
| BALB/c mice treated with 40 mg mL−1 TNBS for 7 days | 20 mg kg−1 | Reduced neutrophil infiltration | 64 | ||
| C57BL/6 mice treated with high-fat chow for 12 weeks | 60 mg kg−1 | Increased expression of tight junction proteins; reduced abundance of harmful bacteria | 65 | ||
| Mice treated with white wine for 21 days | 60 mg kg−1 | Increased expression of tight junction proteins; regulated intestinal flora | 66 | ||
| Restraint stress stimulation rats, 21 days | 100 mg kg−1 | Increased tight junction protein expression; down-regulated TNF-α gene expression | 67 | ||
| C57BL/6 mice treated with 10 mg kg−1 SD | 30 and 60 mg kg−1 | The expression of tight junction proteins ZO-1 and occludin is up-regulated | 68 | ||
| BALB/c mice treated with IL-10 KO | 1, 3 and 50 mg kg−1 | Increased ratio of CD4+/CD8+ T cell subsets, prevented inflammation | 69 | ||
| LPS treatment of Caco-2 cells, 24 h | 35.28, 70.56 and 141.12 μM | Increased expression of tight junction protein, down-regulated expression of pro-inflammatory factors | 70 | ||
| 50 ng mL−1 LPS and 10 ng mL−1 IFN-γ were co-incubated with Caco-2 cells for 24 h | 100 μM | Inflammatory factors TNF-α, IL-8 and IL-6 are down-regulated | 71 | ||
| 2 mM H2O2 and 10 ng mL−1 TNF-α coincubation of Caco-2 cells | 500, 1000 and 2000 μM | Inhibited IL-8 secretion | 72 | ||
| Gallic acid |
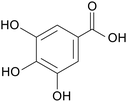
|
Mice treated with 2.5% DSS for 10 days | 20, 100 and 500 mg kg−1 | Increased abundance of beneficial bacteria; increased secretion of anti-inflammatory factors; reduced secretion of pro-inflammatory factors | 73 |
| Mice treated with 3.5% DSS for 10 days | 40, 80 and 120 mg kg−1 | Inhibited inflammatory body stimulation activation; reduced the expression of inflammatory factors | 74 | ||
| BALB/c mice treated with TNBS | 20, 40 and 60 mg kg−1 | Inhibited the NF-κB pathway; inhibited expression of inflammatory factors IL-1, IL-4, IL-6, etc. | 11 | ||
| SD rats fed a HFD for 112 days | 100 mg kg−1 | Increased tight junction protein expression; restored intestinal microbial diversity | 75 | ||
| Wistar rats treated with 40 mg kg−1 DMH for 14 days | 25 mg kg−1 | Reduced goblet cell damage; increased mucin secretion | 76 | ||
| Albino Wistar rats treated with 20 mg kg−1 DMH for 30 weeks | 50 mg kg−1 | Maintained antioxidant factor activity | 77 | ||
| 50 ng mL−1 LPS + 10 ng mL−1 IFN-γ-treated Caco-2 cell and RAW 264.7 cell co-culture model | 100 μM | Enhanced tight junction protein; reduced the expression of inflammatory factors | 78 | ||
| 10 μg mL−1 LPS treatment of Caco-2 cells | 5.88, 29.41 and 58.82 μM | Reduced oxidative stress; reduced inflammation | 79 | ||
| 25 ng mL−1 IL-1b or 50 ng ml−1 TNF-α or 10 mg mL−1 LPS treatment of Caco-2 cells for 24 h | 50 μM | Decreased NF-κB; reduced inflammation | 80 | ||
| Calcium ion depletion treatment of T84 cells | 200 µM | Promoted recombination of tight junction proteins ZO-1 and occludin | 81 | ||
| BALB/c mice treated with 2.5% DSS for 7 days | 10 mg kg−1 | Reduced oxidative stress; inhibited IL-21 and IL-23 expression | 82 | ||
| Caffeic acid |
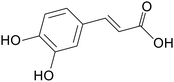
|
ICR mice treated with 3% DSS for 7 days | 251 mg kg−1 | Suppressed inflammation; increased expression of antioxidant factors | 12 |
| Piglets treated with 80 μg kg−1 LPS for 7 days | 500 mg kg−1 | Increased expression of tight junction protein mRNA; modulated intestinal microbial composition | 83 | ||
| PA treatment of LS174T cells | 500 µM | Increased the level of MUC2 | 84 | ||
| Ferulic acid |
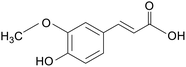
|
Piglets treated with 10 mg kg−1 LPS for 2 days | 4000 mg kg−1 | Increased expression of tight junction protein mRNA; altered gut microbial composition; reduced expression of inflammatory factors | 16 |
| Sprague Dawley rats treated with 100 mg kg−1 TNBS for 1 day | 10, 20 and 40 mg kg−1 | Decreased MDA and NO levels; increased antioxidant enzyme activity | 13 | ||
| Mice treated with 15 mg/LNaAsO2 for 1 day | 100 mg kg−1 | Reduced oxidative stress by activation of the Nrf2/HO-1 pathway; increased expression of tight junction proteins | 85 | ||
| C57BL/6 mice fed high-fat chow for 8 weeks | 50 mg kg−1 | Upregulated MUC2 mRNA expression | 86 | ||
| Wistar rats treated with 7% acetic acid | 20, 40 and 60 mg kg−1 | Decreased expression of inflammatory factors | 87 | ||
| SD rats treated with 100 mg kg−1 TNBS for 14 days | 10, 20 and 250 mg kg−1 | Inhibited the TXNIP/NLRP3 pathway; reduced expression of inflammatory factors | 88 | ||
| Heat stress treatment of IEC-6 cells for 6 hours | 5, 10 and 20 μM | Intercellular gaps became smaller, increased expression of tight junction proteins | 89 | ||
| PA treatment of LS174T cells | 500 µM | Increased the level of MUC2 | 90 | ||
| 50 ng mL−1 TNFα + 50 ng mL−1 IFNγ + 25 ng mL−1 IL- 1β + 10 µg mL−1 LPS mixed treatment of Caco-2 cells for 24 h | 50 μM | Attenuated pro-inflammatory responses in polarized intestinal epithelial cells via IRE1a and PERK pathways; reduced NO levels | 91 | ||
| 10 ng mL−1 TNF-α treated HIMECs | 125, 250 and 500 µM | Reduced expression of inflammatory factors | 92 | ||
| Protocatechuic acid |
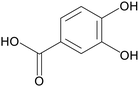
|
Piglets treated with 10 μg kg−1 LPS for 21 days | 4000 mg kg−1 | Upregulated tight junction protein mRNA expression; restored gut microbial diversity | 14 |
| Wistar rats treated with 5% DSS for 5 days | 10 mg kg−1 | Reduced oxidative stress; inhibited plasma inflammatory factor expression; inhibited elevated colonic myeloperoxidase activity | 93 | ||
| C57BL/6 mice treated with 3.5% DSS for 7 days | 5, 10 and 20 mg kg−1 | Inhibited inflammatory factor expression; increased expression of tight junction proteins; restored the relative abundance of intestinal flora; reduced oxidative stress | 94 | ||
| BALB/c mice treated with 20 mg mL−1 TNBS for 1 day | 30 and 60 mg kg−1 | Reduced oxidative stress; increased expression of antioxidant enzymes SOD, CAT; reduced expression of inflammatory factors | 95 | ||
| ETEC K88 infected IPEC-1 cell | 40 μM | Increased expression of tight junction proteins; downregulated inflammatory factor mRNA levels | 96 | ||
| Vanillic acid |
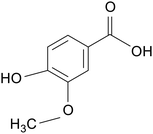
|
Piglets treated with 10 mg kg−1 LPS for 2 days | 4000 mg kg−1 | Reduced inflammation; restored intestinal flora diversity; enhanced tight junction protein mRNA expression | 97 |
| BALB/c mice treated with 5% DSS for 7 days | 200 mg kg−1 | Decreased IL-6 levels; inhibited NF-κB activation | 98 | ||
| PA treatment of LS174T cells | 500 µM | Increased the level of MUC2 | 99 | ||
| Sinapic acid |
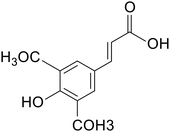
|
Kunming mice treated with 2% DSS for 7 days | 10 and 50 mg kg−1 | Reduced oxidative stress; reduced expression of inflammatory factors; reduced inflammatory vesicle expression | 15 |
| BALB/c mice treated with 30 mg kg−1 TNBS for 1 day | 10, 30 and 100 mg kg−1 | Decreased MPO activity and reduced MDA levels | 100 | ||
| 10 μmol mL−1 LPS treatment of Caco-2 cells | 5, 10 and 15 μM | Enhanced tight junction protein expression and redistribution of injured tight junction proteins | 101 | ||
| 20 μg mL−1 LPS and 20 ng mL−1 TNF-α mixed treatment of Caco-2 cells for 24 h | 12.5, 25 and 50 μM | Inhibited inflammatory factor expression; inhibited tight junction protein delocalization | 102 | ||
| p-Coumaric acid |
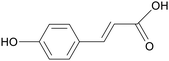
|
c57 mice treated with high-fat chow + STZ for 8 weeks | 200 mg kg−1 | Promoted mucus secretion; enhanced tight junction protein expression | 103 |
| PA treatment of LS174T cells | 500 µM | Increased the level of MUC2 | 104 | ||
| Rosmarinic acid |
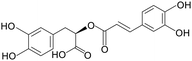
|
Mice treated with 4% DSS for 7 days | 5, 10 and 20 mg kg−1 | Increased cuprocytes, increased mucus secretion; inhibited inflammatory factor expression | 105 |
| Mice treated with 5% DSS for 7 days | 100 mg kg−1 | Regulated the composition of intestinal flora; suppressed inflammation | 106 | ||
| Coumaric acid |
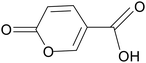
|
Wistar rats treated with 7% acetic acid for 1 day | 50, 100 and 150 mg kg−1 | Inhibited NF-κB expression; reduced inflammation | 107 |
| Wistar rats treated with 7% acetic acid for 1 day | 100 and 150 mg kg−1 | Reduced oxidative stress | 108 | ||
| Syringic acid |
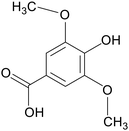
|
C57BL/6 mice treated with 2.5% DSS for 7 days | 50 mg kg−1 | Up-regulated antioxidant indicator expression; increased abundance of intestinal flora | 109 |
| Wistar rats treated with 7% acetic acid for 1 day | 10, 25 and 50 mg kg−1 | Reduced oxidative stress | 108 | ||
| 4-Hydroxybenzoic acid |
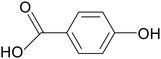
|
C57BL/6 mice treated with 2.5% DSS for 7 days | 100 mg kg−1 | Promoted mucus secretion | 110 |
| PA treatment of LS174T cells | 500 µM | Increased the level of MUC2 | 104 | ||
| Chicory root phenolic acid | Mice treated with 2.5% DSS | 100 and 200 mg kg−1 | Restored intestinal flora composition; reduced expression of inflammatory factors | 111 | |
| Salvinorin | C57BL/6 mice treated with 2% DSS for 7 days | 100 and 200 mg kg−1 | Suppressed inflammatory response | 112 |
3.1 Increased tight junction protein expression
3.2 Regulating intestinal mucus secretion
3.3 Antioxidant effects
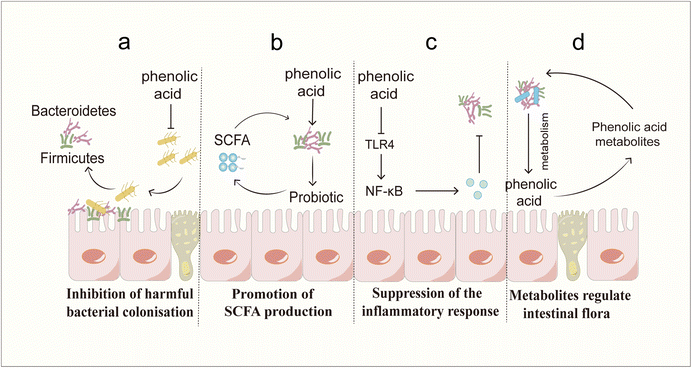
3.4 Regulating the balance of the intestinal flora
3.5 Effects on intestinal immune function
4. Application prospects of phenolic acids in treating intestinal diseases
Emerging evidence highlights the importance of intestinal permeability as a key indicator in the pathogenesis of multiple diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), gastrointestinal infections, and metabolic disorders such as obesity, diabetes and nonalcoholic fatty liver disease. The protective effects of phenolic acids on the intestinal barrier highlight their potential therapeutic applications in gastrointestinal diseases.4.1 Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a complex, multifactorial, chronic, recurring immune-mediated inflammatory disease of the gastrointestinal tract. The two most common forms are Crohn's disease (CD) and ulcerative colitis (UC). CD can affect the entire gastrointestinal tract and manifests as discontinuous transmural inflammation that occurs below the mucosa layer; UC is limited to mucosal inflammation, usually starting from the rectum and extending continuously to the proximal colon. Current treatment strategies for IBD include biologics, immunomodulators, aminosalicylates, and corticosteroids. However, due to the heterogeneity of the disease, the treatment effects vary.174 In addition, existing therapeutic drugs are often accompanied by multiple side effects, such as hyperglycaemia, hypertension, liver necrosis and pancreatitis, limiting their long-term use in patients with IBD. The pathogenesis of IBD is thought to involve multiple mechanisms, including inflammation, oxidative stress, intestinal barrier dysfunction, and intestinal flora dysfunction,175–178 among which impaired intestinal barrier function is an important feature of the disease. As natural plant metabolites, phenolic acids have almost no toxic side effects and have been shown to have a protective effect on the intestinal barrier, highlighting their potential in the treatment of IBD. Therefore, phenolic acids can be used as adjuvant or alternative therapeutic agents to develop safer treatments for IBD. DSS-induced colitis is a classic model of UC in animal experiments. Various phenolic acids and pharmaceutical foods rich in phenolic acids have been shown to have therapeutic effects on DSS-induced colitis in animals. Chlorogenic acid improves the pathological state of colitis in mice by reducing intestinal inflammation, activating the Nrf-2/HO-1 signalling pathway to reduce oxidative stress, and improving intestinal barrier function, as demonstrated by improvements in weight loss, shortening of the colon length and intestinal barrier damage caused by DSS.10 Caffeic acid regulates intestinal flora disorders related to colitis and restores the intestinal barrier to further reduce inflammatory cell infiltration in the colon and alleviate the course of colitis.12 Studies have shown that the consumption of natural products rich in phenolic acids, such as fruits, vegetables, tea, and traditional Chinese medicines, can effectively prevent and relieve typical symptoms of UC. Zheng Li et al. showed that purslane, which is rich in phenolic acids, repairs intestinal barrier damage by inhibiting the NF-κB signalling pathway, increasing the expression of the tight junction proteins claudin-1, occludin, and ZO-1, and increasing the diversity of the intestinal flora, alleviating DSS-induced ulcerative colitis.17 In patients with intestinal inflammation, eating fruits rich in phenolic acids can protect the intestinal barrier and reduce intestinal inflammation by regulating apoptosis and inflammatory factors.18 Given the limited clinical research data on phenolic acid monomers for treating inflammatory bowel disease (IBD), we attempted to extrapolate preclinical findings to humans to offer preliminary insights. However, it is important to note that phenolic acids undergo extensive microbial metabolism in the gut following oral administration. Substantial differences exist between the intestinal microbiota of animal models and humans, complicating the extrapolation of preclinical data. Elucidating species-specific microbial differences or addressing the impact of gut microbiota on drug metabolism may help mitigate these challenges.4.2 Irritable bowel syndrome
Irritable bowel syndrome is a very common functional digestive disease characterized by repeated abdominal pain, abdominal distension, cramps, flatulence, and altered bowel habits according to the Rome IV diagnostic criteria.179 The aetiology and mechanism of IBS are not fully understood but are believed to be caused by interactions among multiple factors, including visceral hypersensitivity, abnormal gastrointestinal motility, nervous system dysfunction, gastrointestinal infections, intestinal flora disorders, and psychological disorders. At present, a radical cure is not available for IBS. The purpose of treatment is to relieve symptoms and improve quality of life. Treatments include reducing food intolerance through diet modifications; medical intervention with anticonvulsants, antidiarrhoeal drugs, laxatives and antidepressants; and maintaining a healthy lifestyle. Studies have shown that some patients with IBS, especially those with the diarrhoea type (IBS-D) and postinfectious type (PI-IBS) subtypes, have impaired intestinal mucosal barrier function.180 A recent animal and human study has shown that inhibiting or restoring barrier dysfunction can correct visceral hypersensitivity reactions181 and pain, respectively, in individuals with irritable bowel syndrome.182 Most studies have shown that a loss of barrier function is positively correlated with symptom severity (such as abdominal pain and changes in bowel habits), suggesting that impaired intestinal mucosal barrier function may exacerbate the severity of IBS symptoms.183,184 When treating IBS, in addition to symptomatic drug treatment, treatment strategies for intestinal barrier dysfunction are sometimes considered. For example, probiotics and prebiotics are used to improve the composition of the intestinal microbiota, and certain drugs are used to enhance intestinal mucosal barrier function. In general, impaired intestinal mucosal barrier function appears to be part of the pathophysiological mechanism of IBS, and targeting this process may represent a new strategy to ameliorate IBS symptoms.Phenolic acids can improve symptoms related to irritable bowel syndrome. Studies have shown that irritable bowel syndrome is strongly associated with visceral pain and depression.185 The occurrence of visceral pain and depression complicated by IBS is related to increased expression of the P2X7 receptor. In a rat model of concurrent visceral pain and depression created by Lequan Wen et al., gallic acid reversed the increases in IL-1β and TNF-α levels and the decreases in IL-10 and BDNF levels caused by the increased expression of the P2X7 receptor in a comorbid model, alleviating the pain threshold and degree of depression in model rats.186 In patients with diarrhoea (IBS-D), the 5-HT3 receptor mediates the release of 5-HT from enterochromaffin (EC) cells, increasing the number of productive colonic contractions.187 5-HT3 receptor antagonists have been shown to be effective at inhibiting IBS-D emergencies, prolonging small and large intestine transit, and alleviating symptoms.188 Talley et al.189 and Steadman et al.190 showed that in patients with irritable bowel syndrome dominated by diarrhoea, the 5-HT3 antagonist ondansetron improved the faecal consistency by reducing rectal movement and sensitivity to balloon inflation in patients with irritable bowel syndrome. Chlorogenic acid is believed to be an antagonist of 5-HT3 and D2 receptors. In a 5-HT-induced IBS-D mouse model, a chlorogenic acid-rich methanol extract of lotus flowers was shown to ameliorate IBS-D by functioning as a 5-HT3 receptor antagonist.191 Depression is a common complication of IBS. The intake of phenolic acids can prevent the development of depressive symptoms, which is related mainly to the anti-inflammatory and antioxidant activities of phenolic acids. Ferulic acid192 and chlorogenic acid193 reduce the protein and mRNA expression of proinflammatory cytokines (IL-6, IL-1, and TNF-α) in depressed rats. In addition, ferulic acid increases the expression of the glucocorticoid receptor (GR) by reducing the serum levels of adrenocorticotropin (ACTH) and corticosterone and regulating the function of the hypothalamic–pituitary–adrenal (HPA) axis.192 In addition, phenolic acids can relieve symptoms of IBS disease by improving the intestinal barrier. CGA alleviates symptoms in mice with postinfectious irritable bowel syndrome (PI-IBS) by reducing inflammation and regulating the intestinal flora to maintain mucosal barrier function.194 In summary, phenolic acids can improve the symptoms of IBS complications and achieve the current treatment goals for IBS.
4.3 Intestinal infection
Intestinal infections are infectious diseases caused by a variety of bacteria, viruses, parasites or fungi and mainly present as digestive tract symptoms. Common intestinal infections include cholera, bacterial diarrhoea, viral hepatitis A, typhoid fever, infectious diarrhoea, and hand, foot and mouth disease. These diseases require urgent attention as they spread quickly, are highly contagious, and highly harmful. If effective prevention and control measures are not implemented in a timely manner, these diseases can easily lead to epidemics. Phenolic acids have shown anti-infective potential in vitro against microorganisms that cause various diseases. Therefore, we believe that phenolic acids have the potential to improve or treat infectious diseases caused by intestinal infections. Nontyphoid Salmonella enteritis (ST) is a prominent pathogen. During infection, ST can adhere to and subsequently invade multiple cell types in the gastrointestinal tract of human hosts, especially intestinal epithelial cells, which are important virulence factors of ST. Studies have shown that gallic acid and vanillic acid display antibacterial activity against ST through their effectiveness in preventing ST adhesion and invasion during the infection of epithelial cell lines.195Candida albicans affects the gastrointestinal tract, and systemic infections mainly originate from the host. Host-related factors can cause harmless Candida to easily transform into opportunistic pathogens, causing infection on the superficial mucosal surface.196 Natural extracts containing phenolic acids have been shown to have antifungal activity against Candida. The main virulence factors of Candida are the production of proteases, biomembrane formation, adhesion and dimorphism. Sung et al. found that caffeic acid derivatives have antibiofilm effects on Candida and can affect the dimorphism of Candida.197 In addition, protocatechuic acid shows broad-spectrum antibacterial activity against species such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus, Streptococcus pneumoniae, Acinetobacter pasteurii, and Helicobacter pylori.198,199 In summary, the antipathogenic microbial activity of phenolic acids makes them valuable for research and use in treating intestinal infectious diseases.4.4 Intestinal barrier-related metabolic diseases
The integrity of the intestinal barrier is crucial for maintaining systemic metabolic homeostasis and damage to this barrier is closely related to the occurrence and progression of multiple metabolic diseases, including diabetes, obesity, and fatty liver disease. Gut barrier dysfunction may trigger or exacerbate these metabolic disorders. Diabetes is one of the major chronic diseases worldwide. It is divided into two types: type 1 diabetes (T1DM) and type 2 diabetes (T2DM). T1DM is an autoimmune disease characterized by damage to pancreatic beta cells and reduced insulin secretion. Studies have shown that a loss of intestinal barrier integrity can cause the symbiotic intestinal microbiota to activate islet-responsive T cells, thereby promoting the development of autoimmune diabetes.200 T2DM is the most common form of diabetes and is characterized by insulin resistance and insufficient insulin secretion, with elevated blood sugar levels. Impaired intestinal barrier function affects the secretion of glucose-dependent insulin-releasing peptide (GIP) and glucagon-like peptide-1 (GLP-1), thereby promoting the occurrence or worsening of T2DM.201,202 Various treatment strategies have been reported to improve disease outcomes by restoring intestinal barrier integrity.203–205p-Coumaric acid ameliorated high-fat diet (HFD)-induced glucose intolerance and hyperglycemia through restoration of small intestinal barrier integrity and improved hepatic glucose handling.103 Phenolic acid-enriched Salvia miltiorrhiza aerial parts repaired ileocolonic mucosal architecture and attenuated hyperglycemia in diabetic murine models.206 Obesity is a complex chronic metabolic disease characterized by the excessive accumulation of adipose tissue in the body, leading to significant weight gain beyond the healthy range. The development of obesity is closely related to an imbalance in the intestinal flora and intestinal barrier damage. Multiple studies demonstrate that enhanced tight junction protein expression improves intestinal barrier dysfunction, thereby reducing lipid accumulation in hepatic and adipose tissues and ameliorating obesity.207,208 Norigool extract (NFE), which is rich in phenolic acids, significantly alleviates obesity induced by a high-fat diet by regulating the intestinal flora, improving intestinal barrier function, and inhibiting inflammatory reactions.209 Dietary ferulic acid supplementation mitigated high-fat diet (HFD)-induced weight gain and significantly reduced plasma lipid profiles, hepatic triglyceride accumulation, and systemic cholesterol levels in murine models.210 Fatty liver disease, characterized by excessive accumulation of fat in the liver, is one of the most common liver diseases worldwide. It is divided into two main categories: alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD). Damage to the intestinal barrier can allow endotoxins such as LPS to enter the blood, triggering systemic inflammatory responses and oxidative stress, thereby disrupting liver metabolism, increasing fat synthesis, reducing fatty acid oxidation, and leading to fat accumulation in the liver. Studies have shown that intestinal farnesol X receptor (FXR) signalling protects intestinal integrity and barrier function and that FXR deficiency in mice exacerbates AFLD.211 These findings highlight the importance of intestinal barrier integrity in the pathogenesis of fatty liver disease. Studies have shown that protection against NAFLD and AFLD is achieved by attenuating intestinal mucosal dysfunction, reducing intestinal mucosal permeability, and regulating intestinal innate immunity.212,213 Phenolic acids and foods rich in phenolic acids have been reported to repair the intestinal barrier and prevent or treat related metabolic diseases. For example, the phenolic acid-rich extract of Citrus trifoliata leaves maintains intestinal barrier integrity and regulates the intestinal flora, thereby alleviating oxidative stress, lipid accumulation, and inflammation in mice with NAFLD.214 Chlorogenic acid increases the relative abundance of beneficial bacteria and reduces alcoholic liver injury in ALD mice by promoting the production of N-butyric acid, which helps maintain the integrity of the intestinal barrier.215 Chlorogenic acid also ameliorates NAFLD by increasing the expression of ZO-1 and occludin and regulating the integrity of the intestinal barrier.65 In summary, maintaining intestinal barrier integrity is critical for overall metabolic health. Restoring intestinal barrier function is a promising treatment strategy for metabolic diseases. Phenolic acids and phenolic-rich foods provide potential benefits in this context by repairing the intestinal barrier and regulating the intestinal flora, thereby alleviating the symptoms of multiple metabolic disorders.5. Clinical applications and safety profiles of phenolic acids
5.1 Clinical applications
In a clinical trial enrolling 85 type-2 diabetic patients, oral administration of phenolic acid complexes (12 or 20 mg kg−1 every 48 hours for 6 months) significantly improved glycemic control in both dosage groups. However, serious adverse events – exclusively diarrhea – were reported in 13% and 16% of participants in the 12 mg kg−1 and 20 mg kg−1 groups, respectively.216 A randomized double-blind trial revealed that acute single-dose administration of 400 mg chlorogenic acid (4.8–6.8 mg kg−1 based on mean body weight 69.9 ± 12.7 kg) significantly reduced systolic and diastolic pressures.217 Similarly, six-week supplementation with grape seed extract (chlorogenic acid: 300 mg day−1 in divided doses) significantly lowered blood pressure in hypertensive patients while elevating plasma concentrations of phenolic acid metabolites, primarily hippuric acid and 3-hydroxyphenylacetic acid.218 Current evidence suggests lower phenolic acid doses confer physiological benefits, whereas higher doses may lead to adverse effects (e.g., diarrhea) in susceptible populations. Notably absent are clinical investigations evaluating phenolic acids’ effects on intestinal barrier function, leaving their therapeutic index undefined for gastrointestinal disorders. Most clinical studies assess phenolic acids through dietary matrices rather than standardized preparations. Despite attempts to extrapolate doses from food phenolic content, significant bioavailability disparities persist between whole-food matrices and purified phenolic acids due to three primary factors: food matrix interactions, digestive liberation kinetics, and variable microbial biotransformation dynamics. Consequently, food-derived dose approximations exhibit compromised translational validity for therapeutic applications, resulting in critical gaps in human dose–response data. To address these limitations, future clinical trials must prioritize four key objectives: establishing intestinal barrier enhancement dose–response relationships; determining maximum tolerated doses; characterizing pharmacokinetic and pharmacodynamic profiles; and validating clinical efficacy in gastrointestinal pathologies.5.2 Safety profiles
High-dose protocatechuic acid administration (500 mg kg−1, intraperitoneal) induced subclinical hepatorenal impairment in ICR mice, significantly elevating plasma ALT and urea levels, consistent with oxidative stress-mediated toxicity.219 Cumulative subcutaneous salicylic acid dosing (428 mg kg−1) produced embryotoxic effects in pregnant rats, manifested as minor skeletal malformations.220 Conversely, oral vanillic acid (1000 mg kg−1 day−1 × 14 days) in Wistar rats elicited no significant alterations in hematological or biochemical parameters, demonstrating favorable toxicological profiles at this dosage.221 Similarly, 2,4-dihydroxybenzoic acid (6000 mg day−1 × 16 days, oral gavage) was well tolerated in rats.222 Collectively, phenolic acid toxicity exhibits significant route dependency, with oral administration conferring substantially enhanced safety margins compared to parenteral routes (intraperitoneal/subcutaneous). Human trials consistently demonstrate excellent tolerability of high oral doses. Ferulic acid (1000 mg day−1 × 6 weeks) improved lipid parameters without adverse events or clinically significant hepatorenal biomarker deviations.223 Critical clinical considerations reveal that while orally administered phenolic acids exhibit minimal intrinsic toxicity, parenteral administration may present safety hazards in clinical settings. Importantly, comorbid populations demonstrate differential susceptibility to phenolic acids, as evidenced by dose-dependent adverse effects in diabetic patients.216 Furthermore, safety profiles in gastrointestinal pathologies remain unestablished, necessitating targeted clinical evaluation. Based on cumulative evidence, oral delivery represents the therapeutically optimal administration route. To address existing knowledge gaps, future investigations must: (1) establish safety thresholds in disease-specific populations; (2) characterize gastrointestinal disorder-associated risk profiles; and (3) quantify comparative bioavailability across administration routes.6. Discussion and outlook
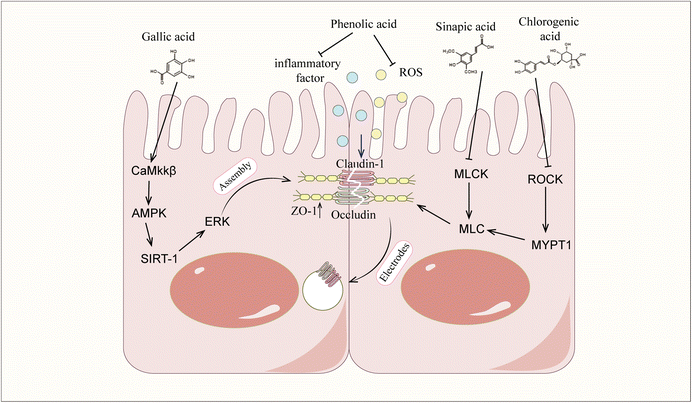
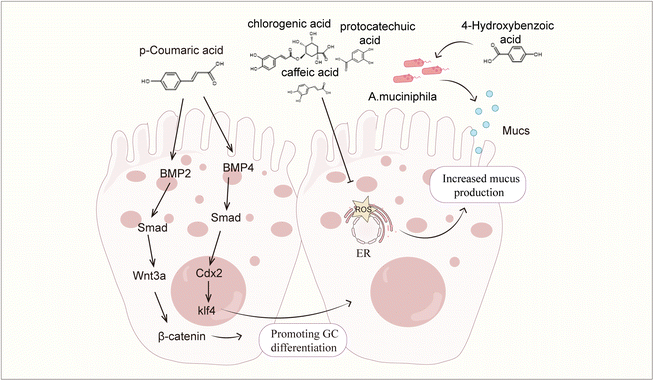
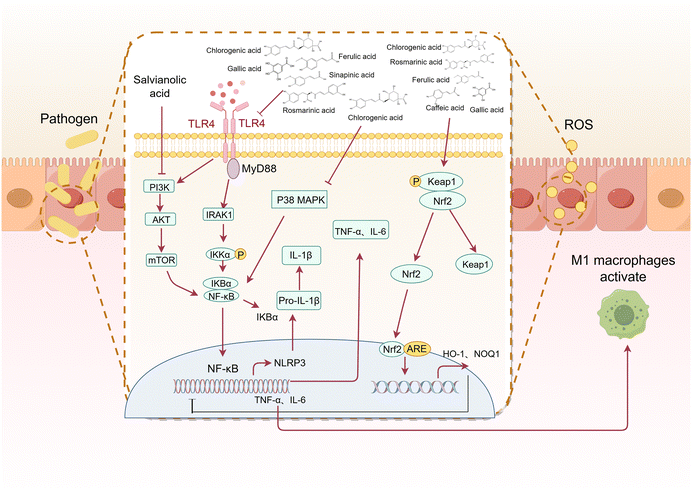
Phenolic acids have been shown to exert protective effects on the intestinal barrier through multiple mechanisms, and these effects can be systematically divided into direct and indirect modes of action. The direct mechanism of action involves the targeted regulation of intestinal barrier components, such as regulating the intestinal microbial composition by promoting the proliferation of beneficial bacteria and inhibiting the growth of conditioned pathogens; enhancing tight junction integrity through dual-pathway regulation/inhibition of the ROCK/MLC and MLCK/MLC signalling cascades; activating the CaMKKβ/AMPK/SIRT-1/ERK axis; upregulating tight junction proteins (claudin-1, claudin-2, occludin and ZO-1); preventing dissociation caused by occludin phosphorylation; and activating the BMP4/Smad1 pathway to stimulate goblet cell differentiation and subsequent MUC2 secretion, ensuring adequate mucus layer formation. Indirect protection appears to reduce barrier damage caused by inflammation and oxidative damage. Mechanistic studies have shown that phenolic acids inhibit the nuclear transport and transcriptional activity of NF-κB by inhibiting upstream regulatory factors such as the TLR4, PI3K and p38MAPK pathways, thereby reducing the production of proinflammatory cytokines. Phenolic acids activate the Nrf2/Keap1/HO-1 signalling axis to combat barrier dysfunction mediated by oxidative stress.Despite the fact that phenolic acid demonstrates better intestinal barrier protection, there are still some challenges in its application. The first is the issue of bioavailability. The bioavailability of phenolic acid compounds is generally poor, which limits their potential to exert biological activities in the body. Numerous in vivo studies have confirmed that phenolic acids are rapidly absorbed and metabolized in the gastrointestinal tract, resulting in low and inconsistent oral bioavailability.41,224–229 Structural modifications during metabolism also have important effects on the biological activity of phenolic acids. Studies have shown that both the methylation and demethylation of phenolic acids lead to a decrease in their antioxidant activity,230–232 which may be related to changes in the number and position of phenolic acid hydroxyl groups during metabolism. In addition to their structures, the form in which phenolic acids exist also affects their bioavailability. When these compounds are present in complex substrates (grains), their absorption and metabolism are inhibited.34,230,231 This may weaken the rapid protective effect of phenolic acids on the intestinal barrier, but has a greater advantage in improving chronic low-grade inflammation related barrier damage.233 Advanced delivery systems offer promising solutions: polycarboxylate-loaded gels increase local drug concentrations,234 calcium-loaded nanoparticles extend drug retention times in the colon,235 and glycerolipid complexes extend serum half-lives in rats.236 Despite these advances, challenges remain in optimizing colloidal delivery systems for clinical translation, requiring a comprehensive assessment of the bioavailability enhancement and safety. The complex dose–response relationship of phenolic acids is the second major challenge for their clinical application. The phenolic acid dose–response relationship shows great individual variability. In preclinical models, the administration of chlorogenic acid at 1 mg kg−1 and 500 mg kg−1 had anti-inflammatory effects but was not effective in all experimental animals.61,69 In diabetic patients, a certain amount of phenolic acids can improve blood sugar control, but individual responses vary.237,238 In addition, in vitro studies have confirmed a concentration-dependent vasodilatory effect of phenolic acids,239 but the vascular effect in vivo is not dose dependent.240 The wide range of therapeutic doses and unpredictable efficacy may stem from changes in microbial metabolism. Since most phenolic acids undergo biotransformation by colonic microorganisms before systemic absorption,237 differences in the microbiota composition between individuals have important impacts on metabolite profiles and pharmacological activity. These metabolic differences pose fundamental challenges in establishing clear dose–response relationships. Although the health benefits of phenolic acids have been observed, conclusive evidence for treatment remains difficult to obtain, and mechanistic research is needed to resolve dose–response uncertainties.
The intestinal barrier-protective effects of phenolic acids are closely associated with their bioavailability and antioxidant activity. However, within food matrices, phenolic acids frequently interact with various nutrients and non-nutrient components, which can either enhance or inhibit their bioavailability and antioxidant potential. Regarding nutrient interactions, β-casein in milk has been shown to reduce the antioxidant capacity of phenolic acids such as gallic acid, kaempferol, caffeic acid, ferulic acid, and chlorogenic acid by binding to hydroxyl groups on their phenolic rings, thereby masking active sites.241 Milk fat is considered a key factor in improving the bioaccessibility of chlorogenic acid from coffee. In contrast, caffeine and high-molecular-weight food components may reduce its bioaccessibility through complex formation with chlorogenic acid or pro-oxidant mechanisms, while calcium content exhibits no significant correlation with chlorogenic acid bioaccessibility.242 Clinical studies further indicate that milk proteins and fats significantly suppress the absorption efficiency and in vivo antioxidant activity of phenolic acids via binding interactions.243 Moreover, both flaxseed and soybean proteins have been found to impair the antioxidant capacity of phenolic acids, with soybean protein demonstrating a more pronounced inhibitory effect.244 Polysaccharides and dietary fiber, on the other hand, hinder the release of phenolic acids and attenuate their antioxidant effects by delaying gastrointestinal transit and engaging in molecular interactions.245,246 Vitamin E exhibits antagonistic effects toward chlorogenic acid activity,247 and vitamin B3 has been shown to inhibit the antioxidant activity of caffeic acid in both CUPRAC and Folin–Ciocalteu assays.248 As for non-nutrient components, carotenoids exert a bidirectional influence on the antioxidant capacity of phenolic acids: when carotenoids are predominant, the activity of phenolic acids is suppressed, whereas a higher proportion of phenolic acids leads to a synergistic enhancement effect with carotenoids.249 This phenomenon may be attributed to the ability of phenolic acids to facilitate the absorption of carotenoids at low concentrations, thereby indirectly amplifying their own antioxidant-related signaling capacity.250 The chemical structure of phenolic acids influences their synergistic effect with carotenoids. Compared with p-coumaric acid, caffeic acid, which possesses a catechol hydroxyl group, exhibits a stronger synergistic interaction with carotenoids, while p-coumaric acid tends to produce additive or mildly antagonistic effects.249 Additionally, non-phenolic constituents in willow bark (e.g., salicin and flavonoids) and phenolic acids in coffee display a complex bidirectional behavior in antioxidant activities: although the former may inhibit phenolic acid activity in certain contexts such as free radical scavenging and reduction reactions, they significantly enhance phenolic acid efficacy in processes such as enzyme inhibition, metal chelation, and hydroxyl radical scavenging.251 In summary, the development of functional foods rich in phenolic acids should strategically leverage synergistic interactions with other food components, while minimizing inhibitory factors through rational formulation design.
Collectively, phenolic acids exhibit substantial therapeutic promise and broad clinical applicability, yet they cannot supplant conventional synthetic pharmaceuticals. To establish clinical safety, comprehensive human trials must characterize in vivo metabolic fate and define safety profiles. A critical limitation remains their inherently poor systemic bioavailability, necessitating advanced delivery system development to enhance bioavailability and targeted food-processing technologies to improve bioaccessibility of matrix-bound compounds. Particularly limited are clinical validations of preclinical findings regarding intestinal barrier protection. Therefore, definitive clinical trials are imperative.
Author contributions
Li Xia: conceptualization, data curation, and writing – original draft. Xiulian Lin: writing – review and editing and conceptualization. Yuanjiao Zhou: writing – review and editing and data curation. Yamei Li: writing – review and editing and supervision. Yingyan Liao: writing – review and editing. Yan Lin: writing – review and editing. Limei Lin: writing – review and editing and supervision. Ping Wu: writing – review and editing and funding acquisition. Jingchen Xie: writing – review and editing and supervision. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interests.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the Provincial Natural Science Foundation Project (2023JJ604787) and the Joint Cultivation Base for Postgraduates’ Top Innovative Talents in Hunan Province (Xiangtong [2023] No. 372).References
- B. Singh, J. P. Singh, A. Kaur and N. Singh, Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel, Food Res. Int., 2020, 132, 109114 CrossRef CAS.
- X. Yang, W. Lan and X. Sun, Antibacterial and Antioxidant Properties of Phenolic Acid Grafted Chitosan and Its Application in Food Preservation: A Review, Food Chem., 2023, 428, 136788 CrossRef CAS.
- A. F. Afonso, O. R. Pereira and S. M. Cardoso, Health-Promoting Effects of Thymus Phenolic-Rich Extracts: Antioxidant, Anti-Inflammatory and Antitumoral Properties, Antioxidants, 2020, 9(9), 814 CrossRef CAS PubMed.
- P. Oka, H. Parr, B. Barberio, C. J. Black, E. V. Savarino and A. C. Ford, Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta-Analysis, Lancet Gastroenterol. Hepatol., 2020, 5(10), 908–917 CrossRef PubMed.
- G. G. Kaplan, The Global Burden of IBD: From 2015 to 2025, Nat. Rev. Gastroenterol. Hepatol., 2015, 12(12), 720–727 CrossRef PubMed.
- Y. Zhang, Y. Zhang, Z. Chen, Z. Jia, Y. Yu, J. Wang and H. Liang, Global Burden, Subtype, Risk Factors and Etiological Analysis of Enteric Infections from 1990–2021: Population Based Study, Front. Cell. Infect. Microbiol., 2025, 15, 1527765 CrossRef PubMed.
- K. Parikh, A. Antanaviciute, D. Fawkner-Corbett, M. Jagielowicz, A. Aulicino, C. Lagerholm, S. Davis, J. Kinchen, H. H. Chen, N. K. Alham, N. Ashley, E. Johnson, P. Hublitz, L. Bao, J. Lukomska, R. S. Andev, E. Björklund, B. M. Kessler, R. Fischer, R. Goldin, H. Koohy and A. Simmons, Colonic Epithelial Cell Diversity in Health and Inflammatory Bowel Disease, Nature, 2019, 567(7746), 49–55 CrossRef CAS PubMed.
- G. Barbara, M. Grover, P. Bercik, M. Corsetti, U. C. Ghoshal, L. Ohman and M. Rajilić-Stojanović, Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome, Gastroenterology, 2019, 156(1), 46–58 CrossRef.
- M. A. Odenwald and J. R. Turner, The Intestinal Epithelial Barrier: A Therapeutic Target?, Nat. Rev. Gastroenterol. Hepatol., 2017, 14(1), 9–21 CrossRef CAS.
- F. Wan, X. Cai, M. Wang, L. Chen, R. Zhong, L. Liu, B. Yi, F. Hou and H. Zhang, Chlorogenic Acid Supplementation Alleviates Dextran Sulfate Sodium (DSS)-Induced Colitis via Inhibiting Inflammatory Responses and Oxidative Stress, Improving Gut Barrier Integrity and Nrf-2/HO-1 Pathway, J. Funct. Foods, 2021, 87, 104808 CrossRef CAS.
- L. Zhu, P. Gu and H. Shen, Gallic Acid Improved Inflammation via NF-κB Pathway in TNBS-Induced Ulcerative Colitis, Int. Immunopharmacol., 2019, 67, 129–137 CrossRef CAS.
- F. Wan, R. Zhong, M. Wang, Y. Zhou, Y. Chen, B. Yi, F. Hou, L. Liu, Y. Zhao, L. Chen and H. Zhang, Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice, Front. Microbiol., 2021, 12, 784211 CrossRef.
- S. S. Sadar, N. S. Vyawahare and S. L. Bodhankar, Ferulic Acid Ameliorates TNBS-Induced Ulcerative Colitis through Modulation of Cytokines, Oxidative Stress, iNOs, COX-2, and Apoptosis in Laboratory Rats, EXCLI J., 2016, 15, 482–499 Search PubMed.
- R. Hu, Z. He, M. Liu, J. Tan, H. Zhang, D.-X. Hou, J. He and S. Wu, Dietary Protocatechuic Acid Ameliorates Inflammation and Up-Regulates Intestinal Tight Junction Proteins by Modulating Gut Microbiota in LPS-Challenged Piglets, J. Anim. Sci. Biotechnol., 2020, 11(1), 92 CrossRef CAS PubMed.
- B. Qian, C. Wang, Z. Zeng, Y. Ren, D. Li and J.-L. Song, Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice, Oxid. Med. Cell. Longevity, 2020, 2020, 1–13 Search PubMed.
- R. Hu, S. Wu, B. Li, J. Tan, J. Yan, Y. Wang, Z. Tang, M. Liu, C. Fu, H. Zhang and J. He, Dietary Ferulic Acid and Vanillic Acid on Inflammation, Gut Barrier Function and Growth Performance in Lipopolysaccharide-Challenged Piglets, Anim. Nutr., 2022, 8, 144–152 CrossRef CAS.
- Z. Li, T. Chu, X. Sun, S. Zhuang, D. Hou, Z. Zhang, J. Sun, Y. Liu, J. Li and Y. Bian, Polyphenols-Rich Portulaca Oleracea L. (Purslane) Alleviates Ulcerative Colitis through Restiring the Intestinal Barrier, Gut Microbiota and Metabolites, Food Chem., 2025, 468, 142391 CrossRef CAS.
- L. Lavefve, L. R. Howard and F. Carbonero, Berry Polyphenols Metabolism and Impact on Human Gut Microbiota and Health, Food Funct., 2020, 11(1), 45–65 RSC.
- A. P. G. Da Silva, W. G. Sganzerla, O. D. John and R. Marchiosi, A Comprehensive Review of the Classification, Sources, Biosynthesis, and Biological Properties of Hydroxybenzoic and Hydroxycinnamic Acids, Phytochem. Rev., 2023, 24, 1061–1090 CrossRef.
- J. León, V. Shulaev, N. Yalpani, M. A. Lawton and I. Raskin, Benzoic Acid 2-Hydroxylase, a Soluble Oxygenase from Tobacco, Catalyzes Salicylic Acid Biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 1995, 92(22), 10413–10417 CrossRef PubMed.
- M. Freitas, D. Ribeiro, J. S. Janela, C. L. Varela, S. C. Costa, E. T. Da Silva, E. Fernandes and F. M. F. Roleira, Plant-Derived and Dietary Phenolic Cinnamic Acid Derivatives: Anti-Inflammatory Properties, Food Chem., 2024, 459, 140080 CrossRef CAS PubMed.
- R. M. Muir, A. M. Ibáñez, S. L. Uratsu, E. S. Ingham, C. A. Leslie, G. H. McGranahan, N. Batra, S. Goyal, J. Joseph, E. D. Jemmis and A. M. Dandekar, Mechanism of Gallic Acid Biosynthesis in Bacteria (Escherichia Coli) and Walnut (Juglans Regia), Plant Mol. Biol., 2011, 75(6), 555–565 CrossRef CAS PubMed.
- L. M. O. Monteiro, C. Del Cerro, T. Kijpornyongpan, A. Yaguchi, A. Bennett, B. S. Donohoe, K. J. Ramirez, A. F. Benson, H. D. Mitchell, S. O. Purvine, L. M. Markillie, M. C. Burnet, K. J. Bloodsworth, B. P. Bowen, T. V. Harwood, K. Louie, T. Northen and D. Salvachúa, Metabolic Profiling of Two White-Rot Fungi during 4-Hydroxybenzoate Conversion Reveals Biotechnologically Relevant Biosynthetic Pathways, Commun. Biol., 2025, 8(1), 224 CrossRef CAS.
- J. Barros, J. C. Serrani-Yarce, F. Chen, D. Baxter, B. J. Venables and R. A. Dixon, Role of Bifunctional Ammonia-Lyase in Grass Cell Wall Biosynthesis, Nat. Plants, 2016, 2(6), 16050 CrossRef CAS.
- J. Barros, L. Escamilla-Trevino, L. Song, X. Rao, J. C. Serrani-Yarce, M. D. Palacios, N. Engle, F. K. Choudhury, T. J. Tschaplinski, B. J. Venables, R. Mittler and R. A. Dixon, 4-Coumarate 3-Hydroxylase in the Lignin Biosynthesis Pathway Is a Cytosolic Ascorbate Peroxidase, Nat. Commun., 2019, 10(1), 1994 CrossRef PubMed.
- R. B. Nair, K. L. Bastress, M. O. Ruegger, J. W. Denault and C. Chapple, The Arabidopsis Thaliana REDUCED EPIDERMAL FLUORESCENCE1 Gene Encodes an Aldehyde Dehydrogenase Involved in Ferulic Acid and Sinapic Acid Biosynthesis, Plant Cell, 2004, 16(2), 544–554 CrossRef CAS.
- K. Ou and L. Gu, Absorption and Metabolism of Proanthocyanidins, J. Funct. Foods, 2014, 7, 43–53 CrossRef CAS.
- H. Gui, L. Sun, R. Liu, X. Si, D. Li, Y. Wang, C. Shu, X. Sun, Q. Jiang, Y. Qiao, B. Li and J. Tian, Current Knowledge of Anthocyanin Metabolism in the Digestive Tract: Absorption, Distribution, Degradation, and Interconversion, Crit. Rev. Food Sci. Nutr., 2023, 63(22), 5953–5966 CrossRef CAS PubMed.
- H. G. Ulusoy and N. Sanlier, A Minireview of Quercetin: From Its Metabolism to Possible Mechanisms of Its Biological Activities, Crit. Rev. Food Sci. Nutr., 2020, 60(19), 3290–3303 CrossRef CAS PubMed.
- J. Tan, Y. Li, D.-X. Hou and S. Wu, The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity, Antioxidants, 2019, 8(10), 479 CrossRef CAS PubMed.
- L. Ho, D. Zhao, K. Ono, K. Ruan, I. Mogno, M. Tsuji, E. Carry, J. Brathwaite, S. Sims, T. Frolinger, S. Westfall, P. Mazzola, Q. Wu, K. Hao, T. E. Lloyd, J. E. Simon, J. Faith and G. M. Pasinetti, Heterogeneity in Gut Microbiota Drive Polyphenol Metabolism That Influences α-Synuclein Misfolding and Toxicity, J. Nutr. Biochem., 2019, 64, 170–181 CrossRef CAS PubMed.
- J. P. N. Rosazza, Z. Huang, L. Dostal, T. Volm and B. Rousseau, Review: Biocatalytic Transformations of Ferulic Acid: An Abundant Aromatic Natural Product, J. Ind. Microbiol., 1995, 15(6), 457–471 CrossRef CAS PubMed.
- J. C. Dacre and R. T. Williams, The Role of the Tissues and Gut Micro-Organisms in the Metabolism of [14C]Protocatechuic Acid in the Rat. Aromatic Dehydroxylation, J. Pharm. Pharmacol., 1968, 20(8), 610–618 CrossRef CAS PubMed.
- M. N. Clifford, A. Kerimi and G. Williamson, Bioavailability and Metabolism of Chlorogenic Acids (Acyl–quinic Acids) in Humans, Compr. Rev. Food Sci. Food Saf., 2020, 19(4), 1299–1352 CrossRef CAS PubMed.
- P.-V. Gemiises , [Jber das Vorkommen von Methyl- und Ethylestern der Hydroxyzimts∼iuren und Hydroxybenzoes∼iuren im Gemiise.
- H. Abe and S. Marumo, Identification of Auxin-Active Substances as Ethyl Chlorogenate and Indolyl-3-Acetic Acid in Immature Seeds of Helianthus Annuus, Agric. Biol. Chem., 1972, 36(1), 42–46 CrossRef CAS.
- B. Schuster and K. Herrmann, Hydroxybenzoic and Hydroxycinnamic Acid Derivatives in Soft Fruits, Phytochemistry, 1985, 24(11), 2761–2764 CrossRef CAS.
- U. Dirks and K. Herrmann, Hochleistungsfliissigkeitschromatographie der Hydroxycinnamoylchinasiiuren und der 4-(/-l)-Glucopyranosyloxy)-benzoesiiure in Gewiirzen.
- S. Klick and K. Herrmann, Glucosides and Glucose Esters of Hydroxybenzoic Acids in Plants, Phytochemistry, 1988, 27(7), 2177–2180 CrossRef CAS.
- S. Klick and K. Herrmann, Bestimmung von Hydroxybenzoesiiure-Verbindungen in Gewiirzen und weiteren pflanzlichen Lebensmitteln.
- A. Crozier, D. Del Rio and M. N. Clifford, Bioavailability of Dietary Flavonoids and Phenolic Compounds, Mol. Aspects Med., 2010, 31(6), 446–467 CrossRef CAS PubMed.
- L. C. Bourne and C. A. Rice-Evans, Urinary Detection of Hydroxycinnamates and Flavonoids in Humans after High Dietary Intake of Fruit, Free Radic. Res., 1998, 28(4), 429–438 CrossRef CAS PubMed.
- A. Adam, M. Leuillet, V. Crespy, M.-A. Levrat-Verny, F. Leenhardt, C. Demigné and C. Rémésy, The Bioavailability of Ferulic Acid Is Governed Primarily by the Food Matrix Rather than Its Metabolism in Intestine and Liver in Rats, J. Nutr., 2002, 132(7), 1962–1968 CrossRef CAS PubMed.
- L. Poquet, M. N. Clifford and G. Williamson, Transport and Metabolism of Ferulic Acid through the Colonic Epithelium, Drug Metab. Dispos., 2008, 36(1), 190–197 CrossRef CAS PubMed.
- Z. Zhao, Y. Egashira and H. Sanada, Ferulic Acid Sugar Esters Are Recovered in Rat Plasma and Urine Mainly as the Sulfoglucuronide of Ferulic Acid, J. Nutr., 2003, 133(5), 1355–1361 CrossRef CAS PubMed.
- L. Rondini, M.-N. Peyrat-Maillard, A. Marsset-Baglieri and C. Berset, Sulfated Ferulic Acid Is the Main in Vivo Metabolite Found after Short-Term Ingestion of Free Ferulic Acid in Rats, J. Agric. Food Chem., 2002, 50(10), 3037–3041 CrossRef CAS PubMed.
- J. Fang, Some Anthocyanins Could Be Efficiently Absorbed across the Gastrointestinal Mucosa: Extensive Presystemic Metabolism Reduces Apparent Bioavailability, J. Agric. Food Chem., 2014, 62(18), 3904–3911 CrossRef CAS PubMed.
- R. M. De Ferrars, C. Czank, Q. Zhang, N. P. Botting, P. A. Kroon, A. Cassidy and C. D. Kay, The Pharmacokinetics of Anthocyanins and Their Metabolites in Humans, Br. J. Pharmacol., 2014, 171(13), 3268–3282 CrossRef CAS PubMed.
- Y. Cao, L. Zhang, C. Ma, B. Chang, Y.-C. Chen, Y. Tang, X. Liu and X. Liu, Metabolism of Protocatechuic Acid Influences Fatty Acid Oxidation in Rat Heart: New Anti-Angina Mechanism Implication, Biochem. Pharmacol., 2009, 77(6), 1096–1104 CrossRef CAS.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: Food Sources and Bioavailability, Am. J. Clin. Nutr., 2004, 79(5), 727–747 CrossRef CAS PubMed.
- J. Zheng, H. Xiong, Q. Li, L. He, H. Weng, W. Ling and D. Wang, Protocatechuic Acid from Chicory Is Bioavailable and Undergoes Partial Glucuronidation and Sulfation in Healthy Humans, Food Sci. Nutr., 2019, 7(9), 3071–3080 CrossRef CAS PubMed.
- Y. Konishi, Z. Zhao and M. Shimizu, Phenolic Acids Are Absorbed from the Rat Stomach with Different Absorption Rates, J. Agric. Food Chem., 2006, 54(20), 7539–7543 CrossRef CAS PubMed.
- S.-J. Wang, J. Zeng, B.-K. Yang and Y.-M. Zhong, Bioavailability of Caffeic Acid in Rats and Its Absorption Properties in the Caco-2 Cell Model, Pharm. Biol., 2014, 52(9), 1150–1157 CrossRef CAS.
- S. Lafay, C. Morand, C. Manach, C. Besson and A. Scalbert, Absorption and Metabolism of Caffeic Acid and Chlorogenic Acid in the Small Intestine of Rats, Br. J. Nutr., 2006, 96(1), 39–46 CrossRef CAS PubMed.
- X. Wang, W. Li, X. Ma, Y. Chu, S. Li, J. Guo, Y. Jia, S. Zhou, Y. Zhu and C. Liu, Simultaneous Determination of Caffeic Acid and Its Major Pharmacologically Active Metabolites in Rat Plasma by LC–MS/MS and Its Application in Pharmacokinetic Study, Biomed. Chromatogr., 2015, 29(4), 552–559 CrossRef CAS PubMed.
- M. H. Omar, W. Mullen, A. Stalmach, C. Auger, J.-M. Rouanet, P.-L. Teissedre, S. T. Caldwell, R. C. Hartley and A. Crozier, Absorption, Disposition, Metabolism, and Excretion of [3-14 C]Caffeic Acid in Rats, J. Agric. Food Chem., 2012, 60(20), 5205–5214 CrossRef CAS PubMed.
- K. Azuma, K. Ippoushi, M. Nakayama, H. Ito, H. Higashio and J. Terao, Absorption of Chlorogenic Acid and Caffeic Acid in Rats after Oral Administration, J. Agric. Food Chem., 2000, 48(11), 5496–5500 CrossRef CAS PubMed.
- M. R. Olthof, M. B. Katan and P. C. H. Hollman, Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans, J. Nutr., 2001, 131(1), 66–71 CrossRef CAS PubMed.
- J. Zeng, D. Zhang, X. Wan, Y. Bai, C. Yuan, T. Wang, D. Yuan, C. Zhang and C. Liu, Chlorogenic Acid Suppresses miR–155 and Ameliorates Ulcerative Colitis through the NF–κB/NLRP3 Inflammasome Pathway, Mol. Nutr. Food Res., 2020, 64(23), 2000452 CrossRef CAS.
- M. Ghasemi-Dehnoo, H. Amini-Khoei, Z. Lorigooini, M. AnjomShoa and M. Rafieian-Kopaei, Ferulic Acid Ameliorates Ulcerative Colitis in a Rat Model via the Inhibition of Two LPS-TLR4-NF-κB and NF-κB-INOS-NO Signaling Pathways and Thus Alleviating the Inflammatory, Oxidative and Apoptotic Conditions in the Colon Tissue, Inflammopharmacology, 2023, 31(5), 2587–2597 CrossRef CAS.
- H. S. Shin, H. Satsu, M.-J. Bae, Z. Zhao, H. Ogiwara, M. Totsuka and M. Shimizu, Anti-Inflammatory Effect of Chlorogenic Acid on the IL-8 Production in Caco-2 Cells and the Dextran Sulphate Sodium-Induced Colitis Symptoms in C57BL/6 Mice, Food Chem., 2015, 168, 167–175 CrossRef CAS.
- Z. Zhang, X. Wu, S. Cao, M. Cromie, Y. Shen, Y. Feng, H. Yang and L. Li, Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice, Nutrients, 2017, 9(7), 677 CrossRef PubMed.
- W. Gao, C. Wang, L. Yu, T. Sheng, Z. Wu, X. Wang, D. Zhang, Y. Lin and Y. Gong, Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway, BioMed Res. Int., 2019, 2019, 1–13 Search PubMed.
- H. Zatorski, M. Sałaga, M. Zielińska, A. Piechota-Polańczyk, K. Owczarek, R. Kordek, U. Lewandowska, C. Chen and J. Fichna, Experimental Colitis in Mice Is Attenuated by Topical Administration of Chlorogenic Acid, Naunyn-Schmiedeberg's Arch. Pharmacol., 2015, 388(6), 643–651 CrossRef CAS.
- A. Shi, T. Li, Y. Zheng, Y. Song, H. Wang, N. Wang, L. Dong and H. Shi, Chlorogenic Acid Improves NAFLD by Regulating Gut Microbiota and GLP-1, Front. Pharmacol., 2021, 12, 693048 CrossRef CAS PubMed.
- H. Zhu, W. Jiang, C. Liu, C. Wang, B. Hu, Y. Guo, Y. Cheng and H. Qian, Ameliorative Effects of Chlorogenic Acid on Alcoholic Liver Injury in Mice via Gut Microbiota Informatics, Eur. J. Pharmacol., 2022, 928, 175096 CrossRef CAS.
- Y. Zhao, C. Wang, T. Yang, G. Feng, H. Tan, X. Piao, D. Chen, Y. Zhang, W. Jiao, Y. Chen, J. Sha and H. Fan, Chlorogenic Acid Alleviates Chronic Stress-Induced Intestinal Damage by Inhibiting the P38MAPK/NF-κB Pathway, J. Agric. Food Chem., 2023, 71(24), 9381–9390 CrossRef CAS PubMed.
- H. Zhu, F. Shen, X. Wang, H. Qian and Y. Liu, Chlorogenic Acid Improves the Cognitive Deficits of Sleep-Deprived Mice via Regulation of Immunity Function and Intestinal Flora, Phytomedicine, 2024, 123, 155194 CrossRef CAS PubMed.
- Y. M. Lee, D. W. Shin and B. O. Lim, Chlorogenic Acid Improves Symptoms of Inflammatory Bowel Disease in Interleukin-10 Knockout Mice, J. Med. Food, 2020, 23(10), 1043–1053 CrossRef CAS.
- H. Wu, T. Luo, Y. M. Li, Z. P. Gao, K. Q. Zhang, J. Y. Song, J. S. Xiao and Y. P. Cao, Granny Smith Apple Procyanidin Extract Upregulates Tight Junction Protein Expression and Modulates Oxidative Stress and Inflammation in Lipopolysaccharide-Induced Caco-2 Cells, Food Funct., 2018, 9(6), 3321–3329 RSC.
- K. Mu and D. D. Kitts, Gallic Acid Mitigates Intestinal Inflammation and Loss of Tight Junction Protein Expression Using a 2D-Caco-2 and RAW 264.7 Co-Culture Model, Arch. Biochem. Biophys., 2024, 756, 109978 CrossRef CAS.
- H. S. Shin, H. Satsu, M.-J. Bae, Z. Zhao, H. Ogiwara, M. Totsuka and M. Shimizu, Anti-Inflammatory Effect of Chlorogenic Acid on the IL-8 Production in Caco-2 Cells and the Dextran Sulphate Sodium-Induced Colitis Symptoms in C57BL/6 Mice, Food Chem., 2015, 168, 167–175 CrossRef CAS PubMed.
- J. Peng, T. Liu, P. Meng, Y. Luo, S. Zhu, Y. Wang, M. Ma, J. Han, J. Zhou, X. Su, S. Li, C.-T. Ho and C. Lu, Gallic Acid Ameliorates Colitis by Trapping Deleterious Metabolite Ammonia and Improving Gut Microbiota Dysbiosis, mBio, 2024, 15(2), e02752–e02723 CrossRef.
- T.-Y. Yu, Y.-M. Feng, W.-S. Kong, S.-N. Li, X.-J. Sun, G. Zhou, R.-F. Xie and X. Zhou, Gallic Acid Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice via Inhibiting NLRP3 Inflammasome, Front. Pharmacol., 2023, 14, 1095721 CrossRef CAS PubMed.
- S. Marinho, M. Illanes, J. Ávila-Román, V. Motilva and E. Talero, Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome, Biomolecules, 2021, 11(2), 162 CrossRef CAS PubMed.
- A. Shree, J. Islam, A. Vafa, S. Mohammad Afzal and S. Sultana, Gallic Acid Prevents 1, 2-Dimethylhydrazine Induced Colon Inflammation, Toxicity, Mucin Depletion, and Goblet Cell Disintegration, Environ. Toxicol., 2020, 35(6), 652–664 CrossRef CAS PubMed.
- J. S. Giftson, S. Jayanthi and N. Nalini, Chemopreventive Efficacy of Gallic Acid, an Antioxidant and Anticarcinogenic Polyphenol, against 1,2-Dimethyl Hydrazine Induced Rat Colon Carcinogenesis, Invest. New Drugs, 2010, 28(3), 251–259 CrossRef CAS.
- K. Mu and D. D. Kitts, Gallic Acid Mitigates Intestinal Inflammation and Loss of Tight Junction Protein Expression Using a 2D-Caco-2 and RAW 264.7 Co-Culture Model, Arch. Biochem. Biophys., 2024, 756, 109978 CrossRef CAS.
- C. Chu, H. Ru, Y. Chen, J. Xu, C. Wang and Y. Jin, Gallic Acid Attenuates LPS-Induced Inflammation in Caco-2 Cells by Suppressing the Activation of the NF-κB/MAPK Signaling Pathway, Acta Biochim. Biophys. Sin., 2024, 56(6), 905–915 CAS.
- B. Romier, J. Van De Walle, A. During, Y. Larondelle and Y.-J. Schneider, Modulation of Signalling Nuclear Factor-κB Activation Pathway by Polyphenols in Human Intestinal Caco-2 Cells, Br. J. Nutr., 2008, 100(03), 542–551 CrossRef CAS PubMed.
- W. Wachiradejkul, P. Sukmak, S. Treveeravoot, L. Yurasakpong, N. Rangchaikul, P. Chatkul, P. Supapol, A. Arinno, N. Teansuk, J. Inchai, S. Phummisutthigoon, M. Phongjit, A. Loungjan, N. Akrimajirachoote, W. Poolsri, C. Aonbangkhen, R. Khumjiang, C. Muanprasat, C. S. Vaddhanaphuti and P. Pongkorpsakol, Enhancing Intestinal Tight Junction Assembly by Gallic Acid as a Subcellular Basis for the Pharmacological Effect of Ocimum Sanctum L, Flower Aqueous Extract, J. Funct. Foods, 2024, 122, 106519 CrossRef CAS.
- A. K. Pandurangan, M. Norhaizan, N. Mohebali and L. C. Yeng, Gallic Acid Attenuates Dextran Sulfate Sodium-Induced Experimental Colitis in BALB/c Mice, Drug Des., Dev. Ther., 2015, 3923 CrossRef PubMed.
- X. Wen, F. Wan, Y. Wu, L. Liu, Y. Liu, R. Zhong, L. Chen and H. Zhang, Caffeic Acid Supplementation Ameliorates Intestinal Injury by Modulating Intestinal Microbiota in LPS-Challenged Piglets, Food Funct., 2023, 14(16), 7705–7717 RSC.
- L. Song, T. Wu, L. Zhang, J. Wan and Z. Ruan, Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways, Food Funct., 2022, 13(8), 4562–4575 RSC.
- S. Wang, Y. Hong, Y. Li, Z. Zhang, J. Han, Z. Yang, Y. Yang, Z. Ma and Q. Wang, Ferulic Acid Inhibits Arsenic–Induced Colon Injury by Improving Intestinal Barrier Function, Environ. Toxicol., 2024, 39(11), 4821–4831 CrossRef CAS PubMed.
- H.-J. Hwang, S. R. Lee, J.-G. Yoon, H.-R. Moon, J. Zhang, E. Park, S.-I. Yoon and J. A. Cho, Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells, Antioxidants, 2022, 11(8), 1448 CrossRef CAS PubMed.
- M. Ghasemi-Dehnoo, H. Amini-Khoei, Z. Lorigooini, M. AnjomShoa and M. Rafieian-Kopaei, Ferulic Acid Ameliorates Ulcerative Colitis in a Rat Model via the Inhibition of Two LPS-TLR4-NF-κB and NF-κB-INOS-NO Signaling Pathways and Thus Alleviating the Inflammatory, Oxidative and Apoptotic Conditions in the Colon Tissue, Inflammopharmacology, 2023, 31(5), 2587–2597 CrossRef CAS.
- S. Yu, H. Qian, D. Zhang and Z. Jiang, Ferulic Acid Relieved Ulcerative Colitis by Inhibiting the TXNIP/NLRP3 Pathway in Rats, Cell Biol. Int., 2023, 47(2), 417–427 CrossRef CAS PubMed.
- S. He, F. Liu, L. Xu, P. Yin, D. Li, C. Mei, L. Jiang, Y. Ma and J. Xu, Protective Effects of Ferulic Acid against Heat Stress-Induced Intestinal Epithelial Barrier Dysfunction In Vitro and In Vivo, PLoS One, 2016, 11(2), e0145236 CrossRef PubMed.
- L. Song, T. Wu, L. Zhang, J. Wan and Z. Ruan, Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways, Food Funct., 2022, 13(8), 4562–4575 RSC.
- H.-J. Hwang, S. R. Lee, J.-G. Yoon, H.-R. Moon, J. Zhang, E. Park, S.-I. Yoon and J. A. Cho, Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells, Antioxidants, 2022, 11(8), 1448 CrossRef CAS PubMed.
- S. Yu, H. Qian, D. Zhang and Z. Jiang, Ferulic Acid Relieved Ulcerative Colitis by Inhibiting the TXNIP/NLRP3 Pathway in Rats, Cell Biol. Int., 2023, 47(2), 417–427 CrossRef CAS.
- E. O. Farombi, I. A. Adedara, O. V. Awoyemi, C. R. Njoku, G. O. Micah, C. U. Esogwa, S. E. Owumi and J. O. Olopade, Dietary Protocatechuic Acid Ameliorates Dextran Sulphate Sodium-Induced Ulcerative Colitis and Hepatotoxicity in Rats, Food Funct., 2016, 7(2), 913–921 RSC.
- X. Yang, X. Sun, F. Zhou, S. Xiao, L. Zhong, S. Hu, Z. Zhou, L. Li and Y. Tan, Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis, Molecules, 2023, 28(9), 3775 CrossRef CAS PubMed.
- I. Crespo, B. San-Miguel, J. Mauriz, J. Ortiz De Urbina, M. Almar, M. Tuñón and J. González-Gallego, Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway, Nutrients, 2017, 9(3), 288 CrossRef PubMed.
- K. Xiao, M. Zhou, Q. Lv, P. He, X. Qin, D. Wang, J. Zhao and Y. Liu, Protocatechuic Acid and Quercetin Attenuate ETEC-Caused IPEC-1 Cell Inflammation and Injury Associated with Inhibition of Necroptosis and Pyroptosis Signaling Pathways, J. Anim. Sci. Biotechnol., 2023, 14(1), 5 CrossRef CAS PubMed.
- R. Hu, S. Wu, B. Li, J. Tan, J. Yan, Y. Wang, Z. Tang, M. Liu, C. Fu, H. Zhang and J. He, Dietary Ferulic Acid and Vanillic Acid on Inflammation, Gut Barrier Function and Growth Performance in Lipopolysaccharide-Challenged Piglets, Anim. Nutr., 2022, 8, 144–152 CrossRef CAS PubMed.
- S.-J. Kim, M.-C. Kim, J.-Y. Um and S.-H. Hong, The Beneficial Effect of Vanillic Acid on Ulcerative Colitis, Molecules, 2010, 15(10), 7208–7217 CrossRef CAS PubMed.
- L. Song, T. Wu, L. Zhang, J. Wan and Z. Ruan, Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways, Food Funct., 2022, 13(8), 4562–4575 RSC.
- J.-Y. Lee, Anti-Inflammatory Effects of Sinapic Acid on 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis in Mice, Arch. Pharmacal Res., 2018, 41(2), 243–250 CrossRef CAS PubMed.
- H. Lan, L.-Y. Zhang, W. He, W.-Y. Li, Z. Zeng, B. Qian, C. Wang and J.-L. Song, Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells, Mediators Inflammation, 2021, 2021, 1–10 Search PubMed.
- S. Jang, S. Kim, B. R. So, Y. Kim, C.-K. Kim, J. J. Lee and S. K. Jung, Sinapic Acid Alleviates Inflammatory Bowel Disease (IBD) through Localization of Tight Junction Proteins by Direct Binding to TAK1 and Improves Intestinal Microbiota, Front. Pharmacol., 2023, 14, 1217111 CrossRef CAS PubMed.
- Z. Liu, Y. Yang, Y. Xu, Z. Zhang, R. Tang, J. Liu, H. Jiang and R. Zhao, Procyanidin B1 and p -Coumaric Acid from Whole Highland Barley Ameliorated HFD-Induced Impaired Glucose Tolerance via Small Intestinal Barrier and Hepatic Glucose Metabolism, Food Funct., 2024, 15(18), 9272–9283 RSC.
- L. Song, T. Wu, L. Zhang, J. Wan and Z. Ruan, Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways, Food Funct., 2022, 13(8), 4562–4575 RSC.
- S. Marinho, M. Illanes, J. Ávila-Román, V. Motilva and E. Talero, Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome, Biomolecules, 2021, 11(2), 162 CrossRef CAS PubMed.
- K. Li, J. Wu, S. Xu, X. Li, Y. Zhang and X. Gao, Rosmarinic Acid Alleviates Intestinal Inflammatory Damage and Inhibits Endoplasmic Reticulum Stress and Smooth Muscle Contraction Abnormalities in Intestinal Tissues by Regulating Gut Microbiota, Microbiol. Spectrum, 2023, 11(5), e01914–e01923 Search PubMed.
- M. Ghasemi-Dehnoo, H. Amini-Khoei, Z. Lorigooini, K. Ashrafi-Dehkordi and M. Rafieian-Kopaei, Coumaric Acid Ameliorates Experimental Colitis in Rats through Attenuation of Oxidative Stress, Inflammatory Response and Apoptosis, Inflammopharmacology, 2022, 30(6), 2359–2371 CrossRef CAS.
- M. Ekhtiar, M. Ghasemi-Dehnoo, Y. Mirzaei, F. Azadegan-Dehkordi, H. Amini-Khoei, Z. Lorigooini, A. Samiei-Sefat and N. Bagheri, The Coumaric Acid and Syringic Acid Ameliorate Acetic Acid-Induced Ulcerative Colitis in Rats via Modulator of Nrf2/HO-1 and pro-Inflammatory Cytokines, Int. Immunopharmacol., 2023, 120, 110309 CrossRef CAS PubMed.
- Q. Luo, P. Gong, R. Shi, W. Chen, C. Wang, C. Zhang and Z. Wu, Syringic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota, J. Agric. Food Chem., 2023, 71(22), 8458–8470 CrossRef CAS PubMed.
- X. Han, M. Li, L. Sun, X. Liu, Y. Yin, J. Hao and W. Zhang, P-Hydroxybenzoic, Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner, Nutrients, 2022, 14(24), 5383 CrossRef CAS PubMed.
- Y. Tian, T. Jian, J. Li, L. Huang, S. Li, H. Lu, G. Niu, X. Meng, B. Ren, H. Liao, X. Ding and J. Chen, Phenolic Acids from Chicory Roots Ameliorate Dextran Sulfate Sodium–Induced Colitis in Mice by Targeting TRP Signaling Pathways and the Gut Microbiota, Phytomedicine, 2024, 128, 155378 CrossRef CAS PubMed.
- K.-Y. Peng, J.-F. Gu, S.-L. Su, Y. Zhu, J.-M. Guo, D.-W. Qian and J.-A. Duan, Salvia Miltiorrhiza Stems and Leaves Total Phenolic Acids Combination with Tanshinone Protect against DSS-Induced Ulcerative Colitis through Inhibiting TLR4/PI3K/AKT/mTOR Signaling Pathway in Mice, J. Ethnopharmacol., 2021, 264, 113052 CrossRef CAS PubMed.
- M. G. Farquhar and G. E. Palade, JUNCTIONAL COMPLEXES IN VARIOUS EPITHELIA, J. Cell Biol., 1963, 17(2), 375–412 CrossRef CAS PubMed.
- C. M. Van Itallie and J. M. Anderson, Architecture of Tight Junctions and Principles of Molecular Composition, Semin. Cell Dev. Biol., 2014, 36, 157–165 CrossRef CAS PubMed.
- L. W. Kaminsky, R. Al-Sadi and T. Y. Ma, IL-1β and the Intestinal Epithelial Tight Junction Barrier, Front. Immunol., 2021, 12, 767456 CrossRef CAS PubMed.
- B. T. Schwarz, F. Wang, L. Shen, D. R. Clayburgh, L. Su, Y. Wang, Y. Fu and J. R. Turner, LIGHT Signals Directly to Intestinal Epithelia to Cause Barrier Dysfunction via Cytoskeletal and Endocytic Mechanisms, Gastroenterology, 2007, 132(7), 2383–2394 CrossRef CAS PubMed.
- M. Nighot, R. Al-Sadi, S. Guo, M. Rawat, P. Nighot, M. D. Watterson and T. Y. Ma, Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression, Am. J. Pathol., 2017, 187(12), 2698–2710 CrossRef CAS PubMed.
- M. Nighot, M. Rawat, R. Al-Sadi, E. F. Castillo, P. Nighot and T. Y. Ma, Lipopolysaccharide-Induced Increase in Intestinal Permeability Is Mediated by TAK-1 Activation of IKK and MLCK/MYLK Gene, Am. J. Pathol., 2019, 189(4), 797–812 CrossRef CAS PubMed.
- F. Wang, B. T. Schwarz, W. V. Graham, Y. Wang, L. Su, D. R. Clayburgh, C. Abraham and J. R. Turner, IFN-γ-Induced TNFR2 Expression Is Required for TNF-Dependent Intestinal Epithelial Barrier Dysfunction, Gastroenterology, 2006, 131(4), 1153–1163 CrossRef CAS PubMed.
- H.-Y. Chang, S.-Y. Chen, J.-A. Lin, Y.-Y. Chen, Y.-Y. Chen, Y.-C. Liu and G.-C. Yen, Phyllanthus Emblica Fruit Improves Obesity by Reducing Appetite and Enhancing Mucosal Homeostasis via the Gut Microbiota–Brain–Liver Axis in HFD-Induced Leptin-Resistant Rats, J. Agric. Food Chem., 2024, 72(18), 10406–10419 CrossRef CAS.
- W. Wachiradejkul, P. Sukmak, S. Treveeravoot, L. Yurasakpong, N. Rangchaikul, P. Chatkul, P. Supapol, A. Arinno, N. Teansuk, J. Inchai, S. Phummisutthigoon, M. Phongjit, A. Loungjan, N. Akrimajirachoote, W. Poolsri, C. Aonbangkhen, R. Khumjiang, C. Muanprasat, C. S. Vaddhanaphuti and P. Pongkorpsakol, Enhancing Intestinal Tight Junction Assembly by Gallic Acid as a Subcellular Basis for the Pharmacological Effect of Ocimum Sanctum L, Flower Aqueous Extract, J. Funct. Foods, 2024, 122, 106519 CrossRef CAS.
- Y. Jin and A. T. Blikslager, The Regulation of Intestinal Mucosal Barrier by Myosin Light Chain Kinase/Rho Kinases, Int. J. Mol. Sci., 2020, 21(10), 3550 CrossRef CAS PubMed.
- S. Olivier, J. Leclerc, A. Grenier, M. Foretz, J. Tamburini and B. Viollet, AMPK Activation Promotes Tight Junction Assembly in Intestinal Epithelial Caco-2 Cells, Int. J. Mol. Sci., 2019, 20(20), 5171 CrossRef CAS.
- H.-Y. Nie, J. Ge, G.-X. Huang, K.-G. Liu, Y. Yue, H. Li, H.-G. Lin, T. Zhang, H.-F. Yan, B.-X. Xu, H.-W. Sun, J.-W. Yang, S.-Y. Si, J.-L. Zhou and Y. Cui, New Insights into the Intestinal Barrier through “Gut-Organ” Axes and a Glimpse of the Microgravity's Effects on Intestinal Barrier, Front. Physiol., 2024, 15, 1465649 CrossRef.
- J.-F. Sicard, G. Le Bihan, P. Vogeleer, M. Jacques and J. Harel, Interactions of Intestinal Bacteria with Components of the Intestinal Mucus, Front. Cell. Infect. Microbiol., 2017, 7, 387 CrossRef PubMed.
- M. E. V. Johansson and G. C. Hansson, Immunological Aspects of Intestinal Mucus and Mucins, Nat. Rev. Immunol., 2016, 16(10), 639–649 CrossRef CAS PubMed.
- J. P. Katz, N. Perreault, B. G. Goldstein, C. S. Lee, P. A. Labosky, V. W. Yang and K. H. Kaestner, The Zinc-Finger Transcription Factor Klf4 Is Required for Terminal Differentiation of Goblet Cells in the Colon, Development, 2002, 129(11), 2619–2628 CrossRef CAS.
- R. Barros, B. Pereira, I. Duluc, M. Azevedo, N. Mendes, V. Camilo, R. Jacobs, P. Paulo, F. Santos-Silva, I. Van Seuningen, G. Van Den Brink, L. David, J. Freund and R. Almeida, Key Elements of the BMP/SMAD Pathway Co–localize with CDX2 in Intestinal Metaplasia and Regulate CDX2 Expression in Human Gastric Cell Lines, J. Pathol., 2008, 215(4), 411–420 CrossRef CAS PubMed.
- M. Yu, K. Qin, J. Fan, G. Zhao, P. Zhao, W. Zeng, C. Chen, A. Wang, Y. Wang, J. Zhong, Y. Zhu, W. Wagstaff, R. C. Haydon, H. H. Luu, S. Ho, M. J. Lee, J. Strelzow, R. R. Reid and T.-C. He, The Evolving Roles of Wnt Signaling in Stem Cell Proliferation and Differentiation, the Development of Human Diseases, and Therapeutic Opportunities, Genes Dis., 2024, 11(3), 101026 CrossRef CAS PubMed.
- G. Aviello and U. Knaus, ROS in Gastrointestinal Inflammation: Rescue Or Sabotage?, Br. J. Pharmacol., 2017, 174(12), 1704–1718 CrossRef CAS PubMed.
- T. Ju, R. P. Aryal, C. J. Stowell and R. D. Cummings, Regulation of Protein O-Glycosylation by the Endoplasmic Reticulum–Localized Molecular Chaperone Cosmc, J. Cell Biol., 2008, 182(3), 531–542 CrossRef CAS PubMed.
- T. Hussain, B. Tan, Y. Yin, F. Blachier, M. C. B. Tossou and N. Rahu, Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?, Oxid. Med. Cell. Longevity, 2016, 2016(1), 7432797 CrossRef.
- Y. Ji, X. Luo, Y. Yang, Z. Dai, G. Wu and Z. Wu, Endoplasmic Reticulum Stress-Induced Apoptosis in Intestinal Epithelial Cells: A Feed-Back Regulation by Mechanistic Target of Rapamycin Complex 1 (mTORC1), J. Anim. Sci. Biotechnol., 2018, 9(1), 38 CrossRef.
- M. Tang, D. Yuan and P. Liao, Berberine Improves Intestinal Barrier Function and Reduces Inflammation, Immunosuppression, and Oxidative Stress by Regulating the NF-κB/MAPK Signaling Pathway in Deoxynivalenol-Challenged Piglets, Environ. Pollut., 2021, 289, 117865 CrossRef CAS PubMed.
- D. E. Iglesias, E. Cremonini, P. I. Oteiza and C. G. Fraga, Curcumin Mitigates TNFα–Induced Caco–2 Cell Monolayer Permeabilization Through Modulation of NF–κB, ERK1/2, and JNK Pathways, Mol. Nutr. Food Res., 2022, 66(21), 2101033 CrossRef CAS.
- G. Zeng, J. Li, Y. Wang, J. Su, Z. Lu, F. Zhang and W. Ding, Polystyrene Microplastic-Induced Oxidative Stress Triggers Intestinal Barrier Dysfunction via the NF-κB/NLRP3/IL-1β/MCLK Pathway, Environ. Pollut., 2024, 345, 123473 CrossRef CAS PubMed.
- Y. Zhuang, H. Wu, X. Wang, J. He, S. He and Y. Yin, Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway, Oxid. Med. Cell. Longevity, 2019, 2019, 1–14 CrossRef.
- Z. Zhao, W. Qu, K. Wang, S. Chen, L. Zhang, D. Wu and Z. Chen, Bisphenol A Inhibits Mucin 2 Secretion in Intestinal Goblet Cells through Mitochondrial Dysfunction and Oxidative Stress, Biomed. Pharmacother., 2019, 111, 901–908 CrossRef CAS PubMed.
- P. Muro, L. Zhang, S. Li, Z. Zhao, T. Jin, F. Mao and Z. Mao, The Emerging Role of Oxidative Stress in Inflammatory Bowel Disease, Front. Endocrinol., 2024, 15, 1390351 CrossRef.
- Y. Cheng, T. Wu, S. Tang, F. Liang, Y. Fang, W. Cao, S. Pan and X. Xu, Fermented Blueberry Pomace Ameliorates Intestinal Barrier Function through the NF-κB-MLCK Signaling Pathway in High-Fat Diet Mice, Food Funct., 2020, 11(4), 3167–3179 RSC.
- Y. Jin, Z. Zhai, H. Jia, J. Lai, X. Si and Z. Wu, Kaempferol Attenuates Diquat-Induced Oxidative Damage and Apoptosis in Intestinal Porcine Epithelial Cells, Food Funct., 2021, 12(15), 6889–6899 RSC.
- P. Zhou, H. Xu and L. Wang, Cardiovascular Protective Effects of Natural Flavonoids on Intestinal Barrier Injury, Mol. Cell. Biochem., 2025, 480(6), 3343–3362 CrossRef CAS.
- R. Saito, T. Suzuki, K. Hiramoto, S. Asami, E. Naganuma, H. Suda, T. Iso, H. Yamamoto, M. Morita, L. Baird, Y. Furusawa, T. Negishi, M. Ichinose and M. Yamamoto, Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response, Mol. Cell. Biol., 2016, 36(2), 271–284 CrossRef CAS PubMed.
- R. Hu, Z. He, M. Liu, J. Tan, H. Zhang, D.-X. Hou, J. He and S. Wu, Dietary Protocatechuic Acid Ameliorates Inflammation and Up-Regulates Intestinal Tight Junction Proteins by Modulating Gut Microbiota in LPS-Challenged Piglets, J. Anim. Sci. Biotechnol., 2020, 11(1), 92 CrossRef CAS PubMed.
- J. Gao, Y. Li, Y. Wan, T. Hu, L. Liu, S. Yang, Z. Gong, Q. Zeng, Y. Wei, W. Yang, Z. Zeng, X. He, S.-H. Huang and H. Cao, A Novel Postbiotic From Lactobacillus Rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function, Front. Microbiol., 2019, 10, 477 CrossRef.
- L. Wrzosek, S. Miquel, M.-L. Noordine, S. Bouet, M. J. Chevalier-Curt, V. Robert, C. Philippe, C. Bridonneau, C. Cherbuy, C. Robbe-Masselot, P. Langella and M. Thomas, Bacteroides Thetaiotaomicron and Faecalibacterium Prausnitziiinfluence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent, BMC Biol., 2013, 11(1), 61 CrossRef PubMed.
- L. Arike, J. Holmén-Larsson and G. C. Hansson, Intestinal Muc2 Mucin O-Glycosylation Is Affected by Microbiota and Regulated by Differential Expression of Glycosyltranferases, Glycobiology, 2017, 27(4), 318–328 CAS.
- A. E. Powell, Y. Wang, Y. Li, E. J. Poulin, A. L. Means, M. K. Washington, J. N. Higginbotham, A. Juchheim, N. Prasad, S. E. Levy, Y. Guo, Y. Shyr, B. J. Aronow, K. M. Haigis, J. L. Franklin and R. J. Coffey, The Pan-ErbB Negative Regulator Lrig1 Is an Intestinal Stem Cell Marker That Functions as a Tumor Suppressor, Cell, 2012, 149(1), 146–158 CrossRef CAS PubMed.
- R. Atreya, J. Mudter, S. Finotto, J. Müllberg, T. Jostock, S. Wirtz, M. Schütz, B. Bartsch, M. Holtmann, C. Becker, D. Strand, J. Czaja, J. F. Schlaak, H. A. Lehr, F. Autschbach, G. Schürmann, N. Nishimoto, K. Yoshizaki, H. Ito, T. Kishimoto, P. R. Galle, S. Rose-John and M. F. Neurath, Blockade of Interleukin 6 Trans Signaling Suppresses T-Cell Resistance against Apoptosis in Chronic Intestinal Inflammation: Evidence in Crohn Disease and Experimental Colitis in Vivo, Nat. Med., 2000, 6(5), 583–588 CrossRef CAS PubMed.
- C. A. Lindemans, M. Calafiore, A. M. Mertelsmann, M. H. O'Connor, J. A. Dudakov, R. R. Jenq, E. Velardi, L. F. Young, O. M. Smith, G. Lawrence, J. A. Ivanov, Y.-Y. Fu, S. Takashima, G. Hua, M. L. Martin, K. P. O'Rourke, Y.-H. Lo, M. Mokry, M. Romera-Hernandez, T. Cupedo, L. E. Dow, E. E. Nieuwenhuis, N. F. Shroyer, C. Liu, R. Kolesnick, M. R. M. Van Den Brink and A. M. Hanash, Interleukin-22 Promotes Intestinal-Stem-Cell-Mediated Epithelial Regeneration, Nature, 2015, 528(7583), 560–564 CrossRef CAS PubMed.
- W. Xu, L. Lin, A. Liu, T. Zhang, S. Zhang, Y. Li, J. Chen, Z. Gong, Z. Liu and W. Xiao, l -Theanine Affects Intestinal Mucosal Immunity by Regulating Short-Chain Fatty Acid Metabolism under Dietary Fiber Feeding, Food Funct., 2020, 11(9), 8369–8379 RSC.
- Q. Zhang, Q. Zhao, T. Li, L. Lu, F. Wang, H. Zhang, Z. Liu, H. Ma, Q. Zhu, J. Wang, X. Zhang, Y. Pei, Q. Liu, Y. Xu, J. Qie, X. Luan, Z. Hu and X. Liu, Lactobacillus Plantarum-Derived Indole-3-Lactic Acid Ameliorates Colorectal Tumorigenesis via Epigenetic Regulation of CD8+ T Cell Immunity, Cell Metab., 2023, 35(6), 943–960 CrossRef CAS PubMed.
- J. Cong, P. Liu, Z. Han, W. Ying, C. Li, Y. Yang, S. Wang, J. Yang, F. Cao, J. Shen, Y. Zeng, Y. Bai, C. Zhou, L. Ye, R. Zhou, C. Guo, C. Cang, D. L. Kasper, X. Song, L. Dai, L. Sun, W. Pan and S. Zhu, Bile Acids Modified by the Intestinal Microbiota Promote Colorectal Cancer Growth by Suppressing CD8+ T Cell Effector Functions, Immunity, 2024, 57(4), 876–889 CrossRef CAS PubMed.
- M. G. Xie, Y. Q. Fei, Y. Wang, W. Y. Wang and Z. Wang, Chlorogenic Acid Alleviates Colon Mucosal Damage Induced by a High–Fat Diet via Gut Microflora Adjustment to Increase Short–Chain Fatty Acid Accumulation in Rats, Oxid. Med. Cell. Longevity, 2021, 2021(1), 3456542 CrossRef PubMed.
- I. Khan, Y. Bai, L. Zha, N. Ullah, H. Ullah, S. R. H. Shah, H. Sun and C. Zhang, Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection, Front. Cell. Infect. Microbiol., 2021, 11, 716299 CrossRef CAS PubMed.
- D. Yi, M. Wang, X. Liu, L. Qin, Y. Liu, L. Zhao, Y. Peng, Z. Liang and J. He, Rosmarinic Acid Attenuates Salmonella Enteritidis-Induced Inflammation via Regulating TLR9/NF-κB Signaling Pathway and Intestinal Microbiota, Antioxidants, 2024, 13(10), 1265 CrossRef CAS PubMed.
- J.-G. Xu, H.-X. Hu, J.-Y. Chen, Y.-S. Xue, B. Kodirkhonov and B.-Z. Han, Comparative Study on Inhibitory Effects of Ferulic Acid and P-Coumaric Acid on Salmonella Enteritidis Biofilm Formation, World J. Microbiol. Biotechnol., 2022, 38(8), 136 CrossRef CAS PubMed.
- M. Liu, Y. Lu, G. Xue, L. Han, H. Jia, Z. Wang, J. Zhang, P. Liu, C. Yang and Y. Zhou, Role of Short–chain Fatty Acids in Host Physiology, Anim. Models Exp. Med., 2024, 7(5), 641–652 CrossRef CAS PubMed.
- M. Y. Zeng, N. Inohara and G. Nuñez, Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut, Mucosal Immunol., 2017, 10(1), 18–26 CrossRef CAS PubMed.
- C. E. Mills, X. Tzounis, M.-J. Oruna-Concha, D. S. Mottram, G. R. Gibson and J. P. E. Spencer, In Vitro Colonic Metabolism of Coffee and Chlorogenic Acid Results in Selective Changes in Human Faecal Microbiota Growth, Br. J. Nutr., 2015, 113(8), 1220–1227 CrossRef CAS PubMed.
- O. J. Lara-Guzmán, Á. Arango-González, D. A. Rivera, K. Muñoz-Durango and J. A. Sierra, The Colonic Polyphenol Catabolite Dihydroferulic Acid (DHFA) Regulates Macrophages Activated by Oxidized LDL, 7-Ketocholesterol, and LPS Switching from pro- to Anti-Inflammatory Mediators, Food Funct., 2024, 15(20), 10399–10413 RSC.
- S. Lu, D. Cheng, H. Yao, Y. Wen, Y. Yu, H. Li, J. Wang and B. Sun, Cascade Microbial Metabolism of Ferulic Acid In Vitro Fermented by the Human Fecal Inoculum, J. Agric. Food Chem., 2024, 72(17), 9807–9817 CrossRef CAS PubMed.
- S. Mukherjee and L. V. Hooper, Antimicrobial Defense of the Intestine, Immunity, 2015, 42(1), 28–39 CrossRef CAS PubMed.
- C. Chelakkot, J. Ghim and S. H. Ryu, Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications, Exp. Mol. Med., 2018, 50(8), 1–9 CrossRef CAS PubMed.
- A. Buckley and J. R. Turner, Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease, Cold Spring Harbor Perspect. Biol., 2018, 10(1), a029314 CrossRef PubMed.
- J. R. Turner, Intestinal Mucosal Barrier Function in Health and Disease, Nat. Rev. Immunol., 2009, 9(11), 799–809 CrossRef CAS PubMed.
- J. Zeng, D. Zhang, X. Wan, Y. Bai, C. Yuan, T. Wang, D. Yuan, C. Zhang and C. Liu, Chlorogenic Acid Suppresses miR–155 and Ameliorates Ulcerative Colitis through the NF–κB/NLRP3 Inflammasome Pathway, Mol. Nutr. Food Res., 2020, 64(23), 2000452 CrossRef CAS PubMed.
- M. Ghasemi-Dehnoo, H. Amini-Khoei, Z. Lorigooini, M. AnjomShoa and M. Rafieian-Kopaei, Ferulic Acid Ameliorates Ulcerative Colitis in a Rat Model via the Inhibition of Two LPS-TLR4-NF-κB and NF-κB-INOS-NO Signaling Pathways and Thus Alleviating the Inflammatory, Oxidative and Apoptotic Conditions in the Colon Tissue, Inflammopharmacology, 2023, 31(5), 2587–2597 CrossRef CAS PubMed.
- B. Romier, J. Van De Walle, A. During, Y. Larondelle and Y.-J. Schneider, Modulation of Signalling Nuclear Factor-κB Activation Pathway by Polyphenols in Human Intestinal Caco-2 Cells, Br. J. Nutr., 2008, 100(03), 542–551 CrossRef CAS PubMed.
- K. K. Nyati, K. Masuda, M. M.-U. Zaman, P. K. Dubey, D. Millrine, J. P. Chalise, M. Higa, S. Li, D. M. Standley, K. Saito, H. Hanieh and T. Kishimoto, TLR4-Induced NF-κB and MAPK Signaling Regulate the IL-6 mRNA Stabilizing Protein Arid5a, Nucleic Acids Res., 2017, 45(5), 2687–2703 CrossRef CAS PubMed.
- H. M. Blevins, Y. Xu, S. Biby and S. Zhang, The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases, Front. Aging Neurosci., 2022, 14, 879021 CrossRef CAS PubMed.
- M. Z. Khan, L. Li, Y. Zhan, H. Binjiang, X. Liu, X. Kou, A. Khan, A. Qadeer, Q. Ullah, K. J. Alzahrani, T. Wang, C. Wang and M. Zahoor, Targeting Nrf2/KEAP1 Signaling Pathway Using Bioactive Compounds to Combat Mastitis, Front. Immunol., 2025, 16, 1425901 CrossRef CAS PubMed.
- D. Han, Y. Wu, D. Lu, J. Pang, J. Hu, X. Zhang, Z. Wang, G. Zhang and J. Wang, Polyphenol-Rich Diet Mediates Interplay between Macrophage-Neutrophil and Gut Microbiota to Alleviate Intestinal Inflammation, Cell Death Dis., 2023, 14(10), 656 CrossRef CAS PubMed.
- M. E. Gerich and D. P. B. McGovern, Towards Personalized Care in IBD, Nat. Rev. Gastroenterol. Hepatol., 2014, 11(5), 287–299 CrossRef CAS PubMed.
- E. C. Martens, M. Neumann and M. S. Desai, Interactions of Commensal and Pathogenic Microorganisms with the Intestinal Mucosal Barrier, Nat. Rev. Microbiol., 2018, 16(8), 457–470 CrossRef CAS PubMed.
- Z. Zhai, F. Zhang, R. Cao, X. Ni, Z. Xin, J. Deng, G. Wu, W. Ren, Y. Yin and B. Deng, Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice With DSS-Induced IBD, Front. Microbiol., 2019, 10, 1595 CrossRef PubMed.
- J. Ni, G. D. Wu, L. Albenberg and V. T. Tomov, Gut Microbiota and IBD: Causation or Correlation?, Nat. Rev. Gastroenterol. Hepatol., 2017, 14(10), 573–584 CrossRef PubMed.
- L. Dong, J. Xie, Y. Wang, H. Jiang, K. Chen, D. Li, J. Wang, Y. Liu, J. He, J. Zhou, L. Zhang, X. Lu, X. Zou, X.-Y. Wang, Q. Wang, Z. Chen and D. Zuo, Mannose Ameliorates Experimental Colitis by Protecting Intestinal Barrier Integrity, Nat. Commun., 2022, 13(1), 4804 CrossRef CAS PubMed.
- B. E. Lacy, F. Mearin, L. Chang, W. D. Chey, A. J. Lembo, M. Simren and R. Spiller, Bowel Disorders, Gastroenterology, 2016, 150(6), 1393–1407 CrossRef PubMed.
- N. Hanning, A. L. Edwinson, H. Ceuleers, S. A. Peters, J. G. De Man, L. C. Hassett, B. Y. De Winter and M. Grover, Intestinal Barrier Dysfunction in Irritable Bowel Syndrome: A Systematic Review, Ther. Adv. Gastroenterol., 2021, 14, 1756284821993586 CrossRef CAS PubMed.
- Y. Long, L. Du, J. J. Kim, B. Chen, Y. Zhu, Y. Zhang, S. Yao, H. He, X. Zheng, Z. Huang and N. Dai, MLCK –mediated Intestinal Permeability Promotes Immune Activation and Visceral Hypersensitivity in PI – IBS Mice, Neurogastroenterol. Motil., 2018, 30(9), e13348 CrossRef CAS PubMed.
- Q. Zhou, M. L. Verne, J. Z. Fields, J. J. Lefante, S. Basra, H. Salameh and G. N. Verne, Randomised Placebo-Controlled Trial of Dietary Glutamine Supplements for Postinfectious Irritable Bowel Syndrome, Gut, 2019, 68(6), 996–1002 CrossRef CAS PubMed.
- Y. Xie, X. Zhan, J. Tu, K. Xu, X. Sun, C. Liu, C. Ke, G. Cao, Z. Zhou and Y. Liu, Atractylodes Oil Alleviates Diarrhea-Predominant Irritable Bowel Syndrome by Regulating Intestinal Inflammation and Intestinal Barrier via SCF/c-Kit and MLCK/MLC2 Pathways, J. Ethnopharmacol., 2021, 272, 113925 CrossRef CAS PubMed.
- N. Hanning, A. L. Edwinson, H. Ceuleers, S. A. Peters, J. G. De Man, L. C. Hassett, B. Y. De Winter and M. Grover, Intestinal Barrier Dysfunction in Irritable Bowel Syndrome: A Systematic Review, Ther. Adv. Gastroenterol., 2021, 14, 1756284821993586 CrossRef CAS PubMed.
- R. D. Moloney, A. C. Johnson, S. M. O'Mahony, T. G. Dinan, B. Greenwood-Van Meerveld and J. F. Cryan, Stress and the Microbiota–Gut–Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome, CNS Neurosci. Ther., 2016, 22(2), 102–117 CrossRef PubMed.
- L. Wen, L. Tang, M. Zhang, C. Wang, S. Li, Y. Wen, H. Tu, H. Tian, J. Wei, P. Liang, C. Yang, G. Li and Y. Gao, Gallic Acid Alleviates Visceral Pain and Depression via Inhibition of P2X7 Receptor, Int. J. Mol. Sci., 2022, 23(11), 6159 CrossRef CAS PubMed.
- Y. W. Min and P.-L. Rhee, The Clinical Potential of Ramosetron in the Treatment of Irritable Bowel Syndrome with Diarrhea (IBS-D), Ther. Adv. Gastroenterol., 2015, 8(3), 136–142 CrossRef CAS PubMed.
- R. C. Spiller, Targeting the 5-HT3 Receptor in the Treatment of Irritable Bowel Syndrome, Curr. Opin. Pharmacol., 2011, 11(1), 68–74 CrossRef CAS PubMed.
- N. J. Talley, S. F. Phillips, A. Haddad, L. J. Miller, C. Twomey, A. R. Zinsmeister, R. L. MacCarty and A. Ciociola, GR 38032F (Ondansetron), a Selective 5HT3 Receptor Antagonist, Slows Colonic Transit in Healthy Man, Dig. Dis. Sci., 1990, 35(4), 477–480 CrossRef CAS PubMed.
- C. J. Steadman, N. J. Talley, S. F. Phillips and A. R. Zinsmeister, Selective 5-Hydroxytryptamine Type 3 Receptor Antagonism With Ondansetron as Treatment for Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Study, Mayo Clin. Proc., 1992, 67(8), 732–738 CrossRef CAS PubMed.
- H.-Y. Kim, C.-E. Kim, D.-R. Oh, Y. Kim, C.-Y. Choi and J. Kim, Development and Validation of a High-Performance Liquid Chromatography Method to Quantify Marker Compounds in Lysimachia Vulgaris Var. Davurica and Its Effects in Diarrhea-Predominant Irritable Bowel Syndrome, Molecules, 2024, 29(7), 1489 CrossRef CAS PubMed.
- X. Zheng, Y. Cheng, Y. Chen, Y. Yue, Y. Li, S. Xia, Y. Li, H. Deng, J. Zhang and Y. Cao, Ferulic Acid Improves Depressive-Like Behavior in Prenatally-Stressed Offspring Rats via Anti-Inflammatory Activity and HPA Axis, Int. J. Mol. Sci., 2019, 20(3), 493 CrossRef CAS PubMed.
- J. Song, N. Zhou, W. Ma, X. Gu, B. Chen, Y. Zeng, L. Yang and M. Zhou, Modulation of Gut Microbiota by Chlorogenic Acid Pretreatment on Rats with Adrenocorticotropic Hormone Induced Depression-like Behavior, Food Funct., 2019, 10(5), 2947–2957 RSC.
- C. Zheng, Y. Zhong, W. Zhang, Z. Wang, H. Xiao, W. Zhang, J. Xie, X. Peng, J. Luo and W. Xu, Chlorogenic Acid Ameliorates Post–Infectious Irritable Bowel Syndrome by Regulating Extracellular Vesicles of Gut Microbes, Adv. Sci., 2023, 10(28), 2302798 CrossRef CAS PubMed.
- Z. Alvarado-Martinez, Z. Tabashsum, A. Aditya, K. Hshieh, G. Suh, M. Wall, A. Scriba, G. Sellers, C. Canagarajah, S. Kapadia and D. Biswas, Plant-Derived Phenolic Acids Limit the Pathogenesis of Salmonella Typhimurium and Protect Intestinal Epithelial Cells during Their Interactions, Molecules, 2024, 29(6), 1364 CrossRef CAS PubMed.
- M. Nucci and E. Anaissie, Revisiting the Source of Candidemia: Skin or Gut?, Clin. Infect. Dis., 2001, 33(12), 1959–1967 CrossRef CAS PubMed.
- W. S. Sung and D. G. Lee, Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption, Pure Appl. Chem., 2010, 82(1), 219–226 CAS.
- Y. Semaming, P. Pannengpetch, S. C. Chattipakorn and N. Chattipakorn, Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine, Evidence Based Complementary Altern. Med., 2015, 2015, 1–11 CrossRef PubMed.
- M. Miklasińska, M. Kępa, R. Wojtyczka, D. Idzik, A. Zdebik, K. Orlewska and T. Wąsik, Antibacterial Activity of Protocatechuic Acid Ethyl Ester on Staphylococcus Aureus Clinical Strains Alone and in Combination with Antistaphylococcal Drugs, Molecules, 2015, 20(8), 13536–13549 CrossRef PubMed.
- Y. Sun, Y. Wang, Z. Lin, F. Zhang, Y. Zhang, T. Ren, L. Wang, Q. Qiao, M. Shen, J. Wang, Y. Song, Y. Sun and P. Lin, Irisin Delays the Onset of Type 1 Diabetes in NOD Mice by Enhancing Intestinal Barrier, Int. J. Biol. Macromol., 2024, 265, 130857 CrossRef CAS PubMed.
- A. M. Martin, E. W. Sun and D. J. Keating, Mechanisms Controlling Hormone Secretion in Human Gut and Its Relevance to Metabolism, J. Endocrinol., 2020, 244(1), R1–R15 CAS.
- M. A. Nauck and J. J. Meier, Incretin Hormones: Their Role in Health and Disease, Diabetes, Obes. Metab., 2018, 20(S1), 5–21 CrossRef CAS PubMed.
- H. Wu, E. Esteve, V. Tremaroli, M. T. Khan, R. Caesar, L. Mannerås-Holm, M. Ståhlman, L. M. Olsson, M. Serino, M. Planas-Fèlix, G. Xifra, J. M. Mercader, D. Torrents, R. Burcelin, W. Ricart, R. Perkins, J. M. Fernàndez-Real and F. Bäckhed, Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug, Nat. Med., 2017, 23(7), 850–858 CrossRef CAS PubMed.
- J. De La Cuesta-Zuluaga, N. T. Mueller, V. Corrales-Agudelo, E. P. Velásquez-Mejía, J. A. Carmona, J. M. Abad and J. S. Escobar, Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia Muciniphila and Several Short-Chain Fatty Acid–Producing Microbiota in the Gut, Diabetes Care, 2017, 40(1), 54–62 CrossRef CAS PubMed.
- D. Liu, S. Zhang, S. Li, Q. Zhang, Y. Cai, P. Li, H. Li, B. Shen, Q. Liao, Y. Hong and Z. Xie, Indoleacrylic Acid Produced by Parabacteroides Distasonis Alleviates Type 2 Diabetes via Activation of AhR to Repair Intestinal Barrier, BMC Biol., 2023, 21(1), 90 CrossRef CAS PubMed.
- J.-F. Gu, S.-L. Su, J.-M. Guo, Y. Zhu, M. Zhao and J.-A. Duan, The Aerial Parts of Salvia Miltiorrhiza Bge. Strengthen Intestinal Barrier and Modulate Gut Microbiota Imbalance in Streptozocin-Induced Diabetic Mice, J. Funct. Foods, 2017, 36, 362–374 CrossRef.
- G. Li, S. Li, H. Liu, L. Zhang, J. Gao, S. Zhang, Y. Zou, X. Xia and X. Ren, Isinglass Polysaccharides Regulate Intestinal-Barrier Function and Alleviate Obesity in High-Fat Diet Mice through the HO-1/Nrf2 Pathway and Intestinal Microbiome Environment, Nutrients, 2022, 14(19), 3928 CrossRef CAS PubMed.
- M. Bai, X. Wang, D. Liu, A. Xu, H. Cheng, L. Li and C. Zhang, Tolypocladium Sinense Mycelium Polysaccharide Alleviates Obesity, Lipid Metabolism Disorder, and Inflammation Caused by High Fat Diet via Improving Intestinal Barrier and Modulating Gut Microbiota, Mol. Nutr. Food Res., 2024, 68(9), 2300759 CrossRef CAS PubMed.
- R. Wang, L. Wang, S. Wang, J. Wang, C. Su, L. Zhang, C. Li and S. Liu, Phenolics from Noni (Morinda Citrifolia L.) Fruit Alleviate Obesity in High Fat Diet-Fed Mice via Modulating the Gut Microbiota and Mitigating Intestinal Damage, Food Chem., 2023, 402, 134232 CrossRef CAS PubMed.
- M. Jin Son, C. W. Rico, S. Hyun Nam and M. Young Kang, Influence of Oryzanol and Ferulic Acid on the Lipid Metabolism and Antioxidative Status in High Fat-Fed Mice, J. Clin. Biochem. Nutr., 2010, 46(2), 150–156 CrossRef PubMed.
- M. Huang, B. Kong, M. Zhang, D. Rizzolo, L. E. Armstrong, J. D. Schumacher, M. D. Chow, Y.-H. Lee, L. B. Joseph, M. Stofan, L. Zhang and G. L. Guo, Enhanced Alcoholic Liver Disease in Mice with Intestine-Specific Farnesoid X Receptor Deficiency, Lab. Invest., 2020, 100(9), 1158–1168 CrossRef CAS PubMed.
- Y. Wang, S. Cui, J. Zheng, Y. Li, P. Li and H. Hou, Berberine Ameliorates Intestinal Mucosal Barrier Dysfunction in Nonalcoholic Fatty Liver Disease (NAFLD) Rats, J. King Saud Univ., Sci., 2020, 32(5), 2534–2539 CrossRef.
- M. Sangineto, C. Grander, F. Grabherr, L. Mayr, B. Enrich, J. Schwärzler, M. Dallio, V. N. Bukke, A. Moola, A. Moschetta, T. E. Adolph, C. Sabbà, G. Serviddio and H. Tilg, Recovery of Bacteroides Thetaiotaomicron Ameliorates Hepatic Steatosis in Experimental Alcohol-Related Liver Disease, Gut Microbes, 2022, 14(1), 2089006 CrossRef PubMed.
- L. Xiao, H. Xiong, Z. Deng, X. Peng, K. Cheng, H. Zhang, L. Jiang and Y. Sun, Tetrastigma Hemsleyanum Leaf Extracts Ameliorate NAFLD in Mice with Low-Grade Colitis via the Gut–Liver Axis, Food Funct., 2023, 14(1), 500–515 RSC.
- H. Zhu, W. Jiang, C. Liu, C. Wang, B. Hu, Y. Guo, Y. Cheng and H. Qian, Ameliorative Effects of Chlorogenic Acid on Alcoholic Liver Injury in Mice via Gut Microbiota Informatics, Eur. J. Pharmacol., 2022, 928, 175096 CrossRef CAS PubMed.
- P. U. Amadi, J. O. Osuoha, C. N. Ekweogu, S. J. Jarad, E. E. Efiong, P. C. Odika, C. Ejiofor, O. Aloy-Amadi, G. S. Gill, C. W. Adumekwe, A. Gaowa, D. Zhang, B. De Courten and E. N. Agomuo, Phenolic Acids from Anisopus Mannii Modulates Phosphofructokinase 1 to Improve Glycemic Control in Patients with Type 2 Diabetes: A Double-Blind, Randomized, Clinical Trial, Pharmacol. Res., 2025, 212, 107602 CrossRef CAS PubMed.
- A. Mubarak, C. P. Bondonno, A. H. Liu, M. J. Considine, L. Rich, E. Mas, K. D. Croft and J. M. Hodgson, Acute Effects of Chlorogenic Acid on Nitric Oxide Status, Endothelial Function, and Blood Pressure in Healthy Volunteers: A Randomized Trial, J. Agric. Food Chem., 2012, 60(36), 9130–9136 CrossRef CAS PubMed.
- E. Park, I. Edirisinghe, Y. Y. Choy, A. Waterhouse and B. Burton-Freeman, Effects of Grape Seed Extract Beverage on Blood Pressure and Metabolic Indices in Individuals with Pre-Hypertension: A Randomised, Double-Blinded, Two-Arm, Parallel, Placebo-Controlled Trial, Br. J. Nutr., 2016, 115(2), 226–238 CrossRef CAS PubMed.
- Y. Nakamura, K. Torikai and H. Ohigashi, Toxic Dose of a Simple Phenolic Antioxidant, Protocatechuic Acid, Attenuates the Glutathione Level in ICR Mouse Liver and Kidney, J. Agric. Food Chem., 2001, 49(11), 5674–5678 CrossRef CAS PubMed.
- H. Saito, A. Yokoyama, S. Takeno, T. Sakai, K. Ueno, H. Masumura and H. Kitagawa, Fetal Toxicity and Hypocalcemia Induced by Acetylsalicylic Acid Analogues, Res. Commun. Chem. Pathol. Pharmacol., 1982, 38(2), 209–220 CAS.
- A. C. Mirza and S. S. Panchal, Safety Assessment of Vanillic Acid: Subacute Oral Toxicity Studies in Wistar Rats, Turk. J. Pharm. Sci., 2020, 17(4), 432–439 CrossRef CAS PubMed.
- N. E. Clarke, C. N. Clarke and R. E. Mosher, Phenolic Compounds in Chemotherapy of Rheumatic Fever, Am. J. Med. Sci., 1958, 235(1), 7–22 CrossRef CAS PubMed.
- A. Bumrungpert, S. Lilitchan, S. Tuntipopipat, N. Tirawanchai and S. Komindr, Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial, Nutrients, 2018, 10(6), 713 CrossRef PubMed.
- Y. Konishi, Y. Hitomi and E. Yoshioka, Intestinal Absorption of p-Coumaric and Gallic Acids in Rats after Oral Administration, J. Agric. Food Chem., 2004, 52(9), 2527–2532 CrossRef CAS PubMed.
- S. Lafay, A. Gil-Izquierdo, C. Manach, C. Morand, C. Besson and A. Scalbert, Chlorogenic Acid Is Absorbed in Its Intact Form in the Stomach of Rats, J. Nutr., 2006, 136(5), 1192–1197 CrossRef CAS PubMed.
- M. Monteiro, A. Farah, D. Perrone, L. C. Trugo and C. Donangelo, Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans, J. Nutr., 2007, 137(10), 2196–2201 CrossRef CAS PubMed.
- A. Farah, M. Monteiro, C. M. Donangelo and S. Lafay, Chlorogenic Acids from Green Coffee Extract Are Highly Bioavailable in Humans, J. Nutr., 2008, 138(12), 2309–2315 CrossRef CAS PubMed.
- K. Kishida and H. Matsumoto, Urinary Excretion Rate and Bioavailability of Chlorogenic Acid, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid in Non-Fasted Rats Maintained under Physiological Conditions, Heliyon, 2019, 5(10), e02708 CrossRef CAS PubMed.
- X. Li, C. Yu, Y. Lu, Y. Gu, J. Lu, W. Xu, L. Xuan and Y. Wang, Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Depside Salts from Salvia Miltiorrhiza in Rats, Drug Metab. Dispos., 2007, 35(2), 234–239 CrossRef CAS PubMed.
- M. Hasyima Omar, R. González Barrio, G. Pereira-Caro, T. M. Almutairi and A. Crozier, In Vitro Catabolism of 3′,4′-Dihydroxycinnamic Acid by Human Colonic Microbiota, Int. J. Food Sci. Nutr., 2021, 72(4), 511–517 CrossRef CAS PubMed.
- M. Naranjo Pinta, I. Montoliu, A. Aura, T. Seppänen-Laakso, D. Barron and S. Moco, In Vitro Gut Metabolism of [U–13 C]–Quinic Acid, The Other Hydrolysis Product of Chlorogenic Acid, Mol. Nutr. Food Res., 2018, 62(22), 1800396 CrossRef PubMed.
- D. Deng, J. Zhang, J. M. Cooney, M. A. Skinner, A. Adaim, D. J. Jensen and D. E. Stevenson, Methylated Polyphenols Are Poor “Chemical” Antioxidants but Can Still Effectively Protect Cells from Hydrogen Peroxide–induced Cytotoxicity, FEBS Lett., 2006, 580(22), 5247–5250 CrossRef CAS PubMed.
- N. Mateo Anson, A.-M. Aura, E. Selinheimo, I. Mattila, K. Poutanen, R. Van Den Berg, R. Havenaar, A. Bast and G. R. M. M. Haenen, Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects Ex Vivo, J. Nutr., 2011, 141(1), 137–143 CrossRef PubMed.
- Z. Li, L. Cao, C. Yang, T. Liu, H. Zhao, X. Luo and Q. Chen, Protocatechuic Acid-Based Supramolecular Hydrogel Targets SerpinB9 to Achieve Local Chemotherapy for OSCC, ACS Appl. Mater. Interfaces, 2022, 14(32), 36379–36394 CrossRef CAS PubMed.
- S. Hu, R. Zhao, T. Chen, X. Chi, Y. Li, D. Wu, B. Zhu and J. Hu, Construction of Chlorogenic Acid Nanoparticles for Effective Alleviation of Ulcerative Colitis, Food Funct., 2024, 15(18), 9085–9099 RSC.
- S. Bhattacharyya, S. M. Ahammed, B. P. Saha and P. K. Mukherjee, The Gallic Acid–Phospholipid Complex Improved the Antioxidant Potential of Gallic Acid by Enhancing Its Bioavailability, AAPS PharmSciTech, 2013, 14(3), 1025–1033 CrossRef CAS PubMed.
- A. Tresserra-Rimbau, M. Guasch-Ferré, J. Salas-Salvadó, E. Toledo, D. Corella, O. Castañer, X. Guo, E. Gómez-Gracia, J. Lapetra, F. Arós, M. Fiol, E. Ros, L. Serra-Majem, X. Pintó, M. Fitó, N. Babio, M. A. Martínez-González, J. V. Sorli, M. C. López-Sabater, R. Estruch and R. M. Lamuela-Raventós, Intake of Total Polyphenols and Some Classes of Polyphenols Is Inversely Associated with Diabetes in Elderly People at High Cardiovascular Disease Risk, J. Nutr., 2016, 146(4), 767–777 CrossRef CAS PubMed.
- on behalf of the TOSCA.IT Study Group, M. Vitale, M. Masulli, A. A. Rivellese, E. Bonora, F. Cappellini, A. Nicolucci, S. Squatrito, D. Antenucci, A. Barrea, C. Bianchi, F. Bianchini, L. Fontana, P. Fornengo, F. Giorgino, A. Gnasso, E. Mannucci, A. Mazzotti, R. Nappo, A. P. Palena, P. Pata, G. Perriello, S. Potenziani, R. Radin, L. Ricci, F. Romeo, C. Santini, M. Scarponi, R. Serra, A. Timi, A. A. Turco, M. Vedovato, D. Zavaroni, S. Grioni, G. Riccardi and O. Vaccaro, Dietary Intake and Major Food Sources of Polyphenols in People with Type 2 Diabetes: The TOSCA.IT Study, Eur. J. Nutr., 2018, 57(2), 679–688 CrossRef PubMed.
- E. Van Rymenant, J. Van Camp, B. Pauwels, C. Boydens, L. Vanden Daele, K. Beerens, P. Brouckaert, G. Smagghe, A. Kerimi, G. Williamson, C. Grootaert and J. Van De Voorde, Ferulic Acid-4-O-Sulfate Rather than Ferulic Acid Relaxes Arteries and Lowers Blood Pressure in Mice, J. Nutr. Biochem., 2017, 44, 44–51 CrossRef CAS PubMed.
- C. E. Mills, A. Flury, C. Marmet, L. Poquet, S. F. Rimoldi, C. Sartori, E. Rexhaj, R. Brenner, Y. Allemann, D. Zimmermann, G. R. Gibson, D. S. Mottram, M.-J. Oruna-Concha, L. Actis-Goretta and J. P. E. Spencer, Mediation of Coffee-Induced Improvements in Human Vascular Function by Chlorogenic Acids and Its Metabolites: Two Randomized, Controlled, Crossover Intervention Trials, Clin. Nutr., 2017, 36(6), 1520–1529 CrossRef CAS PubMed.
- T. Li, X. Li, T. Dai, P. Hu, X. Niu, C. Liu and J. Chen, Binding Mechanism and Antioxidant Capacity of Selected Phenolic Acid - β-Casein Complexes, Food Res. Int., 2020, 129, 108802 CrossRef CAS PubMed.
- D. Tagliazucchi, A. Helal, E. Verzelloni and A. Conte, The Type and Concentration of Milk Increase the in Vitro Bioaccessibility of Coffee Chlorogenic Acids, J. Agric. Food Chem., 2012, 60(44), 11056–11064 CrossRef CAS PubMed.
- M. Serafini, M. F. Testa, D. Villaño, M. Pecorari, K. Van Wieren, E. Azzini, A. Brambilla and G. Maiani, Antioxidant Activity of Blueberry Fruit Is Impaired by Association with Milk, Free Radicals Biol. Med., 2009, 46(6), 769–774 CrossRef CAS PubMed.
- M. H. Alu'datt, T. Rababah, K. Ereifej, S. Brewer and I. Alli, Phenolic–Protein Interactions in Oilseed Protein Isolates, Food Res. Int., 2013, 52(1), 178–184 CrossRef.
- A. E. Quirós-Sauceda, H. Palafox-Carlos, S. G. Sáyago-Ayerdi, J. F. Ayala-Zavala, L. A. Bello-Perez, E. Álvarez-Parrilla, L. A. De La Rosa, A. F. González-Córdova and G. A. González-Aguilar, Dietary Fiber and Phenolic Compounds as Functional Ingredients: Interaction and Possible Effect after Ingestion, Food Funct., 2014, 5(6), 1063–1072 RSC.
- P. Matić, Š. Ukić and L. Jakobek, Interactions of Phenolic Acids and β-Glucan: Studies of Adsorption Isotherms and Thermodynamics, Chem. Biochem. Eng. Q., 2021, 35(2), 177–187 CrossRef.
- G. Neunert, P. Górnaś, K. Dwiecki, A. Siger and K. Polewski, Synergistic and Antagonistic Effects between Alpha-Tocopherol and Phenolic Acids in Liposome System: Spectroscopic Study, Eur. Food Res. Technol., 2015, 241(6), 749–757 CrossRef CAS.
- A. Sentkowska and K. Pyrzyńska, Zwitterionic Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry for Analysis of Beetroot Juice and Antioxidant Interactions between Its Bioactive Compounds, LWT, 2018, 93, 641–648 CrossRef CAS.
- Y. Pan, H. Li, B. Zhang, Z. Deng and F. Shahidi, Antioxidant Interactions among Hydrophilic and Lipophilic Dietary Phytochemicals Based on Inhibition of Low–density Lipoprotein and DNA Damage, J. Food Biochem., 2022, 46(10), e14267 CAS.
- Y. Pan, Z.-Y. Deng, X. Chen, B. Zhang, Y. Fan and H. Li, Synergistic Antioxidant Effects of Phenolic Acids and Carotenes on H2O2-Induced H9c2 Cells: Role of Cell Membrane Transporters, Food Chem., 2021, 341, 128000 CrossRef CAS PubMed.
- A. Durak, U. Gawlik-Dziki and D. Sugier, Coffee Enriched with Willow (Salix Purpurea and Salix Myrsinifolia) Bark Preparation – Interactions of Antioxidative Phytochemicals in a Model System, J. Funct. Foods, 2015, 18, 1106–1116 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |