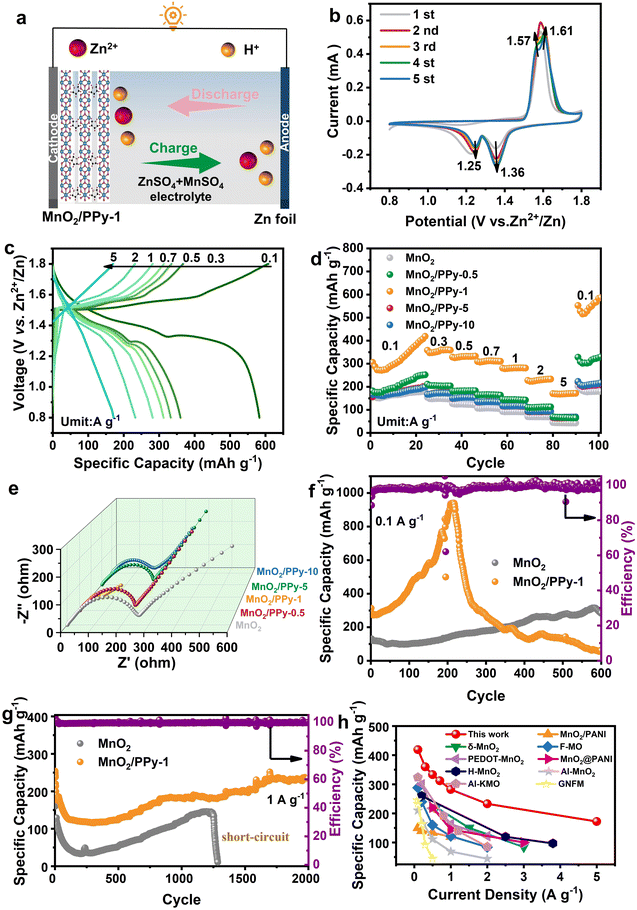
The spatial and electronic effects of polypyrrole between MnO2 layers enhance the diffusion ability of Zn2+ ions†
Le
Li
 a,
Shaofeng
Jia
a,
Yue
Shi
a,
Yuanyuan
Yang
a,
Chao
Tan
b,
Conghui
Wang
b,
Hengwei
Qiu
c,
Yongqiang
Ji
e,
Minghui
Cao
d and
Dan
Zhang
a,
Shaofeng
Jia
a,
Yue
Shi
a,
Yuanyuan
Yang
a,
Chao
Tan
b,
Conghui
Wang
b,
Hengwei
Qiu
c,
Yongqiang
Ji
e,
Minghui
Cao
d and
Dan
Zhang
 *b
*b
aShaanxi Key Laboratory of Industrial Automation, School of Mechanical Engineering, Shaanxi University of Technology, Hanzhong 723001, China
bShaanxi Key Laboratory of Catalysis, School of Chemistry and Environment Science, Shaanxi University of Technology, Hanzhong 723001, China. E-mail: zhangdan@snut.edu.cn
cState Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources, School of New Energy, North China Electric Power University, Beijing 102206, China
dSchool of Electronic and Information Engineering, Qingdao University, Qingdao 266071, China
eInstitute of Physics, Henan Academy of Sciences, Zhengzhou, 450046, China
First published on 3rd December 2024
Abstract
New electrochemical energy storage systems have stringent requirements for energy storage materials, and traditional MnO2 cannot comply with the requirements because of the problems of electrical conductivity and phase transition. In this work, a novel polypyrrole (PPy) intercalation MnO2 (MnO2/PPy-x) material was prepared and proved to be suitable for use in a high performance cathode of aqueous zinc ion batteries (AZIBs). The material characterization results proved that PPy played a key role between MnO2 layers, and the reduction of Mn and extension of Mn–O bonds inhibited the distortion reaction of MnO2, resulting in enhanced structural stability and excellent cycle life. In addition, electrochemical analysis revealed the H+/Zn2+ co-intercalation mechanism, and MnO2/PPy-1 had high electrical conductivity, and fast reaction kinetics. Density functional theory (DFT) calculation proved the change of electron distribution between the MnO2 layers. The PPy endowed MnO2 with excellent electrical conductivity. Moreover, as an interlayer spacer, it hindered charge transfer and decreased the binding ability of Zn2+ and MnO2. As a result, the electrochemical performance of MnO2/PPy-1 was greatly enhanced. The final results demonstrated that MnO2/PPy-1, which has a high conductivity and wide layer spacing, offered a superior capacity of 234 mA h g−1 and a long cycle life of 2000 cycles at a current density of 1 A g−1. In addition, according to the test results of pouch batteries, MnO2/PPy-1 shows great potential for the flexible device market because of its superior flexibility and safety. This work provides a new method and approach for the modification of MnO2-based materials.
1. Introduction
Lithium-ion batteries currently dominate the energy storage field because of their high energy density and stable cycle life. However, as society's requirements for environmental protection, economic benefits, and safety increase, the high cost, safety issues brought about by organic electrolytes, and thermal runaway phenomenon of lithium-ion batteries have become the main factors hindering their further development.1–4 Fortunately, aqueous zinc ion batteries (AZIBs) have attracted the attention of researchers because of abundant zinc reserves, their superior electrochemical energy storage level, and their high safety performance.5–9 AZIBs are considered suitable lithium-ion battery candidates. In recent years, many types of material such as manganese oxides,10,11 vanadium oxides,12,13 Prussian blue analogs,7,14 and organic compounds15,16 have been reported as cathode materials for AZIBs. Among them, MnO2 has a very long history, which can be traced back to the initial zinc–manganese battery system, and the cathode material of the first AZIBs was a MnO2 material.17 Therefore, MnO2 has a relatively mature preparation process and improvement measures, and the advantages of diverse crystal forms, suitable voltage, and high theoretical capacity have become additional advantages for its use as an ideal cathode. Nevertheless, the low conductivity of MnO2 and the easy phase transition problem during the charge and discharge process are still major challenges to the industrialization of AZIBs.Generally, MnO2 with a layered structure is considered to have a positive effect on the inhibition of phase changes during charging and discharging. To obtain a high-performance cathode for AZIBs, previous researchers have chosen to introduce polymers into the interlayers of MnO2 to play a key role between the layers and prevent structural collapse. In addition, the high conductivity and particular functional groups of the polymers effectively promote the diffusion and transfer of ions in the host materials. Similarly, the intercalation of polymers reduces the average oxidation state of the materials and inhibits the disproportionation reaction of Mn3+. For example, Huang et al. introduced polyaniline molecules into the interlayers of δ-MnO2 to limit the phase change caused by the insertion of hydrated H+/Zn2+ and the subsequent structural damage.17,18 Under the action of polyaniline, δ-MnO2 showed an extremely high utilization rate of 90% (280 mA h g−1) and an extremely long cycle life (5000 times). In conclusion, the application of the intercalation strategy brought new possibilities for high-performance AZIBs.
In previous studies, the intercalation of conductive polymers generally occurred during the polymerization process, which led to overly complex reactions between the intercalated species and the host material, making it unfavorable for researchers to analyze the intercalation mechanism. In response to this, we simplified the previous intercalation method. First, the polypyrrole (PPy) was prepared by an ice bath method, and then it was introduced into the interlayers of MnO2 by a one-step hydrothermal method to obtain the final product (MnO2/PPy-x). Characterization results such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman, scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) showed that MnO2/PPy-1 had a widened interlayer spacing and suppressed Mn distortion reaction, which contributed to enhanced ion transport ability and structural stability of the material. Through electrochemical analyses such as cyclic voltammetry (CV), galvanostatic charge and discharge (GCD), galvanostatic intermittent titration technique (GITT), and ex situ XRD, the energy storage mechanism of MnO2/PPy-1 was proposed to be related to the H+/Zn2+ insertion/extraction mechanism, and during the charge and discharge process, the appearance/dissolution of by-products Zn2SO4(OH)6·nH2O (ZSH) and ZnxMnO(OH)2 (ZMO) takes place. Density functional theory (DFT) analysis demonstrated that inserting PPy between the layers of MnO2 will greatly weaken the interaction between Zn2+ and the host material, and change the interlayer electronic environment, thereby enhancing the electrochemical reaction and conductivity of MnO2 (Fig. 1). Finally, the MnO2/PPy-1 achieved a superior capacity of 234 mA h g−1, and the capacity retention rate is 92% after 2000 cycles. In addition, the operation results of pouch batteries proved that MnO2/PPy-1 was suitable as a promising substitute for wearable devices.
2. Results and discussion
2.1. Material characterization
In this work, MnO2-based cathode materials with different proportions of intercalation were prepared, by regulating the content of PPy. In XRD characterization, it can be found that the prepared samples conform to the standard card JCPDS 80–1098 of the layered manganese dioxide phase. Specifically, as shown in Fig. 2a, with the intercalation of the PPy, the diffraction peaks attributed to the layered manganese dioxide phase become wider and weaker, and no new peaks appear, indicating that the introduction of PPy did not change the phase attributed to layered manganese dioxide. It is worth noting that the wide and weak diffraction peaks meant the MnO2/PPy-x had typical nanocrystal characteristics, which contributed to improving the electrical conductivity and enhancing electrochemical reactions. In addition, with the increase of PPy, a gradually strengthening peak in the range of 20–25° in the XRD pattern emerged, which was attributed to the characteristic peak of PPy and served as important evidence for the successful introduction of PPy. | ||
| Fig. 2 (a) XRD of MnO2/PPy-x. (b) FTIR spectra of MnO2/PPy-x. (c) Raman spectra of MnO2/PPy-x. SEM of MnO2/PPy-1 at (d) 1 μm, and (e) 200 nm. (f) SEM–EDX of MnO2/PPy-1. | ||
FTIR is a common tool to explore the composition and structure of materials. As shown in Fig. 2b, the infrared absorption peak at 500–800 cm−1 belongs to the stretching vibration of Mn–O bonds in the MnO6 octahedron.10 The diffraction peaks at 1594 cm−1 and 3426 cm−1 were attributed to the interlayer water of the material. The diffraction peaks of MnO2/PPy-x were enhanced because of the successful intercalation of PPy, which increased the interlayer space of MnO2, thereby resulting in more interlayer water molecules, which was beneficial for the insertion/extraction of Zn2+.11,19 In addition, interlayer water molecules played an important role in stabilizing the material structure and balancing the charge in transition metal oxides. Additionally, the combination of water molecules and confined alkali metal ions achieved high-efficiency charge storage in neutral electrolytes.20,21 The peaks at 1350 cm−1 and 1387 cm−1 correspond to the stretching vibration of the C–N bonds and the deformation and stretching of the pyrrole ring (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).11,22,23 In particular, vibration peaks related to the C–N bonds and C
C).11,22,23 In particular, vibration peaks related to the C–N bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds appeared for MnO2. This may be affected by the elements C and N in the air, which was proved in later elemental mapping. In addition, the stronger vibration peaks in the MnO2/PPy-x indicated that the introduction of a trace amount of PPy increased the content of elements C and N in the material.
C bonds appeared for MnO2. This may be affected by the elements C and N in the air, which was proved in later elemental mapping. In addition, the stronger vibration peaks in the MnO2/PPy-x indicated that the introduction of a trace amount of PPy increased the content of elements C and N in the material.
Raman spectra further revealed the changes in the composition and structure of MnO2 after the introduction of PPy. As shown in Fig. 2c, the vibration peaks of the Mn–O bonds characteristic of MnO2 (570 cm−1 and 640 cm−1) in MnO2/PPy-x still existed but had changed, which was the result of PPy intercalation.11 In addition, the regulation of the Mn–O bonds was conducive to enhancing the storage efficiency of Zn2+ ions.24 The characteristic peaks at 903 cm−1 and 1083 cm−1 were attributed to the in-plane bending and deformation of the C–H bonds and N–H bonds.25 The vibration peak of the pyrrole ring was located at 1273 cm−1, and the vibration peak at 1486 cm−1 was attributed to the stretching mode of the π-conjugated structure of the polymer main chain. The appearance of these characteristic peaks demonstrated the successful intercalation of PPy.26,27 In conclusion, the similar results for the FTIR spectra and Raman spectra indicated that PPy had been successfully introduced into the interlayers of MnO2, effectively expanding the interlayer spacing of the host material and greatly improving the diffusion rate of Zn2+.
SEM and energy dispersive X-ray (EDX) spectroscopy were used to analyze and study the morphological structure and elemental composition of MnO2 and MnO2/PPy-1. Fig. 2d and e exhibit the morphology of MnO2/PPy-1 at scales of 1 μm and 100 nm, respectively. They show a typical nanoflower structure composed of nanosheets with a thickness of 12.9 nm, which is consistent with the structure of MnO2 (Fig. S1†). Compared to MnO2 (15.1 nm), the intercalation of PPy reduced the thickness of the MnO2 nanosheet, resulting in the optimization of interlayer space. Meanwhile, PPy particles were observed on the surface of MnO2/PPy-1 with an uneven distribution (red box), which led to the rapid capacity decay. This finding will be proved in the following text. The EDX analysis of MnO2 and MnO2/PPy-1 is shown in Fig. S2† and Fig. 2f. Through comparison, it was found that the increased content of element O and the appearance of an additional 2.65% of element N provided strong evidence for the introduction of PPy. In addition, the appearance of element C in MnO2 confirmed the appearance of C-related groups in the FTIR spectrum.
The chemical bonds and valence states of the materials were analyzed by XPS. The full XPS spectra of MnO2 and MnO2/PPy-1 are shown in Fig. 3a. This shows the energy spectra of elements Mn, O, N, and C. Fig. 3b shows the high-resolution XPS spectrum of Mn 2p. The Mn 2p3/2 and Mn 2p1/2 features are shown at 642.3 eV and 654.2 eV, respectively. The spin energy difference of MnO2/PPy-1 was 11.9 eV, which is typical of an MnO2 structure and is similar to previous studies.11,19 In addition, compared with MnO2 (11.76 eV), the higher spin energy of MnO2/PPy-1 was attributed to the intercalation of PPy changing the electronic structure of the host material and enhancing its electrical conductivity. The two sets of peaks of Mn 2p can be fitted to Mn3+ and Mn4+. In addition, a characteristic peak attributed to Mn2+ was found at 643.0 eV in the MnO2/PPy-1 material.28–30 The Mn2+ originated from the reduction of the average oxidation state of Mn caused by the intercalation of PPy, which effectively inhibited the distortion reaction of Mn. Therefore, enhanced material structural stability and excellent cycle life were obtained. As shown in Fig. 3c, Mn–O–Mn bonds, Mn–O–H bonds, and H–O–H bonds were detected in the spectrum of O 1s, located at 530.0 eV, 531.9 eV and 533.2 eV, respectively.31 Compared with MnO2, the increased H–O–H bonds in MnO2/PPy-1 demonstrated the introduction of PPy, effectively expanding the interlayer spacing of MnO2 and increasing the content of lattice water. The increase in the content of lattice water contributed to the promotion of the diffusion of H+/Zn2+, reduced the internal resistance of the battery, increased the effective contact area of the electrode, and provided more electrochemical reaction interfaces.32 In addition, the highest binding energy of the H–O–H bonds corresponded to the concentration of adsorbed oxygen, and was an important indicator for evaluating the content of oxygen vacancies.33 The XPS spectrum of C 1s is shown in Fig. S3.† Notably, 284.8 eV, 285.7 eV, and 288.9 eV correspond to C–C bonds, C–N bonds, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively, which is consistent with the results of FTIR and Raman spectra.30,34 In addition, element N, with an atomic ratio of 0.51%, was detected in XPS, and the XPS spectrum is shown in Fig. 3d. The characteristic peaks of 398.3 eV and 399.2 eV were attributed to
O bonds, respectively, which is consistent with the results of FTIR and Raman spectra.30,34 In addition, element N, with an atomic ratio of 0.51%, was detected in XPS, and the XPS spectrum is shown in Fig. 3d. The characteristic peaks of 398.3 eV and 399.2 eV were attributed to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds, and –NH– bonds.26 These results demonstrated the successful insertion of PPy.
N– bonds, and –NH– bonds.26 These results demonstrated the successful insertion of PPy.
 | ||
| Fig. 3 (a) XPS full spectrum of MnO2/PPy-1 and MnO2. (b) Mn 2p, (c) O 1s, and (d) N 1s XPS spectra of MnO2/PPy-1 and MnO2. | ||
2.2. Electrochemical analysis
A CR2032 coin cell was assembled with zinc foil as the anode and a 2 M ZnSO4 + 0.2 M MnSO4 solution as the electrolyte to evaluate the electrochemical performance of the MnO2 and MnO2/PPy-x cathodes. The working mechanism is shown in Fig. 4a. CV of the materials was performed in the voltage range 0.8–1.8 V to evaluate their cycle stability and reaction kinetics (Fig. 4b). The MnO2/PPy-1 exhibited two pairs of redox peaks at 1.25/1.57 V and 1.36/1.61 V, corresponding to the two-step insertion mechanism of Zn2+/H+.35 In addition, the reduced redox potential difference made the charge–discharge process more reversible. In particular, the results of five cycles at a scanning rate of 0.1 mV s−1 verified the reversible performance of the material. Except for the activation in the first cycle, the CV curves of the cycles generally coincide. The GCD of MnO2/PPy-1 was carried out at a current density of 0.1–5 A g−1 (Fig. 4c), and the results were found to be consistent with the CV results. Two charge–discharge platforms were discovered, corresponding to the insertion/extraction of Zn2+/H+. Clearly, as the current density was increased, the discharge platform attributed to Zn2+ insertion disappeared. The reason for this is that at a high current density, the H+ with a smaller ionic radius is more likely to be extracted from the interlayers of MnO2.36,37Rate performance is an important indicator of battery performance. The current density was increased from 0.1 A g−1 to 5 A g−1, and the rate capabilities of the MnO2 and MnO2/PPy-x cathodes were evaluated during this period, as shown in Fig. 4d. The specific capacities of MnO2/PPy-1 were 419, 360, 333, 312, 282, 233, and 172 mA h g−1. When the current was restored to 0.1 A g−1, the MnO2/PPy-1 exhibited an enhanced specific capacity of 587 mA h g−1. The sources of these enhanced capacities will be explained when discussing the cycle performance later. Compared with MnO2, the MnO2/PPy-1 clearly exhibited increased rate performance. This result demonstrated the appropriateness of introducing PPy between the layers of MnO2 to enhance the conductivity and structural stability of the material. To explore the influence of PPy on the electrical conductivity of MnO2, electrochemical impedance spectroscopy (EIS) was performed. As shown in Fig. 4e, the electrochemical impedance of MnO2 was 263 Ω, while the charge transfer resistance of MnO2/PPy-1 was reduced to 37 Ω, much lower than that of MnO2, indicating the effective improvement of the electrical conductivity of MnO2 by PPy intercalation.
Benefiting from the high conductivity and interlayer supporting role of the PPy, the MnO2/PPy-1 exhibited excellent cycling performance. The performance at a current of 0.1 A g−1 is shown in Fig. 4f. The initial capacities of the MnO2 and MnO2/PPy-1 samples were 127 mA h g−1 and 257 mA h g−1, respectively. In particular, the capacity of MnO2/PPy-1 increased significantly during the cycling process, and the highest capacity reached even an astonishing 817 mA h g−1. As found in SEM, in the initial state, the PPy was unevenly coated on the surface of the MnO2, and the unique structural reorganization of δ-MnO2 during the charge and discharge process made PPy insert into MnO2 during the cycling process, resulting in the capacity being increased. However, the MnO2/PPy-1 had a regrettable capacity decay. This may be because the secondary intercalation of the PPy during the structural reorganization process hindered the dissolution of the ZSH by-products. The deposition of a large amount of ZSH hindered the ion transmission and led to a reduction in cathode utilization.38 Surprisingly, after a short activation process (0.1 A g−1), the MnO2/PPy-1 retained a superior specific capacity of 234 mA h g−1 and a high capacity retention rate of 92% after 2000 cycles at a current density of 1 A g−1 (Fig. 4g), which was completely different from the performance at a current of 0.1 A g−1. This may be attributed to the short activation process allowing the material to have a standing process after the secondary intercalation of the PPy, so the ZSH by-product had time to dissolve. In addition, the MnO2 was short-circuited after 1300 cycles, which once again demonstrated the effective improvement of PPy intercalation on the performance of MnO2. Although there were some defects in this work, upon comparing with some published works, it was found that the MnO2/PPy-1 samples still had certain advantages19,39–46 (Fig. 4h and Table S1†).
Wearable devices have strict standards for the flexibility and safety of electronic devices, which is beginning to prompt researchers to pay more attention to the operation of batteries in bent and punctured states.47,48 We prepared a pouch battery with the MnO2/PPy-1 electrode as the cathode and observed its operation in normal, bent, and torn states. As shown in Fig. 5a–c, the prepared pouch battery can operate normally in both normal and bent states, and can even work normally after being cut, and no heating or fire phenomena were observed. The performance of the pouch battery under extreme conditions demonstrated that the MnO2/PPy-1 had excellent flexibility and high safety, reflecting its considerable prospects in the flexible device market.
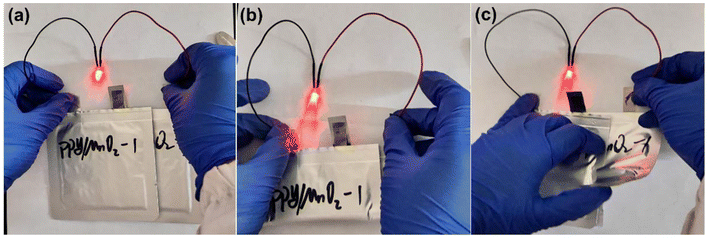 | ||
| Fig. 5 Photographs of the light bulb of a pouch battery prepared with MnO2/PPy-1 as a cathode: (a) normal state, (b) bending state, and (c) puncturing state. | ||
2.3. Mechanism analysis
Reaction mechanism research is an important means to describe the internal connection of electrochemical reactions inside batteries. Therefore, we conducted an in-depth analysis of CV curves at different scanning rates. As shown in Fig. 6a, two pairs of redox peaks corresponded to the two-step insertion mechanism of the MnO2/PPy-1 electrode. At high scanning rates, the oxidation peak at 1.61 V gradually disappeared, which was attributed to the faster diffusion speed of H+, consistent with the GCD results. The value of b was an index for evaluating the capacity contribution in the energy storage process of MnO2/PPy-1, as shown in eqn (1):12,37| i = avb | (1) |
log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) + log(a) log(v) + log(a) | (2) |
| i = k1v + k2v1/2 | (3) |
Ex situ XRD is an important means to demonstrate the structural evolution and energy storage mechanism of the MnO2/PPy-1 electrode during the cycling process. As shown in Fig. 7a, the strong peaks at 26.5° and 54.6° of the MnO2/PPy-1 electrode were attributed to the graphite foil current collector. When charged to 1.4 V, new diffraction peaks appeared at 16.3°, 21.3°, and 58.7° (red boxes), which was attributed to the formation of the by-product ZSH. The embedding of H+ into the host material led to an increase in OH− in the electrolyte, which combined with Zn2+ detached from the anode to generate ZSH.19 In addition, the new peaks located at 32°–36° were attributed to ZMO, which was caused by the combination of Mn2+ and OH− ions in the electrolyte.50 When discharged back to 0.8 V, the peaks related to ZSH and ZMO disappeared, demonstrating the high reversibility of MnO2/PPy-1, and the specific chemical equations are as follows:
| 2Zn2+ + SO42− + 6OH− + nH2O ↔ Zn2SO4(OH)6·nH2O | (4) |
 | (5) |
 | ||
| Fig. 7 (a) Ex situ XRD patterns of the MnO2/PPy-1 cathode in different charge and discharge states. (b) GITT profiles and corresponding diffusion coefficient of H+/Zn2+ for the MnO2/PPy-1 cathode. | ||
Further, the GITT technique was used to evaluate the ion diffusion of the MnO2 and MnO2/PPy-1. The ion diffusion coefficient (DZn2+) can be expressed as eqn (6):
| DZn2+ = 4/πτ·(mBVM/MBA)2·(ΔES/ΔEτ)2 | (6) |
2.4. Theoretical calculation
Theoretical calculations were used to analyze the structural models, density of states (DOS), interaction with Zn2+, and electron density distribution after adsorbing Zn2+ for the MnO2 and MnO2/PPy-1. As shown in Fig. 8a and b, the intercalation of PPy clearly enhanced the interlayer spacing of the MnO2, and optimized the diffusion rate of interlayer ions, consistent with the material characterization results. The adsorption capacity of MnO2 and MnO2/PPy-1 for Zn2+ and their adsorption models are shown in Fig. 8c–e. Compared with MnO2 (−1.13 eV), the MnO2/PPy-1 had a lower Zn2+ adsorption capacity (−0.65 eV). This result indicated that the PPy layer effectively weakened the interaction between Zn2+ and MnO2 and contributed to increased ion diffusion. Fig. 8f shows the DOS of the MnO2 and MnO2/PPy-1. The MnO2 exhibited a wide band gap of 1.482 eV, demonstrating its poor electrical conductivity, consistent with the electrochemical results. After PPy intercalation, the band gap of MnO2/PPy-1 is 1.042 eV. The reduced band gap energy makes the electron transition in the material easier, resulting in more electrons jumping from the valence band to the conduction band and enhancing the conductivity of MnO2. In addition, new DOS appears near the Fermi level, proving that the intercalation of PPy changes the interlayer electronic environment of MnO2 and greatly improves its conductivity. Notably, the DOS of PPy appeared in the range of −10 to −15 eV, demonstrating the successful intercalation of the PPy. The differential charge densities of the MnO2 and MnO2/PPy-1 are shown in Fig. 8g and h. There was a large amount of charge accumulation and dissipation around Zn and O. But for the MnO2/PPy-1, the existence of the PPy layer blocked the charge transfer behavior, and weakened the electrostatic interaction between Zn2+ and MnO2, consistent with the adsorption energy results. The DFT calculation results demonstrated that the PPy intercalation will optimize the interlayer spacing and electronic environment, and enhance the electrical conductivity of the MnO2. In addition, the PPy layer blocked the binding between Zn2+ and MnO2, improving the reaction kinetics of Zn2+, which contributed to the enhanced electrochemical performance of the MnO2/PPy-1.3. Conclusion
In conclusion, a new PPy intercalation method was proposed to improve the electrochemical performance of MnO2. The SEM results showed that PPy intercalation did not change the structure of MnO2, but increased its interlayer spacing compared to the original morphology. The FTIR and XPS results demonstrated the increase in the content of interlayer water molecules, which was beneficial for high-efficiency charge storage in the electrolyte. In addition, the reduction of Mn played an important role in inhibiting its distortion reaction. It is worth noting that the unique electrical conductivity of the PPy made a huge contribution to the electrochemical performance of MnO2/PPy-1. Thanks to this, the MnO2/PPy-1 showed an extraordinary specific capacity (817 mA h g−1), a long life of 2000 cycles, and an excellent retention rate of 92%. The CV, GCD and GITT results revealed the H+/Zn2+ co-insertion mechanism of MnO2/PPy-1. Theoretical calculation results demonstrated that PPy played a role as an interlayer pillar, and the PPy layer as a spacer in MnO2 effectively weakened its electrostatic interaction, and enhanced the diffusion ability of Zn2+. Finally, the good flexibility and satisfactory safety of MnO2/PPy-1 provided a promising candidate for wearable devices.Data availability
All relevant data are within the paper.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported the National Natural Science Foundation of China (Grant No. 62101296 and 52303335), the China Postdoctoral Science Foundation (2021M702656 and 2023M730099), the Natural Science Foundation of Shaanxi Province (Grant No. 2021JQ-756 and 2023-JC-QN0577), the Graduate Innovation Fund of the School of Mechanical Engineering, Shaanxi University of Technology (SLGJX202404).References
- G. Offer, Y. Patel, A. Hales, L. B. Diaz and M. Marzook, Cool metric for lithium-ion batteries could spur progress, Nature, 2020, 582, 485–487 CrossRef CAS PubMed.
- H. Yang, F. He, J. Shen, Z. Chen, Y. Yao, L. He and Y. Yu, 2D Nb2O5@2D metallic RuO2 heterostructures as highly reversible anode materials for lithium-ion batteries, Energy Lab, 2023, 1, 220007 CrossRef.
- S. Zheng, X. Wang, Z. Gu, J. Guo, X. Wu and H. Xu, Advances and Challenges on Recycling the Electrode and Electrolyte Materials in Spent Lithium-Ion Batteries, Mater. Lab, 2022, 1, 220036 Search PubMed.
- H. Ye and Y. Li, A perspective on sulfur-equivalent cathode materials for lithium-sulfur batteries, Energy Lab, 2023, 1, 220003 Search PubMed.
- S. Jia, L. Li, Y. Shi, C. Wang, M. Cao, Y. Ji and D. Zhang, Recent development of manganese dioxide-based materials as zinc-ion battery cathode, Nanoscale, 2024, 16, 1539–1576 RSC.
- L. E. Blanc, D. Kundu and L. F. Nazar, Scientific Challenges for the Implementation of Zn-Ion Batteries, Joule, 2020, 4, 771–799 CrossRef CAS.
- H. Tan, C. Meng, T. Sun, X. Wu, H. Liu and J. Wang, Boosting zinc anode durability through synergistic inner Helmholtz plane and interfacial electric field regulation, Sci. Bull., 2024, 69, 2025–2029 CrossRef CAS PubMed.
- C. Meng, W. He, H. Tan, X. Wu, H. Liu and J. Wang, A eutectic electrolyte for an ultralong-lived Zn//V2O5 cell: an in situ generated gradient solidelectrolyte interphase, Energy Environ. Sci., 2023, 16, 3587–3599 RSC.
- Z. Song, C. Yang, N. T. Kiatwisarnkij, A. Lu, N. Tunghathaithip, K. Lolupiman, T. Bovornratanaraks, X. Zhang, G. He and J. Qin, Polyethylene Glycol-Protected Zinc Microwall Arrays for Stable Zinc Anodes, ACS Appl. Mater. Interfaces, 2024, 16, 64834–64845 CrossRef CAS PubMed.
- W. Lv, J. Meng, X. Li, C. Xu, W. Yang, S. Duan, Y. Li, X. Ju, R. Yuan, Y. Tian, M. Wang, X. Lyu, P. Pan, X. Ma, Y. Cong and Y. Wu, Boosting zinc storage in potassium-birnessite via organic-inorganic electrolyte strategy with slight N-methyl-2-pyrrolidone additive, Energy Storage Mater., 2023, 54, 784–793 CrossRef.
- J. Xu, Q. Gao, Y. Xia, X. Lin, W. Liu, M. Ren, F. Kong, S. Wang and C. Lin, High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole, J. Colloid Interface Sci., 2021, 598, 419–429 CrossRef CAS PubMed.
- Y. Liu, T. Wang, Y. Sun, M. Zhang, G. Gao, J. Yang and K. Cai, Fast and efficient in situ construction of low crystalline PEDOT-intercalated V2O5 nanosheets for high-performance zinc-ion battery, Chem. Eng. J., 2024, 484, 149501 CrossRef CAS.
- R. Li, F. Xing, T. Li, H. Zhang, J. Yan, Q. Zheng and X. Li, Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery, Energy Storage Mater., 2021, 38, 590–598 CrossRef.
- Y. Zeng, J. Xu, Y. Wang, S. Li, D. Luan and X.-W. Lou, Formation of CuMn Prussian Blue Analog Double-shelled Nanoboxes Toward Long-life Zn-ion Batteries, Angew. Chem., Int. Ed., 2022, 61, e202212031 CrossRef CAS PubMed.
- S. Gong, Y. Xie, J. Zhao, Q. Liang, R. Huang, X. Jia, D. Chao and C. Wang, An electrolyte-rich nano-organic cathode constructs an ultra-high voltage Zinc-ion battery, Chem. Eng. J., 2023, 476, 146619 CrossRef CAS.
- Z. Song, L. Miao, H. Duan, Y. Lv, L. Gan and M. Liu, Multielectron Redox-Bipolar Tetranitroporphyrin Macrocycle Cathode for High-Performance Zinc-Organic Batteries, Angew. Chem., Int. Ed., 2024, 63, e202401049 CrossRef CAS PubMed.
- C. Xu, B. Li, H. Du and F. Kang, Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery, Angew. Chem., Int. Ed., 2012, 54, 933–935 CrossRef PubMed.
- J. Huang, Z. Wang, M. Hou, X. Dong, Y. Liu, Y. Wang and Y. Xia, Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery, Nat. Commun., 2018, 9, 2906 CrossRef PubMed.
- N. Li, Z. Hou, S. Liang, Y. Cao, H. Liu, W. Hua, C. Wei, F. Kang and J.-G. Wang, Highly flexible MnO2@polyaniline core-shell nanowire film toward substantially expedited zinc energy storage, Chem. Eng. J., 2022, 452, 139408 CrossRef.
- P. Simon and Y. Gogotsi, Confned water controls capacitance, Nat. Mater., 2021, 20, 1597–1598 CrossRef CAS PubMed.
- S. Boyd, K. Ganeshan, W.-Y. Tsai, T. Wu, S. Saeed, D.-E. Jiang, N. Balke, A. C. T. V. Duin and V. Augustyn, Effects of interlayer confinement and hydration on capacitive charge storage in birnessite, Nat. Mater., 2021, 20, 1689–1694 CrossRef CAS PubMed.
- T. Niu, J. Li, Y. Qi, X. Huang and Y. Ren, Preparation and electrochemical properties of α-MnO2/rGO-PPy composite as cathode material for zinc-ion battery, J. Mater. Sci., 2021, 56, 16582–16590 CrossRef CAS.
- Y. Song, M. Shang, J. Li and Y. Su, Continuous and controllable synthesis of MnO2/PPy composites with core-shell structures for supercapacitors, Chem. Eng. J., 2021, 405, 127059 CrossRef CAS.
- P. G. An, W. Li, Y. Leng, T. Zhang, J. Zhu, T. Li, W. Li and Y. Han, Preparation and electrochemical performances of core-shell microtubular PPy/CNT/MOF ternary composites with spherical Co-MOF as sacrificial template, Polymer, 2024, 300, 127004 CrossRef CAS.
- W. Lv, Z. Shen, X. Li, J. Meng, W. Yang, F. Ding, X. Ju, F. Ye, Y. Li, X. Lyu, M. Wang, Y. Tian and C. Xu, Discovering Cathodic Biocompatibility for Aqueous Zn–MnO2 Battery: An Integrating Biomass Carbon Strategy, Nano-Micro Lett., 2024, 16, 109 CrossRef CAS PubMed.
- S. Rajkumar, S. Dhineshkumar, N. Arunprakash, P. Raychel, S. A. Kumar and J. P. Merlin, Fabrication of SrWO4/PPy composite as electrode material for high-performance supercapacitors, Opt. Mater., 2023, 142, 113934 CrossRef CAS.
- A. Huang, W. Zhou, A. Wang, M. Chen, J. Chen, Q. Tian and J. Xu, Self-initiated coating of polypyrrole on MnO2/Mn2O3 nanocomposite for high-performance aqueous zinc-ion batteries, Appl. Surf. Sci., 2021, 545, 149041 CrossRef CAS.
- H. Yao, H. Yu, Y. Zheng, N. Li, S. Li, D. Luan, X.-W. Lou and L. Yu, Pre-intercalation of Ammonium Ions in Layered δ-MnO2 Nanosheets for High-Performance Aqueous Zinc-Ion Batteries, Angew. Chem., Int. Ed., 2023, 62, e202315257 CrossRef CAS PubMed.
- S. Cui, D. Zhang and Y. Gan, Traditional Electrochemical Zn2+ Intercalation/Extraction Mechanism Revisited: Unveiling Ion-Exchange Mediated Irreversible Zn2+ Intercalation for the δ-MnO2 Cathode in Aqueous Zn Ion Batteries, Adv. Energy Mater., 2024, 14, 2302655 CrossRef CAS.
- D. Li, Q. Gao, H. Zhang, Y. Wang, W. Liu, M. Ren, F. Kong, S. Wang and J. Chang, MnO2 particles grown on the surface of N-doped hollow porous carbon nanospheres for aqueous rechargeable zinc ion batteries, Appl. Surf. Sci., 2020, 510, 145458 CrossRef CAS.
- X. Hu, Y. Liao, M. Wu, W. Zheng, M. Long and L. Chen, Mesoporous copper-doped δ-MnO2 superstructures to enable high-performance aqueous zinc-ion batteries, J. Colloid Interface Sci., 2024, 674, 297–305 CrossRef CAS PubMed.
- W. Lv, Z. Shen, J. Liu, X. Li, F. Ding, D. Zhang, L. Miao, X. Lyu, R. Li, M. Wang, Y. Li, J. Meng and C. Xu, In situ synthetic C-encapsulated δ-MnO2 with O-vacancies: A versatile programming in bio-engineering, Sci. Bull., 2024 DOI:10.1016/j.scib.2024.10.034.
- L. Hu, R. Gao, A. Zhang, R. Yang, X. Zang, S. Wang, S. Yao, Z. Yang, H. Hao and Y.-M. Yan, Cu2+ intercalation activates bulk redox reactions of MnO2 for enhancing capacitive performance, Nano Energy, 2020, 74, 104891 CrossRef CAS.
- X. Li, Q. Zhou, Z. Yang, D. Qiu, H. Qiu, X. Huang and Y. Yu, Unraveling the role of nitrogen-doped carbon nanowires incorporated with MnO2 nanosheets as high performance cathode for zinc ion batteries, Energy Environ. Mater., 2023, 6, e12378 CrossRef CAS.
- A. Zhang, R. Zhao, Y. Wang, J. Yue, J. Yang, X. Wang, C. Wu and Y. Bai, Hybrid Superlattice-Triggered Electron-Entropy Stimulation and Selective Proton Grotthuss-Intercalation in Polymer-Intercalated δ-MnO2 for High-Performance Zn–MnO2 Battery, Angew. Chem., Int. Ed., 2023, 62, e202313163 CrossRef CAS PubMed.
- Y. Zhao, S. Zhang, Y. Zhang, J. Liang, L. Ren, H. J. Fan, W. Liu and X. Sun, Vacancy-rich Al-doped MnO2 cathode breaks the trade-off between kinetics and stability for high-performance aqueous Zn-ion battery, Energy Environ. Sci., 2024, 17, 1279–1290 RSC.
- X. Zhao, F. Zhang, H. Li, H. Dong, C. Yan, C. Meng, Y. Sang, H. Liu, Y. Guo and S. Wang, Dynamic Heterostructure Designing of MnO2 for High-Performance Aqueous Zinc-Ion Batteries, Energy Environ. Sci., 2024, 17, 3629–3640 RSC.
- N. Jiang, Y. Zeng, Q. Yang, P. Lu, K. Qu, L. Ye, X. Lu, Z. Liu, X. Li, Y. Tang, J. Cao, S. Chen, C. Zhi and J. Qiu, Deep ion mass transfer addressing the capacity shrink challenge of aqueous Zn||MnO2 batteries during the cathode scaleup, Energy Environ. Sci., 2024, 17, 8904–8914 RSC.
- C. Zuo, F. Chao, M. Li, Y. Dai, J. Wang, F. Xiong, Y. Jiang and Q. An, Improving Ca-ion Storage Dynamic and Stability by Interlayer Engineering and Mn-dissolution Limitation Based on Robust MnO2@PANI Hybrid Cathode, Adv. Energy Mater., 2023, 13, 2301014 CrossRef CAS.
- Y. Chen, Z. Lu, T. Chen, Y. Liu, G. Han and G. Xu, Template-free hydrothermal synthesis of δ-MnO2 hierarchical nanoflowers with potassium ions intercalation as cathodes for high-performing aqueous zinc ion batteries, J. Electroanal. Chem., 2023, 929, 117084 CrossRef CAS.
- D. Wang, Z. Liu, X.-W. Gao, Q. Gu, L. Zhao and W.-B. Luo, Massive anionic fluorine substitution two-dimensional δ-MnO2 nanosheets for high-performance aqueous zinc-ion battery, J. Energy Storage, 2023, 72, 108740 CrossRef.
- H. Chen, W. Ma, J. Guo, J. Xiong, F. Hou, W. Si, Z. Sang and D. Yang, PEDOT-intercalated MnO2 layers as a high-performance cathode material for aqueous Zn-ion batteries, J. Alloys Compd., 2023, 932, 167688 CrossRef CAS.
- P. Chomkhuntod, K. Hantanasirisakul, S. Duangdangchote, N. Phattharasupakun and M. Sawangphruk, The charge density of intercalants inside layered birnessite manganese oxide nanosheets determining Zn-ion storage capability towards rechargeable Zn-ion batteries, J. Mater. Chem. A, 2022, 10, 5561–5568 RSC.
- S. Zhou, X. Wu, H. Du, Z. He, X. Wu and X. Wu, Dual metal ions and water molecular pre-intercalated δ-MnO2 spherical microflowers for aqueous zinc ion batteries, J. Colloid Interface Sci., 2022, 623, 456–466 CrossRef CAS PubMed.
- B. Cao, H. Liu, P. Zhang, N. Sun, B. Zheng, Y. Li, H. Du and B. Xu, Flexible MXene Framework as a Fast Electron/Potassium-Ion Dual-Function Conductor Boosting Stable Potassium Storage in Graphite Electrodes, Adv. Funct. Mater., 2021, 31, 2102126 CrossRef CAS.
- M. Li, C. Liu, J. Meng, P. Hei, Y. Sai, W. Li, J. Wang, W. Cui, Y. Song and X.-X. Liu, Hydroxylated Manganese Oxide Cathode for Stable Aqueous Zinc-Ion Batteries, Adv. Funct. Mater., 2024, 34, 2405659 CrossRef CAS.
- A. Hu, Y. Hong, S. Feng and S. Bian, Wearable Sweat Biosensors on Sports Analysis, Mater. Lab, 2022, 1, 220028 Search PubMed.
- Q. Zheng, W. Cao and M. Cao, Multifunctional conductive organohydrogel with environmental tolerance for flexible wearable devices, Matter, 2024, 7, 3240–3242 CrossRef.
- Y. Yuan, R. Sharpe, K. He, C. Li, M. T. Saray, T. Liu, W. Yao, M. Cheng, H. Jin, S. Wang, K. Amine, S.-Y. Reza, M. S. Islam and J. Lu, Understanding intercalation chemistry for sustainable aqueous zinc–manganese dioxide batteries, Nat. Sustainability, 2022, 5, 890–898 CrossRef.
- Z. Wang, Y. Fang, J. Shi, Z. Ma, X. Qu and P. Li, Conversion Reaction of the Zinc Sulfate Hydroxide Activated by Voltage Modulation for High-Performance Aqueous Zn/MnO2 Batteries, Adv. Energy Mater., 2024, 14, 2303739 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02739f |
| This journal is © the Partner Organisations 2025 |