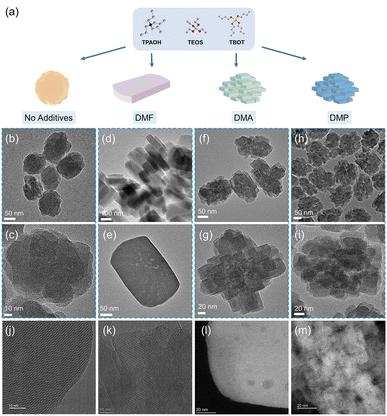
Regulation of TS-1 zeolite morphology by crystallization modifiers to boost the oxidative reactions†
Jiani
Zhang
ab,
Risheng
Bai
a,
Shiyu
Zhou
a and
Jiyang
Li
 *a
*a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China. E-mail: lijiyang@jlu.edu.cn
bInternational Center of Future Science, Jilin University, Qianjin Street 2699, Changchun 130012, China
First published on 14th January 2025
Abstract
TS-1 zeolite is a heterogeneous catalyst renowned for its remarkable catalytic performance in oxidation processes. Thus, tailoring its morphology is crucial for targeted reactions. Herein, we presented a facile and versatile method to regulate the orientation-dependent growth behavior of TS-1 zeolite by utilizing crystal growth modifiers (CGMs). Nanosheet TS-1 zeolites with a short b-axis dimension were synthesized using N,N-dimethylformamide (DMF) as a CGM, while thin-layer assemblies along the c-axis dimension with a hierarchical structure were prepared in the presence of N,N-dimethylacetamide (DMA) or N,N-dimethylpropionamide (DMP) as CGMs. The directional adsorption of different CGMs on specific crystal facets of zeolite was found to be the core reason for modulating the morphology of TS-1. Diverse TS-1 morphologies were suitable for different oxidation reactions: nanosheets enhanced selectivity in 1-hexene epoxidation, while thin-layer assemblies showed excellent activity and stability in dibenzothiophene oxidative desulfurization. This work offers further insights into customizing zeolite properties and optimizing their catalytic performance.
10th anniversary statementIt is a great pleasure for me to contribute an article to Inorganic Chemistry Frontiers on its 10th anniversary. Inorganic Chemistry Frontiers has become an established journal in the field of chemical sciences. In 2016, we published our first article titled “A new methylviologen-templated zinc gallophosphate zeolite with photo-/thermochromism, fluorescent and photoelectric properties” in Inorganic Chemistry Frontiers, and there have been 10 articles published to date. This journal has witnessed the continuous progress of our research on zeolites and related porous materials. I hope that the journal will continue to showcase pioneering research in the field of nanoporous materials. Happy anniversary! |
Introduction
Zeolites, a class of significant microporous crystalline materials, have long been the subject of both fundamental and applied research.1–5 They have become one of the most widely used heterogeneous catalysts across various applications, particularly in the petrochemical industry.6–10 Apart from the inherent catalytic active sites of zeolites, the catalytic rate and selectivity are influenced by the diffusion speed and orientation of reactants, meaning that the catalytic activity of zeolites depends not only on their pore size and cage/cavity structure but also on their crystal morphology. For example, the large, highly alternating twin ZSM-5 zeolite demonstrates a p-xylene selectivity exceeding 99% (compared to 24.1% exhibited by a commercial sample), which is attributable to the increased exposure of (100) faces featuring characteristic sinusoidal channels.11 ZSM-5 nanosheets yield light hydrocarbons (C1–C7) converted from polyethylene at a rate as high as 74.6%, with a selectivity of 83.9% for C3–C6.5 Therefore, the morphology of zeolite should be tailored to align with the specific demands of the target reaction.Titanosilicate-1 (TS-1, MFI zeotype) zeolite has attracted significant attention due to its outstanding catalytic performance in diverse oxidation reactions, such as alkene epoxidation and oxidative desulfurization.12 The structure of TS-1 is isomorphic to ZSM-5, which has 10-ring straight channels aligned parallel to the b-axis and 10-ring sinusoidal channels parallel to the a-axis.13 To optimize mass transfer and product diffusion through the straight-ring channels, TS-1 nanosheets with a short b-axis are highly desired.14–18 Moreover, nanosheet aggregates of TS-1 zeolite may produce rich mesopores, which are more conducive to full contact between the substrate molecules and active sites, resulting in high reactivity.19–21
Crystal growth modifiers (CGMs) such as urea, amino acids and guanidine compounds can alter the growth rates of TS-1 crystals and/or assembly anisotropy, providing an effective method for constructing plate-like TS-1 zeolites.15,17,22–24 Additionally, the introduction of novel bolaform surfactants or ionic liquids can endow TS-1 zeolites with nanosheet-stacked morphologies. However, the synthesis of these modifiers typically involves complex steps or is prohibitively expensive, making them less compelling for industrial applications.14,18,25–28 Currently, it remains challenging to direct the formation of plate-like or nanosheet-stacked TS-1 zeolites by simply incorporating readily accessible CGMs.
In this study, we propose a method to effectively tailor the morphology of TS-1 zeolite solely by CGM within a conventional synthesis system. Commercially available organic small molecules, N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA), and N,N-dimethylpropionamide (DMP), were employed as CGMs. As a result, TS-1 nanosheets with a shortened b-axis dimension and thin-layer assemblies with hierarchical structure were produced. Based on the characterization results and theoretical calculations, the intrinsic reasons for the orientation-dependent growth behavior of TS-1 crystals were elucidated. The as-synthesized TS-1 zeolites with diverse morphologies are suitable for different oxidation reactions.
Results and discussion
Synthesis and characterization of TS-1-CGM zeolites
Achieving uniformly crystallized TS-1 zeolites with specific orientations using inexpensive raw materials through one-pot synthesis is quite difficult. In this study, we implemented a CGM-assisted synthesis strategy to efficiently produce nanosheet zeolite with the addition of DMF (designated as TS-1-DMF) and thin layer-assembled hierarchical TS-1 zeolite with the aid of DMA (designated as TS-1-DMA) or N,N-dimethylpropionamide (DMP) (designated as TS-1-DMP) (Fig. 1a). The structures of these CGMs are depicted in Fig. S1.† The powder X-ray diffraction (PXRD) patterns of all the synthesized TS-1 samples confirm the phase purity (Fig. S2a†). The relative crystallinity of these samples compared with the control TS-1 zeolite without CGMs (designated as TS-1-C) is illustrated in Fig. S2b.† It is evident that the introduction of CGMs decelerates the crystallization process of TS-1. Particularly, the influence of CGMs on the crystal morphology of TS-1 was studied. TS-1-C exhibits a regular ellipsoidal morphology with a particle size of approximately 110 nm (Fig. 1b and c). Conversely, TS-1-DMF displays a nanosheet morphology with a length of about 305 nm and a thickness ranging from 90 to 120 nm, characterized by a relatively flat and smooth surface (Fig. 1d, e, and j). The addition of DMA (Fig. 1f, g, and k) or DMP (Fig. 1h and i) results in samples with stacked thin layers, featuring a very rough surface and an overall particle size of about 150 nm. The HAADF-STEM images reveal a small number of intracrystalline mesopores in TS-1-DMF (Fig. 1l) and distinct stacked mesopores in TS-1-DMA (Fig. 1m).The N2 adsorption–desorption isotherms display typical Type I characteristics (Fig. 2a). TS-1-DMF exhibits a distinct hysteresis loop within the P/P0 ratio range of 0.4–0.8 (Fig. S3†), signifying the presence of intragranular mesopores. The pore size distribution diagram (Fig. 2b) suggests the absence of stacked mesopores.29 On the other hand, TS-1-DMA and TS-1-DMP show a broad pore size distribution, attributable to mesopores between small particles resulting from the orderly stacking of thin crystal layers.30 In comparison to the control sample TS-1-C, TS-1-DMF with larger crystal sizes demonstrates a smaller BET surface area (554 vs. 506 m2 g−1) and less mesoporous volume (0.38 vs. 0.15 cm3 g−1). The hierarchical TS-1-DMA and TS-1-DMP possess larger mesoporous volumes (0.60 and 0.52 cm3 g−1), with no notable reduction in the microporous surface area and microporous volume (Table S1†).
 | ||
| Fig. 2 (a) N2 adsorption–desorption isotherms and (b) pore size distributions of the synthesized samples. | ||
The analysis of the molar composition of TS-1 samples reveals a gradient in titanium content: TS-1-DMA > TS-1-DMF > TS-1-DMP > TS-1-C, with corresponding n(Si/Ti) ratios of 55.4, 63.4, 66.0, and 97.0, respectively (Table S1†). The HAADF–STEM image and elemental mappings confirm the homogeneous dispersion of Ti in the TS-1-DMA crystals (Fig. S4†). In the Fourier transform infrared (FT-IR) spectra (Fig. S5†), the I960/800 ratio demonstrates that these CGMs promote the integration of titanium into the zeolite framework.31 As shown in Fig. 3a, all samples contain a large number of framework TiO4 species (λ = 210 nm) and a little of TiO6 species (λ = 260 nm).16,32,33 The peak at ∼260 nm in TS-1-DMF was notably intense, indicating a higher concentration of TiO6 species. TS-1-C and TS-1-DMF contain some anatase TiO2 (λ = 330 nm), but the amount in TS-1-DMF is much less.32,33 These results indicate that DMA and DMP hinder anatase formation, whereas DMF not only suppresses anatase but also introduces TiO6 species. The titanium species in the surface layer (5–10 nm) of the crystal was also studied by X-ray photoelectron spectroscopy (XPS) (Fig. 3b). The peaks at ∼460.4, ∼459.2, and ∼458.2 eV in the XPS spectra of Ti 2p are attributed to TiO4, TiO6, and anatase TiO2, respectively, and the results are consistent with those of UV-vis spectra.32,34
 | ||
| Fig. 3 (a) UV-vis spectra, (b) XPS spectra of Ti 2p3/2, UV-Raman spectra with 325 nm excitation light (c) and 266 nm excitation light (d) of the synthesized samples. | ||
In UV resonance Raman (UV-Raman) spectroscopy with 325 nm excitation, the bands at 144, 390, 516, and 637 cm−1 are attributed to anatase TiO2, while the 380 cm−1 band is characteristic of the MFI topology (Fig. 3c). The I144/380 ratio is employed to assess the relative content of anatase TiO2.31 Obviously, the content of TiO2 in CGM-assisted samples is lower than that in TS-1-C (Fig. S6†). Under 266 nm excitation (Fig. 3d), the distinct 695 cm−1 peak with high intensity is attributed to hexacoordinated Ti(OH2)2(OH)2(OSi)2 species, which are more active than TiO4 in some oxidation reactions.21,35
Formation mechanism of TS-1-DMF and TS-1-DMA zeolites
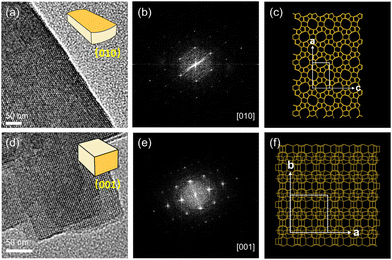
TS-1-DMF and TS-1-DMA were chosen as representative objects to investigate the underlying mechanisms governing their unique morphological characteristics. Fast Fourier transform (FFT) analysis conducted on specific crystal regions provides corresponding selected-area electron diffraction patterns. TS-1-DMF presents a platelet morphology characterized by well-developed ac planes and a short dimension along the b-axis (Fig. 4a and b), thus exposing 10-ring straight channels (Fig. 4c).36 Interestingly, thin crystal layers stacked along the c-axis are observed in TS-1-DMA (Fig. 4d–f). These layers can be peeled by exposing TS-1-DMA to a 0.19 M (tetrapropylammonium hydroxide) TPAOH aqueous solution (Fig. S7†), which display a relatively flat plane (50–80 nm) and a thin thickness (30–50 nm). Although TPAOH may have some etching effects on TS-1 crystals, the mild treatment conditions permit a rough estimation of the outcomes.The crystallization processes of TS-1-C, TS-1-DMF, and TS-1-DMA were examined using scanning electron microscopy (SEM) and PXRD (Fig. S8 and S9†). For the TS-1-C sample (Fig. S8a–c and S9a†), its crystallization starts at 6 hours, and an oval shape is formed after one day of crystallization. Further extending the crystallization time does not notably change the morphology or size of the crystals. For TS-1-DMF, the introduction of DMF slows down the crystallization of TS-1, and the solid product remains amorphous after one day of crystallization (Fig. S8d and S9b†); then, crystals with a particle size of ca. 300 nm form within the amorphous phase at 2 days (Fig. S8e†). After 4 days of crystallization, these amorphous particles rearrange and grow along the a-axis and c-axis, ultimately transforming into nanosheets with a thickness of 90–120 nm (Fig. S8f†). Notably, the amount of DMF affects the crystal growth of TS-1 zeolite. As shown in Fig. S10 and S11,† when n(DMF)/n(SiO2) ≤ 0.27, the samples progressively transition from an elliptical shape to a plate form as the amount of DMF increased. However, when n(DMF)/n(SiO2) reaches 0.38, a significant amount of amorphous solid is observed. As the ratio increases to 0.49, it results in a completely amorphous structure. This suggests that an appropriate amount of DMF addition can effectively guide the formation of nanosheet TS-1. Additionally, varying synthesis conditions can produce TS-1-DMF zeolites with different sizes and b-axis lengths (Fig. S12†). As for TS-1-DMA, the PXRD patterns indicate that DMA has a relatively minor effect on the crystallization rate of TS-1, and crystallization commences after 6 hours and is nearly complete after 12 hours (Fig. S9c†). During the initial stages of crystallization, small crystals with a thin thickness are identified using TEM (Fig. S13†). As the crystallization time extends, a noticeable aggregation of small particles is observed, and such aggregation becomes more pronounced with prolonged crystallization (Fig. S8h and i†). These findings collectively demonstrate that different CGMs significantly influence the growth behaviour of TS-1 crystals.
The results of CHN elemental analysis (Table S2†) and thermogravimetry (TG) (Fig. S14†) reveal that the organic template TPAOH and CGMs jointly contribute to the formation of the crystals. Among these, DMA has a higher content within the crystals compared to other CGMs, suggesting a more extensive interaction with the zeolite crystals. To further elucidate the fundamental reasons behind the alteration of TS-1 crystallization by CGMs, XPS Si 2p measurement was conducted (Fig. S15†). Compared to the calcined TS-1-CGMs, the Si 2p peak of the uncalcined sample shifts significantly to a lower electron energy level. However, such a shift is not observed in TS-1-C. This indicates that DMF, DMA, and DMP have strong interactions with silicon species during the crystallization process.13,32,37 Moreover, compared with TS-1-C, the TS-1-CGM samples exhibit broader and stronger intensity bands in the 3000–3700 cm−1 range of the FT-IR spectra (Fig. S16†), indicating the higher concentration of Si–OH groups and the strong interaction between CGMs and Si.38,39 CGMs bind with Si–OH species during crystallization and are removed through high-temperature calcination after the completion of crystallization, thereby exposing Si–OH groups.
Electrostatic potential (ESP) plays a unique role in the prediction and analysis of molecular recognition, often aiding in the elucidation of the nature of non-covalent molecular interactions. Employing ESP surfaces, we can identify spatial regions within molecular structures where the electrostatic potential is either negative or positive.40 The electrostatic potential involved in the analyses is evaluated by Multiwfn based on the highly effective algorithm proposed in Lu's work.41 As shown in Fig. 5a, the minimum and maximum values of the electrostatic potential on the van der Waals (vdW) surface were mapped onto the molecular surface. All CGMs exhibit negative potential regions (shown in dark blue) near oxygen atoms due to their higher electronegativity. These negatively charged regions are easily attracted by Si–OH on the surface of TS-1 zeolite to form hydrogen bonds (Si–OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).42–44
C).42–44
 | ||
| Fig. 5 (a) Electrostatic potential maps of DMF, DMA and DMP. (b) Schematic of the growth of TS-1-DMF and TS-1-DMA. | ||
Based on the above results, we propose a possible growth mechanism for TS-1-DMF and TS-1-DMA with different morphologies. Under hydrothermal conditions, the continuous growth of TS-1 zeolite depends on the continuous condensation of silicate anions on the crystal surface. The selective adsorption of CGMs on different crystal planes affects silicon species to crystallize on specific crystal planes, which is the reason for the change in the TS-1 morphology. In the presence of DMF, DMF was adsorbed by Si–OH on the (010) surface through hydrogen bonds, which interferes with the adsorption of Si or TPA+, thus leading to the slow deposition of silicate on such a surface, finally resulting in the formation of (010)-oriented plate crystals.45,46 In the presence of DMA, DMA restricts the growth of all crystal planes, with a particularly pronounced adsorption on the (010) plane. Due to the electrostatic interaction between DMA themselves, the thin-layer crystals surrounded by DMA tend to stack growth along the c-axis dimension to form rich mesopores. Interestingly, DMF and DMA, which are structurally different by only one ethyl group, exhibit strikingly distinct behaviors in regulating the zeolite morphology. The underlying reasons for these differences remain to be further explored.
The CGM-assisted synthesis strategy proposed here is also applicable to aluminosilicate ZSM-5 zeolite with the same MFI topology. As shown in Fig. S17,† ZSM-5 without the addition of CGMs has an uneven particle morphology and size. After the addition of DMF, the samples are plate-like with uniform dimensions of approximately 800 × 600 × 300 nm. In contrast, the addition of DMA results in spherical particles with a diameter of about 800 nm. Therefore, by selecting appropriate CGMs, it is possible to effectively tailor the desired zeolite morphology, thereby providing suitable catalysts for different organic reactions.
Catalytic performance of TS-1-CGM zeolites
Considering different morphologies of synthesized TS-1 zeolites, we evaluated their catalytic performance through the oxidative desulfurization of dibenzothiophene (DBT) and epoxidation of 1-hexene reactions. In the oxidative desulfurization of DBT (Fig. 6a), TS-1-DMA exhibits optimal performance, achieving 100% DBT desulfurization within just 20 minutes. This is attributed to its abundant mesoporous structure, which not only facilitates the entry of large DBT molecules into the zeolite but also effectively shortens the diffusion distance, thus enhancing the catalyst's reaction activity. TS-1-DMP shows 87.9% DBT removal rate after 20 minutes due to the lower titanium content (Si/Ti = 66.0), despite possessing similar mesopores to that of TS-1-DMA (0.52 vs. 0.60 cm3 g−1). Conversely, TS-1-C shows only 54.3% and 64.3% conversion after 20 and 40 minutes of reaction, respectively. TS-1-DMF has the poorest activity (52.8% DBT desulfurization within 40 minutes), attributed to its low mesopore volume (Vmeso = 0.15 cm3·g−1), which hinders reactant access to active sites, despite its higher proportion of highly active Ti(OH)2(OH)2(OSi)2 species. The oxidation reaction of DBT involves two sequential steps: initial oxidation to sulfoxide (DBTO), followed by further oxidation to sulfone (DBTO2), as shown in Fig. S18a.† TS-1-DMA shows the highest yield of DBTO2 (62.65%) and the highest selectivity to DBTO2 (62.65%) (Fig. S18b and c†), indicating the high oxidation of DBT on the catalyst. To assess the intrinsic activity of the TS-1 zeolite catalyst, the turnover number (TON) was calculated from DBT conversion data after 20 minutes of reaction (Fig. S19†). The TON values for DBT are 10.8 for TS-1-DMP and 10.4 for TS-1-DMA, higher than those for TS-1-C (9.7) and TS-1-DMF (5.3). This improvement is due to the larger mesoporous volumes in TS-1-DMP (0.52 cm3 g−1) and TS-1-DMA (0.60 cm3 g−1). The apparent activation energy (Ea) of TS-1-DMA was calculated to be 96.44 kJ mol−1 (Fig. 6d and Fig. S20†), and the sample exhibits excellent cyclic stability, maintaining a DBT removal rate of 98.9% even after seven cycles (Fig. 6b). In addition, PXRD and UV-vis spectra (Fig. S21†) as well as ICP result (Si/Ti = 59.2) show that compared with the fresh catalyst, the zeolite structure and most of the Ti species of the used TS-1-DMA are well maintained.In the epoxidation of 1-hexene (Fig. 6c and Fig. S22†), TS-1-DMA also exhibits high reactivity, achieving a conversion rate of 23.8% and a considerable TON of 162.4 after 2 hours but with a lower selectivity of 95.7%. In industrial production, product selectivity is a critical metric as it influences the further purification. In contrast, TS-1-DMF shows significantly improved product selectivity, reaching 97.9%. This enhancement is attributed to the shorter b-axis of TS-1-DMF, which facilitates the rapid desorption of products from the straight pores, reducing the risk of ring-opening reactions due to prolonged contact with active sites and secondary oxidation. Compared to other plate-like TS-1 and stacked morphology TS-1 reported in the literatures, TS-1-DMF consistently demonstrates superior selectivity (Table S3†). Additionally, TS-1-DMF had a lower activation energy, which was calculated to be 38.07 kJ mol−1 (Fig. 6e and Fig. S23†). These results clearly illustrate that the distinct morphological features of TS-1 confer different benefits in various catalytic processes.
Conclusion
In summary, we successfully regulated the morphology of TS-1 zeolites by integrating different crystal growth modifiers into hydrothermal synthesis systems; as a result, nanosheet and thin layer-stacked TS-1 zeolites were produced in the presence of DMF and DMA/DMP, respectively. Zeolite growth studies reveal the different modification effect of DMF and DMA on zeolite crystals. DMF selectively adsorbs on the ac plane of the crystals via H-bonding interaction with the surface Si–OH of the crystal, hindering the deposition of silicon species and TPA+ on this plane, thereby resulting in plate-like crystals with a shorter b-axis. On the other hand, majority of DMA are adsorbed on the (010) crystal plane, with the remaining DMA randomly distributed on other planes, forming thin crystal layers. Meanwhile, the electrostatic interactions between DMA molecules induce these thin layers to undergo directional stacking and structural rearrangement, ultimately generating rich hierarchical porosity. Compared to the control TS-1 zeolites, the nanosheet TS-1 shows significantly enhanced substrate selectivity in the catalytic epoxidation of 1-hexene, while the layer-stacked hierarchical TS-1 zeolites exhibit excellent catalytic activity and cyclic stability in the oxidative desulfurization of DBT.Author contributions
Jiani Zhang: conceptualization, preparing catalysts, formal analysis, investigation, writing original draft. Risheng Bai: preparing catalysts, formal analysis, investigation. Shiyu Zhou: formal analysis, investigation. Jiyang Li: conceptualization, project administration, supervision, writing review & editing, funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Key Research and Development Program of China (grant no. 2023YFA1507703) and the ‘111 Center’ (B17020).References
- Y. Li and J. Yu, Emerging applications of zeolites in catalysis, separation and host-guest assembly, Nat. Rev. Mater., 2021, 6, 1156–1174 CrossRef
.
- Q. Zhang, J. Yu and A. Corma, Applications of zeolites to C1 chemistry: recent advances, challenges, and opportunities, Adv. Mater., 2020, 32, 2002927 CrossRef PubMed
.
- Z. Qu, T. Zhang, X. Yin, J. Zhang, X. Xiong and Q. Sun, Zeolite-encaged ultrasmall Pt-Zn species with trace amount of Pt for efficient propane dehydrogenation, Chem. Res. Chin. Univ., 2023, 39, 870–876 CrossRef
.
- Y. Wei, M. Chen, X. Ren, Q. Wang, J. Han, W. Wu, X. Yang, S. Wang and J. Yu, One-pot three-dimensional printing robust self-supporting MnOx/Cu-SSZ-13 zeolite monolithic catalysts for NH3-SCR, CCS Chem., 2022, 4, 1708–1719 Search PubMed
.
- J. Duan, W. Chen, C. Wang, L. Wang, Z. Liu, X. Yi, W. Fang, H. Wang, H. Wei, S. Xu, Y. Yang, Q. Yang, Z. Bao, Z. Zhang, Q. Ren, H. Zhou, X. Qin, A. Zheng and F.-S. Xiao, Coking-resistant polyethylene upcycling modulated by zeolite micropore diffusion, J. Am. Chem. Soc., 2022, 144, 14269–14277 Search PubMed
.
- E. Perez-Botella, S. Valencia and F. Rey, Zeolites in adsorption processes: State of the art and future prospects, Chem. Rev., 2022, 122, 17647–17695 Search PubMed
.
- B. Wang, S. Zong and J. Li, Recent progress on photoluminescent zeolite-based composite materials, Chem. J. Chin. Univ., 2021, 42, 299–310 Search PubMed
.
- Q. Zhang, S. Gao and J. Yu, Metal Sites in Zeolites: Synthesis, Characterization, and Catalysis, Chem. Rev., 2023, 123, 6039–6106 CrossRef PubMed
.
- A. Velty and A. Corma, Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels, Chem. Soc. Rev., 2023, 52, 1773–1946 Search PubMed
.
- M. Moliner, C. Martinez and A. Corma, Multipore zeolites: synthesis and catalytic applications, Angew. Chem., Int. Ed., 2015, 54, 3560–3579 CrossRef PubMed
.
- C. Wang, L. Zhang, X. Huang, Y. Zhu, G. Li, Q. Gu, J. Chen, L. Ma, X. Li, Q. He, J. Xu, Q. Sun, C. Song, M. Peng, J. Sun and D. Ma, Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene, Nat. Commun., 2019, 10, 4348 CrossRef PubMed
.
- J. Prech, Catalytic performance of advanced titanosilicate selective oxidation catalysts - a review, Catal. Rev.: Sci. Eng., 2018, 60, 71–131 CrossRef
.
- G. Bonilla, I. Díaz, M. Tsapatsis, H. K. Jeong, Y. Lee and D. G. Vlachos, Zeolite (MFI) crystal morphology control using organic structure-directing agents, Chem. Mater., 2004, 16, 5697–5705 CrossRef
.
- I. Khan, X. Chu, Y. Liu, S. Khan, L. Bai and L. Jing, Synthesis of Ni2+ cation modified TS-1 molecular sieve nanosheets as effective photocatalysts for alcohol oxidation and pollutant degradation, Chin. J. Catal., 2020, 41, 1589–1602 CrossRef
.
- Z. Shang, Y. Chen, L. Zhang, X. Zhu, X. Wang and C. Shi, Plate-like MFI crystal growth achieved by guanidine compounds, Inorg. Chem. Front., 2022, 9, 2097–2103 Search PubMed
.
- X. Song, X. Yang, T. Zhang, H. Zhang, Q. Zhang, D. Hu, X. Chang, Y. Li, Z. Chen, M. Jia, P. Zhang and J. Yu, Controlling the morphology and titanium coordination states of TS-1 zeolites by crystal growth modifier, Inorg. Chem., 2020, 59, 13201–13210 CrossRef PubMed
.
- M. Zhang, S. Ren, Q. Guo and B. Shen, Synthesis of sheet-like zeolite TS-1 with short b-axis for epoxidation of 1-hexene, ChemistrySelect, 2023, 8, e202203687 CrossRef
.
- W. Dai, J. Zhao, Z. Liu, J. Zheng and R. Li, Lysine-assisted synthesis of plate-like TS-1 zeolite for 1-hexene epoxidation, Mater. Today Sustain., 2024, 27, 100943 Search PubMed
.
- R. Bai, Y. Song, R. Bai and J. Yu, Creation of hierarchical titanosilicate TS-1 zeolites, Adv. Mater. Interfaces, 2021, 8, 2001095 CrossRef CAS
.
- Y. Song, R. Bai, Y. Zou, Z. Feng and J. Yu, Temperature-regulated construction of hierarchical titanosilicate zeolites, Inorg. Chem. Front., 2020, 7, 1872–1879 RSC
.
- J. Zhang, R. Bai, Z. Feng and J. Li, Amide-assisted synthesis of TS-1 zeolites with active Ti(OH2)2(OH)2(OSi)2 sites toward efficient oxidative desulfurization, Appl. Catal., B, 2024, 342, 123339 CrossRef
.
- C. Ping, Q. Zhu, W. Ma, C. Hu and Y. Zhang, Effect of urea on the morphology of TS-1 and catalytic performance of Au/TS-1 for direct gas phase epoxidation of propylene, J. Porous Mater., 2022, 29, 1919–1928 CrossRef
.
- J. Xu, Z. Zhang, D. Yu, W. Du, N. Song, X. Duan and X. Zhou, Au/TS-1 catalyst for propylene epoxidation with H2 and O2: Effect of surface property and morphology of TS-1 zeolite, Nano Res., 2023, 16, 6278–6289 CrossRef
.
- Z. Shan, H. Wang, X. Meng, S. Liu, L. Wang, C. Wang, F. Li, J. P. Lewis and F.-S. Xiao, Designed synthesis of TS-1 crystals with controllable b-oriented length, Chem. Commun., 2011, 47, 1048–1050 RSC
.
- N. Li, M. Wang, Q. You, C. Bi, H. Chen, B. Liu, M. Sun, Q. Hao, J. Zhang and X. Ma, Bolaform surfactant-directed synthesis of TS-1 zeolite nanosheets for catalytic epoxidation of bulky cyclic olefins, Catal. Sci. Technol., 2020, 10, 1323–1335 RSC
.
- M. Wang, X. Wang, Q. You, Y. Wu, X. Yang, H. Chen, B. Liu, Q. Hao, J. Zhang and X. Ma, Dual-template synthesis of hierarchically layered titanosilicate-1 zeolites for catalytic epoxidation of cyclooctene, Microporous Mesoporous Mater., 2021, 323, 111207 CrossRef
.
- W. Wu, D. T. Tran, X. Wu, S. C. Oh, M. Wang, H. Chen, L. Emdadi, J. Zhang, E. Schulman and D. Liu, Multilamellar and pillared titanium Silicalite-1 with long-range order of zeolite nanosheet layers: Synthesis and catalysis, Microporous Mesoporous Mater., 2019, 278, 414–422 CrossRef
.
- X. Guo, Z. Wang, S. Jiang, M. Li, J. Guo, Y. Cui, X. Wei, B. Qi, Q. Huang, Y. Liu, H. Jiang and Y. Hu, Inductive effect in amino functionalized ionic liquids modified TS-1 nanosheets for efficiently sunlight-driven CO2 reduction, Fuel, 2024, 367, 131504 CrossRef
.
- L.-H. Chen, X.-Y. Li, G. Tian, Y. Li, J. C. Rooke, G.-S. Zhu, S.-L. Qiu, X.-Y. Yang and B.-L. Su, Highly stable and reusable multimodal zeolite TS-1 based catalysts with hierarchically interconnected three-level micro-meso-macroporous structure, Angew. Chem., Int. Ed., 2011, 50, 11156–11161 CrossRef
.
- H. Zhang, H. Zhang, Y. Zhao, Z. Shi, Y. Zhang and Y. Tang, Seeding bundlelike MFI zeolite mesocrystals: a dynamic, nonclassical crystallization via epitaxially anisotropic growth, Chem. Mater., 2017, 29, 9247–9255 CrossRef
.
- T. Zhang, X. Chen, G. Chen, M. Chen, R. Bai, M. Jia and J. Yu, Synthesis of anatase-free nano-sized hierarchical TS-1 zeolites and their excellent catalytic performance in alkene epoxidation, J. Mater. Chem. A, 2018, 6, 9473–9479 RSC
.
- J. Zhang, R. Bai, Y. Zhou, Z. Chen, P. Zhang, J. Li and J. Yu, Impact of a polymer modifier on directing the non-classical crystallization pathway of TS-1 zeolite: accelerating nucleation and enriching active sites, Chem. Sci., 2022, 13, 13006–13014 RSC
.
- W. Xu, T. Zhang, R. Bai, P. Zhang and J. Yu, A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species, J. Mater. Chem. A, 2020, 8, 9677–9683 RSC
.
- W. Xu, L. Li, T. Zhang and J. Yu, Tailoring porosity and titanium species of TS-1 Zeolites via, organic base-assisted sequential post-treatment, Chem. Res. Chin. Univ., 2022, 38, 50–57 Search PubMed
.
- Q. Guo, K. Sun, Z. Feng, G. Li, M. Guo, F. Fan and C. Li, A thorough investigation of the active titanium species in TS-1 zeolite by in situ UV resonance Raman spectroscopy, Chem. – Eur. J., 2012, 18, 13854–13860 CrossRef
.
- I. Díaz, E. Kokkoli, O. Terasaki and M. Tsapatsis, Surface structure of zeolite (MFI) crystals, Chem. Mater., 2004, 16, 5226–5232 CrossRef
.
- J. M. da Silva, R. C. Sousa, J. C. S. Costa, J. L. Magalhaes, G. E. Luz, C. V. Rodarte de Moura and E. M. de Moura, Base-free benzyl alcohol aerobic oxidation catalyzed by AuPdNPs supported on SBA-15 and TiO2/SBA-15 mesoporous materials, Catal. Lett., 2022, 152, 585–599 CrossRef
.
- S. Bordiga, I. Roggero, P. Ugliengo, A. Zecchina, V. Bolis, G. Artioli, R. Buzzoni, G. Marra, F. Rivetti, G. Spanò and C. Lamberti, Characterisation of defective silicalites, J. Chem. Soc., Dalton Trans., 2000, 3921–3929 RSC
.
- P. Wu, T. Komatsu and T. Yashima, IR and MAS NMR studies on the incorporation of Aluminum atoms into defect sites of dealuminated mordenites, J. Phys. Chem., 1995, 99, 10923–10931 CrossRef
.
- F. Zhang, B. Liu, G. Liu, Y. Zhang, J. Wang and S. Wang, Substructure-activity relationship studies on antibody recognition for phenylurea compounds using computational chemistry, Sci. Rep., 2018, 8, 3131 CrossRef PubMed
.
- J. Zhang and T. Lu, Efficient evaluation of electrostatic potential with computerized optimized code, Phys. Chem. Chem. Phys., 2021, 23, 20323–20328 RSC
.
- A. A. Tsyganenko, E. N. Storozheva and O. V. Manoilova, Manifestations of the acidity of adsorbed molecules in H-bonded complexes with silanol groups: Lewis acidity of ozone, Catal. Today, 2001, 70, 59–71 CrossRef
.
- N. N. Chipanina, N. F. Lazareva, T. N. Aksamentova, A. Y. Nikonov and B. A. Shainyan, Apicophilicity versus hydrogen bonding. intramolecular coordination and hydrogen bonds in N- (Hydroxydimethylsilyl)methyl -N,N′-propyleneurea and its hydrochloride. DFT and FT-IR study and QTAIM and NBO analysis, Organometallics, 2014, 33, 2641–2652 CrossRef
.
- R. Ma, W. Chen, L. Wang, X. Yi, Y. Xiao, X. Gao, J. Zhang, X. Tang, C. Yang, X. Meng, A. Zheng and F.-S. Xiao,
N-Oxyl radicals trapped on zeolite surface accelerate photocatalysis, ACS Catal., 2019, 9, 10448–10453 CrossRef
.
- A. I. Lupulescu and J. D. Rimer, Tailoring silicalite-1 crystal morphology with molecular modifiers, Angew. Chem., Int. Ed., 2012, 51, 3345–3349 CrossRef
.
- Q. Yue, C. Liu, H. Zhao, H. Liu, P. Ruterana, J. Zhao, Z. Qin and S. Mintova, Urea-assisted morphological engineering of MFI nanosheets with tunable b-thickness, Nano Res., 2023, 16, 12196–12206 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02626h |
| This journal is © the Partner Organisations 2025 |