Insights into the terahertz response of L-glutamic acid and its receptor†
Yu
Wu
abc,
Zhongjie
Zhu
 *b,
Jinrong
Yang
*b,
Jinrong
Yang
 d,
Jie
Wang
b,
Te
Ji
b,
Huachun
Zhu
b,
Weiwei
Peng
b,
Min
Chen
d,
Jie
Wang
b,
Te
Ji
b,
Huachun
Zhu
b,
Weiwei
Peng
b,
Min
Chen
 ab and
Hongwei
Zhao
ab and
Hongwei
Zhao
 *ab
*ab
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China. E-mail: zhaohw@sari.ac.cn
bShanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China. E-mail: zhuzj@sari.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dEast China Normal University, Shanghai 200241, China
First published on 12th July 2024
Abstract
L-Glutamic acid (L-Glu) is a basic unit of proteins and also serves as an important neurotransmitter in the central nervous system. Its structural properties are critical for biological functions and selective receptor recognition. Although this molecule has been extensively studied, the low frequency vibrational behavior that is closely related to conformational changes and the intermolecular interactions between L-Glu and its receptors are still unclear. In this study, we acquired the fingerprint spectrum of L-Glu by using air plasma terahertz (THz) time-domain spectroscopy in the 0.5–18 THz range. The low frequency vibrational characteristics of L-Glu were investigated through density functional theory (DFT) calculations. The THz responses of the ligand binding domain of the NMDAR–L-Glu complex were studied by the ONIOM method, with a focus on discussing the normal modes and interactions of ligand L-Glu and water molecules. The results illustrate that THz spectroscopy exhibits a sensitive response to the influence of L-Glu on the structure of the NMDAR. The water molecules in proteins have various strong vibration modes in the THz band, showing specificity, diversity and complexity of vibrational behavior. There is potential for influencing and regulating the structural stability of the NMDAR–L-Glu complex through water molecules.
1 Introduction
Amino acids are the basic units of proteins, which play an irreplaceable role in life and are the material basis of organisms. L-Glutamic acid (L-Glu) is an amino acid that is abundant in the brains of animals. Meanwhile, L-Glu is a central excitatory neurotransmitter, which exists in neurons of the central nervous system, mediates most excitatory neurotransmission in the brain, and participates in the cognitive functions of the brain such as learning and memory.1,2L-Glu consists of a flexible carbon skeleton and three ionizable functional groups, one amino group and two carboxyl groups. There are multiple hydrogen bond donors and acceptors in L-Glu, which can form complex hydrogen bond networks. As a neurotransmitter, L-Glu mainly acts on ionotropic or metabotropic receptors.3 Glutamate is the physiological form of glutamic acid. On the postsynaptic membrane, glutamate receptors, such as the N-methyl-D-aspartic acid receptor (NMDAR) or the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR), bind to L-Glu and are activated. Ionotropic receptors are involved in excitatory synaptic transmission and neuronal plasticity, which can affect learning and memory behavior and excitatory neurotoxicity.4,5 In the process of mutual recognition and interaction between L-Glu and its receptors, the conformation of L-Glu directly affects the activation state of the receptor, and high or low activation is closely related to nervous system diseases, including Alzheimer's disease, Parkinson's disease, depression, schizophrenia and ischemic injury associated with stroke.1,6,7 Therefore, glutamate receptors, especially the NMDAR, are important targets for drug research. Studying the interaction between L-Glu and the NMDAR, as well as the conformation characteristics and dynamic changes, will help reveal the mechanisms of diseases and provide inspiration for the search for new drugs.During the process of the protein–ligand complex exerting biological functions, water molecules usually play an essential role, often participating in the change of configuration and conformation as part of the structure and affecting biological activity. The interactions between water molecules and biomolecules are complex and subtle.8–10 Water molecules have the dual ability to act as both hydrogen bond donors and acceptors and can form rich hydrogen bond networks.11,12 They are sometimes regarded as “biomolecules” with a basic structure and function.13 Water molecules located in the internal cavities and cracks of proteins can affect the shape and flexibility of proteins as well as stabilize the structure between the protein and the ligand, improve spatial complementarity,11,14 regulate interactions, and increase affinity.8 Vance et al. studied the structures of the ionotropic glutamate receptor NMDAR in complex with four ligands including L-glutamate and its analogues. Compared with other agonists, the time of receptor inactivation caused by L-Glu is significantly slower, which is due to the unique conformation induced by L-Glu at the backside of the ligand binding site. An atomic-resolution crystallographic study shows that in the complex structure of the NMDAR with L-Glu, there are numerous water molecules embedded, with one water molecule bridging the ligand L-Glu and residues through hydrogen bonding, which is critical in the ligand binding domain.15 However, due to the complexity of the microenvironment in the L-Glu–NMDAR complex, the characteristics of the hydrogen bond network established by water molecules, the impact of variations in water molecules on receptor conformation, and the specificity of interactions involving water molecules remain uncertain.
In protein–ligand complexes, weak interactions such as hydrogen bonding and van der Waals forces play an important role in receptor–ligand recognition and maintaining the structure of biological molecules. Recent studies have shown that these weak interactions are widely distributed in the THz far-infrared region and exhibit unique properties. The low frequency vibrations are dominated by intermolecular interactions, which mainly manifest as collective vibrations involving more atoms, and multiple vibrations are mixed together, which are highly sensitive to intramolecular and intermolecular structures, thus providing characteristic fingerprint information on molecular conformational states.16–18 Terahertz time-domain spectroscopy (THz-TDS) exhibits a highly sensitive response to the conformational changes of molecules, enabling effective detection of the physical and chemical properties of materials and facilitating non-destructive testing of biomolecules in the THz band.19–24 Ruggiero et al. used THz-TDS to distinguish the unique fingerprint spectra of two crystal types of L-Glu in the 0.5–4.0 THz region and evaluated the quality of the crystal intermolecular force model.25 Li et al. found that L-Glu and its structural analog exhibit different vibrational characteristics through THz-TDS and DFT calculations in the 0.5–3.0 THz region, which can be attributed to their distinct hydrogen bonding network structures.26 Hand et al.27 suggested that some of the capabilities of biomolecules depend on their sampling of different conformational states, which are determined by the vibrational dynamics of macromolecules. Broad band THz spectroscopy provides a wealth of low-frequency vibrational information about molecules, and many biomolecules exhibit rich spectra near 6 THz, corresponding to the densest transitions between states. Hence, richer information in the entire THz range is helpful for a comprehensive understanding of biomolecules. The spectral resolution and fingerprint characteristics of THz spectroscopy make it useful for label-free detection in biochemical research.
THz photons have low energy and usually do not cause ionization damage to biomolecules.28 Therefore, THz electromagnetic waves are considered to be relatively safe for living organisms.29 However, some recent research results suggest that the resonant coupling between THz photons and molecules may produce some effects, even unexpected ones, indicating that the biological effects of THz radiation cannot be ignored.30–33 Song et al. revealed the potential mechanism of THz photons released by ion oscillation in the potassium ion channel and their resonant and coherent coupling with other channel oscillations, demonstrating the macroscopic coherent state of multiple ion channels.34 Wu et al. found that THz photons facilitate stretching vibrations on the purine plane, weakening hydrogen bonds near base pairs, thereby accelerating DNA unwinding in an efficient and non-thermal way.35 Zhu et al. successfully induced one-dimensional confined water to transform from a normal permeation state to a superpermeation state by using specific frequency THz waves resonating with water in a confined space and proved that the effect is non-thermal, suggesting the potential application of THz technology in biomedicine.36 Li et al. showed that the emission of THz waves resonating with the –COO− and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups in voltage-gated calcium channels can significantly enhance the selectivity and conductivity of Ca2+ and found that an electromagnetic wave with a frequency of 4.0 THz can expand the exit of the risperidone ligand in the dopamine D2 receptor through conformational modulation and accelerate risperidone dissociation, thereby reducing the toxic side effects of drugs.31,37 As a possible neural information processing mechanism, the generation and signal transmission of biophotons in the nervous system have been supported by a series of studies.38–41 However, because of biological complexity, the mechanisms by which THz electromagnetic waves interact with biomolecules remain poorly understood. The inherent correlations between low frequency vibrations and interactions associated with conformational changes in protein–ligand complexes are still elusive. As an important amino acid and neurotransmitter, despite extensive work on L-Glu, little is known about the intermolecular interactions between this ligand and its receptors.
O functional groups in voltage-gated calcium channels can significantly enhance the selectivity and conductivity of Ca2+ and found that an electromagnetic wave with a frequency of 4.0 THz can expand the exit of the risperidone ligand in the dopamine D2 receptor through conformational modulation and accelerate risperidone dissociation, thereby reducing the toxic side effects of drugs.31,37 As a possible neural information processing mechanism, the generation and signal transmission of biophotons in the nervous system have been supported by a series of studies.38–41 However, because of biological complexity, the mechanisms by which THz electromagnetic waves interact with biomolecules remain poorly understood. The inherent correlations between low frequency vibrations and interactions associated with conformational changes in protein–ligand complexes are still elusive. As an important amino acid and neurotransmitter, despite extensive work on L-Glu, little is known about the intermolecular interactions between this ligand and its receptors.
In this paper, L-Glu and its receptor NMDAR are studied as a model to investigate the signal response in the 0.5–18 THz band. The rich absorption peaks and unique normal modes of L-Glu reveal the characteristics of the interaction between L-Glu and THz electromagnetic waves. In protein–ligand complexes, water molecules dominate THz absorption in multiple frequency bands and are associated with protein and ligand binding. This study may provide a reference for the future use of THz waves to regulate the interaction between the ligand and the receptor.
2 Materials and methods
2.1 Materials and sample preparation
L-Glu (purity 98.5%) was purchased from J&K. L-Glu was mixed with cyclic olefin copolymer powder (COC, obtained from the Shanghai Institute of Nuclear Research) in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The sample was pressed into a pellet with a diameter of 13 mm by a tablet press.
20. The sample was pressed into a pellet with a diameter of 13 mm by a tablet press.
2.2 Terahertz time-domain spectroscopy (THz-TDS)
The THz spectrum of the sample was recorded in the spectral range of 0.5–18 THz using a THz spectrometer based on the broadband air-plasma system.42 The sample tablet was placed into the THz system path, which was purged with dry air continuously during the detection to maintain a relative humidity of less than 1%. The THz spectrum is the average of three measurements with drying air as a reference. All measurements were taken at room temperature.2.3 Fourier transform infrared spectroscopy (FTIR)
The FTIR spectrum of the sample was recorded using a Bruker VERTEX 80v spectrometer in the range of 50–600 cm−1 with a resolution of 4 cm−1 and 64 scans. All measurements were conducted in vacuum and room temperature environments.2.4 Powder X-ray diffraction (PXRD)
The PXRD pattern of L-Glu was obtained using a Bruker D8 Advance diffractometer (Cu source, 40 kV voltage, and 40 mA filament emission). The data were collected within a scan range of 5°–90° (2θ). The scanning rate was 0.2° s−1.2.5 Quantum chemical calculations of the crystal
Considering that different crystal forms of L-Glu exhibit distinct spectra,25 the PXRD pattern was utilized to check the crystal form of our experimental sample. The experimental PXRD pattern of L-Glu in this study is in good agreement with the simulated PXRD pattern using crystal cell parameters from the Cambridge Crystallographic Data Centre43 (see ESI Fig. S1†). Therefore, the data of the crystal structure reported in the literature,43 the space group P212121, Z = 4, a = 5.17(1) Å, b = 17.34(3) Å, c = 6.95(1) Å, α = 90°, β = 90°, and γ = 90°, were taken as the initial values for theoretical calculations. The DFT-based CASTEP44 was used for structural optimization and infrared spectrum calculations.45 The cell structure of the sample was optimized using the Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm. The results of the crystalline state were obtained using the generalized gradient approximation (GGA) within the Perdew–Burke–Ernzerhof (PBE) correlation functional, incorporating DFT-D correction based on Grimme's dispersion correction method, and employing a norm-conserving pseudopotential as implemented in CASTEP. The plane wave cutoff energy was 830.0 eV. Brillouin zone sampling of the electronic states was performed on a 1 × 1 × 1 Monkhorst–Pack grid, the total energy was converged to 5.0 × 10−6 eV per atom, the maximum forces between atoms were 0.01 eV Å−1, and the fast Fourier transform grid was 48 × 48 × 48.2.6 Our own N-layered integrated molecular orbital + molecular mechanics (ONIOM) calculation
The glutamate receptor, NMDAR, was obtained from the RCSB Protein Data Bank, and its PDB ID is 3OEN.15 The protein was cut to retain the L-Glu ligand and surrounding molecules, which contain 14 amino acids and 5 water molecules and are denoted as the NMDAR–L-Glu complex (see ESI Table S1†). In order to reduce the calculation cost of the NMDAR–L-Glu complex without losing calculation accuracy, we adopted the ONIOM method for geometry optimization and frequency calculation. The QM layer contains L-Glu, which was calculated by DFT methods, the B3LYP functional and the 6-31+G(d) basis set. The MM layer contains surrounding molecules and was treated with the universal force field (UFF).46 All the above calculations were performed using Gaussian 16 software.47 The independent gradient model based on the Hirshfeld partition (IGMH) method48 in the Multiwfn quantum chemical wave function analysis program49 was used to analyze the calculated results, and Visual Molecular Dynamics (VMD)50 was used to visualize the molecular interactions between L-Glu and the surrounding molecules.3 Results and discussion
3.1 THz absorption spectrum of L-Glu
The THz spectrum of L-Glu obtained in the THz-TDS experiment is shown as the red solid line in Fig. 1. L-Glu has a series of rich absorption peaks in the detected frequency range. The absorption peaks are basically consistent with the literature51 and are consistent with the FTIR experiment in the 1.5–18 THz band (see ESI Fig. S3†). The THz spectrum obtained by theoretical calculations based on the L-Glu periodic cell structure is shown as the blue dashed line in Fig. 1. It corresponds well with the experimental spectrum to a certain extent, which is helpful in understanding the conformational changes of L-Glu at the molecular level. In the THz band, the difference in the structure and environments will cause a change of vibration, reflecting molecular structural and interaction information. Therefore, the rich absorption peaks of L-Glu in the broadband THz band provide the possibility for its label-free recognition.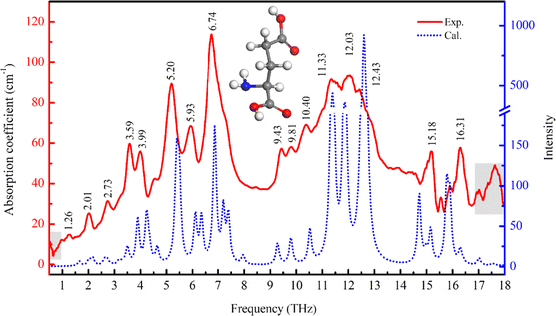 | ||
| Fig. 1 Experimental spectrum (solid red line) and the calculated spectrum (blue dotted line) of L-Glu in the frequency range of 0.5–18 THz. The absorption in the grey area is for reference only. | ||
3.2 Vibrational analysis of L-Glu
The absorption peaks of the substance in the THz band originate from the vibrations of the molecule. Due to the complex mixing between intramolecular and intermolecular vibrations in the low frequency vibration, we used the mode analysis method52 to quantify the normal mode of L-Glu. Considering that four L-Glu molecules in the crystal cell exhibit similar vibrational behavior, one of them is used as an example for analysis (see ESI Fig. S4†).Modes with no infrared activity have no absorption peaks in the THz spectrum, but that does not imply their nonexistence. Therefore, both infrared and non-infrared active modes are considered. Fig. 2a shows the intramolecular vibrational contribution of L-Glu in the range of 0.5–18 THz, in which red dots indicate infrared activity modes and blue dots indicate non-infrared activity modes. It can be seen from the figure that the intramolecular vibrational contribution increases with the increase of frequency. This indicates that the molecular vibrational distribution with frequency in the studied region of 0.5–18 THz has certain regularity,53–55 which is related to the resonance response between different interactions and THz electromagnetic waves. The resonance frequency is positively correlated with the interaction strength. Therefore, the intermolecular interaction response is significant at low frequencies, with a relatively high contribution.
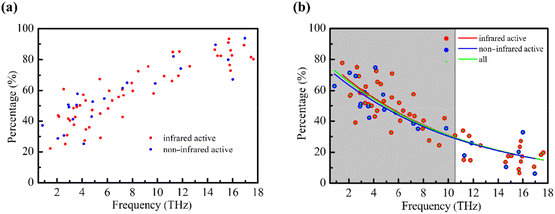 | ||
| Fig. 2 (a) Changes in the intramolecular vibrational contribution and (b) intermolecular vibrational contribution of normal modes of L-Glu in the range of 0.5–18 THz with frequency. | ||
In order to further clarify the relationship between molecular vibration and THz frequency, we used the function P = Ae−λF to fit the relationship between the intermolecular vibrational contribution and frequency, where P represents the intermolecular vibrational contribution, A is a constant, e is a natural constant, F is the frequency, and λ is a constant, which is related to the decay rate of the function. We found that the λ value of all normal modes (green dots in Fig. 2b) is 0.094, and P = 79.13e−0.094F is used for fitting. The λ value of infrared active normal modes (red circle in Fig. 2b) is 0.096, and P = 80.85e−0.096F is used for fitting. The λ value of normal modes without infrared active modes (blue circle in Fig. 2b) is 0.092, and P = 76.43e−0.092F is used for fitting. We used 1/λ to represent the upper limit of the frequency effectively dominated by the intermolecular interaction as a range indicator for evaluating the THz wave response of the intermolecular interaction, which is about 10.63 for all modes, further confirming the uniqueness of the matter's vibration in the THz range. The results show that the low frequency normal modes of THz are complex, but have certain regularity. The intermolecular vibration of biomolecules is dominated by low frequency collective vibration, which involves a large number of atoms and a series of intermolecular interactions. The hydrogen bond plays a major role in the weak intermolecular interaction, and the frequency of vibration led by the hydrogen bond has a large distribution in the THz range.56 In combination with the characteristic frequency distribution of the covalent bond in the mid-infrared band,57 it explains, to a certain extent, the phenomenon that the contribution of intermolecular vibration decreases with the increase of frequency.
3.3 Interactions between the NMDAR and L-Glu
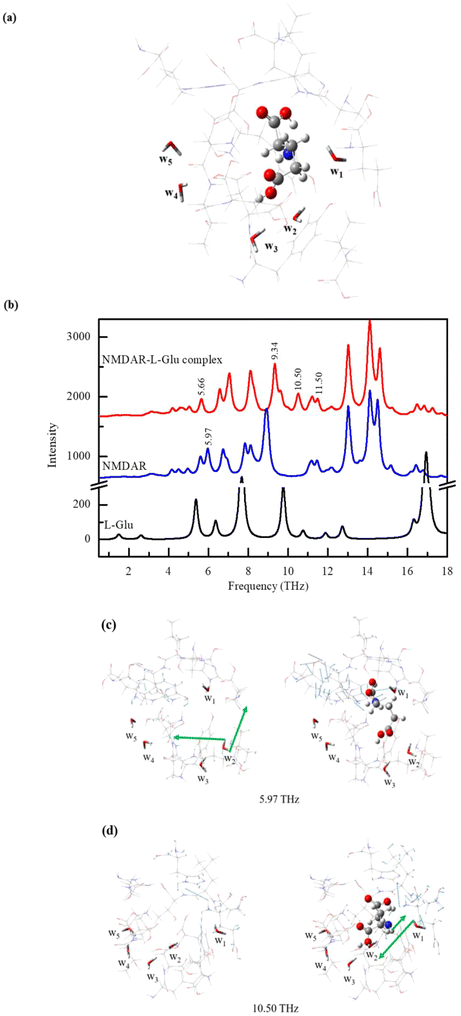
To study the vibrational properties of a ligand, it is usually necessary to consider the protein environment. The ONIOM method provides an effective tool for the study of the interactions between the receptor and the ligand and their infrared spectra.46,58,59 The NMDAR–L-Glu complex model is shown in Fig. 3a, where L-Glu is shown in ball and stick representation, water molecules are shown in stick representation, where wn (n = 1, 2, 3, 4, 5) is the water molecule label, and other parts are shown as thin sticks. L-Glu is encompassed by surrounding molecules such as histidine, serine and leucine, and five water molecules are distributed around L-Glu to participate in its structural composition. The w1, as the closest water molecule to the amino group of the ligand L-Glu, bridges L-Glu to the surrounding molecules. w2 and w3 are close to the γ-COOH of L-Glu, while w4 and w5 are far away from the ligand and blocked by surrounding molecules. The THz spectrum of the NMDAR–L-Glu complex in the range of 0.5–18 THz is shown as a red line in Fig. 3b. The blue and black lines represent the spectra of NMDAR and L-Glu, respectively. It should be noted that L-Glu in this calculation is a single molecule, which is different from the L-Glu in the crystal shown in Fig. S2.† Consequently, their spectra exhibit dissimilarities. From Fig. 3b, it can be seen that the three substances present rich absorption peaks. The number of peaks is in the order NMDAR–L-Glu > NMDAR > L-Glu, which is probably because NMDAR–L-Glu and NMDAR have more atoms involved in vibrations than L-Glu. Among them, the spectra of NMDAR–L-Glu and NMDAR are similar to some extent, for example, the two regions 0.5–5 THz and 11–16 THz have high degrees of coincidence. But the differences are also obvious, such as in the 5–11 THz and 16–18 THz regions. It may be caused by the intermolecular interactions between NMDAR and L-Glu.There are two obvious differences between the spectra of NMDAR–L-Glu and NMDAR at 5.97 THz and 10.50 THz. Their corresponding normal modes are shown in Fig. 3c and d, respectively. The vibrations are collective and mixed with each other, showing complexity. Among them, the vibrations of water molecules are more significant, and the vibrations related to water are highlighted by the green arrow. Considering the special role of water, we focus on the response of water molecules to THz waves in the NMDAR–L-Glu complex. In the NMDAR, the normal mode at 5.97 THz frequency is mainly the rocking vibration of w2 (left panel in Fig. 3c). When L-Glu acts on the receptor, the vibration is significantly inhibited (right panel in Fig. 3c), and the spectrum shows that NMDAR–L-Glu lacks the 5.97 THz absorption peak compared to NMDAR. This may be related to the interaction between γ-COOH in ligand L-Glu and w2. In the normal mode of the NMDAR at a frequency of 10.50 THz, all five water molecules are inactive (left panel in Fig. 3d). When L-Glu acts on the receptor, w1 exhibits an active vibration (right panel in Fig. 3d), mainly a vibration in the opposite direction between the –OH of w1 and the –NH2 of ligand L-Glu. And the absorption peak of NMDAR–L-Glu at 10.50 THz is much higher than that of NMDAR. This is most likely related to the interaction between –NH2 in L-Glu and w1 next to it. It has been suggested that low frequency vibrations and intermolecular interactions may be involved in the biological structure change.60,61 Chou's study has shown that the vibrational signal in IgG antibodies can be transmitted from one location to another via low frequency resonant channels, thus being able to induce relevant conformational changes required for some important biological functions.62 According to González-Jiménez et al., the origin of DNA hydrogen bond breaks in living organisms is believed to be the propagation of low frequency vibrational modes along its length in the form of phonon-like modes that can expand and contract the space between bases.63,64 The use of THz electromagnetic waves with specific frequencies may affect the vibrational behavior of substances and further achieve the effect of regulating biological functions.65,66 In the NMDAR–L-Glu complex system, the state of water molecules can be regarded as that in a certain confined space, different water molecules are present in diverse micro-environments and assume distinct roles. This phenomenon is related to the surrounding molecules, such as charge density, hydrophilicity, steric hindrance and three-dimensional architecture. Owing to environmental variations, the vibrational behavior of one-dimensional confined water differs from that of bulk water in the theoretical calculations conducted by Zhu et al. It is this fact that the absorption of 1.39 THz electromagnetic waves by the confined water can be realized, and then the resonance coupling causes the superpermeation of water, while the response of bulk water to this band is limited.36 Simulations conducted by Okumura et al. demonstrated distinct differences in the absorption spectra between the helical structure and the intermolecular β-sheet structure within amyloid-β amyloid fibrils. Upon resonance with an irradiated laser, numerous stable helical structures are formed subsequent to the destruction of intermolecular β-sheet structures.67 Zhang et al.'s simulation results showed that, under an appropriate THz stimulation, water confined on both sides of an asymmetric wettability membrane channel with different collective vibration modes has dissimilar absorbability to frequency-specific THz stimulation, resulting in a water density gradient that drives the ultrahigh water flux and can effectively penetrate into a bulk water system.68
Fig. 4 and Fig. S5† are the vibrations of water molecules corresponding to specific frequencies. For the same frequency, water molecules at different locations respond differently to THz waves. At a frequency of 5.66 THz, the response mode of w1 is shown in Fig. 4a, where one H atom amplitude is small and the other H atom amplitude is large, and the responses of four water molecules w2–w5 are not obvious. The modes of w2 and w3 at 9.34 THz are shown in Fig. 4b and they are significantly different. The other three water molecules have no obvious response. The modes of w4 and w5 at 11.50 THz are mainly attributed to wagging vibrations, but the directions of vibrations are roughly opposite, as shown by the red and blue arrow heads in Fig. 4c. In addition, an interesting phenomenon is that the same water molecules exhibit distinct response modes at different frequencies, demonstrating a diversity of behaviors. The w5 molecule is inactive at 5.66 THz and 9.34 THz, but active at 11.50 THz. The results show that the response of water molecules to THz waves is spatial and frequency specific. This could be due to the unique environment of the bound water, which leads to distinct responses of the different bound water in the molecule. Therefore, the vibrational behavior of water molecules can serve as valuable indicators for discerning diverse environments and unveiling distinct interactions.
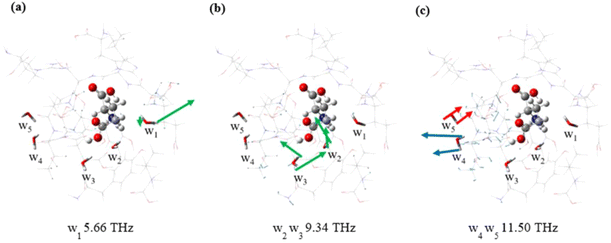 | ||
| Fig. 4 Schematic diagram of normal modes of different water molecules at 5.66 THz (a), 9.34 THz (b) and 11.50 THz (c). | ||
Since the water molecules in the NMDAR–L-Glu complex exhibit significant THz responses, we further studied the influence of the presence or absence of water molecules on the structure of the NMDAR–L-Glu complex. As shown in Fig. 5a, L-Glu is deeply embedded in surrounding molecules, and the green areas represent the locations of interactions between L-Glu and surrounding molecules. In the absence of water molecules (Fig. 5b), the interactions between L-Glu and its surroundings are weakened, as indicated by the arrows, which are largely due to the influence of w1 and w2, while the contributions of other water molecules are relatively small. The interaction change indicated by the red arrow could be related to the change of the w1 normal mode at 10.50 THz (Fig. 3d), while the interaction change indicated by the blue arrow could be related to the change of the w2 normal mode at 5.97 THz (Fig. 3c). The results of further structural optimization are shown in Fig. 5c. The complex shrinks as a whole, and the gaps created by the absence of water molecules are filled by the surrounding molecules. The γ-COOH of L-Glu undergoes torsion, which may be caused by the disappearance of the pulling effect of w2. The interactions between L-Glu and surrounding molecules are adjusted. The calculations show that the binding energy of L-Glu to the surrounding molecules in the initial structure is −27.0 kcal mol−1. In the absence of water molecules, it decreases to −25.0 kcal mol−1. After structural optimization, it increases to −26.2 kcal mol−1, but is still lower than the initial state.
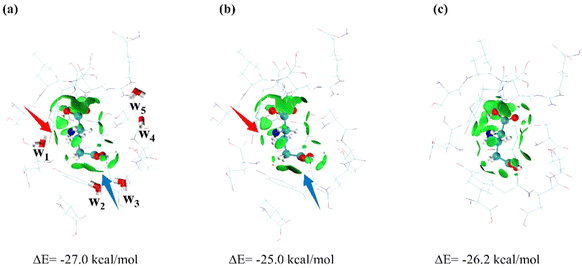 | ||
| Fig. 5 The conformation and binding energy of the NMDAR–L-Glu complex in the presence of water molecules (a), in the absence of water molecules (b) and after further structural optimization (c). | ||
These results suggest that the lack of water molecules leads to a tendency for ligands to detach from or bind less tightly to receptors, indicating that the presence of water molecules can promote the binding of the receptor and the ligand. Water molecules increase the affinity between the protein and the ligand through intermolecular interactions and regulate the structure between the protein and the ligand. This affects the shape and flexibility of protein binding sites, further causing changes in protein configuration and conformation and affects biological activity. Yang et al. studied the influence of water molecules in CDK2 active pockets on the CDK2-ligand binding free energy, and the results showed that the presence of water molecules at the active site helps to increase the value of protein–ligand binding free energy.69 A study by Schiebel et al. showed that water molecules have a key influence on the ligand binding and dissociation process.70 Calculations of Rohani et al. suggest that three specific water molecules near the phylloquinone molecule in the photosystem I protein complex are particularly important and may provide structural factors that increase the strength of hydrogen bonds.59 Arinaminpathy et al. demonstrated a correlation between the migration rate of water within the binding pockets of the glutamate receptor and both the degree of domain closure and the presence of ligands, which has implications for drug design targeting proteins.71,72
Considering that L-Glu is an acidic amino acid, its isoelectric point is less than 7, and it exists as a negative ion in neutral solution, we also investigated two negatively charged L-Glu, and preliminary studies show similar results to neutral L-Glu (see ESI Fig. S5–S11†). Therefore, we conclude that: (1) there are some differences in the THz spectra between the NMDAR and the NMDAR–L-Glu complex, which reflects the ability of the THz spectra to detect non-local conformational changes of proteins. (2) The water molecules in the NMDAR–L-Glu complex have a strong response in the THz band. Water molecules at different locations respond differently to THz waves. Water molecules exhibit distinct response modes at varying frequencies. This shows the specificity of the responses of water molecules to THz waves in a particular confined space. (3) The binding energy of L-Glu to the surrounding molecules decreased after the removal of water, which revealed that water molecules can promote the stability of the structure to a certain extent. Considering water molecules have a small volume, high activity, high flexibility and strong responses to THz waves, it is suggested that water molecules could be used as probes to reveal the structure and interaction of receptor proteins. Numerous studies have demonstrated the crucial and intricate role of water in biological structures; however, the detection of alterations in the molecular structure of these biological systems in solution remains a formidable challenge due to the pronounced absorption of THz waves by water.73,74 González-Jiménez et al., employing femtosecond optical Kerr-effect spectroscopy for the analysis of low frequency vibrational spectra of G-quadruplexes, discovered the presence of strongly underdamped delocalized phonon-like modes that hold potential implications for DNA biology at the atomic level.75 Li et al. present a time-domain terahertz optoacoustics method for the analysis of water-rich samples. Changing the THz photoacoustic signal of water by adjusting the temperature can improve the sensitivity of its analysis. This could provide a powerful tool for exploring molecular interactions and biochemical processes in aqueous solutions.76 The development of these techniques will be helpful to the study of low frequency vibration dynamics of biomolecules under water or physiological conditions.
4 Conclusions
In this study, the THz spectrum of L-Glu was recorded using an air plasma broadband THz-TDS system, and vibrational analysis was conducted based on DFT calculations. The rich characteristic absorption peaks provide valuable references for molecular detection and label-free identification. The intermolecular vibrational contribution shows a natural exponential decline trend with the increase of frequency. This is related to the diverse responses of different intensity interactions in the THz band. The interactions between L-Glu and surrounding molecules in the protein environment were investigated. The results demonstrate that THz spectroscopy exhibits sensitivity towards molecular conformational changes arising from intermolecular interactions. Different water molecules experience distinct microenvironmental constraints and play varying roles. The response of water molecules to THz waves exhibits specificity. The vibrational behavior has the potential to serve as a probe for indicating various environments and revealing diverse interactions. Water molecules act as regulators or adhesives, facilitating L-Glu binding with receptors while promoting structural stability to some extent, leading to changes in the conformational structure of the protein, which may potentially impact biological activity. Furthermore, whether water molecules can serve as relay stations for THz photons to facilitate energy transfer is worth further exploration.Author contributions
H. Z. initiated the research. H. Z. and Z. Z. designed the research. Y. W. prepared the samples and performed the THz-TDS, XRD and FTIR experiments; J. Y., Z. Z., and Y. W. performed the theoretical calculations and analysis. T. J., J. W., H. Z., W. P., and M. C. technically assisted the FTIR measurement; Y. W. and Z. Z. wrote the original manuscript; and H. Z. and Z. Z. revised the manuscript. All authors contributed to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (62075225 and 12204499), the Joint Key Project of the National Natural Science Foundation of China (U2032206), the CAS Project for Young Scientists in Basic Research (YSBR-042), and the Open Project of the State Key Laboratory of Surface Physics at Fudan University (KF2022_05). The authors would like to thank the staff at the BL06B beamline of the Shanghai Synchrotron Radiation Facility for their help in data collection and the staff at the BL17B1 beamline of the National Facility for Protein Science (NFPS) in Shanghai for their guidance in the experiment.References
- E. Karakas and H. Furukawa, Science, 2014, 344, 992–997 CrossRef CAS PubMed.
- W. J. McEntee and T. H. Crook, Psychopharmacology, 1993, 111, 391–401 CrossRef CAS PubMed.
- B. S. Meldrum, J. Nutr., 2000, 130, 1007S–1015S CrossRef CAS PubMed.
- T. E. Bartlett and Y. T. Wang, Neuropharmacology, 2013, 74, 59–68 CrossRef CAS PubMed.
- T. W. Lai, S. Zhang and Y. T. Wang, Prog. Neurobiol., 2014, 115, 157–188 CrossRef CAS PubMed.
- M. F. Cox, E. R. Hascup, A. Bartke and K. N. Hascup, Front. Aging, 2022, 3, 929474 CrossRef PubMed.
- L. Iovino, M. E. Tremblay and L. Civiero, J. Pharmacol. Sci., 2020, 144, 151–164 CrossRef CAS PubMed.
- P. Ball, Chem. Rev., 2008, 108, 74–108 CrossRef CAS PubMed.
- E. Persch, O. Dumele and F. Diederich, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS PubMed.
- R. K. Pal, S. Gadhiya, S. Ramsey, P. Cordone, L. Wickstrom, W. W. Harding, T. Kurtzman and E. Gallicchio, PLoS One, 2019, 14, 20 Search PubMed.
- Z. Li and T. Lazaridis, Phys. Chem. Chem. Phys., 2007, 9, 573–581 RSC.
- S. B. A. de Beer, N. P. E. Vermeulen and C. Oostenbrink, Curr. Top. Med. Chem., 2010, 10, 55–66 CrossRef CAS PubMed.
- F. Mallamace, P. Baglioni, C. Corsaro, S. H. Chen, D. Mallamace, C. Vasi and H. E. Stanley, J. Chem. Phys., 2014, 141, 10 Search PubMed.
- S. Fischer, J. C. Smith and C. S. Verma, J. Phys. Chem. B, 2001, 105, 8050–8055 CrossRef CAS.
- K. M. Vance, N. Simorowski, S. F. Traynelis and H. Furukawa, Nat. Commun., 2011, 2, 11 Search PubMed.
- B. M. Fischer, M. Walther and P. U. Jepsen, Phys. Med. Biol., 2002, 47, 3807–3814 CrossRef CAS PubMed.
- M. T. Ruggiero, J. Sibik, R. Orlando, J. A. Zeitler and T. M. Korter, Angew. Chem., Int. Ed., 2016, 55, 6877–6881 CrossRef CAS PubMed.
- I. V. Lundholm, H. Rodilla, W. Y. Wahlgren, A. Duelli, G. Bourenkov, J. Vukusic, R. Friedman, J. Stake, T. Schneider and G. Katona, Struct. Dyn., 2015, 2, 12 Search PubMed.
- C. Jordens, M. Scheller, S. Wietzke, D. Romeike, C. Jansen, T. Zentgraf, K. Wiesauer, V. Reisecker and M. Koch, Compos. Sci. Technol., 2010, 70, 472–477 CrossRef.
- J. Neu, E. A. Stone, J. A. Spies, G. Storch, A. S. Hatano, B. Q. Mercado, S. J. Miller and C. A. Schmuttenmaer, J. Phys. Chem. Lett., 2019, 10, 2624–2628 CrossRef CAS PubMed.
- L. Chen, G. Ren, L. Liu, P. Guo, E. Wang, L. Zhou, Z. Zhu, J. Zhang, B. Yang, W. Zhang, Y. Li, W. Zhang, Y. Gao, H. Zhao and J. Han, J. Phys. Chem. Lett., 2020, 11, 7146–7152 CrossRef CAS PubMed.
- Y. Ueno, R. Rungsawang, I. Tomita and K. Ajito, Anal. Chem., 2006, 78, 5424–5428 CrossRef CAS PubMed.
- Z. Q. Zhu, Y. J. Bian, X. Zhang, R. N. Zeng and B. Yang, Spectrochim. Acta, Part A, 2022, 275, 9 CrossRef PubMed.
- Z. Zhu, J. Zhang, Y. Song, C. Chang, G. Ren, J. Shen, Z. Zhang, T. Ji, M. Chen and H. Zhao, Analyst, 2020, 145, 6006–6013 RSC.
- M. T. Ruggiero, J. Sibik, J. A. Zeitler and T. M. Korter, J. Phys. Chem. A, 2016, 120, 7490–7495 CrossRef CAS PubMed.
- Y. Li, L. Xu, H. Li, G. Xiong, Q. Liu, W. Yang, S. Yang and X. Deng, J. Mol. Struct., 2019, 1180, 636–641 CrossRef CAS.
- K. Hand and E. Yates, J. R. Soc., Interface, 2017, 14, 4 CrossRef PubMed.
- X. Y. Peng and H. Zhou, Acta Phys. Sin., 2021, 70, 14 Search PubMed.
- H. Hintzsche, C. Jastrow, T. Kleine-Ostmann, U. Karst, T. Schrader and H. Stopper, PLoS One, 2012, 7, 8 CrossRef PubMed.
- X. Liu, Z. Qiao, Y. Chai, Z. Zhu, K. Wu, W. Ji, D. Li, Y. Xiao, L. Mao, C. Chang, Q. Wen, B. Song and Y. Shu, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, 9 Search PubMed.
- Y. Li, C. Chang, Z. Zhu, L. Sun and C. Fan, J. Am. Chem. Soc., 2021, 143, 4311–4318 CrossRef CAS PubMed.
- C. Zhang, Y. F. Yuan, K. J. Wu, Y. Wang, S. T. Zhu, J. Y. Shi, L. H. Wang, Q. Li, X. L. Zuo, C. H. Fan, C. Chang and J. Li, Nano Lett., 2022, 22, 8 Search PubMed.
- J. K. Yin, K. J. Wu, Y. Yu, Y. Zhong, Z. H. Song, C. Chang and G. Z. Liu, ACS Nano, 2024, 18, 4796–4810 CrossRef CAS PubMed.
- B. Song and L. Jiang, Sci. China Mater., 2021, 64, 2572–2579 CrossRef CAS.
- K. Wu, C. Qi, Z. Zhu, C. Wang, B. Song and C. Chang, J. Phys. Chem. Lett., 2020, 11, 7002–7008 CrossRef CAS PubMed.
- Z. Zhu, C. Chang, Y. S. Shu and B. Song, J. Phys. Chem. Lett., 2020, 11, 256–262 CrossRef CAS PubMed.
- Y. M. Li, Z. Zhu, L. Sun, Z. X. Xiang, C. Chang and C. H. Fan, ACS Nano, 2022, 16, 8419–8426 CrossRef CAS PubMed.
- R. D. Tang and J. P. Dai, PLoS One, 2014, 9, 8 Search PubMed.
- Z. Wang, N. T. Wang, Z. H. Li, F. Y. Xiao and J. P. Dai, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8753–8758 CrossRef CAS PubMed.
- W. T. Chai, Z. R. Han, Z. Wang, Z. H. Li, F. Y. Xiao, Y. Sun, Y. F. Dai, R. D. Tang and J. P. Dai, Neurosci. Bull., 2018, 34, 534–538 CrossRef PubMed.
- Z. R. Han, W. T. Chai, Z. Wang, F. Y. Xiao and J. P. Dai, Photochem. Photobiol. Sci., 2021, 20, 343–356 CrossRef CAS PubMed.
- X. Xie, J. M. Dai and X. C. Zhang, Phys. Rev. Lett., 2006, 96, 4 Search PubMed.
- S. Hirokawa, Acta Crystallogr., 1955, 8, 637–641 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- Z. Zhu, C. Cheng, C. Chang, G. Ren, J. Zhang, Y. Peng, J. Han and H. Zhao, Analyst, 2019, 144, 2504–2510 RSC.
- L. Rohani and G. Hastings, Biochim. Biophys. Acta, Bioenerg., 2021, 1862, 9 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, GAUSSIAN 16 (Revision C.01), Gaussian Inc., Wallingford, CT, 2019.
- T. Lu and Q. X. Chen, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS PubMed.
- T. Lu and F. W. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- A. Matei, N. Drichko, B. Gompf and M. Dressel, Chem. Phys., 2005, 316, 61–71 CrossRef CAS.
- F. Zhang, H. W. Wang, K. Tominaga and M. Hayashi, J. Phys. Chem. A, 2015, 119, 3008–3022 CrossRef CAS PubMed.
- M. R. C. Williams, A. B. True, A. F. Izmaylov, T. A. French, K. Schroeck and C. A. Schmuttenmaer, Phys. Chem. Chem. Phys., 2011, 13, 11719–11730 RSC.
- M. R. C. Williams, D. J. Aschaffenburg, B. K. Ofori-Okai and C. A. Schmuttenmaer, J. Phys. Chem. B, 2013, 117, 10444–10461 CrossRef CAS PubMed.
- J. X. Shen, Z. J. Zhu, Z. C. Zhang, C. Guo, J. B. Zhang, G. H. Ren, L. G. Chen, S. P. Li and H. W. Zhao, Spectrochim. Acta, Part A, 2021, 247, 8 Search PubMed.
- S. Bakels, M. P. Gaigeot and A. M. Rijs, Chem. Rev., 2020, 120, 3233–3260 CrossRef CAS PubMed.
- A. Barth, Biochim. Biophys. Acta, Bioenerg., 2007, 1767, 1073–1101 CrossRef CAS PubMed.
- H. P. Lamichhane and G. Hastings, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10526–10531 CrossRef CAS PubMed.
- L. Rohani, H. Makita, A. Levitz, M. Henary and G. Hastings, Biochim. Biophys. Acta, Bioenerg., 2019, 1860, 699–707 CrossRef CAS PubMed.
- D. A. Turton, H. M. Senn, T. Harwood, A. J. Lapthorn, E. M. Ellis and K. Wynne, Nat. Commun., 2014, 5, 6 Search PubMed.
- B. Varvdekar, A. Prabhakant and M. Krishnan, J. Chem. Inf. Model., 2022, 62, 1669–1679 CrossRef CAS PubMed.
- K. C. Chou, Biopolymers, 1987, 26, 285–295 CrossRef CAS PubMed.
- M. González-Jiménez, G. Ramakrishnan, T. Harwood, A. J. Lapthorn, S. M. Kelly, E. M. Ellis and K. Wynne, Nat. Commun., 2016, 7, 6 Search PubMed.
- K. C. Chou, G. M. Maggiora and B. Mao, Biophys. J., 1989, 56, 295–305 CrossRef CAS PubMed.
- Z. Zhu, C. Chen, C. Chang and B. Song, ACS Photonics, 2021, 8, 781–786 CrossRef CAS.
- T. Y. Sun and Z. Zhu, J. Membr. Sci., 2022, 662, 7 CrossRef.
- H. Okumura, S. G. Itoh, K. Nakamura and T. Kawasaki, J. Phys. Chem. B, 2021, 125, 4964–4976 CrossRef CAS PubMed.
- Q.-L. Zhang, T. Zhou, C. Chang, S.-Y. Gu, Y.-J. Wang, Q. Liu and Z. Zhu, Phys. Rev. Lett., 2024, 132, 184003 CrossRef CAS PubMed.
- L. Yang, R. Jia and S. Yang, Acta Chim. Sin., 2009, 67, 255–260 CAS.
- J. Schiebel, R. Gaspari, T. Wulsdorf, K. Ngo, C. Sohn, T. E. Schrader, A. Cavalli, A. Ostermann, A. Heine and G. Klebe, Nat. Commun., 2018, 9, 15 CrossRef PubMed.
- M. L. Mayer, Nature, 2006, 440, 456–462 CrossRef CAS PubMed.
- Y. Arinaminpathy, M. S. P. Sansom and P. C. Biggin, Mol. Pharmacol., 2006, 69, 5–12 CrossRef PubMed.
- H. Y. Ge, Z. Y. Sun, Y. Y. Jiang, X. Y. Wu, Z. Y. Jia, G. Y. Cui and Y. Zhang, Int. J. Mol. Sci., 2023, 24, 23 Search PubMed.
- N. Q. Vinh, L. C. Doan, N. L. H. Hoang, J. R. R. Cui and B. Sindle, J. Chem. Phys., 2023, 158, 9 CrossRef PubMed.
- M. González-Jiménez, G. Ramakrishnan, N. V. Tukachev, H. M. Senn and K. Wynne, Phys. Chem. Chem. Phys., 2021, 23, 13250–13260 RSC.
- J. Li, Y. X. Yao, L. W. Jiang, S. Li, Z. H. Yi, X. Y. Chen, Z. Tian and W. L. Zhang, Adv. Photonics, 2021, 3, 13 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, structure diagram, FTIR spectrum, and normal modes. See DOI: https://doi.org/10.1039/d4an00697f |
| This journal is © The Royal Society of Chemistry 2024 |