Surface plasmon resonance of Au/Ag metals for the photoluminescence enhancement of lanthanide ion Ln3+ doped upconversion nanoparticles in bioimaging
Hao
Peng
ab,
Shunxiang
Li
a,
Jie
Xing
ac,
Fang
Yang
 *ac and
Aiguo
Wu
*ac and
Aiguo
Wu
 *ac
*ac
aCixi Institute of Biomedical Engineering, International Cooperation Base of Biomedical Materials Technology and Application, Chinese Academy of Science (CAS) Key Laboratory of Magnetic Materials and Devices and Zhejiang Engineering Research Center for Biomedical Materials, Ningbo Institute of Materials Technology and Engineering, CAS, Ningbo, 315201, P. R. China. E-mail: yangf@nimte.ac.cn; aiguo@nimte.ac.cn
bUniversity of Chinese Academy of Sciences, No. 1 Yanqihu East Road, Huairou District, Beijing, 101408, China
cAdvanced Energy Science and Technology Guangdong Laboratory, Huizhou, 516000, China
First published on 14th November 2022
Abstract
Deep tissue penetration, chemical inertness and biocompatibility give UCNPs a competitive edge over traditional fluorescent materials like organic dyes or quantum dots. However, the low quantum efficiency of UNCPs becomes an obstacle. Among extensive methods and strategies currently used to prominently solve this concerned issue, surface plasmon resonance (SPR) of noble metals is of great use due to the agreement between the SPR peak of metals and absorption band of UCNPs. A key challenge of this match is that the structures and sizes of noble metals have significant influences on the peak of SPR formants, where achieving an explicit elucidation of relationships between the physical properties of noble metals and their SPR formants is of great importance. This review aims to clarify the mechanism of the SPR effect of noble metals on the optical performance of UCNPs. Furthermore, novel research studies in which Au, Ag or Au/Ag composites in various structures and sizes are combined with UCNPs through different synthetic methods are summarized. We provide an overview of improved photoluminescence for bioimaging exhibited by different composite nanoparticles with respect to UCNPs acting as both cores and shells, taking Au@UCNPs, Ag@UCNPs and Au/Ag@UCNPs into account. Finally, there are remaining shortcomings and latent opportunities which deserve further research. This review will provide directions for the bioimaging applications of UCNPs through the introduction of the SPR effect of noble metals.
10th Anniversary StatementOver the past ten years, Journal of Materials Chemistry B has achieved considerable developments. More than 200 articles related to upconversion nanoparticles (UCNPs) have been published since 2013 when the research was focused on cancer cell imaging and death, and has recently shifted to multimodal theranostics of tumors in vivo. Wu's group has also made some progress in the fluorescence imaging of Ln3+-doped nanoparticles but encountered the problem of insufficient quantum efficiency. In this review, a combination of the surface plasmon resonance effect of novel metals Au/Ag and Ln3+-doped UCNPs has been proved to be practicable in fluorescence enhancement which can be explained using three plausible mechanisms. Au/Ag–UCNP composites fabricated by physical approaches exhibit superior enhancement factors but less flexibility and biocompatibility, while Au/Ag–UCNP systems synthesized chemically improve the disadvantages but with hard-to-control fluorescence enhancement. This manuscript therefore emphasizes the urgent need to develop an efficient and biosafe preparation method. In addition, this review covers the applications of Au/Ag–UCNPs in bioimaging from upconversion luminescence imaging agents to multi-modal imaging agents in tumor-bearing mice. Hence, the strategy of noble metal enhanced UCNPs has been proven to be feasible and has prospects for bioimaging applications like accurate tumor diagnosis and surgery guidance. |
Introduction
The concept of upconversion luminescence was first discovered by Bloembergen in 1959.1 Afterwards, Auzel pointed out in his study that when Tm3+,Er3+ and Ho3+ were co-doped with Yb3+ in ytterbium sodium tungstate glass, the excitation light intensity under infrared light increased by nearly two orders of magnitude, formally proposing the concept of upconversion luminescence.2 However, it was not until the past two decades that the application of upconversion luminescence materials developed rapidly, along with the exponential growth of the publication of relevant papers.3 Upconversion nanoparticles (UCNPs) are a class of materials with anti-Stokes luminescence properties, and exhibit continuous absorption of two or more low-energy pumped photons by excited state absorption (ESA), energy transfer upconversion (ETU), photon avalanche (PA), cooperative energy transfer (CTE) and energy migration-mediated upconversion (EMU), thus releasing photons with higher energy.3,4Compared with conventional organic fluorescent dyes and quantum dots, UCNPs have better chemical stability, reducing the occurrence of photobleaching.5 Meanwhile, multi-wavelength emission can be achieved by adjusting both the matrix and doping ions due to their independence from the crystal size and structure.6–8 On the other hand, UCNPs excited by near-infrared irradiation can effectively solve the problems of poor tissue penetration, biological toxicity and self-fluorescence interference of organic fluorescent dyes and quantum dots.5,9 Considering the above advantages as well as long fluorescence life, UCNPs have been widely applied in biological imaging,9–13 therapy14–17 and biomarkers as ideal functional materials.18–21
One of the most important indexes to evaluate the optical properties of upconversion materials is fluorescence quantum efficiency (QE), which can be calculated by the ratio of emitted to absorbed photons.22 Unfortunately, the limited absorption cross section and large specific surface area of UCNPs, as well as surface defects, result in low luminescence efficiency,23 usually no more than 1% as reported,24 restricting the further application of UCNPs severely. Moreover, there is an intensity threshold (from the ground state to excited states) for the occurrence of nonlinear luminescence upconversion.25 Meanwhile, according to the data from “American National Standard for Safe Use of Lasers”, radiation light in the near-infrared band with power above 0.726 W cm−2 is harmful to biological tissue,26 which sets the maximum value of excitation light utilized in biological applications. Therefore, the development of UCNPs with both low excitation threshold and high luminescence intensity has become a hotspot and difficulty in biomedical research.27
Through years of exploration, researchers have discovered ways to effectively modify UCNPs, briefly divided into two types. One approach is internal regulation, including matrix lattice28–30 and doping ions.9,31 The other is to regulate the external environment of UCNPs, such as surface passivation32,33 commonly and metal surface plasmon resonance (SPR).34–36 Zhan37 utilized gold nanorods (GNRs) to enhance, by up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, the upconversion emission, demonstrating the great potential of the metal-SPR method.
000 times, the upconversion emission, demonstrating the great potential of the metal-SPR method.
Mechanism of SPR enhanced upconversion
Mechanism of upconversion luminescence
UCNPs are generally composed of a host matrix, a sensitizer and an activator. The most widely used host matrix is NaYF4 in the hexagonal phase38 because of its ample interspace for doping ions, favourable light transmission and low phonon energy, which reduce the energy loss of the exciting radiation. Trivalent lanthanide ions are ideal elements for both a sensitizer (such as Neodymium, Nd3+ for 808 nm;39 Ytterbium, Yb3+ for 980 nm38) and an activator (such as Holmium, Ho3+,40 Thulium, Tm3+,41 and Erbium,Er3+,42). The occurrence of upconversion luminescence requires that the doping ions inside UCNPs have abundant long-life intermediate energy levels (Fig. 1(a)) to promote the ETU process between sensitizers and activators.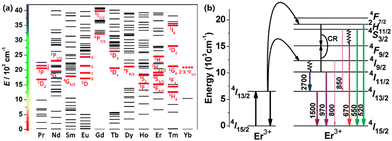 | ||
| Fig. 1 (a) Partial energy level diagrams of Ln3+. Corresponding typical UC emissive excited levels are highlighted with red bold lines; reproduced from ref. 4 with permission from the Royal Society of Chemistry. (b) Diagram of the energy levels of the Er3+ ion and the proposed mechanisms of upconversion. Adapted with permission from ref. 43. Copyright {2022} American Chemical Society. | ||
The outermost electron configuration of Er, one of the lanthanide elements, is 4f125s25p66s2. Under the combination of Coulomb interaction, spin–orbital coupling and crystal field disturbance, the 4fn electron configuration is divided into several intermediate energy levels.38 When Er single-doped UCNPs are exposed to 1550 nm NIR-II photons, Er3+ ions can be excited from the ground state 4I15/2 to 4I13/2 through the ESA process,4 whereafter, another absorbed photon can excite the ions into 4I9/2, some of which are dropped to the ground state, resulting in the emission of 800 nm photons. The further ESA process stimulates 4I9/2 ions to higher 2H11/2. As a consequence, the excited ions emit green photons through a radiative transition to the ground state or transit to the 4F9/2 state and perform red emission (Fig. 1(b)).43 As for Yb3+/Er3+ co-doped UCNPs, Yb3+ ions are firstly excited to 2F5/2 by the ESA process with absorption at 980 nm. Since the energy barrier in Yb3+ ions from 2F5/2 down to the ground state 2F7/2 matches the energy difference between intermediate energy levels (4I15/2 5/4I11/2, 4I15/2 → 4I11/2) in Er3+ ions, an effective ET process helps to excite nearby Er3+ ions into 4I11/2 or one of the higher energy levels 4F7/2 through a continuous process.44 Hence, for co-doping UCNPs, sensitizers can absorb quantities of photons, increasing the ESA process which is weak and even negligible in single doped UCNPs45 while the ET between sensitizer ions and activator ions causes energy loss as well. Corresponding to the statement above, it is significant to develop practical routes to improve the luminescence efficiency of UCNPs.
Mechanism of the plasmon resonance effect
The plasmon resonance effect of noble metals with nano sizes (usually Silver, Ag and Gold, Au), also known as localized surface plasmon resonance (LSPR), refers to the continuous oscillation of electrons in the conduction band of noble metals under the circumstance of illumination with electromagnetic radiation, resulting in a dense wave of electrons propagating along the metal surface.46 In the case of light radiation or an applied electric field, the surface electrons collectively vibrate and emit an electromagnetic wave. When the free electron field produced by the electron vibration couples with the wavelength of incident light, the LSPR effect whereby the energy of the incident light is focused or transferred on the metal surface takes place,47 and scattering or absorption enhancement occurs at the particle. It can be noticed that LSPR will occur only if the incident light wavelength exceeds the particle size. As an example, bulk Au material has a golden color, whereas Au NPs appear deeply ruby-red.48 Here, the absorption of photons exceeds light reflection.49The absorption and scattering characteristics of noble metal particles in the visible band have been discovered and utilized by intelligent humans since ancient Rome, where wine glass doped with noble metal particles revealed gorgeous colors in the sunlight.50 But it was not until 1908 that Gustav Mie explained the chromatic dispersion properties of noble metal ions through Maxwell's equations.51 The Mie theory is an analytical solution to Maxwell's equations for electric field scattering operation, mainly used for spherical noble metal particles with wavelength-similar sizes.52 In addition to calculating the scattering of a single spherical noble metal particle, Mie theory can also be applied to calculate multiple metal particles. Since Pines proposed the term ‘Plasmon’ to describe this phenomenon,53 mathematical methods such as finite difference time domain (FDTD), discrete dipole approximation (DDA) and finite element method (FEM) have been commonly used to study its nature. In 1957, Swan and Powell experimentally confirmed the surface electron oscillation mode of noble metals calculated by Ritchie,54 after which the name ‘surface plasmon’ formally appeared.55
According to theoretical calculation models like the Drude model,56,57 the oscillation frequency of metal particles is related to the dielectric constant and the damping coefficient of metals. In addition, Mie's equation also indicates the connection between the position of the LSPR spectrum and the size and morphology of metal particles. The position of the resonance peak appears to red shift with the increase of particle size as reported.58,59
Mechanism of plasmon enhancement of UCNPs
Enhancing upconversion materials by using plasmon resonance is recognized as one of the most effective methods to improve luminescence intensity. Yan Chunhua et al. first reported the application of SPR enhancement in upconversion luminescence of rare earth fluorides60 where they fabricated Ag nanowire-NaYF4: Yb3+,Er3+ composites by a self-assembly approach. The absorption peak of Ag nanowires lay around 420 nm which was different from the spectrum of pure NaYF4: Yb3+,Er3+ particles. The results showed that the emission intensity of composites at 543 nm (green emission) and 659 nm (red emission) under 980 nm excitation increased by 3.7 and 2.3 times, respectively.Actually, SPR-enhanced upconversion luminescence refers to the phenomenon that when UCNPs are in proximity to the surface of noble metals, the metal LSPR interacts with the UCNPs, resulting in a significant increase in the photoluminescence intensity of the UCNPs compared to that of the free state.61 In general, the enhancement can be achieved only if the metal is kept at an appropriate distance from the UCNPs. Shen et al.62 found that the best thickness of a SiO2 layer sandwiched between Ag nanoparticles and NaYF4: Yb,Er,Gd was 15 nm with an enhancement factor of 3.4. When Ag particles and NaYF4 UCNPs approached a distance below 5 nm, the activator ions in UCNPs easily diffuse to the surface,63 leading to electron countercurrent with metal ions at the Fermi level,64 causing a severe surface quenching effect. As for a far distance, the Ag SPR effect did not interact with both the absorption and emission of NaYF4 UCNPs.
So far, SPR enhanced upconversion can be basically explained by the following three mechanisms:65–69 excitation enhancement, emission enhancement and energy transfer, as shown in Fig. 2.
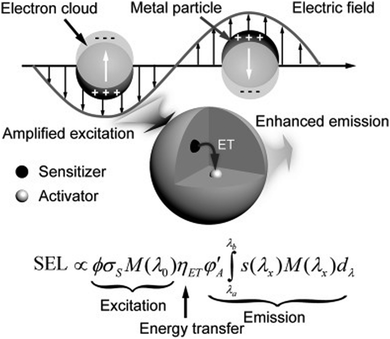 | ||
| Fig. 2 Schematic illustration showing the plausible mechanism that governs the plasmonic enhancement of upconversion luminescence.65 | ||
The SPR effect of metal nanostructures enhances the local electric field, which is coupled with UCNPs, thus amplifying the absorption of the sensitizer.70 Better performance can be realized if the excitation wavelength matches the strongest extinction of the SPR effects. The luminescence intensity I of UCNPs is estimated using the following equation
| I ∝ |E|2 × σs × ηET × ψA |
Therefore, when UCNPs are anchored near metal nanoparticles, the SPR effect of the metal generates a strong local electric field, and the boosted electric field intensity E expands the flux of incident light ϕ, thus increasing the light absorption by UCNPs, and finally improving the luminescence intensity.
Emission enhancement will take place when the oscillation frequency of metal SPR overlaps with the luminous band of UCNPs. The coupling of the emitted light and the SPR effect can magnify the local state density of photons near the metal surface.65 Subsequently, the radiation decay rate of the activator is improved so as to increase luminous intensity.71,72 The addition of metal nanoparticles fastens the radiative transition rate of the UCNP activator, advancing the quantum yield while reducing the fluorescence lifetime, and eventually enhancing upconversion emission.
Besides, Nagpal et al.73 coupled NaYF4:Yb3+,Er3+ nanoparticles with Au pyramid nanostructures, which implies that the SPR effect can accelerate the energy transfer between sensitizer and activator pairs in UNCPs. For a certain SPR–UCNP system, the upconversion enhancement is conventionally controlled by one of three mechanisms. For instance, Zhang74 synthesized Au–NaYF4 core–shell nanostructures where the emission enhancement mechanism by the Au nanoshell contributed to upconversion improvement. Besides, there may be two or even three mechanisms working together to promote the enhancement. Both Kang75 and He76 reported that Au nanorod plasma can enhance excitation and emission simultaneously in Au nanorod@SiO2@UCNP composites (Fig. 3). By adjusting the aspect ratio of Au nanorods, the longitudinal SPR wavelength can be efficiently shifted from visible to near-infrared light fitting the excitation wavelength of sensitizers. The transverse SPR remains at 530 nm, matching the green emission band of the activator Er3+, thus selectively accelerating the radiative decay in green upconversion. However, the three mechanisms stated above are still related to the interaction between the SPR effect and electromagnetic waves substantially. At present, there is no recognized explanation for the deep mechanism of the metal SPR effect enhancing UCNP luminescence.
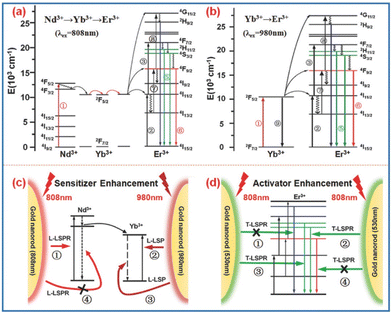 | ||
| Fig. 3 (a and b) The energy diagrams of Nd3+, Yb3+, and Er3+ ions undergoing ETU upon excitation at 808 and 980 nm, respectively. (c) The GNR Longitudinal-LSPR-induced absorption enhancement for an Nd3+ ion upon excitation at 808 nm and for a Yb3+ ion upon excitation at 980 nm. The radiative decay rate of the Yb3+ ion is also accelerated by the GNR Longitudinal-LSPR (due to energy matching) while that of the Nd3+ ion is not (denoted by “ ×”) because of the fast phonon-mediated relaxation and energy migration to the Yb3+ ion. (d) The GNR Transverse-LSPR-induced green-emission enhancement for both ions upon excitation at 808 nm. The blue and red emissions are not affected by the GNR Transverse-LSPR due to energy mismatch (denoted by “ ×”).75 | ||
Construction methods of the metal-UCNP system
As described above, the SPR–UCNP interaction is closely related to the distance. In order to accurately control the distance between UCNPs and metal, most of the literature adjusts the concentration and size of the metal77–80 or introduces an intermediate layer74,81–84 to achieve the best enhancement performance. So far, SPR–UCNP systems are mostly built either by chemical methods or physical approaches.Physical construction of SPR–UCNPs
Self-assembly method
The most basic physical construction method is to deposit metal and UCNPs directly on the surface of the substrate (generally glass). Metal-UCNP nanocomposites can be self-assembled after the solvent evaporates.85 Zhang and co-workers86 took an optical microfiber prepared by flame heating as a template, sprayed the Au nanofilm onto its surface by rotation and then dropped the aqueous solution of UCNPs. UCNPs/Au samples were fabricated after evaporation. The UCNPs/Au composites displayed enhanced emission at 523 nm under 980 nm excitation, 35 times stronger than that of the original UCNPs. The mechanism could be attributed to the excitation enhancement from the SPR effect instead of emission enhancement because there was only a slight change in the lifetimes of emissions after the Au modification, standing for the invariant radiative decay rate (Fig. 4b–d).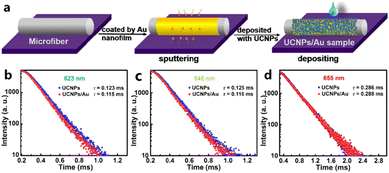 | ||
| Fig. 4 (a) Formation process of the UCNPs/Au sample. (b–d) Decay curves of 2H11/2–4I15/2 (523 nm), 4S3/2–4I15/2 (540 nm) and 4F9/2–4I15/2 (655 nm) transition of Er3+ ions in UCNPs/Au and UCNPs samples, respectively. Adapted with permission from ref. 86. Copyright {2022} American Chemical Society. | ||
Subtly, Xue78 and Shao87 spin-coated the solution of Au nanorods and UCNPs on the same glass substrate and evaporated it in a vacuum. Then, Xue used atomic force microscopy (AFM) to identify the locations of gold nanorods and UCNPs, and propelled UCNPs with AFM probes until the gold nanorods joined. The whole process was monitored under a confocal laser microscope, which assisted in optical emission spectrum measurement, revealing that the red upconversion intensity was increased by 110 fold and 19 fold joined with Au nanorods with a large diameter (46.7 nm) or a small diameter (27.3 nm). The experimental data and finite-difference-time-domain calculation ensure the combination of excitation and emission enhancement mechanisms. The Shao group first synthesized porous Ag nanofilms based on polystyrene microspheres and dispersed NaYF4: Yb3+,Er3+ nanoparticles, fabricated in the traditional way,88 using a spin coater. After the solvent evaporated, a 60-fold increase in green emission (540 nm) and a nearly 50-fold increase in red emission (668 nm) were detected. Lee89 designed a plasmonic Ag nanodome array (NDA)-UCNP structure by spin coating as well, which could attain as high SPR enhancement as 800 and 340 times at 656 nm and 542 nm under weak excitation power, separately, as shown in Fig. 5.
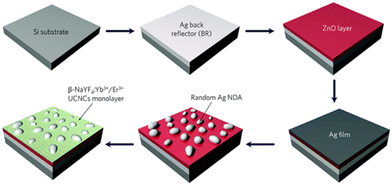 | ||
| Fig. 5 A schematic illustration of the fabrication procedure of the plasmonic back-reflector substrate on which a dewetted Ag nanodome array (NDA) and a monolayer of upconversion nanocrystals are mounted. Reproduced from ref. 89 with permission from the Royal Society of Chemistry. | ||
Liu et al.90 fabricated a hybrid composed of Al disk/SiO2/UCNPs/Al film through a series of physical processes like electron beam deposition, spin coating and developing processing. The maximum enhancement factors of the final products in green (545 nm) and red bands (655 nm) were 90 and 180, respectively. In addition, they have noticed that the SPR effect of Al disks has significantly different influences on green and red emissions (Fig. 6). The hybrids exhibit the strongest red signals at 170 nm diameter of Al disks. However, when the diameter of the Al disks is enlarged to 210 nm, green emission dominates the luminescence with the highest enhancement factor.
 | ||
| Fig. 6 SEM (i), reflected bright field (ii), and upconversion luminescence (iii) color patches of pixel arrays with D = 90–220 nm and s = 100 nm.90 | ||
Periodic metal nanostructures
The direct deposition or spin-coating method is convenient but the nanoparticles tend to agglutinate during the solvent volatilization process, which makes it difficult to ensure uniformity on the substrate. Therefore, periodic metal nanostructures such as nanogratings, nanoholes, nanorods and triangular arrays prepared by template etching and nano-imprint technology have been introduced into the SPR–UCNP nanocomposites. Good periodicity, controllable geometry and nanoscale precision can better exert the metal SPR effect due to a transverse momentum.91 As reported, both periodic gold nanorods or gold nanoholes enhanced upconversion intensity over 35 fold.92,93A nanohole array is one familiar periodic nanostructure. As shown in Fig. 7a, Zhan coupled plasmonic Au-nanohole–nanoplate bilayer arrays (PABAs) with core–shell nanoparticles.94 The results demonstrated a 6-fold increase in the green emission of core–shell UCNPs after coupling, which was proportional to the irradiation intensity from 12.5 to 50 mW mm−2, arising from a stimulated local electric field of PABAs and coherent interference of the SPR effect with the emission band. Similarly, but further, Das and his group synthesized dispersible metal–insulator–metal (MIM) structures (inset in Fig. 7b),95 where Au nanohole arrays were constructed utilizing the template method. UCNPs were encapsulated in an insulator layer and coupled with top and bottom Au layers. From the upconversion spectra in Fig. 7b, the photoluminescence intensity was demonstrated to be strongly heightened at the 250 nm diameter of MIM composites. Moreover, it is noteworthy that a relatively low excitation power density (<50 W cm−2) induced green emission with over 1000 times enhancement while the factor was no more than 200 at a power density exceeding 20 kW cm−2 (Fig. 7c), which shows potential in sensitive and effective theranostics of lesions with mild side effects.
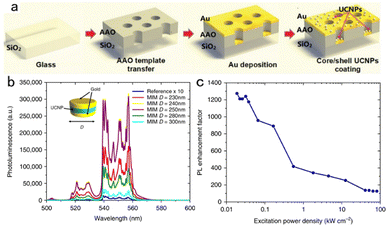 | ||
Fig. 7 (a) The fabrication process of the AAO@Au@CS-UCNPs.94 (b) Green emission spectra of the reference sample and the (MIM) structure of varying diameters under 980![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm excitation. The reference sample emission is magnified by 10 to show the relevant features. Inset: Schematic of the MIM nanostructure. (c) 250 nm excitation. The reference sample emission is magnified by 10 to show the relevant features. Inset: Schematic of the MIM nanostructure. (c) 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm diameter MIM PL enhancement as a function of power density.95 nm diameter MIM PL enhancement as a function of power density.95 | ||
Chu96 obtained a periodic Ag nanograting through photochemical reduction on a polyimide layer and a Ag nanofilm as a control. The period of the Ag nanograting is precisely managed by the regulation of the angle at which the Ag stock solution is exposed to the laser and reduced. The strongest enhancement of the SPR effect on UCNP luminescence is at the nanograting period of 700 nm, with a factor of 4.5 totally, two times higher than that of non-periodic Ag nanofilms. It can be attributed to a broad absorption of Ag nanograting-UCNPs at the NIR region, resulting from the Ag SPR effect.
Lanthanide ion doped UCNPs uniformly coated on the neatly arranged vertical Au nanorod (GNR) substrate under rotation also show upconversion emission improvement with 8.8-fold and 35-fold maxima, adjusted by the thickness of the intermediate layer (SiO2 and MoO3 shown in Fig. 8) between the GNR and UCNPs under 980 nm excitation,92,97 respectively. GNR absorption peaks are located at 520 nm and 680 nm, which are approximately coupled to Er3+ emission wavelengths of 540 nm and 640 nm. Besides, due to the vertical alignment characteristics of GNRs, the coupling effect between the SPR effect and the emission wavelength of red light (4F9/2–4I15/2) is larger than that of green light.75
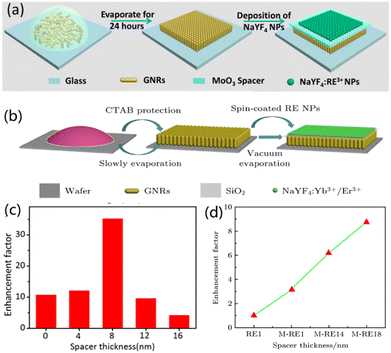 | ||
| Fig. 8 (a and b) Schematic illustration of the fabrication process of NaYF4:Yb3+,Er3+/MoO3/GNRs and GNRs@SiO2@NaYF4:Yb3+/Er3+, respectively. (c and d) The enhancement factors as a function of the MoO3 or SiO2 spacer thickness. Adapted with permission from ref. 92. Copyright {2022} American Chemical Society and ref. 97. | ||
Except for the standard circular arrays, other periodic metal nano-arrays in various shapes perform well in SPR enhancement too. Square aperture arrays of Au metal are reported to have an enhancement factor of 450 on the upconversion emission of Er3+ doped UCNPs, even better than the performance of Au annular aperture arrays, which is 370.98 A fascinating Au pyramid array coupled with NaYF4: Yb3+/Er3+ nanoparticles was synthesized by Sun,73 displaying a minimum factor of 6 of the SPR effect enhancement. Chen36 prepared islands Au–Ag alloy films via the PMMA removal method with UCNPs self-assembled onto the surface and explored an optimal upconversion enhancement of the composites which is 180-fold.
However, though periodic metal structures have an outstanding upconversion enhancement effect on UCNPs, their processing equipment is expensive and not suitable for mass production. More critically, physical methods are mostly carried out on rigid substrates such as glass and silicon wafers, which lack flexibility and biocompatibility. Hence, the applications in biomedical fields are greatly limited.
Chemical synthesis of SPR–UCNPs
The simplest chemical synthesis method is to directly dope metal ions into UNCPs, currently used in rare earth ions doped phosphors.99 With advanced research work, it is found that the morphology, size and concentration of metal particles as well as the distance between metal and UCNPs will affect the emission intensity, as mentioned above. Therefore, SPR–UCNP systems with core–shell structures synthesized in chemical approaches are mostly considered100 which can make better use of the SPR effect of metal while reducing the energy loss from surface defects of UCNPs.101Core–shell is an ordered structure formed by covering nanomaterials through chemical bonds, Coulomb electrostatic attraction, adsorption layer media and other interaction forces. Based on the different materials of the core and shells, SPR–UCNP systems can be separated into two types (Fig. 9): one is the core composed of UCNPs and the shell layer is composed of metal, while the inverted structures also hold. The former one is the current mainstream of plasmon-enhanced upconversion materials, since the surface defects are weakened and the SPR effect is greatly brought into play.
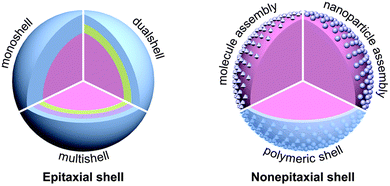 | ||
| Fig. 9 Schematic illustration of the typical architectures of UC core–shell nanoparticles. Reproduced from ref. 100 with permission from the Royal Society of Chemistry. | ||
Duan's group studied the SPR effect of gold particle coverage on NaYF4: Yb3+, Tm3+ core102 and the results revealed that with the increase of attached Au particles, the upconversion luminescence intensity gradually increases to the maximum value (1.5 times more than pure UCNPs). However, after the formation of an Au shell in Fig. 10, intensity started to decline with a thicker Au shell. Considering the distance between metals and UCNPs affects the enhancement,62 an intermediate isolation layer is widely introduced in the compounds.
 | ||
| Fig. 10 Illustration of surface functionalization, gold NP attachment, and gold nanoshell growth on the upconversion nanoparticles.102 | ||
Ding and co-workers103 took NaYF4: Yb3+,Er3+ nanoparticles as the core and SiO2-encapsulated Ag nanoparticles as the shell, fabricating the hybrid material with almost 3.5 times luminescence enhancement in both green and red emission. Yuan104 set Ag nanoparticles free from the SiO2 shell and designed a NaYF4: Yb3+,Er3+@SiO2@Ag nanostructure. The addition of an Ag shell improved the photoluminescence intensity of NaYF4: Yb3+,Er3+, which depended on the thickness of SiO2. The most significant enhancement appeared when the SiO2 layer is regulated to 10 nm. Soon after, Shen62 and Qin81 proved the SPR enhancement of the Ag shell and the negative influence of isolation layer thickness on the UCNPs core (Fig. 11), which can be attributed to the competition between energy transfer and radiation attenuation rate.
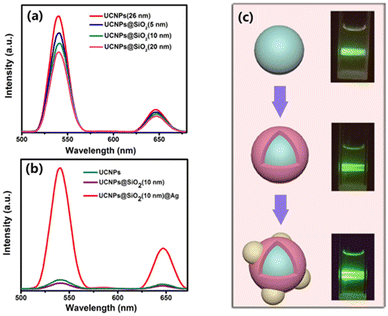 | ||
| Fig. 11 (a) UC emission spectra of pure UCNPs and UCNPs@SiO2 core/shell NPs. (b) UC emission spectra of the UCNPs, UCNPs@SiO2(10 nm) NPs and UCNPs@SiO2(10 nm)@Ag NPs. (c) Schematic illustrations of these three types of NPs and their digital luminescence photos.81 | ||
More interestingly, studies have revealed that the thickness of the metal shell which is usually made up of Ag and Au along with the thickness of the intermediate layer composed of SiO2 affects the upconversion enhancement.105 When the Au shell is 8 nm thick, its SPR peak located at 540 nm corresponds to the emission wavelength of UCNPs thus contributing to the enhancement factor of 9.1.74 However, the SPR peak shifts to 900 nm with a 3 nm thick Au shell, enhancing full-wave luminescence by a factor of 2–3.106
Currently, for the sake of a higher enhancement factor of metal-UCNPs core–shell structures, Kang75 regarded a sensitizer-doped host matrix NaGdF4: Yb3+, Nd3+ as another intermediate layer coating the luminous core NaGdF4: Yb3+,Er3+. Inside the core–shell UCNPs, the energy transfer can occur between Nd3+–Yb3+–Er3+ or Yb3+–Er3+ ion pairs. Besides, on the surface of UCNPs, Au nanorods alongside SiO2 layers are attached due to electrostatic interaction. Kang named the final Au–UCNPs composites core–shell–shell upconversion nanocrystals (UCNC). The Au Longitudinal-SPR wavelength couples with the irradiation wavelength at 808 nm if the thickness of the SiO2 layer is 28 nm, increasing the local electrical field which is proportional to the square root of light flux. Thus because of the excitation enhancement mechanism, the signal intensity in green emission is 18.85 times greater than that of UCNPs without Au@SiO2 shells.
To avoid the adverse absorption of irradiation and emission of the metal shell existing in the metal shell-UCNPs core structure, some researchers fabricated metal particles as the core materials while UCNPs acted as the shell part. Fig. 12 displays a Ag core coated with SiO2 isolation layers by Yin.72 UCNPs were synthesized in situ from precursors (LnCl3) in Ag@SiO2 solution and precipitated on the surface to constitute the outermost shell. The absorption spectrum ensured the SPR effect of the Ag core, without a SiO2@UCNPs peak being detected. Additionally, compared with pure Ag nanoparticles, Ag@SiO2@UCNPs showed a redshift of the SPR wavelength from 350–665 nm to a broader range at 400–850 nm, resulting in a 30-fold enhancement of red emission under the excitation of 980 nm. Afterwards, Yin107 carried out another attractive research work where Ag/graphene nanocomposites played the role of a core, while outside of a 10 nm thick SiO2 isolation layer, the UCNP (NaLuF4: Yb3+, Gd3+, Er3+) shell existed. By graphene addition, the enhancement factor of the UCNPs-metal composites rose up to 52, and hence even higher than the former work.
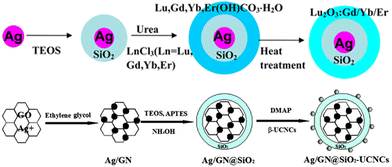 | ||
| Fig. 12 Examples of the fabrication process of the metal core-UCNP shell nanostructures. Adapted with permission from ref. 72. Copyright {2022} American Chemical Society. Reproduced from ref. 107 with permission from the Royal Society of Chemistry. | ||
Based on the core–shell mode, Hinamoto deposited a metal (Ag) cap above the Au-core@UCNPs-shell structures.108 The Ag cap has a weak influence on the excitation enhancement of the SPR effect because of the mismatch of SPR peak at 735 nm and irradiation at 980 nm. Yet, the scattering wavelength of the Ag cap from 500 nm to 850 nm overlapped the emission bands of Au@UCNPs. Then the emission intensities of a number of Au@UCNPs and Ag-cap@UCNPs@Au nanoparticles were measured and shown in Fig. 13(b). Consequently, the average green and red emissions calculated were further enhanced by factors 3.5 and 6, respectively, by virtue of dual SPR–UCNP coupling.
 | ||
| Fig. 13 (a) Schematic illustration, TEM image, EDS mapping image of Au core/Er3+ and Yb3+ co-doped yttrium silicate shell/Ag shell (CSCap) composite nanoparticles. (b) Upconversion PL peak intensities obtained for a 50 Au core/Er3+ and Yb3+ co-doped yttrium silicate shell (CS) and 24 CSCap particles. Adapted with permission from ref. 108. Copyright {2022} American Chemical Society. | ||
However, chemical synthesis methods show disadvantages, like complex preparation processes. Besides, reaction parameters such as surfactant, reaction temperature, time or speed may exert effects on the surface defects of NPs.109 So the enhancement factor is hard to catch up with that of the physical method.
Applications in bioimaging
On account of advances in the metal SPR effect enhancement on UCNPs photoluminescence intensity achieved in recent years, the application of plasmon resonance materials can be witnessed in solar cells,110 photocatalysis,111 anti-counterfeiting,90 sensors,112 photothermal therapy,113 bioimaging114 and other fields. Combined with our own research background, this review focuses on the application of SPR-enhanced upconversion luminescence in bioimaging.The NaYF4: Yb3+,Er3+@SiO2@Ag nanocomposites prepared by Yuan104 exhibited an obvious upconversion luminescence enhancement because of the coupling between UCNPs and Ag nanoparticles, as mentioned in the Section “Chemical construction of SPR–UCNPs”. The cytotoxicity of nanocomposites is weakened through the modification with DNA materials, seen from the increase of cell viability from 50% to 92% after 24 h incubation in Fig. 14. After co-incubation with murine melanoma cells B16F0, confocal images showed bright green fluorescence corresponding to the emission band of Er3+ (4S3/2–4I15/2) inside the cells under the excitation of 980 nm at 500 mW power. The irradiation led to little cellular lethality only, confirming the potential of NaYF4: Yb3+,Er3+@SiO2@Ag as an optical imaging agent.
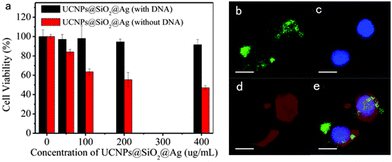 | ||
| Fig. 14 (a) Viability of cells incubated with metal@UCNP nanocomposites before (red) and after (black) modification with DNA. (b) Upconversion luminescence image of live B16F0 cells after incubation with DNA-modified nanocomposites (green). (c and d) Fluorescence images of the cells counterstained with DAPI (blue) and Concanavalin A (red) to show the nucleus and cell membranes, respectively. The merged images are shown in (e). (Scale bar: 20 μm.) Reproduced from ref. 104 with permission from the Royal Society of Chemistry. | ||
Another kind of cancer cell line (HeLa), which is artificially cultured with infinite multiplication capacity, was first derived from cervical cancer from an American woman Henrietta Lacks, also known as experimental proliferating epidermal cancer cells. The studies on the fluorescence imaging ability of metal-UCNPs nanomaterials with HeLa cells in vitro have been carried out abundantly over the past five years.114–119 Observable upconversion fluorescence under near-infrared excitation is dependent on the lanthanide ions doped inside UCNPs. The inverted fluorescence microscope displayed prominent red and green signals inside HeLa cells under 980 nm laser,120 corresponding to the energy level 4F9/2–4I15/2 and 4S3/2–4I15/2 of activator ions Er3+ doped in Au nanorods@NaGdF4 hybrids with SPR enhancement,114 separately. Furthermore, He76 discovered that the enhancement factor of Au nanorods in red emission exceeds that in the green region. The overlay images of HeLa cells by confocal laser scanning microscopy displayed a color change from green when incubated with SiO2@UCNPs to yellow after the incubation with modified Au nanorods, as shown in Fig. 15. Another important discovery in HeLa upconversion luminescence experiments was the uptake of metal-UCNP nanocomposites. Neither under the confocal microscope nor the inverted fluorescence microscope, researchers observed the fluorescence signals in the culture medium surrounding HeLa cells. However, the fluorescence distributed among cellular cytoplasm and nucleus randomly showed up.115 Wang114 demonstrated the continuous ingestion and accumulation of UNCPs coated with shell layer formed by Au nanorods, as incubation time with HeLa cells was prolonged. Furthermore, the quantitative calculation of the intracellular content of Au, determined by ICP-MS technique also ensured its accuracy. Cell uptake and a relatively long period of stay made metal-UCNP hybrid materials satisfy the basic requirements of in vitro and in vivo bioimaging.
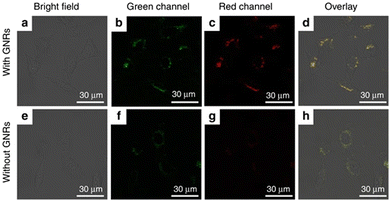 | ||
| Fig. 15 Confocal laser scanning microscopy images of HeLa cells incubated with GNR@UCNPs hybrid nanostructures (top row: (a) bright field image; (b) green channel image; (c) red channel image and (d) overlay imaging.) and UCNPs (bottom row: (e) bright field image; (f) green channel image; (g) red channel image and (h) overlay imaging).76 | ||
Fig. 16 displays apparent green light located at the tumor site under near-infrared excitation (980 nm) which can downshift tissue overheating and autofluorescence interference. The green emission intensity reached the maximum of 4 h after the injection of Au nanorods @NaGdF4: Yb3+,Er3+ in mice.114 Considering the aspect ratio of Au nanorods, a low aspect ratio is beneficial to the transportation and uptake of nanomaterials. However, a high length-diameter ratio strengthens the longitudinal instead of the transverse SPR effect and enhances red emission. The red shift of the integral emission wavelength to the near-infrared region increases the penetration depth of upconversion imaging in vivo which was certified by Liu and co-workers.113 After the modification with Au/Ag nanocages, NaYF4: Yb3+, Tm3+@Yb3+, Nd3+ nanoparticles exhibited yellow fluorescence penetration 2 mm deeper than unmodified ones in Fig. 17.
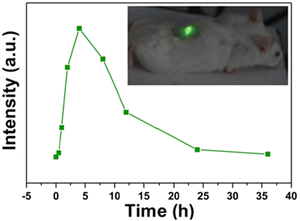 | ||
| Fig. 16 The integrated intensity of UCL emission as a function of the tumor-bearing site injected time. (An in vivo merged images of a mouse injected with Au nanorods@UCNPs under NIR laser excitation is inset.)114 | ||
 | ||
| Fig. 17 Penetration changes when UCNPs are modified with Au/Ag metals. Adapted with permission from ref. 113. Copyright {2022} American Chemical Society. | ||
Apart from the fundamental upconversion luminescence property, metal-UCNP conjugates generally work as multifunctional contrast agents in the bioimaging field (Fig. 18).121 Noble metals like Ag nanowires119 turn out to be excellent computed tomographic (CT) agents with a Hounsfield unit (HU) value of 53.29 HU L g−1 which is almost twice as much as that of the commercial CT agent iohexol (29.67 HU L g−1)122 because of high absorption of X-ray.123 The Gd element doped in the metal-UCNPs composites has an intrinsic CT imaging performance but also shows a high magnetic resonance imaging (MRI) contrast either in T1-weighted imaging119 with relaxivity of 10.39 mM−1 s−1 or in T2-weighted imaging114 with 15.625 mM−1 s−1 relaxivity. It is unusual that the metal shell coating UCNP core structures attenuate imaging resolution mainly because the metals affect the paramagnetic properties of Gd-doped UCNPs. What is more, the addition of antibodies or targeting molecules towards metal-UCNPs composites prompts hybrids to act as bioimaging probes labelling tumor sites9 or studying intermediate products of biological activities.118
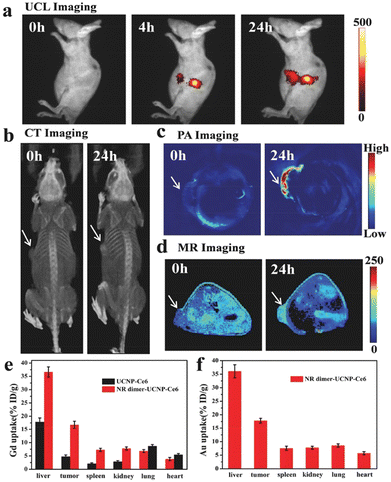 | ||
| Fig. 18 In vivo multimodal imaging. (a) UCL imaging, (b) CT imaging, (c) photoacoustic imaging, and (d) T1-MR imaging of HeLa tumor-bearing mice taken at different time points after injection with Au@UCNPs.121 | ||
Bioimaging is vital to the development of life sciences and medicine because of its ability to monitor various physiological and pathological processes in real-time with high clarity and visualize various biological entities. The metal SPR effect enhanced UCNPs photoluminescence with higher sensitivity is a promising strategy for applications in bioimaging. However, though Das95 and Yin92 respectively indicated that the SPR effect induced more intense enhancement to the photoluminescence of UCNPs at the lower excitation intensities compared with stronger irradiation, absolute emission intensities might be decreased because of less absorption. Therefore, it is significant to find the balance between the large enhancement factor by SPR effect and the high emission intensity of UCNPs at the safe dose26 of 980 nm excitation (no more than 0.726 W cm−2). Some researchers have realized this issue and mentioned it in the studies where the 980 nm laser intensity utilized is 600 mW cm−2 for the in vitro cellular experiments but is not indicated for in vivo bioimaging.114,120 The question remains whether the bioimaging of UCNPs in vivo can be realized at a safe power density of 980 nm with the aid of the Au/Ag SPR effect, which is believed to be solvable.
Conclusion
UCNPs have low upconversion luminescence efficiency, limiting their further application. The UCNP luminescence can be effectively enhanced by the metal SPR effect. In this review, mechanisms of the SPR effect regulating UCNP luminescence, and preparation methods of the SPR–UCNP system along with its applications in the bioimaging field are elaborated in sequence.However, there are still some pressing issues in the field: (1) as photoluminescence enhancement involves the complex interaction of excitation light, rare earth ions and metal SPR in different states, there is still a lack of accepted theoretical models to explain the interaction mechanism between metal SPR and UCNPs. Therefore, the theory of UCNP luminescence enhanced by metal SPR needs to be further improved and corroborated in the experiment. (2) The chemical synthesis methods have complex steps, multiple reaction parameters along with limited enhancement factors while physical ones are mostly completed on a rigid substrate which results in poor biocompatibility. Therefore, how to prepare an effective and repeatable SPR–UCNP system is still a big challenge. (3) Compared with the preparation and mechanism research of the SPR–UCNP system, the application research in bioimaging of the SPR–UCNP system is relatively rare but has potential and is attractive.
Author contributions
Hao Peng: conceptualization, investigation, writing – original draft, and writing – review and editing. Shunxiang LI: resources and writing – original draft. Jie Xing: investigation and writing – original draft. Fang Yang: conceptualization, funding acquisition, supervision, and writing – review and editing. Aiguo Wu: funding acquisition, supervision, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (32111540257, 32025021, and 31971292), the Science & Technology Bureau of Ningbo City (2020Z094), and Funding in Zhejiang Province (2020C03110). The authors acknowledge the support from the Youth Innovation Promotion Association, Chinese Academy of Sciences (2022301) and the 3315 Innovative Talent Project of Ningbo (2018-05-G).Notes and references
- N. Bloembergen, Phys. Rev. Lett., 1959, 2, 84–85 CrossRef CAS.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- J. F.-C. Loo, Y.-H. Chien, F. Yin, S.-K. Kong, H.-P. Ho and K.-T. Yong, Coord. Chem. Rev., 2019, 400, 213042 CrossRef CAS.
- H. Dong, L.-D. Sun and C.-H. Yan, Chem. Soc. Rev., 2015, 44, 1608–1634 RSC.
- B. Yang, H. Chen, Z. Zheng and G. Li, J. Lumin., 2020, 223, 117226 CrossRef CAS.
- H. S. Mader, P. Kele, S. M. Saleh and O. S. Wolfbeis, Curr. Opin. Chem. Biol., 2010, 14, 582–596 CrossRef CAS PubMed.
- Y. Fan, P. Wang, Y. Lu, R. Wang, L. Zhou, X. Zheng, X. Li, J. A. Piper and F. Zhang, Nat. Nanotechnol., 2018, 13, 941–946 CrossRef CAS PubMed.
- Y. Lu, J. Lu, J. Zhao, J. Cusido, F. M. Raymo, J. Yuan, S. Yang, R. C. Leif, Y. Huo, J. A. Piper, J. Paul Robinson, E. M. Goldys and D. Jin, Nat. Commun., 2014, 5, 3741 CrossRef CAS PubMed.
- G. Xiang, X. Liu, Q. Xia, X. Liu, S. Xu, S. Jiang, X. Zhou, L. Li, D. Wu, L. Ma, X. Wang and J. Zhang, Talanta, 2021, 224, 121832 CrossRef CAS PubMed.
- N. Kang, Y. Liu, Y. Zhou, D. Wang, C. Chen, S. Ye, L. Nie and L. Ren, Adv. Healthcare Mater., 2016, 5, 1356–1363 CrossRef CAS PubMed.
- Y. Liu, N. Kang, J. Lv, Z. Zhou, Q. Zhao, L. Ma, Z. Chen, L. Ren and L. Nie, Adv. Mater., 2016, 28, 6411–6419 CrossRef CAS.
- Y. Luo, W. Zhang, Z. Liao, S. Yang, S. Yang, X. Li, F. Zuo and J. Luo, Nanomaterials, 2018, 8, 466 CrossRef PubMed.
- F. He, G. Yang, P. Yang, Y. Yu, R. Lv, C. Li, Y. Dai, S. Gai and J. Lin, Adv. Funct. Mater., 2015, 25, 3966–3976 CrossRef CAS.
- S. Chen, A. Z. Weitemier, X. Zeng, L. He, X. Wang, Y. Tao, A. J. Y. Huang, Y. Hashimotodani, M. Kano, H. Iwasaki, L. K. Parajuli, S. Okabe, D. B. L. Teh, A. H. All, I. Tsutsui-Kimura, K. F. Tanaka, X. Liu and T. J. McHugh, Science, 2018, 359, 679–684 CrossRef CAS PubMed.
- Y. Ma, J. Bao, Y. Zhang, Z. Li, X. Zhou, C. Wan, L. Huang, Y. Zhao, G. Han and T. Xue, Cell, 2019, 177, 243–255 CrossRef CAS PubMed.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- Y. Ouyang, Y. Liu, Z. M. Wang, Z. Liu and M. Wu, Nano-Micro Lett., 2021, 13, 133 CrossRef CAS PubMed.
- M. Huang, L. Wang, X. Zhang, J. Zhou, L. Liu, Y. Pan, B. Yu and Z. Yu, Nano, 2017, 12, 1750057 CrossRef CAS.
- D. T. Vu, T. T. Vu-Le, V. N. Nguyen, Q. M. Le, C.-R. C. Wang, L.-K. Chau, T.-S. Yang, M. W. Y. Chan, C.-I. Lee, C.-C. Ting, J.-Y. Lin, H.-C. Kan and C. C. Hsu, Int. J. Smart Nano Mater., 2021, 12, 49–71 CrossRef.
- G. Yang, Y. Cao, B. Yan, Q. Lv, J. Yu, F. Zhao, Z. Chen, H. Yang, M. Chen and Z. Jin, Oncotarget, 2018, 9, 16758–16774 CrossRef PubMed.
- X. Zou, M. Xu, W. Yuan, Q. Wang, Y. Shi, W. Feng and F. Li, Chem. Commun., 2016, 52, 13389–13392 RSC.
- D. M. Wu, A. García-Etxarri, A. Salleo and J. A. Dionne, J. Phys. Chem. Lett., 2014, 5, 4020–4031 CrossRef CAS PubMed.
- A. Gupta, S. Ghosh, M. K. Thakur, J. Zhou, K. Ostrikov, D. Jin and S. Chattopadhyay, Prog. Mater. Sci., 2021, 121, 100838 CrossRef CAS.
- G. Chen, T. Y. Ohulchanskyy, S. Liu, W.-C. Law, F. Wu, M. T. Swihart, H. Ågren and P. N. Prasad, ACS Nano, 2012, 6, 2969–2977 CrossRef CAS PubMed.
- Z. Chen, W. Sun, H.-J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165–9170 CrossRef CAS PubMed.
- Laser Institute of America, SPIE Medical Imaging, 2007.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Nat. Commun., 2018, 9, 2415 CrossRef.
- Q. Cheng, J. Sui and W. Cai, Nanoscale, 2012, 4, 779–784 RSC.
- Y. Sun, H. Bi, T. Wang, L. Sun, Z. Li, H. Song, F. Sun, H. Zhou, G. Zhou and J. Hu, Mater. Sci. Eng., B, 2020, 261, 114674 CrossRef CAS.
- H. Lin, Z. Wang and Y. Hong, J. Nanomater., 2020, 2020, 8509380 Search PubMed.
- R. Luo, L. Chen, Q. Li, J. Zhou, L. Mei, Z. Ning, Y. Zhao, M. Liu, X. Lai, J. Bi, W. Yin and D. Gao, Inorg. Chem., 2020, 59, 17906–17915 CrossRef CAS PubMed.
- H. Rabie, Y. Zhang, N. Pasquale, M. J. Lagos, P. E. Batson and K.-B. Lee, Adv. Mater., 2019, 31, 1806991 CrossRef.
- Z. Meng, S. Zhang and S. Wu, J. Lumin., 2020, 227, 117566 CrossRef CAS.
- B. Li, T. Gu, T. Ming, J. Wang, P. Wang, J. Wang and J. C. Yu, ACS Nano, 2014, 8, 8152–8162 CrossRef CAS PubMed.
- Z. Yin, X. Zhang, D. Zhou, H. Wang, W. Xu, X. Chen, T. Zhang and H. Song, RSC Adv., 2016, 6, 86297–86300 RSC.
- X. Chen, W. Xu, L. Zhang, X. Bai, S. Cui, D. Zhou, Z. Yin, H. Song and D.-H. Kim, Adv. Funct. Mater., 2015, 25, 5462–5471 CrossRef CAS.
- Q. Zhan, X. Zhang, Y. Zhao, J. Liu and S. He, Laser Photonics Rev., 2015, 9, 479–487 CrossRef CAS.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS.
- D. Yang, C. Cao, W. Feng, C. Huang and F. Li, J. Rare Earths, 2018, 36, 113–118 CrossRef CAS.
- W. Shao, G. Chen, A. Kuzmin, H. L. Kutscher, A. Pliss, T. Y. Ohulchanskyy and P. N. Prasad, J. Am. Chem. Soc., 2016, 138, 16192–16195 CrossRef CAS.
- H. Wang, Z. Wang, Y. Tu, Y. Li, T. Xu, M. Yang, P. Wang and Y. Gu, Biomaterials, 2020, 235, 119765 CrossRef CAS PubMed.
- C. Liu, B. Liu, J. Zhao, Z. Di, D. Chen, Z. Gu, L. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 2634–2638 CrossRef CAS PubMed.
- G. Chen, T. Y. Ohulchanskyy, A. Kachynski, H. Ågren and P. N. Prasad, ACS Nano, 2011, 5, 4981–4986 CrossRef CAS PubMed.
- C. Strohhofer and A. Polman, J. Appl. Phys., 2001, 90, 4314–4320 CrossRef CAS.
- D. Gao, X. Zhang and W. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 9732–9739 CrossRef CAS.
- H. Atwater and A. Polman, Nat. Mater., 2010, 9, 865 CrossRef CAS.
- H. Yang, L.-Q. He, Y.-W. Hu, X. Lu, G.-R. Li, B. Liu, B. Ren, Y. Tong and P.-P. Fang, Angew. Chem., Int. Ed., 2015, 54, 11462–11466 CrossRef CAS PubMed.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS.
- C. Cretu and E. van der Lingen, Gold Bull., 1999, 32, 115–126 CrossRef CAS.
- D. Compton, L. Cornish and E. van der Lingen, Gold Bull., 2003, 36, 10–16 CrossRef CAS.
- Y. A. Eremin, in Encyclopedia of Modern Optics, ed., R. D. Guenther, Elsevier, Oxford, 2005, pp. 326–330 Search PubMed.
- G. Mie, Ann. Phys., 1908, 330, 377–445 CrossRef.
- D. Pines, Rev. Mod. Phys., 1956, 28, 184–198 CrossRef CAS.
- R. H. Ritchie, Phys. Rev., 1957, 1, 874–881 CrossRef.
- C. Powell and J. Swan, Phys. Rev., 1959, 116, 81–83 CrossRef CAS.
- M. M. Alvarez, J. T. Khoury, T. G. Schaaff, M. N. Shafigullin, I. Vezmar and R. L. Whetten, J. Phys. Chem. B, 1997, 101, 3706–3712 CrossRef CAS.
- P. Chang, K. Liu, J. Jiang, T. Xu, Z. Zhang, J. Ma, Y. Zhao, J. Zhang, X. Li and T. Liu, Sens. Actuators, A, 2020, 309, 112022 CrossRef CAS.
- P. K. Baruah, A. K. Sharma and A. Khare, Opt. Laser Technol., 2018, 108, 574–582 CrossRef CAS.
- D. Mann, D. Nascimento-Duplat, H. Keul, M. Möller, M. Verheijen, M. Xu, H. P. Urbach, A. J. L. Adam and P. Buskens, Plasmonics, 2017, 12, 929–945 CrossRef CAS PubMed.
- W. Feng, L.-D. Sun and C.-H. Yan, Chem. Commun., 2009, 4393–4395 RSC.
- W. Park, D. Lu and S. Ahn, Chem. Soc. Rev., 2015, 44, 2940–2962 RSC.
- J. Shen, Z. Q. Li, Y. R. Chen, X. H. Chen, Y. W. Chen, Z. Sun and S. M. Huang, Appl. Surf. Sci., 2013, 270, 712–717 CrossRef CAS.
- K. Zheng, K. Y. Loh, Y. Wang, Q. Chen, J. Fan, T. Jung, S. H. Nam, Y. D. Suh and X. Liu, Nano Today, 2019, 29, 100797 CrossRef CAS.
- G. Schneider, G. Decher, N. Nerambourg, R. Praho, M. H. V. Werts and M. Blanchard-Desce, Nano Lett., 2006, 6, 530–536 CrossRef CAS.
- S. Han, R. Deng, X. Xie and X. Liu, Angew. Chem., Int. Ed., 2014, 53, 11702–11715 CrossRef CAS PubMed.
- K. Aslan and C. D. Geddes, Met.-Enhanced Fluoresc., 2010, pp. 1–23 Search PubMed.
- E. C. L. Ru, J. Grand, N. Félidj, J. Aubard, G. Lévi, A. Hohenau, J. R. Krenn, E. Blackie and P. G. Etchegoin, Me.-Enhanced Fluoresc., 2010, pp. 25–65 Search PubMed.
- D. J. Ross, N. P. W. Pieczonka and R. F. Aroca, Met.-Enhanced Fluoresc., 2010, pp. 67–90 Search PubMed.
- K. Munechika, Y. Chen, J. M. Smith and D. S. Ginger, Met.-Enhanced Fluoresc., 2010, pp. 91–118 Search PubMed.
- W. Deng, F. Xie, H. T. M. C. M. Baltar and E. M. Goldys, Phys. Chem. Chem. Phys., 2013, 15, 15695–15708 RSC.
- P. Xu, Q. Li, T. Li, W. Rao, Y. Wang, S. Lan and L. Wu, Plasmonics, 2014, 9, 1039–1047 CrossRef CAS.
- D. Yin, C. Wang, J. Ouyang, X. Zhang, Z. Jiao, Y. Feng, K. Song, B. Liu, X. Cao, L. Zhang, Y. Han and M. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 18480–18488 CrossRef CAS PubMed.
- Q.-C. Sun, H. Mundoor, J. C. Ribot, V. Singh, I. I. Smalyukh and P. Nagpal, Nano Lett., 2014, 14, 101–106 CrossRef CAS PubMed.
- A. Priyam, N. M. Idris and Y. Zhang, J. Mater. Chem., 2012, 22, 960–965 RSC.
- F. Kang, J. He, T. Sun, Z. Y. Bao, F. Wang and D. Y. Lei, Adv. Funct. Mater., 2017, 27, 1701842 CrossRef.
- J. He, W. Zheng, F. Ligmajer, C.-F. Chan, Z. Bao, K.-L. Wong, X. Chen, J. Hao, J. Dai, S.-F. Yu and D. Y. Lei, Light: Sci. Appl., 2017, 6, e16217 CrossRef CAS PubMed.
- A. L. Feng, M. Lin, L. Tian, H. Y. Zhu, H. Guo, S. Singamaneni, Z. Duan, T. J. Lu and F. Xu, RSC Adv., 2015, 5, 76825–76835 RSC.
- Y. Xue, C. Ding, Y. Rong, Q. Ma, C. Pan, E. Wu, B. Wu and H. Zeng, Small, 2017, 13, 1701155 CrossRef.
- D. Mendez-Gonzalez, S. Melle, O. G. Calderón, M. Laurenti, E. Cabrera-Granado, A. Egatz-Gómez, E. López-Cabarcos, J. Rubio-Retama and E. Díaz, Nanoscale, 2019, 11, 13832–13844 RSC.
- R. Lv, F. Yang, X. Jiang, B. Hu, X. Zhang, X. Chen and J. Tian, J. Lumin., 2020, 220, 116974 CrossRef CAS.
- Y. Qin, Z. Dong, D. Zhou, Y. Yang, X. Xu and J. Qiu, Opt. Mater. Express, 2016, 6, 1942–1955 CrossRef CAS.
- H. Wang, J. Cui, Z. Zheng, Q. Shi, T. Sun, X. Liu, Q. Huang and T. Fukuda, ACS Appl. Mater. Interfaces, 2017, 9, 41669–41679 CrossRef CAS PubMed.
- F. Xu, H. Gao, J. Liang, S. Jin, J. Zhao, Y. Liu, H. Zhang, Z. Zhang and Y. Mao, Ceram. Int., 2019, 45, 21557–21563 CrossRef CAS.
- L. Lin, Z. Yu, Z. Wang, Z. Feng, F. Huang, L. Huang, Q. Dai, F. Zhang and Z. Zheng, J. Lumin., 2019, 214, 116598 CrossRef CAS.
- C. Zhao, N. Chen, S. Liu, Z. Chen and T. Wang, J. Phys. Conf. Ser., 2017, 844, 012055 CrossRef.
- W. Zhang, J. Li, H. Lei and B. Li, ACS Appl. Mater. Interfaces, 2017, 9, 42935–42942 CrossRef CAS PubMed.
- B. Shao, Z. Yang, J. Li, J. Yang, Y. Wang, J. Qiu and Z. Song, Opt. Mater. Express, 2017, 7, 1188–1197 CrossRef CAS.
- L. Wang and Y. Li, Chem. Mater., 2007, 19, 727–734 CrossRef CAS.
- G. Y. Lee, K. Jung, H. S. Jang, J. Kyhm, I. K. Han, B. Park, H. Ju, S. J. Kwon and H. Ko, Nanoscale, 2016, 8, 2071–2080 RSC.
- H. Liu, J. Xu, H. Wang, Y. Liu, Q. Ruan, Y. Wu, X. Liu and J. K. W. Yang, Adv. Mater., 2019, 31, 1807900 CrossRef PubMed.
- J. Dong, W. Gao, Q. Han, Y. Wang, J. Qi, X. Yan and M. Sun, Rev. Phys., 2019, 4, 100026 CrossRef.
- Z. Yin, D. Zhou, W. Xu, S. Cui, X. Chen, H. Wang, S. Xu and H. Song, ACS Appl. Mater. Interfaces, 2016, 8, 11667–11674 CrossRef CAS PubMed.
- M. Saboktakin, X. Ye, U. K. Chettiar, N. Engheta, C. B. Murray and C. R. Kagan, ACS Nano, 2013, 7, 7186–7192 CrossRef CAS PubMed.
- S. Zhan, J. Xiong, G. Nie, S. Wu, J. Hu, X. Wu, S. Hu, J. Zhang, Y. Gao and Y. Liu, Adv. Mater. Interfaces, 2019, 6, 1802089 CrossRef.
- A. Das, C. Mao, S. Cho, K. Kim and W. Park, Nat. Commun., 2018, 9, 4828 CrossRef PubMed.
- A. Chu, H. He, Z. Yin, R. Peng, H. Yang, X. Gao, D. Luo, R. Chen, G. Xing and Y. J. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 1292–1298 CrossRef CAS PubMed.
- W. Gao, B. Y. Wang, Q. Y. Han, S. S. Han, X. T. Cheng, C. X. Zhang, Z. Y. Sun, L. Liu, X. W. Yan, Y. K. Wang and J. Dong, Acta Phys. Sin., 2020, 69, 184213 CrossRef.
- E. Verhagen, L. Kuipers and A. Polman, Opt. Express, 2009, 17, 14586–14598 CrossRef CAS PubMed.
- S. K. Maurya, S. P. Tiwari, A. Kumar and K. Kumar, J. Rare Earths, 2018, 36, 903–910 CrossRef CAS.
- X. Chen, D. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318–1330 RSC.
- G.-S. Yi and G.-M. Chow, Chem. Mater., 2007, 19, 341–343 CrossRef CAS.
- H. Zhang, Y. Li, I. A. Ivanov, Y. Qu, Y. Huang and X. Duan, Angew. Chem., Int. Ed., 2010, 49, 2865–2868 CrossRef CAS PubMed.
- Y. Ding, X. Zhang, H. Gao, S. Xu, C. Wei and Y. Zhao, J. Lumin., 2014, 147, 72–76 CrossRef CAS.
- P. Yuan, Y. H. Lee, M. K. Gnanasammandhan, Z. Guan, Y. Zhang and Q.-H. Xu, Nanoscale, 2012, 4, 5132–5137 RSC.
- W. Xu, X. Chen and H. Song, Nano Today, 2017, 17, 54–78 CrossRef CAS.
- W. Deng, L. Sudheendra, J. Zhao, J. Fu, D. Jin, I. M. Kennedy and E. M. Goldys, Nanotechnology, 2011, 22, 325604 CrossRef PubMed.
- D. Yin, X. Cao, L. Zhang, J. Tang, W. Huang, Y. Han and M. Wu, Dalton Trans., 2015, 44, 11147–11154 RSC.
- T. Hinamoto, H. Sugimoto and M. Fujii, J. Phys. Chem. C, 2018, 122, 17465–17472 CrossRef CAS.
- W. Xu, S. Xu, Y. Zhu, T. Liu, X. Bai, B. Dong, L. Xu and H. Song, Nanoscale, 2012, 4, 6971–6973 RSC.
- Y. Ding, H. Qiao, T. Yang, N. Yin, P. Li, Y. Zhao and X. Zhang, Opt. Mater., 2017, 73, 617–622 CrossRef CAS.
- Z. Zhang, Y. Liu, Y. Fang, B. Cao, J. Huang, K. Liu and B. Dong, Adv. Sci., 2018, 5, 1800748 CrossRef PubMed.
- K. Zhang, F. Lu, Z. Cai, S. Song, L. Jiang, Q. Min, X. Wu and J.-J. Zhu, Anal. Chem., 2020, 92, 11795–11801 CrossRef CAS PubMed.
- J. Liu, F. Yang, M. Feng, Y. Wang, X. Peng and R. Lv, ACS Biomater. Sci. Eng., 2019, 5, 5051–5059 CrossRef CAS PubMed.
- C. Wang, C. Xu, L. Xu, C. Sun, D. Yang, J. Xu, F. He, S. Gai and P. Yang, J. Mater. Chem. B, 2018, 6, 2597–2607 RSC.
- B. Kumar, A. Murali, I. Mattan and S. Giri, J. Phys. Chem. B, 2019, 123, 3738–3755 CrossRef CAS PubMed.
- M. Kataria, K. Yadav, A. Nain, H.-I. Lin, H.-W. Hu, C. R. Paul Inbaraj, T.-J. Chang, Y.-M. Liao, H.-Y. Cheng, K.-H. Lin, H.-T. Chang, F.-G. Tseng, W.-H. Wang and Y.-F. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 19840–19854 CrossRef CAS PubMed.
- S. S. Syamchand, R. S. Aparna and G. Sony, Microchim. Acta, 2017, 184, 2255–2264 CrossRef CAS.
- S. Ghosh, Y.-F. Chang, D.-M. Yang and S. Chattopadhyay, Biosens. Bioelectron., 2020, 155, 112115 CrossRef CAS PubMed.
- S. Yamini, M. Gunaseelan, G. A. Kumar, S. Singh, G. C. Dannangoda, K. S. Martirosyan, D. K. Sardar, S. Sivakumar, A. Girigoswami and J. Senthilselvan, Microchim. Acta, 2020, 187, 317 CrossRef CAS PubMed.
- R. Lv, P. Yang, Y. Dai, S. Gai, F. He and J. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 15550–15563 CrossRef CAS PubMed.
- M. Sun, L. Xu, W. Ma, X. Wu, H. Kuang, L. Wang and C. Xu, Adv. Mater., 2016, 28, 898–904 CrossRef CAS PubMed.
- Z. Zhou, J. Xie, S. Ma, X. Luo, J. Liu, S. Wang, Y. Chen, J. Yan and F. Luo, ACS Omega, 2021, 6, 10723–10734 CrossRef CAS PubMed.
- X. Hu, Y. Zhang, T. Ding, J. Liu and H. Zhao, Front. Bioeng. Biotechnol., 2020, 8, 990 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |





