Open Access Article
Open Access ArticleSustainability applications of rare earths from metallurgy, magnetism, catalysis, luminescence to future electrochemical pseudocapacitance energy storage
Shan-Shan
Chai
a,
Wei-Bin
Zhang
 *a,
Jing-Lei
Yang
a,
Lun
Zhang
a,
Myat Myintzu
Theint
b,
Xian-Li
Zhang
a,
Shao-Bo
Guo
a,
Xia
Zhou
a and
Xue-Jing
Ma
*a,
Jing-Lei
Yang
a,
Lun
Zhang
a,
Myat Myintzu
Theint
b,
Xian-Li
Zhang
a,
Shao-Bo
Guo
a,
Xia
Zhou
a and
Xue-Jing
Ma
 *a
*a
aCollege of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China. E-mail: zhangweibin17@cdut.edu.cn; maxuejing17@cdut.edu.cn
bMineral Development Section for International Relation, Department of Mines, Ministry of Natural Resources and Environmental, Nay Pyi Taw 15011, Myanmar
First published on 21st December 2022
Abstract
Rare Earths (REs) are referred to as ‘industrial vitamins’ and play an indispensable role in a variety of domains. This article reviews the applications of REs in traditional metallurgy, biomedicine, magnetism, luminescence, catalysis, and energy storage, where it is surprising to discover the infinite potential of REs in electrochemical pseudocapacitive energy storage. The use of REs in the field of pseudocapacitance is an important opportunity to link resources with burgeoning electrochemical energy storage. On the basis of the electrochemical energy storage potential of REs, typical rare earth oxides are selected as research objects to provide a comprehensive overview of their research progress in the field of supercapacitors. The future challenges and opportunities that these compounds will encounter in the field of pseudocapacitance energy storage are evaluated with a summary and discussion of existing research outcomes.
Sustainability spotlightBased on the current situation of increasing energy demand and environmental pollution, the development of new sustainable energy sources has become a key issue. In accordance with the UN SDG “Ensuring Access to Affordable, Reliable, and Sustainable Modern Energy for All”, this paper investigates the unlimited potential of abundant and environmentally friendly rare-earth-based compounds for sustainable electrochemical storage applications. Rare earth compounds are ideal electrode materials due to their environmental friendliness, abundant supply, easy preparation, and excellent redox capacitance properties. This paper presents a comprehensive overview and future application prospects of rare earth compounds in the field of pseudocapacitance to provide readers with more effective preparation strategies and future development directions for pseudocapacitive electrode materials based on rare-earth-based compounds. |
1 Introduction
Sustainability of the energy supply and resolving the problem of environmental pollution are challenging issues that must be resolved in today's and future societies.1–3 In light of the current situation of rising energy demand and worsening environmental pollution, the development of new sustainable energy sources has emerged as a paramount concern in the energy sector.4,5 Right now the ideal replacement for nonrenewable energy sources like fossil fuels is hydrogen energy because of its high calorific value (120 MJ kg−1) and lack of pollutants.6,7 However, present hydrogen storage technologies have inherent flaws, such as low efficiency and a propensity to leak.8 It is critical to create innovative and effective energy storage technologies given the present issues with hydrogen energy storage and the intermittent release of energy from renewable energy sources.9,10Current representative electrochemical energy storage devices include lithium-ion batteries and supercapacitors.11 Lithium-ion batteries will not be able to meet future needs for more energy use, faster power transfer, and better cycle stability because they have a low power density and are unstable.12 Supercapacitors make up for this lack and have been extensively studied.13–15 Electrodes in supercapacitor energy storage devices determine electrochemical performance.16 Consequently, the development of novel electrode materials is the next research priority.
Rare Earths (REs), commonly referred to as rare earth metals, comprise a total of 17 elements, including scandium (Sc), yttrium (Y), and lanthanides.17 Due to their unique electronic structure, rare earth elements feature optical, electrical, magnetic, and catalytic capabilities not found in other elements; consequently, they are used in practically every industry.18 Sc and Y, for instance, are essential components for improving the crystal structure and mechanical properties of steels and alloys.19 Nd and Sm are the necessary raw materials for the manufacture of permanent magnets.20 Ce and Eu are highly efficient luminous materials for activating ions due to their unusual 4f–5d electronic transitions, which allow them to emit light at all wavelengths.21 In the sectors of air purification and ammonia production, the Ce-based catalyst has an indispensable position.22 In recent years, REs have been extensively utilized in the energy storage industry. LaNi5 is a solid-state hydrogen storage material of superior efficiency.23 REs affect the cathode materials of lithium-ion batteries (such as LiCoO2 and LiMn2O4) to distort the lattice, hence enhancing the conductivity of the electrode material, and restricting the dissolution of Mn to enhance the electrode material's electrochemical performance.24,25 Some researchers have uncovered the inexhaustible potential of rare earths in the realm of pseudocapacitive energy storage through wide application and in-depth investigation.26,27 In prior research, rare earth elements were frequently considered electrochemically inert. Therefore, the abundant rare earth resources have been neglected in the field of pseudocapacitors. However, the majority of rare earth elements show RE3+/RE2+ or RE4+/RE3+ redox coupling, laying the thermodynamic groundwork for their employment in pseudocapacitive energy storage.28 In addition, the aforementioned redox couples are active in organic solvents and aqueous environments and can be utilized as redox-active components in pseudocapacitive energy storage.
In this review, we introduce the applications of rare earths in traditional metallurgy, biomedicine, magnetism, luminescence, catalysis, and energy storage. The research advances of typical oxides in rare earth compounds in the field of pseudocapacitors is highlighted, including the energy storage mechanism and electrochemical performance. In addition, future challenges and opportunities for rare earth compounds in the realm of pseudocapacitive energy storage are elaborated upon.
2 Elementary rare earths
2.1 Elementary rare earth elements
Rare earth elements (REs), also known as rare earth metals, are made up of 17 different elements, including scandium (Sc), yttrium (Y), and lanthanide elements from the third subgroup.29 According to the regulations of the International Union of Pure and Applied Chemistry (IUPAC), the elements with atomic numbers 57–71 (blue in Fig. 1): lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu), are called the lanthanide elements.30 When the electrons of lanthanide atoms occupy the 4f orbital of the inner layer, an electron in the same 4f orbital cannot be completely shielded by another electron.31 Consequently, when the atomic number increases, the number of effective nuclear charges acting on each 4f electron increases, resulting in a decrease in radius; this phenomenon is known as ‘lanthanide shrinkage’. Table 1 displays the atomic mass, outer electron, spectral ground state, and other characteristics of rare earth elements.29–31 16 of the 17 rare earth elements, except scandium (Sc), are separated into two categories based on the electronic layer structure and the physical and chemical properties of the reaction: light rare earths and heavy rare earths. La, Ce, Pr, Nd, Pm, Sm, and Eu are cerium-group rare earths, that is, light rare earths (L-RE); Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y are yttrium-group rare earths, that is, heavy rare earths (H-RE).| Element | Atomic number | Relative atomic mass | Electronic configuration of atoms | Electronic configuration of RE2+ | Electronic configuration of RE3+ | Spectral ground state notation | Electronic configuration of RE4+ |
|---|---|---|---|---|---|---|---|
| Sc | 21 | 44.95591 | 3d14s2 | — | — | — | — |
| Y | 39 | 88.90585 | 4d15s2 | — | — | — | — |
| La | 57 | 138.9055 | 5d16s2 | 5d1 | — | — | — |
| Ce | 58 | 140.115 | 4f26s2 | 4f2 | 4f1 | 2F5/2 | 5s25p6 |
| Pr | 59 | 140.90765 | 4f36s2 | 4f3 | 4f2 | 3H4 | 4f15s65p6 |
| Nd | 60 | 144.24 | 4f46s2 | 4f4 | 4f3 | 4I9/2 | 4f25s25p6 |
| Pm | 61 | 145 | 4f56s2 | 4f5 | 4f4 | 5I4 | |
| Sm | 62 | 150.36 | 4f66s2 | 4f6 | 4f5 | 6H5/2 | — |
| Eu | 63 | 151.965 | 4f76s2 | 4f7 | 4f6 | 7F0 | — |
| Gd | 64 | 157.25 | 4f75d16s2 | 4f8 | 4f7 | 8S7/2 | — |
| Tb | 65 | 158.92534 | 4f96s2 | 4f9 | 4f8 | 7F6 | 4f75s25p6 |
| Dy | 66 | 162.50 | 4f106s2 | 4f10 | 4f9 | 6H15/2 | 4f85s25p6 |
| Ho | 67 | 164.93032 | 4f116s2 | 4f11 | 4f10 | 5I8 | — |
| Er | 68 | 167.26 | 4f126s2 | 4f12 | 4f11 | 4I15/2 | — |
| Tm | 69 | 168.93421 | 4f136s2 | 4f13 | 4f12 | 3H6 | — |
| Yb | 70 | 173.04 | 4f146s2 | 4f14 | 4f13 | 2F7/2 | — |
| Lu | 71 | 174.967 | 4f145d16s2 | — | 4f14 | — | — |
The earth's crust contains up to 153 g t−1 of rare earth elements, the most abundant of which is cerium. The distribution of rare earth elements conforms to the Oddo–Harkins rule, which states that elements with even atomic numbers are more abundant than nearby elements with odd atomic numbers. Rare earth elements exist in three distinct forms: I. those that participate in lattice and rare earth symbiosis to form minerals containing rare earth elements, such as monazite and bastnasite; II. those that undergo isomorphic substitution, Ca, Sr, Ba, Mn, Zr, etc., are scattered throughout minerals such as apatite, europium titanite, etc.; III. those whose ions are adsorbed on the surface of minerals or between grains, including clay minerals, mica ore, etc. In minerals, rare earth elements are mostly found as ionic compounds, which make up the coordination polyhedrons.
2.2 Typical applications fields
Due to their distinctive electronic structure, rare earth elements possess optical, electrical, and magnetic capabilities not seen in other elements.32 As ‘contemporary industrial vitamins’, rare earths and their derivatives are widely utilized in traditional materials and emerging industries.33The vast majority of research on rare earth alloys focuses on lightweight magnesium (Mg) alloys, which are widely employed in high-performance automobiles and the aerospace industry.42 Mg-based alloys are suited for application in gravity-sensitive devices due to Mg's low density of 1.7 g cm−3, which is the lowest among typical structural metals. However, their poor ductility and susceptibility to corrosion in liquid environments restrict their widespread utilization.43 Adjusting the metallurgical conditions can successfully improve the plasticity issue, whereas the corrosion issue continues to be the primary focus and challenge of current research in the field of Mg-alloys. The large negative free energy on the surface of Mg-alloys might facilitate the rapid formation of an oxidation/hydroxide layer, but the oxidation/hydroxide layer cannot prevent corrosion due to the following issues: I. the majority of the oxidation/hydroxide film is soluble in water. II. The oxidation/hydroxide film cannot completely cover the alloy. III. Galvanic corrosion cannot be avoided in magnesium alloys with high negative potential.44–46
Doping with rare earth elements presents a potential solution to the problems outlined.47 The effect of doping with rare earth elements is clarified below, using magnesium-based alloys as an illustration. In 2003, Morales et al. investigated the corrosion behavior of magnesium alloys containing rare earth metals in NaCl solution.48 Studies indicate that rare earth elements can effectively block the hydrogen evolution process in corrosion and significantly enhance the corrosion resistance of magnesium alloys. Hort et al. also confirmed the influence of rare earth elements on the improvement of magnesium alloys' corrosion resistance.49 Notably, only when the rare earth element Gd occurs as a solid solution in the magnesium alloy is its performance significantly enhanced. When the precipitation phase appears, in contrast, it promotes the occurrence of corrosion. Consequently, Schlüter and his team fabricated MgY and MgGd alloy thin films by sputter deposition.50 The film is not only resistant to corrosion but also exhibits remarkable mechanical properties. Due to the fact that the atomic radius and electronegativity of rare earth elements are comparable to those of magnesium, it is simple for them to create solid solutions with magnesium alloys. The rare earth oxides and sulfides produced by eliminating O, S, and other impurities from magnesium alloys have a minor potential difference with the magnesium matrix, thereby preventing corrosion. Doping with rare earths can effectively inhibit the formation of precipitates and produce solid-solution strengthening. Therefore, doping with rare earth elements can not only effectively improve the corrosion resistance of magnesium alloys but also change the texture of the alloy, refine the grains, and improve its mechanical properties.51,52 This opens up a new vista for the improvement of established metallurgical procedures in order to produce high-performance magnesium alloys. Based on the corrosion resistance and excellent mechanical properties of the RE-Mg alloys mentioned above, RE-Mg alloys show great application potential as degradable alloys in the field of biomedicine.53 Except for Pm, which is radioactive, the remaining rare earth elements have excellent biocompatibility at low doses. Numerous studies have determined that the rare earth elements (Y, Nd, Gd, Dy, Ho, etc.) have no effect on cellular metabolism, although La and Ce should be added with caution to avoid adverse reactions, such as gas embolism and tissue necrosis. Table 2 is a summary of the research that has been done on rare earth Mg-alloys used in medicine.54–61
| REEs | Alloys | Research object | Biocompatibility | Application | Ref. |
|---|---|---|---|---|---|
| Y, Sc | Mg-3Sc-3Y | Mouse osteoblast cells | No cytotoxicity | Degradable implant material | 54 |
| Y, Nd | Mg-Nd-Y-Zr-Ca | Subcutaneous rat tissue | No toxicological or pathophysiological risks | Degradable implant material | 55 |
| Y, Nd | Mg–Zn–Y–Nd–Zr | L929 and MC3T3-E1 cells | Minimal cytotoxicity | Orthopaedic implant material | 56 |
| Y, Nd, Ce | Mg-RE | Vascular smooth muscle cells | Little effect on biological activity of vascular cells at low dose | Cardiovascular stent | 57 |
| Nd, Gd, Dy | Mg–Y–Zr–RE | Aortic endothelial cells | No deleterious effect on platelets and reduces platelet adhesion and activation | Vascular stent | 58 |
| Gd | Mg–Gd | Rat tissues | Accumulate in animal organs | Use with caution | 59 |
| Gd, Dy | WE43 | RAW 264.7, MG63 and HUCPV cells | No cytotoxicity | High solid solution element | 60 |
| Nd, Pr, Eu | No cytotoxicity | Low solid solution element | |||
| Ce, La | Influences viability of cells | Use with caution | |||
| Ho | Mg–Zr–Sr–Ho | SaOS2 cells | Little effect on cells at low dose | Orthopedic degradable material | 61 |
In general, the addition of rare earths to magnesium alloys not only provides advantages in reducing pollution, alleviating corrosion, and improving mechanical properties, but also plays an important role in the application of alloys in the circular economy in terms of low cost and efficient recycling. Current market developments indicate that the use of La and Ce instead of Mg in future alloy design concepts has incomparable advantages in terms of reducing environmental pollution and improving cost-effectiveness.
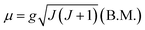 | (1) |
The value of the Lande factor, denoted by g, is:
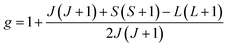 | (2) |
The value of the Bohr magneton B.M. is:
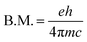 | (3) |
There are as many as seven unpaired electrons in the 4f configuration of rare earth elements.63 The orbital motion, spin motion, and spin–orbit coupling of these 4f electrons distinguish them from transition metals in their magnetism. Rare earth elements are the best option for permanent magnet materials and magnetic refrigeration materials due to their great paramagnetic susceptibility, saturation magnetization, magnetic anisotropy and magnetocaloric effect.64–66 A permanent magnet material is a material that converts mechanical energy to electrical energy via a magnetic field, and it is a typical material for achieving high-efficiency and lightweight energy conversion.67 They have widespread use in refrigerators, air conditioners, wind turbines, and other small electrical appliances and electric equipment.68 Nd and Sm are the rare earth elements utilized most typically in permanent magnets.69 Due to the extraordinary magnetic anisotropy and high energy product, nanostructured SmCo5 and Nd2Fe14B have become the key research objects among the numerous Nd-based and Sm-based compounds.70 For the transition from rare earth resources to clean energy, SmCo5 and Nd2Fe14B are essential application materials, fully aligning with the Sustainable Development Goal (SDG): ensuring access to affordable, reliable, and sustainable modern energy for all. It has been discovered that the microstructures of SmCo5 and Nd2Fe14B, such as particle size, shape, and interface, determine the coercivity and maximum energy product.71 The most effective way of optimizing the magnetic characteristics of SmCo5 and Nd2Fe14B is, therefore, exact control over the microstructure. The control structure focuses mostly on the following three actions: I. regulating the grain size of the oxidation precursor; II. regulating the reduction–diffusion process of the oxidation precursor; and III. regulating the oxidation precursor's thermal stability, getting rid of reaction byproducts.72 The majority of studies are centered on grain refining, grain boundary phase change, and grain boundary diffusion processes based on the preceding notions. Hou et al. pioneered the manufacture of SmCo5 by annealing core/shell-structured Co/Sm2O3 nanoparticles at high temperatures, utilizing KCl as a dispersion medium to enhance the reduction of Sm2O3, prevent SmCo5 from developing into single crystals, and address the question of refining grain size.73 The synthesized SmCo5 demonstrates a Curie temperature (Tc) of 1020 K, and a coercivity of up to 24 kOe at 100 K. Ma et al. proposed a novel strategy for precisely controlling the precursor size in order to produce highly magnetically anisotropic SmCo5, employing a hydrothermal process to synthesize precursors consisting of sheet-like Co(OH)2 (250–400 nm) and rod-like Sm(OH)3 (15–20 nm), which were subsequently reduced to SmCo5 nanosheets.74 SmCo5 nanosheets show substantial magnetic anisotropy and excellent magnetic characteristics at room temperature. The coercivity of the SmCo5 nanosheets was 19.3 kOe, and the maximal energy product reached 14.4 MGOe, which is the best performance among the SmCo5 nanosheets yet synthesized. Nd2Fe14B is prepared in a manner similar to SmCo5, in which oxide precursors are reduced and transformed.75 Notably, the saturated vapor pressure of Nd is higher than that of Sm, necessitating the addition of additional Nd2O3 to the precursor to compensate for the loss of Nd element during the evaporation process.76 Current research findings on Nd2Fe14B-based permanent magnet materials are presented in Table 3, demonstrating that the selection of appropriate dispersants can effectively alter the kinetics of the reduction–diffusion reaction and optimize the crystal size of Nd2Fe14B.77–80 Current research reveals that coupled Nd2Fe14B/α-Fe nanocomposites created by the reduction–diffusion process have higher coercivity than Nd2Fe14B, but the manufacture of homogenous Fe nanoparticles remains challenging.81,82
| Material | Preparation | Coercivity | Reference |
|---|---|---|---|
| Nd2Fe14B | R-D process | 10 KOe | 77 |
| Nd2Fe14B | High-energy ball milling | 8 KOe | 78 |
| Nd2Fe14B | Mechanochemical processing | 12 KOe | 79 |
| Nd2Fe14B | High-energy ball milling | 6.8 KOe | 80 |
| Nd2Fe14B/α-Fe | Thermal decomposition, annealing | 12 KOe | 81 |
| Nd2Fe14B/α-Fe | Microwave assisted combustion process | 9 KOe | 82 |
A magnetic refrigeration (MR) technique based on the magnetocaloric effect (MCE) has become a possibility for replacing gas compression refrigeration technology because of its high conversion efficiency, lack of gas pollution, safety, and other advantages.83 The peculiar magnetic characteristics of rare-earth-based compounds have incomparable advantages in the realm of magnetic refrigeration. Changes in magnetic moiety (ΔSM) and refrigerant capacity (RC) are the main parameters for evaluating the performance of magnetic refrigeration. ΔSM in response to variations in magnetic field (ΔH) can directly characterize the MCE. Based on Maxwell's magnetization experiment, eqn (4) establishes the link between temperature (T), ΔH, and ΔSM.84
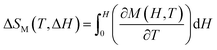 | (4) |
RC is calculated from eqn (5):85
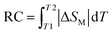 | (5) |
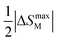 on both sides of the −ΔSM(T) curve.
on both sides of the −ΔSM(T) curve.
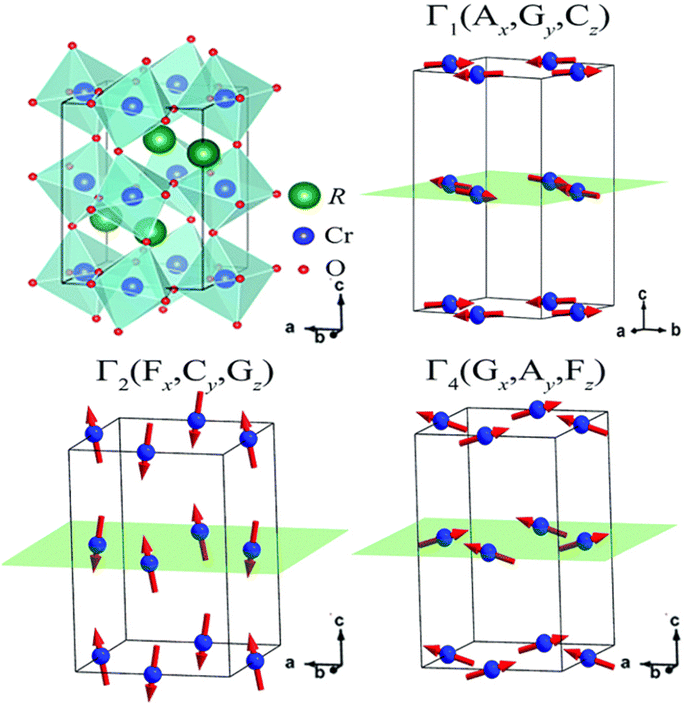
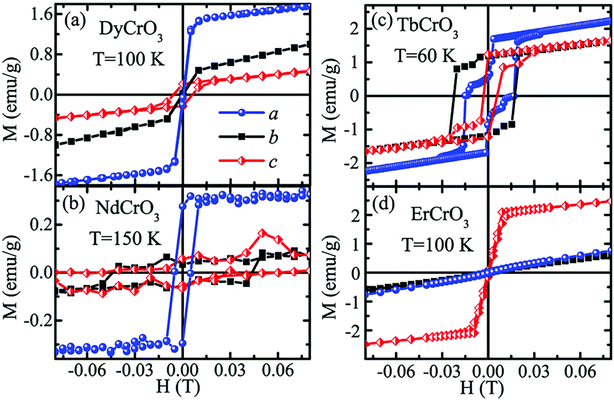
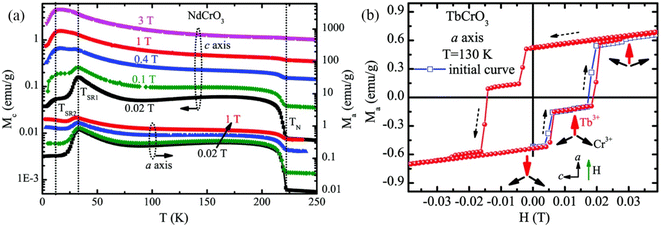
In the following sections, RECrO3 is used as a study object to show how rare earths can be used in the field of magnetic refrigeration. Magnetic phenomena, such as temperature-induced magnetization reversal and temperature-induced magnetization hopping, were reported in the RECrO3 system due to the presence of RE–RE, RE–Cr, and Cr–Cr magnetic interactions.86Fig. 2 depicts both the crystal structure and the spin structure of RECrO3. Yin et al. synthesized single crystals of RECrO3 (R: Dy, Tb, Er) via a solvent method.86,87 The relationship between the magnetization and temperature (M–T) of RECrO3 (R = Dy, Nd, Tb, Er) crystal and the evolution of the crystal's spin structure along various axes is depicted graphically in Fig. 3a–d. The Cr3+ in each RECrO3 crystal exhibits magnetic anisotropy within a particular temperature range. In terms of magnetization anisotropy, RECrO3 (R = Dy, Nd, Tb, Er) demonstrates a notable degree of anisotropy (Fig. 4a–d). These findings are congruent with those of prior research. Interestingly, Fig. 5a reveals that the decrease in NdCrO3 magnetization along the c-axis (Mc) coincides with the increase along the a-axis (Ma), a phenomenon that persists even in the presence of a 3 T magnetic field. On the saturation magnetization–magnetic field (M–H) relationship curve of the TbCrO3 crystal (Fig. 5b), a unique behavior of stepwise magnetization rise can be observed. Even in the absence of a magnetic field, the original curve demonstrates stepwise growth, demonstrating that this is a unique attribute of the TbCrO3 crystal itself. The magnetic moiety (ΔSM), maximum magnetic moiety (ΔSmaxM), and refrigerant capacity (RC) of RECrO3 were computed using eqn (4) and (5), and the results are presented in Table 4. This work describes how the rare earth affects the spin effect of RECrO3 and the two-step-up behavior of saturation magnetization. This suggests that the RECrO3 system has great magnetic anisotropy and magnetocaloric properties and could be used for magnetic refrigeration.
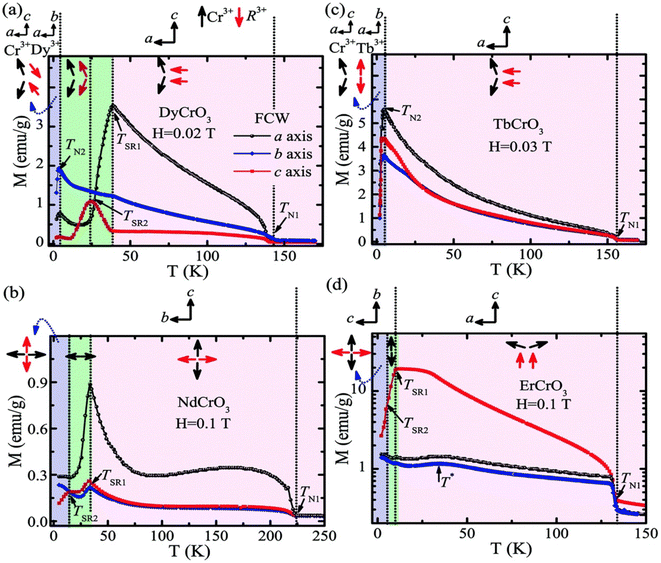 | ||
| Fig. 3 The relationship between the magnetization and temperature of the RECrO3 crystals and the evolution of the crystal's spin structure along various axes. | ||
| Material | −ΔSM (J Kg K) | −ΔSmaxM (J Kg K) | RC (J Kg−1) | ||
|---|---|---|---|---|---|
| ΔH = 2T | ΔH = 4.5T | ΔH = 2T | ΔH = 4.5T | ||
| DyCrO3 | 11.5 | 15.2 | 11.4 | 14.8 | 300 |
| NdCrO3 | −0.2 | −1.1 | — | — | — |
| TbCrO3 | 5.0 | 12.2 | 0.7 | 5.4 | 125 |
| ErCrO3 | 10.1 | 20.6 | 9.5 | 16.9 | 276 |
| GdCrO3 | 14.1 | 31.6 | 4.3 | 3.8 | — |
Despite having stronger magnetic characteristics than nickel–cobalt alloys, ferrite, and steel magnets, the recovery of rare earth magnetic materials is a significant obstacle to the long-term sustainable use of rare earths.88 The separation stage is a crucial processes that impacts the recovery efficiency. At the moment, leaching for chemical separation using hydrometallurgy is a particularly efficient approach for separating rare earth elements. Sarfo et al. used a novel hydrometallurgical process to recover rare earth magnet scrap, which consisted of H2SO4 leaching, NH4OH precipitation and NH4F·HF reaction.89 The magnet scrap was converted into rare earth fluoride, which was then converted into rare earth metals by high-temperature calcination. The process of this work not only avoids the use of hydrofluoric acid, but also optimizes the process through statistics and modeling to achieve high-purity rare earth resource recovery, which lays a solid foundation for the sustainable development of rare earth magnetic materials.
 | (6) |
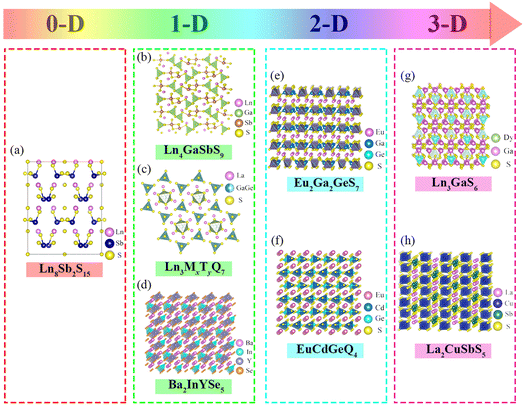
Rare earth luminescent materials (RELs) can be divided into different excitation methods: cathode ray, photoluminescence, electroluminescence, X-ray luminescence, thermoluminescence, and laser materials, etc.96 They have the incomparable advantages of radiation resistance, resistance to light decay, good temperature resistance, high visual efficiency function value, high color purity, etc., and are thus widely used in lighting, display, radiology, national defense, and other fields. Eu3+-activated YVO4, Y2O3, and Y2O2S are currently the most efficient cathode-ray luminous materials, replacing the original Mn3+-activated Zn3(PO4)2 used in TVs, greatly improving the distortion of TV images, brightness–current saturation, and other characteristics, a leap forward in the color TV industry.97 With in-depth research into rare earth trichromatic (red, blue, and green) phosphors, rare earth photoluminescent materials have been rapidly developed. Y2O3:Eu3+, with quantum efficiency of up to 100%, is nearly ideal for red phosphors; Tb3+ is mostly chosen as the activating ion for green phosphors. For use of 5D4–7Fj energy level transition luminescence, subsequent research proved that doping with sensitizer Ce3+ can achieve radiation-free energy transfer between Ce3+ and Tb3+, greatly improving the luminous efficiency.98,99 Currently developed rare earth electroluminescent materials (RE-EL) are mainly Eu2+ and Ce3+ activated CaS or SrS, where the CaS or SrS matrix allows high-energy electron transport inside the crystal. Eu2+ and Ce3+ based on (4f)n−15d–(4f)n transitions preserve structural stability at high electric field intensities; therefore CaS:(Eu2+/Ce3+) and SrS:(Eu2+/Ce3+) should in future realize the unique color luminescence potential of RE-EL.100,101 For rare earth laser materials, garnet-type Nd3+-activated yttrium aluminum garnet (YAG:Nd3+) with a narrow fluorescence spectral line, low threshold, and high power has shown the best performance. Nd3+ single replacement of Al3+ in the lattice in YAG, where the emission spectrum can broaden uniformly, is currently the best option for laser guidance, laser ranging, laser medical treatment, and other fields.102 A variety of non-linear optical crystals have been made to meet more civilian and military laser needs. Fig. 6 shows the crystal structure of the currently used RE-sulfide (RE-S).103Fig. 6a shows the crystal structures of 0-dimensional Ln8Sb2S15; Fig. 6b–d represent the 1-dimensional structures including Ln4GaSbS9, Ln3MxTyS9, and Ba2InYSe5; Fig. 6e and f show the crystal structures of 2-dimensional Eu2Ga2GeS7 and EuCdGeQ4; and Fig. 6g and h depict the crystal structures of 3-dimensional Ln3GaS6 and La2CuSbS5. The RE-S active group is extremely distorted and can greatly raise the second harmonic generation (SHG), but the band gap does not surpass the 3 eV threshold. The combination of RE with other reactive groups X–O (X = P, B, C and N) enables tuning of the band gap and SHG effects to achieve optimum laser performance. Subsequent studies discovered that the unique synergy between the RE-S and B–O/C–O groups improved SHG effects and widened the band gap. Future research on RELs should therefore concentrate on rare earth borides and rare earth carbides.
RELs have become a pillar material in the display, lighting, and optoelectronic industries, and their continual development and organic integration with other fields of technology has resulted in the development of new high-precision technologies and industries.104 Nevertheless, it is indisputable that the direct excitation of RE3+ ions is a relatively inefficient luminescence process due to forbidden jump of the rare earth ion 4f jump and the quenching promoted by the –OH, –NH and–CH bond vibrations in the adjacent ligands.105 Development of quantum dots with exceptional electronic characteristics could provide a remedy. Wang et al. synthesized SnS and SnS: Ce3+ quantum dots and used the density generalized first principle to compute the energy band structure, total density of states (TDO), and partial density of states (PDO); the findings are depicted in Fig. 7a–d.106 The spin polarization caused by Ce ions was analyzed using the spin density distribution (Fig. 7e and f), and for the Sn36S36, the majority of the spin density is located on S, yet that of Sn36Ce1S36 is found on the Ce ions. The energy level provided by the 4f orbital of the Ce ions effectively promotes the electron jump, and the strong coupling between the carrier and the 4f state considerably boosts the photoluminescence intensity of the SnS quantum dots.
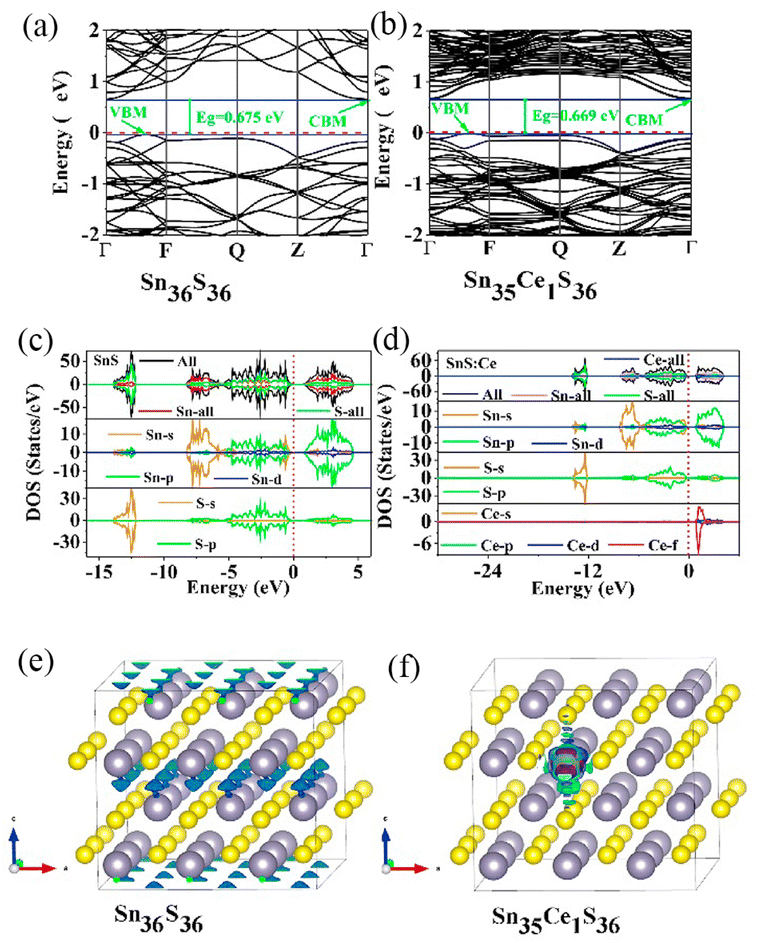 | ||
| Fig. 7 Band structures of the structural models of SnS (Sn36S36) and SnS: Ce3+ (Sn35CeS36): (a and b) energy band diagrams, (c and d) total and partial density maps, (e and f) spin density maps. | ||
In addition to quantum dots, researchers are becoming increasingly concerned with the potential uses of quantum wires, superlattices, and ordered nanostructure arrays.107,108 Most of the research going on right now is about how quantum confinement and quantum scale affect the structure of energy bands and spectral features. New methods for the preparation of rare earth luminous materials should be developed and studied in the future, beginning with the materials' preparation and processing. For instance, microwave sintering, ultrasonic dispersion, and further synthesis processes will be integrated to promote the development of rare earths in the luminescence field.
As the industrialization process accelerates, volatile organic compounds, such as automotive exhaust and formaldehyde, are polluting the atmosphere more and more.113 Strictly regulating the emission of volatile organic compounds is a significant environmental concern. Due to its low flame-out temperature, consistent combustion, high efficiency, and high-temperature oxidation resistance, rare earth–precious metal oxidation catalysis has been extensively investigated in comparison to other methods for removing organic compounds.114,115 As illustrated in Fig. 8a, Hu et al. fabricated Pt-deposited CeO2 nanospheres (Pt-CeO2-NS) and nanorods (Pt-CeO2-NR) as catalysts and evaluated the reaction mechanism of Pt-loaded CeO2.116 CeO2 considerably improves the catalytic activity for the CO reduction of NO, while Pt provides the NO conversion activity. Compared to rod-shaped CeO2, spherical CeO2 contains more oxygen vacancies and demonstrates a stronger interfacial interaction with Pt particles, which further stimulates the surface reaction sites and effectively lowers the CO poisoning effect of platinum. Jiang's investigation revealed the Frankel oxygen vacancy as the crucial active site for catalytic oxidation.117 MnOx–CeO2 produced by the sacrificial template approach possesses exceptional catalytic oxidation performance for ethyl acetate (T99 = 210 °C, which is the temperature required for 99% conversion), and Fig. 8b depicts the catalytic oxidation mechanism. In rare earth–precious metal catalysts, the rare earth can enhance the oxygen storage capacity and lattice oxygen reaction activity of the catalyst, promote the uniform dispersion of precious metals on the carrier, and increase the catalytic efficiency, while decreasing the amounts of precious metals needed.
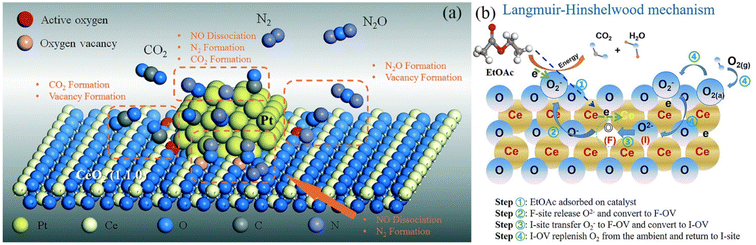 | ||
| Fig. 8 Catalytic reaction mechanism of rare earth catalysts: (a) reaction mechanism of CO reduction of NO with Pt/CeO2-NR catalyst, (b) catalyzed oxidation processes mediated by Ce-based catalysts. | ||
Furthermore, rare earth catalysts play an important and wide-ranging role in making and breaking down ammonia. Ammonia (NH3) is the primary source of nitrogen fertilizer and nitrogen compounds used in agriculture, and its synthesis is depicted in eqn (7).118 It is thermodynamically reactive, but because N2 is an inert gas, it is difficult to obtain the kinetic conditions of ammonia synthesis at low temperatures.119 Therefore, scientists are committed to inventing catalysts that facilitate ammonia synthesis:
| N2 + 3H2 → 2NH3 | (7) |
Currently, molten iron catalysts still occupy an absolute position in ammonia synthesis, and it has been found that their activity decreases when the R-value (Fe2+/Fe3+) is higher or lower than 0.5.120 This led to a broad consensus among researchers that the composition of molten iron catalysts is set, and that consequently changing the composition would not improve the catalytic performance of such catalysts. The classical theory that the catalytic activity is optimal at an R value of 0.5 for molten iron catalysts was followed for more than 80 years until a new cobalt-containing molten iron catalytic system with better performance was found to break this classical conclusion.121 Cobalt-containing catalysts are an important development of traditional Fe-based fused iron catalysts, marking a new period of development for ammonia catalysts. ICI (UK) first successfully developed an Fe–Co catalyst (type 74-1 containing drill catalyst), which led to a certain improvement in its activity and was applied in the ICI-AMV process. China has also successfully developed Fe–Co catalysts (type A201 and A202) and applied them to industrial production with good production benefits.122
In 1976, Takeshita was the first to discover that rare earth intermetallic compounds absorb hydrogen faster, indicating that they have increased catalytic activity for the dissociation of hydrogen molecules and for diatomic molecules such as N2.123 Compared to commercially available iron catalysts with high cobalt content, the use of rare earth blends can reduce the cobalt content or even replace it completely, which significantly reduces the cost of molten iron catalysts. Subsequently, Niwa discovered in 1998 that the rare earth element La had a considerable impact on the ammonia synthesis activity of MgO-supported ruthenium.124 And noble metal catalysts doped by rare earth composite oxides demonstrated superior catalytic performance in terms of reactivity, selectivity, and stability under high-temperature settings. Since then, rare earth catalyst research has entered a new era. Okura investigated the effects of various rare earth oxide supported Ni catalysts on the breakdown of ammonia.125 In a study of reaction kinetics, rare earth oxide supports can effectively ameliorate the phenomenon of hydrogen inhibition in the reaction process. The Y2O3–Ni catalyst shows the highest catalytic activity at elevated temperatures, and research indicates that the optimal Ni loading is 40% (wt%), and Ni can be supported uniformly on the Y2O3 carrier under these conditions. Moreover, the perovskite-type rare earth composite catalyst showed outstanding reaction kinetics during ammonia production. Li produced BaCeO3 modified with rare earth elements (La, Y, Pr), and the ammonia synthesis rate of BaCe0.9La0.1O3−δ supporting 5% Ru at 3 MPa and 450 °C was as high as 34 (mmol (g−1 h−1)), with no loss of catalytic performance after 100 h of continuous reaction.126 The addition of La3+ increases oxygen vacancies in the lattice and decreases the particle size of Ru, which is advantageous for the increase in electron density on the Ru surface and the production of more b5-type active sites that promote N–N dissociation.
In conclusion, the ammonia synthesis reaction is of high industrial importance and the fused iron catalyst has excellent properties that cannot be matched by some other ammonia synthesis catalysts.127 However, in achieving the sustainable development of materials and processes, the aim is to minimize the consumption of non-renewable resources and the negative impact on the environment. In addition, minimizing dependence on a particular raw material is essential to sustaining high-tech industries and maintaining economic progress.128 Considering the non-renewable nature of iron-bearing ores and the cost of Co resources, a logical step for the industrial economy is to promote the substitution of reserve-rich, environmentally friendly and low-cost rare earth resources, and therefore research and development of rare earth catalysts is crucial for the current and future ammonia industry to comply with the concept of sustainable green development.
Among the various candidate materials for hydrogen storage, ionic hydrides (e.g. LiH and MgH2 based on first and second main group elements) are gaining interest due to their high hydrogen storage capacity (7.7 wt%), low raw material cost, and great cycle reversibility.135,136 There are downsides, however, such as poor hydrogen absorption and discharge kinetics, high operating temperature, and poor stability in air. From a practical standpoint, such hydrogen storage materials require further improvement in terms of hydrogen discharge temperature, hydrogen discharge rate, and reversible hydrogen storage performance, and the strong chemical bonding interactions between the atoms of constituent elements in Li- and Mg-based hydrides present significant thermodynamic and kinetic challenges for hydrogen storage applications.137,138
Against the backdrop of contemporary research on hydrogen storage, AB5-type (A: RE metal, B: transition metal) RE-based alloys exhibit exceptional performance. The hydrogen storage capacity of LaNi5 is as high as 190–200 cm3 g−1.139 It features easy activation, a moderate equilibrium pressure, minor hysteresis, rapid hydrogen absorption and desorption rates, and nontoxicity, so it has become a representative hydrogen storage material of high quality.140 Since 1970, when Van Vucht first documented the behavior of LaNi5 reversibly absorbing and releasing hydrogen at ambient temperature,141,142 LaNi5 hydrogen storage materials have experienced fast growth. Due to the limitations of the initial experimental conditions, it is difficult to set up a prolonged isothermal reaction; hence, there are a variety of differing opinions regarding the primary factors governing the reaction kinetics of LaNi5: I. it is controlled by the chemical reaction of hydrogen atoms with the α-phase solid solution at the two-phase interface.142 II. It is controlled by the diffusion of hydrogen molecules in the beta phase.143 III. There is chemisorption control of hydrogen molecules on surfaces.144 IV. Controlled variables fluctuate with degrees of response.145 On this basis, Silin et al. systematically studied the reaction kinetics of LaNi5 hydrogen storage by the two-phase coexistence initial rate method. They assumed that LaNi5 was a spherical particle with a constant density before and after hydrogenation; the reaction's apparent activation energy is 4700 cal (g−1 mol−1), and the reaction rate equation is as follows:146
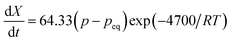 | (8) |
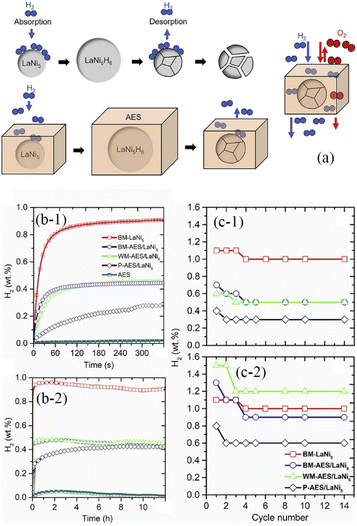
Knowing that pH2 = p0, pH2eq = peq, 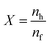 , represents the ratio between the amount of hydrogen absorbed (nh) and the entire amount of hydrogen that can be absorbed in the two-phase zone (nf). This model correlates well with the kinetic data of LaNi5 during the reaction process, demonstrating that the chemisorption of hydrogen molecules on the alloy's surface is the primary factor governing the kinetics of the hydrogenation reaction. However, LaNi5 undergoes a substantial volume expansion of 23.5% during the hydrogen absorption and desorption process, and the usage of pure metal as a raw material is expensive, hence limiting its implementation on a broad scale in the field of hydrogen storage.147 Therefore, further research focuses primarily on three aspects: enhancing reaction kinetics, cycle stability, and cost reduction.148 The internal stress caused by the volume expansion and contraction of LaNi5 during the hydrogen absorption and desorption processes will damage the material's internal structure, and the newly exposed surface will be oxidized to form an impurity phase, which hinders the hydrogen absorption and desorption processes. Adding polymer to form composites is the optimal solution for resolving this problem of cycling stability.149 The existing series of LMBNH-TPX, LMNH-TPX,150 Mg-NCs/PMMA,151 Mg-PMMA,152 SP-MnO6,153 SPMnO2 (A, B, E)154 alloy-polymers provides a good barrier for hydrides, preventing oxidation and corrosion of the material, considerably increasing the reversible cycle life, and inhibiting side reactions of metal hydrides during hydrogen absorption and dehydrogenation reactions. By ball milling and wet milling, Almeida Neto et al. made BM-AES/LaNi5 and WM-AES/LaNi5 composites with AES-polymer-doped LaNi5.155 The molten BM-AES/LaNi5 was generated into filaments signified as P-AES/LaNi5, and the preparation procedure is depicted in Fig. 9a. Fig. 9b(1 and 2) depict the sample's short- and long-term hydrogen absorption capacity. AES-LaNi5 has the best hydrogen absorption kinetics. P-AES/LaNi5 has a poor short-term capacity for hydrogen absorption, but after 12 h, its hydrogen capacity is comparable to that of other samples. The changes in hydrogen capacity during cycling are depicted in Fig. 9c(1 and 2). All samples exhibited a declining and then stabilizing trend. During cycling, defects that trap hydrogen atoms in the hydride structure may account for the loss in capacity. In sum, this study demonstrates that LaNi5 particles with superior particle dispersion have superior hydrogen storage capabilities. Adding AES can effectively increase the performance of the composite's hydrogen absorption/dehydrogenation cycle and prevent the negative effect of air on the kinetics of hydrogen uptake.
, represents the ratio between the amount of hydrogen absorbed (nh) and the entire amount of hydrogen that can be absorbed in the two-phase zone (nf). This model correlates well with the kinetic data of LaNi5 during the reaction process, demonstrating that the chemisorption of hydrogen molecules on the alloy's surface is the primary factor governing the kinetics of the hydrogenation reaction. However, LaNi5 undergoes a substantial volume expansion of 23.5% during the hydrogen absorption and desorption process, and the usage of pure metal as a raw material is expensive, hence limiting its implementation on a broad scale in the field of hydrogen storage.147 Therefore, further research focuses primarily on three aspects: enhancing reaction kinetics, cycle stability, and cost reduction.148 The internal stress caused by the volume expansion and contraction of LaNi5 during the hydrogen absorption and desorption processes will damage the material's internal structure, and the newly exposed surface will be oxidized to form an impurity phase, which hinders the hydrogen absorption and desorption processes. Adding polymer to form composites is the optimal solution for resolving this problem of cycling stability.149 The existing series of LMBNH-TPX, LMNH-TPX,150 Mg-NCs/PMMA,151 Mg-PMMA,152 SP-MnO6,153 SPMnO2 (A, B, E)154 alloy-polymers provides a good barrier for hydrides, preventing oxidation and corrosion of the material, considerably increasing the reversible cycle life, and inhibiting side reactions of metal hydrides during hydrogen absorption and dehydrogenation reactions. By ball milling and wet milling, Almeida Neto et al. made BM-AES/LaNi5 and WM-AES/LaNi5 composites with AES-polymer-doped LaNi5.155 The molten BM-AES/LaNi5 was generated into filaments signified as P-AES/LaNi5, and the preparation procedure is depicted in Fig. 9a. Fig. 9b(1 and 2) depict the sample's short- and long-term hydrogen absorption capacity. AES-LaNi5 has the best hydrogen absorption kinetics. P-AES/LaNi5 has a poor short-term capacity for hydrogen absorption, but after 12 h, its hydrogen capacity is comparable to that of other samples. The changes in hydrogen capacity during cycling are depicted in Fig. 9c(1 and 2). All samples exhibited a declining and then stabilizing trend. During cycling, defects that trap hydrogen atoms in the hydride structure may account for the loss in capacity. In sum, this study demonstrates that LaNi5 particles with superior particle dispersion have superior hydrogen storage capabilities. Adding AES can effectively increase the performance of the composite's hydrogen absorption/dehydrogenation cycle and prevent the negative effect of air on the kinetics of hydrogen uptake.
To lower costs, mixed rare earth elements (La, Ce, Pr, Nd) are used to replace high-valent La,156,157 and Al, Mn, Cr, Fe, and Co are used to replace Ni. In these investigations, it has been discovered that the stability of hydrides can be enhanced without incurring any capacity loss. Increasing the amount of Ce and Pr in a misch metal can accelerate reaction kinetics and improve temperature stability. In contrast, the presence of a lot of La and Nd decreases the performance. The mixed rare earth RENi5 with the ratio La0.1645Ce0.7277Pr0.0234Nd0.0845Ni5 has the highest hydrogen storage capacity, according to numerous experiments.158–161 Al and Mn partially replacing Ni can boost cycle stability and decrease reaction equilibrium pressure; Fe, Co, and Cr can partially replace Ni to increase hydrogen storage capacity, as Fe, Co, and Cr exhibit greater electron attraction and considerably increase the number of hydrogen atoms.
The aforementioned research strategies are efficient in enhancing the hydrogen storage performance and reducing the cost of RE-based alloys, but they are insufficient to suit the needs of practical industrial applications. To further enhance the hydrogen storage capacity as well as the kinetics and thermodynamics of rare-earth-based compounds, the following modification methods can be used: nanosizing, synthesizing sub-stable phases, doping with catalytic additives, forming nanocomposites, changing the reaction mode, etc.162 However, it is important to note that none of the aforementioned modification techniques can entirely meet the requirements of real applications. Doping, synthesis of sub-stable phases, and altering the reaction path, for instance, can effectively reduce the enthalpy of generation; however, not all reactions are totally reversible, resulting in a lower hydrogen storage capacity.163 Although rare earths still face serious challenges in the practical application of hydrogen storage, they offer a new idea in the development of hydrogen storage materials.
In light of the existing limitations associated with hydrogen energy storage and the intermittent release of energy from renewable energy sources, it is vital to create new and efficient energy storage devices, among which lithium-ion batteries (LIB) and supercapacitors are representative.164–166 In electrochemical energy storage, RE-based compounds also show amazing promise. Typically, rare earth elements are doped in LIB to modify the electrodes (common ones are LiCoO2 and LiMn2O4).167,168 The large-radius rare earth ions are introduced into the lattice of the electrode material to induce distortion, thereby decreasing the charge transfer resistance and enhancing the electrode material's conductivity.169,170 LiCoO2 is a common cathode material for LIB with a theoretical specific capacity of up to 274 mA h g−1. It is currently the cathode material with the highest compaction density and thus the manufactured lithium-ion battery has the largest volume specific energy, and it has become the primary material for batteries used in tablet computers and mobile smart terminals.171 However, when the voltage exceeds 4.35 V, LiCoO2's capacity diminishes dramatically.172 The highest capacity of commercial LiCoO2 is therefore just 165 mA h g−1. Liu et al. fabricated LiCoO2 doped with La and Al (D-LCO) via liquid-phase crystallization; the structure diagram is shown in Fig. 10a.173 La functions as a pillar to support the structure and suppress the phase transition during cycling. Al, on the other hand, acts as a charge center to allow Li to diffuse even at 4.5 V. When D-LCO was charged to 4.5 V, three distinct phase transitions of Li/Li+ occurred at 4.1 V, 4.2 V, and 4.46 V along the dQ/dV curves (Fig. 10b). In situ testing (Fig. 11a–e) reveals that Li+ intercalation/deintercalation is extremely reversible and there is no discernible phase transition. The maximum volume change of the P-LCO battery is 3.63%, as depicted in the voltage and volume change curve (Fig. 11f).
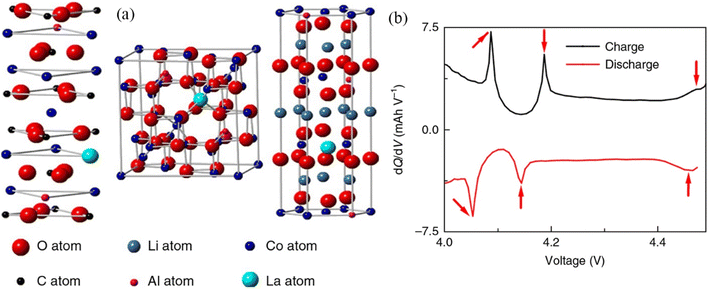 | ||
| Fig. 10 Structural diagrams and dQ/dV curves: (a) diagrammatic representation of the structures of doped CoCO3 and Co3O4, (b) dQ/dV curves of pristine LiCoO2 during charging and discharging. | ||
LiMn2O4 is also a typical cathode material for batteries, but the performance degradation brought on by manganese dissolution restricts its practical application.174 Cationic doping modification can effectively inhibit manganese dissolution, stabilize the surface crystal structure, and produce a protective layer to prevent corrosion by the electrolyte.175 Ram synthesized LiMn2O4 doped with various rare earth elements (Gd, Tb, Dy, Yb) using the sol–gel method.176 According to studies, doping LiMnO4 with Dy and Tb can successfully improve its conductivity, while Gd and Dy can greatly enhance its cycle performance. In a study, Singhal et al. improved the cycling stability of LiMn2O4 by supplementing with Nd.177 Other cathode materials doping rare earth elements provide a variety of performance enhancements. Doping LiNi0.5Co0.2Mn0.3O2 with Ce, for instance, can increase the initial discharge capacity and cycle stability.178 Comparative research was conducted on the impact of Y doping on the performance enhancement of LiMn2O4, Li1+xV3O8, and LiFePO4.179 Doping with Y decreases the crystal parameters of LiMn2O4, increases the (100) interplanar spacing of Li1+xV3O8, and decreases the grain size of LiFePO4. Various Y doping mechanisms result in distinct performance enhancements. The appropriate amount of doped Y enhances the initial discharge capacity of LiFePO4 and Li1+xV3O8 as well as the cycling performance of LiMn2O4.
However, due to the low power density and poor cycle stability of lithium-ion batteries, they will not be able to meet the demand for higher energy consumption, faster power transfer, and higher stability during cycling in the future.180 Supercapacitors compensate for this deficiency and are being intensively explored.181 Supercapacitors are divided into electric double-layer capacitors and pseudocapacitors according to their mechanism. The majority of double-layer capacitor electrode materials are carbon and carbon derivatives, which are outside the focus of this research. Therefore, the following sections describe the application of rare earths in the field of pseudocapacitors.
3 Pseudocapacitive applications
In the 1960s, David C. Graham used the word “pseudocapacitance” to describe extra electrochemical capacity that is not caused by the formation of an electric double layer. This is where the concept of ‘pseudocapacitance’ originated.182 In the early 1970s, B.E. Conway first defined pseudocapacitance in his work. Conway proposed: pseudocapacitance describes an electrochemical mechanism similar to capacitance but is actually a charge transfer process at the electrode–electrolyte interface, a fast and reversible redox reaction, which is the reason for decorating with the prefix ‘pseudo’.183 As illustrated in Fig. 12, the pseudocapacitance mechanism can be broken down into three sub-mechanisms: underpotential deposition, redox, and intercalation pseudocapacitance.184 According to this description, the pseudocapacitive electrode material and the double layer electric electrode material are clearly defined. Despite the fact that the energy storage behavior of the electric double-layer electrode material also occurs on the surface of the electrode material, the entire process involves only the simple faradaic reaction of charge adsorption.185,186 The cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) patterns of pseudocapacitors are comparable to those of EDLC due to the rapid surface or near-surface charge transfer.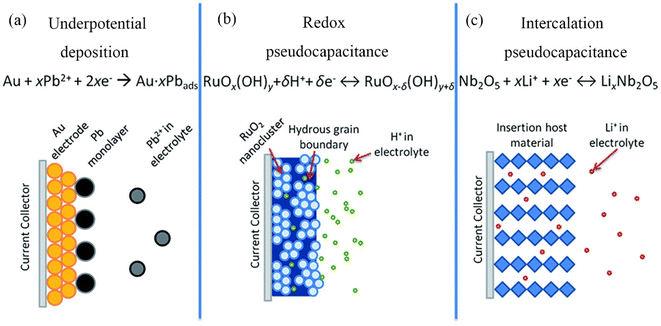 | ||
| Fig. 12 Different pseudocapacitance mechanisms: (a) underpotential deposition, (b) redox pseudocapacitance, and (c) intercalation pseudocapacitance. | ||
However, pseudocapacitive materials that store energy through redox reactions behave like batteries in some ways.187,188 When the particle size of the cathode LiCoO2 block, which is predominantly used in lithium-ion batteries, reaches the critical nanometer size of 10 nm, the battery characteristics vanish and the voltage distribution transitions from the platform to ramp form, exhibiting miraculous capacitance characteristics.189 The major difference between pseudocapacitive and battery materials is that pseudocapacitive materials are not constrained by ion diffusion within the electrode material, whereas battery materials are governed by the diffusion process. With an in-depth study of how pseudocapacitance stores charge and the widespread use of nanomaterials and thin-film materials, more and more studies have shown that the pseudocapacitance mechanism and the diffusion process control each other in pseudocapacitive materials.190 This is due to the fact that nano-scale electrode materials have greater specific surface areas and shorter ion diffusion paths than traditional bulk materials, and this difference can even be of several orders of magnitude, making it increasingly difficult to distinguish between the surface and bulk of materials. This synergistic effect happens not just with traditional battery materials, but also with intercalation materials like TiO2, Nb2O5, metal oxides, MoOx, MXene, and emerging cationic intercalation materials.191–194 Therefore, intercalated pseudocapacitance combines the advantages of batteries and supercapacitors, namely massive charge storage and storage that is not governed by diffusion.
In many intercalation pseudocapacitive materials, rare earth compounds have become an important area of study in the past few years.195 Since rare earth elements are often regarded as electrochemically inactive, abundant rare earth resources have been neglected in the field of pseudocapacitors in previous studies. However, the majority of rare earth elements show RE3+/RE2+ or RE4+/RE3+ redox coupling, laying the thermodynamic groundwork for their employment in pseudocapacitive energy storage.196 Rare earth oxides are the largest group of rare earth compounds that are easily manufactured, structurally stable, corrosion-resistant, and harmless to the environment.197 Their electrochemical activity can be changed by changing the amount of oxygen vacancies and oxygen in the lattice, which is a simple step in the manufacturing process. Thus, rare earth oxides have tremendous practical potential in the pseudocapacitive field.198 In subsequent sections, we will discuss the application and compound strategies of rare earth oxides in pseudocapacitors in recent years, clarify the energy storage mechanism of rare earth oxides, and provide references for enhancing the performance of rare earth oxide pseudocapacitors.
3.1 Typical oxides for pseudocapacitance
| CeO2(surface) + M+ ↔ CeO2·M+(surface) | (9) |
| CeO2 + M+ + e− ↔ CeO·OM | (10) |
| Materials | Synthesis method | Electrolyte | Potential (V) | Capacitance (F g−1) | Capacitance retention (%) | Reference |
|---|---|---|---|---|---|---|
| Hexagonal CeO2 nanoparticles | Hydrothermal method | 1 M NaCl | 0–0.8 | 457.0 (2 A g−1) | 82.0 (2000 cycles) | 202 |
| CeO2–W nanocrystals | Epoxide precipitation reaction | 3 M KOH | 0–0.5 | 372.6 (6.3 A g−1) | 91.0 (4000 cycles) | 203 |
| CeO2 nanocrystal | Supercritical hydrothermal method | 6 M KOH | 0–0.4 | 339.5 (1 A g−1) | 85.7 (1000 cycles) | 204 |
| CeO2 mesoporous microsphere | Assisted microwave method | 3 M KOH | 0–0.45 | 773.0 (5 mV s−1) | 56.3 (3000 cycles) | 205 |
| CeO2 nanostructures | Combustion method | 1 M KOH | −0.3–0.45 | 114.6 (10 mV s−1) | 92.5 (1000 cycles) | 206 |
| CeO2@20 nanoparticles | One step solvothermal method | 1 M Na2SO4 | 0–0.8 | 143.0 (0.2 mA g−1) | 75.0 (1000 cycles) | 207 |
| CeO2 nanoparticles | Precipitation and the hydrothermal method | 1 M LiCl | −0.8–0.8 | 877.5 (3 A g−1) | 96.5 (5000 cycles) | 208 |
| Hexagonal CeO2 | Hydrothermal method | 1 M HCl | 0–0.8 | 889.0 (2 mV s−1) | ∼100 (1500 cycles) | 209 |
Nanostructured CeO2 is a significant application-relevant electrode material. The specific surface area and particle morphology are crucial elements that influence pseudocapacitive performance. The further development direction of nanoparticles is to use a large specific surface area and special morphology to achieve optimum pseudocapacitive dynamic performance and optimize the theoretical specific capacity. In order to investigate high-performance CeO2 electrode materials, Wang et al. using propylene oxide as a precipitant, prepared spherical and linear CeO2 nanoparticles in aqueous and ethanolic solutions, respectively.203 The acidity of the cation in the solution affects how propylene oxide reacts. Since Ce3+ has greater acidity in ethanol solution, it can rapidly produce CeO2. CeO2 was orientated on the high surface energy {110} plane to grow and link, resulting in wire-like crystals. In an aqueous solution, its growth process follows an isotropic growth mechanism, thus forming spherical crystals. Electrochemical tests reveal that CeO2 nanospheres have better pseudocapacitive properties than CeO2 nanowires. To investigate the effect of surfactants on the morphology, size, and exposed crystal faces of CeO2 nanocrystals in greater depth, Hao et al. synthesized CeO2 nanocrystals using supercritical hydrothermal synthesis.204 The results indicate that cubic CeO2 has a greater pseudocapacitive performance because of the highly active {100} plane on the exposed surface and the nanoparticle size. This study elucidates the crystal plane evolution and self-assembly mechanism of CeO2, introducing a fresh concept for the construction of high-performance novel CeO2.
In addition, controlling the morphology of nano-functional materials is one of the most effective strategies for enhancing their performance. Cheng et al. achieved high-efficiency energy storage by effectively controlling the morphology of CeO2 nanoparticles.205 They synthesized CeO2 mesoporous nanospheres using a simple microwave process and sodium citrate as a chelating agent. As sodium citrate formed citric acid during hydrolysis and created alkaline conditions, it was economical because no urea was required. After 3000 cycles at 0.1 mA cm−2, the as-prepared CeO2 still exhibits a high specific capacitance of 415 F g−1, an exceptional lithium storage capacity of 529 mA h g−1 after 100 cycles at 0.5 mA g−1. CeO2's superior electrochemical performance is due to its unique mesoporous microsphere structure. On the basis of this research, Abdul Jabbar Khan et al. synthesized porous CeO2 nanoparticles as electrode materials and used highly conductive carbon cloth as a current collector.206 CeO2 nanoparticles supported on carbon cloth exhibit excellent energy storage performance, with a specific capacitance of 877.5 F g−1 at 3 A g−1 and capacity retention of 96.52% after 5000 cycles. This further demonstrates the critical role of a porous structure in enhancing CeO2 pseudocapacitive performance. On the basis of the aforementioned research findings, Nallappan Maheswari and Gopalan Muralidharan developed ultra-high-performance CeO2 electrode materials, which represented a significant milestone in the development of CeO2 electrode materials.207 They prepared hexagonal nanostructured CeO2 by a one-step hydrothermal method using cetyltrimethylammonium bromide (CTAB) as a surfactant; the formation mechanism is shown in Fig. 13a. They maintained the reaction environment's pH at 10. The OH− content is saturated under these conditions, and CTAB ionizes CTA+ and regulates the rate at which NaOH ionizes OH−, resulting in the formation of well-defined hexagonal CeO2. In this work, the effects of different calcination temperatures on the shape, structure, and electrochemical performance of CeO2 were looked at in greater detail. CeO2 calcined at 500 °C, named C-500, has the greatest active surface area and porosity, which promotes the rapid diffusion of electrolyte ions and has fast kinetics, resulting in the best electrochemical performance. When the charge–discharge current density was increased from 2 to 10 A g−1, the specific capacity declined from 927 to 475 F g−1 at a retention rate of 51.24% (Fig. 13b). After 1500 cycles at a high current density of 20 A g−1, the capacity can still be maintained at over 100%, as shown in Fig. 13c. Additionally, an asymmetric supercapacitor utilizing activated carbon as the anode was fabricated. The device had a high energy density of 45.6 W h kg−1 at a power density of 187.5 W kg−1 with good cycle stability (Fig. 13d). The pseudocapacitive properties of hexagonal CeO2 nanoparticles were further investigated in aqueous solutions of NaCl, KCl, Na2SO4, and K2SO4, the four most common neutral electrolytes.202 At a current density of 2 A g−1, the specific capacitances in the four electrolytes were 457, 395, 400, and 320 F g−1, respectively. This is because Na+ has a lower radius than K+, and SO42− has a substantially higher ionic radius than Cl−. When the hydration radius is similar, ions with a smaller radius spread more rapidly. This shows the crucial function of selecting an appropriate electrolyte for improving the pseudocapacitive performance of CeO2 electrode materials.
Finally, researchers investigated the mechanism by which oxygen vacancies affect the electrochemical performance. Prasanna and his team synthesized ultra-porous CeO2 nanoparticles containing oxygen vacancies (CeO2 NS) with a fast and low-cost combustion method.208 Capacitance retention after 1000 cycles was 92.5% at 1 A g−1. The resistances to charge transfer were 2.01 Ω and 2.86 Ω, respectively, before and after cycling. The CeO2 NS exhibits outstanding kinetics and cycling stability thanks to the oxygen vacancies and porous structure. Manjeet Kumar et al. investigated the influence of oxygen vacancies on electrochemical performance in detail.209 The oxygen vacancy content of CeO2 was quantified using XPS. Experiments demonstrate that raising the concentration of Ce3+ in CeO2 stimulates the formation of additional oxygen vacancies on the surface, which is essential for redox activity.
But enabling CeO2 to have a high capacitance and be stable during cycling are still big problems that make it hard to use it as an ideal electrode material. The disparity in electrochemical performance between theory and practice is related to the low conductivity of CeO2 (8.24 × 10−8 S cm−1) and the lattice expansion that happens during the reduction of Ce4+ to Ce3+.210 As a result, compositing CeO2 with conductive polymers, carbon materials and their derivatives provides another effective and convenient method of increasing pseudocapacitive energy storage ability.211 In Table 6, the typical electrochemical parameters of CeO2 composites are compared to those of pure CeO2. It can be seen that CeO2 composites have a greater specific capacitance than pure CeO2.212–216
| Materials | Potential (V) | Capacitance (F g−1) | Capacitance retention (%) | Reference |
|---|---|---|---|---|
| CeO2/CNTs | 0.3–0.81 | 818 (1 mV s−1) | 95.3 (5000 cycles) | 212 |
| CeO2/GC | 0–0.55 | 501 (1 A g−1) | 93.0 (5000 cycles) | 213 |
| CNTs@CeO2-HTs | 0–1.55 | 70.7 mA h cm−2 (1 mA cm−2) | 86.4 (3000 cycles) | 214 |
| PANI/rGO/CeO2 | −0.2–0.8 | 684 (1 A g−1) | 92.0 (3000 cycles) | 215 |
| rGO-CeO2/PANI | −1.4–0 | 454.8 (1 A g−1) | 70.2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
216 |
Unfortunately, carbon compounds and their derivatives are difficult to produce, costly, and difficult to process.217,218 The cycle stability of conductive polymers is poor and harmful to the environment. Because of this, it is necessary to explore other strategies to enhance the capacitance and cycling stability of CeO2. Despite the immaturity of current research in this field, fortunately, significant progress has been achieved in the study of the conductivity of transition metal oxides (TMO) with similar properties to CeO2.219 Numerous studies have demonstrated that the key to enhancing the electrical conductivity of TMO is to regulate their electronic structure, energy band structure, and reactive sites. To begin with, a Schottky heterojunction is built by tweaking the electronic structure in order to lower the amount of energy that is released while carriers are being transmitted.220 In addition, the energy band is changed to generate a z-type heterojunction in order to boost the separation rate of hole–electron pairs.221 In conclusion, the development of a distinct p–n junction by an internal electric field accelerates the movement of hole–electron pairs.222 Improving electrical conductivity by promoting electron transport by doping and defect engineering is still the prevalent strategy at the present time. In addition, by modifying the electron and crystal structure, more reactive sites can be exposed, and the hole–electron pair can be split through the heterojunction, thereby promoting redox processes and providing extra charges for pseudocapacitive storage. Currently, improving electrical conductivity by promoting electron transport by doping and defect engineering is still the prevalent strategy at the present time. Furthermore, the conductivity can be enhanced by modifying the electronic and energy band structures, producing heterogeneous knots, introducing interface effects, and other strategies. The preceding investigations have significant reference value for further boosting the conductivity of CeO2.
In addition, the effect of the electrolyte on the electrochemical performance of a CeO2 electrode cannot be ignored. In addition to selecting an electrolyte with the most appropriate ionic radius for the material pores, it is essential to enhance the electrode/electrolyte interface interaction. The degree of electrode material immersion in the electrolyte is paramount for defining the pseudocapacitance kinetics.195 When electrolyte ions are well immersed in the electrode material, cations can quickly and reversibly intercalate and deintercalate, and strong interfacial contacts greatly increase the ionic conductivity of the material. However, CeO2 is not hydrophilic; therefore inadequate infiltration will result in a drop in pseudocapacitive kinetic properties in aqueous electrolytes, thus combining with hydrophilic substances is an efficient remedy. Layered silicate minerals possess exceptional wettability, a distinct interlayer environment, and a stable layered structure, making them perfect composite materials. Zhang et al. synthesized transition metal boride–clay composites by liquid-phase reduction and investigated the effect of the clay's structural type and microstructure on its electrochemical characteristics.223 The composites exhibited exceptional electrochemical kinetics and cycling stability, according to electrochemical measurements. However, research in this field is still in its infancy, and the augmentation of electrochemical characteristics by clay minerals and the underlying mechanisms of action require additional investigation.
| La2O3 + nM+ + ne− ↔ MnLa2O3 | (11) |
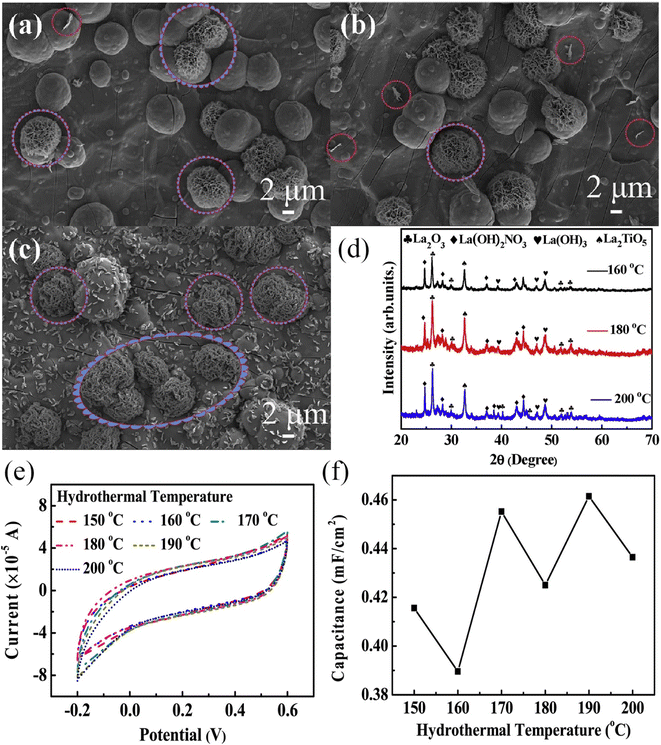
As early as 2016, Yadav et al. used a one-step hydrothermal method to grow rod-like La2O3 films on stainless steel substrates as electrode materials for flexible supercapacitors.224 The film has a specific surface area of 45.39 m2 g−1 and a pore size of 6.9 nm according to the BET calculation method. These films have a higher specific surface area and are comparable to those made by chemical deposition of La2O3, Ni(OH)2, and Fe2O3.225–227 Electrochemical tests were performed on rod-like La2O3 films, which exhibited a high specific capacity of 250 F g−1 and a power density of up to 1.5 kW kg−1 at an energy density of 80 W h kg−1. A symmetric supercapacitor was assembled using PVA/LiClO4 gel as the electrolyte, exhibiting a specific capacitance of 10 F g−1 at a scan rate of 5 mV s−1, and a capacity retention of 79% after 1000 cycles. In the same year, Yadav et al. used one-step spray pyrolysis to fabricate cellular La2O3 porous films for supercapacitors and gas sensors.228 After 3000 cycles, the La2O3 film retains 85% of its specific capacitance (166 F g−1), indicating good cycling stability. According to the research outlined above, Wang et al. synthesized La2O3 thin films using a straightforward one-step hydrothermal method for the purpose of investigating electrowetting responses for high-performance supercapacitors.229 The study discovered that by adjusting the hydrothermal temperature and time, the surface structure of the La2O3 thin film can be precisely controlled. SEM images of La2O3 thin films hydrothermally heated for 20 hours at various temperatures are shown in Fig. 14a–f. As the temperature is increased from 150 to 160 °C, the microspheres grown on the titanium substrate become denser. At 170 °C, the microspheres form a dense membrane structure; at 180 °C, an La2O3 hierarchical structure with vertically arranged flower-like nanoparticles is formed. When the temperature is increased to 190 °C, the microspheres aggregate into a dense microsphere film, indicating that 180 °C is the critical temperature for the formation of a flower-like hierarchical structure. The hydrothermal reaction time (10, 15, and 20 h) was changed at the optimal temperature of 180 °C. The morphologies of the prepared samples are shown in Fig. 15a–c. Based on the XRD patterns of samples made under different reaction conditions (shown in Fig. 15d), the hydrothermal method was able to make an La2O3 film with dense microspheres that look like flowers and nano-metal structures that are arranged in a hierarchical way.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) No effect was observed on the ingredients under different reaction conditions. The CV curves clearly illustrate the pseudocapacitive features of the samples (Fig. 15e), and the specific capacitance changes with hydrothermal temperature, as illustrated in Fig. 15f, indicating that the prepared La2O3 film has a high specific capacitance and that hydrothermal temperature has a significant effect on its capacitance. Additionally, Yadav et al. investigated the practicality of La2O3.230 Chemical water bath deposition was used to fabricate Co3O4 and La2O3 thin films. In 1 mol L−1 KOH, the specific capacitances are 415 F g−1 and 288 F g−1, respectively. A highly conductive oxide film of Co3O4 was used as the anode, an La2O3 film was used as the cathode, and PVA-KOH gel was used as the solid electrolyte. The device has a high specific capacitance of 15 F g−1 and retains 92% of its high cycling performance after 2000 cycles. When the power density is 108.2 W kg−1, the energy density is as high as 42.9 W h kg−1. When two asymmetric capacitors are connected in series, the LED can remain illuminated for 45 seconds after being fully charged.
No effect was observed on the ingredients under different reaction conditions. The CV curves clearly illustrate the pseudocapacitive features of the samples (Fig. 15e), and the specific capacitance changes with hydrothermal temperature, as illustrated in Fig. 15f, indicating that the prepared La2O3 film has a high specific capacitance and that hydrothermal temperature has a significant effect on its capacitance. Additionally, Yadav et al. investigated the practicality of La2O3.230 Chemical water bath deposition was used to fabricate Co3O4 and La2O3 thin films. In 1 mol L−1 KOH, the specific capacitances are 415 F g−1 and 288 F g−1, respectively. A highly conductive oxide film of Co3O4 was used as the anode, an La2O3 film was used as the cathode, and PVA-KOH gel was used as the solid electrolyte. The device has a high specific capacitance of 15 F g−1 and retains 92% of its high cycling performance after 2000 cycles. When the power density is 108.2 W kg−1, the energy density is as high as 42.9 W h kg−1. When two asymmetric capacitors are connected in series, the LED can remain illuminated for 45 seconds after being fully charged.
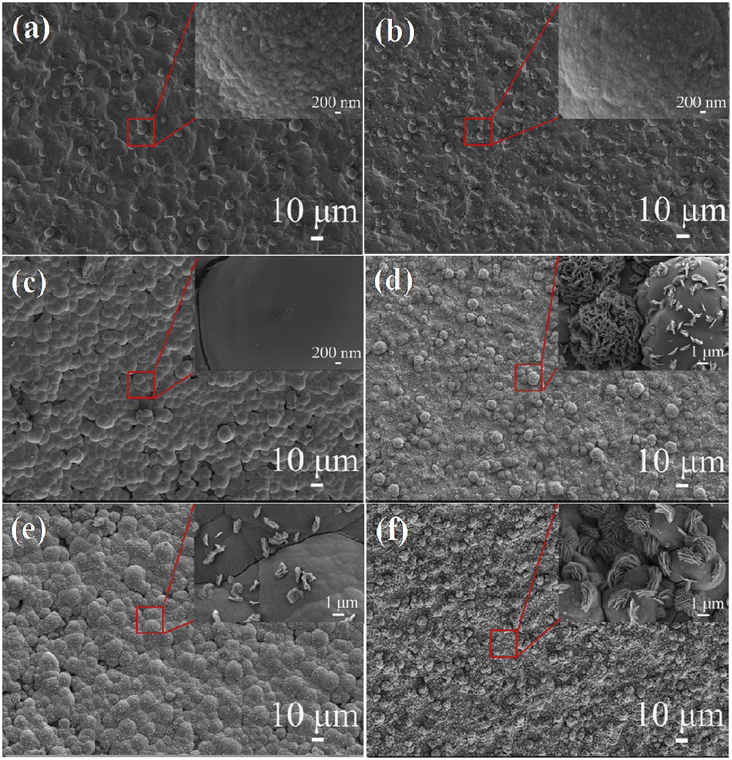 | ||
| Fig. 14 SEM images of samples with different hydrothermal times: (a) 150 °C, (b) 160 °C, (c) 170 °C, (d) 180 °C, (e) 190 °C and (f) 180 °C. SEM magnified images of the inset marked areas. | ||
Graphene and its derivatives (r-GO) have emerged as star materials in the field of supercapacitor energy storage in recent years due to their large specific surface area, high conductivity, high electron mobility, and chemical stability.231 As a result, composites of La2O3@G(r-GO) have a greater energy storage capacity and a more stable cycle life than pure La2O3. Zhang and his peers synthesized reduced graphene oxide/lanthanum oxide (r-GO/La2O3) nanocomposites using a straightforward and cost-effective reflow method.232 Throughout the process of condensation reflow, graphene oxide (GO) was broken down, and La2O3 was successfully added to the surface of GO. Electrochemical tests were conducted in a 3 mol L−1 KOH electrolyte. At a current density of 0.1 A g−1, the r-GO/La2O3 composite had a specific capacitance of 156.25 F g−1 and a capacity retention of 78% after 500 cycles. The composite material's superior electrochemical performance is due to the fact that the addition of La2O3 reduces the degree of aggregation of the graphene sheets and increases their effective active specific surface area, thereby increasing the electrode material's specific capacitance. The r-GO/La2O3 composites were used to assemble button cells. When the power density is increased to 15.62 kW kg−1, the energy density value increases to 13.02 W h kg−1, indicating the r-GO/La2O3 composite's suitability for practical applications. Similarly, Karthikeyan et al. synthesized lanthanum oxide/reduced graphene oxide (La2O3/r-GO) nanocomposites by a simple hydrothermal method.233 The interfacial effect of La2O3 and r-GO significantly improves the specific capacitance and cycling stability of electrode materials, as demonstrated in this work. The specific capacitance of La2O3/r-GO is as high as 546 F g−1 (at 2 mA g−1) in an electrochemical test using the traditional three-electrode system, and the capacity remains at 92.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating extraordinary cycle stability. R-GO increases the La2O3/r-GO composites' effective specific surface area, improves their electrical conductivity, and accelerates charge transport, thus enhancing the composites' redox activity. The interfacial effect of r-GO and La2O3 boosts electrolyte adsorption at the interface, increases the wettability of the electrode material and electrolyte, and reduces the ion transport distance, effectively improving the composites' pseudocapacitive performance. Additionally, the author proposes a cost-effective way to combine supercapacitors and clean energy. Solar energy's application value will increase even further if it can be converted to electricity via supercapacitors. Miah et al. also investigated the temperature dependency of capacitance by utilizing the interface effect between GO and La2O3.234 High-performance supercapacitors degrade the active material at elevated temperatures, restricting their applicability range. As a result, it is vital to enhance supercapacitors' thermal stability. Miah prepared GO-La2O3 composites exhibiting 158% and 205% increases in specific capacitance at 30 °C and 70 °C, respectively, compared to pure GO. When the current density was 1 A g−1 and the temperature was 70 °C, the specific capacitance increased to a maximum of 751 F g−1. After 2000 cycles, the electrode capacity retention rate was 78% at 30 °C and 67% even at 70 °C, indicating excellent thermal stability. The effect of temperature on the charge transport properties of La2O3 was investigated by examining the current–voltage characteristics of samples at temperatures ranging from 0 to 70 °C. Fig. 16(a and b) depict the J–V curve. The J–V curve is nearly symmetrical across the entire voltage range, and the current is linear with the bias voltage at low voltages (±0.5 V), indicating that the conduction process is ohmic. However, as the bias high voltage increases, a nonlinear relationship emerges, with the degree of nonlinearity increasing with temperature. This is because high temperature increases the conductivity of the electrode material, and high temperature induces internal field emission, resulting in a linear deviation. Eqn (12) can be utilized to determine the voltage contribution (Vm) of the inner field emission:235
000 cycles, demonstrating extraordinary cycle stability. R-GO increases the La2O3/r-GO composites' effective specific surface area, improves their electrical conductivity, and accelerates charge transport, thus enhancing the composites' redox activity. The interfacial effect of r-GO and La2O3 boosts electrolyte adsorption at the interface, increases the wettability of the electrode material and electrolyte, and reduces the ion transport distance, effectively improving the composites' pseudocapacitive performance. Additionally, the author proposes a cost-effective way to combine supercapacitors and clean energy. Solar energy's application value will increase even further if it can be converted to electricity via supercapacitors. Miah et al. also investigated the temperature dependency of capacitance by utilizing the interface effect between GO and La2O3.234 High-performance supercapacitors degrade the active material at elevated temperatures, restricting their applicability range. As a result, it is vital to enhance supercapacitors' thermal stability. Miah prepared GO-La2O3 composites exhibiting 158% and 205% increases in specific capacitance at 30 °C and 70 °C, respectively, compared to pure GO. When the current density was 1 A g−1 and the temperature was 70 °C, the specific capacitance increased to a maximum of 751 F g−1. After 2000 cycles, the electrode capacity retention rate was 78% at 30 °C and 67% even at 70 °C, indicating excellent thermal stability. The effect of temperature on the charge transport properties of La2O3 was investigated by examining the current–voltage characteristics of samples at temperatures ranging from 0 to 70 °C. Fig. 16(a and b) depict the J–V curve. The J–V curve is nearly symmetrical across the entire voltage range, and the current is linear with the bias voltage at low voltages (±0.5 V), indicating that the conduction process is ohmic. However, as the bias high voltage increases, a nonlinear relationship emerges, with the degree of nonlinearity increasing with temperature. This is because high temperature increases the conductivity of the electrode material, and high temperature induces internal field emission, resulting in a linear deviation. Eqn (12) can be utilized to determine the voltage contribution (Vm) of the inner field emission:235
| J = σV + CVm | (12) |
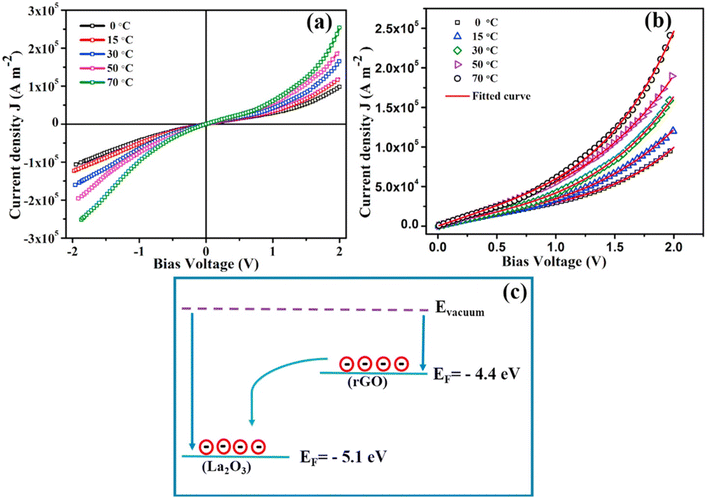 | ||
| Fig. 16 J–V characteristics of G-La2O3 at different temperatures: (a) full-range experimental J–V plots, (b) half-range J–V fitting, (c) schematic of charge transfer from rGO to La2O3. | ||
| Temperature (°C) | σ (S m−1) | C | m |
|---|---|---|---|
| 0 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 891 891 |
1778 | 3.10 |
| 15 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238 238 |
5301 | 3.27 |
| 30 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 004 004 |
6972 | 3.75 |
| 50 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969 969 |
8290 | 4.04 |
| 70 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095 095 |
8743 | 4.61 |
Since Trasatti S studied the pseudocapacitive properties of RuO2 and found that its theoretical specific capacitance is as high as 1300–2200 F g−1, more and more studies have been devoted to transition metal compounds (TMs).236 The highly reversible redox reaction between transition metal compounds and electrolyte ions provides ultra-high capacitance, but RuO2 is expensive and toxic, so Ni-based, Mn-based, and Co-based compounds have become promising pseudocapacitive electrode materials.237 Sun et al. deposited MnO2 and La2O3 on carbon paper as working electrodes, and assembled an asymmetric supercapacitor.238 By effectively increasing the potential range of the working electrode, the capacitor's energy density was greatly increased, and the range of uses for the capacitor grew. In 0.5 mol L−1 Na2SO4 electrolyte, the asymmetric supercapacitor exhibits a mass energy density of 80.56 W h kg−1 and a volumetric energy density of 0.74 mW h cm−3. After further encapsulation into a device, the power density remains at 94.29 mW cm−3 while the energy density is 0.49 mW h cm−3. Duan et al. synthesized ultrathin triangular sheet Ni(OH)2 and La2O3 composites (LONH) by a simple solvothermal method.239 LONH exhibits excellent electrochemical energy storage performance. This study shows that the addition of La2O3 to transition metal compounds (TMs) can effectively change the morphology and structure of the composites, providing a new vision to develop novel La-TM electrode materials with excellent performance. Li et al. added La2O3 as an electrocatalyst to MnO2, and prepared waxberry-shaped Mn–La composite microspheres by a two-step hydrothermal method.240 The lattice defects and electrocatalytic functions of La2O3, which improve the active specific surface area of the electrode material and speed up the rate of charge transfer, give the composite its excellent rate performance and ultra-high cycle stability.
| RE2O3 + nM+ + ne− ↔ MnRE2O3 | (13) |
| 2RE(OH)2 + 2OH− ↔ RE2O3 + 2e− + 3H2O | (14) |
Mohammad Shiri et al. fabricated Nd2O3 nanorod composite conductive polymer films by ultrasonic-assisted pulsed electrochemical deposition, which exhibited significantly higher rate capability and superior cycling stability than conductive polymer films.242 Liu et al. prepared polypyrrole/Sm2O3 (PPy/Sm2O3) nanocomposites by in situ chemical oxidative polymerization, and the half-cell exhibited a large capacitance of 771 F g−1 in 1 M NaNO3.243 This demonstrates that Sm2O3 is an energy storage material with potential electrochemical applications. Similarly, Shiri and his research team prepared polyaniline-derivative/Sm2O3 (POAP/Sm) composite films by electrochemical deposition.244 The electrode material exhibits excellent cycling stability, with a capacity retention of 91% and a coulombic efficiency of 95% after 1000 cycles. Further, Mohammadian-Sarcheshmeh et al. prepared p-phenylenediamine/Sm2O3 (PPDA/Sm2O3) cross-linked graphene oxide (GO) aerogels by a simple hydrothermal method.245 Through the nucleophilic addition reaction of amino groups on PPDA and carboxy groups on GO, the stacking of graphene oxide layers can be stopped, and the space between graphene oxide layers can be altered to greatly improve the composite's electrochemical performance. This porous three-dimensional PPDA/Sm2O3 cross-linked GO aerogel structure greatly improves the specific capacity of the electrode material, and the specific capacitance is as high as 591 F g−1 at a scan rate of 5 mV s−1, which is about nine times higher than that of pure Sm2O3, and it has good cycle stability, with a capacity retention of 92.7% after 4000 cycles. The porous three-dimensional structure proposed in this study can be extended to other electrode material designs, which is beneficial to improving the electrochemical kinetics and greatly improving the energy storage efficiency of pseudocapacitors. On this basis, Shiri et al. prepared POAP/Eu2O3 and POAP/Gd2O3 by the pulsed electrochemical deposition method.246,247 The enhanced electrochemical performance of the composites is due to the special surface structure as well as the synergistic effect of the two components.
| Pr6O11 + nM+ + ne− ↔ MnPr6O11 | (15) |
| 6Pr(OH)2 + 10OH− ↔ Pr6O11 + 6e− + 11H2O | (16) |
As early as 2011, Wang et al. prepared Pr6O11/polypyrrole (Pr6O11/PPy) with a core–shell structure, whose specific capacitance can reach 400 F g−1 at a current density of 10 mA cm−2.249 Similarly, Kubra et al. presented Pr6O11/Mn3O4 composites by a simple hydrothermal method, exhibiting a high 794.58 F g−1 ratio capacitance at 0.5 A g−1 current densities.250 Based on the research cited above, Chen et al. prepared a Pr6O11–NiCo2O4 core–shell structure with better performance, coating Pr6O11 as a growth framework on nickel foam (NF), and Pr6O11–NiCo2O4 exhibited an ultra-high specific capacitance of 1635 F g−1 at 0.5 mA cm−2.251 Paravannoor et al. prepared PrOx/unzipped multi-walled carbon nanotubes (PrOx/UZCNT) and presented the unique insight that the anion intercalation effect generated by surface oxygen vacancies enhances the electrochemical performance.252Fig. 17a lists the work functions of different metal oxides. The assembly of low-work-function Pr6O11 (2.8 eV) with high-work-function compounds (V2O5, 6.85 eV) can prepare supercapacitors with a wide potential window exceeding 4 V, thereby significantly improving the energy density of the supercapacitor. The value is calculated from eqn (17) and (18):252
| E = E0 + E1 + E2 | (17) |
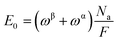 | (18) |
The above research on the pseudocapacitive properties of rare earth oxides opens up new horizons for the future development of high-performance nanocomposites.
4 Conclusion and outlook
This article has described the applications of rare earths in traditional metallurgy, biomedicine, magnetism, luminescence, catalysis, and the energy storage field. In recent years, the rare earth industry's new development model has been based on the economics of sustainable development, clean production, and industrial ecology, shifting from traditional high energy consumption, resource waste, and environmental pollution to green ecological development, which is a green resource that can be utilized in new energy storage fields. On the basis of the electrochemical energy storage potential of rare earths, typical rare earth oxides were selected as research objects to provide a comprehensive overview of their research progress in the field of pseudocapacitors, including energy storage mechanisms, electrochemical performance, and application prospects. As pseudocapacitive electrode materials, rare earth oxides have the following advantages: I. they are simple to prepare, have abundant resources, and are environmentally friendly. II. Excellent redox properties are possessed by RE3+/RE2+ or RE4+/RE3+ couples of rare earth oxides. III. Oxygen vacancies exist in the lattice of rare earth oxides, which serve as effective reactive sites. IV. They show acid and alkaline corrosion resistance, compatible with a variety of electrolytes. V. They have high volume energy density, suitable for use in small mobile devices.However, the low conductivity and lattice expansion of rare earth oxides restrict their development as ideal electrode materials. Based on current research results, we highlight the following concerns and challenges rare earth oxides will encounter in the future energy storage field:
I. Improve conductivity. Currently, combining with highly conductive materials is an effective method for improving electrical conductivity (such as conductive polymers, carbon materials and their derivatives). However, carbon compounds and their derivatives are difficult to produce, costly, and difficult to process. The cycle stability of conductive polymers is poor and harmful to the environment. As a result, it is necessary to explore other strategies to enhance the conductivity of rare earth oxides, such as atomic and vacancy doping; heterojunction engineering; exposing active crystal planes; and introducing interfacial effects.
II. Optimize structure. The future development path of efficient RE-oxide-based pseudocapacitive materials will be to pursue stable structures, such as ultra-high-porosity structures, core–shell structures, and layered structures. In energy storage research involving transition metal oxides, several existing research designs have been modified into a core–shell structured composite that efficiently tackles the issues of volume expansion and poor electrical conductivity while elucidating the mechanism of improved electrochemical performance through first-principles and kinetic analysis. Through structural optimization, this kind of work gives a theoretical basis for and technical help in making rare earth oxide electrode materials that work better.
III. Strengthened electrode/electrolyte interface interaction. The hydrophilicity of the electrode material and electrolyte is the key point for pseudocapacitive dynamics. The adequate wetting of the electrode material by electrolyte ions is conducive to the rapid and reversible intercalation/deintercalation of cations, and high interfacial interaction substantially enhances the ionic conductivity. However, rare earth oxides are not hydrophilic, so inadequate infiltration will result in a drop in pseudocapacitive kinetic properties in aqueous electrolytes, thus combining with hydrophilic substances is an efficient remedy. Layered silicate minerals possess exceptional wettability, a distinct interlayer environment, and a stable layered structure, making them perfect composite materials. However, research in this area is still in its infancy, and the improvement in electrochemical performance and the mechanism of action of clay minerals remain obscure.
IV. Enhance comprehension of the energy storage mechanism. Despite the fact that a generally consistent understanding of the energy storage mechanism of rare earth oxides has been attained, there are still shortcomings. In particular, additional research is required to explore the mechanism by which the physical and chemical properties of electrolyte cations affect electrochemical performance and to identify whether electrolyte anions contribute to pseudocapacitance. Existing research has demonstrated that the energy storage mechanism of transition metal nitrides in KOH electrolyte is the synergistic effect of K+ intercalation and OH− surface electrochemical adsorption, but whether RE oxides exhibit a similar mechanism has not yet been concluded.
We believe that this review will help researchers realize the potential of rare earth compounds in pseudocapacitive energy storage and build on prior research to develop a fundamental insight into the role of rare earth compounds in pseudocapacitive energy storage. Finally, it is hoped that this article will provide readers with more effective preparation strategies and future directions for the development of rare earth compound pseudocapacitive electrode materials. Even though there are still some problems with the current study, we think that through more research, rare earth compounds will soon have better pseudocapacitive properties and more uses.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Science and Technology Project of Sichuan province (No. 2020YJ0163).References
- M. T. Costa Campi, P. del Rio and E. Trujillo-Baute, Trade-offs in energy and environmental policy, Energy Policy, 2017, 104, 415 CrossRef.
- F. S. Chien, How renewable energy and non-renewable energy affect environmental excellence in N-11 economies?, Renewable Energy, 2022, 196, 526 CrossRef.
- M. Hu, Balance between energy conservation and environmental impact: life-cycle energy analysis and life-cycle environmental impact analysis, Energy Build., 2017, 140, 131 CrossRef.
- I. Yildiz, E. Acikkalp, H. Caliskan and K. Mori, Environmental pollution cost analyses of biodiesel and diesel fuels for a diesel engine, J. Environ. Manage., 2019, 243, 218 CrossRef CAS PubMed.
- V. Dusastre and L. Martiradonna, Materials for sustainable energy, Nat. Mater., 2016, 16, 15 CrossRef PubMed.
- L. G. Kong, L. Y. Li, G. W. Cai, C. Liu, P. Ma, Y. D. Bian and T. Ma, Techno-economic analysis of hydrogen energy for renewable energy power smoothing, Int. J. Hydrogen Energy, 2021, 46, 2847 CrossRef CAS.
- X. Zhou, W. B. Zhang, X. Han, S. Chai, S. Guo, X. Zhang, L. Zhang, X. Bao, Y. Guo and X. Ma, Principles and Materials of Mixing Entropy Battery and Capacitor for Future Harvesting Salinity Gradient Energy, ACS Appl. Energy Mater., 2022, 5, 3979 CrossRef CAS.
- M. D. Scovell, Explaining hydrogen energy technology acceptance: a critical review, Int. J. Hydrogen Energy, 2022, 47, 10441 CrossRef CAS.
- A. G. Olabi, Energy quadrilemma and the future of renewable energy, Energy, 2016, 108, 1 CrossRef.
- A. G. Olabi and M. A. Abdelkareem, Renewable energy and climate change, Renewable Sustainable Energy Rev., 2022, 158, 112111 CrossRef CAS.
- J. R. Miller, Perspective on electrochemical capacitor energy storage, Appl. Surf. Sci., 2018, 460, 3 CrossRef CAS.
- A. Cresce and K. Xu, Aqueous lithium-ion batteries, Carbon Energy, 2021, 3, 721 CrossRef.
- J. Libich, J. Máca, J. Vondrák, O. Čech and M. Sedlaříková, Supercapacitors: properties and applications, J. Energy Storage, 2018, 17, 224 CrossRef.
- S. S. Chai, L. Zhang, W. B. Zhang, X. Bao, Y. W. Guo, X. W. Han and X. J. Ma, Acid etching halloysite loaded cobalt boride material for supercapacitor electrode application, Appl. Clay Sci., 2022, 218, 106426 CrossRef CAS.
- X. L. Zhang, W. B. Zhang, X. W. Han, L. Zhang, X. Bao, Y. W. Guo, S. S. Chai, S. B. Guo, X. Zhou and X. J. Ma, Review-Pseudocapacitive Energy Storage Materials from Hägg-Phase Compounds to High-Entropy Ceramics, J. Electrochem. Soc., 2021, 168, 120521 CrossRef CAS.
- Poonam, K. Sharma, A. Arora and S. K. Tripathi, Review of supercapacitors: materials and devices, J. Energy Storage, 2019, 21, 801 CrossRef.
- X. P. Luo, Y. B. Zhang, H. P. Zhou, K. Z. He, C. G. Luo, Z. S. Liu and X. K. Tang, Review on the Development and Utilization of Ionic Rare Earth Ore, Minerals, 2022, 12, 554 CrossRef CAS.
- T. Kegl, A. Kosak, A. Lobnik, Z. Novak, A. K. Kralj and I. Ban, Adsorption of rare earth metals from wastewater by nanomaterials: a review, J. Hazard. Mater., 2020, 386, 121632 CrossRef CAS PubMed.
- G. Lin, Q. L. Pan, W. Y. Wang, B. Liu, Z. Q. Huang, S. Q. Xiang, Y. R. Liu, Y. Q. Sun and J. Ye, The role of trace Sc and Y in the corrosion resistance of cold drawn Al-0.2Ce based alloys with high electrical conductivity, J. Alloys Compd., 2021, 882, 160708 CrossRef CAS.
- P. Tozman, H. Sepehri Amin and K. Hono, Prospects for the development of SmFe12-based permanent magnets with a ThMn12-type phase, Scr. Mater., 2021, 194, 113686 CrossRef CAS.
- Q. G. Huang, H. Lin, B. Wang, S. S. Lin, P. F. Wang, P. Sui, J. Xu, Y. Cheng and Y. S. Wang, Patterned glass ceramic design for high-brightness high-color-quality laser-driven lightings, J. Adv. Ceram., 2022, 11, 862 CrossRef CAS.
- L. Zhang, Q. Li, Y. C. Qin, X. T. Zhang, X. H. Gao and L. J. Song, Investigation on the mechanism of adsorption and desorption behavior in cerium ions modified Y-type zeolite and improved hydrocarbons conversion, J. Rare Earths, 2016, 34, 1221 CrossRef CAS.
- U. R. Singh and S. Bhogilla, Performance analysis of LaNi5 added with expanded natural graphite for hydrogen storage system, Int. J. Hydrogen Energy, 2022, 5, 244 Search PubMed.
- B. Nageswara Rao, M. Venkateswarlu and N. Satyanarayana, Electrical and dielectric properties of rare earth oxides coated LiCoO2 particles, Ionics, 2013, 20, 175 CrossRef.
- X. Y. Li, S. Hao, Z. W. Wang, C. L. Zhao and Z. B. Wang, Improving the electrical conductivity and electrochemical performance of LiMn2O4 by Sm gaseous penetration technology, Appl. Surf. Sci., 2022, 599, 153923 CrossRef CAS.
- X. Y. Li, H. M. Chen, C. Y. Yang, Y. F. Li and M. D. Wei, A new neodymium-phosphine compound for supercapacitors with long-term cycling stability, Chem. Commun., 2021, 57, 5933 RSC.
- J. Prakash, Samriti, A. Kumar, H. L. Dai, B. C. Janegitz, V. Krishnan, H. C. Swart and S. H. Sun, Novel rare earth metal-doped one-dimensional TiO2 nanostructures: fundamentals and multifunctional applications, Mater. Today Sustain., 2021, 13, 100026 Search PubMed.
- J. Liang, H. Peng, Z. M. Wang, R. Zhao, W. X. Zhang, G. F. Ma and Z. Q. Lei, Rare earth metal lanthanum–organic frameworks derived three-dimensional mesoporous interconnected carbon nanosheets for advanced energy storage, Electrochim. Acta, 2020, 353, 136597 CrossRef CAS.
- M. Asadollahzadeh, R. Torkaman and M. Torab Mostaedi, Extraction and Separation of Rare Earth Elements by Adsorption Approaches: Current Status and Future Trends, Sep. Purif. Rev., 2020, 50, 417 CrossRef.
- A. Akah, Application of rare earths in fluid catalytic cracking: a review, J. Rare Earths, 2017, 35, 941 CrossRef CAS.
- C. L. Owens, G. R. Nash, K. Hadler, R. S. Fitzpatrick, C. G. Anderson and F. Wall, Apatite enrichment by rare earth elements: a review of the effects of surface properties, Adv. Colloid Interface Sci., 2019, 265, 14 CrossRef CAS.
- M. Traore, A. J. Gong, Y. W. Wang, L. N. Qiu, Y. Z. Bai, W. Y. Zhao, Y. Liu, Y. Chen, Y. Liu, H. L. Wu, S. L. Li and Y. Y. You, Research progress of rare earth separation methods and technologies, J. Rare Earths, 2022, 4, 009 Search PubMed.
- Z. Y. Chen, Z. Li, J. Chen, P. Kallem, F. Banat and H. D. Qiu, Recent advances in selective separation technologies of rare earth elements: a review, J. Environ. Chem. Eng., 2022, 10, 107104 CrossRef CAS.
- J. L. Wang, M. Y. Guo, M. M. Liu and X. Q. Wei, Long-term outlook for global rare earth production, Resour. Policy, 2020, 65, 101569 CrossRef.
- P. E. Waudby, Rare earth additions to steel, Int. Met. Rev., 1978, 23, 74 CAS.
- L. M. Wang and T. Du, Thermodynamics of Fe–Y–S, Fe–Y–O and Fe–Y–S–O metallic solution, J. Less Common Met., 1985, 110, 179 CrossRef.
- W. L. M. Wu Yie Min and T. Du, Thermodynamics of rare earth elements in liquid iron, J. Less Common Met., 1985, 110, 187 CrossRef.
- J. Y. Mao, G. Y. Chen, J. Zhao, Y. Y. He and J. B. Luo, An investigation on the tribological behaviors of steel/copper and steel/steel friction pairs via lubrication with a graphene additive, Friction, 2020, 9, 228 CrossRef.
- F. Pan, J. Zhang, H. L. Chen, Y. H. Su, C. L. Kuo, Y. H. Su, S. H. Chen, K. J. Lin, P. H. Hsieh and W. S. Hwang, Effects of Rare Earth Metals on Steel Microstructures, Materials, 2016, 9, 417 CrossRef.
- Y. P. Ji, M. X. Zhang and H. P. Ren, Roles of Lanthanum and Cerium in Grain Refinement of Steels during Solidification, Metals, 2018, 8, 884 CrossRef CAS.
- C. Liu, Z. H. Jiang, J. B. Zhao, X. Q. Cheng, Z. Y. Liu, D. W. Zhang and X. G. Li, Influence of rare earth metals on mechanisms of localised corrosion induced by inclusions in Zr-Ti deoxidised low alloy steel, Corros. Sci., 2020, 166, 108463 CrossRef CAS.
- A. Biesiekierski, Y. C. Li and C. Wen, The Application of the Rare Earths to Magnesium and Titanium Metallurgy in Australia, Adv. Mater., 2020, 32, e1901715 CrossRef PubMed.
- F. Y. Cao, Z. M. Shi, G. L. Song, M. Liu, M. S. Dargusch and A. Atrens, Influence of hot rolling on the corrosion behavior of several Mg-X alloys, Corros. Sci., 2015, 90, 176 CrossRef CAS.
- K. Gusieva, C. H. J. Davies, J. R. Scully and N. Birbilis, Corrosion of magnesium alloys: the role of alloying, Int. Mater. Rev., 2014, 60, 169 CrossRef.
- O. Bouaziz, Revisited Storage and Dynamic Recovery of Dislocation Density Evolution Law: Toward a Generalized Kocks-Mecking Model of Strain-Hardening, Adv. Eng. Mater., 2012, 14, 759 CrossRef CAS.
- D. S. Gandel, M. A. Easton, M. A. Gibson and N. Birbilis, Calphad simulation of the Mg-(Mn, Zr)-Fe system and experimental comparison with as-cast alloy microstructures as relevant to impurity driven corrosion of Mg-alloys, Mater. Chem. Phys., 2014, 143, 1082 CrossRef CAS.
- Z. W. Geng, D. H. Xiao and L. Chen, Microstructure, mechanical properties, and corrosion behavior of degradable Mg–Al–Cu–Zn–Gd alloys, J. Alloys Compd., 2016, 686, 145 CrossRef CAS.
- E. D. Morales, E. Ghali, N. Hort, W. Dietzel and K. U. Kainer, Corrosion Behaviour of Magnesium Alloys with RE Additions in Sodium Chloride Solutions, Mater. Sci. Forum, 2003, 419, 867 Search PubMed.
- N. Hort, Y. Huang, D. Fechner, M. Stormer, C. Blawert, F. Witte, C. Vogt, H. Drucker, R. Willumeit, K. U. Kainer and F. Feyerabend, Magnesium alloys as implant materials-principles of property design for Mg-RE alloys, Acta Biomater., 2010, 6, 1714 CrossRef CAS PubMed.
- K. Schlüter, Z. Shi, C. Zamponi, F. Cao, E. Quandt and A. Atrens, Corrosion performance and mechanical properties of sputter-deposited MgY and MgGd alloys, Corros. Sci., 2014, 78, 43 CrossRef.
- W. Y. Wang, J. L. Zhu, N. N. Qin, Y. F. Zhang, S. Y. Li, Z. Xiao, Q. Lei and Z. Li, Effects of minor rare earths on the microstructure and properties of Cu–Cr–Zr alloy, J. Alloys Compd., 2020, 847, 155762 CrossRef CAS.
- M. F. Wang, D. H. Xiao, P. F. Zhou, W. S. Liu, Y. Z. Ma and B. R. Sun, Effects of rare earth yttrium on microstructure and properties of Mg Al Zn alloy, J. Alloys Compd., 2018, 742, 232 CrossRef CAS.
- H. F. Li, P. Y. Wang, G. C. Lin and J. Y. Huang, The role of rare earth elements in biodegradable metals: a review, Acta Biomater., 2021, 129, 33 CrossRef CAS PubMed.
- H. S. Brar, J. P. Ball, I. S. Berglund, J. B. Allen and M. V. Manuel, A study of a biodegradable Mg–3Sc–3Y alloy and the effect of self-passivation on the in vitro degradation, Acta Biomater., 2013, 9, 5331 CrossRef CAS PubMed.
- E. Aghion, G. Levy and S. Ovadia, In vivo behavior of biodegradable Mg–Nd–Y–Zr–Ca alloy, J. Mater. Sci.: Mater. Med., 2012, 23, 805 CrossRef CAS PubMed.
- X. Z. Song, L. Chang, J. Wang, S. J. Zhu, L. G. Wang, K. Feng, Y. G. Luo and S. K. Guan, Investigation on the in vitro cytocompatibility of Mg–Zn–Y–Nd–Zr alloys as degradable orthopaedic implant materials, J. Mater. Sci.: Mater. Med., 2018, 29, 44 CrossRef PubMed.
- A. Drynda, N. Deinet, N. Braun and M. Peuster, Rare earth metals used in biodegradable magnesium-based stents do not interfere with proliferation of smooth muscle cells but do induce the upregulation of inflammatory genes, J. Biomed. Mater. Res., Part A, 2009, 91, 360 CrossRef PubMed.
- N. Zhao, N. Watson, Z. G. Xu, Y. J. Chen, J. Waterman, J. Sankar and D. H. Zhu, In vitro biocompatibility and endothelialization of novel magnesium-rare Earth alloys for improved stent applications, PLoS One, 2014, 9, e98674 CrossRef PubMed.
- A. Myrissa, S. Braeuer, E. Martinelli, R. Willumeit-Romer, W. Goessler and A. M. Weinberg, Gadolinium accumulation in organs of Sprague-Dawley(R) rats after implantation of a biodegradable magnesium-gadolinium alloy, Acta Biomater., 2017, 48, 521 CrossRef CAS PubMed.
- F. Feyerabend, J. Fischer, J. Holtz, F. Witte, R. Willumeit, H. Drucker, C. Vogt and N. Hort, Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines, Acta Biomater., 2010, 6, 1834 CrossRef CAS PubMed.
- Y. F. Ding, J. X. Lin, C. Wen, D. M. Zhang and Y. C. Li, Mechanical properties, in vitro corrosion and biocompatibility of newly developed biodegradable Mg–Zr–Sr–Ho alloys for biomedical applications, Sci. Rep., 2016, 6, 31990 CrossRef CAS PubMed.
- E. Mendive-Tapia and J. B. Staunton, Theory of Magnetic Ordering in the Heavy Rare Earths: Ab Initio Electronic Origin of Pair- and Four-Spin Interactions, Phys. Rev. Lett., 2017, 118, 197202 CrossRef PubMed.
- K. P. Skokov and O. Gutfleisch, Heavy rare earth free, free rare earth and rare earth free magnets-Vision and reality, Scr. Mater., 2018, 154, 289 CrossRef CAS.
- S. B. Cheng, C. Berkdemir and A. W. Castleman Jr, Mimicking the magnetic properties of rare earth elements using superatoms, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4941 CrossRef CAS PubMed.
- S. Gabani, K. Flachbart, K. Siemensmeyer and T. Mori, Magnetism and superconductivity of rare earth borides, J. Alloys Compd., 2020, 821, 153201 CrossRef CAS.
- Y. Hinatsu and Y. Doi, Magnetic interactions in new fluorite-related rare earth oxides LnLn′2RuO7 (Ln, Ln′ = rare earths), J. Solid State Chem., 2016, 239, 214 CrossRef CAS.
- P. L. Gao, Y. H. Xia, J. Gong and X. Ju, Structure and Magnetic Properties of ErFexMn12−x (7.0 ≤ x ≤ 9.0, Deltax = 0.2), Nanomaterials, 2022, 9, 1586 CrossRef PubMed.
- A. S. Patil, A. V. Patil, C. G. Dighavkar, V. A. Adole and U. J. Tupe, Synthesis techniques and applications of rare earth metal oxides semiconductors: a review, Chem. Phys. Lett., 2022, 796, 139555 CrossRef CAS.
- T. H. Wang, Z. K. Ma, Q. H. Zhu, L. J. Yang, B. S. Liu, Y. Zhao, C. Xu, B. Z. Zheng, F. Q. Hu, J. Z. Li, Q. F. Huang and Z. L. Song, Effect of aluminum on microstructure and magnetic properties of sintered Nd-Fe-B magnets processed by grain boundary diffusion of Tb-Al, J. Rare Earths, 2022, 4, 017 Search PubMed.
- H. Nakamura, The current and future status of rare earth permanent magnets, Scr. Mater., 2018, 154, 273 CrossRef CAS.
- H. Wakayama and H. Yonekura, Use of block copolymer templates for chemical synthesis of Nd2Fe14B nanocomposites with controlled magnetic properties, Mater. Chem. Phys., 2019, 227, 265 CrossRef CAS.
- J. J. Xu, K. Zhu, S. Gao and Y. L. Hou, Rare earth permanent magnetic nanostructures: chemical design and microstructure control to optimize magnetic properties, Inorg. Chem. Front., 2021, 8, 383 RSC.
- Y. L. Hou, Z. C. Xu, S. Peng, C. Rong, J. P. Liu and S. Sun, A Facile Synthesis of SmCo5 Magnets from Core/Shell Co/Sm2O3 Nanoparticles, Adv. Mater., 2007, 19, 3349 CrossRef CAS.
- Z. H. Ma, S. X. Yang, T. L. Zhang and C. B. Jiang, The chemical synthesis of SmCo5 single-crystal particles with small size and high performance, Chem. Eng. J., 2016, 304, 993 CrossRef CAS.
- F. Lu, S. Xu and L. H. Wang, Pressure-induced magnetic transition in Nd2Fe14B based on two-sublattice model, Rare Met., 2019, 41, 232 CrossRef.
- P. K. Deheri, V. Swaminathan, S. D. Bhame, Z. W. Liu and R. V. Ramanujan, Sol-Gel Based Chemical Synthesis of Nd2Fe14B Hard Magnetic Nanoparticles, Chem. Mater., 2010, 22, 6509 CrossRef CAS.
- C. Q. Chen, D. Kim and C. Choi, Influence of Ca amount on the synthesis of Nd2Fe14B particles in reduction–diffusion process, J. Magn. Magn. Mater., 2014, 355, 180 CrossRef CAS.
- K. Zhu, J. J. Xu, X. B. Wang, W. Li, K. S. Tian and Y. L. Hou, Chemical synthesis and coercivity enhancement of Nd2Fe14B nanostructures mediated by non-magnetic layer, Nano Res., 2020, 13, 1141 CrossRef CAS.
- A. Pal, A. Gabay and G. C. Hadjipanayis, Mechanochemical synthesis of Nd2Fe14B alloy with high coercivity, J. Alloys Compd., 2012, 543, 31 CrossRef CAS.
- B. Z. Cui, L. Y. Zheng, W. F. Li, J. F. Liu and G. C. Hadjipanayis, Single-crystal and textured polycrystalline Nd2Fe14B flakes with a submicron or nanosize thickness, Acta Mater., 2012, 60, 1721 CrossRef CAS.
- L. Q. Yu, C. Yang and Y. L. Hou, Controllable Nd2Fe14B/α-Fe nanocomposites: chemical synthesis and magnetic properties, Nanoscale, 2014, 6, 10638 RSC.
- H. Parmar, T. Xiao, V. Chaudhary, Y. Zhong and R. V. Ramanujan, High energy product chemically synthesized exchange coupled Nd2Fe14B/α-Fe magnetic powders, Nanoscale, 2017, 9, 13956 RSC.
- K. A. Gschneidner and V. K. Pecharsky, Rare Earths and Magnetic Refrigeration, J. Rare Earths, 2006, 24, 641 CrossRef.
- L. W. Li and M. Yan, Recent progresses in exploring the rare earth based intermetallic compounds for cryogenic magnetic refrigeration, J. Alloys Compd., 2020, 823, 153810 CrossRef CAS.
- L. W. Li and M. Yan, Recent progress in the magnetic and cryogenic magnetocaloric properties of RE2TMTMO6 double perovskite oxides, J. Mater. Sci. Technol., 2022, 1, 041 CrossRef.
- L. H. Yin, J. Yang, P. Tong, X. Luo, C. B. Park, K. W. Shin, W. H. Song, J. M. Dai, K. H. Kim, X. B. Zhu and Y. P. Sun, Role of rare earth ions in the magnetic, magnetocaloric and magnetoelectric properties of RCrO3 (R = Dy, Nd, Tb, Er) crystals, J. Mater. Chem. C, 2016, 4, 11198 RSC.
- L. H. Yin, J. Yang, X. C. Kan, W. H. Song, J. M. Dai and Y. P. Sun, Giant magnetocaloric effect and temperature induced magnetization jump in GdCrO3 single crystal, J. Appl. Phys., 2015, 117, 133901 CrossRef.
- Y. He, S. H. Guo, K. H. Chen, S. W. Li, L. B. Zhang and S. H. Yin, Sustainable green production: a review of recent development on rare earths extraction and separation using microreactors, ACS Sustainable Chem. Eng., 2019, 7, 17616 CrossRef CAS.
- P. Sarfo, T. Frasz, A. Das and C. Young, Hydrometallurgical Recovery and Process Optimization of Rare Earth Fluorides from Recycled Magnets, Minerals, 2020, 21, 17616 Search PubMed.
- H. G. Danielmeyer, Efficiency and fluorescence quenching of stoichiometric rare earth laser materials, J. Lumin., 1976, 12, 179 CrossRef.
- H. Kang, J. L. Peng, Z. Zhang and W. Zhou, Fluorescent strengthening effect of co-doped inert rare earth ions (La3+, Gd3+, Lu3+) on white-light-emitting of Eu-Tb coordination polymers, J. Lumin., 2022, 247, 118904 CrossRef CAS.
- S. K. Gupta, K. Sudarshan and R. M. Kadam, Optical nanomaterials with focus on rare earth doped oxide: a review, Mater. Today Commun., 2021, 27, 112277 Search PubMed.
- C. Kränkel, D. T. Marzahl, F. Moglia, G. Huber and P. W. Metz, Out of the blue: semiconductor laser pumped visible rare-earth doped lasers, Laser Photonics Rev., 2016, 10, 548 CrossRef.
- Z. F. Wang, W. W. Ye, X. R. Luo and Z. G. Wang, Fabrication of Superhydrophobic and Luminescent Rare Earth/Polymer complex Films, Sci. Rep., 2016, 6, 24682 CrossRef PubMed.
- L. J. He, J. L. Meng, J. Feng, Z. X. Zhang, X. J. Liu and H. J. Zhang, Insight into the Characteristics of 4f-Related Electronic Transitions for Rare-Earth-Doped KLuS2 Luminescent Materials through First-Principles Calculation, J. Phys. Chem. C, 2019, 124, 932 CrossRef.
- S. V. Eliseeva and J. C. G. Bünzli, Rare earths: jewels for functional materials of the future, New J. Chem., 2011, 35, 1165 RSC.
- Q. S. Wang, J. J. Li, M. N. Zhang and X. Li, A luminescent Eu(III)-based metal–organic framework as a highly effective sensor for cation and anion detections, Sens. Actuators, B, 2018, 258, 358 CrossRef CAS.
- R. Shi, B. H. Zhu, M. X. Hu, H. F. Zhou, J. M. Fan, Z. Tao, F. Chang and S. Y. Yu, Graphene oxide induced multi-layered six-petal flower-shaped rare earth Tb3+ hybrid luminescent material: synthesis, characterization, luminescence and fluorescence anti-counterfeiting properties, J. Mater. Chem. C, 2020, 8, 2336 RSC.
- Y. Wang, L. H. Wang, S. Y. Bao, L. Xu, J. D. Zhang, Y. Y. Liang, L. S. Wang, X. J. Liang and W. D. Xiang, High-performance and heat-resistant Ce:YAG phosphor in glass for laser lighting, J. Alloys Compd., 2022, 921, 166083 CrossRef CAS.
- S. Mukherjee, N. Pathak, K. Ali, D. Das and D. Dutta, Tailoring defect structure and dopant composition and the generation of various color characteristics in Eu3+ and Tb3+ doped MgF2 phosphors, Phys. Chem. Chem. Phys., 2022, 24, 10915 RSC.
- K. C. Wang, B. J. Zhao and L. Gao, X-ray photoemission spectroscopy investigation of CaTiO3:Eu for luminescence property: effect of Eu3+ ion, Mater. Res. Bull., 2016, 78, 31 CrossRef CAS.
- X. Pan, L. F. Mei, Y. H. Wang, T. Seto, Y. X. Zhuang, Q. F. Guo, M. Plyaskin, W. Xi, C. Li, Y. S. Guo and L. B. Liao, Activators lattice migration strategy customized for tunable luminescence of Ce3+ doped β-Ca3(PO4)2, Chem. Eng. J., 2022, 446, 137271 CrossRef CAS.
- J. Zhao, D. J. Mei, W. K. Wang, Y. D. Wu and D. F. Xue, Recent advances in nonlinear optical rare earth structures, J. Rare Earths, 2021, 39, 1455 CrossRef CAS.
- T. Justel, C. R. Ronda and H. Nikol, Rare earth phosphors: fundamentals and applications, J. Alloys Compd., 1998, 275, 669 Search PubMed.
- A. Escudero, A. I. Becerro, C. Carrillo-Carrión, N. O. Núñez, M. V. Zyuzin, M. Laguna, D. González-Mancebo, M. Ocaña and W. J. Parak, Rare earth based nanostructured materials: synthesis, functionalization, properties and bioimaging and biosensing applications, Nanophotonics, 2017, 6, 881 CAS.
- Z. Y. Wang, L. S. Huang, C. C. Zhang, M. Y. Chuai, X. H. Zhang and M. Z. Zhang, The optical properties of Ce-doped SnS quantum dots with fast nanosecond lifetime, J. Alloys Compd., 2019, 799, 425 CrossRef CAS.
- S. Silvi, M. Baroncini, M. La Rosa and A. Credi, Interfacing Luminescent Quantum Dots with Functional Molecules for Optical Sensing Applications, Top. Curr. Chem., 2016, 374, 65 CrossRef PubMed.
- B. Yan, H. C. Wu, P. T. Ma, J. Y. Niu and J. P. Wang, Recent advances in rare earth co-doped luminescent tungsten oxygen complexes, Inorg. Chem. Front., 2021, 8, 4158 RSC.
- W. C. Zhan, Y. Guo, X. Q. Gong, Y. L. Guo, Y. Q. Wang and G. Z. Lu, Current status and perspectives of rare earth catalytic materials and catalysis, Chin. J. Catal., 2014, 35, 1238 CrossRef CAS.
- Z. Q. Hou, W. B. Pei, X. Zhang, K. F. Zhang, Y. X. Liu, J. G. Deng, L. Jing and H. X. Dai, Rare earth oxides and their supported noble metals in application of environmental catalysis, J. Rare Earths, 2020, 38, 819 CrossRef CAS.
- S. Zhang, S. E. Saji, Z. Yin, H. Zhang, Y. Du and C. H. Yan, Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications, Adv. Mater., 2021, 33, e2005988 CrossRef PubMed.
- X. C. Sun, K. Yuan and Y. W. Zhang, Advances and prospects of rare earth metal–organic frameworks in catalytic applications, J. Rare Earths, 2020, 38, 801 CrossRef CAS.
- R. Burch, Knowledge and Know-How in Emission Control for Mobile Applications, Catal. Rev., 2011, 46, 271 CrossRef.
- T. Fujita, H. Abe, T. Tanabe, Y. Ito, T. Tokunaga, S. Arai, Y. Yamamoto, A. Hirata and M. Chen, Earth-Abundant and Durable Nanoporous Catalyst for Exhaust-Gas Conversion, Adv. Funct. Mater., 2016, 26, 1609 CrossRef CAS.
- H. Tanaka, I. Tan, M. Uenishi, M. Taniguchi, M. Kimura, Y. Nishihata and J. i. Mizuki, LaFePdO3 perovskite automotive catalyst having a self-regenerative function, J. Alloys Compd., 2006, 408, 1071 CrossRef.
- Q. Hu, K. Cao, Y. Lang, R. Chen, S. Q. Chu, L. W. Jia, J. Yue and B. Shan, Improved NO-CO reactivity of highly dispersed Pt particles on CeO2 nanorod catalysts prepared by atomic layer deposition, Catal. Sci. Technol., 2019, 9, 2664 RSC.
- Y. W. Jiang, J. H. Gao, Q. Zhang, Z. Y. Liu, M. L. Fu, J. L. Wu, Y. Hu and D. Q. Ye, Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template, Chem. Eng. J., 2019, 371, 78 CrossRef CAS.
- Y. Y. Wang, D. H. Wu, P. Lv, B. L. He, X. Li, D. W. Ma and Y. Jia, Theoretical insights into the electroreduction of nitrate to ammonia on the graphene-based single-atom catalyst, Nanoscale, 2022, 14(30), 10862 RSC.
- C. X. Wang, W. Z. Xia, D. X. Yang, T. T. Zheng, Y. J. Rong, J. C. Du, B. X. Wu and Y. K. Zhao, Understanding ammonia and nitrous oxide formation in typical three-way catalysis during the catalyst warm-up period, J. Hazard. Mater., 2022, 438, 129553 CrossRef CAS PubMed.
- H. Liu, Ammonia synthesis catalyst 100 years: practice, enlightenment and challenge, Chin. J. Catal., 2014, 35, 1619 CrossRef CAS.
- J. Fuller, A. Fortunelli, W. A. Goddard and Q. An, Reaction mechanism and kinetics for ammonia synthesis on the Fe(211) reconstructed surface, Phys. Chem. Chem. Phys., 2019, 21, 11444 RSC.
- J. Ji, X. Yan, G. Qian, C. Peng, X. Duan and X. Zhou, Morphology and location manipulation of Fe nanoparticles on carbon nanofibers as catalysts for ammonia decomposition to generate hydrogen, Int. J. Hydrogen Energy, 2017, 42, 17466 CrossRef CAS.
- W. W. E. Takeshita T and R. S. Craig, Rare earth intermetallics as synthetic ammonia catalysts, J. Catal., 1976, 44, 236 CrossRef.
- A. K. I. Niwa Y, The promoter effect of lanthana on MgO supported ruthenium catalysts for ammonia synthesis, Res. Chem. Intermed., 1998, 24, 593 CrossRef.
- K. Okura, T. Okanishi, H. Muroyama, T. Matsui and K. Eguchi, Ammonia Decomposition over Nickel Catalysts Supported on Rare-Earth Oxides for the On-Site Generation of Hydrogen, ChemCatChem, 2016, 8, 2988 CrossRef CAS.
- W. Li, S. Wang and J. P. Li, Effect of rare earth elements (La, Y, Pr) in multi-element composite perovskite oxide supports for ammonia synthesis, J. Rare Earths, 2021, 39, 427 CrossRef CAS.
- J. Baltrusaitis, Sustainable Ammonia Production, ACS Sustainable Chem. Eng., 2017, 5, 9527 CrossRef CAS.
- S. Ghavam, M. Vahdati, I. A. G. Wilson and P. Styring, Sustainable Ammonia Production Processes, Front. Energy Res., 2021, 9, 580808 CrossRef.
- A. Kovač, M. Paranos and D. Marciuš, Hydrogen in energy transition: a review, Int. J. Hydrogen Energy, 2021, 46, 10016 CrossRef.
- J. O. Abe, A. P. I. Popoola, E. Ajenifuja and O. M. Popoola, Hydrogen energy, economy and storage: review and recommendation, Int. J. Hydrogen Energy, 2019, 44, 15072 CrossRef CAS.
- T. Y. Wei, K. L. Lim, Y. S. Tseng and S. L. I. Chan, A review on the characterization of hydrogen in hydrogen storage materials, Renewable Sustainable Energy Rev., 2017, 79, 1122 CrossRef CAS.
- A. Kumar, P. Muthukumar, P. Sharma and E. A. Kumar, Absorption based solid state hydrogen storage system: a review, Sustain. Energy Technol. Assessments, 2022, 52, 102204 CrossRef.
- M. Bououdina, D. Grant and G. Walker, Review on hydrogen absorbing materials-structure, microstructure, and thermodynamic properties, Int. J. Hydrogen Energy, 2006, 31, 177 CrossRef CAS.
- L. Z. Ouyang, F. Liu, H. Wang, J. W. Liu, X. S. Yang, L. X. Sun and M. Zhu, Magnesium-based hydrogen storage compounds: a review, J. Alloys Compd., 2020, 832, 154865 CrossRef CAS.
- S. Kumar, M. Samolia and T. J. Dhilip Kumar, Hydrogen Storage in Sc and Li Decorated Metal–Inorganic Framework, ACS Appl. Energy Mater., 2018, 1, 1328 CrossRef CAS.
- T. Sadhasivam, H. T. Kim, S. Jung, S. H. Roh, J. H. Park and H. Y. Jung, Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: a review, Renewable Sustainable Energy Rev., 2017, 72, 523 CrossRef CAS.
- H. Chaib, L. Mohammedi, L. Benmebrouk, A. Boukraa, B. Daoudi and A. Achouri, Effect of metal atom substitutions in Li based hydrides for hydrogen storage, Int. J. Hydrogen Energy, 2020, 45, 28920 CrossRef CAS.
- Y. D. Chen, S. Yu, W. H. Zhao, S. F. Li and X. M. Duan, A potential material for hydrogen storage: a Li decorated graphitic-CN monolayer, Phys. Chem. Chem. Phys., 2018, 20, 13473 RSC.
- C. Briki, P. de Rango, S. Belkhiria, M. H. Dhaou and A. Jemni, Measurements of expansion of LaNi5 compacted powder during hydrogen absorption/desorption cycles and their influences on the reactor wall, Int. J. Hydrogen Energy, 2019, 44, 13647 CrossRef CAS.
- W. Liu and K. F. Aguey-Zinsou, Low temperature synthesis of LaNi5 nanoparticles for hydrogen storage, Int. J. Hydrogen Energy, 2016, 41, 1679 CrossRef CAS.
- Y. Wu, H. G. Yu, Y. R. Guo, Y. X. Zhang, X. J. Jiang, B. X. Sun, K. Fu, J. Chen, Y. Qi, J. Zheng and X. G. Li, Promoting hydrogen absorption of liquid organic hydrogen carriers by solid metal hydrides, J. Mater. Chem. A, 2019, 7, 16677 RSC.
- H. Shi, G. L. Liu and X. H. Hu, Accelerating electrochemical hydrogen evolution kinetics in alkaline media using LaNi5 as a hydrogen reservoir, Chem. Commun., 2022, 58, 7289 RSC.
- T. von Waldkirch and P. Zürcher, Surface segregation in LaNi5 induced by oxygen, Appl. Phys. Lett., 1978, 33, 689 CrossRef CAS.
- K. Goto, T. Hirata, I. Yamamoto and W. Nakao, Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In Situ Displacement Measurement during Hydrogen Pressure Change, Molecules, 2019, 24, 2420 CrossRef CAS PubMed.
- H. G. Yu, Y. Wu, S. P. Chen, Z. W. Xie, Y. M. Wu, N. Cheng, X. Yang, W. Lin, L. Xie, X. G. Li and J. Zheng, Pd-modified LaNi5 nanoparticles for efficient hydrogen storage in a carbazole type liquid organic hydrogen carrier, Appl. Catal., B, 2022, 317, 121720 CrossRef CAS.
- M. Melnichuk, D. J. Cuscueta and N. Silin, LaNi5 hydride powder flowability as a function of activation and hydrogen content, Int. J. Hydrogen Energy, 2017, 42, 15799 CrossRef CAS.
- Y. Ye, Y. Yue, J. F. Lu, J. Ding, W. L. Wang and J. Y. Yan, Enhanced hydrogen storage of a LaNi5 based reactor by using phase change materials, Renewable Energy, 2021, 180, 734 CrossRef CAS.
- J. M. Joubert, V. Paul-Boncour, F. Cuevas, J. Zhang and M. Latroche, LaNi5 related AB5 compounds: structure, properties and applications, J. Alloys Compd., 2021, 862, 158163 CrossRef CAS.
- H. Cao, P. Georgopanos, G. Capurso, C. Pistidda, F. Weigelt, A. L. Chaudhary, V. Filiz, J. C. Tseng, M. T. Wharmby, M. Dornheim, V. Abetz and T. Klassen, Air-stable metal hydride-polymer composites of Mg(NH2)2-LiH and TP, Mater. Today Energy, 2018, 10, 98 CrossRef.
- L. Schlapbach, A. Züttel, P. Gröning, O. Gröning and P. Aebi, Hydrogen for novel materials and devices, Appl. Phys. A: Mater. Sci. Process., 2001, 72, 245 CrossRef CAS.
- K. J. Jeon, H. R. Moon, A. M. Ruminski, B. Jiang, C. Kisielowski, R. Bardhan and J. J. Urban, Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts, Nat. Mater., 2011, 10, 286 CrossRef CAS PubMed.
- H. Liang, D. D. Chen, M. F. Chen, W. J. Li and R. Snyders, Study of the synthesis of PMMA-Mg nanocomposite for hydrogen storage application, Int. J. Hydrogen Energy, 2020, 45, 4743 CrossRef CAS.
- R. Pedicini, B. Schiavo, P. Rispoli, A. Saccà, A. Carbone, I. Gatto and E. Passalacqua, Progress in polymeric material for hydrogen storage application in middle conditions, Energy, 2014, 64, 607 CrossRef CAS.
- R. Pedicini, A. Saccà, A. Carbone and E. Passalacqua, Hydrogen storage based on polymeric material, Int. J. Hydrogen Energy, 2011, 36, 9062 CrossRef CAS.
- G. R. d. Almeida Neto, C. A. Gonçalves Beatrice, D. R. Leiva and L. A. Pessan, Polymer-based composite containing nanostructured LaNi5 for hydrogen storage: improved air stability and processability, Int. J. Hydrogen Energy, 2020, 45, 14017 CrossRef CAS.
- K. C. Majhi and M. Yadav, Facile hydrothermal synthesis of rare earth phosphate for boosting hydrogen evolution reaction, Int. J. Hydrogen Energy, 2022, 47, 14092 CrossRef CAS.
- S. Suwarno, M. V. Lototskyy and V. A. Yartys, Thermal desorption spectroscopy studies of hydrogen desorption from rare earth metal trihydrides REH3 (RE = Dy, Ho, Er), J. Alloys Compd., 2020, 842, 155530 CrossRef CAS.
- J. J. Liu, K. Li, H. H. Cheng, K. Yan, Y. Wang, Y. Liu, H. M. Jin and Z. Zheng, New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5-Al alloys, Int. J. Hydrogen Energy, 2017, 42, 24904 CrossRef CAS.
- L. J. Y. Han J I, Hybriding kinetics of LaNi5 and LaNi4.7Al0.3, Int. J. Hydrogen Energy, 1989, 14, 181 CrossRef.
- S. Todorova, B. Abrashev, V. Rangelova, L. Mihaylov, E. Vassileva, K. Petrov and T. Spassov, Hydrogen Gas Phase and Electrochemical Hydriding of LaNi5−xMx (M = Sn, Co, Al) Alloys, Materials, 2020, 14, 14 CrossRef PubMed.
- Z. Łodziana, A. Dębski, G. Cios and A. Budziak, Ternary LaNi4.75M0.25 hydrogen storage alloys: surface segregation, hydrogen sorption and thermodynamic stability, Int. J. Hydrogen Energy, 2019, 44, 1760 CrossRef.
- F. F. Azenwi, H. W. Langmi and G. Sean McGrady, Hydrogenation of LiH/Al catalyzed with TiN, TiMn2 and LaNi5, Int. J. Hydrogen Energy, 2012, 37, 10210 CrossRef CAS.
- X. Ma, X. Wei, H. Dong and Y. Liu, The relationship between discharge capacity of LaNi5 type hydrogen storage alloys and formation enthalpy, J. Alloys Compd., 2010, 490, 548 CrossRef CAS.
- S. Nowak and M. Winter, Elemental analysis of lithium ion batteries, J. Anal. At. Spectrom., 2017, 32, 1833 RSC.
- T. C. Ouyang, B. L. Liu, P. H. Xu, C. C. Wang and J. Ye, Electrochemical-thermal coupled modelling and multi-measure prevention strategy for Li-ion battery thermal runaway, Int. J. Heat Mass Transfer, 2022, 194, 123082 CrossRef CAS.
- S. Sahoo, R. Kumar, E. Joanni, R. K. Singh and J. J. Shim, Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors, J. Mater. Chem. A, 2022, 10, 13190 RSC.
- L. Z. Zhou, H. Yang, T. T. Han, Y. Z. Song, G. T. Yang and L. S. Li, Carbon-Based Modification Materials for Lithium-ion Battery Cathodes: Advances and Perspectives, Front. Chem., 2022, 10, 914930 CrossRef CAS PubMed.
- S. Zhang, W. T. Deng, R. Momen, S. Y. Yin, J. Chen, A. Massoudi, G. Zou, H. S. Hou, W. N. Deng and X. B. Ji, Element substitution of a spinel LiMn2O4 cathode, J. Mater. Chem. A, 2021, 9, 21532 RSC.
- Z. Wang, J. T. Zhang, F. H. Dong, P. D. Liu, Y. M. Zhu, P. Gao, X. X. Huang and G. W. Wen, Density functional theory guidance on rare earth doping-inhibition of lattice oxygen evolution in lithium-rich layered manganese oxide materials, J. Alloys Compd., 2022, 899, 163311 CrossRef CAS.
- J. Henao, O. Sotelo, M. Casales-Diaz and L. Martinez-Gomez, Hydrogen storage in a rare-earth perovskite-type oxide La0.6Sr0.4Co0.2Fe0.8O3 for battery applications, Rare Met., 2018, 37, 1003 CrossRef CAS.
- L. Xue, Q. H. Zhang, X. H. Zhu, L. Gu, J. L. Yue, Q. Y. Xia, T. Xing, T. T. Chen, Y. Yao and H. Xia, 3D LiCoO2 nanosheets assembled nanorod arrays via confined dissolution-recrystallization for advanced aqueous lithium-ion batteries, Nano Energy, 2019, 56, 463 CrossRef CAS.
- Q. H. Zhou, H. H. Xu, l. Lu, W. H. Liu, Y. Liang, H. L. Li and T. Chen, Study on the decline mechanism of cathode material LiCoO2 for Li-ion battery, Vacuum, 2020, 177, 109313 CrossRef CAS.
- Q. Liu, X. Su, D. Lei, Y. Qin, J. G. Wen, F. M. Guo, Y. A. Wu, Y. C. Rong, R. H. Kou, X. H. Xiao, F. Aguesse, J. Bareño, Y. Ren, W. Q. Lu and Y. X. Li, Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping, Nat. Energy, 2018, 3, 936 CrossRef CAS.
- R. Kataoka, N. Taguchi, T. Kojima, N. Takeichi and T. Kiyobayashi, Improving the oxygen redox stability of NaCl-type cation disordered Li2MnO3 in a composite structure of Li2MnO3 and spinel-type LiMn2O4, J. Mater. Chem. A, 2019, 7, 5381 RSC.
- J. Lu, C. Zhan, T. Wu, J. Wen, Y. Lei, A. J. Kropf, H. Wu, D. J. Miller, J. W. Elam, Y. K. Sun, X. Qiu and K. Amine, Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach, Nat. Commun., 2014, 5, 5693 CrossRef CAS PubMed.
- P. Ram, A. Gören, S. Ferdov, M. M. Silva, R. Singhal, C. M. Costa, R. K. Sharma and S. Lanceros-Méndez, Improved performance of rare earth doped LiMn2O4 cathodes for lithium-ion battery applications, New J. Chem., 2016, 40, 6244 RSC.
- R. Singhal, S. R. Das, M. S. Tomar, O. Ovideo, S. Nieto, R. E. Melgarejo and R. S. Katiyar, Synthesis and characterization of Nd doped LiMn2O4 cathode for Li-ion rechargeable batteries, J. Power Sources, 2007, 164, 857 CrossRef CAS.
- L. Xia, K. H. Qiu, Y. Y. Gao, X. He and F. D. Zhou, High potential performance of Cerium-doped LiNi0.5Co0.2Mn0.3O2 cathode material for Li-ion battery, J. Mater. Sci., 2015, 50, 2914 CrossRef CAS.
- Y. W. Tian, X. X. Kang, L. Y. Liu, C. Q. Xu and T. Qu, Research on cathode material of Li-ion battery by yttrium doping, J. Rare Earths, 2008, 26, 279 CrossRef.
- I. Rabani, Y. J. Park, J. W. Lee, M. S. Tahir, A. Kumar and Y. S. Seo, Ultra-thin flexible paper of BNNT–CNF/ZnO ternary nanostructure for enhanced solid-state supercapacitor and piezoelectric response, J. Mater. Chem. A, 2022, 10(29), 15580 RSC.
- K. F. Chen, G. Li and D. F. Xue, Architecture engineering of supercapacitor electrode materials, Funct. Mater. Lett., 2016, 9, 1640001 CrossRef CAS.
- D. C. Grahame, Properties of the electrical double layer at a mercury surface. I. Methods of measurement and interpretation of results, J. Am. Chem. Soc., 1941, 63, 1207 CrossRef CAS.
- B. E. Conway, Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage, J. Electrochem. Soc., 1991, 138, 1539 CrossRef CAS.
- V. Augustyn, P. Simon and B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage, Energy Environ. Sci., 2014, 7(5), 1597 RSC.
- Y. Zhou, H. L. Qi, J. Y. Yang, Z. Bo, F. Huang, M. S. Islam, X. Y. Lu, L. M. Dai, R. Amal, C. H. Wang and Z. J. Han, Two-birds-one-stone: multifunctional supercapacitors beyond traditional energy storage, Energy Environ. Sci., 2021, 14, 1854 RSC.
- G. Z. Chen, Supercapacitor and supercapattery as emerging electrochemical energy stores, Int. Mater. Rev., 2016, 62, 173 CrossRef.
- A. Laheäär, P. Przygocki, Q. Abbas and F. Béguin, Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors, Electrochem. Commun., 2015, 60, 21 CrossRef.
- P. Cai, K. Y. Zou, X. L. Deng, B. W. Wang, M. Zheng, L. H. Li, H. S. Hou, G. Q. Zou and X. B. Ji, Comprehensive Understanding of Sodium-Ion Capacitors: Definition, Mechanisms, Configurations, Materials, Key Technologies, and Future Developments, Adv. Energy Mater., 2021, 11, 2003804 CrossRef CAS.
- T. Schoetz, L. W. Gordon, S. Ivanov, A. Bund, D. Mandler and R. J. Messinger, Disentangling faradaic, pseudocapacitive, and capacitive charge storage: a tutorial for the characterization of batteries, supercapacitors, and hybrid systems, Electrochim. Acta, 2022, 412, 140072 CrossRef CAS.
- B. E. Conway and E. Gileadi, Kinetic theory of pseudo-capacitance and electrode reactions at appreciable surface coverage, Trans. Faraday Soc., 1962, 58, 2494 Search PubMed.
- H. L. Yang, H. H. Xu, L. B. Wang, L. Zhang, Y. L. Huang and X. H. Hu, Microwave-Assisted Rapid Synthesis of Self-Assembled T-Nb2O5 Nanowires for High-Energy Hybrid Supercapacitors, Chemistry, 2017, 23, 4203 CrossRef CAS PubMed.
- X. Q. Bin, Y. P. Tian, Y. Y. Luo, M. H. Sheng, Y. J. Luo, M. M. Ju and W. X. Que, High-performance flexible and free-standing N-doped Ti3C2T/MoO films as electrodes for supercapacitors, Electrochim. Acta, 2021, 389, 38774 CrossRef.
- R. Ma, Z. T. Chen, D. N. Zhao, X. J. Zhang, J. T. Zhuo, Y. J. Yin, X. F. Wang, G. W. Yang and F. Yi, Ti3C2Tx MXene for electrode materials of supercapacitors, J. Mater. Chem. A, 2021, 9, 11501 RSC.
- M. M. Hu, H. Zhang, T. Hu, B. B. Fan, X. H. Wang and Z. J. Li, Emerging 2D MXenes for supercapacitors: status, challenges and prospects, Chem. Soc. Rev., 2020, 49, 6666 RSC.
- H. Y. Zhao, J. L. Xia, D. D. Yin, M. Luo, C. H. Yan and Y. P. Du, Rare earth incorporated electrode materials for advanced energy storage, Coord. Chem. Rev., 2019, 390, 32 CrossRef CAS.
- D. B. Bailmare, P. Tripathi, A. D. Deshmukh and B. K. Gupta, Designing of two dimensional lanthanum cobalt hydroxide engineered high performance supercapacitor for longer stability under redox active electrolyte, Sci. Rep., 2022, 12, 3084 CrossRef CAS PubMed.
- K. M. Thulasi, S. T. Manikkoth, A. Paravannoor, S. Palantavida, M. Bhagiyalakshmi and B. K. Vijayan, Ceria deposited titania nanotubes for high performance supercapacitors, J. Phys. Chem. Solids, 2019, 135, 109111 CrossRef CAS.
- A. K. Das, U. N. Pan, V. Sharma, N. H. Kim and J. H. Lee, Nanostructured CeO2/NiV–LDH composite for energy storage in asymmetric supercapacitor and as methanol oxidation electrocatalyst, Chem. Eng. J., 2021, 417, 128019 CrossRef CAS.
- S. F. Liang, H. Y. Wang, Y. Li, H. Z. Qin, Z. Y. Luo, B. Huang, X. Zhao, C. L. Zhao and L. Y. Chen, Rare-earth based nanomaterials and their composites as electrode materials for high performance supercapacitors: a review, Sustainable Energy Fuels, 2020, 4, 3825 RSC.
- J. D. Wang, X. Xiao, Y. Liu, K. M. Pan, H. Pang and S. Z. Wei, The application of CeO2-based materials in electrocatalysis, J. Mater. Chem. A, 2019, 7, 17675 RSC.
- C. W. Sun, H. Li and L. Q. Chen, Nanostructured ceria-based materials: synthesis, properties, and applications, Energy Environ. Sci., 2012, 5, 8475 RSC.
- N. Maheswari and G. Muralidharan, Supercapacitor Behavior of Cerium Oxide Nanoparticles in Neutral Aqueous Electrolytes, Energy Fuels, 2015, 29, 8246 CrossRef CAS.
- L. Wang, Y. H. Li, M. Shi, W. L. Ma and H. T. Cui, Synthesis of CeO2 nanocrystals with controlled size and shape and their influence on electrochemical performance, J. Sol-Gel Sci. Technol., 2017, 83(2), 308 CrossRef CAS.
- X. D. Hao, S. Zhang, Y. Xu, L. Y. Tang, K. Inoue, M. Saito, S. F. Ma, C. L. Chen, B. S. Xu, T. Adschiri and Y. Ikuhara, Surfactant-mediated morphology evolution and self-assembly of cerium oxide nanocrystals for catalytic and supercapacitor applications, Nanoscale, 2021, 13, 10393 RSC.
- C. X. Cheng, F. Chen, H. Y. Yi and G. S. Lai, CeO2 mesoporous microspheres for high performance supercapacitors and lithium-ion batteries, J. Energy Storage, 2021, 35, 102305 CrossRef.
- A. J. Khan, M. Hanif, M. S. Javed, S. Hussain, W. J. Zhong, M. Saleem and Z. W. Liu, Energy storage properties of hydrothermally processed, nanostructured, porous CeO2 nanoparticles, J. Electroanal. Chem., 2020, 865, 114158 CrossRef CAS.
- N. Maheswari and G. Muralidharan, Hexagonal CeO2 nanostructures: an efficient electrode material for supercapacitors, Dalton Trans., 2016, 45, 14352 RSC.
- K. Prasanna, P. Santhoshkumar, Y. N. Jo, I. N. Sivagami, S. H. Kang, Y. C. Joe and C. W. Lee, Highly porous CeO2 nanostructures prepared via combustion synthesis for supercapacitor applications, Appl. Surf. Sci., 2018, 449, 454 CrossRef CAS.
- M. Kumar, J. H. Yun, V. Bhatt, B. Singh, J. Kim, J. S. Kim, B. S. Kim and C. Y. Lee, Role of Ce3+ valence state and surface oxygen vacancies on enhanced electrochemical performance of single step solvothermally synthesized CeO2 nanoparticles, Electrochim. Acta, 2018, 284, 709 CrossRef CAS.
- G. Nabi, W. Ali, A. Majid, T. Alharbi, S. Saeed and M. A. Albedah, Controlled growth of Bi-functional La doped CeO2 nanorods for photocatalytic H2 production and supercapacitor applications, Int. J. Hydrogen Energy, 2022, 47, 15480 CrossRef CAS.
- T. Kar, M. Casales-Diaz, J. J. Ramos-Hernandez, O. Sotelo-Mazon, J. Henao, S. Valdez Rodriguez, S. Godavarthi, S. Liu, Y. Yamauchi and M. K. Kesarla, CeO2−x quantum dots decorated nitrogen-doped hollow porous carbon for supercapacitors, J. Colloid Interface Sci., 2022, 622, 147 CrossRef CAS PubMed.
- Y. Luo, T. Y. Yang, Q. Zhao and M. Z. Zhang, CeO2/CNTs hybrid with high performance as electrode materials for supercapacitor, J. Alloys Compd., 2017, 729, 64 CrossRef CAS.
- W. J. Wu, W. T. Qi, Y. F. Zhao, X. Tang, Y. F. Qiu, D. W. Su, H. B. Fan and G. X. Wang, Hollow CeO2 spheres conformally coated with graphitic carbon for high-performance supercapacitor electrodes, Appl. Surf. Sci., 2019, 463, 244 CrossRef CAS.
- B. Ramulu, G. Nagaraju, S. C. Sekhar and J. S. Yu, Highly porous CNTs knotted cerium oxide hollow tubes with exalted energy storage performance for hybrid supercapacitors, J. Alloys Compd., 2020, 819, 152942 CrossRef CAS.
- A. Jeyaranjan, T. S. Sakthivel, C. J. Neal and S. Seal, Scalable ternary hierarchical microspheres composed of PANI/rGO/CeO2 for high performance supercapacitor applications, Carbon, 2019, 151, 192 CrossRef CAS.
- A. J. Xie, F. Tao, T. Li, L. Wang, S. J. Chen, S. P. Luo and C. Yao, Graphene-cerium oxide/porous polyaniline composite as a novel electrode material for supercapacitor, Electrochim. Acta, 2018, 261, 314 CrossRef CAS.
- M. Z. Zhong, M. Zhang and X. F. Li, Carbon nanomaterials and their composites for supercapacitors, Carbon Energy, 2022, 1, 36 Search PubMed.
- M. Nallappan and M. Gopalan, Fabrication of CeO2/PANI composites for high energy density supercapacitors, Mater. Res. Bull., 2018, 106, 357 CrossRef CAS.
- D. Biswas, A. S. Das, R. Mondal, A. Banerjee, A. Dutta, S. Kabi, D. Roy and L. S. Singh, Structural properties and electrical conductivity mechanisms of semiconducting quaternary nanocomposites: effect of two transition metal oxides, J. Phys. Chem. Solids, 2020, 144, 109505 CrossRef CAS.
- K. Chen, X. M. Zhang, X. F. Yang, M. G. Jiao, Z. Zhou, M. H. Zhang, D. H. Wang and X. H. Bu, Electronic structure of heterojunction MoO2/g-C3N4 catalyst for oxidative desulfurization, Appl. Catal., B, 2018, 238, 263 CrossRef CAS.
- Z. Jiang, W. Wan, H. Li, S. Yuan, H. Zhao and P. K. Wong, A Hierarchical Z-Scheme alpha-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction, Adv. Mater., 2018, 30(10), 1706108 CrossRef PubMed.
- J. Y. Tang, R. T. Guo, W. G. Zhou, C. Y. Huang and W. G. Pan, Ball-flower like NiO/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction, Appl. Catal., B, 2018, 237, 802 CrossRef CAS.
- L. Zhang, S. S. Chai, W. B. Zhang, S. B. Guo, X. W. Han, Y. W. Guo, X. Bao and X. J. Ma, Cobalt boride on clay minerals for electrochemical capacitance, Appl. Clay Sci., 2022, 218, 802 Search PubMed.
- A. A. Yadav, A. C. Lokhande, J. H. Kim and C. D. Lokhande, Supercapacitive activities of porous La2O3 symmetric flexible solid-state device by hydrothermal method, Int. J. Hydrogen Energy, 2016, 41, 18311 CrossRef CAS.
- A. A. Yadav, A. C. Lokhande and C. D. Lokhande, A simple chemical route for synthesis of microrods-like La2O3 thin films, Mater. Lett., 2015, 160, 500 CrossRef CAS.
- G. S. Gund, D. P. Dubal, S. B. Jambure, S. S. Shinde and C. D. Lokhande, Temperature influence on morphological progress of Ni(OH)2 thin films and its subsequent effect on electrochemical supercapacitive properties, J. Mater. Chem. A, 2013, 1, 4793 RSC.
- K. L. Fang, J. Z. Chen, X. Y. Zhou, C. T. Mei, Q. H. Tian, J. L. Xu and C. P. Wong, Decorating biomass-derived porous carbon with Fe2O3 ultrathin film for high-performance supercapacitors, Electrochim. Acta, 2018, 261, 198 CrossRef CAS.
- A. A. Yadav, A. C. Lokhande, R. B. Pujari, J. H. Kim and C. D. Lokhande, The synthesis of multifunctional porous honey comb-like La2O3 thin film for supercapacitor and gas sensor applications, J. Colloid Interface Sci., 2016, 484, 51 CrossRef CAS PubMed.
- J. Wang, J. w. Zhang, S. Liu, X. y. Pei, Y. Li and C. w. Wang, The accurate control of the La2O3 hierarchical structure of the flower-like microspheres accompanied with vertical-standing nanopetals and the electrowetting responses with low voltage actuation, Ceram. Int., 2020, 46, 11232 CrossRef CAS.
- A. A. Yadav, A. C. Lokhande, J. H. Kim and C. D. Lokhande, High electrochemical performance asymmetric supercapacitor based on La2O3//Co3O4 electrodes, J. Ind. Eng. Chem., 2017, 56, 90 CrossRef CAS.
- J. Sun, A. Iakunkov, A. T. Rebrikova and A. V. Talyzin, Exactly matched pore size for the intercalation of electrolyte ions determined using the tunable swelling of graphite oxide in supercapacitor electrodes, Nanoscale, 2018, 10, 21386 RSC.
- J. X. Zhang, Z. Z. Zhang, Y. T. Jiao, H. X. Yang, Y. Q. Li, J. Zhang and P. Gao, The graphene/lanthanum oxide nanocomposites as electrode materials of supercapacitors, J. Power Sources, 2019, 419, 99 CrossRef CAS.
- S. Karthikeyan, M. Selvapandiyan and A. Sankar, Electrochemical performance of reduced graphene oxide (rGO) decorated lanthanum oxide (La2O3) composite nanostructure as asymmetric supercapacitors, Inorg. Chem. Commun., 2022, 139, 109331 CrossRef CAS.
- M. Miah, S. Bhattacharya, D. Dinda and S. K. Saha, Temperature dependent supercapacitive performance in La2O3 nano sheet decorated reduce graphene oxide, Electrochim. Acta, 2018, 260, 449 CrossRef CAS.
- A. J. Webb, D. Bloor, M. Szablewski and D. Atkinson, Temperature dependence of electrical transport in a pressure-sensitive nanocomposite, ACS Appl. Mater. Interfaces, 2014, 6, 12573 CrossRef CAS PubMed.
- B. G. Trasatti S, Ruthenium dioxide: a new interesting electrode material. Solid state structure and electrochemical behaviour, J. Electroanal. Chem. Interfacial Electrochem., 1971, 29, A1 CrossRef.
- R. Liu, A. Zhou, X. R. Zhang, J. B. Mu, H. W. Che, Y. M. Wang, T. T. Wang, Z. X. Zhang and Z. K. Kou, Fundamentals, advances and challenges of transition metal compounds-based supercapacitors, Chem. Eng. J., 2021, 412, 128611 CrossRef CAS.
- C. X. Sun, W. X. Pan, D. Y. Zheng, R. B. Yao, Y. H. Zheng, J. H. Zhu and D. W. Jia, Low-Cost MnO2 Nanoflowers and La2O3 Nanospheres as Efficient Electrodes for Asymmetric Supercapacitors, Energy Fuels, 2020, 34, 14882 CrossRef CAS.
- H. Y. Duan, M. Shi, M. F. Zhang, G. Y. Feng, S. L. Liu and C. Y. Chen, Lanthanum Oxide Nickel Hydroxide Composite Triangle Nanosheets for Energy Density Asymmetric Supercapacitors, Front. Chem., 2021, 9, 783942 CrossRef CAS PubMed.
- Y. Li, B. Guan, A. Maclennan, Y. F. Hu, D. D. Li, J. Zhao, Y. Q. Wang and H. H. Zhang, Porous waxberry-like MnO2/La2O3 microspheres for high performance asymmetric supercapacitor, Electrochim. Acta, 2017, 241, 395 CrossRef CAS.
- M. Majumder, R. B. Choudhary, A. K. Thakur, C. S. Rout and G. Gupta, Rare earth metal oxide (RE2O3; RE = Nd, Gd, and Yb) incorporated polyindole composites: gravimetric and volumetric capacitive performance for supercapacitor applications, New J. Chem., 2018, 42, 5295 RSC.
- H. Mohammad Shiri and A. Ehsani, Electrosynthesis of neodymium oxide nanorods and its nanocomposite with conjugated conductive polymer as a hybrid electrode material for highly capacitive pseudocapacitors, J. Colloid Interface Sci., 2017, 495, 102 CrossRef CAS PubMed.
- P. Liu, Y. J. Wang, X. Wang, C. Yang and Y. F. Yi, Polypyrrole-coated samarium oxide nanobelts: fabrication, characterization, and application in supercapacitors, J. Nanopart. Res., 2012, 14, 1232 CrossRef.
- H. Mohammad Shiri, A. Ehsani and M. Jalali Khales, Electrochemical synthesis of Sm2O3 nanoparticles: application in conductive polymer composite films for supercapacitors, J. Colloid Interface Sci., 2017, 505, 940 CrossRef CAS PubMed.
- M. Mazloum-Ardakani, F. Sabaghian, H. Naderi, A. Ebadi and H. Mohammadian-Sarcheshmeh, Electrochemical and theoretical study of novel functional porous graphene aerogel-supported Sm2O3 nanoparticles for supercapacitor applications, J. Solid State Electrochem., 2020, 24, 571 CrossRef CAS.
- H. Mohammad Shiri and A. Ehsani, A simple and innovative route to electrosynthesis of Eu2O3 nanoparticles and its nanocomposite with p-type conductive polymer: characterisation and electrochemical properties, J. Colloid Interface Sci., 2016, 473, 126 CrossRef CAS PubMed.
- H. M. Shiri and A. Ehsani, Pulse electrosynthesis of novel wormlike gadolinium oxide nanostructure and its nanocomposite with conjugated electroactive polymer as a hybrid and high efficient electrode material for energy storage device, J. Colloid Interface Sci., 2016, 484, 70 CrossRef CAS PubMed.
- V. Thangadurai, R. A. Huggins and W. Weppner, Mixed ionic-electronic conductivity in phases in the praseodymium oxide system, J. Solid State Electrochem., 2001, 5, 531 CrossRef CAS.
- X. Wang, C. Yang, T. M. Wang and P. Liu, Praseodymium oxide/polypyrrole nanocomposites for electrochemical energy storage, Electrochim. Acta, 2011, 58, 193 CrossRef CAS.
- K. T. Kubra, A. Javaid, B. Patil, R. Sharif, A. Salman, S. Shahzadi, S. Siddique and S. Ghani, Synthesis and characterization of novel Pr6O11/Mn3O4 nanocomposites for electrochemical supercapacitors, Ceram. Int., 2019, 45, 6819 CrossRef CAS.
- N. Chen, A. Younis, S. H. Huang, D. W. Chu and S. Li, Advanced three-dimensional hierarchical Pr6O11@Ni-Co oxides-based core–shell electrodes for supercapacitance application, J. Alloys Compd., 2019, 783, 772 CrossRef CAS.
- A. Paravannoor, C. A. Augustine and N. Ponpandian, Rare earth nanostructures based on PrOx/CNT composites as potential electrodes for an asymmetric pseudocapacitor cell, J. Rare Earths, 2020, 38, 625 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |