Open Access Article
Open Access ArticleCritical overview on the green synthesis of carbon quantum dots and their application for cancer therapy
Liam Joseph
Desmond
,
Anh N.
Phan
* and
Piergiorgio
Gentile
 *
*
School of Engineering, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: anh.phan@ncl.ac.uk; piergiorgio.gentile@ncl.ac.uk
First published on 10th March 2021
Abstract
Cancer therapeutic systems can help to confront and overcome many different strands of tumours in clinical patients. Despite the benefits, current therapeutic systems present clear unmet challenges, such as their toxicity and damaging effects to human body cells and the host. The need for easy-to-fabricate, safe and non-toxic therapeutic systems has driven the research field towards the use of carbon quantum dots. Their bioimaging with green, non-toxic, biocompatible and attractive quantum properties present them as distinctively advantageous over conventional/metal-based quantum dots. In this review, the current progress in the development of quantum dots, focusing on carbon quantum dots for use in nanotheranostics for different tumours, will be critically analysed. The advantages of applying carbon quantum dots by green bottom-up approaches into nanotherapy systems will be highlighted. Finally, ongoing challenges and opportunities associated with a green and sustainable synthesis of carbon quantum dots with specific functional groups to aid the next stages in the development of the nanotherapy system will also be discussed.
Environmental significanceCurrent cancer nanotherapy systems studied have only reported on the use of quantum dots (QDs) for bioimaging of the tumour and not for therapeutic reasons because these systems can damage human body cells while targeting the tumour. To overcome this issue, the goal is to develop a safer nanotherapy system. Herein lies a novel use of a nanosystem composed of carbon QDs (CQDs) to tackle the tumour whilst creating minimal damage to the surrounding cells. This hypothesis is based on the non-toxicity and overall safety of CQDs. In addition, the biomass waste products used to synthesise CQDs are readily available, cheap and pose minimal to no risk in creating suitable nanotherapy systems. The direct comparison of different types of biomass waste and their opposing advantages are discussed in this review, which is something that has not been seen in previous literature. These biomass waste materials employed are described as ‘green’, which means that their use in synthesising CQDs pose no risk to the environment, further showing their advantages over previous less green methods. The future use of these non-toxic generated CQDs will be to combine them with drug molecules, such as by layer-by-layer assembly, to generate a unique drug delivery system. |
1. Introduction: quantum dots (QDs) definition and description
Quantum dots (QDs), a heterogenous group of small brightly fluorescent nanoparticles,1 have emerged as a new class of material beneficial for sensing, medical and imaging applications.2 A QD is a miniscule spot of matter that is effectively concentrated into a single point. Particles inside it that carry electricity are trapped and have well-defined energy levels according to the laws of quantum theory, similar to individual atoms. QDs typically have a diameter ranging between 1 nm and a few tens of nanometres, grown from or etched into semiconductor materials.3 Consequently they are typically a few dozen atoms across and contain from anywhere between a hundred to a few thousand atoms. They are made from a semiconductor such as silicon, and although they are crystals, they behave more like individual atoms and hence are referred to as “artificial atoms”, semiconductor particles sufficiently tiny such that charge and energy levels are quantized.4 Because of this precision they are generated in the same fashion that other precise semiconductor nanocrystals are generated. These methods include molecular beam epitaxy, ion implementation and X-ray lithography.5 These nanoparticles are highly luminescent and monodisperse nanocrystals that are currently of great interest due to their use as labels in bioanalytical applications.6 In recent years, the unique properties of these QDs have attracted major attention in the biomedical field to enable real-time tissue imaging (referred to as bioimaging), diagnostics, single-molecule probes, and drug delivery, among many other areas.7 The use of QDs in therapeutic drug delivery systems is a better approach rather than polymer or silica spheres as they are green and less toxic reagents.8 Further advantages of semi-conducting QDs include their tuneable bandgap and high absorption coefficients,9,10 multiple exciton generation with single-photon absorption,11,12 easy formation of different forms in sheets or in 3D arrays, low cost and can be produced in bulk.13,14 Also, QDs are good for chemical sensing due to their high surface-to-volume ratio, high reactivity, small size and geometry-dependent electronic properties.15Although the nature of precursors for QD synthesis plays a role in determining the intrinsic energy signature of the particle, the size of the dots mostly affects the optical properties. Different-sized QDs change the colour emitted or absorbed by the crystal due to the energy levels within the crystal. In the fluorescence spectrum, the colour of the light differs according to the energy emitted by the crystal, the colours of which are seen in Fig. 1(a). Red light is associated with lower energy and blue light with higher energy. The bandgap energy of a QD is the difference in energy level between the dot's excited energy state and its resting state. The QD can absorb fluorescent light at the frequency of its bandgap to become excited or emit the same frequency of light to return to its resting state, as seen in Fig. 1(b).16 The size of a QD is inversely proportional to the bandgap energy level and therefore alters the frequency of light emitted and has an effect on the colour. Smaller dots emit higher-energy light that is bluer in colour, whereas larger dots emit lower-energy red light.17 It is also possible for larger QDs to possess several energy levels that are more closely aligned. This allows for the absorption of photons with different frequency levels, such as those on the red end of the light spectrum. Additionally, due to these additional energy levels electron–hole pairs can become trapped inside larger QDs. Over the long term, this causes larger QDs to have a longer lifespan than small QDs.18Fig. 1b denotes all of these properties and shows the outcome of each, factoring the colour depending on its initial size.
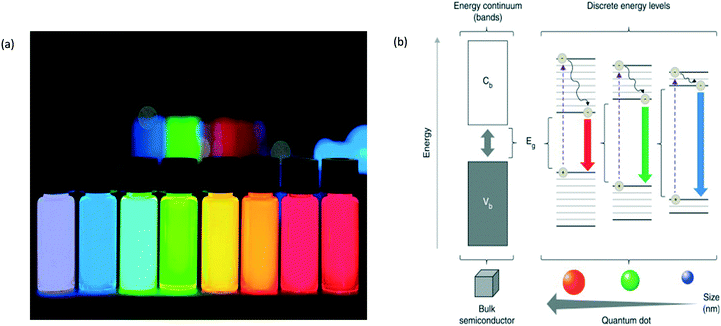 | ||
| Fig. 1 (a) The different colours that QDs give rise to according to the energy emitted by the crystal; (b) the colour and size of the QD based on the energy level properties.3,16 | ||
When excited by electricity or light, QDs produce a brilliant, single-colour glow. They typically contain relatively few atoms, ranging from about 1000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.3 This means that the crystals are large enough to be useful in the laboratory but small enough to exhibit quantum behaviours associated with individual atoms. Movement of electrons is confined in all three directions so tightly that QDs are said to be “zero-dimensional”. As a result, changing the size of a QD controls how it absorbs and emits energy. This behaviour makes them appealing for a wide range of photonic and electronic applications. A quantum well is a sandwich of thin, stacked slices of semiconductor materials with different bandgaps. The bandgap is the energy required for an electron to jump from one energy level to another.3 In a quantum well, the central layer has the smallest bandgap, which means that the larger gaps of the outer layers act like barriers, restricting the electrons in the middle one. This structure vertically confines the energy levels of the middle layer in one dimension. By controlling the size of that layer, scientists realized in the 1970s that they could tune the wavelengths—corresponding to colour—of photons emitted by the electrons. When a QD is provided with energy (i.e. it is in an excited state) the electron is boosted to a higher energy level, generating an exciton. When the electron returns to a lower level, the atom emits a photon of light with the same energy that the atom originally absorbed. The biggest QDs produce the longest wavelengths/lowest frequencies, while the smallest QDs make shorter wavelengths/higher frequencies. This indicates that big dots make red light and small dots make blue light, with intermediate-sized dots producing green light (and the familiar spectrum of other colours too). This effect is due to the bandgap size: a small dot has a bigger bandgap (the minimum energy it takes to free electrons so they will carry electricity through a material), so it takes more energy to excite it; because the frequency of emitted light is proportional to the energy, smaller dots with higher energy produce higher frequencies and shorter wavelengths. Larger dots have more (and more closely) spaced energy levels, so they give out lower frequencies and therefore longer wavelengths.19
000.3 This means that the crystals are large enough to be useful in the laboratory but small enough to exhibit quantum behaviours associated with individual atoms. Movement of electrons is confined in all three directions so tightly that QDs are said to be “zero-dimensional”. As a result, changing the size of a QD controls how it absorbs and emits energy. This behaviour makes them appealing for a wide range of photonic and electronic applications. A quantum well is a sandwich of thin, stacked slices of semiconductor materials with different bandgaps. The bandgap is the energy required for an electron to jump from one energy level to another.3 In a quantum well, the central layer has the smallest bandgap, which means that the larger gaps of the outer layers act like barriers, restricting the electrons in the middle one. This structure vertically confines the energy levels of the middle layer in one dimension. By controlling the size of that layer, scientists realized in the 1970s that they could tune the wavelengths—corresponding to colour—of photons emitted by the electrons. When a QD is provided with energy (i.e. it is in an excited state) the electron is boosted to a higher energy level, generating an exciton. When the electron returns to a lower level, the atom emits a photon of light with the same energy that the atom originally absorbed. The biggest QDs produce the longest wavelengths/lowest frequencies, while the smallest QDs make shorter wavelengths/higher frequencies. This indicates that big dots make red light and small dots make blue light, with intermediate-sized dots producing green light (and the familiar spectrum of other colours too). This effect is due to the bandgap size: a small dot has a bigger bandgap (the minimum energy it takes to free electrons so they will carry electricity through a material), so it takes more energy to excite it; because the frequency of emitted light is proportional to the energy, smaller dots with higher energy produce higher frequencies and shorter wavelengths. Larger dots have more (and more closely) spaced energy levels, so they give out lower frequencies and therefore longer wavelengths.19
QDs as a group of materials can have many differing structures and properties based on each component (Fig. 2).20 They vary in their core and shell composition, in their size and shape, and in their surface chemistry. QD surfaces can have hydrophobic ligands that make them soluble in the organic phase. Their surfaces can be modified to render them water-soluble and biocompatible. Their surface can have bifunctional molecules such as mercaptoacetic acid, can be coated with amphiphilic polymers such as poly(acrylic acid)-octadecyl-amine polymers, or can be trapped within micelles. QDs can be further modified with targeting ligands such as oligonucleotides, peptides, or antibodies that will direct the QDs to specific sites within the body or to specific locations within a cell.20
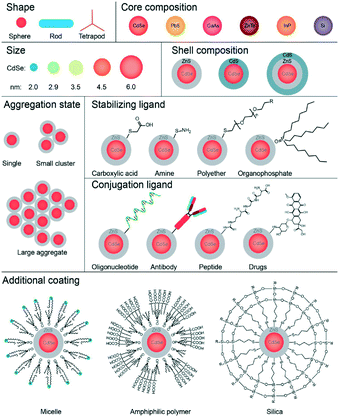 | ||
| Fig. 2 The different components/shapes of those components in QDs which generate different QDs. Due to the number of different components, it means that they differ in shape/size/properties.20 | ||
The use of QDs in many different scientific fields, in particular, biomedical application, has been extensively studied owing to their useful properties as highlighted above. Despite these benefits, there are still shortcomings to using them, such as their toxicity.20 This has required attention to be refocused on developing safer and just as useful alternatives, which has led to carbon-based quantum dots (CQDs). A number of competitive advantages makes QDs desirable in numerous biomedical applications. In comparison to common organic dyes and luminescent proteins, QDs have broad excitation spectra, narrow emission spectra and large Stokes shifts. QDs also possess superior optical properties including increased intensity (up to nearly 100 times brighter) and significantly improved stability against photo-bleaching.7
Despite the interest in QDs for biomedical applications, there is concern that they will not be viable for use in treatment of patients due to their potential toxicity. This is due to a clear factor. As previously established, common QDs in the market such as cadmium-containing QDs (CdQDs) can kill cells in culture, as the Cd2+ ions released from the surface of QDs into the environment are highly toxic to cells and tissues. The assumption is that if they are toxic to these cells, the same must apply to human cells.20 The assumption that QDs are toxic to humans as they are toxic to cells in culture is not concrete as results from cell-based toxicity studies are rarely transferable to more complex biological systems. Notably, QD toxicity has not been demonstrated in animal models after single-cell dosing. Nevertheless, it is highly important to focus on Cd-free and non-metallic QDs for their potential applications in biological systems on the assumption that they will be a cleaner safer approach without the risk of harming patients/human cells.1 Furthermore, it has been demonstrated that “naked” quantum dots are cytotoxic by induction of reactive oxygen species, resulting in damage to plasma membranes, mitochondria, and nuclei.21 As it is the bioactive coating which allows the use of QDs for specific targeting to cells and/or cell organelles, attention is warranted in using the surface molecules in terms of induction of toxic effects.21
As highlighted in Fig. 2, QDs as a group of materials can have many differing structures and properties based on each component; therefore it is difficult to make general statements regarding their toxicity. A vast number of QD formulations are possible, each with a unique combination of physicochemical properties that dictate the QD's interaction with a biological entity, meaning that the toxicity of each different QD is uncertain. Despite this, CQDs have proved to be safer alternatives in biological systems tested.22 The essence of this review aims to highlight the use of QDs in cancer therapy systems due to their tuneable properties and discover why CQDs present a clearly advantageous use as opposed to quantum dots. This is due to their lack of toxicity. Recent publications denote the use of CQDs in cancer therapeutics such as bioimaging, and the use of QDs in biosensors and biomolecular/drug delivery for cancer therapeutics, which could be replaced by CQDs due to their similar properties, will be discussed. This review will look into the development, use and advantages of utilising CQDs in biomedical applications, proposing the limitations and the future perspectives in the use of CQDs.
2. Carbon-based quantum dots
CQDs (their general chemical structure is shown in Fig. 3) have been considered to be greener and safer in medicine and biology.1,23 Similar to their QD counterparts, they show bright fluorescence but do not exhibit toxicity or harm to the environment and are less expensive to generate. Moreover, CQDs exhibit commercial potential superior to that of their semiconductor counterparts owing to their low toxicity, good biocompatibility, superb solubility, strong and stable photoluminescence, high specific surface area and tuneable bandgap.24 Over the past few years, CQDs, also known as carbon dots/nanostructured carbons,25 have gained considerable attention towards this branch of science due to their advantages such as exceptional water solubility, ease of synthesis/functionalization, exceptional photoluminescence (PL) and optical properties and large-scale production with low cost. CQDs have been applied in several fields such as carbon fixation, gas storage, cell biology and, most interestingly, cancer imaging and drug delivery.26–28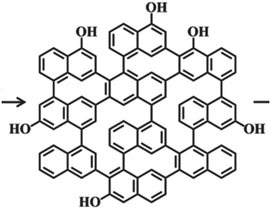 | ||
| Fig. 3 The general chemical structure of CQDs.1 | ||
As reported previously,1,29 compared to traditional semiconductor QDs and organic dyes, the use of CQDs in biomedical applications is an advantageous approach for several reasons. Firstly, the production of CQDs is generally a facile and inexpensive process, whilst at the same time a high-yield process, in an environmentally friendly way.27,30 These factors render them advantageous in terms of cost, time and safety (Table 1).25 CQDs also exhibit several characteristics that make them valuable tools in biomedical applications, such as their ultra-small sizes, good biocompatibility, high chemical inertness, tuneable hydrophilicity, rich surface functional groups and antifouling characteristics.15,25,26,31,32 CQDs display less toxicity than their traditional QD counterparts as they are void of heavy metals.27,33 Their extensive surface33 ensures that they can bind to many different compounds, an attractive property useful in a drug delivery system, i.e. can bind to many anti-cancer drug molecules.26 In particular, fluorescent CQDs of size less than 10 nm have attracted considerable research interest due to their small size which gives them advantages such as low photobleaching, favourable biocompatibility, facile modification, chemical inertness, good water solubility and excellent cell-membrane permeability. These properties make CQDs superior to organic dyes and semiconductor quantum dots with heavy metal cores in biological systems, inspiring the dominant applications in biosensors and bioimaging.28 These finer biological properties of CQDs, such as low toxicity and good biocompatibility, entrust them with potential applications in bioimaging, biosensors and biomolecule/drug delivery. The outstanding electronic properties of carbon-based quantum dots as electron donors and acceptors, causing chemiluminescence and electrochemical luminescence, endow them with wide potential in optronics, catalysis and sensors.34
| Method (top-down/bottom-up) | Procedure | Advantages | Disadvantages |
|---|---|---|---|
| Arc discharge method (top-down)44,45 | Two graphite rods in an open vessel are used as anode and cathode electrodes, respectively, with an arc of current voltage produced in between the two | This method produces MWCNTs, which in theory could be broken down further to produce CQDs | Nanotube soot contains a variety of impurities. The graphitic sheets in the soot have a greater oxidative stability than the nanotubes |
| Laser ablation (top-down)36,49–53 | Toluene is used as the carbon source via laser irradiation technique. The size of CQDs is controlled using laser furnace | The size/photoluminescence properties of the CQDs can easily be controlled by changing the parameters such as irradiation time | This is not a green method; a low yield of CQDs is generated (4–10%). Low QY, poor control over sizes, modification is needed |
| Acidic oxidation (top-down)29 | Carbon nanoparticles are oxidised by a mixture solution of HNO3, H2SO4 and NaClO3, then hydrothermally reacted with DMF, NaHS and NaHSe | The generated CQDs exhibit tuneable PL performance, high quantum yield and longer fluorescence lifetime than other CQDs | The heavily doped heteroatoms can affect the PL properties due to the electronegativity of N, S, and Se |
| Plasma induced pyrolysis (bottom-up)54,55 | A negative electrode and a platinum disc are connected to a negative power supply to ignite and sustain the plasma. The CQDs are generated from here | The amphiphilicity of the CQDs make them dispersible in water/most organic solvents. This method can be used to produce CQDs from a wide range of carbon sources | Losses to the reactor walls in the form of tar as well as gas leakage to the atmosphere as this uses higher temperatures than conventional pyrolysis |
| Combustion/thermal routes (bottom-up)56–58 | Combustion of a carbon source followed by functionalization with carboxyl groups through conjugation of acetic acid moieties under a high temperature | The obtained CQDs possess a uniform particle size and rich carboxyl groups on the surface. Facile, ease of scale-up production | Small polycyclic aromatic hydrocarbons (PAHs) are generated in soot formation; an environmental hazard |
| Hydrothermal/solvothermal synthesis (bottom-up)59–61 | Small organic molecules/polymers are dissolved in water/organic solvent then transferred to a Teflon-lined stainless-steel autoclave at high temperature to form CQDs | Quantum yield of the CQDs can reach 80%, facile synthetic process, green method, many carbon sources can be used | Low quantum yield. Losses via the reactor wall can occur; gas is a side product. Poor control over size |
| Microwave pyrolysis (bottom-up)41,62 | Combining a carbon source and a saccharide in water to form a transparent solution, in an inert environment, followed by heating in a microwave oven | A rapid synthesis route, great commercialization, simple and environmentally friendly method | The maximum QY reported is 34%, lower than those of other methods reported in the table |
2.1 CQD synthesis
There have been many differently demonstrated methods to synthesise CQDs. Much research mainly focuses into each synthetic scheme to determine the most efficient, clean and high-yielding method.35 CQDs are generated mainly via two routes: (i) top-down approach and (ii) bottom-up approach.36 The top-down approach refers to the breaking down/cleaving of larger carbonaceous materials/structures via methods such as chemical oxidation, discharge, electrochemical oxidation, and ultrasonic methods (Fig. 4).23,29 However, the drawbacks of this approach include the requirement of expensive materials, harsh reaction conditions, and long reaction time.35 On the other hand, the bottom-up approach refers to the conversion of smaller carbon structures into CQDs of the desired size. Typical bottom-up methods are hydrothermal treatment, ultrasonic treatment, thermal decomposition, pyrolysis, carbonization, microwave synthesis and solvothermal methods which synthesize CQDs (Fig. 4).36 The chemical difference between the two approaches is emphasised in Fig. 5, highlighting how the top-down approach breaks down the bulk material to generate the CQDs, whereas the bottom-up approach builds the material from molecules.36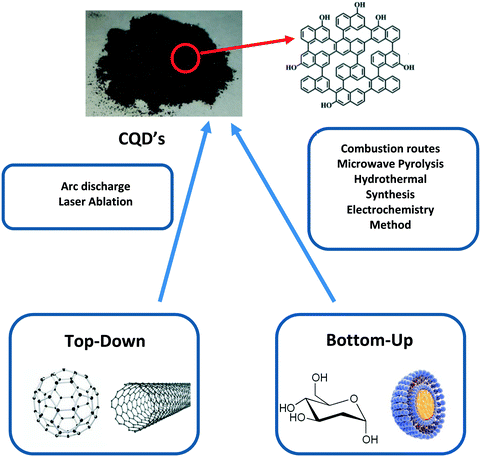 | ||
| Fig. 4 A general scheme outlining how CQDs are generated from different successful top-down and bottom-up methods.29 The top-down and bottom-up boxes display typical starting materials used for the methods shown in the boxes (centre). The top of the scheme shows the final CQD product and its general chemical structure. | ||
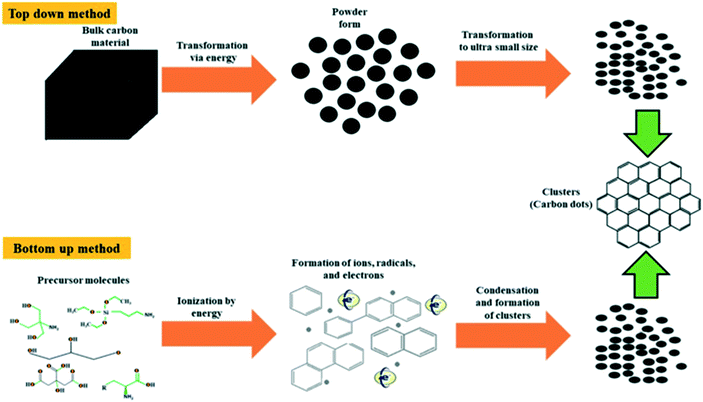 | ||
| Fig. 5 A route depicting the two different routes that can be taken to generate CQDs and the difference between them. The top-down approach breaks down bulk carbon material to generate the CQDs, whereas the bottom-up approach builds the material from precursor molecules,36 with both routes obtaining the same product. | ||
Top-down approaches usually result in smaller flakes or particles with a wide size distribution, whereas bottom-up approaches generally generate particles with a uniform size, which is preferred in the use in biomedical applications. It is possible to overcome that disadvantage and synthesize nanoparticles of precise size using a focused ion beam or lithography, but it requires expensive equipment. For this reason, the bottom-up approach is considered simpler and more precise in the synthesis of small nanoparticles less than 100 nm, and the top-down approach is preferred for the synthesis of thin films and nanoparticles larger than 100 nm.37
Several top-down methods have been established for the successful preparation of CQDs, including etching with larger carbon materials, such as laser ablation, electrochemical oxidation, thermal oxidation, microwave irradiation, hot injection, and pyrolysis.38–41 However, these reported methods have several limitations, such as limited spectral efficiency, low product yield, lack of size control, and the use of toxic chemicals or high temperature for experiments.42 For this reason, there is an urgent need to establish new methods which are facile and environmentally low load. Consideration of preserving a green environment and preventing increasing global warming whilst ensuring sustainable and renewable sources of energy and cost effectivity must be brought toward the preparation of the CQDs.43
Table 1 reports more advantages and disadvantages of top-down vs. bottom-up procedures, as reported in the literature. In particular, as can be seen in the top half of the table, impure products, low yield and not green clean methods plague top-down methods, signifying that the use of these in generating CQDs is not advisable as compared to the bottom-up methods reported in the table which have great control over size, higher yield and clean green method and use materials that are in abundance. This indirectly has a positive correlation between top-down vs. bottom-up methods and overall cost, with it once again favouring bottom-up methods, highlighting that it is more advantageous to use the latter approach. Furthermore, top-down methods initiated from materials such as graphite powder or multi-walled carbon nanotubes (MWCNTs) are usually conducted under harsh physical or chemical conditions,44,45 which bring into question the viability of carrying out the synthesis of CQDs via this way if safer alternatives are available. Opposingly, preparation of CQDs via a bottom-up approach applies external energy such as ultra-sonification, microwave pyrolysis and heating46 which are much less harsh conditions, providing a safer alternative approach. The dots derived from top-down methods almost all had fluorescence emission in relatively short wavelengths and lacked effective control on morphology and size distribution. The bottom-up approach, meanwhile, provides more space to design products with the desired performance. Bottom-up methods, relying on the chemical reaction from precursors or assembly of small building blocks, are convenient in controlling the morphologies and structures of nanomaterials.47 Generally speaking, the bottom-up method has a high yield and it is convenient to introduce heteroatom doping in the synthesis process.48
The hydrothermal/pyrolysis methods outlined in Table 1 overshadow the other methods due to their advantages of potential high quantum yields, facile synthetic method and green, environmental procedure. Not only that, but the use of biomass waste in these two procedures is applicable, which means that they can rely on an abundant clean source. Whilst it is clear from Table 1 that there are downsides to bottom-up methods, such as low quantum yield with methods such as hydrothermal/pyrolysis, the overall benefits of bottom-up methods outweigh those of top-down methods.
At present, the steps for the hydrothermal/pyrolysis methods using carbonaceous starting materials to generate CQDs remain consistent in all recent literature.29,40,60,61,63 For these steps, the biomass waste/starting material is combined with deionised water in different sized Teflon-lined stainless-steel autoclaves,33 at a range of temperatures and periods of time depending on controlling the outcome of the product generated. Whilst this has been proven to be a successful synthetic process, several setbacks must be considered for future reactions to operate more effectively. Firstly, whilst a larger Teflon-lined stainless-steel autoclave can hold more biomass waste and deionised water, this will in fact mean that it is harder for the materials to react with each other as there is too high of a quantity, which will in effect lower the yield. This can be tackled by employing a magnetic stirrer large enough to interact with all the material inside the autoclave. Another setback witnessed in the hydrothermal setup arises from a small amount of leakage from the autoclave. This can be tackled by using cotton wool/tin foil and wrapping it around the system to prevent, or significantly reduce the volume of, gas or crude material escaping.
3. Biomass waste in synthesising CQDs
Biomass waste such as food waste and agricultural residues has gained considerable attention in recent years as a source for CQD synthesis,26,48 the different types of which are highlighted in Fig. 6.64 It is rich in carbon (up to 40–50%) with abundant oxygen and nitrogen and thus can be used as a successful precursor for the valorisation of CQDs. Previous successfully used biomass waste products to generate CQDs have included watermelon peel,65 lemon peel,66,67 orange peel,43 fennel seeds,68 orange juice69 and coffee grounds70 (Fig. 6). Many of these “green” CQDs without any post-treatment exhibited outstanding performances in biosensing, cellular imaging and chemical sensing applications.71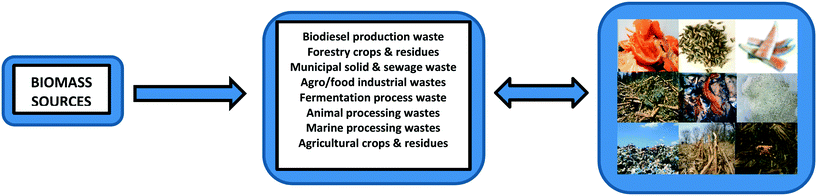 | ||
| Fig. 6 The different types of biomass sources that can be used as biomass waste precursors for CQD synthesis. In particular, agro/food industrial wastes such as orange peels (top left), fennel seeds (top centre) and watermelon peel (top right) are all established examples of biomass waste that have successfully generated CQDs.42,64,64,67 | ||
The route of applying biomass waste in bottom-up methods to generate CQDs is detailed in Table 1 where the biomass waste is used as the carbon source starting material and the relevant steps are performed on it. One particularly successfully applied biomass waste in the synthesis of CQDs was walnut shell, as shown in Fig. 7.71 Its CQDs exhibited better photostability and photoluminescence properties and no toxicity as compared to standard metal-based QDs, indicating that future use in bioimaging applications will be deemed safe.71
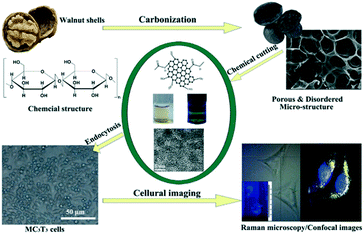 | ||
| Fig. 7 A synthetic route by use of biomass waste precursors, walnut shells, which generates CQDs. This scheme shows the outcome and bioimaging of these CQDs.71 | ||
Chitin is yet another biomass waste that has seen recent successful attempts in converting into CQDs via bottom-up approaches.63 Chitin can be converted into chitosan gel, which can be stable for years without any degradation to the material, thus increasing the shelf life of the source material for preparing CQDs.72 This is just one example of successfully employed biomass waste in the synthesis of CQDs. Table 2 outlines the use of other biomass waste products in synthesising CQDs, all via bottom-up methods like pyrolysis, hydrothermal carbonization and microwave treatment, where the biomass waste is usually an advantageous starting material.
| Biomass waste | Advantages | Disadvantages | Evidence of use in synthesising CQDs |
|---|---|---|---|
Walnut shell71 |
Generated CQDs exhibited photostability and photoluminescence properties | Walnut shells have to be ground initially for efficiency, increasing the time scale of the overall reaction | ✓ |
| Facile method, uniform-sized CQDs | |||
Chitin63,72 |
Can be stored as chitosan gel, increasing the shelf life of the material | Most methods in the literature reported an initial conversion step to chitosan gel, increasing the overall time scale. CQDs range in size | ✓ |
Coffee grounds70 |
Uniform-sized CQDs/narrow size distribution generated | Yield reported in literature is 33%, rather lower than those of other methods with other biomass waste precursors | ✓ |
Watermelon peel65 |
The CQDs possess good water-solubility, strong blue luminescence and acceptable fluorescence lifetime | Broad` size distribution of CQDs generated. Low quantum yield (7.1%) | ✓ |
Orange peel43 |
Generated CQDs have high solubility in aqueous medium and luminescence property | The orange peels need to be washed in water and dried overnight initially. Low yield (12.3%). Size distribution 2–7 nm | ✓ |
Fennel seeds68 |
No initial steps needed, can be used as they are | The use of fennel seeds is a recent phenomenon; there is a lack of clear results | ✓ (only recently employed) |
All of these biomass wastes in Table 2 reported facile bottom-up synthesis methods of CQDs, with all reports indicating the use of the generated CQDs in bioimaging and biosensing applications. Despite this, each biomass waste product reported opposing advantages and disadvantages; therefore the decision of which biomass waste to employ must be carefully considered. Particular attention is paid to employing the biomass waste(s) which will give the best yield/quantum yield with optimum photostability and photoluminescence properties, whilst maintaining a uniform size of CQDs. As seen in Table 2, whilst the CQDs generated from watermelon peel and orange peel both have high water solubility and good luminescence properties, they are both plagued with yielding low amounts of CQDs, evidenced by their low yields/quantum yields. This is the same problem which has been seen when using the bottom-up methods on coffee grounds, with a low yield of CQDs from coffee grounds as the starting material (33%). Despite this, coffee grounds do produce CQDs with a uniform size; however, other biomass wastes manage to achieve this uniform size characteristic whilst generating a high yield. This is clear with employing walnut shells in which the literature has reported that it maintains great photostability and photoluminescence properties, generates CQDs with uniform size and is high yielding.71 From this evidence in Table 2 it is apparent that it would be wise to employ walnut shells in synthesising CQDs via a bottom-up method.
4. Application of CQDs in cancer therapy systems
The development of biocompatible nanoparticles for molecular targeted diagnosis and treatment is an area of considerable interest. The basic rationale is that nanoparticles have unique structural and functional properties different from those of discrete molecules or bulk materials.Previous disadvantageous nanoparticle-based systems that are currently being used in cancer therapy that need an overhaul are modelled on nanoparticles such as carbon nanotubes (Fig. 4, top-down box, right). These help to identify the exact location of the cancer-related DNA changes, thereby aiding in diagnosis of the disease. While this is a promising strategy, further in vitro work on carbon nanotubes has showed that they may cause reactive oxygen species production, oxidative stress, lipid peroxidation, mitochondrial dysfunction and changes in morphology of the cells. They may also induce platelet aggregation. Furthermore, their intratracheal instillation at high doses may result in chronic lung inflammation and lung toxicity.73
Some studies have also exposed currently used nanosystems that incorporate fullerenes (Fig. 4, top-down box, left) to cause brain damage due to the fullerenes causing lipid peroxidation. Silica nanoparticles which bind favourably to the cancer cell, thus providing the ability to see cells and molecules that cannot be otherwise detected through conventional imaging, have been shown to be toxic and non-toxic under different instances. The toxicity increases with the dose and the exposure time. Silica exposure also causes oxidative stress. At high doses, it induces membrane damage and a reduction in cell viability/proliferation. From this information it is clear that the need for a safer nanoparticle system is required.73
QDs are such nanoparticles which means that they have been used in the past few years to facilitate this theory.5 Used as in vitro and in vivo fluorophores, QDs are semiconductor nanocrystals that emit fluorescence on excitation with a light source. Recent developments in surface modification of QDs enable their potential application in cancer imaging.5 Because of this, investigations in the past decade has commenced into the use of QDs in the investigation of tumour environments,74 in addition to the networks of healthy blood vessels and cells that surround cancerous growth. It has been determined from earlier studies that there is a strong advantage for their use in tumours3,75 such as glioblastoma multiforme (GBM)76 and prostate and breast cancer.74 Tumours interact with their environment in many ways: blood vessels feed the tumour, and the tumour may release chemical signals or other cancer cells into the rest of the body. Recent work by Jain et al. implies that QDs will help inform the design of drugs that can travel through the often-leaky blood vessels of tumours and reach malignant cells directly.75 The method starts by injecting QDs of different sizes into animal cancer models. Because different-sized dots have different colours, Jain et al.75 have managed to estimate the sizes of blood vessels by observing which coloured dots get into the tumour.3 Furthermore, QDs have excellent optical properties, including high brightness, resistance to photobleaching and tunable wavelength.5
Other cancer researchers are investigating ways to use the glowing dots to identify cancerous cells in pathology studies by tagging cells that have particular receptor types on their surfaces, which may show that the tumour is vulnerable to targeted therapies. Still other researchers77,78 argue that QDs may even play a role one day as a drug delivery system by ferrying treatment (such as anti-cancer compounds and oncology treatments) directly to the cancer cells. Although QDs can be made from many different semiconductors, many require heavy metals, like cadmium, that are toxic to living cells. Even so, researchers are looking to develop non-toxic shells to protect the QDs and prevent leakage; some have demonstrated long-lasting non-toxicity in animal models.3 Whilst this advancement in findings in cancer therapy has been indispensable, recent work has inferred that the use of QDs can potentially be critical due to their cytotoxicity properties,79 and despite this advancement in discovery, QDs have still not been successfully utilised as of 201980 in cancer therapy. In spite of this, their characteristics such as bioimaging for locating tumours (Fig. 8) are too important to overlook, which has driven research towards the safer, much less toxic and facile synthesised CQDs.23,35,71
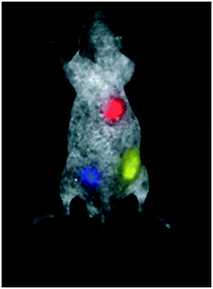 | ||
| Fig. 8 Multicolour quantum dot (QD) capability of QD imaging in in vivo animal studies.86 | ||
4.1 CQDs applied in bioimaging
CQDs are widely used in the area of bioimaging both in vitro and in vivo and in diagnosis purposes36 due to their fine-tuned properties which are very similar to those of standard QDs, such as their ability to emit fluorescence,81 whilst being non-toxic like QDs. It is because of this that their potential use in cancer therapy is appealing. Bioimaging is a powerful and irreplaceable tool for visualising the abnormal state at the target site of the related disease.82 In cancer, this tool helps to visualise the tumour, provide a more detailed image and help to plan a course of action.A recent study of the use of CQDs in bioimaging was carried out by C. Huang et al.83 to emphasize the significance of CQDs in in vivo optical bioimaging studies. A nude mouse was inoculated with Smmc-7721 tumour cells; following this, the optical imaging of the CQDs (generated from wheat straw (WS), a biomass waste) was investigated by an intravenous injection of 200 μL of the synthesized CQDs into a mouse that had a tumour via the tail vein. Optical images of distribution of the CQD-WS in the tumour-bearing mouse over increasing time periods are shown in Fig. 9a. In addition, optical images of fluorescence intensities within the harvested organs are shown in Fig. 9b. Without the injection of the CQDs, no fluorescence signals were detected in the tumour-bearing mouse's body (Fig. 9a, 0 min). Five minutes after injection of the CQD solution, fluorescence signals gradually became detectable, first at the head of the mouse. As time progressed towards three hours, the CQDs circulated in the mouse's body and gradually congregated at the location of the tumour. This trial exposed that CQDs can cycle in a mouse's body and also aggregate where they are intended to. After 12 hours, it was found that the CQDs stabilized almost exclusively at the location of the tumour. This is another valid demonstration that CQDs have good biocompatibility and could be used for tumour bioimaging over prolonged periods of time. Fig. 9b shows that the dissected kidney, liver, and tumour from the euthanized mouse showed fluorescence, revealing that the CQDs were taken up by these organs.84,85 However, no fluorescence signals were detected in the heart, lung, and spleen. Overall, most of the CQDs aggregated in the tumour and fluoresced intensely, indicating that the as-synthesized CQDs are a viable bioimaging agent and thus have immense potential in related fields such as phototherapy and bioimaging.83 The findings as carried out on the mouse have now brought into question if these can findings can be transferred to tumours in humans and work in the same fashion as in the mice.
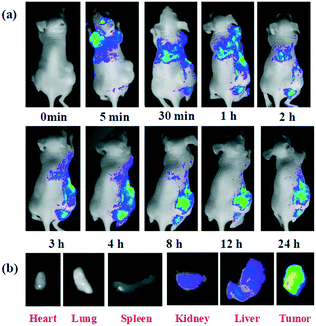 | ||
| Fig. 9 (a) In vivo fluorescence imaging of mice after an intravenous injection of the CQD solution synthesised; (b) representative fluorescence images of dissected organs of the mouse after the intravenous injection of the CQD solution for 24 hours.83 | ||
The significance of these findings makes it clear that CQDs are a useful tool in bioimaging. A study carried out by A. Bagher5 proposed that QDs with near-infrared emission could be applied to sentinel lymph-node mapping to aid biopsy and surgery,86 as shown in Fig. 8. As reported by Zhang et al.,77 conjugation of QDs with biomolecules, including peptides and antibodies, could be used to target tumours in vivo. This infers that the use of QDs as biosensors and for biomolecule/drug delivery to target cancer sites is possible; therefore the substitution of QDs for CQDs should be the next task, as CQDs demonstrated that they were just as capable as QDs of performing bioimaging successfully, as seen in the study by C. Huang et al.,83 inferring that if they can carry out the same task as QDs for bioimaging they should be able to replicate QDs for use as biosensors and for biomolecule/drug delivery.87,88
4.2 Nanotheranostics and CQDs applied in cancer treatment
As discussed previously, QDs and, in particular due to lack of metal atoms, CQDs have been established as a useful tool in nanotheranostics sensing, medical and imaging applications.2 Their advantageous properties such as their high luminescence characteristic provide a great interest in utilising them as labels in bioanalytical applications.6 Recent research commencing into the use of CQDs to identify cancerous cells in pathology studies by tagging cells that have particular receptor types on their surfaces may show that the tumour is vulnerable to targeted therapies.3 One key component of a CQD's properties that is particularly useful for use in nanotheranostics/drug delivery is that their shell can bind to a number of different structures, including antibodies, peptides, proteins and most interestingly, drug molecules, highlighted in Fig. 10.89 Given this property, it can be proposed that should CQDs bind to specific anti-cancer drugs and provided they remain attached, they could be transported to the tumour site and could help target the cancer cells in a safe way. This necessitates designing a drug delivery system which combines the CQDs, anti-cancer drugs and something to ensure that they are kept bound until they reach the required site.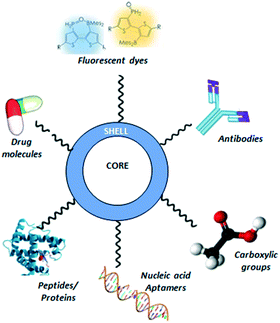 | ||
| Fig. 10 A CQD (centre, core and shell) showing the different types of structures that can be bound to its shell. | ||
4.3 Environmental risks of nanotheranostics
One downfall of employing nano-based therapies is the fate of the residues of the nanomedicine once they have performed in the relevant area and are released into the environment.90 It is not difficult to imagine that residues of nanomedicine or nano-sized drug carriers could have unexpected effects in the environment because it has been already observed for conventional medicine, for which several laboratory studies reported ecotoxicological effects,91 which is caused by the release of chemicals that have been broken down in the drug. Despite this concerning factor, no research studies into exploring the environmental effects of nanomedical products have been published to date.90,92 This makes it imperative to ensure that these potential effects of nanomedicines are investigated fully. Indeed, it is required that when creating a nano-based therapy system, the risks to humans need to be avoided as much as possible, which is achieved by preclinical research using in vivo and in vitro experiments. Because no studies have been done on the experimental risks, this hazard identification strategy should be broadened to include also the environmental fate and effects.90 Although environmental exposures may be very low due to the size of the nanomedicine, they should nonetheless not be discounted when trying to generate an overall environmentally safe and clean nano-based therapy system. Residues of medical products have been recorded in a number of monitoring studies by conventional pharmaceuticals in wastewater, surface water, groundwater and drinking water.91 Hence, the presence of nanomedicine residues in the environment is a challenge that will have to be confronted as soon as possible.90Despite the lack of studies for environmental effects in nanomedicine, the potential adverse effects of nanomaterials on the environment and human health have recently been addressed by research initiatives organized under the National Science Foundation and the U.S. Environmental Protection Agency,93 which can be used as a base foundation for nanomedicines. On the other hand, no obtainable information is currently available regarding routes of QD exposure. QD stability, aerosolization, half-lives, and how they partition into environmental media are currently poorly understood.93 Potential routes of QD exposure are environmental, workplace, and therapeutic or diagnostic administration. Workplace exposures (e.g., engineers, researchers, clinicians) may result from inhalation, dermal contact, or ingestion. In particular, for the inhalation routes, an extensive body of toxicologic research exists on other nanoscale particles (e.g., asbestos, ultrafine particles) that may provide a foundation from which to approach QD inhalation studies. QDs vary in size, ranging from approximately 2.5 nm up to 100 nm, depending on coating thickness due to surface functionalisation, and vary in their sites of deposition in pulmonary tissues once aerosolized. For instance, QDs <2.5 nm may reach the deep lung and interact with the alveolar epithelium, whereas larger aerosolized QDs deposit in bronchial spaces. However, under what conditions QDs aerosolize and whether they form aggregates in ambient air are not known.94 Inhalation exposures may pose potential risks given that QDs have been shown to be incorporated via endocytosis by a variety of cell types and may reside in cells for weeks to months. What risks exposures via dermal absorption and accidental ingestion may pose are currently unknown.93
Another aspect that needs to be considered is the introduction of QDs into environmental media that may occur via waste streams from industries that synthesize or use QDs and via clinical and research settings. Consequently, disposal of QD materials and the risks of leakage and spilling during manufacturing and transport are potential sources of environmental exposure/damage. Environmental exposures are a significant source for several reasons: (a) the environmental concentration of anthropogenic substances increases in direct proportion to their use in society, and QDs, given their wide range of applications, may see substantial production volumes; (b) the half-lives of these materials may be quite long (months to possibly years); and (c) environmental exposure will depend on where these materials partition (e.g., air, water, soil types). Because of the diversity of physicochemical properties among varied types of QDs (Fig. 2), it is likely that exposing the environmental risks of them will be difficult. These are important considerations given that degradation of these materials in environmental media, in the event they reach environmental compartments, will undoubtedly occur, and their rates of decay are likely to be highly variable, depending on both QD physicochemical characteristics and the environmental media in which they partition.93 Due to all of this information that we know, it correlates that as CQDs are a safer alternative to QDs as discussed previously, they must therefore be safer after environmental release.
At present, studies on the toxicity of CQDs are limited to zebrafish (Danio rerio), zooplankton (Daphnia magna), and phytoplankton (Scenedesmus obliquus) which have been assessed for the first time.95 The results of this study indicated that CQDs (up to 200 mg L−1) could be depurated by D. rerio with negligible toxicity. In comparison, CQDs induced mortality and immobility in D. magna with a 48 h EC50 value and LC50 value of 97.5 and 160.3 mg L−1, respectively. In S. obliquus, CQDs inhibited photosynthesis and nutrition absorption in a dose- and time-dependent manner, and the growth of algae was also inhibited with a 96 h EC50 value of 74.8 mg L−1, suggesting that S. obliquus, which belongs to the lowest trophic level in this study, was most sensitive to CQD exposure. Further investigations revealed that CQDs induced an increase in oxidative stress in algae cells and a decrease in pH value of an algae medium, implying that oxidative stress and water acidification may be the mechanisms underlying the toxicity of CQDs to S. obliquus.95 This indicates that CQDs still present a risk to the environment; however, as the safest nanoparticle option for a nanotherapy-based system as well as a safer alternative to QDs, the goal will be to fully study the environmental risks of CQDs and determine a safer release of CQDs into the environment to ensure that they pose little to no environmental risk.
5. Conclusion, CQD challenges and future perspective
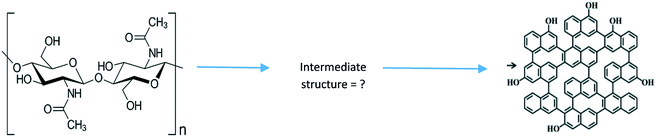
Despite the recent advancements in developing nanotheranostics for cancer therapy, there are still limitations that need to be considered before proposing an overall scheme. Optimistically, the use of QDs for bioimaging has successfully transitioned to using CQDs, as reported by C. Huang et al.83 This is one aspect of cancer therapy that has proved successful by use of CQDs; the question that now arises is whether biosensors and drug delivery will be just as successful as bioimaging by use of CQDs. Another potential downside is that many synthetic methods of CQDs (both top-down and bottom-up) find it hard to have control over the size of CQDs generated;35 if there is no uniformity within the CQDs, there will be difficulty in generating a cancer therapy system. Furthermore, another challenge faced in generating the CQDs comes from the synthesis steps themselves. The overall yield of CQDs produced from recently employed synthetic schemes by use of biomass waste rarely reports a yield higher than 80%.96 While this is a respectably high yield, this is only seen in the most successful synthetic steps and rarely reaches this high in the majority of synthetic schemes.59–61 This can come from several reasons which include that the design of the equipment can sometimes result in leakage. This is a man-made problem which needs to be tackled before commencing the reaction. In addition, in all the synthetic schemes reported, by-products such as bio-oil are generated,55,97 which explains the reduction in yield of CQDs. A future potential trial would be to obtain the bio-oil and recycle it through the reaction conditions that generate CQDs, with slightly altered variables to determine whether more CQDs can be obtained, ensuring that less waste is left and increasing the yield.A prominent question in all of the current literature of synthesis of CQDs from biomass waste is the absence of a general mechanism which converts the starting material into the CQDs by breaking/formation of chemical bonds. By establishing the mechanism that these chemicals go through to form the CQDs (such as using chitin as the precursor to synthesise CQDs, as seen in Fig. 11), then not only could the science behind the CQDs be understood more fully, it would also present clear advantages. One of these is that in future reactions, different reactants could be employed in order to drive the reaction in a certain way required. This would then help dictate what functional groups are present in the CQDs which would give rise to certain properties of the CQDs. Despite this, there are recent advancements which will help to develop a therapeutic system. Much progress, for example, has been made in engineering bright CQD bio-probes with good stability. The downside, however, is that the biocompatibility of the functionalized CQDs is still a critical issue for further applications in live cells, tissues and animals,35 which in essence poses a potential drawback when it will come to trial these CQDs in cancer therapy. Although there is clear evidence that the shell of CQDs can successfully bind to molecules such as drugs/anti-cancer drugs,89 a problem that arises is how successfully will they manage to stay bound whilst travelling though the host, so that when they arrive at the tour site, the CQD can offload the drugs at the intended site. This has proved to be a problem in recent investigations,98 thus the need for further modification to the CQD–drug system to ensure the drugs arrive at the intended site. This in theory can be tackled by the use of layer-by-layer assembly (LbL), a thin film fabrication technique, where the films are formed by depositing alternate layers of oppositely charged materials with wash steps in between.99
This has already been successfully applied with CQDs recently,100 where the CQDs were embedded in 2D layered double hydroxide (LDH) nanosheets through LbL assembly. The resulting ultrathin films (UTFs) presented a long-range ordered structure and also maintained the luminescence properties of the CQDs. Therefore, given that overall this was a facile method and successful with CQDs, it is apparent that this assembly method could help overcome the issue of the drugs detaching from the CQDs at the wrong time. Once the CQDs are synthesised and anti-cancer drugs are determined, this will be the next step in an overall nanotheranostic drug delivery system.
Finally, in this review the recent progress in shifting from the use of QDs to CQDs in cancer therapy due to their advantageous qualities over QDs such as their non-toxicity and facile and inexpensive synthetic methods has been outlined, with clear bias towards the use of CQDs. Though several different methods of synthesising CQDs via a bottom-up or a top-down approach have been proposed and executed successfully by several different sources, the overall consensus concurs that the bottom-up approach to synthesising CQDs is more beneficial over the top-down approach due to its much less toxic, greener and more facile methods. Many studies have argued successfully for the use of biomass waste as precursor for synthesising CQDs as the most favourable due to its abundance in nature and commercially as well as being green/eco-friendly. As proved by recent studies, QDs and CQDs have been successfully utilised in bioimaging of tumours in animals such as mice, and therefore there is valid reason to believe that (1) not only could this test be performed on humans and (2) CQDs could be used for the purpose of biosensors and biomolecular/drug delivery, given that QDs have performed exceptionally in these areas, with minor problems such as toxicity that CQDs could remove.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge support from the EPSRC for the Ph.D. studentship (EPSRC Grant EP/R51309X/1).Notes and references
- N. Kumar and R. Kumar, Chapter 1 – Introduction, in Nanotechnology and Nanomaterials in the Treatment of Life-threatening Diseases, ed. N. Kumar and R. Kumar, William Andrew Publishing, Oxford, 2014, pp. 1–51 Search PubMed.
- A. P. Demchenko and M. O. Dekaliuk, Methods Appl. Fluoresc., 2013, 1(4), 042001 CrossRef CAS PubMed.
- S. Ornes, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(11), 2796–2797 CrossRef CAS.
- L. P. Kouwenhoven, C. M. Marcus, P. L. McEuen, S. Tarucha, R. M. Westervelt and N. S. Wingreen, Electron Transport in Quantum Dots, in Mesoscopic Electron Transport, ed. L. L. Sohn, L. P. Kouwenhoven and G. Schön, Springer Netherlands, Dordrecht, 1997, pp. 105–214 Search PubMed.
- A. M. Bagher, Sens. Transducers J., 2016, 198(3), 37–43 Search PubMed.
- S. Rauf, A. Glidle and J. M. Cooper, Langmuir, 2010, 26(22), 16934–16940 CrossRef CAS PubMed.
- A. M. Wagner, J. M. Knipe, G. Orive and N. A. Peppas, Acta Biomater., 2019, 94, 44–63 CrossRef CAS PubMed.
- P. Juzenas, W. Chen, Y.-P. Sun, M. A. N. Coelho, R. Generalov, N. Generalova and I. L. Christensen, Adv. Drug Delivery Rev., 2008, 60(15), 1600–1614 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. C, 2008, 112(48), 18737–18753 CrossRef CAS.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15(14), 2854–2860 CrossRef CAS.
- M. C. Beard, J. Phys. Chem. Lett., 2011, 2(11), 1282–1288 CrossRef CAS PubMed.
- S. R. Kantelhardt, W. Caarls, A. H. B. de Vries, G. M. Hagen, T. M. Jovin, W. Schulz-Schaeffer, V. Rohde, A. Giese and D. J. Arndt-Jovin, PLoS One, 2010, 5(6), e11323 CrossRef PubMed.
- P. K. De and D. C. Neckers, J. Photochem. Photobiol., A, 2013, 252, 8–13 CrossRef CAS.
- Z. Zhang, K. Edme, S. Lian and E. A. Weiss, J. Am. Chem. Soc., 2017, 139(12), 4246–4249 CrossRef CAS PubMed.
- N. U. H. Alvi, P. E. D. S. Rodriguez, V. J. Gómez, P. Kumar, M. Willander and R. Nötzel, Appl. Phys. Express, 2013, 6(11), 115201 CrossRef.
- L. A. Bentolila, 5 - Photoluminescent quantum dots in imaging, diagnostics and therapy, in Applications of Nanoscience in Photomedicine, ed. M. R. Hamblin and P. Avci, Chandos Publishing, Oxford, 2015, pp. 77–104 Search PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4(6), 435–446 CrossRef CAS PubMed.
- W. C. Chan and S. Nie, Science, 1998, 281(5385), 2016–2018 CrossRef CAS PubMed.
- W. J. Parak, L. Manna and T. Nann, Fundamental Principles of Quantum Dots, In Nanotechnology, 2010 Search PubMed.
- K. M. Tsoi, Q. Dai, B. A. Alman and W. C. W. Chan, Acc. Chem. Res., 2013, 46(3), 662–671 CrossRef CAS PubMed.
- J. Lovric, S. J. Cho, F. M. Winnik and D. Maysinger, Chem. Biol., 2005, 12(11), 1227–1234 CrossRef CAS PubMed.
- K. M. Tsoi, Q. Dai, B. A. Alman and W. C. Chan, Acc. Chem. Res., 2013, 46(3), 662–671 CrossRef CAS PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44(1), 362–381 RSC.
- L. Hu, C. Zhang, G. Zeng, G. Chen, J. Wan, Z. Guo, H. Wu, Z. Yu, Y. Zhou and J. Liu, RSC Adv., 2016, 6(82), 78595–78610 RSC.
- D. L. Zhao and T.-S. Chung, Water Res., 2018, 147, 43–49 CrossRef CAS PubMed.
- A. Abbas, L. T. Mariana and A. N. Phan, Carbon, 2018, 140, 77–99 CrossRef CAS.
- F. Wu, L. Yue, L. Yang, K. Wang, G. Liu, X. Luo and X. Zhu, Colloids Surf., B, 2019, 175, 272–280 CrossRef CAS PubMed.
- Y. Qiao, D. Luo, M. Yu, T. Zhang, X. Cao, Y. Zhou and Y. Liu, Chem. – Eur. J., 2018, 24(9), 2257–2263 CrossRef CAS PubMed.
- X. Wang, Y. Feng, P. Dong and J. Huang, Front. Chem., 2019, 7(671) Search PubMed , https://www.frontiersin.org/articles/10.3389/fchem.2019.00671/full.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22(18), 8764–8766 RSC.
- Y. Qiu, B. Zhou, X. Yang, D. Long, Y. Hao and P. Yang, ACS Appl. Mater. Interfaces, 2017, 9(20), 16848–16856 CrossRef CAS PubMed.
- K. Hola, Y. Zhang, Y. Wang, E. P. Giannelis, R. Zboril and A. L. Rogach, Nano Today, 2014, 9(5), 590–603 CrossRef CAS.
- K. S. Prasad, G. Shruthi and C. Shivamallu, Inorg. Chem. Commun., 2019, 101, 11–15 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48(31), 3686–3699 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2(34), 6921–6939 RSC.
- A. Sharma and J. Das, J. Nanobiotechnol., 2019, 17(1), 92 CrossRef PubMed.
- K. Habiba, V. Makarov, B. Weiner and G. Morell, Fabrication of Nanomaterials by Pulsed Laser Synthesis, 2014, pp. 263–291 Search PubMed.
- B. R. Selvi, D. Jagadeesan, B. S. Suma, G. Nagashankar, M. Arif, K. Balasubramanyam, M. Eswaramoorthy and T. K. Kundu, Nano Lett., 2008, 8(10), 3182–3188 CrossRef CAS PubMed.
- S.-T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y.-P. Sun, J. Am. Chem. Soc., 2009, 131(32), 11308–11309 CrossRef CAS PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22(6), 734–738 CrossRef CAS PubMed.
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, J. Mater. Chem., 2011, 21(8), 2445–2450 RSC.
- H. Ming, Z. Ma, Y. Liu, K. Pan, H. Yu, F. Wang and Z. Kang, Dalton Trans., 2012, 41(31), 9526–9531 RSC.
- A. Prasannan and T. Imae, Ind. Eng. Chem. Res., 2013, 52(44), 15673–15678 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126(40), 12736–12737 CrossRef CAS PubMed.
- R. Sharma, A. K. Sharma and V. Sharma, Cogent Engineering, 2015, 2(1), 1094017 CrossRef.
- P. Roy, P.-C. Chen, A. P. Periasamy, Y.-N. Chen and H.-T. Chang, Mater. Today, 2015, 18(8), 447–458 CrossRef CAS.
- D. Huang, H. Zhou, Y. Wu, T. Wang, L. Sun, P. Gao, Y. Sun, H. Huang, G. Zhou and J. Hu, Carbon, 2019, 142, 673–684 CrossRef CAS.
- W. Meng, X. Bai, B. Wang, Z. Liu, S. Lu and B. Yang, Energy Environ. Mater., 2019, 2(3), 172–192 CrossRef CAS.
- H. Yu, X. Li, X. Zeng and Y. Lu, Chem. Commun., 2016, 52(4), 819–822 RSC.
- V. Nguyen, L. Yan, J. Si and X. Hou, J. Appl. Phys., 2015, 117(8), 084304 CrossRef.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128(24), 7756–7757 CrossRef CAS PubMed.
- X. Li, H. Wang, Y. Shimizu, A. Pyatenko, K. Kawaguchi and N. Koshizaki, Chem. Commun., 2011, 47(3), 932–934 RSC.
- J. Chrzanowska, J. Hoffman, A. Małolepszy, M. Mazurkiewicz, T. A. Kowalewski, Z. Szymanski and L. Stobinski, Phys. Status Solidi B, 2015, 252(8), 1860–1867 CrossRef CAS.
- J. Wang, C.-F. Wang and S. Chen, Angew. Chem., Int. Ed., 2012, 51(37), 9297–9301 CrossRef CAS PubMed.
- C. J. Lupa, R. S. Wylie, A. Shaw, A. Al-Shamma'a, J. A. Sweetman and B. M. J. Herbert, Fuel Process. Technol., 2012, 97, 79–84 CrossRef CAS.
- Q. Li, S. Zhang, L. Dai and L.-S. Li, J. Am. Chem. Soc., 2012, 134(46), 18932–18935 CrossRef CAS PubMed.
- D. Tang, J. Liu, X. Wu, R. Liu, X. Han, Y. Han, H. Huang, Y. Liu and Z. Kang, ACS Appl. Mater. Interfaces, 2014, 6(10), 7918–7925 CrossRef CAS PubMed.
- C. Russo, B. Apicella and A. Ciajolo, Sci. Rep., 2019, 9(1), 14566 CrossRef PubMed.
- S. Ahirwar, S. Mallick and D. Bahadur, ACS Omega, 2017, 2(11), 8343–8353 CrossRef CAS PubMed.
- P. A. N. D. Yro, G. M. O. Quaichon, R. A. T. Cruz, C. S. Emolaga, M. C. O. Que, E. R. MagdaluyoJr and B. A. Basilia, AIP Conf. Proc., 2019, 2083(1), 020007 CrossRef.
- T. Shen, Q. Wang, Z. Guo, J. Kuang and W. Cao, Ceram. Int., 2018, 44(10), 11828–11834 CrossRef CAS.
- C. Zhu, J. Zhai and S. Dong, Chem. Commun., 2012, 48(75), 9367–9369 RSC.
- Y. A. Shchipunov, O. N. Khlebnikov and V. E. Silant'ev, Polym. Sci., Ser. B, 2015, 57(1), 16–22 CrossRef CAS.
- S. Kaur, G. S. Dhillon, S. J. Sarma, S. K. Brar, K. Misra and H. S. Oberoi, Waste Biomass: A Prospective Renewable Resource for Development of Bio-Based Economy/Processes, in Biotransformation of Waste Biomass into High Value Biochemicals, ed. S. K. Brar, G. S. Dhillon and C. R. Soccol, Springer New York, New York, NY, 2014, pp. 3–28 Search PubMed.
- J. Zhou, Z. Sheng, H. Han, M. Zou and C. Li, Mater. Lett., 2012, 66(1), 222–224 CrossRef CAS.
- A. Su, D. Wang, X. Shu, Q. Zhong, Y. Chen, J. Liu and Y. Wang, Chem. Res. Chin. Univ., 2018, 34(2), 164–168 CrossRef CAS.
- S. Ghosh, K. Ghosal, S. A. Mohammad and K. Sarkar, Chem. Eng. J., 2019, 373, 468–484 CrossRef CAS.
- A. Dager, T. Uchida, T. Maekawa and M. Tachibana, Sci. Rep., 2019, 9(1), 14004 CrossRef PubMed.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48(70), 8835–8837 RSC.
- H. Xu, L. Xie and M. Hakkarainen, ACS Sustainable Chem. Eng., 2017, 5(6), 5360–5367 CrossRef CAS.
- C. Cheng, Y. Shi, M. Li, M. Xing and Q. Wu, Mater. Sci. Eng., C, 2017, 79, 473–480 CrossRef CAS PubMed.
- D. Chowdhury, N. Gogoi and G. Majumdar, RSC Adv., 2012, 2(32), 12156–12159 RSC.
- K. Shubhika, Int. J. Drug Dev. Res., 2013, 5, 1–10 Search PubMed.
- M. Fang, C.-W. Peng, D.-W. Pang and Y. Li, Cancer Biol. Med., 2012, 9(3), 151–163 CAS.
- M. Stroh, J. P. Zimmer, D. G. Duda, T. S. Levchenko, K. S. Cohen, E. B. Brown, D. T. Scadden, V. P. Torchilin, M. G. Bawendi, D. Fukumura and R. K. Jain, Nat. Med., 2005, 11(6), 678–682 CrossRef CAS.
- J. Wang, W. H. Yong, Y. Sun, P. T. Vernier, H. P. Koeffler, M. A. Gundersen and L. Marcu, J. Biomed. Opt., 2007, 12(4), 044021 CrossRef PubMed.
- H. Zhang, D. Yee and C. Wang, Nanomedicine, 2008, 3(1), 83–91 CrossRef CAS PubMed.
- M. Fang, M. Chen, L. Liu and Y. Li, J. Biomed. Nanotechnol., 2017, 13(1), 1–16 CrossRef CAS PubMed.
- A. M. Derfus, W. Chan and S. N. Bhatia, Nano Lett., 2004, 4(1), 11–18 CrossRef CAS PubMed.
- J. Ashree, Q. Wang and Y. Chao, Front. Chem. Sci. Eng., 2020, 14(3), 365–377 CrossRef CAS.
- B. Cui, X.-T. Feng, F. Zhang, Y.-L. Wang, X.-G. Liu, Y.-Z. Yang and H.-S. Jia, New Carbon Materials, 2017, 32(5), 385–401 CrossRef.
- S. Shaikh, F. U. Rehman, T. Du, H. Jiang, L. Yin, X. Wang and R. Chai, ACS Appl. Mater. Interfaces, 2018, 10(31), 26056–26063 CrossRef CAS.
- C. Huang, H. Dong, Y. Su, Y. Wu, R. Narron and Q. Yong, Nanomaterials, 2019, 9(3), 387 CrossRef CAS PubMed.
- T. He, N. Niu, Z. Chen, S. Li, S. Liu and J. Li, Adv. Funct. Mater., 2018, 28(11), 1706196 CrossRef.
- X. Zhang, H. Wang, C. Ma, N. Niu, Z. Chen, S. Liu, J. Li and S. Li, Mater. Chem. Front., 2018, 2(7), 1269–1275 RSC.
- D. Hu, P. Zhang, P. Gong, S. Lian, Y. Lu, D. Gao and L. Cai, Nanoscale, 2011, 3(11), 4724–4732 RSC.
- M. J. Molaei, RSC Adv., 2019, 9(12), 6460–6481 RSC.
- Q. Zeng, D. Shao, X. He, Z. Ren, W. Ji, C. Shan, S. Qu, J. Li, L. Chen and Q. Li, J. Mater. Chem. B, 2016, 4(30), 5119–5126 RSC.
- M. E. Díaz-García and R. Badía-Laíño, Fluorescence | Fluorescence Labeling☆, in Encyclopedia of Analytical Science, ed. P. Worsfold, C. Poole, A. Townshend and M. Miró, Academic Press, Oxford, 3rd edn, 2019, pp. 266–280 Search PubMed.
- A. Baun and S. F. Hansen, Nanomedicine, 2008, 3(5), 605–608 CrossRef PubMed.
- E. Zuccato, S. Castiglioni, R. Fanelli, G. Reitano, R. Bagnati, C. Chiabrando, F. Pomati, C. Rossetti and D. Calamari, Environ. Sci. Pollut. Res., 2006, 13(1), 15–21 CrossRef CAS PubMed.
- S. Foss Hansen, B. H. Larsen, S. I. Olsen and A. Baun, Nanotoxicology, 2007, 1(3), 243–250 CrossRef.
- R. Hardman, Environ. Health Perspect., 2006, 114(2), 165–172 CrossRef.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113(7), 823–839 CrossRef PubMed.
- K. Yao, X. Lv, G. Zheng, Z. Chen, Y. Jiang, X. Zhu, Z. Wang and Z. Cai, Environ. Sci. Technol., 2018, 52(24), 14445–14451 CrossRef CAS PubMed.
- S. Jing, Y. Zhao, R.-C. Sun, L. Zhong and X. Peng, ACS Sustainable Chem. Eng., 2019, 7(8), 7833–7843 CrossRef CAS.
- C.-M. Liu and S.-Y. Wu, Renewable Energy, 2016, 96, 1056–1062 CrossRef CAS.
- J. Pardo, Z. Peng and R. M. Leblanc, Molecules, 2018, 23(2), 378 CrossRef PubMed.
- F. Hua and Y. M. Lvov, Chapter 1 - Layer-by-layer assembly, in The New Frontiers of Organic and Composite Nanotechnology, ed. V. Erokhin, M. K. Ram and O. Yavuz, Elsevier, Amsterdam, 2008, pp. 1–44 Search PubMed.
- W. Liu, S. Xu, Z. Li, R. Liang, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2016, 28(15), 5426–5431 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |