 Open Access Article
Open Access ArticleAlkyl chain elongation and acyl group effects in a series of Eu/Tb complexes with hexadentate π-electronic skeletons and their enhanced luminescence in solutions†
Shuhei
Ogata
a,
Naoto
Goto
a,
Shoya
Sakurai
a,
Ayumi
Ishii
 ab,
Miho
Hatanaka
ab,
Miho
Hatanaka
 *bc,
Koushi
Yoshihara
a,
Ryota
Tanabe
a,
Kyosuke
Kayano
a,
Ryo
Magaribuchi
a,
Kenta
Goto
d and
Miki
Hasegawa
*bc,
Koushi
Yoshihara
a,
Ryota
Tanabe
a,
Kyosuke
Kayano
a,
Ryo
Magaribuchi
a,
Kenta
Goto
d and
Miki
Hasegawa
 *a
*a
aCollege of Science and Engineering, Aoyama Gakuin University, 5-10-1 Fuchinobe, Chuo-ku, Sagamihara, Kanagawa 252-5258, Japan. E-mail: hasemiki@chem.aoyama.ac.jp; Fax: +81-42-759-6221
bJST, PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
cInstitute for Research Initiatives, Division for Research Strategy, Graduate School of Materials Science, Data Science Center, Nara Institute for Science and Technology, 8916-5 Takayama-cho, Ikoma, Nara 630-0192, Japan. E-mail: hatanaka@ms.naist.ac.jp
dInstitute for Materials Chemistry and Engineering, Kyushu University, 744 Moto-oka, Fukuoka 819-0395, Japan
First published on 14th May 2018
Abstract
Five Eu complexes with long alkyl chain groups, abbreviated as EuLCx (“x” indicates the number of methylene groups: x = 8, 12, 14, 18, and 22), were synthesized to evaluate their structural and luminescence properties in chloroform. The mother helicate Eu complex, EuL, which has two bipyridine moieties bridged by an ethylenediamine, has been previously reported. A reduced form in which the azomethine groups of L also coordinated to the Eu ion, EuLH, was newly prepared. EuLH also adopts a helicate molecular structure based on single crystal X-ray structural analysis. The amine hydrogens of the bridging ethylenediamine of LH are active sites for substitution and were exchanged with five different alkyl chains to form EuLCx. Luminescence band positions and shapes of EuLCx in chloroform were completely identical, with a quantum yield of 37.1 ± 1.2 and a lifetime of around 1.25 ms. This indicates that the environments surrounding the Eu ion in the various complexes are all similar. Luminescence quantum yields of TbLH and TbLC18 are also strengthened, 48.7% in acetonitrile and 55% in chloroform, respectively. Potential energy surfaces were also described by using density functional theory, suggesting the possibility of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex of Eu and the ligand as a main luminescent species in solutions. This 1
2 complex of Eu and the ligand as a main luminescent species in solutions. This 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexation forms Eu–oxygen coordination using acyl groups. It indicates that the acyl group modification results in a different structure from the mother complexes.
2 complexation forms Eu–oxygen coordination using acyl groups. It indicates that the acyl group modification results in a different structure from the mother complexes.
1. Introduction
An advantage of lanthanide (Ln) complexes with π-electronic ligands is their stable luminescence colours originating from f–f transitions of Ln ions, which are strengthened by an antenna effect.1,2 Based on this feature, luminescent Ln complexes have attracted much interest in applications for counterfeiting tags, devices, sensors, bioimaging and luminescent probes.3–5Alkyl chain groups attached to a molecular structure can control not only the molecular size but also the chemical/physical properties such as hydrophilicity/hydrophobicity and solubility. It is well known that alkyl chain groups play an important role in biological systems.6–8 For instance, phospholipids such as sphingomyelin and glycerophospholipid possess hydrophobic and hydrophilic moieties, and form phospholipid bilayers based on hydrophobic forces. The hydrophobic forces not only control the folding of proteins, but also help in maintaining their unique structures against deformation. Such a surfactant behaviour of the alkyl groups also has much potential in the fabrication of hybridized materials. In fact, we recently reported9 a red-emissive graphene sheet hybridized with a Eu complex, EuLC18, and studied its fundamental chemical properties.
Previously, we reported the mother complexes of Ln with helicate skeletons (LnL; Ln = Nd, Eu, Gd, Tb, Dy, and Ho).10,11 The hexadentate ligand, L (Scheme 1), consists of two bipyridine (bpy) moieties bridged with an ethylenediamine. From single crystal X-ray structural analyses, all LnL complexes are isostructural. EuL in acetonitrile shows luminescence bands at 580, 595, 615, 650, and 685 nm by the irradiation of UV light. The luminescence and structural behaviours of LnL were stable even in acetonitrile due to the chelate effect. After the reduction of the azomethine moieties of LnL, derivatives that retained the luminescence were synthesized.12 For instance, EuLCOOH shows high water solubility and luminescence with the same luminescence properties in the pH range of 2.6–9.7. The two carboxylic groups play different roles for the solubility and half-capsulation of the molecular form. We could also obtain EuLCOOH, which was derived from the reduced form of EuL. Here, a series of lanthanide complexes with various lengths of alkyl chains combined with LH (abbreviated as EuLCx, x = 8, 12, 14, 18, and 22) were synthesized (EuLC8, EuLC12, EuLC14, EuLC18, and EuLC22) and examined (Scheme 1). The mother molecule, EuLH, was also newly prepared in order to clarify the effect of the alkyl chain groups. Each ligand acts as a photo-antenna to sensitize the f–f emission localized on EuIII. The electronic absorption spectra, luminescence spectra, absolute quantum yields and lifetimes of EuLH, EuLC8, EuLC12, EuLC14, EuLC18, and EuLC22 were examined to elucidate their luminescence behaviour. Furthermore, the luminescent species and its stoichiometry of the Eu complex with LC2 as a model molecule were elucidated by using density functional theory (DFT) calculations supporting mass spectroscopy.
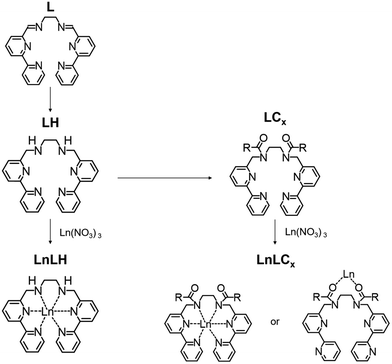 | ||
| Scheme 1 Syntheses of LnLCx and LnLH. R and x indicate the alkyl group and its number of hydrocarbons, respectively. | ||
2 Results and discussion
2.1 Structural analyses of Eu complexes with LH and LC8
Each bpy skeleton is planar and the distance between the two nearest carbon atoms located on the pyridine edges is ca. 3.28 Å. This value is higher than that of EuL by 0.1 Å, suggesting that the structures of EuL and EuLH are slightly different due to the azomethine reduction.
The Z-value of the unit cell was 4 (Table 1 and Fig. S1†), indicating that a couple of different structures of EuLH exist within the cell. Furthermore, four nitrate ions as counterions and six acetonitrile molecules are included in the unit cell. From the above results, there is enough space at the hydrogen atoms of azomethine's nitrogen to add alkyl chains due to the sp3 hybridization of nitrogen atoms, as described above.
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2. | |
|---|---|
| Formula | C27H28.5EuN10.5O9 |
| Formula weight | 796.05 |
| Crystal size (mm) | 0.137 × 0.162 × 0.063 |
| Crystal system | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 11.3057(4) |
| b (Å) | 15.635(2) |
| c (Å) | 19.340(2) |
| α (°) | 106.1440(10) |
| β (°) | 91.3930(10) |
| γ (°) | 105.6950(10) |
| V (Å3) | 3143.02 |
| Z value | 4 |
| D calcd (mg m−3) | 1.682 |
| μ (Mo Kα) (mm−1) | 2.065 |
| F(000) | 1596 |
| λ (Mo Kα) (Å) | 0.71073 |
| Temperature (K) | 90 |
| R 1 (I > 2.00σ(I)) | 0.0309 |
| wR2b (I > 2.00σ(I)) | 0.0677 |
| Goodness of the fit | 1.023 |
| Largest peak and hole (e Å−3) | 1.071, −0.609 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of Eu and the ligand. The latter one corresponds to a 1
1 stoichiometry of Eu and the ligand. The latter one corresponds to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric species of Eu and LC8. As described later, the electronic absorption band and luminescence properties of the Eu complex with LC8 differ from those of EuLH in solutions. Especially, there is only single component of the Eu complex with LC8 in the luminescence lifetime. Thus, the stoichiometry or coordination sphere of Eu complexes with LCx in solutions should be defined.
2 stoichiometric species of Eu and LC8. As described later, the electronic absorption band and luminescence properties of the Eu complex with LC8 differ from those of EuLH in solutions. Especially, there is only single component of the Eu complex with LC8 in the luminescence lifetime. Thus, the stoichiometry or coordination sphere of Eu complexes with LCx in solutions should be defined.
According to the DFT calculations, the Gibbs free energy of the model 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex Eu(LC2)2(NO3)2 was about 60 kcal mol−1 more stable than those of the model 1
2 complex Eu(LC2)2(NO3)2 was about 60 kcal mol−1 more stable than those of the model 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes EuLC2(NO3)2, which indicated that EuLCx could exist as 1
1 complexes EuLC2(NO3)2, which indicated that EuLCx could exist as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes. In the 1
2 complexes. In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex, Eu was coordinated by two oxygen atoms of acyl groups instead of the nitrogen atoms of bpy moieties (Fig. 2).
2 complex, Eu was coordinated by two oxygen atoms of acyl groups instead of the nitrogen atoms of bpy moieties (Fig. 2).
2.2 Electronic absorption and luminescence of Eu complexes
The excited triplet level of the ligand, which is the key to sensitize the f–f emission, can be estimated from the phosphorescence bands of the Gd complexes (Fig. 3).13–15 The phosphorescence band of GdLH in ethanol appears at 410 nm (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cm−1). The ligand LH acts as a sensitizer for Eu. The emission bands of EuLH were observed at 580.5, 592.3, 616.6, 649.7, and 686.2 nm in acetonitrile, assigned to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions of EuIII, respectively.
400 cm−1). The ligand LH acts as a sensitizer for Eu. The emission bands of EuLH were observed at 580.5, 592.3, 616.6, 649.7, and 686.2 nm in acetonitrile, assigned to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions of EuIII, respectively.
The excitation spectrum of EuLH corresponds to its absorption spectrum (Fig. S3†). The absolute luminescence quantum yield (ϕL–Ln) and luminescence lifetime (τobs) of EuLH in acetonitrile are 5.3% and 0.27 ms, respectively (Fig. S4†). The radiative rate constant (kR), the non-radiative rate constant (kNR), the efficiency of the metal centred luminescence (ϕLn–Ln) and the efficiency of energy transfer from the triplet state of the ligand to Eu (ηEnT) are calculated from the equations shown in the ESI† using the above values. The values of kR, kNR, and ηEnT of EuLH are 267 (s−1), 3437 (s−1), and 74%, respectively. The value of kNR is much higher than that of EuLCx as described later.
 | ||
| Fig. 4 Electronic absorption spectra of Eu (red line) and Gd (gray line) complexes with LC8 (a), LC12 (b), LC14 (c), LC18 (d), and LC22 (e). Dotted lines correspond to free ligands. | ||
The luminescence spectra of EuLC8, EuLC12, EuLC14, EuLC18, and EuLC22 in chloroform are shown in Fig. 5. EuLCx also luminesces in the red wavelength region. EuLC8 shows luminescence bands at 581.0, 592.5, 615.3, 649.8, and 685.0 nm in chloroform and these bands are assigned to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions of EuIII, respectively. The corresponding bands of EuLC12, EuLC14, EuLC18, and EuLC22 with different chain lengths appeared at the same positions. The spectral shapes of the series of EuLCx are completely identical indicating that the environment surrounding the Eu ion is similar for all the members of the series. Each excitation spectrum monitored at the f–f emission band positions reproduces well each electronic absorption spectrum assigned to the lowest excited state of LCx (Fig. S5†).
 | ||
| Fig. 5 Luminescence spectra of EuLC8 (a), EuLC12 (b), EuLC14 (c), EuLC18 (d), and EuLC22 (e) in chloroform. λex = 290 nm. | ||
To evaluate the energy donor level of each ligand as well as EuLH, Gd complexes with LCx were used to determine the excited triplet state by the measurement of phosphorescence at a low temperature (Fig. 6). The band of GdLC18 in solutions at 77 K appears around 425–600 nm as a broad band with some peaks and is slightly red-shifted more than that of GdLH. The band is reproduced well for other Gd complexes with LCx, meaning that a series of different alkylation ligands LCx can maintain the triplet state in their π-electronic systems. The fluorescence bands localized on the ligand of GdLCx at 332 nm in solutions reproduces that of GdLH which remains on the blue-side in GdL (Fig. S6†). GdLCx has a relatively clear shoulder around 360 nm. These results will maintain the possibility of a quasi-sp2 conformation around the reduced azomethine moieties of the ligand LCx after the complexation.
 | ||
| Fig. 6 Phosphorescence spectra of GdLC8 (a), GdLC12 (b), GdLC14 (c), GdLC18 (d), and GdLC22 (e) in ethanol at 77 K. λex = 290 nm. | ||
The photophysical properties of EuLC8, EuLC12, EuLC14, EuLC18, and EuLC22 are summarized in Table 2 and Fig. S7.† After the introduction of two long alkyl chain groups into the ligand, the values of ϕL–Ln were over 30% in a series of EuLCx which drastically changed compared with that in EuLH.
| φ L–Ln | τ (ms) | k R (s−1) | k NR (s−1) | φ Ln–Ln | η EnT | |
|---|---|---|---|---|---|---|
| EuLC8 | 38.6% | 1.24 | 539 | 261 | 67% | 57% |
| EuLC12 | 37.4% | 1.25 | 539 | 261 | 67% | 56% |
| EuLC14 | 35.6% | 1.25 | 543 | 257 | 68% | 52% |
| EuLC18 | 38.6% | 1.25 | 538 | 262 | 68% | 58% |
| EuLC22 | 35.2% | 1.25 | 543 | 258 | 68% | 52% |
| EuLH | 5.3% | 0.27 | 267 | 3437 | 7% | 74% |
The τobs value of EuLC8 is 1.24 ms as a single component and corresponds to those of EuLC12, EuLC14, EuLC18, and EuLC22 in chloroform. The value of kNR drastically decreased after the introduction of alkyl chain groups. Thus, the two long alkyl chain groups are important to suppress the non-radiative relaxation process and sensitize the luminescence of the Eu ion. The kR values of the series of EuLCx are similar, indicating that the environments around the Eu ion of EuLCx are the same. This is supported by spectral measurements. The ηEnT values of EuLCx are lower than that of EuLH. The molecular size-controlled Eu complexes with long alkyl chains were developed while maintaining the luminescence of the Eu ion.
The difference of luminescence properties between EuLCx and EuLH could be explained by the potential energy profiles (Fig. 7). The triplet energy levels of LC2 and LH were similar because both of their excitations were localized on one of the bpy moieties.
From the triplet state, the energy transfer to Eu and quenching via the intersystem crossing could take place through the minimum energy crossing points between potential energy surfaces. The activation barrier for the quenching via the intersystem crossing between the triplet state and the ground state of EuLH was larger than that of EuLC2, which resulted in a larger kNR of EuLH. The activation barriers for the energy transfer were almost zero both for EuLCx and EuLH. However, the rate of the energy transfer for EuLCx could be slower than that of EuLH because the distance between Eu and excited bpy moieties in EuLC2 was longer than that in EuLH,16 which is consistent with a smaller ηEnT of EuLCx.
2.3 Luminescence properties of TbLH in acetonitrile and TbLC18 in chloroform
As reported previously, Eu and Tb complexes with the mother ligand L adopt the same molecular shape in the helicate form and luminesce from the center metals by UV excitation. To explore the possibilities of other Ln metals, we synthesized a Tb complex with LC18 as a white powder (see the Experimental section).The electronic absorption and luminescence spectra of TbLH and TbLC18 in chloroform are shown in Fig. 8. The π–π* absorption bands of TbLH in acetonitrile appear at the corresponding positions of EuLH. TbLC18 shows absorption bands at 290, 300 (sh) and 315 (sh) nm assigned to the π–π* transition. The absorption band position of TbLC18 appeared at a shorter wavelength than that of TbL,10 indicating that the electronic state of the ligand changed after the reduction of the azomethine moieties and the introduction of the alkyl chain groups.
 | ||
| Fig. 8 Electronic absorption and luminescence spectra of TbLH (top: λex = 305 nm) in acetonitrile and TbLC18 (bottom: λex = 290 nm) in chloroform. | ||
We recently suggested that the reduction of L to LH will theoretically enhance the strong luminescence of the terbium ion.17 Consequently, we experimentally realized the strong f–f emission of TbLH in acetonitrile. Thus, the band positions at 491.0, 545.0, 583.5, 624.5, and 647.5 nm are assigned to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, 5D4 → 7F3, and 5D4 → 7F2 transitions of TbIII, respectively, and the luminescence quantum yield becomes 48.7%, which is much higher than that of TbL (<0.1%) in acetonitrile.10 In the case of TbL, the lowest minimum energy crossing from T1 to S0 is only 0.4 kcal mol−1 and the relaxation from the T1 to S0 occurs preferentially to transfer energy from the T1 to 5D4 resulting in a low quantum yield. In contrast, the energy level of the T1 state of TbLH becomes higher than that of TbL as mentioned above (section 2.2.1). The energy barrier between the T1 and S0 also becomes higher. Thus, the absolute quantum yield in TbLH remarkably increased with the comparison of that in TbL. The luminescence decay curve demonstrates a single component with a lifetime of 1.66 ms (Fig. S8(a)†).
The luminescence bands of TbLC18 in chloroform observed at 492.3, 547.2, 584.1, 625.9, and 650.1 nm are assigned to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, 5D4 → 7F3, and 5D4 → 7F2 transitions of TbIII, respectively. The mother molecule TbL shows negligibly weak luminescence in acetonitrile,10 whereas the absolute luminescence quantum yield of TbLC18 in chloroform is 55%, which is significantly higher than that of TbLH. The luminescence lifetime of this compound is 1.48 ms (Fig. S8(b)†). Thus, the reduction of the azomethine moieties and the introduction of the alkyl chain groups clearly affect the luminescence properties of TbIII in solutions. The alkylation of the ligand in the Tb complex such as TbLC18 results in more efficient luminescence than those in EuLC18. It was caused by a higher energy donor level of the ligand LC18 positioning at the acceptor level of TbIII compared with the mother molecule L.
3. Experimental
3.1 Reagents and materials
Commercially available reagents and spectral-grade solvents (Wako Pure Chemical Industries Ltd, Tokyo Chemical Industry Ltd, and Kanto Chemical Co. Inc.) were generally used without further purification.1H-NMR spectra were recorded on a JEOL JMN-500 II spectrometer in CDCl3 with tetramethylsilane. The X-ray structural data for EuLH were collected on a Bruker Smart APEX-II CCD diffractometer equipped with graphite monochromated Mo Kα radiation at 90 K. The data were collected to a maximum 2 h value of 55° and processed using the Bruker APEX-II software package.18 The structure was solved by direct methods and refined by full-matrix least-squares calculations using a SHELX-97.19–21 All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were located at the idealized positions. A summary of the fundamental crystal data and experimental parameters used to determine the structure of EuLH is given in the ESI.† CCDC 1813448.† Fast Atomic Bombardment (FAB-MS) was performed using the MStation JMS-700A (JEOL). Electron spray ionization mass spectroscopy (ESI-MS) was carried out by using JMS-T100CS (JEOL) and LCMS-8040 (Shimadzu) for EuLC8 in acetonitrile and EuLC18 and TbLC18 in chloroform. Electronic absorption spectra were observed by using a UV-3600S (Shimadzu). Luminescence and excitation spectra were recorded on a Fluorolog 3–22 (Horiba Jobin Yvon). Absolute luminescence quantum yields and luminescence lifetimes were determined using an absolute luminescence quantum yield C9920-02 spectrometer (Hamamatsu Photonics K. K.) and a Quantaurus-Tau C11367-12 spectrometer (Hamamatsu Photonics K. K.), respectively, with pulsed excitation light sources.
3.2 Syntheses
TbLH was prepared similarly to EuLH using Tb(NO3)3·6H2O (50.4 mg, 111.2 μmol). Yield: 58.1 mg (76%). MS (FAB+); m/z, 679 [M − (NO3−)]+ (calcd. 679.43).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Yield: 215.7 mg (72%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(a)†]: δ 8.66 (m, 2H), 8.30 (m, 4H), 7.75 (m, 4H), 7.29 (m, 4H), 4.78 (m, 4H), 3.70 (s, 4H), 2.38 (t, 4H), 1.60 (m, 4H), 1.25 (m, 16H), 0.88 (t, 6H). MS (FAB+): m/z 648 [M + H+]+ (calcd. 648.42). Elemental analysis, calcd for [LC8] (C40H52N6O2): C 74.04, H 8.08, N 12.95; found: C 73.89, H 7.91, N 12.89.
3). Yield: 215.7 mg (72%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(a)†]: δ 8.66 (m, 2H), 8.30 (m, 4H), 7.75 (m, 4H), 7.29 (m, 4H), 4.78 (m, 4H), 3.70 (s, 4H), 2.38 (t, 4H), 1.60 (m, 4H), 1.25 (m, 16H), 0.88 (t, 6H). MS (FAB+): m/z 648 [M + H+]+ (calcd. 648.42). Elemental analysis, calcd for [LC8] (C40H52N6O2): C 74.04, H 8.08, N 12.95; found: C 73.89, H 7.91, N 12.89.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 288.5 mg (82%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(b)†]: δ 8.67 (m, 2H), 8.31 (m, 4H), 7.75 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.71 (s, 4H), 2.41 (t, 4H), 1.64 (m, 4H), 1.21 (m, 32H), 0.87 (t, 6H). MS (FAB+): m/z 760 [M + H+]+ (calcd. 760.54).
4). Yield: 288.5 mg (82%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(b)†]: δ 8.67 (m, 2H), 8.31 (m, 4H), 7.75 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.71 (s, 4H), 2.41 (t, 4H), 1.64 (m, 4H), 1.21 (m, 32H), 0.87 (t, 6H). MS (FAB+): m/z 760 [M + H+]+ (calcd. 760.54).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 216.7 mg (80%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(c)†]: δ 8.66 (m, 2H), 8.32 (m, 4H), 7.79 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.70 (s, 4H), 2.38 (t, 4H), 1.62 (m, 4H), 1.24 (m, 40H), 0.87 (t, 6H). MS (FAB+): m/z 816 [M + H+]+ (calcd. 816.60).
4). Yield: 216.7 mg (80%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(c)†]: δ 8.66 (m, 2H), 8.32 (m, 4H), 7.79 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.70 (s, 4H), 2.38 (t, 4H), 1.62 (m, 4H), 1.24 (m, 40H), 0.87 (t, 6H). MS (FAB+): m/z 816 [M + H+]+ (calcd. 816.60).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 253.5 mg (66%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(d)†]: δ 8.68 (m, 2H), 8.32 (m, 4H), 7.80 (m, 4H), 7.31 (m, 4H), 4.79 (m, 4H), 3.72 (s, 4H), 2.40 (t, 4H), 1.64 (m, 4H), 1.27 (d, 56H), 0.89 (t, 6H). MS (FAB+): m/z 930 [M + H+]+ (calcd. 929.73).
4). Yield: 253.5 mg (66%). 1H-NMR [500.00 MHz, CDCl3, Fig. S9(d)†]: δ 8.68 (m, 2H), 8.32 (m, 4H), 7.80 (m, 4H), 7.31 (m, 4H), 4.79 (m, 4H), 3.72 (s, 4H), 2.40 (t, 4H), 1.64 (m, 4H), 1.27 (d, 56H), 0.89 (t, 6H). MS (FAB+): m/z 930 [M + H+]+ (calcd. 929.73).
LH (166.2 mg, 419.1 μmol) was dissolved in tetrahydrofuran (10 mL) and n-docosanoyl chloride in tetrahydrofuran (2 mL) was slowly added. Next, triethylamine (1030 mg, 10.1 mmol) in tetrahydrofuran (1 mL) was added to the mixture and the reaction mixture was stirred overnight. A white precipitate was filtered out and the filtrate was evaporated to dryness. The product was purified by column chromatography (chloroform/ethyl acetate, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Yield: 346.3 mg (79%). 1H-NMR (500.00 MHz, CDCl3, Fig. S9(e)†): δ 8.66 (m, 2H), 8.31 (m, 4H), 7.79 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.70 (m, 4H), 2.39 (m, 4H), 1.62 (m, 4H), 1.26 (m, 72H), 0.88 (t, 6H). MS (FAB+): m/z 1041 [M + H]+ (calcd. 1040.85).
4). Yield: 346.3 mg (79%). 1H-NMR (500.00 MHz, CDCl3, Fig. S9(e)†): δ 8.66 (m, 2H), 8.31 (m, 4H), 7.79 (m, 4H), 7.30 (m, 4H), 4.78 (m, 4H), 3.70 (m, 4H), 2.39 (m, 4H), 1.62 (m, 4H), 1.26 (m, 72H), 0.88 (t, 6H). MS (FAB+): m/z 1041 [M + H]+ (calcd. 1040.85).
GdLC8 was prepared similarly to EuLC8 using Gd(NO3)3·6H2O (13.6 mg, 31.3 μmol). Yield: 21.3 mg (71%). MS (FAB+): m/z 930 [M − (NO3−)]+ (calcd. 930.15). Elemental analysis, calcd for [GdLC8] (C40H52GdN9O11): C 48.42, H 5.28, N 12.71; found: C 48.79, H 5.50, N 12.52.
GdLC12 was prepared similarly to EuLC12 using Gd(NO3)3·6H2O (47.4 mg, 105 μmol). Yield: 71.2 mg (67%). MS (FAB+): m/z 1042 [M − (NO3−)]+ (calcd. 1042.37).
GdLC14 was prepared similarly to EuLC14 using Gd(NO3)3·6H2O (27.5 mg, 60.9 μmol). Yield: 38.3 g (98%). MS (FAB+): m/z 1098 [M − (NO3−)]+ (calcd. 1098.48). Elemental analysis, calcd for [GdLC14] (C52H76GdN9O11): C 54.20, H 6.86, N 10.61; found: C 53.82, H 6.60, N 10.86.
GdLC18 was prepared similarly to EuLC18 using Gd(NO3)3·6H2O (19.2 mg, 42.7 μmol). Yield: 13.0 mg (25%). MS (FAB+): m/z 1211 [M − (NO3−)]+ (calcd. 1210.69).
TbLC18 was also synthesized by using Tb(NO3)3·6H2O (16.0 mg, 35.7 μmol). Yield: 10.0 mg (26%), MS (FAB+): m/z 1212 [M − (NO3−)]+ (calcd. 1211.63). ESI-MS in chloroform (Fig. S2(c)†): m/z 1211.7 [M − (NO3−)]+ (calcd. 1211.63).
GdLC22 was prepared similarly to EuLC22 using Gd(NO3)3·6H2O (11.1 mg, 24.7 μmol). Yield: 25.7 mg (75%). MS (FAB+): m/z 1323 [M − (NO3−)]+ (calcd. 1322.91).
3.3 Computational
All the potential energy surfaces (PESs) were described using density functional theory (DFT) with the ωB97XD functional.22 The basis sets for Eu3+ and others were the (7s6p5d)/[5s4p3d] Stuttgart-Dresden large-core RECP basis set23 and cc-pVDZ,24 respectively. The PESs of Eu3+-centered excited states (5DJ; J = 0, 1, and 2) were approximately represented by that of the ground state corrected by energy shift parameters25 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 cm−1, 19
250 cm−1, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, and 21
000 cm−1, and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 for J = 0, 1, and 2, respectively). The details of this approximation, called the energy shift method, are shown in ref. 26 and 27. The geometry optimizations of local minima and minimum energy crossing points were performed via the global reaction route mapping (GRRM) program28 using the energies and energy derivatives computed using the Gaussian09 program.29
500 cm−1 for J = 0, 1, and 2, respectively). The details of this approximation, called the energy shift method, are shown in ref. 26 and 27. The geometry optimizations of local minima and minimum energy crossing points were performed via the global reaction route mapping (GRRM) program28 using the energies and energy derivatives computed using the Gaussian09 program.29
4. Conclusions
Five europium complexes with long alkyl chain groups were successfully synthesized and their luminescence spectra were obtained quantitatively by means of luminescence lifetimes and quantum yields. Their periodical size change upon alkyl-derivatization on the π-electronic skeleton did not influence the luminescence of the resulting Eu complexes, EuLCx, except for EuLH in solutions. Based on the DFT calculation concerned with the luminescence properties, it was found that the acyl groups of LCx coordinate to Eu instead of bpy moieties in solutions. Finally, it is known that the environments surrounding the Eu ion are similar to each other in solutions.TbLH in acetonitrile shows enhanced luminescence based on the theoretical approaches in our previous report. The quantum yield of TbLH is quite high at 48.7% in comparison with the mother compound TbL. Furthermore, after the alkylation of TbLH to form TbLC18, the f–f emission ability in solutions increases drastically.
Author contributions
M. Hasegawa conceived and designed the study with A. Ishii and K. Goto, S. Ogata, N. Goto, S. Sakurai, K. Yoshihara, R. Tanabe, K. Kayano, and R. Magaribuchi performed the experiments. M. Hatanaka provided the theoretical aspects to improve molecular designs.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge Prof. Makoto Handa and Prof. Michiko Egawa (Shimane University) for their kind discussions. This work was partly supported by grants-in-aid from Scientific Research on Innovative Areas of “Soft Crystals (area number: 2903)” (no. 17H06374; MH), MEXT Supported Program for the Strategic Research Foundation at Private Universities (2013–2017; MH), Network Joint Research Centre for Materials and Devices (2017; MH) and a SOKEN Project provided by Aoyama Gakuin University Research Institute (2016–2017; MH). MH also acknowledges Izumi Science and Technology Foundation for their financial support. SO thanks the Early Eagle Program for a subsidy from Aoyama Gakuin University. AI and Miho Hatanaka acknowledge the support of JST PRESTO.Notes and references
- S. I. Weissman, J. Chem. Phys., 1942, 10, 214 CrossRef.
- S. Tobita, M. Arakawa and I. Tanaka, J. Phys. Chem., 1985, 89, 5649 CrossRef.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189 RSC.
- X. Wang, H. Chang, J. Xie, B. Zhao, B. Liu, S. Xu, W. Pei, N. Ren, L. Huang and W. Huang, Coord. Chem. Rev., 2014, 273–274, 201 CrossRef.
- H. Xu, C.-S. Cao, X.-M. Kanga and B. Zhao, Dalton Trans., 2016, 45, 18003 RSC.
- C. Tanford, Science, 1978, 200, 1012 Search PubMed.
- R. G. Snyder, H. L. Strauss and C. A. Elliger, J. Phys. Chem., 1982, 86, 5145 CrossRef.
- H. R. Marsden, I. Tomatsu and A. Kros, Chem. Soc. Rev., 2011, 40, 1572 RSC.
- Y. Hara, K. Yoshihara, K. Kondo, S. Ogata, T. Watanabe, A. Ishii, M. Hasegawa and S. Koh, Appl. Phys. Lett., 2018, 112, 173103 CrossRef.
- M. Hasegawa, H. Ohtsu, D. Kodama, T. Kasai, S. Sakurai, A. Ishii and K. Suzuki, New J. Chem., 2014, 38, 1225 RSC.
- H. Wada, S. Ooka, D. Iwasawa, M. Hasegawa and T. Kajiwara, Magnetochemistry, 2016, 2, 43 CrossRef.
- S. Ogata, T. Shimizu, T. Ishibashi, Y. Ishiyone, M. Hanami, M. Ito, A. Ishii, S. Kawaguchi, K. Sugimoto and M. Hasegawa, New J. Chem., 2017, 41, 6385 RSC.
- G. H. Dieke and H. M. Crosswhite, Appl. Opt., 1963, 2, 675 CrossRef.
- S. Comby, D. Imbert, A.-S. Chauvin and J.-C. G. Bünzli, Inorg. Chem., 2006, 45, 732 CrossRef PubMed.
- C. Y. Chow, S. V. Eliseeva, E. R. Trivedi, T. N. Nguyen, J. W. Kampf, S. Petoud and V. L. Pecorato, J. Am. Chem. Soc., 2016, 138, 5100 CrossRef PubMed.
- O. L. Malta, J. Non-Cryst. Solids, 2008, 354, 4770 CrossRef.
- M. Hatanaka, A. Osawa, T. Wakabayashi, K. Morokuma and M. Hasegawa, Phys. Chem. Chem. Phys., 2018, 20, 3328 RSC.
- SMART, SAINT, XPREP and SADABS, Bruker AXS Inc., Madision, Wisconsin, 2004 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef PubMed.
- G. M. Sheldrick, SHELXS-97, University of Göttingen, Göttingen, 1997 Search PubMed.
- G. M. Sheldrick, SHELX-97, University of Göttingen, Göttingen, 1997 Search PubMed.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta, 1993, 85, 441 CrossRef.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007 CrossRef.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1 CrossRef.
- M. Hatanaka and K. Morokuma, J. Chem. Theory Comput., 2014, 10, 4184 CrossRef PubMed.
- M. Hatanaka, Y. Hirai, Y. Kitagawa, T. Nakanishi, Y. Hasegawa and K. Morokuma, Chem. Sci., 2017, 8, 423 RSC.
- S. Maeda, Y. Osada, T. Taketsugu, K. Morokuma and K. Ohno, GRRM14, http://iqce.jp/GRRM/index_e.shtml Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, A. Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 09, Rev. E.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Packing structure with detailed structural information of EuLH, excitation spectra in solutions, calculation of rate constants for energy relaxation, luminescence decay profiles, and 1H-NMR. CCDC 1813448. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt04899h |
| This journal is © The Royal Society of Chemistry 2018 |




