Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Point-and-shoot: rapid quantitative detection methods for on-site food fraud analysis – moving out of the laboratory and into the food supply chain
David I.
Ellis
*a,
Howbeer
Muhamadali
a,
Simon A.
Haughey
b,
Christopher T.
Elliott
b and
Royston
Goodacre
a
aSchool of Chemistry, The University of Manchester, Manchester Institute of Biotechnology, University of Manchester, 131 Princess Street, Manchester M1 7ND, UK. E-mail: D.Ellis@manchester.ac.uk
bQueens University Belfast, School of Biological Sciences, Institute of Global Food Security, Antrim, Belfast BT9 5AG, North Ireland, UK
First published on 1st September 2015
Abstract
Major food adulteration and contamination events occur with alarming regularity and are known to be episodic, with the question being not if but when another large-scale food safety/integrity incident will occur. Indeed, the challenges of maintaining food security are now internationally recognised. The ever increasing scale and complexity of food supply networks can lead to them becoming significantly more vulnerable to fraud and contamination, and potentially dysfunctional. This can make the task of deciding which analytical methods are more suitable to collect and analyse (bio)chemical data within complex food supply chains, at targeted points of vulnerability, that much more challenging. It is evident that those working within and associated with the food industry are seeking rapid, user-friendly methods to detect food fraud and contamination, and rapid/high-throughput screening methods for the analysis of food in general. In addition to being robust and reproducible, these methods should be portable and ideally handheld and/or remote sensor devices, that can be taken to or be positioned on/at-line at points of vulnerability along complex food supply networks and require a minimum amount of background training to acquire information rich data rapidly (ergo point-and-shoot). Here we briefly discuss a range of spectrometry and spectroscopy based approaches, many of which are commercially available, as well as other methods currently under development. We discuss a future perspective of how this range of detection methods in the growing sensor portfolio, along with developments in computational and information sciences such as predictive computing and the Internet of Things, will together form systems- and technology-based approaches that significantly reduce the areas of vulnerability to food crime within food supply chains. As food fraud is a problem of systems and therefore requires systems level solutions and thinking.
Background
Major food fraud and contamination events occur with alarming regularity, and are known to be episodic, with the question being not if but when another large-scale food safety/integrity incident will occur. The increase in scale and spread of these events could be said to be directly related to globalization and rapid distribution systems, with the result that major events can now have international impacts, with far-reaching and sometimes lethal consequences.1 It is perhaps not surprising then that as the modern phase of globalization is relatively recent (from the latter part of the 20th century), issues related to large-scale food adulteration and contamination events are only now beginning to be realised, discussed and analysed in far more detail, by the food industry, regulators, as well as by consumers. These discussions could be said to include the parts played by the drivers of global population and consumer/supplier demand for an increasingly wide-range of food products year round, regardless of seasonality or local availability, resulting in the ever increasing scale and complexity of food supply networks. This not only leads to food supply networks becoming significantly more vulnerable to fraud and contamination (as well as potentially fragmented and dysfunctional2–4); it also makes the task of deciding which analytical methods are more suitable to collect and analyse data within the component parts, or targeted points of vulnerability along complex and dynamic supply chains, that much more challenging.The vulnerabilities currently inherent within complex international food supply chains were very publically demonstrated by the horsemeat scandal (so-called ‘Horsegate’ scandal) in 2013 centred in the UK and Europe, which also focused the attention of governments, industry, researchers and regulatory bodies across the world onto the subject of food fraud (food crime) and contamination. The events are well documented but primarily involved the large-scale replacement of processed beef products with horsemeat and other undeclared meat products, such as pork, sometimes up to levels of 100% substitution.5 Of course this form of adulteration (or contamination) of the food supply is nothing new, and is probably as old as the food production systems themselves and continues unabated.1Table 1 contains examples of the adulterants found by Accum and Hassall in some of the first studies of food adulteration and contamination in the first half of the 19th century and published in The Lancet.6,7 A short list of relative recent high impact examples that have affected global food security would include: widespread adulteration of milk products with melamine in China in 2008; PCBs and dioxins in pork via industrial oil contaminated animal feed in Ireland in 2008 (and Belgium in 1999); carcinogenic Sudan I–IV dyes in chilli powder and tomato-based products leading to EU regulation in 2003; scrapie-infected feed for cattle leading to BSE in the late 1980s/early 1990s in the UK; wine adulteration, e.g. by methanol in Italy in 1986; diethylene glycol (used in some anti-freeze products) in Austrian wines in 1985; and Toxic Oil Syndrome in Spain in 1981 which unfortunately killed over 600 people.8
| Food product | Adulterant | |
|---|---|---|
| To increase the bulk/weight | To alter the appearance/flavour | |
| Cayenne pepper | Bulked out with the addition of a variety of compounds such as ground rice, mustard seed husks, sawdust, and salt | Coloured with red lead, vermillion, venetian red (from ferric oxide, also known commonly as rust), turmeric |
| Cocoa and chocolate | Arrowroot, wheat, maize, sago, potato, tapioca, flour, chicory were commonly used to increase weight and volume | Venetian red, red ochre, and other iron compounds added to effect the colour |
| Coffee | Chicory, roasted wheat, rye flour, potato flour, roasted beans, and acorns were added to bulk out the volume | Burnt sugar, which was also referred to as black jack, was used as a darkener |
| Confectionery | No bulking agents found | Sweets coloured with Gamboge, a Southeast Asian tree sap/resin, traditionally used to dye Buddhist monks robes. White comfits were coloured with clay from Cornwall, red sweets with red lead and vermillion, green sweets were often found to be coloured with copper salts and Scheele's green, a compound which used to be used to colour paints and also known as copper arsenite (a compound famously linked to the death of Napoleon) |
| Custard powders | Bulked out with wheat, potato, or rice flour | Lead chromate, and turmeric were used to enhance the yellow colour |
| Gin | Diluted with water | Cayenne, cassia, cinnamon, sugar, alum (aluminium sulfate), and so-called salt of tartar (potassium carbonate) used to change taste |
| Olive oil | No bulking agents found | Olive oil was reported to contain lead from the olive presses |
| Pickles | No bulking agents found | Toxic copper salts were used as a green colourant |
| Porter and stout | Diluted with water | Adulterated with fishberry (also known as Levant nut), a poisonous picrotoxin. And many other adulterants such as brown sugar, capsicum, salt, wormwood, ginger, caraway seeds, highly poisonous Nux vomica seeds from the strychnine tree, brucine, cream of tartar, shavings from the horns of male red deer, treacle, coriander, liquorice and honey |
| Red cheese | No bulking agents found | Red lead (lead tetroxide), and vermillion (mercury sulfide), used as colourants |
| Tea | Used tea leaves, as well as a variety of leaves from plants not related to tea. Starch, sand, China clay | The pigment Prussian blue (ferric ferrocyanide) in black tea, turmeric, orpiment (arsenic sulfide), and copper salts for green tea. Plumbago, gum, and Indigofera |
| Vinegar | No bulking agents found | Vinegar was found to contain dissolved tin and lead after being boiled in pewter vessels. It could also undergo a process known as sharpening, using sulfuric acid |
Whilst these are just a few of the recent major incidents, the engagement with fraudsters and detection of adulterated and contaminated food is continuous and becoming increasingly more sophisticated, with the leading food categories of reported food fraud including adulteration and mislabelling of dairy produce, meat, seafood, wines, spirits, edible oils, honey, fruit juices, coffee and tea, organic food and products, and clouding agents.9 More recently, the adulteration of various herbs10 and spices,11–13 the so-called gutter oil scandal in Asia,14,15 and fake rice, for example, are causing significant or potential problems. One recent report stated that in the UK alone, food and drink companies lose an estimated £11.2 billion per annum to food fraud and that tackling fraud within this industry could boost profitability by £4.48 bn (34%).16 Worldwide, this is a huge and growing problem with sophisticated and well connected networks of fraudsters becoming involved and seeing opportunities in existing and rapidly expanding sectors such as whole meats and fish into newly affluent markets, the burgeoning halal market, where the consumption of haram food is highly undesirable,17–19 and organic food sectors,20 through to low-value but very high turnover products such as processed foods, as was the case with beef substitution with horse etc. in ready meals.
Spectrometry versus spectroscopy
Following the horsemeat scandal, as well as the other food fraud and contamination events mentioned above (in addition to many others not mentioned here), it is evident that those working within and associated with the food industry, such as producers, retailers and regulators, are seeking rapid, user-friendly methods to detect food fraud and contamination, and rapid/high-throughput screening methods for the analysis of food in general (i.e. quality indicators). These methods should be portable, ruggedized, and ideally handheld and/or remote sensor devices that can be taken to or positioned on/at/in-line at points of vulnerability along (complex) food supply networks. It is also essential that these approaches require a minimum amount of background training in order to allow the users to acquire information rich and reproducible data rapidly (ergo point-and-shoot). These rapid methods could include any one, or a combination of, spectroscopies as well as many other methods (see below). Of course much more sophisticated and sensitive, though considerably more expensive, techniques already exist in the form of mass spectrometry and related hyphenated approaches which incorporate prior chromatographic separations. Here we will briefly discuss a range of spectrometry and spectroscopy approaches.Mass spectrometry
Mass spectrometry (MS) is a powerful technology which offers a number of advantages and, as inferred above, some current limitations for applications within the food supply chain (though not within food industry and food regulatory laboratories). Targeted analytical methods such as MS offer high chemical specificity and sensitivity for example, enabling the accurate identification and quantification of known analytes within complex food matrices at sub-μg concentrations. Considered to be the gold standard within many industries, and research fields, including the agri-food as well as the pharmaceutical, petrochemical, and defence industries, these methods are usually coupled with chromatographic techniques. The chromatography column chemistry needs to be carefully chosen in order to separate out the complex components of food adequately and thus comes with an additional analytical cost as well as being relatively slow (min–h).That being said, some forms of MS can be considered as fingerprinting techniques21 as they involve the direct introduction of samples into the mass spectrometer without the requirement for prior chromatographic separation. Some recent examples of these MS fingerprinting techniques have included direct infusion (or injection) mass spectrometry (DIMS) for the characterization of the foodborne pathogen Campylobacter jejuni,22 desorption electrospray ionisation (DESI) for the analysis of melamine migration into foods from melamine tableware,23 matrix-assisted laser desorption (MALDI) MS for the detection of hazelnut oil in extra virgin olive oil (EVOO) down to levels of 1%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and direct analysis in real-time (DART) MS for the direct swabbing of fruit and vegetables for the detection of pesticides,25 amongst many others. For a more technical explanation of these, and other, lab-based MS methods for the authentication and analysis of food adulteration and contamination, such as isotope ratio mass spectrometry (IRMS),26 the reader is directed to our more comprehensive review on fingerprinting food in Chemical Society Reviews,1 as well as other reviews on the potential of ambient mass spectrometry for high-throughput analyses,27 and DART-MS for food analysis.28
24 and direct analysis in real-time (DART) MS for the direct swabbing of fruit and vegetables for the detection of pesticides,25 amongst many others. For a more technical explanation of these, and other, lab-based MS methods for the authentication and analysis of food adulteration and contamination, such as isotope ratio mass spectrometry (IRMS),26 the reader is directed to our more comprehensive review on fingerprinting food in Chemical Society Reviews,1 as well as other reviews on the potential of ambient mass spectrometry for high-throughput analyses,27 and DART-MS for food analysis.28
Whilst the MS methods mentioned thus far are all relatively bulky and therefore confined to conventional laboratories, there is obviously huge potential for these techniques outside the lab and out in the food supply chain, with research and development into the portability and miniaturization of ambient MS29 having been underway for some considerable time30 (at least two decades31). These developments have primarily been based around point-of-care clinical applications (such as the Mini 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32) or in-field chemical detection33 applications, though the broader potential of ‘handheld’ MS in other areas such as food analysis has of course been recognised.32,34,35 Very significant and progressive steps in both portability and the less relative term, miniaturization, during the last decade have been achieved with reductions in size to less than 4 kg by Graham Cooks and Zheng Ouyang36 (and for the purposes of this article, we consider any instrument weighing 4 kg or less to be handheld,37 and above 4 kg as portable). Cooks and Ouyang have also been at the forefront in a multitude of other areas of broader MS research such as DESI, low temperature plasma, and paper spray handheld/portable mass spectrometry.38–40 Miniaturization of MS has continued to evolve by addressing technical challenges such as the development of compact low-power pumping systems suitable for miniature MS and the reduction in size of ion traps. These have led to the development of discontinuous atmospheric pressure interface (DAPI), as well as rectilinear ion traps as mass analysers, the optimisation, miniaturization, and simulation of which are still on-going.41,42
32) or in-field chemical detection33 applications, though the broader potential of ‘handheld’ MS in other areas such as food analysis has of course been recognised.32,34,35 Very significant and progressive steps in both portability and the less relative term, miniaturization, during the last decade have been achieved with reductions in size to less than 4 kg by Graham Cooks and Zheng Ouyang36 (and for the purposes of this article, we consider any instrument weighing 4 kg or less to be handheld,37 and above 4 kg as portable). Cooks and Ouyang have also been at the forefront in a multitude of other areas of broader MS research such as DESI, low temperature plasma, and paper spray handheld/portable mass spectrometry.38–40 Miniaturization of MS has continued to evolve by addressing technical challenges such as the development of compact low-power pumping systems suitable for miniature MS and the reduction in size of ion traps. These have led to the development of discontinuous atmospheric pressure interface (DAPI), as well as rectilinear ion traps as mass analysers, the optimisation, miniaturization, and simulation of which are still on-going.41,42
Whilst these developments are extremely encouraging, to date these systems still require much further optimization for them to be tolerant to and tested in a wide-range of environmental conditions outside of the laboratory, cost-effective, and importantly, the ability to be used and the data presented in a way that is readily interpretable by those not expert or with a background in MS. The development of totally self-sustained, integrated, and truly handheld MS sensors may yet be sometime in the future. Yet this could still be possible with simplified user interfaces, and as some have reported, perhaps with the same MS core but with any number of interchangeable sample cartridges for a variety of on-site applications.31 Innovations such as these would allow for the true democratisation of MS methods in becoming universal techniques able to be routinely used by non-specialists within a wide range of applications outside of laboratories, such as food supply chains.
Infrared spectroscopy
Spectroscopy includes a wide range of methods which involve the measurement of the interaction of matter with electromagnetic (EM) radiation. Here we will briefly focus on a subset of these methods, specifically those termed vibrational spectroscopies, and their current and potential future use within food supply chains. Infrared spectroscopies include near infrared (NIR) and Fourier transform infrared (FT-IR) spectroscopy, the latter operating in the mid-infrared (MIR) part of the EM spectrum.43 Both these methods involve measuring the absorption of an infrared beam within a sample, with every sample having its own unique spectral fingerprint.44 Unlike the predominantly lab-bound MS methods mentioned above, on/at-line NIR instrumentation has been used within the food processing industry for four decades, particularly for the monitoring and control of product and process quality.1,45The use of NIR hyperspectral imaging as an analytical tool for process control, food safety and quality has also been well recognised, more so during the last decade,46 as well as more recently,47–49 accompanied by the application of chemometrics for data pre-treatment and analysis50,51 and multivariate screening and modelling.52 Other recent reports include the application of NIR to melamine adulteration of soya bean meal53,54 and non-targeted analysis of the adulteration of milk powders.55 Even more relevant to our focus here, are reports such as advancements in the miniaturization of these methods using handheld micro-electrical-mechanical-systems (MEMS) based NIR online in abattoirs for the in situ classification of several different high-value gourmet meat carcass types on the slaughterhouse line56 with further reports into the algorithms used for the rapid transfer of large databases from at-line high performance NIR monochromators downloaded directly to handheld MEMS-NIR.57 These methods have been reported to enable a new approach and confirm the suitability of handheld MEMS-NIR for the rapid, low-cost, on-line/in situ analysis of meat products. For a recent review of the applications of portable NIR in the agro-food industry the reader is directed to dos Santos and co-workers.58
Whilst not having the on-site history that NIR applications have had within the food processing and related industries over the last four decades, the potential of FT-IR spectroscopy for food analysis (and many other forms of rapid bioanalysis44,59,60) has been recognised for some considerable time. As mentioned above, FT-IR operates within the mid-infrared range of the EM spectrum, and along with NIR, it readily presents itself as a rapid, high-throughput at/in/on-line screening technology for food and feed. As well as operating within the mid-infrared, FT-IR (like NIR) uses a broadband source, though the resultant data collected contains fundamental vibrations of the sample under analysis from the entire wavenumber range (unlike NIR which corresponds to vibrational overtones and combination modes, which are consequently broader in nature and not so information rich). Consequently, whilst NIR technology is still improving and is an extremely convenient technology within the agri-food sector (predominantly due to the perceived lack of water interference) for rapid, bulk and high-throughput screening, FT-IR is more sensitive and perhaps more suited to the detection of low-level compounds within complex food matrices and subtle differences between samples from very similar backgrounds.
Within research laboratories FT-IR has a long history of published food-based research applications, such as food and food ingredient authentication,61 with work by Gerard Downey and co-workers contributing a great deal to this, and indeed, other areas of vibrational spectroscopy for food analysis and authentication.62,63 The range of applications of food-based FT-IR research are considerably broad (and increasing) and include the rapid detection of food spoilage bacteria (an indicator of food quality as well as shelf-life estimation) at ambient temperatures in meat,64–67 and detecting food spoilage microorganisms68 on meat in different forms of conventional and vacuum packaging,69 as well as dairy produce.70 Others include the monitoring of bacterial interactions within milk,71 speciation in meat and dairy produce,72,73 and more recently, brand authentication of a range of Trappist beers,74 adulteration of milk75 and of highly processed foods with complex chemical and physical matrices, such as fresh/frozen/thawed beef burgers.76 For a review of FT-IR for rapid authentication and detection of food adulteration, the reader is directed to Rodriguez-Saona and Allendorf.77
Again, as with NIR, the suitability and utility of portable/handheld FT-IR spectroscopy within the food supply chain has become increasingly more evident; with portable and handheld spectroscopy having already been demonstrated for its potential to the move from the confines of the relatively stable and controlled laboratory environment and out into the potentially more challenging and dynamic environs of the food supply chain. Indeed, very recently a considerable body of work by Rodriguez-Saona and co-workers has shown the utility and efficacy of portable and handheld FT-IR for a range of food-based applications. This growing body of work includes monitoring oxidative stability78 as well as measuring trans fat content in edible oils,79 showing that handheld FT-IR can be a simple and rapid alternative to MS for on-site analysis of acrylamides in potato chips,80 and in situ discrimination and authentication of conventionally produced and organic butter.81
Combined NIR/FT-IR
Interestingly, one study by the Rodriguez-Saona group compared and evaluated the use of both portable mid-IR and handheld NIR for determining the levels of sucrose coatings in infant cereals, and found that superior predictive capability was obtained with their portable mid-IR unit.82 We consider this evaluation and direct comparison of both NIR and MIR methods of particular interest, as we believe that the ability to combine both these methods (and others, see below) into one unit, ideally handheld, would be a very useful tool indeed for a range of applications across the food supply chain (as well as countless other applications). This is due to the fact that both NIR and MIR spectroscopy have their own advantages and limitations. FT-IR is more sensitive to vibrations from liquid, bound and atmospheric water than NIR, which can be overcome to some extent via the use of very narrow path lengths or attenuated total reflectance (ATR).83,84 Conversely NIR, whilst less sensitive to water than FT-IR (and the resultant data less information rich), is able to penetrate much deeper into the surface of samples.Lab-based benchtop combined mid-IR/NIR spectroscopy already exists and allows for the selection of the most appropriate range to be chosen according to context, what is fit-for-purpose, allowing for a broader and more diverse range of samples to be analysed rapidly by a single instrument. It is only a matter of time before such benchtop innovations are significantly reduced in size and available on/at-line, and handheld combination single package MIR/NIR instruments can be used within the food supply chain. These would be simple to use, truly democratised analytical technology much closer to development and commercialisation than handheld MS for use in the food supply chain, with the ability to switch to reduced wavenumber ranges when and if required and generate highly reproducible and easily interpretable data.
Raman spectroscopy
Raman spectroscopy is another vibrational technique, which has to a large degree been made portable by many manufacturers (vide infra) and we consider it to have exceptional potential for use within food supply chains. Related and complementary to both forms of infrared spectroscopy discussed above, in simplistic terms, whilst infrared spectroscopy measures the absorption of energy, Raman spectroscopy involves the measurement of the exchange of energy with EM radiation of a particular wavelength, which is usually provided by a monochromatic light source such as a laser of any wavelength from the deep UV (e.g. 244 nm) to visible (405–633 nm) and into the NIR (785 nm, 830 nm, or 1064 nm are currently popular).1 The measurement in the shift of the incident laser light (the Raman shift) is observed, which is also known as the inelastic light scattering effect. Infrared and Raman spectroscopy are complementary due to the selection rules, whereby molecules are either infrared or Raman active, with molecules being infrared active only if the vibration induced by infrared light causes a change in the dipole moment, with Raman spectroscopy detecting changes in the polarizability of molecules. Therefore, there is a rule of mutual exclusion meaning that the two methods can provide complementary (bio)chemical information, with bands in Raman typically being a lot sharper and hence more characteristic than in the infrared. Whilst the Raman effect is an inherently weaker process than infrared, and to date, the equipment more expensive to produce, the materials used to construct Raman devices are becoming gradually less expensive. In addition, the detection responses in Raman devices are faster than infrared techniques, and indeed the detectors themselves are charge-coupled devices (CCDs) similar to those found in digital cameras and therefore within every smart phone and home.In terms of food analysis, Raman spectroscopy also offers other distinct advantages to infrared spectroscopy, with water being a weak Raman scatterer for example, which is always an advantage when the vast majority of foods or feed contain water in some form. It is also a confocal method. Being a confocal technique means that Raman spectroscopy measures precisely at the point where the laser is focused on/within a sample, with any out-of-focus signal being eliminated. This in itself is highly significant as it means that as long as the material the laser is passing through is transparent to laser light, conventional Raman spectroscopy can readily analyse samples through glass or plastic bottles/bags and other forms of transparent packaging (used in abundance throughout the food industry), focusing directly on the contents inside (including liquids) and collect a (bio)chemical fingerprint within seconds; this eliminates the need to remove the sample from the container which is very important if the sample is highly hazardous. Therefore, Raman affords the user some advantages over the infrared methods above. Several groups have undertaken direct comparative studies of infrared and Raman spectroscopies for the investigation of food samples including meat speciation,72 detection of meat spoilage,85 and the detection, enumeration and growth interactions of bacterial species in milk.71 The benefits of direct comparative studies of infrared and Raman have also been recognised in other areas more recently.86 It is interesting to note that as well as these lab-based benchtop comparative studies, a handheld combined FT-IR/Raman spectrometer is already commercially available (see Table 2), in addition to a wide-range of the other handheld spectroscopy devices discussed here.
| Company | Product | Spectral range (cm−1) | Weight (kg) | Size (cm) | Laser (nm) | |
|---|---|---|---|---|---|---|
| a ns = not specified. | ||||||
| Raman | Metro-Ohm | Mira M-1 | 400–2300 | 0.54 | 12.5 × 8.5 × 3.9 | 785 |
| Mira M-2* | 400–2300 | 0.82 | 14.4 × 9.3 × 6.4 | 1064 | ||
| Ocean Optics | ID Raman Mini | 400–2300 | 0.33 | 9.1 × 7.1 × 3.8 | 785 | |
| Rigaku | Progeny | 200–2500 | 1.6 | 29.9 × 8.1 × 7.4 | 1064 | |
| Thermo Scientific | First Defender RM | 250–2875 | 0.82 | 4.4 × 19.3 × 10.7 | 785 | |
| First Defender RMX | 250–2875 | 0.92 | 19.6 × 11.4 × 6.1 | 785 | ||
| TruNarc | 250–2875 | 0.505 | 16.3 × 10.4 × 5.1 | 785 | ||
| TruScan GP | 250–2875 | 0.9 | 20.8 × 10.7 × 4.3 | 785 | ||
| TruScan RM | 250–2875 | 0.9 | 20.8 × 10.7 × 4.3 | 785 | ||
| Snowy Range | CBEx | 400–2300 | 0.33 | 9.1 × 7.1 × 3.8 | 785 | |
| CBEx 1064 | 400–2300 | 0.77 | 11.3 × 7.9 × 5.7 | 1064 | ||
| Sciaps | Inspector 300 | 175–2875 | 1.7 | 19 × 17.5 × 4.3 | 785 | |
| Inspector 500 | 100–2500 | 2.7 | 20 × 17.5 × 4.3 | 1030 | ||
| Airsense Analytics | LS-ID | ns | 0.4 | 13 × 7 × 4 | 785 | |
| Chemring Detection Systems | THOR-1064 | 160–2200 | 1.5 | 22.9 × 11.5 × 5.1 | 1064 | |
| PGR-1064 | ns | 1 | 6.4 × 19 × 16.7 | 1064 | ||
| BWTEK | NanoRam | 176–2900 | 1.2 | 22 × 10 × 5 | 785 | |
| TacticID | 176–2900 | 0.9 | 19 × 10 × 5 | 785 | ||
| Wasatch Photonics | NOVA | 200–2500 | 0.82 | 16.2 × 13.2 × 3.7 | 785 | |
| Agiltron | Pin Pointer | 200–3000 | 1.36 | 21.4 × 10.8 × 6.3 | 785 | |
| TSI | ASSURX | 250–2350 | 1.9 | 23.1 × 10.1 × 22.2 | 785 | |
| Bruker | BRAVO | 300–3200 | 1.5 | 27 × 15.6 × 6.2 | 700–1100 | |
| FT-IR/Raman | Thermo Scientific | Gemini Analyzer | 250–2875 (Raman) | 1.9 | 25.6 × 14.6 × 6.1 | 785 |
| 650–4000 (IR) | ||||||
| FT-IR | Agilent | 4300 | 650–4500 | 2.2 | 10 × 19 × 35 | Not applicable |
| 4100 Exoscan | 650–4000 | 3.2 | 17.1 × 11.9 × 22.4 | |||
| Thermo Scientific | TruDefender | 650–4000 | 1.3 | 5.3 × 19.6 × 11.2 | ||
| Pyreos | Mid-IR | 909–1818 or 2000–4000 | 0.71 | 16.5 × 7.4 × 3.5 | ||
| Arcoptix | FTIR-Rocket | 1700–5000 or 830–4000 | 1.2 | 18 × 16 × 8 | ||
| NIR | Sentronic | SentroID | 5800–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.1 | 23 × 8 × 4.2 | |
| BWTEK | i-Spec nano | 4500–7700 | ns | 12 × 6 × 3 | ||
| Thermo | microPHAZIR | 4100–6250 | 1.8 | 26.6 × 25.1 × 10.9 | ||
| JDSU | MicroNIR Pro | 6000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.06 | 4.5 × 4.4 | ||
| ASD | QualitySpec | 4000–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.5 | 31 × 10 × 30 | ||
| Ocean Optics | NIRQUEST256-2.5 | 4000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.18 | 18.2 × 11 × 4.7 | ||
| Avantes | AvaSpec-NIR256-2.5-HSC | 4000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.5 | 18.5 × 14.5 × 18.5 | ||
| Brimrose | Luminar 5030 | 4300–9000 (others available) | ns | ns | ||
| Arcoptix | FT-NIR Rocket | 3800–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.7 | 18 × 12.6 × 7.8 | ||
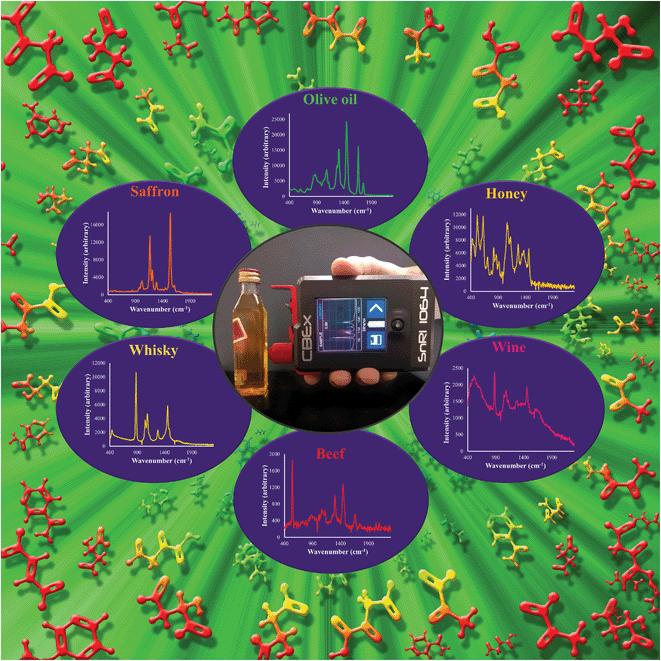
The advantages of portable/handheld Raman spectroscopy are being increasingly recognised by the pharmaceutical, materials, biosecurity87 and other sectors, as well as their potential applications within clinical settings.88 In terms of food analysis, relatively recent studies involving portable Raman spectroscopy have included the screening of melamine adulteration in milk powder89 and in other multiple sample matrices such as infant formula, lactose, whey protein, wheat bran and wheat gluten and povidone (which can have contraindications and severe allergic reactions).90 Portable Raman devices have been used successfully for the detection of the organophosphate and organothiophosphate pesticides phorate and fenthion on apple skins91 and the fungicide and parasiticide thiabendazole applied on citrus fruits and bananas,92 authenticity and origin of vegetable and essential oils,93 detection of marker compounds for illegal (non-commercial) alcoholic beverages.94 Detection and discrimination of pathogenic bacteria on food crops in the field,95 detection of offal adulteration in beef burgers;96 rapid meat spoilage identification,97 and finally, a similar study to the one above using MEMS-NIR, for prediction of pork quality on a slaughterhouse line, here using a portable Raman device. Fig. 1 shows a commercially available handheld 1064 nm Raman spectrometer (Snowy Range Instruments, Laramie, USA), with a range of spectra acquired by us from several different foods and beverages including extra virgin olive oil, honey, red wine, beef, whisky, and saffron. For a review of infrared and Raman spectroscopy for the verification of food origin, the reader is, perhaps not surprisingly, directed to Downey.62 In addition, several of the more recent studies have employed surface enhanced Raman scattering (SERS) techniques98,99 and for a more in-depth review of SERS and its application to food safety, specifically in terms of foodborne pathogen detection and food fraud and contamination, the reader is directed to Craig et al.100
Another more recent and exciting innovation and variant of Raman spectroscopy is spatially offset Raman spectroscopy (SORS).101 With SORS, Raman spectra are collected from locations within a sample at depth that are spatially separate from the point at which the sample is illuminated by the laser on the sample's surface. SORS can be undertaken in seconds, by shining a laser light onto a surface/container and detecting the Raman signal at the point of excitation and one or more offset positions, the resultant spectra subtracted using a scaled subtraction, which produce two spectra representing the surface and subsurface of samples.102 Therefore, SORS enables the user to isolate and retrieve chemically rich spectral information from distinct layers, substructures, and indeed through other barriers, which would not be accessible even via conventional Raman spectroscopy, or indeed, any of the other techniques (handheld or otherwise) mentioned thus far. When commenting from the perspective of its potential use for food product analysis, the ability of SORS to penetrate through barriers/packaging and retrieve chemically rich information is especially pertinent and it appears to be a readily transferable technology, and one may even suggest it has the potential to be a highly disruptive technology.
The range of potential SORS applications demonstrated to date include the determination and fast screening of genuine and counterfeit pharmaceuticals (including anti-malarials) through translucent plastic, paper sacks, coloured glass bottles,103,104 and tablet blister packs,105 the latter study by Ricci et al. combining SORS with ATR. This ability to see through and penetrate layers and packaging not transparent to the human eye has led to its use for the screening of liquids, aerosols and gels (LAGs) at multiple international airports,106 concealed liquid explosives detection,107 and other concealed substances in sealed opaque plastic and coloured glass bottles and containers several millimetres thick. These are compared with reference libraries of pure materials, to enable the rapid and unambiguous identification of the containers contents,108 with a reported inherently high probability of detection and low false alarm rate.106 Concealed contents identification has also included the determination of fake and genuine ivory through paint, plastic, varnishes and cloth.109 More recent emerging applications of SORS include those within the clinical sciences and the reader is directed to an excellent review of this area by Pavel Matousek (the co-inventor of this technique) and co-workers including the non-invasive diagnosis of bone disease, cancer, and non-invasive monitoring of glucose levels.110
Being such a recent innovation, the only food-related SORS applications to date include one to demonstrate the potential utility of subsurface detection of lycopene and product quality through the pericarp of tomato fruit,111 and more recently, the qualitative and quantitative characterization of quality parameters of salmon through the skin.112 Whilst there appears to be a paucity of published food-based SORS studies, to date at least, the wide range of applications published thus far in the other areas mentioned above show the specific and seemingly unique combined capabilities of this technique. All of which keenly illustrate that SORS remains an exciting area, ripe for further exploration, development, and detailed investigation within the area of food authenticity, wider food analysis in general within supply chains/networks and its use within other forms of logistic networks.
Future perspective
The emphasis in this short review has predominantly been toward an optics based approach to food fraud and contamination detection (Table 3), due in part to the commercial availability and ubiquitous nature of these methods, but also their ease of use and rapidity, chemical sensitivity, and the continued innovation and development of these spectroscopic devices and their components. In addition to the advantages of portable/handheld spectrometers being taken out into the supply chain, optics/photonic technologies in general also readily lend themselves to be utilized as fixed/embedded on/at-line technologies. Therefore, one must not overlook ongoing technological developments within the whole suite of optical technologies, and the future potential of these within food supply chains, such as the use of vertical-cavity surface emitting lasers (VCSELs) as minute low-power light sources,113 UV-Vis,114 and blue LED115 amongst others. With further innovation and future developments in sensor technologies and computing, such as wired or wireless connectivity (i.e. Wi-Fi, Bluetooth) and/or remote access capability116 built into the portable and handheld devices discussed here, these rapid methods could be networked and thus used to detect trends in the food market (perhaps even before any food security threat/event is acknowledged by regulators) and thus could very easily sit within the umbrella of the Internet of Things (IoT). Which, like cloud computing, will not be one but rather a series of paradigms and platforms which are set to explode and impact on all our lives within the next decade.117–119| Term | Definition |
|---|---|
| Food fraud | Committed when food is deliberately placed on the market for financial gain, with the intent of deception of consumers.* Referred to in the USA and occasionally elsewhere as economically motivated adulteration (EMA). Two of the main types include: trading of food which is unfit for consumption or harmful, or deliberately misdescribing or mislabelling food. The latter can include false statements regarding geographical origin, ingredients, or substitution with lower value (i.e. myrtle instead of oregano), or sometimes even dangerous contents not intended for human consumption (i.e. industrial dyes). The terms food fraud and food adulteration can be used to mean the same thing, when adulteration is intentional |
| Contamination | Can involve unwanted and usually unintentional physical, chemical, or biological contamination. Examples could include metal or plastic fragments (physical) or chemicals used for cleaning from food processing equipment, or microbial (bacterial/fungal/toxins) from microbes. If on rare occasions any of these are intentional, then it would be food crime, and depending on the intention and extent of deliberate contamination, bioterrorism |
| Food spoilage | Usually described as any changes in organoleptic characteristics which make a food undesirable for consumption. These may include changes in appearance (discoloration), development of off-odours, slime formation, or changes in taste. In meat and poultry for example it is generally accepted that detectable organoleptic spoilage is a result of decomposition and the formation of metabolites caused by the growth of microorganisms |
| Food crime | Food crime has been described as the point when food fraud is no longer just random acts caused by so-called ‘rogues’ within the food industry, but when this activity becomes organised and is undertaken by groups who knowingly set out with the intention to deceive, or injure, those purchasing a food product |
| Food security | Concerns the food supply, and ensuring access to a secure, sufficient quantity of safe, nutritious food to maintain a healthy and active life |
| Food authenticity | Is reflective of a reasonable assumption that the description of the labelling, or the menu section, of a finished food product purchased by the consumer is correct. Reasonableness should be a Wednesbury test in that it assumes no specialist knowledge of the food industry |
| Food integrity | Ensuring that food products that are sold or offered are of the quality, substance, and nature expected by the consumer. To sections of society which eschew certain types of foods, due to religious, medical, or dietary considerations, this can be of particular importance |
Indeed, it is worth remembering that the first use of the term the ‘Internet of Things’ (by the British technology pioneer Kevin Ashton) was for its direct application to supply chains.120 IoT is comprised of networks of interconnected communicating sensor/actuating physical objects (Things) able to identify each other, and generate, analyse, share and act upon information across common operating platforms and applications. At its current stage of evolution, at the forefront of IoT sensor technologies are radio frequency identification (RFID) tags/togs/labels, characterized by unique identifiers which can be passive, semi-passive and active, as small as an adhesive sticker, and used to monitor objects in real-time;117e.g., to track luggage at airports. Within food supply chains RFID approaches can be used to monitor product quality in terms of expiry dates on perishable goods,121 determine the probability of goods such as RFID-tagged oils as being counterfeit using mathematical algorithms,122 establish traceability systems,123 enable low cost and ultra-low power food logistics,124 low-cost chipless short range ID and temperature/humidity monitoring,125 and the detection of food freshness and bacterial growth.126
However, as the IoT continues to evolve it will be comprised of many more sensor modalities and innovations in addition to RFID and become fully formed via a much larger analytical sensor,127 biosensor128,129 and computational toolbox.130,131 These will include machine-to-machine communicating fixed/embedded as well as portable/handheld sensor devices with direct human input such as those discussed here. These people-centric (participatory) sensing platforms are able to acquire rapid, timely, and context specific data associated with predicted or anticipated events, compared to data from fixed sensor networks alone,118 and particularly so when in the hands of operatives with experience of supply chains and non-specialists in spectroscopy or science in general. This ability to ensure at the developmental stage that handheld detection methods can be used by non-specialists is in itself an extremely important part of the research and development process of these rapid devices. It forms a part of the knowledge exchange process, is an exercise in mutual learning, and allows the translation of research into practical applications, with positive impacts on the food supply chains and therefore society as a whole.
In addition, whilst fixed or benchtop spectroscopic devices could be based at major distribution and transport nodes/hubs within complex food supply networks, the handheld devices can be taken to changing points of vulnerability to fraud within these increasingly complex and dynamic networks. Points of vulnerability in food supply networks that, in the future, may well have been automatically identified/predicted and targeted for further investigation by pervasive and automated computational systems analysis embedded within an IoT network. As stated elsewhere, pervasive computing in conjunction with sensor technology platforms offers considerable potential for the improvement and efficiency of food supply chains/systems.117
Therefore, the future analytical toolbox will also include a combination of an increasingly innovative sensor portfolio, with methods able to track, trace and detect within food supply chains. These could include microfluidic132,133 and nanofluidic devices such as Nanopore,134 active and intelligent packaging135 and containers,136 DNA barcoding,137 edible tags,138 3D-printed smart caps139 and novel adaptations to existing technologies, such as turning a handheld personal blood glucose meter into a melamine detector for milk for one very recent example.140 In addition to computational tools for data analysis, simulation and fusion, as well as visualisation and interpretation of food supply chains and systems.141–143
Concluding remarks
In this review we have focussed, and some may say ‘shone a light on’, one small subset of potentially very useful handheld detection methods for on-site food fraud detection, namely vibrational spectroscopy. It is our sincere hope that, along with the many other methods currently in development, mobile handheld (as well as static, benchtop, fixed at-line) spectroscopy, will play a far greater role within the area of food and feed fraud detection, and indeed food analysis/screening in general, within increasingly complex and globalized food supply chains. As it is our firm belief that the ever expanding portfolio of sensor platforms and technologies and future pervasive, predictive computation will together take on the role of a technology-based capable guardian for food systems.144,145 Able to increase the resilience of food systems, and reduce the areas of vulnerability within complex food supply chains significantly, as well as the space within which the opportunities for food crime currently exist (Fig. 2). As food fraud has repeatedly been shown to be a problem of systems, and it therefore requires systems level solutions and thinking,146 holism, as opposed to one-eyed reductionism. | ||
| Fig. 2 Adapted from a graphical model of Routine Activity Theory,138,139 which is based on the three necessary conditions for many forms of crime (such as food fraud) to occur, converging in time and space. These three conditions require: (i) a likely offender (potential adulterer/fraudster); (ii) a suitable target (food supply network); and (iii) the absence of a capable guardian (detection technologies). The opportunities for, and vulnerabilities to crime (food fraud) occur in the areas where the so-called capable guardian is absent. We propose a technology-based capable guardian system, whereby static and mobile/handheld sensor platforms and technologies and future pervasive, predictive computation will together take on this role and assist in significantly reducing the areas of vulnerability to food crime within food supply chains. | ||
Acknowledgements
DIE and RG would like to thank Matt Cude at RSC for his hard work and assistance with the development of the themed collection dedicated to food authenticity and integrity which this article will hopefully form a part of. DIE/RG also thank the EPS Faculty Deans Fund at the University of Manchester for funding for the Snowy Range Instruments handheld Raman spectrometer. RG is also indebted to UK BBSRC (BB/L014823/1) for Raman microspectroscopy.References
- D. I. Ellis, V. L. Brewster, W. B. Dunn, J. W. Allwood, A. P. Golovanov and R. Goodacre, Chem. Soc. Rev., 2012, 41, 5706–5727 RSC.
- A. Bowman, J. Froud, S. Johal, J. Law and A. Leaver, Bringing home the bacon: from trader mentalities to industrial policy, CRESC, 2012 Search PubMed.
- A. Bowman, J. Froud, S. Johal, J. Law, A. Leaver, M. Moran and K. Williams, in The end of the experiment? From competition to the foundational economy, ed. A. Bowman, J. Froud, S. Johal, J. Law, A. Leaver, M. Moran and K. Williams, Manchester University Press, Manchester, 2014, ch. 3 Search PubMed.
- A. Bowman, J. Froud, S. Johal, A. Leaver and K. Williams, Account. Forum, 2013, 37, 300–314 CrossRef PubMed.
- C. Elliott, Elliott Review into the Integrity and Assurance of Food Supply Networks-Final Report-A National Food Crime Prevention Framework, 2014 Search PubMed.
- F. Accum, A Treatise On Adulterations Of Food and Culinary Poisons, and Methods Of Detecting Them, Evergreen Review, Inc., New York, 1820 Search PubMed.
- A. H. Hassall, Food and its adulterations; comprising the reports of the analytical sanitary commission of 'The Lancet' for the years 1851 to 1854, Longman, Brown, Green, and Longmans, London, 1855 Search PubMed.
- A. Pestana and E. Munoz, Nature, 1982, 298, 608 CrossRef CAS PubMed.
- R. Johnson, Food Fraud and "Economically Motivated Adulteration" of Food and Food Ingredients, Congressional Research Service, Washington, D.C., 2014 Search PubMed.
- C. Black, S. A. Haughey, O. P. Chevallier, P. Galvin-King and C. T. Elliott, Analyst, 2015 Search PubMed , submitted.
- F. Gao, Y. Hu, D. Chen, E. C. Y. Li-Chan, E. Grant and X. Lu, Talanta, 2015, 143, 344–352 CrossRef CAS.
- S. A. Haughey, P. Galvin-King, Y.-C. Ho, S. E. J. Bell and C. T. Elliott, Food Control, 2015, 48, 75–83 CrossRef CAS PubMed.
- E. A. Petrakis, L. R. Cagliani, M. G. Polissiou and R. Consonni, Food Chem., 2015, 173, 890–896 CrossRef CAS PubMed.
- F. Q. Lu and X. L. Wu, Food Control, 2014, 41, 134–138 CrossRef PubMed.
- T. T. Ng, P. K. So, B. Zheng and Z. P. Yao, Anal. Chim. Acta, 2015, 884, 70–76 CrossRef CAS PubMed.
- J. Gee, L. Jack and M. Button, Minimising Fraud and Maximising Value in the UK Food and Drink Sector, 2014 Search PubMed.
- J. Lever and M. Miele, J. Rural Stud., 2012, 28, 528–537 CrossRef PubMed.
- K. Nakyinsige, Y. B. Man and A. Q. Sazili, Meat Sci., 2012, 91, 207–214 CrossRef PubMed.
- M. van der Spiegel, H. J. van der Fels-Klerx, P. Sterrenburg, S. M. van Ruth, I. M. J. Scholtens-Toma and E. J. Kok, Trends Food Sci. Technol., 2012, 27, 109–119 CrossRef CAS PubMed.
- R. S. Hughner, P. McDonagh, A. Prothero, C. J. Shultz and J. Stanton, J. Consum. Behav., 2007, 6, 94–110 CrossRef.
- D. I. Ellis, W. B. Dunn, J. L. Griffin, J. W. Allwood and R. Goodacre, Pharmacogenomics, 2007, 8, 1243–1266 CrossRef CAS PubMed.
- R. M. Howlett, M. P. Davey, W. P. Quick and D. J. Kelly, Metabolomics, 2014, 10, 887–896 CrossRef CAS.
- M. Mattarozzi, M. Milioli, C. Cavalieri, F. Bianchi and M. Careri, Talanta, 2012, 101, 453–459 CrossRef CAS PubMed.
- C. D. Calvano, C. de Ceglie, L. D'Accolti and C. G. Zambonin, Food Chem., 2012, 134, 1192–1198 CrossRef CAS.
- E. Crawford and B. Musselman, Anal. Bioanal. Chem., 2012, 403, 2807–2812 CrossRef CAS PubMed.
- M. Scampicchio, T. Mimmo, C. Capici, C. Huck, N. Innocente, S. Drusch and S. Cesco, J. Agric. Food Chem., 2012, 60, 11268–11273 CrossRef CAS PubMed.
- L. P. Li, B. S. Feng, J. W. Yang, C. L. Chang, Y. Bai and H. W. Liu, Analyst, 2013, 138, 3097–3103 RSC.
- J. Hajslova, T. Cajka and L. Vaclavik, TrAC, Trends Anal. Chem., 2011, 30, 204–218 CrossRef CAS PubMed.
- M. E. Monge, G. A. Harris, P. Dwivedi and F. M. Fernandez, Chem. Rev., 2013, 113, 2269–2308 CrossRef CAS PubMed.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Science, 2006, 311, 1566–1570 CrossRef CAS PubMed.
- X. Y. Zhou, J. J. Liu, R. G. Cooks and Z. Ouyang, Bioanalysis, 2014, 6, 1497–1508 CrossRef CAS.
- L. F. Li, T. C. Chen, Y. Ren, P. I. Hendricks, R. G. Cooks and Z. Ouyang, Anal. Chem., 2014, 86, 2909–2916 CrossRef CAS.
- J. N. Smith, R. J. Noll and R. G. Cooks, Rapid Commun. Mass Spectrom., 2011, 25, 1437–1444 CrossRef CAS PubMed.
- C. J. Pulliam, R. M. Bain, J. S. Wiley, Z. Ouyang and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2015, 26, 224–230 CrossRef CAS.
- Z. P. Zhang, R. G. Cooks and Z. Ouyang, Analyst, 2012, 137, 2556–2558 RSC.
- Z. Ouyang and R. G. Cooks, Annu. Rev. Anal. Chem., 2009, 2, 187–214 CrossRef CAS PubMed.
- G. Huang, W. Xu, M. A. Visbal-Onufrak, Z. Ouyang and R. G. Cooks, Analyst, 2010, 135, 705–711 RSC.
- J. S. Wiley, J. T. Shelley and R. G. Cooks, Anal. Chem., 2013, 85, 6545–6552 CrossRef CAS.
- S. Soparawalla, F. K. Tadjimukhamedov, J. S. Wiley, Z. Ouyang and R. G. Cooks, Analyst, 2011, 136, 4392–4396 RSC.
- H. Wang, J. J. Liu, R. G. Cooks and Z. Ouyang, Angew. Chem., Int. Ed., 2010, 49, 877–880 CrossRef CAS PubMed.
- C. H. Chen, T. C. Chen, X. Y. Zhou, R. Kline-Schoder, P. Sorensen, R. G. Cooks and Z. Ouyang, J. Am. Soc. Mass Spectrom., 2015, 26, 240–247 CrossRef CAS PubMed.
- G. Huang, Y. Chen, F. Tang, L. T. Liu and X. H. Wang, AIP Adv., 2015, 5, 6 Search PubMed.
- D. Ellis, G. Harrigan and R. Goodacre, in Metabolic Profiling: Its Role in Biomarker Discovery and Gene Function Analysis, ed. G. Harrigan and R. Goodacre, Springer, US, 2003, ch. 7, pp. 111–124 Search PubMed.
- D. I. Ellis and R. Goodacre, Analyst, 2006, 131, 875–885 RSC.
- G. Downey, R. Briandet, R. H. Wilson and E. K. Kemsley, J. Agric. Food Chem., 1997, 45, 4357–4361 CrossRef CAS.
- A. A. Gowen, C. P. O'Donnell, P. J. Cullen, G. Downey and J. M. Frias, Trends Food Sci. Technol., 2007, 18, 590–598 CrossRef CAS PubMed.
- G. Elmasry, M. Kamruzzaman, D. W. Sun and P. Allen, Crit. Rev. Food Sci. Nutr., 2012, 52, 999–1023 CrossRef PubMed.
- D. Wu and D. W. Sun, Talanta, 2013, 111, 39–46 CrossRef CAS PubMed.
- Y. Z. Feng and D. W. Sun, Crit. Rev. Food Sci. Nutr., 2012, 52, 1039–1058 CrossRef PubMed.
- J. H. Cheng and D. W. Sun, Compr. Rev. Food Sci. Food Saf., 2015, 14, 478–490 CrossRef CAS.
- C. Esquerre, A. A. Gowen, J. Burger, G. Downey and C. P. O'Donnell, Chemom. Intell. Lab. Syst., 2012, 117, 129–137 CrossRef CAS.
- M. I. Lopez, E. Trullols, M. P. Callao and I. Ruisanchez, Food Chem., 2014, 147, 177–181 CrossRef CAS.
- S. A. Haughey, S. F. Graham, E. Cancouet and C. T. Elliott, Food Chem., 2013, 136, 1557–1561 CrossRef CAS.
- S. A. Haughey, P. Galvin-King, A. Malechaux and C. T. Elliott, Anal. Methods, 2015, 7, 181–186 RSC.
- L. L. Botros, J. Jablonski, C. Chang, M. M. Bergana, P. Wehling, J. M. Harnly, G. Downey, P. Harrington, A. R. Potts and J. C. Moore, J. Agric. Food Chem., 2013, 61, 9810–9818 CrossRef CAS PubMed.
- E. Zamora-Rojas, D. Perez-Marin, E. De Pedro-Sanz, J. E. Guerrero-Ginel and A. Garrido-Varo, Meat Sci., 2012, 90, 636–642 CrossRef CAS PubMed.
- E. Zamora-Rojas, D. Perez-Marin, E. De Pedro-Sanz, J. E. Guerrero-Ginel and A. Garrido-Varo, Chemom. Intell. Lab. Syst., 2012, 114, 30–35 CrossRef CAS.
- C. A. T. dos Santos, M. Lopo, R. Pascoa and J. A. Lopes, Appl. Spectrosc., 2013, 67, 1215–1233 CrossRef.
- M. J. Baker, J. Trevisan, P. Bassan, R. Bhargava, H. J. Butler, K. M. Dorling, P. R. Fielden, S. W. Fogarty, N. J. Fullwood, K. A. Heys, C. Hughes, P. Lasch, P. L. Martin-Hirsch, B. Obinaju, G. D. Sockalingum, J. Sule-Suso, R. J. Strong, M. J. Walsh, B. R. Wood, P. Gardner and F. L. Martin, Nat. Protoc., 2014, 9, 1771–1791 CrossRef CAS.
- C. L. Pickering, J. R. Hands, L. M. Fullwood, J. A. Smith and M. J. Baker, Forensic Sci. Int., 2015, 249, 189–196 CrossRef CAS PubMed.
- G. Downey, TrAC, Trends Anal. Chem., 1998, 17, 418–424 CrossRef CAS.
- G. Downey, in New Analytical Approaches for Verifying the Origin of Food, ed. P. Brereton, Woodhead Publ Ltd, Cambridge, 2013, pp. 94–116 Search PubMed.
- L. M. Reid, C. P. O'Donnell and G. Downey, Trends Food Sci. Technol., 2006, 17, 344–353 CrossRef CAS.
- D. Alexandrakis, G. Downey and A. G. M. Scannell, Food Bioprocess Technol., 2012, 5, 338–347 CrossRef CAS.
- D. I. Ellis, D. Broadhurst and R. Goodacre, Anal. Chim. Acta, 2004, 514, 193–201 CrossRef CAS PubMed.
- D. I. Ellis, D. Broadhurst, D. B. Kell, J. J. Rowland and R. Goodacre, Appl. Environ. Microbiol., 2002, 68, 2822–2828 CrossRef CAS.
- D. I. Ellis and R. Goodacre, Trends Food Sci. Technol., 2001, 12, 414–424 CrossRef CAS.
- D. I. Ellis and R. Goodacre, in Food Spoilage Microorganisms, ed. C. W. Blackburn, Woodhead, Cambridge, UK, 2006, ch. 1, pp. 3–27 Search PubMed.
- M. S. Ammor, A. Argyri and G. J. E. Nychas, Meat Sci., 2009, 81, 507–514 CrossRef CAS.
- N. Nicolaou and R. Goodacre, Analyst, 2008, 133, 1424–1431 RSC.
- N. Nicolaou, Y. Xu and R. Goodacre, Anal. Chem., 2011, 83, 5681–5687 CrossRef CAS.
- D. I. Ellis, D. Broadhurst, S. J. Clarke and R. Goodacre, Analyst, 2005, 130, 1648–1654 RSC.
- N. Nicolaou, Y. Xu and R. Goodacre, J. Dairy Sci., 2010, 93, 5651–5660 CrossRef CAS PubMed.
- J. Engel, L. Blanchet, L. M. C. Buydens and G. Downey, Talanta, 2012, 99, 426–432 CrossRef CAS.
- P. M. Santos, E. R. Pereira and L. E. Rodriguez-Saona, J. Agric. Food Chem., 2013, 61, 1205–1211 CrossRef CAS PubMed.
- M. Zhao, G. Downey and C. P. O'Donnell, Meat Sci., 2014, 96, 1003–1011 CrossRef CAS.
- L. E. Rodriguez-Saona and M. E. Allendorf, in Annual Review of Food Science and Technology, Vol 2, ed. M. P. Doyle and T. R. Klaenhammer, Annual Reviews, Palo Alto, 2011, vol. 2, pp. 467–483 Search PubMed.
- M. Allendorf, A. Subramanian and L. Rodriguez-Saona, J. Am. Oil Chem. Soc., 2012, 89, 79–88 CrossRef CAS.
- E. Birkel and L. Rodriguez-Saona, J. Am. Oil Chem. Soc., 2011, 88, 1477–1483 CrossRef CAS.
- H. Ayvaz and L. E. Rodriguez-Saona, Food Chem., 2015, 174, 154–162 CrossRef CAS PubMed.
- M. P. Pujolras, H. Ayvaz, M. L. Shotts, R. A. Pittman, S. Herringshaw and L. E. Rodriguez-Saona, J. Am. Oil Chem. Soc., 2015, 92, 175–184 CrossRef.
- C. A. Lin, H. Ayvaz and L. E. Rodriguez-Saona, Food Anal. Meth., 2014, 7, 1407–1414 CrossRef.
- D. M. Hashim, Y. B. C. Man, R. Norakasha, M. Shuhaimi, Y. Salmah and Z. A. Syahariza, Food Chem., 2010, 118, 856–860 CrossRef CAS PubMed.
- N. Koca, N. A. Kocaoglu-Vurma, W. J. Harper and L. E. Rodriguez-Saona, Food Chem., 2010, 121, 778–782 CrossRef CAS PubMed.
- A. A. Argyri, R. M. Jarvis, D. Wedge, Y. Xu, E. Z. Panagou, R. Goodacre and G. J. E. Nychas, Food Control, 2013, 29, 461–470 CrossRef CAS PubMed.
- H. Muhamadali, M. Chisanga, A. Subaihi and R. Goodacre, Anal. Chem., 2015, 87, 4578–4586 CrossRef CAS PubMed.
- D. P. Cowcher, Y. Xu and R. Goodacre, Anal. Chem., 2013, 85, 3297–3302 CrossRef CAS PubMed.
- D. I. Ellis, D. P. Cowcher, L. Ashton, S. O'Hagan and R. Goodacre, Analyst, 2013, 138, 3871–3884 RSC.
- Y. Cheng, Y. Y. Dong, J. H. Wu, X. R. Yang, H. Bai, H. Y. Zheng, D. M. Ren, Y. D. Zou and M. Li, J. Food Compos. Anal., 2010, 23, 199–202 CrossRef CAS.
- L. C. Mecker, K. M. Tyner, J. F. Kauffman, S. Arzhantsev, D. J. Mans and C. M. Gryniewicz-Ruzicka, Anal. Chim. Acta, 2012, 733, 48–55 CrossRef CAS.
- X. Z. Li, S. Zhang, Z. Yu and T. Y. Yang, Appl. Spectrosc., 2014, 68, 483–487 CrossRef CAS.
- C. Muller, L. David, V. Chis and S. C. Pinzaru, Food Chem., 2014, 145, 814–820 CrossRef CAS PubMed.
- P. V. Jentzsch and V. Ciobota, Flavour Fragrance J., 2014, 29, 287–295 CrossRef PubMed.
- A. Kwiatkowski, M. Czerwicka, J. Smulko and P. Stepnowski, J. Forensic Sci., 2014, 59, 1358–1363 CrossRef CAS PubMed.
- X. M. Wu, C. Xu, R. A. Tripp, Y. W. Huang and Y. P. Zhao, Analyst, 2013, 138, 3005–3012 RSC.
- M. Zhao, G. Downey and C. P. O'Donnell, J. Agric. Food Chem., 2015, 63, 1433–1441 CrossRef CAS.
- K. Sowoidnich, H. Schmidt, H. D. Kronfeldt and F. Schwagele, Vib. Spectrosc., 2012, 62, 70–76 CrossRef CAS.
- W. Cheung, I. T. Shadi, Y. Xu and R. Goodacre, J. Phys. Chem. C, 2010, 114, 7285–7290 CAS.
- K. Gracie, E. Correa, S. Mabbott, J. A. Dougan, D. Graham, R. Goodacre and K. Faulds, Chem. Sci., 2014, 5, 1030–1040 RSC.
- A. P. Craig, A. S. Franca and J. Irudayaraj, Annu. Rev. Food Sci. Technol., 2013, 4, 369–380 CrossRef CAS PubMed.
- P. Matousek, I. P. Clark, E. R. C. Draper, M. D. Morris, A. E. Goodship, N. Everall, M. Towrie, W. F. Finney and A. W. Parker, Appl. Spectrosc., 2005, 59, 393–400 CrossRef CAS.
- P. Matousek, M. D. Morris, N. Everall, I. P. Clark, M. Towrie, E. Draper, A. Goodship and A. W. Parker, Appl. Spectrosc., 2005, 59, 1485–1492 CrossRef CAS PubMed.
- M. Bloomfield, D. Andrews, P. Loeffen, C. Tombling, T. York and P. Matousek, J. Pharm. Biomed. Anal., 2013, 76, 65–69 CrossRef CAS PubMed.
- C. Eliasson and P. Matousek, Anal. Chem., 2007, 79, 1696–1701 CrossRef CAS PubMed.
- C. Ricci, C. Eliasson, N. A. Macleod, P. N. Newton, P. Matousek and S. G. Kazarian, Anal. Bioanal. Chem., 2007, 389, 1525–1532 CrossRef CAS PubMed.
- P. W. Loeffen, G. Maskall, S. Bonthron, M. Bloomfield, C. Tombling and P. Matousek, Optics and Photonics for Counterterrorism and Crime Fighting Vii, Optical Materials in Defence Systems Technology Viii and Quantum-Physics-Based Information Security, 2011, vol. 8189, p. 10 Search PubMed.
- C. Eliasson, N. A. Macleod and P. Matousek, Anal. Chem., 2007, 79, 8185–8189 CrossRef CAS PubMed.
- M. Bloomfield, P. W. Loeffen and P. Matousek, Optics and Photonics for Counterterrorism and Crime Fighting Vi and Optical Materials in Defence Systems Technology Vii, 2010, vol. 7838, p. 15 Search PubMed.
- M. D. Hargreaves, N. A. Macleod, V. L. Brewster, T. Munshi, H. G. M. Edwards and P. Matousek, J. Raman Spectrosc., 2009, 40, 1875–1880 CrossRef CAS PubMed.
- P. Matousek and N. Stone, J. Biophotonics, 2013, 6, 7–19 CrossRef CAS.
- J. W. Qin, K. L. Chao and M. S. Kim, J. Food Eng., 2011, 107, 277–288 CrossRef CAS PubMed.
- N. K. Afseth, M. Bloomfield, J. P. Wold and P. Matousek, Appl. Spectrosc., 2014, 68, 255–262 CrossRef CAS PubMed.
- A. Dalla Mora, D. Contini, S. Arridge, F. Martelli, A. Tosi, G. Boso, A. Farina, T. Durduran, E. Martinenghi, A. Torricelli and A. Pifferi, Biomed. Opt. Express, 2015, 6, 1749–1760 CrossRef PubMed.
- U. Souto, M. J. C. Pontes, E. C. Silva, R. K. H. Galvao, M. C. U. Araujo, F. A. C. Sanches, F. A. S. Cunha and M. S. R. Oliveira, Food Chem., 2010, 119, 368–371 CrossRef CAS PubMed.
- V. Ghate, A. L. Leong, A. Kumar, W. S. Bang, W. B. Zhou and H. G. Yuk, Food Microbiol., 2015, 48, 49–57 CrossRef CAS.
- M. J. Evans, G. Clemens, C. Casey and M. J. Baker, Vib. Spectrosc., 2014, 72, 37–43 CrossRef CAS.
- L. Atzori, A. Iera and G. Morabito, Comput. Network, 2010, 54, 2787–2805 CrossRef.
- J. Gubbi, R. Buyya, S. Marusic and M. Palaniswami, Future Generat Comput. Syst., 2013, 29, 1645–1660 CrossRef PubMed.
- D. Miorandi, S. Sicari, F. De Pellegrini and I. Chlamtac, Ad Hoc Networks, 2012, 10, 1497–1516 CrossRef PubMed.
- K. Ashton, That ‘Internet of Things’ Thing, RFID Journal, http://www.rfidjournal.com/articles/view?4986, 2009 Search PubMed.
- A. Dada and F. Thiesse, in Internet of Things, Proceedings, ed. C. Floerkemeier, M. Langheinrich, E. Fleisch, F. Mattern and S. E. Sarma, Springer-Verlag Berlin, Berlin, 2008, vol. 4952, pp. 140–154 Search PubMed.
- A. Dada and C. Magerkurth, RFID based anti-counterfeiting utilizing supply chain proximity, Insticc-Inst Syst Technologies Information Control & Communication, Setubal, 2008 Search PubMed.
- H. Zhang and X. Chen, in Research on Food Packaging Technology, ed. O. Yun, X. Min, Y. T. Li and L. Xunting, 2014, vol. 469, pp. 481–485 Search PubMed.
- Z. Zou, Q. Chen, I. Uysal and L. R. Zheng, Philos. Trans. R. Soc., A, 2014, 372, 16 CrossRef.
- Y. Feng, L. Xie, Q. Chen and L. R. Zheng, IEEE Sens. J., 2015, 15, 3201–3208 CrossRef.
- R. A. Potyrailo, N. Nagraj, Z. X. Tang, F. J. Mondello, C. Surman and W. Morris, J. Agric. Food Chem., 2012, 60, 8535–8543 CrossRef CAS PubMed.
- A. Ilic, T. Staake and E. Fleisch, IEEE Pervasive Comput., 2009, 8, 22–29 CrossRef.
- G. Zhao, Y. Guo, X. Sun and X. Wang, Int. J. Electrochem. Sci., 2015, 10, 3387–3399 CAS.
- T. F. McGrath, C. T. Elliott and T. L. Fodey, Anal. Bioanal. Chem., 2012, 403, 75–92 CrossRef CAS.
- R. Y. Chen, Food Control, 2015, 51, 70–84 CrossRef.
- M. Wu, Y. Weng and Z. Liao, in Advances in Mechatronics, Automation and Applied Information Technologies, Pts 1 and 2, ed. Q. Lu and C. G. Zhang, 2014, vol. 846–847, pp. 1830–1835 Search PubMed.
- A. Escarpa, Chem. Rec., 2012, 12, 72–91 CrossRef CAS PubMed.
- A. Martin, D. Vilela and A. Escarpa, Electrophoresis, 2012, 33, 2212–2227 CrossRef CAS.
- G. F. Schneider and C. Dekker, Nat. Biotechnol., 2012, 30, 326–328 CrossRef CAS PubMed.
- D. A. P. de Abreu, J. M. Cruz and P. P. Losada, Food Rev. Int., 2012, 28, 146–187 CrossRef.
- R. Haass, P. Dittmer, M. Veigt and M. Luetjen, Int. J. Prod. Econ., 2015, 164, 400–408 CrossRef PubMed.
- V. A. Parvathy, V. P. Swetha, T. E. Sheeja, N. K. Leela, B. Chempakam and B. Sasikumar, Food Biotechnol., 2014, 28, 25–40 CrossRef CAS PubMed.
- M. Freebody, Photonics Spectra, 2010, 44, 20–21 Search PubMed.
- S.-Y. Wu, C. Yang, W. Hsu and L. Lin, Microsystems & Nanoengineering, 2015, 1, 1–9 Search PubMed.
- C. Gu, T. Lan, H.-C. Shi and Y. Lu, Anal. Chem., 2015, 87, 7676–7682 CrossRef CAS PubMed.
- W. Han, Y. Gu, W. Wang, Y. Zhang, Y. Yin, J. Wang and L.-R. Zheng, Inform. Syst. Front., 2015, 17, 275–287 CrossRef.
- Z. B. Pang, Q. Chen, W. L. Han and L. R. Zheng, Inform. Syst. Front., 2015, 17, 289–319 CrossRef.
- R. Shi, K. Yang, Q. Su, X. Zhou and H. Shi, in Research on Food Packaging Technology, ed. O. Yun, X. Min, Y. T. Li and L. Xunting, 2014, vol. 469, pp. 467–472 Search PubMed.
- M. E. Hollis-Peel and B. C. Welsh, Secur. J., 2014, 27, 320–337 CrossRef.
- L. E. Cohen and M. Felson, Am. Socio. Rev., 1979, 44, 588–608 CrossRef.
- F. Capra and P. L. Luisi, The Systems View of Life: A Unifying Vision, Cambridge University Press, Cambridge, UK, 2014 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |