Cheng Zhang, Yun-Ze Hui, De-Yang Zhang and Xiang-Ping Hu
RSC Adv., 2016,6, 14763-14767
DOI:
10.1039/C5RA25627E,
Communication
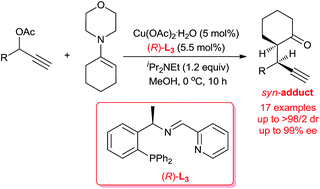
Highly diastereo- and enantioselective copper-catalyzed propargylic alkylations of propargylic acetates with morpholine-derived cyclic enamines for the construction of vicinal tertiary stereocenters have been developed. By the employment of a less sterically hindered chiral tridentate P,N,N-ligand, good to excellent diastereo- (up to >98 : 2 dr) and enantioselectivity (up to 99% ee) could be achieved for a wide range of substrates.