Theoretical study of Cu-based alloy catalysts for oxidative coupling of methane†
Abstract
Methane, the main component of natural gas, is abundant but difficult to activate due to its stable structure and strong C–H bond. The oxidative coupling of methane (OCM) offers a direct route for methane conversion to valuable C2 hydrocarbons such as ethane (C2H6) and ethylene (C2H4), providing both economic and environmental benefits. In this study, the catalytic activity of screened Cu-based intermetallic compounds (Cu3Ir) and surface alloy (Cu3Ir@Cu) catalysts as well as comprehensive reaction mechanisms for OCM were systematically evaluated by combining density functional theory (DFT) calculations and microkinetic modeling. The results showed that both Cu3Ir and Cu3Ir@Cu possessed low energy barriers for methane dissociation. Further electronic structure analysis reveals that the Ir atoms in Cu3Ir and Cu3Ir@Cu exhibit a stronger interaction with methane, facilitating the cleavage of the C–H bond to form methyl groups. In addition, the subsequent formation of both C2H6 and C2H4 follows the surface Langmuir–Hinshelwood mechanism, where ethane production is accomplished through the coupling of adsorbed 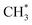 with low activation energy (0.20 eV on Cu3Ir and 0.38 eV on Cu3Ir@Cu), while ethylene is generated mainly through the coupling of
with low activation energy (0.20 eV on Cu3Ir and 0.38 eV on Cu3Ir@Cu), while ethylene is generated mainly through the coupling of 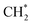 rather than
rather than 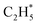 dehydrogenation. Comprehensive microkinetic simulations revealed the distribution of reactive species by using the surface coverage, degree of rate control (DRC) of elementary steps and turnover frequency (TOF) for C2 hydrocarbon production over screened alloy catalysts, where Cu3Ir@Cu exhibited higher TOF over a wide range of temperatures and pressures compared to Cu3Ir, especially at low temperatures. This study reveals the excellent activity of Cu3Ir and Cu3Ir@Cu alloys in methane activation and C2 hydrocarbon generation, which provides a theoretical basis for the development of high-efficiency methane conversion catalysts and a potential direction for industrial applications.
dehydrogenation. Comprehensive microkinetic simulations revealed the distribution of reactive species by using the surface coverage, degree of rate control (DRC) of elementary steps and turnover frequency (TOF) for C2 hydrocarbon production over screened alloy catalysts, where Cu3Ir@Cu exhibited higher TOF over a wide range of temperatures and pressures compared to Cu3Ir, especially at low temperatures. This study reveals the excellent activity of Cu3Ir and Cu3Ir@Cu alloys in methane activation and C2 hydrocarbon generation, which provides a theoretical basis for the development of high-efficiency methane conversion catalysts and a potential direction for industrial applications.



 Please wait while we load your content...
Please wait while we load your content...