Hierarchical Cu-MOF hollow nanowire modified copper mesh for efficient antibacterial PM filtration†
Haiyan
Li
a,
Tao
Wang
*a,
Yulong
Ying
 a,
Zhiqi
Wang
a,
Lianjun
Pan
b and
Sheng
Wang
a,
Zhiqi
Wang
a,
Lianjun
Pan
b and
Sheng
Wang
 *a
*a
aSchool of Materials Science and Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, China. E-mail: wangtao@zstu.edu.com; taotao571@hotmail.com; wangsheng@zstu.edu.com; wangsheng571@hotmail.com
bZhejiang Light Industrial Products Inspection and Research Institute, Hangzhou 310000, China
First published on 17th March 2023
Abstract
The filtration of fine particulate matter (PM) particles poses a significant threat to public health, with potentially adverse effects on the lungs and blood. Metal mesh filters have shown potential as multifunctional PM filters; however, filtration of fine PM particles is still challenging. In this study, we developed a hierarchical metal–organic-framework-based structure (Cu-MOF hollow nanowires) on copper mesh for PM filtration. The in situ grown three-dimensional (3D) Cu-MOF hollow nanowires effectively reduced the pore size and provided an enhanced electrostatic surface with maximum MOF exposure. This Cu-MOF coated metal mesh demonstrated excellent filtration efficiencies in a small-size PM regime (89.1% for PM0.3, 90.4% for PM0.5, 93.8% for PM1.0, and 98.1% for PM2.5). Furthermore, the filter exhibited excellent antibacterial performance against both S. aureus and E. coli. These attractive metal-based materials show potential in developing energy-saving and high-efficient metal-based filters for various applications.
1. Introduction
Air pollution caused by particulate matter (PM) is a significant environmental issue that seriously threatens human health.1–3 Generally, PM particles are classified into three types based on their aerodynamic equivalent diameter (AED): coarse, fine, and ultrafine particles (AED > 2.5 μm, AED = 0.1–2.5 μm, and AED < 0.1 μm, respectively).4 Among these particles, fine particles are particularly hazardous as they tend to carry toxic organics, heavy metals, and harmful microorganisms due to their higher specific surface area and active adsorption sites.5 Therefore, there is a pressing need to design an advanced filter that combines high fine PM removal efficiency with bacterial inhibition properties.According to classical filtration theory, the filtration mechanism can be either passive or proactive, depending on the interaction between the filter materials and PM particles.6–8 In the passive process, the primary mechanism is structure-based filtration, where the filter materials act as an “obstacle” to the particle-transport path and achieve filtration through physical collision, attachment, and trapping. In contrast, interaction-based filtration, i.e., the chemical and electrostatic interactions between PM particles and filter materials, becomes dominant in the proactive process. Most fiber-based filters are designed based on the passive trapping mechanism, i.e., blocking off the contaminants. While nanofiber-based air filters have been proven to be superior to microfibers in various aspects,9 the clogging effect has significantly shortened their lifetime due to their narrow pore structures.10,11
In a proactive filtration method, electrostatic precipitation and centrifugal collection are the most common ways to remove PMs. However, these methods require large energy consumption and take up large volume space. Recently, researchers have realized that surface modification of high-polarity materials in the filter can remarkably enhance the electrostatic interactions and improve the filtration efficiency.12,13 Corona charging or tribocharging of electret nanofibrous membranes using high-polarity polymer materials can efficiently capture PMs.14 Nylon-6 and poly(vinylidene fluoride) (PVDF) are representative high-polarity polymer materials with a high dipole moment.15,16 Gu et al. developed a high-efficiency rotating TENG (R-TENG)-enhanced PI-nanofiber air filter for PM removal under an ambient atmosphere, around which an electric field was formed and led to a significantly improved removal efficiency.17 However, achieving the charge stability of electret materials for air filtration is still a big challenge.14
Metal–organic frameworks (MOFs) have recently attracted great attention because of their ultrahigh porosity, high thermal and chemical stability, and abundant functionalities.18–21 The pioneering work of Wang et al. has led to a series of follow-up studies on the incorporation of MOFs into filtration systems for enhanced PM capture via electrostatic forces.22–24 However, the exposure degree of MOFs remains challenging due to the limited loading process.22,24 To date, loading MOFs on substrates has mainly included in situ growth,25,26 mechanical loading,27,28 binder-assistant binding,29,30 and electrospinning.31,32In situ growth is the most convenient method, but most supports are chemically inert and not suitable for loading. Severe agglomeration of MOFs often occurs during the operation of mechanical loading. Adding binders can improve the loading and robustness of the MOF coating, but both the porosity and active sites will be significantly reduced.29 The application of the electrospinning method often results in MOF nanocrystals being wrapped into nanofibers. As such, achieving appropriate bonding between highly effective MOFs and the substrate is crucial to realizing high filtration efficiency, longevity, reusability, and low cost.
Metal mesh filters are widely used in the industry due to their excellent durability and reliability.33,34 However, their filtration precision and performance on fine particles remain relatively low. Typically, electric or magnetic devices are necessary to aid in capturing fine PMs with metal filters, but this consumes high levels of energy.35 Few reports have been published thus far on metal mesh filters capable of filtering fine PMs without energy consumption.36 Given that MOFs can form and grow directly on the mesh surface, we introduce a novel self-sacrificial template strategy in this work to fabricate a PM filter utilizing 3D Cu-MOF hollow nanowires (NWs) on a commercial copper mesh. The physical PM screening of hierarchically structured and electrostatic PM screening of the partially charged surface of Cu-MOFs played critical roles in improving air filter properties. Furthermore, the composite mesh filter exhibited impressive antibacterial performance. In contrast to previous metal mesh filters, our work provides insight into the production of efficient antibacterial PM filters based on metal meshes. This approach can effectively protect individuals from the threat posed by pathogenic aerosols and airborne PM.
2. Experimental
2.1. Materials
2,3,6,7,10,11-Hexahydroxytriphenylene (HHTP, 95%) and ammonium persulfate ((NH4)2S2O8, 98%) were acquired from Macklin Biochemical Co., Ltd. Copper mesh (400 mesh size) was purchased from Changzhou Sa Small wire Mesh Co., Ltd. N,N-Dimethylformamide (DMF, 99%) was supplied by Hangzhou Shuanglin Chemical Reagent Co., Ltd. All chemicals and solvents were of analytical grade and used as received without further purification.2.2. Methods
Here, the resultant modified meshes obtained at different temperatures were named “CMM-T”, where T denotes the specific reaction temperature (30, 50, 70, and 90 °C) used for the growth of MOFs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–500
000–500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), with a mass mean diameter of 0.3–10 μm, were delivered through the filter (an effective area of 100 cm2). All test conditions strictly followed the international standards. The filtration test was repeated three times for each test filter.
000), with a mass mean diameter of 0.3–10 μm, were delivered through the filter (an effective area of 100 cm2). All test conditions strictly followed the international standards. The filtration test was repeated three times for each test filter.
The filtration efficiency (E) could be calculated from the PM particle concentration between upstream (C0) and downstream (C) using eqn (1). The pressure drop (ΔP) was obtained from the pressure difference between upstream (P1) and downstream (P2) using eqn (2). The quality factor (Qf) could be calculated using eqn (3).
 | (1) |
| ΔP = P1 − P2 | (2) |
 | (3) |
2.3. Characterization
The structure and surface morphology of the filter sample were examined with a field emission scanning electron microscope (FESEM, ZEISS VLTRA-55, Germany) and transmission electron microscope using a JEOL instrument (TEM, JEM-2010 HR) operated at 200 kV. The composition of the mesh sample was studied using energy-dispersive X-ray analysis (EDX, IncaEnergy-200). Fourier-transform infrared (FT-IR) spectrum of the mesh sample were recorded using a Nicolet 5700 FT-IR spectrometer. The crystalline structure of the mesh sample was analyzed by X-ray diffraction (XRD) measurements (Rigaku D/max-2200 diffractometer employing Cu-Kα radiation (λ = 0.154 nm)). The scanning rate was 2° min−1 at 2θ angles from 3° to 80°. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha) with a monochromatized Al-Kα X-ray source operated at 12 kV and 20 mA was applied for elemental analysis. N2 adsorption and desorption analyses of the mesh sample were performed on a nitrogen adsorption analyzer (BeiShiDe 3H-2000BET-A, China).3. Results and discussion
3.1. Characterization of Cu-MOF covered copper mesh
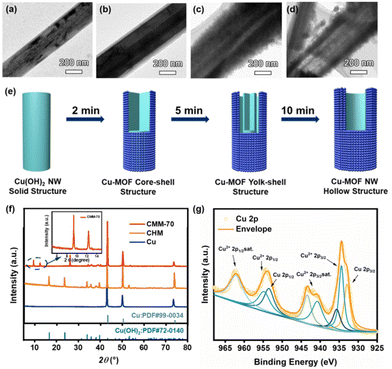
The self-sacrificial template strategy for preparing the Cu-MOF covered metal mesh filter is presented in Scheme 1. It involves two straightforward steps: the in situ growth of Cu(OH)2 NW arrays on the copper mesh (CHM), followed by the in situ transformation of Cu(OH)2 NWs to Cu-MOF hollow structures (CMM).38–40 The Cu(OH)2 NWs act as self-sacrificial templates and deprotonating agents. The CMM formation process can be explained by the Kirkendall effect, which is a widely used method to construct micro/nano-hollow structures via the diffusion rate difference of two ion species.41–43First, the morphologies of the copper mesh, CHM, and CMM were characterized by scanning electron microscopy (SEM) (for EDX spectra, see Fig. S2a–c†). The pristine copper mesh consisted of interwoven smooth copper microwires with a diameter of approximately 25 μm, exhibiting a net-like structure with a pore diameter of 40 μm (Fig. 1a and e). After the oxidation process, large amounts of Cu(OH)2 NWs, with a length of around 10 μm and a diameter of approximately 200–500 nm, grew on the surfaces of Cu wires, forming numerous micro/nano-scale bark-like grooves and filling the empty spaces of the copper mesh (Fig. 1b and f). The resulting CHM showed a pale blue appearance, suggesting the formation of Cu(OH)2 (inset in Fig. 1b).
Then, the CHM was immersed in HHTP solution, and the color of the mesh quickly turned black-green. The SEM images clearly showed the rough coral branch-like structure of the obtained Cu-MOF, with a larger diameter of 400–600 nm than that of the Cu(OH)2 NWs (Fig. 1c and g), led to a further space decrease in the copper mesh. The transmission electron microscopy (TEM) image revealed that the Cu-MOF has a hollow structure containing many intergrown nanoparticles of approximately 20–40 nm (Fig. 1d). The tubular-shaped NWs with a well-defined hollow interior were also distinguishable. The high-resolution transmission electron microscopy (HRTEM) image revealed that the lattice distance of 0.181 nm corresponds to the (100) lattice face of the hexagonal Cu-MOF.44,45 Elemental distribution mapping confirmed the uniform carbon, oxygen, and copper distribution of the CMM (Fig. S2b†). Fig. 2a–d illustrates the time-dependent formation process of CMM-70. The formation of the hollow structure is attributed to the Kirkendall effect, whereby the ionic size of the deprotonated ligand anion is larger than that of Cu2+. As a result, the diffusion rate of Cu2+ is faster than that of the ligand anions, leading to the formation of a hollow structure. After a reaction time of 2 min, a layer of the solid Cu-MOF shell was observed on the surface of the Cu(OH)2 NWs, resulting in a core–shell structure as shown in Fig. 2b. This MOF shell acts both as a template to maintain the wirelike morphology and a barrier to prevent the inward diffusion of the ligand anions.41,43
As the reaction time increased to 5 minutes, voids appeared inside the Cu(OH)2 NWs, with unconverted Cu(OH)2 remaining in the core, leading to a yolk–shell structure, as shown in Fig. 2c. This indicates that the growth of the Cu-MOF shell is dominated by the diffusion of Cu2+ from solid Cu(OH)2. After a reaction time of 10 minutes, the voids grew larger and a complete hollow structure with a rough surface composed of Cu-MOF nanoparticles was formed, as shown in Fig. 2d. The diameter of the Cu-MOF hollow NWs was larger than that of the corresponding Cu(OH)2 NWs, possibly due to the outward growth of the porous Cu-MOF shell. Based on the above analysis, the evolution mechanism of CMM-70 is elucidated and presented in Fig. 2e.
X-ray diffraction (XRD) analysis was performed to confirm the crystal structure of the products obtained at different stages of the reaction. As shown in Fig. 2f, all samples exhibited three strong diffraction peaks at 2θ = 43.3°, 50.4°, and 74.1°, which can be attributed to the metal copper (JCPDS No. 04-0836).46 The diffraction pattern of CHM also showed additional characteristic peaks at 16.7°, 23.9°, 34.1°, 35.9°, 38.3°, 39.9°, and 63.5°, which are consistent with the orthorhombic-phase Cu(OH)2 crystal (JCPDS No. 13-0420).47 In contrast, the diffraction pattern of CMM-70 showed new peaks at 2θ = 9.49, 12.59, and 27.09°, which correspond to the (200), (210), and (002) lattice planes of the Cu3(HHTP)2 crystals, respectively.48–50 Furthermore, the diffraction pattern of CMM-70 also exhibited residual diffraction peaks of Cu(OH)2, indicating the incomplete transformation of Cu(OH)2 to the Cu-MOF.
The FT-IR spectra of the pristine copper mesh, CHM, and CMM-70 were analysed, as shown in Fig. S3.† The CHM displayed absorption peaks at 3553, 3291, 942, and 680 cm−1. The bands at 3553 and 3291 cm−1 can be attributed to the O–H stretching vibration of Cu(OH)2, while the bands at 942 and 680 cm−1 may correspond to the O–H bending vibration of Cu(OH)2.51 In contrast, the FT-IR spectrum of CMM-70 showed strong characteristic peaks at 1219, 1289, and 1430 cm−1, which can be assigned to the C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C tensile vibration of the aromatic ring.52 Additionally, the characteristic peaks in the fingerprint region (800–500 cm−1) may correspond to the C–H bending mode and the ring out-of-plane bending vibrations of the substituted benzene.
C tensile vibration of the aromatic ring.52 Additionally, the characteristic peaks in the fingerprint region (800–500 cm−1) may correspond to the C–H bending mode and the ring out-of-plane bending vibrations of the substituted benzene.
To study the chemical state of elements in CMM-70, X-ray photoelectron spectroscopy (XPS) was performed, as shown in Fig. S4† and Fig. 2g. The survey spectrum of CMM-70 revealed the binding energies (BEs) of C 1s, O 1s, and Cu 2p at 282, 539, and 938 eV, respectively (Fig. S4†). Fig. 2g displays the high-resolution XPS spectra of Cu 2p, showing peaks at 934.8 eV and 954.3 eV that correspond to 2p3/2 and 2p1/2 of Cu2+, respectively.50,51 The Cu2+ 2p3/2 signals can be deconvoluted into two peaks (BEs at 934.5 and 935.5 eV), which can be assigned to the coordination of Cu2+ with HHTP. Moreover, the BEs at 940.8 eV, 944.6 eV, and 964.4 eV correspond to satellite peaks, indicating the paramagnetic chemical state of Cu2+.50,51
3.2. Temperature-dependent growth of the Cu-MOF
The influence of reaction temperature on the morphology of the Cu-MOF is presented in Fig. 3, where the diameter of the products increases gradually with the increase of the reaction temperature. The outward diffusion rate of Cu2+ is found to increase with the increase of the reaction temperature, leading to a gradual transformation of the structure from solid NWs to completely hollow NWs and even partial collapse. Although both CMM-70 and CMM-90 displayed well-defined hollow structures, the surface of CMM-90 exhibited partial collapse of the hollow structures, as observed in Fig. S5.† | ||
| Fig. 3 SEM and TEM images of modified mesh samples obtained at different reaction temperatures: (a, e and i) CMM-30, (b, f and j) CMM-50, (c, g and k) CMM-70, and (d, h and l) CMM-90. | ||
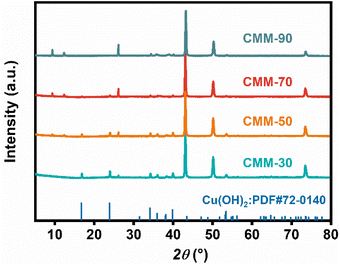
The XRD measurements shown in Fig. 4 reveal that as the reaction temperature increases from 50 to 90 °C, the peak intensity of Cu(OH)2 gradually decreases, while that of the Cu-MOF increases, indicating a smooth structural transformation. Furthermore, EDX analysis confirms the transformation processes, as shown in Fig. S6,† where the percentage of the C element observed in the Cu-MOF increases with higher reaction temperature.
The N2 adsorption–desorption results presented in Fig. S7† (with the corresponding data in Table S1†) demonstrate the adsorption isotherm of the CHM and CMM-T filters. The pristine copper mesh displayed almost no specific surface area. However, the specific surface area of the CMM-T showed a progressively increasing trend with the gradual formation of hollow structures. The SBET values of the obtained CMM-30, CMM-50, CMM-70, and CMM-90 filters were 9.19, 15.52, 25.34, and 22.53 m2 g−1, respectively, all of which were higher than that of CHM (5.13 m2 g−1). The reduced SBET of CMM-90 was due to the collapse of the hollow structures.
3.3. Filtration performance of the modified copper mesh filters
The filtration properties of the modified mesh filters were evaluated using two filtration tests.The filtration efficiency and pressure drop of the pristine copper mesh, CHM, and CMM were evaluated and the results are presented in Fig. 5a, b and Table S2.† The PM2.5 filtration efficiency of the CHM improved significantly from 20.1% of the pristine copper mesh to 78.8% with the growth of Cu(OH)2 NWs. The CMM showed further improvement in PM2.5 filtration efficiency, with the highest filtration efficiency observed in CMM-70 (96.0% for PM2.5 and 98.5% for PM10), which was significantly higher than those of the pristine copper mesh and CHM.
The PM2.5 filtration efficiency of CMM-70 gradually decreased from 96% to 85% after 10 cycles in long-term recycling tests (Fig. S9†). The SEM images revealed a slight collapse (Fig. S10 and S11†). The PMs attached to the surface could be easily washed out with a DI-water/ethanol mixture after PM removal in multiple cycling measurements. The FT-IR spectra of CMM-70 after filtration for 1 h show a significantly increased peak assigned to C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and C
O, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonding, indicating efficient PM2.5 adsorption from the cigarette smoke on CMM-70 (Fig. 5d). The peaks at 1757, 1643, and 1386 cm−1 are assigned to C
C bonding, indicating efficient PM2.5 adsorption from the cigarette smoke on CMM-70 (Fig. 5d). The peaks at 1757, 1643, and 1386 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–N. The peak at 1076 cm−1 is ascribed to C–O stretching vibrations. The peaks at 875 and 712 cm−1 are due to the out-of-plane bending vibration of C–H linked to a benzene ring.
C, and C–N. The peak at 1076 cm−1 is ascribed to C–O stretching vibrations. The peaks at 875 and 712 cm−1 are due to the out-of-plane bending vibration of C–H linked to a benzene ring.
The filtration efficiencies of the pristine copper mesh, CHM, and CMM-70 were investigated and are presented in Fig. S12 and S13.† The copper mesh showed poor filtration performance, while the CHM exhibited good filtration performance for large-size particles (96.9% for PM5.0 and 100% for PM10 at an airflow rate of 12 L min−1). CMM-70 showed excellent filtration performance across all particle size regimes (Table S3†), demonstrating that the Cu-MOF could significantly improve the filtration efficiency, particularly for fine PM particles.
Furthermore, the filtration performance of the CMM filters for fine particles (PM1.0 and PM2.5) at various air flow rates was investigated. The filtration efficiency of all the samples increased and then decreased with an increase in the airflow rate, while the pressure drop tended to increase linearly with the airflow rate (Fig. 6a and b). At an airflow rate of 12 L min−1, all the samples showed the optimal filtration efficiency for PM1.0 and PM2.5. Specifically, CMM-70 exhibited the highest filtration efficiency (93.8% for PM1.0 and 98.1% for PM2.5) compared to the others (PM1.0, 89.6%, 92.4%, and 88% for CMM-30, CMM-50, and CMM-90, and PM2.5, 93.5%, 94.3%, and 92.6% for CMM-30, CMM-50, and CMM-90) (Fig. 6a). Compared to the CHM, all CMM-T types demonstrated significantly higher filtration efficiency for small-size particles with the AED being lower than 2.5 μm. It is well-known that passive filtration mechanisms, such as interception, inertial impaction, and diffusion, have less influence on the capture of small-size PM particles.52–54 Therefore, the electrostatic interaction between the Cu-MOF and PM is likely the dominant factor in the capture enhancement.
It is noteworthy that CMM-70 consistently exhibits the lowest pressure drop across all airflow rates compared to CMM-30 and CMM-50, both of which have solid structures (Fig. 6b). The hollow structure of CMM-70 is believed to be responsible for the increased airflow passage. Although CMM-90 also has a hollow structure, it may experience a collapse of its hollow structure, leading to an increased pressure drop and decreased filtration efficiency.
The filtration efficiency of the CMM-T filters for different sizes of PM particles at an airflow rate of 12 L min−1 is depicted in Fig. 6c. All the filters demonstrate excellent filtration performance for large particles (100% for PM5.0 and PM10), with the efficiency decreasing as the particle size decreases, reaching its lowest point at 0.3 μm. The filters exhibit different particle capture abilities, with CMM-70 displaying the highest filtration efficiency among all CMM-T filters, particularly for fine particles (89.1% for PM0.3, 90.4% for PM0.5, 93.8% for PM1.0, and 98.1% for PM2.5). Consequently, CMM-70 exhibits the highest quality factor of 0.015 and 0.011 Pa−1 for PM2.5 and PM1.0, respectively, at an airflow rate of 12 L min−1 (Fig. 6d). It is worth noting that the quality factors obtained using the commercial filtration system are much lower than those obtained using the homemade smoke system, possibly due to the higher airflow rate in commercial filtration system.
In our study, the main air filtration mechanism of the Cu-MOF loaded copper mesh for submicron PMs is attributed to passive trapping including mechanical filtration, such as Brownian diffusion, direct interception, and inertial impaction.3 However, the filtration efficiency of the mechanical filtration mechanism is limited, especially for fine PM sizes. Therefore, in addition to mechanical filtration, the Cu-MOF loaded copper mesh shows the following additional filtration mechanisms: at first, the surface roughness of the Cu(OH)2 NWs can be improved by in situ growth of Cu-MOFs, which produces much more micro/nanoscale pores, and when the airflow containing PMs passes, the PMs are captured in the pores due to the inertial force and insufficient time to bypass the filter along the streamline of the airflow. Secondly, the in situ grown Cu-MOFs on 3D Cu(OH)2 NWs could provide a large amount of adsorption sites for fine PMs. The PMs will easily interact with the Cu-MOFs that have positive charges provided by the unbalanced metal ions, thereby enhancing the capacity of the filter to capture PMs. Furthermore, the SBET value of the copper wire can be improved after the in situ growth of Cu-MOFs due to their high SBET, enhancing the surface energy of the filter and increasing the deposition probability of fine aerosol particles. Finally, as the density of the Cu-MOFs increases, the airflow is blocked, while the hollow structure could facilitate airflow passage, reducing the pressure drop of the filtration system to some extent.
3.4. Antibacterial properties of mesh filters
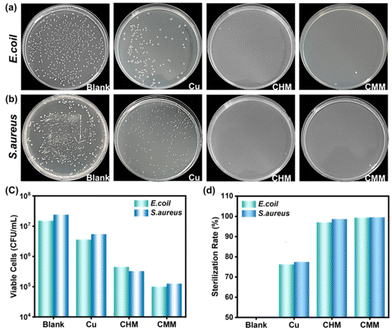
The antibacterial efficacy of the filters was evaluated through the plate counting method (Fig. 7a–d). The results showed that all the modified mesh filters, except the pristine copper mesh, exhibited sterilization rates higher than 95% against E. coli and S. aureus, respectively. The CHM showed higher antibacterial properties against E. coli (97.0%) and S. aureus (98.6%). CMM-70 demonstrated the highest antibacterial properties against E. coli (99.3%) and S. aureus (99.5%). It is reported that for copper containing surfaces, the antibacterial activity is strongly correlated with the release of copper ions from the surface into the surrounding media. It is generally accepted that copper ions cause a sequence of events that eventually lead to cell death.55–58 The antibacterial mechanism of the CHM and CMM here could also be attributed to the release of Cu2+ ions, which initially adsorb on the cell membrane of bacteria and then infiltrate into the cells through interactions with the hydroxyl groups of peptidoglycans, causing damage to the cell membranes and DNA breakage. The SEM images were further used to observe visually the morphological changes of E. coli and S. aureus after antibacterial experiments for CMM-70 (Fig. S14†). Both bacteria were damaged by Cu2+-induced antibacterial performance, showing extensive collapse and damage.4. Conclusions
In this study, a hierarchical 3D Cu-MOF hollow NW coated copper mesh filter was fabricated using a self-sacrificial template method, and its efficient PM-filtering effect was demonstrated. The CMM filter displayed improved filtration performance for PM particles in all size regimes, with particular emphasis on PM2.5 and PM1.0. The MOF-loaded filter also demonstrated excellent antibacterial activity against both E. coli and S. aureus. Overall, this study offers a straightforward preparation strategy for designing multi-functional MOF-loaded metal filters with high filtration performance across all particle size regimes. These highly efficient and antibacterial air filters are anticipated to have a wide range of applications in energy-efficient metal-based purification/separation devices and indoor air cleaning systems.Author contributions
Supervision, resources, writing – review, and editing: Tao Wang, Yulong Ying, and Sheng Wang. Methodology, experiments, and data curation: Haiyan Li, Zhiqi Wang, and Lianjun Pan. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Natural Science Foundation of Zhejiang province (LZ22C100002 and LQ22B010006), and 521 Talent Project of Zhejiang Sci-Tech University for providing financial support. The authors would like to thank Suzhou Huada Instrument Equipment Co., Ltd for the support with PM filtration tests.References
- Q. Zhang, X. Jiang, D. Tong, S. J. Davis, H. Zhao, G. Geng, T. Feng, B. Zheng, Z. Lu, D. G. Streets, R. Ni, M. Brauer, A. V. Donkelaar, R. V. Martin, H. Huo, Z. Liu, D. Pan, H. Kan, Y. Yan, J. Lin, K. He and D. Guan, Nature, 2017, 543, 705–709 CrossRef CAS PubMed.
- S. Zhang, H. Liu, N. Tang, S. Zhou, J. Yu and B. Ding, Spider-web-inspired PM0.3 filters based on self-sustained electrostatic nanostructured networks, Adv. Mater., 2020, 32, 2002361 CrossRef CAS PubMed.
- Z. Li, Q. Wen and R. Zhang, Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review, Sci. Total Environ., 2017, 586, 610–622 CrossRef CAS PubMed.
- J. O. Anderson, J. G. Thundiyil and A. Stolbach, Clearing the Air: A review of the effects of particulate matter air pollution on human health, J. Med. Toxicol., 2020, 8, 166–175 CrossRef PubMed.
- A. Nel, Air pollution-related illnesses: Effects of particles, Science, 2005, 308, 804–806 CrossRef CAS PubMed.
- S. Han, J. Kim and S. H. Ko, Advances in air filtration technologies: structure-based and interaction-based approaches, Mater. Today Adv., 2021, 9, 100134 CrossRef CAS.
- J. Xiao, J. Liang, C. Zhang, Y. Tao, G. W. Ling and Q. H. Yang, Advanced materials for capturing particulate matter: Progress and perspectives, Small Methods, 2018, 2, 1800012 CrossRef.
- S. Han, J. Kim and S. H. Ko, Advances in air filtration technologies: structure-based and interaction-based approaches, Mater. Today Adv., 2021, 9, 100134 CrossRef CAS.
- Kenry and C. T. Lim, Nanofiber technology: current status and emerging developments, Prog. Polym. Sci., 2017, 70, 1–17 CrossRef CAS.
- T. Xia and C. Chen, Evolution of pressure drop across electrospun nanofiber filters clogged by solid particles and its influence on indoor particulate air pollution control, J. Hazard. Mater., 2021, 402, 123479 CrossRef CAS PubMed.
- T. Xia, Y. Bian, S. Shi, L. Zhang and C. Chen, Influence of nanofiber window screens on indoor PM2.5 of outdoor origin and ventilation rate: An experimental and modeling study, Build. Simul., 2020, 13, 873–886 CrossRef.
- S. Mohankumar and P. Senthilkumar, Particulate matter formation and its control methodologies for diesel engine: A comprehensive review, Renewable Sustainable Energy Rev., 2017, 80, 1227–1238 CrossRef CAS.
- C. Liu, P.-C. Hsu, H.-W. Lee, M. Ye, G. Zheng, N. Liu, W. Li and Y. Cui, Transparent air filter for high-efficiency PM2.5 capture, Nat. Commun., 2015, 6, 6205 CrossRef CAS PubMed.
- X. Ma, Y. Chai, P. Li and B. Wang, Metal−organic framework films and their potential applications in environmental pollution control, Acc. Chem. Res., 2019, 52, 1461–14705 CrossRef CAS PubMed.
- J. Xu, C. Liu, P. C. Hsu, K. Liu, R. Zhang, Y. Liu and Y. Cui, Roll-to-roll transfer of electrospun nanofiber film for high-efficiency transparent air filter, Nano Lett., 2016, 16, 1270–1275 CrossRef CAS PubMed.
- S. Wang, X. L. Zhao, X. Yin, J. Y. Yu and B. Ding, Electret polyvinylidene fluoride nanofibers hybridized by polytetrafluoroethylene nanoparticles for high-efficiency air filtration, ACS Appl. Mater. Interfaces, 2016, 8, 23985–23994 CrossRef CAS PubMed.
- G. Q. Gu, C. B. Han, C. X. Lu, C. He, T. Jiang, Z. L. Gao, C. J. Li and Z. L. Wang, Triboelectric nanogenerator enhanced nanofiber air filters for efficient particulate matter removal, ACS Nano, 2017, 11, 6211–6217 CrossRef CAS PubMed.
- T. Kitao, Y. Zhang, S. Kitagawa, B. Wang and T. Uemura, Hybridization of MOFs and polymers, Chem. Soc. Rev., 2017, 46, 3108–3133 RSC.
- Y. Qian, F. Zhang and H. Pang, A review of MOFs and their composites-based photocatalysts: Synthesis and applications, Adv. Funct. Mater., 2021, 31, 2104231 CrossRef CAS.
- L. Ji, H. Zheng, Y. Wei, S. Gong, T. Wang, S. Wang and Z. Chen, Temperature-controlled fabrication of Co-Fe-based nanoframes for efficient oxygen evolution, Sci. China Mater., 2022, 65, 431–441 CrossRef CAS.
- H. Huang, J. Li, M. Yuan, H. Yang, Y. Zhao, Y. Ying and S. Wang, Large-scale self-assembly of MOFs colloidosomes for bubble-propelled micromotors and stirring-free environmental remediation, Angew. Chem., Int. Ed., 2022, 61, e202211163 CAS.
- Y. Zhang, S. Yuan, X. Feng, H. Li, J. Zhou and B. Wang, Preparation of nanofibrous metal–organic framework filters for efficient air pollution control, J. Am. Chem. Soc., 2016, 138, 5785–5788 CrossRef CAS PubMed.
- X. Ji, J. Huang, L. Teng, S. Li, X. Li, W. Cai, Z. Chen and Y. Lai, Advances in particulate matter filtration: Materials, performance, and application, Green Energy Environ., 2022 DOI:10.1016/j.gee.2022.03.012.
- D. K. Yoo, H. C. Woo and S. H. Jhung, Removal of particulate matter with metal–organic framework-incorporated materials, Coord. Chem. Rev., 2020, 422, 213477 CrossRef CAS.
- Z. Su, M. Zhang, Z. Lu, S. Song, Y. Zhao and Y. Hao, Functionalisation of cellulose fiber by in situ growth of zeolitic imidazolate framework-8 (Zif-8) nanocrystals for preparing a cellulose-based air filter with gas adsorption ability, Cellulose, 2018, 25, 1997–2008 CrossRef CAS.
- S. Ma, M. Zhang, J. Nie, J. Tan, B. Yang and S. Song, Design of double-component metal-organic framework air filters with PM2.5 capture, gas adsorption and antibacterial capacities, Carbohydr. Polym., 2019, 203, 415–422 CrossRef CAS PubMed.
- Z. Li, G. Zhou, H. Dai, M. Yang, Y. Fu, Y. Ying and Y. Li, Biomineralization-mimetic preparation of hybrid membranes with ultra-high loading of pristine metal−organic frameworks grown on silk nanofibers for hazard collection in water, J. Mater. Chem. A, 2018, 6, 3402–3413 RSC.
- Y. Chen, S. Zhang, S. Cao, S. Li, F. Chen, S. Yuan, C. Xu, J. Zhou, X. Feng, X. Ma and B. Wang, Roll-to-roll production of metal-organic framework coatings for particulate matter removal, Adv. Mater., 2017, 29, 1606221 CrossRef PubMed.
- Y.-S. Li, F.-Y. Liang, H. Bux, A. Feldhoff, W.-S. Yang and J. Caro, Molecular sieve membrane: supported metal-organic framework with high hydrogen selectivity, Angew. Chem., Int. Ed., 2010, 49, 548–551 CrossRef CAS PubMed.
- A. Huang, W. Dou and J. Caro, Steam-stable zeolitic imidazolate framework ZIF-90 membrane with hydrogen selectivity through covalent functionalization, J. Am. Chem. Soc., 2010, 132, 15562–15564 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, X. Wang, J. Yu and B. Ding, Ultrahigh metal−organic framework loading and flexible nanofibrous membranes for efficient CO2 capture with long-term, ultrastable recyclability, ACS Appl. Mater. Interfaces, 2018, 10, 34802–34810 CrossRef CAS PubMed.
- M. Lee, G. P. Ojha, H. J. Oh, T. Kim and H. Y. Kim, Copper//terbium dual metal organic frameworks incorporated side-by-side electrospun nanofibrous membrane: A novel tactics for an efficient adsorption of particulate matter and luminescence property, J. Colloid Interface Sci., 2020, 578, 155–163 CrossRef CAS PubMed.
- D. Wang, B. Zhu, X. He, Z. Zhu, G. Hutchins, P. Xu and W. N. Wang, Iron oxide nanowires based filter for inactivation of airborne bacteria, Environ. Sci.: Nano, 2018, 5, 1096–1106 RSC.
- S. J. Dunnett and C. F. Clement, Numerical investigation into the loading behaviour of filters operating in the diffusional and interception deposition regimes, J. Aerosol Sci., 2012, 53, 85–99 CrossRef CAS.
- S. I. Choi, J. Feng, S. B. Kim and Y. M. Jo, Magnetization of metal mesh for fine dust capture, Aerosol Air Qual. Res., 2018, 18, 1932–1943 CrossRef CAS.
- K. Yang, Z. Yu, C. Yu, H. Chen and F. Pan, An electrically renewable air filter with integrated 3D nanowire networks, Adv. Mater. Technol., 2019, 4, 1900101 CrossRef CAS.
- Z. Wang, T. Wang, Z. Zhang, L. Ji, L. Pan and S. Wang, ZIF-67 grown on a fibrous substrate via a sacrificial template method for efficient PM2.5 capture and enhanced antibacterial performance, Sep. Purif. Technol., 2022, 280, 119814 CrossRef CAS.
- J. Liu, L. Wang, N. Wang, F. Guo, L. Hou, Y. Chen, J. Liu, Y. Zhao and L. Jiang, A robust Cu(OH)2 nanoneedles mesh with tunable wettability for nonaqueous multiphase liquid separation, Small, 2017, 13, 1600499 CrossRef PubMed.
- F. Chen, J. Ma, Y. Zhu, X. Li, H. Yu and Y. Sun, Biodegradation Performance and anti-fouling mechanism of an ICME/electro-biocarriers-MBR system in livestock wastewater (antibiotic-containing) treatment, J. Hazard. Mater., 2022, 426, 128064 CrossRef CAS PubMed.
- S. Zhu, Y. Chen, M. A. Khan, H. Xu, F. Wang and M. Xia, In-depth study of heavy metal removal by an etidronic acid-functionalized layered double hydroxide, ACS Appl. Mater. Interfaces, 2022, 14, 7450–7463 CrossRef CAS PubMed.
- H. J. Fan, M. Knez, R. Scholz, D. Hesse, K. Nielsch, M. Zacharias and U. Gösele, Influence of surface diffusion on the formation of hollow nanostructures induced by the Kirkendall effect: The basic concept, Nano Lett., 2007, 7, 993–997 CrossRef CAS PubMed.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Synthesis, properties, and applications of hollow micro-/nanostructures, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- X. Zhang, C. Y. Chuah, P. Dong, Y. H. Cha, T. H. Bae and M. K. Song, Hierarchically porous Co-MOF-74 hollow nanorods for enhanced dynamic CO2 separation, ACS Appl. Mater. Interfaces, 2018, 10, 43316–43322 CrossRef CAS PubMed.
- S. Gu, Z. Bai, S. Majumder, B. Huang and G. Chen, Conductive metal–organic framework with redox metal center as cathode for high rate performance lithium ion battery, J. Power Sources, 2019, 429, 22–29 CrossRef CAS.
- R. Li, S. Li, Q. Zhang, Y. Li and H. Wang, Layer-by-layer assembled triphenylene-based MOFs films for electrochromic electrode, Inorg. Chem. Commun., 2021, 123, 108354 CrossRef CAS.
- Y. Yin, L. Zhu, T. Guo, X. Qiao, S. Gan, X. Chang, X. Li, F. Xia and Q. Xue, Microphone-like Cu-CAT-1 hierarchical structures with ultra-low oil adhesion for highly efficient oil/water separation, Sep. Purif. Technol., 2020, 241, 116688 CrossRef CAS.
- G. N. Ren, Y. M. Song, X. M. Li, Y. L. Zhou, Z. Z. Zhang and X. T. Zhu, A superhydrophobic copper mesh as an advanced platform for oil-water separation, Appl. Surf. Sci., 2018, 428, 520–525 CrossRef CAS.
- Q. Ma, P. Yin, M. Zhao, Z. Luo, Y. Huang, Q. He, Y. Yu, Z. Liu, Z. Hu, B. Chen and H. Zhang, MOF-based hierarchical structures for solar-thermal clean water production, Adv. Mater., 2019, 31, 1808249 CrossRef PubMed.
- M. S. Yao, X. J. Lv, Z. H. Fu, W. H. Li, W. H. Deng, G. D. Wu and G. Xu, Layer-by-Layer Assembled Conductive Metal-Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing, Angew. Chem., 2017, 129, 16510–16514 CrossRef PubMed.
- M. Hmadeh, Z. Lu, Z. Liu, F. Gándara, H. Furukawa, S. Wan, V. Augustyn, R. Chang, L. Liao, F. Zhou, E. Perre, V. Ozolins, K. Suenaga, X. Duan, B. Dunn, Y. Yamamto, O. Terasaki and O. M. Yaghi, New porous crystals of extended metal-catecholates, Chem. Mater., 2012, 24, 3511–3513 CrossRef CAS.
- Z. Shami, A. Gharloghi and S. M. Amininasab, Multifunctional pH-switched superwetting copolymer nanotextile: Surface engineered toward on-demand light oil-water separation on super-hydrophilic-underwater low-adhesive superoleophobic nonwoven mesh, ACS Sustainable Chem. Eng., 2019, 7, 8917–8930 CrossRef CAS.
- S. Hwang, W. S. Chi, S. J. Lee, S. H. Im, J. H. Kim and J. Kim, Hollow ZIF-8 nanoparticles improve the permeability of mixed matrix membranes for CO2/CH4 gas separation, J. Membr. Sci., 2015, 480, 11–19 CrossRef CAS.
- Y. Bian, Z. Niu, S. Wang, Y. Pan, L. Zhang and C. Chen, Removal of size-dependent submicron particles using metal−organic framework-based nanofiber air filters, ACS Appl. Mater. Interfaces, 2022, 14, 23570–23576 CrossRef CAS PubMed.
- Y. V. Kaneti, J. Tang, R. R. Salunkhe, X. Jiang, A. Yu, K. C. Wu and Y. Yamauchi, Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks, Adv. Mater., 2017, 29, 1604898 CrossRef PubMed.
- H. N. Rubin, B. H. Neufeld and M. M. Reynolds, Surface-anchored metal−organic framework−cotton material for tunable antibacterial copper delivery, ACS Appl. Mater. Interfaces, 2018, 10, 15189–15199 CrossRef CAS PubMed.
- J. Xiao, Y. Zhu, S. Huddleston, P. Li, B. Xiao, O. K. Farha and G. A. Ameer, Copper metal–organic framework nanoparticles stabilized with folic acid improve wound healing in diabetes, ACS Nano, 2018, 12, 1023–1032 CrossRef CAS PubMed.
- Z. Chen, J. Wang, M. Hao, Y. Xie, X. Liu, H. Yang, G. I. N. Waterhouse, X. Wang and S. Ma, Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance, Nat. Commun., 2023, 14, 1106 CrossRef CAS PubMed.
- M. Hao, Y. Xie, X. Liu, Z. Chen, H. Yang, G. I. N. Waterhouse, S. Ma and X. Wang, JACS Au, 2023, 3, 239–251 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi00349c |
| This journal is © the Partner Organisations 2023 |