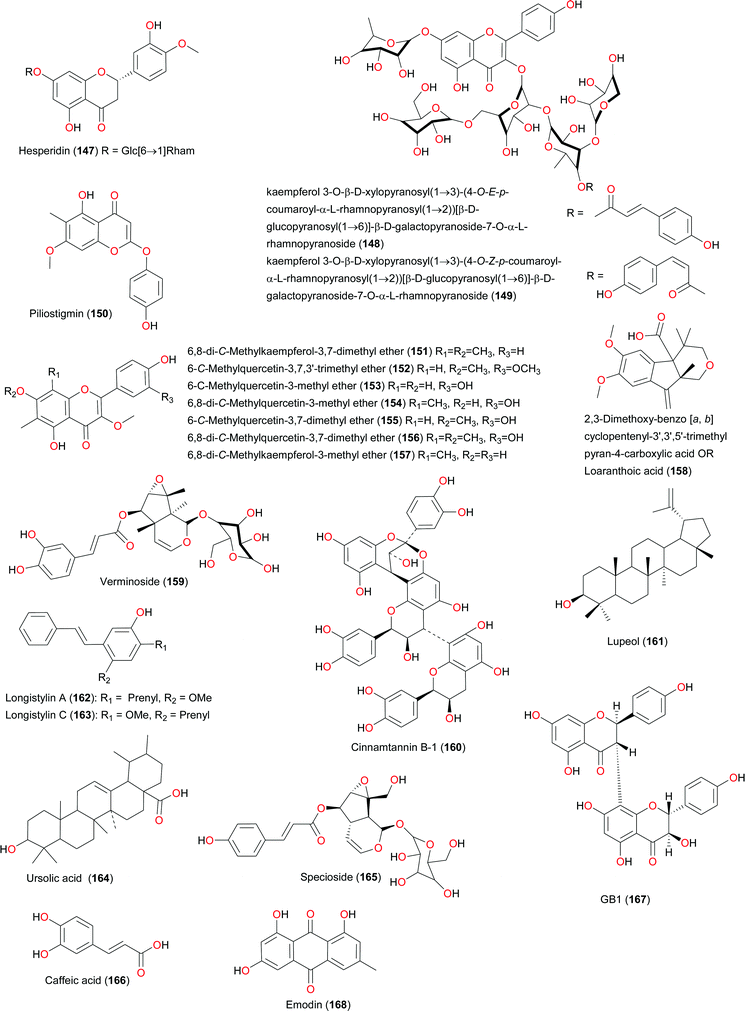
The uniqueness and therapeutic value of natural products from West African medicinal plants. Part I: uniqueness and chemotaxonomy
Fidele Ntie-Kang
ab,
Lydia L. Lifongo
a,
Conrad V. Simoben
a,
Smith B. Babiaka
a,
Wolfgang Sippl
b and
Luc Meva'a Mbaze
*c
aChemical and Bioactivity Information Centre, Department of Chemistry, Faculty of Science, University of Buea, P. O. Box 63, Buea, Cameroon
bDepartment of Pharmaceutical Sciences, Martin-Luther University of Halle-Wittenberg, Wolfgang-Langenbeck Str. 4, Halle, Saale 06120, Germany
cDepartment of Chemistry, Faculty of Science, University of Douala, P. O. Box 24157, Douala, Cameroon. E-mail: lmbazze@yahoo.fr; Tel: +237 99232190
First published on 15th May 2014
Abstract
This review gives an in depth coverage of the natural products derived from West African medicinal plants with diverse biological activities. Unique compound classes from West African flora having remarkable biological activities have been highlighted, as well as a correlation between the biological activities of the derived compounds and the uses of the plants in traditional African medicine, and their chemotaxonomic classifications have been included in the discussion. In the first part of the review, the focus is on alkaloids and flavonoids.
1 Introduction
Traditional medicine is known to cater to the health care needs of a significant proportion of the world's population, particularly in the developing economies of Africa, Asia and Latin America because of the limited availability of pharmaceutical medicines and the low purchasing power of these populations.1 The use of plants in the treatment of several diseases is a common practice in Africa2 and it is believed that the derived natural products (NPs) hold enormous potential for drug discovery.3 This has also been encouraged by the diverse use of a plethora of these plants in traditional medicinal practices. Hence, research groups in Africa have embarked on the extraction, bioassay-guided fractionation, isolation and characterisation of the bioactive metabolites from plants commonly used in African traditional medicine (ATM), with the view of identifying the active ingredients, which might have implications in drug discovery programs. This could either be directly as drug molecules or as hits/leads for synthetic modifications that lead to more potent or less toxic analogues with improved drug metabolism and pharmacokinetic (DMPK) profiles.1,2bHowever, some of the important data on bioactive metabolites derived from African medicinal plants with implications in ATM are dispersed in journal articles, as well as in MSc and PhD theses in university libraries (which, most often than not, are without online internet access). This renders such information inaccessible to a wider scientific community. Moreover, the efforts of African researchers have been limited to the random screening of crude extracts, essential oils and isolated metabolites from plants used in ATM using diverse bioassays in the search for hits and leads with promising activities, particularly against the neglected tropical diseases that affect the vast majority of the African population. Unfortunately, such efforts have not been complemented with similar efforts from the industrial sector towards transforming the research results into drug discovery/development programs aimed at manufacturing drugs for the sick populations. It therefore becomes imperative to summarise the most important findings for drug discovery from the dispersed data on African medicinal plants, critically analyse such data and hence make suggestions to pave the way forward.
Recent review papers on the potential of NPs, and in particular those isolated from African medicinal plants, have been focused on particular plant families, genera or species,4 particular diseases,2a,5 particular countries6 and particular sub-regions.7 Our recent review series have been focused on bioactive metabolites derived from medicinal plants growing in Central Africa,6b,7b including the development of NP databases7,8 and the pharmacokinetic profiling of NPs derived from plant materials.9 This has received significant attention from the readership and consequently motivated similar efforts for the other regions in the continent, knowing that the West African region has not been investigated thoroughly, in spite of its rich floral biodiversity and phytochemistry.
According to the United Nations, the West Africa sub region includes the following sixteen (16) countries: Benin, Burkina Faso, Cape Verde, Ivory Coast, the Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone and Togo. These countries occupy an area of over 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140
140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 and the natural environment in this area consists of subtropical and tropical regions with semi-arid and humid climates.10 In these communities, traditional herbalists operate closer to people, taking advantage of the biodiversity of the plant species present to cure various diseases and ailments. Numerous varieties of medicinal plants growing in West Africa are widely used against many diseases ranging from endemic tropical diseases like malaria,5d,5m,6c,11 trypanosomiasis5i and leishmaniasis12 to complex illnesses such as asthma,13 psychosis,14 hepatitis15 and even cancer.16 In the first part of the present review, the unique compound classes from West African flora will be highlighted, along with a correlation between the biological activities of the derived alkaloids and flavonoids and the uses of the plants in ATM. The second part will be focused on the huge class of terpenoids along with the remaining classes, the impact of the geographical distribution of plants on the chemical contents, a study of selected genera will also be covered and an insight on how the available data could be exploited in drug discovery.
000 km2 and the natural environment in this area consists of subtropical and tropical regions with semi-arid and humid climates.10 In these communities, traditional herbalists operate closer to people, taking advantage of the biodiversity of the plant species present to cure various diseases and ailments. Numerous varieties of medicinal plants growing in West Africa are widely used against many diseases ranging from endemic tropical diseases like malaria,5d,5m,6c,11 trypanosomiasis5i and leishmaniasis12 to complex illnesses such as asthma,13 psychosis,14 hepatitis15 and even cancer.16 In the first part of the present review, the unique compound classes from West African flora will be highlighted, along with a correlation between the biological activities of the derived alkaloids and flavonoids and the uses of the plants in ATM. The second part will be focused on the huge class of terpenoids along with the remaining classes, the impact of the geographical distribution of plants on the chemical contents, a study of selected genera will also be covered and an insight on how the available data could be exploited in drug discovery.
2 Unique natural products from West Africa
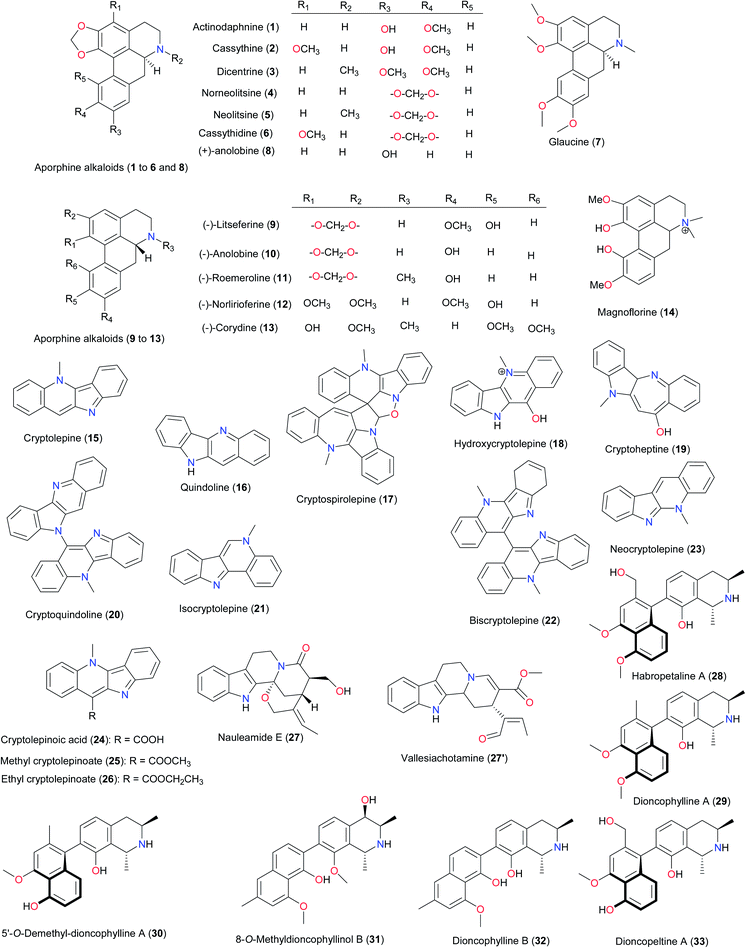
Among the promising compounds derived from West African medicinal plants, for the very first time are the aporphine alkaloids (1–7) from Cassytha filiformis (Lauraceae).17 These compounds have been shown to be cytotoxic agents, in addition to the in vitro antitrypanosomal properties on Trypanosoma brucei brucei, exhibited by actinodaphnine (1), cassythine (2), and dicentrine (3). The in vitro anticancer test on HeLa cells showed that glaucine (7) was the most cytotoxic compound (IC50 = 8.2 μM) in the series. Mechanistic studies using optical measurements indicated that all seven aporphines effectively bind to DNA and behave as typical intercalating agents. Biochemical experiments have also showed that actinodaphnine, cassythine and dicentrine interfere with the catalytic activity of topoisomerases in contrast to the four other aporphines. These interactions with DNA may explain, at least in part, the effects observed on cancer cells and trypanosomes. Even though aporphinoids are also known to have been isolated from several species,18 including Siparuna sp. from French Guyana,19 Spirospermum penduliflorum from Madagascar,20 Glaucium sp. from diverse regions,21 Corydalis yanhusuo22 and Croton lechleri from China,23 Papaver aculeatum from South Africa,24 Enantia chlorantha from Cameroon,25 and Artabotrys brachypetalus from Zimbabwe;26 those from the West African Cassytha filiformis are remarkable for their cytotoxic/antitrypanosomal activities.27 The aporphines (+)-anolobine (8), (−)-litseferine (9), (−)-anolobine (10), (−)-roemeroline (11), (−)-norlirioferine (12) and (−)-corydine (13) have also been unusually found recently in Monodora sp. from the Ivory Coast (M. crispata and M. brevipes, Annonaceae).28 Aporphines 9–12 are new to the Monodora genus, while 8 and 13 have been observed in only one Monodoreae (M. tenuifolia)29 and their analogues have been identified in M. angolensis,30 M. junodii31 and M. tenuifolia29 harvested in other parts of the world.Another remarkable and unique subclass of alkaloids from West Africa is the indoloquinoline alkaloids, which exhibit anti-malarial, antitrypanosomal and cytotoxic properties. Cryptolepine (15) was isolated from Sida acuta (Malvaceae) harvested in the Ivory Coast32 and from Cryptolepis sanguinolenta (Periplocaceae) growing in diverse regions of West Africa.33 The cryptolepine derivatives (16–26) isolated from the stems, roots and root bark of Cryptolepis sanguinolenta have also exhibited potent anti-malarial properties.34 Cryptolepine is one of those rare highly potent anti-malarial agents unique for its structure and biological activty. It is currently used as an anticancer drug because of its ability to intercalate into DNA at the cytosine–cytosine sites.35
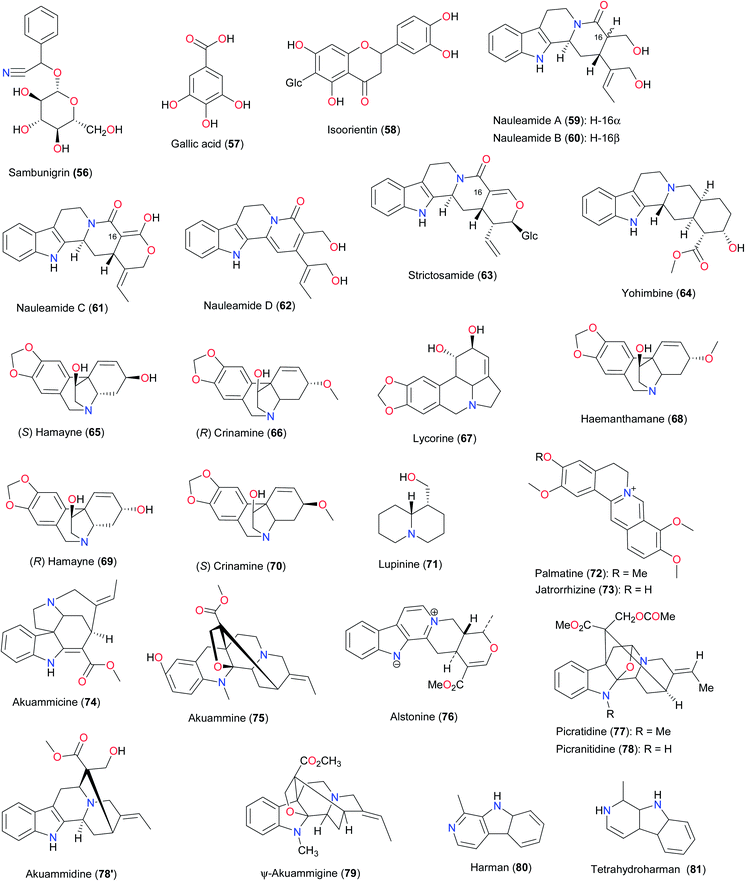
Nauleamide E (27) is a unique monoterpene indole alkaloid possessing a pentacyclic ring system with an amino acetal bridge and has been isolated from Nauclea latifolia (Rubiaceae) harvested in Calabar, Nigeria.36 Even though this type of monoterpene indole alkaloid is rare, vallesiachotamine (27′) has been obtained from the Peruvian plant, Vallesia dichotoma.37
Naphthylisoquinoline alkaloids represent another set of potent and naturally occurring anti-malarial/antileishmanial agents from West African flora. Habropetaline A (28),38 dioncophylline A (29) and its 5′-O-demethylated analogue (30),39 8-O-methyldioncophyllinol B (31),40 dioncophylline B (32) and dioncopeltine A (33)39,41 from Triphyophyllum peltatum (Dioncophyllaceae) belong to this category. Korupensamine A (34) and B (35) have been isolated from both Ancistrocladus guineensis (Nigeria)42 and its sister species A. korupensis (Cameroon),43 while ancistroguineines A (36) and B (37), ancistrotectorine (38) and ancistrobrevine B (39) were unique to the Nigerian species.44 The chemistry and biological activities of the other naphthyl isoquinolines isolated from Central and East Africa have been discussed in recently published reviews.5d,7
Other rare compound types identified from West African flora are the unusual furanone-substituted flavones44 and divanilloylquinic acids.45 The former has been isolated from the leaves of Hoslundia opposita (Lamiaceae) while the latter was derived from Fagara zanthoxyloides (Rutaceae), a plant species used in folk medicine for its antisickling properties in Burkina-Faso and other West African countries. The NPs isolated from Fagara zanthoxyloides; burkinabins A (40), B (41) and C (42) showed promising activities against sickle cell anaemia.45 The unusual 6-furanoflavones, hoslunfuranine (43) and 5-O-methylhoslunfuranine (44), isolated from H. opposita, are characterised by a furanone moiety that is branched at the C-4′′ position. Compound 44 exhibited leishmanicidal potential in the micromolar range.44 Another aporphine alkaloid magnoflorine (14), isolated from a sister Fagara species (F. macrophylla) in the Republic of Guinea, exhibited antifeedant properties against S. frugiperda.46
3 Analysis of plant families, compound types and attempted chemotaxonomic classification
In the current survey, a literature search on the major natural product journal websites was carried out based on country names in the search engine of each journal. The article hits were filtered based on the geographical location of the harvested plant materials and articles only describing NPs isolated from plants harvested in West Africa were retained. The data from each article was extracted on an excel sheet with several fields, including plant species names, family names, ethnobotanical uses, compound types identified (e.g. alkaloids, flavonoids, terpenoids, etc.), biological activities of the isolated metabolites, collection dates of the plant samples, authors, references, etc. Our collection was composed of around 700 input compounds, previously isolated/derived from 97 plant species belonging to 41 families. The data was collected from 5 MSc theses, 9 PhD theses and 445 articles from 134 peer reviewed journals. We have carried out an analysis on the collected data, based on the number of compounds isolated per plant species and per family. The majority of the metabolites are shown to have been previously isolated from plants harvested in Nigeria.In this analysis, an emphasis was placed on those plant families from which at least ∼2% of the secondary metabolites have been isolated. The majority of the compounds were isolated from the Euphorbiaceae family, constituting 13.67%. This was followed by the Annonaceae (10.83%), Leguminosae (9.67%), Guttiferae (8.67%), Rubiaceae (4.50%), Loganiaceae and Meliaceae (both 4.17%), Compositae (3.61%), Combretaceae and Loranthaceae (both 3.50%), Rutaceae (2.83%), Lamiaceae (2.50%), and finally the Apocynaceae and Asteraceae (both 2.00%) families (Fig. 1).
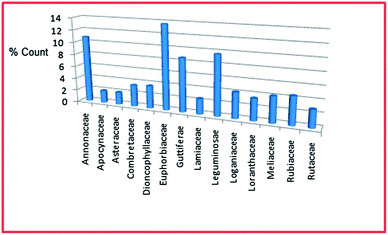 | ||
| Fig. 1 Bar chart showing the distribution of percentage number of compounds isolated per plant family. | ||
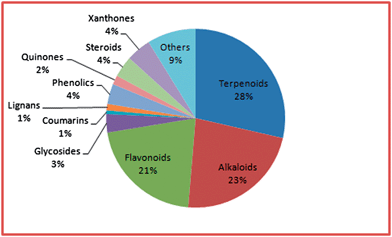
An overall distribution by compound type (based solely on unique compounds and not the compound concentration in the plants) is shown in Fig. 2. Our results showed that the terpenoids were the most abundant among the isolated compounds, constituting 29.91% of the isolated compounds, a similar picture to our data previously analysed from Central Africa.7b This was followed by the alkaloids (23.83%), flavonoids (22.09%), xanthones (4.70%), steroids (4.00%), phenolics (3.83%), and glycosides (3.48%). The remaining compounds classes constituted <2.00% of each of the isolated compounds in terms of numbers.
Table 1 shows a summary of the dominant compound types isolated versus the recorded biological activities for the most outstanding plant families. The Euphorbiaceae family is dominated by terpenoids (39.51%) and flavonoids (24.70%). Alkaloids dominated the Annonaceae family, constituting 49.21%, while the Leguminosae were as usual dominated by flavonoids (23.68%).6b,7b The other alkaloid-rich families were the usual Apocynaceae (75.00%), Rutaceae (40.00%) and Rubiaceae (43.75%). The Asteraceae, Combretaceae, Euphorbiaceae, Loganiaceae, Loranthaceae and Meliaceae families were seen to contain large numbers of terpenoids, when compared with the other classes of compound, constituting 63.64%, 85.00%, 39.51%, 56.00%, 33.33% and 100%, respectively. The Compositae family was equally rich in terpenoids and flavonoids, while xanthones and flavonoids were dominantly present among the Guttiferae. The above percentages could be seriously affected by the limited number of NPs in this study, when compared with our previous Central African study, even though the overall classification is similar.7b
| Plant family | % of isolated compounds | Remarkable compound classes (% composition) | Genera studied | Recorded biological activities of the isolated compounds |
|---|---|---|---|---|
| Annonaceae | 10.83 | Alkaloids (49.21%) | Enantia, Dennettia, Monodora, Uvaria, Freisodielsia, Piptostigma and Annona | Antiplasmodial |
| Apocynaceae | 2.00 | Alkaloids (75.00%) | Picralima, Rauwolfia and Strophanthus | Antiplasmodial and inhibition of binding of 1H-diazepam to the benzodiazepine sites within the rat GABA receptor complex |
| Asteraceae | 2.00 | Terpenoids (63.64%) | Acanthospermum, Chromolaena, Dicoma and Struchium | Antiplasmodial, antileishmanial, antitrypanosomal, antimicrobial, cytotoxicity and anticancer properties |
| Combretaceae | 3.50 | Terpenoids (85.00%) | Guiera, Pteleopsis and Combretum | Antiplasmodial, antibacterial and cytotoxic |
| Compositae | 2.00 | Terpenoids (50.00%) | Tithonia, Centaurea and Laggera | Antiplasmodial and antimicrobial |
| Flavonoids (50.00%) | ||||
| Dioncophyllaceae | 3.67 | Alkaloids (100.00%) | Triphyophyllum | Antiplasmodial and antiparasitic |
| Euphorbiaceae | 13.67 | Terpenoids (39.51%) | Securinega, Alchornea, Jatropha, Croton and Elaeophorbia | Cytotoxic, anti-inflammatory, antioxidant and antimicrobial |
| Flavonoids (24.70%) | ||||
| Guttiferae | 8.67 | Xanthones (52.00%) | Garcinia, Tithonia, Centaurea and Laggera | Antimicrobial |
| Flavonoids (32.70%) | ||||
| Lamiaceae | 2.50 | Flavonoids (85.71%) | Hyptis, Hoslundia and Platostoma | Antiplasmodial, antiparasitic, cytotoxicity, anti-inflammatory and antioxidant |
| Terpenoids (14.30%) | ||||
| Leguminosae | 9.67 | Flavonoids (23.68%) | Abrus, Cassia, Erythrina, Millettia, Cajanus, Abrus, Russelia, Baphia and Leptoderris | Antiplasmodial, cytotoxicity, antibacterial, anti-inflammatory, anti-inflammatory, antinociceptive, antinociceptive, antioxidant and antioxidant |
| Terpenoids (15.79%) | ||||
| Alkaloids (8.00%) | ||||
| Loganiaceae | 4.17 | Terpenoids (56.00%) | Strychnos and Anthocleista | Antitrypanosomal |
| Loranthaceae | 3.50 | Terpenoids (33.33%) | Loranthus | Antioxidant, immunostimulatory and proliferative |
| Meliaceae | 4.17 | Terpenoids (100%) | Azadirachta and Khaya | Antitrypanosomal |
| Rubiaceae | 4.50 | Flavonoids (43.33%) | Nauclea, Mitracarpus and Ixora | Antimicrobial and antioxidant activities |
| Alkaloids (40.00%) | ||||
| Rutaceae | 2.83 | Alkaloids (43.75%) | Murraya and Fagara | Antisickling and antifeedant activities |
Previous attempts towards the taxonomic classification of West African medicinal plants have been carried out.47 However, a vivid discussion of the classification of plant families by compound type has not been presented to date. In addition to the above mentioned main compound classes, we provide a summary of the compound subclasses that characterise the selected genera and species. Within the Annonaceae family, for example, the isolation of the acyclic diterpene alcohol trans-phytol (45) from Piptostigma fasciculate, harvested from Ghana, is of taxonomic interest (unique to the genus),48 since the diterpenes encountered so far in the family are clerodane, trachylobane, kolavane and predominantly kaurane derivatives.49 This compound is rather most commonly found in various species of marine algae,50 as well as in a number of higher plants, including Fatsia japonica (Araliaceae),51 Tetragonia tetragonioides (Aizoaceae)52 and Artemisia annua (Compositae)53 Moreover, the morphinandienones (−)-mocrispatine (46) and pallidine (47) obtained from Monodora crispata, harvested from the South of the Ivory Coast are unusual in Annonaceae,28 pallidine having been encountered as the major aporphine in several tribes within Annonoideae.54 Kablan et al. also studied another species within the Monodora genus from the Ivory Coast.28 It was observed that compounds 46 and 47 were absent in the batch of M. brevipes, confirming that morphinandienones are rare compounds within Monodora sp., with no chemotaxonomical value at the supraspecific level. It is noteworthy that the morphinandienones from Annonaceae bear an S configuration at C-9, as observed in other Magnoliids (Magnoliales, Laurales), contrary to their counterparts isolated from Papaveraceae (Ranunculales, Eudicots).55 Within the Asteraceae family, for example, the presence of a number of acetyl chromenes in Ageratum conyzoides is believed to be of chemotaxonomic significance. It indicates that the genus is chemically closer to the Ageritanae subtribe, as opposed to the Piqueriiae group to which it was previously assigned.56 Among the plants of the Vernonia genus studied so far for biological activity, a good number of the biological activity claims have been associated with the presence of terpenoids. Despite the fact that the literature reports the isolation of several terpenoids from the Vernonia genus, only a limited number have been tested for bioactivity.4c
Paulo and Houghton have extensively discussed the chemotaxonomy of the Cryptolepis genus,57 which is mostly represented in West Africa by C. sanguinolenta, noted for the presence of a special class of indole alkaloids (cryptolepines), exhibiting antiplasmodial activities, named after this genus.32,33 According to the authors, the Cryptolepis genus could be carefully placed under the subfamily of Periplocoideae and the related families Asclepiadaceae and Apocynaceae. This is because the chemistry of the Cryptolepis genus is in agreement with its taxonomic position within the Periplocaceae/Periplocoideae taxon. Additionally, chemical evidence obtained so far is consistent with the idea that the Periplocaceae/Periplocoideae taxon is an evolutionary link between the families Apocynaceae and Asclepiadaceae. The authors further advance strong arguments that the Periplocaceae/Periplocoideae taxon could be considered an independent family.57 The taxonomy of the Echium genus (Boraginaceae) is known to be quite complex.58 The exploration of two endemic species, E. Stenosiphon and E. hypertropicum, from Cape Verde has led to the identification of the hepatotoxic diesters echimidine (48) and 7-(2-methylbutyryl)-9-echimidinylretronecine (49) in both species. Echimidine was the major component in the diethyl ether fraction from the leaves of E. hypertropicum, whereas 7-(2-methylbutyryl)-9-echimidinylretronecine was the major component in the dichloromethane fraction from the leaves of E. stenosiphon.59 According to the study by Carvalho et al., E. stenosiphon subsp. stenosiphon and E. hypertropicum were found to be rich in pyrrolizidine alkaloids (PAs),59 having common structural features i.e. 1,2 unsaturation, an esterified allylic hydroxyl group at C9 and an esterified alcoholic hydroxyl group at C7, which generally make the PAs potentially toxic.59,60 The authors cautioned that these two species be regarded as potentially hepatotoxic, thus discouraging their use in traditional medicine. However, PAs could be used as chemotaxonomic markers for the Echium genus.59
The investigations of Niassy et al. on the aerial parts of two species of the Tephrosia genus (T. deflexa and T. albifoliolis, Leguminosae), harvested from the Nature Reserve of Niokolo-Koba in the South-East of Senegal, made a significant contribution towards the understanding of the chemistry of this genus.61 Although the presence of C-prenylflavonoids appears to be widespread in this genus,62 these authors could demonstrate only the presence of rotenone (50) in T. deflexa, along with other common flavonoids. No prenylated flavonoids, considered intermediates in the synthesis of the rotenoids, were detected in either of the two species. The investigations of Niassy et al. thus led to the first report on the occurrence of the flavonols in the Tephrosia genus. Moreover, until the time of the publication of their results, the four quercetagetin derivatives—jacein, eupatolin, quercetagetin-3,3′-dimethylether-7-O-glucoside and quercetagetin 3′-methylether-7-O-glucoside—had been encountered in Compositae, but not in Leguminosae.63 Within the Loganiaceae family, lichexanthone (51), previously known as a fungal metabolite,64 has been isolated from Anthocleista djalonensis, collected in Ibadan, Nigera.65 This compound co-occurs with alternariol methyl ether within this plant. The above observations have led to the conclusion that alternariol (52) and thus its mono methyl ether are biosynthesized via nor-lichexanthone. The co-occurrence of 3,4′-dihydroxy-5-methoxy-6′-methyl-dibenzo-α-pyrone (mono methyl ether alternariol) with lichexanthone in A. djalonensis could be of chemotaxonomic importance as supportive evidence in favour of nor-lichexanthone as the precursor of alternariol methyl ether.66 In the Loranthaceae family, the presence of the unusual dihydroxylated lupeol-based fatty acid esters (53–55) in mistletoes (Loranthus micranthus), harvested in Eastern Nigeria,67 as well as from mistletoes growing in Japan68 is an indication that they may be mistletoe-specific. This however warrants further investigation.
As a family, the Meliaceae are known for the presence of an abundance of limonoids.69 In this family, attempts to classify the genus Trichilia have often led to conflicting conclusions.70 As an example, it is believed that the antischistosomal and antiplasmodial properties and a thorough biosystematic study of T. emetica, possibly including T. dregeana, should provide valuable insights on the chemotypic variation and the intraspecific taxonomy of these two ethnobotanically important species.71 Bero et al. carried out a study of the leaf extracts of T. emetica subsp. suberosa collected from Benin and these showed no activity on Plasmodium falciparum, except for the dichloromethane extract, which had a very moderate effect (IC50 = 59.2 μg mL−1). These results confirmed the results obtained by Traore et al.72 In Mali, another study on this subspecies showed antiplasmodial activity for the dichloromethane extract, with an IC50 of 11.9 μg mL−1.73 This activity could be due to variations in the chemical content of the samples from different localities. The other subspecies, Trichilia emetica subsp. emetica was active in various studies.74 However, the taxonomic differentiation is proof of the different biological properties linked with different chemical compositions.75
Sonibare et al. have identified the chemotaxonomic significance of leaf alkanes in a species of Ficus (Moraceae) from Nigeria.76 The alkane pattern of the leaf waxes from twenty-four tropical Ficus species in Nigeria was determined by gas chromatography and gas chromatography-mass spectrometry. Of the twelve alkanes identified, hentriacontane and tritriacontane were the major components in all the species studied. This indicates that the occurrence of alkanes in Ficus could provide useful information to the understanding of the species variability. Le et al. have identified various bioactive polyphenols in Ximenia americana (Olacaceae) used traditionally among Malian healers for throat infections, amenorrhea, and as a tonic for wound healing and pain relief.77 Sambunigrin (56) was the main compound in the EtOAc soluble fraction of the alcoholic extract of Ximenia americana leaves. Nine other compounds, including gallic acid (57), two gallotannins and six flavonoids were identified for the first time in the Ximenia genus. While Sambunigrin was previously known from Ximenia americana leaves,78 the other nine compounds had not been previously reported in the Ximenia genus. While some of these compounds are nearly ubiquitous, others (such as the galloylated flavonol glycosides) have a limited distribution in nature. Their presence in this plant species may therefore be of chemotaxonomical interest.
The flavonoids isolated from Vetiveria zizanioides and Vetiveria nigritana (Poaceae), harvested in Koulikoro, near Bamako (Mali), also have a significance for the chemotaxonomy of West African medicinal plants.79 Apart from isoorientin (58), which is only present in V. zizanioides, the same flavonoids were identified in both species. The aerial parts of both species contained mainly 6,8-di-C-heterosides of luteolin. These flavonoids share a common structural motif, probably indicating the existence of a C-glycoside step during the flavone biosynthesis system. Some authors have considered C-glycosyl flavones to be the basic flavonoids in many Poaceae,80 including species of the Vetiveria genus. However, flavone-C-glycosides cannot be considered specific to Poaceae, since they are also found in many other families such as Passifloraceae, Orchidaceae, and Caryophyllaceae. Moreover, the report of Gluchoff-Fiasson et al. was the first time 6,8-di-C-arabinopyranosylluteolin was isolated in the Poaceae family. The compound tricin-5-O-glucoside has been already reported in the other species of Poaceae: Triticum spp.80b and Oryza sativa.81 Its presence in the roots of the two Vetiveria species could be significant from a chemotaxonomic point of view.
Within the Rubiaceae family, naucleamides are monoterpene indole alkaloids isolated for the very first time from Nauclea latifolia (Rubiaceae) from Calabar, Nigeria.36 This type of monoterpene indole alkaloid is rare, and Nauleamide E (27) is a unique monoterpene indole alkaloid possessing a pentacyclic ring system with an amino acetal bridge, used to identify the species. Biosynthetically, naucleamides A–E (27, 59–62) may be derived from strictosamide (63) through reductive and/or oxidative cleavage of ring E.
4 Ethnobotany versus bioactivity survey
The biological activities of the selected compounds, along with the ethnobotanical uses of the plants from which they were derived have been summarised in Tables 2 and 3. Whenever there is a correlation between bioactivity and ethnobotany, these have been highlighted in bold in the tables. The discussion that follows is arranged according to the various compound classes identified, with a focus on the most abundant classes—alkaloids and flavonoids.| Compound | Plant species (country) | Family | Ethnobotanical use | Measured activity | References |
|---|---|---|---|---|---|
| 1–7 | Cassytha filiformis (Benin) | Lauraceae | Used in African folk medicines to treat cancer, African trypanosomiasis and other diseases | Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases | Hoet et al.17 |
| 8–13 | Monodora sp. (Ivory Coast) | Annonaceae | Not reported | Not tested | Kablan et al.,28 Spiff et al.29 |
| 14, 97–102 | Fagara macrophylla (Guinea) | Rutaceae | Used as a remedy for several afflictions, in particular the cure of toothache, rheumatism and urogenital affections, as well as to prepare poisonous arrows | Antifeedant against S. frugiperda. | Tringali et al.46 |
| 15, 16 | Sida acuta (Burkina Faso, Ivory Coast) | Malvaceae | Treatment of malaria, diarrhea and many other diseases | Antiplasmodial and anticancer activities | Banzouzi et al.,32 Karou et al.33g |
| 16–26 | Cryptolepis sanguinolenta (Ghana, Cape Verde, Guinea Bissau, etc.) | Periplocaceae | Treatment of various fevers, including malaria and hepatitis | Antiplasmodial activity | Barku et al., Cimanga et al., Ablordeppey et al., Paulo et al., Hadden et al., Karou et al.33 |
| 64 | Pausinystalia johimbe | Rubiaceae | Treatment of erectile dysfunction | Blockade of presynaptic α-adrenoceptors in rabbits | Vasisht and Kumar,82 Morales et al.,83 Susset et al.,84 Starke et al.85a |
| 28–33, 84, 86–91 | Triphyophyllum peltatum (Ivory Coast) | Dioncophyllaceae | Treatment of malaria, leishmaniasis, dysentery and elephantiasis | Activity against P. falciparum and other parasites | Bringmann et al.40–43 |
| 85 | Mixture of Triphyophyllum peltatum, Dioncophyllum tholloni and Habropetalum dawei (Ivory Coast, Sierra Leone) | Dioncophyllaceae | Treatment of malaria | Anti-malarial activity | Bringmann et al.86 |
| 34–39 | Ancistrocladus guineensis (Nigeria) | Ancistrocladaceae | Not reported | Not tested | Bringmann et al.42 |
| 93–95 | Ancistrocladus spp (Ivory Coast) | Ancistrocladaceae | Treatment of malaria, dysentery and elephantiasis | Antiparasitic activity | François et al.41 |
| 40–42 | Fagara zanthoxyloides (Burkina Faso) | Rutaceae | Used in folk medicine for its antisickling properties in Burkina-Faso and other West African countries | Antisickling activity | Ouattara45 |
| 65–67 | Crinum glaucum (Nigeria) | Amaryllidaceae | Used in the treatment of cough, asthma, and convulsions | Acetylcholinesterase inhibition | Okpo and Adeyemi,95 Houghton et al.97 |
| 68–70 | Crinum jagus (Nigeria) | Amaryllidaceae | Treatment of all forms of convulsions | Acetylcholinesterase inhibition | Houghton et al.,97 Azikiwe et al.96 |
| 71 | Loranthus micranthus (Nigeria) | Loranthaceae | Treatment of several diseases including immune-modulating diseases | Immunostimulatory activity | Omeje et al.99 |
| 72, 73 | Enantia chlorantha (Nigeria) | Annonaceae | Treatment of malaria, jaundice, dysentery, hypertension, skin, gastric and duodenal ulcers, inflammation, and liver-related diseases | Antiplasmodial and antiviral activities | Adebayo et al.,6c Bhadra and Kumar,100 Bidla et al.,101 Jia et al.102 |
| 74–79 | Picralima nitida (Nigeria) | Apocynaceae | Treatment of malaria, diarrhea and used as a painkiller | Antiplasmodial activity, antipsychotic and anxiolytic properties and known potent μ-opioid agonists | Adebayo et al.,6c Ezeamuzie et al.,103 Okokon et al.104 Elisabetsky and Costa-Campos105 |
| 80, 81 | Guiera senegalensis (Nigeria) | Combretaceae | Treatment of malaria, diarrhea, dysentery, venereal diseases and microbial infections | Antiplasmodial and antifungal activities | Iwalewa et al.,106 Ancolio et al.,107 Combier et al.,108 Silva and Gomes109 |
| 82 | Fagara zanthoxyloides (Nigeria) | Rutaceae | Treatment of malaria. The stem and the root of the plant are used as a chewing stick in Nigeria particularly among the Yoruba ethnic group in South-Western Nigeria | Antiplasmodial activity | Odebiyi and Sofowora110 |
| 83 | Cassia siamea (Nigeria) | Leguminosae | Treatment of malaria. In Asia, the stem bark is used as a mild, pleasant, safe purgative; to treat diabetes; a paste is used as a dressing for ringworm and chilblains; the roots are used as an antipyretic; and the leaves are used for the treatment of constipation, hypertension, and insomnia | Antiplasmodial activity, vasodilator effect | Ajaiyeoba et al.,111 Morita et al.,112 Oshimi et al.,113 Matsumoto et al.114 |
| 103 | Rauwolfia vomitoria (Ghana) | Apocynaceae | Treatment of sexual weakness | Inhibition of the in vitro binding of 3H-diazepam to the benzodiazepine sites within the rat GABAA receptor complex | Ai et al.115 |
| Compound | Plant species (Country) | Family | Ethnobotanical use | Measured Activity | References |
|---|---|---|---|---|---|
| 43, 44 | Hoslundia opposita (Ivory Coast) | Lamiaceae | Various parts of the plant are used against snake bites, herpes, conjunctivitis, epilepsy, chest pain, yellow fever, stomach troubles, and mental disorders. Infusions of the leaves are used as a purgative, diuretic, febrifuge, antibiotic and antiseptic | Leishmanicidal potential in the micromolar range, cytotoxicity | Salame et al.,44 Tringali et al.46 |
| 96, 142–146 | Ixora coccinea (Nigeria) | Rubiaceae | Treatment of a variety of infections; hypertension, menstrual irregularities, sprains, chronic ulcers and skin diseases | Antioxidant activity | Idowu et al.120 |
| 105 | Pavetta crassipes (Nigeria) | Rubiaceae | Management of respiratory infections and abdominal disorders | Antimicrobial activity | Bello et al.121 |
| 107–111, 114 | Ximenia americana (Mali) | Olacaceae | Treatment throat infection, malaria, dysmenorrhea, malaria, leprotic, ulcers, skin diseases and for wound healing | Antioxidant activity | Le et al.77 |
| 112 | Bryophyllum pinnatum (Nigeria) | Crassulaceae | Treatment of ulcers, allergic inflammation and epilepsy | Antibacterial activity | Ogungbamila et al.122 |
| 113–115, 141 | Byrsocarpus coccineus (Nigeria) | Connaraceae | Leaf decoction for venereal diseases and as an antidote to arrow poisoning, also used as a remedy for piles, while the decoction of the whole plant is applied to swelling and tumours. Also used to arrest bleeding. The plant has also been reported as a remedy for diarrhea | Not tested | Ahmadu et al.130 |
| 116–124 | Chrozophora senegalensis (Mali) | Euphorbiaceae | Treatment of diarrhea, rheumatism, teniasis, stomach ache, rachitis, and venereal diseases. The leaf and root decoctions are also drunk for hair loss | Antioxidant activity | Vassallo et al.131 |
| 125, 126 | Cajanus cajan (Nigeria) | Fabaceae | The leaves are used as a weak decoction for the treatment of measles, malaria, catarrh, hepatitis and cancer. An aqueous infusion of the seeds is sometimes mixed with the leaves and dispensed for the management of sickle-cell anaemia | Cytotoxicity and antiplasmodial activity | Ashidi et al.,133 Ajaiyeoba et al.138 |
| 127, 128 | Chromolaena odorata (Ivory Coast) | Asteraceae | Aqueous extracts used for the treatment of malaria, abdominal, cervical pain, and of wounds as a local antiseptic and antiinflammatory agent | Cytotoxicity and anticancer properties | Kouamé et al.140 |
| 129–132 | Hoslundia opposita (Ivory Coast) | Lamiaceae | The leaves are infused to treat a wide range of ailments, from wounds, fractures, skin and eye infections, to psychiatric and convulsive illnesses, jaundice, and snake bites | Antiparasitic and cytotoxicity activity | Salame et al.44 |
| 133 | Spathodea campanulata (Nigeria) | Bignoniaceae | Treatment of diseases (ulcers, dysentery, oedemas, skin eruptions, scabies, wound healing and urethral discharge) and veterinary applications have been attributed to the plant in different cultures | Antioxidant activity | Elusiyan et al.141 |
| 134–139 | Securinega virosa (Mali) | Euphorbiaceae | Used in traditional medicine for many diseases, including diarrhea, rheumatism, malaria, liver disease, inflammation and pain Extracts of the plant are used for the expulsion of worms, the treatment of bilharziasis, and for other urinary and genital tract disorders | Antioxidant Activity | Sanogo et al.142 |
| 140 | Alchornea floribunda (Nigeria) | Euphorbiaceae | Leaves are traditionally used as a remedy for arthritis, muscle pain and other inflammatory disorders | Anti-inflammatory activity | Okoye et al.,144 Okoye and Osadebe.145 |
| 147 | Fagara macrophylla (Guinea) | Rutaceae | Used to cure of toothache, rheumatism and urogenital affections, as well as to prepare poisonous arrows | Antifeedant activity | Tringali et al.46 |
| 148, 149 | Baphia nitida (Ivory Coast) | Fabaceae | For gastro-intestinal complaints among other uses | Antioxidant activity | Chaabi et al.146 |
| 150–157 | Piliostigma thonningii (Nigeria) | Caesalpiniaceae | Used to treat a variety of infections, fever and inflammatory conditions | Anti-inflammatory and antibacterial activities | Ibewuike et al.147 |
| 167 | Garcinia kola | Clusiaceae | The roots and stems are used as a chewing stick, while the seeds are also chewed | Antibacterial, α-glucosidase inhibitory, aromatase inhibitory, and antimalarial activities | Antia et al., Lee et al., Xe et al.148 |
4.1 Alkaloids
The bioactive alkaloids identified from West African flora have been isolated from a broad range of plant species and families (Table 2). The measured biological activities are mostly antiparasitic, the dominant activity being anti-malarial.Yohimbine (64) is another unique alkaloid derived from a plant growing in West Africa (Pausinystalia johimbe). This plant is commonly used to treat erectile dysfunction in ATM in West and Central Africa.82 Both yohimbine83 and its hydrochloride84 have proven to be potent in the treatment of erectile dysfunction by preferential blockade of presynaptic α-adrenoceptors in rabbits.85a Yohimbine (64) has received great attention from chemists and the total synthesis of this alkaloid has been achieved.85b–d
Cassytha filiformis (Lauraceae) is a widely distributed antiparasitic plant containing several aporphine alkaloids. This plant has been used in African folk medicine to treat cancer, African trypanosomiasis and other diseases.17 Six (6) aporphines have been isolated by Hoet et al. from samples of C. filiformis harvested in Benin. The compounds have been tested for in vitro cytotoxic properties on different cancer and non-cancer cell lines. The major alkaloids actinodaphnine (1), cassythine (2), and dicentrine (3) were also shown to possess in vitro antitrypanosomal properties on Trypanosoma brucei, thus showing that the use of this plant in traditional medicine is coherent with its phytochemical content. The cytotoxicity of compound 7 demonstrates that it is the active ingredient in this plant, justifying the use of the plant in cancer treatment in ATM.
Another set of aporphine alkaloids (8–13) has been isolated from Monodora sp. from the Ivory Coast. These include M. crispata and M. brevipes.28 The plant species have not been reported to be used in ATM and the isolated compounds have not been tested to date, although they may serve in the chemotaxonomic classification of the plant species of the Monodora genus. In contrast to the other genera of the Monodoreae tribe (Isolona, Hexalobus, Monocyclanthus and Uvariopsis), Monodora sp. shows a strong tendency towards the production of quaternary ammonium derivatives, which could be proposed as a distinctive generic trait.28 Fagara macrophylla, harvested from the Republic of Guinea, is known to be used to cure toothache, rheumatism and urogenital affections as well as to prepare poisonous arrows, among other uses. The poisonous substances in the plant may explain why insects do not feed on it. As an example, Tringali et al. have isolated the aporphine magnoflorine (14), along with the acridones 1-hydroxy-3-methoxy-N-methyl-acridone (97), arborinine (98) and the aporphine tembetarine (102), which have all demonstrated antifeedant properties against Spodoptera frugiperda.46
The indoloquinoline alkaloid cryptolepine (15) has been isolated from Sida acuta (Malvaceae) from the Ivory Coast and Burkina Faso,32,33 while other derivatives (16–26) have been isolated from Cryptolepis sanguinolenta (Periplocaceae) from Ghana, Cape Verde, Guinea Bissau, and other countries in the West African region.33 Banzouzi et al. carried out an anti-malarial assay on the extracts of Sida acuta. The IC50 values obtained ranged from 3.9 to 5.4 μg mL−1. Cryptolepine was identified as the active anti-plasmodial constituent of the plant after purification of the active fraction. This compound showed IC50 values against the chloroquine-sensitive strain (at 0.13 and 0.17 μg mL−1 after 24 and 72 hours, respectively) from Nigeria and the Fcm29 chloroquine-resistant strain (at 0.17 and 0.17 μg mL−1 after 24 and 72 hours, respectively) from Cameroon. The cryptolepine derivatives (16–26), which were isolated from the stems, roots and root bark of Cryptolepis sanguinolenta,33 showed antiplasmodial activities as well. Cimanga and his coworkers also observed that cryptolepine and its hydrochloride salt, 11-hydroxycryptolepine (18) and neocryptolepine (23) showed strong in vitro antiplasmodial activities against P. falciparum chloroquine-resistant strains (D-6), while quindoline (16) was less active. The highest activity was obtained with cryptolepine. In vivo tests on infected mice showed that cryptolepine exhibited a significant chemosuppressive effect against Plasmodium yoelii and Plasmodium berghei, while cryptolepine had the same effect against P. yoelii only. Compounds 16 and 18 did not show activity in the in vivo test system.33c Another study by Paulo et al. on the roots of Cryptolepis sanguinolenta harvested from Guinea-Bissau led to the isolation of cryptolepinoic acid (24) and methyl cryptolepinoate (25) in addition to compounds 15, 16 and 17 from the ethanol and chloroform extracts of the leaves.33e The isolated compounds and extracts were tested in vitro against P. falciparum K1 (a multidrug-resistant strain) and T996 (a chloroquine-sensitive clone). All extracts had 90% inhibition of P. falciparum K1 growth at concentrations of <23 μg mL−1. Cryptolepine was the most active alkaloid tested with IC50 values (0.23 μM to K1; 0.059 μM to T996), compared to chloroquine (0.26 μM to K1; 0.019 μM to T996). The indolobenzazepine alkaloid cryptoheptine (19) was the second most active with IC50 values of 0.8 μM (K1) and 1.2 μM (T996). Cryptolepinoic acid (24) showed no significant activity while its ethyl ester derivative (26) was active against P. falciparum K1 (IC50 = 3.7 μM). All the indoloquinoline alkaloids showed cross-resistance with chloroquine but not the indolobenzazepine cryptoheptine (19). It was noticed that alkaloids with weakly basic characteristics were active whereas the other structurally related alkaloids with different acid–base profiles were inactive. These observations are in agreement with the anti-malarial mechanism of action for quinolines. According to Hadden et al., the unusual incorporation of the isopropyl group at the C11-position of the indolo[3,2-b]quinoline nucleus in 11-isopropylcryptolepine is suggestive of a mixed biosynthetic origin for the alkaloid.33f
The Dioncophyllaceae and Ancistrocladaceae (the only genus is Ancistrocladus) families are closely related and represent rich sources of naphthylisoquinoline alkaloids. In West Africa, Ancistrocladaceae are present in Nigeria (A. uncinatus and A. guineensis), Ghana (A. abbreviatus) and the Republic of Guinea (A. barteri), while the carnivorous Triphyophyllum peltatum (Dioncophyllaceae) is a native of the Ivory Coast. From T. peltatum, several naphthyl isoquinolines (28–33, 84, 86–91) have been isolated. These compounds have demonstrated activities against P. falciparum and other parasites, supporting the use of the plant in the treatment of malaria, leishmaniasis, dysentery and elephantiasis, among other uses.40–43 Jozipeltine A (85) was later isolated from a mixture of T. peltatum, Dioncophyllum tholloni and Habropetalum dawei, harvested from the Ivory Coast and Sierra Leone. The anti-malarial property of this compound supports the use of these plants, in combination, for the treatment of malaria. Although this compound showed some in vitro anti-plasmodial activity against P. falciparum (K1 = 875 ng mL−1, NF54 = 2530 ng mL−1), it is significantly less active than its monomeric precursor, dioncopeltine A (33) (K1 = 4.8 ng mL−1, NF54 = 3.3 ng mL−1). This observation could lead to the conclusion that only naphthyl isoquinolines containing one phenolic OH group each, such as dioncophylline A (29) and ancistrocladine (95), can easily undergo the required dimerization reaction, implying that doubling of the number of free OH groups would increase the antiplasmodial activity.86 Additionally, the C5,8′-coupled naphthyl isoquinolines, ancistroguineines A (36) and B (37), were isolated from the Nigerian species (A. guineensis), along with the 7,3′-coupled ancitrotectorine (38),44 which is dominantly present in the South-East Asian species (A. tectorius).87 Korupensamines A (34) and B (35) are known to contain the anti-malarial “halves” of the anti-HIV michellamines, derived from the Cameroonian species (A. korupensis).88 Both species (A. guineensis and A. korupensis) grow in Cameroon,89 the former being more dominant in Nigeria, even though its traditional use is not reported and the biological activities of the isolated ancistriguineines have not been assessed. The Ghanaian species (A. abbreviatus) has been used traditionally as treatment against measles and fever, the active ingredient being ancistrobrevine D (94).90 A full discussion of the naphthyl isoquinolines has been presented in a separate review.91
The root bark of Fagara zanthoxyloides or Zanthoxylum zanthoxyloides (Rutaceae) is widely used in folk medicine for its antisickling properties in Burkina Faso and other West African countries.45,47,92 Ouattara et al. have isolated three (3) isomeric divanilloylquinic acids, 40 to 42 (3,4-O-divanilloylquinic acid or burkinabin A; 3,5-O-divanilloylquinic acid or burkinabin B and 4,5-O-divanilloylquinic acid or burkinabin C respectively), with antisickling properties.45 The investigations of Ouattara et al. have also demonstrated that burkinabin C, the most abundant burkinabin in the plant, has the same range of activity as the reference drug, cromoglycate. These results could further validate the hypothesis of Elujoba and Sofowora,93 who stipulated that the antisickling compounds in Fagara sp. require a single benzene ring, a carboxylic acid and an electron rich group in the benzoic acid series. However, Ouattara et al. could further show that compounds with two aromatic rings are also active. Even though other phenolics contained in the plant could also participate in the antisickling activity,94 such compounds were only present in minute quantities in the plant material investigated.45 The report of Ouattara et al. was the first report of the antisickling properties of these divanilloylquinic acid derivatives. The conclusions drawn could further support the traditional use of F. zanthoxyloides and would encourage the development of “improved traditional medicines” containing this plant in the management of sickle cell disease.
Alkaloids (65–70) derived from Crinum sp. (C. glaucum and C. jagus) of the Amaryllidaceae family have demonstrated acetylcholinesterase inhibition.95–97 This may be a justifiable reason why the plants are being used for the treatment of convulsions among other ailments. Specifically, C. glaucum is used in the treatment of cough, asthma, and convulsions in Nigeria,95,96 while C. jagus is used either alone or in a combination with Chromoleana odorata and Emilia prateramisa (both belonging to the Asteraceae family) in the treatment of all forms of convulsion.97 The most active alkaloid isolated is hamayne (69, IC50 = 250 μM) and lycorine (67, IC50 = 450 μM), while the other alkaloids were comparatively inactive, with haemanthamane (68) inducing 3% inhibition and crinamine (70) inducing 4.4% inhibition at 50 mg mL−1 (174 μM). These results contrast with the positive control physostigmine, which gave an IC50 of 0.25 μM. Thus, the cholinesterase activity appears to be associated with the presence of two free hydroxy groups in this structural type of the Amaryllidaceae alkaloids. Crinamine has also been isolated from the aerial parts of the Asian subspecies C. asiaticum var. japonicum, together with lycorine, norgalanthamine and epinorgalanthamine.98 The compound showed potent dose-dependent inhibition (IC50 = 2.7 μM) of hypoxia-inducible factor (HIF-1α) in a cell-based reporter gene assay.98 The other components of the Asian subspecies (from Korea) showed no significant inhibition of HIF-1α induced transcriptional activity.
As part of the investigations into the medicinal value of plants from the Loranthaceae family in Nigeria, the results of Omeje et al. showed that the immunostimulatory activities of lupinine (71) and the sesquiterpene 2,3-dimethoxy-benzo[a,b]cyclopentenyl-3′,3′,5′-trimethylpyran-4-carboxylic acid (158) from Loranthus micranthus could justify the use of the plant leaves in the treatment of several diseases including immune-modifying diseases.99
Enantia chlorantha is an ornamental tree in the Annonaceae family, whose stem bark is used against fever/malaria by traditional medicine practitioners in the forest regions,6c in addition to its use in the treatment of jaundice, dysentery, hypertension, inflammation, and liver-related diseases.100 The isolated compounds palmatine (72) and jatrorrhizine (73) are known to exhibit anti-malarial activity,101 while palmatine (72) also has weak in vitro activity against flavivirus.102 From the stem bark and seeds of Picralima nitida (Apocynaceae), a plant used in the treatment of malaria and in the management of pains and other ailments,103,104 seven (7) compounds with anti-malarial properties, including akuammicine (74), akuammine (75), alstonine (76), picratidine (77) picranitidine (78) and ψ-akuammigine (79) have been isolated. The extract showed potent and dose-dependent anti-inflammatory, anti-pyretic and anti-malarial activities. Given intraperitoneally, this extract inhibited carrageenan-induced rat paw oedema with IC50 of 102 mg kg−1, and with the highest dose tested (300 mg kg−1), it produced 72.2% inhibition. In rabbits with LPS-induced pyrexia, 50 mg kg−1 of the extract produced a mean percentage antipyrexia of 38.7% compared with 29.0% by 200 mg kg−1 of aspirin. In a 4 day in vivo schizontocidal test in mice infected with P. berghei, up to 300 mg kg−1 daily for 4 days was ineffective in preventing the development of parasitaemia or the consequent mortality. However, marked inhibitory activity was obtained on multi-drug resistant human P. falciparium parasites cultured in vitro. The dose causing 50% inhibition of parasite growth was 1.75 μg mL−1, compared with 0.14 μg mL−1 for chloroquine. The results justify the use of this plant by natives of West Africa in the treatment of malaria. Akuammidine (78′) and ψ-akuammigine (79) are known to be potent μ-opioid agonists, although not particularly selective.105 An enterprising Ghanaian hospital has started manufacturing standardised 250 mg capsules of powdered P. nitida seed, and they are being commercialised around the country, where they have become widely accepted as a safe and effective pain relief product.
Guiera senegalensis (Combretaceae) is often used in Nigeria for the treatment of malaria. The leaf extract of the plant harvested in Nigeria showed positive anti-malarial activity in vitro in Plasmodium yoelii nigeriensis.106 The alkaloids harman (80) and tetrahydroharman (81) and the methoxylated naphthalene derivative guieranone A (159) were shown to be the active principles from this species harvested in Mali and São Tomé107,108 in addition to the antifungal activity of compound 159.109 The presence and anti-plasmodial property of the alkaloid fagaronine (82), with an IC50 of 0.018 μM against the 3D7 strain of P. falciparum, in Fagara zanthoxyloides (Rutaceae), could explain why the roots of this plant are used in preparations against malaria, among other applications in ATM.110
Ajaiyeoba et al. also reported the use of the leaves and stem bark of Cassia siamea in the treatment of malaria.111 Investigation of the leaves of this plant led to the isolation of the active ingredient cassiarin A (83), along with emodin (168) and lupeol (161). The IC50 values of the isolated compounds were 5.0 μg mL−1 against the K1 strain for both emodin and lupeol, while an IC50 value of 0.02 μM was recorded for cassiarin A.112,113 In Asian traditional folk medicine, the stem bark of Cassia siamea is used as a mild, pleasant, safe purgative; a decoction of the bark is given to treat diabetes; its paste is used as a dressing for ringworm and chilblains; the roots are used as an antipyretic; and the leaves are used for the treatment of constipation, hypertension, and insomnia.112 The vasodilator effect of cassiarin A (83) can explain the use of this plant in the treatment of hypertension, amongst other ailments.113,114 Mayumbine (103), an isomer of ajmalicine (104), is a naturally occurring heteroyohimbine, which was isolated from Rauwolfia vomitoria extracts, a plant used in Ghana to treat sexual weakness. This compound was shown to have potency (IC50 = 76 ± 3.5 nM) against the in vitro binding of 3H-diazepam to the benzodiazepine sites within the rat gamma-amino-butyric acid (GABAA) receptor complex.115 From the study of Ai et al.,115 it is obvious that the substitutions on the E-ring of heteroyohimbine structure determines the binding activity towards the GABAA/benzodiazepine (BZD) receptor, since structurally related compounds such as yohimbine (64) and reserpine are inactive at the BZD receptor.116 The potency of mayumbine (103) to displace 3H-Diaz binding in the cortex, cerebellum and hippocampus suggests that mayumbine does not distinguish between different BZD receptor subtypes expressed in these brain areas. This could offer some explanation why the plant is used in the treatment of sexual weakness in ATM.115
4.2 Flavonoids
The summary of the most important findings on the bioactive flavonoids from West African flora is given in Table 3. Hoslundia opposita (Lamiacea) is a widely distributed shrub in West Africa.46,117 This plant is also found in Southern Africa118 and Central Africa.119 Various parts of the plant are popular remedies for inter alia, snake bites, herpes, conjunctivitis, epilepsy, chest pain, yellow fever, stomach troubles, and mental disorders. Infusions of its leaves have found wide use in traditional medicine as a purgative, diuretic, febrifuge, antibiotic and antiseptic. From the leaves of the plants harvested from West Africa, pyrone and unusual furanone substituted flavones (43 and 44) have been isolated by Salame et al., along with the known methylpyranoflavonic analogues hosloppin (129), hoslundin (130), 5-O-methylhoslundin (131) and oppositin (132).46 The Southern African species gave three known compounds—5,7-dimethoxy-6-methylflavone, hoslunddiol and euscaphic acid118 while the Central African species originally gave hoslundin (130), hoslundal and hoslunddiol.119 It was observed that 5,7-dimethoxy-6-methylflavone inhibits the HIV-1 reverse transcriptase enzyme by 52% at 100 μg mL−1 while euscaphic acid was found to exhibit a minimum inhibitory concentration (MIC) of 50 μg mL−1 against a drug-sensitive H37Rv reference strain (27294) of Mycobacterium tuberculosis. Of the compounds isolated from the plant harvested from West Africa, oppositin and 5-O-methylhoslunfuranine (44) exhibited leishmanicidal potential in the micromolar range.48 Both compounds also demonstrated significant activity against Trypanosoma brucei brucei.48Ixora coccinea (Rubiaceae) is used to treat a variety of infections, including hypertension, menstrual irregularities, sprains, chronic ulcers and skin diseases.120 Idowu et al. identified a doubly linked, A-type proanthocyanidin trimer (ixoratannin A2, 96), along with other constituents from the leaves of the plant.120 The antioxidant and antibacterial properties of the identified compounds (96, 105, 142–146) were also investigated. All tested compounds inhibited the growth of B. subtilis, while only epicatechin (142) and quercetin-3-O-α-L-rhamnopyranoside (105) inhibited the growth of E. coli. Antioxidant evaluation of the isolated compounds revealed that ixoratannin A-2 (96) and cinnamtannin B-1 (160) were the most active compounds in DPPH, inhibition of lipid peroxidation and nitric oxide radical scavenging assays. This could explain why the plant is effective in the treatment of chronic ulcers. Pavetta crassipes (Rubiaceae) has been used in handling respiratory infections and abdominal disorders.121 A bioactive flavonoid (quercetin-3-O-rutinoside, 105) has been isolated from the aqueous extract of P. crassipes leaves, which showed activity against some pathogenic microorganisms, including Streptococcus pyogenes, Corynebacterium ulcerans, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Escherichia coli at a concentration <50 μg mL−1.121 Compound 105 had MIC values ranging from 6.25 to 12.5 μg mL−1 and minimum bactericidal concentrations (MBC) ranging from 12.5 to 25 μg mL−1. This supports the ethnomedicinal use of the plant in the treatment of respiratory infections and abdominal disorders.121
Ximenia americana is a medicinal, bushy, spiny shrub or a small tree used in Mali and other West African countries for the treatment of various diseases, the most common being infectious and inflammatory ailments.77 Fractionation of the ethanol extract led to the isolation and identification of the cyanogenic glycoside sambunigrin (58), along with gallic acid (57) and the gallotannins—β-glucogalline and 1,6-digalloyl-β-glucopyranose. The flavonoids quercetin (114), quercitrin or quercetin-3-O-α-rhamnopyranoside (107), avicularin or quercetin-3-O-α-arabinofuranoside (108), quercetin-3-O-β-xylopyranoside (109), quercetin-3-O-(6′′-galloyl)-β-glucopyranoside (110) and kaempferol-3-O-(6′′-galloyl)-β-glucopyranoside (111) were also isolated. The flavonoids were active as both enzyme inhibitors and DPPH radical scavengers. Sambunigrin (58) was the main compound in the EtOAc soluble fraction of the alcoholic extract of X. americana leaves and the identified compounds may give a rationale for the traditional use of X. americana in Mali, since the traditional healers interviewed reported its use for throat infections, amenorrhea, and as a tonic for wound healing and pain relief.77
Bryophyllum pinnatum (Crassulaceae) has diverse uses in ATM. The flavonoid luteolin (141), epigallocatechin 3-O-syringate (112) and gallic acid (57) have been identified as the active principles responsible for the antibacterial activity of this plant, which explains why it is used in many West African traditional medicinal recipes for the treatment of ulcers.122 The main antibacterial constituent was found to be free gallic acid (57), which accounted for about 0.014% w/w of the fresh aerial part. However, luteolin (141) and a new acylated flavan-3-ol, epigallocatechin-3-O-syringate, were isolated as the minor constituents in the active fraction. Luteolin exhibits a wide range of biological activities, including antioxidant activity, promotion of carbohydrate metabolism, and immune system modulation. Other in vitro studies suggest that luteolin has anti-inflammatory activity,123,124 and acts as a monoamine transporter activator,125 phosphodiesterase inhibitor126 and interleukin 6 inhibitor.123 In vivo studies show that luteolin affects xylazine/ketamine-induced anesthesia in mice.126 In vitro and in vivo experiments also suggest that the compound may inhibit the development of skin cancer.127
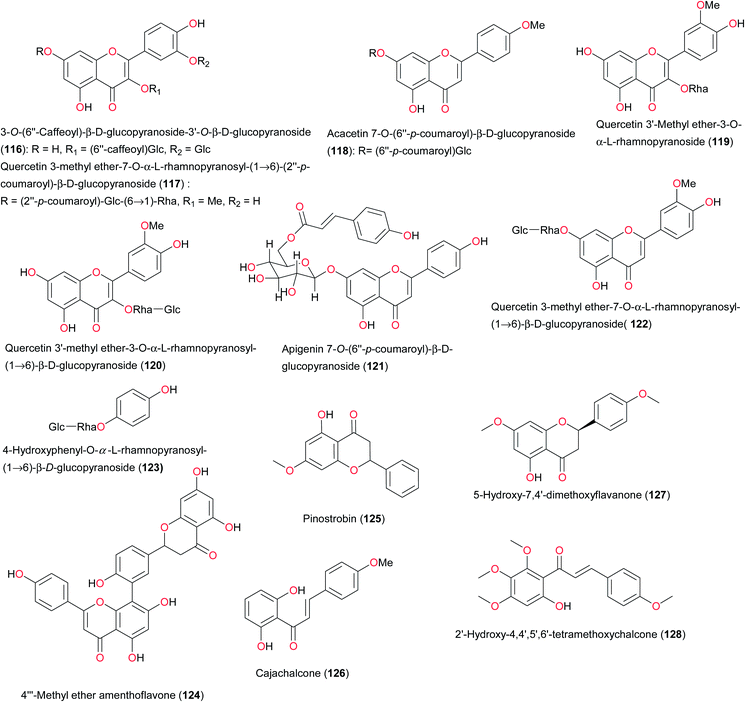
The plant Byrsocarpus coccineus (Connaraceae) is indigenous to Africa, especially Togo, Ghana and Nigeria.128 This plant has diverse uses in ATM, including the treatment of venereal diseases, as an antidote to arrow poisoning and as a remedy for diarrhoea.129 Ahmadu et al. have investigated the bioactive ethyl acetate and n-butanol soluble parts of an ethanolic extract of the leaves of this plant and led to the isolation of three flavonoid glycosides identified as quercetin 3-O-α-arabinoside (113), quercetin (114) and quercetin 3-β-D-glucoside (115).130 It may be interesting to test these compounds against a wide range of bacteria responsible for the aforementioned ailments. Vassallo et al. also investigated the antioxidant flavonoid glycosides isolated from Chrozophora senegalensis, also known as Croton senegalensis (Euphorbiaceae), harvested in Mali.131 It is a small tree widely distributed in Mali where it grows wild and is used in folk medicine for the treatment of diarrhea, rheumatism, teniasis, stomach ache, rachitis, and venereal diseases. The leaf and root decoctions are also consumed to treat hair loss.132 In order to justify the ethnobotanical use of C. senegalensis, the leaf extracts were assayed for in vitro antioxidant activity. Bioassay-guided fractionation revealed the methanol extract to be active. Separation of this extract led to the isolation of three new flavonoids (116–118), along with known flavonoids (119–122 and 124), a phenolic derivative and three megastigmane glycosides. All isolated compounds were tested for their antioxidant activity on DPPH stable radical, superoxide anion, metal chelating activity, and DNA cleavage induced by the photolysis of H2O2. Compound 116, quercetin 3′-methyl ether-3-O-α-L-rhamnopyranoside (119), and 4′′′-methyl ether amenthoflavone (124) exhibited the highest antioxidant capacity also being able to modulate hydroxyl radical formation more efficiently than other compounds acting as direct hydroxyl radical scavengers and iron chelators.131
Cajanus cajan or pigeon pea is a perennial member of the family Fabaceae. The leaves are used as a weak decoction for the treatment of measles, malaria, catarrh, hepatitis and cancer.133 An aqueous infusion of the seeds sometimes mixed with the leaves is dispensed for the management of sickle-cell anaemia.134 The seed extract has been shown to possess hypoglycaemic and antimicrobial activities,135 as well as demonstrate activity against the chloroquine-sensitive P. falciparum strain (3D7).136 Shode et al. have demonstrated that phenylalanine is the predominant antisickling agent in the seed extract of C. cajan.137 In the course of examining the rationale behind the use of this plant in the treatment of cancer, Ashidi et al. isolated six compounds from the dichloromethane fraction; hexadecanoic acid methyl ester, α-amyrin, β-sitosterol, the flavonoid pinostrobin (125), as well as the stilbenoids longistylin A (162) and longistylin C (163).133 Pinostrobin and longistylins A and C were tested for cytotoxicity on cancer cell lines. In addition, an adriamycin-sensitive acute T-lymphoblastic leukaemia cell line (CCRF-CEM) and its multidrug-resistant sub-line (CEM/ADR5000) were used in an XTT assay to evaluate the activity of the pure compounds obtained. It was observed that the dichloromethane fraction of C. cajan had IC50 value 5–10 μg mL−1, with the two constituent stilbenes, longistylins A and C, being primarily responsible, with IC50 values of 0.7–14.7 μM against the range of cancer cell lines. Ajaiyeoba et al. recently examined the antiplasmodial components of the plant and their study led to the isolation of cajachalcone or 2′,6′-dihydroxy-4-methoxy chalcone (126), as the biologically active constituent from the ethyl acetate fraction. Cajachalcone had an IC50 value of 2.0 μg mL−1 (7.4 μM) and could be a lead for anti-malarial drug discovery.138
The leaves of Chromolaena odorata (Asteraceae) are exploited extensively in West and Central African ethnopharmacy for the treatment of a wide range of conditions, including the treatment of malaria, abdominal, cervical pain, and wounds as a local antiseptic and antiinflammatory agent.139 Kouamé et al. isolated 5-hydroxy-7,4′-dimethoxyflavanone (127) and 2′-hydroxy-4,4′,5′,6′-tetramethoxychalcone (128), along with 1,6-dimethyl-4-(1-methylethyl)naphthalene (cadalene) from the hexane-soluble fraction of the leaf extract of the plant and tested their impact on the viability and clonogenicity of cancer cell lines.140 All three compounds were tested for their cytotoxicity and anticancer properties. Compound 128 was found to be both cytotoxic and anticlonogenic at 20 μM in Cal51, MCF7 and MDAMB-468 cell lines, and acts synergistically with the Bcl2 inhibitor ABT737 to enhance apoptosis in Cal51 breast cancer cells.140
The flowers, fruits, leaves and stem bark of Spathodea campanulata (Bignoniaceae), popularly known as the African tulip tree, are used in the treatment of several diseases (ulcers, dysentery, oedemas, skin eruptions, scabies, wound healing and urethral discharge), in addition to veterinary applications.6c Kaempferol-3-O-β-D-(2-O-β-D-glucopyranosyl) glucopyrano-side (133) has been isolated from this plant, along with ursolic acid (164), verminoside (159), specioside (165) and caffeic acid (166).141 The antioxidant activities of these compounds, isolated from the flowers, fruits, leaf and stem bark of the same plant have been investigated by Elusiyan et al.141 The results show that the antioxidant principles isolated from the various parts of the plant are verminoside from the leaves, stem bark and flowers (EC50 = 2.04 μg mL−1), specioside from the flowers (EC50 = 17.44 μg mL−1), kaempferol diglucoside (133) from the leaves (EC50 = 8.87 μg mL−1) and caffeic acid (166) from the leaves and fruits.141 Flavonoid glycosides exhibiting antioxidant activities have also been isolated from Securinega virosa (Euphorbiaceae) harvested in Mali.142 This plant has been used traditionally in the treatment of many diseases, including diarrhea, rheumatism, malaria, liver disease, inflammation and pain. Extracts of the plant are used for the expulsion of worms, in the treatment of bilharziasis, and for other urinary and genital tract disorders.143 Kaempferol 3-O-(4-galloyl)-β-D-glucopyranoside (134), quercetin-3-O-β-D-glucopyranoside (135), corilagin (136), 11-O-caffeoylbergenin (137), glucogallin (138), and geraniin (139) were isolated. In vitro biological analysis of the isolated compounds showed that they were able to quench DPPH radicals and had a direct scavenging activity on the superoxide anion. Kaempferol-3-O-(4-galloyl)-β-D-glucopyranoside, 11-O-caffeoylbergenin, and glucogallin exhibited the highest antioxidant capacity, being also able to modulate hydroxyl radical formation more efficiently than the other compounds, acting as direct hydroxyl radical scavengers and iron chelators.142 The flavonoid glycoside AFF1 or 3,5,7,3′-tetrahydroxyflavone-3-O-α-L-rhamnoside (140) was isolated from Alchornea floribunda (Euphorbiaceae) from Nigeria. The leaves of this plant are traditionally used as a remedy for arthritis, muscle pain and other inflammatory disorders. Okoye et al. demonstrated that the anti-inflammatory activity of this compound could justify the aforementioned uses of this plant in ATM.144,145 The anti-inflammatory activity (50 mg kg−1) of this compound was higher than that of the standard anti-inflammatory drug, aspirin (100 mg kg−1). The compound also significantly (p < 0.001) inhibited heat-induced haemolysis of human erythrocytes in vitro.109 These results demonstrated that the anti-inflammatory activity of A. floribunda leaves may be, in part, a result of the flavonol glycoside, compound 140. The antifeedant activity of hesperidin (147), isolated from Fagara macrophylla from Guinea, gives further arguments towards the justification of the use of the plant in arrow poisoning among other uses.46
Chaabi et al. have also isolated some acylated flavonol pentaglycosides from the leaves of Baphia nitida (Fabaceae), a plant whose leaves are used traditionally in many African countries, particularly for gastro-intestinal complaints.146 Two new acylated flavonol pentaglycosides were isolated from the butanolic extract of B. nitida leaves and identified to be kaempferol 3-O-β-D-xylopyranosyl(1→3)-(4-O-E-p-coumaroyl-α-L-rhamnopyranosyl(1→2))[β-D-glucopyranosyl(1→6)]-β-D-galactopyranoside-7-O-α-L-rhamnopyranoside (148) and kaempferol-3-O-β-D-xylopyranosyl(1→3)-(4-O-Z-p-coumaroyl-α-L-rhamnopyranosyl(1→2))[β-D-glucopyranosyl(1→6)]-β-D-galactopyranoside-7-O-α-L-rhamnopyranoside (149). The antioxidant activity of the two compounds was assessed in the peroxynitrite assay. Compounds 148 and 149 displayed mild antioxidant activities in the in vitro peroxynitrite assay with EC50 values of 62 ± 9.3 μM and 19 ± 2.9 μM, respectively. These values were higher than those of the reference compound, gallic acid (4.9 ± 0.4 μM). The isomeric difference of activity might be explained by the higher reactivity of cis, compared to trans bonds.146
Piliostigma thonningii (Caesalpiniaceae) is a tropical African plant used to treat a variety of infections, fever and inflammatory conditions.89 Ibewuike et al. investigated the anti-inflammatory and antibacterial activities of C-methylflavonols (150–157) from the leaves of the plant and tested the isolated compounds for their ability to inhibit prostaglandin synthesis in vitro and antibacterial activity against Staphylococcus aureus.147 From their results, it was observed that the influence of the B ring 3′,4′ diol group on the activity of C-methylflavonols in the inhibition of prostaglandin synthesis differ from that observed for a series of flavonoids without C-methyl groups. The antibacterial activity in the series mirrors those of methylated antimicrobial flavonoids. The traditional uses of the plant in the treatment of infections and inflammatory conditions were rationalized on the basis of the activities of the isolated compounds.147
Garcinia kola (Clusiaceae) is an African medicinal plant known for its use as chewing sticks in maintaining oral health.148 The use of the trunk and roots as chewing sticks is reported to lower the rate of dental caries and provide better general oral health to its users than non-users. It is believed that the positive effects of chewing sticks could be partly attributed to the antimicrobial substances present in the sticks. The biflavonoid 3′′,4′,4′′′,5,5′′,7,7′′′-heptahydroxy-3,8′′-biflavanone, otherwise known as GB1 (167) has been isolated from this plant, as the major constituent of the antibacterial fraction of the stem bark.148a This biflavonoid has shown antibacterial activities against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) with MIC of 32 and 128 μg mL−1, respectively,148b as well as activity against Streptococcus mutans and other oral bacteria with minimum inhibitory concentration (MIC) values from 32 to 64 μg mL−1.148c GB1 also exhibited α-glucosidase and aromatase inhibitory activities, as well as antiplasmodial activity, but was not toxic against cell lines tested.148a It could therefore be inferred that GB1 may be a potential dietary supplement or phytomedicine for the prevention of breast cancer and type 2 diabetes mellitus.
5 Conclusions
This review focused on NPs derived from medicinal plants from West Africa. The entire study showed that about 700 NPs from 97 plant species grouped into 41 families exhibiting diverse biological activities have been identified. The claim that medicinal plants from West Africa hold a huge potential for drug discovery cannot be disputed. How this potential could be released remains the driving theme of current synergistic efforts for drug discovery by Africans for Africans, both on the continent and in the Diaspora. The exploitation of data from medicinal plants for drugs will require a synergy between academia and industry, since this is currently almost inexistent in this part of the World. Our observations are that almost all of the research is inadequately funded. Existing funding sources include university grants to research staff, funding schemes targeting laboratories in the South like the Third World Academy of Sciences (TWAS), the International Foundation for Science (IFS), and the International Society for Infectious Diseases (ISID), just to mention some. Such funding programs are usually geared towards providing basic supplies like the procurement of plant samples, solvents, reagents, basic tools and national travel costs. The acquisition and/or repair of heavy instrumentation are not often considered. Some of the research is also funded via travel grants for intermittent visits of senior staff and graduate students to research laboratories in the North, mainly for sample analysis, since modern analytical instrumentation like IR, Mass, NMR, etc., spectrometers are grossly absent in African laboratories.Much of the research efforts, whose results have been discussed in this review paper, were also funded via collaborative programs tailored such that the African researchers play the role of plant sample collectors. This is because the most equipped laboratories in Africa barely host enough instrumentation to be able to perform extractions and purifications. A bulk of the published work from West Africa has followed the last mentioned scheme. The authors of this review do not envisage this approach as research towards the development of African researchers and African institutions, since the analysis and almost all the screening results are determined in the laboratories of developed countries (Europe and North America) and some countries with transition economies like Brazil, India and China via the TWAS funding scheme. An African representative often travels abroad with samples from the entire research team for analysis and/or screening (samples are often barely enough for both purposes) and returns home with a scientific publication, most often without samples for further analysis/testing. This may partly explain why so much data has been made available, but very little exploitation and implementation has followed. A detailed analysis of the African scene in terms of biomedical and NP research is beyond the scope of this review. However, the empowerment of African researchers and research institutions via synergistic networks like the African network for drugs and diagnostics innovation (ANDI)149,150 may be a promising way forward. This entails the strengthening of intra-continental efforts and adopting governmental funding schemes that target research towards the validation and implementation of results aimed at making Africa-driven research products available to the local populations at an affordable cost. One laudable effort of the African continent has been to collect physical samples of NPs at a site which could be directly available for bioassays.151 Such an agenda will greatly enhance drug discovery efforts from the continent.
Acknowledgements
Financial support is acknowledged from Lhasa Ltd, Leeds, UK through the Chemical and Bioactivity Information Centre (CBIC), University of Buea, Cameroon. Dr Kerstin Andrae-Marobela (University of Botswana) is acknowledged for proofreading the draft manuscript.Notes and references
- (a) S. Tagboto and S. Townson, Adv. Parasitol., 2001, 50, 199 CrossRef CAS PubMed; (b) O. Akerele, HerbalGram, 1993, 28, 13 Search PubMed.
- (a) S. Schwikkard and R. F. van Heerden, Nat. Prod. Rep., 2002, 19, 675 RSC; (b) S. M. N. Efange, Natural products: a continuing source of inspiration for the medicinal chemist, in Advances in Phytomedicine, vol. 1, Ethnomedicine and Drug Discovery, ed. M. M. Iwu and J. C. Wootton, Amsterdam, The Netherlands, Elsevier Science, 2002, pp. 61–69 Search PubMed; (c) K. Chibale, M. Davies-Coleman and C. Masimirembwa, Drug discovery in Africa: impacts of genomics, natural products, traditional medicines, insights into medicinal chemistry, and technology platforms in pursuit of new drugs, Springer, 2012 Search PubMed.
- K. Hostettmann, A. Marston, K. Ndjoko and J. L. Wolfender, Curr. Org. Chem., 2000, 4, 973 CrossRef CAS.
- (a) D. A. Mulholland, S. L. Schwikkard and N. R. Crouch, Nat. Prod. Rep., 2013, 30, 1165 RSC; (b) K. P. Devkota, B. N. Lenta, P. A. Fokou and N. Sewald, Nat. Prod. Rep., 2008, 25, 612 RSC; (c) N. J. Toyang and R. Verporte, J. Ethnopharmacol., 2013, 146, 681 CrossRef; (d) U. Wölfe, G. Seelinger and C. M. Schempp, Planta Med., 2014, 80, 109 Search PubMed.
- (a) J. Bero, M. Frédérich and J. Quetin-Leclercq, J. Pharm. Pharmacol., 2009, 61, 1401 CrossRef CAS PubMed; (b) S. Saxena, N. Pant, D. C. Jain and R. S. Bhakuni, Curr. Sci., 2003, 85, 1314 CAS; (c) M. Frédérich, M. Tits and L. Angenot, Trans. R. Soc. Trop. Med. Hyg., 2008, 102, 11 CrossRef PubMed; (d) P. Amoa Onguéné, F. Ntie-Kang, L. L. Lifongo, J. C. Ndom, W. Sippl and L. Mbaze Meva'a, Malar. J., 2013, 12, 449 CrossRef PubMed; (e) M. d. R. Camacho, S. L. Croft and J. D. Phillipson, Curr. Opin. Invest. Drugs, 2000, 2, 47 Search PubMed; (f) A. Fournet and V. Muñoz, Curr. Top. Med. Chem., 2002, 2, 1215 CrossRef CAS PubMed; (g) O. Keyser, A. F. Kiderlen and S. L. Croft, Parasitol. Res., 2003, 90, S55 CrossRef; (h) M. J. Chan-Bacab and L. M. Peña-Rodriguez, Nat. Prod. Rep., 2001, 18, 674 RSC; (i) S. Hoet, F. Opperdoes, R. Brun and J. Quertin-Leclerg, Nat. Prod. Rep., 2004, 21, 353 RSC; (j) C. L. Cantrell, S. G. Franzblau and N. H. Fischer, Planta Med., 2001, 67, 685 CrossRef CAS PubMed; (k) L. Pan, H. Chai and A. D. Kinghorn, Phytochem. Lett., 2010, 3, 1 CrossRef CAS PubMed; (l) B. R. Copp and A. N. Pearce, Nat. Prod. Rep., 2007, 24, 278 RSC; (m) F. Ntie-Kang, P. Amoa Onguéné, L. L. Lifongo, J. C. Ndom, W. Sippl and L. Mbaze Meva'a, Malar. J., 2014, 13, 81 CrossRef PubMed; (n) J. N. Yong and F. Ntie-Kang, Curr. Med. Chem.: Anti-Infect. Agents Search PubMed , in press; (o) A. Mohammed, M. A. Ibrahim and M. S. Islam, Planta Med., 2014, 80(05), 354 CrossRef CAS PubMed.
- (a) V. Kuete and T. Efferth, Front. Pharmacol., 2010, 1, 123 Search PubMed; (b) F. Ntie-Kang, L. L. Lifongo, L. M. Mbaze, N. Ekwelle, L. C. Owono Owono, E. Megnassan, P. N. Judson, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 147 CrossRef PubMed; (c) J. O. Adebayo and A. U. Krettli, J. Ethnopharmacol., 2011, 133, 289 CrossRef CAS PubMed; (d) V. Kuete, Planta Med., 2010, 76, 1479 CrossRef CAS; (e) L. L. Lifongo, C. V. Simoben, F. Ntie-Kang, S. B. Babiaka and P. N. Judson, Nat. Prod. Bioprospect., 2014, 4, 1 CrossRef PubMed.
- (a) J. J. Magdula and P. Erasto, Nat. Prod. Rep., 2009, 26, 1535 RSC; (b) D. Zofou, F. Ntie-Kang, W. Sippl and S. M. N. Efange, Nat. Prod. Rep., 2013, 30, 1098 RSC.
- (a) F. Ntie-Kang, J. A. Mbah, L. M. Mbaze, L. L. Lifongo, M. Scharfe, J. Ngo Hanna, F. Cho-Ngwa, P. A. Onguéné, L. C. O. Owono, E. Megnassan, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 88 CrossRef PubMed; (b) F. Ntie-Kang, P. Amoa Onguéné, M. Scharfe, L. M. Mbaze, L. C. O. Owono, E. Megnassan, W. Sippl and S. M. N. Efange, RSC Adv., 2014, 4, 409 RSC; (c) F. Ntie-Kang, D. Zofou, S. B. Babiaka, R. Meudom, M. Scharfe, L. L. Lifongo, J. A. Mbah, L. M. Mbaze, W. Sippl and S. M. N. Efange, PLoS One, 2013, 8(10), e78085 CAS.
- (a) F. Ntie-Kang, J. A. Mbah, L. L. Lifongo, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, Org. Med. Chem. Lett., 2013, 3, 1 CrossRef PubMed; (b) F. Ntie-Kang, L. L. Lifongo, J. A. Mbah, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, In Silico Pharmacol., 2013, 1, 12 CrossRef PubMed.
- P. S. Njomnang and F. Benoit-Vical, J. Ethnopharmacol., 2007, 114, 130 CrossRef PubMed.
- A. Ramazani, S. Zakeri, S. Sardari, N. Khodakarim and N. Djadidt, Malar. J., 2010, 9, 124 CrossRef PubMed.
- O. Kayser, A. F. Kiderlen, H. Laatsch and S. L. Croft, Acta Trop., 2000, 77, 307 CrossRef CAS PubMed.
- M. A. Sonibare and Z. O. Gbile, Afr. J. Tradit. Complement. Altern. Med., 2008, 5(4), 340 CAS.
- A. Adamu, E. M. Abdurahman, H. Ibrahim, M. S. Abubakar, M. G. Magaji and A. H. Yaro, Niger. J. Pharm. Sci., 2007, 6(2), 1 Search PubMed.
- O. Silva, A. Duarte, M. Pimentel, S. Viegas, H. Barroso and J. Machado, J. Ethnopharmacol., 1997, 57, 203 CrossRef CAS PubMed.
- M. S. Abubakar, A. M. Musa, A. Ahmed and I. M. Hussaini, J. Ethnopharmacol., 2007, 111, 625 CrossRef CAS PubMed.
- (a) S. Hoet, C. Stévigny, S. Block, F. Opperdoes, P. Colson, B. Baldeyrou, A. Lansiaux, C. Bailly and J. Quetin-Leclercq, Planta Med., 2004, 70, 407 CrossRef CAS PubMed; (b) J. Quetin-Leclercq, S. Hoet, S. Block, M. C. Wautier and C. Stévigny, Studies on Cassytha filiformis from Benin: isolation, biological activities and quantification of aporphines. In African folk medicine, used to treat cancer and African trypanosomiasis, Proceedings of Bioresources Towards Drug Discovery and Development, 2004, pp. 81–104 Search PubMed.
- (a) M. Shamma and W. A. Slusarchyk, Chem. Rev., 1964, 64, 59 CrossRef CAS; (b) I. A. Israilov, S. U. Karimova, M. S. Yunusov and S. Y. Yunusov, Chem. Nat. Compd., 1980, 16, 197 CrossRef.
- (a) G. Marti, V. Eparvier, B. Morleo, J. Le Ven, C. Apel, B. Bodo, S. Amand, V. Dumontet, O. Lozach, L. Meijer, F. Guéritte and M. Litaudon, Molecules, 2013, 18, 3018 CrossRef CAS PubMed; (b) R. Braz-F, S. J. Gabriel, C. M. R. Gomes, O. R. Gottlieb, M. D. G. A. Bichara and J. G. S. Maia, Phytochemistry, 1976, 15, 1187 CrossRef; (c) H. Guinaudeau, M. Leboeuf and A. Cavé, J. Nat. Prod., 1979, 42, 325 CrossRef CAS; (d) H. Guinaudeau, M. Leboeuf and A. Cavé, J. Nat. Prod., 1983, 46, 761 CrossRef CAS; (e) H. Guinaudeau, M. Leboeuf and A. Cavé, J. Nat. Prod., 1994, 57, 1033 CrossRef CAS.
- M. H. Rafamantanana, B. Debrus, G. E. Raoelison, E. Rozet, P. Lebrun, S. Uverg-Ratsimamanga, P. Hubert and J. Quetin-Leclercq, J. Pharm. Biomed. Anal., 2012, 62, 23 CrossRef CAS PubMed.
- G. B. Lapa, O. P. Sheichenko, A. G. Serezhechkin and O. N. Tolkachev, Pharm. Chem. J., 2004, 38, 441 CrossRef CAS.
- (a) X. H. Xu, G. D. Yu and Z. T. Wang, Zhongguo Zhongyao Zazhi, 2004, 29, 399 Search PubMed; (b) K. Morteza-Semnani, G. Amin, M. R. Shidfar, H. Hadizadeh and A. Shafiee, Fitoterapia, 2003, 74, 493 CrossRef CAS PubMed.
- D. J. Milanowski, R. E. Winter, M. P. Elvin-Lewis and W. H. Lewis, J. Nat. Prod., 2002, 65, 814 CrossRef CAS.
- A. Langlois, D. A. Mulholland, N. R. Crouch and O. M. Grace, Biochem. Syst. Ecol., 2004, 32, 1087 CrossRef CAS.
- P. Wafo, B. Nyasse, C. Fontaine and B. L. Sondengam, Fitoterapia, 1999, 70, 157 CrossRef CAS.
- A.-L. Sagen, S. Sahpaz, S. Mavi and K. Hostettmann, Biochem. Syst. Ecol., 2003, 31, 1447 CrossRef CAS.
- C. Stévigny, C. Bailly and J. Quetin-Leclercq, Curr. Med. Chem.: Anti-Cancer Agents, 2005, 5, 173 CrossRef.
- L. Kablan, J. Dade, T. Okpekon, F. Roblot, L. A. Djakouré and P. Champy, Biochem. Syst. Ecol., 2013, 46, 162 CrossRef CAS.
- A. I. Spiff, F. K. Duah, D. J. Slatkin and P. L. Schiff Jr, Planta Med., 1984, 50, 455 CrossRef CAS.
- M. Leboeuf, A. Cavé, P. K. Bhaumik, B. Mukherjee and R. Mukherjee, Phytochemistry, 1982, 21, 2783 CrossRef CAS.
- Y. Nishiyama, M. Moriyasu, M. Ichimura, K. Iwasa, A. Kato, S. G. Mathenge, P. B. Chalo Mutiso and F. D. Juma, Nat. Med., 2000, 2000(54), 338 Search PubMed.
- J. T. Banzouzi, R. Prado, H. Menan, A. Valentin, C. Roumestan, M. Mallié, Y. Pelissier and Y. Blache, Phytomedicine, 2004, 11, 338 CrossRef CAS.
- (a) V. Y. A. Barku, Y. Opoku-Boahen and E. Y. Dzotsi, Int. Res. J. Biochem. Bioinform., 2012, 2, 58 Search PubMed; (b) K. Cimanga, T. De Bruyne, L. Peters, M. Claeys and A. Vlietinck, Tetrahedron Lett., 1996, 37, 1703 CrossRef CAS; (c) K. Cimanga, T. De Bruyne, L. Peters and A. J. Vlietinck, J. Nat. Prod., 1997, 60, 688 CrossRef CAS; (d) S. Y. Ablordeppey, C. D. Hufford, R. F. Bourne and D. Dwuma-Badu, Planta Med., 1990, 56, 416 CrossRef CAS; (e) A. Paulo, E. T. Gomes, J. Steele, D. C. Warhurst and P. J. Houghton, Planta Med., 2000, 66, 30 CrossRef CAS; (f) C. E. Hadden, M. H. M. Sharaf, J. E. Guido, R. H. Robins, A. N. Tackie, C. H. Phoebe Jr, P. L. Schiff Jr and G. E. Martin, J. Nat. Prod., 1999, 62, 238 CrossRef CAS; (g) D. Karou, A. Savadogo, A. Canini, S. Yameogo, C. Montesano, J. Simpore, V. Colizzi and A. S. Traore, Afr. J. Biotechnol., 2005, 4, 1452 CAS.
- (a) C. W. Wright, J. Addae-Kyereme, A. G. Breen, J. E. Brown, M. F. Cox, S. L. Croft, Y. Gökçek, H. Kendrick, R. M. Phillips and P. L. Pollet, J. Med. Chem., 2001, 44, 3187 CrossRef CAS; (b) O. Onyeibor, S. L. Croft, H. I. Dodson, M. Feiz-Haddad, H. Kendrick, N. J. Millington, S. Parapini, R. M. Phillips, S. Seville, S. D. Shnyder, D. Taramelli and C. W. Wright, J. Med. Chem., 2005, 48, 2701 CrossRef CAS.
- J. N. Lisgarten, M. Coll, J. Portugal, C. W. Wright and J. Aymami, Nat. Struct. Biol., 2002, 9, 57 CrossRef.
- H. Shigemori, T. Kagata, H. Ishiyama, F. Morah, A. Ohsaki and J. Kobayashi, Chem. Pharm. Bull., 2003, 51, 58 CrossRef CAS.
- C. Djerassi, H. J. Monteiro, A. Walser and L. J. Durham, J. Am. Chem. Soc., 1966, 88, 1792 CrossRef CAS.
- G. Bringmann, K. Messer, B. Schwöbel, R. Brun and L. Aké Assi, Phytochemistry, 2003, 62, 345 CrossRef CAS.
- G. Bringmann, W. Saeb, R. God, M. Schaèffer, G. François, K. Peters, E.-M. Peters, P. Proksch, K. Hostettmann and L. Aké Assi, Phytochemistry, 1998, 49, 1667 CrossRef CAS.
- G. Bringmann, C. Günther, W. Saeb, J. Mies, R. Brun and L. Aké Assi, Phytochemistry, 2000, 54, 337 CrossRef CAS.
- G. François, G. Bringmann, C. Dochez, C. Schneider, G. Timperman and L. Aké Assi, J. Ethnopharmacol., 1995, 46, 115 CrossRef.
- G. Bringmann, C. Günther, S. Busemann, M. Schaèffer, J. D. Olowokudejo and B. I. Alo, Phytochemistry, 1998, 47, 37 CrossRef CAS.
- Y. F. Hallock, K. P. Manfredi, J. W. Blunt, J. H. Cardellina, M. Schaffer, K.-P. Gulden, G. Bringmann, A. Y. Lee, J. Clardy, G. François and M. R. Boyd, J. Org. Chem., 1994, 59, 6349 CrossRef CAS.
- R. Salame, Z. Cheikh-Ali, C. Bories, M. Adiko, E. Poupon and P. Champy, Planta Med., 2012, 78(16), 1777 CrossRef.
- (a) B. Ouattara, L. Angenot, I. P. Guissou, P. Fondu, J. Dubois, M. Frédérich, O. Jansen, J. C. Van Heugen, J. N. Wauters and M. Tits, Phytochemistry, 2004, 65, 1145 CrossRef CAS; (b) B. Ouattara, O. Jansen, L. Angenot, I. P. Guissou, M. Frédérich, P. Fondu and M. Tits, Phytomedicine, 2009, 16, 125 CrossRef CAS.
- C. Tringali, C. Spatafora, V. Cali and M. S. J. Simmonds, Fitoterapia, 2001, 72, 538 CrossRef CAS.
- O. Omotoye, Taxonomy of West African flowering plants, Longman Group Ltd, USA, 1984, p. 24 Search PubMed.
- V. Seidel, F. Bailleul and P. G. Waterman, Biochem. Syst. Ecol., 1999, 27, 543 CrossRef CAS.
- M. Leboeuf, A. Cave, P. K. Bhaumik, B. Mukherjee and R. Mukherjee, Phytochemistry, 1982, 21, 2783 CrossRef CAS.
- (a) I. Itawa and Y. Sakurai, Agric. Biol. Chem., 1963, 27, 253 CrossRef; (b) N. J. De Souza and W. R. Nes, Phytochemistry, 1969, 8, 819 CrossRef CAS; (c) J. J. Sims and J. A. Pettus, Jr, Phytochemistry, 1976, 15, 1076 CrossRef CAS.
- T. Suga and T. Aoki, Phytochemistry, 1974, 13, 1623 CrossRef CAS.
- T. Aoki, K. Takagi, T. Hirata and T. Suga, Phytochemistry, 1982, 21, 1361 CrossRef CAS.
- G. D. Brown, Phytochemistry, 1994, 36, 1553 CrossRef CAS.
- (a) M. Leboeuf, A. Cavé, M. El Tohami, J. Pusset, P. Forgacs and J. Provost, J. Nat. Prod., 1982, 45, 617 CrossRef CAS; (b) F. Roblot, R. Hocquemiller, A. Cavé and C. Moretti, J. Nat. Prod., 1983, 46, 862 CrossRef CAS; (c) L. Castedo, J. A. Granja, A. Rodriguez de Lera and M. C. Villaverde, Phytochemistry, 1991, 30, 2781 CrossRef CAS; (d) A. Cavé, M. Leboeuf and P. G. Waterman, The aporphinoid alkaloids of the Annonaceae, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, John Wiley and Sons, New York, 1987, vol. 5, p. 134 Search PubMed; (e) L. C. Caetano and H. Dadoun, J. Nat. Prod., 1987, 50, 330 CrossRef CAS; (f) F.-R. Chang, T.-L. Hwang, Y.-L. Yang, C.-E. Li, C.-C. Wu, H. H. Issa, W.-B. Hsieh and Y.-C. Wu, Planta Med., 2006, 72, 1344 CAS.
- J. Ziegler, P. J. Facchini, R. Geibler, J. Schmidt, C. Ammer, R. Kramell, S. Voigtländer, A. Gesell, S. Pienkny and W. Brandt, Phytochemistry, 2009, 70, 1696 CrossRef CAS.
- O. Ekundayo, I. Laasko and R. Hiltunen, Planta Med., 1988, 54, 55 CrossRef CAS.
- A. Paulo and P. J. Houghton, Biochem. Syst. Ecol., 2003, 31, 155 CrossRef CAS.
- (a) E. Roeder, K. Liu and T. Bourauel, Phytochemistry, 1991, 30(9), 3107 CrossRef CAS; (b) M. Romeiras, L. Ascensão, M. Duarte, M. Diniz and M. Pais, Aust. Syst. Bot., 2008, 21, 26 CrossRef.
- J. C. B. Carvalho, H. dos Santos Almeida, J. F. R. Lobo, J. L. P. Ferreira, A. P. Oliveira and L. Rocha, Biochem. Syst. Ecol., 2013, 50, 1 CrossRef CAS.
- P. P. Fu, Q. Xia, G. Lin and M. W. Chou, Drug Metab. Rev., 2004, 36, 1 CrossRef CAS.
- B. Niassy, A. Lobstein, B. H. Um, R. Anton and M. E. K. Koné, Biochem. Syst. Ecol., 2005, 33, 309 CrossRef CAS.
- (a) F. Gómez-Garibay, L. Quijano, G. Garcia, J. S. Calderón and T. Riso, Phytochemistry, 1983, 22, 1305 CrossRef; (b) F. Gómez-Garibay, L. Quijano, J. S. Calderón, J. S. Rodriguez, C. Rodriguez and T. Riso, Phytochemistry, 1985, 24, 1057 CrossRef; (c) F. Gómez-Garibay, L. Quijano, J. S. Calderón, S. Morales and T. Riso, Phytochemistry, 1988, 27, 2971 CrossRef; (d) F. Gómez-Garibay, J. S. Calderón, L. Quijano, V. O. Téllez, S. M. Olivares and T. Rios, Phytochemistry, 1997, 46, 1285 CrossRef.
- J. B. Harborne and H. Baxter, The Handbook of Natural Flavonoids, Wiley, Chichester, 1999 Search PubMed.
- J. C. Frisvad and O. Filtenborg, Mycologia, 1989, 81, 837 CrossRef CAS.
- P. A. Onocha, D. A. Okorie, J. D. Connolly and D. S. Roycroft, Phytochemistry, 1995, 40, 1183 CrossRef CAS.
- E. E. Stinson, W. B. Wise, R. A. Moreau, A. Z. Jurewilz and P. E. Pfeffer, Can. J. Chem., 1985, 64, 1590 CrossRef.
- E. O. Omeje, P. O. Osadebe, C. O. Esimone, C. S. Nworu, A. Kawamura and P. Proksch, Nat. Prod. Res., 2012, 26, 1775 CrossRef CAS.
- T. Fukunaga, I. Kajikawa, K. Nishiya, K. Takeya and H. Itokawa, Chem. Pharm. Bull., 1989, 37, 1543 CrossRef CAS.
- W. S. Judd, C. S. Campbell, E. A. Kellog and P. F. Stevens, Plant Systematics: A Phytogenetic Approach, Sinauer Associates Incorporation, Massachusetts, 1999.
- B. M. Komane, E. I. Olivier and A. M. Viljoen, Phytochem. Lett., 2011, 4, 1 CrossRef CAS.
- J. Beroa, H. Ganfon, M.-C. Jonville, M. Frédérich, F. Gbaguidi, P. DeMol, M. Moudachirou and J. Quetin-Leclercq, J. Ethnopharmacol., 2009, 122, 439 CrossRef.
- M. Traore, L. Zhai, M. Chen, C. E. Olsen, N. Odile, G. I. Pierre, O. J. Bosco, G. T. Robert and S. B. Christensen, Nat. Prod. Res., 2007, 21, 13 CrossRef CAS.
- S. Bah, A. K. Jager, A. Adsersen, D. Diallo and B. S. Paulsen, J. Ethnopharmacol., 2007, 110, 451 CrossRef.
- (a) A. El Tahir, G. M. H. Satti and S. A. Khalid, J. Ethnopharmacol., 1999, 64, 227 CrossRef CAS; (b) E. A. Prozesky, J. J. M. Meyer and A. I. Louw, J. Ethnopharmacol., 2001, 76, 239 CrossRef CAS; (c) C. Clarkson, V. J. Maharaj, N. R. Crouch, W. M. Grace, P. Pillay, M. G. Matsabisa, N. Bhagwandin, P. J. Smith and P. I. Folb, J. Ethnopharmacol., 2004, 92, 177 CrossRef.
- S. Hoet, F. Opperdoes, R. Brun, V. Adjakidjé and J. Quetin-Leclercq, J. Ethnopharmacol., 2004, 91, 37 CrossRef.
- M. A. Sonibare, A. A. Jayeola and A. Egunyomi, Biochem. Syst. Ecol., 2005, 33, 79 CrossRef CAS.
- N. H. T. Le, K. E. Malterud, D. Diallo, B. S. Paulsen, C. S. Nergård and H. Wangensteen, J. Ethnopharmacol., 2012, 139, 858 CrossRef CAS.
- H. Finnemore and J. M. Cooper, J. Soc. Chem. Ind., 1938, 57, 162 CAS.
- P. Champagnat, A. Heitz, A. Carnat, D. Fraisse, A.-P. Carnat and J.-L. Lamaison, Biochem. Syst. Ecol., 2008, 36, 68 CrossRef CAS.
- (a) K. Gluchoff-Fiasson, M. Jay and M. R. Viriciel, Phytochemistry, 1989, 28, 2471 CrossRef CAS; (b) J. B. Harborne, M. Boardley, S. Froest and G. Holm, Plant Syst. Evol., 1986, 154, 251 CrossRef CAS; (c) M. Jay and A. Ismaili, Phytochemistry, 1989, 28, 2035 CrossRef.
- M. Kim, H. S. Koh and H. Fukami, J. Chem. Ecol., 1985, 11(4), 441 CrossRef CAS.
- K. Vasisht and V. Kumar, Compendium of Medicinal and aromatic plants, ICS-UNIDO, Africa, Trieste, 2004, vol. 1, pp. 23–56 Search PubMed.
- A. Morales, M. Condra, J. A. Owen, D. H. Surridge, J. Fenemore and C. Harris, J. Urol., 1987, 137, 1168 CAS.
- J. G. Susset, C. D. Tessier, J. Wincze, S. Bansal, C. Malhotra and M. G. Schwacha, J. Urol., 1989, 141, 1360 CAS.
- (a) K. Starke, E. Borowski and T. Endo, Eur. J. Pharmacol., 1975, 34, 385 CrossRef CAS; (b) S. F. Martin and H. Rüeger, Tetrahedron Lett., 1985, 26(43), 5227 CrossRef CAS; (c) G. Blaskó, H. Knight, K. Honty and C. Szántay, Liebigs Ann. Chem., 1986, 198(4), 655 CrossRef; (d) D. J. Mergott, S. J. Zuend and E. N. Jacobsen, Org. Lett., 2008, 10(5), 745 CrossRef CAS.
- G. Bringmann, W. Saeb, M. Wohlfarth, K. Messer and R. Brun, Phytochemistry, 2000, 56, 5871 CAS.
- N. Ruangungsi, V. Wongpanich, P. Tantivatana, H. J. Cowe, P. J. Cox, S. Funayama and G. A. Cordell, J. Nat. Prod., 1985, 48, 529 CrossRef.
- M. R. Boyd, Y. F. Yallock, J. H. Cardellina II, K. P. Manfredi, J. W. Blunt, J. B. McMahon, R. W. Buckheit Jr, G. Bringmann, M. Schaffer, G. M. Cragg, D. W. Thomas and J. G. Jato, J. Med. Chem., 1994, 37, 1740 CrossRef CAS.
- J. Hutchinson and J. M. Dadziel, in Flora of West Tropical Africa, Vol. 1. Part I, ed. R. W. J. Keay, Crown Agents for Oversea Governments and Administrations, London, 1954, p. 233 Search PubMed.
- (a) G. Bringmann, F. Pokorny, M. Stäblein, M. Schäffer and L. Aké Assi, Phytochemistry, 1993, 33, 1511 CrossRef CAS; (b) K. P. Manfredi, J. W. Blunt, J. H. Cardellina II, J. B. McMahon, L. L. Pannell, G. M. Cragg and M. R. Boyd, J. Med. Chem., 1991, 34(12), 3402 CrossRef CAS.
- K. W. Bently, Nat. Prod. Rep., 1999, 16, 367 RSC.
- (a) A. Ouattara, I. P. Guissou, A. Sawadogo and M. Sawadogo, Pharmacien d'Afrique, 199(268), 19 Search PubMed; (b) Contribution aux études ethnobotaniques et floristiques au Togo, ed. E. Adjanohoun, A. M. Alyi, L. Aké Assi, J. Baniakina, P. Chibon, G. Cusset, V. Doulou, A. Enzanza, J. Eymé, E. Gondoté, A. Kéita, C. Mbemba and J. Mollet, et al., ACCT, Paris, 1986, p. 327, ISBN:92-9028-096.4 Search PubMed; (c) Contribution aux etudes ethnobotaniques et floristiques au Bénin, ed. E. Adjanohoun, A. M. Alyi, L. Aké Assi, J. Baniakina, P. Chibon, G. Cusset, V. Doulou, A. Enzanza, J. Eymé, E. Gondoté, A. Kéita, C. Mbemba and J. Mollet, et al., ACCT, Paris, 1989, p. 481, ISBN:92-9028-152.9 Search PubMed.
- A. A. Elujoba and E. A. Sofowora, Planta Med., 1977, 32, 54 CrossRef CAS.
- S. K. Adesina, Afr. J. Tradit. Complement. Altern. Med., 2005, 2(3), 282 CAS.
- S. O. Okpo and O. O. Adeyemi, West Afr. J. Pharmacol. Drug Res., 2008, 24, 17 Search PubMed.
- C. C. A. Azikiwe, I. M. Siminialayi, N. Brambaifa, L. U. Amazu, J. C. Enye and M. C. Ezeani, Asian Pac. J. Trop. Dis., 2012, S, 446 CrossRef.
- P. J. Houghton, J. M. Agbedahunsi and A. Adegbulugbe, Phytochemistry, 2004, 65, 2893 CrossRef CAS.
- Y. H. Kim, E. J. Park, M. H. Park, U. Badarch, G. M. Woldemichael and J. A. Beutler, Biol. Pharm. Bull., 2006, 29(10), 2140 CAS.
- E. O. Omeje, P. O. Osadebe, C. S. Nworu, J. N. Nwodo, W. O. Obonga, A. Kawamura, C. O. Esimone and P. Proksch, Pharm. Biol., 2011, 49, 1271 CrossRef CAS.
- K. Bhadra and G. S. Kumar, Med. Res. Rev., 2011, 31(6), 821 CrossRef CAS.
- G. Bidla, V. P. Titanji, B. Joko, G. E. Ghazali, A. Bolad and K. Berzins, Ind. J. Pharmacol., 2004, 36, 245 Search PubMed.
- F. Jia, G. Zou, J. Fan and Z. Yuan, Arch. Virol., 2010, 155(8), 1325 CrossRef CAS.
- K. Ezeamuzie, M. C. Ojinnaka, E. O. Uzogara and S. E. Orji, Afr. J. Med. Med. Sci., 1994, 23, 85 Search PubMed.
- J. E. Okokon, B. S. Antia, A. C. Igboasoiyi, E. E. Essien and H. O. Mbagwu, J. Ethnopharmacol., 2007, 111, 464 CrossRef.
- E. Elisabetsky and L. Costa-Campos, eCAM, 2006, 3(1), 39 CAS.
- E. O. Iwalewa, L. Lege-Oguntoye, P. P. Rai, T. T. Iyaniwura and N. L. Etkin, West Afr. J. Pharmacol. Drug Res., 1990, 9, 19 Search PubMed.
- C. Ancolio, N. Azas, V. Mahiou, E. Ollivier, C. Di Giorgio, A. Keita, P. Timon-David and G. Balansard, Phytother. Res., 2002, 16, 646 CrossRef CAS.
- H. Combier, M. Becchi and A. Cavé, Plantes Médicinales et Phytothérapie, 1977, vol. 11, p. 251 Search PubMed.
- O. Silva and E. T. Gomes, J. Nat. Prod., 2003, 66(3), 447 CrossRef CAS.
- O. O. Odebiyi and E. S. Sofowora, Planta Med., 1979, 36, 204 CrossRef CAS.
- E. O. Ajaiyeoba, J. S. Ashidi, L. C. Okpako, P. J. Houghton and C. W. Wright, Phytother. Res., 2008, 22, 254 CrossRef CAS.
- H. Morita, S. Oshimi, Y. Hirasawa, K. Koyama, T. Honda, W. Ekasari, G. Indrayanto and N. C. Zaini, Org. Lett., 2007, 9, 3691 CrossRef CAS.
- S. Oshimi, Y. Tomizawa, Y. Hirasawa, T. Honda, W. Ekasari, A. Widyawaruyanti, M. Rudyanto, G. Indrayanto, N. C. Zaini and H. Morita, Bioorg. Med. Chem. Lett., 2008, 18, 3761 CrossRef CAS.
- T. Matsumoto, T. Kobayashi, K. Ishida, Y. Hirasawa, H. Morita, T. Honda and K. Kamata, Biol. Pharm. Bull., 2010, 33(5), 844 CAS.
- J. Ai, K. Dekermendjian, M. Nielsen and M. R. Witt, Nat. Prod. Lett., 1997, 11, 73 CrossRef CAS.
- C. R. Mackener, R. L. Kochiman, B. A. Bierschenk and S. S. Bremner, J. Pharmacol. Exp. Therap., 1978, 206, 405 Search PubMed.
- J. F. Morton, Atlas of medicinal plants of Middle America: Bahamas to Yucatan, Charles Thomas, Springfield Illinois, 1981, pp. 745–750 Search PubMed.
- S. F. Mujovo, A. A. Hussein, J. J. M. Meyer, B. Fourie, T. Muthivhi and N. Lall, Nat. Prod. Res., 2008, 22(12), 1047 CrossRef CAS.
- B. T. Ngadjui, J. F. Ayafor, B. L. Sondengam, J. D. Connolly and D. S. Rycroft, Tetrahedron, 1991, 47, 3555 CrossRef CAS.
- T. O. Idowu, A. O. Ogundaini, A. O. Salau, E. M. Obuotor, M. Bezabih and B. M. Abegaz, Phytochemistry, 2010, 71, 2092 CrossRef CAS.
- I. A. Bello, G. I. Ndukwe, O. T. Audu and J. D. Habila, Org. Med. Chem. Lett., 2011, 1, 14 CrossRef.
- F. O. Ogungbamila, G. O. Onawunmi and O. Adeosun, Nat. Prod. Lett., 1997, 10, 201 CrossRef CAS.
- S. Jang, K. W. Kelley and R. W. Johnson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(21), 7534 CrossRef CAS.
- T. C. Theoharides, J. Neuroinflammation, 2009, 6, 29 CrossRef.
- G. Zhao, G. W. Qin, J. Wang, W. J. Chu and L. H. Guo, Neurochem. Int., 2010, 56(1), 168 CrossRef CAS.
- M. C. Yu, J. H. Chen, C. Y. Lai, C. Y. Han and W. C. Ko, Eur. J. Pharmacol., 2010, 10627(1–3), 269 CrossRef.
- (a) S. Byun, K. W. Lee, S. K. Jung, E. J. Lee, M. K. Hwang, S. H. Lim, A. M. Bode, H. J. Lee and Z. Dong, Cancer Res., 2010, 70, 2415 CrossRef CAS; (b) M. López-Lázaro, Mini-Rev. Med. Chem., 2009, 9(1), 31 CrossRef.
- J. M. Dalziel, The useful plants of West Tropical Africa, A Crown Agent for Oversea Publication, London, 1955, pp. 568–570 Search PubMed.
- (a) F. R. Irvine, Woody plants of Ghana, An Oxford University Press Publication, London, 1961, pp. 568–570 Search PubMed; (b) C. O. Okunji and M. M. Iwu, Int. J. Crude Drug Res., 1988, 26(4), 246 Search PubMed; (c) A. J. Akindele and O. O. Adeyemi, J. Ethnopharmacol., 2006, 108(1), 20 CrossRef CAS.
- A. A. Ahmadu, H. S. Hassan, M. U Abubakar and I. N. Akpulu, Afr. J. Tradit. Complement. Altern. Med., 2007, 4(3), 257 CAS.
- A. Vassallo, G. Cioffi, F. De Simone, A. Braca, R. Sanogo, A. Vanella, A. Russo and N. De Tommasi, Nat. Prod. Commun., 2006, 1(12), 1089 CAS.
- (a) Pharmacopée Sénégalaise traditionelle: plantes médicinales et toxicologiques, ed. J. Kerharo and J. C. Adam, Vigot et Frères, Paris, 1974, p. 1011 Search PubMed; (b) H. D. Neuwinger, African traditional medicine. A dictionary of plant use and application, Medpharm Scientific Publisher, 2000 Search PubMed.
- J. S. Ashidi, P. J. Houghton, P. J. Hylands and T. Efferth, J. Ethnopharmacol., 2010, 128, 501 CrossRef CAS.
- A. O. Akinsulie, E. O. Temiye, A. S. Akanmu, F. E. Lesi and C. O. Whyte, J. Trop. Pediatr., 2005, 51, 200 CrossRef CAS.
- M. M. Iwu, Handbook of African medicinal plants, C.R.C. Press Inc, Boca Raton, 1993, p. 23 Search PubMed.
- G. Duker-Eshun, J. W. Jaroszewski, W. A. Asomaning, F. Oppong-Boachie and S. Brøgger Christensen, Phytother. Res., 2004, 18(2), 128 CrossRef CAS.
- F. O. Shode, N. Koorbanally, V. Mudogo, P. T. Mpiana, J. P. K. Nbgolua and G. I. Ekeke, Planta Med., 1990, 56, 41 CrossRef.
- E. O. Ajaiyeoba, O. O. Ogbole, O. O. Abiodun, J. S. Ashidi, P. J. Houghton and C. W. Wright, J. Parasitol. Res., 2013, 703781 CAS.
- (a) S. I. Agu, Phytochemical investigation of Nigerian medicinal plants used in the treatment of skin disease, MSc thesis, University of Ife, Nigeria, 1980; (b) E. S. Ayensu, Medicinal plant of West Africa, Reference Publications Inc, Algonac, Michigan, 1978, Search PubMed; (c) V. B. Owoyele, J. O. Adediji and A. O. Soladoye, Inflammopharmacology, 2005, 13, 479 CrossRef.
- P. B.-K. Kouamé, C. Jacques, G. Bedi, V. Silvestre, D. Loquet, S. Barillé-Nion, R. J. Robins and I. Tea, Phytother. Res., 2013, 27(6), 835 CrossRef.
- C. A. Elusiyan, N. C. Ani, C. O. Adewunmi and T. A. Olugbade, Afr. J. Tradit. Complement. Altern. Med., 2011, 8(1), 27 CAS.
- R. Sanogo, A. Vassallo, N. Malafronte, S. Imparato, A. Russo and F. Dal Piaz, Nat. Prod. Commun., 2009, 4(12), 1645 CAS.
- (a) Contribution aux études ethnobotaniques et floristiques au Mali, Médecine traditionnelle et pharmacopée, ed. E. J. Adjanohoun, L. Aké Assi, J. J. Floret, S. Guindo, M. Koumaré, A. M. R. Ahyi and J. Raynal, ACCT, Paris, 1985, p. 250 Search PubMed; (b) Pharmacopée sénégalaise traditionnelle: Plantes médicinales et toxicologiques, ed. J. Kerharo and J. C. Adam, Paris Vigot et Frères, 1974, p. 1011 Search PubMed.
- F. B. C. Okoye, P. O. Osadebe, C. S. Nworu, N. N. Okoye, E. O. Omeje and C. O. Esimone, Nat. Prod. Lett., 2011, 25(20), 1941 CrossRef CAS.
- F. B. C. Okoye and P. O. Osadebe, Nat. Prod. Lett., 2010, 24(3), 266 CrossRef CAS.
- M. Chaabi, P. Chabert, C. Vonthron-Sénécheau, B. Weniger, M. Ouattara, H. Corstjens, I. Sente, L. Declercq and A. Lobstein, Phytochem. Lett., 2010, 3, 70 CrossRef CAS.
- J. C. Ibewuike, F. O. Ogungbamila, A. O. Ogundaini, I. N. Okeke and L. Bohlin, Phytother. Res., 1997, 11, 281 CrossRef CAS.
- (a) B. S. Antia, A. Pansanit, O. D. Ekpa, U. J. Ekpe, C. Mahidol, P. Kittakoop and Q. B. Han, Planta Med., 2010, 76(3), 276 CrossRef CAS; (b) S. F. Lee, C. F. Qiao, Z. D. He, J. Z. Song, H. D. Sun and H. X. Xu, Chem. Pharm. Bull., 2005, 53(8), 1034 CrossRef; (c) H. X. Xu, S. Mughal, O. Taiwo and S. F. Lee, J. Ethnopharmacol., 2013, 147(2), 497 CrossRef CAS PubMed.
- T. Mboya-Okeyo, R. G. Ridley and S. Nwaka, Lancet, 2009, 373, 1507 CrossRef.
- S. Nwaka, T. B. Ilunga, J. S. Da Silva, E. R. Verde, D. Hackley, R. D. Vré, T. Mboya-Okeyo and R. G. Ridley, PLoS Med., 2010, 7, e1000293 Search PubMed.
- F. Ntie-Kang, P. Amoa Onguéné, G. W. Fotso, K. Andrae-Marobela, M. Bezabih, J. C. Ndom, B. T. Ngadjui, A. O. Ogundaini, B. M. Abegaz and L. M. Mbaze, PLoS One, 2014, 9(3), e90655 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |