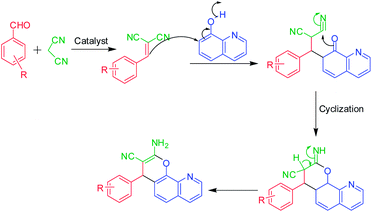
Ce–Zr/SiO2: a versatile reusable heterogeneous catalyst for three-component synthesis and solvent free oxidation of benzyl alcohol†
Ramakanth Pagadala,
Suresh Maddila,
Surjyakanta Rana and
Sreekantha B. Jonnalagadda*
School of Chemistry & Physics, University of KwaZulu-Natal, Westville Campus, Chiltern Hills, Durban-4000, South Africa. E-mail: jonnalagaddas@ukzn.ac.za; Fax: +27 31 260 3091; Tel: +27 31 260 7325/3090
First published on 24th December 2013
Abstract
Ce–Zr loaded on SiO2 as catalyst provides an extremely efficient method to synthesize pyranoquinolines. This catalyst is found to be superb for the three-component synthesis of target compounds and for the solvent-free liquid-phase oxidation of benzyl alcohols. The catalyst is fully recoverable and reusable with no loss of catalytic activity even after multiple cycles.
The importance and popularity of green chemistry principles introduced significant advances in organic synthesis, such as combinatorial chemistry, multicomponent processes, organofluorine chemistry, organocatalysis, microwave synthesis and sonochemistry to mention a few. Among these developments, multicomponent reaction techniques played a leading role and the field experienced tremendous progresses.1–7 It is pertinent to note that multi-component reactions have added advantages such as high bond forming efficiency, convergence, operational simplicity, and reduction in waste generation and hence conform to the principles of green chemistry. Consequently, they are regarded as viable synthetic routes toward both economic and environmental benefits of chemical transformations.8,9 Multicomponent cascade reactions, which are ideal synthetic methods, play an important role in the synthesis of heterocyclic compounds.10–20 The oxidation of benzyl alcohol to benzaldehyde, is also an important organic conversion because it is a valuable chemical in the perfumery, dyestuffs, and agrochemical industries.21 Heterogeneous catalysts also have received sizeable attention in organic synthesis because of environmental, economic and industrial aspects. The use of solid heterogeneous catalysts for the development of clean processes is a theme of extreme importance in the chemical industry. These catalysts make the synthetic process economically viable as they can be easily recovered from the reaction mixture by simple filtration and can be reused several times to achieve very high turnover numbers compared to homogeneous catalysts.22 Recently, heterogeneous catalysts have received recognition as the new generation of catalysts having their dual organic and inorganic natures allows them to establish nearly all kinds of interactions with reacting species including transition states, and hence sometimes give rise to improved yields and rate enhancements. In addition, silica supported reagents are promising for both academic and industrial applications due to their high mechanical and thermal stabilities, non-corrosive and easy separation of the catalyst from the reaction mixture.23,24
Functionalized heterocyclic building blocks are of great importance in both medicinal and synthetic chemistry and development of new efficient synthetic methodologies for these scaffolds remains a great challenge in modern organic synthesis.25,26 The importance of quinoline and its annulated derivatives is well recognized by synthetic and biological chemists.27–30 Compounds possessing this motif have found broad application in drug development material science31–35 bioorganometallic processes36,37 and biological activities covering antimalarial, anticancer, antifungal, antibacterial, antiprotozoic antibiotic and anti-HIV effects.38–40
In continuation of our effort toward the development of new protocols for the expeditious synthesis of biologically relevant heterocyclic compounds,41–44 keeping these aspects in mind, we have designed a series of 2-amino-4H-pyranoquinoline derivatives with using Ce–Zr/SiO2 as a heterogeneous solid catalyst for the first time, with no earlier report of its kind. In green chemistry point of view, the design of multicomponent reactions using recyclable heterogeneous catalyst and ethanol as a sole solvent is ideally the best choice owing to the easier workup procedure and the inherent environmental and economic advantages of such process.
To develop a clean three component protocol for the formation of 2-amino-4H-pyranoquinolines, we have installed a large number of Brønsted and Lewis acid catalysts for the optimization of the reaction condition. In a pilot experiment, benzaldehyde (1 mol), malononitrile (1 mol), and 8-hydroxyquinoline (1 mol) were stirred in ethanol at 70 °C temperature (Scheme 1). It is evident that the three component coupling product, pyranoquinoline derivative was not obtained in ethanol after 8 h at room temperature as well as 70 °C temperature (Table 1, entries 1 & 2). To accelerate the three-component reaction, we have applied a wide spectrum of Brønsted and Lewis acid catalysts like NaOH, p-toluene sulphonic acid (p-TsOH) and [Bmim]BF4 were selected (Table 1). This group of catalysts except [Bmim]BF4 has the advantage low impact in the environment. We found that for the three-component coupling protocol, Brønsted acids were not very much efficient as compared to the Lewis acid catalysts desiring the synthesis of the heterocyclic scaffold under the particular experimental condition.
 | ||
| Scheme 1 One-pot three-component reaction of aromatic aldehydes, malononitrile and 8-hydroxyquenoline catalyzed by Ce–Zr/SiO2 in ethanol media. | ||
| Entry | Product no. | Catalyst | Amount | Solvent | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|---|
| a Isolated yields.b Products was not found.c Trace. | |||||||
| 1 | 4a | — | — | EtOH | Room temp. | 8.0 | b |
| 2 | 4a | — | — | EtOH | 70 | 8.0 | b |
| 3 | 4a | NaOH | 1.0 (eq.) | H2O | Room temp. | 6.0 | c |
| 4 | 4a | [Bmim]BF4 | 6 drops | EtOH | Room temp. | 10.0 | b |
| 5 | 4a | [Bmim]BF4 | 6 drops | — | Room temp. | 8.0 | b |
| 6 | 4a | [Bmim]BF4 | 6 drops | — | 70 | 5.0 | 50 |
| 7 | 4a | PTSA | 30.0 mg | EtOH | 70 | 5.0 | 30 |
| 8 | 4a | Ce–Zr/SiO2 | 20.0 mg | EtOH | 70 | 5.0 | 55 |
| 9 | 4a | Ce–Zr/SiO2 | 30.0 mg | EtOH | 70 | 4.0 | 70 |
| 10 | 4a | Ce–Zr/SiO2 | 40.0 mg | EtOH | 70 | 4.0 | 94 |
| 11 | 4a | Ce–Zr/SiO2 | 50.0 mg | EtOH | 70 | 4.0 | 95 |
Developing environmentally benign and economical syntheses is an area of research that is being vigorously pursued, and avoiding the use of harmful organic solvents is a fundamental strategy to achieving this. Hence, we have tried to synthesize the heterocyclic scaffold in less amount of ethanol applying suitable catalyst combination and we have applied Ce–Zr/SiO2 catalyst combination to enhance the rate of the reaction. The improvement of the yield of the desired product was noticed by using the catalyst combination (Table 1, entry 8–11). With the success, focus was then shifted to optimization. The above model reaction was carried out under different Ce–Zr/SiO2 quantities (20 mg, 30 mg, 40 mg and 50 mg Table 1). It was observed that 40 mg loading of the Ce–Zr/SiO2 provided the best yield (Table 1, entry 10).
Under the optimal reaction conditions, a range of aldehydes were treated with malononitrile and 8-hydroxyquenoline in order to examine the scope of the reaction, and several representative examples are summarized in Table 2. Notably, even sterically hindered substrates also participated effectively to afford the desired 2-amino-4H-pyranoquinoline derivatives in good yield (Table 2). The reaction mechanism may cautiously be visualized to occur via a tandem sequence of the reactions depicted in the reaction scheme in Scheme 2. The resulting products were characterized by FTIR, 1H NMR, 13C NMR and MS. Compounds were further identified by 15N NMR (GHSQC) to confirm the presence of the –NH2 group in their structure.
The corresponding N2 adsorption–desorption distribution isotherms of the catalysts (Fig S1 in ESI†). The isotherms showed the hysteric loop (type IV) which demonstrates the presence of mesoporous character in the catalyst. The catalyst showed the pore diameter of 151 Å along with high pore volume of 1.055 cm3 g−1 and surface area of 275 m2 g−1, which demonstrates its mesoporus character. The Ce (0.49 wt%) and Zr (0.48 wt%) obtained from Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) are in correlation with the nominal weight loading. SEM-EDX (Fig S2 in ESI†) data also showed presence of Ce and Zr in the catalysts with the appropriate nominal loading on the surface of the support.47 In order to understand the pore structure of the catalysts, the N2 adsorption–desorption measurements were carried out. This could be attributed to the clogging of the narrow pores of the support with the cerium–zirconium making it inaccessible to nitrogen molecules. The Ce–Zr loaded on SiO2 support catalyst exhibited high surface area due to the weak interaction between the Ce–Zr and, Ce–Zr particles may be agglomerated on the surface of the SiO2. The mesoporous size has significant role on the adsorption properties of reactant molecules and thereby it can play a major role in the activity. The wide range distribution in the pore size may be correlated with the activity exhibited by the catalyst.
The oxidation of benzyl alcohol is often used as an ideal reaction for alcohol oxidation. The catalytic activities of Ce–Zr/SiO2 for the oxidation of benzyl alcohol in the presence H2O2 are presented in Table 3. The conversion of benzyl alcohol and the selectivity to benzaldehyde increased to 87% and 97%, respectively, the oxidation reaction proceeding through the formation of a peroxo complex by a reaction between the catalyst and H2O2. This peroxo compound reacts with benzyl alcohol and gives the product. Activity of the catalyst remained unchanged up to 6th cycle in oxidation reaction. After that the activity diminished due to possible coke formation of the catalyst.
| Catalyst | Conversion of benzyl alcohol | Selectivity (%) | |
|---|---|---|---|
| Benzaldehyde | Benzoic acid | ||
| Ce–Zr/SiO2 | 87 | 97 | 3 |
| Ce–Zr/SiO2 (1st Cycle) | 87 | 97.2 | 2.8 |
| Ce–Zr/SiO2 (2nd Cycle) | 87 | 97 | 3 |
| Ce–Zr/SiO2 (3rd Cycle) | 86.5 | 97 | 3 |
| Ce–Zr/SiO2 (4th Cycle) | 86.8 | 96 | 4 |
| Ce–Zr/SiO2 (5th Cycle) | 87 | 97 | 3 |
| Ce–Zr/SiO2 (6th Cycle) | 87 | 97 | 3 |
| Ce–Zr/SiO2 (7th Cycle) | 78 | 86 | 14 |
In multicomponent reaction, catalytic activity does not change up to 5th cycle (Fig. 1). The activity loss observed with the regenerated catalyst later could be due to partial loss of acid sites or surface area of the catalyst during reaction/regeneration. The filtrate after reaction was analyzed for leached metal content by ICP-OES. No trace of metal was detected, confirming no leaching.
The X-ray diffraction patterns of prepared 1 wt% Ce–Zr supported on SiO2 catalysts are shown in Fig. 2. The phases of support silica are correlating with literature.47,48 The d-spacing's of ceria and zirconia phases are in agreement with the ICDD files no's 01-078-0047, 01-73-6328, 38-1436 for CeO2, ZrO2 and Ce–ZrO2 respectively. There is an evidence of the mixed metal oxide phase i.e. Ce50–Zr50O2, where d-spacing are in correlation with ICDD file no: 28-0271. The catalyst showed the polycrystalline nature, and the crystalline nature.
Fig. 3 shows the SEM images of the Ce–Zr supported on silica catalyst. In the presence of Ce–Zr is distributed over silica surface. The Ce–Zr particles are agglomerated on the surface of silica. A possible reason could be that silica has a large surface area and therefore requires more Ce–Zr particles to cover its surface.47,48 The SEM images confirm the polycrystalline nature of the catalyst, which is not affected by the different loadings of cerium-zirconium. SEM-EDX shows that the cerium–zirconium is evenly distributed on the surface of the supports, and SEM-EDX results are in correlation with ICP-OES elemental analysis.
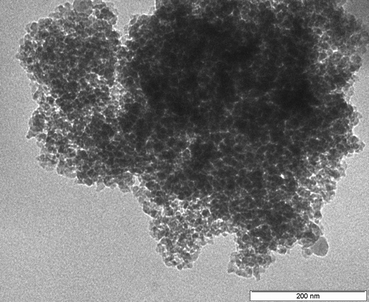
The TEM images of the catalysts showed an agglomeration of particles on the surface of the supports (Fig. 4). Agglomeration of particles is the result of exposure of the samples to a beam with the high energy resulting in the loss of hydroxyl groups. The agglomeration of the particles is high on the surface of the silica. On high magnification, the particles appear as irregular a needle-like shape which was also observed in literature.48–50 The darker parts of the images show the presence of Ce–Zr dispersed evenly on the surface which appear as elongated rod-like particles in the TEM images with particle sizes around 40–80 nm.
Conclusions
In summary, we have developed a very practical carbon–carbon and carbon–oxygen bond forming reaction in ethanol. The key is the use of newly synthesized Lewis acid heterogeneous solid catalyst, Ce–Zr/SiO2. A heterogeneous catalyst is of more utility, when it can be easily recovered and is reusable and the proposed catalyst meets those criteria excellently. From green chemistry point of view, to develop a work up procedure without using large amount of any organic solvent is desirable. This simple, environmentally benign and convenient methodology extends the scope towards a wide spectrum of novel compounds possessing an important structural subunit of a variety of biologically active molecules. The solid support Lewis acid catalyst, Ce–Zr/SiO2 reported in this article will positively contribute to green chemical processes by reducing the use of organic solvents and reaction time.Catalyst preparation
The preparation of metal oxide supported catalysts was synthesized by wet impregnation method.47,51 The catalysts were prepared by dissolving appropriate amount of Ce [cerium nitrate, Aldrich-99%], Zr [zirconium nitrate, Aldrich-99%] in distilled water [50.0 mL] and adding it to 10 g of silica [SiO2, Aldrich] stirring for 4 h using a magnetic stirrer at room temperature and again at room temperature for overnight. Catalysts are further dried in an oven at 110–120 °C for 12 h. Then the catalysts are calicined in the presence of air, at 550 °C for 3 h to obtain the 1% w/w catalysts.Typical procedure for the synthesis of pyranoquinolines catalyzed by Ce–Zr/SiO2 (4a–f)
A mixture of freshly distilled benzaldehyde (1.0 mol) in ethanol (3 mL) at room temperature, maloninitrile (1.0 mol), 8-hydroxyquinoline (1 mol) and Ce–Zr/SiO2 (40.0 mg) were added to a round bottom flask equipped with a magnetic bar and condenser. Then, the reaction mixture was heated at 70 °C for appropriate time as shown in Table 2. The reaction progress was monitored by TLC (EtOAc/hexane = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). After completion of the reaction, the mixture was cooled to room temperature and extracted with dichloromethane. After filtering the Ce–Zr/SiO2 solid, the solvent layer was washed with water, dried with anhydrous sodium sulfate and the solvent removed to obtain essentially pure product 4a (94% yield) as off-white solid. The recovered Ce–Zr/SiO2 solid was washed with dichloromethane and evaporated solvent under reduced pressure and reused in the next cycles.
5). After completion of the reaction, the mixture was cooled to room temperature and extracted with dichloromethane. After filtering the Ce–Zr/SiO2 solid, the solvent layer was washed with water, dried with anhydrous sodium sulfate and the solvent removed to obtain essentially pure product 4a (94% yield) as off-white solid. The recovered Ce–Zr/SiO2 solid was washed with dichloromethane and evaporated solvent under reduced pressure and reused in the next cycles.
Compound 4a
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 4.94 (1H, s), 7.15 (2H, s, NH2), 7.19–7.33 (6H, m), 7.57–7.63 (2H, m), 8.30 (1H, dd, J = 8.2 Hz), 8.92 (1H, dd, J = 4.2 Hz); 13C NMR (100 MHz, CDCl3): δ 41.07, 55.99, 120.41, 121.93, 122.13, 123.56, 126.91, 126.99, 127.63, 128.72, 136.0, 137.43, 142.93, 145.57, 150.22, 160.27; IR (KBr, cm−1): 3319 (NH2), 2190 (CN); LCMS of [C19H13N3O + Na] (m/z): 322 (100%); anal. calcd: C 76.24, H 4.38, N 14.04%. Found: C 76.18, H 4.40, N 14.01%.Compound 4b
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 3.70 (3H, s), 4.89 (1H, s), 6.86 (2H, d, J = 8.5 Hz), 7.10 (2H, s, NH2), 7.18 (3H, t, J = 8.2 Hz), 7.57–7.63 (2H, m), 8.31 (1H, d, J = 7.4 Hz), 8.92 (1H, d, J = 2.8 Hz); 13C NMR (100 MHz, CDCl3): δ 40.26, 55.0, 56.27, 114.06, 120.45, 122.07, 122.24, 123.46, 126.98, 127.59, 128.73, 135.97, 137.46, 137.76, 142.81, 150.16, 158.19, 160.10; IR (KBr, cm−1): 3298 (NH2), 2187 (CN); LCMS of [C20H15N3O2 + H+] (m/z): 330 (100%); anal. calcd: C 72.94, H 4.59, N 12.76%. Found: C 72.89, H 4.52, N 12.80%.Compound 4c
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 4.99 (1H, s), 7.18–7.24 (5H, m, Ar-H and NH2), 7.50–7.66 (4H, m), 8.32 (1H, dd, J = 8.3 Hz), 8.93 (1H, dd, J = 4.0 Hz); 13C NMR (100 MHz, CDCl3): δ 40.43, 55.50, 120.14, 120.25, 121.31, 122.23, 123.69, 126.78, 127.76, 129.94, 131.24, 131.63, 136.02, 137.43, 142.99, 144.97, 150.27, 160.26; IR (KBr, cm−1): 3299 (NH2), 2193 (CN); LCMS of [C19H12BrN3O + Na] (m/z): 400 (100%); anal. calcd: C 60.34, H 3.20, N 11.11%. Found: C 60.39, H 3.27, N 11.08%.Compound 4d
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 4.82 (1H, s), 6.6 (2H, d, J = 8.3 Hz), 7.03–7.20 (5H, m, Ar-H and NH2), 7.57–7.63 (2H, m), 8.31 (1H, d, J = 8.1 Hz), 8.92 (1H, d, J = 3.2 Hz), 9.35 (1H, s, OH); 13C NMR (100 MHz, CDCl3): δ 40.32, 56.44, 115.37, 120.52, 122.02, 122.48, 123.40, 127.03, 127.54, 128.68, 129.94, 135.96, 136.10, 137.46, 142.77, 150.12, 156.30, 160.07, 161.13; IR (KBr, cm−1): 3331 (NH2), 3210 (OH), 2199 (CN); LCMS of [C19H13N3O2 + H+] (m/z): 316 (100%); anal. calcd: C 72.37, H 4.16, N 13.33%. Found: C 72.32, H 4.21, N 13.38%.Compound 4e
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 5.45 (1H, s), 7.07 (1H, d, J = 8.5 Hz); 7.21 (2H, s, NH2), 7.26–7.64 (6H, m), 8.31 (1H, dd, J = 8.2 Hz), 8.93 (1H, dd, J = 4.0 Hz); 13C NMR (100 MHz, CDCl3): δ 38.48, 54.60, 120.04, 120.52, 122.25, 123.74, 126.12, 127.84, 127.95, 128.95, 129.84, 131.25, 131.99, 136.0, 137.33, 142.01, 143.27, 150.30, 160.47; IR (KBr, cm−1): 3320 (NH2), 2195 (CN); LCMS of [C19H12ClN3O + H+] (m/z): 334 (100%); anal. calcd: C 68.37, H 3.62, N 12.59%. Found: C 68.42, H 3.60, N 12.67%.Compound 4f
Off-white solid: 1H NMR (400 MHz, DMSO-d6) δ = 5.46 (1H, s), 7.06 (1H, d, J = 8.5 Hz), 7.16–7.35 (5H, m, Ar-H and NH2), 7.59–7.63 (3H, m), 8.31 (1H, dd, J = 8.3 Hz), 8.93 (1H, dd, J = 4.0 Hz); 13C NMR (100 MHz, CDCl3): δ 40.58, 54.86, 119.95, 120.63, 122.27, 122.38, 123.78, 125.98, 127.86, 128.61, 129.22, 131.48, 133.0, 136.01, 137.37, 143.15, 143.78, 150.31, 160.37; IR (KBr, cm−1): 3319 (NH2), 2195 (CN); LCMS of [C19H12BrN3O + Na] (m/z): 400 (100%); anal. calcd: C 60.34, H 3.20, N 11.11%. Found: C 60.42, H 3.16, N 11.18%Catalytic activity towards oxidation reaction
The catalytic oxidation of benzyl alcohol was carried out in a magnetically stirred round-bottom flask fitted with a reflux condenser. The reaction mixture contain 200 mmol of benzyl alcohol, 200 mmol of hydrogen peroxide (30%), 0.1 g of catalyst; temperature, 80 °C; and reaction time, 2 h. The catalyst was recovered from the reaction mixture by centrifugation, and the reaction products were analyzed by gas chromatography.Acknowledgements
The authors are grateful to the School of Chemistry & Physics and the College of Agriculture, Engineering & Science, University of KwaZulu-Natal, Westville campus, Durban, South Africa for the facilities and financial support.Notes and references
- R. Pagadala, S. Maddila and S. B. Jonnalagadda, Ultrason. Sonochem., 2014, 21, 472 CrossRef CAS PubMed.
- M. B. Gawande, V. D. B. Bonifacio, R. Luque, P. S. Brancoa and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC.
- F. M. Kerton and R. Marriott, Alternative Solvents for Green Chemistry, 2013, FP005–FP007, 10.1039/9781849736824-FP005.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef CAS.
- A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed.
- A. Domling and Y. Huang, Synthesis, 2010, 2010, 2859 CrossRef PubMed.
- M. A. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. Bonacorso, Chem. Rev., 2008, 108, 2015 CrossRef CAS PubMed.
- J. D. Holbrey, M. B. Turner, W. M. Reichert and R. D. Rogers, Green Chem., 2003, 5, 731 RSC.
- J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed.
- D. M. D'Souza and T. J. J. Muller, Chem. Soc. Rev., 2007, 36, 1095 RSC.
- J. Zhu, Eur. J. Org. Chem., 2003, 1133 CrossRef CAS.
- B. A. Granger, K. Kaneda and S. F. Martin, Org. Lett., 2011, 13, 4542 CrossRef CAS PubMed.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, J. Org. Chem., 2011, 76, 8394 CrossRef CAS PubMed.
- H. Zhu, J. Stcckigt, Y. Yu and H. Zou, Org. Lett., 2011, 13, 2792 CrossRef CAS PubMed.
- L. V. Frolova, N. M. Evdokimov, K. Hayden, I. Malik, S. Rogelj, A. Kornienko and I. V. Magedov, Org. Lett., 2011, 13, 1118 CrossRef CAS PubMed.
- S. Hardy and S. F. Martin, Org. Lett., 2011, 13, 3102 CrossRef CAS PubMed.
- L. E. Kaim, L. Grimaud and S. R. Purumandla, J. Org. Chem., 2011, 76, 4728 CrossRef PubMed.
- K. Worrall, B. Xu, S. Bontemps and B. A. Arndtsen, J. Org. Chem., 2011, 76, 170 CrossRef CAS PubMed.
- H. R. Pan, Y. J. Li, C. X. Yan, J. Xing and Y. Cheng, J. Org. Chem., 2010, 75, 6644 CrossRef CAS PubMed.
- N. Lingaiah, K. M. Reddy, N. Seshu Babu, K. Narasimha Rao, I. Suryanarayana and P. S. Sai Prasad, Catal. Commun., 2006, 7, 245 CrossRef CAS PubMed.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed.
- P. M. Price, J. H. Clark and D. J. Macquarrie, J. Chem. Soc., Dalton Trans., 2000, 101 RSC.
- R. K. Sharma, S. Mittal and M. Koel, Crit. Rev. Anal. Chem., 2003, 33, 183 CrossRef CAS.
- A. Nefzi, J. M. Ostresh and R. A. Houghten, Chem. Rev., 1997, 97, 449 CrossRef CAS PubMed.
- S. J. Teagues, A. M. Davis, P. D. Leeson and T. Opera, Angew. Chem., Int. Ed., 1999, 38, 3743 CrossRef.
- C. W. Wright, J. Addae-Kyereme, A. G. Breen, J. E. Brown, M. F. Cox, S. L. Croft, Y. Gokcek, H. Kendrick, R. M. Phillips and P. L. Pollet, J. Med. Chem., 2001, 44, 3187 CrossRef CAS PubMed.
- N. P. Sahu, C. Pal, N. B. Mandal, S. Banerjee, M. Raha, A. P. Kundu, A. Basu, M. Ghosh, K. Roy and S. Bandyopadhyay, Bioorg. Med. Chem., 2002, 10, 1687 CrossRef CAS.
- G. Bringmann, Y. Reichert and V. V. Kane, Tetrahedron, 2004, 60, 3539 CrossRef CAS PubMed.
- V. V. Kournetsov, L. Y. V. Mendez and C. M. M. Gomez, Curr. Org. Chem., 2005, 9, 141 CrossRef.
- S. A. Jenekhe, L. Lu and M. M. Alam, Macromolecules, 2001, 34, 7315 CrossRef CAS.
- A. K. Agrawal, S. A. Jenekhe, H. Vanher zeele and J. S. Meth, J. Phys. Chem., 1992, 96, 2837 CrossRef CAS.
- G. Jegou and S. A. Jenekhe, Macromolecules, 2001, 34, 7926 CrossRef CAS.
- L. Lu and S. A. Jenekhe, Macromolecules, 2001, 34, 6249 CrossRef CAS.
- S. A. Jenekhe, X. Zhang, X. X. L. Chen, V. E. Choong, Y. Gao and B. R. Hsieh, Chem. Mater., 1997, 9, 409 CrossRef CAS.
- K. Nakatani, S. Sando and I. Saito, Bioorg. Med. Chem., 2001, 9, 2381 CrossRef CAS.
- C. He and S. J. Lippard, J. Am. Chem. Soc., 2001, 40, 1414 CAS.
- A. Mahamoud, J. Chevalier, A. Davin-Regli, J. Barbe and J. M. Pages, Curr. Drug Targets, 2006, 7, 843 CrossRef CAS.
- N. Ahmed, K. G. Brahmbhatt, S. Sabde, D. Mitra, I. P. Singh and K. K. Bhutani, Bioorg. Med. Chem., 2010, 18, 2872 CrossRef CAS PubMed.
- N. C. Desai, K. M. Rajpara, V. V. Joshi, H. V. Vaghani and H. M. Satodiya, Med. Chem. Res., 2013, 22, 1172 CrossRef CAS.
- R. Pagadala, J. S. Meshram, H. N. Chopde, V. Jetti, Ch. Praveen and U. Kusampally, J. Heterocycl. Chem., 2012, 49, 1151 CrossRef CAS.
- S. Maddila, P. Lavanya, K. Naga Raju, S. B. Jonnalagadda and C. Venkata Rao, Org. Commun., 2011, 4, 33 Search PubMed.
- S. Maddila and S. B. Jonnalagadda, Lett. Drug Des. Discovery, 2012, 9, 687 CrossRef CAS.
- R. Pagadala, J. S. Meshram, H. N. Chopde, V. Jetti, U. Kusampally and V. Udayini, Med. Chem., 2011, 7, 325 CAS.
- Z. H. Khalil, A. A. Abdel-Hafez, A. A. Geies and A. M. Kamal El-Dean, Bull. Chem. Soc. Jpn., 1991, 64, 668 CrossRef CAS.
- W. Xiang-Shan, S. Da-Qing and T. Shu-Jian, Chin. J. Chem., 2003, 21, 1114 Search PubMed.
- S. Maddila, V. D. B. C. Dasireddy, E. O. Oseghe and S. B. Jonnalagadda, Appl. Catal., B, 2013, 142-143, 129 CrossRef CAS PubMed.
- E. C. Chetty, S. Maddila, V. B. Dasireddy and S. B. Jonnalagadda, Appl. Catal., B, 2012, 117-118, 18–28 CrossRef CAS PubMed.
- S. Maddila, V. D. B. C. Dasireddy and S. B. Jonnalagadda, Appl. Catal., B, 2013, 138-139, 149 CrossRef CAS PubMed.
- B. M. Reddy, A. Khan, Y. Yamada, T. Kobayashi, S. Loridant and J. C. Volta, Langmuir, 2003, 19, 3025 CrossRef CAS.
- E. C. Chetty, S. Maddila, C. Southway and S. B. Jonnalagadda, Ind. Eng. Chem. Res., 2012, 51, 2864 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47145d |
| This journal is © The Royal Society of Chemistry 2014 |