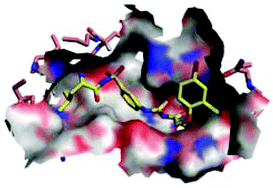
Human African trypanosomiasis (HAT) is one of the most neglected diseases in the tropic regions, which is fatal if not treated in time. There is an urgent need for new therapeutics, especially those in new chemical classes. Leucyl-tRNA synthetase (LeuRS) has been paid much attention as a recently clinically validated antimicrobial target. Our group has previously reported T. brucei LeuRS (TbLeuRS) inhibitors, including benzoxaboroles targeting the editing site and pyrrolinones targeting the synthetic site. Here we report the discovery of N-(4-sulfamoylphenyl)thioureas as a new class of TbLeuRS inhibitors. The R1 and R2 groups, reminiscent of the leucyl and adenyl regions of aa-AMP and aa-AMS, were optimized to result in a significant 13-fold increase of inhibitory activity (compound 19, IC50 = 13.7 μM). Aided by ligand–protein docking, the 1,3-substitution at the central phenyl ring was predicted and proved to give significantly improved activity (59, IC50 = 1.1 μM). This work provided a new scaffold for the exploration of novel inhibitors against TbLeuRS, which may become potential therapeutics for the treatment of HAT.