Emerging trends in membrane-based wastewater treatment: electrospun nanofibers and reticular porous adsorbents as key components
Manish
Kumar
 a,
Sumanta
Chowdhury
a,
Sumanta
Chowdhury
 b and
Jaspreet Kaur
Randhawa
b and
Jaspreet Kaur
Randhawa
 *a
*a
aSchool of Mechanical & Materials Engineering, Indian Institute of Technology Mandi, India. E-mail: jaspreet@iitmandi.ac.in
bSchool of Chemical Sciences, Indian Institute of Technology Mandi, India
First published on 9th November 2023
Abstract
The increasing global population and its reliance on water-based activities have led to freshwater scarcity and unequal distribution. Researchers have responded to the pressing need for effective wastewater treatment by developing new adsorbent materials. However, practical application of these nanomaterials is hindered by challenges in scalability, regeneration, and agglomeration. The integration of nano-adsorbents into polymeric membranes, particularly electrospun nanofibrous membranes, holds potential for improving their performance, reusability, and durability, while also addressing separation concerns. This comprehensive review examines the selection and application of innovative adsorbent materials in membrane technology for wastewater treatment. It explores synthetic techniques for membrane fabrication, emphasizes the impact of materials like 2D materials, MOFs, and COFs, and also addresses problems such as biofouling. Additionally, this study highlights the potential of zwitterionic materials in mitigating biofouling and discusses the concept of biomineralization for water remineralization. Altogether, this review provides valuable insights into the current progress and prospects of material selection in membrane technology for water remediation.
Water impactThe current status of technologies for wastewater remediation is reviewed. Membrane technologies and their functionalization strategies for organic dye, hazardous heavy metal, and salt rejection are examined critically. The prospects of membrane biofouling mitigation and purified water remineralization techniques are conferred in relation to the functionalization of cutting-edge membranes. The knowledge gap in advanced material selection for hybrid membrane design has been identified critically. |
Introduction
Water plays a crucial role in various human activities. With the ongoing increase in the world population, a substantial volume of wastewater is generated on a daily basis across several sectors including households, industry, and agriculture. Freshwater supplies, though, do not undergo replenishment at a rate sufficient to satisfy the continually expanding population and its corresponding demands for water usage. Consequently, there has been a proliferation of fierce competition and inequitable allocation of the few freshwater resources across different sectors. The significance of water was underscored in the World Bank study. At present, a substantial population of over four billion individuals resides in regions characterised by limited access to water resources. Among these individuals, two billion depend on groundwater as their primary source of potable water. Furthermore, it is noteworthy that one out of every four cities across the globe has varying degrees of water insecurity.1 Industrial organic waste, plastics and microplastics, heavy metals, pathogens, suspended particles, emulsified oils, and other pollutants are common types of wastewater contaminants in freshwater sources. These contaminants are often reduced through sedimentation, coagulation, and, most critically, filtration-based techniques. Among these, adsorption is critical in filtration-based processes. As a result, over the last few decades, there has been an increase in interest in the creation of efficient adsorbents for the aim of cleaning wastewater. In this context, the discovery of innovative materials to filter wastewater sources is critical.2,3A material's effectiveness as an adsorbent is predominantly determined by its surface area and anchoring sites, such as functional groups. In order to augment the surface area of adsorbents, two potential approaches can be considered: exterior and internal surface area augmentation. The enhancement of nanomaterials' exterior surface area and subsequent improvement in efficiency can be achieved through the synthesis of these materials using either bottom-up or top-down methodologies. A wide array of cost-effective and environmentally sustainable nanomaterials possessing unique properties have been proposed for application in the treatment of industrial wastewater, groundwater, surface water, and seawater.4 The scientific literature extensively acknowledges nanotechnology as an innovative and effective approach for the treatment of wastewater. Nano-adsorbents are well acknowledged for their substantial external surface area and their ability to undergo functionalization through various means. The exterior morphology, observable dimensions, and intrinsic and extrinsic composition, as well as the physical, chemical, and material characteristics of these entities exhibit notable differences. Nanomaterials have been employed in the field of wastewater treatment through many methods, such as adsorption,5–7 photocatalysis,8–10 membrane filtration,11–13 and chemical disinfection.14–16 The global nanotechnology market was valued at $1.76 billion in 2020 and is predicted to reach $33.63 billion by 2030, expanding at a CAGR of 36.4% from 2021 to 2030.17 Water and wastewater treatment enabled by nanotechnology has the ability to not only overcome fundamental obstacles confronting present treatment methods, but also to create unique treatment capabilities that could enable cost-effective, highly efficient, and adaptable wastewater treatment solutions.18–20 Yet, the application of nanomaterials as nanoadsorbents (NAs) is limited due to challenges such as regeneration, agglomeration/aggregation, and instability in the waste stream.21
The assessment of internal surface area in adsorbents holds vital importance and should not be overlooked, in conjunction with the control of external surface areas of nanomaterials. As a result, there has been a considerable amount of academic research conducted on the advancement of porous adsorbents, including activated carbon, charcoal, mesoporous silica, and zeolites. The primary objective of these investigations is to tackle the issue of water remediation needs.22 But the challenge of attaining the desired attributes, such as adjustable pore size, form, functionality, particle size, and morphology, in these porous materials has presented significant difficulties. In recent times, there has been significant progress in the advancement of reticular porous materials, particularly metal–organic frameworks (MOFs). MOFs,23 covalent organic frameworks (COFs),24 hydrogen bonded organic frameworks (HOFs),25etc. have seen an exponential growth of interest in a variety of science disciplines. In this regard, the science of linking molecular building blocks using bonds as glue to create extended crystalline structures is called reticular chemistry.26,27 Reticular chemistry has also had an impact on the development of advanced water purification technology, owing to its architectural, chemical, and thermal stability, all of which could be combined to preserve the porous nature of reticules as well as their hydrolytic stability. Starting from the discovery of an extended crystal structure of manganese germanium sulfide reported by O. M. Yaghi and co-workers in 1994,28 reticular chemistry has developed into one of the fastest growing fields of science that is being currently practiced over hundreds of countries worldwide.29 The synergistic combination of large surface area, adjustable pore size, and surface design capabilities can effectively address the prevalent issues encountered in water remediation using membrane technology.
Adsorbent materials are frequently utilised in the process of water purification to eliminate contaminants. Nonetheless, it is important to acknowledge that these materials do possess certain constraints. Powdered nano or micro materials occasionally depend on chemical coagulants or flocculants, hence resulting in increased operating expenses.30 Furthermore, extracting powdered components from treated water might be difficult, potentially resulting in recontamination.31 Membrane technology, on the other hand, provides significant advantages. Membranes act as selective barriers, enabling water molecules to pass but preventing contaminants from passing.32 This methodology ensures comprehensive and reliable processing, leading to the production of uncontaminated water without the need for further chemical substances. In addition, membrane technology possesses a reduced carbon footprint, exhibits a modular architecture, and offers enhanced control over the treatment process, rendering it a favored solution for desalination and wastewater treatment.33
The utilization of polymer-based membrane technology presents a promising solution for addressing diverse remediation challenges, including but not limited to air purification and wastewater treatment, as evidenced by recent research conducted by Goh et al.34 Tan and Rodrigue conducted a study on the production of polymeric membranes. These membranes are created using materials that can be handled in solution. Subsequently, they are shaped into several membrane morphologies, including fiber mats, hollow fibers, and flat sheet membranes.35 Accordingly, the research for materials that work well together as better adsorbents for membranes has led researchers to look for new ways to combine materials science and membrane technology.36 Hybrid nano composites have better qualities than their pristine counterparts alone. Functional nanomaterials attached to a membrane structure improve the membrane stability, functionality, tunable rejection, mechanical strength, fouling liability and beyond.37–40 Among the various manufacturing techniques, electrospinning produces high-quality fibres with a three-dimensional network, making it ideal for filtration. Electrospun nanofibrous membranes (ENMs) have outstanding mechanical properties, a high flux, up to 80% porosity, and configurable pore sizes in the sub-micron range. Because of their large external surface area combined with increased mass transfer aided by their internal surface areas, including porosity, ENMs provide a substantial advantage. This characteristic allows for more effective interactions with the environment, increasing mass transfer efficiency and aiding successful filtration operations. Furthermore, because of their tunable pore sizes and high surface-to-volume ratio, ENMs are well suited for microfiltration applications and constitute attractive candidates for pollutant exclusion. Core–shell, porous, and hollow nanofibers with high porosity and low tortuosity could be synthesized using the process of electrospinning.41,42
Nevertheless, the utilization of polymeric membranes in wastewater treatment poses several significant drawbacks that must be addressed. These include challenges such as unstable morphology, limited reusability, and the potential for fouling, along with inconsistent or non-uniform filtration.43 These issues demand careful attention and innovative solutions to optimize the performance and durability of polymeric membranes in wastewater treatment processes. Furthermore, fouling control is another difficulty to be addressed in most of the membrane-based separation applications.44 Feed composition and foulant characteristics, as well as hydrodynamic circumstances, draw solution composition, and membrane orientation, all impact fouling in membranes.45–47 Antifouling modifications include polymer brushes, zwitterions, ultra-hydrophilic nanomaterials, etc.48–50 The integration of nanomaterials into pre-existing membranes is expected to enhance the efficiency of wastewater treatment due to their distinctive attributes, including but not limited to their hydrophilicity, thermal stability, surface roughness, hydraulic stability, heightened permeability, fouling control, and superior selectivity.4
This comprehensive review article offers a detailed examination of the selection and utilization of new and efficient adsorbent materials in the realm of membrane technology within the current global context. The article commences by analyzing the extent of the market and the notable influence of membrane technology in the context of wastewater cleanup. Subsequently, the focus shifts towards the methodologies employed in the manufacturing of membranes, with particular emphasis on the application of synthetic approaches. Significantly, there exists a specific focus on the development of environmentally friendly and readily scalable engineered nanomaterials. The study also provides a comprehensive analysis of recent developments in material selection and their subsequent application in hybrid membranes. Moreover, this current research explores the incorporation of diverse materials, such as transition metal dichalcogenides, (TMDs), graphene, and graphitic carbon nitride (GCN), and offers an in-depth examination of their respective influences. Moreover, this research extensively examines the utilization of reticular porous materials, specifically metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), as advanced additives in membranes. The primary focus is on evaluating their efficacy in the removal of dyes and heavy metals from wastewater, as well as their potential application in desalination processes. Furthermore, this scholarly review article explores the matter of biofouling in membrane-based water filtration systems. This statement emphasizes the potential of zwitterionic materials as a promising solution to address and alleviate this difficulty. The present discourse provides an elucidation of the idea of biomineralization, emphasizing its importance in the process of water remineralization subsequent to water purification. This paper provides a comprehensive and instructive analysis of the advancements achieved in the utilization of fillers as material selections and the prospective trajectory of membrane technology in the field of water treatment.
Membrane technology for water treatment and its market and upcoming techniques
In an era characterized by rapid technological progress, there exists a compelling imperative to modernise conventional as well as nascent separation methodologies. Furthermore, it is imperative to acknowledge the current obstacles in the realm of separation, as they significantly contribute to the preservation of resources and the attainment of sustainable development. The purpose of this paper is to assess the current obstacles in fundamental separation processes and propose a plan for future investigation in the advancement of innovative separation technologies as alternatives to obsolete approaches.The use of traditional chemical substances in the process of membrane manufacturing poses a significant threat to the preservation of the environment, hence raising concerns regarding the classification of membrane technology as an environmentally friendly separation alternative. Consequently, there exists a necessity to transition membrane production techniques towards environmentally sustainable alternatives, characterised by reduced utilization of hazardous solvents and adherence to principles of sustainability.51–55 Furthermore, in order to optimize the effectiveness of the separation process, a range of modifications and manufacturing techniques have been employed with regard to the membrane. Significant enhancements in membrane performance have been achieved by modifying surface properties using various techniques, including plasma etching,56 thermal crosslinking,57 and oxidation.58 Furthermore, the direct integration of various nanoparticles, such as MOFs,59 SiO2,60 TiO2,61 graphene oxide (GO),62 zeolites,63 and MXenes,64 during the electrospinning process has exhibited promising outcomes in terms of improving membrane rejection and augmenting flux.
Two factors distinguish water-treatment membranes: action mechanism and pore size (Fig. 1). Membrane separation technologies are classified as pressure-driven microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), etc.65,66 In this regard, the estimated market size of MF membranes was expected to reach 12.8 billion USD in 2022, while the market for UF membranes is projected to reach 5.5 billion USD by 2025. In the same timeframe, the market size for RO membranes is anticipated to reach 13.5 billion USD, and the market for NF membranes is predicted to be around 950 million USD.67,68
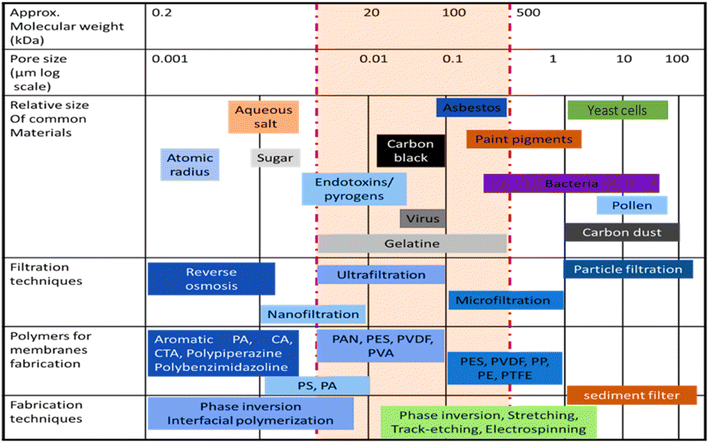 | ||
| Fig. 1 Comparison of the filtration technique spectrum of RO, NF, UF, and MF, based on size exclusion. | ||
The MF method is used to remove turbidity, color, and pathogens. The MF technique can reduce the greatest amount of turbidity (more than 97%) while restricting just around 10% of natural organic matter. UF can remove practically all microorganisms and suspended particles with pore dimensions ranging from 0.01–0.1 μm. Due to the wide pore size of MF and UF membranes, the transmembrane pressure required for operation is minimal, resulting in low energy consumption. For the treatment of surface and groundwater, MF and UF have been widely used. NF membranes are highly flexible, cost-effective, and simple to manufacture. Polymeric membranes and ceramic membranes are the two most common types of NF membranes. Polymeric membranes have a short lifetime due to their poor chemical resistance and high fouling rate. NF is known as a more advanced filtration method than UF; it can be used to separate small colloids to small molecular ions with pressures ranging from 4–20 MPa. RO is a type of reverse osmosis that operates at pressures ranging from 7–100 bar.69 RO pressure separates smaller molecules with a semi-permeable membrane (pore size 0.5–1.5 nm). RO removed 95–99% inorganic salts and charged organics. FO membranes strike a balance between selectivity and penetrating water flux. Semi-permeable membranes are used for FO feed/draw. Draw solutions have a higher osmotic potential than feed solutions. Water is transported from the feed solution to the draw solution by osmotic pressure differentials, which retain rejected solutes on the feed side and purified water on the draw side. Energy-saving FO does not require hydraulic pressure. For wastewater treatment, FO is safe and easy to clean and has a low fouling rate. FO is plagued by draw solution re-concentration, membrane selection, and internal and external concentration polarization. The membrane distillation (MD) method uses a microporous hydrophobic membrane and a temperature difference between the wastewater stream and the permeate stream to evaporate and condense water across the membrane. Only vapor can pass through MD pores, while other molecules are blocked. The MD method has been shown to remove over 96% of Ca2+, Mg2+, Fe3+, and Fe2+, and more than 99% of As3+ and As5+.
Membranes differ primarily in the material used to make them and the type of technique involved. Water treatment membranes can be made of a wide range of inorganic and organic materials, such as polymers, composite materials, and natural materials.70,71 There are two modes of membrane filtration, dead-end and cross-flow membrane filtration.72 In the process of dead-end filtration, the complete feed flow is propelled through the membrane, resulting in the accumulation of filtered materials on the membrane's surface. Dead-end filtration is considered a batch technique due to the fact that the filtration capability of the filter diminishes as materials accumulate on its surface. The presence of a consistent turbulent flow adjacent to the membrane surface hinders the build-up of substances in the process of cross-flow filtration. The membranes employed in this methodology often consist of tubular structures with an inner tube wall coated with a membrane layer. The flow velocity of the feed passing through the membrane tube is maintained at a high level in order to induce turbulent conditions and generate a significant pressure differential, which in turn facilitates the filtration process. The process is referred to as cross flow filtration due to the perpendicular orientation of the feed flow and the filtration flow. Crossflow filtration is a very efficient technique utilized for the filtration of liquid substances that possess a substantial concentration of filterable constituents.
Fig. 2a depicts the existing technologies for global water purification in terms of their economic turnover contribution to the water purification industry. Some of the reported removal efficiencies for a variety of heavy metals for four well-known technologies are shown in Fig. 2b. The entire cost per million liters of treated water is shown in Fig. 2c. Despite substantial variation in the statistics, it becomes clear that techniques including adsorption and ion exchange tend to give the most cost-effective options for water treatment. To offer a comprehensive explanation, it is imperative to differentiate between investments and operational costs, which are detailed in Fig. 2d and e, respectively. Based on the provided data, it is clear that the two examined techniques require the least quantity of initial capital investment out of the five under consideration. In terms of operational expenses, the sorption process is also notable for its cost-effectiveness. Fig. 2f depicts the average quantities of energy required for the operation of these techniques, which are supported by research. Consequently, the quantitative evaluation of emerging effluent technologies and the diversity of their sources constitute a major global concern.
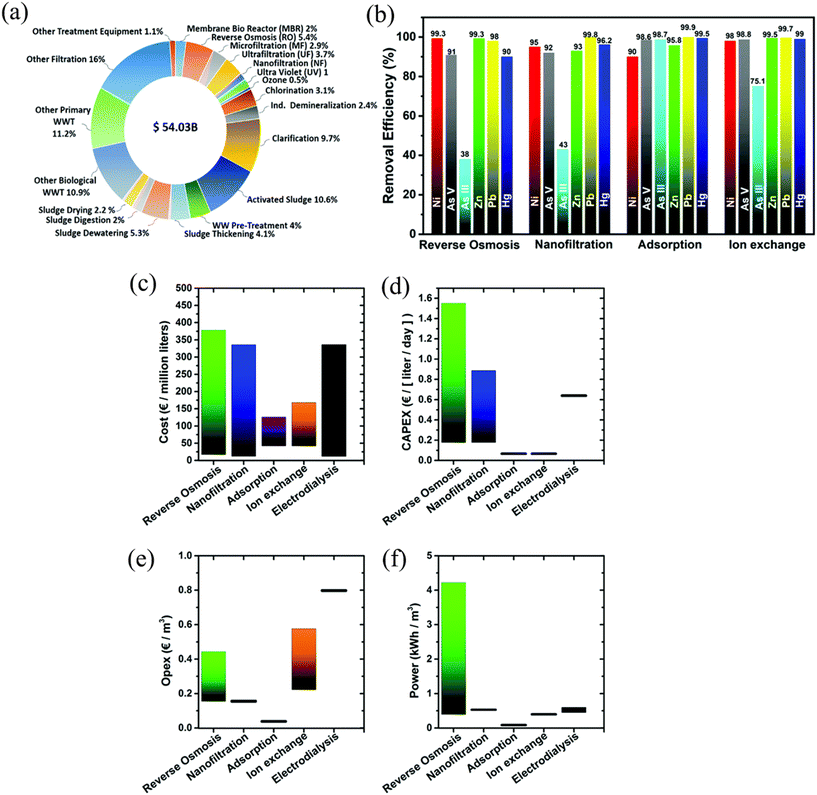 | ||
| Fig. 2 Current water purification technologies: (a) by global economic turnover by sector; (b) by efficiency (up to the first decimal) for a selected number of technologies and a small representative selection of heavy metal ions; cost (upper and lower limits) of wastewater and drinking water treatment technologies: (c) total cost per volume of treated water, (d) installation costs, (e) operating expenses and (f) energy consumption [reproduced from ref. 73 with permission from The Royal Society of Chemistry, copyright 2023]. | ||
Natural colors or dyes originating from plants and animals are thought to be environmentally friendly. The market was then revolutionized by the discovery of the synthetic dye mauvine in 1856. The industrial sector is anticipated to use more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different dyes and pigments, with over 7 × 107 tons of synthetic dyes generated annually.74 Synthetic dyes are widely available, inexpensive, and colorfast. Table 1 also contributes to the basic characteristics of today's salt and dye removal membranes.
000 different dyes and pigments, with over 7 × 107 tons of synthetic dyes generated annually.74 Synthetic dyes are widely available, inexpensive, and colorfast. Table 1 also contributes to the basic characteristics of today's salt and dye removal membranes.
| Entry | Membrane | Membrane materials | Producer | Permeability (L m−2 h−1 bar−1) | MWCO (Da) | Salt rejection | Dye removal efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | NF 90 | Polyamide (PA) thin film composite (TFC) | Dow Filmtec | 5.3 | 200–400 | NaCl 90–96% | 100% Remazol Turquoise Blue G, Remazol Yellow GR and Lanaset Blue 2R | 77 |
| MgSO4 98% | ||||||||
| 2. | DK | PA thin film | Osmonics | 3.3 | 150–300 | MgSO4 98% | 100% | |
| 3. | Desal-5 DK | Polysulfone (PSF)/PA | Osmonics | 5.1 | 150–300 | NaCl 17.1–47.8% | Reactive black 5 (RB5) | 78 |
| 4. | NF 20 | TFC | Sepro | 4.1 | NaCl 10.7–34.8% | — | 79 | |
| 5. | NF 270 | PS | Dow Filmtec | 13.3 | 270 | NaCl 7–21.8% | — | |
| 6. | ESNA-1-LF | meta-Phenylene diamine-based PA | Nitto Denko Hydranautics | 4.25 | 100–300 | NaCl 29–94.8% | — | |
| 7. | TFCSR | PS/PA thin film | Koch | — | — | NaCl 58–64.3% | — | 80 |
| 8. | TR 60 | PA spiral wound | Toray | 3.9 | 400 | NaCl 23–86.3% | — | 81 |
| 9. | Sepro NF 6 | PA | Ultura | 13.7 | 862 | NaCl 2.6–17.9% | 99% direct red 80, direct red 23, and Congo red (CR) | 82 |
| 10. | Sepro NF 2A | PA | Ultura | 10.5 | 493 | NaCl 6.9–33.3% | 99.9% | |
| 11. | K-5 | PA | KEKI, Hungary | ∼5 | 500 | — | 76% | 83 |
| 12. | NP010 | PES | Microdyn Nadir | >5 | 1000 | MgSO4 35–75% | 64.7% cyanidin-3 | 84 |
| 13. | NP030 | PES | Microdyn Nadir | >1 | 400 | MgSO4 80–95% | 89.8% cyanidin-3 | |
| 14. | NTR-7450 | Sulfonated PES | Nitto-Denko | 14.6 | 600–800 | Sucrose retention 80% | 95% molasses | 85 |
| 15. | TS-40 | PA | MEY | 5.2 | 400 | Sucrose retention 80% | 96% | 86 |
| 16. | SW30HRLE-400 | PA TFC | Dow Filmtec, USA | — | — | NaCl 99.8% | — | 87 |
| 17. | HB10255 | CTA hollow fiber | Toyobo, Japan | — | — | NaCl >99.4% | ||
| 18. | NFX | Proprietary PA TFC | Synder, USA | NaCl >40% | ||||
| MgSO4>99 | ||||||||
| Lactose >99% |
The use of artificial dyes is crucial in various sectors, such as textile manufacturing, dyeing processes, paper production, pulp industry, tannery operations, and paint applications. Dyes, which are organic substances, exhibit significant water solubility, hence presenting difficulties in their removal and control. Polar dyes, in particular, demonstrate a notable inclination to cling to polar surfaces of fabrics with a considerable level of easiness. According to Katheresan et al.,75 the textile industry accounts for approximately 55% of the dye effluents that are released. During the normal process of dyeing textiles, approximately 80% of the dye molecules are absorbed by the fabric while the remaining 20% are released into the surrounding water. The presence of pigments in water impedes the penetration of light, resulting in a decrease in the rate of photosynthesis and the concentration of dissolved oxygen, which impacts the entire aquatic ecosystem. Furthermore, dyes are carcinogenic and toxic substances that can spread throughout the food chain. Therefore, expedient measures and innovative technologies are required for the effective removal of dyes from effluent. It has been determined that adsorption is the most effective method for dye removal due to its high removal efficiency and low manufacturing cost. The adsorption process is dependent on the constitution of the dyes, which can be categorized as either ionic or nonionic. The efficacy of ion exchange depends on the adsorption and subsequent release of dyes.76
Another key socioeconomic challenge that developing countries must address is a sizable rural or distant population. One of the most significant impediments to the nation's adoption of such technology is consumers' capacity to acquire more advanced membrane-based filtering systems. Installation of regulated decentralized water treatment plants to serve a local population is one technique for addressing clean water shortages in rural areas. Furthermore, a pre-treatment system utilized before membrane filtration improves the efficacy and lifespan of the membrane by reducing fouling. Table 2 lists the various membrane systems used in developing countries. According to the literature, a range of over 200 synthetic and natural polymers have been utilized in the creation of nanofiber membranes through the electrospinning process.88 The aforementioned membranes have been employed in diverse separation and filtration applications, including but not limited to the elimination of dyes and heavy metals, separation of oil and water, capture of toxic gases, and removal of bacteria.89–91
| Type of technology | Pre-treatment (capacity) | Nations | Source of water | Characteristics | Ref. |
|---|---|---|---|---|---|
| UF | NA (not applicable) (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 ML d−1) 536 ML d−1) |
Malaysia | Surface water and groundwater | Cost-effective, 92–100% efficient heavy metal removal | 92 |
| Gravity driven UF | NA (5000 L d−1) | South Africa | Groundwater | Elimination of all coliform bacteria (from 2419.2 to 7 cfu/100 mL), E. coli, and enterococci membrane demonstrates prolonged durability | 93 |
| RO and desalination | Double media sand using 2.5 μm cassette filter | South Africa | Borehole water | RO TDS was reduced from 1292 ppm to 24 ppm, and nitrate-nitrogen from 42.5 to 0.9 ppm | 94 |
| Low pressure UF | NA | South Africa | Surface water | Clean water requires 100–150 kPa pressure in UF systems. Effective turbidity and coliform removal | 95 |
| AGMD (air gap MD) | MF (552 L per day) | Vietnam | Seawater | Using 87 kW h m−3 of water, produce 46 litres per hour of distillate without fouling or moisture issues in membranes | 96 |
| MF, UF, NF, RO, FO and MD | Purification | Vietnam | Wastewater and seawater | A membrane is compact, modular, and efficient | 97 |
| NA | |||||
| NF and ED hybrid process | Electrodialysis | Vietnam | Surface water | Small water plants in rural Vietnam can use ED-NF. Water quality exceeds VN norms | 98 |
| NA | |||||
| Decentralized membrane filtration | Purification | Southern India | Household wastewater and membrane-filtered | Fecal coliform bacteria were reduced by membrane filters. The setup and maintenance costs are affordable | 99 |
| NA | |||||
| NF and RO | Adsorption and coagulation | India | Pesticide contaminated surface water | Hardness, chemical oxygen demand, total organic carbon, and microbes decreased with NF | 100 |
| NF | NA | India | Arsenic contaminated water | NF eliminates arsenic by 99.80%, meeting WHO standards | 101 |
| UF | Sand mesh of 25 μm and 150 μm | Mozambique | Freshwater | To meet microbiological regulations, the permeate tank must be post-chlorinated before distribution. Permeate flux has not changed | 102 |
| Ozonation submerged ceramic MD and UF | Mesh with 50 μm (5 m3 h−1) | Thailand | Freshwater | Multi-step process ensures virus- and pathogen-free water. Compact treatment ideal for decentralized sites | 103 |
| RO-desalination | Coagulation, degasification, and a dual-media filter | Indonesia | Brackish water | Solar or reverse osmosis can treat groundwater. Modular methods that are cost-effective and easy to modify and maintain | 104 |
| Hollow fiber UF | Purification (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 d−1) 000 m3 d−1) |
China | Reservoir | No organic leakage in 7 year operation, confirming UF membrane effectiveness | 105 |
| UF | Coagulation | China | Raw water | Effectively eliminates metals, coliforms, and turbidity | 106 |
| NA | |||||
| RO and NF-desalination | NF | Turkey | Seawater | NF as pre-treatment for seawater RO solves scale, cuts costs, boosts yield | 107 |
| NF | Precision filter (20 m3 d−1) cation exchange resin, sand filters | Sri Lanka | Ground water | In permeation, NF plant reduces hardness, fluoride, and organic carbon | 108 |
| RO-desalination | NA | Brazil | Brackish water | The desalinated water had 94% rejection rates for SO42−, 97% for TDS, and 100% for F− | 109 |
The fundamental driving force behind the creation of high-performing and durable membrane materials is the fine-tuning of surface shape and/or chemistry through in situ or ex situ alteration processes. The alteration of membrane materials takes place simultaneously with the production of the membrane in the process of direct membrane material development. Following the formation of the membrane, the subsequent step involves the alteration of the said membrane. Enhancing the wettability of a membrane, as quantified by the contact angle exhibited by a liquid droplet (such as water or oil) placed on the membrane's surface, is the principal strategy employed to alter its surface chemistry. The significance of membrane porosity and pore size distribution holds considerable importance within the realm of efficient wastewater treatment. The performance of the membrane is affected by a range of factors associated with the chemical properties and composition of the feed, including pH, ionic strength, and the presence of foulants. Furthermore, there are concerns regarding the wettability and antifouling properties of the membrane.
The total membrane resistance (Rtot) is used to describe fouling in membranes.
| Rtot = Rm + Rc + Rp + Ra + Rcp |
Synthetic strategies to form membranes
Polymer membranes possess the capacity to comprise either naturally occurring or artificially synthesized polymers, including but not limited to polysulfone, polyether sulfone, polyacrylonitrile, polyvinylidene fluoride, polyamide, polyimide, and various others. There are three distinct classifications for membranes: hollow membranes, core–shell membranes, and functionalized membranes. The cylindrical form of the hollow fibre membranes is characterized by a porous nature. The water that permeates is able to travel through the wall, but the supply water runs through the fibre. Hollow fibre membranes are utilised in several separation processes, encompassing microfiltration, ultrafiltration, nanofiltration, reverse osmosis, forward osmosis, pressure retarded osmosis, membrane distillation, and hybrid forward-reverse osmosis. The hollow fibre membrane has significant attributes, including a substantial surface area, little fouling propensity, effective module manufacturing, and low energy consumption. A core–shell membrane consists of two layers with contrasting characteristics. The shell layer separates and performs distinct functions, whereas the core layer provides mechanical strength and stability. Electrospinning, interfacial polymerization, self-assembly, and coating are a few of the techniques that can be used to create core–shell membranes. Improving the layer structure and composition of core–shell membranes increases their efficacy and longevity. Functionalized membranes are distinguished by the strategic attachment of functional groups or nanoparticles to their surfaces. It has been demonstrated that functionalized membranes improve many properties, including selectivity, affinity, catalytic activity, antifouling capabilities, and biocompatibility. Several methods, including grafting, coating, combining, doping, and embedding, can be used to produce functionalized membranes. In wastewater, functionalized membranes can adsorb, degrade, segregate, and detect contaminants. There has been significant research of novel substances in the field of potential membrane materials, including graphene and waste styrofoam. The method is chosen based on numerous aspects, including the polymer used, the desired features and structure of the membrane, as well as the economic and environmental ramifications of the procedure. Membranes are made using four different synthetic techniques: (1) electrospinning, (2) phase inversion, (3) interfacial polymerization (IP), and (4) track-etching. Fig. 3 depicts a methodical construction strategy.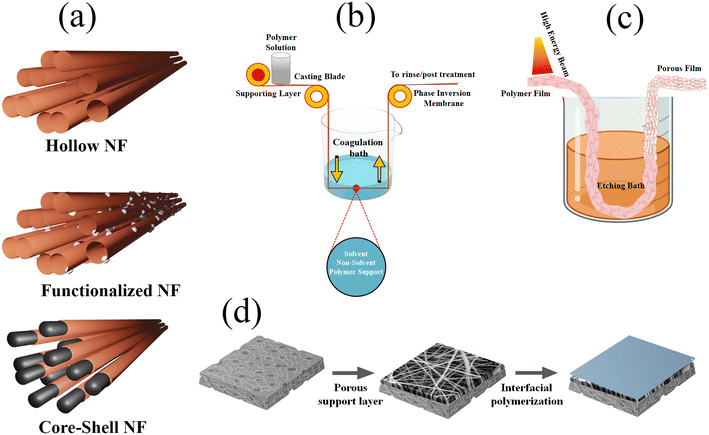 | ||
| Fig. 3 Schematic representation of (a) electrospun nanofibers with varying (hollow, functionalized and core–shell) structures, (b) PI process, (c) track etching process and (d) IP process. | ||
Electrospinning technique
Researchers have focused on the applications of nanofibers and their composites in this field, as well as current trends in energy conservation and environmental cleanup, since they have achieved some major results with diverse materials and designs along the road. It is a low-cost, high-efficiency electrohydrodynamic technology for creating micron, submicron, ultrathin, and nanoscale electrospun polymers. Electrospun fibres have a low density, a high porosity, with pore diameters ranging from nano to micrometre domains. Furthermore, the ability to modify their thickness, length, area-to-volume ratio, surface chemistry, and composition enables us to customise their attributes to specific applications. The constructed ENMs were thought to be excellent platforms for combining filtration and adsorption procedures with high purification efficiency in order to remove organic contaminants and heavy metal ions from contaminated water. As a result, scientists are excited about the prospects of developing revolutionary nanofiber designs. The electrospinning process involves a grounded collector, such as a metallic needle or capillary tube, to be used in the electrospinning process to collect the ENMs. The collector is connected to a high-voltage power source (5–30 kV), which creates a strong electric field (typically 1–5 kV cm−1) between the needle and collector. An infusion pump infuses the polymer solution through the tip by pressing a syringe piston or tubing to hold the solubilized or melted polymer.65–67 The formation of electrospun fibers is caused by a high-voltage electric field on the surface of polymer solution droplets, which causes a liquid jet to be ejected through a spinneret. The surface charges interact with the external field, causing the droplet to orient into a Taylor cone.68 This is primarily due to two significant electrostatic forces (the electrostatic repulsion of similar charges and the coulombic force of the external electric field). As soon as electrostatic force overcomes surface tension, a charged polymer jet is ejected from the tip of the Taylor cone. As the jet is propelled towards the collector, the unevenly distributed charges cause whipping or bending motion. Solid polymer fibers are collected as a randomly or oriented electrospun mat on the grounded collector because of the elongation of the jet and the rapid evaporation of the solvent.69,70 The collector's orientation has an effect on the electrospun fiber alignment. It also has an impact on the shape and properties of the nanofibers produced. There are plain plate collectors, drum rotatory collectors, grid type collectors, and edge type collectors. As shown in Fig. 3a, the process settings could be adjusted to produce hollow, core–shell, porous, and yarn-like electro spun nanofibers.71,72 These ultrathin nanomaterials can be several centimeters long and can be made by variety of techniques, including uniaxial and coaxial spinnerets. Furthermore, using such innovative approaches and scalable routes to create novel materials may reduce society's reliance on traditional filtration techniques, as well as their material and energy consumption.In 1963, the invention of electric current and the formation of fibres from viscous liquid using an air stream assisted route were reported.111 One of the factors considered in the electrospinning technique is electric current. The electric current is determined by the movement of the charge carrier through the electrospinning distance. Furthermore, most charge carriers are obtained by ionising the air and exposing it to the sharp needle and polymer jet solution. The concentration of charge carriers is measured using the amount of salt in the polymer solution. Some independent parameters influence the electric current in the jet, including solution feed, relative humidity, applied voltage, solution conductivity, needle (hollow) diameter, and a few geometrical properties. The current in a jet can be calculated by using the theoretical equation mentioned below:
| Itotal ≈ EQ0.5K0.4 |
Recent developments in the field have demonstrated that both pristine and functionalized electrospun polymeric nanofibrous membranes have enormous potential in water treatment applications. These membranes may consist of natural and synthetic polymers, functionalized polymers, and (inorganic/organic) additives.
In light of the current global environmental crisis and the escalation of water, it is essential to develop innovative ENMs with enhanced efficacy. Electrospinning has a number of limitations that must be addressed in order to address environmental concerns. These limitations include bulk productivity, tip obstruction during fabrication, unwanted repulsive forces leading to waste in the case of multiple syringes, electrostatic charge disturbance, and the requirement for real-time active system monitoring of parameters to ensure reproducibility. This matter has been highlighted in recent studies by Li et al.112
Fuat Topuz et al. fabricated a very effective adsorbent platform composed of hyper crosslinked cyclodextrin networks (HCNs) based on ENMs. After being treated with acidic methanol, the HCN membrane was employed to scavenge textile dyes and polycyclic aromatic hydrocarbons from polluted water, and it displayed great sorption performance (Qmax = 692 mg g −1 dye) and remarkable reusability.121
Additionally, polymeric ENMs are also found to be excellent adsorbents for toxic heavy metal adsorption. For example, Li et al. created a chitosan-based poly(methyl acrylate) electrospun membrane for the removal of chromium Cr6+ from polluted water in another research study.122 Aliabadi et al. developed a hybrid mesoporous membrane of polyethylene oxide (PEO) and chitosan nanofibers using the electrospinning technique.123 The study showed that the high surface area (312.2 m2 g−1) of the PEO/chitosan-based ENMs was responsible for the selective adsorption of heavy metal ions. The measured removal efficiency was in the order 89%, 82%, 72%, and 68% for Pb2+ < Cd2+ < Cu2+ < Ni2+, respectively. The charge density of metal ions decreases as the ionic radius increases. As a result, the number of active sites on the membrane available for adsorption decreases, leading to a decrease in the efficiency of removal. Yang et al. prepared a functionalized chitosan electrospun membrane using poly(glycidyl methacrylate) (PGMA) and polyethyleneimine (PEI) and utilized it for heavy metal removal.124 The study showed maximum adsorption capacities of 138.96, 69.27 and 68.31 mg g−1 for Cr6+, Cu2+, and Co2+, respectively. Zhao et al. developed a branched PEI embedded polyacrylonitrile (PAN) nanofibrous membrane using electrospinning.125 The study showed potential for Cr6+ removal from water with improved hydrophilicity of the membrane. An adsorption capacity of Cr6+ as high as 637.46 mg g−1 could be obtained. The adsorption capacity increased along with decreasing fiber-diameter. In another study, Zhao et al. prepared a phosphorylated PAN membrane using electrospinning and further modified it using chemical grafting.126 The membrane exhibits high adsorption affinity towards selected heavy metal ions. The adsorption amount was calculated to be 98.06, 78.03, 102.40 and 18.89 mg g−1 for Pb2+, Cu2+, Ag+ and Cd2+, respectively. Karim et al. used the electrospinning method to create ENMs from PVA/chitosan (PVA/Chi) for selective and high adsorption of Pb2+ and cadmium Cd2+ ions depending on the acidity of the solution.127 The manufactured ENMs have proven to be a superior material due to their high functionality and numerous active sites for interaction with Pb2+ and Cd2+ ions that provided a high adsorption capacity of 266.0 and 148.0 mg g−1, respectively.
Hence, along this line of discussion, it could be well understood that ENMs can act as superior adsorbents owing to their capillary action. Furthermore, the tunability of their fiber diameter along with the wide choice of polymeric backbone makes them highly desirable in the field of water purification. Additionally, it is simple to functionalize the pristine polymeric ENMs to create hybrid membranes that can target a particular pollutant to be adsorbed from wastewater sources in addition to amplifying their physicochemical qualities.
For example, novel hollow α-Fe2O3 nanofibers of rice-like nanorods were successfully fabricated by Gao et al. on an electrospun PVA nanofiber template using a simple hydrothermal process followed by calcination.135 Furthermore, hollow α-Fe2O3 fiber arrangements exhibited a magnetic response as well as effective methyl orange (MO) dye adsorption in water. Du et al. created a conductive CNT nanofiber hybrid hollow fiber membrane (CNT-HFM) by coating and crosslinking CNTs on an electrospun PAN hollow nanofiber support layer with considerable permeability and mechanical strength enhancements.136 The CNC-HFMs had 3.06 and 12.7 times the tensile stress and Young's modulus of commercial phase inverted HFMs. The CNC-HFMs also had 7.3 times the permeability of commercial materials. The membrane removed turbidity and total organic carbon (TOC) efficiently. According to the study, the synergy of positive electro-assistance and single membrane separation reduced fouling on the CNC-HFMs and enhanced turbidity and TOC removal efficiency. Another study by Xizi Xu fabricated MgO/PAN functional core–shell nanofibers for the removal of copper with a maximum adsorption capacity of 354 mg g−1 at pH 5.137
Mousa et al. used the electrospinning process to create a coaxial nanofibrous membrane.138 In their study, they employed hydrophilic cellulose acetate (CA) as the core and hydrophobic polysulfone (PSf) polymers as the shell for creating a core–shell membrane, supplemented with 0.1 wt% ZnO nanoparticles. The membranes were also treated with 2 M NaOH to increase hydrophilicity and hence water separation flow. The mechanical parameters of the polymeric membranes in terms of Young's modulus, tensile strength, and toughness are higher than those of the NaOH-treated membranes. Furthermore, the membranes have strong antimicrobial properties and have successfully isolated water from oil–water effluent. Moreover, when compared to untreated membranes, the water flux for the modified membranes was increased by 1.6 times. Tijing et al. designed and prepared a superhydrophobic PVDF-co-hexafluoropropylene nanofiber membrane doped with CNTs by the electrospinning process.139 The mechanical strength and hydrophobicity of the membrane depended on the concentration of CNTs. These properties were controlled by different concentrations of the incorporated CNTs. The 5% CNT concentration showed the highest water flux (24–29.5 L m−2) and high salt rejection under an external pressure of 99 kPa. Deng et al. synthesized a novel nanocomposite MWCNT–PEI embedded with PAN nanofibers.140 Their study showed higher mechanical strength and improved hydrophilicity of the membrane with 145.8 L m−2 h−1 water flux. The membrane exhibited more than 93% removal efficiency of Cu2+ and Pb2+ heavy metals.
Regarding the fact that electrospinning technology offers a fascinating perspective on the creation of novel nanofiber-based materials, a substantial obstacle remains. The current limitations of electrospinning in terms of providing reliable processing of nanofibers have resulted in instability and inconsistency. As a result, there are significant limits on existing developments and the range of spinnable fluids. Due to the presence of these impediments, there exists an important reason to enhance manufacturing efficiency with the aim of mitigating the interconnected issues posed by macro-level production limitations.
Phase inversion (PI) technique
The majority of commercially accessible membranes are fabricated using phase inversion (PI). The phase change of the dope solution or casting solution, which consists of a homogeneously dissolved polymer in a solvent, gives rise to polymer-deficient and polymer-rich phases during the solidification process, causing PI (Fig. 3b). A miscibility gap in the phase diagram is necessary for phase separation. As a result of the solution's instability in the miscibility gap, liquid–liquid separation easily creates a membrane structure with pores (derived from the polymer-rich phase). In order to create UF, MF, and RO filtering membranes, thermally induced phase separation and immersion precipitation techniques are applied.141,142 The conditions that produce solution demixing can be used to classify phase separation into three groups. PI can manufacture hydrophilic industrial membranes with high flux and long life which are easy to fabricate and have low environmental impact. With the PI method, flat-sheet and tubular membranes with controlled thickness and aperture and high porosity (around 80%) can be achieved.With the PI method, Sharma et al. fabricated a highly efficient graphene-oxide doped CA-based IEM.143 Cation exchange membranes' ion exchange capacity (IEC), fixed charged density, water uptake, swelling degree, and proton conductivity were optimized by adjusting the compositional ratio of GO/CA/polyethylene glycol. The maximum GO content led to a membrane with a proton conductivity of 0.273 S cm−1 and an ionic exchange capacity of 1.08 m mol g−1 at 30 °C (CAG3). The WCA of the modified CAG3 membrane was only 53 degrees, making it more hydrophilic. The rejection rate was low for cationic dye (20%) but high for anionic dye (92.5%). Improvements in acid recovery and selectivity for anionic dye indicate the development of a more efficient IEM with increased proton conductivity. PI was used by Bhran et al.144 to create a membrane using PVC and PVP. Increased membrane wettability and decreased filter pressure are what the hydrophobicity results show. This may be because the membrane in question has a lower WCA, a factor known to significantly impact the drop in capillary pressure experienced by filter media. Salt rejection (NaCl) by the membranes was measured at 98% at a very high flux.
Using the PI method, Aji et al. created an UF membrane from recycled polyvinyl chloride.145 In addition to a BSA rejection rate of over 90% for the neat PVC membrane, the results showed that the membrane had a PWF of 85 L m−2 h−1. The WCA was reduced by about 25%, the hydrophilicity was improved, and the equilibrium water content was increased by about 19%. Han et al. created Mg(OH)2/PES (magnesium hydroxide/polyethersulfone) hybrid membranes using synthetic PI.146 The hybrid Mg(OH)2/PES membrane showed the best performance, the pure water flux increased from 430 to 720 L m−2 h−1 MPa, the egg albumin rejection rate was 94.58%, and the recovery flux ratio was around 70%. Another study from Masheane et al. showcased the fabrication of Fe–Ag/functionalized-multiwalled carbon nanotube (Fe–Ag/fMWCNT/PES) nanostructured hybrid membranes via modified PI.147 The addition of Fe–Ag/f-MWCNTs to the PES polymer boosted the membrane hydrophilicity, thermal stability, crystallinity, and fouling resistance. Nevertheless, the Fe–Ag/f-MWCNTs did not leach from the PES membranes. In crossflow systems, adding Fe–Ag/f-MWCNTs to the PES polymer matrix increased water flux from 26.5 to 36.9 L m−2 h−1 and improved Cr6+ ion rejection up to 94%.
Interfacial polymerization (IP) technique
The process of interfacial polymerization (IP) involves the creation of ultrathin functional layers, capsules, or fibres at the interface between two phases (see Fig. 3d). Polymerization commonly refers to a polycondensation reaction that occurs between two monomers with high reactivity, which are dispersed in two liquids that are not capable of being mixed together. Due to the confinement of polymer formation inside the interface, there is an increased likelihood for reactants to encounter the expanding polymer chain rather than other monomers. Consequently, milder reaction conditions can yield larger molecular weights compared to those achieved through bulk polymerization. The phenomenon of polymer precipitation at the interface can manifest within a certain range of molecular weights, leading to a particular polydispersity that differs from that observed in bulk polymerization. The characteristics of the resulting polymer are significantly impacted by the reactivity and local concentration of the monomers, the stability of the solvent interface, and the quantity of reactive groups present on each monomer. The reaction of di-, tri-, or multi-functionalized monomers results in IP. A schematic diagram of the process is shown in Fig. 3d. The IP process can be used to create membranes by using various polymers such as PANI, polysulfonamides, polycarbonates with controlled mean size and mechanical and chemical stability.148,149 The PHGH–TMC/PSF composite membrane had a water flow of 17.2 L m−2 h−1 and a MgCl2 rejection of 86.2%. This composite membrane rejected MgCl2 > MgSO4 > Na2SO4 > NaCl. Moreover, the composite membrane could separate tiny organic molecules or contaminants with MWCOs of approximately 700 Da, based on the dye rejection results. Using an IP process, Kang et al. created an NF membrane with a slick surface made of PIP and trimesoyl chloride (TMC).150 The optimized fabrication conditions resulted in a membrane with high dye rejection (e.g., 99.6% for CR) and low salt rejection (e.g., 6.3% for NaCl) in addition to excellent pure water permeance (20.2 L m−2 h−1 bar−1). With a high flux recovery rate (FRR) of 87.1% and a low irreversible fouling (Rir) of 12.9% following fouling by bovine serum albumin, the membrane displayed superior antifouling properties to the commercial NF membrane (NF90). By combining IP and zwitterionic copolymer building components, Duong et al. created membranes with a selective thin PA layer.151 Kang et al. created a TFC loose NF membrane of poly(piperazine-amide) on a PSF support membrane using electrospray interfacial polymerization (EIP).150 The developed EIP membrane has excellent pure water permeance (20.2 L m−2 h−1 bar−1), strong dye rejection (99.6% for CR), and low salt rejection (6.3% for NaCl). Furthermore, the EIP membrane excelled the commercial NF90 membrane in terms of antifouling, with a high flux recovery rate (FRR) of 87.1 percent and a low irreversible fouling of 12.9% after being fouled by bovine serum albumin (BSA). NF has an opportunity to provide energy-efficient, cost-effective, and space-efficient wastewater treatment solutions for industrial sites. However, traditional TFC-NF membranes deteriorate in acidic environments, severely limiting their use in industrial wastewater treatment.152 Hongling Lan et al. fabricated a TFC membrane with a Noria–2,2′-benzidinedisulfonic acid nanoparticle interlayer using IP with a thin and crumpled PA layer, which can reduce the hydraulic resistance and improve the effective area during the separation process. The permeability of this TFC membrane (24.01 L m−2 h−1 bar−1) was achieved about 3.5 times compared to the conventional TFC membrane.153Track-etching technique
Track-etching membranes (TEMs) possess the ability to be fabricated with meticulous control over pore dimensions, morphology, and spatial arrangement. Consequently, these membranes find extensive application in various specialised domains, including biomedical and industrial separation methodologies. TEMs are created by irradiating thin polymer films with a heavy ion beam at a DC-60 heavy ion accelerator. The irradiated polymer is then chemically etched, yielding membranes with cylindrical pores ranging in diameter from 0.02–5.0 μm and pore numbers up to 1010 pores per cm2. The regular geometry of pores, with the ability to control their number per unit area and narrow pore size distribution, is a distinguishing feature and advantage of TEMs (Fig. 3c). This, in turn, improves the membranes' selectivity and specific performance. They can be made from various polymers like PPMA, PVA, and PEO and are widely used, for example, in precision UF and MF of liquid and gas cleaning, analytical substance control systems, food, pharmaceutical, and chemical industries, microelectronics, and other scientific and industrial fields.Korolkov et al. demonstrated that hydrophobic TEMs with pore sizes of 180 nm and thicknesses of 12 mm based on polyethylene terephthalate (PET) were prepared using photo-induced graft polymerization of silicon monomers such as triethoxyvinylsilane (TEVS), resulting in a WCA of up to 104°.154 Korolkov et al. performed additional hydrophobization of PET TEMs by polycondensation reaction on the membrane surface via soaking in trichloro(octyl)silane solution for separation of oil–water emulsion.155 The best pressure for maximal flux and separation efficiency was 700 mbar. Membranes with pore diameters of 350 nm and vacuum pressures of 700 mbar demonstrated fluxes of 305 mL m−2 h−1 for chloroform–water emulsions and 75 mL m−2 h−1 for cetane–water emulsions. Choi et al. fabricated track etched porous MF membranes for filtration which overcome the fouling issue in a submerged MBR system.156 They fabricated three different membranes PETE, PCTE and PTFE. Their result shows that the filtration resistance increased the fastest for the PETE membrane with the lowest PWP value, and the slowest for the PCTE membrane with the intermediate PWP value. With the greatest PWP and a microstructure resembling a sponge, the PTFE membrane demonstrated a faster increase in filtration resistance than the PCTE. This was probably because of the PTFE membrane's rougher surface, which facilitated the foulant's quicker deposition. Zdorovets et al. prepared a hydrophobized PET track etched membrane for low level radioactive waste treatment and salt rejection.157 UV-induced grafting was used to modify PET track etched with styrene and TEVS. The alteration led to an increase in the wetting contact angle (WCA) of polyethylene terephthalate (PET) track-etched membranes to 99° when the pore size was increased from 150 to 300 nm. The results indicate that the rejection rates for Cs, Sb, Mo, Al, Ca, Sr, Mg, Na, K, and Fe ions were consistently high, approaching 100% in the majority of cases.
Based on the aforementioned instances, it is evident that the utilization of diverse fabrication techniques in the creation of unspoiled membranes shows potential for their application in water restoration activities. Nevertheless, via meticulous selection of tailor-made materials, there is potential for substantial enhancement in both performance and fouling characteristics. Therefore, the integration of high-performance materials with self-supporting membranes may serve as a crucial factor in the development of cost-effective and exceptionally efficient water treatment systems.
Recent advancements in hybrid membranes for wastewater treatment
Adsorption by nanosized adsorbents has been the most studied and applied technique to treat even trace amounts of heavy metal ions due to its many benefits, including its ease of design, high efficiency, low cost, high selectivity, and low production of secondary waste.158 However, this treatment method's utility may be constrained by issues like regeneration, agglomeration, instability of the applied NAs, and the difficulty of separating the adsorbing particles from the waste stream. The complexity of industrial effluents means that no single method is currently adequate for treating wastewater effectively. In practice, a number of different treatment processes are typically used in tandem to achieve a level of water quality that is drinkable.In this regard, the incorporation of organic/inorganic NAs into the polymer matrix has led to the development of a new generation of membranes.159 The nanoparticles (NAs) possess distinct features that confer selectivity towards contaminants, while the polymer matrices serve as a supportive medium for the NAs. The behaviour of numerous interfacial atoms distributed as nanoparticles (0D, 1D, 2D) at the interface effects the behaviour of the bulk material in a system. In nanoparticles, this behaviour manifests as changes in optical, magnetic, thermodynamic, thermomechanical, electrical, and structural properties. As a result, the intended electrical, thermal, mechanical, and rheological properties of the targeted nanocomposite may be increased based on the size, shape, and composition of these nanomaterials, as well as their interactions with the host polymeric matrix. In total, adding nanoparticles to the membrane may help lower the amount of energy required, the quantity of chemicals necessary to clean the membrane, and the total operating cost. Nonetheless, they face a number of challenges, including fouling, limited flux, and the trade-off between flow and selectivity.
TiO2 and silver (Ag) with their 0D nanostructures are among the most frequently investigated metals and metal-oxides in order to address energy and environmental challenges.160–162 Peydayesh et al. constructed a composite NF membrane from polyethersulfone (PES) and ethylenediamine (ED) grafted MWCNTs.163 The membrane surface roughness, hydrophilicity, isoelectric point, and thermal and mechanical properties were optimized using different loading concentrations of the CNTs. The hybrid membrane reveals excellent heavy metal removal in the order Zn2+ (96.7%) > Mg2+ (95.01%) > Cd2+ (92.4%) > Cu2+ (91.9%) > Ca2+ (91.3%) > Ni2+ (90.7%) > Pb2+ (90.5%). Additionally, the water flux of the hybrid membrane was enhanced by 122% compared to the pure PES membrane and achieved around 80.5 L m−2 h−1. In another study, Orudzhev et al. produced a novel piezophotocatalytically active membrane based on PVDF nanofibers with α-Fe2O3 nanoparticles integrated into them via electrospinning.164 Immobilization of α-Fe2O3 nanoparticles in PVDF nanofibers improves crystallization and promotes increased self-polarization of PVDF nanofibers into the electroactive phase due to ion–dipole interphase interaction. The rate of MB breakdown during piezophotocatalysis employing α-Fe2O3/PVDF increased 4.7 times when compared to piezocatalysis due to the piezophototronic effect. Xiao-Qiong Wu et al. established an effective approach for fabricating a high-performance triple-layer (PVDF-HFP and SiNPs) superhydrophobic/hydrophobic/hydrophilic membrane for potential practical MD applications for industrial wastewater treatment.165 The triple-layer membrane exhibited stable MD performances when using real seawater and industrial flue gas desulfurization wastewater as the feed solutions, while no obvious fouling and wetting were observed even at 60% water recovery. In contrast, the TL membrane maintained a stable water flux of ∼15.1 kg m−2 h−1, indicating a better fouling resistance towards inorganic scalants of the TL membrane than that of the DL membrane. The WCAs of the TL membrane remained above 150° even after 1 h ultrasonication or 40 abrasion cycles. Similarly, Chenyi Fang et al. prepared a PVDF membrane where the surface chemistry was altered by Teflon coating that showed high WCA (142.5 ± 2.3°), low water sliding angle (25.8 ± 3.8°) and fouling/scaling resistance in the presence of salt and surfactants.166 In another study, the interlayer of the TFC membrane is improved by incorporating doping sulfonated polyaniline (SPANI) nanofibers. These nanofibers exhibit exceptional hydrophilicity and distinctive chemical characteristics. The membrane exhibits a commendable permeate flux of 35.35 L m−2 h−1 bar−1, along with satisfactory retention capacities for Na2SO4 (98%) and MgSO4 (95%).167 Cong Yang et al. fabricated a solely green TFC system that contains a biodegradable green electrospun support layer (PLA), green solvents, and green monomers (genipin–priamine) using a scalable electrospinning technique. A gelatin interlayer between the PLA support and the selective layer (genipin–priamine) was utilized to improve the compatibility of these two layers.168 Further, the robustness of the fabricated PLA support was enhanced by hot annealing, as the tensile strength and Young's modulus of the support increased from 1.62 to 4.55 MPa and from 25.36 to 60.12 MPa, respectively. The prepared green TFC membrane also exhibited excellent oil removal efficiency (99.6%) with a water permeance of 5.6 L m−2 h−1 bar−1. The incorporation of zero-dimensional (0D) and one-dimensional (1D) nanoparticles into the polymeric membrane matrix has been attempted to improve its performance. However, the lack of appropriate interfacial compatibility between the nanofillers and polymers can lead to the formation of defects, which in turn create non-selective channels. As a result, achieving a high level of selectivity becomes challenging. A noteworthy attribute of these entities is their proclivity to aggregate upon dissolution, leading to a substantial reduction in surface area. However, the objective of attaining accurate separation of molecules with similar sizes from a wide variety of solutes is a considerable obstacle when utilising zero-dimensional (0D) and one-dimensional (1D) nanomaterials. Therefore, there exists a significant need for the development of novel and advanced membrane materials that demonstrate both rapid and accurate ion/molecular transport. In the context of this paradigm, 2D nanomaterials possess structures that are atomically thin and demonstrate potential in terms of low material utilisation, quick manipulation, and extensive treatment adaptability.
2D material-based hybrid membranes
The family of two-dimensional (2D) materials has garnered significant attention as promising candidates for membrane separation, owing to their captivating physical and chemical properties. Several unique two-dimensional (2D) materials have been synthesised, such as layered double hydroxides, graphene oxide (GO), MXenes, and transition metal dichalcogenides (TMDs). The construction of membranes composed of 2D materials commonly involves the utilisation of vacuum-assisted filtering techniques. These procedures facilitate the assembly of 2D nanosheets into lamellar membranes, where the interlayer galleries function as pathways for molecular transport. The membranes composed of 2D inorganic materials demonstrate remarkable permeation properties that are selective based on size. These properties are attributed to the extremely thin nature of the membranes, the ability to adjust transport channels by modifying the spacing between layers, and the robustness of the membranes in terms of chemical and mechanical stability. Significant advancements have been achieved in the field of design and fabrication of membranes based on 2D polymer nanosheets in recent years.In TMDs, two chalcogen layers are divided by a transition metal atomic layer, resulting in a sandwich-like structure.169 Foreign molecules can easily intercalate between the layers due to their weak interlayer van der Waals contact. As a result of the simple permeability of guest molecules across TMD interlayers, efforts have been made to develop TMD-based hybrid membranes.170 In this regard, TMD materials based on MoS2 and WS2 have shown tremendous promise in hybrid membrane-based water treatment systems. For example, Liang et al. used a reverse atom transfer radical polymerization method to prepare a MoS2-PSBMA/PES composite membrane using the PI method.171 The zwitterionic PSBMA compound modified the hydrophilicity of the MoS2 nanosheets as fillers. This approach resulted in a water flux of 108.3 L m−2 h−1 at 0.6 MPa pressure. Furthermore, dyes like reactive black 5% and reactive green 19 could be efficiently rejected from water with an efficiency of 98.2% and 99.3%, respectively. However, the fabricated membrane showed insufficient rejection of salts. In another study, Yang et al. fabricated an oxidized MoS2 (O-MoS2) based TFN membrane using a PA layer for salt (Na2SO4, MgSO4, MgCl2 and NaCl) rejection from water.172 The few-layered O-MoS2 nanosheets with strong hydrophilicity and negative charge provided a theoretical foundation for developing NF membranes with good selectivity, permeability, and antifouling performance. TMD-based 2D hybrid membranes could also be beneficial for the adsorption of heavy metal ions from water owing to their chalcogen rich atomic layers. For instance, Zhao et al. prepared a MoS2 nanosheet decorated PVDF membrane to remove toxic Hg2+ ions from water with a maximum adsorption capacity of 578 mg g−1.173 In addition, the study revealed the role of sulfur in such a high adsorption capacity.
Apart from TMDs, carbon based 2D materials like pristine graphene and its analogues GO and reduced graphene oxide (rGO) are extensively studied for their use in hybrid membranes to mitigate water contaminants.174 Their excellent robustness, tunable hydrophilicity, interlayer transport channels and facile surface functionalization make them appealing as fillers in hybrid membrane technology. However, tunability in surface functionalization, hydrophilicity and surface charge is challenging for pristine graphene as compared to oxidized graphene. Furthermore, large-scale graphene production is complex, whereas industrial-scale GO production is feasible. Hence, research related to GO based hybrid membranes is more explored than that related to pristine graphene-based membranes. For example, Liu et al. fabricated about 100 nm thick layers of GO based nanocellulose hybrid membrane to reject more than 90% positively and negatively charged dye molecules (Victoria Blue 2B, methyl violet 2B and Rhodamine 6G) with a water flux as high as 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 ± 574 L m−2 h−1 bar−1.175 Additionally, the membranes exhibited significantly improved mechanical stability in both dry and wet states and exceptionally high water flux. Another study from Tan et al. demonstrated the capability of GO based membranes in heavy metal removal applications.176 According to the study, PVA/GO@PTFE hybrid membranes could effectively separate toxic metal ions like Cu2+ and Cd2+ from water with 72.6 and 83.8 mg g−1 adsorption capacities with six regeneration cycles. Moreover, GO based hybrid membranes can efficiently reject salts from water, featuring them as promising candidates for desalination.177
123 ± 574 L m−2 h−1 bar−1.175 Additionally, the membranes exhibited significantly improved mechanical stability in both dry and wet states and exceptionally high water flux. Another study from Tan et al. demonstrated the capability of GO based membranes in heavy metal removal applications.176 According to the study, PVA/GO@PTFE hybrid membranes could effectively separate toxic metal ions like Cu2+ and Cd2+ from water with 72.6 and 83.8 mg g−1 adsorption capacities with six regeneration cycles. Moreover, GO based hybrid membranes can efficiently reject salts from water, featuring them as promising candidates for desalination.177
Another type of 2D material is GCN, which has a sp2 layered structure with weak van der Waals forces.178 GCN is made up of two essential structural (triazine and heptazine) units made up of N-heterocyclic rings. The principal components are carbon and nitrogen, and at least certain portions of the structure can be compared to elongated planes of graphite. When compared to other carbon-based materials, GCN delivers outstanding properties in terms of tunable band structure, nitrogen rich sites, tunable defects and tailor-made functionalization.179 Furthermore, the evenly distributed tiny triangular nanopores (3.11 Å) in GCN allow for the preferential transport of small molecules such as H2O (2.65 Å kinetic diameter). As a result, GCN has been regarded as an advanced material with potential applications in membrane based water remediation.180 Fabrication techniques such as vacuum filtration, dip coating, blending, IP, 3D printing, and electrospinning could all be used to fabricate GCN-based membranes. Nevertheless, the relationship between the GCN morphology and membrane performance was not established in prior research. In this regard, Ge et al. constructed three different g-C3N4 nanostructures including 1D nanorods, 2D nanosheets, and a 1D/2D nanohybrid, to fill a PA layer and create hybrid TFN membranes. The 1D/2D nanohybrid like structure aided in creating more open channels. Moreover, g-C3N4 nanohybrid membranes exhibited good antifouling properties in response to BSA and silica foulants.181 In a study conducted by Shahabi et al., similar outcomes such as enhanced surface roughness and hydrophilicity improved the salt rejection efficiency of GCN-based hybrid membranes. With only 0.015 wt% of GCN nanosheets as fillers in the PA membrane, it was possible to achieve a NaCl rejection performance of up to 99.7%.182 On the other hand, owing to its rich nitrogen-rich structure GCN can improve membrane hydrophilicity, surface charge density and adsorption sites. In this regard, the adsorptive capacity of pristine membranes could be enhanced using fillers like GCN.183 Very recently, Nadig et al. fabricated a PSF based MMM using 2D nanosheets of GCN to separate heavy metal ions like Pb (95%), Cd (80%) and As (70%) from water.183
Apart from these examples, 2D materials like hexagonal boron nitrides, black phosphorous, layered double hydroxides, and MXenes have also gained a lot of attention due to their outstanding physicochemical properties.184–186 In general, recent developments in hybrid membranes based on their designs, morphologies, physical properties, and chemical characteristics display a range of performance enhancements, including enhanced water permeability, rejection of pollutants and salts, antichlorine behavior and antifouling activity. Unfortunately, despite their intriguing membrane separation properties, their ability to achieve a higher flux is severely limited by their inherent nonporousness and the ensuing excessively long permeation pathways along the nanosheet interlayers. This is primarily due to lack of vertical transport pathways which limits their water permeability performance. Although drilling with chemical etching or electron beams has created pores in these 2D materials, the pore-forming conditions are too harsh for practical use. Porous materials with tunable nanopores facilitate mass transport of smaller molecules such as water, whereas they act as molecular sieves to reject pollutants. Thus, it is anticipated that adding reticular porous materials to the polymer matrix will significantly increase the water flux and selectivity of traditional polymeric membranes. In 2019, Jiang and Wu's group developed mixed nanosheet membranes using GO and covalent triazine frameworks (CTFs) to reject organic dyes (>90%) from water.187 According to their report, exfoliated CTFs were chemically grafted with GO by forming amide bonds between amidine terminated CTFs and COOH terminated GO to form GO-CTF membranes. The GO-CTF membranes showed a thickness of 32 nm in the layered configuration that exhibited a 12-fold increment in water flux compared to pure GO membranes. As a result, novel and ground-breaking materials with intrinsically porous structures could further enhance the water flux and may provide better permeation pathways.
Reticular membranes
Over the past three decades, reticular chemistry has outgrown into two main fronts: inorganic–organic crystalline porous solids or MOFs188,189 and crystalline organic porous solids or COFs.190 Linking of two building units to form reticules are done via co-ordination bonding for MOFs with charged organic linkers and metallic clusters or secondary building units (SBUs), whereas strong covalent bonds are used for COFs. The rapid development in the discovery of isoreticular series,191 pore-wall functionalization192 post-synthetic modification193,194 and multivariate synthetic strategies195,196 has made MOFs and COFs highly desirable in the field of water remediation29,197 (Fig. 4). Adjoining the advantages of reticular chemistry and membrane technology, there exists a plethora of reports that have been published over the last decade.198–202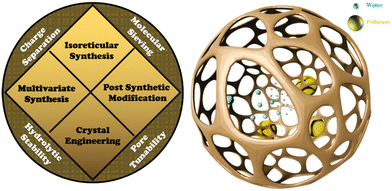 | ||
| Fig. 4 Schematic representation of the reticular-porous material-based synthetic advantages, as well as the molecular sieving mechanism in the pore channels. | ||
MOFs are a fascinating class of porous materials with numerous uses in a variety of fields.23 The synthesis of MOFs typically involves mixing metal salts and organic ligands in a suitable solvent under controlled conditions, such as temperature, pH, and time, resulting in a three-dimensional framework with high surface areas and tunable pore sizes.203 A number of techniques, including X-ray diffraction and gas sorption studies, can be used to better characterize MOF crystals. MOFs have shown potential as heterogeneous catalysts, gas storage materials, drug delivery vehicles, and sensing and imaging agents, among other applications. MOFs also represent a significant breakthrough in realizing the prospects of harvesting water from air. Notably, Yaghi et al. demonstrated that certain MOF materials, when utilized in an electrified device with frequent operation, can achieve a tenfold enhancement in water productivity.204 This research finding highlights the potential of MOFs as a promising technology for addressing global water scarcity issues. Ongoing research in MOF synthesis and applications is expected to lead to further advances and innovations in these fields.
Apart from inorganic–organic porous hybrids like MOFs, a class of highly organized, porous, and crystalline materials known as COFs are made of organic building units that are connected by covalent bonds.24 As the glue between the knots and linkers is made up of covalent bonds, the kinetics of the bond formation is faster, resulting in mostly amorphous polymeric networks. Nevertheless, the choice of linking covalent bonds is dynamic in nature and thus brings reversibility in the reaction media via dynamic covalent chemistry,205 yielding crystalline porous solids via the error–correction mechanism.206 COFs have gained increasing attention in recent years due to their unique properties, including organic skeletons, high surface area, tunable pore size and shape and pore-interface, along with excellent stability under various conditions.207 These frameworks are identified by their precise spatial structures and distribution of building blocks, forming either extended two-dimensional (2D) or three-dimensional (3D) frameworks. COFs can be synthesized through different methods, including Schiff base condensation, boronate ester formation, and beyond.208 In addition to these common linkages, other types of linkages have also been used for COF synthesis, including triazine formation, acetylene linkage formation, etc.208 The choice of linkages used for COF synthesis can influence the properties and performance of the resulting COF, making it an important consideration in COF design. These methods offer flexibility in controlling the structure and properties of COFs, enabling the design of materials for specific applications.
The formation of pores in COFs is intricately linked to the stacking of 2D layers in the case of 2D COFs or the folding and interpenetration of 3D networks in 3D COFs. These architectural features are primarily governed by non-covalent interactions, including π–π and hydrogen bonding interactions. Such interactions play a crucial role in tailoring the properties of COFs for various applications. In the realm of 2D COFs, the face-to-face stacking of sheets (AA, AB, or ABC mode) offers an interlayer distance ranging from 3.2 to 5 Å.207 This stacking arrangement allows for precise control over the interlayer spacing and thus influences the resulting porosity. Meanwhile, in 3D COFs, the intricate folding and interpenetration of networks contribute to their unique porous structures. However, the design and synthesis of 3D COFs pose challenges due to the high cost of knots/linkers and the complexity of achieving desired structural arrangements.
Researchers have investigated the functionalization of COF backbones or skeletons with various functional groups or heteroatoms to improve the function of COFs.207 This approach not only enables the specific adsorption of guest molecules or ions but also allows for the development of biomimetic systems, reminiscent of ion-pump channels such as Na+, K+, and Ca2+. This tailored functionalization opens up exciting opportunities for applications ranging from water remediation to catalysis and gas storage. Given their selective adsorption of gases like carbon dioxide, methane, and hydrogen, COFs have been proven to exhibit remarkable gas storage and separation capabilities.209 Additionally, COFs have been explored as heterogeneous catalysts, where their high surface area and ordered structure allow for efficient mass transport and high catalytic activity.210The chemical tunability, highly π-conjugated electronic structure and high surface area of COFs also enable their use in selective sensing applications for the detection of various analytes.211 Finally, because of their improved charge transport and accessible surface area for electrochemical reactions, COFs have been investigated as electrode materials for energy conversion as well as storage devices, such as batteries, supercapacitors, and fuel cells.212 However, the scope of this review article is limited to water purification. Hence, detailed discussions pertaining to other applications are beyond the scope of this article.
While both 2D and 3D COFs hold promise for a wide range of applications, the extensive exploration of 2D COFs in water remediation applications is primarily driven by practical considerations. The cost of knots/linkers used in constructing 3D COFs and the challenges associated with tailor-made design hinder their widespread application. However, ongoing research efforts continue to tackle these obstacles, paving the way for future advancements in 3D COFs.
In the future, MOFs and COFs have the potential to revolutionize the field of water remediation by providing new and innovative solutions to address pressing environmental challenges. The unique structural properties of MOFs and COFs offer several advantages for water treatment applications, including high surface areas, tailored pore sizes, and selective adsorption properties. These materials can selectively adsorb organic pollutants, including dyes, pesticides, and pharmaceuticals, due to their high surface area and functionalized pore structures. Additionally, MOFs and COFs can be modified to enhance their adsorption properties, providing new opportunities for the development of more efficient and selective adsorbents. Moreover, MOFs and COFs can be designed to have high stability and reusability, which can reduce the cost and environmental impact of water treatment processes. Looking ahead, further research is needed to fully explore the potential of MOFs and COFs in water remediation applications. This includes the development of new MOFs and COFs with tailored properties for specific water treatment applications, as well as the optimization of existing materials to enhance their performance and reduce their cost.
Utilizing MOFs for membrane fabrication
To facilitate the development of MOF-based membranes, it is imperative to satisfy certain requirements. These include achieving high water flow, effectively rejecting solutes, ensuring long-term mechanical, chemical, and thermal stability, controlling the crystallite size of MOF particles, implementing cost-effective working conditions, and employing environmentally friendly synthetic methods. In conjunction with the aforementioned parameters, the dispersibility of MOF crystallites, the aqueous stability, and the hydrophobicity/hydrophilicity of the membranes play a crucial role in achieving enhanced membrane separation performances for applications in water treatment. Metal–organic frameworks (MOFs) are predominantly synthesised in the form of microcrystalline powders, rendering them highly suitable for utilisation in packed bed systems, in contrast to membrane-based separation techniques. To date, two fabrication strategies have been evolved, namely, continuous growth and MMM based composite systems.198 Herein, we have provided a brief discussion on the MOF based membrane fabrication techniques along with their advantages and shortcomings. The detailed comprehensive methodology for the construction of these aforementioned methods is outlined elsewhere.199,213,214Continuous MOF membranes
In the continuous growth strategy, membranes are formed from pure MOF particles without the aid of any polymer binders.215 Generally, continuous MOF membranes are grown using solvothermal or hydrothermal methods along with the liquid phase epitaxy technique. In the solvothermal or hydrothermal continuous growth method, MOFs are typically formed in the presence of a substrate to support the growth phenomenon (Fig. 5a). Using this method, molecular sieving could be achieved by tuning the pore windows of the MOF materials as the MOF pores are the only possible permeation pathways being present in these membranes. However, generation of defects (cracks and voids) often leads to compromised membrane performances originating from imperfect intergrowth of the film or insufficient surface coverage on the substrate. Another shortcoming which is encountered more often is the nucleation and growth of MOFs in free solution along with substrate surfaces. This leads to wastage of huge amounts of free and unusable MOF by-products. | ||
| Fig. 5 Schematic illustration of design strategies for MOF-based membranes in liquid separation: (a) in situ preparative route; (b) blending method; (c) IP process [reproduced from ref. 226 with permission from The Royal Society of Chemistry, copyright, 2023]. MOF membrane fabrication methods. (d) One-step growth of ZIF-8 membranes on an AAO support using PDA.11. (e) Secondary development of ZIF-8 membranes (in situ). (f) ZIF-8 membrane counter-diffusion growth. (g) Asynchronous development of ZIF-8 membranes with a sol–gel method. (h) ZIF-8 membrane fabrication using the atomic layer deposition (ALD) technique. (i) Gel-vapor deposition for the manufacture of ultrathin ZIF-8 membranes. (j) An electric current drives the production of ZIF-8 membranes. (k) Microfluidic interfacial processing of ZIF-8 fibers [reproduced from ref. 227 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
Another route to obtain continuous MOF films is the liquid phase epitaxy method (e.g., layer-by-layer method) resulting in surface-attached MOFs (SURMOFs).216,217 In this method, a substrate is repeatedly exposed to the metal precursor solution and ligand precursor solution (Fig. 5c) alternately with intermedial washing. Although a higher degree of control and a smaller number of defects could be achieved by this method, SURMOF films suffer from scalability issues. Additionally, the interfacial diffusion technique has also been employed for the selective growth of continuous MOF films.218,219 In this technique, precursor solutions of both the metal and organic linker are introduced to the two opposite sides of a porous substrate (Fig. 5). Hence, the precursors meet each other by diffusion through the voids of the substrate and react, resulting in a MOF film at the interface. Furthermore, the MOF nucleation and growth only takes place at the interface of the two precursor solutions forming continuous films with improved uniformity. So far, this technique has been mostly applied to MOFs (ZIF-8, ZIF-71 and HKUST-1) that are formed under ambient conditions.
Hybrid MOF membranes
To overcome the limitations of continuous MOF film fabrication, a new strategy has been developed where polymers are combined with solid microcrystalline MOF powders as fillers to synthesize MOF based MMM composite systems.220 Notably, MMM based materials have both mechanical flexibility of polymer binders and intrinsic porosity of MOFs. To fabricate MOF based MMMs, MOF particles are generally dispersed in a polymer solution producing an ‘ink’.221 The ink is typically cast on a film by using the doctor blading or spin coating technique followed by solvent evaporation to generate self-standing membranes (Fig. 5). For this method, pre-synthesized MOF particles can be incorporated while avoiding the synthetic conditions for the fabrication of the membranes. Moreover, different types of MOFs and MOF mixtures could also be incorporated in the MMMs.222 Hence, via this method MOFs which are not known to form continuous MOF films can also be formulated to membranes. Nevertheless, there are still some challenges and limitations left to be addressed.223,224 In hybrid MOF membranes, improving adhesion between the polymeric matrix and MOF fillers is a significant challenge. Carja et al. addressed this issue by means of hybrid computational–experimental research, revealing that polymer functionalization is a reliable method for enhancing the adhesion between the pure MOF infill and the polymer matrix.225 Their research on UiO-66 MOFs as illustrative cases led to the development of MMMs devoid of defects. On the other hand, in most cases, the polymer binders are of non-porous nature resulting in reduced permeability compared to their pure MOF analogues. This challenge can be overcome by increasing the MOF loading in the MOF based MMMs. However, the increment in MOF loading can again lead to aggregation of MOF crystallites leading to poor dispersion of MOFs and creating void defects in the MMMs.Aggregation of MOF crystallites takes place when the loading% of MOFs is higher in the MOF based MMMs, which leads to low water flux and solute rejection performance, swelling and poor long term operational capability. To address this problem, there should be a compatibility between the polymer and MOF crystallites present in the MMMs. Further, the hydrolytic stability of the MOFs is another factor to consider. Originating from the labile nature of metal–ligand coordinative bonds, the sensitivity of most of the MOFs to water or moisture is a well-known problem to reticular chemists. In order to address water sensitivity issues, researchers have designed MOFs with hard–hard or soft–soft interactions between SBUs and ligand donor sites. For example, Zr4+ with oxygen donor sites of carboxylates (UiO series, CAU series, etc.)228–230 and Zn2+ with azole-based (imidazole, triazole, tetrazole) nitrogen donor sites [zeolitic imidazolate frameworks (ZIFs)] are evident in the literature.231 MOFs with these attributes are more desired for water treatment-based applications. Nevertheless, post synthetic modification (PSM) has also been used to modulate the key properties of MOF based membranes in water treatment applications.232–236 To summarize, water stable MOFs that could be used as continuous membranes or fillers in MMMs are generally applied for water treatment applications such as dye removal, heavy metal removal and desalination of saline water sources based on their NF, UF and RO-based mechanisms, respectively. Selective and careful design of monomers results in variation of pore windows and diameter of MOFs. So far, pore windows of MOFs may vary from 0.3 nm to 10 nm. Hence, MOF membranes can act in the nano/UF regime and could act as RO membranes.
Removal of dyes from water
Zeolitic imidazolate frameworks (ZIFs) have garnered significant attention in the realm of metal–organic framework (MOF) based membranes for water remediation. This is primarily due to their straightforward synthesis process, narrow pore size distribution, enhanced hydrophilic properties, and notable stability when exposed to neutral water conditions. In 2014, Zhang et al. developed a coordination-driven in situ assembly for the fabrication of MOF hybrid membranes.237 In their study, ZIF-8/PSS was grown in situ into a NaOH modified PAN membrane using vacuum filtration. Under optimized conditions, the double layered membrane exhibited a high flux of 265 L m−2 h−1 MPa−1 and a 98.6% MB retention which were higher than those of the pristine PSS membrane (13.5 L m−2 h−1 bar−1 and 90.4%). According to the authors, the even dispersion of ZIF-8 particles in the PSS polymer, the better compatibility due to coordination of sulfonate groups with Zn2+ of ZIF-8 particles and the improved hydrophilicity of ZIF-8 resulted in the high dye-retention rate and water flux. In another study by Wang et al., a PA/ZIF-8 membrane was fabricated with the combination of in situ growth of ZIF-8 and IP.238 The authors prepared a series of PA/ZIF-8 membranes by increasing the number of in situ growths from one to four. The PA/ZIF-8 (LBL-#3) membrane exhibited the best combination of water flux and CR removal of 20.1 kg m−2 h−1 and >99%, respectively. The performance of the PA/ZIF-8 (LBL-#3) membrane was slightly higher than that of the PA membrane (99.6%) with a flux enhancement of 179%. Later on, Yang et al. used a chelation-assisted interfacial reaction (CAIR) to fabricate a ZIF-8/PEI hybrid layer on a hydrolyzed PAN substrate.239 The abundant amine groups of the PEI matrix assisted to graft the Zn2+ metal ions to provide nucleation sites for the ZIF-8 growth. The excessive growth of ZIF-8 led to reduced mass transfer, thereby compromising water permeance. The optimal ZIF-8/PEI-HPAN composite membrane achieved a high permeance of 75, 78, 97, and 115 L m−2 h−1 bar−1 for MB, CR, AF, and CV aqueous solutions, with rejections ranging from 98.9%, 99.2%, 87.2%, and 45.8% respectively, with a good long-term operation (60 h). Ting et al. constructed a ZIF-L based porous α-alumina membrane to adsorb rose bengal (RB) dye from water using a surfactant assisted method (Fig. 6).240 Moreover, the external surface of ZIF-L possessed high affinity for the dye. The use of cetyltrimethylammonium bromide (CTAB) as a surfactant greatly reduced the ZIF-L particle size which in turn facilitated the ZIF-L loading in the membrane. ZIF-8 was synthesized by the authors using varying amounts of CTAB, resulting in different zeta potentials for the respective samples. Specifically, the measured zeta potentials for ZIF-L (CTAB, 1×), ZIF-L (CTAB, 2×), and ZIF-L (CTAB, 8×) were found to be 56.6 mV, 50.2 mV, and 30.9 mV, respectively. The presence of a positive surface charge facilitated the adsorption of the negatively charged RB dye. Further, the ZIF-L (CTAB, 8×)/α-alumina membrane showed a superior water permeance of 2 × 104 L m−2 h−1 bar−1. However, the as-prepared membranes showed negligible amounts of RB adsorption. In another study, the addition of 0.3 wt% microemulsion to Ag@ZIF-8 resulted in good permeation (Jw = 222 L m−2 h−1 and FRR = 82%). When treating high-salinity wastewater, the even dispersion of Ag@ZIF-8 within the PVDF polymer matrix increased the dye retention of the mixed-matrix membranes.241 | ||
| Fig. 6 SEM images of (a) ZIF-8, (b) ZIF-L (no CTAB), (c) ZIF-L (CTAB, 1×), (d) ZIF-L (CTAB, 2×), and (e) ZIF-L (CTAB, 8×); (f) molecular structures and dimensions of the adsorbents and adsorbate; (g) water permeability during the dynamic adsorption of RB; (h) suggested microstructure of the membrane (ZIF-8 at the top, ZIF-L without CTAB in the middle, and ZIF-L synthesized with CTAB at the bottom) [reproduced from ref. 240 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
In 2018, Chi et al. identified the key factors affecting the dye adsorption performance of ZIF-L.242 The authors found that, during sorption free imidazole molecules from ZIF-L leached into water and exhibited the highest adsorption capacity for RB dye. Zhu et al. constructed a PSS modified ZIF-8 based TFN membrane in a PA layer using the IP method.243 The as-synthesized membrane showed ultrahigh retention (>99%) of reactive black 5 and reactive black 2 dyes. Li et al. constructed a robust ZIF based JUC-160 membrane (approx. 2.5 μm thick) as a molecular sieving membrane for dye molecules from water.244 The membrane possessed uniform pores which were larger than water molecules and smaller than dye molecules at the same time. Owing to the pore structure, the JUC-160 membrane exhibited higher rejection rates for different dye molecules (>99.8%) with a water flux of 100 L m−2 h−1. The as-constructed membrane was assessed for a total duration of 150 hours with uncompromised rejection rates and water flux. This indicated the superior recyclability of the membrane.
The utilization of ZIFs as fillers or adsorbents in membranes for water remediation presents certain inherent pitfalls. ZIFs typically exhibit inadequate chemical stability especially under slightly acidic conditions, thereby compromising their long-term performance and durability in water treatment applications. Excessive ZIF growth is another concern, leading to diminished mass transfer rates and consequent compromised water permeance. Additionally, fouling issues may arise, impeding the effective removal of contaminants from water. These scientific challenges underscore the imperative for continued research and development to surmount these limitations and fully unlock the potential of ZIFs in membrane-based water remediation systems.
Apart from ZIFs, UiO-66 based isoreticular MOFs have also been widely explored in the field of dye removal from wastewater owing to their high surface area, small pore aperture, high hydrolytic stability and hydrophilicity. Most of the reports related to MOF based membranes for dye removal were focused on utilizing a variety of MOFs. However, very few were focused on the loading% of MOFs in the membranes. Denny et al. achieved the introduction of MOF particles in a MMM with a high MOF loading (approx. 67%) using a wide range of MOFs [UiO-66, UiO-66-NH2, MIL-101-(Cr), MIL-101-(Fe), HKUST-1, MIL-53(Fe), ZIF-8] to prepare MMMs that could be fabricated on a large scale using PVDF as a support material.245 All of the as-prepared MMMs retained the high surface areas of the parent MOFs and were also subjected to PSM with promising results. The as-prepared UiO-66-MMMs showed >99% removal of Coomassie brilliant blue (CBB) dye from water, whereas the PVDF support didn't allow analyte solution to pass through. In another follow up study, Moreton et al. utilized styrene/butadiene polymer based MMMs with up to 90% UiO-66 loading.246 In contrast to the previous work, the SBS MMMs achieved higher MOF loadings than PVDF and also provided better mechanical tunability than PVDF (Fig. 7). Nevertheless, the MOF loading of <70% was devoid of any cracks and showed homogeneous particle size distribution over the MMMs. In addition, the authors demonstrated selective retention of CBB (60%) over MO (22%) from aqueous solutions using the 80 wt% UiO-66/SBS MMM. In 2016, Yao et al. developed UiO-66-MOF-urea based MMMs by post synthetic polymerization of UiO-66-NH2 and polyurethane oligomer to yield 50, 60 and 70 wt% loading of the UiO-66-NH2 MOF in the MMMs.247 The 70% UiO-66-urea based MMM exhibited different adsorption affinities to hydrophilic dyes (EY, RB, MG and MB) in aqueous solutions. The authors showed that the as-prepared MMMs acted as superior adsorbents to totally separate EY/MB, RB/MG and RB/MB mixtures owing to their preferential adsorption of EY and RB over MG and MB in aqueous solutions.
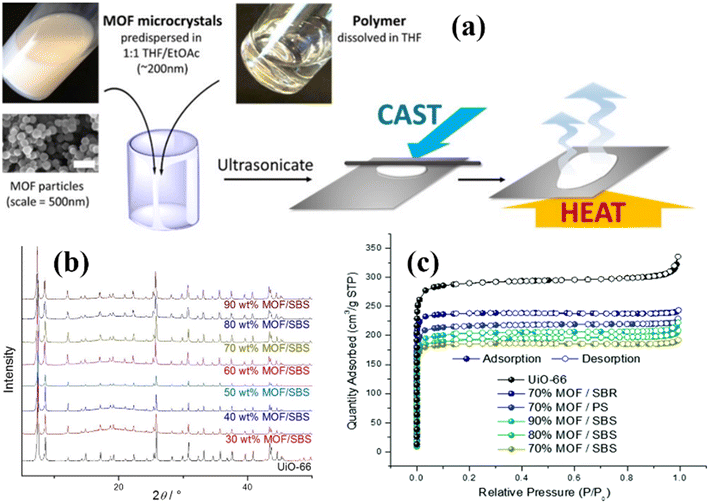 | ||
| Fig. 7 UiO-66 based MMM membranes made up of polystyrene-block-polybutadiene (SBS) polymers: (a) schematic showing fabrication techniques, (b) PXRD patterns and (c) N2 sorption studies of the UiO-66 based MMMs [reproduced from ref. 246 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
Nonsolvent-induced phase separation (NIPS) and thermally induced phase separation (TIPS) techniques are widely recognized in the realm of large-scale membrane manufacturing processes. Nonetheless, membranes produced through the NIPS or TIPS technique commonly exhibit a tendency to exclude sizable macromolecules and particulate substances such as proteins, suspended solids, bacteria, viruses, and colloids. However, they struggle to effectively segregate small organic molecules with precision. The addition of MOFs with customized functionalities and adsorption sites to NIPS or TIPS membranes may enhance the current MF and UF membrane technology. In this regard, Wang et al. reported a thermally induced phase separation-hot pressing (TIPS-HoP) strategy to produce 10 distinct MOF-membranes (e.g., UiO-66-NH2, ZIF-8, MOF-5, MOF-801, MOF-808, HKUST-1, MIL-100(Cr), BIT-72, Mg-MOF-74, and Zn-BLD) with up to 86 wt% loading of MOFs.248 The authors used ultrahigh-molecular weight polyethylene for the interweaving of MOF particles which in turn increased their mechanical strength. A CR rejection of >99.0% with 126.9 L m−2 h−1 bar−1 water permeance was achieved using UiO-66-NH2-PE MMM-92% in a cross-flow system. According to the study, even after ten CR removal cycles (5 h for each cycle), the membrane did not show any obvious compromise in the water flux and CR rejection performance. This work has shown great promise for the large-scale roll-to-roll production of MOF based membranes with easy retrofitting possibility in existing industrial processes to exclude small molecules such as organic dyes.
In addition to employing ZIFs and UiO-66, several other MOF structures including two-dimensional MOFs have been documented for the purpose of dye removal. For example, Ang et al. utilized polycationic polymers like PEI or PDDA for regulating the assembly of 2D Zn-TCP(Fe) nanosheets by crosslinking of polycationic polymers and the surface terminated carboxylic groups of the 2D MOF nanosheets.249 The lamellar NF membrane (thickness 48 nm) exhibited superior BB dye separation (>90%) with an ultrahigh water permeance of 4243 L m−2 h−1 bar−1 (2-fold higher than the control adsorbent). Moreover, the membrane prepared was exposed to BB dye separation to assess its long-term stability in the presence of the fouling agent BSA. Results revealed that the membrane exhibited exceptional stability for a duration of 8 hours, experiencing a marginal decrease of about 1% in rejection rate. Furthermore, the permeance rate reached a plateau after 1.5 hours. El-Mehalmey described an efficient pathway to fabricate a UiO-66-NH2 based MMM comprising CA as a polymer matrix for superior dye (both cationic and anionic) rejection performance.250 According to the report, CaCO3 was used as a sacrificial porogen which can be removed post synthetically to allow better water permeation by generating a network of channels inside the membrane. The formulated MMM showed superior mechanical durability, high MB and MO uptake capacity and recyclability. Zhao et al. fabricated Zr-porphyrin MOF-based (2D and 3D) photocatalytic self-cleaning TFN membranes using PAN as a substrate.251 The formulated membrane exhibited self-cleaning properties owing to its photocatalytic activity. Moreover, the optimum membrane (TFC-3D-2) showed a high water permeate flux of 110.4 L m−2 h−1 with very high dye rejection (approx. 100%) for five distinct dye molecules viz. MB, RhB, CR, DR 23, and RB 5. The Zr-porphyrin MOF-based TFN membrane consisting of 1D triangular channels with improved hydrophilicity and surface charge resulted in the superior separation and self-cleaning ability of the membrane. Nevertheless, fabrication of membranes utilizing these processes possesses intrinsic limitations, such as substrate stability requirements during MOF growth, limited selection of mild-condition-grown MOFs, and the resulting membranes' rigidity and brittleness. Table 3 provides a comprehensive summary of recent advancements in the performance of MOF-based membranes for dye removal.
| MOF | Metal | Linker | Polymer support | Membrane | Fabrication method | Pore aperture (nm) & size (nm) | Stability | Dye and removal % | Water permeance (L m−2 h−1 bar−1) | Water flux (L m−2 h−1) | Recyclability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIF-8 | Zn | 2-Methylimidazole (Hmim) | NaOH treated PAN and poly(sodium 4-styrenesulfonate) [PSS] | ZIF-8/PSS | Co-ordination driven in situ self-assembly | — | — | MB 98.6% | 26.5 | — | — | 237 |
| ZIF-8 | Zn | Hmim | PSF as a support and PA as a coating layer | PA/ZIF-8 (LBL#3) | Layer-by-layer (LBL) | — | CR 99.8% | — | 20.1 | 238 | ||
| PA/ZIF-8 (LBL#4) | CR 99.2% | — | 27.1 | 238 | ||||||||
| ZIF-8 | Zn | Hmim | NaOH treated PAN as a support and PEI as a coating layer | ZIF-8/PEI-HPAN | Chelation assisted interfacial reaction (CAIR) | — | 60 h operation | CR 99.8% | 78 | — | — | 239 |
| MB 98.9% | 75 | |||||||||||
| AF 87.2% | 97 | |||||||||||
| CV 45.8% | 115 | |||||||||||
| JUC-160 | Zn | 2-Methylbenzimidazole and benzimidazole | α-Alumina support | JUC-160 membrane | Seed mediated secondary growth method | 0.33–0.36 nm | Soaked in HCl, NaOH and boiling water, for 7 days | CR 99.8% | — | 102.3 | 50 h of continuous operation with negligible change in water flux and dye rejection | 244 |
| CBB R250 99.8% | — | 98.6 | ||||||||||
| RhB 99.8% | — | 103.1 | ||||||||||
| UiO-66 | Zr | Terephthalate acid | PVDF | UiO-66 MMM | Drawdown coating | 0.6 | Organic solvents, mechanically stable | CBB 99% | — | — | Two cycles | 246 |
| Zr | 2-Amino terephthalate acid | High density polyethylene (HDPE) and paraffin | NH2-UiO-66 PE MMM-86% | TIPS-HoP strategy | — | — | CR 99% | 126.9 | — | 5 h cycle for 10 times | 248 | |
| FA 99.1% | 111.4 | |||||||||||
| OG 99% | 115.9 | |||||||||||
| Cr | Benzene-tricarboxylic acid | High density polyethylene (HDPE) and paraffin | MIL-100(Cr) PE MMM-86% | CV 99% | 112.5 | |||||||
| RB 99.2% | 108 | |||||||||||
| MB 99.2% | 120 | |||||||||||
| UiO-66-NH2, MIL-100 (Cr) | Zr, Cr | 2-Amino terephthalate acid, benzene-tricarboxylic acid | High density polyethylene (HDPE) and paraffin | NH2-UiO-66-MIL-100(Cr) PE MMM-86% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
CR, FA, OG, CV, RB, MB >99% | >100 |
Removal of heavy metals from water
Metal–organic frameworks (MOFs) exhibit considerable potential as adsorbents in the context of eliminating heavy metal ions from aqueous solutions. However, the practical application of these substances is very hard due to their limited solubility in water and their tendency to adhere to equipment surfaces. Hence, immobilizing MOFs onto diverse substrates to form membranes is a promising approach.252 In this regard, various MOFs have been used as fillers to create polymeric membranes. However, the choice of MOFs according to their pore window, pore size, surface charge, functionality, defect sites, particle size and morphology plays critical roles in adsorbing heavy metal ions. Extensive investigations have been conducted to comprehend the aforementioned attributes and their relevance in addressing the challenge of eliminating heavy metal ions from water. Therefore, it is strongly recommended that the authors refer to the cited articles for further exploration and comprehension of the topic.199,253–257The most frequently utilized approaches involve increasing the number of adsorptive sites within the frameworks to enhance the capacity for adsorbing various toxic heavy metal ions. This can be achieved through several means, including electrostatic interactions, acid–base interactions, hydrogen-bonding interactions, and the presence of coordinatively unsaturated adsorptive sites or defect sites. For example, Chen et al. prepared formic acid modulated UiO-66-NH2 to adsorb Cr6+ ions from aqueous solution. The pH dependent surface charge modulation resulted in the enhanced adsorption of Cr6+ ions. At pH 2, the pendant amino groups were protonated resulting in a zeta-potential of >30 mV. Hence, under the acidic pH conditions the Form-UiO-66-NH2 adsorbed 169.4 mg g−1 Cr6+ ions [HCrO4−, CrO42−], whereas under neutral pH conditions the adsorptive capacity was limited to only 28.1 mg g−1.258 Hence, this work showcases the role of electrostatic interactions in enhancing the adsorptive properties of MOFs as heavy metal adsorbents. Acid–base interactions also play a key role in the adsorption of heavy metal ions. Metal ions that are chemically hard (Fe3+, Mn2+, Co2+, etc.), specifically acids, have small sizes and are not easily polarized. On the other hand, soft metal ions (Hg2+, Cd2+, Cu+, Hg+, Ag+, etc.) are larger in size and are readily polarized. Meanwhile, Pb2+, Cu2+, Ni2+, Zn2+, etc. are considered as borderline acids. Ligands containing highly electronegative donor atoms like oxygen or nitrogen are classified as hard bases, while ligands with sulfur donors are considered soft bases due to their higher polarizability. On this basis, numerous functionalities have been designed in order to selectively capture heavy metal ions in functionalized MOF pores. Different types of functional groups such as thiols, amines, sulfamine, and others can serve as chelating agents to increase the adsorption capacity of MOF-based adsorbent materials for Hg2+. For example, Zhao et al. demonstrated that introducing cysteine moieties (pendant –NH2, –SH and –COO− functional groups) onto UiO-66 surfaces can enhance the removal efficiency of Hg2+ ions from water.259 To highlight the role of coordinative interactions between the functional groups and metal ions, Ahmadijokani et al. used an ethylene diamine functionalized UiO-66 MOF for the removal of Pb2+, Cd2+and Cu2+ ions from aqueous solutions.260 The UiO-66-EDA membrane exhibited maximum adsorption capacities of 243.90, 217.39 and 208.33 mg g−1 for Pb2+, Cd2+and Cu2+ ions, respectively. According to the study, electron exchange, electron sharing, and electrostatic and coordinative interactions between the metal ions and the abundant amine groups were key to the adsorption of the metal cations on the UiO-66-EDA surface. In addition to functional groups, metal oxo-clusters are also proven to be beneficial for the adsorption of heavy metal ions.261 Often open metal sites (OMSs) as defect sites in MOFs play a crucial role in adsorbing toxic heavy metal ions from wastewater sources. To highlight this, Xu et al. created oxygen vacancies in a UiO-66 MOF to create open metal sites that acted as active sites to adsorb As5+ with an ultrahigh adsorption capacity of 248.75 mg g−1 under neutral pH conditions.262 Also, the external surfaces of MOF particles could enhance the adsorption efficiency towards heavy metal removal from water. To highlight this, Huang et al. synthesized a ZIF-8/hyphae membrane utilizing hyphae fungus (Mucor) as a robust scaffold to immobilize ZIF-8 by an in situ growth method followed by vacuum filtration.263 According to the study, the adsorption capacities of ZIF8@Mucor-0, ZIF8@Mucor-3 and ZIF8@Mucor-4 were found to be 973.53, 1443.29 and 1013.47, respectively. Owing to its smaller size and increased active sites, ZIF-8@Mucor-3 exhibited the maximum adsorption capacity.
However, when it comes to large-scale applications, neither pristine nor modified MOFs alone are capable of effectively driving heavy metal ion-based wastewater remediation, unlike their membrane counterparts. Nonetheless, the utilization of electrospun MOF-based fiber composites offers a promising solution for wastewater treatment, particularly in the removal of heavy metal ions. Therefore, this section explores the key findings regarding the adsorptive removal of heavy metals using MOF-based electrospun nanofibers. This is due to the fact that these nanofibers possess appropriate functionalities that can work in harmony to both anchor MOFs and adsorb toxic metal ions, potentially overcoming the traditional challenges associated with MOF-based membranes. As already discussed in the Synthetic strategies to form membranes section, ENMs that are produced by electrospinning have been widely explored for heavy metal ion removal from aqueous solutions.264–266 In addition to their porous nature, easy separation from water, high flux, and cost-effectiveness are some key benefits being offered by ENMs. Thus, ENMs could act as supports for the fabrication of MOF based nanofiber composites for the removal of heavy metal ions from water (Fig. 8). MOFs are generally fabricated into ENMs by direct electrospinning in the presence of MOF nanoparticles or in situ growth of MOFs on ENM substrates.267,268
Recent advancements in this research area have been focused on introducing various kinds of MOFs onto different polymeric nanofibers using the aforementioned strategies.268 Efome et al. fabricated a zirconium-based MOF-808/PAN composite membrane using electrospinning and studied its removal efficiencies for the filtration of Cd2+ and Zn2+ ions from water.269 A MOF loading of 20% could be achieved in the composite membrane which could remove almost 60–70% of the Cd2+ and Zn2+ ions. In this study, the authors achieved adsorption capacities as high as 225.1 and 287.1 mg g−1 for Cd2+ and Zn2+, respectively. Meanwhile, the pristine PAN membrane without MOF-808 as a filler adsorbed 21.6 and 4.9 mg g−1 for Cd2+ and Zn2+, respectively. In their study, the authors demonstrated that as compared to conventional thermal activation, the ‘hydractivation’ method regenerated an expanded MOF without further crystal downsizing and pore shrinkage. From the same research group, another study was conducted where process parameters like feed concentration, transmembrane pressure and membrane thickness could play a vital role in determining the efficiency of heavy metal ion removal from water.270 The authors also investigated the effect of competitive co-existing ions like Na+, Mg2+, and Ca2+ on the removal efficiencies of Zn2+, Cd2+, Pb2+ and Hg2+ ions. In another study, Jamshidifard et al. synthesized a zirconium based UiO-66-NH2 MOF using microwave heating and introduced it onto electrospun PAN/chitosan nanofibrous membranes.271 The as-prepared membranes were employed for the removal of Pb2+, Cd2+ and Cr6+ ions via both adsorption and membrane filtration. In their study, under optimized conditions (pH, temperature, MOF content and equilibrium time) the maximum adsorption capacity of the PAN/chitosan/UiO-66-NH2 membrane was 441.2, 415.6 and 372.6 mg g−1 for Pb2+, Cd2+ and Cr6+ ions, respectively, and the membrane could be recycled up to 5 cycles without significant loss in water flux and heavy metal removal capacities.
Hashem et al. used filter paper as a source of cellulose fibers to graft UiO-66-NH2 nanoparticles using a one-pot strategy.272 The grafting process became facile when chloroacetic acid was used for creating carboxylate anchoring sites on the cellulose nanofibers. Together with the UiO-66-NH2 nanoparticles, the porous support rejected 78.2% of Cr6+ ions from water. Li et al. proposed biomineralization-mimetic fabrication of ZIF-8 and -67 based electrospun-silk-nanofibers (ESF@MOFs) for As5+ and Cr6+ removal from water with ion rejection >99%.273 In 2018, Hou et al. fabricated ZIF-67 based “pearl-necklace-like” composite membranes by in situ growth of ZIF-67 on a 2-methylimidazole/CA (MIM/CA) ENM.274 The BET surface area of the control membrane increased after the grafting of ZIF-67 from 6.9 to 463.1 m2 g−1. The as-synthesized composites demonstrated exclusion of 18.9 and 14.5 mg g−1 for Cu2+ and Cr6+ ions, respectively. According to the study, electrostatic adsorption and ion-exchange were the important factors to adsorb such quantities of metal ions. Nevertheless, the recyclability studies revealed that the removal efficiency decreased to 30% after three cycles, showing incompatibility in their long-term performance. Another study from Efome et al. was done by selecting two water-stable MOFs (MIL-100-Fe and MOF-808) for fabricating electrospun PAN and PVDF based nanofibrous MOF membranes (NMOMs).275 The MIL-100-Fe/PAN based NMOM showed water flux as high as 348 L m−2 h−1 with a permeance of 870 L m−1 h−1 bar−1. Owing to the high affinity between the MOFs and electrospun PVDF nanofibers, the NMOM retained its adsorption capability to 90% even after four cycles of sorption experiments. According to the study, removal of Pb2+ ions from water as high as 248.6 L m−2 h−1 bar−1 could be achieved with a membrane thickness of 560 μm. Chen et al. developed an EDTA modified MOF-808@PAN based PME membrane via the electrospinning method to remove heavy metal ions from water.276 A MOF loading of 60 wt% (PME-60) was found to be the optimum loading% for the removal of Cu2+ and Cd2+ ions with adsorption efficiencies of 81.9% and 85.5%, respectively. The as-prepared PME membranes can perform in an acidic environment with good corrosion resistance. Furthermore, the PME membrane could be easily reused by treating with EDTA-2Na solution.
As shown in Table 4, MOFs are a promising class of materials for removing heavy metal ions from wastewater. However, there are several challenges that need to be addressed before MOFs can be widely adopted for this application. One challenge is the stability of MOFs in water and under harsh chemical conditions. MOFs are typically made up of metal ions and organic linkers, and these components can be susceptible to degradation in the presence of water and other chemicals. This can lead to the loss of the MOF-structure and its ability to adsorb heavy metal ions. Another challenge is the synthesis of MOFs. The synthesis of MOFs can be complex and time-consuming, and it is often difficult to scale up the production of MOFs to meet the needs of industrial applications. MOFs must also be selective in their ability to adsorb heavy metal ions. This means that they should preferentially adsorb heavy metal ions over other ions that may be present in wastewater, such as sodium or chloride ions. MOFs should also have a high adsorption capacity, meaning that they should be able to remove a large number of heavy metal ions from a given volume of wastewater. The kinetics and mass transfer properties of MOFs also need to be improved. The kinetics of adsorption refers to the rate at which heavy metal ions are adsorbed onto the MOF surface. The mass transfer properties of MOFs refer to the rate at which heavy metal ions diffuse through the MOF's structure. Both of these properties need to be improved in order to achieve faster adsorption rates. Finally, regeneration methods that effectively remove adsorbed metal ions without damaging the MOF structure are necessary for reuse. MOFs can be regenerated by exposing them to heat, light, or other stimuli. However, it is important to ensure that these regeneration methods do not damage the MOF structure, as this could reduce its ability to adsorb heavy metal ions in the future. The cost-effectiveness of MOFs, including their synthesis and raw materials, also needs to be addressed for large-scale implementation. MOFs are typically made from expensive metal ions and organic linkers. This can make them prohibitively expensive for large-scale use. Real-world wastewater is often complex, containing a mixture of heavy metal ions and other contaminants. It is important to understand how MOFs perform in such environments. For example, some MOFs may be more effective at adsorbing certain heavy metal ions than others. It is also important to consider the effects of other contaminants in wastewater on the adsorption of heavy metal ions by MOFs.
| MOF with loading% | Metal & linker | Support membrane | Functionality in MOF | Pore size (MOF) (nm) | Heavy metals | Maximum removal capacity (mg g−1) | Equilibrium time (min) | Recyclability | Water flux (L m−2 h−1) | Water permeance (L m−2 h−1 bar−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MOF-808, 20 wt% | Zr4+ and 1,3,5-benzene tricarboxylic acid | PAN ENM on PVDF ENM | –COOH, –OH | — | Cd2+ | 225.05 | 180 | 4 cycles | — | 139.2 ± 22 | 269 |
| Zn2+ | 287.06 | ||||||||||
| UiO-66-NH2, 10 wt% | Zr4+ and 2-aminoterephthalic acid | PAN/chitosan ENM on PVDF | –NH2, –OH | 0.9–1.3 | Pb2+ | 441.2 | 60 | 5 cycles | 452 | — | 271 |
| Cd2+ | 415.6 | 463 | — | ||||||||
| Cr6+ | 372.6 | 479 | — | ||||||||
| ZIF-8, 36 wt% | Zn2+, 2-methylimidazole | ESF | –Imidazolyl nitrogen, –CH3 | — | As5+ | 50 | — | — | — | — | 273 |
| ZIF-67, 34 wt% | Co2+, 2-methylimidazole | ESF | –Imidazolyl nitrogen, –CH3 | — | Cr6+ | 15.4 | 1400 | — | — | — | |
| F300, 20 wt% | Fe3+, BTC | PAN | –COO− | 2.1 | Hg2+ | 229.66 | 120 | 4 cycles | 348 ± 25.8 | 870 | 275 |
| PVDF | Pb2+ | 148.13 | — | — | |||||||
| MOF-808, 20 wt% | Zr4+, BTC | PAN | –COO− | 0.5–1.8 | Hg2+ | 276.96 | — | — | |||
| PVDF | Pb2+ | 170.74 | — | — |
The challenges described above can be addressed through interdisciplinary collaboration between chemists, engineers, and materials scientists. By working together, new MOFs can be developed that are more stable, selective, and cost-effective. By overcoming these challenges, MOFs could become valuable tools in tackling water pollution and ensuring cleaner water resources.
Removal of salts from water
Na+/K+ ion pumps actively transport sodium ions (Na+) out of cells while bringing in potassium ions (K+), maintaining cellular balance. Using ATP energy, these pumps create an electrochemical gradient, essential for nerve impulse transmission and cellular processes. The pump has binding sites for both Na+ and K+, allowing them to be transported against their concentration gradients. These voids (made out of protein self-assembly) ensure that the correct ions are transported in the appropriate ratios, maintaining the appropriate ion balance necessary for cellular function and signaling. The precision and specificity of these voids are crucial for the pump's overall effectiveness and cellular homeostasis. Reticular porous materials with systematic voids can also a play key role in specifically binding salt ions from saline water leading to desalination. However, a close scrutiny of specific functionalities, pore size, aperture, etc. should be considered.In the last two decades, the rapid development and advancements in the field of MOFs have led researchers to utilize MOFs in various water treatment processes. Despite synthesizing countless MOFs, their application to wastewater treatment is limited to only water stable MOFs among which very few could be used for removing salts from water. Until now, the selection of MOFs to be applied in water treatment has mostly relied upon water stability, pore size and aperture along with hydrophilicity/hydrophobicity. According to the discoveries made so far, UiO-66 and ZIF-8 are the most effective MOFs for separating monovalent ions from water. Their robust nature and high-water stability allow them to be operational for longer times. On the other hand, their smaller pore apertures allow them to retain the dissolved ions in the voids.
MOF based membranes have shown potential for desalination processes and their water desalination capability was investigated by Hu et al. by a molecular simulation study.277 ZIF-8 was investigated in this study because of their thermal/chemical stability and water/moisture tolerance. The computational study was performed using NaCl solution having concentration equivalent to seawater. The very small pore apertures of ZIF-8 membranes (3.4 Å) were successful to sieve Na+ or Cl− and allowed transport of water through the membrane. However, it has to be reminded that in contrast to experimental conditions, the simulated study was done without taking the defects of the membrane into consideration. In 2015, another simulation study was conducted by the same research group, using a series of ZIFs as potential RO membranes for seawater desalination.278 The ZIFs were ZIF-25, -71, -93, -96, and -97 with similar topology but varying in imidazolate organic linkers with different functional groups. The presence of different functional groups resulted in variation of aperture size and polarity difference in the ZIFs which in turn affected the desalination performance. For instance, the smaller aperture size of ZIF-93 and -97 (3.5–3.7 Å) demonstrated decreased water flux compared to ZIF-25, -71 and -96 (5.1–5.5 Å). Also, the authors have shown by analyzing the radial distribution functions that the framework affinity for water follows the trend ZIF-25 < -71 < -96 and the trend for water flux follows the order ZIF-25 > -71 > -96. In conclusion, the most hydrophobic ZIF-25 showed the highest water flux. This simulation study from Gupta et al. showed that ZIF-25 could be a potential RO membrane for water desalination.
The group of Liu et al. was one of the first groups to develop an alumina based UiO-66 membrane in 2015.279 The authors fabricated UiO-66 MOFs on hollow alumina fibers using an in situ solvothermal approach. Outstanding multivalent ion rejection performance (86.3% for Ca2+, 98.0% for Mg2+, and 99.3% for Al3+) with moderate permeance (0.14 L m−2 h−1 bar−1) and good permeability (0.28 L m−2 h−1 bar−1 μm) was exhibited by the as-prepared membrane. In particular, originating from the better water stability of UiO-66 MOF particles, the membrane showed long term operational stability even after 170 h of operation. In another study, Duan et al. employed ZIF-8 nanoparticles on a PA layer to investigate the desalination performance of the membrane. The increased interaction between PA and ZIF-8 could eliminate non-selective voids and thus maintained good rejection of NaCl (98%) with better water permeance (162% better than only PA membrane) under brackish water RO conditions. Recently, Bonnett et al. synthesized zirconium based PCN 222 MOF nanorods (300 nm) and incorporated them into an RO based TFN membrane.280 According to this work, MOFs containing relatively larger pores increased the water flux whereas at the same time decreased salt rejection. Nevertheless, PSM could achieve better tunability of the pore sizes of MOFs and hence contribute to better salt rejection with reduced water flux. In their seminal work, myristic acid was employed for the modification of PCN-222 MOF particles which could achieve >95.5% salt rejection with almost 100% water flux. Park et al. introduced a H2SO4 treated HKUST MOF into a PSF substrate to fabricate RO membranes.281 The agglomeration tendency of the MOF particles was reduced after acid treatment and finally the surface roughness of the substrate was lowered. The acid modified membrane has shown a 33% increment in water flux with uncompromised salt rejection performance. The membrane fouling resistance was also improved displaying stable permeance under longer operational conditions.
While most of the studies were focused on substrate-MOF compatibility or pore tunability of MOF particles to increase water flux and salt rejection, studies on the effect of MOF particle size were infrequent. Lee et al. demonstrated the effect of ZIF-8 particle size on the desalination performance of the ZIF-8/PSF membrane.282 The authors synthesized various sizes of ZIF-8 nanoparticles (60, 150 and 250) on the microporous PSF substrate. Employing different ZIF-8 sized particles, the interfacial area of the PA matrix and ZIF-8 particles affected the membrane performance. Analysis of AFM studies showed that the 150 nm sized ZIF-8 possessed the highest ZIF-8/PA interfacial area. This result can be attributed to the balance between the outside surface area of the nanoparticles and surface coverage by ZIF-8 based on the size of the MOF. Also, the size of the ZIF-8 nanofillers could affect the IP process which in turn influences the RO performance. The ZIF-8 (150 nm)/PA TFN membrane showed the highest water permeance of 3.95 L m−2 h−1 bar−1 with the highest NaCl rejection of 99.2% when compared to the pristine TFC and other sizes (60, 250 nm) of ZIF-8/PA TFN membranes.
MOF based membranes could also be utilized to sieve divalent cations of saline water like MgCl2, Na2SO4, MgSO4, etc. by the NF technique. For instance, Liu et al. post synthetically modified UiO-66-NH2 by palmitoyl chloride (UiO-66-NH2-PC) to increase the dispersibility of nanofillers within the PA layer in cyclohexane medium (Fig. 9).283 The aggregation tendency was drastically reduced due to the polarity enhancement with the organic solvent after modification by palmitoyl chloride. In their study, the pure water permeance was found to increase from 8.1 to 12.4 L m−2 h−1 bar−1 with 95% Na2SO4 rejection performance. All the as-prepared membranes showed negative zeta potential and exhibited rejection properties in the order MgSO4 > Na2SO4 > NaCl following the mechanism of NF membranes. The modified UiO-66-NH2 based membrane offered better membrane performance with outstanding long-term operation for continuous filtration of 80 h.
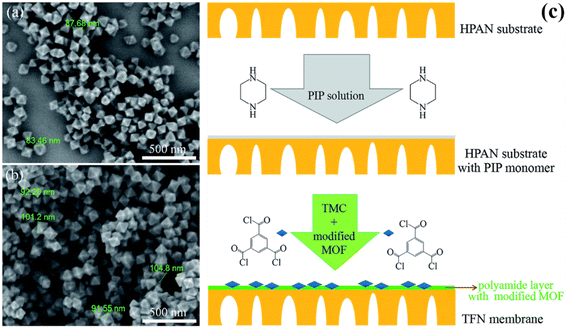 | ||
| Fig. 9 FESEM images of UiO-66-NH2 (a) and UiO-66-NH2-PC (b) along with the schematic representation of the preparation process of UiO-66-NH2 based TFN membranes [reproduced from ref. 284 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
In most of the TFC NF membranes, the permeance and selectivity are primarily based upon the active PA layer. Efforts have been directed towards the enhancement of permeance of the PA active layer without compromising the selectivity. To address this, tailoring of intrinsic properties of the active layer is necessary. This could be achieved by the judicious design of the polymer structure itself or with the introduction of hydrophilic nanomaterials into the layer. In addition to that, an alternative strategy could be to make ultrathin active layers to decrease the water transport resistance. Following these strategies, Wang et al. reported a novel strategy on the nanostructure mediated IP process by using preloaded PDA/ZIF-8 nanoparticles as sacrificial templating materials supported on a porous SWCNT/PES membrane.285 IP was used to fabricate the PA layer on supported PD/ZIF-8 nanoparticles. After the completion of the IP process, the synthesized NF composite membrane was exposed to water to remove PD/ZIF-8 nanoparticles by dissolution to form a thin PA layer consisting crumpled nanoscale structures. Additionally, fine tuning of the crumpled surface morphology and the surface area could be achieved by controlling the loading% and particle size of sacrificial nanoparticles. By this strategy, the authors could achieve significant water permeation as high as 53.2 L m−2 h−1 bar−1 with 95.2% Na2SO4 rejection. Furthermore, owing to the geometric structure of ZIF-67, the use of it as a sacrificial templating material has contributed towards a larger effective surface area. This in turn affected the water permeance and a water flux of 1831 L m−2 h−1 was achieved with 97.2% Na2SO4 rejection. In another study, Zhao et al. used three different water stable MOFs (MIL-53(Al), UiO-66-NH2 and ZIF-8) to fabricate TFN NF membranes by both blending and preloading IP methods.286 The introduction of MOFs successfully decreased the degree of cross-linking and increased the membrane thickness, surface negative charge and roughness of the PA active layer. MIL-53 was found to interact more strongly to the PA layer than UiO-66-NH2 and ZIF-8. Stronger interaction along with MOF hydrophilicity could explain the variation in permselectivity of the as-prepared TFN NF membranes. In comparison to the control TFC membrane, the MOF incorporated TFN membranes showed better water permeability without compromising their permselectivity to reject NaCl (>40%). Among the three membranes, UiO-66-NH2 showed the highest permeability, almost 1.3 times higher than the other two MOF (MIL-53, ZIF-8) based TFN membranes.
Following this, the applicability and practicality of MOF based membranes are found to be more promising than those of commercial membranes. However, the large-scale synthesis of defect free MOF-based membranes still remains a challenging task. As the literature suggests, MOFs as fillers are more widely applied for water remediation than continuous MOF membranes. Hence in order to enable the purification of significant quantities of waste or saline water, it is necessary to synthesize MOFs on a large scale which is a challenging task to accomplish. This is due to their high production cost, which is mainly associated with the high cost of starting materials, solvents, and energy consumption required for their synthesis.287 The high costs of MOF production are also attributed to the absence of efficient production methods for large-scale manufacturing and the difficulties encountered in reproducing the desired MOF properties at such a scale.287
Although various synthesis methods have been proposed to address these issues, such as continuous flow synthesis, microwave-assisted synthesis, mechanochemistry and the spray drying technique, the feasibility of these methods for industrial-scale production is yet to be demonstrated. Moreover, the lack of a standardized synthesis protocol and the need for skilled personnel to carry out the synthesis further increase the cost of production. Despite these challenges, there is growing interest in developing low-cost synthesis methods for MOFs and COFs to enhance their economic viability. For instance, most of the MOFs reported to date are synthesized in DMF solvents at elevated temperatures. Some of them even require prolonged time duration to ensure crystallinity. In this regard, attempts have been made to use low-cost starting materials and solvents, such as biomass and water, waste plastics, etc. for the synthesis of MOFs. The market size of metal–organic frameworks (MOFs) is experiencing substantial growth, driven by the contributions of key industry players such as BASF, MOFapps, MOF Technologies,287 Framergy Inc., Lumtec, etc. The collective efforts of these companies have propelled the commercialization and utilization of MOFs and COFs across various sectors.
Utilizing COFs for membrane fabrication
Previously, crystalline porous materials with uniform pore sizes like MOFs and zeolites have shown remarkable water treatment performance over conventional membranes based on amorphous polymers. Nevertheless, ultrathin membranes based on MOFs are tedious to synthesize via the IP approach as both the organic and inorganic parts belong to the same solvent. Even, layer-by-layer stacking is difficult to perform for MOFs as they are mostly 3D in nature. Additionally, the vulnerability of MOFs at varying pH is another drawback for the use of MOFs as membranes for water treatment applications.Recently, COFs have emerged as one of the best alternatives to overcome these existing problems.190 The inception of COFs and COF based thin films and membranes can solve the problem of attaining high permeability with good selectivity. COFs, which were discovered by Omar M. Yaghi and co-workers in 2005, have shown tremendous prospects in water treatment and separation processes so far.288 Considering the mighty possibility of structural designability, easy tunability of pore dimensions, excellent stability under harsh conditions, high surface areas, and tailored functionalities at external surfaces and pores, low density COFs could be a breakthrough material of this century (Fig. 10).289–291 So far, a wide diversity of COFs has been explored involving three broad classes: imine-based, triazine based, and boron-based COFs.208
 | ||
| Fig. 10 Schematic representation of water filtration of polluted water using the molecular sieving mechanism by a covalent-organic framework based membrane. | ||
Separation processes involving membranes contribute to small carbon footprint and consume less energy than adsorption-based technologies. Considering the advantages that membrane-based technology could provide, a surge in reports regarding COF based membranes can be found in the literature starting from 2009.200 Mostly, COF based membrane fabrication involves IP, layer-by-layer stacking and in situ growth (Fig. 11). The detailed methodology for the fabrication of these aforementioned methods is summarized elsewhere.200–202,292–294 So far, a plethora of reports on COF based membranes are found in the literature; however, shortcomings like the chemical instability, complexity in synthesis and scalability still remain to be addressed.
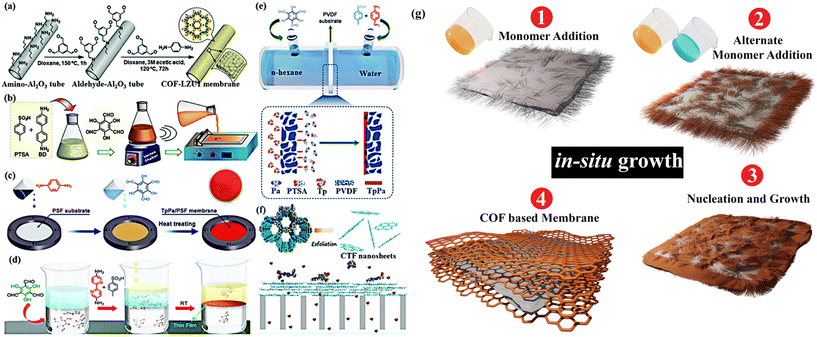 | ||
| Fig. 11 Schematic diagrams of the various approaches for the fabrication of ultrathin COF membranes: (a) creating tubular COF-LZU1 membranes through an in situ solvothermal synthesis approach. (b) Crafting COF membranes using the blade-casting technique. (c) Immobilizing COF membranes onto polymeric substrates through interfacial polymerization. (d) Self-standing COF membrane production through liquid–liquid-phase interface-assisted polymerization. (e) Producing COF membranes via unidirectional diffusion synthesis. (f) Employing vacuum assistance to assemble single-layered CTF nanosheets into membranes. (g) Schematic representation of in situ grown COF based membrane. [reproduced from ref. 227 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
Additionally, most of the reports related to COF based membranes as adsorbents are based on the discovery of new structures, PSM, molecular sieving and the charge separation mechanism.187–189,295,296 Very less is explored on their mechanical behaviour or film-to substrate adhesion strength. To address this, Banerjee's group has recently reported the underlying structure–mechanical property relationships of porous and crystalline frameworks for the construction of COF based thin films and membranes using self-assembly of crystalline COF particles.297 The authors have also shown that the mechanical characteristics of COF based thin films are majorly regulated by packing efficiency, i.e., the quality and quantity of the defects.
The challenges associated with the large-scale fabrication of COFs are even more different than MOFs. Dynamic covalent chemistry plays a crucial role in the crystallization of COFs, as it facilitates the formation of bonds and the transition from amorphous to crystalline phases, leading to the repair of structural defects. However, the solvothermal synthetic strategy to yield high-quality COFs using dynamic covalent chemistry and error–correction mechanisms can be difficult without prolonged reaction times, organic solvents, and complex freeze–pump–thaw cycles. In this regard, the Banerjee research group has addressed this challenge by demonstrating a scalable, time-saving method for synthesizing highly crystalline COFs using a mechanochemical approach.298,299 Apart from this, the choice of solvents greatly affects the quality of the produced COFs. In this aspect, water is not commonly used as a solvent for COF synthesis because organic monomers have very low solubility in aqueous solutions. Nevertheless, the idea of synthesizing water-based COFs is ground breaking because the production of water as an end-product has the potential to facilitate the dynamic and reversible formation of imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bonds in an aqueous environment.300 By using an inexpensive catalyst like p-toluene sulfonic acid and solvent like water, crystalline COFs with high surface area can be formed in just 60 seconds.299 The extruder-based “terracotta process” shows promise for scaling up COF synthesis; however, it may not be suitable for synthesizing ionic COFs (iCOFs) and ionic covalent organic nanosheets (iCONs).
N) bonds in an aqueous environment.300 By using an inexpensive catalyst like p-toluene sulfonic acid and solvent like water, crystalline COFs with high surface area can be formed in just 60 seconds.299 The extruder-based “terracotta process” shows promise for scaling up COF synthesis; however, it may not be suitable for synthesizing ionic COFs (iCOFs) and ionic covalent organic nanosheets (iCONs).
Removal of dyes from water
Loh's group developed a salicylideneaniline-based COF (SA-COF) functionalized with chemoresponsive pores and reversible proton tautomerism which could efficiently adsorb dye molecules based on size exclusion [pore size of SA-COF, 1.43 nm], charge and functionality.301 The size of these dye molecules follows the increasing order MB < RhB ≲ Chrome Azurol S. The tiniest sized dye MB was adsorbed in just 10 minutes, whereas the larger sized dye RB took almost 60 minutes. The tendency of the SA-COF to adsorb cationic molecules at high pH and exclude them at low pH is apparent from the studies, demonstrating the charge selective separation mechanism. Removal efficiency as high as 99.8% for MB could be achieved in just 10 seconds at pH = 12 (0.01 M NaOH) and also could be selectively separated (95.2%) from anthraflavic acid using the base treated SA-COF as a filter. However, the study failed to demonstrate any recyclability studies, which constrains the material's practicality. In essence, this study showcases the effectiveness of functionalized COFs in removing dyes from wastewater, demonstrating their potential in addressing water purification needs. However, in order to tackle the large-scale demands of industrial water purification, it is imperative to adopt membrane technology. Consequently, endeavours have been undertaken to develop COF-based membranes specifically designed for the removal of dyes from wastewater.A simple methodology using the liquid–liquid interfacial technique was demonstrated by Banerjee's group for the synthesis of COF thin films with thickness control.302 The authors have synthesized Tp-Bpy, Tp-Azo, Tp-Ttba, and Tp-Tta COF thin films using triformylphloroglucinol (Tp) as the trialdehyde with di- and tritopic amines (Fig. 12). Moreover, both the Tp-Bpy and Tp-Azo COF thin films show excellent rejection values as high as 94% (brilliant blue-G), 80% (CR), 97% (AF), and 98% (RhB) and 90% (BB), 79% (CR), 99% (AF), and 99% (RhB), respectively. The long-term applicability and recyclability of the COF thin films were well established by the negligible changes in rejection performance over five cycles.
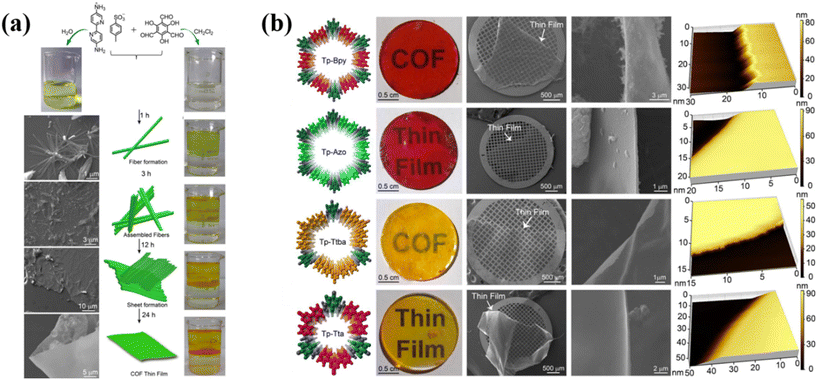 | ||
| Fig. 12 COF thin-films and their mechanism of formation from fibers to an assembled sheet like structure: (a) illustrated is the suggested mechanism for creating COF thin films (Tp-Bpy). On the left, SEM images are presented, while on the right, digital images show different stages of thin-film development, encompassing fiber formation, assembly of fibers, and sheet formation. (b) In the subsequent section, transparent COF thin films are characterized, featuring digital images on the left, SEM images in the middle, and AFM images on the right. [reproduced from ref. 302 with permission from American Chemical Society, copyright, 2023]. | ||
In their follow up work, Banerjee's group developed a scalable, simple strategy to obtain self-standing covalent organic membranes (COMs) like M-TpBD-OMe2, M-TpBD-Me2, M-TpAQ and M-TpAD by knife casting of COF precursors with a PTSA catalyst on a glass plate and then baking for 12–72 hours.303 The ordered porous framework structure of the as-obtained COMs led to high permeance of organic solvents like acetone and acetonitrile. The acetonitrile permeance of M-TpTD was almost 2.5 times higher than the pre-existing PA-based NF membranes with comparable solute rejection performance. Additionally, the ultrahigh stability of the as-prepared COMs allowed them to function in water, organic solvents and also under acidic conditions (3N HCl). More significantly, the as-prepared M-TpBD COM rejected RB dye up to 99%, whereas 94% and 96% for MB and CR, respectively, with an excellent water permeance of 120 L m−2 h−1 bar−1. Further, the COFs showed excellent recyclability of up to five cycles without a considerable drop in their performance.
In 2016, Yu and coworkers304 developed a polycationic 2D COF, with a pore size of 5.8 nm. The polycationic COF consisted of bipyridinium (BIPY) based aldehyde monomers which demonstrated high uptake (>97%) of anionic dye pollutants from water even at very low concentrations like 3.2 × 10−5 M. The uptake of the anionic dyes by the COF matrix was possible due to the presence of bipyridinium motifs in the framework where the exchange of the Cl− ions with the anionic dyes took place.
COF membranes could also be developed using a support material. For example, Caro's group synthesized a COF-LZU1 membrane of only 400 nm thickness using amine modified ceramic tubes, which not only ensured mechanical stability but also allowed better water permeance.305 The dye rejection performance of the COF-LZU1 membrane (pore size = 1.8 nm) was evaluated using different dye solutions (chrome black T (CB-T), MB, AF, CR, and RB). Rejection rates greater than 90% were observed for all dye molecules except for molecular dimensions less than 1.2 nm. Among other dyes, the lowest rejection rate of 91.4% for fuchsin was observed due to the spherical shape (1.13 nm × 1.17 nm × 1.17 nm) of the dye molecule. No notable decrease in permeation flux and rejection rate was observed over 80 h. According to their work, the COF-LZU1 membrane had superior permeation to other commercial NF membranes. Wang's group synthesized a composite COF-based membrane on PSF substrates using Tp and Pa as monomers.306 The proportion of COF crystallites was shown to be the critical aspect for developing permselective composite membranes. The resulting COF/PSF composite membrane demonstrates extraordinary rejection of CR up to 99.5%. The optimized TpPa/PSF membranes can reject MB (799.80 Da), CR (696.66 Da) AF (585.54 Da), CB-T (461.38 Da) and AO7 (350.32 Da) with a rate of 94.4%, 99.5%, 52.6%, 96.3% and 14.7%, respectively. Despite the good recyclability and stability under acidic/basic conditions, a low water permeability of 50 L m−2 h−1 bar−1 was obtained. Lai's group developed a 2D covalent organic thin film with a pore size of 1.5 nm by utilizing an amphiphilic truxene based amine and a simple dialdehyde as building blocks at the air/water interface by the Langmuir–Blodgett method.307 The 2D membrane supported on a porous PSF substrate separates organic dye molecules on the basis of their smallest projection size. The cut off molecular size is around 1.3 nm related to the membrane pore size. Thus, a molecular sieving phenomenon could be performed using the 2D COF membrane where smaller MO and RB molecules pass through whereas larger molecules like DR80 and PEG (MW 4000) could be absolutely rejected.
Charged (cationic and anionic) COFs are also desired in the field of water remediation. The intrinsic charge residing inside the pores and surfaces could increase the adsorption of oppositely charged pollutants leading to an enhanced dye rejection performance. In this regard, Ma & Li groups synthesized the first 2D cationic EB-COF:Br based membrane using ethidium bromide (EB) and Tp as the monomers for interfacial crystallization.308 The covalent organic nanosheets were restacked using a layer-by-layer assembly method to assemble a continuous and dense membrane on a nylon 66 support. The accurately prepared 2D cationic EB-COF:Br membrane displayed superior selectivity to sieve ionic pollutants of varying molecular dimensions and charges assisted with high solvent permeability. The imine linked cationic EB-COF:Br membrane demonstrated better solvent permeability performance than GO-membranes and other commercial NF membranes due to its intrinsic porous nature and hence better mass transport in the vertical direction. The cationic 1D pore channels in the EB-COF:Br based membrane successfully rejected dyes in the following order: anionic dyes > cationic dyes > neutral dyes. The recyclability studies exhibited outstanding rejection efficiency (>99%) even after six cycles of adsorption and after 10 hours of continuous processing. Moreover, the EB-COF:Br cationic nanochannels can be easily restored using 2 mol L−1 NaBr solution.
In addition, the role of functionality may often cause the performance of isostructural COFs to vary. For instance, He et al. synthesized two imine-based isostructural COF films by the introduction of H-bonding between COF crystallites, namely COF-TBDH and COF-TBDM.309 Both the COFs were synthesized using the same amine but with different functionalities present in the dialdehyde monomers namely –OH and –OCH3. Implementation of both the COF films in dye adsorption revealed the underlying role of H-bonding and indeed the COF-TBDH/nylon membrane consisting –OH groups showed outstanding performance in CR rejection (100%) and exhibited a water permeation of 4394 L m−2 h−1 MPa−1. According to the authors, the –OH groups in the COF-TBDH had an impact on both the quality of the COF film and the enhancement of rejection performance.
Creation of defects in microporous materials often leads to high adsorption capacities compared to their pristine counterparts. This enhancement may be linked to unsaturated adsorption sites, increased pore sizes and highly energetic surfaces. In this context, Siril and colleagues have devised a continuous flow-based synthetic approach for an imine-linked DAB-TFP COF that includes defects in the COF matrix (Table 5).310 These defects were discovered to be quite useful for the separation of textile industry-based MB dye. The authors demonstrated a recyclability of ten cycles without a noticeable reduction in dye removal performance.
| COF | Functionality | Pore size (nm) | Surface charge | pH stability | Removal percentage | Recyclability | Ref. |
|---|---|---|---|---|---|---|---|
| SA-COF | Imine/enamine, enol/keto | 1.43 | −ve at pH 12 and +ve at pH 1 | 1–12 | MB (98.3%, 10 min) > RB (94.1%, 60 min) ≫ CA (none) | — | 301 |
| MB (99.8%) > AA (none) at pH 12 | — | ||||||
| Tp-Bpy (thin film) | Enamine, keto & pyridinic nitrogen | 2.5 | — | pH 1 | 94% (BB), 80% (CR), 97% (AF), 98% (RH), and 96% (TB) | 5 cycles | 302 |
| Tp-Azo (thin film) | Enamine, keto & azo | 2.6 | — | pH 1 | 90% (BB), 79% (CR), 99% (AF), 99% (RH) and 96% (TB) | 5 cycles | |
| Tp-BD (thin film) | Enamine, keto | 2.2 | — | 3 M HCl | 99% (RB), 96% (MB), 94% (CR) | 5 cycles | 303 |
| Tp-TD (thin film) | Enamine, keto | 3.0 | — | 3 M HCl | 84% (RB), 80% (MB), 83% (CR) | 5 cycles | |
| PC-COF | Imine, bipyridinium | 5.8 | +ve | — | 96.9% (MO), 99.6% (AG-25), 60.3% (DFBM), 99.7% (IC), 97.8% (AR-27) | — | 311 |
| COF-LZU-1 (membrane) | Imine | 1.8 | — | pH 7, 30 days | 99.2% (MB), 98.6% (CR), 98.2% (CB-T), 99.1% (RB), 91.4% (AF) | — | 305 |
| 84.5% (RhB), <30% (MB, MO) | |||||||
| EB-COF: Br@GO (membrane) | Enamine, keto, phenanthridinium-bromide | 1.68 | +ve | — | 99.6% (MO), 99.2% (FS) and 98.1% (PP) | 6 cycles (MO) | 308 |
| 91.2% (RB), 87.2% (MB) and 84.9% (DMPD) | |||||||
| 74.4% (CAc), 22.3% (NR) and 15.7% (NA) | |||||||
| Tru(NH2)3-TPA@PSF (thin film) | Truxene | 1.5 | — | 2.4% (MO, RhB), 14.5% (BB), 46.1% (RB5), 97.8% (DR80) | — | 307 | |
| GO@CTF membrane | Triazine | 1.2 | — | pH 7–11, unstable under acidic conditions | 98.8% (EBT), 92.6% (CR), 93.3% (MB), and 93.1% (AB) | 30 cycles | 187 |
| COF-TBDH/nylon | Imine, hydroxy | 2.0 | −ve (−15.4 eV) | — | 100% (CR), 93.1% (CBB) | 5 cycles | 309 |
| COF-TBDM/nylon | Imine, methoxy | 2.0 | −ve (−13 mV) | — | 86.1% (CR), 85% (CBB) | 5 cycles | |
| DAB-TFP-flow | Enamine, keto | 0.5–1.0 | −31 mV | — | 99.3% (MB) | 10 cycles | 310 |
| DAB-TFP-batch | Enamine, keto | 0.5–1.0 | −27 mV | — | 39.4% (MB) | — |
Hybrid reticular membranes made up of both MOFs and COFs are a new area of research that has the potential to revolutionize the field of water remediation. The best of both worlds, i.e., hierarchical porous networks and hydrolytic stability, could aid their water treatment performance. Rafiee's group developed a MOF/COF composite (M5C) using MOF-5 and a melamine-terephthaldehyde based COF for the rapid removal of auramine O and RB cationic dyes from water by electrostatic and other weak interplay like H-bonding, Lewis acid–base and π–π stacking interactions.312 According to the authors, at pH 7 the adsorption capacity of M5C for AO and RB dyes was found to be 17.95 and 16.18 mg g−1, respectively.
Removal of heavy metals from water
Owing to the high surface area, hydrophilicity, and tethered functional groups, COF-based membranes could address the pressing issue of toxic heavy metal ion pollution in water. COFs could be created to focus on a specific heavy metal ion's adsorption from contaminated water using the HSAB (hard-soft acid base theory) approach. To boost hydrophilicity and get beyond the limitations of crystalline COF powders, for instance, a hydrazone-based Tp-ODH COF was constructed onto a monolithic chitosan membrane.313 With a maximum capacity of 131 mg g−1, the CM@COF membrane was able to efficiently adsorb Cu2+, outperforming Tp-ODH COF powders. Recently, a porphyrin-based COF-carbon fiber hybrid membrane was created by Jin et al. using a mechanical pressing technique.314 The porphyrin moieties showed a dual function by adsorbing Cd2+ from water and detecting it. However, the latest developments in COF-based membranes to address heavy metal pollution are still in their infancy, and the area is anticipated to expand significantly in the years to come.315 In order to illustrate how COFs may be able to remove heavy metals from water, we have solely utilised COF powders as examples. We did this by choosing some of the prime aspects that are currently accessible in the literature.Thiols and thioether functionalities are known to specifically adsorb mercury ions from water. Hence, porous COFs being functionalized with these sulfur functionalities are expected to capture Hg2+ ions from water. In this context, Wang's group was one of the first groups to report the application of fluorescent COFs to sense and remove Hg2+ ions and other metals.316 They have designed and constructed thioether based hydrazone linked COF-LZU8 where continuous π-conjugation present in the COF matrix behaves as an outstanding signal transducer. This was made possible by emphasizing the alteration in fluorescence signals and also the soft thioether nucleophilic centres as side arms to adsorb Hg2+. Hence, by applying both the bottom-up approach and PSM, the COF scaffold could provide fluorescence sensing, soft ionophores and high surface area for its utilization in heavy metal sensing and removal from water. Quenching of fluorescence emission, the resultant colour change detectable in a UV-lamp (λ = 365 nm) was found to be selective for Hg2+ among other metal cations. Thus, the easy synthesis, low cost, excellent robustness, pH independent performance and long 1D channels of COF-LZU8 with a pore diameter of 1.3 nm work in unison to outperform most of the reported materials for the related detection and removal of Hg2+ ions from aqueous solutions. Jiang's group also developed a thioether based imine linked highly robust 2D COF to remove Hg2+ ions from water.317 The structural design enhances the adsorption on the basis of large mesopores and soft-nucleophilic thioether centres that can efficiently adsorb Hg2+ ions with better selectivity and recyclability. The TAPB-BMTTPA-COF possesses benchmark mercury adsorption capacity (734 mg g−1) outperforming most of the reported adsorbents in the literature.318–320 The methyl groups attached to the thioether moieties presented in the 13C CP/MAS NMR spectra of the adsorbent COF shifted downfield after adsorption, indicating an interaction between Hg2+ and the thioether group. Additionally, the distribution co-efficient (Kd) was found to be comparable (7.82 × 105 mL g−1) to those of other state-of-the-art materials such as UiO-66-(SH)2 (ref. 318) and sulphur functionalised porous carbon.319 Moreover, pH dependent stability and adsorption are one of the key factors to be addressed while working with porous adsorbents. Nevertheless, the TAPB-BMTTPA-COF stability and adsorption capability were found to have a negligible effect over a broad pH range. The TAPB-BMTTPA-COF could be easily regenerated using 6 M HCl solution and recyclable up to six cycles with 92% capacity. Ma's group constructed COF-S-SH using simple thiol–ene click reactions by post synthetically modifying vinyl functionalised 2D imine linked COF-V with 1,2-ethanedithiol (Fig. 13a).321 COF-S-SH effectively removed both Hg2+ (1350 mg g−1) and Hg0 (863 mg g−1) from water and air, respectively, twice as much as sulphur functionalized mesoporous carbon. The superior performance of the COF is because of the intramolecular cooperativity between two sulphur atoms and one mercury atom as revealed by the XAFS study. Moreover, COF-S-SH displayed a high distribution co-efficient value (Kd) as high as 2.3 × 109 mL g−1 such that the Hg2+ concentration goes down from 5 ppm to well below the acceptable limit of drinking water (2 ppb) after just 30 min. The presence of free sulphur atoms could eliminate Hg2+, Pb2+ and Cu2+ below the U.S. Environment Protection Agency elemental limits for drinking water, within just 10 min. More significantly, COF-S-SH excluded most of the non-toxic ions like Na+, Ca2+, Mg2+, and Zn2+ creating a possibility to eliminate toxic ions in the presence of competitive non-toxic ions. According to the authors, the flexibility of the 1,2-dithiol moieties along with the appended thiol functionalities inside the porous matrix was key to this ultrahigh adsorption capacities of COF-S-SH as compared to COF-S-Ph-SH and COF-S-Et (Fig. 13b) adsorbents. The COF-S-SH adsorbent was regenerated using acetone and it was further recycled for four cycles without significant loss of Hg uptake capacity.
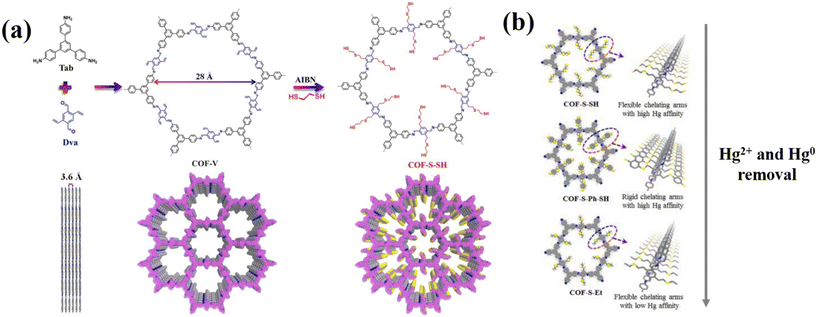 | ||
| Fig. 13 (a) Post synthetically modified vinyl functionalized COF (COF-V) for mercury removal. (b) Schematic depicting the trade-off between flexibility and rigidity of thiol linked COFs for the capture of mercury from water [reproduced from ref. 321 with permission from American Chemical Society, copyright, 2023]. | ||
In another seminal study from Mancheño's group, thiol-based TPB-DMTP-COF-SH was developed by PSM of ethynyl functionalised [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–]0.5-TPB-DMTP-COF using simple click-chemistry.322 The post-synthetically modified COF was very efficient and selective for the removal of mercury from water. Even the Hg2+ concentration as high as 10 mg L−1 (in water) has been reduced to <2 μg L−1 in just a few minutes (Kd = 3.23 × 109) in the presence of the thiol modified COF. The TPB-DMTP-COF-SH has shown a record uptake capacity of 4395 mg g−1 with a Hg2+ retention value of 99.98% in just 2 minutes. The thiol functionalised COF also showed good selectivity while capturing toxic heavy metal ions such as Sn2+ and Pb2+ in comparison with Cd2+ or As3+ ions. More significantly, TPB-DMTP-COF-SH could remove mercury ions even in the presence of non-toxic metal ions and also from seawater. Recyclability studies (using 6 M HCl) further showed that the TPB-DMTP-COF-SH COF could be reused up to four consecutive cycles without significant loss in the adsorption performance.
C–]0.5-TPB-DMTP-COF using simple click-chemistry.322 The post-synthetically modified COF was very efficient and selective for the removal of mercury from water. Even the Hg2+ concentration as high as 10 mg L−1 (in water) has been reduced to <2 μg L−1 in just a few minutes (Kd = 3.23 × 109) in the presence of the thiol modified COF. The TPB-DMTP-COF-SH has shown a record uptake capacity of 4395 mg g−1 with a Hg2+ retention value of 99.98% in just 2 minutes. The thiol functionalised COF also showed good selectivity while capturing toxic heavy metal ions such as Sn2+ and Pb2+ in comparison with Cd2+ or As3+ ions. More significantly, TPB-DMTP-COF-SH could remove mercury ions even in the presence of non-toxic metal ions and also from seawater. Recyclability studies (using 6 M HCl) further showed that the TPB-DMTP-COF-SH COF could be reused up to four consecutive cycles without significant loss in the adsorption performance.
Imine-linked COFs are one of the most explored COFs in the water remediation field. They could be broadly subclassified as β-ketoenamine linked and imine-linked COFs. Imine COFs are vulnerable towards acidic water environments whereas β-ketoenamine linked COFs are robust under acidic as well as alkaline aqueous conditions. In this regard, Li et al. has developed hydrazone linked COFs, namely TpODH and TFBODH with two different aldehydes as building blocks (triformylphloroglucinol and 1,3,5-triformyl benzene respectively).323 The TpODH COF had better crystallinity and hence greater surface area than the TFBODH COF due to extensive irreversible keto–enol/imine–enamine tautomerism and intramolecular hydrogen bonding. The presence of functional groups and the higher surface area (835 m2 g−1) of the TpODH COF were found to be the key factors for its enhanced adsorption capacities of Hg2+ and Cu2+ cationic adsorbates. The TpODH COF had high adsorption capacities of 324 and 1692 mg g−1 for Cu2+ and Hg2+ respectively, exceeding most of the earlier reported COFs. In contrast, the adsorption capacity of the TFBODH COF was found to be only 23 mg g−1 for Cu2+. TpODH was regenerated using 6 M acid solutions (HCl/HNO3) and was further subjected to Hg2+ and Cu2+ adsorption to achieve only 51 and 67% of the original adsorption capacities. This demonstrates their poor recyclability when compared to other state-of-the-art adsorbents in the literature.
Apart from the studies related to appended functionalities and different covalent linkages, the nature of the organic linkers (aliphatic/aromatic) also plays a key role in adsorbing heavy metal ions. In light of this, Liu's group developed amide-linked COFs viz. COF-TP and COF-TE from diamines and acyl chloride by mechanical ball milling at room temperature for Pb2+ removal.324 Two different kinds of diamine monomers were selected to generate both the COFs i.e., COF-TP was constructed from aromatic diamines whereas COF-TE was prepared using aliphatic diamines. This approach resulted in different adsorption capacities of Pb2+ in the COFs. COF-TE being constructed from aliphatic diamines resulted in higher adsorption capacities of Pb2+ than COF-TP. This is due to the missing π–π stacking interaction in the adsorbent, resulting in better diffusion of Pb2+ ions. Moreover, the authors showcased recyclability experiments where 1% HCl solutions were employed to regenerate the adsorbents. Remarkably, even after undergoing 10 cycles of adsorption and desorption, nearly 95% of the initial adsorption capacity could be preserved.
Most recently, Khojastehnezhad et al. demonstrated the significance of PSM of a COF (covalent organic framework) in enhancing the heavy metal removal performance.325 Initially, they synthesized an imine-linked TFPOT-PDA COF on the surface of spherical Fe3O4 nanoparticles with a particle size of 200 nm. Subsequently, a three-step PSM was carried out to incorporate thiourea units into the COF structure. These thiourea units exhibited exceptional activity as selective sites for adsorbing Hg2+ ions from aqueous solutions, achieving an extraordinarily high uptake capacity of 1400.9 mg g−1. The study revealed that other functional groups such as imine and secondary amine groups played a minor role in capturing Hg2+, emphasizing the crucial role of the thiocarbonyl group in the adsorption process. Furthermore, the adsorbent demonstrated excellent recyclability, maintaining its effectiveness for up to 5 cycles without significant loss of activity. Furthermore, the incorporation of a Fe3O4 core in the core–shell adsorbents facilitated the convenient retrieval of the adsorbents following each utilization (Table 6).
| Adsorbent | Linkage | Pore size | Functional group | Heavy metals | Maximum removal capacity | Equilibrium time of adsorption | Optimum pH | Competitive ions | Recyclability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| TAPB-BMTTPA-COF | Imine | 3.2 | Imine, thioether | Hg(II) and Pb(II) | 734 and 726 mg g−1 | 10 min | 3–4 | Zn(II), Fe(III), Mg(II), Ca(II), and K(I) | 6 cycles | 317 |
| COF-S-SH | Imine | <2.8 | Imine, thiol and thioether | Hg(II) and Hg(0) | 1350 and 863 mg g−1 | 30 min | 3–10 | Hg(II), Pb(II), Cu(II), Ca(II), Mg(II), Zn(II), Hg(0), and Na(I) | 321 | |
| TPB-DMTP-COF-SH | Imine | 2.14 | Imine, methoxy, triazole, thiol | Hg(II), Sn(II) | 4395 and 4350 mg g−1 | 2 min | 5.6 | Sn(II), Pb(II), Cd(II), As(III), Hg(II) | 322 | |
| TpODH | Ketoenamine | 0.5–1.2 | Enamine, keto, urea | Cu(II) and Hg(II) | 324 and 1692 mg g−1 | 250 min | 4 | Hg(II), Cu(II), Pb(II), Cr(II), Cd(II) | 4 cycles | 326 |
| COF-TP | Amide | — | Aromatic amide | Pb(II) | 140 mg g−1 | 25 min | 6.5 | — | 10 cycles | 324 |
| COF-TE | Amide | — | Aliphatic amide | Pb(II) | 185.7 mg g−1 | 25 min | 6.5 | — | 10 cycles | |
| Fe3O4@RCOF-EBH-TSC | Imine | 4.01 | Hydroxyl, amine and thiocarbonyl | Hg2+ | 1400.9 | 5 min | 6 | Pb(II), Zn(II), Fe(III), Cd(II), Ni(II), Mn(II), Ag(I) | 5 cycles | 325 |
Altogether, COFs as a subclass of organic polymers have shown tremendous potential in heavy metal removal from water. As evident from the above discussions, there are very few reports summarizing the benefits of COF based membranes for the separation of heavy metal ions. Nevertheless, a thorough examination of the literature reveals that the effectiveness of heavy metal adsorption is significantly influenced by various factors, namely linking units, interlayer stacking, functional groups, hydrophilicity, and surface area. Therefore, when designing a membrane based on COFs for heavy metal adsorption, it is crucial to take into account these aforementioned factors, as well as their structural stability under different pH conditions. Additionally, another important aspect that requires careful consideration is the regeneration of the adsorbents. In many large-scale processes, the use of corrosive acidic solutions or sodium sulphide solutions can introduce complexities and increase the cost of the process. In addition, it is worth mentioning that a majority of the existing literature focuses on batch adsorption processes, which not only result in compromised removal of heavy metal ions but also lead to a more cumbersome and space-intensive adsorption procedure. However, it should be noted that these issues can be readily addressed by employing continuous flow techniques, which offer a viable solution to enhance the efficiency and streamline the adsorption process. Considering the progress made in COF-particle based adsorbents so far, a surge in the development of COF based membranes for the removal of heavy metal ions could be expected in the near future.
Removal of salts from water
The shortage of freshwater resources in many regions of this world aided by the depletion of groundwater sources and water pollution makes desalination one of the best alternatives to increase freshwater supply. To date, separation processes based on RO and NF membranes are considered as the supreme substitutes for seawater desalination. However, NF membranes being capable of rejecting multivalent ions and better salt rejection with high water flux and high stability are rare. On the other hand, polymeric membranes easily suffer from fouling. Again, inorganic membranes are stable but being brittle makes them difficult to scale-up. Covalent organic framework based membranes have a substantial capability in the desalination processes owing to their high stability, ultrahigh surface area, tuneable pore sizes and surface charge as well as highly hydrophilic nature. Several COF based membranes are reported in the literature for desalination applications. TpPa-1 is an imine based microporous 2D COF that has been applied to several applications. However, in 2017, Jiang's group reported a molecular simulation study of TpPa-X COFs including various functional groups, such as TpPa-AM2(NHCOCH2CH3), TpPa-AM3(–NHCOCH2CH2CH3), TpPa-AMC2NH2(–NHCOCH2CH2CH3), TpPa-AMCOOH(–NHCOCH2COOH), TpPa-OBn(–OCH2C6H5), TpPa-OC3OH(–OCH2CH2CH2OH), and TpPa-OC4H9(–OCH2CH2CH2CH3).327 The authors defined a diameter of an assumed ball as da that can fit into the aperture of the COF pore without contact with any framework atoms. The computed da ranged from 5.17 to 7.64 Å and followed the order TpPa-AM3 ≈ TpPa-AMCOOH < TpPa-OBn ≈ TpPa-AMC2NH2 < TpPa-OC4H9 ≈ TpPa-OC3OH < TpPa-AM2. The computationally determined results demonstrated that all of the TpPa-X membranes were capable of outstanding water permeation values which were superior to most of the commercial RO membranes. Both the pore size (the larger, the better) and functionality (hydrophilic > hydrophobic) were key to the high-water flux through the membranes. Notably, TpPa-OC3OH exhibited the highest water permeance and outstanding salt rejection among others. The NaCl rejections of these TpPa-X membranes were more than 95% with water flux ranging from 1216 to 3375 L m2 h−1 bar−1. More significantly, in line with the simulated results, the TpPa-X COFs showed better water flux when these COFs existed as monolayers. Nevertheless, using monolayers of COFs in RO membranes is practically a difficult approach to implement.In 2019, Wang and Wei groups came up with an idea to investigate the conduct of transportation of water and salt ions based on multilayer stacking.328 The authors showed that with an increase in the number of layers, the water permeation decreased whereas the salt ion rejection performance increased. The number of water-hydrogen bonding interactions increases with the increase in the number of layers, which results in decreased water permeance. Generally, COF monolayers tend to stack for the formation of laminated multi-layers to minimise the surface energy. Hence, there is a possibility that the way of stacking might affect the water transport and salt rejection performance. To gain further insight, the authors have modelled 25 monolayers of TpPa-1 COF, with a pore size of 1.58 nm. They have found that when the monolayers were stacked in a fully eclipsed fashion, the water permeation was 3201 L m−2 h−1 bar−1 whereas the MgCl2 rejection was restricted to 42%. In contrast, when the COF monolayers were stacked in an offset-eclipsed fashion, with an effective pore diameter of 0.89 nm, the water permeation decreased to 1118 L m−2 h−1 bar−1 while the MgCl2 rejection increased to 100%. Hence, a clear idea on the impact of layer thickness and subsequent arrangement of COF layers can be drawn from this work.
Implementation of MMMs for desalination by the introduction of COFs into TFC or TFN membranes is another research direction explored in the literature.329–332 However, poor pore connectivity, the presence of irregular pore channels and poor control over active COF layer thickness restrict the water permeance. For instance, in 2016, the Wu group developed TFN NF membranes using COFs as additives for the first time.331 The TFN membranes exhibited outstanding rejection performance (>90%) of NaSO4 from water. Interfacial assembly of a TpPa-COF interlayer on a PSF substrate followed by PA skin layer grafting by the interfacial polymerisation process led Gao’s group to achieve a sandwich-like TFC RO membrane which could reject NaCl as high as 99.2% with compromised (16.78 L m−2 h−1 MPa−1) water permeance.332 The introduction of the COF active layer on the substrate system resulted in 33.8% better water permeance and marginally increased NaCl rejection performance. However, COF-based membranes have been used as adsorbents to remove bivalent ions from water.
In another study, to overcome the problem of larger pore aperture in the COF matrix, Huang and Jiang groups developed a PSM strategy to narrow the pore apertures of IISERP-COF1 (Fig. 14a), resulting in restricted accessibility of ions inside the pores.333 The simple PSM approach was done using a ring opening reaction with a cyclic anhydride molecule converting the pore size from 12.7 to 6.5 Å (Fig. 14b and c). The post functionalization strategy not only constricted the pore size, but also reduced the transport through intracrystalline defects. As evident from the studies, the ion rejection rates for the desalination of Na2SO4, MgSO4, FeCl3, MgCl2 and NaCl solutions increased from 73.1%, 76.5%, 89.8%, 64.6%, and 56.4% to 96.3%, 97.2%, 99.6%, 90.6%, and 82.9%, respectively (Fig. 14d).
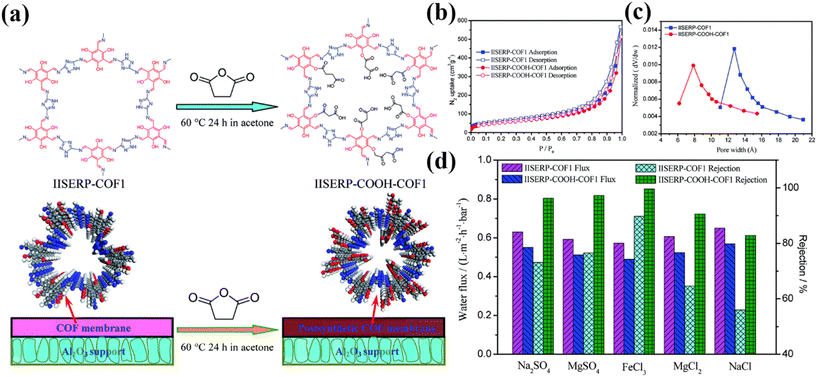 | ||
| Fig. 14 Salt rejection performance of IISERP-COF1 and IISERP-COOH-COF1: (a) schematic showing the PSM and membrane fabrication process; (b) N2 sorption isotherms and (c) pore-size distribution of unmodified and modified IISERP-COF1along with (d) salt rejection performance [reproduced from ref. 333 with permission from The Royal Society of Chemistry, copyright, 2023]. | ||
So far, careful investigation of the literature has indicated two key aspects to look for when designing COF-based membranes for improved desalination performance, which are smaller pore apertures and defect-free ultrathin COF layers. By considering both factors, Wang and Wei groups obtained an imine-based 2D COF (TpHz) on a PEI modified PES substrate by using a secondary growth approach.334 The authors grew TpHz on top of the PES substrate by a unidirectionally diffused growth strategy such that the overall thickness of the COF layer was adequate for the fast permeation of water. The as-prepared TpHz membrane, with a pore aperture of 0.8 nm, exhibited a Na2SO4 rejection of 58.3% and a water permeance of 40.5 L m−1 h−1 MPa−1, being one order of magnitude better than other reported COF membranes with similar salt rejection performance.
COFs being made of solely organic building blocks are capable of better dispersibility and compatibility in polymer solutions, making them a good candidate for MMMs. More significantly, COFs can be loaded as high as 50% in the MMMs being difficult for most of the other 2D materials and porous materials.335 In addition, COFs are better suitable as molecular sieves in water treatment owing to their larger pore sizes (1–4 nm). However, COFs with an ultra-microporous nature are infrequent in the literature, making COFs even harder to utilize as RO membranes for desalination. Currently it is challenging to fabricate COF-based membranes at the large scale due to the complex and sluggish fabrication methods. Hence, advancements in cost friendly fabrication methods need to be evolved to use COFs as membranes at the industrial scale.
Biofouling and leaching issues
The application of anti-scalants and biocides (such as oxidizing and non-oxidizing biocides), feed pretreatments (such as coagulation, flocculation, media-filtration, and membrane pretreatment), membrane surface modification, and chemical cleaning are some of the methods that have been suggested to control membrane fouling.336 Chemical cleaning is essential to prevent membrane fouling. There may be differences between foulant generation on membranes used in full-scale plants and those used in experiments. Chemical cleaning employing mixtures of acidic and alkaline chemicals has often been used in full-scale RO systems.337 Trans-membrane pressure and flux were frequently recorded in these plants to gauge the efficiency of the cleaning. Nevertheless, the effects of chemical cleaning on the elimination of genuine foulants are still unknown. The elimination of foulants should be prioritized when evaluating the efficacy of RO membrane cleaning, and it may also aid in optimizing the design of cleaning or pretreatment processes. Additionally, after cleaning with sodium hypochlorite (NaOCl), microbial community structure modifications on the MF membrane have been documented.338A comparison of RO membranes before and after ordinary alkaline and acid washing was conducted by Yu et al. to examine the impact of chemical cleaning on the elimination of membrane foulants.339 Due to its highest content in the feed water, calcium was the main inorganic component of the foulants. Due to their high deposition ratios and low removal efficiency, aluminum and iron were also prevalent components on the membranes. Before cleaning, the two greatest dissolved organic matter (DOM) fractions on the membranes were hydrophilic neutrals (HIN) and hydrophobic neutrals (HON). Hydrophilic acids (HIA) and HIN could not be sufficiently eliminated. 90% and 94% of all bacteria on the lead and tail membranes, respectively, were eliminated by chemical cleaning, which also significantly altered the composition of the microbial populations.
A UF hollow-fiber membrane based on PAN was developed by Kim et al. for use in water filtration. In comparison to a virgin UF hollow-fiber membrane, the modified membrane displayed increased negative charge and hydrophilicity and decreased surface roughness.340 In a lab setting, humic acid and sodium alginate were used to mimic the effects of fouling and performance in a cross-flow system operating in in-out mode. Humic acid and sodium alginate were removed with 65% and 73% efficiency, respectively, in the unaltered hollow-fiber membrane, while the proposed membrane achieved 93% and 95%. They used sodium NaOCl as a chemically improved backwashing agent to reduce organic fouling on the suggested membrane. The membrane flux recovery was maximized at 92.1% of the initial permeability of the PAN-NF hollow-fiber membrane at a NaOCl concentration of 1 mg L−1 with a backwashing period of 30 s, with minimal membrane degradation.
Nilusha et al. developed and optimized a unique backwashing, filtration, and relaxation approach for steady operation of a side stream tubular anaerobic ceramic membrane bioreactor handling domestic wastewater at room temperature.341 Two in situ backwashing schedules were examined, one that backwashed (once a day at 60 s per day, and twice a day at 60 s × 2 per day) rest times at 55 minutes and 5 minutes, respectively. Permeate backwashing in situ stabilized the flow at a level greater than 70% of the original flux across the membrane. After 60 days of operation, ex situ chemical cleaning was performed utilizing a sequence of pure water, NaOCl, and citric acid. In situ backwashing was able to successfully thin the dominating cake layer, and fulvic acid analogues and humic acid analogues were found to be the most pervasive organic foulants. The most common microbes adhered to the ceramic membrane fouling layer were proteobacteria, firmicutes, epsilon bacteria, and bacteroides, all of which were easily washed away by NaOCl.
Membrane fouling has a negative effect on process efficacy, resulting in increased use of energy and a shorter membrane lifetime. Variations in light/dark photoperiods have been introduced to optimize treatment performance and improve biomass properties, with the goal of minimizing membrane contamination, in order to mitigate this issue. E. Segredo-Morales et al. observed the effect of photoperiod on the efficacy of a membrane bioreactor (MBR), specifically membrane fouling.342 Excellent amounts of biomass concentration (3.21 ± 0.45 g L−1) and nutrient elimination rates (4.71 mg N L−1 d−1 and 0.67 mg P L−1 d−1, respectively) were attained under a moderate photoperiod of 16/8 h.
There are significant technical concerns regarding the potential for secondary contamination due to nanoparticles leaking from polymeric membranes (such as polyethersulfone (PES) UF membranes) during filtering. Studies have demonstrated that nanoparticles have the capacity to leach from the membrane polymer matrix, despite the fact that this has not been widely documented. The antibacterial and antibiofouling capabilities of PES membranes treated with biogenic silver nanoparticles, for instance, were studied by Zhang et al.343 Silver nanoparticles enhanced the hydrophilicity and permeated flux of membranes. The maximal leaching rate of the nanoparticles from the polymer matrix was reported to be 92.0 g m−2 h−1. In a different study, Liu et al.344 examined silver leaching from nanocomposite membranes using a quartz crystal microbalance with dissipation. The initial leaching rate from the nanocomposite membrane reached 800 ng cm−2 h−1, but it gradually dropped over time, according to the authors. Given that it is unknown how poisonous nanomaterials are to humans and other living things, leaching of nanoparticles could be hazardous to health. According to several investigations, the membrane polymer matrix does not allow nanoparticles anchored in GO sheets to leak.345 The majority of the time, however, nanoparticles are integrated directly into the membrane matrix rather than being anchored in GO.
The integration of nanoparticles on membranes is still the subject of several investigations; however, most studies are primarily concerned with enhancing separation qualities rather than long-term adherence of the nanoparticles to the membrane polymer. In addition to long-term use, when the retentate is washed on the membrane surface during filtering in cross-flow mode, leaching could arise during membrane cleaning, where the cleaning solution may impair connections between the membrane polymer and nanoparticles. The effluent (permeate) from the membrane filtration system enters the water distribution system directly during membrane filtration. If nanoparticles are present in the effluent and manage to pass through the membrane during the filtration process, they could cause secondary contamination.346 The nanoparticles may enter the retained water and cause further pollution even if they do not pass through the membrane. It is thought that changes in the membrane surface characteristics and membrane filtration efficiency would indicate the leaching of nanoparticles from the membrane surface. For instance, when the nanoparticles have been washed from the membrane matrix, a change in the membrane surface's shape, hydrophobicity, roughness, and permeability to pure water is anticipated. Previous research has demonstrated that after being exposed to pH = 8 for 144 h, the polymeric membrane permeability rose to almost double its starting value.347 After washing with NaOCl and NaOH, the membrane pore size increased from around 200 to 640 nm, demonstrating the influence of membrane wear out due to cleaning.348 Nanoplastics and microplastics may leach from membrane systems due to mechanical stress, chemical agents, physical flushing, ageing, wear, and other reasons, according to a recent paper by Ding et al.349 This is a dilemma because poisonous organic molecules might adhere to the hydrophobic plastics. The disposal of old membranes is seriously hampered by the presence of nanoparticles in the polymer matrix. Only by minimizing the wastewater produced during the membrane manufacturing process will membrane technology become green and sustainable. For the first time, Mayamin Razali et al. demonstrated and confirmed a realistic and successful method for continuously recycling membrane effluent, lowering waste creation by 99%. Using membrane production wastewater contaminated with DMF and NMP, seven different classes of adsorbent materials charcoal, MIPs, zeolites, MOFs, graphene, PIMs, and resins were fully screened, with the lowest consumption of energy of about 1200 kJ per m2 of the membrane manufactured.68
Zwitterionic materials
Using zwitterionic polymers like carboxybetaine and sulfobetaine to modify surfaces has shown to be a successful method for lowering bacterial adhesion, protein adsorption, and marine creature settlement.350 Fouling-resistant membrane properties are exhibited by cross-linkable copolymers with a high zwitterionic content and tunable pore size.351 The introduction of bio-inspired prebiotic chemistry to membrane functionalization creates new opportunities for effective and efficient wastewater treatment in the textile sector by solving the challenge of separating dyes and ions from complex wastewater matrices.352 Positive and negative charges are both present in the repeating unit of zwitterionic polymers, which also have excellent hydration properties and stable charge stoichiometry.353As an alternative to zwitterions, mixed-charge materials have also been investigated. Chen et al. demonstrated that when the surface composition of counter charges is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mixed self-assembled monolayers (SAMs) containing tetraalkylammonium groups and monovalent acid thiols resist fibrinogen adsorption.354 Bernards et al. demonstrated that when the monomer ratio of positively charged [2-(meth-acryloyloxy)ethyl]trimethylammonium chloride and negatively charged 3-sulfopropyl methacrylate potassium salt in the feed solution is 1
1, mixed self-assembled monolayers (SAMs) containing tetraalkylammonium groups and monovalent acid thiols resist fibrinogen adsorption.354 Bernards et al. demonstrated that when the monomer ratio of positively charged [2-(meth-acryloyloxy)ethyl]trimethylammonium chloride and negatively charged 3-sulfopropyl methacrylate potassium salt in the feed solution is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mixed-charge polymer brushes grafted from surfaces via surface-initiated atom transfer polymerization (SI-ATRP) can eliminate protein adsorption.355 Furthermore, Chen et al. demonstrated that hydrogels made from valence-balanced polyampholyte aminoethyl methacrylate hydrochloride and 2-carboxyethyl acrylate are highly resistant to protein adsorption.356 These findings suggested that zero charge valence from either zwitterion or mixed-charge groups is key to these surfaces' antifouling properties, as electrostatic interaction is presumably minimized in this way. Several studies reported adjustment of the isoelectric point (IsP) of surfaces using mixed-charge groups via SAMs. Lin et al. co-utilized carboxylic acid (from 16-mercaptohexa-decanoic acid) and amine groups (from 8-amino-1-octanethiol) in solution and achieved PIs from 3.2 to7.3.357 Kuo et al. used mixed thiol (from 3-mercaptopropyltrimethoxysilane) and amine (from 3-aminopropyltrimethoxysiliane) groups to obtain IsPs from <2 to 7.358 SAMs are useful for molecular-level studies but they have limited stability to resist protein adsorption and bacterial adhesion. Alternatively, polymer brushes formed using SI-ATRP have shown to be more chemically stable and more densely packed. They also exhibit stronger resistance to protein adsorption and bacterial adhesion than their SAM counterparts. Thus, SI-ATRP is an attractive polymerization method to introduce charged groups to surfaces. The difficulty to control a single surface property while maintaining other essential physicochemical properties unchanged has been pointed out by several reports.48 This interdependency of surface properties makes it hard to isolate the effect of a single property in antifouling studies. When controlling surface IsPs is the objective, this problem proves to be difficult to resolve as well. For example, recently Peng et al. used photoinitiated graft polymerization to introduce mixed-charge SO3− and N(CH3)3+ groups onto cyclic olefin copolymer microchips.359 They demonstrated that the PIs of the surfaces can be tuned by varying ratios of these two monomers and obtained three PIs of <3, 5 and 7. However, the variation in contact angles of these surfaces can reach around 15, thus the effect of surface charge on fouling cannot be disentangled from the effect of surface energy. Nevertheless, the ideal protocol for controlling surface PIs should be capable of isolating this material parameter from other properties.
1, mixed-charge polymer brushes grafted from surfaces via surface-initiated atom transfer polymerization (SI-ATRP) can eliminate protein adsorption.355 Furthermore, Chen et al. demonstrated that hydrogels made from valence-balanced polyampholyte aminoethyl methacrylate hydrochloride and 2-carboxyethyl acrylate are highly resistant to protein adsorption.356 These findings suggested that zero charge valence from either zwitterion or mixed-charge groups is key to these surfaces' antifouling properties, as electrostatic interaction is presumably minimized in this way. Several studies reported adjustment of the isoelectric point (IsP) of surfaces using mixed-charge groups via SAMs. Lin et al. co-utilized carboxylic acid (from 16-mercaptohexa-decanoic acid) and amine groups (from 8-amino-1-octanethiol) in solution and achieved PIs from 3.2 to7.3.357 Kuo et al. used mixed thiol (from 3-mercaptopropyltrimethoxysilane) and amine (from 3-aminopropyltrimethoxysiliane) groups to obtain IsPs from <2 to 7.358 SAMs are useful for molecular-level studies but they have limited stability to resist protein adsorption and bacterial adhesion. Alternatively, polymer brushes formed using SI-ATRP have shown to be more chemically stable and more densely packed. They also exhibit stronger resistance to protein adsorption and bacterial adhesion than their SAM counterparts. Thus, SI-ATRP is an attractive polymerization method to introduce charged groups to surfaces. The difficulty to control a single surface property while maintaining other essential physicochemical properties unchanged has been pointed out by several reports.48 This interdependency of surface properties makes it hard to isolate the effect of a single property in antifouling studies. When controlling surface IsPs is the objective, this problem proves to be difficult to resolve as well. For example, recently Peng et al. used photoinitiated graft polymerization to introduce mixed-charge SO3− and N(CH3)3+ groups onto cyclic olefin copolymer microchips.359 They demonstrated that the PIs of the surfaces can be tuned by varying ratios of these two monomers and obtained three PIs of <3, 5 and 7. However, the variation in contact angles of these surfaces can reach around 15, thus the effect of surface charge on fouling cannot be disentangled from the effect of surface energy. Nevertheless, the ideal protocol for controlling surface PIs should be capable of isolating this material parameter from other properties.
Biomineralization
Water sources differ in their features, and each requires a different treatment, which is outside the scope of this review. However, they are all being investigated in order to create drinking freshwater. In terms of processability, a lack of secondary nutrients such as calcium, magnesium, and sulphate reduces crop quality. Furthermore, minerals essential for human health are deficient in desalinated water, indicating an obvious necessity for the addition of calcium and magnesium during the post-treatment process. Tang et al. discovered a membrane-based process using the NF–NF–DiaNF combination,360 a membrane-based process highly selectively separating divalent ions (Ca2+, Mg2+, and SO42−) from seawater. The main goal is to selectively and cost-effectively separate Mg2+ from seawater by either dosing it into desalinated water during the post-treatment stage of desalination or generating a Mg2+/Ca2+/SO42− solution that can be used for precipitating high-strength wastewater prior to anaerobic digestion with minimal addition of harmful Cl−/Na+ ions. The procedure is divided into three steps: first, the seawater is passed through a high-recovery NF membrane to reduce the molar ratio of divalent-cations to SO42−. This step's retentate is then subjected to a further 65% recovery (conventional) NF step to increase the Mg2+ concentration in the retentate, which is then subjected to a diananofiltration (DNF) step to reduce the monovalent ion concentrations while maintaining high Mg2+/SO42− concentrations in the product solution. Birnhack et al. employed a series of processes, including UF, to reject sulphate and achieve a total hardness to sulphate ratio close to one, followed by cycles of combined NF–DNF to separate monovalent ions from divalent ions.361 As a result, it produces a Mg2+/Ca2+/SO42− solution that can be used for post-treatment. Tang et al. created a new process that combines cation exchange Resin and DNF.362 The goal of the cation exchange step is to get the total hardness to sulphate ratio close to unity, whereas the goal of the DNF step is to get rid of singly charged ions like chloride, sodium, and potassium. The concentrated magnesium sulphate solution can be used to remineralize the seawater desalination permeates. Apart from polymeric traditional membranes, ENM based membranes are also explored in order to remineralize purified-wastewater sources. Calcium phosphate coatings have been achieved on various types of electrospun nanofibers, including poly(L-lactic acid), poly(-caprolactone), and poly(-caprolactone) (lactic-co-glycolic acid).363Apart from these examples, the use of CA is also evident in the literature owing to its hydrophilicity, environmental friendliness, biodegradability and easy processability. Additionally, CA is an ester of naturally occurring polysaccharide polymers.364 Hence, it leaves a lower carbon footprint as it does not rely on non-renewable sources. As a result, electrospun CA nanofibers may be an ideal matrix for directing CaCO3 mineralization, and the templating effect of CA fibres is anticipated for large-scale synthesis of CaCO3 tubular structures under mild conditions.365Conclusion
In the realm of wastewater treatment, the selection of membrane materials stands as a pivotal determinant in combating both biological and chemical impurities across industrial and domestic sectors. The careful selection of materials is crucial in achieving a balance between chemical resistance, selectivity, and compatibility with operational conditions, taking into account the specific challenges encountered in each particular context. The successful management of industrial wastewater requires the use of resilient materials capable of withstanding various chemicals and challenging environments, while also ensuring the effective removal of pollutants. In contrast, within domestic settings, the essential considerations of biocompatibility, energy efficiency, and ease of upkeep become relevant. The growing popularity of hybrid membrane solutions underscores the fluidity of material selection, offering innovative strategies to tackle intricate contaminants. The selection of membrane materials is of utmost relevance in facilitating effective and ecologically responsible wastewater treatment solutions across many contexts, given the increasing significance of water resource management. The convergence of chemistry, nanotechnology, and chemical engineering has facilitated cooperative initiatives within the discipline, resulting in notable progress in membrane technology. Although multifunctional nanomaterials have been discovered, their practical integration into membrane production procedures has been limited to only a few cases. The commercial viability of promising materials has been impeded by the exorbitant costs associated with their synthesis procedures. Hence, the investigation of economically viable alternatives for adsorptive membranes and their potential for reusability emerges as a compelling field of study.Future outlook
Membrane technology has risen in importance in providing safe drinking water as well as wastewater treatment and reuse since its introduction more than five decades ago. The difficulties are both technological and societal in nature, and they act as catalysts for new discoveries and advancements. There are at least three major technological challenges: energy consumption, greenhouse gas (GHG) emissions and product quality and productivity (in terms of fouling). The enhancement of wastewater treatment can potentially be achieved by the utilisation of electrospinning techniques to fabricate multifunctional membranes with increased surface areas (Fig. 15). Tailored functional components, such as sorbents at the nanoscale and materials for ion-exchange, have the potential to enhance the effectiveness of remineralization processes by selectively removing undesired contaminants. In addition, the advancement of energy-efficient techniques and the optimisation of membrane structures will enhance the viability of hybrid membranes in the context of large-scale implementations. | ||
| Fig. 15 A conclusive schematic of the current trends in membrane modifications towards sustainable and highly efficient wastewater remediation. | ||
List of abbreviations (dyes)
| MB | Methylene blue |
| MO | Methyl orange |
| CR | Congo red |
| RB | Rose bengal |
| AF | Acid fuchsin |
| CV | Crystal violet |
| CBB | Coomassie brilliant blue R250 |
| EY | Eosin yellow |
| MG | Malachite green |
| RhB | Rhodamine B |
| RB5 | Reactive black 5 |
| OG | Orange G |
| MR | Methyl red |
| CB-T | Chrome black T |
| CA | Cellulose acetate |
| CA-dye | Chrome azurol |
| AA | Anthraflavic acid |
| BB | Brilliant blue G |
| TB | Thymolphthalein blue |
| AG-25 | Acid green 25 |
| DFBM | Direct fast brown M |
| IC | Indigo carmine |
| AR-27 | Acid blue 27 |
| FS | Fluorescein sodium salt |
| PP | Potassium permanganate |
| DFPB | N,N-Dimethyl-p-phenylene-diamine dihydrochloride |
| NR | Nile red |
| NA | p-Nitroaniline |
| CAc | Calcein |
| DR 80 | Direct red 80 |
| EBT | Eriochrome black T |
| AB | Alcian blue |
Author contributions
M. K. and S. C. contributed equally to this work. Manish Kumar: conceptualization, data curation, writing – review & editing. Sumanta Chowdhury: data curation, visualization, artwork, writing – review & editing. Jaspreet Kaur Randhawa: supervision, conceptualization, funding acquisition, resources, rephrasing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Mission on Himalayan Studies (NMHS), Govt. of India for the project funding and IIT Mandi, India for providing facilities.References
- A. Boretti and L. Rosa, Reassessing the Projections of the World Water Development Report, npj Clean Water, 2019, 2(1), 15, DOI:10.1038/s41545-019-0039-9
.
- A. Nagar and T. Pradeep, Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment, ACS Nano, 2020, 14(6), 6420–6435, DOI:10.1021/acsnano.9b01730
.
- X. Qu, J. Brame, Q. Li and P. J. J. Alvarez, Nanotechnology for a Safe and Sustainable Water Supply: Enabling Integrated Water Treatment and Reuse, Acc. Chem. Res., 2013, 46(3), 834–843, DOI:10.1021/ar300029v
.
- M. Anjum, R. Miandad, M. Waqas, F. Gehany and M. A. Barakat, Remediation of Wastewater Using Various Nano-Materials, Arabian J. Chem., 2019, 12(8), 4897–4919, DOI:10.1016/j.arabjc.2016.10.004
.
- F. Lu and D. Astruc, Nanocatalysts and Other Nanomaterials for Water Remediation from Organic Pollutants, Coord. Chem. Rev., 2020, 408, 213180, DOI:10.1016/j.ccr.2020.213180
.
- S. Kim, S.-N. Nam, A. Jang, M. Jang, C. M. Park, A. Son, N. Her, J. Heo and Y. Yoon, Review of Adsorption–Membrane Hybrid Systems for Water and Wastewater Treatment, Chemosphere, 2022, 286, 131916, DOI:10.1016/j.chemosphere.2021.131916
.
- W. S. Chai, J. Y. Cheun, P. S. Kumar, M. Mubashir, Z. Majeed, F. Banat, S.-H. Ho and P. L. Show, A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application, J. Cleaner Prod., 2021, 296, 126589, DOI:10.1016/j.jclepro.2021.126589
.
- L. Zhao, J. Deng, P. Sun, J. Liu, Y. Ji, N. Nakada, Z. Qiao, H. Tanaka and Y. Yang, Nanomaterials for Treating Emerging Contaminants in Water by Adsorption and Photocatalysis: Systematic Review and Bibliometric Analysis, Sci. Total Environ., 2018, 627, 1253–1263, DOI:10.1016/j.scitotenv.2018.02.006
.
- S. A. Kumar, M. Jarvin, S. S. R. Inbanathan, A. Umar, N. P. Lalla, N. Y. Dzade, H. Algadi, Q. I. Rahman and S. Baskoutas, Facile Green Synthesis of Magnesium Oxide Nanoparticles Using Tea (Camellia Sinensis) Extract for Efficient Photocatalytic Degradation of Methylene Blue Dye, Environ. Technol. Innovation, 2022, 28, 102746, DOI:10.1016/j.eti.2022.102746
.
- M. Kumar, A. Tiwari and J. K. Randhawa, Electrospun Nanofibers of α-Hematite/Polyacrylonitrile/Calcium Carbonate/Cellulose Triacetate as a Multifunctional Platform in, Wastewater Treatment and Remineralisation, Desalination, 2022, 541, 116030, DOI:10.1016/j.desal.2022.116030
.
- S. Hube, M. Eskafi, K. F. Hrafnkelsdóttir, B. Bjarnadóttir, M. Á. Bjarnadóttir, S. Axelsdóttir and B. Wu, Direct Membrane Filtration for Wastewater Treatment and Resource Recovery: A Review, Sci. Total Environ., 2020, 710, 136375, DOI:10.1016/j.scitotenv.2019.136375
.
- G. Pearce, Introduction to Membranes: Filtration for Water and Wastewater Treatment, Filtr. Sep., 2007, 44(2), 24–27, DOI:10.1016/S0015-1882(07)70052-6
.
- W. Fu and W. Zhang, Microwave-Enhanced Membrane Filtration for Water Treatment, J. Membr. Sci., 2018, 568, 97–104, DOI:10.1016/j.memsci.2018.09.064
.
- S. Hand and R. D. Cusick, Electrochemical Disinfection in Water and Wastewater Treatment: Identifying Impacts of Water Quality and Operating Conditions on Performance, Environ. Sci. Technol., 2021, 55(6), 3470–3482, DOI:10.1021/acs.est.0c06254
.
- S. AlZain, Effect of Chemical, Microwave Irradiation, Steam Autoclave, Ultraviolet Light Radiation, Ozone and Electrolyzed Oxidizing Water Disinfection on Properties of Impression Materials: A Systematic Review and Meta-Analysis Study, Saudi Dent. J., 2020, 32(4), 161–170, DOI:10.1016/j.sdentj.2019.12.003
.
- A. Najafpoor, R. Norouzian-Ostad, H. Alidadi, T. Rohani-Bastami, M. Davoudi, F. Barjasteh-Askari and J. Zanganeh, Effect of Magnetic Nanoparticles and Silver-Loaded Magnetic Nanoparticles on Advanced Wastewater Treatment and Disinfection, J. Mol. Liq., 2020, 303, 112640, DOI:10.1016/j.molliq.2020.112640
.
- C. Cardoza, V. Nagtode, A. Pratap and S. N. Mali, Emerging Applications of Nanotechnology in Cosmeceutical Health Science: Latest Updates, Health Sci. J., 2022, 4, 100051, DOI:10.1016/j.hsr.2022.100051
.
- H. Saleem and S. J. Zaidi, Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment, Nanomaterials, 2020, 10(9) DOI:10.3390/nano10091764
.
- M. Kamali, K. M. Persson, M. E. Costa and I. Capela, Sustainability Criteria for Assessing Nanotechnology Applicability in Industrial Wastewater Treatment: Current Status and Future Outlook, Environ. Int., 2019, 125, 261–276, DOI:10.1016/j.envint.2019.01.055
.
- K. Jain, A. S. Patel, V. P. Pardhi and S. J. Flora, Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment, Molecules, 2021, 26(6) DOI:10.3390/molecules26061797
.
- J. Yang, B. Hou, J. Wang, B. Tian, J. Bi, N. Wang, X. Li and X. Huang, Nanomaterials for the Removal of Heavy Metals from Wastewater, Nanomaterials, 2019, 9(3), 424, DOI:10.3390/nano9030424
.
- S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, A Review of Potentially Low-Cost Sorbents for Heavy Metals, Water Res., 1999, 33(11), 2469–2479, DOI:10.1016/S0043-1354(98)00475-8
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341(6149), 1230444, DOI:10.1126/science.1230444
.
- K. T. Tan, S. Ghosh, Z. Wang, F. Wen, D. Rodríguez-San-Miguel, J. Feng, N. Huang, W. Wang, F. Zamora, X. Feng, A. Thomas and D. Jiang, Covalent Organic Frameworks, Nat. Rev. Methods Primers, 2023, 3(1), 1, DOI:10.1038/s43586-022-00181-z
.
- B. Wang, R.-B. Lin, Z. Zhang, S. Xiang and B. Chen, Hydrogen-Bonded Organic Frameworks as a Tunable Platform for Functional Materials, J. Am. Chem. Soc., 2020, 142(34), 14399–14416, DOI:10.1021/jacs.0c06473
.
- O. M. Yaghi, Reticular Chemistry in All Dimensions, ACS Cent. Sci., 2019, 5(8), 1295–1300, DOI:10.1021/acscentsci.9b00750
.
- O. M. Yaghi, The Reticular Chemist, Nano Lett., 2020, 20(12), 8432–8434, DOI:10.1021/acs.nanolett.0c04327
.
- O. M. Yaghi, Z. Sun, D. A. Richardson and T. L. Groy, Directed Transformation of Molecules to Solids: Synthesis of a Microporous Sulfide from Molecular Germanium Sulfide Cages, J. Am. Chem. Soc., 1994, 116(2), 807–808, DOI:10.1021/ja00081a067
.
- B. Hosseini Monjezi, K. Kutonova, M. Tsotsalas, S. Henke and A. Knebel, Current Trends in Metal–Organic and Covalent Organic Framework Membrane Materials, Angew. Chem., Int. Ed., 2021, 60(28), 15153–15164, DOI:10.1002/anie.202015790
.
- A. A. Yaqoob, T. Parveen, K. Umar and M. N. Mohamad Ibrahim, Role of Nanomaterials in the Treatment of Wastewater: A Review, Water, 2020, 12(2) DOI:10.3390/w12020495
.
-
A. K. Patel, D. Gupta, A. Singh, V. K. Mishra and N. K. Sharma, Green-synthesized nanoparticles for treatment of wastewater: an environmentally sustainable pollution remediation technology, Sustainable Environmental Clean-up, ed. V. Kumar Mishra and A. Kumar, Elsevier, 2021, ch. 2, pp. 29–70, DOI:10.1016/B978-0-12-823828-8.00002-5
.
- N. L. Le and S. P. Nunes, Materials and Membrane Technologies for Water and Energy Sustainability, Sustainable Mater. Technol., 2016, 7, 1–28, DOI:10.1016/j.susmat.2016.02.001
.
- E. Obotey Ezugbe and S. Rathilal, Membrane Technologies in Wastewater Treatment: A Review, Membranes, 2020, 10(5) DOI:10.3390/membranes10050089
.
- P. S. Goh, K. C. Wong and A. F. Ismail, Membrane Technology: A Versatile Tool for Saline Wastewater Treatment and Resource Recovery, Desalination, 2022, 521, 115377, DOI:10.1016/j.desal.2021.115377
.
- X. Tan and D. Rodrigue, A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride), Polymers, 2019, 11(7) DOI:10.3390/polym11071160
.
- Y. Wen, J. Yuan, X. Ma, S. Wang and Y. Liu, Polymeric Nanocomposite Membranes for Water Treatment: A Review, Environ. Chem. Lett., 2019, 17(4), 1539–1551, DOI:10.1007/s10311-019-00895-9
.
- H. Watson, A. J. Cockbain, J. Spencer, A. Race, M. Volpato, P. M. Loadman, G. J. Toogood and M. A. Hull, Measurement of red blood cell eicosapentaenoic acid (EPA) levels in a randomised trial of EPA in patients with colorectal cancer liver metastases, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2016, 115, 60–66, DOI:10.1016/j.plefa.2016.10.003
.
- S. Xiao, H. Ma, M. Shen, S. Wang, Q. Huang and X. Shi, Excellent Copper(II) Removal Using Zero-Valent Iron Nanoparticle-Immobilized Hybrid Electrospun Polymer Nanofibrous Mats, Colloids Surf., A, 2011, 48–54, DOI:10.1016/j.colsurfa.2011.03.005
.
- S. R. Lakhotia, M. Mukhopadhyay and P. Kumari, Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment, Sci. Rep., 2018, 8, 4976, DOI:10.1038/s41598-018-23188-7
.
- S. Vetrivel, D. Rana, M. S. Sri Abirami Saraswathi, K. Divya, N. J. Kaleekkal and A. Nagendran, Cellulose Acetate Nanocomposite Ultrafiltration Membranes Tailored with Hydrous Manganese Dioxide Nanoparticles for Water Treatment Applications, Polym. Adv. Technol., 2019, 30(8), 1943–1950, DOI:10.1002/pat.4626
.
- K. T. Alali, J. Liu, J. Yu, D. Moharram, R. Chen, H. Zhang, Q. Liu, M. Zhang and J. Wang, HFIP-Functionalized Electrospun WO3 Hollow Nanofibers/RGO as an Efficient Double Layer Sensing Material for Dimethyl Methylphosphonate Gas under UV-Light Irradiation, J. Alloys Compd., 2020, 832, 1–12, DOI:10.1016/j.jallcom.2020.154999
.
- D. Li, J. T. McCann and Y. Xia, Use of Electrospinning to Directly Fabricate Hollow Nanofibers with Functionalized Inner and Outer Surfaces, Small, 2004, 1(1), 83–86, DOI:10.1002/smll.200400056
.
- M. A. Abdulhamid and K. Muzamil, Recent Progress on Electrospun Nanofibrous Polymer Membranes for Water and Air Purification: A Review, Chemosphere, 2023, 310, 136886, DOI:10.1016/j.chemosphere.2022.136886
.
- N. H. Barbhuiya, U. Misra and S. P. Singh, Biocatalytic Membranes for Combating the Challenges of Membrane Fouling and Micropollutants in Water Purification: A Review, Chemosphere, 2022, 286, 131757, DOI:10.1016/j.chemosphere.2021.131757
.
- S. Kalla, K. S. Piash and O. Sanyal, Anti-Fouling and Anti-Wetting Membranes for Membrane Distillation, J. Water Process. Eng., 2022, 46, 102634, DOI:10.1016/j.jwpe.2022.102634
.
- S. F. Anis, B. S. Lalia, R. Hashaikeh and N. Hilal, Titanium Coating on Ultrafiltration Inorganic Membranes for Fouling Control, Sep. Purif. Technol., 2022, 282, 119997, DOI:10.1016/j.seppur.2021.119997
.
- S. F. Ahmed, F. Mehejabin, A. Momtahin, N. Tasannum, N. T. Faria, M. Mofijur, A. T. Hoang, D.-V. N. Vo and T. M. I. Mahlia, Strategies to Improve Membrane Performance in Wastewater Treatment, Chemosphere, 2022, 306, 135527, DOI:10.1016/j.chemosphere.2022.135527
.
- W. Yang and F. Zhou, Polymer Brushes for Antibiofouling and Lubrication, Biosurface and Biotribology, 2017, 3(3), 97–114, DOI:10.1016/j.bsbt.2017.10.001
.
- M. Rezakazemi, A. Dashti, H. Riasat Harami, N. Hajilari and Inamuddin, Fouling-Resistant Membranes for Water Reuse, Environ. Chem. Lett., 2018, 16(3), 715–763, DOI:10.1007/s10311-018-0717-8
.
- Z. Guo, M. Yao, H. Sun, M. Shi, X. Dong, S. He, B. Guo, F. Yao, H. Zhang and J. Li, Tyramine-Enhanced Zwitterion Hyaluronan Hydrogel Coating for Anti-Fouling and Anti-Thrombosis, Sci. China: Technol. Sci., 2022, 65(8), 1828–1844, DOI:10.1007/s11431-022-2048-1
.
- C. Zhang, Y. Zhang, X. Xiao, G. Liu, Z. Xu, B. Wang, C. Yu, R. H. A. Ras and L. Jiang, Efficient Separation of Immiscible Oil/Water Mixtures Using a Perforated Lotus Leaf, Green Chem., 2019, 21(24), 6579–6584, 10.1039/C9GC03254A
.
- A. Venault, H. N. Aini, T. A. Galeta and Y. Chang, Using the Dimethyl Sulfoxide Green Solvent for the Making of Antifouling PEGylated Membranes by the Vapor-Induced Phase Separation Process, J. Membr. Sci. Lett., 2022, 2(2), 100025, DOI:10.1016/j.memlet.2022.100025
.
- K. R. Balaji, M. H. Abdellah, V. G. D. Kumar, M. S. Santosh, R. Reddy, S. Kumar and G. Szekely, Nanofiltration Membranes Composed of Carbonized Giant Cane and Pongamia Meal Binder for Ion Sieving in Water and Molecular Sieving in Organic Solvents, Sustainable Mater. Technol., 2023, 35, e00517, DOI:10.1016/j.susmat.2022.e00517
.
- D. G. Oldal, F. Topuz, T. Holtzl and G. Szekely, Green Electrospinning of Biodegradable Cellulose Acetate Nanofibrous Membranes with Tunable Porosity, ACS Sustainable Chem. Eng., 2023, 11(3), 994–1005, DOI:10.1021/acssuschemeng.2c05676
.
- W. Xie, G. Chen, C. Chen, Z. Song, Q. Wu, L. Tian, Z. Dai, S. Liang, P. Tang, X. Zhang, J. Ma and B. Liu, Polydopamine/ Polyethyleneimine/ MOF Ternary-Coated Poly (Vinyl Chloride) Nanocomposite Membranes Based on Green Solvent for Shale Gas Wastewater Treatment, J. Membr. Sci., 2023, 665, 121100, DOI:10.1016/j.memsci.2022.121100
.
- M. Dufay, M. Jimenez and S. Degoutin, Effect of Cold Plasma Treatment on Electrospun Nanofibers Properties: A Review, ACS Appl. Bio Mater., 2020, 3(8), 4696–4716, DOI:10.1021/acsabm.0c00154
.
- Y. Han, Y. Xu, S. Zhang, T. Li, S. Ramakrishna and Y. Liu, Progress of Improving Mechanical Strength of Electrospun Nanofibrous Membranes, Macromol. Mater. Eng., 2020, 305(11), 2000230, DOI:10.1002/mame.202000230
.
- P. Sagitha, C. R. Reshmi, S. P. Sundaran and A. Sujith, Recent Advances in Post-Modification Strategies of Polymeric Electrospun Membranes, Eur. Polym. J., 2018, 105, 227–249, DOI:10.1016/j.eurpolymj.2018.05.033
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Metal-Organic Frameworks Supported on Nanofibers to Remove Heavy Metals, J. Mater. Chem. A, 2018, 6(10), 4550–4555, 10.1039/c7ta10428f
.
- S. Yang, Y. Si, Q. Fu, F. Hong, J. Yu, S. S. Al-Deyab, M. El-Newehy and B. Ding, Superwetting Hierarchical Porous Silica Nanofibrous Membranes for Oil/Water Microemulsion Separation, Nanoscale, 2014, 6(21), 12445–12449, 10.1039/C4NR04668D
.
- M. Y. Haddad, H. F. Alharbi, M. R. Karim, M. O. Aijaz and N. H. Alharthi, Preparation of TiO2 Incorporated Polyacrylonitrile Electrospun Nanofibers for Adsorption of Heavy Metal Ions, J. Polym. Res., 2018, 25(10), 218, DOI:10.1007/s10965-018-1613-4
.
- H. H. Najafabadi, M. Irani, L. R. Rad, A. Sojoudi and I. Haririan, Correction: Removal of Cu2+, Pb2+ and Cr6+ from Aqueous Solutions Using a Chitosan/Graphene Oxide Composite Nanofibrous Adsorbent, RSC Adv., 2015, 5(29), 22390, 10.1039/C5RA90015H
.
- S. F. Anis and R. Hashaikeh, Electrospun Zeolite-Y Fibers: Fabrication and Morphology Analysis, Microporous Mesoporous Mater., 2016, 233, 78–86, DOI:10.1016/j.micromeso.2015.11.022
.
- E. A. Mayerberger, R. M. Street, R. M. McDaniel, M. W. Barsoum and C. L. Schauer, Antibacterial Properties of Electrospun Ti3C2Tz (MXene)/Chitosan Nanofibers, RSC Adv., 2018, 8(62), 35386–35394, 10.1039/C8RA06274A
.
- E. O. Ezugbe and S. Rathilal, Membrane Technologies in Wastewater Treatment: A Review, Membranes, 2020, 10(5) DOI:10.3390/membranes10050089
.
- H. Chen, M. Huang, Y. Liu, L. Meng and M. Ma, Functionalized Electrospun Nanofiber Membranes for Water Treatment: A Review, Sci. Total Environ., 2020, 739, 139944, DOI:10.1016/j.scitotenv.2020.139944
.
- K. Zuo, K. Wang, R. M. DuChanois, Q. Fang, E. M. Deemer, X. Huang, R. Xin, I. A. Said, Z. He, Y. Feng, W. Shane Walker, J. Lou, M. Elimelech, X. Huang and Q. Li, Selective Membranes in Water and Wastewater Treatment: Role of Advanced Materials, Mater. Today, 2021, 50, 516–532, DOI:10.1016/j.mattod.2021.06.013
.
- M. Razali, J. F. Kim, M. Attfield, P. M. Budd, E. Drioli, Y. M. Lee and G. Szekely, Sustainable Wastewater Treatment and Recycling in Membrane Manufacturing, Green Chem., 2015, 17(12), 5196–5205, 10.1039/C5GC01937K
.
- M. F. Abid, M. A. Zablouk and A. M. Abid-Alameer, Experimental Study of Dye Removal from Industrial Wastewater by Membrane Technologies of Reverse Osmosis and Nanofiltration, Iran. J. Environ. Health Sci. Eng., 2012, 9(1), 17, DOI:10.1186/1735-2746-9-17
.
- E. I. El-Aswar, H. Ramadan, H. Elkik and A. G. Taha, A Comprehensive Review on Preparation, Functionalization and Recent Applications of Nanofiber Membranes in Wastewater Treatment, J. Environ. Manage., 2022, 301, 113908, DOI:10.1016/j.jenvman.2021.113908
.
- A. K. Singh, P. Singh, S. Mishra and V. K. Shahi, Anti-Biofouling Organic-Inorganic Hybrid Membrane for Water Treatment, J. Mater. Chem., 2012, 22(5), 1834–1844, 10.1039/C1JM14250J
.
-
E. Nagy, Membrane Materials, Structures, and Modules, in Basic Equations of Mass Transport Through a Membrane Layer, ed. E. Nagy, Elsevier, 2nd edn, 2019, ch. 2, pp. 11–19, DOI:10.1016/B978-0-12-813722-2.00002-9
.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis, Chem. Soc. Rev., 2019, 48(2), 463–487, 10.1039/c8cs00493e
.
- R. Al-Tohamy, S. S. Ali, F. Li, K. M. Okasha, Y. A.-G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu and J. Sun, A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety, Ecotoxicol. Environ. Saf., 2022, 231, 113160, DOI:10.1016/j.ecoenv.2021.113160
.
- V. Katheresan, J. Kansedo and S. Y. Lau, Efficiency of Various Recent Wastewater Dye Removal Methods: A Review, J. Environ. Chem. Eng., 2018, 6(4), 4676–4697, DOI:10.1016/j.jece.2018.06.060
.
- M. M. Hassan and C. M. Carr, A Critical Review on Recent Advancements of the Removal of Reactive Dyes from Dyehouse Effluent by Ion-Exchange Adsorbents, Chemosphere, 2018, 209, 201–219, DOI:10.1016/j.chemosphere.2018.06.043
.
- C. Hildebrand, V. B. Kuglin, H. L. Brandão, V. J. P. Vilar, S. M. A. Guelli Ulson De Souza and A. A. Ulson De Souza, Insights into Nanofiltration of Textile Wastewaters for Water Reuse, Clean Technol. Environ. Policy, 2014, 16(3), 591–600, DOI:10.1007/s10098-013-0665-8
.
- I. Koyuncu and D. Topacik, Effects of Operating Conditions on the Salt Rejection of Nanofiltration Membranes in Reactive Dye/Salt Mixtures, Sep. Purif. Technol., 2003, 33(3), 283–294, DOI:10.1016/S1383-5866(03)00088-1
.
- J. Tanninen, M. Mänttäri and M. Nyström, Effect of Salt Mixture Concentration on Fractionation with NF Membranes, J. Membr. Sci., 2006, 283(1–2), 57–64, DOI:10.1016/j.memsci.2006.06.012
.
- L. Shu, T. D. Waite, P. J. Bliss, A. Fane and V. Jegatheesan, Nanofiltration for the Possible Reuse of Water and Recovery of Sodium Chloride Salt from Textile Effluent, Desalination, 2005, 172(3), 235–243, DOI:10.1016/j.desal.2004.07.037
.
- J. Cuhorka and P. Mikulášek, Performance Evaluation of Nanofiltration Membranes for Diafiltration of Dye/Salt Mixtures: Experimental Observations and Model Verification, Desalin. Water Treat., 2010, 16(1–3), 110–119, DOI:10.5004/dwt.2010.1087
.
- J. Lin, W. Ye, H. Zeng, H. Yang, J. Shen, S. Darvishmanesh, P. Luis, A. Sotto and B. Van der Bruggen, Fractionation of Direct Dyes and Salts in Aqueous Solution Using Loose Nanofiltration Membranes, J. Membr. Sci., 2015, 477, 183–193, DOI:10.1016/j.memsci.2014.12.008
.
- G. S. Vieira, F. K. V. Moreira, R. L. S. Matsumoto, M. Michelon, F. M. Filho and M. D. Hubinger, Influence of Nanofiltration Membrane Features on Enrichment of Jussara Ethanolic Extract (Euterpe Edulis) in Anthocyanins, J. Food Eng., 2018, 226, 31–41, DOI:10.1016/j.jfoodeng.2018.01.013
.
- G. S. Vieira, F. K. V. Moreira, R. L. S. Matsumoto, M. Michelon, F. M. Filho and M. D. Hubinger, Influence of Nanofiltration Membrane Features on Enrichment of Jussara Ethanolic Extract (Euterpe Edulis) in Anthocyanins, J. Food Eng., 2018, 226, 31–41, DOI:10.1016/j.jfoodeng.2018.01.013
.
- S. Guo, J. Luo, Q. Yang, X. Qiang, S. Feng and Y. Wan, Decoloration of Molasses by Ultrafiltration and Nanofiltration: Unraveling the Mechanisms of High Sucrose Retention, Food Bioprocess Technol., 2019, 12(1), 39–53, DOI:10.1007/s11947-018-2189-z
.
- J. Gyura, Z. Šereš and M. Eszterle, Influence of Operating Parameters on Separation of Green Syrup Colored Matter from Sugar Beet by Ultra-and Nanofiltration, J. Food Eng., 2005, 66(1), 89–96, DOI:10.1016/j.jfoodeng.2004.02.038
.
- Z. Yang, Y. Zhou, Z. Feng, X. Rui, T. Zhang and Z. Zhang, A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification, Polymers, 2019, 11(8), 1–22, DOI:10.3390/polym11081252
.
-
M. Wasim, A. Sabir, M. Shafiq and T. Jamil, Electrospinning: A Fiber Fabrication Technique for Water Purification, in Micro and Nano Technologies, Nanoscale Materials in Water Purification, ed. S. Thomas, D. Pasquini, S.-Y. Leu and D. A. Gopakumar, Elsevier, 2019, ch. 11, pp. 289–308, DOI:10.1016/B978-0-12-813926-4.00016-1
.
- X. Wang and B. S. Hsiao, Electrospun Nanofiber Membranes, Curr. Opin. Chem. Eng., 2016, 12, 62–81, DOI:10.1016/j.coche.2016.03.001
.
- B. Li, J. Zhao, X. Lin, D. Tu, Y. Meng, Y. Li, P. Huang and H. Zhang, Highly Efficient Sunlight-Driven Self-Cleaning Electrospun Nanofiber Membrane NM88B@HPAN for Water Treatment, J. Cleaner Prod., 2022, 355, 131812, DOI:10.1016/j.jclepro.2022.131812
.
- L. Cseri, F. Topuz, M. A. Abdulhamid, A. Alammar, P. M. Budd and G. Szekely, Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater, Adv. Mater. Technol., 2021, 6(10), 2000955, DOI:10.1002/admt.202000955
.
- N. H. Ab Razak, S. M. Praveena, A. Z. Aris and Z. Hashim, Drinking Water Studies: A Review on Heavy Metal, Application of Biomarker and Health Risk Assessment (a Special Focus in Malaysia), J. Epidemiol. Glob. Health, 2015, 5(4), 297–310, DOI:10.1016/j.jegh.2015.04.003
.
- G. F. Molelekwa, M. S. Mukhola, B. Van Der Bruggen and P. Luis, Preliminary Studies on Membrane Filtration for the Production of Potable Water: A Case of Tshaanda Rural Village in South Africa, PLoS One, 2014, 9(8), e105057, DOI:10.1371/journal.pone.0105057
.
- J. J. Schoeman and A. Steyn, Nitrate Removal with Reverse Osmosis in a Rural Area in South Africa, Desalination, 2003, 155(1), 15–26, DOI:10.1016/S0011-9164(03)00235-2
.
- M. J. Pryor, E. P. Jacobs, J. P. Botes and V. L. Pillay, A Low Pressure Ultrafiltration Membrane System for Potable Water Supply to Developing Communities in South Africa, Desalination, 1998, 119(1–3), 103–111, DOI:10.1016/S0011-9164(98)00126-X
.
- H. C. Duong, L. T. T. Tran, H. T. Truong and B. Nelemans, Seawater Membrane Distillation Desalination for Potable Water Provision on Remote Islands − A Case Study in Vietnam, Case Studies in Chemical and Environmental Engineering., 2021, 100110, DOI:10.1016/j.cscee.2021.100110
.
- D. C. Hung and N. C. Nguyen, Membrane Processes and Their Potential Applications for Fresh Water Provision in Vietnam, Vietnam J. Chem., 2017, 55(5), 533, DOI:10.15625/2525-2321.2017-00504
.
- L. D. Nguyen, S. Gassara, M. Q. Bui, F. Zaviska, P. Sistat and A. Deratani, Desalination and Removal of Pesticides from Surface Water in Mekong Delta by Coupling Electrodialysis and Nanofiltration, Environ. Sci. Pollut. Res., 2019, 26(32), 32687–32697, DOI:10.1007/s11356-018-3918-6
.
- M. R. Francis, R. Sarkar, S. Roy, S. Jaffar, V. R. Mohan, G. Kang and V. Balraj, Effectiveness of Membrane Filtration to Improve Drinking Water: A Quasi-Experimental Study from Rural Southern India, Am. J. Trop. Med. Hyg., 2016, 95(5), 1192–1200, DOI:10.4269/ajtmh.15-0675
.
- B. Sarkar, N. Venkateshwarlu, R. Nageswara Rao, C. Bhattacharjee and V. Kale, Potable Water Production from Pesticide Contaminated Surface Water-A Membrane Based Approach, Desalination, 2007, 204(1–3), 368–373, DOI:10.1016/j.desal.2006.02.041
.
- R. S. Harisha, K. M. Hosamani, R. S. Keri, S. K. Nataraj and T. M. Aminabhavi, Arsenic Removal from Drinking Water Using Thin Film Composite Nanofiltration Membrane, Desalination, 2010, 252(1–3), 75–80, DOI:10.1016/j.desal.2009.10.022
.
- J. M. Arnal, B. García-Fayos, M. Sancho, G. Verdú and J. Lora, Design and Installation of a Decentralized Drinking Water System Based on Ultrafiltration in Mozambique, Desalination, 2010, 613–617, DOI:10.1016/j.desal.2009.09.035
.
- M. Sartor, B. Schlichter, H. Gatjal and V. Mavrov, Demonstration of a New Hybrid Process for the Decentralised Drinking and Service Water Production from Surface Water in Thailand, Desalination, 2008, 222(1–3), 528–540, DOI:10.1016/j.desal.2007.03.013
.
- R. W. Finansyah and W. Hadi, Appropriate Desalination Technology, Focusing for Low Income Communities Drinking Water in Indonesia, World Appl. Sci. J., 2009, 7(9), 1188–1194 Search PubMed
.
- H. Yu, X. Li, H. Chang, Z. Zhou, T. Zhang, Y. Yang, G. Li, H. Ji, C. Cai and H. Liang, Performance of hollow fiber ultrafiltration membrane in a full-scale drinking water treatment plant in China: A systematic evaluation during 7-year operation, J. Membr. Sci., 2020, 613, 118469, DOI:10.1016/j.memsci.2020.118469
.
- S. Xia, J. Nan, R. Liu and G. Li, Study of Drinking Water Treatment by Ultrafiltration of Surfacewater and Its Application to China, Desalination, 2004, 170(1), 41–47, DOI:10.1016/j.desal.2004.03.014
.
- C. Kaya, G. Sert, N. Kabay, M. Arda, M. Yüksel and Ö. Egemen, Pre-Treatment with Nanofiltration (NF) in Seawater Desalination-Preliminary Integrated Membrane Tests in Urla, Turkey, Desalination, 2015, 369, 10–17, DOI:10.1016/j.desal.2015.04.029
.
- T. Cooray, Y. Wei, J. Zhang, L. Zheng, H. Zhong, S. K. Weragoda and R. Weerasooriya, Drinking-Water Supply for CKDu Affected Areas of Sri Lanka, Using Nanofiltration Membrane Technology: From Laboratory to Practice, Water, 2019, 11(12), 1–15, DOI:10.3390/w11122512
.
- V. B. Brião, J. Magoga, M. Hemkemeier, E. B. Brião, L. Girardelli, L. Sbeghen and D. P. C. Favaretto, Reverse Osmosis for Desalination of Water from the Guarani Aquifer System to Produce Drinking Water in Southern Brazil, Desalination, 2014, 402–411, DOI:10.1016/j.desal.2014.04.008
.
- S. Saufi and A. Ismail, Fabrication of Carbon Membranes for Gas Separation––a Review, Carbon, 2004, 42(2), 241–259, DOI:10.1016/j.carbon.2003.10.022
.
- S. Kailasa, M. S. B. Reddy, M. R. Maurya, B. G. Rani, K. V. Rao and K. K. Sadasivuni, Electrospun Nanofibers: Materials, Synthesis Parameters, and Their Role in Sensing Applications, Macromol. Mater. Eng., 2021, 306(11), 2100410, DOI:10.1002/mame.202100410
.
- Y. Li, J. Zhu, H. Cheng, G. Li, H. Cho, M. Jiang, Q. Gao and X. Zhang, Developments of Advanced Electrospinning Techniques: A Critical Review, Adv. Mater. Technol., 2021, 6(11), 2100410, DOI:10.1002/admt.202100410
.
- I. I. Cárdenas Bates, É. Loranger, A. P. Mathew and B. Chabot, Cellulose Reinforced Electrospun Chitosan Nanofibers Bio-Based Composite Sorbent for Water Treatment Applications, Cellulose, 2021, 28(8), 4865–4885, DOI:10.1007/s10570-021-03828-4
.
- R. C. da Silva, S. B. de Aguiar, P. L. R. da Cunha, R. C. M. de Paula and J. P. A. Feitosa, Effect of Microwave on the Synthesis of Polyacrylamide-g-Chitosan Gel for Azo Dye Removal, React. Funct. Polym., 2020, 148, 104491, DOI:10.1016/j.reactfunctpolym.2020.104491
.
- C. Li, T. Lou, X. Yan, Y. Long, G. Cui and X. Wang, Fabrication of Pure Chitosan Nanofibrous Membranes as Effective Absorbent for Dye Removal, Int. J. Biol. Macromol., 2018, 106, 768–774, DOI:10.1016/j.ijbiomac.2017.08.072
.
- C. Feng, K. C. Khulbe, T. Matsuura, R. Gopal, S. Kaur, S. Ramakrishna and M. Khayet, Production of Drinking Water from Saline Water by Air-Gap Membrane Distillation Using Polyvinylidene Fluoride Nanofiber Membrane, J. Membr. Sci., 2008, 311(1–2), 1–6, DOI:10.1016/j.memsci.2007.12.026
.
- Y. Yu, R. Ma, S. Yan and J. Fang, Preparation of Multi-Layer Nylon-6 Nanofibrous Membranes by Electrospinning and Hot Pressing Methods for Dye Filtration, RSC Adv., 2018, 8(22), 12173–12178, 10.1039/c8ra01442f
.
- A. Celebioglu, Z. I. Yildiz and T. Uyar, Electrospun Crosslinked Poly-Cyclodextrin Nanofibers: Highly Efficient Molecular Filtration Thru Host-Guest Inclusion Complexation, Sci. Rep., 2017, 7(1), 7369, DOI:10.1038/s41598-017-07547-4
.
- D. Zamel, A. H. Hassanin, R. Ellethy, G. Singer and A. Abdelmoneim, Novel Bacteria-Immobilized Cellulose Acetate/Poly(Ethylene Oxide) Nanofibrous Membrane for Wastewater Treatment, Sci. Rep., 2019, 9(1), 18994, DOI:10.1038/s41598-019-55265-w
.
- J. Yan, Y. Huang, Y.-E. Miao, W. W. Tjiu and T. Liu, Polydopamine-Coated Electrospun Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Membranes as Efficient Dye Adsorbent with Good Recyclability, J. Hazard. Mater., 2015, 283, 730–739, DOI:10.1016/j.jhazmat.2014.10.040
.
- F. Topuz, T. Holtzl and G. Szekely, Scavenging Organic Micropollutants from Water with Nanofibrous Hypercrosslinked Cyclodextrin Membranes Derived from Green Resources, Chem. Eng. J., 2021, 419, 129443, DOI:10.1016/j.cej.2021.129443
.
- Q.-H. Li, M. Dong, R. Li, Y.-Q. Cui, G.-X. Xie, X.-X. Wang and Y.-Z. Long, Enhancement of Cr(VI) Removal Efficiency via Adsorption/Photocatalysis Synergy Using Electrospun Chitosan/g-C3N4/TiO2 Nanofibers, Carbohydr. Polym., 2021, 253, 117200, DOI:10.1016/j.carbpol.2020.117200
.
- M. Aliabadi, M. Irani, J. Ismaeili, H. Piri and M. J. Parnian, Electrospun Nanofiber Membrane of PEO/Chitosan for the Adsorption of Nickel, Cadmium, Lead and Copper Ions from Aqueous Solution, Chem. Eng. J., 2013, 220, 237–243, DOI:10.1016/j.cej.2013.01.021
.
- D. Yang, L. Li, B. Chen, S. Shi, J. Nie and G. Ma, Functionalized Chitosan Electrospun Nanofiber Membranes for Heavy-Metal Removal, Polymer, 2019, 163, 74–85, DOI:10.1016/j.polymer.2018.12.046
.
- R. Zhao, X. Li, B. Sun, Y. Li, Y. Li, R. Yang and C. Wang, Branched Polyethylenimine Grafted Electrospun Polyacrylonitrile Fiber Membrane: A Novel and Effective Adsorbent for Cr(VI) Remediation in Wastewater, J. Mater. Chem. A, 2017, 5(3), 1133–1144, 10.1039/c6ta09784g
.
- R. Zhao, X. Li, B. Sun, M. Shen, X. Tan, Y. Ding, Z. Jiang and C. Wang, Preparation of Phosphorylated Polyacrylonitrile-Based Nanofiber Mat and Its Application for Heavy Metal Ion Removal, Chem. Eng. J., 2015, 268, 290–299, DOI:10.1016/j.cej.2015.01.061
.
- M. R. Karim, M. O. Aijaz, N. H. Alharth, H. F. Alharbi, F. S. Al-Mubaddel and M. R. Awual, Composite Nanofibers Membranes of Poly(Vinyl Alcohol)/Chitosan for Selective Lead(II) and Cadmium(II) Ions Removal from Wastewater, Ecotoxicol. Environ. Saf., 2019, 169, 479–486, DOI:10.1016/j.ecoenv.2018.11.049
.
- P. Bahmani, A. Maleki, H. Daraei, R. Rezaee, M. Khamforoush, S. Dehestani Athar, F. Gharibi, A. H. Ziaee and G. McKay, Application of Modified Electrospun Nanofiber Membranes with α-Fe2O3 Nanoparticles in Arsenate Removal from Aqueous Media, Environ. Sci. Pollut. Res., 2019, 26(21), 21993–22009, DOI:10.1007/s11356-019-05228-5
.
- J. Chang, J. Wang, J. Qu, Y. Vivian Li, L. Ma, L. Wang, X. Wang and K. Pan, Preparation of α-Fe2O3/Polyacrylonitrile Nanofiber Mat as an Effective Lead Adsorbent, Environ. Sci.: Nano, 2016, 3(4), 894–901, 10.1039/c6en00088f
.
- U. Habiba, A. M. Afifi, A. Salleh and B. C. Ang, Chitosan/(Polyvinyl Alcohol)/Zeolite Electrospun Composite Nanofibrous Membrane for Adsorption of Cr6+, Fe3+ and Ni2+, J. Hazard. Mater., 2017, 322, 182–194, DOI:10.1016/j.jhazmat.2016.06.028
.
- W. Guo, R. Guo, H. Pei, B. Wang, N. Liu and Z. Mo, Electrospinning PAN/PEI/MWCNT-COOH Nanocomposite Fiber Membrane with Excellent Oil-in-Water Separation and Heavy Metal Ion Adsorption Capacity, Colloids Surf., A, 2022, 641, 128557, DOI:10.1016/j.colsurfa.2022.128557
.
- R. HMTShirazi, T. Mohammadi and A. A. Asadi, Incorporation of amine-grafted halloysite nanotube to electrospun nanofibrous membranes of chitosan/poly (vinyl alcohol) for Cd (II) and Pb(II) removal, Appl. Clay Sci., 2022, 220, 106460, DOI:10.1016/j.clay.2022.106460
.
- A. Mohamed, S. Yousef, S. Ali, M. Sriubas, S. Varnagiris, S. Tuckute, M. A. Abdelnaby and B. M. Kamel, Highly Efficient Visible Light Photodegradation of Cr(Vi) Using Electrospun Mwcnts-Fe3o4@pes Nanofibers, Catalysts, 2021, 11(7), 1–10, DOI:10.3390/catal11070868
.
- M. G. Yazdi, M. Ivanic, A. Mohamed and A. Uheida, Surface Modified Composite Nanofibers for the Removal of Indigo Carmine Dye from Polluted Water, RSC Adv., 2018, 8(43), 24588–24598, 10.1039/c8ra02463d
.
- Q. Gao, J. Luo, X. Wang, C. Gao and M. Ge, Novel Hollow α-Fe2O3 Nanofibers via Electrospinning for Dye Adsorption, Nanoscale Res. Lett., 2015, 10(1), 1–8, DOI:10.1186/s11671-015-0874-7
.
- L. Du, X. Quan, X. Fan, G. Wei and S. Chen, Conductive CNT/Nanofiber Composite
Hollow Fiber Membranes with Electrospun Support Layer for Water Purification, J. Membr. Sci., 2020, 596, 117613, DOI:10.1016/j.memsci.2019.117613
.
- X. Xu, M. Zhang, H. Lv, Y. Zhou, Y. Yang and D.-G. Yu, Electrospun Polyacrylonitrile-Based Lace Nanostructures and Their Cu(II) Adsorption, Sep. Purif. Technol., 2022, 288, 120643, DOI:10.1016/j.seppur.2022.120643
.
- H. M. Mousa, H. Alfadhel and E. A. Nasr, Engineering and Characterization of Antibacterial Coaxial Nanofiber Membranes for Oil/Water Separation, Polymers, 2020, 12(11), 1–16, DOI:10.3390/polym12112597
.
- L. D. Tijing, Y. C. Woo, W. G. Shim, T. He, J. S. Choi, S. H. Kim and H. K. Shon, Superhydrophobic Nanofiber Membrane Containing Carbon Nanotubes for High-Performance Direct Contact Membrane Distillation, J. Membr. Sci., 2016, 502, 158–170, DOI:10.1016/j.memsci.2015.12.014
.
- S. Deng, X. Liu, J. Liao, H. Lin and F. Liu, PEI Modified Multiwalled Carbon Nanotube as a Novel Additive in PAN Nanofiber Membrane for Enhanced Removal of Heavy Metal Ions, Chem. Eng. J., 2019, 375, 122086, DOI:10.1016/j.cej.2019.122086
.
- M. Sadrzadeh and S. Bhattacharjee, Rational Design of Phase Inversion Membranes by Tailoring Thermodynamics and Kinetics of Casting Solution Using Polymer Additives, J. Membr. Sci., 2013, 441, 31–44, DOI:10.1016/j.memsci.2013.04.009
.
- M. Safarpour, A. Khataee and V. Vatanpour, Thin Film Nanocomposite Reverse Osmosis Membrane Modified by Reduced Graphene Oxide/TiO2 with Improved Desalination Performance, J. Membr. Sci., 2015, 489, 43–54, DOI:10.1016/j.memsci.2015.04.010
.
- M. Sharma, P. Mondal, A. D. Sontakke, A. Chakraborty and M. K. Purkait, High Performance Graphene-Oxide Doped Cellulose Acetate Based Ion Exchange Membrane for Environmental Remediation Applications, Int. J. Environ. Anal. Chem., 2021, 1–22, DOI:10.1080/03067319.2021.1975276
.
- A. Bhran, A. Shoaib, D. Elsadeq, A. El-gendi and H. Abdallah, Preparation of PVC/PVP Composite Polymer Membranes via Phase Inversion Process for Water Treatment Purposes, Chin. J. Chem. Eng., 2018, 26(4), 715–722, DOI:10.1016/j.cjche.2017.09.003
.
- M. M. Aji, S. Narendren, M. K. Purkait and V. Katiyar, Utilization of Waste Polyvinyl Chloride (PVC) for Ultrafiltration Membrane Fabrication and Its Characterization, J. Environ. Chem. Eng., 2020, 8(2), 103650, DOI:10.1016/j.jece.2019.103650
.
- S. Han, L. Mao, T. Wu and H. Wang, Homogeneous Polyethersulfone Hybrid Membranes Prepared with In-Suit Synthesized Magnesium Hydroxide Nanoparticles by Phase Inversion Method, J. Membr. Sci., 2016, 516, 47–55, DOI:10.1016/j.memsci.2016.05.040
.
- M. L. Masheane, L. N. Nthunya, S. P. Malinga, E. N. Nxumalo, B. B. Mamba and S. D. Mhlanga, Synthesis of Fe-Ag/f-MWCNT/PES Nanostructured-Hybrid Membranes for Removal of Cr(VI) from Water, Sep. Purif. Technol., 2017, 184(Vi), 79–87, DOI:10.1016/j.seppur.2017.04.018
.
- B. Khorshidi, T. Thundat, B. A. Fleck and M. Sadrzadeh, A Novel Approach toward Fabrication of High Performance Thin Film Composite Polyamide Membranes, Sci. Rep., 2016, 6, 1–10, DOI:10.1038/srep22069
.
- B. Khorshidi, T. Thundat, B. A. Fleck and M. Sadrzadeh, Thin Film Composite Polyamide Membranes: Parametric Study on the Influence of Synthesis Conditions, RSC Adv., 2015, 5(68), 54985–54997, 10.1039/c5ra08317f
.
- Y. Kang, J. Jang, S. Kim, J. Lim, Y. Lee and I. S. Kim, PIP/TMC Interfacial Polymerization with Electrospray: Novel Loose Nanofiltration Membrane for Dye Wastewater Treatment, ACS Appl. Mater. Interfaces, 2020, 12(32), 36148–36158, DOI:10.1021/acsami.0c09510
.
- P. H. H. Duong, K. Daumann, P.-Y. Hong, M. Ulbricht and S. P. Nunes, Interfacial Polymerization of Zwitterionic Building Blocks for High-Flux Nanofiltration Membranes, Langmuir, 2019, 35(5), 1284–1293, DOI:10.1021/acs.langmuir.8b00960
.
- J. Lee, Y. Shin, C. Boo and S. Hong, Performance, Limitation, and Opportunities of Acid-Resistant Nanofiltration Membranes for Industrial Wastewater Treatment, J. Membr. Sci., 2023, 666, 121142, DOI:10.1016/j.memsci.2022.121142
.
- H. Lan, Y. Zhai, K. Chen, Z. Zhai, C. Jiang, P. Li, Y. Hou and Q. Jason Niu, Fabrication of High Performance Nanofiltration Membrane by Construction of Noria Based Nanoparticles Interlayer, Sep. Purif. Technol., 2022, 290, 120781, DOI:10.1016/j.seppur.2022.120781
.
- I. V. Korolkov, Y. G. Gorin, A. B. Yeszhanov, A. L. Kozlovskiy and M. V. Zdorovets, Preparation of PET Track-Etched Membranes for Membrane Distillation by Photo-Induced Graft Polymerization, Mater. Chem. Phys., 2018, 205, 55–63, DOI:10.1016/j.matchemphys.2017.11.006
.
- I. V. Korolkov, A. R. Narmukhamedova, G. B. Melnikova, I. B. Muslimova, A. B. Yeszhanov, Z. K. Zhatkanbayeva, S. A. Chizhik and M. V. Zdorovets, Preparation of Hydrophobic PET Track-Etched Membranes for Separation of Oil–Water Emulsion, Membranes, 2021, 11(8), 637, DOI:10.3390/membranes11080637
.
- J. H. Choi, S. K. Park and H. Y. Ng, Membrane Fouling in a Submerged Membrane Bioreactor Using Track-Etched and Phase-Inversed Porous Membranes, Sep. Purif. Technol., 2009, 65(2), 184–192, DOI:10.1016/j.seppur.2008.10.019
.
- M. V. Zdorovets, A. B. Yeszhanov, I. V. Korolkov, O. Güven, S. S. Dosmagambetova, D. I. Shlimas, Zh. K. Zhatkanbayeva, I. S. Zhidkov, P. V. Kharkin, V. N. Gluchshenko, D. A. Zheltov, N. A. Khlebnikov and I. E. Kuklin, Liquid low-level radioactive wastes treatment by using hydrophobized track-etched membranes, Prog. Nucl. Energy, 2020, 118, 103128, DOI:10.1016/j.pnucene.2019.103128
.
- E. Priya, S. Kumar, C. Verma, S. Sarkar and P. K. Maji, A Comprehensive Review on Technological Advances of Adsorption for Removing Nitrate and Phosphate from Waste Water, J. Water Process. Eng., 2022, 49, 103159, DOI:10.1016/j.jwpe.2022.103159
.
- J. Yin and B. Deng, Polymer-Matrix Nanocomposite Membranes for Water Treatment, J. Membr. Sci., 2015, 256–275, DOI:10.1016/j.memsci.2014.11.019
.
- N. A. Ahmad, P. S. Goh, K. C. Wong, A. K. Zulhairun and A. F. Ismail, Enhancing Desalination Performance of Thin Film Composite Membrane through Layer by Layer Assembly of Oppositely Charged Titania Nanosheet, Desalination, 2020, 476, 114167, DOI:10.1016/j.desal.2019.114167
.
- C. Chambers, S. B. Stewart, B. Su, H. F. Jenkinson, J. R. Sandy and A. J. Ireland, Silver Doped Titanium Dioxide Nanoparticles as Antimicrobial Additives to Dental Polymers, Dent. Mater., 2017, 33(3), e115–e123, DOI:10.1016/j.dental.2016.11.008
.
- N. A. Ahmad, P. S. Goh, A. K. Zulhairun and A. F. Ismail, Antifouling Property of Oppositely Charged Titania Nanosheet Assembled on Thin Film Composite Reverse Osmosis Membrane for Highly Concentrated Oily Saline Water Treatment, Membranes, 2020, 10, 237, DOI:10.3390/membranes10090237
.
- M. Peydayesh, T. Mohammadi and S. K. Nikouzad, A Positively Charged Composite Loose Nanofiltration Membrane for Water Purification from Heavy Metals, J. Membr. Sci., 2020, 611(1), 118205, DOI:10.1016/j.memsci.2020.118205
.
- F. Orudzhev, S. Ramazanov, D. Sobola, P. Kaspar, T. Trčka, K. Částková, J. Kastyl, I. Zvereva, C. Wang, D. Selimov, R. Gulakhmedov, M. Abdurakhmanov, A. Shuaibov and M. Kadiev, Ultrasound and water flow driven piezophototronic effect in self-polarized flexible α-Fe2O3 containing PVDF nanofibers film for enhanced catalytic oxidation, Nano Energy, 2021, 90(Part B), 106586, DOI:10.1016/j.nanoen.2021.106586
.
- X.-Q. Wu, X. Wu, H.-W. Huo, Q.-B. Zhao, Y.-M. Zheng and Z. Xie, Designing Triple-Layer Superhydrophobic/Hydrophobic/Hydrophilic Nanofibrous Membrane via Electrohydrodynamic Technique for Enhanced Anti-Fouling and Anti-Wetting in Wastewater Treatment by Membrane Distillation, J. Membr. Sci. Lett., 2022, 2(2), 100030, DOI:10.1016/j.memlet.2022.100030
.
- C. Fang, O. Garcia-Rodriguez, C. Shang, J. Imbrogno, T. M. Swenson, O. Lefebvre and S. Zhang, An Omniphobic Membrane with Macro-Corrugation for the Treatment of Real Pharmaceutical Wastewater via Membrane Distillation, J. Membr. Sci., 2023, 676, 121582, DOI:10.1016/j.memsci.2023.121582
.
- Y. Guo, S. Wei, Y. Chen, H. Ye, S. Xue and Q. J. Niu, Sulfonated Polyaniline Interlayer with Controllable Doping Conditions for High-Performance Nanofiltration, J. Membr. Sci., 2023, 672, 121478, DOI:10.1016/j.memsci.2023.121478
.
- C. Yang, F. Topuz, S.-H. Park and G. Szekely, Biobased Thin-Film Composite Membranes Comprising Priamine–Genipin Selective Layer on Nanofibrous Biodegradable Polylactic Acid Support for Oil and Solvent-Resistant Nanofiltration, Green Chem., 2022, 24(13), 5291–5303, 10.1039/D2GC01476A
.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, 2D Transition Metal Dichalcogenides, Nat. Rev. Mater., 2017, 2(8), 17033, DOI:10.1038/natrevmats.2017.33
.
- Y. Liu, Y. Zhao, X. Zhang, X. Huang, W. Liao and Y. Zhao, MoS2-Based Membranes in Water Treatment and Purification, Chem. Eng. J., 2021, 422, 130082, DOI:10.1016/j.cej.2021.130082
.
- X. Liang, P. Wang, J. Wang, Y. Zhang, W. Wu, J. Liu and B. Van der Bruggen, Zwitterionic Functionalized MoS2 Nanosheets for a Novel Composite Membrane with Effective Salt/Dye Separation Performance, J. Membr. Sci., 2019, 573, 270–279, DOI:10.1016/j.memsci.2018.12.015
.
- S. Yang, Q. Jiang and K. Zhang, Few-Layers 2D O–MoS2 TFN Nanofiltration Membranes for Future Desalination, J. Membr. Sci., 2020, 604, 118052, DOI:10.1016/j.memsci.2020.118052
.
- X. Zhao, J. Li, S. Mu, W. He, D. Zhang, X. Wu, C. Wang and H. Zeng, Efficient Removal of Mercury Ions with MoS2-Nanosheet-Decorated PVDF Composite Adsorption Membrane, Environ. Pollut., 2021, 268, 115705, DOI:10.1016/j.envpol.2020.115705
.
- P. Bhol, S. Yadav, A. Altaee, M. Saxena, P. K. Misra and A. K. Samal, Graphene-Based Membranes for Water and Wastewater Treatment: A Review, ACS Appl. Nano Mater., 2021, 4(4), 3274–3293, DOI:10.1021/acsanm.0c03439
.
- P. Liu, C. Zhu and A. P. Mathew, Mechanically Robust High Flux Graphene Oxide - Nanocellulose Membranes for Dye Removal from Water, J. Hazard. Mater., 2019, 371, 484–493, DOI:10.1016/j.jhazmat.2019.03.009
.
- P. Tan, J. Sun, Y. Hu, Z. Fang, Q. Bi, Y. Chen and J. Cheng, Adsorption of Cu2+, Cd2+ and Ni2+ from Aqueous Single Metal Solutions on Graphene Oxide Membranes, J. Hazard. Mater., 2015, 297, 251–260, DOI:10.1016/j.jhazmat.2015.04.068
.
- H.-H. Huang, R. K. Joshi, K. K. H. De Silva, R. Badam and M. Yoshimura, Fabrication of Reduced Graphene Oxide Membranes for Water Desalination, J. Membr. Sci., 2019, 572, 12–19, DOI:10.1016/j.memsci.2018.10.085
.
- Z. Zhao, Y. Sun and F. Dong, Graphitic Carbon Nitride Based Nanocomposites: A Review, Nanoscale, 2015, 7(1), 15–37, 10.1039/C4NR03008G
.
- J. Liu, H. Wang and M. Antonietti, Graphitic Carbon Nitride “Reloaded”: Emerging Applications beyond (Photo)Catalysis, Chem. Soc. Rev., 2016, 45(8), 2308–2326, 10.1039/C5CS00767D
.
- Y. Wang, B. Gao, Q. Yue and Z. Wang, Graphitic Carbon Nitride (g-C3N4)-Based Membranes for Advanced Separation, J. Mater. Chem. A, 2020, 8(37), 19133–19155, 10.1039/D0TA06729F
.
- M. Ge, Z. Jia, Q. Jiang, G. Ying, Y. Yang, S. Wu, T. Goto and J. Zhang, Highly-Permeable and Antifouling Thin-Film Nanocomposite Reverse Osmosis Membrane: Beneficial Effects of 1D/2D g-C3N4 Nanohybrids, J. Environ. Chem. Eng., 2022, 10(6), 108902, DOI:10.1016/j.jece.2022.108902
.
- S. Seyyed Shahabi, N. Azizi and V. Vatanpour, Synthesis and Characterization of Novel G-C3N4 Modified Thin Film Nanocomposite Reverse Osmosis Membranes to Enhance Desalination Performance and Fouling Resistance, Sep. Purif. Technol., 2019, 215, 430–440, DOI:10.1016/j.seppur.2019.01.031
.
- A. R. Nadig, N. S. Naik, M. Padaki, R. K. Pai and S. Déon, Impact of Graphitic Carbon Nitride Nanosheets in Mixed- Matrix Membranes for Removal of Heavy Metals from Water, J. Water Process. Eng., 2021, 41, 102026, DOI:10.1016/j.jwpe.2021.102026
.
- G. Liu, W. Jin and N. Xu, Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes, Angew. Chem., Int. Ed., 2016, 55(43), 13384–13397, DOI:10.1002/anie.201600438
.
- Y. A. J. Al-Hamadani, B.-M. Jun, M. Yoon, N. Taheri-Qazvini, S. A. Snyder, M. Jang, J. Heo and Y. Yoon, Applications of MXene-Based Membranes in Water Purification: A Review, Chemosphere, 2020, 254, 126821, DOI:10.1016/j.chemosphere.2020.126821
.
- M. Sajid, S. M. Sajid Jillani, N. Baig and K. Alhooshani, Layered Double Hydroxide-Modified Membranes for Water Treatment: Recent Advances and Prospects, Chemosphere, 2022, 287, 132140, DOI:10.1016/j.chemosphere.2021.132140
.
- N. A. Khan, J. Yuan, H. Wu, L. Cao, R. Zhang, Y. Liu, L. Li, A. U. Rahman, R. Kasher and Z. Jiang, Mixed Nanosheet Membranes Assembled from Chemically Grafted Graphene Oxide and Covalent Organic Frameworks for Ultra-High Water Flux, ACS Appl. Mater. Interfaces, 2019, 11(32), 28978–28986, DOI:10.1021/acsami.9b09945
.
- H. Zhou, J. R. Long and O. M. Yaghi, Introduction to Metal–Organic Frameworks, Chem. Rev., 2012, 112(2), 673–674, DOI:10.1021/cr300014x
.
- H.-C. J. Zhou and S. Kitagawa, Metal–Organic Frameworks (MOFs), Chem. Soc. Rev., 2014, 43(16), 5415–5418, 10.1039/C4CS90059F
.
- C. S. Diercks and O. M. Yaghi, The atom, the molecule, and the covalent organic framework, Science, 2017, 355(6328), eaal1585, DOI:10.1126/science.aal1585
.
- E. Mohamed, K. Jaheon, R. Nathaniel, V. David, W. Joseph, O. Michael and O. M. Yaghi, Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage, Science, 2002, 295(5554), 469–472, DOI:10.1126/science.1067208
.
- S. Chowdhury, P. Sharma, K. Kundu, P. P. Das, P. Rathi and P. F. Siril, Systematic Thiol Decoration in a Redox-Active UiO-66-(SH)2 Metal–Organic Framework: A Case Study under Oxidative and Reductive Conditions, Inorg. Chem., 2023, 62(9), 3875–3885, DOI:10.1021/acs.inorgchem.2c04233
.
- J. L. Segura, S. Royuela and M. Mar Ramos, Post-Synthetic Modification of Covalent Organic Frameworks, Chem. Soc. Rev., 2019, 48(14), 3903–3945, 10.1039/C8CS00978C
.
- M. Kalaj and S. M. Cohen, Postsynthetic Modification: An Enabling Technology for the Advancement of Metal–Organic Frameworks, ACS Cent. Sci., 2020, 6(7), 1046–1057, DOI:10.1021/acscentsci.0c00690
.
- A. Helal, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Multivariate Metal-Organic Frameworks, Natl. Sci. Rev., 2017, 4(3), 296–298, DOI:10.1093/nsr/nwx013
.
- M. Mon, R. Bruno, E. Tiburcio, M. Viciano-Chumillas, L. H. G. Kalinke, J. Ferrando-Soria, D. Armentano and E. Pardo, Multivariate Metal–Organic Frameworks for the Simultaneous Capture of Organic and Inorganic Contaminants from Water, J. Am. Chem. Soc., 2019, 141(34), 13601–13609, DOI:10.1021/jacs.9b06250
.
- L. Niu, X. Zhao, F. Wu, Z. Tang, H. Lv, J. Wang, M. Fang and J. P. Giesy, Hotpots and Trends of Covalent Organic Frameworks (COFs) in the Environmental and Energy Field: Bibliometric Analysis, Sci. Total Environ., 2021, 783, 146838, DOI:10.1016/j.scitotenv.2021.146838
.
- M. S. Denny, J. C. Moreton, L. Benz and S. M. Cohen, Metal–organic frameworks for membrane-based separations, Nat. Rev. Mater., 2016, 1(12), 1–17, DOI:10.1038/natrevmats.2016.78
.
- J. Li, H. Wang, X. Yuan, J. Zhang and J. W. Chew, Metal-Organic Framework Membranes for Wastewater Treatment and Water Regeneration, Coord. Chem. Rev., 2020, 404, 213116, DOI:10.1016/j.ccr.2019.213116
.
- Z. Wang, S. Zhang, Y. Chen, Z. Zhang and S. Ma, Covalent Organic Frameworks for Separation Applications, Chem. Soc. Rev., 2020, 49(3), 708–735, 10.1039/C9CS00827F
.
- J. Li, X. Zhou, J. Wang and X. Li, Two-Dimensional Covalent Organic Frameworks (COFs) for Membrane Separation: A Mini Review, Ind. Eng. Chem. Res., 2019, 58(34), 15394–15406, DOI:10.1021/acs.iecr.9b02708
.
- Z. Xia, Y. Zhao and S. B. Darling, Hall of Fame Article: Covalent Organic Frameworks for Water Treatment (Adv. Mater. Interfaces 1/2021), Adv. Mater. Interfaces, 2021, 8(1), 2170005, DOI:10.1002/admi.202170005
.
-
J. G. Vitillo, C. Atzori, B. Civalleri, N. Barbero, C. Barolo and F. Bonino, Design and Characterization of MOFs (Metal–Organic Frameworks) for Innovative Applications, in Hybrid Organic-Inorganic Interfaces, 2018, pp. 459–495, DOI:10.1002/9783527807130.ch10
.
- W. Xu and O. M. Yaghi, Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime, ACS Cent. Sci., 2020, 6(8), 1348–1354, DOI:10.1021/acscentsci.0c00678
.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Recent Advances in Dynamic Covalent Chemistry, Chem. Soc. Rev., 2013, 42(16), 6634–6654, 10.1039/C3CS60044K
.
- L. Bourda, C. Krishnaraj, P. Van Der Voort and K. Van Hecke, Conquering the Crystallinity Conundrum: Efforts to Increase Quality of Covalent Organic Frameworks, Mater. Adv., 2021, 2(9), 2811–2845, 10.1039/D1MA00008J
.
- L. Deng, Z. Ding, X. Ye and D. Jiang, Covalent Organic Frameworks: Chemistry of Pore Interface and Wall Surface Perturbation and Impact on Functions, Acc. Mater. Res., 2022, 3(8), 879–893, DOI:10.1021/accountsmr.2c00108
.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120(16), 8814–8933, DOI:10.1021/acs.chemrev.9b00550
.
- M.-X. Wu and Y.-W. Yang, Applications of Covalent Organic Frameworks (COFs): From Gas Storage and Separation to Drug Delivery, Chin. Chem. Lett., 2017, 28(6), 1135–1143, DOI:10.1016/j.cclet.2017.03.026
.
- J. Guo and D. Jiang, Covalent Organic Frameworks for Heterogeneous Catalysis: Principle, Current Status, and Challenges, ACS Cent. Sci., 2020, 6(6), 869–879, DOI:10.1021/acscentsci.0c00463
.
- X. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, H. Wang, H. Yi, B. Li, S. Liu, M. Zhang, R. Deng, Y. Fu, L. Li, W. Xue and S. Chen, Recent Advances in Covalent Organic Frameworks (COFs) as a Smart Sensing Material, Chem. Soc. Rev., 2019, 48(20), 5266–5302, 10.1039/C9CS00299E
.
- X. Zhao, P. Pachfule and A. Thomas, Covalent Organic Frameworks (COFs) for Electrochemical Applications, Chem. Soc. Rev., 2021, 50(12), 6871–6913, 10.1039/D0CS01569E
.
- M. Kalaj, K. C. Bentz, S. Ayala, J. M. Palomba, K. S. Barcus, Y. Katayama and S. M. Cohen, MOF-Polymer Hybrid Materials: From Simple Composites to Tailored Architectures, Chem. Rev., 2020, 120(16), 8267–8302, DOI:10.1021/acs.chemrev.9b00575
.
- B. M. Jun, Y. A. J. Al-Hamadani, A. Son, C. M. Park, M. Jang, A. Jang, N. C. Kim and Y. Yoon, Applications of Metal-Organic Framework Based Membranes in Water Purification: A Review, Sep. Purif. Technol., 2020, 247, 116947, DOI:10.1016/j.seppur.2020.116947
.
- R. Castro-Muñoz and V. Fíla, Effect of the ZIF-8 Distribution in Mixed-Matrix Membranes Based on Matrimid® 5218-PEG on CO 2 Separation, Chem. Eng. Technol., 2019, 744–752, DOI:10.1002/ceat.201800499
.
- X.-J. Yu, Y.-M. Xian, C. Wang, H.-L. Mao, M. Kind, T. Abu-Husein, Z. Chen, S.-B. Zhu, B. Ren, A. Terfort and J.-L. Zhuang, Liquid-Phase Epitaxial Growth of Highly Oriented and Multivariate Surface-Attached Metal–Organic Frameworks, J. Am. Chem. Soc., 2019, 141(48), 18984–18993, DOI:10.1021/jacs.9b08169
.
- S.-M. Chen, L.-M. Chang, X.-K. Yang, T. Luo, H. Xu, Z.-G. Gu and J. Zhang, Liquid-Phase Epitaxial Growth of Azapyrene-Based Chiral Metal–Organic Framework Thin Films for Circularly Polarized Luminescence, ACS Appl. Mater. Interfaces, 2019, 11(34), 31421–31426, DOI:10.1021/acsami.9b11872
.
- S. Jiang, X. Shi, Y. Zu, F. Sun and G. Zhu, Interfacial Growth of 2D MOF Membranes via Contra-Diffusion for CO2 Separation, Mater. Chem. Front., 2021, 5(13), 5150–5157, 10.1039/D1QM00154J
.
- H. Wang, S. He, X. Qin, C. Li and T. Li, Interfacial Engineering in Metal–Organic Framework-Based Mixed Matrix Membranes Using Covalently Grafted Polyimide Brushes, J. Am. Chem. Soc., 2018, 140(49), 17203–17210, DOI:10.1021/jacs.8b10138
.
- A. M. Marti, S. R. Venna, E. A. Roth, J. T. Culp and D. P. Hopkinson, Simple Fabrication Method for Mixed Matrix Membranes with in Situ MOF Growth for Gas Separation, ACS Appl. Mater. Interfaces, 2018, 10(29), 24784–24790, DOI:10.1021/acsami.8b06592
.
- M. S. Denny, M. Kalaj, K. C. Bentz and S. M. Cohen, Multicomponent Metal–Organic Framework Membranes for Advanced Functional Composites, Chem. Sci., 2018, 9(47), 8842–8849, 10.1039/C8SC02356E
.
- R. Lin, B. Villacorta Hernandez, L. Ge and Z. Zhu, Metal Organic Framework Based Mixed Matrix Membranes: An Overview on Filler/Polymer Interfaces, J. Mater. Chem. A, 2018, 6(2), 293–312, 10.1039/C7TA07294E
.
- L. Cseri, R. Hardian, S. Anan, H. Vovusha, U. Schwingenschlögl, P. M. Budd, K. Sada, K. Kokado and G. Szekely, Bridging the Interfacial Gap in Mixed-Matrix Membranes by Nature-Inspired Design: Precise Molecular Sieving with Polymer-Grafted Metal–Organic Frameworks, J. Mater. Chem. A, 2021, 9(42), 23793–23801, 10.1039/D1TA06205K
.
- D. Fan, A. Ozcan, O. Shekhah, R. Semino, M. Eddaoudi and G. Maurin, Engineering MOF Surface Defects in Mixed Matrix Membranes: An Effective Strategy to Enhance MOF/Polymer
Adhesion and Control Interfacial Gas Transport, J. Membr. Sci. Lett., 2022, 2(2), 100029, DOI:10.1016/j.memlet.2022.100029
.
- I.-D. Carja, S. R. Tavares, O. Shekhah, A. Ozcan, R. Semino, V. S. Kale, M. Eddaoudi and G. Maurin, Insights into the Enhancement of MOF/Polymer Adhesion in Mixed-Matrix Membranes via Polymer Functionalization, ACS Appl. Mater. Interfaces, 2021, 13(24), 29041–29047, DOI:10.1021/acsami.1c03859
.
- X. Li, Y. Liu, J. Wang, J. Gascon, J. Li and B. Van der Bruggen, Metal–Organic Frameworks Based Membranes for Liquid Separation, Chem. Soc. Rev., 2017, 46(23), 7124–7144, 10.1039/C7CS00575J
.
- H. Dou, M. Xu, B. Wang, Z. Zhang, G. Wen, Y. Zheng, D. Luo, L. Zhao, A. Yu, L. Zhang, Z. Jiang and Z. Chen, Microporous Framework Membranes for Precise Molecule/Ion Separations, Chem. Soc. Rev., 2021, 50(2), 986–1029, 10.1039/D0CS00552E
.
- X. Liu, Metal-Organic Framework UiO-66 Membranes, Front. Chem. Sci. Eng., 2020, 14(2), 216–232, DOI:10.1007/s11705-019-1857-5
.
- H. Jin, K. Mo, F. Wen and Y. Li, Preparation and Pervaporation Performance of CAU-10-H MOF Membranes, J. Membr. Sci., 2019, 577, 129–136, DOI:10.1016/j.memsci.2019.02.008
.
- H. J. Yu, D.-S. Chiou, C.-H. Hsu, H.-Y. Tsai, M.-Y. Kan, J. S. Lee and D.-Y. Kang, Engineering CAU-10-H in the Preparation of Mixed Matrix Membranes for Gas Separation, J. Membr. Sci., 2022, 663, 121024, DOI:10.1016/j.memsci.2022.121024
.
- M. S. Mirqasemi, M. Homayoonfal and M. Rezakazemi, Zeolitic Imidazolate Framework Membranes for Gas and Water Purification, Environ. Chem. Lett., 2020, 18(1), 1–52, DOI:10.1007/s10311-019-00933-6
.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications, Adv. Funct. Mater., 2021, 31(4), 2006291, DOI:10.1002/adfm.202006291
.
- Z. Yin, S. Wan, J. Yang, M. Kurmoo and M.-H. Zeng, Recent Advances in Post-Synthetic Modification of Metal–Organic Frameworks: New Types and Tandem Reactions, Coord. Chem. Rev., 2019, 378, 500–512, DOI:10.1016/j.ccr.2017.11.015
.
- H. Saleem, U. Rafique and R. P. Davies, Investigations on Post-Synthetically Modified UiO-66-NH2 for the Adsorptive Removal of Heavy Metal Ions from Aqueous Solution, Microporous Mesoporous Mater., 2016, 221, 238–244, DOI:10.1016/j.micromeso.2015.09.043
.
- A. A. Alqadami, M. A. Khan, M. R. Siddiqui and Z. A. Alothman, Development of Citric Anhydride Anchored Mesoporous MOF through Post Synthesis Modification to Sequester Potentially Toxic Lead (II) from Water, Microporous Mesoporous Mater., 2018, 261, 198–206, DOI:10.1016/j.micromeso.2017.11.016
.
- H. Zhang, J. James, M. Zhao, Y. Yao, Y. Zhang, B. Zhang and Y. S. Lin, Improving Hydrostability of ZIF-8 Membranes via Surface Ligand Exchange, J. Membr. Sci., 2017, 532, 1–8, DOI:10.1016/j.memsci.2017.01.065
.
- R. Zhang, S. Ji, N. Wang, L. Wang, G. Zhang and J.-R. Li, Coordination-Driven In Situ Self-Assembly Strategy for the Preparation of Metal–Organic Framework Hybrid Membranes, Angew. Chem., Int. Ed., 2014, 53(37), 9775–9779, DOI:10.1002/anie.201403978
.
- L. Wang, M. Fang, J. Liu, J. He, J. Li and J. Lei, Layer-by-Layer Fabrication of High-Performance Polyamide/ZIF-8 Nanocomposite Membrane for Nanofiltration Applications, ACS Appl. Mater. Interfaces, 2015, 7(43), 24082–24093, DOI:10.1021/acsami.5b07128
.
- L. Yang, Z. Wang and J. Zhang, Highly Permeable Zeolite Imidazolate Framework Composite Membranes Fabricated via a Chelation-Assisted Interfacial Reaction, J. Mater. Chem. A, 2017, 5(29), 15342–15355, 10.1039/C7TA03244G
.
- H. Ting, H.-Y. Chi, C. H. Lam, K.-Y. Chan and D.-Y. Kang, High-Permeance Metal–Organic Framework-Based Membrane Adsorber for the Removal of Dye Molecules in Aqueous Phase, Environ. Sci.: Nano, 2017, 4(11), 2205–2214, 10.1039/C7EN00639J
.
- X. Zhang, T. Wang, L. Wu and H.-C. Guo, Construction of Ag@ZIF-8/PVDF Mixed-Matrix Ultrafiltration Membranes with High Separation Performance for Dye from High-Salinity Wastewater by Microemulsion Coupling with Blending, J. Membr. Sci., 2023, 670, 121371, DOI:10.1016/j.memsci.2023.121371
.
- H.-Y. Chi, S.-H. Hung, M.-Y. Kan, L.-W. Lee, C. H. Lam, J.-J. Chen and D.-Y. Kang, Metal–Organic Frameworks for Dye Sorption: Structure–Property Relationships and Scalable Deposition of the Membrane Adsorber, CrystEngComm, 2018, 20(36), 5465–5474, 10.1039/C8CE01066H
.
- J. Zhu, L. Qin, A. Uliana, J. Hou, J. Wang, Y. Zhang, X. Li, S. Yuan, J. Li, M. Tian, J. Lin and B. Van der Bruggen, Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration, ACS Appl. Mater. Interfaces, 2017, 9(2), 1975–1986, DOI:10.1021/acsami.6b14412
.
- Z. Li, P. Yang, Z. Gao, M. Song, Q. Fang, M. Xue and S. Qiu, A New ZIF Molecular-Sieving Membrane for High-Efficiency Dye Removal, Chem. Commun., 2019, 55(24), 3505–3508, 10.1039/C9CC00902G
.
- M. S. Denny Jr. and S. M. Cohen, In Situ Modification of Metal–Organic Frameworks in Mixed-Matrix Membranes, Angew. Chem., Int. Ed., 2015, 54(31), 9029–9032, DOI:10.1002/anie.201504077
.
- J. C. Moreton, M. S. Denny and S. M. Cohen, High MOF Loading in Mixed-Matrix Membranes Utilizing Styrene/Butadiene Copolymers, Chem. Commun., 2016, 52(100), 14376–14379, 10.1039/c6cc07329h
.
- B.-J. Yao, W.-L. Jiang, Y. Dong, Z.-X. Liu and Y.-B. Dong, Post-Synthetic Polymerization of UiO-66-NH2 Nanoparticles and Polyurethane Oligomer toward Stand-Alone Membranes for Dye Removal and Separation, Chem. – Eur. J., 2016, 22(30), 10565–10571, DOI:10.1002/chem.201600817
.
- H. Wang, S. Zhao, Y. Liu, R. Yao, X. Wang, Y. Cao, D. Ma, M. Zou, A. Cao, X. Feng and B. Wang, Membrane Adsorbers with Ultrahigh Metal-Organic Framework Loading for High Flux Separations, Nat. Commun., 2019, 10(1), 4204, DOI:10.1038/s41467-019-12114-8
.
- H. Ang and L. Hong, Polycationic Polymer-Regulated Assembling of 2D MOF Nanosheets for High-Performance Nanofiltration, ACS Appl. Mater. Interfaces, 2017, 9(33), 28079–28088, DOI:10.1021/acsami.7b08383
.
- W. A. El-Mehalmey, Y. Safwat, M. Bassyouni and M. H. Alkordi, Strong Interplay between Polymer Surface Charge and MOF Cage Chemistry in Mixed-Matrix Membrane for Water Treatment Applications, ACS Appl. Mater. Interfaces, 2020, 12(24), 27625–27631, DOI:10.1021/acsami.0c06399
.
- P. Zhao, J. Wang, X. Han, J. Liu, Y. Zhang and B. Van der Bruggen, Zr-Porphyrin Metal–Organic Framework-Based Photocatalytic Self-Cleaning Membranes for Efficient Dye Removal, Ind. Eng. Chem. Res., 2021, 60(4), 1850–1858, DOI:10.1021/acs.iecr.0c05583
.
- K. Zhu, C. Chen, H. Xu, Y. Gao, X. Tan, A. Alsaedi and T. Hayat, Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres, ACS Sustainable Chem. Eng., 2017, 5(8), 6795–6802, DOI:10.1021/acssuschemeng.7b01036
.
- H. Shayegan, G. A. M. Ali and V. Safarifard, Recent Progress in the Removal of Heavy Metal Ions from Water Using Metal-Organic Frameworks, ChemistrySelect, 2020, 5(1), 124–146, DOI:10.1002/slct.201904107
.
- Y. Cheng, S. J. Datta, S. Zhou, J. Jia, O. Shekhah and M. Eddaoudi, Advances in Metal–Organic Framework-Based Membranes, Chem. Soc. Rev., 2022, 51(19), 8300–8350, 10.1039/D2CS00031H
.
- F. Zadehahmadi, N. T. Eden, H. Mahdavi, K. Konstas, J. I. Mardel, M. Shaibani, P. C. Banerjee and M. R. Hill, Removal of Metals from Water Using MOF-Based Composite Adsorbents, Environ. Sci.: Water Res. Technol., 2023, 9(5), 1305–1330, 10.1039/D2EW00941B
.
- H. I. Adil, M. R. Thalji, S. A. Yasin, I. A. Saeed, M. A. Assiri, K. F. Chong and G. A. M. Ali, Metal–Organic Frameworks (MOFs) Based Nanofiber Architectures for the Removal of Heavy Metal Ions, RSC Adv., 2022, 12(3), 1433–1450, 10.1039/D1RA07034G
.
- N. Abdollahi, G. Moussavi and S. Giannakis, A Review of Heavy Metals' Removal from Aqueous Matrices by Metal-Organic Frameworks (MOFs): State-of-the Art and Recent Advances, J. Environ. Chem. Eng., 2022, 10(3), 107394, DOI:10.1016/j.jece.2022.107394
.
- P. Chen, Y. Wang, X. Zhuang, H. Liu, G. Liu and W. Lv, Selective Removal of Heavy Metals by Zr-Based MOFs in Wastewater: New Acid and Amino Functionalization Strategy, J. Environ. Sci., 2023, 124, 268–280, DOI:10.1016/j.jes.2021.10.010
.
- M. Zhao, Z. Huang, S. Wang, L. Zhang and Y. Zhou, Design of L-Cysteine Functionalized UiO-66 MOFs for Selective Adsorption of Hg(II) in Aqueous Medium, ACS Appl. Mater. Interfaces, 2019, 11(50), 46973–46983, DOI:10.1021/acsami.9b17508
.
- F. Ahmadijokani, S. Tajahmadi, A. Bahi, H. Molavi, M. Rezakazemi, F. Ko, T. M. Aminabhavi and M. Arjmand, Ethylenediamine-Functionalized Zr-Based MOF for Efficient Removal of Heavy Metal Ions from Water, Chemosphere, 2021, 264, 128466, DOI:10.1016/j.chemosphere.2020.128466
.
- C. O. Audu, H. G. T. Nguyen, C.-Y. Chang, M. J. Katz, L. Mao, O. K. Farha, J. T. Hupp and S. T. Nguyen, The Dual Capture of AsV and AsIII by UiO-66 and Analogues, Chem. Sci., 2016, 7(10), 6492–6498, 10.1039/C6SC00490C
.
- R. Xu, Q. Ji, P. Zhao, M. Jian, C. Xiang, C. Hu, G. Zhang, C. Tang, R. Liu, X. Zhang and J. Qu, Hierarchically Porous UiO-66 with Tunable Mesopores and Oxygen Vacancies for Enhanced Arsenic Removal, J. Mater. Chem. A, 2020, 8(16), 7870–7879, 10.1039/C9TA13747E
.
- L. Huang, B. Wu, Y. Wu, Z. Yang, T. Yuan, S. I. Alhassan, W. Yang, H. Wang and L. Zhang, Porous and Flexible Membrane Derived from ZIF-8-Decorated Hyphae for Outstanding Adsorption of Pb2+ Ion, J. Colloid Interface Sci., 2020, 565, 465–473, DOI:10.1016/j.jcis.2020.01.035
.
- S. Wu, F. Li, Y. Wu, R. Xu and G. Li, Preparation of Novel Poly(Vinyl Alcohol)/SiO2 Composite Nanofiber Membranes with Mesostructure and Their Application for Removal of Cu2+ from Waste Water, Chem. Commun., 2010, 46(10), 1694–1696, 10.1039/B925296G
.
- Q. Feng, D. Wu, Y. Zhao, A. Wei, Q. Wei and H. Fong, Electrospun AOPAN/RC Blend Nanofiber Membrane for Efficient Removal of Heavy Metal Ions from Water, J. Hazard. Mater., 2018, 344, 819–828, DOI:10.1016/j.jhazmat.2017.11.035
.
- Y. Ren, L. Mei, Y. Gu, N. Zhao, Y. Wang, R. Fan, A. Tong, H. Chen, H. Yang, B. Han and G. Guo, Stereocomplex Crystallite-Based Eco-Friendly Nanofiber Membranes for Removal of Cr(VI) and Antibacterial Effects, ACS Sustainable Chem. Eng., 2019, 7(19), 16072–16083, DOI:10.1021/acssuschemeng.9b02828
.
- Y. Dou, W. Zhang and A. Kaiser, Electrospinning of Metal–Organic Frameworks for Energy and Environmental Applications, Adv. Sci., 2020, 7(3), 1902590, DOI:10.1002/advs.201902590
.
- F. Ahmadijokani, H. Molavi, A. Bahi, R. Fernández, P. Alaee, S. Wu, S. Wuttke, F. Ko and M. Arjmand, Metal-Organic Frameworks and Electrospinning: A Happy Marriage for Wastewater Treatment, Adv. Funct. Mater., 2022, 32(51), 2207723, DOI:10.1002/adfm.202207723
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Insight Studies on Metal-Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution, ACS Appl. Mater. Interfaces, 2018, 10(22), 18619–18629, DOI:10.1021/acsami.8b01454
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Effects of Operating Parameters and Coexisting Ions on the Efficiency of Heavy Metal Ions Removal by Nano-Fibrous Metal-Organic Framework Membrane Filtration Process, Sci. Total Environ., 2019, 674, 355–362, DOI:10.1016/j.scitotenv.2019.04.187
.
- S. Jamshidifard, S. Koushkbaghi, S. Hosseini, S. Rezaei, A. Karamipour, A. Jafari rad and M. Irani, Incorporation of UiO-66-NH2 MOF into the PAN/Chitosan Nanofibers for Adsorption and Membrane Filtration of Pb(II), Cd(II) and Cr(VI) Ions from Aqueous Solutions, J. Hazard. Mater., 2019, 368, 10–20, DOI:10.1016/j.jhazmat.2019.01.024
.
- T. Hashem, A. H. Ibrahim, C. Wöll and M. H. Alkordi, Grafting Zirconium-Based Metal–Organic Framework UiO-66-NH2 Nanoparticles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water, ACS Appl. Nano Mater., 2019, 2(9), 5804–5808, DOI:10.1021/acsanm.9b01263
.
- Z. Li, G. Zhou, H. Dai, M. Yang, Y. Fu, Y. Ying and Y. Li, Biomineralization-Mimetic Preparation of Hybrid Membranes with Ultra-High Loading of Pristine Metal–Organic Frameworks Grown on Silk Nanofibers for Hazard Collection in Water, J. Mater. Chem. A, 2018, 6(8), 3402–3413, 10.1039/C7TA06924C
.
- X. Hou, H. Zhou, J. Zhang, Y. Cai, F. Huang and Q. Wei, High Adsorption Pearl-Necklace-Like Composite Membrane Based on Metal–Organic Framework for Heavy Metal Ion Removal, Part. Part. Syst. Charact., 2018, 35(6), 1700438, DOI:10.1002/ppsc.201700438
.
- J. E. Efome, D. Rana, T. Matsuura and C. Q. Lan, Metal–Organic Frameworks Supported on Nanofibers to Remove Heavy Metals, J. Mater. Chem. A, 2018, 6(10), 4550–4555, 10.1039/C7TA10428F
.
- X. Chen, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Modified-MOF-808-Loaded Polyacrylonitrile Membrane for Highly Efficient, Simultaneous Emulsion Separation and Heavy Metal Ion Removal, ACS Appl. Mater. Interfaces, 2020, 12(35), 39227–39235, DOI:10.1021/acsami.0c10290
.
- Z. Hu, Y. Chen and J. Jiang, Zeolitic Imidazolate Framework-8 as a Reverse Osmosis Membrane for Water Desalination: Insight from Molecular Simulation, J. Chem. Phys., 2011, 134(13), 134705, DOI:10.1063/1.3573902
.
- K. M. Gupta, K. Zhang and J. Jiang, Water Desalination through Zeolitic Imidazolate Framework Membranes: Significant Role of Functional Groups, Langmuir, 2015, 31(48), 13230–13237, DOI:10.1021/acs.langmuir.5b03593
.
- X. Liu, N. K. Demir, Z. Wu and K. Li, Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination, J. Am. Chem. Soc., 2015, 137(22), 6999–7002, DOI:10.1021/jacs.5b02276
.
- B. L. Bonnett, E. D. Smith, M. De La Garza, M. Cai, J. V. Haag, J. M. Serrano, H. D. Cornell, B. Gibbons, S. M. Martin and A. J. Morris, PCN-222 Metal–Organic Framework Nanoparticles with Tunable Pore Size for Nanocomposite Reverse Osmosis Membranes, ACS Appl. Mater. Interfaces, 2020, 12(13), 15765–15773, DOI:10.1021/acsami.0c04349
.
- H. M. Park, K. Y. Jee and Y. T. Lee, Preparation and Characterization of a Thin-Film Composite Reverse Osmosis Membrane Using a Polysulfone Membrane Including Metal-Organic Frameworks, J. Membr. Sci., 2017, 541, 510–518, DOI:10.1016/j.memsci.2017.07.034
.
- T. H. Lee, J. Y. Oh, S. P. Hong, J. M. Lee, S. M. Roh, S. H. Kim and H. B. Park, ZIF-8 Particle Size Effects on Reverse Osmosis Performance of Polyamide Thin-Film Nanocomposite Membranes: Importance of Particle Deposition, J. Membr. Sci., 2019, 570–571, 23–33, DOI:10.1016/j.memsci.2018.10.015
.
- H. Liu, M. Zhang, H. Zhao, Y. Jiang, G. Liu and J. Gao, MOFs ) in the Organic Phase via Surface Modification for TFN Nanofiltration Membrane Prepration, RSC Adv., 2020, 10, 4045–4057, 10.1039/c9ra09672h
.
- H. Liu, M. Zhang, H. Zhao, Y. Jiang, G. Liu and J. Gao, Enhanced Dispersibility of Metal–Organic Frameworks (MOFs) in the Organic Phase via Surface Modification for TFN Nanofiltration Membrane Preparation, RSC Adv., 2020, 10(7), 4045–4057, 10.1039/C9RA09672H
.
- Z. Wang, Z. Wang, S. Lin, H. Jin, S. Gao, Y. Zhu and J. Jin, Nanoparticle-Templated Nanofiltration Membranes for Ultrahigh Performance Desalination, Nat. Commun., 2018, 9(1), 2004, DOI:10.1038/s41467-018-04467-3
.
- Y. Zhao, Y. Liu, X. Wang, X. Huang and Y. F. Xie, Impacts of Metal–Organic Frameworks on Structure and Performance of Polyamide Thin-Film Nanocomposite Membranes, ACS Appl. Mater. Interfaces, 2019, 11(14), 13724–13734, DOI:10.1021/acsami.9b01923
.
- J. Casaban, Y. Zhang, R. Pacheco, C. Coney, C. Holmes, E. Sutherland, C. Hamill, J. Breen, S. L. James, D. Tufano, D. Wong, E. Stavrakakis, H. Annath and A. Moore, Towards MOFs' Mass Market Adoption: MOF Technologies' Efficient and Versatile One-Step Extrusion of Shaped MOFs Directly from Raw Materials, Faraday Discuss., 2021, 231, 312–325, 10.1039/D1FD00025J
.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Porous, Crystalline, Covalent Organic Frameworks, Science, 2005, 310(5751), 1166–1170, DOI:10.1126/science.1120411
.
- Z. Xia, Y. Zhao and S. B. Darling, Covalent Organic Frameworks for Water Treatment, Adv. Mater. Interfaces, 2021, 8(1), 2001507, DOI:10.1002/admi.202001507
.
- A. K. Mohammed and D. Shetty, Macroscopic Covalent Organic Framework Architectures for Water Remediation, Environ. Sci.: Water Res. Technol., 2021, 7(11), 1895–1927, 10.1039/D1EW00408E
.
- I. Ahmed and S. H. Jhung, Covalent Organic Framework-Based Materials: Synthesis, Modification, and Application in Environmental Remediation, Coord. Chem. Rev., 2021, 441, 213989, DOI:10.1016/j.ccr.2021.213989
.
- R. Wang, J. Guo, J. Xue and H. Wang, Covalent Organic Framework Membranes for Efficient Chemicals Separation, Small Struct., 2021, 2(10), 2100061, DOI:10.1002/sstr.202100061
.
- C.-H. Yang, J.-S. Chang and D.-J. Lee, Chemically Stable Covalent Organic Framework as Adsorbent from Aqueous Solution: A Mini-Review, J. Taiwan Inst. Chem. Eng., 2020, 110, 79–91, DOI:10.1016/j.jtice.2020.02.008
.
- S. Yuan, X. Li, J. Zhu, G. Zhang, P. Van Puyvelde and B. Van der Bruggen, Covalent Organic Frameworks for Membrane Separation, Chem. Soc. Rev., 2019, 48(10), 2665–2681, 10.1039/C8CS00919H
.
- A. K. Mohammed and D. Shetty, Macroscopic Covalent Organic Framework Architectures for Water Remediation, Environ. Sci.: Water Res. Technol., 2021, 7(11), 1895–1927, 10.1039/D1EW00408E
.
- Z. Xia, Y. Zhao and S. B. Darling, Covalent Organic Frameworks for Water Treatment, Adv. Mater. Interfaces, 2021, 8(1), 2001507, DOI:10.1002/admi.202001507
.
- K. Dey, S. Bhunia, H. S. Sasmal, C. M. Reddy and R. Banerjee, Self-Assembly-Driven Nanomechanics in Porous Covalent Organic Framework Thin Films, J. Am. Chem. Soc., 2021, 143(2), 955–963, DOI:10.1021/jacs.0c11122
.
- S. Karak, S. Kumar, P. Pachfule and R. Banerjee, Porosity Prediction through Hydrogen Bonding in Covalent Organic Frameworks, J. Am. Chem. Soc., 2018, 140(15), 5138–5145, DOI:10.1021/jacs.7b13558
.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process, J. Am. Chem. Soc., 2017, 139(5), 1856–1862, DOI:10.1021/jacs.6b08815
.
- J. Thote, H. Barike Aiyappa, R. Rahul Kumar, S. Kandambeth, B. P. Biswal, D. Balaji Shinde, N. Chaki Roy and R. Banerjee, Constructing Covalent Organic Frameworks in Water via Dynamic Covalent Bonding, IUCrJ, 2016, 3(6), 402–407, DOI:10.1107/S2052252516013762
.
- G.-H. Ning, Z. Chen, Q. Gao, W. Tang, Z. Chen, C. Liu, B. Tian, X. Li and K. P. Loh, Salicylideneanilines-Based Covalent Organic Frameworks as Chemoselective Molecular Sieves, J. Am. Chem. Soc., 2017, 139(26), 8897–8904, DOI:10.1021/jacs.7b02696
.
- K. Dey, M. Pal, K. C. Rout, H. S. Kunjattu, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films, J. Am. Chem. Soc., 2017, 139(37), 13083–13091, DOI:10.1021/jacs.7b06640
.
- S. Kandambeth, B. P. Biswal, H. D. Chaudhari, K. C. Rout, H. S. Kunjattu, S. Mitra, S. Karak, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes, Adv. Mater., 2017, 29(2), 1603945, DOI:10.1002/adma.201603945
.
- S.-B. Yu, A polycationic covalent organic framework: a robust adsorbent for anionic dye pollutants, Polym. Chem., 2016, 3392–3397, 10.1039/C6PY00281A
.
- H. Fan, J. Gu, H. Meng, A. Knebel and J. Caro, High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration, Angew. Chem., Int. Ed., 2018, 57(15), 4083–4087, DOI:10.1002/anie.201712816
.
- R. Wang, X. Shi, A. Xiao, W. Zhou and Y. Wang, Interfacial Polymerization of Covalent Organic Frameworks (COFs) on Polymeric Substrates for Molecular Separations, J. Membr. Sci., 2018, 566, 197–204, DOI:10.1016/j.memsci.2018.08.044
.
- I. Gadwal, G. Sheng, R. L. Thankamony, Y. Liu, H. Li and Z. Lai, Synthesis of Sub-10 Nm Two-Dimensional Covalent Organic Thin Film with Sharp Molecular Sieving Nanofiltration, ACS Appl. Mater. Interfaces, 2018, 10(15), 12295–12299, DOI:10.1021/acsami.7b19450
.
- W. Zhang, L. Zhang, H. Zhao, B. Li and H. Ma, A Two-Dimensional Cationic Covalent Organic Framework Membrane for Selective Molecular Sieving, J. Mater. Chem. A, 2018, 6(27), 13331–13339, 10.1039/C8TA04178D
.
- Y. He and X. Lin, Fabricating Compact Covalent Organic Framework Membranes with Superior Performance in Dye Separation, J. Membr. Sci., 2021, 637, 119667, DOI:10.1016/j.memsci.2021.119667
.
- A. Singh, R. Gogoi, K. Sharma, R. Kumar and P. F. Siril, Continuous Flow Synthesis of Disordered Covalent Organic Framework for Ultra-High Removal of Industrial Pollutants in Flow, Sep. Purif. Technol., 2023, 307, 122739, DOI:10.1016/j.seppur.2022.122739
.
- S.-B. Yu, H. Lyu, J. Tian, H. Wang, D.-W. Zhang, Y. Liu and Z.-T. Li, A Polycationic Covalent Organic Framework: A Robust Adsorbent for Anionic Dye Pollutants, Polym. Chem., 2016, 7(20), 3392–3397, 10.1039/C6PY00281A
.
- M. Firoozi, Z. Rafiee and K. Dashtian, New MOF/COF Hybrid as a Robust Adsorbent for Simultaneous Removal of Auramine O and Rhodamine B Dyes, ACS Omega, 2020, 5(16), 9420–9428, DOI:10.1021/acsomega.0c00539
.
- L. Zhang, Y. Li, Y. Wang, S. Ma, J. Ou, Y. Shen, M. Ye and H. Uyama, Integration of Covalent Organic Frameworks into Hydrophilic Membrane with Hierarchical Porous Structure for Fast Adsorption of Metal Ions, J. Hazard. Mater., 2021, 407, 124390, DOI:10.1016/j.jhazmat.2020.124390
.
- W.-L. Jin, X. Ji, X.-L. Hou, S.-Y. Ji, W. Li, X. Yu, X.-W. Liu, L.-N. Zhu, H.-X. Jiang and D.-M. Kong, Porphyrin COF and Its Mechanical Pressing-Prepared Carbon Fiber Hybrid Membrane for Ratiometric Detection, Removal and Enrichment of Cd2+, J. Hazard. Mater., 2022, 439, 129574, DOI:10.1016/j.jhazmat.2022.129574
.
- E. A. Gendy, J. Ifthikar, J. Ali, D. T. Oyekunle, Z. Elkhlifia, I. I. Shahib, A. I. Khodair and Z. Chen, Removal of Heavy Metals by Covalent Organic Frameworks (COFs): A Review on Its Mechanism and Adsorption Properties, J. Environ. Chem. Eng., 2021, 9(4), 105687, DOI:10.1016/j.jece.2021.105687
.
- S.-Y. Ding, M. Dong, Y.-W. Wang, Y.-T. Chen, H.-Z. Wang, C.-Y. Su and W. Wang, Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II), J. Am. Chem. Soc., 2016, 138(9), 3031–3037, DOI:10.1021/jacs.5b10754
.
- N. Huang, L. Zhai, H. Xu and D. Jiang, Stable Covalent Organic Frameworks for Exceptional Mercury Removal from Aqueous Solutions, J. Am. Chem. Soc., 2017, 139(6), 2428–2434, DOI:10.1021/jacs.6b12328
.
- K.-K. Yee, N. Reimer, J. Liu, S.-Y. Cheng, S.-M. Yiu, J. Weber, N. Stock and Z. Xu, Effective Mercury Sorption by Thiol-Laced Metal–Organic Frameworks: In Strong Acid and the Vapor Phase, J. Am. Chem. Soc., 2013, 135(21), 7795–7798, DOI:10.1021/ja400212k
.
- Y. Shin, G. E. Fryxell, W. Um, K. Parker, S. V. Mattigod and R. Skaggs, Sulfur-Functionalized Mesoporous Carbon, Adv. Funct. Mater., 2007, 17(15), 2897–2901, DOI:10.1002/adfm.200601230
.
- J. Liu, X. Feng, G. E. Fryxell, L.-Q. Wang, A. Y. Kim and M. Gong, Hybrid Mesoporous Materials with Functionalized Monolayers, Adv. Mater., 1998, 10(2), 161–165, DOI:10.1002/(SICI)1521-4095(199801)10:2<161::AID-ADMA161>3.0.CO;2-Q
.
- Q. Sun, B. Aguila, J. Perman, L. D. Earl, C. W. Abney, Y. Cheng, H. Wei, N. Nguyen, L. Wojtas and S. Ma, Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal, J. Am. Chem. Soc., 2017, 139(7), 2786–2793, DOI:10.1021/jacs.6b12885
.
- L. Merí-Bofí, S. Royuela, F. Zamora, M. L. Ruiz-González, J. L. Segura, R. Muñoz-Olivas and M. J. Mancheño, Thiol Grafted Imine-Based Covalent Organic Frameworks for Water Remediation through Selective Removal of Hg(Ii), J. Mater. Chem. A, 2017, 5(34), 17973–17981, 10.1039/C7TA05588A
.
- Y. Li, C. Wang, S. Ma, H. Zhang, J. Ou, Y. Wei and M. Ye, Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions, ACS Appl. Mater. Interfaces, 2019, 11(12), 11706–11714, DOI:10.1021/acsami.8b18502
.
- G. Li, J. Ye, Q. Fang and F. Liu, Amide-Based Covalent Organic Frameworks Materials for Efficient and Recyclable Removal of Heavy Metal Lead (II), Chem. Eng. J., 2019, 370, 822–830, DOI:10.1016/j.cej.2019.03.260
.
- A. Khojastehnezhad, F. Moeinpour, M. Jafari, M. K. Shehab, A. Samih ElDouhaibi, H. M. El-Kaderi and M. Siaj, Postsynthetic Modification of Core–Shell Magnetic Covalent Organic Frameworks for the Selective Removal of Mercury, ACS Appl. Mater. Interfaces, 2023, 15(23), 28476–28490, DOI:10.1021/acsami.3c02914
.
- Y. Li, C. Wang, S. Ma, H. Zhang, J. Ou, Y. Wei and M. Ye, Fabrication of Hydrazone-Linked Covalent Organic Frameworks Using Alkyl Amine as Building Block for High Adsorption Capacity of Metal Ions, ACS Appl. Mater. Interfaces, 2019, 11(12), 11706–11714, DOI:10.1021/acsami.8b18502
.
- K. Zhang, Z. He, K. M. Gupta and J. Jiang, Computational Design of 2D Functional Covalent–Organic Framework Membranes for Water Desalination, Environ. Sci.: Water Res. Technol., 2017, 3(4), 735–743, 10.1039/C7EW00074J
.
- W. Zhou, M. Wei, X. Zhang, F. Xu and Y. Wang, Fast Desalination by Multilayered Covalent Organic Framework (COF) Nanosheets, ACS Appl. Mater. Interfaces, 2019, 11(18), 16847–16854, DOI:10.1021/acsami.9b01883
.
- J. Yuan, M. Wu, H. Wu, Y. Liu, X. You, R. Zhang, Y. Su, H. Yang, J. Shen and Z. Jiang, Covalent Organic Framework-Modulated Interfacial Polymerization for Ultrathin Desalination Membranes, J. Mater. Chem. A, 2019, 7(44), 25641–25649, 10.1039/C9TA08163A
.
- L. Xu, B. Shan, C. Gao and J. Xu, Multifunctional Thin-Film Nanocomposite Membranes Comprising Covalent Organic Nanosheets with High Crystallinity for Efficient Reverse Osmosis Desalination, J. Membr. Sci., 2020, 593, 117398, DOI:10.1016/j.memsci.2019.117398
.
- C. Wang, Z. Li, J. Chen, Z. Li, Y. Yin, L. Cao, Y. Zhong and H. Wu, Covalent Organic Framework Modified Polyamide Nanofiltration Membrane with Enhanced Performance for Desalination, J. Membr. Sci., 2017, 523, 273–281, DOI:10.1016/j.memsci.2016.09.055
.
- C. Li, S. Li, J. Zhang, C. Yang, B. Su, L. Han and X. Gao, Emerging Sandwich-like Reverse Osmosis Membrane with Interfacial Assembled Covalent Organic Frameworks Interlayer for Highly-Efficient Desalination, J. Membr. Sci., 2020, 604, 118065, DOI:10.1016/j.memsci.2020.118065
.
- C. Liu, Y. Jiang, A. Nalaparaju, J. Jiang and A. Huang, Post-Synthesis of a Covalent Organic Framework Nanofiltration Membrane for Highly Efficient Water Treatment, J. Mater. Chem. A, 2019, 7(42), 24205–24210, 10.1039/C9TA06325K
.
- R. Wang, M. Wei and Y. Wang, Secondary Growth of Covalent Organic Frameworks (COFs) on Porous Substrates for Fast Desalination, J. Membr. Sci., 2020, 604, 118090, DOI:10.1016/j.memsci.2020.118090
.
- B. P. Biswal, H. D. Chaudhari, R. Banerjee and U. K. Kharul, Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation, Chem. – Eur.
J., 2016, 22(14), 4695–4699, DOI:10.1002/chem.201504836
.
- A. Gul, J. Hruza and F. Yalcinkaya, Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review, Polymers, 2021, 13(6), 846, DOI:10.3390/polym13060846
.
- S. S. Madaeni and S. Samieirad, Chemical Cleaning of Reverse Osmosis Membrane Fouled by Wastewater, Desalination, 2010, 257(1), 80–86, DOI:10.1016/j.desal.2010.03.002
.
- A. Piasecka, R. Bernstein, F. Ollevier, F. Meersman, C. Souffreau, R. M. Bilad, K. Cottenie, L. Vanysacker, C. Denis and I. Vankelecom, Study of Biofilms on PVDF Membranes after Chemical Cleaning by Sodium Hypochlorite, Sep. Purif. Technol., 2015, 141, 314–321, DOI:10.1016/j.seppur.2014.12.010
.
- T. Yu, L. Meng, Q.-B. Zhao, Y. Shi, H.-Y. Hu and Y. Lu, Effects of Chemical Cleaning on RO Membrane Inorganic, Organic and Microbial Foulant Removal in a Full-Scale Plant for Municipal Wastewater Reclamation, Water Res., 2017, 113, 1–10, DOI:10.1016/j.watres.2017.01.068
.
- H. Kim, I. Shim and M. Zhan, Chemical Enhanced Backwashing for Controlling Organic Fouling in Drinking Water Treatment Using a Novel Hollow-Fiber Polyacrylonitrile Nanofiltration Membrane, Appl. Sci., 2021, 11(15), 6764, DOI:10.3390/app11156764
.
- R. Thejani Nilusha, T. Wang, H. Wang, D. Yu, J. Zhang and Y. Wei, Optimization of In Situ Backwashing Frequency for Stable Operation of Anaerobic Ceramic Membrane Bioreactor, Processes, 2020, 8(5), 545, DOI:10.3390/pr8050545
.
- E. Segredo-Morales, E. González, C. González-Martín and L. Vera, Evaluation of Membrane Fouling in a Microalgal-Bacterial Membrane Photobioreactor Treating Secondary Wastewater Effluent: Effect of Photoperiod Conditions, Environ. Sci.: Water Res. Technol., 2023, 9(6), 1672–1682, 10.1039/D3EW00138E
.
- M. Zhang, K. Zhang, B. De Gusseme and W. Verstraete, Biogenic Silver Nanoparticles (Bio-Ag 0) Decrease Biofouling of Bio-Ag 0/PES Nanocomposite Membranes, Water Res., 2012, 46(7), 2077–2087, DOI:10.1016/j.watres.2012.01.015
.
- Y. Liu, E. Rosenfield, M. Hu and B. Mi, Direct Observation of Bacterial Deposition on and Detachment from Nanocomposite Membranes Embedded with Silver Nanoparticles, Water Res., 2013, 47(9), 2949–2958, DOI:10.1016/j.watres.2013.03.005
.
- E. Mahmoudi, L. Y. Ng, W. L. Ang, Y. T. Chung, R. Rohani and A. W. Mohammad, Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles, Sci. Rep., 2019, 9(1), 1216, DOI:10.1038/s41598-018-38060-x
.
- J. Li, X. Liu, J. Lu, Y. Wang, G. Li and F. Zhao, Anti-Bacterial Properties of Ultrafiltration Membrane Modified by Graphene Oxide with Nano-Silver Particles, J. Colloid Interface Sci., 2016, 484, 107–115, DOI:10.1016/j.jcis.2016.08.063
.
- B. Pellegrin, F. Mezzari, Y. Hanafi, A. Szymczyk, J.-C. Remigy and C. Causserand, Filtration Performance and Pore Size Distribution of Hypochlorite Aged PES/PVP Ultrafiltration Membranes, J. Membr. Sci., 2015, 474, 175–186, DOI:10.1016/j.memsci.2014.09.028
.
- S. Robinson, S. Z. Abdullah, P. Bérubé and P. Le-Clech, Ageing of Membranes for Water Treatment: Linking Changes to Performance, J. Membr. Sci., 2016, 503, 177–187, DOI:10.1016/j.memsci.2015.12.033
.
- H. Ding, J. Zhang, H. He, Y. Zhu, D. D. Dionysiou, Z. Liu and C. Zhao, Do Membrane Filtration Systems in Drinking Water Treatment Plants Release Nano/Microplastics?, Sci. Total Environ., 2021, 755(Pt 2), 142658, DOI:10.1016/j.scitotenv.2020.142658
.
- G. Cheng, G. Li, H. Xue, S. Chen, J. D. Bryers and S. Jiang, Zwitterionic Carboxybetaine Polymer Surfaces and Their Resistance to Long-Term Biofilm Formation, Biomaterials, 2009, 30(28), 5234–5240, DOI:10.1016/j.biomaterials.2009.05.058
.
- S. J. Lounder, P. T. Wright, L. Mazzaferro and A. Asatekin, Fouling-Resistant Membranes with Tunable Pore Size Fabricated Using Cross-Linkable Copolymers with High Zwitterion Content, J. Membr. Sci. Lett., 2022, 2(1), 100019, DOI:10.1016/j.memlet.2022.100019
.
- P. Gao, P. Jin, R. Dumas, J. Huang, A. B. Asha, R. Narain, B. Van der Bruggen and X. Yang, A Prebiotic Chemistry Inspired One-Step Functionalization of Zwitterionic Nanofiltration Membranes for Efficient Molecular Separation, J. Membr. Sci. Lett., 2022, 2(1), 100013, DOI:10.1016/j.memlet.2022.100013
.
- K. Qu, Z. Yuan, Y. Wang, Z. Song, X. Gong, Y. Zhao, Q. Mu, Q. Zhan, W. Xu and L. Wang, Structures, Properties, and Applications of Zwitterionic Polymers, ChemPhysMater, 2022, 1(4), 294–309, DOI:10.1016/j.chphma.2022.04.003
.
- S. Chen, F. Yu, Q. Yu, Y. He and S. Jiang, Strong Resistance of a Thin Crystalline Layer of Balanced Charged Groups to Protein Adsorption, Langmuir, 2006, 22(19), 8186–8191, DOI:10.1021/la061012m
.
- M. T. Bernards, G. Cheng, Z. Zhang, S. Chen and S. Jiang, Nonfouling Polymer Brushes via Surface-Initiated, Two-Component Atom Transfer Radical Polymerization, Macromolecules, 2008, 41(12), 4216–4219, DOI:10.1021/ma800185y
.
- S. Chen and S. Jiang, An New Avenue to Nonfouling Materials, Adv. Mater., 2008, 20(2), 335–338, DOI:10.1002/adma.200701164
.
- Y.-C. Lin, B.-Y. Yu, W.-C. Lin, S.-H. Lee, C.-H. Kuo and J.-J. Shyue, Tailoring the Surface Potential of Gold Nanoparticles with Self-Assembled Monolayers with Mixed Functional Groups, J. Colloid Interface Sci., 2009, 340(1), 126–130, DOI:10.1016/j.jcis.2009.08.014
.
- C.-H. Kuo, H.-Y. Chang, C.-P. Liu, S.-H. Lee, Y.-W. You and J.-J. Shyue, Effect of Surface Chemical Composition on the Surface Potential and Iso-Electric Point of Silicon Substrates Modified with Self-Assembled Monolayers, Phys. Chem. Chem. Phys., 2011, 13(9), 3649–3653, 10.1039/C0CP02615H
.
- X. Peng, L. Zhao, G. Du, X. Wei, J. Guo, X. Wang, G. Guo and Q. Pu, Charge Tunable Zwitterionic Polyampholyte Layers Formed in Cyclic Olefin Copolymer Microchannels through Photochemical Graft Polymerization, ACS Appl. Mater. Interfaces, 2013, 5(3), 1017–1023, DOI:10.1021/am3027019
.
- S. C. N. Tang, L. Birnhack, P. Nativ and O. Lahav, Highly-Selective Separation of Divalent Ions from Seawater and Seawater RO Retentate, Sep. Purif. Technol., 2017, 175, 460–468, DOI:10.1016/j.seppur.2016.10.030
.
- L. Birnhack, S. C. N. Tang and O. Lahav, Implementation, Design and Cost Assessment of a Membrane-Based Process for Selectively Enriching Desalinated Water with Divalent Seawater Ions, ChemEngineering, 2018, 2(3), 41, DOI:10.3390/chemengineering2030041
.
- S. C. N. Tang, L. Birnhack, Y. Cohen and O. Lahav, Selective Separation of Divalent Ions from Seawater Using an Integrated Ion-Exchange/Nanofiltration Approach, Chem. Eng. Process., 2018, 126, 8–15, DOI:10.1016/j.cep.2018.02.015
.
- D. Kulkarni, S. Musale, P. Panzade, A. C. Paiva-Santos, P. Sonwane, M. Madibone, P. Choundhe, P. Giram and S. Cavalu, Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications, Nanomaterials, 2022, 12(21), 3899, DOI:10.3390/nano12213899
.
- L. Liu, D. He, G.-S. Wang and S.-H. Yu, Bioinspired Crystallization of CaCO3 Coatings on Electrospun Cellulose Acetate Fiber Scaffolds and Corresponding CaCO3 Microtube Networks, Langmuir, 2011, 27(11), 7199–7206, DOI:10.1021/la200738n
.
- L. Liu, D. He, G. S. Wang and S. H. Yu, Bioinspired Crystallization of CaCO3 Coatings on Electrospun Cellulose Acetate Fiber Scaffolds and Corresponding CaCO3 Microtube Networks, Langmuir, 2011, 27(11), 7199–7206, DOI:10.1021/la200738n
.
| This journal is © The Royal Society of Chemistry 2024 |