Self-healing materials in biomedicine and the circular economy
Meenakshi R.
Venkateswaran
ab,
Arezoo
Khosravi
c,
Atefeh
Zarepour
d,
Siavash
Iravani
 *e and
Ali
Zarrabi
*e and
Ali
Zarrabi
 *bf
*bf
aDepartment of Environmental Biotechnology, Bharathidasan University, Tiruchirappalli-620024, Tamil Nadu, India
bDepartment of Biomedical Engineering, Faculty of Engineering and Natural Sciences, Istinye University, 34396, Istanbul, Türkiye
cDepartment of Genetics and Bioengineering, Faculty of Engineering and Natural Sciences, Istanbul Okan University, Istanbul 34959, Türkiye
dDepartment of Research Analytics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai – 600 077, India
eIndependent Researcher, W Nazar ST, Boostan Ave, Isfahan, Iran. E-mail: siavashira@gmail.com
fGraduate School of Biotechnology and Bioengineering, Yuan Ze University, Taoyuan 320315, Taiwan. E-mail: alizarrabi@gmail.com
First published on 7th May 2024
Abstract
Self-healing (bio)materials represent a cornerstone in the transition towards a circular economy in healthcare. These materials possess the remarkable ability to autonomously repair damage, thereby extending the lifespan of medical devices, implants, sensors, wound dressings, and drug delivery systems. By extending the lifespan of biomedical products, they can significantly reduce waste generation and minimize the environmental impact associated with frequent replacement. In addition, the integration of self-healing properties into drug delivery systems can enhance their efficacy and reduce the need for frequent administration, resulting in a more sustainable healthcare system. Notably, self-healing polymers and hydrogels have the potential to improve the durability and lifespan of wound dressings, providing extended protection and support throughout the healing process. The development and implementation of self-healing biomaterials signify a shift towards a more environmentally conscious and resource-efficient healthcare sector. By adopting a circular approach, healthcare facilities can optimize the use of resources throughout the product lifecycle. This includes designing medical devices with self-healing capabilities, implementing efficient recycling systems, and promoting the development of new materials from recycled sources. Such an approach not only reduces the environmental footprint of the healthcare sector but also contributes to a more sustainable and resilient supply chain. The adoption of self-healing (bio)materials offers numerous benefits for the healthcare industry. These materials not only can reduce the environmental impact of medical practices by extending the lifecycle of products but also enhance patient safety and treatment outcomes. The integration of self-healing materials in the healthcare industry holds promise for supporting a more circular economy by extending the product lifespan, reducing waste generation, and fostering sustainable practices in medical settings. However, additional explorations are warranted to optimize the performance and stability of self-healing (bio)materials, ensuring their long-term effectiveness. One of the primary challenges in the adoption of self-healing materials is the cost associated with their production. Notably, the exploration of specific self-healing mechanisms will be crucial in expanding their applications. This review examines the intersection of self-healing materials, biomedicine, and the circular economy, focusing on the challenges, advantages, and future perspectives associated with their implementation.
Environmental significanceSelf-healing materials have immense environmental significance in biomedicine and the circular economy. By autonomously repairing damage, these materials extend their lifespan, reducing the need for replacement and minimizing waste generation. In biomedicine, self-healing materials reduce medical waste by minimizing frequent device replacement. By promoting longevity, resource conservation, and waste reduction, they play a vital role in creating a more environmentally conscious and sustainable future for the medical industry. In the circular economy, they promote resource preservation by enabling reuse and recycling. Longer product lifecycles reduce the demand for new resources and minimize the environmental impact of extraction and manufacturing. Integrating self-healing materials in these industries contributes to a greener future, with reduced waste, resource conservation, and improved environmental stewardship. |
1. Introduction
In recent years, there has been a growing focus on sustainability and the need for innovative solutions in various industries. One such area is biomedicine, where the development of self-healing materials holds great promise. These materials have the remarkable ability to repair themselves when damaged, making them ideal for biomedical applications.1 Furthermore, the concept of the circular economy has gained attention as a means to achieve sustainability by minimizing waste and maximizing resource efficiency.2,3 Self-healing materials are a class of substances that possess the remarkable ability to repair damage with minimal external intervention.4 Inspired by nature's ability to heal wounds, scientists and engineers have been working to mimic this phenomenon in artificial materials. These materials can detect and respond to damage by initiating a healing process, thereby restoring their structural integrity and functionality.5,6 Self-healing materials represent a cutting-edge technological advancement with significant potential benefits across various industries. One primary advantage lies in their ability to autonomously repair damage, reducing the need for frequent maintenance and replacement. This capability can lead to substantial cost savings, especially in sectors where materials undergo wear and tear, such as aerospace, automotive, and infrastructure.7 The self-healing properties ensure a longer lifespan for products and structures, contributing to sustainability by minimizing resource consumption and waste.8–10 Moreover, self-healing materials enhance safety by addressing structural issues promptly. In applications like construction and manufacturing, where structural integrity is critical, the ability of materials to self-repair micro-cracks or other forms of damage can prevent catastrophic failures.11 This not only safeguards human lives but also protects valuable assets. In addition, self-healing materials can be instrumental in reducing the environmental impact of industries, as they contribute to a circular economy by promoting the reuse and longevity of materials.12–14Creating self-healing materials presents a design challenge in developing a composite material with the ability to autonomously heal damage or respond to external stimuli, thereby prolonging the performance lifespan of the material or product. The existence of localized regions within the material exhibiting inferior or compromised performance compared to the surrounding areas can be classified as damage.1 The versatility of self-healing materials extends to various forms, including polymers, metals, concretes, ceramics, and their composites.15 This diversity allows for widespread applications, from everyday consumer products to high-performance components in advanced technologies.16–21 To enhance the use and extend the lifespan of self-healing materials, various modifications are being implemented in the fundamental materials. These modifications involve the development of self-healing materials utilizing dynamic covalent bonds, non-covalent bonds, and multi-cross-linked materials. It is essential to have materials that possess the ability to self-heal while preserving their structural integrity. It is crucial to produce self-healing materials that have a long shelf life and promote environmental awareness. This is particularly important due to the recycling challenges associated with certain polymers such as elastomers or thermosets, which necessitate the exploration of promising emerging trends.22
In the context of a circular economy, the extension of product lifetimes emerges as a pivotal strategy aimed at conserving and enhancing the value of products and their components within the system, and could be achieved via various tactics such as reuse, maintenance, repair, and remanufacturing.23,24 Given the susceptibility of materials and components to degradation, both natural and artificial, over time, the integration of self-healing technology holds promise for enhancing product reliability and longevity. Indeed, self-healing materials have the capability of repairing damage autonomously, thereby extending their lifespan and reducing the need for frequent replacement.25,26 By implementing these materials in various industries, from construction to electronics, the circular economy is reinforced as products become more durable and less prone to disposal, leading to significant reductions in waste generation and resource depletion.27,28 Furthermore, a fundamental principle of the circular economy is to optimize the value of materials, components, and products by prolonging their cycling within the system, hence technologies like self-healing could contribute to this objective by maintaining product integrity.29 Thus, it addresses economic imperatives by mitigating the costs associated with maintenance, repair, and replacement. In other words, in traditional linear models, products are discarded once they reach the end of their lifespan, resulting in a constant demand for new resources and increased disposal costs. However, by integrating self-healing properties into materials, the need for frequent replacement diminishes, thereby reducing the strain on raw material reserves and lowering the overall expenditure associated with product lifecycle management. This shift towards a circular economy of self-healing materials not only improves the environmental supervision but also promotes economic resilience through enhanced resource efficiency and cost savings.30
Self-healing materials in biomedicine have the potential to develop healthcare by improving the lifespan and performance of medical devices, systems, and implants. These materials possess the ability to autonomously repair small cracks or damage, thus extending the functional life of biomedical products.31 For instance, self-healing polymers can be employed to create implantable devices that can heal themselves over time, reducing the need for frequent replacement and minimizing medical waste.32,33 One area where self-healing materials are particularly valuable is in drug delivery systems. By incorporating self-healing properties into drug carriers, researchers can create vehicles that can release drugs in a controlled manner while also repairing any damage caused during their journey through the body. This not only improves the efficacy of drug delivery but also reduces the need for frequent administration, thus minimizing the overall environmental impact.34 However, there are challenges that need to be addressed for the widespread adoption of self-healing materials in biomedicine and the circular economy. One such challenge is the cost of production, as manufacturing self-healing materials can be more expensive compared to traditional materials. In addition, the development of efficient and scalable production methods is crucial to ensure the widespread availability of these materials.1,35 As research continues to push the boundaries of materials engineering, the quest for cost-effective production methods remains a key focus. Streamlining manufacturing processes and optimizing material compositions are crucial steps towards making self-healing materials more economically competitive with traditional alternatives. Ensuring the performance and long-term stability of self-healing materials is another challenge. These materials need to exhibit reliable self-healing capabilities over an extended period, withstanding various environmental conditions and mechanical stresses. Achieving a balance between self-healing properties and other desired material attributes, such as strength and durability, is essential.5,31,36
The purpose of this review is to explore the potential of self-healing materials in biomedicine and their role in promoting sustainability within the circular economy. By examining the advancements and applications of these materials, we aim to shed light on their potential to develop healthcare and contribute to a more sustainable future. Through a comprehensive analysis of their properties, benefits, and challenges, this review seeks to provide valuable insights into the integration of self-healing materials in biomedicine and their role in supporting the principles of the circular economy. By understanding the possibilities and limitations of these materials, researchers can find innovative solutions that prioritize longevity, resource efficiency, and reduced waste generation in the field of biomedicine.
2. Implications for sustainability
In the context of self-healing materials, the circular economy approach involves considering the entire lifecycle of a product, from its design and production to its use and eventual disposal. By incorporating self-healing properties into materials, products can be designed to withstand damage and maintain their functionality over a longer period. This reduces the need for frequent replacement and contributes to resource conservation. Furthermore, the circular economy approach encourages the use of recycled or up-cycled materials in the production of self-healing materials. By utilizing waste materials as feedstock, the environmental impact of extracting and processing virgin resources can be reduced. This not only minimizes waste but also reduces energy consumption and greenhouse gas emissions associated with traditional manufacturing processes.2.1. Waste reduction
By enhancing the durability and lifespan of products, self-healing materials contribute to the reduction of waste generation.27,37 This is particularly relevant in industries like electronics, where frequent replacement and disposal are common. Electronic devices often contain intricate components that can be prone to wear and damage over time. Self-healing nanomaterials, with their ability to autonomously repair micro-scale defects, can contribute to the prolonged lifespan of electronic products. This can lead to a reduction in the volume of electronic waste generated, as devices can withstand more extended use without the need for frequent replacement.38–40 In addition, self-healing polymer materials, designed to independently repair damage triggered by external triggers, represent a cutting-edge focus in sustainable materials investigation. Their capacity to uphold product integrity and functionality and extend product lifespan significantly contributes to reducing the ecological impact of plastic waste.37 Notably, self-healing materials can contribute to the development of more sustainable and eco-friendly manufacturing processes. By prolonging the lifespan of products, the overall demand for raw materials and energy-intensive production processes can be reduced. While the lifespan of regular materials can vary depending on factors such as use, maintenance, and environmental conditions, self-healing materials have the potential to extend the product lifespan significantly. Regular materials may require replacement or repair due to wear and tear, leading to waste production. In contrast, self-healing materials can autonomously repair minor damage, thereby delaying the need for replacement and reducing waste generation. The continuous self-restoration ability of self-healing materials can contribute to a prolonged lifespan compared to traditional materials, ultimately promoting sustainability and resource efficiency.30 The production of regular materials often generates a significant amount of waste due to manufacturing processes, material inefficiencies, and the disposal of unused or excess materials. In contrast, the fabrication of self-healing materials typically involves more precise processes that minimize waste generation. The design of self-healing materials aims to optimize material use and reduce the need for frequent replacement or repairs, thereby decreasing the overall waste output associated with the lifecycle of the material. By promoting a more efficient use of resources and minimizing the generation of waste during fabrication and maintenance, self-healing materials contribute to a more sustainable and eco-friendly approach to material production.62.2. Energy efficiency
Self-healing materials can contribute to energy efficiency, eliminating or reducing the need for energy-intensive repair processes or the production of new materials.30,37 Traditional repair processes often involve energy-intensive methods such as welding, gluing, or thermal treatments, which consume significant amounts of energy. In contrast, self-healing materials exhibit inherent mechanisms that allow them to autonomously repair damage with minimal intervention or energy input. The recovery process in self-healing materials can occur either spontaneously or through the application of particular stimuli like radiation, heat, temperature, pressure, and humidity. This self-healing mechanism draws inspiration from the self-awareness, automatic response, and damage recovery properties observed in living organisms within the field of bionics.34 Zhu et al.41 have reviewed about self-healing polymer materials for flexible electronic devices. Implementing self-healing materials in flexible electronic devices can diminish mechanical damage resulting from bending, folding, and scratching. This integration has the potential to enhance the durability and lifespan of such devices significantly.412.3. Resource conservation
The incorporation of self-healing capabilities in materials reduces the demand for virgin resources/raw materials. This aligns with the principles of the circular economy, where resources are kept in use for as long as possible.30,42 In a traditional linear economy, materials are extracted, manufactured into products, used, and eventually discarded as waste. This linear approach leads to the depletion of finite resources and generates significant amounts of waste. In contrast, the circular economy aims to close the loop by keeping resources in use for as long as possible and minimizing waste generation. By integrating self-healing capabilities into materials, the lifespan of products can be significantly extended, reducing the need for frequent replacement. Rather than discarding damaged or worn-out products, self-healing materials have the ability to repair themselves, allowing them to remain in use for longer periods. The circular economy is commonly defined as a regenerative system focused on reducing resource input, waste, and energy loss by slowing down and closing material and energy loops.43 Given the substantial environmental consequences of global material extraction, prolonging the lifespan of materials emerges as a crucial tactic to address these challenges.44 Integrating self-healing capabilities into materials aligns with the principles of the circular economy by enhancing the durability and lifespan of products. This innovative approach not only extends the useful life of materials but also reduces the overall consumption of resources and energy required for manufacturing new products. Self-healing materials contribute to a more sustainable production and consumption system by promoting resource efficiency and waste reduction.302.4. Maintenance cost reduction
Self-healing materials can reduce the need for costly maintenance and repairs, minimizing the financial burden associated with maintaining infrastructure, machinery, and other products.45 In industries and sectors where maintenance and repair expenses can be substantial, the incorporation of self-healing materials can have a profound impact on cost savings. Traditional maintenance and repair processes often require skilled labor, specialized equipment, and the procurement of replacement parts, all of which can be costly. However, self-healing materials have the ability to autonomously repair damage, eliminating or reducing the need for extensive manual intervention and costly repairs. Furthermore, the implementation of self-healing materials in consumer products can also provide financial benefits to end-users. For instance, electronic devices often require repairs or replacement due to screen damage, battery issues, or other common problems. With self-healing materials, these issues can be rectified autonomously, eliminating the need for expensive repairs or the purchase of new devices. This not only saves consumers' money but also reduces electronic waste.9 Traditional electronic devices often require costly repairs or replacement when components such as screens or batteries become damaged or degraded over time. These repairs not only burden users with additional expenses but also contribute to the growing issue of electronic waste accumulation. By incorporating self-healing capabilities into electronic devices, the need for external repairs is significantly reduced. Self-healing materials can autonomously mend minor damage, such as scratches or cracks on screens, or restore functionality to deteriorating battery components.46 This self-repair mechanism not only enhances the longevity of electronic devices but also minimizes the environmental impact associated with electronic waste disposal. Furthermore, the adoption of self-healing materials in electronic devices aligns with the growing trend towards sustainable technology solutions. By extending the lifespan of electronic devices through self-repairing materials, consumers can reduce their carbon footprint and contribute to an eco-friendlier approach to technology consumption. Notably, the cost savings from fewer repairs and replacement can benefit both consumers and manufacturers alike, fostering a more economically sustainable electronic industry.462.5. Improved safety
The use of self-healing materials in critical applications, such as aerospace and automotive industries, can enhance safety.45,47 By repairing damage automatically, these materials can prevent catastrophic failures and ensure the reliability of key components. For instance, self-healing materials can be applied in advanced energy-storage devices to significantly improve their lifespan, durability, and safety.48 In safety-critical industries like aerospace and automotive, the integrity of components and structures is of utmost importance. The failure of critical components can have severe consequences, including accidents, injuries, or even loss of life. Self-healing materials offer an innovative solution to address these risks. By incorporating self-healing properties into materials used in the construction of aircraft, spacecraft, or vehicles, the likelihood of catastrophic failures can be significantly reduced. These materials have the ability to autonomously repair damage caused by impacts, fatigue, or other stressors, ensuring that structural integrity is maintained. In electronic systems used in aerospace or automotive applications, self-healing materials can repair damage to circuits or connections, ensuring the consistent operation of these systems. By utilizing self-healing materials in critical applications, the aerospace and automotive industries can minimize the risk of catastrophic failures, enhance safety, and ensure the reliability of key components. However, it is important to note that extensive testing, certification, and validation processes are necessary to ensure the effectiveness and performance of self-healing materials in these demanding environments. Notably, understanding the composition of self-healing materials is crucial in evaluating their safety profile. Assessing the toxicity levels of components used in the manufacturing process is essential to prevent any harmful effects on the environment or human health. By prioritizing non-toxic, sustainable, and environmentally friendly materials, the overall safety of self-healing materials can be enhanced.49Self-healing materials could cover nearly all of these sectors of the circular economy and appropriate them for applications in different fields, especially in biomedicine, which is described in the following section.
3. Biomedical applications
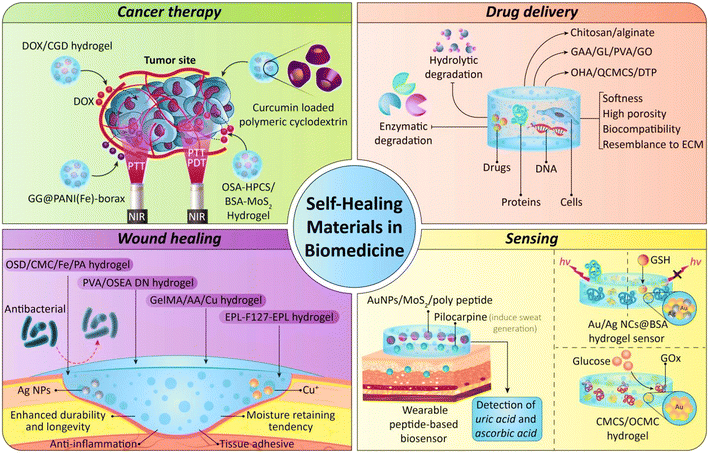
One of the interesting applications of self-healing materials is their use in different sectors of the biomedicine field (Fig. 1). These innovative materials possess the ability to autonomously repair damage, enhancing the durability and longevity of biomedical devices within the human body. In the context of implantable medical devices, such as pacemakers or biosensors, self-healing materials can mitigate the risk of device failure due to wear and tear over time. Moreover, these materials may contribute to reducing the frequency of surgical interventions needed for device replacement. The application of self-healing materials in biomedicine aligns with the growing demand for more reliable and long-lasting medical technologies, ultimately improving patient outcomes and minimizing the burden on healthcare systems.32,33 In this case, different types of polymeric-based materials with different aims have been introduced which are mentioned in the following section.3.1. Drug delivery
Self-healing materials have emerged as a promising avenue for innovative drug delivery systems, offering unique advantages in terms of sustained release and localized therapeutic effects. These materials can be designed to encapsulate drug payloads and, in response to specific environmental triggers such as changes in pH, temperature, or the presence of enzymes, undergo self-repair, enabling controlled and targeted drug release.34 Different strategies have been used for the fabrication of self-healable drug delivery formulation, especially in the form of hydrogels. Stimuli-responsive hydrogels, particularly, play an indispensable role in the realm of drug delivery technologies, leveraging their distinct characteristics such as water abundance, softness, high porosity, biocompatibility, and close resemblance to extracellular matrices (ECMs).50–53 These hydrogels possess the capability to maintain the inherent properties of entrapped therapeutic factors like drugs, proteins, DNA, and cells, creating biomimetic wet conditions that could protect the bioactive molecules from undesirable enzymatic or hydrolytic degradation. The induction of self-healing ability in the structure of these hydrogels leads to overcoming the limitations of these hydrogels such as the need for invasive surgical implantation, improper adaptation to the defect site, potential loss of structural integrity and functionality under frequent stress within the human body, and an elevated risk of infections.54–57 The application of these materials could reduce the cost of treatment via reduction in the dosage and targeted delivery of therapeutic compounds and enhance the performance, while decreasing probable side-effects. The self-healing properties of these materials reduce the use of resources needed for their preparation and also diminish their waste. For instance, Wu and coworkers fabricated a self-healing injectable drug loaded hydrogel via incorporating amine-modified silica nanoparticles inside aldehyde-functionalized polymers using Schiff base reactions. It was a rapid self-healable hydrogel that had good stability under normal conditions while a little change in the pH of the microenvironment led to a change in the stability, degradability, and drug release pattern of the hydrogel. Bringing two pieces of this hydrogel into contact activated dynamic Schiff base interactions at the contact area and led to a considerable strength that facilitated the integration of the pieces into a cohesive and free-standing hydrogel with structural integrity. Therefore, this self-repair ability led to reduction in the amounts of materials and energy that are used for the preparation of normal carriers. The pH-responsiveness of the fabricated hydrogel was confirmed via evaluating the release of drugs at different pH values. Results of this test showed the lowest release at normal pH and the highest amount at pH 6.4, which could reduce the drug dosage, enhance the therapeutic effect, for example in the case of cancer therapy, and reduce the probable side-effects.58The Schiff-base reaction was used in another study to fabricate a self-healing chitosan/alginate hydrogel containing magnetic gelatin microspheres (MGMs). This formulation showed a concentration dependent gelation time; thereby increasing the concentration of MGMs led to a decrease in gelation time due to the interaction between the amine groups of MGMs and the aldehyde groups of the alginate. In addition, this improved the stability of the hydrogel and reduced its swelling ratio and degradation percentage. It also had robust self-healing properties so its storage moduli before and after the healing process were nearly the same when the concentration of MGMs was between 30 and 59 mg. These self-healing properties resulted from the Schiff-base reaction and dynamic reversible covalent bonding occurring in the structure of the hydrogel. The healing process was completed within 2 h and the fabricated hydrogel showed resistance against external stretching force; thus it could maintain its structure which led to reduction in cost, energy, and materials.59
A composite of gelatin, gum Arabic aldehyde (GAA), graphene oxide (GO), polyvinyl alcohol (PVA), and boric acid was used to fabricate an injectable nanocomposite hydrogel (GAA/GL/PVA/GO) with self-healing ability. The self-healing ability was provided via the hydrogen and carboxyl bonds of boric acid and GO with PVA chains and the Schiff base interaction between the gelatin's amine groups and the aldehyde groups of GAA. Excellent mechanical properties of the hydrogel were confirmed with different tests (including tensile, toughness, and strength tests). The swelling ratio of this hydrogel was found to be related to two main factors, the pH of the microenvironment and the amount of GO. In other words, increasing the concentration of GO led to an increase in the swelling ratio at first and then a decrease. Indeed, at first and by increasing the GO concentration, its interactions with polymeric chains were improved, increasing the hydrophilicity of GO. Addition of more amounts of GO led to its aggregation and decreased the swelling ratio due to the formation of a compact hydrogel. On the other hand, increasing the pH from acidic to neutral led to an increase in the swelling ratio as well, due to the generation of hydrolyzed acetate groups of the PVA. Increasing the concentration of GO also increased the stability of the hydrogel and decreased its degradation rate due to increasing condensation of the hydrogel. Rivastigmine was loaded as a drug model inside the hydrogel and the results of drug release showed sustained release of drug molecules in 7 days in the case of the complete hydrogel (Fig. 2), which could improve therapeutic performance and decrease the amounts of drug use.52
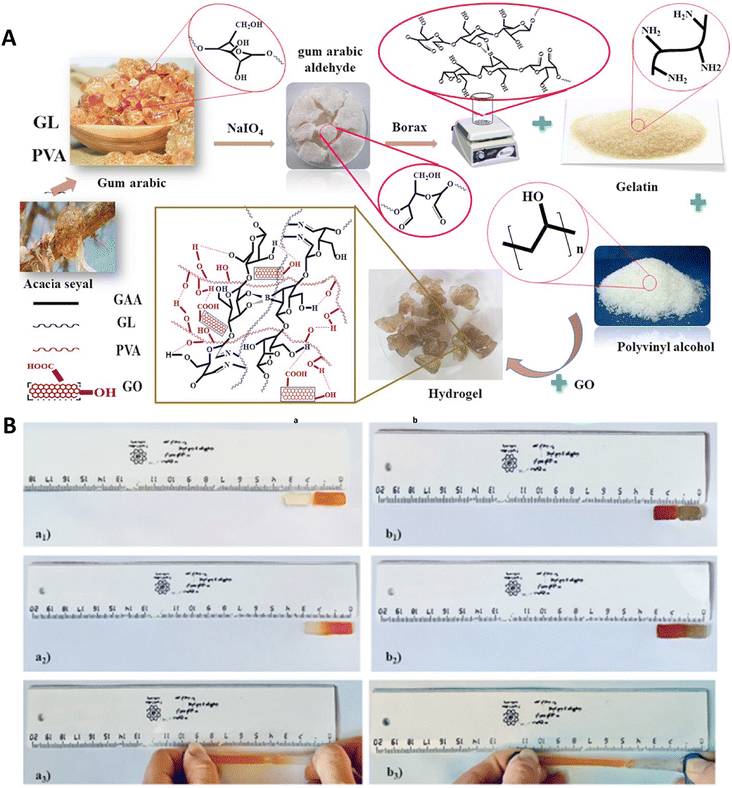 | ||
| Fig. 2 (A) Schematic image of fabrication of the GAA/GL/PVA/GO nanocomposite hydrogel. (B) Self-healing ability of GAA/GL/PVA (a1–a3) and GAA/GL/PVA/GO (b1–b3) hydrogels (a) and GAA/GL/PVA/GO (b) hydrogels. Reprinted with permission from ref. 52. Copyright 2022, Springer Nature. | ||
The Diels–Alder reaction is another method for the fabrication of self-healing materials that has found widespread application in the preparation of dynamic covalent hydrogels, primarily due to its thermal responsiveness, the strength of the covalent bond formed, high selectivity, high chemical yields, and the absence of by-products. Notably, the D–A reaction is categorized as click chemistry with the ability to occur in a “one-pot” fashion, meaning that the reaction can take place without interference from water. This characteristic is particularly advantageous for applications in biological environments, making D–A-based hydrogels attractive for various biomedical applications, including drug delivery.60,61 Li et al. employed this reaction to fabricate a self-healable smart hydrogel composed of furan-modified pectin (PF) and maleimide-modified chitosan (CA). Heating the cut parts at 37 °C for 5 h led to occurrence of the healing reaction by employing D–A and electrostatic reactions. It also showed a high swelling ratio using non-Fickian diffusion. This swelling ratio had dual pH responsivity; thus the swelling was increased by increasing the pH until neutral, and then decreased by increasing the pH to 9, which was due to the amphoteric nature of hydrogel precursors. Results of the release test showed a burst release (near 30%) at first 4 h followed by a controlled sustained release pattern. Results of the cytotoxicity test also confirmed the cell viability of the hydrogel. The self-healing ability of this hydrogel could improve its lifetime and reduce the time, energy, and materials needed to produce new carriers, while its sustainable behavior in drug release could enhance its therapeutic performance.53
Oxidized hyaluronic acid (OHA), quaternized carboxymethyl chitosan (QCMCS), and 3,3′-dithiobis-(propionohydrazide) (DTP) were used in a recent study to fabricate an injectable hydrogel for the aim of drug delivery. A simple “one-pot” method was used in this study to fabricate the hydrogel with hydrogen and dynamic covalent bonds (imine bonds, disulfide bonds, and acylhydrazone bonds). The fabricated hydrogel showed rapid healing (30 min) with the same storage modulus and without any remaining cracks or using energy, which prolonged its lifetime use. This self-healing activity was related to the synergistic affinity of free reactive groups of the broken bonds (imine, acylhydrazone, disulfide, and hydrogen bonds) to rearrange the conformation of the hydrogel and heal the broken part. It showed good mechanical and thermal stability, a high swelling ratio (related to the presence of different hydrophilic groups) and pH-dependent swelling ability. It also exhibited pH-dependent degradability and it was degraded under acidic pH conditions (due to the breaking of imide and hydrazone bonds) for 28 days, while it was stable at neutral and alkaline pH. The results of the drug release study at different pH values showed a sustainable release pattern based on the Fick diffusion mechanism that was affected by the pH so the fastest drug release occurred at acidic pH. It was also shown that increasing the crosslinking density of the hydrogel led to reduction of the degradation rate and a decrease in the amounts of the released drug via delaying drug release. Besides, this hydrogel exhibited high biocompatibility and good antimicrobial activity (related to the presence of quaternary ammonium groups in the structure of QCMCS) (Fig. 3).62
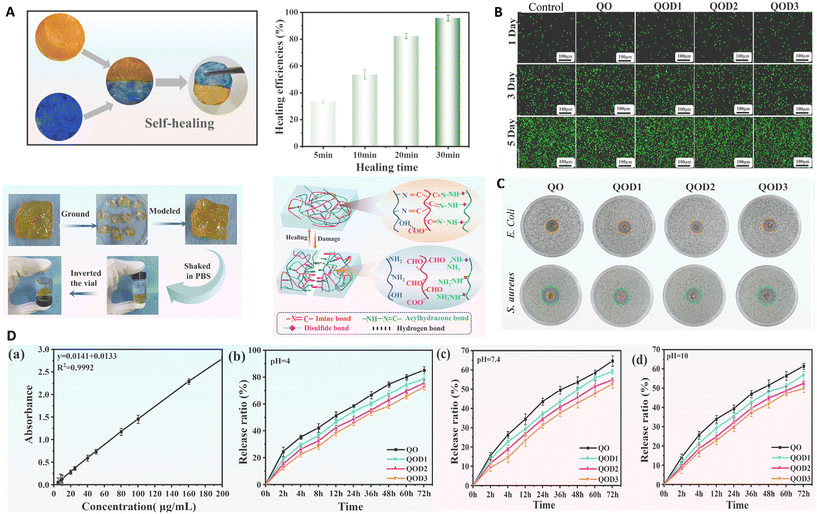 | ||
| Fig. 3 (A) Self-healing ability of a hydrogel. Results of cell viability (B) and antibacterial activity (C) of the hydrogel containing different amounts of DTP. (D) Standard curve related to acetylsalicylic acid (ASA) (a) and release of ASA from different hydrogels at acidic pH (4) (b), normal pH (7.4) (c), and basic pH (10) (d). Reprinted with permission from ref. 62. Copyright 2023, Elsevier. | ||
3.2. Sensing
In recent years, lots of researchers have consistently looked to natural systems to derive innovative and groundbreaking design concepts for fabricating materials in different sectors. In the field of soft sensor research, the focus has been on creating biomimetic devices, electronics, and sensors inspired by nature via mimicking properties like softness, flexibility, stretchability, self-healing, and self-cleaning.63–68 The notion of self-healing, initially conceptualized in science fiction, has recently garnered significant attention in scientific exploration. The basic idea is clear: providing electronic devices with the ability to heal, the same as the natural recovery and repair processes observed in living organisms when faced with structural damage such as scratches and cuts, thereby restoring their functionality. This not only leads to reduction in the time, energy, and materials used for the fabrication of sensors, but also makes them cost-effective and appropriates them for wide use. The first type of self-healable sensor was fabricated by Bao and co-workers in 2012, and from then several types of these sensors have been fabricated and introduced.69–71The majority of existing self-healing sensing platforms rely on electronic/ionic devices that convert physical and chemical signals into electrical signals. These systems primarily utilize three types of materials: insulators/dielectrics, conductors, and semiconductors. The advancement of self-healing materials with diverse electronic, chemical, and mechanical properties plays a crucial role in the evolution of these sensors.72–75 Currently, self-healing materials, whether natural or synthetic, exhibit great versatility, spanning a broad range of mechanical properties. These properties range from very soft, gel-like materials (e.g., Young's modulus ≈ 50 Pa) to tough and stiff ones (e.g., Young's modulus ≈ 109 Pa). The desired set of properties and specifications, encompassing chemical, mechanical, and electrical aspects, is typically determined based on the specific application of these materials.76 Accordingly, several types of self-healing sensors have been introduced some of them are as follows.
Electrochemical biosensors are compact analytical devices that integrate a biological sensing element with an electrochemical transducer to detect and quantify specific biochemical substances. These biosensors rely on the interaction between the biological recognition element, such as enzymes, antibodies, or nucleic acids, and the target analyte to produce a measurable electrochemical signal. The transducer converts the biological response into an electrical signal, allowing for sensitive and selective detection of various biomolecules. The combination of high specificity, rapid response, and miniaturized design makes electrochemical biosensors valuable tools for real-time and point-of-care detection. As the demand for rapid and accurate analytical methods continues to grow, electrochemical biosensors play a crucial role in advancing biosensing technologies.77,78 This technique was used to fabricate wearable sweat sensors to fabricate intelligent devices for non-invasive monitoring of health.79–82 Composite of these sensors with self-healing hydrogels led to producing biosensors with long-term monitoring of their target on the skin. This approach was used by Qiao et al. to fabricate a wearable peptide-based biosensor composed of a stretchable thermoplastic polyurethane (TPU) thin film in a three-electrode system and an iontophoresis (IP) electrode in which the working electrode was functionalized with gold nanoparticles (AuNPs)/MoS2/poly peptide (Pep), serving as storage for sweat as well as a sensing surface. To induce sweat generation, the pilocarpine drug was loaded on the IP anode. The hydrogel was composed of 2-Nap-k-(2-Nap)-kf with d-type amino acid that were attached to each other via π–π interaction between the Nap groups. The presence of MoS2 sheets induced self-healing ability (within 5 min) to the hydrogel via supplying stronger π–π force, on the one hand, and acted as a catalyst for detection of uric acid (UA) and ascorbic acid (AA), on the other hand. AuNPs also were used to improve the conductivity and sensitivity of the sensor. Evaluating the anti-fouling ability of the hydrogel in the presence of sweat (with different purity) confirmed the effectiveness of D-peptide in preventing fouling formation (4.1% signal suppression in the differential pulse voltammetry (DPV) response curve). These anti-fouling properties resulted from the presence of several amphoteric hydrophilic groups in the structure of the hydrogel, which prevented the adsorption of contaminants. Evaluation of the sensing performance of the hydrogel modified screen-printed electrodes (AuNPs/MoS2/Pep/SPE) at different pH values showed that the detection ability of this formulation was pH dependent so it was optimum at pH 6 and then decreased with increasing pH. This sensor showed detection limits of about 6.0 nM and 0.67 μM for UA and AA, respectively, selectivity, and excellent reproducibility (Fig. 4).83
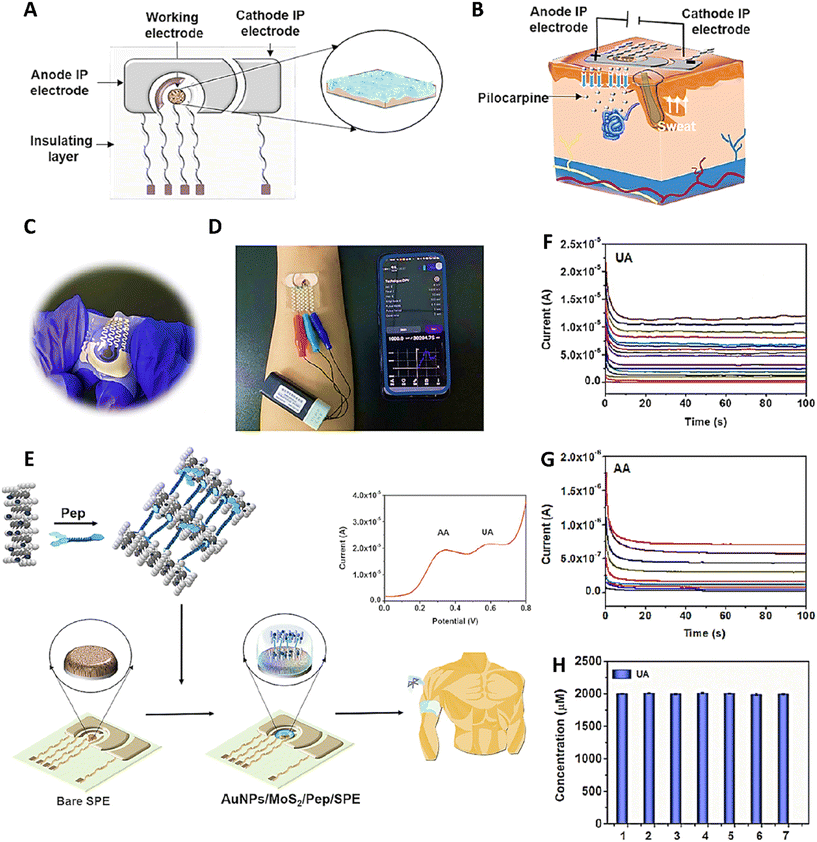 | ||
| Fig. 4 Schematic image of (A) a wearable sensor and (B) iontophoresis operation used for sweating induction. (C) Real image of the fabricated wearable sensor. (D) In situ utilization of the wearable sensor connected to a wireless mobile. (E) Schematic image related to the application of the fabricated wearable sensor for detection of AA and UA. Results of current response of the sensor in response to different concentrations of (F) UA, and (G) AA. (H) Sensor reproducibility results related to detection of 2.0 mM UA prepared in artificial sweat. Reprinted with permission from ref. 83. Copyright 2023, American Chemical Society. | ||
Quaternized chitosan (QCS) and oxidized dextran (OD) were used to fabricate a hydrogel, which were covalently linked to CeO2/MnO2 hollow nanospheres (MOx), as the electrocatalytic medium, which was then functionalized with glucose oxidase (GOx) via electrostatic interaction (QCS-MOx-OD/GOx). A polyethylene terephthalate film coated with an indium tin oxide (ITO) conductive coating (PET) electrode functionalized with (3-aminopropyl)triethoxysilane (APTES) was then functionalized with QCS-MOx-OD/GOx (via a Schiff base bond) and a covering agent (PET/QCS-MOx-OD/GOx) to fabricate a continuous glucose monitoring system (CGMS). To evaluate the responsivity of the hydrogel to H2O2, PET/QCS-MOx-OD/BSA was fabricated and it showed rapid excellent amperometric responses against H2O2 with a linearity (R2) of about 0.9961 in the range of 0.05 μM–112.7 mM H2O2 with a detection limit of about 2.4 μM. It also showed strong selectivity and anti-interference ability. In the next step, the PET/QCS-MOx-OD/GOx electrode was used to detect its sensitivity against glucose, and it showed good sensitivity with 0.9931 linearity in the glucose range of 1–111 mM and a detection limit of about 32.4 μM. The same as PET/QCS-MOx-OD/BSA, this electrode also showed good anti-interference ability. The fabricated electrode showed stability for more than 30 days which was more than that of the current available sensors. Moreover, the electrochemical response of the sensor was completely recovered after self-healing occurred.84
Electrochemiluminescence (ECL) is a highly sensitive method that has gained prominence as a robust analytical approach in recent decades. This method uses the production of luminescence signals on an electrode surface through electrochemical processes and shows considerable potential in diverse analytical applications, with a particular emphasis on the examination of biological samples. This method was used in a study to fabricate a new type of self-healing anti-biofouling biosensor hydrogel composed of a bovine serum albumin (BSA) matrix containing fluorescent Au/Ag alloy nanoclusters (Au/Ag NCs). Indeed, hydrogels are ideal structures to fabricate biosensors that could be used for biological formulation. Their porous structure provides the capability of penetrating small, targeted molecules while hindering the penetration of macromolecules. Moreover, their different non-covalent crosslinking interactions (such as hydrophobic, electrostatic, and hydrogen bonds) as well as the physical entanglement of their chains grant them self-healing ability. The fabricated hydrogel could be excited at 540 nm and emitted red light at 620 nm and the intensity of emission was directly related to the Au/Ag molar ratio so the maximum intensity was related to the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The hydrogel modified glassy carbon electrode (GCE) showed repeatable and stable sensing under optimal conditions (−1.3 to 1.3 V). Besides, the thickness of the hydrogel also played a pivotal role in the intensity of ECL and the anti-biofouling performance so the thickness of about 120 μm was the optimum thickness for the best anti-biofouling and ECL intensity. The anti-biofouling ability of this hydrogel was assessed in the presence of different compounds, and it was confirmed that except hemoglobin, which has a little quenched fluorescence intensity, none of the other compounds affected ECL. It also exhibited self-healing ability within 10 min showing that the ECL response of the hydrogel decreased by about 17% from the cut part and increased during the healing process to reach the optimum amounts, again. These self-healing properties resulted from the physical interactions between protein molecules as well as dynamic interactions between the hydrogel and thiol groups of Au/Ag NCs. The fabricated sensor was then used for detecting the amounts of glutathione of serum as the indicator of different diseases, and the results showed a detection limit of about 8.7 × 10−6 m and good stability and reusability (Fig. 5).85
1 molar ratio. The hydrogel modified glassy carbon electrode (GCE) showed repeatable and stable sensing under optimal conditions (−1.3 to 1.3 V). Besides, the thickness of the hydrogel also played a pivotal role in the intensity of ECL and the anti-biofouling performance so the thickness of about 120 μm was the optimum thickness for the best anti-biofouling and ECL intensity. The anti-biofouling ability of this hydrogel was assessed in the presence of different compounds, and it was confirmed that except hemoglobin, which has a little quenched fluorescence intensity, none of the other compounds affected ECL. It also exhibited self-healing ability within 10 min showing that the ECL response of the hydrogel decreased by about 17% from the cut part and increased during the healing process to reach the optimum amounts, again. These self-healing properties resulted from the physical interactions between protein molecules as well as dynamic interactions between the hydrogel and thiol groups of Au/Ag NCs. The fabricated sensor was then used for detecting the amounts of glutathione of serum as the indicator of different diseases, and the results showed a detection limit of about 8.7 × 10−6 m and good stability and reusability (Fig. 5).85
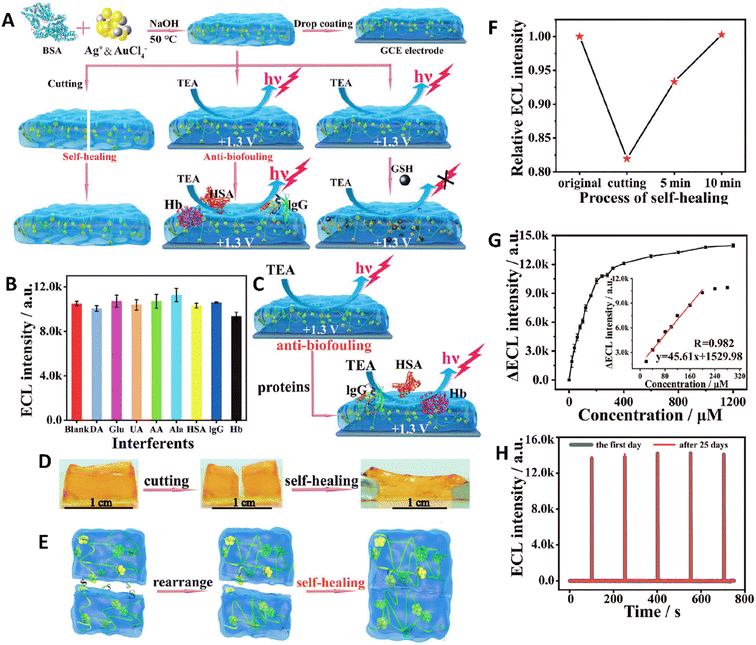 | ||
| Fig. 5 (A) Schematic illustration of fabrication of the Au/Ag NCs@BSA hydrogel sensor with anti-biofouling and self-healing ability used for the detection of GSH. (B) Effect of different samples (dopamine (DA), glucose (Glu), uric acid (UA), ascorbic acid (AA), alanine (Ala), human serum protein (HSA), immunoglobulin (IgG), and hemoglobin (Hb)) on the ECL intensity of the fabricated biosensor. (C) Schematic image of the antifouling mechanism of the hydrogel biosensor. Image (D) and scheme (E) showing the self-healing of the Au/Ag NCs@BSA hydrogel sensor. (F) Change in the ECL intensity during the healing process. (G) ΔECL (ΔECL = I0 − I) curve of the biosensor in response to increasing the concentration of glutathione. (H) Result of stability of the biosensor for 25 days. Reprinted with permission from ref. 85. Copyright 2020, Wiley-VCH GmbH. | ||
Oxidized sodium alginate/hydrazide polyethylene glycol (OSA/PEG-DH) and 4-amino-DL-phenylalanine (4a-Phe) were used to fabricate a self-healing hydrogel which then was functionalized with an ionic liquid (IL) (hydroxyethyltrimethylammonium chloride) and the luminescent molecule N-(aminobutyl)-N-(ethyl isoluminol) (ABEI). In here, 4a-Phe not only reduced the gelation time via elevating the reactions between the hydrazide and aldehyde groups, but also improved the self-healing rate of the hydrogel (from 4 h in the absence of 4a-Phe to 20 min in the presence of this compound), which was due to the enhanced exchange of the acylhydrazone bonds. The fabricated ABEI/IL/OSA/PEG-DH conductive hydrogel was then added onto the surface of a flexible screen-printed electrode (SPEC) to fabricate the ECL hydrogel sensor. Due to its high amounts of water, the hydrogel could act as a semi-solid electrolyte that prepares a good environment for conducting ions. In the presence of H2O2, as a co-reactant and one of the key risk indicators of different diseases, the fabricated sensor could produce ECL signals with a limit of detection of about 0.033 μM, high selectivity, high sensing stability, and good reproducibility (about 90.3% of the first ECL response).86
Fluorescence sensing is another method that could be used for the fabrication of biosensors. In a study, carboxymethyl chitosan (CMCS) and oxidized carboxymethyl cellulose (OCMC) were used to fabricate a self-healable hydrogel which incorporated gold nanoclusters (AuNCs) and glucose oxidase (GOx) as fluorescent bioprobes and was used for the detection of glucose. The precursors of the hydrogel were reacted to each other using the dynamic Schiff-base reaction and it showed self-healing ability within 5 h without any external stimulation and without any remaining signs in the cut/healed part. The self-healing ability of the fabricated hydrogel was related to the reversible Schiff-base as well as the abundant hydroxyl and carboxyl groups present in the structure of the hydrogel. It also showed a good swelling ratio as well as good mechanical stability related to the presence of strong connections between the several amine and aldehyde groups. Due to the effect of PBS on fluorescence intensity under UV irradiation, the optimum time for the fluorescence measurement was determined to be 5–10 min. Besides, it was shown that H2O2 molecules could affect the fluorescent probe and quench its photoirradiation in a concentration dependent manner (with a detection limit of about 0.029 mM). This ability was used to detect glucose amounts; the glucose molecules were oxidized after exposure to this hydrogel, due to the presence of GOx, which led to production of H2O2 molecules, thus reducing the fluorescence intensity of the hydrogel (detection limit 0.029 mM). Interestingly, a blue-shift occurred by the addition of glucose which was used for differentiating between the presence of glucose and H2O2 molecules. Results of the in vivo test also confirmed the high accuracy of this biosensor in detecting the amounts of blood glucose and the calculated amount using this sensor was very near to the amounts measured using a glucometer.87
Inspired from jellyfish, a new type of self-healable fluorescence biosensor was fabricated via polymerization and self-assembly of a 2-ureido-4-[1H] pyrimidone (UPy) core embedded in polyelectrolyte–surfactant micelles (ESMs, containing acrylamide (AM), 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS), and sodium dodecyl sulfate (SDS)), which exhibited interesting properties like ideal toughness and good self-healing ability resulting from reversible physical crosslinks created by the self-complementary dimers through the quadruple hydrogen bonds. The nanoscale micelle cores create a unique chromophore through hydrogen-bonded networks and interactions of functional groups with electron stacking. The tightly packed hydrophobic core of the micelle, characterized by restricted intramolecular motion due to UPy dimer aggregation, serves to safeguard the chromophore fluorescence from water-induced quenching, leading to a significant enhancement in fluorescence emission. The amphiphilic PAMPS incorporated in the structure of micelles could mimic the transmembrane proteins' function and could enlarge the size of micelle nanostructures and improve both fluorescence emission and toughness. Indeed, the intermolecular quadruple hydrogen bonds of UPy confined within the nanosized micelle core result in a significant limitation of intramolecular motion (RIM), ultimately boosting light emission. This led to emission of strong blue fluorescence with quantum yields of about 9.2 and 23.5% for the hydrogel and dry forms, respectively. Moreover, the dense hydrogen-bonded network within the micelle core played a crucial role in shielding chromophore fluorescence from being quenched by water, the same as what happened in jellyfish. It also had the capability of emitting different light in response to excitation with different wavelengths which was related to the presence of different emission species in the structure of this hydrogel. The phenomenon of clustering-triggered emission (CTE) has been suggested as a mechanism to elucidate the AIE behavior observed in nonaromatic luminogens. This concept revolves around the belief that clusters are created through the aggregation of electron-rich groups, leading to the generation of delocalized electrons and an enhancement in emission. In here, the presence of amine, carbonyl, and sulfonic groups could induce photoluminescence ability to the structure of this hydrogel. Moreover, the presence of reversible physical cross-linked quadruple hydrogen bonds provided the self-healing ability to the hydrogel; the cut pieces could attach to each other in 3 min without using external stimulation. These H-bonds also improved the mechanical properties of the hydrogel, so increasing the number of UPy dimers with quadruple hydrogen bonds exhibited better mechanical properties via facilitating the effective energy dissipation through H-bond rearrangement and chain alignment during stretching. It could also be fully ionized and exhibit an electric field-responsive bending property without affecting its fluorescence properties, which appropriates it for applications in under water soft robotics or as artificial muscles.88
The electronic skin (e-skin) represents a sophisticated electronic system that adeptly transforms diverse external stimuli, such as pressure, deformation, and humidity, into electronic signals. Moreover, it possesses the ability to replicate fundamental aspects of the human skin, including stretching, self-healing, and versatile sensory capabilities. Its considerable potential extends to applications in wearable healthcare sensors, tactile devices, robotic artificial skin, prostheses, and implantable medical devices. As of now, the e-skin has realized flexibility, reduced weight, miniaturization, and multifunctionality.89
In research done by Liu et al., a new type of e-skin with tactile and non-contact sensing ability was fabricated, which had a 3 μm thickness and could exhibit high transparency, good flexibility, and stable operation, simultaneously. It was a sandwich like elastomer, acting as a triboelectric layer, composed of polytetramethylene ether glycol (PTMEG), as the soft component that provided the elastic matrix, and hydrogenated 4,4′-methylenediphenyl diisocyanate (HMDI) and aliphatic disulfide bis(2-hydroxyethyl) disulfide (HEDS), as the stiff compound with self-healing ability resulting from dynamic aliphatic disulfide bonding interactions. Then, a layer of polystyrene nanospheres was coated on the elastomer followed by coating this substrate with a layer of transparent conductive polymer, poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) that acted as the electrode. It was an ultrathin triboelectric nanogenerator (TENG) with interesting properties like transparency, flexibility, reduced size and weight, self-healing ability, cost-effectiveness, feasible fabrication, and high-power output. The fabricated formulation could heal completely within 5 min under a hot airflow (50 °C), due to the presence of dynamic disulfide and hydrogen bonds, without any change in its transparency and elasticity or changing its electrical output. It also had mechanical durability, without any performance decay even after 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles, and operational stability so it could maintain consistent electrical output even after undergoing 146
000 bending cycles, and operational stability so it could maintain consistent electrical output even after undergoing 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of contact–separation operation conducted by a linear motor. These outstanding mechanical performances were credited to its ultrathin design and the inherent robustness of the elastomer. The presence of nanospheres improved the sensitivity of the chip via generating additional space during the contact–separation process since it could experience greater mechanical deformation at specific pressures before reaching saturation. Therefore, the fabricated chip could act as both an energy harvester and a self-powered tactile sensor.90
000 cycles of contact–separation operation conducted by a linear motor. These outstanding mechanical performances were credited to its ultrathin design and the inherent robustness of the elastomer. The presence of nanospheres improved the sensitivity of the chip via generating additional space during the contact–separation process since it could experience greater mechanical deformation at specific pressures before reaching saturation. Therefore, the fabricated chip could act as both an energy harvester and a self-powered tactile sensor.90
Recently, a new type of triboelectric self-powered sensor with self-healing ability was fabricated using two layers of self-healing polyurea (SH-PUrea) films (with a size of about 30 mm × 30 mm) incorporated with Ag nanowires (AgNWs), in which each of the AgNWs was connected to copper wires. The strong affinity between the silicon element and SH-PUrea could effectively function as the electrification layer in the triboelectric sensor which could attract electrons upon contact with other objects, relying on the triboelectrification effect. The self-healing ability of the sensor was assessed at room temperature which showed healing in 30 min without any remaining cracks, even in a harsh environment that was accelerated by increasing temperature. This self-healing ability was related to the presence of physical entanglements that could tolerate various harsh environments. The fabricated sensor possessed the capability for both mechanical and electrical self-healing, thereby reducing device maintenance costs and extending its overall service life. When the skin made full contact with the sensor, the skin became positively charged, while the sensor surface acquired an equal negative charge. SH-PUrea exhibited a superior ability to capture electrons compared to the human skin, allowing electrons on the skin to transfer to the sensor's friction layer upon contact. Upon separation of the skin from the friction layer, a potential difference arose, and the negative charge in the friction layer was neutralized by the electrode layer, causing electrons to move out of the electrode layer to the ground. The electrical potential equilibrium was only achieved when the skin was completely separated from the sensor. Upon reattachment of the skin to the friction layer, the potential difference was reestablished, leading electrons to flow back to the electrode. The repetitive contact and separation movements, driven by external mechanical forces, created a continuous flow of electrons between the electrode layer and the ground, which converted external mechanical energy into electrical energy, generating a low-frequency alternating current (AC). The sensing activity of the fabricated self-powered E-skins was assessed via synthesizing a patch comprising nine sensing units and tested under sweaty environments on the hand. Results confirmed the capability of this sensor to identify pressure positions while maintaining its self-healing functionality, ensuring stable output. In addition, the encapsulated sensor array exhibited effective operation for transmitting information both in air and underwater (Fig. 6).91
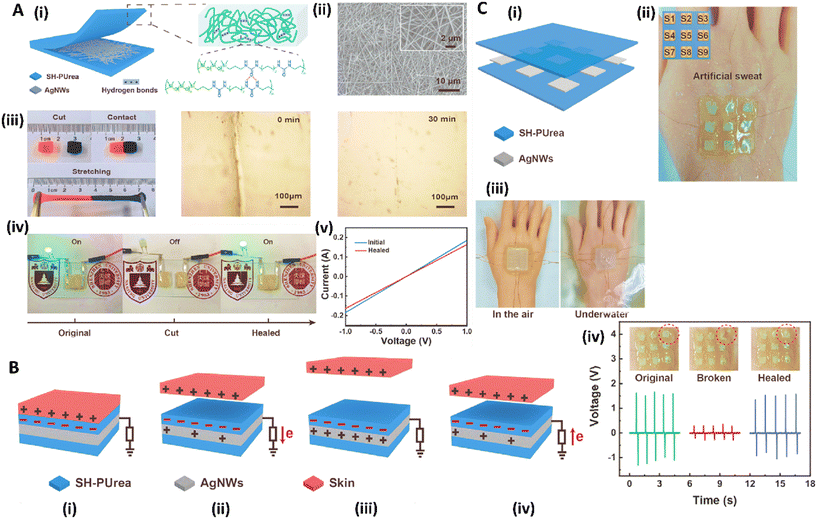 | ||
| Fig. 6 (A) Schematic image of sandwich like hydrogel fabricated from SH-PUrea and AgNWs (i), AgNWs SEM result (ii), Self-healing ability of hydrogel (iii), changes in the electrical ability of hydrogel during healing process (iv), results of I–V curve before and after healing process (v). (B) Results of working mechanism of the hydrogel, (i) inducing charge in the skin by the attachment of sensor to the skin, (ii) separating skin and balancing the negative charge of friction layer by the electrode layer, (iii) gaining the electrical potential equilibrium state after completely separating the skin, (iv) remodeling the potential difference after reattachment of skin to the friction layer. (C) Schematic (i) and real (ii) image of sandwich like sensor. Image of sensor attached to the hand under air and water condition (iii), recovery of the output signals of the sensor array attached on the hand during healing process (iv). Reprinted with permission from ref. 91. Copyright 2024, Elsevier. | ||
3.3. Wound dressings
Wound healing is a systematic process that involves production of growth factors, inflammatory cytokines, fibroblasts, and epithelial cells and tissue regeneration.92 Wounds can be of acute or chronic type, where the healing of acute wounds occurs within 4 weeks following the phase of haemostasis, inflammation, tissue proliferation and skin remodeling. Though the normal mechanism of wound healing gets initiated, angiogenesis gets stalled during the chronic wound healing especially during the interlinked microvascular complications.93,94 Wound dressings provide an environment for healing of the wounds and tissue regeneration, via possessing antibacterial properties to prevent infection, maintaining the moisture environment of the wound, and promoting rapid healing.95,96Self-healing hydrogels are of great choice due to their moisture retaining tendency, repairing and restoring the damage. Dynamic networking using metal ion coordination bonds and Schiff base bonds is generally used in fabrication to enhance the self-healing properties of the hydrogel.97,98 The incorporation of self-healing polymers and hydrogels can contribute to enhanced durability and longevity of wound dressings, ensuring prolonged protection and support during the healing process, and preventing the need for renewing the hydrogels. This innovative approach aligns with the principles of biomimicry, drawing inspiration from natural healing mechanisms. Such materials offer the potential to reduce the frequency of dressing changes, minimize infection risks, and improve patient comfort. The field is evolving, and ongoing research focuses on tailoring self-healing materials to meet specific wound healing requirements, emphasizing biocompatibility and therapeutic efficacy.99,100
Qiao et al. adopted a sequential procedure to prepare sodium alginate-grafted dopamine/carboxymethyl chitosan/Fe3+ (OSD/CMC/Fe hydrogel)/polydopamine-encapsulated poly(thiophene-3-acetic acid) (OSD/CMC/Fe/PA hydrogel), which possessed tissue adhesive, anti-bacterial, and antioxidant properties. Sodium alginate exhibits good biocompatibility and coordination properties with metals. In order to impart good adhesion and antioxidant action to OSA, dopamine-grafted oxidized sodium alginate (OSD) was prepared. Further, poly(thiophene-3-acetic acid) was coated over polydopamine (PDA) to impart water solubility and photothermal activities. Schiff base and metal based Fe3+ coordination bonds were responsible for the self-healing properties of the hydrogel. Since carboxymethyl chitosan (CMC) has carboxyl and amino groups, it was used to introduce the Schiff base bond with OSD. The mechanical strength of the hydrogel was improved through the addition of Fe3+ ions that could bind with carboxyl groups in the CMC and catechol groups in dopamine. Analysis of the swelling ratio showed a gradual decrease with the addition of Fe3+ and PA. The fabricated hydrogel showed fast healing ability within 10 min at 25 °C resulting from the ligand and Schiff base bonds. Major application of wound healing was tested on a mouse with a full-thickness defect using the OSD/CMC/Fe/PA hydrogel in the presence and absence of near-infrared (NIR) light, which confirmed better wound healing properties in the presence of light after 14 days of treatment of healing (97.02%). It also showed improved anti-inflammatory and photothermal-antibacterial properties tested against Escherichia coli (E. coli) and methicillin-resistant Staphylococcus aureus (MRSA). This study made use of polydopamine (PDA) that acts as a natural and biocompatible adhesive and mimics the environment of the extracellular matrix. Its derivatives have near-infrared (NIR) absorption and exert excellent biocompatibility along with antioxidant and antibacterial properties.101
Green chemistry was adopted to prepare a poly(vinyl alcohol) oxidized salep double-network (PVA/OSEA DN) hydrogel via Schiff-base cross-linking between oxidized salep (OSa) and ethylene diamine-modified salep (SaHEA). The self-healing characteristic was attained through the utilization of reversible Schiff-base and hydrogen bonds, which in turn increased the structure strength, stability and rigidity. This network was combined with Arnebia euchroma extract and AgNPs to study the wound healing nature. This extract was rich in bioactive compounds that could exhibit anti-microbial and anticancer properties on the one hand and was used as a reducing agent for the AgNP synthesis on the other hand. It could be observed that this self-healing hydrogel had better tensile strength with mechanical properties such as compression, stretchability and adhesive nature that was majorly dependent on the concentrations of OSa and SaHEA. In vitro cytotoxicity were assessed for PVA/OSEA DN (OSa: SaEA concentrations – (P0–0: 0 wt%), (P3–3: 3 wt%), (P6–6: 6 wt%), and (P9–9: 9 wt%)) hydrogels using MTT assay in A375 mouse fibroblasts cell lines. The study shows that the percentage of cell viability was higher (80%) than the cell toxicity. Furthermore, the in vivo effect of the hydrogel on burn and full-thickness wound healing was evaluated in male Wistar rats. The observational and histomorphological study showed the rapid healing efficiency of the wound with a considerable reduction in the size of the wound after 21 days of treatment when compared to the untreated control rats. It was expected that salep being a non-toxic, swelling agent rich in glucomannan might have contributed to the wound healing action.102
Gelatin methacrylate (GelMA), adenine acrylate (AA), and CuCl2 were used to fabricate a new type of hydrogel with antibacterial, tissue adhesive, and self-healing abilities. In here, the concentration of Cu2+ ions could affect different properties of the hydrogel so increasing the amounts of ions could increase the swelling ratio of the hydrogel since it could impose a constraint on the movement of the molecular chains with double bonds in GelMA and AA. Besides, increasing Cu2+ ions led to an increase in the pore size of the hydrogel. It also had excellent self-healing stability and efficiency so the rheological properties of the hydrogel were reformed after the repeatable healing process. These self-healing properties were related to the inter/intramolecular hydrogen bonds between the carboxyl groups and AA as well as the coordination of interactions between Cu2+ ions and carboxyl ligands. The fabricated hydrogel had stable mechanical properties so no plastic deformation was observed after different compression and recovery cycles, which was related to the presence of ionic and covalent bonds. Cu2+ ions also led to exhibiting antibacterial activity (via affecting the membrane of bacteria and changing the structure of proteins and enzymes) in a concentration dependent manner so increasing the amounts of Cu2+ ions increased the antibacterial activity of the hydrogel. The occurrence of hydrogen and ionic bonds between the substrate and hydrogel provided the tissue adhesiveness capability. Results of the in vivo test also confirmed the effectiveness of the hydrogel in accelerating wound healing so animals treated with the hydrogel were completely healed. Presence of amine groups in the structure of gelatin improve the healing process via providing a more regular epithelial layer, enhancing collagen deposition, reducing inflammatory reactions, and promoting angiogenesis (Fig. 7).103
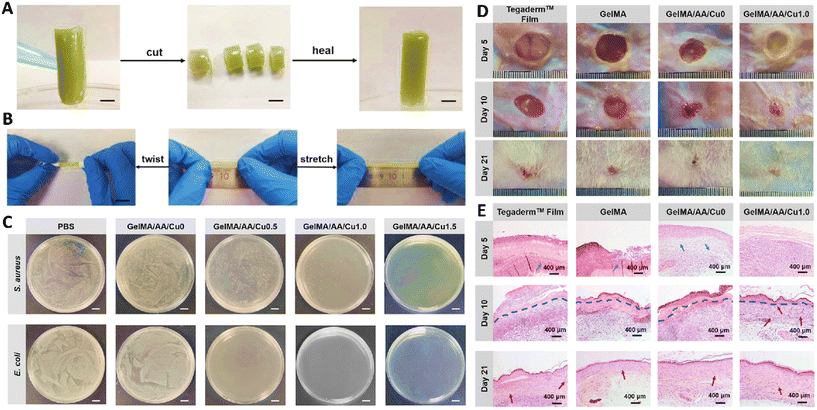 | ||
| Fig. 7 Results of (A) self-healing ability and (B) stretchability of the GelMA/AA/Cu hydrogel. (C) Antibacterial activity of the GelMA/AA/Cu hydrogel against E. coli and S. aureus. Wound healing (D) and histopathological (E) results of different treatments in 5, 10, and 21 days. Reprinted with permission from ref. 103. Copyright 2022, Elsevier. | ||
Drug loaded (antibiotic/anti-microbial) self-healing biomaterials get released without accurate tracing about the intensity of the bacterial infection, which might contribute to issues like drug resistance, overdose, elevated costs, and a diminished rate of healing. It is important to note that the generation of reactive oxygen species, pH, and temperature vary with bacterial loading and population, hence external response based wound dressings can provide personalized medications and reduce the cost and use.104 For instance, Wang et al. fabricated a type of zwitterionic hydrogel composed of poly[(N-isopropyl acrylamide)-co-(butyl acrylate)-co-(sulfobetaine methacrylate)]-b-poly(ethylene glycol)-b-poly[(N-isopropyl acrylamide)-co-(butyl acrylate)-co-(sulfobetaine methacrylate)] (PZOPZ). The fabricated hydrogel showed reversible thermoresponsive sol/gel properties; it was in the sol form at low temperature and changed to a gel form at 37 °C. The self-healing ability of the hydrogel was confirmed by the strain amplitude sweep measurement that showed recovery of G′ and G′′ after healing. The self-healing ability of this hydrogel was related to the strong dipole–dipole interactions between sulfobetaine components. Results of the antibacterial test also showed anti-bacterial adhesion capability via producing a stable hydration layer that prevented bacterial adhesion. The wound healing ability results of the hydrogel showed faster healing in the presence of the fabricated hydrogel that provides a moist favorable environment for the wound to heal and prevents contaminant adhesion. This hydrogel could induce tissue regeneration and granulation and collagen deposition, and enhance neovascularization (Fig. 8).105
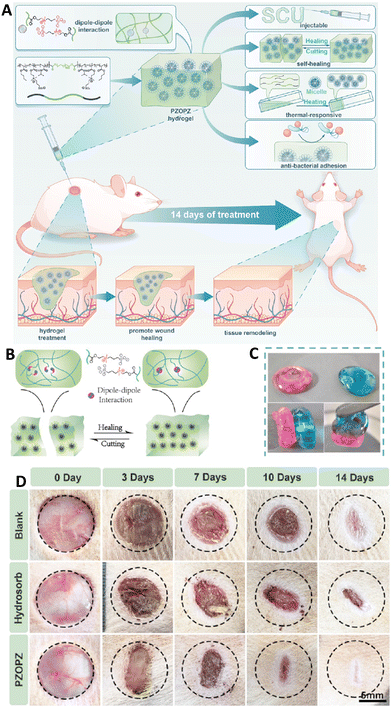 | ||
| Fig. 8 (A) Schematic image of fabrication and wound dressing application of the PZOPZ hydrogel. Schematic (B) and optical image (C) related to the self-healing ability of the PZOPZ hydrogel. (D) Results of the wound healing test for 14 days using different treatments. Reprinted with permission from ref. 105. Copyright 2022, American Chemical Society. | ||
Diabetic wounds associated with hypoxia, insufficient angiogenesis and free radical production at the chronic state might lead to surgery, huge cost live stem cell therapy and medications that affect the patient's wellbeing and increase the healthcare burden.106 The current demand calls for self-healing materials possessing multiple functionalities that address issues associated with diabetes, oxidative stress, and microbial infection. Exosomes derived from mesenchymal stem cells serve as a promising, biocompatible application for wound healing by enhancing the rate of angiogenesis.107 However, the utility of injectable exosomes is mainly influenced by their quick clearance rate, where carriers for sustained and controlled release are needed.108 Pluronic F127 (F127), oxidative hyaluronic acid (OHA), and poly-ε-L-lysine (EPL) were used to fabricate a smart self-healing hydrogel (using reversible Schiff base) loaded with adipose mesenchymal stem cell (AMSC)-derived exosomes that could accelerate diabetic wound healing. The self-healing ability of this hydrogel was assessed using different tests. At first, the rheological ability of the hydrogel before and after healing was assessed, which had no change. The healing process occurred in 16 h due to the presence of dynamic Schiff base bonds in the structure of the hydrogel. The presence of antibacterial polypeptide (EPL) in the structure of this hydrogel provided fast antibacterial activity against both Gram-positive and Gram-negative bacteria in 2 h. The fabricated hydrogel also showed pH-responsive sustainable exosome release, in which the amounts of released exosomes were higher at acidic pH compared to those at normal pH. This sustainable exosome release led to improvement of the proliferation of human umbilical vein endothelial cells (HUVECs) during the time of exposure to the hydrogel containing exosomes. Results of the in vivo test confirmed the capability of the fabricated hydrogel to promote the healing rate via improving collagen deposition, neovascularization, re-epithelization, and tissue granulation.109
Recently, Leng et al. (2022) reported a self-healing and stimuli responsive hydrogel, FEM constituting ε-poly-L-lysine-F127-ε-poly-L-lysine (EPL-F127-EPL) and metformin (antidiabetic drug) for wound healing. Multifaceted qualities such as anti-inflammatory, antidiabetic and anti-microbial activities were attained. Particularly, controlled release of the antidiabetic drug was achieved due to the utilization of the drug loaded hydrogel, and the in vivo study on a full thickness wound mouse model showed that the hydrogel could significantly repair the wound and promote angiogenesis.110
Interestingly, the self-healing ability was used to introduce a new type of healing process without using suture. In a study, a type of self-healing elastomer (PUIDE-CTAB) was fabricated via polycondensation of hydroxyl-terminated polybutadiene (HTPB), 1,10-decanediol (DE), and isophorone diisocyanate (IPDI) catalyzed by dibutyltin dilaurate (DBTDL). This compound was then interacted with cetyltrimethylammonium bromide (CTAB) to fabricate the PUIDE-CTAB elastomer. In here, the presence of HTPB controlled the flexibility of the hydrogel while IPDI and DE were used for controlling the mechanical and self-healing properties of the hydrogel through providing the hydrogen bonds in urea/urethane linkages. The robust mechanical characteristics of PUIDE-CTAB were related to the development of hierarchical hydrogen bond combinations and enhanced phase separation between the rigid and flexible segments. It showed self-surface regeneration at 40 °C in 2 h which confirmed its healing ability, and the healed part could tolerate large stretching deformation. It showed high cytocompatibility and hemocompatibility effects. Moreover, it exhibited effective antibacterial activity against both Gram-positive and Gram-negative bacteria via changing the membrane integrity resulting from the interaction between CTAB and the bacterial membrane. The in vivo wound healing ability of this formulation was then compared with that of sutured and controlled wound which confirmed the effectiveness of the fabricated elastomer in closing and healing wounds. In mice treated with the elastomer and suture, the wound was completely closed and some skin appendages were created (like hair follicles and collagen deposition) (Fig. 9).111
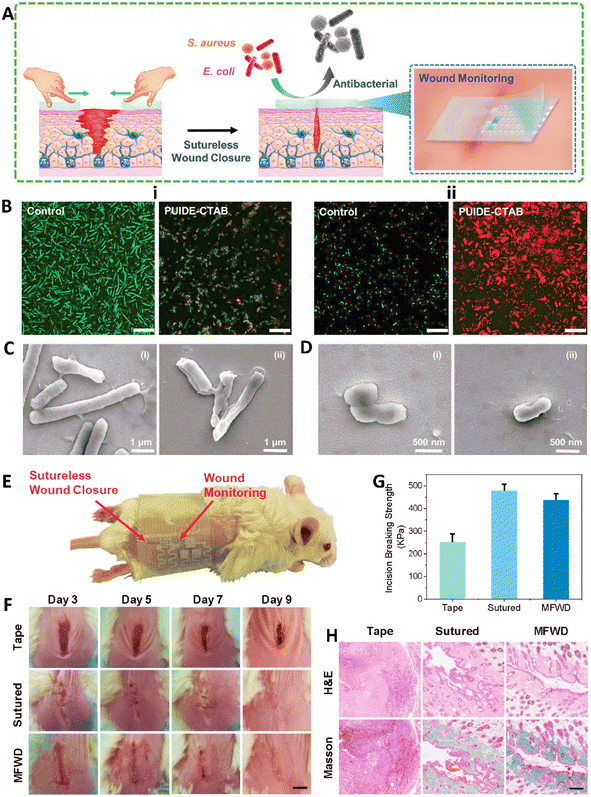 | ||
| Fig. 9 (A) Schematic image of self-healing antibacterial wound closure. (B) Results of live/dead staining of E. coli (i) and S. aureus (ii) in the presence and absence of PUIDE-CTAB (scale bar: 20 μm). SEM images of E. coli (C) and S. aureus (D) in the absence (i) and presence (ii) of PUIDE-CTAB. (E) Image of a mouse treated with sutureless wound closure. (F) In vivo results of wound healing for 9 days treated with tape, suture, and PUIDE-CTAB (MFWD). Result of (G) breaking strengths and (H) histopathological analysis in mice treated with different formulations. Reprinted with permission from ref. 111. Copyright 2021, Wiley-VCH GmbH. | ||
3.4. Cancer therapy
Self-healing materials have potential application in the field of cancer therapy through targeted delivery of therapeutic compounds. They help to release anticancer drugs in a controlled manner at the specific tumor site with response to external stimuli such as pH, temperature, or enzymes.112 Self-healing materials, particularly injectable hydrogels encapsulated with chemotherapeutic drug carriers such as cyclodextrin, chitosan, and cellulose (natural cationic polymers), retain the properties of sustained drug release with bioavailability and aid in site specific drug delivery and killing of malignant cells. This leads to high therapeutic performance in the presence of lower amounts of drugs, which reduces the treatment cost and prevents side-effects on normal tissues. Besides, the self-healing ability of these materials led to a decrease in the amounts of used materials and energy that should be spent for the fabrication of nanocarriers.113A thermosensitive hydrogel (CGD) comprising dialdehyde-functionalized polyethylene glycol (DF-PEG) and β-glycerophosphate (GP) cross-linked chitosan (CS) was synthesized for the targeted and controlled release of the anti-cancer drug doxorubicin (DOX). It was based on the dynamic covalent bond interaction which has the attributes of sol–gel conversion based on the body temperature. It was observed through SEM analysis that there was no major difference between the healed hydrogels and their initial form. Cross-linking between CS and DF-PEG confers mechanical properties to the gel to attain elasticity and strength. In vivo self-healing analysis showed that the destroyed CGD upon implantation into rats could restore the uniform gel like shape compared to the CG gels. It was observed that the rats treated with DOX/CGD hydrogels had enhanced survival rates and the tumor size could be significantly reduced when compared with the DOX/CG hydrogels (DOX/CG: 1.33 ± 0.13 and DOX/CGD: 0.95 ± 0.11 g).114
Iron ion-doped polyaniline (PANI(Fe)) and guar gum (GG) chains were used in a study to fabricate a dual responsive hydrogel (GG@PANI(Fe)-borax) that had simultaneous photothermal (PTT)/chemodynamic (CDT)/chemotherapy under NIR-II irradiation. The presence of dynamic reversible borate/didiol bonds in the structure of this hydrogel provided the sol–gel transition capability in response to changing the temperature and pH; thus by increasing the temperature until 46 °C and also at acidic pH, the gel form of the hydrogel converted to the sol form which could be effective in controlling drug release from the hydrogel. Besides, the presence of several hydrogen bonds between GG and PANI as well as iron-doping induced light absorption ability to the hydrogel in the range of near infrared (NIR) I and II which appropriates it for photothermal therapy (PTT) as well as sol–gel transition that triggers drug release. Induction by NIR light also had other advantages like deep penetration in the inner sites of the body and no side-effects on other parts of the body. This formulation showed a stable repeatable photothermal effect. The presence of iron ions in the structure of this hydrogel could induce Fenton reactions in the tumor microenvironment (due to the presence of high amounts of H2O2) which appropriate it for CDT. The fabricated hydrogel showed self-healing ability without using any external stimulation that was related to the presence of hydrogen bonds and borate/didiol crosslinking. The anticancer activity of the hydrogel was confirmed using in vitro and in vivo tests and the results confirmed the effectiveness of this hydrogel to be applied for cancer treatment.115
Oxidized sodium alginate-hydroxypropyl/bovine serum albumin molybdenum disulfide (OSA-HPCS/BSA-MoS2) was used to fabricate a self-healing hydrogel that was used for the photothermal and photodynamic treatment of breast cancer. The fabricated hydrogel showed rapid healing (within 5 min) related to the presence of Schiff base bonds in the structure of the hydrogel. Besides, the injectable ability of this hydrogel appropriated it for local drug delivery via injecting the hydrogel solution directly inside the cancerous tissue. Evaluating the rheological properties of the hydrogel before and after the healing process also confirmed its rapid self-healing process. The presence of BSA-MoS2 in the structure of this hydrogel induced the photothermal ability to this hydrogel under NIR irradiation (808 nm) which was recovered to room temperature within 10 min. MoS2 also produced high amounts of ROS under NIR irradiation. Results of cytotoxicity tests against normal cells showed no toxic results while cancer cells could be significantly affected under NIR irradiation related to the PTT/PDT effects. The in vivo test also confirmed the effectiveness of this hydrogel against tumor model mice through reducing the size of the tumor while it had no effect on other organs (Fig. 10).116
 | ||
| Fig. 10 (A) Schematic image of fabrication and application of the OSA-HPCS/BSA-MoS2 nanocomposite hydrogel. (B) Self-healing ability of the OSA-HPCS/BSA-MoS2 nanocomposite hydrogel (i–vi). (C) Results of photothermal therapy of mice treated with i – PBS, ii – BSA-MoS2, and iii – OSA-HPCS/BSA-MoS2 nanocomposite hydrogel under NIR irradiation. (D) Change in the size of the tumor treated with different samples (i – PBS, ii – hydrogel, iii – OSA-HPCS/BSA-MoS2 nanocomposite hydrogel, iv – BSA-MoS2 + NIR, and v – OSA-HPCS/BSA-MoS2 nanocomposite hydrogel + NIR). Reprinted with permission from ref. 116. Copyright 2022, Wiley-VCH GmbH. | ||
Self-repairing, injectable pH-responsive, cellulose-based hydrogels (CAAs) were prepared for local and site-specific bone cancer therapy by Jiang and coworkers. CAA was mixed with adipic dihydrazide-grafted carboxyethyl cellulose (CEC-ADH), ethyl-1-adamantane 4-formylbenzoate (AD-CHO) and carboxyethyl cellulose-grafted β-cyclodextrin (CEC-CD). The pH responsive properties and hydrolysis degradation resistance were conferred through aryl hydrazone bonds. A dynamic self-healing hydrogel was established through host–guest interaction between the AD and CD groups. Increased cell viability of 143B cells treated with the DOX encapsulated hydrogel was observed even after 7 days of incubation. Difference in the cell viability was noted in the cells treated at different pH values, where the viability was considerably reduced at pH 6.2 compared to that at 7.4. This might be due to the presence of the acyl hydrazone bond that creates an acidic environment and increases the drug release. The effect of the hydrogel was studied on 143B cell injected BALB/C nude mice (osteosarcoma mice model), which had long term retention capacity and significantly reduced the tumor size when compared with the DOX alone treated mice. Observational changes such as body weight and nutritional intake of the animals were considerably improved with the hydrogel treatment which was able to mask the emerging side effects as analyzed through biochemical studies (liver and kidney markers: creatinine, urea). This study strongly suggests the application of the biocompatible DOX-gel in the treatment of bone cancer.117
Curcumin loaded polymeric cyclodextrin was prepared and encapsulated inside a self-healable hydrogel composed of amino-gelatin (Agel) interacting with oxidized starch (OS) to be applied against osteosarcoma. The presence of Schiff-base interaction between the gelatin and starch and the host–guest interaction between β-cyclodextrin and the aromatic residues of gelatin induced self-healing ability in the structure of this hydrogel. Besides, no change was observed in the weight and pore size of the hydrogel before and after the healing process. The presence of curcumin in the structure of this hydrogel provided antioxidant ability. The protonation of imine groups present in the structure of the hydrogel at acidic pH led to an increase in the amounts of released drug at acidic pH compared to normal pH which induced pH responsiveness for this hydrogel. The hydrogel also showed a selective toxicity effect against cancer cells while a very low toxicity effect was shown against the normal cell line.118
Some of the other types of self-healable materials used in biomedical applications are summarized in Table 1.
| Formulation | Target | Results | Ref. |
|---|---|---|---|
| Self-healing gelatin (GE)/oxidized alginate (OSA)/adipic acid dihydrazide (ADH) hydrogels | Drug delivery/tissue engineering | • Fabrication of the in situ injectable hydrogel using the Schiff base reaction and acylhydrazone bonds | 119 |
| • Sol form at room temperature and gel at body temperature | |||
| • Changing the gelation time via changing the ratio of GE, OSA, and ADH | |||
| • Having an interconnected macroporous structure and self-healing ability | |||
| • Sustainable growth factor release pattern | |||
| • Accelerating tissue engineering via activating immune response, replacing the hydrogel with vascularized connective tissue | |||
| Magnetic chitosan microspheres (MCMs) embedded in the chitosan/carboxymethyl cellulose hydrogel | Drug delivery | • Exhibiting thermal reversibility, self-healing ability (due to utilizing Schiff-base crosslinking), stability, magnetic responsiveness, homogeneity | 120 |
| • Good swelling ratio | |||
| • Low degradability | |||
| • Controlled drug release pattern under external magnetic force | |||
| Multiple-responsive hyaluronic acid (HA)–histamine (His)/Zr4+ hydrogel | Drug delivery/wound healing | • pH and enzyme (hyaluronidase) responsiveness | 121 |
| • Strong antibacterial and antibiofilm activity via production of reactive oxygen species (ROS) that affect DNA and proteins of bacteria | |||
| • Good biocompatibility | |||
| • Inducing collagen expression | |||
| TEMPO-oxidized cellulose nanofibrils (TOCNF) and tannin | Wound dressing/drug delivery | • Excellent antioxidant and antibacterial properties | 122 |
| • Good mechanical stability and biocompatibility | |||
| • Rapid self-healing without using any stimulation | |||
| Gelatin-oxidized tannic acid (GLT-OTAs) hydrogel loaded with indomethacin (IDMC) | Drug delivery | • Making crosslinking reaction due to the Michael addition and Schiff-base reaction | 123 |
| • Self-healing ability at 37 °C in 4 h without remaining cracks | |||
| • Good mechanical properties and mechanical stability that was improved via increasing the amounts of OTAs due to increasing crosslinking density | |||
| • pH and OTAs dependent swelling ratio, thus the swelling ratio was decreased by increasing the pH and OTAs concentration due to the presence of amino groups of the gelatin in the –NH2 form not the ionic form and increasing condensation of the hydrogel, respectively | |||
| • Increasing drug release by increasing the pH and decreasing the amounts of OTAs | |||
| Diselenide-containing carbon dots (dsCD) incorporated in ureidopyrimidinone-conjugated gelatin (Gel-UPy) electrochemical wireless hydrogel biosensors | Cancer biosensor | • High self-healing ability under tumor conditions due to the presence of higher amounts of ROS. The cleavage of dsCD after treating with ROS or glutathione led to creation of intermolecular bonding that improved the self-healing capability of the hydrogel | 124 |
| • Recovery of elastic response in the presence of GSH and H2O2 (ROS) after healing | |||
| • Reversible electronic properties in the presence of GSH and H2O2 | |||
| • Repeatable self-healing performance | |||
| • High biocompatibility | |||
| • Exhibiting self-healing ability in the presence of cancer cells using a smartphone | |||
| • Increasing the electrical conductivity of the hydrogel in the presence of cancer cells that appropriates this biosensor for detecting cancer cells | |||
| Polyaniline–cellulose nanofiber (PANI@CNF) nanocomplexes incorporated in a borax-crosslinked polyvinyl alcohol (PVA) hydrogel | Electrochemical biosensor | • Improving the compression stress (3.5 fold) and storage modulus (400 fold) in comparison with the PVA | 125 |
| • Rapid self-healing in 15 s without any stimulation due to the reversible didiol–borax complex and hydrogen bonds (related to the mobility and hydrophilic nature of polymer chains) | |||
| • Providing ideal electrochemical ability for the hydrogel in a concentration dependent manner (depending on the amounts of CNFs that not only led to homogeneous dispersion of PANI but also could provide paths for transporting electrons and ions) | |||
| • The ability to recover the original conductivity, shape, and strength after healing | |||
| • Repeatable self-healing ability | |||
| • Ideal biocompatibility and cell attachment | |||
| Polyvinyl alcohol (PVA)/poly(N-(2-hydroxyethyl)acrylamide) (pHEAA) polymer matrix containing dopamine (DA)-modified polypyrrole (PPy) coated Sb2S3 nanorods (Sb2S3@PPy-DA) | Electrochemical biosensor | • Show robust tensile strength (1.25 MPa), outstanding sensitivity (GF = 4.97), high interfacial toughness (251 J m−2), and large elongation (620%) due to the presence of Sb2S3@PPy-DA | 126 |
| • Rapid self-healing in 90 s after NIE irradiation that increased the temperature which led to producing a mobile phase and promoting polymeric chain recombination | |||
| • Recovery of tensile stress and strain after healing | |||
| • Anti-freezing capability due to the application of glycerin (and its interaction with water molecules that reduced the freezing point of water) that appropriates it for applying under extreme temperature conditions | |||
| • Good electrochemical conductivity due to the presence of Sb2S3 | |||
| • Suitable for wireless detection of motion, management of healthcare, and energy harvesting applications | |||
| Conductive polyaniline (PANI) precursor infiltrated into the self-healable hydrophobic association poly(acrylic acid) (HAPAA) hydrogel matrix | Deformation detection via electrical signals | • Decreasing swelling ratio compared to HAPAA due to the more compact structure and higher amounts of crosslinking | 127 |
| • Superior mechanical properties related to the dynamic interactions between PANI and HAPAA networks, reversible hydrophobic micelles, and PANI chains' internal fracture | |||
| • Self-healing ability in 30 min which was related to the reversible hydrophobic micelles present in the HAPAA network as well as reformation of dynamic interfacial interactions between free amine, hydroxyl, and carbonyl groups of PANI and HAPAA, and could be accelerated via heating | |||
| • Recovery of electrical conductivity after healing and independent to the PANI concentration | |||
| • Exhibiting superior tensile properties and sensitivity that appropriate it for stretchable electronics | |||
| • Capability of detecting deformation with a detection limit of about 0.05% strain | |||
| • Stable electrical signal (even after 100 cycles) resulting from the interconnected nature of the PANI network, the deformation responsibility of PAAN hydrogels through breaking hydrophobic micelles and hydrogen bonds within the gel network | |||
| Ureidopyrimidinone/tyramine (Upy/Tyr) difunctionalized gelatin incorporated in Tyr-doped poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) | Health monitoring wearable biosensor | • Exhibiting low impedance, high charge storage and conductivity, and injection capability that appropriate it for bioelectronic applications | 128 |
| • Appropriate for long term application in bioelectronics due to the stable mechanical durability | |||
| • Appropriate for irregular interface application due to the reversible dissociation–re-formation ability of hydrogen bonds | |||
| • Self-healing ability in 10 min without using any external force as well as 94% electrical recovery | |||
| • Good tissue adhesiveness resulting from different interactions including electrostatic and cation–π interactions as well as hydrogen bonds | |||
| • Temperature responsive tissue adhesiveness and the adhesive ability was increased by increasing the temperature resulting from weakening the physical bond and increasing the fluidity within the hydrogel | |||
| Waterborne polyurethane carbohydrazide (CHZ) and N,N-bis(2-hydroxyethyl)-3-amino propionyl glycinamide (HO-NAGA-OH) groups (WPU-CHZ-NAGA) | Strain sensor | • Excellent mechanical features including 125.82 MJ m−3 toughness, 36.58 MPa tensile strength, and 81.2 kJ m−2 tearing energy | 129 |
| • Fast self-healing within 8 h at room temperature and in the presence of ethanol resulting from the hierarchical band | |||
| • Excellent tissue adhesion resulting from abundant hydrogen bonds | |||
| • Exhibiting blue fluorescence emission resulting from the aggregation-induced emission effect of tertiary amine | |||
| • Could be applied as a flexible strain sensor for accurate human finger monitoring | |||
| Multifunctional double network ionogel using polyacrylamide, amino-modified agarose, 1,3,5-benzenetricarboxaldehyde and 1-ethyl-3-methylimidazoliumchloride | Strain sensor | • Exhibiting excellent transparency (>95%), nonflammability, strong adhesion, and good temperature tolerance (about 96–260 °C) | 130 |
| • Accelerating self-healing ability resulting from dynamic imine bonds as well as high amounts of hydrogen bonds | |||
| • Mechanical stability | |||
| • Emission of fluorescence light resulting from the gelation-induced emission phenomenon | |||
| • Monitoring various human motions, along with a fast response time (38 ms), high sensitivity, and excellent durability (>1000 cycles) and stability over a wide temperature range (30–80 °C) | |||
| Tetraphenylethylene (TPE) modified poly(L-glutamic acid) (PLGA–TPE) with acrylate γ-cyclodextrin (Ac-γ-CD) hydrogel | α-Amylase detection via a fluorescence sensor | • Degradation of the dual-network of the hydrogel in the presence of α-amylase that led to decreasing the mechanical stability of the hydrogel while increasing its fluorescence | 131 |
| • Exhibiting fast self-healing ability within 3 min resulting from the dynamic host–guest interaction | |||
| • Temperature responsive fluorescence ability resulted from the exit of TPE from the cyclodextrin cavity | |||
| Polyacrylic acid/collagen/oxidized hyaluronic acid/black wattle bark tannin@AgNP/ethylene glycol (PAA/Col/OHA/BWT/EG) (PCOBE) organohydrogel e-skin | Strain resistance sensor | • Capability of about 9 times stretching of its initial length, compressing to about 30% of its initial height, and returning to its original state | 132 |
| • Excellent mechanical properties (tensile fracture strength = 2.15 MPa, ductility = 880%, and toughness = 7.63 MJ m−3) | |||
| • Anti-fatigue, high environmental stability and electrical conductivity (which were related to the presence of anti-freezing solvent EG and Zr4+ ion, respectively), long-term water retention capacity and environmental stability | |||
| • Capability of maintaining its softness and stability even at −50 °C | |||
| • Anti-freezing and moisturizing capacity related to the presence of EG and fabrication of hydrogen bonds with water molecules | |||
| • Good electrical conductivity, fast strain-resistance response capability, and stable electrical signals during 1000 s of the cyclic stretching introduced it as a flexible sensor for long-term motion monitoring (detection limit of about 1% and linearity between 1 and 600%) with fast stress–strain response ability (9 ms) | |||
| • Certain stable adhesion ability (due to the presence of biomass materials and PAA chains) with the capability of attachment to different surfaces | |||
| • Good and repeatable self-healing ability within 24 h, with good electrical and mechanical abilities, without remaining cracks | |||
| • Free radical scavenging capability (due to the presence of collagen), nontoxicity, and antibacterial activity | |||
| • Capability of detecting electrical signals even those resulting from slight movements | |||
| • Capability of real-time monitoring of bioelectric signals | |||
| Exosomes encapsulated with carboxymethyl chitosan (CMCS), chitosan nanoparticles (CS-NPs), BG, and TiO2 nanoparticle-CMCS-CEBT hydrogel | • CEBT enhanced the production of VEGFR and possessed angiogenic, cell proliferative and adhesive properties. RAW cells treated with LPS + CEBT showed downregulated levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, thus acting as a potent anti-inflammatory agent | 133 | |
| • CMCS-CEBT significantly reduced the wound size in the full-thickness skin defect mice model and prevented the microbial entry with 14 days of treatment | |||
| • TiO2 nanoparticles were expected to promote the antibacterial and wound repairing properties and CMCS-CEBT was responsible for collagen production and vascularization | |||
| CK@BBR&Exo self-healing hydrogel containing carboxymethyl chitosan and oxidized konjac glucomannan polysaccharides. This was constituted by berberine (BBR) and stem cell-derived exosomes (Exo) | • The wound healing properties of exosomes were enhanced through addition of crosslinking of polymers through Schiff base bonds | 134 | |
| • The hydrogel exhibited rapid gelation due to introduction of the weakly alkaline BBR, this also enhanced the pH and created an environment for the crosslinking of polymers | |||
| • CK@BBR&Exo showed angiogenic, anti-inflammatory activity and microbicidal action against S. aureus and E. coli | |||
| • Rapid wound repair by providing a moist environment and tissue regeneration with vascularization and collagen deposition was observed in adult SD rats | |||
| pH-sensitive peptide hydrogel carrier with insulin and a glucose-specific enzyme | • Biocompatibility | 135 | |
| • Increased blood glucose levels cause enzymatic reaction, where the pH responsive hydrogel releases insulin (due to inter and intramolecular electrostatic interaction) | |||
| • In vivo study on streptozocin-induced type 1 diabetic rats showed that the hydrogel could monitor and maintain the blood glucose | |||
| Self-healing PEG-CMC/HAP/GO hydrogel – polyethylene glycol (PEG) carboxymethyl chitosan (CMC)/with needle-like nano-hydroxyapatite (HAP)/graphene oxide (GO) | Cancer therapy | • The hydrogel exhibited combinatorial therapy comprising chemotherapy and photothermal therapy in a breast cancer induced mice model | 136 |
| • GO exerted photothermal activity that is toxic to the cancer cells and HAP exerted site specific anticancer action by preventing the cell proliferation | |||
| Pectin aldehyde (pectin-CHO) and acylhydrazide functionalized polymer poly(N-isopropylacrylamide-stat-acylhydrazide) P(NIPAM-stat-AH) loaded with chemotherapeutic drugs doxorubicin and combretastatin A-4 (CA4) | Cancer therapy | • Biodegradable hydrogel with self-healing nature due to the establishment of acylhydrazone bonds | 51 |
| • In vivo study showed that there was sustained, controlled release of drugs, which exhibited synergistic interaction against CT26 tumors | |||
| Gum Arabic with multi-aldehyde group (GAMA) and smart succinic anhydride-modified chitosan (SCS)-hydrogels loaded with the anticancer drug, nanocurcumin | Cancer therapy | • The biodegradable and biocompatible hydrogel was prepared using GAMA and SCS that was linked through Schiff base bonds | 137 |
| • Hydrogel with improved mechanical strength | |||
| • The in vitro MTT assay showed that the hydrogel was nontoxic to the human embryonic kidney cell line (HEK-293) but was toxic against the breast cancer cells (MCF-7) |
4. Applications beyond biomedicine
Self-healing materials have garnered significant attention and application across various fields of science, including electronics, aerospace, and coatings, due to their remarkable ability to autonomously repair damage and extend the lifespan of products. In the realm of electronics, self-healing materials play a crucial role in enhancing the durability and reliability of electronic devices.9 By incorporating self-repair mechanisms into circuit boards, conductive coatings, and flexible electronics, these materials can mitigate the impact of wear and tear, prolonging the operational lifespan of electronic components.9 In the aerospace industry, self-healing materials offer innovative solutions for improving the structural integrity and performance of aircraft components.138 Self-repairing composites and coatings can effectively seal cracks, punctures, and damage caused by environmental factors, reducing maintenance costs and enhancing safety in aerospace applications. The integration of self-healing technologies in aircraft structures and coatings contributes to increased longevity, resilience, and operational efficiency in the aerospace sector.138 Furthermore, for coatings and surface treatments, self-healing materials provide a sustainable and cost-effective solution for maintaining the appearance and functionality of surfaces.139 Self-repairing coatings can autonomously mend scratches, abrasions, and corrosion, preserving the aesthetic appeal and protective properties of surfaces in various environments. By incorporating self-healing capabilities into coatings for automotive, marine, and architectural applications, these materials offer enhanced durability, reduced maintenance requirements, and improved sustainability.139 Across these diverse fields of science, the adoption of self-healing materials represents a paradigm shift towards more resilient, efficient, and sustainable technological solutions. By employing the intrinsic properties of self-repair and regeneration, these materials hold the potential to develop industries by minimizing maintenance costs, reducing waste, and enhancing the performance and longevity of products in electronics, aerospace, the automotive industry, coatings, and beyond.The integration of self-healing materials in various products holds great promise for extending their lifespan, offering both economic and environmental benefits. For instance, self-healing materials can be incorporated into the manufacturing of electronic devices, such as smartphones and laptops, to address wear and tear issues.41 This approach enhances the durability of electronic components, reducing the frequency of replacement, and contributing to a more sustainable electronic waste management system.140 Furthermore, self-healing materials play a crucial role in infrastructure and construction projects. The ability of building materials to autonomously repair cracks and damage over time can lead to a considerable increase in the lifespan of structures.141 This phenomenon has the potential to develop the construction industry by reducing maintenance costs and the need for frequent renovations, ultimately contributing to sustainable and resilient infrastructure. For instance, the use of self-healing materials has gained attention in the field of textiles. Fabrics incorporated with self-healing properties can withstand prolonged use, resisting wear and tear. This has implications for the fashion industry, where durable and long-lasting clothing items could reduce the environmental impact associated with fast fashion and frequent clothing disposal.142–145 By integrating these materials into concrete or other construction materials, the formation of cracks and deterioration due to environmental factors can be mitigated. This has the potential to enhance the durability of structures, reduce maintenance requirements, and decrease the demand for new construction materials, contributing to sustainable construction practices.146,147
5. Challenges and future perspectives
A primary goal in the development of synthetic self-healing materials is to replicate the remarkable healing capabilities observed in biological systems. However, it is vital to acknowledge that researchers have a considerable journey ahead to even begin to imitate the most basic biological healing processes. Unlike synthetic systems, biological systems employ a multitude of healing mechanisms concurrently to fully restore their original properties, a level of healing that is currently beyond the realm of imagination for synthetic materials.141 One notable drawback of existing synthetic self-healing materials is that the developments in this field are still in their early stages, primarily theoretical. Consequently, the practical application of products utilizing such technology is currently impractical due to the uncertainties surrounding their healing capabilities. Factors such as the surrounding environment and the size of the damage greatly affect the performance of these materials, leading to inconsistent healing results. For self-healing products to be genuinely practical, it is essential for the healing efficiency, healing rate, and healing capability to remain unaffected by environmental conditions, including pressure, elevated temperatures, and mechanical stress, as well as damage size and material aging. The healing process should be controlled and swift, as the typical healing time ranging from a few hours to several days is insufficient to prevent catastrophic material failure. In addition, aging poses another challenge, gradually diminishing the effectiveness of the healing components over time. However, there is a notable absence of time series analysis specifically addressing the issue of aging.141,148,149 In various methodologies, the activation of healing in composites relies on the need for the composites to come into contact with each other. This requirement poses a significant obstacle for implementing these approaches in practical applications, particularly in the case of materials used in load-bearing engineering structures. Furthermore, in certain processes, the introduction of self-healing properties into a material leads to substantial alterations in its physical and mechanical properties. This transformation occurs due to the incorporation of additional healing components, fibers, microcapsules, or microvascular networks, resulting in a significant structural modification of the original material.7Intrinsic healing methods offer impressive healing efficiency when dealing with small damaged areas. Approaches such as reversible Diels–Alder, hydrogen bonding, and other supramolecular chemistry techniques are particularly advantageous for synthesizing self-healable polymers with high orientation.141 Polymers created through these interactions possess the ability to fully restore their original properties at the molecular level multiple times, without the need for additional chemicals (resulting in high healing efficiency). However, these approaches encounter limitations when it comes to larger areas of damage. Manual contact with the damaged regions is necessary, and the initiation of healing requires external stimuli such as heat, light, or pH changes. Furthermore, the efficiency of healing diminishes with repeated healing attempts due to the occurrence of side reactions. In addition to intrinsic healing, self-healing materials can also be developed using extrinsic healing mechanisms such as capsule-based, hollow fiber-based, and vascular-based approaches. Capsule-based healing materials can be easily incorporated into the polymer matrix, but they are primarily suitable for addressing small-scale damage. However, their healing capability in a specific location diminishes after a single repairing cycle.141 On the other hand, vascular-based healing approaches offer the advantage of multiple healing attempts for larger damaged areas, as the healing components can be repeatedly delivered to the affected regions. Nevertheless, a significant challenge lies in integrating a vascular network into composite materials without adversely affecting their properties. Factors such as optimizing the tube diameter, capillary forces, and flowability of the healing components need to be carefully considered. In addition to the aforementioned approaches, there are various other methods that show great promise in the field of self-healing materials. These include physical interactions, remote self-healing, shape-memory assisted self-healing, and nanoparticle-based self-healing. Among these methods, shape-memory assisted self-healing can be combined with other healing approaches to effectively reduce crack diameter and enhance healing efficiency. Furthermore, self-healing properties can be incorporated not only in polymers and their composites but also in other materials such as concretes, ceramics, and metals. In the case of self-healable concrete materials, several approaches have been explored, and bacteria-based healing has proven to be particularly effective in addressing wider cracks. Self-healable metal matrix composites hold significant potential for applications in components such as cylinder liners, pistons, constant-velocity joints, gears, and sliding surfaces, which are prone to damage caused by friction, creep, and wear. Incorporating healing properties in ceramics can improve their structural integrity and reduce costs associated with machining and non-destructive inspections.2,5,141,148 However, the incorporation of self-healing components in metals and ceramics presents greater complexity, and progress in this area is still in its early stages.15
Self-healing polymers and smart materials draw inspiration from the regenerative capabilities observed in biological membranes of both humans and plants.35,37 Among different healing strategies, microencapsulated healing materials exhibit superior efficiency compared to vascular and shape-memory techniques. The self-healing process involves a range of physical and chemical reactions, such as Diels–Alder conjugation, π–π bonding, randomization, and diffusion, enhancing stiffness and healing efficacy in nanocomposites. Widely utilized in the medical field due to their accessibility and cost-effectiveness, polymeric materials play a crucial role. The integration of self-healing composites not only enhances fatigue resistance and tensile strength but also expands applications in materials science and biomedicine, facilitating tissue engineering, wound healing, and implantation without the need for replacement. These nanocomposites find utility in drug delivery systems, enabling targeted and sustained drug release triggered by pH, heat, or light stimuli. While existing research highlights the self-healing properties of polymers, there remains a scarcity of evidence specifically focusing on self-healing polymeric materials.35 Addressing ambiguous principles and mechanisms is essential to advance the analysis of composites in biological systems and broaden their applications in polymer science, engineering, and biomedicine. The ongoing pursuit of ideal self-healing polymers is crucial to unlock materials with enhanced physical and chemical attributes. By incorporating healing agents into next-generation polymeric materials, the potential for self-diagnosis, self-regulation, and self-healing capabilities emerges, positioning these materials as versatile assets across diverse applications. These innovative concepts offer a promising pathway for the development of advanced materials and hold immense potential for addressing various conditions, including bone and dental injuries, and advancing cancer therapy.35
The production of self-healing materials can be expensive, primarily due to the incorporation of specialized healing agents or the need for complex manufacturing processes;45 finding cost-effective solutions is essential to make these materials commercially viable. In addition, scaling up the fabrication of self-healing materials can be challenging. The integration of self-healing capabilities into existing manufacturing techniques requires careful consideration and optimization to ensure efficient and large-scale production.150 Notably, the effectiveness of self-healing materials in repairing damage varies depending on the type and severity of the damage. Some materials may struggle to heal larger or more complex fractures, limiting their applicability in certain scenarios.37 While self-healing materials have the potential to contribute to sustainability, the environmental impact of their production and disposal needs to be considered. The use of healing agents or additives may introduce new challenges in terms of waste management and potential environmental harm. In the future, advancements in materials science and manufacturing techniques are expected to overcome current limitations and expand the capabilities of self-healing materials. Research efforts are focused on improving the healing efficiency, reducing costs, and ensuring the long-term stability and performance of these materials.
Overall, in advancing the circular economy of self-healing materials, several challenges and future perspectives come to the forefront. One key challenge lies in the sustainable sourcing and recycling of raw materials used in self-healing technologies. Developing efficient methods for the recovery and reuse of components from end-of-life self-healing materials is essential to close the loop and minimize environmental impact. Notably, enhancing the durability and longevity of self-healing mechanisms to prolong the lifecycle of products poses a significant challenge, as this requires continuous innovation and optimization of material properties. Looking ahead, the future of self-healing materials in the circular economy holds immense promise. By integrating the principles of reuse, repair, and recycling, self-healing materials can contribute to a more sustainable and resource-efficient economy. Potential applications in environmental fields are vast, ranging from self-healing coatings that reduce the need for frequent repainting to self-repairing structures that minimize maintenance requirements and extend the lifespan of infrastructure. These materials have the potential to develop waste management practices by enabling the creation of products that can repair themselves, reducing the volume of discarded materials and promoting a more sustainable approach to consumption. Furthermore, self-healing materials offer innovative solutions for environmental remediation efforts, such as self-repairing membranes for water filtration systems or self-healing barriers for containment of hazardous substances. By harnessing the self-repair capabilities of these materials, researchers can improve the efficiency and effectiveness of environmental protection measures, leading to cleaner and safer ecosystems. Embracing the circular economy principles in the development and utilization of self-healing materials opens up new avenues for sustainable innovation and environmental stewardship, paving the way for a greener and more resilient future.
In the context of biomedicine, the circular economy of self-healing materials presents crucial challenges and promising future perspectives. One significant challenge lies in the development of sustainable sourcing and recycling practices for materials used in biomedical applications. Ensuring that self-healing materials are sourced responsibly and can be efficiently recycled or repurposed after use is crucial to minimize waste and environmental impact in the healthcare sector. Notably, the biocompatibility and biosafety of self-healing materials ought to be rigorously assessed to ensure their suitability for biomedical applications, posing a challenge in maintaining high standards of patient care while advancing sustainable practices. Looking towards the future, there are exciting prospects for improving the circular economy of self-healing materials in biomedicine. Innovations in material recycling and reprocessing technologies can pave the way for closed-loop systems that enable the efficient recovery and reuse of self-healing materials in medical devices, implants, drug delivery systems, biosensors, and other healthcare applications. By integrating principles of sustainability and circularity into the design of self-healing materials, the healthcare industry can reduce its environmental footprint and contribute to a more resource-efficient healthcare system. Furthermore, the potential applications of self-healing materials in biomedicine are vast, ranging from self-repairing implants and medical devices to regenerative tissue engineering scaffolds. These materials have the potential to enhance the longevity and effectiveness of medical treatments, reducing the need for frequent replacement and surgery. By optimizing the design and performance of self-healing materials in biomedical settings, researchers can unlock new possibilities for sustainable healthcare practices and improve patient outcomes while minimizing environmental impact.
The integration of self-healing non-biomaterials with circular economy principles and biomedical self-healing materials represents a significant opportunity for advancing sustainable practices and innovation in healthcare. By bridging these diverse material domains, a cohesive approach can be established to drive efficiency, reduce waste, and enhance the longevity of products within the biomedical field. Self-healing non-biomaterials in the forms of polymers and composites offer unique self-repair capabilities that can be harnessed to extend the lifespan of medical devices, implants, and equipment. These materials, when integrated with circular economy principles, enable a more sustainable approach to healthcare by promoting reuse, repair, and recycling. By incorporating self-healing mechanisms into non-biomaterials, the need for frequent replacement and disposal is reduced, leading to a more resource-efficient and environmentally friendly healthcare ecosystem. When connected with biomedical self-healing materials, such as self-repairing scaffolds or implants, the synergy between self-healing technologies across different material types becomes apparent. The application of self-healing principles in biomaterials not only enhances the durability and performance of medical devices but also aligns with circular economy principles by promoting material reuse and minimizing waste generation. This integrated approach fosters a more holistic and sustainable healthcare system that prioritizes environmental responsibility and long-term resource conservation. In essence, by intertwining the concepts of self-healing non-biomaterials, circular economy principles, and biomedical self-healing materials, a more resilient and environmentally conscious approach to healthcare innovation can be obtained. Through collaborative efforts and cross-disciplinary research, the potential for driving positive change in the healthcare industry while advancing sustainability goals is immense. Embracing the interconnectedness of these material domains provides new opportunities for sustainable materials development, circular economy practices, and enhanced patient care within the biomedical field.
6. Conclusion
Self-healing materials offer significant advantages in terms of extended lifespan, reduced maintenance costs, enhanced safety, and resource conservation. However, challenges such as cost, scalability, and healing efficiency need to be addressed. Despite these challenges, ongoing explorations hold promising prospects for the future of self-healing materials. By overcoming limitations and harnessing their potential, these materials can contribute to a more sustainable and resilient world. Self-healing materials have tremendous potential in biomedicine, offering a wide range of applications that can improve patient care, enhance medical treatments, and reduce waste. These materials can be employed to develop implantable devices, such as pacemakers or artificial joints, with improved durability and longevity. By incorporating self-healing properties into the materials used for these devices, the occurrence of structural damage or wear can be mitigated. This can reduce the need for frequent replacement or revision surgery, leading to improved patient outcomes and reduced medical waste. In addition, self-healing materials offer opportunities to develop advanced drug delivery systems with enhanced functionality. These materials present a promising opportunity for developing smart drug delivery systems, providing distinct benefits in terms of sustained release and localized therapeutic effects. They have the ability to encapsulate drug payloads and respond to specific environmental cues like pH variations, temperature changes, or the presence of enzymes. As a result, they can undergo self-repair, enabling precise and controlled drug release at targeted sites. By incorporating self-healing polymers into drug carriers or nanoparticles, the materials can autonomously repair damage incurred during the delivery process. This ensures the stability and integrity of the drug payload and improves the efficiency of drug delivery, leading to better therapeutic outcomes.In the field of tissue engineering and regenerative medicine, self-healing materials can be used to develop scaffolds or matrices that mimic the natural extracellular environment, promoting cell adhesion, proliferation, and tissue regeneration. The self-healing properties of these materials can help to maintain the structural integrity of the engineered tissues, facilitating the healing process and improving the success rate of tissue engineering approaches. Self-healing materials can also be employed to develop innovative wound dressings or bandages with the ability to repair themselves when damaged. These materials can accelerate the healing process by providing a protective barrier, promoting tissue regeneration, and reducing the risk of infection. In addition, self-healing properties can improve the overall durability and lifespan of wound dressings, reducing the frequency of dressing changes and minimizing waste. Notably, the durability and functionality of surgical instruments or implantable devices can be enhanced by incorporating self-healing properties into the materials, minimizing the risk of structural damage or failure. This improves the safety and reliability of these medical devices, reducing the need for replacement or repairs. Furthermore, self-healing materials offer exciting opportunities in the development of biosensors for various diagnostic applications. By incorporating self-healing polymers into the sensor platforms, the materials can repair damage caused by repeated use or harsh conditions. This ensures the reliability and longevity of the biosensors, enabling accurate and consistent detection of biomarkers or analytes.
Conflicts of interest
The author(s) declare no competing interest.Acknowledgements
Authors would like to acknowledge the Indian Council of Medical Research, India for the funding support through Research Associate fellowship (ICMR RA Sanction order No: 3/1/2/253/2021-Nut dt. 20.05.2022).References
- D. G. Bekas, K. Tsirka, D. Baltzis and A. S. Paipetis, Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques, Composites, Part B, 2016, 87, 92–119 CrossRef CAS.
- D. Jayabalakrishnan, D. B. Naga Muruga, K. Bhaskar, P. Pavan, K. Balaji, P. S. Rajakumar, C. Priya, R. A. B. Deepa, S. Sendilvelan and M. Prabhahar, Self-Healing materials–A review, Mater. Today: Proc., 2021, 45, 7195–7199 CAS.
- S. Jadoun, Synthesis, Mechanism, and Applications of Self-healing Materials, Biomedical Materials & Devices, 2024, 2, 225–240 Search PubMed.
- S. R. M. Paladugu, P. S. R. Sreekanth, S. K. Sahu, K. Naresh, S. A. Karthick, N. Venkateshwaran, M. Ramoni, R. A. Mensah, O. Das and R. Shanmugam, A Comprehensive Review of Self-Healing Polymer, Metal, and Ceramic Matrix Composites and Their Modeling Aspects for Aerospace Applications, Materials, 2022, 15, 8521 CrossRef CAS PubMed.
- S. Wang and M. W. Urban, Self-healing polymers, Nat. Rev. Mater., 2020, 5, 562–583 CrossRef CAS.
- R. Ikura, J. Park, M. Osaki, H. Yamaguchi, A. Harada and Y. Takashima, Design of self-healing and self-restoring materials utilizing reversible and movable crosslinks, NPG Asia Mater., 2022, 14, 10 CrossRef CAS.
- N. K. Guimard, K. K. Oehlenschlaeger, J. Zhou, S. Hilf, F. G. Schmidt and C. Barner-Kowollik, Current Trends in the Field of Self-Healing Materials, Macromol. Chem. Phys., 2012, 213, 131–143 CrossRef CAS.
- D. Chen, D. Wang, Y. Yang, Q. Huang, S. Zhu and Z. Zheng, Self-healing materials for next-generation energy harvesting and storage devices, Adv. Energy Mater., 2017, 7, 1700890 CrossRef.
- F. Mashkoor, S. J. Lee, H. Yi, S. M. Noh and C. Jeong, Self-healing materials for electronics applications, Int. J. Mol. Sci., 2022, 23, 622 CrossRef CAS PubMed.
- N. Wen, T. Song, Z. Ji, D. Jiang, Z. Wu, Y. Wang and Z. Guo, Recent advancements in self-healing materials: Mechanicals, performances and features, React. Funct. Polym., 2021, 168, 105041 CrossRef CAS.
- S. An, S. S. Yoon and M. W. Lee, Self-Healing Structural Materials, Polymers, 2021, 13, 2297 CrossRef CAS PubMed.
- T. Song, B. Jiang, Y. Li, Z. Ji, H. Zhou, D. Jiang, I. Seok, V. Murugadoss, N. Wen and H. Colorado, Self-healing materials: A review of recent developments, ES Mater. Manuf., 2021, 14, 1–19 CAS.
- A. Cseke, M. Haines-Gadd, P. Mativenga, F. Charnley, B. Thomas and J. Perry, Modelling of environmental impacts of printed self-healing products, Sci. Total Environ., 2022, 807, 150780 CrossRef CAS PubMed.
- X. Huang, Z. Huang, J.-C. Lai, L. Li, G.-C. Yang and C.-H. Li, Self-healing improves the stability and safety of polymer bonded explosives, Compos. Sci. Technol., 2018, 167, 346–354 CrossRef CAS.
- K. Mphahlele, S. S. Ray and A. Kolesnikov, Self-Healing Polymeric Composite Material Design, Failure Analysis and Future Outlook: A Review, Polymers, 2017, 9, 535 CrossRef PubMed.
- J. Ekeocha, C. Ellingford, M. Pan, A. M. Wemyss, C. Bowen and C. Wan, Challenges and opportunities of self-healing polymers and devices for extreme and hostile environments, Adv. Mater., 2021, 33, 2008052 CrossRef CAS PubMed.
- R. Trask, H. R. Williams and I. Bond, Self-healing polymer composites: mimicking nature to enhance performance, Bioinspiration Biomimetics, 2007, 2, P1 CrossRef CAS PubMed.
- Y. J. Tan, G. J. Susanto, H. P. Anwar Ali and B. C. Tee, Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics, Adv. Mater., 2021, 33, 2002800 CrossRef CAS PubMed.
- N. van Dijk and S. van der Zwaag, Self-healing phenomena in metals, Adv. Mater. Interfaces, 2018, 5, 1800226 CrossRef.
- S. Sharma, G. Nandan, P. Rohatgi and R. Prakash, Recent advances in self-healing materials, Mater. Today: Proc., 2019, 18, 4729–4737 CAS.
- S. M. Aouadi, J. Gu and D. Berman, Self-healing ceramic coatings that operate in extreme environments: A review, J. Vac. Sci. Technol., A, 2020, 38(5), 050802 CrossRef CAS.
- S. Utrera-Barrios, R. Verdejo, M. A. López-Manchado and M. H. Santana, Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review, Mater. Horiz., 2020, 7, 2882–2902 RSC.
- J. L. Nußholz, Circular business models: Defining a concept and framing an emerging research field, Sustainability, 2017, 9, 1810 CrossRef.
- M. Geissdoerfer, P. Savaget, N. M. Bocken and E. J. Hultink, The Circular Economy–A new sustainability paradigm?, J. Cleaner Prod., 2017, 143, 757–768 CrossRef.
- R. Geitner, F. B. Legesse, N. Kuhl, T. W. Bocklitz, S. Zechel, J. Vitz, M. Hager, U. S. Schubert, B. Dietzek and M. Schmitt, Do you get what you see? Understanding molecular self-healing, Chem. – Eur. J., 2018, 24, 2493–2502 CrossRef CAS PubMed.
- J. Araujo-Morera, R. Verdejo, M. A. López-Manchado and M. Hernández Santana, Sustainable mobility: The route of tires through the circular economy model, Waste Manage., 2021, 126, 309–322 CrossRef PubMed.
- Y. Liu, Y. Zhuge, W. Fan, W. Duan and L. Wang, Recycling industrial wastes into self-healing concrete: A review, Environ. Res., 2022, 214, 113975 CrossRef CAS PubMed.
- J. Liu, H. Jing, Z. Wang, X. Wang and L. Zhang, Recycling of steel slag in sustainable bituminous mixtures: Self-healing performance, mechanism, environmental and economic analyses, J. Cleaner Prod., 2023, 429, 139496 CrossRef.
- G. Griffini, B. Rigatelli and S. Turri, Diels-Alder Macromolecular Networks in Recyclable, Repairable and Reprocessable Polymer Composites for the Circular Economy–A Review, Macromol. Mater. Eng., 2023, 308, 2300133 CrossRef CAS.
- M. Haines-Gadd, F. Charnley and A. Encinas-Oropesa, Self-healing materials: A pathway to immortal products or a risk to circular economy systems?, J. Cleaner Prod., 2021, 315, 128193 CrossRef.
- S. D. Menikheim and E. B. Lavik, Self-healing biomaterials: The next generation is nano, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2020, 12, e1641 CrossRef PubMed.
- J. Chen, Y. Huang, X. Ma and Y. Lei, Functional self-healing materials and their potential applications in biomedical engineering, Adv. Compos. Hybrid Mater., 2018, 1, 94–113 CrossRef CAS.
- Y. Tu, N. Chen, C. Li, H. Liu, R. Zhu, S. Chen, Q. Xiao, J. Liu, S. Ramakrishna and L. He, Advances in injectable self-healing biomedical hydrogels, Acta Biomater., 2019, 90, 1–20 CrossRef CAS PubMed.
- M. Zhu, J. Liu, L. Gan and M. Long, Research progress in bio-based self-healing materials, Eur. Polym. J., 2020, 129, 109651 CrossRef CAS.
- N. Pathan and P. Shende, Strategic conceptualization and potential of self-healing polymers in biomedical field, Mater. Sci. Eng., C, 2021, 125, 112099 CrossRef CAS PubMed.
- A. Zarepour, S. Ahmadi, N. Rabiee, A. Zarrabi and S. Iravani, Self-Healing MXene- and Graphene-Based Composites: Properties and Applications, Nano-Micro Lett., 2023, 15, 100 CrossRef CAS PubMed.
- K. Choi, A. Noh, J. Kim, P. H. Hong, M. J. Ko and S. W. Hong, Properties and Applications of Self-Healing Polymeric Materials: A Review, Polymers, 2023, 15, 4408 CrossRef CAS PubMed.
- Y. Gai, H. Li and Z. Li, Self-healing functional electronic devices, Small, 2021, 17, 2101383 CrossRef CAS PubMed.
- Y. J. Tan, J. Wu, H. Li and B. C. Tee, Self-healing electronic materials for a smart and sustainable future, ACS Appl. Mater. Interfaces, 2018, 10, 15331–15345 CrossRef CAS PubMed.
- S. Latif, S. Amin, S. S. Haroon and I. A. Sajjad, Self-healing materials for electronic applications: an overview, Mater. Res. Express, 2019, 6, 062001 CrossRef CAS.
- S. Zhu, Z. Liu, W. Li, H. Zhang, G. Dai and X. Zhou, Research progress of self-healing polymer materials for flexible electronic devices, J. Polym. Sci., 2023, 61, 1554–1571 CrossRef CAS.
- J. Nilimaa, Smart materials and technologies for sustainable concrete construction, Dev. Built Environ., 2023, 15, 100177 CrossRef.
- M. Geissdoerfer, P. Savaget, N. M. P. Bocken and E. J. Hultink, The Circular Economy – A new sustainability paradigm?, J. Cleaner Prod., 2017, 143, 757–768 CrossRef.
- E. Olivetti and J. Cullen, Toward a sustainable materials system, Science, 2018, 360, 6396 CrossRef PubMed.
- N. N. F. Nik Md Noordin Kahar, A. F. Osman, E. Alosime, N. Arsat, N. A. Mohammad, A. Syamsir, Z. Itam and Z. A. Abdul, The Versatility of Polymeric Materials as Self-Healing Agents for Various Types of Applications: A Review, Polymers, 2021, 13, 1194 CrossRef PubMed.
- Y. J. Tan, J. Wu, H. Li and B. C. K. Tee, Self-Healing Electronic Materials for a Smart and Sustainable Future, ACS Appl. Mater. Interfaces, 2018, 10, 15331–15345 CrossRef CAS PubMed.
- M. Goyal, S. N. Agarwal and N. Bhatnagar, A review on self-healing polymers for applications in spacecraft and construction of roads, J. Appl. Polym. Sci., 2022, 139, e52816 CrossRef CAS.
- W. Mai, Q. Yu, C. Han, F. Kang and B. Li, Self-Healing Materials for Energy-Storage Devices, Adv. Funct. Mater., 2020, 30, 1909912 CrossRef CAS.
- A. Kausar, I. Ahmad, M. Maaza and P. Bocchetta, Self-Healing Nanocomposites—Advancements and Aerospace Applications, J. Compos. Sci., 2023, 7, 148 CrossRef CAS.
- D. Rybak, C. Rinoldi, P. Nakielski, J. Du, M. A. H. Bayan, S. S. Zargarian, M. Pruchniewski, X. Li, B. Strojny-Cieślak and B. Ding, Injectable and self-healable nano-architectured hydrogel for NIR-light responsive chemo-and photothermal bacterial eradication, J. Mater. Chem. B, 2024, 12, 1905–1925 RSC.
- H. An, Y. Yang, Z. Zhou, Y. Bo, Y. Wang, Y. He, D. Wang and J. Qin, Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy, Acta Biomater., 2021, 131, 149–161 CrossRef CAS PubMed.
- S. Rahmani, A. Olad and Z. Rahmani, Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: Study of drug delivery behavior, Polym. Bull., 2023, 80, 4117–4138 CrossRef CAS.
- D. q. Li, S. y. Wang, Y. j. Meng, Z. w. Guo, M. m. Cheng and J. Li, Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility, Carbohydr. Polym., 2021, 268, 118244 CrossRef CAS PubMed.
- W. Wang, L. Xiang, L. Gong, W. Hu, W. Huang, Y. Chen, A. B. Asha, S. Srinivas, L. Chen, R. Narain and H. Zeng, Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties, Chem. Mater., 2019, 31, 2366–2376 CrossRef CAS.
- D. L. Taylor and M. in het Panhuis, Self-healing hydrogels, Adv. Mater., 2016, 28, 9060–9093 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- M. Norouzi, B. Nazari and D. W. Miller, Injectable hydrogel-based drug delivery systems for local cancer therapy, Drug Discovery Today, 2016, 21, 1835–1849 CrossRef CAS PubMed.
- M. Wu, J. Chen, W. Huang, B. Yan, Q. Peng, J. Liu, L. Chen and H. Zeng, Injectable and self-healing nanocomposite hydrogels with ultrasensitive pH-responsiveness and tunable mechanical properties: implications for controlled drug delivery, Biomacromolecules, 2020, 21, 2409–2420 CrossRef CAS PubMed.
- X. Chen, M. Fan, H. Tan, B. Ren, G. Yuan, Y. Jia, J. Li, D. Xiong, X. Xing, X. Niu and X. Hu, Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery, Mater. Sci. Eng., C, 2019, 101, 619–629 CrossRef CAS PubMed.
- Y. Wang, C. K. Adokoh and R. Narain, Recent development and biomedical applications of self-healing hydrogels, Expert Opin. Drug Delivery, 2018, 15, 77–91 CrossRef CAS PubMed.
- R. K. Kramer, M. N. Belgacem, A. J. F. Carvalho and A. Gandini, Thermally reversible nanocellulose hydrogels synthesized via the furan/maleimide Diels-Alder click reaction in water, Int. J. Biol. Macromol., 2019, 141, 493–498 CrossRef CAS PubMed.
- F. Zhang, S. Zhang, S. Cui, X. Jing, Y. Feng and S. Coseri, Rapid self-healing carboxymethyl chitosan/hyaluronic acid hydrogels with injectable ability for drug delivery, Carbohydr. Polym., 2024, 328, 121707 CrossRef CAS PubMed.
- X. Ye, Y. Li, Y. Zhang and P. Wang, A comprehensive review: recent developments of biomimetic sensors, J. Bionic Eng., 2022, 19, 853–876 CrossRef.
- W. Wang, S. Yang, K. Ding, L. Jiao, J. Yan, W. Zhao, Y. Ma, T. Wang, B. Cheng and Y. Ni, Biomaterials-and biostructures Inspired high-performance flexible stretchable strain sensors: A review, Chem. Eng. J., 2021, 425, 129949 CrossRef CAS.
- S. Li, Y. Zhang, Y. Wang, K. Xia, Z. Yin, H. Wang, M. Zhang, X. Liang, H. Lu and M. Zhu, Physical sensors for skin-inspired electronics, InfoMat, 2020, 2, 184–211 CrossRef CAS.
- A. Georgopoulou, J. Brancart, S. Terryn, A. W. Bosman, S. Norvez, G. Van Assche, F. Iida, B. Vanderborght and F. Clemens, Soft self-healing resistive-based sensors inspired by sensory transduction in biological systems, Appl. Mater. Today, 2022, 29, 101638 CrossRef.
- M. Qi, R. Yang, Z. Wang, Y. Liu, Q. Zhang, B. He, K. Li, Q. Yang, L. Wei and C. Pan, Bioinspired Self-healing Soft Electronics, Adv. Funct. Mater., 2023, 33, 2214479 CrossRef CAS.
- G. Sun, P. Wang, Y. Jiang, H. Sun, T. Liu, G. Li, W. Yu, C. Meng and S. Guo, Bioinspired flexible, breathable, waterproof and self-cleaning iontronic tactile sensors for special underwater sensing applications, Nano Energy, 2023, 110, 108367 CrossRef CAS.
- T. P. Huynh and H. Haick, Autonomous flexible sensors for health monitoring, Adv. Mater., 2018, 30, 1802337 CrossRef PubMed.
- Y. Liu, K. He, G. Chen, W. R. Leow and X. Chen, Nature-inspired structural materials for flexible electronic devices, Chem. Rev., 2017, 117, 12893–12941 CrossRef CAS PubMed.
- B. Tee, C. Wang, R. Allen and Z. Bao, Pressure and flexion-sensitive electronic skin with repeatable ambient self-healing capability, Nat. Nanotechnol., 2012, 7, 825–832 CrossRef CAS PubMed.
- X. Wu, J. Wang, J. Huang and S. Yang, Room temperature readily self-healing polymer via rationally designing molecular chain and crosslinking bond for flexible electrical sensor, J. Colloid Interface Sci., 2020, 559, 152–161 CrossRef CAS PubMed.
- T. Yu, X. Lü and W. Bao, High electrical self-healing flexible strain sensor based on MWCNT-polydimethylsiloxane elastomer with high gauge factor and wide measurement range, Compos. Sci. Technol., 2023, 238, 110049 CrossRef CAS.
- Z. Deng, H. Wang, P. X. Ma and B. Guo, Self-healing conductive hydrogels: preparation, properties and applications, Nanoscale, 2020, 12, 1224–1246 RSC.
- F. Wang, C. Zhang and X. Wan, Carbon nanotubes-coated conductive elastomer: electrical and near infrared light dual-stimulated shape memory, self-healing, and wearable sensing, Ind. Eng. Chem. Res., 2021, 60, 2954–2961 CrossRef CAS.
- M. Khatib, O. Zohar and H. Haick, Self-healing soft sensors: from material design to implementation, Adv. Mater., 2021, 33, 2004190 CrossRef CAS PubMed.
- M. R. Hasan, M. S. Ahommed, M. Daizy, M. S. Bacchu, M. R. Ali, M. R. Al-Mamun, M. A. S. Aly, M. Khan and S. I. Hossain, Recent development in electrochemical biosensors for cancer biomarkers detection, Biosens. Bioelectron.: X, 2021, 8, 100075 CAS.
- M. L. Mujica, P. A. Gallay, F. Perrachione, A. E. Montemerlo, L. A. Tamborelli, V. M. Vaschetti, D. F. Reartes, S. Bollo, M. C. Rodríguez and P. R. Dalmasso, New trends in the development of electrochemical biosensors for the quantification of microRNAs, J. Pharm. Biomed. Anal., 2020, 189, 113478 CrossRef CAS PubMed.
- Y. Song, J. Min, Y. Yu, H. Wang, Y. Yang, H. Zhang and W. Gao, Wireless battery-free wearable sweat sensor powered by human motion, Sci. Adv., 2020, 6, eaay9842 CrossRef CAS PubMed.
- F. Mirlou and L. Beker, Wearable electrochemical sensors for healthcare monitoring: A review of current developments and future prospects, IEEE Trans. Mol. Biol. Multi-Scale Commun., 2023, 9, 364–373 Search PubMed.
- F. Criscuolo, I. N. Hanitra, S. Aiassa, I. Taurino, N. Oliva, S. Carrara and G. De Micheli, Wearable multifunctional sweat-sensing system for efficient healthcare monitoring, Sens. Actuators, B, 2021, 328, 129017 CrossRef CAS.
- A. V. Mohan, V. Rajendran, R. K. Mishra and M. Jayaraman, Recent advances and perspectives in sweat based wearable electrochemical sensors, TrAC, Trends Anal. Chem., 2020, 131, 116024 CrossRef CAS.
- X. Qiao, Y. Cai, Z. Kong, Z. Xu and X. Luo, A Wearable Electrochemical Sensor Based on Anti-Fouling and Self-Healing Polypeptide Complex Hydrogels for Sweat Monitoring, ACS Sens., 2023, 8, 2834–2842 CrossRef CAS PubMed.
- Z. Liang, J. Zhang, C. Wu, X. Hu, Y. Lu, G. Wang, F. Yu, X. Zhang and Y. Wang, Flexible and self-healing electrochemical hydrogel sensor with high efficiency toward glucose monitoring, Biosens. Bioelectron., 2020, 155, 112105 CrossRef CAS PubMed.
- C. Han and W. Guo, Fluorescent Noble Metal Nanoclusters Loaded Protein Hydrogel Exhibiting Anti-Biofouling and Self-Healing Properties for Electrochemiluminescence Biosensing Applications, Small, 2020, 16, 2002621 CrossRef CAS PubMed.
- X. Liu, Y. Bai, X. Zhao, J. Chen, X. Chen and W. Yang, Conductive and self-healing hydrogel for flexible electrochemiluminescence sensor, Microchim. Acta, 2023, 190, 123 CrossRef CAS PubMed.
- Y. Shen, Z. Wang, Y. Wang, Z. Meng and Z. Zhao, A self-healing carboxymethyl chitosan/oxidized carboxymethyl cellulose hydrogel with fluorescent bioprobes for glucose detection, Carbohydr. Polym., 2021, 274, 118642 CrossRef CAS PubMed.
- T. Liu, F. Wang, Q. Wu, T. Chen and P. Sun, Fluorescent, electrically responsive and ultratough self-healing hydrogels via bioinspired all-in-one hierarchical micelles, Mater. Horiz., 2021, 8, 3096–3104 RSC.
- J. Chen, L. Wang, X. Xu, G. Liu, H. Liu, Y. Qiao, J. Chen, S. Cao, Q. Cha and T. Wang, Self-healing materials-based electronic skin: mechanism, development and applications, Gels, 2022, 8, 356 CrossRef CAS PubMed.
- R. Liu, Y. Lai, S. Li, F. Wu, J. Shao, D. Liu, X. Dong, J. Wang and Z. L. Wang, Ultrathin, transparent, and robust self-healing electronic skins for tactile and non-contact sensing, Nano Energy, 2022, 95, 107056 CrossRef CAS.
- X. Dai, Q. Liang, Z. H. Zhao, Y. Wu, J. Yang, J. Han, Y. Cao, Y. Wang, C. H. Li, A. Zhong and L.-B. Huang, Self-powered sensors for flexible electronic skins capable of self-healing under multiple extreme environments, Nano Energy, 2024, 121, 109239 CrossRef CAS.
- S.-K. Han, Basics of wound healing, in Innovations and Advances in Wound Healing, Springer, 2023, pp. 1–42 Search PubMed.
- A. Martin, M. R. Komada and D. C. Sane, Abnormal angiogenesis in diabetes mellitus, Med. Res. Rev., 2003, 23, 117–145 CrossRef CAS PubMed.
- K. Raziyeva, Y. Kim, Z. Zharkinbekov, K. Kassymbek, S. Jimi and A. Saparov, Immunology of acute and chronic wound healing, Biomolecules, 2021, 11, 700 CrossRef CAS PubMed.
- G. Yaşayan, O. Nejati, A. F. Ceylan, Ç. Karasu, P. K. Ugur, A. Bal-Öztürk, A. Zarepour, A. Zarrabi and E. Mostafavi, Tackling chronic wound healing using nanomaterials: advancements, challenges, and future perspectives, Appl. Mater. Today, 2023, 32, 101829 CrossRef.
- H. E. Gültekin, G. Yaşayan, A. Bal-Öztürk, A. Bigham, A. A. Simchi, A. Zarepour, S. Iravani and A. Zarrabi, Advancements and applications of upconversion nanoparticles in wound dressings, Mater. Horiz., 2024, 11, 363–387 RSC.
- Y. Yang and M. W. Urban, Self-healing polymeric materials, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC.
- S. Talebian, M. Mehrali, N. Taebnia, C. P. Pennisi, F. B. Kadumudi, J. Foroughi, M. Hasany, M. Nikkhah, M. Akbari and G. Orive, Self-healing hydrogels: the next paradigm shift in tissue engineering?, Adv. Sci., 2019, 6, 1801664 CrossRef PubMed.
- M. Li, Y. Liang, Y. Liang, G. Pan and B. Guo, Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds, Chem. Eng. J., 2022, 427, 132039 CrossRef CAS.
- P. Yang, Z. Li, B. Fang and L. Liu, Self-healing hydrogels based on biological macromolecules in wound healing: A review, Int. J. Biol. Macromol., 2023, 253, 127612 CrossRef CAS PubMed.
- L. Qiao, Y. Liang, J. Chen, Y. Huang, S. A. Alsareii, A. M. Alamri, F. A. Harraz and B. Guo, Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing, Bioact. Mater., 2023, 30, 129–141 CAS.
- G. R. Bardajee, Z. Hooshyar, M. Farsi, A. Mobini and G. Sang, Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release, Mater. Sci. Eng., C, 2017, 72, 558–565 CrossRef CAS PubMed.
- J. Chen, J. He, Y. Yang, L. Qiao, J. Hu, J. Zhang and B. Guo, Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing, Acta Biomater., 2022, 146, 119–130 CrossRef CAS PubMed.
- F. Pahlevanzadeh, M. Setayeshmehr, H. R. Bakhsheshi-Rad, R. Emadi, M. Kharaziha, S. A. Poursamar, A. F. Ismail, S. Sharif, X. Chen and F. Berto, A review on antibacterial biomaterials in biomedical applications: from materials perspective to bioinks design, Polymers, 2022, 14, 2238 CrossRef CAS PubMed.
- Y. Wang, C. He, C. Chen, W. Dong, X. Yang, Y. Wu, Q. Kong and B. Yan, Thermoresponsive self-healing zwitterionic hydrogel as an in situ gelling wound dressing for rapid wound healing, ACS Appl. Mater. Interfaces, 2022, 14, 55342–55353 CrossRef CAS PubMed.
- S. A. Guo and L. A. DiPietro, Factors affecting wound healing, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- J. Zhang, J. Guan, X. Niu, G. Hu, S. Guo, Q. Li, Z. Xie, C. Zhang and Y. Wang, Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis, J. Transl. Med., 2015, 13, 1–14 CrossRef CAS PubMed.
- D. Feng, W. L. Zhao, Y. Y. Ye, X. C. Bai, R. Q. Liu, L. F. Chang, Q. Zhou and S. F. Sui, Cellular internalization of exosomes occurs through phagocytosis, Traffic, 2010, 11, 675–687 CrossRef CAS PubMed.
- C. Wang, M. Wang, T. Xu, X. Zhang, C. Lin, W. Gao, H. Xu, B. Lei and C. Mao, Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration, Theranostics, 2019, 9, 65 CrossRef CAS PubMed.
- T. Leng, Y. Wang, W. Cheng, W. Wang, X. Qu and B. Lei, Bioactive anti-inflammatory antibacterial metformin-contained hydrogel dressing accelerating wound healing, Biomater. Adv., 2022, 135, 212737 CrossRef CAS PubMed.
- N. Tang, R. Zhang, Y. Zheng, J. Wang, M. Khatib, X. Jiang, C. Zhou, R. Omar, W. Saliba and W. Wu, Highly efficient self-healing multifunctional dressing with antibacterial activity for sutureless wound closure and infected wound monitoring, Adv. Mater., 2022, 34, 2106842 CrossRef CAS PubMed.
- M. T. Amjad, A. Chidharla and A. Kasi, Cancer chemotherapy, 2020 Search PubMed.
- S. S. Das, D. Sharma, B. V. K. Rao, M. K. Arora, J. Ruokolainen, M. Dhanka, H. Singh and K. K. Kesari, Natural cationic polymer-derived injectable hydrogels for targeted chemotherapy, Mater. Adv., 2023, 4, 6064–6091 RSC.
- X. Han, X. Meng, Z. Wu, Z. Wu and X. Qi, Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection, Mater. Sci. Eng., C, 2018, 93, 1064–1072 CrossRef CAS PubMed.
- X. Huang, L. Tang, L. Xu, Y. Zhang, G. Li, W. Peng, X. Guo, L. Zhou, C. Liu and X.-C. Shen, A NIR-II light-modulated injectable self-healing hydrogel for synergistic photothermal/chemodynamic/chemo-therapy of melanoma and wound healing promotion, J. Mater. Chem. B, 2022, 10, 7717–7731 RSC.
- Y. Qi, Y. Yuan, Z. Qian, X. Ma, W. Yuan and Y. Song, Injectable and Self-Healing Polysaccharide Hydrogel Loading Molybdenum Disulfide Nanoflakes for Synergistic Photothermal-Photodynamic Therapy of Breast Cancer, Macromol. Biosci., 2022, 22, 2200161 CrossRef CAS PubMed.
- X. Jiang, F. Zeng, X. Yang, C. Jian, L. Zhang, A. Yu and A. Lu, Injectable self-healing cellulose hydrogel based on host-guest interactions and acylhydrazone bonds for sustained cancer therapy, Acta Biomater., 2022, 141, 102–113 CrossRef CAS PubMed.
- L. Yuan, Z. Li, X. Li, S. Qiu, J. Lei, D. Li, C. Mu and L. Ge, Functionalization of an Injectable Self-Healing pH-Responsive Hydrogel by Incorporating a Curcumin/Polymerized β-Cyclodextrin Inclusion Complex for Selective Toxicity to Osteosarcoma, ACS Appl. Polym. Mater., 2022, 4, 1243–1254 CrossRef CAS.
- L. Wang, F. Deng, W. Wang, A. Li, C. Lu, H. Chen, G. Wu, K. Nan and L. Li, Construction of Injectable Self-Healing Macroporous Hydrogels via a Template-Free Method for Tissue Engineering and Drug Delivery, ACS Appl. Mater. Interfaces, 2018, 10, 36721–36732 CrossRef CAS PubMed.
- Z. Wang, X. Zhai, M. Fan, H. Tan and Y. Chen, Thermal-reversible and self-healing hydrogel containing magnetic microspheres derived from natural polysaccharides for drug delivery, Eur. Polym. J., 2021, 157, 110644 CrossRef CAS.
- S. Zhou, D. Yang, D. Yang, Y. Guo, R. Hu, Y. Li, X. Zan and X. Zhang, Injectable, Self-Healing and Multiple Responsive Histamine Modified Hyaluronic Acid Hydrogels with Potentialities in Drug Delivery, Antibacterial and Tissue Engineering, Macromol. Rapid Commun., 2023, 44, 2200674 CrossRef CAS PubMed.
- M. Li, Y. Mu, Q. Xu, L. Jin and Y. Fu, Injectable, rapid self-healing, antioxidant and antibacterial nanocellulose-tannin hydrogels formed via metal-ligand coordination for drug delivery and wound dressing, Ind. Crops Prod., 2024, 208, 117876 CrossRef CAS.
- Y. Tang, X. Sun, J. Ma and Q. Yan, Mussel-inspired self-healing hydrogel based on gelatin and oxidized tannic acid for pH-responsive controlled drug release, J. Biomater. Sci., Polym. Ed., 2023, 1–22 Search PubMed.
- H. J. Won, B. Ryplida, S. G. Kim, G. Lee, J. H. Ryu and S. Y. Park, Diselenide-Bridged Carbon-Dot-Mediated Self-Healing, Conductive, and Adhesive Wireless Hydrogel Sensors for Label-Free Breast Cancer Detection, ACS Nano, 2020, 14, 8409–8420 CrossRef CAS PubMed.
- J. Han, Q. Ding, C. Mei, Q. Wu, Y. Yue and X. Xu, An intrinsically self-healing and biocompatible electroconductive hydrogel based on nanostructured nanocellulose-polyaniline complexes embedded in a viscoelastic polymer network towards flexible conductors and electrodes, Electrochim. Acta, 2019, 318, 660–672 CrossRef CAS.
- L. Xu, Y. Chen, M. Yu, M. Hou, G. Gong, H. Tan, N. Li and J. Xu, NIR light-induced rapid self-healing hydrogel toward multifunctional applications in sensing, Nano Energy, 2023, 107, 108119 CrossRef CAS.
- G. Su, S. Yin, Y. Guo, F. Zhao, Q. Guo, X. Zhang, T. Zhou and G. Yu, Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications, Mater. Horiz., 2021, 8, 1795–1804 RSC.
- J. Sun, X. Wu, J. Xiao, Y. Zhang, J. Ding, J. Jiang, Z. Chen, X. Liu, D. Wei, L. Zhou and H. Fan, Hydrogel-Integrated Multimodal Response as a Wearable and Implantable Bidirectional Interface for Biosensor and Therapeutic Electrostimulation, ACS Appl. Mater. Interfaces, 2023, 15, 5897–5909 CrossRef CAS PubMed.
- Y. Li, Y. Jin, W. Zeng, H. Jin, X. Shang and R. Zhou, Bioinspired Fast Room-Temperature Self-Healing, Robust, Adhesive, and AIE Fluorescent Waterborne Polyurethane via Hierarchical Hydrogen Bonds and Use as a Strain Sensor, ACS Appl. Mater. Interfaces, 2023, 15, 35469–35482 CrossRef CAS PubMed.
- X. Zhao, J. Xu, J. Zhang, M. Guo, Z. Wu, Y. Li, C. Xu, H. Yin and X. Wang, Fluorescent double network ionogels with fast self-healability and high resilience for reliable human motion detection, Mater. Horiz., 2023, 10, 646–656 RSC.
- L. Cai, X. Xiong, M. Qiao, J. Guo, H. Zhang, J. Lin, S. Liu and Y.-G. Jia, Aggregation-induced emission luminogen based self-healing hydrogels fluorescent sensors for α-amylase, Polym. Chem., 2022, 13, 819–828 RSC.
- B. Song, X. Fan, J. Shen and H. Gu, Ultra-stable and self-healing coordinated collagen-based multifunctional double-network organohydrogel e-skin for multimodal sensing monitoring of strain-resistance, bioelectrode, and self-powered triboelectric nanogenerator, Chem. Eng. J., 2023, 474, 145780 CrossRef CAS.
- S. Shang, K. Zhuang, J. Chen, M. Zhang, S. Jiang and W. Li, A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds, Bioact. Mater., 2024, 34, 298–310 CAS.
- P. Yang, Y. Ju, X. Liu, Z. Li, H. Liu, M. Yang, X. Chen, L. Lei and B. Fang, Natural self-healing injectable hydrogels loaded with exosomes and berberine for infected wound healing, Mater. Today Bio, 2023, 23, 100875 CrossRef CAS PubMed.
- M. Fu, C. Zhang, Y. Dai, X. Li, M. Pan, W. Huang, H. Qian and L. Ge, Injectable self-assembled peptide hydrogels for glucose-mediated insulin delivery, Biomater. Sci., 2018, 6, 1480–1491 RSC.
- Y. Qi, Z. Qian, W. Yuan and Z. Li, Injectable and self-healing nanocomposite hydrogel loading needle-like nano-hydroxyapatite and graphene oxide for synergistic tumour proliferation inhibition and photothermal therapy, J. Mater. Chem. B, 2021, 9, 9734–9743 RSC.
- A. H. Pandit, N. Mazumdar, K. Imtiyaz, M. M. Alam Rizvi and S. Ahmad, Self-healing and injectable hydrogels for anticancer drug delivery: A study with multialdehyde gum arabic and succinic anhydride chitosan, ACS Appl. Bio Mater., 2020, 3, 8460–8470 CrossRef CAS PubMed.
- L. Pernigoni, U. Lafont and A. M. Grande, Self-healing materials for space applications: overview of present development and major limitations, CEAS Space J., 2021, 13, 341–352 CrossRef.
- F. Zhang, P. Ju, M. Pan, D. Zhang, Y. Huang, G. Li and X. Li, Self-healing mechanisms in smart protective coatings: A review, Corros. Sci., 2018, 144, 74–88 CrossRef CAS.
- C.-H. Li, C. Wang, C. Keplinger, J.-L. Zuo, L. Jin, Y. Sun, P. Zheng, Y. Cao, F. Lissel and C. Linder, A highly stretchable autonomous self-healing elastomer, Nat. Chem., 2016, 8, 618–624 CrossRef CAS PubMed.
- S. Islam and G. Bhat, Progress and challenges in self-healing composite materials, Mater. Adv., 2021, 2, 1896–1926 RSC.
- Y. Ma, Y. Zou, Z. Zhang, J. Fang, W. Liu, Y. Ni, L. Fang, C. Lu and Z. Xu, Luminescent and hydrophobic textile coatings with recyclability and self-healing capability against both chemical and physical damage, Cellulose, 2020, 27, 561–573 CrossRef CAS.
- S. Ramesh, S. Khan, Y. Park, E. Ford, S. Menegatti and J. Genzer, Self-healing and repair of fabrics: A comprehensive review of the application toolkit, Mater. Today, 2022, 54, 90–109 CrossRef CAS.
- Y. Zhao, E. Liu, J. Fan, B. Chen, X. Hu, Y. He and C. He, Superhydrophobic PDMS/wax coated polyester textiles with self-healing ability via inlaying method, Prog. Org. Coat., 2019, 132, 100–107 CrossRef CAS.
- H. Žáková, J. Pazderka and P. Reiterman, Textile reinforced concrete in combination with improved self-healing ability caused by crystalline admixture, Materials, 2020, 13, 5787 CrossRef PubMed.
- N. De Belie, E. Gruyaert, A. Al-Tabbaa, P. Antonaci, C. Baera, D. Bajare, A. Darquennes, R. Davies, L. Ferrara and T. Jefferson, A review of self-healing concrete for damage management of structures, Adv. Mater. Interfaces, 2018, 5, 1800074 CrossRef.
- C. S. S. Durga and N. Ruben, Assessment of various self healing materials to enhance the durability of concrete structures, Ann. Chim.-Sci. Mat., 2019, 43, 75–79 CrossRef.
- Y. Wang, D. T. Pham and C. Ji, Self-healing composites: A review, Cogent Eng., 2015, 2, 1–28 CrossRef.
- D. Y. Zhu, M. Z. Rong and M. Q. Zhang, Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation, Prog. Polym. Sci., 2015, 49–50, 175–220 CrossRef CAS.
- Y. Jiang, E. L. L. Ng, D. X. Han, Y. Yan, S. Y. Chan, J. Wang and B. Q. Y. Chan, Self-Healing Polymeric Materials and Composites for Additive Manufacturing, Polymers, 2023, 15, 4206 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |