Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fate and biological uptake of polystyrene nanoparticles in freshwater wetland ecosystems†
Franca
Stábile
 *ab,
Mikael T.
Ekvall‡
*ab,
Mikael T.
Ekvall‡
 ab,
Julián A.
Gallego-Urrea§
ab,
Julián A.
Gallego-Urrea§
 c,
Temitope
Nwachukwu
c,
W. G. Chalani
U. Soorasena
a,
Pierina I.
Rivas-Comerlati¶
c,
Temitope
Nwachukwu
c,
W. G. Chalani
U. Soorasena
a,
Pierina I.
Rivas-Comerlati¶
 a and
Lars-Anders
Hansson
a and
Lars-Anders
Hansson
 ab
ab
aAquatic Ecology, Department of Biology, Lund University, Sweden. E-mail: franca.stabile@biol.lu.se
bNanoLund, Lund University, Sweden
cDepartment of Marine Sciences, University of Gothenburg, Sweden
First published on 18th June 2024
Abstract
Little is known about the fate and uptake of nanoplastics (NPs) in natural ecosystems, mainly due to analytical limitations in measuring NPs in complex environmental matrices. Our aim was to quantitatively assess the transport, fate and biological uptake of NPs in freshwater ecosystems by using replicated wetland mesocosms and gold-doped polystyrene nanoparticles. We showed that 97% of the NPs were retained in the wetlands, with most of them found in the sediment of the mesocosm's lake compartment. A small fraction (3%) of the NPs left the system through the outlet. After 10 weeks of exposure, both filter feeders (Daphnia magna) and detritivores (Asellus aquaticus) had taken up NPs, with D. magna showing a 5 times higher uptake than A. aquaticus. Moreover, NPs were detected in macrophyte roots and their leaves, with significantly higher values in the roots. NP distribution was negatively related with distance from the point of addition, a relation observed both for sediments and macrophytes. Both with respect to the experimental set-up and NP concentrations, our study provides novel insights to the understanding of the fate and uptake of NPs, a contaminant of emerging concern, in natural scenarios. In a broader context, our study also provides crucial knowledge for risk assessment and support for decision-makers and ongoing legislative work regarding nanoplastics.
Environmental significanceOur understanding of the fate of nanoplastics in natural ecosystems is negligible, and studies addressing this in complex settings are still scarce. Here, we show that most nanoplastics (97%) were retained in freshwater wetland mesocosms, and their distribution was negatively related with distance from the addition point. Nanoplastics mainly ended up in the sediments of the water compartment, where uptake by biota and hetero-aggregation processes might have been crucial. Nanoplastics were taken up by freshwater invertebrates and macrophytes. Among invertebrates, filter feeders showed a higher nanoplastic uptake than detritivores, highlighting the different risks nanoplastics represent to different taxa of aquatic organisms; with potential negative consequences at higher levels of biological organization and freshwater ecosystem function. |
Introduction
Plastic pollution is a global environmental problem which has become tremendously widespread, being reported in almost all ecosystems of the biosphere, even remote ones with limited human activities.1–3 Freshwater ecosystems play a considerable role in the plastic cycle; besides transporting plastic to the ocean, they also transform it and act as sinks of plastic pollution.4,5 Recently, it was found that plastic concentrations in freshwater lakes can be even higher than those reported in the subtropical oceanic gyres.6Plastic material enters freshwater ecosystems from diffuse inputs (e.g., through runoff and atmospheric deposition4) and from point sources (e.g., landfills and wastewater treatment plants7). After disposal, degradation and fragmentation processes reduce the particle size into smaller particles at the micro- (1 to <1000 μm) and nano-scale (<1 μm).8 Plastic particles are also manufactured at the nano-scale and added to different products, such as cosmetics,9 shampoos,10 laundry detergents and softeners,11 which are often released to the aquatic environment through the sewage systems.7,12
Small-sized plastics, and in particular nanoplastics (NPs), have been in the focus for research during the last decade. One unique property of nano-sized particles is that they exhibit strong surface reactivity per given mass due to their large surface to volume ratio.13 NPs have been shown to be toxic to several freshwater organisms, such as phyto- and zooplankton,14–17 benthic macroinvertebrates18 and fish.19,20 Moreover, NPs can also cross cell membranes,21 affect cell metabolism,22 and be transferred through the food web from algae to top predators, such as fish.19,23 Size has been noted as an important feature for toxicity and smaller NPs often show higher toxicity than larger ones.16,19,24 Other characteristics affecting their toxicity are particle shape, surface charge, as well as dose.12
Most previous studies on NPs have been performed at the lab scale, in simplified experimental designs using specific target organisms, which have contributed considerably to our fundamental understanding of NP behaviour and toxicity. However, despite the rapidly increasing amount of plastic material entering natural ecosystems,3,25 little is known about the fate, biological uptake and effects of nanoplastics in nature. This is particularly true for nanoplastic transport and distribution in natural ecosystems, which we still know very little about, in comparison with several different studies aimed to explore nanoplastic effects. The reason for this may be that quantification of NPs in complex environmental matrices is analytically challenging, and reliable detection and quantification methods are still in its infancy.
Recently, considerable advances have been made thanks to tracing methods using metal-doped nanoplastics,26 which allow tracing the metal core of the NP that can be quantified using standard methods for trace metal analysis. Using this technique, different systems with varying complexity have been studied, examining for instance: NP uptake and effects on Gammarus pulex27 and Daphnia magna,28 acquisition and excretion in rainbow trout29 or removal during drinking water treatment.30 In particular, the studies which addressed NP distribution and effects in aquatic systems, using metal-doped particles, explored it using simplified food chains: as the 14 days assay of a periphyton-snail food chain studied by Holzer et al.,31 and the short-term (48 h) sediment-algae-D. magna setup investigated by Tamayo-Belda et al.32
Although our knowledge on nanoplastic transport, behaviour and toxicity has advanced enormously during the last decade, the real magnitude of nanoplastics pollution is still poorly known.33 The recent quantifications of nanoplastics in nature2,34,35 provided needed information regarding the exposure, and a first glimpse of what can be expected. The reported nanoplastic concentrations for surface inland waters showed an average of 563 μg L−1, ranging from 180 to 1588 μg L−1, considering all types of nanoplastics found.2 Currently, there is a call for understanding exposure to nanoplastics in realistic scenarios, considering both the material and concentrations used, and also the set up tested.33
To contribute towards filling this knowledge gap and provide relevant scenarios, we quantitatively assessed the transport, fate and biological uptake of nanoplastics in different entities of freshwater ecosystems. Specifically, we exposed freshwater wetland mesocosms to polystyrene nanoparticles doped with a gold core, allowing us to track them over time, assessing their distribution in different organisms and in the ecosystem. The wetland mesocosms were exposed to low environmental concentrations of nanoplastics, to provide a realistic scenario in terms of the exposure. Furthermore, studies performed at the mesocosm scale allow a higher degree of complexity and realism than laboratory scale experiments, with the advantage that mesocosms can be replicated, which is rarely possible in natural ecosystems.36
We chose wetlands as our model ecosystem because these ecosystems interconnect, and highly influence, what happens in the adjacent terrestrial and aquatic ecosystems, due to their role in flood protection, carbon sequestration and water quality improvement by retention of nutrients and sediments.37 Moreover, these ecosystems sustain high biodiversity and provide other ecosystem services, for example, as water reservoirs.37 Therefore, understanding how novel pollutants, as nanoplastics, are transported and distributed in wetlands is highly relevant, both for the ecosystem itself and also for the water quality of downstream aquatic ecosystems.
The ultimate goal of our study was to improve the understanding on how nano-sized plastic material entering natural ecosystems are transported and taken up by organisms, which, in a broader context provides crucial knowledge for risk assessment and decision support for legislators.38
Materials and methods
1. Nanoplastic particles
Following Hartmann et al.'s definition,8 we here refer to nanoplastic (NP) as any synthetic or semisynthetic, solid and insoluble polymers, with their largest dimension between 1 to 1000 nm. The nanoplastic particles used in our study were polystyrene (PS) nanoparticles (size: 88 ± 11 nm). Inside, the particles had a gold (Au) core (size: 13 ± 1 nm) surrounded by a silica (SiO2) layer (Table 1, Fig. 1). The gold core allowed us to assess their transport, fate and uptake in the wetlands using inductively coupled plasma mass spectrometry (ICP-MS), but presented as a polystyrene particle, since the core is completely incorporated in the polystyrene layer. To improve the readability, the gold-doped polystyrene nanoparticles are hereafter referred to as nanoplastics (NPs).| Batch 1 | Batch 2 | ||
|---|---|---|---|
| Morphological chacaterization by TEM | With 1 Au core per particle (%) | 84.3 | 95.8 |
| Particle size distribution (nm) | 88.6 (11.2) | 86.9 (9.8) | |
| Au NPs cores size distribution (nm) | 13.6 (1.6) | 13.3 (1.1) | |
| Dynamic Light Scattering (nm) | Intensity | 139.1 (3.8) | 131.7 (2.1) |
| Number | 108.7 (21.07) | 89.1 (3.5) | |
| Pdl | 0.030 (0.024) | 0.085 (0.013) | |
| ζ-Potential (mV) | −68.4 (5.5) | −65.2 (5.2) | |
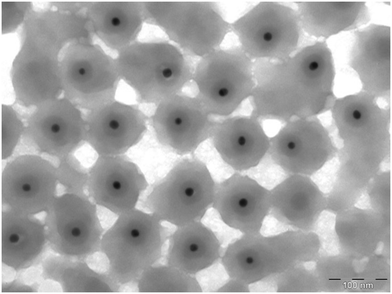 | ||
| Fig. 1 Transmission Electron Microscopy images of the gold-doped polystyrene nanoparticles used, where the gold core and silica layer, incorporated into the polystyrene particle, can be noted. | ||
The gold-doped polystyrene nanoparticles were purchased from Applied Nanoparticles SL (https://www.appliednanoparticles.eu/). The particles had a negatively charged outer PS surface (Table 1), and the PS surface had similar properties as a pure PS particle, assessed by Fourier-transform infrared spectroscopy (FTIR) (ESI† Fig. S1). Two nanoparticle batches were obtained from the company which characteristics are detailed in Table 1. The nanoparticle batches were dispersed in MilliQ water. Before performing the experiment, the nanoparticles were dialyzed in Standard RC Tubing, Dialysis Membrane (MWCO: 3.5 kD) for 24 h in 10 L of MilliQ water, which was changed four times.
The gold and silica core in the particle implies a higher density (1256 kg m−3) than a pure polystyrene nanoparticle (1060 kg m−3), and hence a faster sedimentation. However, in pure water it will take 940 days for the gold-doped polystyrene nanoparticle to reach the bottom of our mesocosms (mesocosm's lake average depth: 8.7 cm), see supplementary material Tables S2 and S3.† This is more than 13 times longer than our experimental time of 70 days. Therefore, if not taken up by biota or adsorbed to other particles, the gold-doped polystyrene particles would stay in suspension in water throughout our experiment.
The main particle characterization was performed by the manufacturer (Applied Nanoparticles SL, https://www.appliednanoparticles.eu/) and is summarized in Table 1. The size distribution characterization by Transmission Electron Microscopy (TEM) was performed using a JEOL 1010 Transmission Electron Microscope working at 80 keV. ImageJ software (NIH, USA) was used to process the acquired TEM images to calculate size distribution.
Dynamic Light Scattering (DLS) and ζ-Potential were performed with Malvern Zetasizer Nano ZS90. The equipment is regularly calibrated with polystyrene standard beads and Surface Charge Transfer Standard supplied by Malvern Instruments Ltd (Worcestershire, UK). Finally, the Au content in the stock solutions was determined using a Microwave Anton Paar Multiwave 7000 and an ICP-MS Agilent 7500, and the assay was performed in duplicate. Quantification was done by interpolation using a calibration curve obtained from a commercial and certificated standard of gold. This determination is certified by LEITAT Technological Center (Terrassa, Spain).
2. Wetland mesocosms
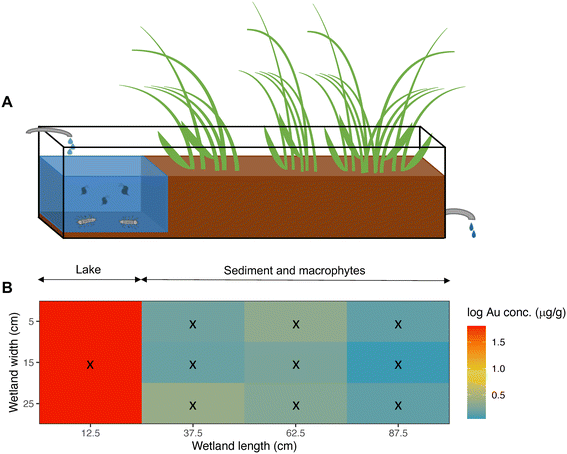
Our wetland mesocosms were set in a greenhouse and consisted of 12 glass aquaria (1.0 × 0.3 × 0.2 m: L × W × H), each continuously fed with tap water at a flow rate of 3 mL min−1. The inlet water dripped directly into the “lake” (Fig. 3A), while the outlet was placed at the opposite end. The outlet water from each wetland was collected in individual 5 L glass bottles and the estimated retention time of each wetland was 6.35 days.Each mesocosm was divided into two sections: an area with water close to the inlet which, despite its small size, carries the same features as a lake and is, accordingly, denoted “the lake” (volume = 6.97 ± 0.58 L). The lake is followed by a sediment section which was planted with macrophytes (mainly Carex rostrata and Juncus sp.) covering approximately three quarters of the aquarium (Fig. 3A). The total volume of the mesocosm was, on average, 27.42 ± 0.98 L. Macrophytes were retrieved from a natural wetland and planted three months before the start of the experiment to allow proper establishment. Sediment tufts with plants were divided in smaller pieces and randomly distributed among the 12 wetlands. Sediment was not frozen after collection, so a natural community of organisms came with it (e.g., copepods, chironomids and tubificid worms). The sediment and macrophytes originated from a natural wetland close to Hästveda, southern Sweden (56.2843° N, 13.9353° E). The original wetland is characterized by an organic wetland soil composed of the remains of plants in various stages of decomposition.
A week before the start of the experiment, each wetland's lake section was inoculated with an algae culture of Tetradesmus obliquus; and 24 h prior the experiment started, both benthic and pelagic feeders, represented by 10 benthic detritivores (Asellus aquaticus) and 20 pelagic filter feeders (D. magna) were added to each wetland's lake section. Before introducing the invertebrates, they were acclimatized during 24 h to the greenhouse temperature and wetland water. Although each mesocosm had a natural community from the original wetland, the addition of a known number of A. aquaticus and D. magna allowed us to use these species as our focal organisms for assessing NP uptake, while also ensuring enough sample size for ICP-MS analysis.
The procedure described above aimed for making the mesocosm wetlands as similar as possible. Considering that each mesocosm represents a small ecosystem, we decided to maximise the number of replicates in our experimental setup (6 replicates), in order to be able to draw firm conclusions, beyond the variability that might exist between replicates.
3. Experimental setup
Six mesocosms were randomly assigned as nanoplastic exposed (NP) and six to be non-exposed controls (C). Once a week, during 10 weeks, 2.28 mL of a colloidal dispersion of gold doped polystyrene nanoparticles (Au content of the dispersion: 49.5 mg of Au L−1) were added to the lake area of each NP wetland using a pipette. Simultaneously, the same volume of MilliQ water was added to each control wetland. When adding the NP and the MilliQ water, respectively, the water was gently mixed with the pipette tip to disperse the additions.The theoretical concentration of gold in each wetland's lake after NP addition was 16.21 μg Au/L (representing 226.32 μg PS L−1). This represents a theoretical number of particles of 6.60 × 1011 Au cored-PS particles per liter of lake water, after each NPs addition. Considering the total volume of the wetland mesocosm, the theoretical concentration of gold in each wetland after NP addition was 4.12 μg Au L−1 (representing 57.53 μg PS L−1). The gold core allowed us to track and quantify the nanoplastics in the system, being our proxy for nanoplastic concentration. Therefore, theoretical and measured gold concentrations can be expressed as polystyrene concentrations following the equation:
| PS concentration = Au concentration × 13.9579 |
4. Sample collection and analyses
Water samples (50 mL) were collected weekly throughout the experiment from the wetland's lake section and three times per week from the outlet. From the wetland's lake, two water samples were taken, one immediately before the NP addition, and the other 1 h after the addition. The outlet water was sampled three times per week: 1 day, 4 days, and 6 days after the NPs addition. Samples were kept in a fridge at 4 °C for later analysis of Au content by ICP-MS (see ESI†). Moreover, oxygen concentration and pH from the wetland's lake and outlet water, and water turbidity of the lake water were measured weekly. Oxygen was measured using an Oxyguard Handy Polaris 2 probe (Copenhagen, Denmark) and pH was assessed using a pHTestr 30 (Thermo Fisher Scientific, Mass., U.S.A.). For turbidity measurements a HI 93703 microprocessor turbidity meter from Hanna Instruments (Woonsocket RI-USA) was used.At the end of the experiment (week 10 and 11), D. magna, A. aquaticus, macrophytes and sediment were sampled. D. magna was collected using a filter (mesh size: 100 μm) and A. aquaticus was collected using a sieve (mesh size: 1 mm) and all animals were rinsed with tap water on a mesh filter. Macrophytes were manually collected, rinsed with tap water, and then roots and leaves were separated. Regarding sediment, 10 samples were taken from each wetland: one in the lake's sediment, collected with a syringe, and 9 surface sediment samples were taken from the sediment area using a core (cut syringes, 20 mm diameter). The position of the sampled macrophytes and sediment was registered as x and y coordinates using the aquarium inlet left corner as the reference point (Fig. 3B). All solid samples (i.e., sediment and biological samples) were frozen at −25 °C, and later, freeze dried using a freeze dryer (Thermo Fisher Scientific Heto Power Dry LL3000). Afterwards, samples were ground, weighted and transferred to 2 mL-vials and sealed in plastic bags. Solid samples were kept in a desiccator until Au content analysis.
Au concentrations in water and solid samples were assessed by ICP-MS. Samples were digested following a microwave-assisted acid digestion. Acidified samples were measured with an ICP-MS Perkin Elmer Nexion 350 D. For the ICP-MS analysis and results, the natural material in which the NPs are enclosed or embedded is referred to as “matrix” (e.g., sediment, water, invertebrate's species, etc.). For water samples, recoveries of Au nanoparticles and dissolved gold (0.1 ppb and 1 ppb) were generally at 75% and 95–105% levels, respectively. For solid samples, recoveries were around 97% for dissolved samples (dissolved spike on the sample or acids before digestion) and 75% for nanoparticles with respect to nominal concentrations. In both water and solid samples, Au concentrations used for calculations were adjusted using the 75% of recovery, following the equation:
| Estimated Au concentration = Measured Au concentration/recovery of the method |
Although testing nanoplastic effects on the exposed communities was beyond the scope of this study, we assessed D. magna and A. aquaticus populations as number of individuals or dry biomass in each wetland at the end of the experiment. Moreover, water samples from the Lake were taken the last 4 weeks of experiment, using a 100 μm filter to avoid the presence of filamentous algae, and were analyzed with the spectrofluorometer AlgaeLabAnalyser® (ALA, BBE Moldaenke GmbH, Germany).
5. Data analysis
All analyses were performed using R version 3.6.139 and R studio version 1.2.5001,40 and figures were drawn using the package ‘tidyverse’.41 The Au concentration in the different matrices, our proxy for nanoplastic concentration, was analysed as dependant variable, using a Generalized linear model (GLM) or a Generalized linear mixed model (GLMM, performed using the package ‘lme4’42), all with Gamma error distribution. Akaike index (AIC) was used as the selection criteria when more than one model was performed. When ΔAIC < 2 when comparing models for the same response variable and data-set, they were considered not different and, in that case, the simplest model was chosen. When performing statistical analysis, or drawing plots, including data which Au concentrations were below detection limit, the value was referred to as the limit of detection.Au concentration in water was analysed with a GLMM where “Time”, “Treatment” and “Water sampling site” were used as explanatory variables, and mesocosm identity (ID) was modelled as random effect with random intercepts and slopes. Moreover, for understanding how NPs are transported through time in the wetlands, Au concentrations in water from NP exposed wetlands were analysed with a GLMM where “Time”, “Label” and their interaction were used as explanatory variables. “Label” refers to either outlet water; lake water before NP additions or lake water after NP additions. In this case, mesocosm ID was also modelled as a random effect with random intercepts and slopes.
The Au concentrations in sediments from NP exposed wetlands were analysed with a GLMM with “distance” as the explanatory variable and mesocosm ID as random effect (random intercepts). Similarly, the Au concentrations in macrophytes from NP exposed wetlands were analysed with a GLMM with “distance”, “matrix” and “plant part” as explanatory variables, and mesocosm ID as random effect, with random intercepts. A GLM was used to analyse the Au concentrations in invertebrates, with “matrix” and “treatment” modelled as explanatory variables.
Linear Models (LM) were performed for the number of individuals of D. magna, D. magna biomass (g of dry weight) and A. aquaticus biomass (g of dry weight) between treatments at the end of the experiment. Moreover, total algae in lake water, oxygen concentration, pH and water turbidity were analysed with linear mixed models (LMM) with time and treatment as explanatory variables, and also the water sampling site when modelling oxygen and pH. All mixed models included mesocosm ID as random effect (random intercepts).
Results
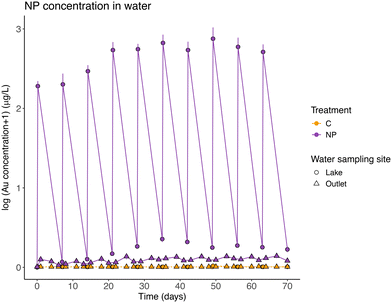
The measured nanoplastic concentrations in the lake water after each particles addition (mean 18.33 ± 3.95 μg Au L−1), which were similar to the theoretically expected concentrations, showed that NPs were in suspension in the lake water after the additions. After a week of exposure, and before the next NP addition, the measured concentrations in the lake water (mean 0.35 ± 0.15 μg Au L−1) showed that most nanoplastics were no longer in the water. It was possible to observe that NP concentrations in the lake water fluctuated in connection with the residence time of the system (6.35 days) and the weekly additions. However, and interestingly, as a result of the gold-core, the NPs could be tracked through the wetlands and were also recorded in the outlet water, although the concentrations were here 10–20 times lower than in the lake section (Table 2, model 1; Fig. 2).| Model | Model type | Dependent variable | Explanatory variable (fixed effects) | χ 2 | d.f. | p value |
|---|---|---|---|---|---|---|
| a Water sampling site: at the lake or the outlet. b Label: lake water before nanoplastic addition; lake water after nanoplastic addition; outlet water. | ||||||
| 1 | GLMM gamma (link: log) | Au concentration in water | Time | 2.5639 | 1 | 0.1093 |
| Treatment | 726.017 | 1 | <0.001 | |||
| Water sampling sitea | 336.692 | 1 | <0.001 | |||
| 2 | GLMM gamma (link: log) | Au concentration in water of NP exposed wetlands | Time | 28.793 | 1 | <0.001 |
| Labelb | 2710.588 | 2 | <0.001 | |||
| Time × Label | 11.801 | 2 | <0.01 | |||
| 3 | GLMM gamma (link: log) | Au concentration in sediments from NP exposed wetlands | Distance | 96.901 | 1 | <0.001 |
| 4 | GLMM gamma (link: log) | Au concentration in macrophytes from NP exposed wetlands | Distance | 49.75 | 1 | <0.001 |
| Matrix | 0.719 | 1 | 0.3965 | |||
| Plant part | 122.155 | 1 | <0.001 | |||
| 5 | GLM gamma (link: log) | Au concentration in invertebrates | Treatment | 87.768 | 1 | <0.001 |
| Matrix | 57.379 | 1 | <0.001 | |||
Mass balance calculations considering all particles introduced during the experiment, showed that, on average, 96.52 ± 1.76% of the particles were retained in the wetland. NPs left the system through the outlet following the input pulses (Table 2, model 2 and post-hoc Tukey test, p = 0.26; Fig. 2), and they represented 3.48% (±1.76 standard deviation) of the total NPs added. The concentration of NPs found in suspension in the lake water before each weekly NP addition, represents on average, 0.50 ± 0.23% of the total amount of NPs added to the system. The pattern of a slight increase in NP concentration in water (average model slope = 0.018), was different between the NP concentrations before and after the particles addition (Table 2, model 2 and post-hoc Tukey test, p < 0.01, significant interaction between the explanatory variables, model slopes 0.029 and 0.008, respectively).
After 10 weeks of exposure, most NPs were retained in the sediment of the lake section (Fig. 3 & S2†), and the mean concentration in the lake's sediment of NP exposed wetlands was 6.72 ± 2.57 μg Au g−1 (or 93.76 ± 35.86 μg PS g−1), with almost 20 times higher concentrations than in the rest of the sediment samples (Table S8†). The mean concentration of particles at the sediment, but in the area covered with macrophytes, was on average, 0.34 ± 0.48 μg Au g−1 (or 4.70 ± 6.66 μg PS g−1). Moreover, the NP concentrations in sediments decreased significantly with distance from the inlet (Table 2, model 3; Fig. 3 & S3†).
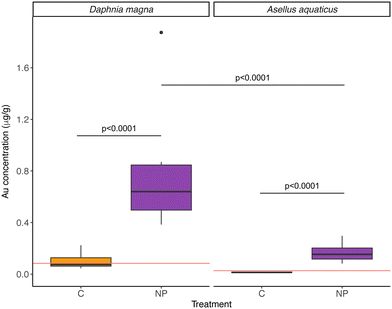
Furthermore, NPs were taken up by both D. magna and A. aquaticus, but the filter feeder D. magna had a significantly higher concentration than A. aquaticus (Table 2, model 5; Fig. 4). The mean concentration of particles found in NPs exposed D. magna was 1.09 ± 0.73 μg Au g−1, and for A. aquaticus, 0.22 ± 0.10 μg Au g−1 (15.20 ± 10.23 μg PS g−1 and 3.13 ± 1.46 μg PS g−1, respectively, and considering the recovery of the method). At the end of the experiment, the biomass of A. aquaticus per mesocosm was 9 times higher than D. magna (unpaired t test, t = −3.7523, d.f.: 11.09, p < 0.01). Considering the total biomass of invertebrates in the system, the population of D. magna accumulated, on average, 0.0019 ± 0.0026% of the total mass of NPs added to the NP exposed wetlands, and for A. aquaticus this was, on average, 0.0024 ± 0.0030%; values which are not significantly different (LM, F = 0.071, d.f.: 10, p = 0.796).
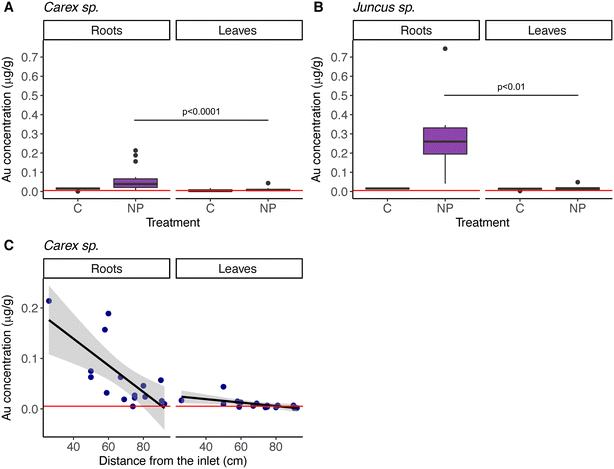
In addition, at the end of the experiment, both macrophyte species had NPs in their roots and in their leaves, although significantly more in their roots (model 4 in Table 2; Fig. 5A & B; Table S8†). As was the case for the sediment, NP concentrations in the macrophytes decreased with distance from the inlet (model 4 in Table 2; Fig. 5C). Juncus sp. had higher concentrations of particles than Carex sp., but according to the GLMM, this difference is explained by their position in the system, closer to the lake, and not due to different macrophyte genus (variable defined as “Matrix” in the model 4, Table 2).
Although the effects of NPs on biota was not the major scope of this study, no significant differences between treatments were found in the population sizes of D. magna either A. aquaticus at the end of the experiment (Table S6 in ESI†). On the other hand, at the end of the experiment, the mean concentration of phytoplankton in control wetlands was 2-fold higher than in nanoplastic exposed wetlands (LMM, significant interaction between Treatment and Time, χ2 = 11.072, d.f.: 45, p < 0.001, model slopes: 0.41 and −0.11 for control and NP exposed wetlands respectively; Fig. S4 in ESI†). Oxygen concentration and pH did not differ between treatments during the experimental time (Table S7†), lake water turbidity was different between treatments throughout the experimental time (LMM, significant interaction between Treatment and Time, χ2 = 5.14, d.f.: 129, p < 0.05, model slopes: 0.06 and 0.02 for control and NP exposed wetlands respectively; Table S7 in ESI†).
Discussion
Nanoplastics are a potentially strong environmental threat, but despite the amount of plastics entering the natural environment is extremely high and continuously increasing,3,43 our knowledge on how nanoplastic particles are transported and where they are ending up in natural ecosystems is still negligible. Our approach, using replicated wetland mesocosms and gold-doped polystyrene nanoparticles, provides novel understanding and shows that ca. 97% of the NPs were retained in the wetlands and only around 3% left the wetlands through the outlet (i.e., on average, 96.52 ± 1.76% of NP stayed into the wetlands). Moreover, most of the NPs were found in the sediment of the lake section, close to where they were added. In general, the distribution of NPs was negatively related to the distance from the addition point. This suggests that the main bulk of NPs are trapped close to where they are deposited, a notion which is in agreement with Pulido-Reyes et al.,30 who showed that metal-doped polystyrene NPs (around 200 nm in diameter) were mostly retained in the first layers of the filtration media (aged sand), used to mimic part of the water potabilization process. Similar results have been shown for microplastics from car tires released into marine coastal ecosystems, where those particles mainly accumulated in the sediment relatively close to the source of pollution, compared to other lighter microplastics.44The residence time of the water in the mesocosms was 6.35 days, which theoretically means that before each weekly NP addition, all the water in the system was replaced. According to our calculations, the NPs would theoretically have stayed in suspension during the experimental time (see materials and methods section for details). The small fraction of NPs that left the wetlands through the outlet water may represent particles dispersed in the water, either monodispersed or forming small aggregates that were not trapped in biota or the sediment. Considering that in our study around 3% of all NPs left the wetlands through the outlet, and the small portion (0.5%) that was found in suspension in the lake water before each weekly addition, we may conclude that most of the NPs were retained in different biotic and abiotic compartments of the ecosystem.
According to both theoretical and experimental studies, it is likely that NPs that entered our wetlands underwent particle hetero-aggregation with humic substances and organic matter,45–48 and due to an increased density, sank to the sediment. In a study addressing the fate and transport of nano- to millimetre sized plastics (density of 1040 kg m−3) using a hydrological model, Besseling et al.48 showed that small plastic particles, between 100 and 2000 nm, are likely to be retained in a river system, and that particle aggregation with suspended solids plays a more important role in nanoplastic removal from the water column than direct settling.48 Furthermore, NPs may also have attached to the phytoplankton's cell walls and their exopolymer substances,49,50 or have been internalized by them,50 and in that way being ingested by zooplankton (D. magna). Moreover, part of the NPs ingested by zooplankton could have been released via excretion, as has been shown for a diverse set of organisms, including D. magna and G. pulex,27,51 and thereby sinking to the bottom. This notion is strengthened by previous studies showing that the presence of both algae and D. magna, may increase the settling of metal-doped NPs, compared to when the organisms are absent.32 All these processes likely explain why most NPs stayed in the lake section of the wetlands.
Our results demonstrate a considerable NP uptake by both filter feeders (D. magna) and detritivores (A. aquaticus), but we cannot exclude that some nanoplastics might have been taken up by organisms that were not investigated (e.g., chironomids or tubificid worms). NP uptake by the same, or similar, organisms have previously been demonstrated in single-species small-scale lab studies, for D. magna28,32 and the detritivore G. pulex.27 In our study, where organisms were part of the natural community, a significantly higher uptake per unit mass was observed for D. magna than for A. aquaticus, and the exposure route and feeding mode might explain this difference. NPs were added directly into the water column of the lake section, where the phyto- and zooplankton community may have primarily interacted with the NPs in the various ways mentioned above. As a benthic organism, A. aquaticus was probably exposed later than the pelagic organisms, after NPs had settled to the sediment, likely hetero-aggregated with organic matter, phytoplankton and their exudates or into zooplankton's fecal pellets.27,45–47,49,51 The differential uptake is interesting considering that A. aquaticus is associated with the sediment compartment, where we found the highest NP concentration at the end of the experiment, where they could have taken up nanoplastics both by active feeding or passive uptake through the gills. However, the higher A. aquaticus biomass has to be considered, and when accounting for the biomass of invertebrates, the NP accumulation was similar for both populations.
On the other hand, it is worth noting that D. magna can feed on periphyton as an alternative food source, although not preferred.52 Periphyton effectively accumulates nanoplastics,31 therefore, D. magna feeding on both food sources, could have accumulated more nanoplastics than the benthic feeder A. aquaticus. Further studies focusing on single-species and assessing excretion and detoxification rates should be carried out to explain the observed result. Besides that, as others have suggested,33,53 when nanoplastics hetero-aggregate, their transport and uptake seems to be governed by the fate of the larger hetero-aggregate, and not to the intrinsic properties of the nanoplastic. In this context, the understanding of basic aspects of the biology of the organisms is crucial to explain the differential uptake. We observed that, in the last weeks of the experiment, the biomass of phytoplankton was lower in the nanoplastic exposed wetlands, which could also explain the marginal difference found in turbidity values in the lake water. We also saw that D. magna populations did not differ between treatments. It is, however, difficult to disentangle if the difference in the concentration of phytoplankton was due to a negative effect of nanoplastics on algae cells, as has been previously reported,14,17 or a D. magna response to nanoplastic exposure, increasing feeding rates, or a combination of both. Further studies should be carried out to explore these effects in detail.
Distance from the point of nanoplastic addition played a major role in explaining the distribution of nanoplastics in macrophytes, being a more important factor than genus-specific differences. It is worth noting that we analyzed two different genera of macrophytes showing that after 10 weeks of exposure, nanoplastics were found both in roots and leaves, which suggests that 88 nm nanoplastics are taken up and transported within the plants. Moreover, the nanoplastic concentration was higher in the roots than in their leaves. This may mirror uptake and incorporation rates, since roots are directly exposed to the water, and thereby to the nanoplastics, whereas it may take longer for the nanoplastics to be incorporated in leaves. Hence, we may predict that the distribution of nanoplastics within the macrophyte may change with time. The presence of submicron plastics inside the plant tissue have been demonstrated by Li et al. for crops,54 using a higher concentration (50 mg L−1) and particle size (200 and 2000 nm). Future studies, including single-species and transmission electron microscopy, may unravel what happens at the root level, how nanoplastics are entering and the role of the root exudates.
Theoretically, the maximum nanoplastic concentration in this study would have been 2263 μg PS L−1 considering only the lake volume, or 575 μg PS L−1 at the whole wetland, counting the 10 nanoplastic additions together. The nanoplastic concentration used in this study was lower than in similar studies conducted with metal-doped nanoplastics,31,32 and closer to concentrations occurring in natural ecosystems, considering that the mean concentration of nanoplastics measured in natural surface waters has been found to be around 560 μg L−1 (ranging from 180 to 1588 μg L−1).2 Furthermore, our study also has a higher degree of realism regarding the complexity of the system, the longer exposure time and the temporal fluctuations in nanoplastic concentrations, thereby mimicking nanoplastic pulses reaching natural freshwater ecosystems, for example, after rainfalls. This is important considering that storm events55 and rainfall56 are significant drivers of microplastic pollution to inland surface waters.
In our study, the use of metal-doped nanoplastics was an effective method for studying the transport of nanoplastics in complex environmental compartments, and the wetland mesocosms constitute a promising tool for assessing the fate of nanoplastics by mirroring natural freshwater ecosystems. It has been shown that nanoplastics could negatively affect ecosystem services, for example the nitrogen removal efficiency.57 Following a similar approach, future studies could focus on nanoplastic exposure and responses of the natural communities at different levels of biological organization.
Conclusions
Given the urgent need to assess the fate of nanoplastics in natural ecosystems, we quantitatively assessed this, using freshwater wetland mesocosms and polystyrene nanoplastics doped with a gold core, which allowed us to track the fate of the nanoplastic over time. The set-up performed beyond lab-scale, with natural invertebrate and plant communities, and a fluctuating nanoplastic inflow, may represent a close to natural degree of realism regarding the complexity of the system, compared to previous studies.We conclude that most of the nanoplastics (97%) were retained within the wetlands, and only a small fraction (3%) left the system. Most nanoplastics retained were found in the sediment of the mesocosm's lake section; however, nanoplastics were also taken up by living organisms (pelagic zooplankton, benthic invertebrates and macrophytes), with different amounts of uptake depending on the organism. Such differences in nanoplastic exposure, may have consequences for community composition and ecosystem function, which needs to be addressed. In this sense, mesocosm-scale studies are promising tools for addressing effects at different levels of biological organization.
Wetlands are key ecosystems that sustain biodiversity and provide a multitude of ecosystem services. The capacity of retaining nanoplastics observed in this study suggest that wetlands can be used as a management tool, e.g., collect nanoplastics prior to reaching downstream waterbodies or the ocean; but this possibility has to be further explored. Finally, and in a broader context, our study provides novel understanding on how nanoplastics are distributed in natural ecosystems, knowledge that is urgently needed for environmental management, risk assessment and legislation, for example in the European Chemicals Agency58 and in OECD's guidance document on aquatic and sediment toxicological testing of nanomaterials.59
Author contributions
Conceptualization: L.-A. H., F. S. & M. T. E. Investigation: F. S., L.-A. H., J. A. G.-U., T. N., W. G. C. U. S., P. I. R.-C. & M. T. E. Methodology: F. S., L.-A. H., J. A. G-U. & M. T. E. Data curation, formal analysis & visualization: F. S. Funding acquisition: L.-A. H. & J. A. G.-U. Project administration: F. S., L.-A. H. & M. T. E. Supervision: L.-A. H. & M. T. E. Writing – original draft: F. S. Writing – review & editing: F. S., L.-A. H., J. A. G.-U., T. N., W. G. C. U. S., P. I. R.-C. & M. T. E.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are very grateful for funding from MISTRA (The Swedish foundation for strategic environmental research) via the Mistra Environmental NanoSafety Program phase II. F. S. acknowledge financial support by Kungliga Fysiografiska Sällskapet i Lund (Royal Physiographic Society). L.-A. H. acknowledge financial support from the Swedish research council FORMAS, Sydvatten AB and Sweden Water Research. J. A. G.-U. acknowledge the support from the Centre for Future Chemical Risk Assessment and Management strategies – UGOT Challenges, from the University of Gothenburg. M. T. E. acknowledge financial support from the Swedish research council FORMAS through grant no.: 2018-00908.References
- M. S. Bank and S. V. Hansson, The Microplastic Cycle: An Introduction to a Complex Issue. In Microplastic in the Environment: Pattern and Process, ed. M. S. Bank, Springer International Publishing, Cham, 2022, pp. 1–16 (Environmental Contamination Remediation and Management). Available from: https://link.springer.com/10.1007/978-3-030-78627-4_1 Search PubMed.
- D. Materić, M. Peacock, J. Dean, M. Futter, T. Maximov and F. Moldan, et al., Presence of nanoplastics in rural and remote surface waters, Environ. Res. Lett., 2022, 17(5), 054036 CrossRef.
- M. Eriksen, W. Cowger, L. M. Erdle, S. Coffin, P. Villarrubia-Gómez and C. J. Moore, et al., A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required, PLoS One, 2023, 18(3), e0281596 CrossRef CAS PubMed.
- E. M. F. Kallenbach, E. S. Rødland, N. T. Buenaventura and R. Hurley, Microplastics in Terrestrial and Freshwater Environments. In: Microplastic in the Environment: Pattern and Process, ed. M. S. Bank, Springer International Publishing, Cham, 2022, pp. 87–130 (Environmental Contamination Remediation and Management). Available from: https://link.springer.com/10.1007/978-3-030-78627-4_4 Search PubMed.
- F. M. Windsor, I. Durance, A. A. Horton, R. C. Thompson, C. R. Tyler and S. J. Ormerod, A catchment-scale perspective of plastic pollution, Global Change Biol., 2019, 25(4), 1207–1221 CrossRef PubMed.
- V. Nava, S. Chandra, J. Aherne, M. B. Alfonso, A. M. Antão-Geraldes and K. Attermeyer, et al., Plastic debris in lakes and reservoirs, Nature, 2023, 619(7969), 317–322 CrossRef CAS PubMed.
- R. C. Hale, A. E. King, J. M. Ramirez, M. La Guardia and C. Nidel, Durable Plastic Goods: A Source of Microplastics and Chemical Additives in the Built and Natural Environments, Environ. Sci. Technol. Lett., 2022, 9(10), 798–807 CrossRef CAS.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor and A. E. Daugaard, et al., Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53, 1039–1047 CrossRef CAS PubMed.
- B. Hosseinkhani, C. Callewaert, N. Vanbeveren and N. Boon, Novel biocompatible nanocapsules for slow release of fragrances on the human skin, New Biotechnol., 2015, 32(1), 40–46 CrossRef CAS PubMed.
- K. A. Günay, D. L. Berthier, H. A. Jerri, D. Benczédi, H. A. Klok and A. Herrmann, Selective Peptide-Mediated Enhanced Deposition of Polymer Fragrance Delivery Systems on Human Hair, ACS Appl. Mater. Interfaces, 2017, 9(28), 24238–24249 CrossRef PubMed.
- D. S. Murphy, Fabric Softener Technology: A Review, J. Surfactants Deterg., 2015, 18(2), 199–204 CrossRef CAS.
- K. Mattsson, S. Jocic, I. Doverbratt and L. A. Hansson, Nanoplastics in the Aquatic Environment. In: Microplastic Contamination in Aquatic Environments, Elsevier, 2018, pp. 379–399. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128137475000138 Search PubMed.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles, Environ. Health Perspect., 2005, 113(7), 823–839 CrossRef PubMed.
- H. Zhu, S. Fu, H. Zou, Y. Su and Y. Zhang, Effects of nanoplastics on microalgae and their trophic transfer along the food chain: recent advances and perspectives, Environ. Sci.: Processes Impacts, 2021, 23(12), 1873–1883 RSC.
- E. Besseling, B. Wang, M. Lürling and A. A. Koelmans, Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna, Environ. Sci. Technol., 2014, 48(20), 12336–12343 CrossRef CAS PubMed.
- E. Kelpsiene, O. Torstensson, M. T. Ekvall, L. A. Hansson and T. Cedervall, Long-term exposure to nanoplastics reduces life-time in Daphnia magna, Sci. Rep., 2020, 10(1), 5979 CrossRef CAS PubMed.
- C. Larue, G. Sarret, H. Castillo-Michel and A. E. Pradas del Real, A Critical Review on the Impacts of Nanoplastics and Microplastics on Aquatic and Terrestrial Photosynthetic Organisms, Small, 2021, 17(20), 2005834 CrossRef PubMed.
- P. E. Redondo-Hasselerharm, G. Gort, E. T. H. M. Peeters and A. A. Koelmans, Nano- and microplastics affect the composition of freshwater benthic communities in the long term, Sci. Adv., 2020, 6(5), eaay4054 CrossRef CAS PubMed.
- K. Mattsson, E. V. Johnson, A. Malmendal, S. Linse, L. A. Hansson and T. Cedervall, Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain, Sci. Rep., 2017, 7(1), 11452 CrossRef PubMed.
- J. A. Pitt, J. S. Kozal, N. Jayasundara, A. Massarsky, R. Trevisan, N. Geitner, M. Wiesner, E. D. Levin and R. T. Di Giulio, et al., Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio), Aquat. Toxicol., 2018, 194, 185–194 CrossRef CAS PubMed.
- L. Liu, K. Xu, B. Zhang, Y. Ye, Q. Zhang and W. Jiang, Cellular internalization and release of polystyrene microplastics and nanoplastics, Sci. Total Environ., 2021, 779, 146523 CrossRef CAS PubMed.
- T. Cedervall, L. A. Hansson, M. Lard, B. Frohm and S. Linse, Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish, PLoS One, 2012, 7(2), e32254 CrossRef CAS PubMed.
- K. Mattsson, M. T. Ekvall, L. A. Hansson, S. Linse, A. Malmendal and T. Cedervall, Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles, Environ. Sci. Technol., 2015, 49(1), 553–561 CrossRef CAS PubMed.
- M. T. Ekvall, I. Gimskog, J. Hua, E. Kelpsiene, M. Lundqvist and T. Cedervall, Size fractionation of high-density polyethylene breakdown nanoplastics reveals different toxic response in Daphnia magna, Sci. Rep., 2022, 12, 3109 CrossRef CAS PubMed.
- S. Wingfield and M. Lim, The United Nations Basel Convention's Global Plastic Waste Partnership: History, Evolution and Progress. In: Microplastic in the Environment: Pattern and Process, ed. M. S. Bank, Springer International Publishing, Cham, 2022, pp. 323–331 (Environmental Contamination Remediation and Management). Available from: https://link.springer.com/10.1007/978-3-030-78627-4_10 Search PubMed.
- D. M. Mitrano, A. Beltzung, S. Frehland, M. Schmiedgruber, A. Cingolani and F. Schmidt, Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems, Nat. Nanotechnol., 2019, 14, 362–368 CrossRef CAS PubMed.
- P. E. Redondo-Hasselerharm, G. Vink, D. M. Mitrano and A. A. Koelmans, Metal-doping of nanoplastics enables accurate assessment of uptake and effects on Gammarus pulex, Environ. Sci.: Nano, 2021, 8, 1761–1770 RSC.
- D. S. Vicentini, D. J. Nogueira, S. P. Melegari, M. Arl, J. S. Köerich and L. Cruz, et al., Toxicological Evaluation and Quantification of Ingested Metal-Core Nanoplastic by Daphnia magna Through Fluorescence and Inductively Coupled Plasma-Mass Spectrometric Methods, Environ. Toxicol. Chem., 2019, 38(10), 2101–2110 CrossRef CAS PubMed.
- N. J. Clark, F. R. Khan, C. Crowther, D. M. Mitrano and R. C. Thompson, Uptake, distribution and elimination of palladium-doped polystyrene nanoplastics in rainbow trout (Oncorhynchus mykiss) following dietary exposure, Sci. Total Environ., 2023, 854, 158765 CrossRef PubMed.
- G. Pulido-Reyes, L. Magherini, C. Bianco, R. Sethi, U. von Gunten and R. Kaegi, et al., Nanoplastics removal during drinking water treatment: Laboratory- and pilot-scale experiments and modeling, J. Hazard. Mater., 2022, 436, 129011 CrossRef CAS PubMed.
- M. Holzer, D. M. Mitrano, L. Carles, B. Wagner and A. Tlili, Important ecological processes are affected by the accumulation and trophic transfer of nanoplastics in a freshwater periphyton-grazer food chain, Environ. Sci.: Nano, 2022, 9(8), 2990–3003 RSC.
- M. Tamayo-Belda, A. V. Pérez-Olivares, G. Pulido-Reyes, K. Martin-Betancor, M. González-Pleiter and F. Leganés, et al., Tracking nanoplastics in freshwater microcosms and their impacts to aquatic organisms, J. Hazard. Mater., 2023, 445, 130625 CrossRef CAS PubMed.
- D. M. Mitrano, P. Wick and B. Nowack, Placing nanoplastics in the context of global plastic pollution, Nat. Nanotechnol., 2021, 16(5), 491–500 CrossRef CAS PubMed.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono and L. Brach, et al., Nanoplastic in the North Atlantic Subtropical Gyre, Environ. Sci. Technol., 2017, 51(23), 13689–13697 CrossRef CAS PubMed.
- D. Materić, H. A. Kjær, P. Vallelonga, J. L. Tison, T. Röckmann and R. Holzinger, Nanoplastics measurements in Northern and Southern polar ice, Environ. Res., 2022, 208, 112741 CrossRef PubMed.
- E. P. Odum, The Mesocosm, BioScience, 1984, 34(9), 558–562 CrossRef.
- J. B. Zedler and S. Kercher, WETLAND RESOURCES: Status, Trends, Ecosystem Services, and Restorability, Annu. Rev. Environ. Resour., 2005, 30(1), 39–74 CrossRef.
- F. Abdolahpur Monikh, S. F. Hansen, M. G. Vijver, E. Kentin, M. B. Nielsen and A. Baun, et al., Can Current Regulations Account for Intentionally Produced Nanoplastics?, Environ. Sci. Technol., 2022, 56(7), 3836–3839 CrossRef CAS PubMed.
- R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2019, Available from: https://www.R-project.org/ Search PubMed.
- RStudio Team, RStudio: Integrated Development Environment for R, RStudio, Inc., Boston, MA, 2019, Available from: https://www.rstudio.com/ Search PubMed.
- H. Wickham, M. Averick, J. Bryan, W. Chang, L. McGowan and R. François, et al., Welcome to the Tidyverse, JOSS, 2019, 4(43), 1686 CrossRef.
- D. Bates, M. Mächler, B. Bolker and S. Walker, Fitting Linear Mixed-Effects Models Using lme4, Journal of Statistical Software, 2015, 67(1), 1–48 CrossRef.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- K. Mattsson, J. A. De Lima, T. Wilkinson, I. Järlskog, E. Ekstrand and Y. A. Sköld, et al., Tyre and road wear particles from source to sea, Micropl. Nanopl., 2023, 3(1), 14 CrossRef.
- G. V. Lowry, E. M. Hotze, E. S. Bernhardt, D. D. Dionysiou, J. A. Pedersen and M. R. Wiesner, et al., Environmental Occurrences, Behavior, Fate, and Ecological Effects of Nanomaterials: An Introduction to the Special Series, J. Environ. Qual., 2010, 39(6), 1867–1874 CrossRef CAS PubMed.
- C. D. Rummel, A. Jahnke, E. Gorokhova, D. Kühnel and M. Schmitt-Jansen, Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment, Environ. Sci. Technol. Lett., 2017, 4(7), 258–267 CrossRef CAS.
- J. Saavedra, S. Stoll and V. I. Slaveykova, Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton, Environ. Pollut., 2019, 252, 715–722 CrossRef CAS PubMed.
- E. Besseling, J. T. K. Quik, M. Sun and A. A. Koelmans, Fate of nano- and microplastic in freshwater systems: A modeling study, Environ. Pollut., 2017, 220, 540–548 CrossRef CAS PubMed.
- G. Liu, R. Jiang, J. You, D. C. G. Muir and E. Y. Zeng, Microplastic Impacts on Microalgae Growth: Effects of Size and Humic Acid, Environ. Sci. Technol., 2020, 54(3), 1782–1789 CrossRef CAS PubMed.
- N. Yan, B. Z. Tang and W. X. Wang, Cell Cycle Control of Nanoplastics Internalization in Phytoplankton, ACS Nano, 2021, 15(7), 12237–12248 CrossRef CAS PubMed.
- F. Pérez-Guevara, P. D. Roy, G. Kutralam-Muniasamy and V. C. Shruti, A central role for fecal matter in the transport of microplastics: An updated analysis of new findings and persisting questions, J. Hazard. Mater. Adv., 2021, 4, 100021 CrossRef.
- S. Siehoff, M. Hammers-Wirtz, T. Strauss and H. T. Ratte, Periphyton as alternative food source for the filter-feeding cladoceran Daphnia magna, Freshwater Biol., 2009, 54(1), 15–23 CrossRef.
- J. Gigault, H. El Hadri, B. Nguyen, B. Grassl, L. Rowenczyk and N. Tufenkji, et al., Nanoplastics are neither microplastics nor engineered nanoparticles, Nat. Nanotechnol., 2021, 16(5), 501–507 CrossRef CAS PubMed.
- L. Li, Y. Luo, R. Li, Q. Zhou, W. J. G. M. Peijnenburg and N. Yin, et al., Effective uptake of submicrometre plastics by crop plants via a crack-entry mode, Nat. Sustain., 2020, 3(11), 929–937 CrossRef.
- J. N. Hitchcock, Storm events as key moments of microplastic contamination in aquatic ecosystems, Sci. Total Environ., 2020, 734, 139436 CrossRef CAS PubMed.
- W. Xia, Q. Rao, X. Deng, J. Chen and P. Xie, Rainfall is a significant environmental factor of microplastic pollution in inland waters, Sci. Total Environ., 2020, 732, 139065 CrossRef CAS PubMed.
- X. Yang, Q. He, F. Guo, X. Sun, J. Zhang and M. Chen, et al., Nanoplastics Disturb Nitrogen Removal in Constructed Wetlands: Responses of Microbes and Macrophytes, Environ. Sci. Technol., 2020, 54(21), 14007–14016 CrossRef CAS PubMed.
- ECHA, 2022. Guidance on Information Requirements and Chemical Safety Assessment - Appendix R7–1 for nanomaterials applicable to Chapter R7a Endpoint specific guidance. Version 4.0. December 2022. Available online: https://echa.europa.eu/documents/10162/17224/appendix_r7a_nanomaterials_en.pdf/1bef8a8a-6ffa-406a-88cd-fd800ab163ae?t=1671610722346.
- OECD, 2021a. Guidance document on aquatic and sediment toxicological testing of nanomaterials - Series on Testing and Assessment (OECD GD 317) - ENV/JM/MONO(2020)8, 2021. [Online]. Available: https://one.oecd.org/document/env/jm/mono(2020)8/en/pdf.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00628j |
| ‡ Current affiliation: Biochemistry and Structural Biology, Department of Chemistry, Lund University, Sweden. |
| § Current affiliation: HORIBA Europe Gmbh, Filial Sweden, Gothenburg, Sweden. |
| ¶ Current affiliation: Aquatic Ecology and Water Quality Management (AEW), Wageningen University & Research, Wageningen, Netherlands. |
| This journal is © The Royal Society of Chemistry 2024 |