Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conjugation through Si–O–Si bonds, silsesquioxane (SQ) half cage copolymers, extended examples via SiO0.5/SiO1.5 units: multiple emissive states in violation of Kasha's rule†
Zijing
Zhang
a,
Jose Jonathan Rubio
Arias
a,
Hana
Kaehr
a,
Yujia
Liu
 b,
Ryoga
Murata
b,
Masafumi
Unno
b,
Ryoga
Murata
b,
Masafumi
Unno
 b,
Nuttapon
Yodsin
c,
Pimjai
Pimbaotham
d,
Siriporn
Jungsuttiwong
b,
Nuttapon
Yodsin
c,
Pimjai
Pimbaotham
d,
Siriporn
Jungsuttiwong
 d,
Matt
Rammo
e,
Jung-Moo
Heo
a,
Jinsang
Kim
d,
Matt
Rammo
e,
Jung-Moo
Heo
a,
Jinsang
Kim
 a,
Aleksander
Rebane
f and
Richard M.
Laine
a,
Aleksander
Rebane
f and
Richard M.
Laine
 *a
*a
aDepartment of Mater. Sci. and Engin., Macromolecular Science and Eng. Center University of Michigan, Ann Arbor, 48109-21236, USA. E-mail: talsdad@umich.edu
bDepartment of Chemistry and Chemical Biology, Graduate School of Science and Technology Gunma University, Kiryu 376-8515, Japan
cDepartment of Chemistry, Faculty of Science, Silpakorn University, Nakorn Pathom 73000, Thailand
dDepartment of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand
eNational Institute of Chemical Physics and Biophysics, Tallinn, Estonia
fMontana State University, Bozeman, MT 59717, USA
First published on 30th May 2024
Abstract
We previously reported that phenyl- and vinyl-silsesquioxanes (SQs), [RSiO1.5]8,10,12 (R = Ph or vinyl) functionalized with three or more conjugated moieties show red-shifted absorption- and emission features suggesting 3-D conjugation via a cage centered LUMOs. Corner missing [PhSiO1.5]7(OSiMe3)3 and edge opened, end capped [PhSiO1.5]8(OSiMe2)2 (double decker, DD) analogs also offer red shifted spectra again indicating 3-D conjugation and a cage centered LUMO. Copolymerization of DD [PhSiO1.5]8(OSiMevinyl)2 with multiple R–Ar–Br gives copolymers with emission red-shifts that change with degree of polymerization (DP), exhibit charge transfer to F4TNCQ and terpolymer averaged red-shifts suggesting through chain conjugation even with two (O–Si–O) end caps possibly via a cage centered LUMO. Surprisingly, ladder (LL) SQ, (vinylMeSiO2)[PhSiO1.5]4(O2SiMevinyl) copolymers offer emission red-shifts even greater for analogous copolymers requiring a different explanation. Here we assess the photophysical behavior of copolymers of a more extreme SQ form: the half cage [PhSiO1.5]4(OSiMe2Vinyl)4, Vy4HC SQs. We again see small red-shifted absorptions coupled with significant red-shifted emissions, even with just a half cage, thus further supporting the existence of pπ–dπ and/or σ*–π* conjugation through Si–O–Si bonds and contrary to most traditional views of Si–O–Si linked polymers. These same copolymers donate an electron to F4TCNQ generating the radical anion, F4TCNQ−. as further proof of conjugation. Column chromatographic separation of short from longer chain oligomers reveals a direct correlation between DP and emission λmax red-shifts as another indication of conjugation. Further, one- and two-photon absorption and emission spectroscopy reveals multiple excited fluorescence-emitting states in a violation of Kasha's rule wherein emission occurs only from the lowest excited state. Traditional modeling studies again find HOMO LUMO energy levels residing only on the aromatic co-monomers rather than through Si–O–Si bonds as recently found in related polymers.
Introduction
Silsesquioxane (SQ) cages, oligomers and polymers are well recognized to offer a wide variety of properties including high temperature stability, biocompatibility, hydrophobicity, transparency, and insulating properties. Their presence in commercial applications is widespread and the subject of multiple reviews and one book.1–21 They have been compared to organic decorated silica; however, in multiple papers we demonstrated that T8, T10 and T12 phenyl and vinyl SQs functionalized with three or more conjugated moieties demonstrate photophysical properties best explained by formation of a cage centered LUMO conjugated with these appended moieties.22–32Modeling finds cage centered LUMOs in the T8, T10 and T12 SQs.33,34 Modified cages and polymers made from them also exhibit 3-D conjugation. SQs exposed to intense laser light generate a spherical magnetic field inside the functionalized cages supporting formation of a LUMO.29
Efforts to identify limiting structures wherein cage LUMOs do not form led to syntheses of corner missing, doubly open, then “double decker” (DD) and finally ladder (LL) SQs with [O–Si(Mevinyl)–O] endcaps, Scheme 1a.30,31,35
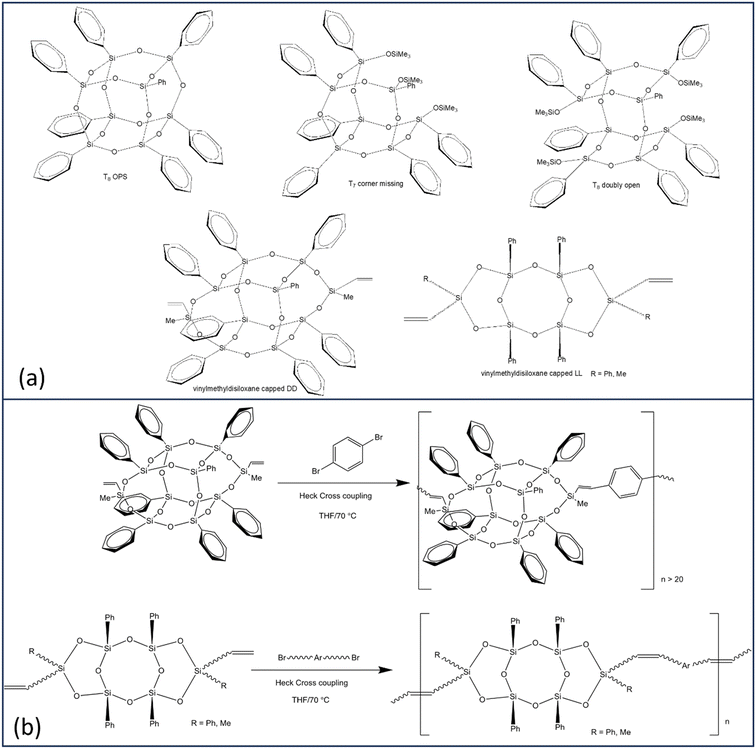 | ||
| Scheme 1 (a) Selected SQ structural formats. (b) Copolymerization of vinylmethylsiloxane capped DD and LL monomers. | ||
Bromination/iodination of all phenyl SQs followed by Heck catalytic cross-coupling, Scheme 1b, gives 4-R-stilbene SQs that also exhibit significantly red-shifted emissions versus model disiloxane compounds with the same substituents.35 While main absorption onsets for almost all compounds show very small red-shifts, these may be accompanied by red-shifted lower-intensity features thus deviating from what is expected based on well-studied conjugated organic polymers.
More recently we copolymerized MeVinylSi(O–)2 end-capped DD and LL systems with multiple aromatics per Scheme 1b31,32 to test conjugation limits using i.e. divinylbenzene tethers building on previous studies wherein phenyl T10 and T12 cage divinylbenzene copolymers exhibited through chain conjugation.24
In particular, we assumed that the absence of a cage in LL SQ copolymers would obviate formation of a cage centered LUMO and therefore conjugation. However both DD and LL SQ copolymers show conjugation even with vinyldisiloxane end caps and for LL systems without a cage.32 In addition to photophysical evidence for conjugation, DD and LL polymers donate electrons to F4TCNQ exhibiting integer electron transfer forming F4TCNQ˙−; also proof of conjugation. Alternating terpolymers also show red shifted emission averages of the two homo copolymers.26,36
Here, we explore further deviations from initial structures searching for limiting siloxane/SQ oligomers/polymers where conjugation is absent. To this end, we synthesized half cages (Vy4HC) capped with pendant OSiMe2Vinyls, Vy4HC, Scheme 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37,38 followed by addition of simple monobromo (model compounds) and then dibromo–aromatics (1
37,38 followed by addition of simple monobromo (model compounds) and then dibromo–aromatics (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-polymerization) via Heck catalytic cross coupling (see experimental) to assess their photophysical properties. We again find conjugation via Si–O–Si bonds based on: (1) comparison with model compounds, (2) red-shifted emission λmaxvs. degree of polymerization (DP), (3) electron transfer to F4TCNQ, and (4) 1PA and 2PA photophysics.
1 co-polymerization) via Heck catalytic cross coupling (see experimental) to assess their photophysical properties. We again find conjugation via Si–O–Si bonds based on: (1) comparison with model compounds, (2) red-shifted emission λmaxvs. degree of polymerization (DP), (3) electron transfer to F4TCNQ, and (4) 1PA and 2PA photophysics.
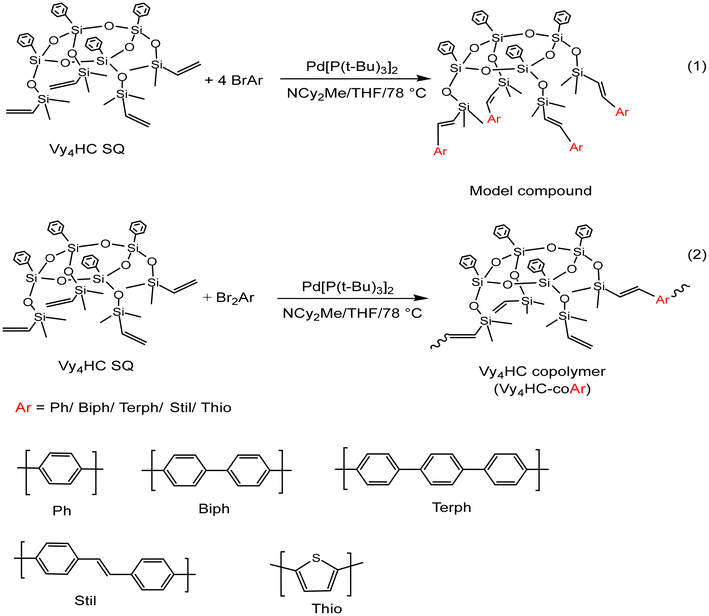 | ||
| Scheme 2 Vinyldimethylsiloxane capped half cage (Vy4HC) model compound reaction (1) and copolymerization reaction (2). Ar: selection of aromatic moieties. | ||
Results and discussion
The objectives of the work reported here are to elaborate on our understanding of “unconventional conjugation” in SQs via efforts to identify copolymer systems with backbones consisting in part of Si–O–Si bonds that show photophysical and electrochemical behavior typically associated with conjugation in traditional conjugated organic polymers.31,39 The approach chosen is to eliminate the cage but retain multiple, pendant Si–O–SiMe2vinyl links, with the idea that making the system look more like a polysiloxane would engender “insulating” properties in copolymers with aromatic and heteroaromatic co-monomers.In the following sections, we first discuss synthesis methods and copolymer characterization, followed by photophysical studies on absorption and emission behavior especially vs. DP. Thereafter, complementary studies are undertaken to assess photophysical behavior vis a vis one photon absorption (1PA) and two photon absorption (2PA) spectroscopy in part demonstrating non-Kasha's rule behavior. Then, electron transfer to F4TCNQ studies is used to assess selected copolymers’ abilities to donate an electron, essentially a form of doping. Finally, we present an approach to theoretically modeling copolymer behavior and give an estimation of the HOMO LUMO levels using a traditional approach. A conclusions section follows.
Synthesis of Vy4HC-copolymers and model compounds
The first step in these studies was to synthesize model compounds by reacting Vy4HC with four equivalents of Br–Ar (phenyl, biphenyl, stilbene, thiophene). Thereafter, copolymers were synthesized using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios Vy4HC
1 molar ratios Vy4HC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br–Ar–Br typical for condensation polymerization studies. Vy4HC copolymer and model compound syntheses and purification often gives liquids. Chromatography via a 5 cm silica gel column allows removal of remaining base and residual catalyst.
Br–Ar–Br typical for condensation polymerization studies. Vy4HC copolymer and model compound syntheses and purification often gives liquids. Chromatography via a 5 cm silica gel column allows removal of remaining base and residual catalyst.
Table 1 presents estimated molar masses, and approximate proportions of fractions of eluted products estimated from deconvoluted GPC profiles (Fig. S1–S4†). Fig. S5† displays stacked multimodal GPC traces for Vy4HC copolymers. The GPC traces suggest chain growth is influenced by co-monomer structure with sulfur containing aromatics reacting more slowly because sulfur binds to the catalyst sufficiently strongly to reduce reactivity as seen previously.26–28 As expected, non-sulfur containing copolymers offer higher average molecular weights (MWs) in accord with our previous reports.30–32 Copolymer structures are confirmed by Fig. S6–8† MALDI data. As an example, Fig. S9† provides before and after chromatography FTIRs proving base removal. Fig. S10† provides an example1H NMR of an Vy4HC-copolymer.30,32,35
| Copolymer | Crude yield (%M) | GPC | Td5% (°C) | Theoretical CY (wt%) | Found CY (wt%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Greatest MW fraction | 2nd MW fraction | 3rd MW fraction | ||||||||
| Mna (kg mol−1) | (%) total area | Mna (kg mol−1) | (%) total area | Mna (kg mol−1) | % total area | |||||
| a Estimated from deconvoluted GPC traces, Fig. S1–S4.† CY = ceramic yield, Fig. S11.† | ||||||||||
| Vy4HC-coThio | 94 | 3.5 | 72 | 2.5 | 5 | 1.7 | 15.7 | 253 | 50 | 33 |
| Vy4HC-coPh | 82 | 8.1 | 1 | 2.6 | 68 | 2.1 | 16.8 | 294 | 50 | 37 |
| Vy4HC-coBiph | 35 | 15.8 | 40 | 6.1 | 5 | 3.5 | 39.9 | 174 | 46 | 34 |
| Vy4HC-coTerph | 56 | 9.9 | 21 | 5 | 22 | 3.5 | 49.9 | 335 | 43 | 35 |
| Vy4HC-coStil | 77 | 9.6 | 4 | 3.9 | 82 | 2.6 | 0.41 | 487 | 45 | 40 |
Fig. S11† presents the corresponding Vy4HC copolymer TGAs. Crucial data are summarized in Table 1. The found ceramic yields for the HC copolymers are quite reduced vs. those calculated as might be expected given that copolymerization is at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Vy4HC
1 Vy4HC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br–Ar–Br ratios. Thus, each HC will have two unreacted vinylMe2SiO groups that likely volatilize easily rather than convert to SiO2 on oxidation. The 5% mass degradation temperature (Td 5%) for Vy4HC-coStil is notably high enough to consider use for solution processing of electrooptical devices.
Br–Ar–Br ratios. Thus, each HC will have two unreacted vinylMe2SiO groups that likely volatilize easily rather than convert to SiO2 on oxidation. The 5% mass degradation temperature (Td 5%) for Vy4HC-coStil is notably high enough to consider use for solution processing of electrooptical devices.
Once the copolymers of both systems and their model compounds were reproducibly synthesized and fully characterized, photophysical characterization studies were undertaken at two levels; one consisting of simply evaluating their one photon absorption and emission behavior using traditional Uv-Vis methods followed by more exacting 1PA and 2PA characterization.
Photophysical characterization of Vy4HC-copolymers and model compounds
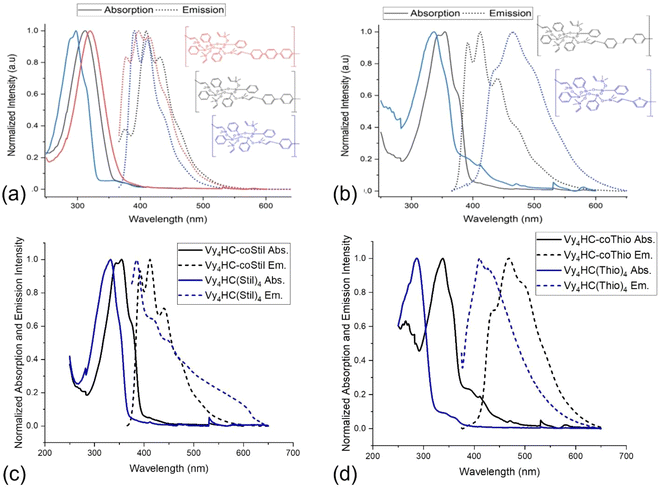
The copolymers isolated from solution all show emission behavior suggesting both conjugation and differences in band gaps that are comonomer dependent, Fig. S12.†Fig. 1a and b present normalized absorption-emission spectra for Vy4HC-copolymers. Their photophysical parameters are summarized in Table 2. All Vy4HC-copolymers offer significant emission red-shifts in λmaxvs. model compounds, the Vy4HC-coStil and Vy4HC-coThio are shown as examples in Fig. 1c and d respectively. Individual absorption and emission data for the Vy4HC-copolymer systems are presented in Fig. S13–S17.† Vy4HC-coPh/Biph/Terph show quantum efficiencies (ΦF) ≥ 70% offering potential for applications in the OLEDs, white light or possibly as hybrid photovoltaic cells.| Absorption, λmax (nm) | Emission, λmax (nm) | E stokes (eV) | Molar Absorptivity, ε (L mol−1 cm−1) | Quantum efficiency, ΦF (%) | τ avg. (ns) | ||
|---|---|---|---|---|---|---|---|
| a Underlined value is λmax; τavg: average excited state lifetime. | |||||||
| Copolymers | Vy4HC-coPh | 289, ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif) ![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif) |
390![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
1.00 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
86 ± 1 | 1.2 |
| Vy4HC-coBiph | 313 | ![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif) , 432 , 432 |
0.93 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
88 ± 1 | 1.3 | |
| Vy4HC-coTerph | 320 | 415 | 0.89 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
75 ± 1 | 1.5 | |
| Vy4HC-coStil | 344, ![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) ![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif) |
392, ![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif) ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) , 440 , 440 |
0.49 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
50 ± 3 | 1.8 | |
| Vy4HC-coThio | 338 | 469 | 1.03 | 5900 | 3.0 ± 0.2 | 1.2 | |
| Model compounds | Vy4HC(Ph)4 | 252 | 390, ![[4 with combining low line]](https://www.rsc.org/images/entities/char_0034_0332.gif) ![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) ![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif) , 431 , 431 |
1.89 | — | 0.08 ± 0.01 | — |
| Vy4HC(Biph)4 | 288 | ![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ![[8 with combining low line]](https://www.rsc.org/images/entities/char_0038_0332.gif) ![[5 with combining low line]](https://www.rsc.org/images/entities/char_0035_0332.gif) , 404 , 404 |
1.09 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
3.0 ± 0.5 | 2.1 | |
| Vy4HC(Stil)4 | 332 | 385 | 0.51 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.2 ± 0.2 | 0.1 | |
| Vy4HC(Thio)4 | 287 | 412 | 1.31 | — | 0.04 ± 0.02 | — | |
Note that in Table 2, the model compounds present lower ΦF, implying oligomerization improves ΦF perhaps by reducing non-radiative decay processes. Compared to the single aromatics, all Vy4HC-copolymers and model compounds show an increasing molar absorption coefficient, which may arise from the presence of Vy4HC enhancing π to π* transitions. The excited state lifetimes (in Fig. S18†) of all Vy4HC-copolymers and model compounds are within the scale of ns which indicates quick recombination happens defining luminescence behavior as fluorescence. The radiative constants of all copolymers (in Table S1†), with the exception of coThio, are enhanced, whereas the non-radiative constants are reduced. This suggests that the copolymer systems are likely effective in suppressing non-radiative processes, thereby enhancing photoluminescence (PL) emission.
Example of the effects of DP on photophysics
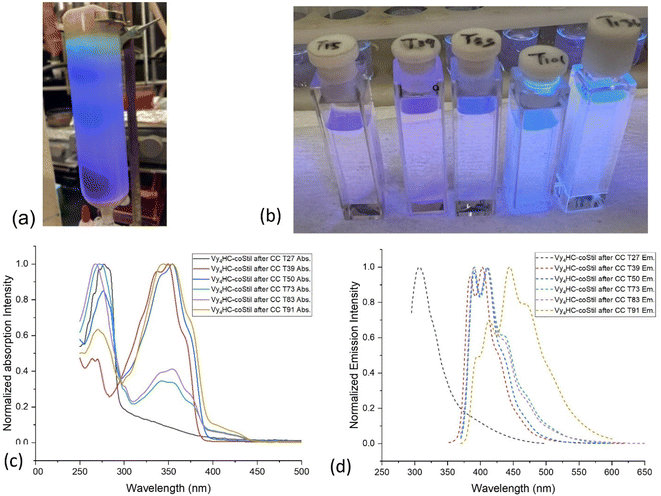
To further explore conjugation in these compounds, column chromatography was used to separate Vy4HC-coStil fractions, to assess DP effects on photophysical properties. Fig. 2a and b provide optical images of the column separation process and the individual samples collected as elution continues. Table 3 provides detailed absorption, emission and MALDI data. Original MALDI figures of selected eluate samples are shown in Fig. S19–S24.† Higher DP materials may also be present but may not ionize efficiently.Samples marked with a larger number contain oligomers with greater DPs. Color changes under 365 nm irradiation vary from purple to blue to green within the column and isolated samples with increasing DP. In isolated samples, absorption λmax exhibit red-shifts from 277 (monomer) to 354 nm (oligomers) in Fig. 2c. Likewise, emission λmax red-shifts from 307 to 444 nm in Fig. 2d. Table 3 summarizes photophysical behavior of selected samples.
From original half cages to oligomers to hexamers, the absorption λmax red shifts from 277 to 354 nm and the emission λmax shifts from 307 to 470 nm. As the structure changes from Vy4HC to Vy4HC-Stil to Vy4HC-Stil-Vy4HC, λmax absorption red shifts from 277 to 349 to 354 nm. These compounds can be viewed as models. At greater DPs the absorption λmax centers at 344–354 nm.
Comparing HC emission spectra with associated MALDI, the new emission peaks appearing at 409, 444, 470 nm likely result from domination by dimers, then trimers and thereafter tetramers. These results offer additional proof of DP dependent extended conjugation, a typical result for conjugated polymers. A series of relatively weak absorption peaks located at 260–280 nm likely derive from vibronic progression of the attached phenyl groups.
Complementary to the more traditional UV-vis absorption and emission studies above, it was also important to explore photophysics in more detail given the unexpected behavior of these new copolymers, hence the following efforts.
2D excitation–emission spectral maps
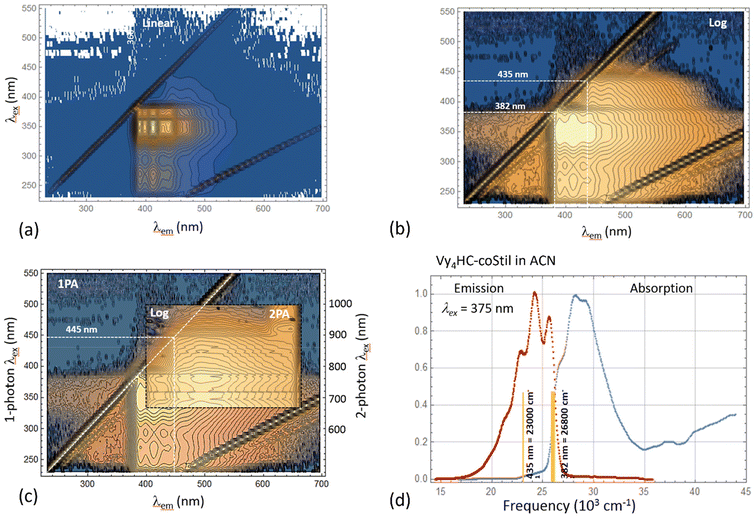
Kasha's rule states that on photoexcitation, molecules/polymers in the excited state can be expected to relax to the lowest energy excited state before relaxing to the ground state with coincident emission of a lower energy photon.40,41 A majority of the polymers synthesized here seem to violate this rule as exemplified by the following.Vy4HC-coStil was chosen as an example to explore its photophysics in further detail by measuring 2D excitation–emission spectral maps for both one-photon excited fluorescence (1PEF) and two-photon excited fluorescence (2PEF).
Fig. 3a and b present the one-photon 2D spectral map for Vy4HC-coStil in ACN spanning the excitation wavelengths, λex = 200–550 nm (vertical axis), and the emission wavelengths, λem = 230–700 nm (horizontal axis). The diagonal lines are spectrometer artifacts corresponding to the 1st and 2nd diffraction order Rayleigh scattering. Weaker lines are Raman scattering from the solvent. When both the excitation and emission wavelengths approach, λem ∼ λex, ∼382 nm, a distinct features called “origin” can be observed corresponding to the excited energy level that emits fluorescence with only a minimal amount of Stokes shift. Normally, i.e. when Kasha's rule is fulfilled, each type of fluorophore shows only one such origin. However, when the same data is presented as a logarithmic intensity scale, Fig. 3b, then a second weaker origin at 435 nm is also observed. This feature appears to correspond in the linear absorption spectrum of Vy4HC-coStil (Fig. 1d) to a relative weak but distinct red-shifted shoulder at λ1PA = 400–450 nm. Multiple origins were observed in several systems studied here which indicates the presence of systematic non-Kasha behavior in Vy4HC-copolymers. It should also be noted that similar excitation- and emission features have been observed previously, even though in variable degrees, in other related polymers, as well as in some model compounds.31
Complementing (linear) one-photon spectroscopy with nonlinear two-photon absorption spectroscopy is often advantageous because juxtaposition of the two alternative pathways between the ground- and excited state allows better revealing potential underlying symmetries.42Fig. 3c (insert) shows the 2-photon excited 2D emission intensity map of Vy4HC-coStil dissolved in DMSO. The 2-photon excitation wavelengths cover the range, λ2PA = 680–1000 nm, and are shown on the right vertical axis using the corresponding 1-photon transition wavelength scale, λex = 340–500 nm. The corresponding emission wavelength is shown on the horizontal axis, λem = 400–660 nm. To better compare the 2PEF and 1PEF spectra, the 2-photon measurement is superimposed over the underlying 1PEF 2D spectra map of Vy4HC-coStil in DMSO but spanning a broader range of wavelengths. The 2-photon excitation map reveals qualitatively similar features as the corresponding 1-photon case; however, the lower-energy origin at ≈445 nm displays a relatively even higher intensity which we may attribute to ∼10 nm red-shifted 1PA version of the 435 nm origin. It could serve to indicate a lower-energy electronic transition accompanied by an increasingly larger change of the permanent electric dipole moment.43 Even though the exact nature of associated excited electronic states is currently under discussion, it is remarkable that the 2- photon excitation produces a picture that is, in many respects, similar to that observed in the 1-photon excitation. In particular, the appearance of an origin in the 2-photon spectral map indicates that the excited species most likely lack center(s) of inversion because otherwise the emission would emanate from a different excited state.
Fig. 3d summarizes the most prominent features of origins observed in the absorption- and emission spectra of Vy4HC-coStil (right) in ACN. To facilitate better comparison of the corresponding transition energies, the spectra are plotted using the cm−1 frequency scale. In this case, the data shows a higher-energy origin at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 cm−1, which could be associated with chromophore-centered vibronic transitions. In addition, there exist origins at 23
800 cm−1, which could be associated with chromophore-centered vibronic transitions. In addition, there exist origins at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 pointing at lower-energy excited state (or states). The fact that these low-lying and often only weakly absorbing features appear to be responsible for highly efficient emission e.g. ΦF = 88% in Vy4HC-coBiph, indicate that role of any potential highly fluorescent aggregates or impurities in this unusual behavior is most likely insignificant.
000 cm−1 pointing at lower-energy excited state (or states). The fact that these low-lying and often only weakly absorbing features appear to be responsible for highly efficient emission e.g. ΦF = 88% in Vy4HC-coBiph, indicate that role of any potential highly fluorescent aggregates or impurities in this unusual behavior is most likely insignificant.
Charge transfer (CT)
Given the unusual nature of the compounds developed in these studies, further proofs are needed to give credence to the proposed existence of conjugation via Si–O–Si bonds as a stepping-stone towards the modeling studies below. Hence our efforts to demonstrate CT behavior.Optoelectronic devices require CT between an electron donor and an acceptor. CT would be in response to absorption of a photon and subsequent electron excitation (photovoltaics) or to an applied potential and subsequent photon emission (OLEDs). To find practical applications for developed materials as reported here, demonstrating CT is of utmost importance. CT can be proven by mixing materials with a strong electron donor, e.g. F4TCNQ. DCM solutions of the co-polymer and F4TCNQ mixed at ambient (0.5 h) lead to visible CT changes (Fig. S25†).
As demonstrated previously, CT can be observed via FTIR, UV-vis absorption spectra and to some extent, it is reflected in a change of color to green even brown (color of anionic F4TCNQ).32 FTIR spectra for Vy4HC copolymers doped with F4TCNQ are shown in Fig. S26.†
On doping with 50mol% F4TCNQ, for Vy4HC-coPh and Vy4HC-coBiph, νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band shifts from 2227 to 2194 cm−1 are observed for the strongest νC
N band shifts from 2227 to 2194 cm−1 are observed for the strongest νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bands of anionic F4TCNQ, indicating integer charge transfer, error in peak value is likely attributable to peak width. Integer charge transfer allows estimating copolymer HOMOs if it is higher than the LUMO of F4TCNQ at −5.3 eV. Vy4HCcoTerph, Vy4HC-coStil and Vy4HC-coThio show both partial and integer CT, the degree of partial CT is presented in Table 4 below. Eqn (1) permits calculation of CT degree.
N bands of anionic F4TCNQ, indicating integer charge transfer, error in peak value is likely attributable to peak width. Integer charge transfer allows estimating copolymer HOMOs if it is higher than the LUMO of F4TCNQ at −5.3 eV. Vy4HCcoTerph, Vy4HC-coStil and Vy4HC-coThio show both partial and integer CT, the degree of partial CT is presented in Table 4 below. Eqn (1) permits calculation of CT degree.
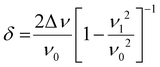 | (1) |
| F4TCNQ Mol % | Cyano- ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
CT degree (δ) | |
|---|---|---|---|
| Vy4HC-coPh | 50 | 2192 | ∼1 |
| Vy4HC-coBiph | 50 | 2196 | ∼1 |
| Vy4HC-coTerph | 50 | 2221, 2195 | 0.18, ∼1 |
| Vy4HC-coStil | 50 | 2222, 2210, 2196 | 015, 0.52, ∼1 |
| Vy4HC-coThio | 50 | 2222, 2189 | 0.15, ∼1 |
| F4TCNQ | 2227 | 0 |
υ
1: νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band value of anionic F4TCNQ, 2194 cm−1.
N band value of anionic F4TCNQ, 2194 cm−1.
υ
0: νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band value of neutral F4TCNQ, 2227 cm−1.
N band value of neutral F4TCNQ, 2227 cm−1.
Δυ: νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N band shift value.
N band shift value.
Absorption spectra for F4TCNQ + Vy4HC-copolymers are shown in Fig. S27.† The absorption peaks at 760 and 860 nm for anionic F4TCNQ correspond to the D0 → D1 transition and are seen for all copolymers with different intensities which indicates varying doping efficiencies.
Modeling studies
As we discovered previously, traditional modeling approaches cannot explain the photophysical results we find with these molecules. Thus, the optimized structures in Fig. S28† represent a starting point for modeling studies using Gaussian 16 methods.As with our previous publications,32,35 examples of calculated HOMO and LUMO for Vy4HC-copolymers systems and corresponding absorption spectra presented in Fig. S29–33† indicate localization on aromatic components belying the above evidence of extended conjugation. This extends to calculated emission behavior. Furthermore, we observe that the trends exhibited by the adsorption spectra align with those observed in experimental studies, as tabulated in Table 5. We are still working to establish more encompassing modeling approaches that are ongoing.
| Molecules | Abs.exp, λmax (nm) | Abs.calc, λmax (nm) | Transition | HOMO, LUMO, Egap |
|---|---|---|---|---|
| Vy4HC-coStil | 354 | 323.09 | HOMO–>LUMO (96%) | −6.64, −0.58, 6.05 |
| Vy4HC-coThio | 335 | 273.32 | HOMO–>LUMO (96%) | −7.20, −0.11, 7.09 |
| Vy4HC-coTerph | 320 | 289.22 | HOMO–>LUMO (91%) | −6.90, −0.29, 6.61 |
| Vy4HC-coBiph | 312 | 275.06 | HOMO–>LUMO (95%) | −7.07, −0.16, 6.91 |
| Vy4HC-coPh | 297 | 247.86 | HOMO–>LUMO (88%) | −7.43, 0.13, 7.56 |
In a recently published paper, using a different modeling approach, we were able to determine the existence of dπ–pπ and/or σ*–π* enables conjugation via Si–O–Si bonds.44
Conclusions
Above, we continue to attempt to define the extent of and source of unexpected conjugation in siloxane and SQ compounds linked by vinylMe2Si–O–SiMe2vinyl tethers exploring unique configurations attempting to identify limiting structures; again, finding conjugation. In the examples presented here, we identify conjugation both by red-shifts in emission in 1PA and 2PA studies, charge transfer from polymeric species to the electron acceptor F4TCNQ, correlation of degrees of polymerization (DPs) with extent of red-shift. We also find unexpected failures of Kasha's rule in that many of the compounds studied exhibit multiple emitting states, an uncommon feature of conjugated polymers. These studies have provided the basis for next generation studies on simple polysiloxane copolymers of the type shown in Scheme 3. | ||
| Scheme 3 Polysiloxane copolymers also show conjugation likely dπ–pπ and/or σ*–π*.45 | ||
Author contributions
R.M. Laine conceived of experiments, directed synthetic efforts and co-wrote the manuscript. Z.Z., J.J.R.A. and H.K. conducted experiments at UM. M.U. and Y.L. conceived of experiments, directed synthesis efforts and co-wrote the manuscript and R.M. conducted syntheses done at Gunma University. S.J. conceived of experiments, directed modeling efforts and N.Y. and P.P. conducted the modeling experiments at Ubon Ratachani and Silpakorn Universities; A.R. conceived of and directed experiments and co-wrote the manuscript, M.R. conducted experiments at Montana State and at National Institute of Chemical Physics and Biophysics. J.H. and J.K. conducted the excited state lifetime measurement at UM.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Laine and Rebane groups gratefully thank NSF Chemistry for a collaborative research award no. 1610344. Support from the Estonian National Science Foundation grant PRG661 is acknowledged (Ramo and Rebane). The Unno/Liu group is grateful for support from NEDO project (JPNP06046). Professor Jungsuttiwong thanks NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [B16F640099] for funding work done by her team.References
- M. G. Voronkov and V. I. Lavrent'yev, in Inorganic Ring Systems, Springer Berlin Heidelberg, Berlin, Heidelberg, 1982, vol. 102, pp. 199–236 Search PubMed.
- J. J. Schwab, J. D. Lichtenhan, K. P. Chaffee, P. T. Mather and A. Romo-Uribe, MRS Proc., 1998, 519, 21 CrossRef CAS.
- R. H. Baney, M. Itoh, A. Sakakibara and T. Suzuki, Chem. Rev., 1995, 95, 1409–1430 CrossRef CAS.
- G. Calzaferri, in Tailor-made Silicon-Oxygen Compounds, Friedr. Vieweg & SohnmbH, 1996, pp. 149–169 Search PubMed.
- J. Lichtenhan, in Polymeric Materials Encyc, CRC Press, NY, 1996, vol. 10, pp. 7768–7777 Search PubMed.
- A. Provatas and J. G. Matisons, in Trends Polym. Sci, 1997, vol. 5, pp. 327–322 Search PubMed.
- G. Li, L. Wang, H. Ni and C. U. Pittman Jr., J. Inorg. Organomet. Polym., 2001, 11, 123–154 CrossRef CAS.
- R. Duchateau, Chem. Rev., 2002, 102, 3525–3542 CrossRef CAS PubMed.
- Y. Abe and T. Gunji, Prog. Polym. Sci., 2004, 29, 149–182 CrossRef CAS.
- S. H. Phillips, T. S. Haddad and S. J. Tomczak, Curr. Opin. Solid State Mater. Sci., 2004, 8, 21–29 CrossRef CAS.
- R. Y. Kannan, H. J. Salacinski, P. E. Butler and A. M. Seifalian, Acc. Chem. Res., 2005, 38, 879–884 CrossRef CAS PubMed.
- R. M. Laine, J. Mater. Chem., 2005, 15, 3725 RSC.
- P. D. Lickiss and F. Rataboul, in Advances in Organometallic Chemistry, Elsevier, 2008, vol. 57, pp. 1–116 Search PubMed.
- K. L. Chan, P. Sonar and A. Sellinger, J. Mater. Chem., 2009, 19, 9103 RSC.
- J. Wu and P. T. Mather, Polym. Rev., 2009, 49, 25–63 CrossRef CAS.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- R. M. Laine and M. F. Roll, Macromolecules, 2011, 44, 1073–1109 CrossRef CAS.
- Applications of polyhedral oligomeric silsesquioxanes, ed. C. Hartmann-Thompson, Springer, Dordrecht, 2011 Search PubMed.
- J. Guan, Z. Zhang and R. M. Laine, Macromolecules, 2022, 55, 5403–5411 CrossRef CAS.
- Y. Du and H. Liu, ChemCatChem, 2021, 13, 5178–5190 CrossRef CAS.
- M. Soldatov and H. Liu, Prog. Polym. Sci., 2021, 119, 101419 CrossRef CAS.
- S. Sulaiman, J. Zhang, T. Goodson III and R. M. Laine, J. Mater. Chem., 2011, 21, 11177 RSC.
- S. Sulaiman, A. Bhaskar, J. Zhang, R. Guda, T. Goodson and R. M. Laine, Chem. Mater., 2008, 20, 5563–5573 CrossRef CAS.
- M. Z. Asuncion and R. M. Laine, J. Am. Chem. Soc., 2010, 132, 3723–3736 CrossRef CAS PubMed.
- J. H. Jung and R. M. Laine, Macromolecules, 2011, 44, 7263–7272 CrossRef CAS.
- J. H. Jung, J. C. Furgal, S. Clark, M. Schwartz, K. Chou and R. M. Laine, Macromolecules, 2013, 46, 7580–7590 CrossRef CAS.
- J. C. Furgal, J. H. Jung, S. Clark, T. Goodson and R. M. Laine, Macromolecules, 2013, 46, 7591–7604 CrossRef CAS.
- J. C. Furgal, J. H. Jung, T. Goodson and R. M. Laine, J. Am. Chem. Soc., 2013, 135, 12259–12269 CrossRef CAS PubMed.
- J. Guan, K. Tomobe, I. Madu, T. Goodson, K. Makhal, M. T. Trinh, S. C. Rand, N. Yodsin, S. Jungsuttiwong and R. M. Laine, Macromolecules, 2019, 52, 4008–4019 CrossRef CAS.
- J. Guan, K. Tomobe, I. Madu, T. Goodson, K. Makhal, M. T. Trinh, S. C. Rand, N. Yodsin, S. Jungsuttiwong and R. M. Laine, Macromolecules, 2019, 52, 7413–7422 CrossRef CAS.
- J. Guan, J. J. R. Arias, K. Tomobe, R. Ansari, M. de F. V. Marques, A. Rebane, S. Mahbub, J. C. Furgal, N. Yodsin, S. Jungsuttiwong, D. Hashemi, J. Kieffer and R. M. Laine, ACS Appl. Polym. Mater., 2020, 2(9), 3894–3907 CrossRef CAS.
- J. Guan, Z. Sun, R. Ansari, Y. Liu, A. Endo, M. Unno, A. Ouali, S. Mahbub, J. C. Furgal, N. Yodsin, S. Jungsuttiwong, D. Hashemi, J. Kieffer and R. M. Laine, Angew. Chem., Int. Ed., 2021, 60, 11115–11119 CrossRef CAS PubMed.
- R. M. Laine, S. Sulaiman, C. Brick, M. Roll, R. Tamaki, M. Z. Asuncion, M. Neurock, J.-S. Filhol, C.-Y. Lee, J. Zhang, T. Goodson, M. Ronchi, M. Pizzotti, S. C. Rand and Y. Li, J. Am. Chem. Soc., 2010, 132, 3708–3722 CrossRef CAS PubMed.
- M. Bahrami, H. Hashemi, X. Ma, J. Kieffer and R. M. Laine, Phys. Chem. Chem. Phys., 2014, 16, 25760–25764 RSC.
- J. Guan, K. Tomobe, I. Madu, T. Goodson, K. Makhal, M. T. Trinh, S. C. Rand, N. Yodsin, S. Jungsuttiwong and R. M. Laine, Macromolecules, 2019, 52, 4008–4019 CrossRef CAS.
- Z. Zhang, J. Guan, R. Ansari, J. Kieffer, N. Yodsin, S. Jungsuttiwong and R. M. Laine, Macromolecules, 2022, 55(18), 8106–8116 CrossRef CAS.
- Y. Liu, N. Takeda, A. Ouali and M. Unno, Inorg. Chem., 2019, 58, 4093–4098 CrossRef CAS PubMed.
- H. Endo, N. Takeda and M. Unno, Organometallics, 2014, 33, 4148–4151 CrossRef CAS.
- R. M. Laine, Chem. Commun., 2022, 58, 10596–10618 RSC.
- J. C. Del Valle and J. Catalán, Phys. Chem. Chem. Phys., 2019, 21, 10061–10069 RSC.
- A. P. Demchenko, V. I. Tomin and P.-T. Chou, Chem. Rev., 2017, 117, 13353–13381 CrossRef CAS PubMed.
- C. W. Stark, M. Rammo, A. Trummal, M. Uudsemaa, J. Pahapill, M. Sildoja, S. Tshepelevitsh, I. Leito, D. C. Young, B. Szymański, O. Vakuliuk, D. T. Gryko and A. Rebane, Angew. Chem., Int. Ed., 2022, 61, e202212581 CrossRef CAS PubMed.
- A. Rebane, N. S. Makarov, M. Drobizhev, B. Spangler, E. S. Tarter, B. D. Reeves, C. W. Spangler, F. Meng and Z. Suo, J. Phys. Chem. C, 2008, 112, 7997–8004 CrossRef CAS.
- J. J. R. Arias, Z. Zhang, M. Takahashi, P. Mahalingam, P. Pimbaotham, N. Yodsin, M. Unno, Y. Liu, S. Jungsuttiwong, J. Azoulay, M. Rammo, A. Rebane and R. M. Laine, Polym. J., 2024, 56, 577–588 CrossRef.
- Z. Zhang, H. Kaehr and R. M. Laine, 2023, unpublished work.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt00567h |
| This journal is © The Royal Society of Chemistry 2024 |

![[D with combining low line]](https://www.rsc.org/images/entities/char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/char_006d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) , trimers
, trimers![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif)