Open Access Article
Open Access ArticlePhoton-gated foldaxane assembly/disassembly†
Chenhao Yao‡
a,
Bappaditya Gole‡§
 a,
Anh Thy Bui
a,
Anh Thy Bui b,
Brice Kauffmann
b,
Brice Kauffmann c,
Ivan Huc
c,
Ivan Huc d,
Nathan D. McClenaghan
d,
Nathan D. McClenaghan *b and
Yann Ferrand
*b and
Yann Ferrand *a
*a
aUniv. Bordeaux, CNRS, Bordeaux INP, CBMN (UMR 5248), 2 rue Escarpit, 33600 Pessac, France. E-mail: yann.ferrand@u-bordeaux.fr
bUniv. Bordeaux, CNRS, Institut des Sciences Moléculaires (UMR5255), 351 cours de la Libération, 33405 Talence cedex, France. E-mail: nathan.mcclenaghan@u-bordeaux.fr
cUniv. Bordeaux, CNRS, INSERM, Institut Européen de Chimie Biologie (UAR3033/US001), 2 rue Escarpit, 33600 Pessac, France
dDepartment of Pharmacy Ludwig-Maximilians-Universität München Butenandtstr. 5–13, 81377 Munich, Germany
First published on 19th July 2024
Abstract
Integrating multiple anthracene motifs into aromatic oligoamide sequences gives rise to photoactive foldamers that can sequester a molecular thread forming helix-on-axle assemblies. Photoirradiation is shown to distort the helical host and drive dissociation of the supramolecular assembly and thread liberation as signalled by a photonic output, while thermal reversion regenerates the assembly.
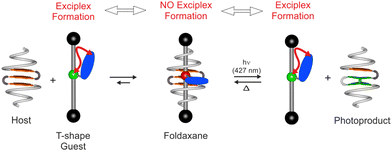
Aromatic oligoamide foldamers have emerged as a versatile class of functional molecules, which adopt robust folded conformations whose properties can be finely tuned based on the chosen sequence of monomers.1 Judicious design of such foldamers has been shown to offer cavities for strong and selective binding of a range of guest molecules2 and, more recently, photoactivity has been introduced both in terms of emission and photoreaction.3 Foldaxanes,4,5 supramolecular architectures comprising aromatic helices on a linear molecular axle, represent a recent addition to the field of interpenetrating molecules.6 Reported examples focus on their formation evoking specific complementary hydrogen-bonding motifs and on-axle helix translation dynamics7 and assembly rates.7,8 Herein, we consider a supramolecular two-component system, where light can be used to both control the assembly and act as a signalling mechanism of association (or dissociation) through a fluorescence output. This functioning is reminiscent of a 2-stroke molecular piston,9 based on a unique foldaxane architecture, combining both light- and thermally-driven steps.
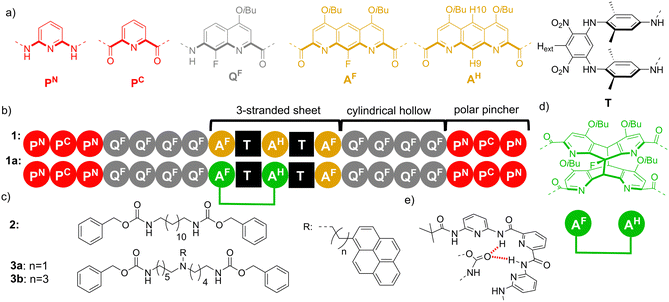
In the current design, as shown schematically in Fig. 1, an unbound T-shaped guest is anticipated to give a specific optical response, a characteristic exciplex emission resulting from an intramolecular interaction between an amine (green) and a fluorophore (blue). Binding and interpenetration within a helix-sheet-helix foldamer3,10 would result in the guest adopting a more extended conformation, spatially decoupling amine and fluorophore and modulating the observed emission. While a light output would signal the state of the system, a light input could also drive guest photoexpulsion based on steric demands mediated by a reversible [4π+4π] photocycloaddition reaction. The structural formulas of molecules developed and studied herein (Fig. 2), include a symmetrical host sequence 1, integrating diazaanthracene units in a bent three stranded sheet AF–T–AH–T–AF, flanked with two helical segments comprising a tetrameric amide sequence of 7-amino-8-fluoro-2-quinolinecarboxylic acid (QF4) providing a cylindrical cavity which is large enough to accommodate a linear alkyl chain. Terminal polar pinchers (P3) comprising pyridine dicarboxamides are capable of binding to each carbonyl of the guest. Sequence 1a corresponds to a photoproduct implying a photocycloisomerisation of two parallel anthracene-like moieties, while 2 is a symmetrical dye-free thread.
Helix-sheet-helix 1 was obtained via a convergent synthetic strategy, as detailed in the ESI† (Schemes S1–S3). Briefly, the conical P3QF4 segment was first prepared and transiently functionalised with a labile dimethoxybenzyl (DMB) group able to prevent the aggregation of the aromatic strands during the synthesis.11 The amino group of the helical segment was connected at each end of the AF–T–AH–T–AF bent sheet diacid and after purification the two DMB groups were removed under acidic conditions.
Evidence for host–guest association in solution was provided by 1H NMR spectroscopy in CD2Cl2 (Fig. 3a–d). Mixing 1 with a linear 2 or T-shaped 3a thread led to significant deshielding of N–H proton resonances, indicative of hydrogen bonding between the carbamate groups of the thread and the amide groups of the host located in the terminal P3 polar pinchers, as represented in Fig. 2e. The NMR spectrum of complex 1⊃3 was globally similar to that of aforementioned 1⊃2, but given the non-symmetrical nature of the thread and hence non-equivalence of these functional groups, the number of resonances was doubled. Equally, shifting of aromatic proton signals associated with the foldamer was observed upon binding. In particular, shifts of CH aromatic protons from both the T turn unit (Hext) and protons on the central ring of the AH unit (H9 and H10) were diagnostic of strong interaction and modification of the internal cavity environment.
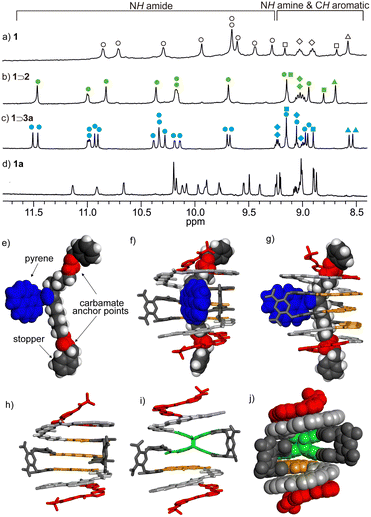 | ||
| Fig. 3 Part of the 1H NMR spectra (CD2Cl2, 400 MHz) of: (a) 0.5 mM 1 in absence of guest; (b) 0.5 mM 1 in the presence of 2 (5 equiv.); (c) 0.5 mM 1 in the presence of 3a (5 equiv.); (d) 1a. NH amide protons, NH amine protons, CH aromatic protons from AH unit (H9 and H10) and Hext are marked as circles (O), diamonds (◊), squares (□) and triangles (Δ), respectively. Signals of the free symmetrical host, symmetrical foldaxane 1⊃2 and unsymmetrical foldaxane 1⊃3a are marked and filled with empty, green, and blue circles, respectively. Structures in the solid state analysed by X-ray crystallography of: (e) T-shape guest in the crystal packing; and (f) front view and (g) side view of foldaxane 1⊃3a. The guest is shown as a CPK representation and the host is shown as a stick representation. (h) Host 1 as it exists in the structure of 1⊃3a. Front view of the energy-minimised molecular models (using Merck molecular force field static, MMFFs) of 1a in stick representation (i) and CPK representation (j). The carbamate and pyrene in the T-shape guest are marked in red and blue, respectively. The monomers in the host are colour coded as in Fig. 2. Side chains (OiBu groups) of host and included solvent molecules have been removed for clarity. | ||
Titrations of host 1 with guest 2 in CD2Cl2 gave an affinity constant (Ka) of 6.47 × 104 L mol−1 and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, while a slightly lower value of 2.63 × 104 L mol−1 was obtained for 1⊃3a (Fig. S1–S3, ESI†). A single crystal X-ray analysis of 1⊃3a (Fig. 3e–g and Fig. S4, ESI†) gave conclusive evidence of the interpenetrating nature of the foldaxane assembly and indications on the key binding features. Short C
1 stoichiometry, while a slightly lower value of 2.63 × 104 L mol−1 was obtained for 1⊃3a (Fig. S1–S3, ESI†). A single crystal X-ray analysis of 1⊃3a (Fig. 3e–g and Fig. S4, ESI†) gave conclusive evidence of the interpenetrating nature of the foldaxane assembly and indications on the key binding features. Short C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N contact distances of 2.13 Å and 2.06 Å designate the carbamate interaction with the polar pinchers, while the pendant pyrene sub-unit occupied the void in the helical backbone. Additionally, in 1⊃3a the π-system of the appended pyrene adopted a face-to-face orientation with a proximal electron-poor dinitroaromatic of one of the turn units T, which is consistent with the shielding of the Hext proton resonance (Fig. 3c vs. Fig. 3b, triangles).
O⋯H–N contact distances of 2.13 Å and 2.06 Å designate the carbamate interaction with the polar pinchers, while the pendant pyrene sub-unit occupied the void in the helical backbone. Additionally, in 1⊃3a the π-system of the appended pyrene adopted a face-to-face orientation with a proximal electron-poor dinitroaromatic of one of the turn units T, which is consistent with the shielding of the Hext proton resonance (Fig. 3c vs. Fig. 3b, triangles).
Concerning the optical properties of 3a and 3b, the parent 1-alkylpyrene chromophore typically gives intense structured absorption and fluorescence in the near UV spectral region.12 However, incorporating such a fluorophore into the thread via an alkyl tether in close proximity to a tertiary amine group may be anticipated to modify the observed excited-state properties. Indeed, thermodynamically and kinetically-favourable photoinduced electron transfer may occur from amines to excited fused aromatics across simple methylene spacers, as popularised by de Silva and coworkers,13 whereas when longer linkers are incorporated different responses may be observed. Notably, De Schryver and coworkers showed that an N-pyrene excited-state complex/exciplex was observed in specific cases in solvents of low-to-medium polarity, and optimal with 2 or 3 methylene group spacers.14 Disruption of the exciplex on foldaxane formation, as well as modulation of electronic energy transfer, was targeted as the optical signalling mechanism in the current work and as such, 3a and 3b integrating a 2 and 4-methylene spacer, respectively, between amine and pyrene were synthesised. In homogeneous and dilute chloroform (or dichloromethane) solution, structured fluorescence as well as a dominant broad lower energy emission band was observed in the case of 3a in toluene and dichloromethane solvents (ϕF = 0.26) but is greatly diminished in intensity in highly polar DMSO (Fig. S5, ESI†). Further, varying concentration had minimal effect on the relative intensities of each emission band in 3a consistent with the emission being intramolecular in origin, ruling out excimer formation at these concentrations. In contrast, in the case of 3b (ϕF = 0.05) at similar (sub-mM) concentration, non-structured emission was minimal (Fig. S6, ESI†), while at concentrations higher than 1 mM the excimer emission became increasingly intense.
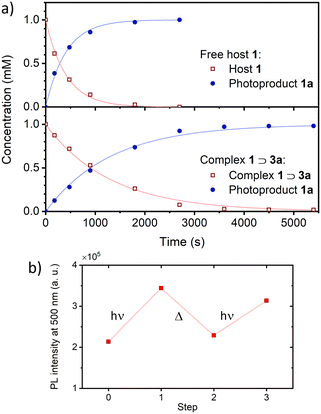
Foldamer host 1 fluorescence was typically broad, short-lived and very weak (ϕF = ca. 0.001, Fig. S7 and S8, ESI†), anthracene quenching being attributed to downhill electronic energy transfer. Diazaanthracence (AF–AH–AF) sub-units were integrated in the host capsule in a parallel orientation at distances (<4 Å) compatible with a AF–AH cycloisomerisation reaction, as shown in the single crystal X-ray structure (Fig. 3h). Diazaanthracene units have a high propensity to undergo homo- and hetero-photocyclisation reactions, often exhibiting higher reaction quantum yields and resistance to secondary photoreactions than parent anthracene.3,15 Photoirradiation of 1 in argon-bubbled halogenated solvents cleanly gave 1a as denoted by shifting bridgehead proton resonances accompanying the C sp2-to-sp3 conversion, notably the appearance of 1H resonances between 5–5.5 ppm further exhibiting coupling with the fluorine, while the 19F resonance at 160 ppm is characteristic of a photocyclised AF–AH pair of anthracenes (see Fig. S11 and S15, ESI†).3b The model of the photoproduct was obtained via molecular mechanics (Fig. 3i), revealing the formation of 1a and 2 C–C bonds between the central anthracene AF and one of the AH central cycles. With photoproduct 1a in hand, binding studies using either 1 or 1a with threads were performed (Fig. S9, ESI†). While titrating guest 3a with helix 1 in CH2Cl2 led to a marked decrease of the exciplex emission band ascribed to complexation (Fig. 4a; ϕF dropping from 0.26 to 0.06), fluorescence emission showed virtually no capsule-induced quenching when adding 1a (Fig. 4b), consistent with the absence of guest binding, further corroborated by NMR (Fig. S11–S13, ESI†). Consequently, photoswitching the foldaxane assembly would result in a lessened guest affinity and light-driven guest release. 1H NMR spectroscopy showed that in 1⊃3a, the cycloisomerised photoproduct was formed more slowly upon irradiation than in the free host 1 (Fig. 5a), as revealed by quantum yields obtained from photoconversion kinetics fits (ϕPC = 8.3 × 10−4 and 2.8 × 10−4, for 1 and 1⊃3a respectively; Fig. S10, ESI†). The thermal reversibility of the reaction was further demonstrated, with the shifted proton resonances being restored to their original position on heating (Fig. S11 and S12, ESI†). Tests of reversible foldaxane assembly and disassembly cycles were carried out, and two full ejection-uptake cycles were successfully performed and monitored by NMR (Fig. S13, ESI†) and fluorescence switching (Fig. 5b), albeit with a small amount of unidentified secondary photoproduct.
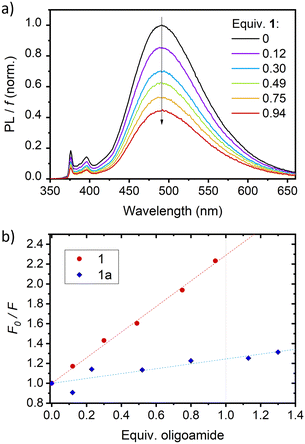 | ||
| Fig. 4 Quenching effect of host 1 on the fluorescence of a 0.5 mM solution of 3a in CH2Cl2 (λexc = 335 nm) in the presence of Et3N (1.5 mM). (a) Fluorescence emission spectra of 3a with increasing number of equivalents of 1 at constant volume, corrected by f, the amount of light absorbed by 3a (see ESI†). (b) Quenching plots obtained upon addition of 1 or 1a to 3a. F is the integral of the corrected fluorescence, and F0 is the value measured in the absence of foldamer (lines are guides for the eye). | ||
In conclusion, a triple decker anthracene-containing foldamer capsule was designed and synthesised, which acted as a host for a T-shaped guest molecule. Photoirradiation of the host resulted in [4π+4π] photocyclisation within the capsule backbone, lowering guest affinity and provoking its photoexpulsion. Thermal reversion of the supramolecular assembly in successive cycles was demonstrated, making this a unique example of a photo- and thermo-reversible foldaxane assembly. Further work is in progress to develop photoactivated foldaxane-based molecular machines.
Financial support from the China Scholarship Council, for a predoctoral fellowship (C. Y.), the French National Research Agency (grant ANR-18-CE6-0018, FORESEE) and the France-Germany International Research Project “Foldamers Structures and Functions” (IRP FoldSFun) are gratefully acknowledged. The work benefited from the facilities and expertise of the Biophysical and Structural Chemistry platform at IECB, CNRS UMS3033, INSERM US001, Univ. Bordeaux.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for single crystals of 1⊃3a, which were obtained via liquid–liquid diffusion, has been deposited at the CCDC under 2162063 (CIF in ESI†).Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D.-W. Zhang, X. Zhao, J.-L. Hou and Z.-T. Li, Chem. Rev., 2012, 112, 5271 CrossRef CAS PubMed; (b) G. Guichard and I. Huc, Chem. Commun., 2011, 47, 5933 RSC; (c) T. A. Sobiech, Y. L. Zhong and B. Gong, Org. Biomol. Chem., 2022, 20, 6962 RSC; (d) K. M. Kim, G. Song, S. Lee, H.-G. Jeon, W. Chae and K.-S. Jeong, Angew. Chem., Int. Ed., 2020, 132, 22661 CrossRef.
- Y. Ferrand and I. Huc, Acc. Chem. Res., 2018, 51, 970 CrossRef CAS PubMed.
- (a) B. Gole, B. Kauffmann, V. Maurizot, I. Huc and Y. Ferrand, Angew. Chem., Int. Ed., 2019, 58, 8063 CrossRef CAS PubMed; (b) B. Gole, B. Kauffmann, A. Tron, V. Maurizot, N. McClenaghan, I. Huc and Y. Ferrand, J. Am. Chem. Soc., 2022, 144, 6894 CrossRef CAS PubMed.
- V. Koehler, A. Roy, I. Huc and Y. Ferrand, Acc. Chem. Res., 2022, 55, 1074 CrossRef CAS PubMed.
- (a) A. Petitjean, L. A. Cuccia, M. Schmutz and J.-M. Lehn, J. Org. Chem., 2008, 73, 2481 CrossRef CAS PubMed; (b) T. A. Sobiech, Y. Zhong, L. S. Sanchez, B. B. Kauffmann, J. K. McGrath, C. Scalzo, D. P. Miller, I. Huc, E. Zurek, Y. Ferrand and B. Gong, Chem. Commun., 2021, 57, 11645 RSC; (c) Y. Zhong, T. A. Sobiech, B. Kauffmann, B. Song, X. Li, Y. Ferrand, I. Huc and B. Gong, Chem. Sci., 2023, 14, 4759 RSC.
- (a) R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef CAS PubMed; (b) J. D. Crowley, S. M. Goldup, A. L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530 RSC.
- (a) Q. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grélard, H. Jiang and I. Huc, Science, 2011, 331, 1172 CrossRef CAS PubMed; (b) X. Wang, B. Wicher, Y. Ferrand and I. Huc, J. Am. Chem. Soc., 2017, 139, 9350 CrossRef CAS PubMed.
- (a) Q. Gan, X. Wang, B. Kauffmann, F. Rosu, Y. Ferrand and I. Huc, Nat. Nanotechnol., 2017, 12, 447 CrossRef CAS PubMed; (b) S. A. Denisov, Q. Gan, X. Wang, L. Scarpantonio, Y. Ferrand, B. Kauffmann, G. Jonusauskas, I. Huc and N. D. McClenaghan, Angew. Chem., Int. Ed., 2016, 55, 1328 CrossRef CAS PubMed.
- (a) L. Scarpantonio, A. Tron, C. Destribats, P. Godard and N. D. McClenaghan, Chem. Commun., 2012, 48, 3981 RSC; (b) P. R. Ashton, V. Balzani, O. Kocian, L. Prodi, N. Spencer and J. F. Stoddart, J. Am. Chem. Soc., 1998, 120, 11190 CrossRef CAS.
- A. Lamouroux, L. Sebaoun, B. Wicher, B. Kauffmann, Y. Ferrand, V. Maurizot and I. Huc, J. Am. Chem. Soc., 2017, 139, 14668 CrossRef CAS PubMed.
- C. Yao, B. Kauffmann, I. Huc and Y. Ferrand, Chem. Commun., 2022, 58, 5789 RSC.
- J. B. Birks, D. J. Dyson and I. H. Munro, Proc. R. Soc. London, Ser. A, 1963, 275(1360), 575 CAS.
- (a) B. Daly, J. Ling and A. P. de Silva, Chem. Soc. Rev., 2015, 44, 4203 RSC; (b) A. P. de Silva, J. Phys. Chem. Lett., 2011, 2, 2865 CrossRef CAS.
- (a) A. M. Swinnen, M. Van der Auweraer, F. C. De Schryver, K. Nakatani, T. Okada and N. Mataga, J. Am. Chem. Soc., 1987, 109, 321 CrossRef CAS; (b) A. K. Purkayastha and S. Basu, J. Photochem., 1979, 11, 261 CrossRef CAS.
- M. Li, A. D. Schlüter and J. Sakamoto, J. Am. Chem. Soc., 2012, 134, 11721 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2162063. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03218g |
| ‡ C. Y. and B. G. contributed equally. |
| § Current address: Department of Chemistry, School of Natural Sciences, Shiv Nadar Institution of Eminence, Greater Noida, Uttar Pradesh 201314, India. |
| This journal is © The Royal Society of Chemistry 2024 |