Open Access Article
Open Access ArticleAdsorbate-dependent phase switching in the square lattice topology coordination network [Ni(4,4′-bipyridine)2(NCS)2]n†
Shi-Qiang
Wang
 *ab,
Shaza
Darwish
a and
Michael J.
Zaworotko
*ab,
Shaza
Darwish
a and
Michael J.
Zaworotko
 *a
*a
aBernal Institute, Department of Chemical Sciences, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: xtal@ul.ie
bInstitute of Materials Research and Engineering, Agency for Science, Technology and Research, 138634, Singapore. E-mail: wangsq@imre.a-star.edu.sg
First published on 8th December 2022
Abstract
Switching coordination networks (CNs) featuring stepped sorption isotherms that are accompanied by phase changes offer promise for gas storage and separation applications. However, their responsiveness to different adsorbates remains largely understudied. Herein, we report the variable switching behaviour of a previously known square lattice (sql) topology CN, [Ni(4,4′-bipyridine)2(NCS)2] (sql-1-Ni-NCS), with respect to nine gaseous adsorbates.
The increasing use of gases as fuels or chemical feedstocks has resulted in the “age of gas”.1 However, the dispersive nature of gases means that they tend to have low densities and form mixtures under ambient and typical industrial process conditions.2 This creates several challenges for gas storage and/or separation processes with respect to the associated energy footprint and hazards.3–6 Porous materials such as activated carbons and zeolites were deployed in the 20th century to mitigate such energy penalty,3,4 even though their gas sorption uptakes are often modest. In the 1990s, a new class of metal–organic materials (i.e., porous coordination polymers/networks, PCPs/PCNs, and metal–organic frameworks, MOFs) were introduced and it was quickly realized that their inherent modularity enables tunable pore size and chemistry.7–11 The majority of such materials are first or second-generation CPs, i.e. upon sorbate removal they undergo structural collapse (first generation) or possess rigid structures like classical zeolites (second generation).8,12 Second-generation CPs typically exhibit Langmuir (type I) sorption isotherms, which necessarily reduces working capacity for gas storage including natural gas storage.13
Seminal studies pioneered by Kitagawa, Férey and others introduced “third generation” flexible CPs or “soft porous crystals” in the early 2000s.14–17 Third generation CPs exhibit structural flexibility when exposed to guest molecules and feature stepped sorption isotherms that are yet to be classified by IUPAC.17–19 A small but growing subset of flexible CPs are the switching coordination networks (CNs) that can undergo extreme structural transition(s) between “closed” nonporous and “open” porous phases. We have classified the resulting stepped isotherms as type F-IV isotherms.17,18 Such switching CNs can enable higher working capacity and better thermal management than rigid sorbents with type I isotherms.13 Furthermore, switching pressure and adsorption enthalpy can be readily calculated by applying the Clausius–Clapeyron equation,17 allowing for the strength comparison of different adsorbate–adsorbent interactions.
The switching pressure and sorption uptake of switching CNs can be influenced by various factors.17 The effects of temperature/pressure, metal–ion and linker on switching CNs have been studied.13,20–24 The adsorbate also plays a key role in triggering the phase transformations that accompanies switching since adsorbate–adsorbent interactions are the main driving force.25–27 In general, nonpolar gases such as N2 and CH4 tend to exhibit relatively weak interactions with CNs, while hydrocarbons with unsaturated bonds and/or more carbon atoms may induce switching by providing stronger host–guest interactions (Tables S1 and S2, ESI†).17 However, such empirical rules-of-thumb need to be experimentally verified using a systematic approach.
To our knowledge, there are around 70 switching CNs reported in the literature for which N2 and CO2 are the dominant adsorbates, usually studied at their boiling point temperatures of 77 K and 195 K, respectively.17 Only a handful of switching CNs have been examined across a range of adsorbates at multiple conditions (Table S1, ESI†).13,20–23,25–28 This situation hinders our understanding of the adsorbate dependence of switching CNs and in turn limits their potential utility. We address this matter herein through a study of the effect of nine adsorbates on the switching parameters associated with a square lattice (sql) topology coordination network.
Switching CNs often feature square or rhombic cavities that exploit the flexible nature of a rhombus from a mechanical perspective as exemplified by sql CNs with general formula [M(L)2(A)2]n (M = divalent metal cation, L = ditopic linker ligand, A = axial counter anion).† Such CNs are modular and highlight the “node and linker” design strategy developed by Robson and Hoskins over 30 years ago.29,30 The first reported sorption study on switching sql CNs was conducted on [Cu(bpy)2(BF4)2] (bpy = 4,4′-bipyridine), ELM-11.31,32 It was observed to exhibit single or multi-step type F-IV sorption isotherms via layer expansion (Fig. 1a) when triggered by gases such as N2, O2, Ar, CO2 and C2H2.33–35 Recently, we studied the sorption properties of three previously known sql CNs [M(bpy)2(NCS)2] (M = Fe, Co, or Ni),36–39sql-1-M-NCS, which are isostructural to the ELM family. Their CO2 sorption isotherms under low or high temperatures/pressures were observed to exhibit single-step type F-IV isotherms and the switching pressures were found to be metal–ion controlled.38 Amongst the three sql-1-M-NCS CNs, sql-1-Ni-NCS was found to be the “softest” switching CN based on the CO2 gate-adsorption pressure (Pga).38 It was later found that sql-1-Ni-NCS exhibits even lower Pga value and higher sorption uptake for C2H2 when compared to its CO2 sorption.39 These studies prompted us to study the effect of a wider range of gaseous adsorbates on sql-1-Ni-NCS to determine their switching pressures and sorption uptakes.
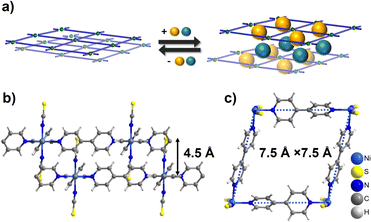 | ||
| Fig. 1 (a) Schematic illustration of the switching mechanism of sql CNs triggered by guest sorption; (b and c) crystal structures of sql-1-Ni-NCS. | ||
sql-1-Ni-NCS was initially synthesized hydrothermally,40 and we have recently adopted an alternate route by heating its 1D chain CP precursor that can be obtained by water slurry.38sql-1-Ni-NCS is sustained by Ni(II) ions coordinated equatorially to bpy linker ligands with terminal NCS− anions occupying the axial positions. The interlayer distance is 4.5 Å (Fig. 1b) and the effective dimension of the square cavity is 7.5 Å × 7.5 Å (Fig. 1c). The cavity void is blocked by the interdigitated NCS− ligands (Fig. 1b). sql-1-Ni-NCS is therefore non-porous and is thermally stable up to 180 °C,38 which is sufficient for most practical applications. In contrast to its hydrophilic analogue ELM-11, sql-1-Ni-NCS is hydrophobic towards humidity,38 an important consideration given that water vapour degrades the performance of many adsorbents.
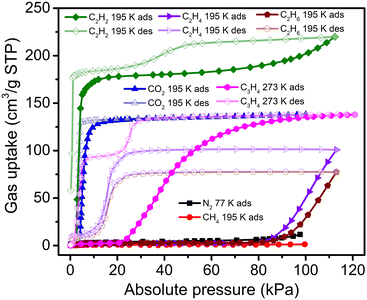
While sql-1-Ni-NCS is ‘‘softer’’ than its Fe and Co analogues, its 77 K N2 and 195 K CH4 sorption revealed negligible uptakes (Fig. 2). In contrast, its 195 K C2H4 and C2H6 isotherms revealed switching behaviour but uptake did not reach saturation at 113 kPa (close to the maximum measurable pressure of the sorption instrument). The corresponding Pga values for C2H4 and C2H6 were determined to be 86 and 91 kPa, respectively, much higher than those for CO2 (4.0 kPa) and C2H2 (2.9 kPa) at the same temperature.38,39 In addition, C3H4 (propyne) sorption on sql-1-Ni-NCS exhibited switching behaviour (Pga = 21.5 kPa) at 273 K with a saturation uptake of 138 cm3 g−1, although its C3H6 (propylene) and C3H8 (propane) sorption uptakes were negligible (Fig. S1, ESI†). This C3H4 uptake matches its CO2 uptake and corresponds to three C3H4 molecules per formula unit (sql-1-Ni-NCS·3C3H4). Interestingly, the desorption branch of the C3H4 sorption isotherm featured two steps, consistent with a new phase with 2/3 of the saturation uptake (ca. 94 cm3 g−1) between 8 and 20 kPa at 273 K. This data suggests that, before transforming to the closed phase, sql-1-Ni-NCS·3C3H4 converted to sql-1-Ni-NCS·2C3H4 (two C3H4 molecules per formula unit) during desorption. Such a phenomenon (i.e., the desorption branch has more steps than the corresponding adsorption branch) is, to the best of our knowledge, reported herein for the first time in switching CNs.
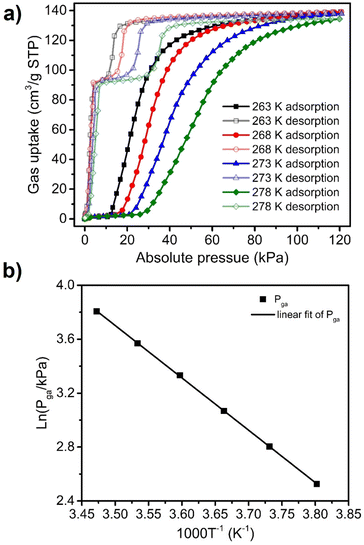
To further study the C3H4 sorption behaviour on sql-1-Ni-NCS, C3H4 sorption isotherms were collected between 263 and 298 K with a 5 K interval (Fig. 3a and Fig. S2, ESI†). The Pga values were observed to be 12.5, 16.5, 21.5, 28.0, 35.5 and 45.0 kPa at 263, 268, 273, 278, 283 and 288 K, respectively. These temperatures and Pga values were fitted to the Clausius–Clapeyron equation (Fig. 3b and Fig. S3, ESI†), to calculate the adsorption enthalpy (ΔH, absolute value) of ca. 32.3 kJ mol−1. This ΔH value is higher than those calculated for the corresponding CO2 (28.4 kJ mol−1) and C2H2 (28.5 kJ mol−1) induced phase transformations.38,39
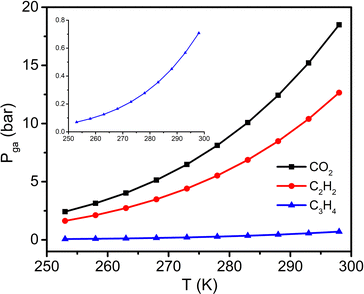
P ga can be calculated at given temperatures once ΔH has been determined.36,38,39 We therefore plotted the Pgavs. temperature from 253 to 298 K for C3H4 and compared it with those for CO2 and C2H2 (Fig. 4 and Table S3, ESI†). The plot reveals that the C3H4 switching pressure increases at elevated temperature in a manner similar to that of its CO2 and C2H2 counterparts.38,39 However, the Pga values for C3H4 are much lower than those for CO2 and C2H2. For example, the Pga value for C3H4 at 298 K is only 0.71 bar, while it reaches 12.66 and 18.49 bar for C2H2 and CO2, respectively. Such a large difference in Pga values suggests that adsorbate–adsorbent interactions strongly affect the switching pressure as reflected in their ΔH values. With respect to the sorption uptake, it is also affected by the adsorbate. For instance, the saturation uptakes of CO2 and C3H4 are both 138 cm3 g−1 (3 mol mol−1), while C2H2 uptake reaches 185 cm3 g−1 (4 mol mol−1) at the first plateau, a 33.3% increase in capacity. The adsorbate impact upon switching easiness for the studied gases can be ordered as follows: C3H4 > C2H2 > CO2 > C2H4 > C2H6 > CH4 ≥ N2 (Fig. S4, ESI†). This order generally agrees with that reported for other switching CNs (Table S2, ESI†) and follows the trend of the boiling point and vaporisation enthalpy of the gases (Table S4, ESI†).
Structural analysis of previously reported sql-1-M-NCS·xG (Tables S5, S6 and Fig. S5, S6, ESI†) reveals that phase switching involves guest intercalation and/or inclusion phenomena and enables volume expansion of 23.5–114.9%, which can be classified into five distinct phase categories, A–E (Table S6 and Fig. S5, ESI†). The stoichiometric ratio (x) of G: M was found to be 2, 3 or 4. The identical stoichiometric ratio (x = 3) of C3H4 and CO2 and their similar shapes prompted us to conduct molecular simulations to predict the location of C3H4 molecules by assuming that the crystal structure of sql-1-Ni-NCS·3C3H4 is isostructural to the previously reported structure of sql-1-Ni-NCS·3CO2.36,38 The resulting calculations of these “category A” phases indicate that C3H4 molecules occupy interlayer voids and internetwork cavities (Figs S6b, ESI†) with C–H⋯π and π⋯π host–guest interactions (Fig. S7, ESI†). Future studies will focus upon in situ PXRD experiments to verify the nature of these structural transformations.
In summary, we herein present the sorption properties of nine gases (N2, CH4, CO2, C2H2, C2H4, C2H6, C3H4, C3H6, and C3H8) of a prototypal switching sql CN, sql-1-Ni-NCS. C3H4 sorption was studied at eight temperatures and compared with the previously reported CO2 and C2H2 sorption properties. C3H4 induced switching had not been previously reported for CNs, although it has been studied in a soft organic cage.41 Our results indicate that both the switching pressure and sorption uptake are strongly influenced by the adsorbate. The switching thresholds for each adsorbate are generally compatible with the rules-of-thumb abovementioned. It should be noted that, whereas CH4, C3H6 and C3H8 did not trigger switching of sql-1-Ni-NCS at 195 or 273 K and 1 bar, this does not mean that they cannot do so at lower temperatures and/or higher pressures. Overall, the primary message from this study is that nonporous structures (as determined by their crystal structures and/or 77 K N2 sorption data) should not be discarded as candidates for sorption-based applications as first suggested by Barrer's studies on molecular compounds.42 Future studies will explore the sorption behaviour of sql-1-Ni-NCS and related switching adsorbent layered materials (SALMAs) for other adsorbates including the effect of pressure.43
M. J. Z. gratefully acknowledges the support of the Irish Research Council (IRCLA/2019/167) and Science Foundation Ireland (16/IA/4624).
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Kitagawa, Angew. Chem., Int. Ed., 2015, 54, 10686–10687 CrossRef CAS PubMed.
- B. E. Poling, J. M. Prausnitz and J. P. O’connell, Properties of gases and liquids, McGraw-Hill Education, 2001 Search PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- K. V. Kumar, K. Preuss, M.-M. Titirici and F. Rodriguez-Reinoso, Chem. Rev., 2017, 117, 1796–1825 CrossRef CAS PubMed.
- B. Li, H.-M. Wen, W. Zhou and B. Chen, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, EnergyChem, 2019, 1, 100006 CrossRef.
- J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- S. Kitagawa, R. Kitaura and S. I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- S. R. Batten, S. M. Neville and D. R. Turner, Coordination polymers: design, analysis and application, Royal Society of Chemistry, 2009 Search PubMed.
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366–2388 RSC.
- L. R. MacGillivray, Metal–organic frameworks: design and application, John Wiley & Sons, 2010 Search PubMed.
- S. Kitagawa and M. Kondo, Bull. Chem. Soc. Jpn., 1998, 71, 1739–1753 CrossRef CAS.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi and J. R. Long, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- S.-Q. Wang, S. Mukherjee and M. J. Zaworotko, Faraday Discuss., 2021, 231, 9–50 RSC.
- Q. Y. Yang, P. Lama, S. Sen, M. Lusi, K. J. Chen, W. Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry IV, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- N. Klein, H. C. Hoffmann, A. Cadiau, J. Getzschmann, M. R. Lohe, S. Paasch, T. Heydenreich, K. Adil, I. Senkovska, E. Brunner and K. Stefan, J. Mater. Chem., 2012, 22, 10303–10312 RSC.
- C. M. McGuirk, T. Runčevski, J. Oktawiec, A. Turkiewicz, M. K. Taylor and J. R. Long, J. Am. Chem. Soc., 2018, 140, 15924–15933 CrossRef CAS PubMed.
- A.-X. Zhu, Q.-Y. Yang, A. Kumar, C. Crowley, S. Mukherjee, K.-J. Chen, S.-Q. Wang, D. O’Nolan, M. Shivanna and M. J. Zaworotko, J. Am. Chem. Soc., 2018, 140, 15572–15576 CrossRef CAS PubMed.
- A.-X. Zhu, Q.-Y. Yang, S. Mukherjee, A. Kumar, C.-H. Deng, A. A. Bezrukov, M. Shivanna and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 18212–18217 CrossRef CAS PubMed.
- N. Kumar, S.-Q. Wang, S. Mukherjee, A. A. Bezrukov, E. Patyk-Kaźmierczak, D. O'Nolan, A. Kumar, M.-H. Yu, Z. Chang, X.-H. Bu and M. J. Zaworotko, Chem. Sci., 2020, 11, 6889–6895 RSC.
- P. L. Llewellyn, P. Horcajada, G. Maurin, T. Devic, N. Rosenbach, S. Bourrelly, C. Serre, D. Vincent, S. Loera-Serna, Y. Filinchuk and G. Ferey, J. Am. Chem. Soc., 2009, 131, 13002–13008 CrossRef CAS PubMed.
- H. Wu, R. S. Reali, D. A. Smith, M. C. Trachtenberg and J. Li, Chem. – Eur. J., 2010, 16, 13951–13954 CrossRef CAS PubMed.
- N. Nijem, H. Wu, P. Canepa, A. Marti, K. J. Balkus, T. Thonhauser, J. Li and Y. J. Chabal, J. Am. Chem. Soc., 2012, 134, 15201–15204 CrossRef CAS PubMed.
- A. Kondo, S.-I. Noro, H. Kajiro and H. Kanoh, Coord. Chem. Rev., 2022, 471, 214728 CrossRef CAS.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962–5964 CrossRef CAS.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- D. Li and K. Kaneko, Chem. Phys. Lett., 2001, 335, 50–56 CrossRef CAS.
- A. Kondo, H. Noguchi, S. Ohnishi, H. Kajiro, A. Tohdoh, Y. Hattori, W.-C. Xu, H. Tanaka, H. Kanoh and K. Kaneko, Nano Lett., 2006, 6, 2581–2584 CrossRef CAS PubMed.
- H. Kanoh, A. Kondo, H. Noguchi, H. Kajiro, A. Tohdoh, Y. Hattori, W.-C. Xu, M. Inoue, T. Sugiura, K. Morita, H. Tanaka, T. Ohba and K. Kaneko, J. Colloid Interface Sci., 2009, 334, 1–7 CrossRef CAS PubMed.
- H. Kajiro, A. Kondo, K. Kaneko and H. Kanoh, Int. J. Mol. Sci., 2010, 11, 3803–3845 CrossRef CAS PubMed.
- S.-Q. Wang, X.-Q. Meng, M. Vandichel, S. Darwish, Z. Chang, X.-H. Bu and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2021, 13, 23877–23883 CrossRef CAS PubMed.
- S.-Q. Wang, Q.-Y. Yang, S. Mukherjee, D. O'Nolan, E. Patyk-Kazmierczak, K.-J. Chen, M. Shivanna, C. Murray, C. C. Tang and M. J. Zaworotko, Chem. Commun., 2018, 54, 7042–7045 RSC.
- S.-Q. Wang, S. Mukherjee, E. Patyk-Kaźmierczak, S. Darwish, A. Bajpai, Q.-Y. Yang and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 6630–6634 CrossRef CAS PubMed.
- S.-Q. Wang, S. Darwish, D. Sensharma and M. J. Zaworotko, Mater. Adv., 2022, 3, 1240–1247 RSC.
- S.-Q. Wang, S. Darwish, X.-Q. Meng, Z. Chang, X.-H. Bu and M. J. Zaworotko, Chem. Commun., 2022, 58, 1534–1537 RSC.
- Z. Yugen, J. Li, W. Deng, N. Masayoshi and I. Tsuneo, Chem. Lett., 1999, 195–196 Search PubMed.
- Z. Wang, N. Sikdar, S.-Q. Wang, X. Li, M. Yu, X.-H. Bu, Z. Chang, X. Zou, Y. Chen, P. Cheng, K. Yu, M. J. Zaworotko and Z. Zhang, J. Am. Chem. Soc., 2019, 141, 9408–9414 CrossRef PubMed.
- S. A. Allison and R. M. Barrer, J. Chem. Soc. A, 1969, 1717–1723 RSC.
- E. Patyk-Kaźmierczak, M. Kaźmierczak, S.-Q. Wang and M. J. Zaworotko, Cryst. Growth Des., 2022 DOI:10.1021/acs.cgd.2c00982.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, sorption isotherms, etc. See DOI: https://doi.org/10.1039/d2cc06549e |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)