Microneedle systems: cell, exosome, and nucleic acid based strategies
Shufei
Zhang†
,
Lian
Yang†
,
Jianfeng
Liu
,
Hanyue
Li
,
Shasha
Hong
and
Li
Hong
*
Department of Obstetrics and Gynecology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, People's Republic of China. E-mail: dr_hongli@whu.edu.cn
First published on 25th September 2023
Abstract
Cells, exosomes, and nucleic acids play crucial roles in biomedical engineering, holding substantial clinical potential. However, their utility is often hindered by various drawbacks, including cellular immunogenicity, and instability of exosomes and nucleic acids. In recent years, microneedle (MN) technology has revolutionized drug delivery by offering minimal invasiveness and remarkable versatility. MN has emerged as an ideal platform for the extraction, storage, and delivery of these biological components. This review presents a comprehensive overview of the historical progression and recent advances in the field of MN. Specifically, it highlights the current applications of cell-, exosome-, and nucleic acid-based MN systems, while presenting prevailing research challenges. Additionally, the review provides insights into the prospects of MN in this area, aiming to provide new ideas for researchers and facilitate the clinical translation of MN technology.
1. Introduction
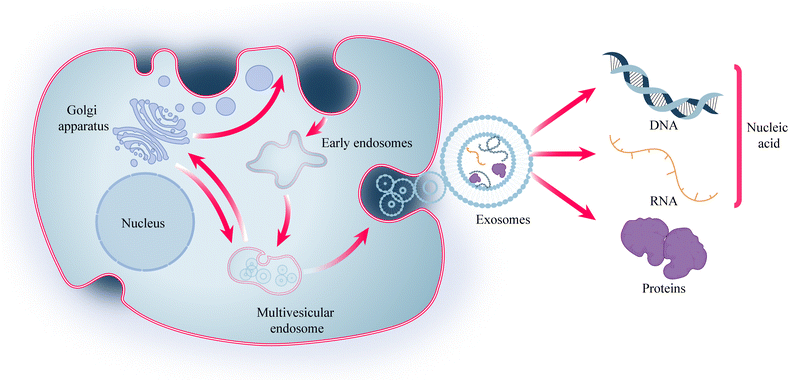
Cells play a crucial role in biomedical engineering, and cellular therapies, such as Chimeric Antigen Receptor (CAR)-T cell therapy and stem cell therapy, have emerged as important therapeutic modalities for various diseases.1 However, these therapies often face challenges related to immunogenicity. To address this issue, researchers have explored the potential of exosomes, which are nanoscale lipid bilayer-encapsulated vesicles secreted by various cell types, as a means of intercellular communication.2 Exosomes, with a diameter ranging from 30 to 150 nm, have demonstrated the ability to reduce immunogenicity while retaining some cellular functions. They have been used in diverse fields, including disease diagnosis, treatment, and drug delivery. The effectiveness of exosomes lies in their cargo, which includes proteins and nucleic acids such as DNA, mRNA, and non-coding RNA (Fig. 1). Among these, microRNA (miRNA) has garnered significant interest due to its role in precision medicine. miRNA function by binding to target genes, thereby exerting inhibitory effects and reducing the expression of these target genes.3,4Cell-based therapies, exosomes, and nucleic acids have emerged as important components in biomedical engineering, showing great potential for disease treatment. However, ensuring effective transplantation and action of these seeds remains a key challenge. While the use of biocompatible materials like hydrogels has partially addressed the issue of exogenous delivery, repeated invasive procedures such as hydrogel injections contradict the concept of minimally invasive treatments. Therefore, it is imperative to explore approaches that can ensure therapeutic effectiveness while minimizing invasiveness. Microneedle (MN) systems present a promising solution.
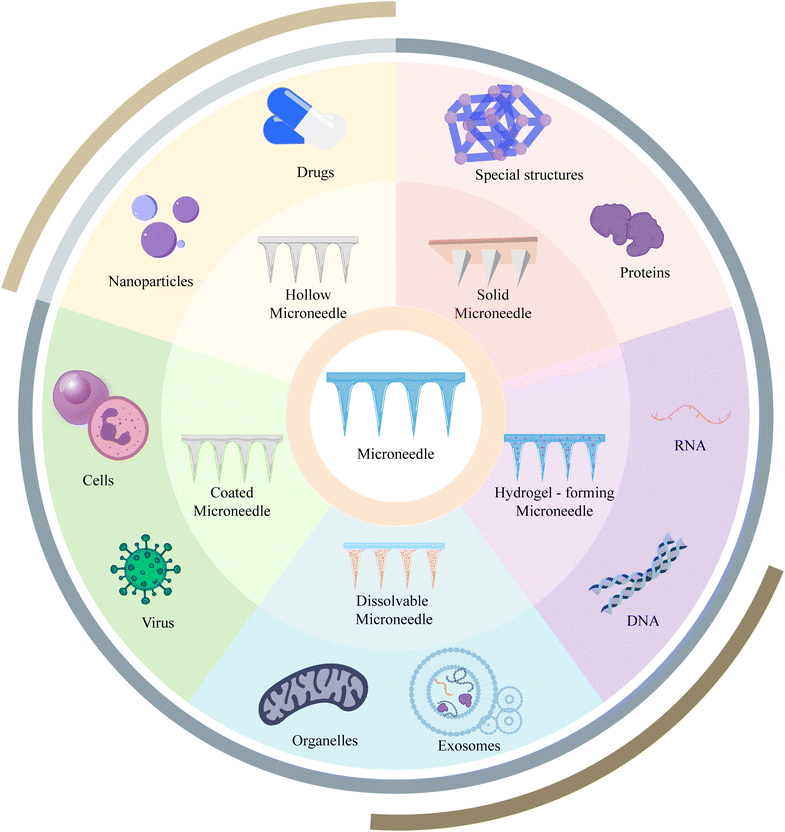
MN systems are novel drug delivery systems consisting of micron-sized needles. By forming an array of MNs, these systems enable minimally invasive transdermal drug delivery with virtually no pain, bridging the gap between non-invasive and invasive therapies.5 MNs have been extensively studied in the fields of drug delivery, vaccination, and disease monitoring.6–8 Based on different fabrication methods and structures, MNs can be classified into solid, hollow, coated, soluble, and hydrogel types and these MNs have found widespread applications in transdermal drug delivery and other areas (Fig. 2).9 This review aims to provide an overview of the historical development and recent advancements of MN applications in the context of cell-based therapies, exosomes, and nucleic acids. It also discusses the current challenges and prospects, aiming to inspire new ideas and provide references for researchers in the field.
2. Research progress of various types of MN
2.1 Research progress on various types of MN materials and manufacturing processes
In the early stages of MN development, teams from Georgia Tech fabricated solid MNs and hollow MNs using etching or stencil-filling methods, which served as the basis for solid, hollow, and hydrogel MN fabrication processes and dominated the field for a long time.10 However, achieving all aspects of the MN manufacturing process has been challenging. The introduction of technologies such as stereolithography laser cutting and ablation, as well as Xenon Difluoride Dry Etching, has optimized the MN manufacturing process and enabled microfabrication and mass production of MNs. However, there are still limitations such as high cost, complex processes, and potential chemical contamination.11,12Silicon, metal, and glass are common MN raw materials with excellent mechanical properties. However, their potential for fracturing after insertion into tissues can lead to complications, limiting the application of MN. As a result, sugar glass MNs and biodegradable polymers such as polylactic acid and polycaprolactone have emerged as new options for MN materials. The choice of biocompatible and biodegradable MN materials aligns more closely with the original design intention of minimally invasive procedures.13–17
Coated MNs are composite structures consisting of a solid MN core and a coating layer. The quality of the coating, process reproducibility, and delivery efficiency are crucial for their application. Unlike MN fabrication techniques such as etching, the dip coating method is the simplest way to fabricate coated MNs. In this method, the solid MN is immersed in a solution and the coated MN is obtained by drying and molding. However, this construction method faces challenges in precisely controlling the dose and is time-consuming. It is also difficult to achieve a uniform coating on the inner tip surface due to surface tension. Ideally, the coating should only cover the MN tip uniformly without unnecessarily wasting coating material.18 Several spraying-based coating methods, such as Gas Jet Drying, Spray Coating, electrohydrodynamic atomization, and Piezoelectric Inkjet Printing, have been developed to achieve uniform coating by replacing liquid immersion with droplet spraying (Fig. 3).19,20 The use of microforming further ensures precise dose control of the coating. When combined with industrialization tools like ultrasonic spraying technology, it facilitates the industrialization and clinical translation of coated MNs.21,22
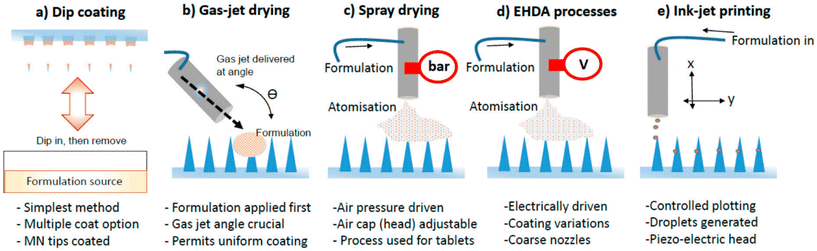 | ||
| Fig. 3 Illustrated examples of techniques used to coat MNs. (a) Dip coating; (b) gas-jet drying; (c) spray drying; (d) EHDA processes; (e) ink-jet printing. Reprinted with permission from MDPI,20 Copyright 2015. | ||
2.2 Research progress of various MN application methods
The traditional method of MN application is finger pressure. Although simple and easy to use, this method lacks control over the magnitude and angle of applied pressure, leading to uneven force distribution and difficulty in handling larger MN patches or shorter MN needles. As a result, finger pressure is gradually being replaced by devices such as electronic applicators and impact-insertion applicators. Digitally controlled MN applicators allow precise control of applied pressure and impact speed, ensuring repeatability in MN applications. The impact force provided by these applicators also enables shorter micro-needle tips (<300 μm) to overcome skin elasticity, enhancing the penetration ability of MNs. However, the MN application method has been neglected for a long time, with only a few studies focusing on its improvement. Further research is needed to address issues such as differences in puncture performance and uneven force distribution in MN patches.23 As MN treatments become more popular, MN administration may need to evolve towards an intelligent and personalized patient self-management model.2.3 Advantageous applications of various types of MN
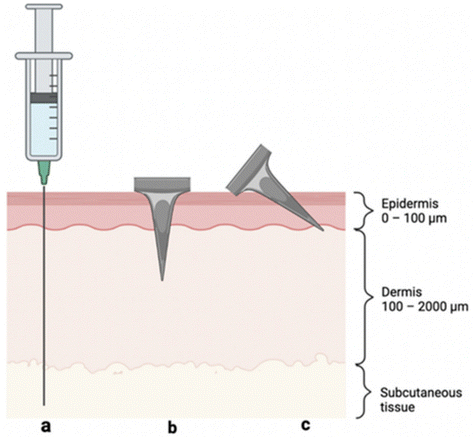 | ||
| Fig. 4 Visual representation of skin layers and three methods of antigen delivery: (a) hypodermic needle injection to the subcutaneous space, (b) perpendicular (i.e., 90°) MN insertion crossing epidermis and into the dermis, and (c) angled MN insertion targeting the epidermis. Reprinted with permission from MDPI,32 Copyright 2022. | ||
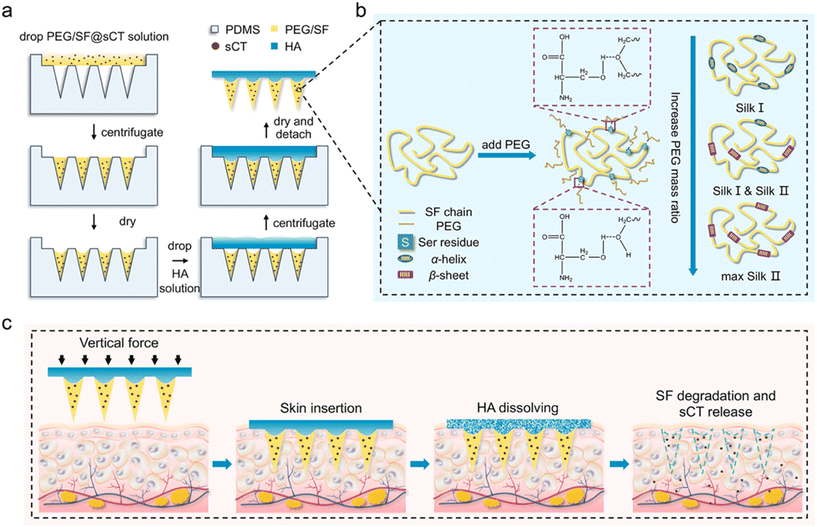 | ||
| Fig. 5 Schematic illustrations of the fabrication process of HA-PEG/SF@sCT MNs (a), PEG-induced conformational transition of SF (b), and transmucosal delivery of sCT using the HA-PEG/SF@sCT MNs (c). Silk fibroin (SF); poly(ethylene glycol) (PEG); salmon calcitonin (sCT). Reprinted with permission from American Chemical Society,38 Copyright 2023. | ||
3. MN system: cell, exosome, and nucleic acid-based treatment strategy
3.1 Extraction and detection
Whether it is cell therapy or exosome delivery, the extraction of isolated seed cells is crucial for subsequent treatment and detection. Stem cells play a key role in cell therapy, but their clinical application is hindered by the challenges of purification and low viability during in vitro transplantation.48 Traditional methods, such as flow cytometry, are often used to purify heterogeneous cells after isolation, but the mechanical pressure exerted on cells during high-speed flow can negatively affect their state, compromising further research or therapeutic use. To address this, Yuli Wang et al. explored an alternative strategy for isolating stem cells using PLGA degradable microrafts and a magnetic needle system, which could be effective for small sample sizes or fragile and sensitive transplanted cells.49 However, this method is currently only applicable to adherent cells and involves a complex procedure, limiting its translational application. Therefore, the next research direction is to achieve targeted binding of microrafts to suspension cells and develop high-throughput screening methods.Similarly, the first step in exosome research is often the extraction, isolation, and identification of exosomes. Common methods for exosome extraction, such as ultracentrifugation and ultrafiltration, are time-consuming and yield low concentrations of exosomes50 (Fig. 6A). In contrast, some researchers have successfully used hollow MNs to extract trace amounts of dermal interstitial fluid exosomes. This extraction process is simple, minimally invasive, and provides a higher concentration of exosomes suitable for proteomic sequencing and detecting circulating biomarkers for disease diagnosis (Fig. 6B). However, these extracted exosomes are still limited in quantity and cannot be directly used for disease treatment. Moreover, although the simplification of the extraction process is beneficial, it does not address the issue of time-consuming exosome extraction or enable the direct extraction of MN-derived exosomes. Future research may focus on combining exosome-specific targets with hollow MNs to achieve direct extraction and arranging individual MNs into matrices for high-throughput collection of exosomes, advancing disease treatment strategies.
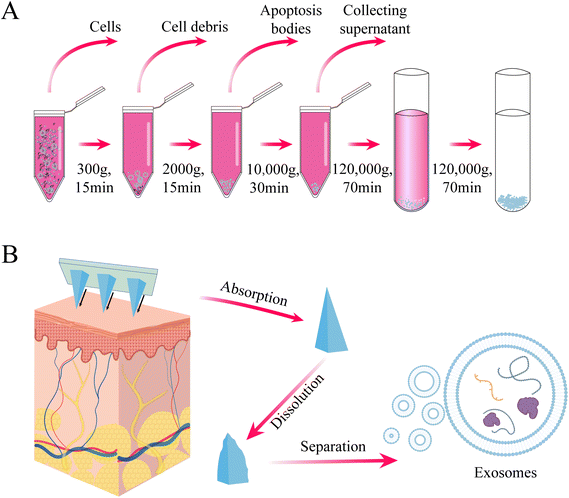 | ||
| Fig. 6 (A) Conventional procedure for the extraction of exosomes by ultracentrifugation (B) extraction of skin tissue fluid for exosome isolation using hydrogel MNs. | ||
Interesting findings have been made regarding the application of MN in nucleic acid extraction. Initially, these applications were focused on the plant, animal, and food sectors. Polyvinyl alcohol (PVA) MN patches were utilized to extract DNA without relying on tissue and cell lysis, resulting in a significant reduction in plant DNA extraction time from 3 to 4 hours to approximately 1 minute. This approach also reduced extraction costs, making it a transformative strategy for the rapid detection of plant diseases.51,52
In the realm of human nucleic acid extraction, there have been notable studies. For instance, Yuchun Qiao et al. developed MN patches composed of methacrylamide gelatin (GelMA) and graphene oxide (GO) for the enrichment and detection of multiple miRNA biomarkers in skin interstitial fluid. These patches exhibited good mechanical strength, rapid sampling ability, and a high swelling rate to enrich miRNA fragments in interstitial fluid. The team also achieved simultaneous fluorescence detection of three psoriasis-specific miRNAs by combining the isothermal catalytic hairpin assembly signal amplification technique (Fig. 7).53 Furthermore, Bin et al. took a step further and constructed an online CRISPR-Cas9-activated wearable MN patch. This innovative patch leverages the synergy between CRISPR-Cas9 and graphene biointerfaces, as well as a conductive MN patch with reverse ion electroosmosis therapy. It enables efficient extraction and real-time monitoring of different DNA targets in a minimally invasive manner, exhibiting excellent electrochemical properties and up to 10 days of in vivo stability. This wearable MN patch holds great potential for early screening and treatment of diseases.54
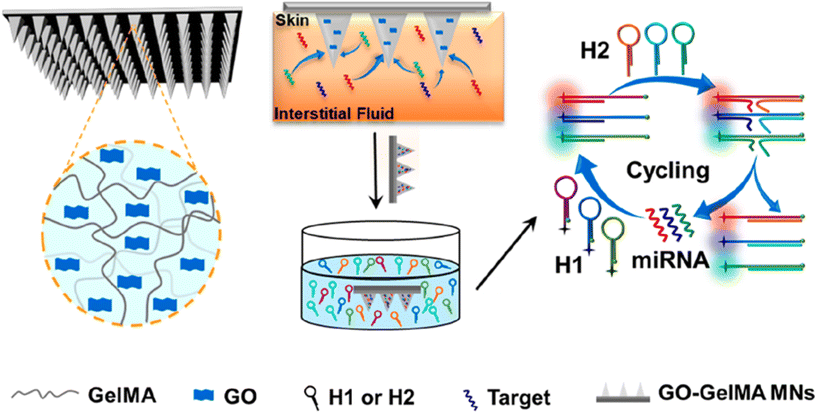 | ||
| Fig. 7 Schematic illustration of GO-GelMA MNs used for interstitial fluid extraction and the detection of miRNA. Reprinted with permission from American Chemical Society,53 Copyright 2023. | ||
In general, MNs have been extensively utilized in the field of cell, exosome, and nucleic acid extraction and detection. However, direct extraction of cells and exosomes remains a challenge. Although MNs can enrich higher concentrations of exosomes, their limited size restricts the total amount available, making it difficult to apply them in subsequent disease treatments. To overcome this limitation, the development of high-throughput continuous sampling MNs is crucial for expanding their application in the future.
Surprisingly, most studies have focused on using hydrogel MNs rather than hollow MNs for nucleic acid extraction. Nowadays, the use of MNs extends beyond nucleic acid extraction to include enrichment, extraction, and in situ detection of nucleic acids in combination with probes, fluorescence techniques, gene editing, and other technologies. Hydrogels offer good biocompatibility and editing properties, making them well-suited for these applications. However, one challenge is that while MNs are minimally invasive, they have limitations in terms of volume and scalability. This may necessitate the use of additional devices, such as fluorescence detection devices, to achieve all the expected functions, thereby adding redundancy to the detection process. Simplifying the steps and transforming nucleic acid extraction tests into individual test kits holds great potential in the field of disease diagnosis, treatment, and early prevention. Moreover, the development of wearable devices for real-time long-term disease monitoring places higher demands on the structure of MNs. Wearable devices are exposed to harsh conditions that can affect hydrogel properties and bioactivity over an extended period. A possible solution strategy could involve adding a layer of isolation colloid at the contact interface to mitigate these effects. In Table 1, MNs for extraction and detection of cells, exosomes, and nucleic acids are presented.
| Seeds | Type | MN design | Application | Advantages | Drawbacks | |
|---|---|---|---|---|---|---|
| Materials | Height/diameter | |||||
| Stem cell49 | Solid MN | Anodized steel | NA/150 μm | Isolation of microrafts | Allows sorting and purification of small samples of walled cells under delicate conditions and maintains cell viability | Only walled cells can be sorted; the process is complex |
| Exosome55 | Hollow MN | Glass | 1500 μm/2.8 mm | Extraction of dermal interstitial fluid for exosome isolation and identification | Simple, minimally invasive, and high enough concentration of exosomes for transcriptomic or proteomic analysis | Low volume for therapeutic use in a single extraction; requires exosome extraction process |
| Plant DNA51 | Hydrogel MN | PVA | 800 μm/150 μm | Instant extraction of plant DNA for rapid pest detection | Fast, economical, minimally invasive, no lysis, no purification | Relatively low early detection rate of pathogen infection |
| Food DNA52 | Hydrogel MN | PVA | 840 μm/280 μm | Instant extraction of food DNA, rapid detection of food allergens | Fast, economical, simple | Difficult to handle complex food products |
| miRNA53 | Hydrogel MN | Gelma/GO | 600 μm/370 μm | Detection of psoriasis-related miRNA | Sensitive, automated | Longer detection time compared to other MN patches |
| DNA54 | Hydrogel MN | Synthesis of polymethyl vinyl ether-alt-maleic acid (PMVE/MA) hydrogel/chitosan/graphene | 600 ± 50 μm/300 ± 10 μm | Long-term capture and real-time monitoring of free DNA | Wearable, efficient extraction, real-time monitoring | Interface receptor immobilization and sensitivity to be improved |
3.2 Seed activity retention
Studies have shown the potential of MN to facilitate the delivery of cellular and bioactive substances. However, it is critical to maintain the activity of cells, exosomes, and other components throughout the manufacturing, storage, and transport processes. To protect the activity of cells during MN manufacturing, KangJu Lee et al. proposed the design of a detachable hybrid MN receptacle. The mechanical strength of the reservoir was ensured by using a PLGA MN shell, and a biocompatible and biodegradable GelMA pre-mixed with cell culture medium was used to deliver bone marrow MSCs. This approach effectively reduces cell loss during MN manufacturing and provides valuable cells, offering a promising strategy for cell therapy applications involving challenging cells. However, the activity of MSCs tends to decline rapidly after 24 hours, posing a challenge for the long-term storage of these cells.56Despite the beneficial biological functions of exosomes, they cannot be stored for long periods, even at −80 °C, resulting in the loss of bioactive material. In contrast, MN not only facilitates the delivery of exosomes but also serves as a long-term storage method for exosomes. For example, delivery of exosomes has been successfully achieved using HA MN, which can be stored at 4 °C for 6 months.57
In addition, Hao Chang et al. developed Cryomicroneedles utilizing a cryogenic medium consisting of 2.5% DMSO and 100 mM sucrose solution with gradient cooling in the mold to incorporate cells into Cryomicroneedles.58 Furthermore, by modifying the formulation, Cryomicroneedles can enable the delivery of various bioactive substances, such as live bacteria and nucleic acids, thus expanding its applicability in delivering various bioactive substances (Fig. 8).59–61 Cryomicroneedles not only have excellent mechanical properties but also dissolve within tissues to facilitate the rapid and efficient delivery of bioactive substances. Importantly, they possess long-term preservation capabilities not available with normothermic MNs, representing an important milestone in advancing the clinical translation of MN technology.
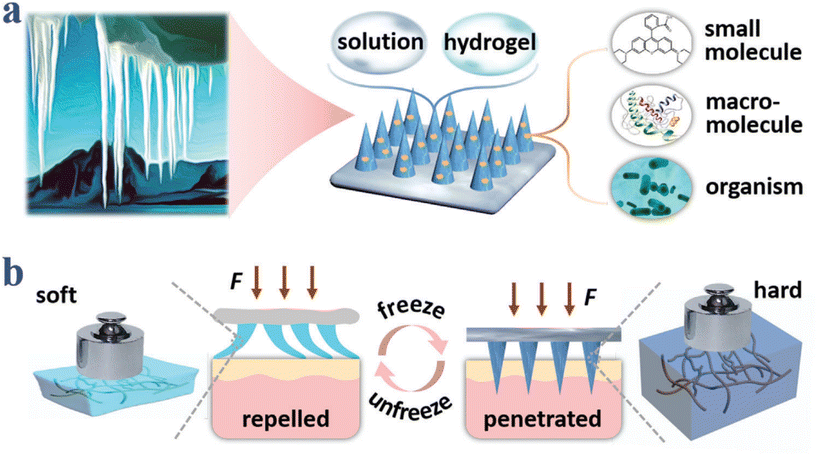 | ||
| Fig. 8 Schematic illustrations of compositions and properties of ice MNs. (a) Ice–formation–inspired ice MNs can be made from soft materials such as solutions and hydrogels, and can carry diverse actives including small molecules, macromolecules, and living organisms. (b) Scheme of transformation from softness to hardness realized by freezing processes. Reprinted with permission from American Chemical Society Advanced Science published by Wiley-VCH GmbH,61 Copyright 2021. | ||
The issue of bioactive component deactivation during MN manufacturing and storage has been partially addressed through the use of Cryomicroneedles. However, there are still some common problems associated with these Cryomicroneedles. Firstly, MN loses its mechanical properties shortly after being removed from the cryogenic environment, making it challenging to achieve transdermal delivery. Secondly, deep cryogenic MNs may cause sensory discomfort. One potential solution is to construct the PLGA shell in the template first and then freeze-form the soluble active ingredients. This approach ensures the activity of the contents during MN fabrication and storage while maintaining the mechanical strength of the MN. It also extends the time window between Cryomicroneedle removal from deep cryogenic temperatures and application, reducing the possibility of discomfort. Although this solution may not be optimal, with the rapid development of technologies like 3D printing, finding new manufacturing processes that can maintain the activity of cells, exosomes, and nucleic acids under more general conditions is a future direction worth exploring.62 In Table 2, MNs for maintaining the biological activity of cells, exosomes, and nucleic acids are presented.
| Seeds | Type | MN design | Application | Advantages | Drawbacks | |
|---|---|---|---|---|---|---|
| Materials | Height/diameter | |||||
| MSC56 | Hydrogel MN | Gelma/PLGA | 700 μm/NA | Local delivery of MSCs | Removable, avoiding the effects of the drying process on cellular activity in traditional MN manufacturing processes | Cellular activity declines precipitously after more than 24 hours of storage |
| Exosome57 | Soluble MN | HA | 600 μm | Long-term storage and transdermal delivery of extracellular vesicles | Can maintain the biological activity of extracellular vesicles for more than 4 months under mild storage conditions | Does not solve the problem of short duration of action after extracellular vesicle administration |
| Cell58 | Soluble MN | DMSO/succrose | 1200 μm/400 μm | Delivers bioactive and maintains bioactivity | Enables long-term storage and transport of actives | Loses mechanical properties quickly after removal from the cryogenic environment; slight discomfort may occur at low temperatures |
| Bacteria59 | Soluble MN | PBS//glycerol | 600 μm/250 μm | Delivers live bacteria | Enables long-term storage and transport of actives | Loses mechanical properties quickly after removal from the cryogenic environment; slight discomfort may occur at low temperatures |
| mRNA60 | Soluble MN | HA | 900 μm/350 μm | Intradermal delivery of mRNA | Enables long-term storage and transport of active material | Loses mechanical properties quickly after removal from the cryogenic environment; may be slightly uncomfortable at low temperatures; no means to facilitate gene transfection |
3.3 Local delivery
After solving the problem of cell, exosome, and nucleic acid extraction and preservation, local delivery of seeds is the most important step for clinical therapeutic translation. To summarize, MNs that can achieve topical delivery have the following basic characteristics: (1) good mechanical strength to penetrate the stratum corneum or tissues; (2) the ability to release the contained components effectively and exert therapeutic effects; and (3) no adverse reactions caused by the MN components.In 2018, B. Gualeni et al. successfully delivered melanocytes targeted to the skin for vitiligo treatment using hollow MN. This approach allows flexibility in treating different areas while minimizing pain and discomfort. It enables direct entry of cells into the skin without significant cell loss, providing a model for cellular delivery using MNs.63 Different application scenarios and MN contents pose additional demands on MN technology.
In the case of CAR-T cell therapy, for example, the abnormal vascular system, dense extracellular matrix, and tumor microenvironment cytokines in solid tumors hinder its effectiveness. To overcome these challenges, Hongjun et al. designed porous MNs that utilize an acid–base chemical reaction to etch the MN exterior and create pores for loading CAR-T cells (Fig. 9). This approach provides a multi-point, dispersed delivery vehicle for CAR-T cells, enhancing their infiltration within the tumor. The porous MNs can be applied to the resection cavity to prevent local tumor recurrence and potential metastatic dissemination.64
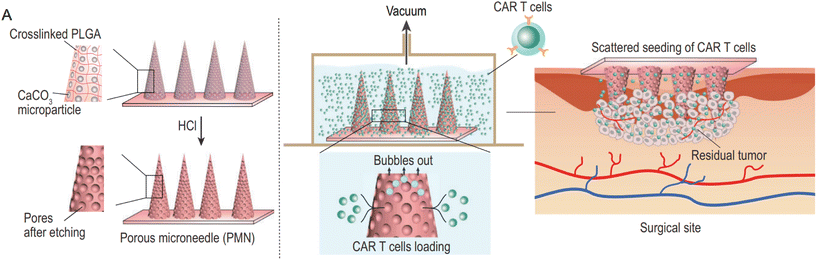 | ||
| Fig. 9 Characterization of porous MN (PMN) patch for CAR T cell loading and delivery. (A) Schematic of PMN fabrication, CAR T cell loading, and implant of CAR-T-cell-loaded PMN (PMN@CAR T) within the tumor bed after surgery. Reprinted with permission from National science review,64 Copyright 2021. | ||
The application of MN for cell delivery extends beyond the skin and includes important areas such as the heart.65 However, delivering cells to the myocardium poses specific challenges. The limited injected dose and the potential leakage of injected fluid and gel due to the beating of the heart put additional demands on MN-based cell delivery systems. There are two approaches for myocardial MN delivery. (1) Cell-loaded MN: this approach involves rapidly inserting the needle tip and inducing rapid substrate lysis to allow cell growth into the myocardium. Shiqi Hu et al. used a soluble HA substrate to achieve rapid separation of the MN needle from the substrate and implant cells into the myocardium.66 (2) Cell-free approach: Tang et al. used PVA MN to establish a channel between cardiac stromal cells (CSC) and host cells, allowing therapeutic effects while providing nutrients for implanted seed cells through an ingenious design (Fig. 10).67 Both approaches require precise administration using specialized instruments and often involve open-heart surgery to expose the heart, making clinical application challenging. Therefore, it is necessary to explore minimally invasive methods for cell delivery to the heart with the assistance of MNs. Mark R. Prausnitz et al. proposed a solution using a luminal unfolding MN injector to deliver MN-loaded capsules to the heart through natural lumens such as large blood vessels. The capsules release MNs for insertion into the myocardium. However, controlling the site and precision of MN release may be challenging.68
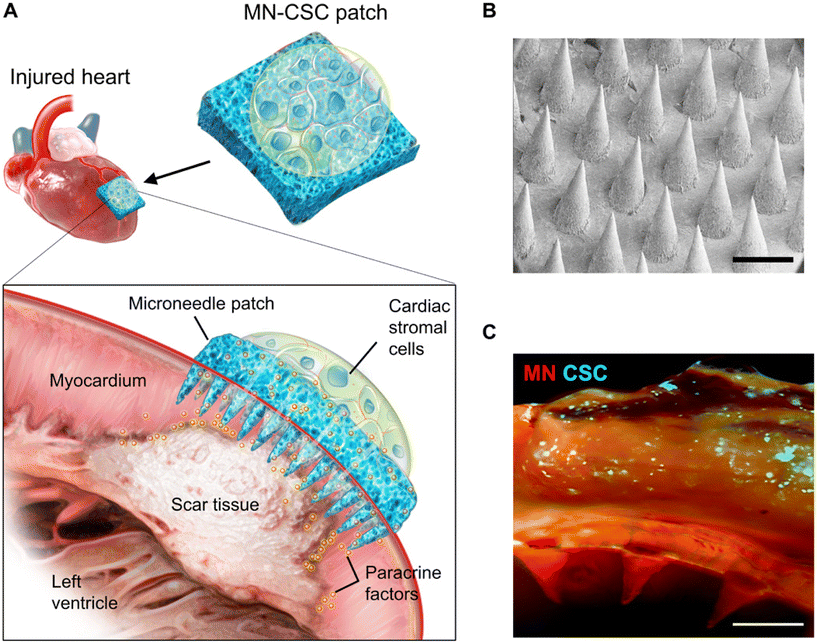 | ||
| Fig. 10 Characterization of MN-CSCs. (A) Schematic showing the overall design used to test the therapeutic benefits of MN-CSCs on the infarcted heart. (B) SEM image of MN. Scale bar, 500 μm. (C) Representative fluorescent image indicating that DiO-labeled CSCs (green) were encapsulated in fibrin gel and then integrated onto the top surface of MN array (red). Scale bar, 500 μm. Reprinted with permission from Science Advances,67 Copyright 2018. | ||
As previously mentioned, certain cells produce exosomes with therapeutic effects, and MSC exosomes are considered as a promising cell-free therapeutic strategy for tissue repair and regeneration.69 In a brilliant study conducted by Min Han et al. they utilized MN as a delivery vehicle and employed 3D culture to generate high-quality exosomes (Fig. 11). This approach facilitated in situ production and release of exosomes, eliminating the need for exosome extraction. In addition, exosomes from cells cultured in a 3D environment exhibited higher therapeutic efficiency. However, it may be wiser to consider the use of soluble material as a substrate for MN.70
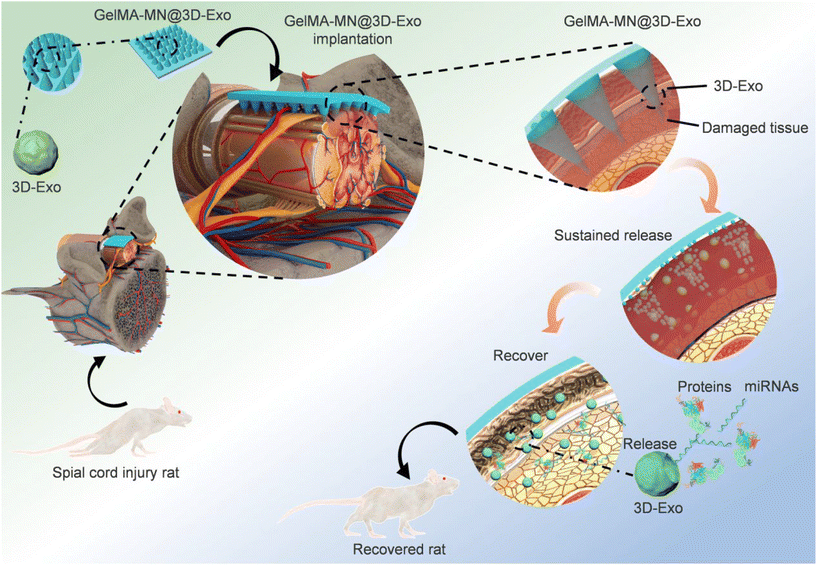 | ||
| Fig. 11 Flow diagram of the overall study design of GelMA-MN@3D-Exo for the treatment of SCI in rats. Reprinted with permission from American Chemical Society,70 Copyright 2022. | ||
In addition to their cellular functions, exosomes possess several advantages including low immunogenicity, low cytotoxicity, high circulating stability, and the ability to address issues related to hydrophobicity and low cellular uptake of drugs due to their phospholipid bilayer structure. As a result, exosomes can serve as carriers and form a dual carrier system with MN systems to facilitate efficient drug delivery and enhance cellular uptake. Yerneni et al. demonstrated improved drug uptake and skin-targeted delivery by utilizing MN-carrying exosomes containing curcumin in a dual-carrier system.71 Yuan et al. successfully prevented cardiac fibrosis after myocardial infarction by delivering microRNA-29b-loaded exosomes via gelatin MN patches.72
To further enhance the effectiveness of exosome-mediated delivery, researchers have combined nanomotors with MN systems to drive the movement of exosomes using self-propelled forces generated through chemical reactions with the surrounding environment (Fig. 12). For instance, the conversion of L-arginine to nitric oxide by NOS or ROS can serve as the driving force for the nanomotors. This shift from a passive to an active mode of transport makes exosomes more effective in their therapeutic effects. However, it should be noted that the efficacy of this nanomotor-based approach may depend on the specific components of the environment and may not be highly compatible for application in different diseases.73
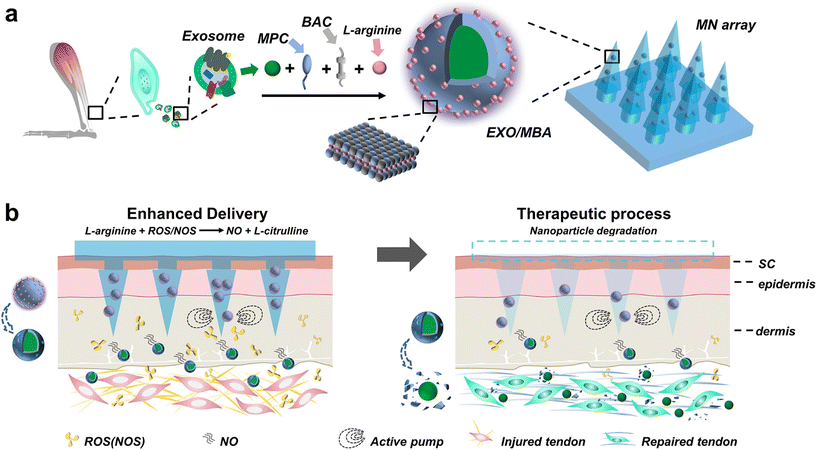 | ||
| Fig. 12 Nitric oxide nanomotor driving exosomes-loaded microneedles schematic illustrations. (a) The manufacturing procedure of EXO/MBA-loaded MN array. (b) The healing process of Achilles tendinopathy after application of EXO/MBA-loaded MN array. Reprinted with permission from American Chemical Society,73 Copyright 2021. | ||
The initial gene delivery methods, such as microinjection, were inefficient due to the limitations of microscopic manipulation.74,75 In contrast, the use of MN for exogenous nucleic acid delivery ensures effective uptake and utilization while meeting basic requirements. Physical techniques like electroporation, iontophoresis, and ultrasound have been shown to enhance nucleic acid uptake and utilization.76 Previous studies utilized hollow conductive MN combined with electrical pulses for DNA electroporation. However, these early studies encountered challenges such as electric field conditions and high viscosity of DNA solutions, resulting in suboptimal delivery capacity and efficiency.77 Therefore, subsequent studies focused on optimizing DNA delivery flux and electrode design for MN. The combination of soluble MNs and electrodes, known as hybrid electro-MN, increased the delivery flux by acting as a gene pool and facilitated in situ transfection through electrical stimulation. Additionally, the integration of metal transfer microforming technology improved the suitability of MN electrode arrays for electroporation, significantly enhancing nucleic acid delivery and utilization efficiency.78,79
Nevertheless, crossing the barrier of nucleic acids is just the first step, and escaping from the endosome-lysosome degradation pathway is crucial for their effectiveness. Coating nucleic acids with SiO2 shells or using amphiphilic peptide RALA has been commonly employed to facilitate intracellular transmembrane delivery and endosomal escape, thereby improving transfection efficiency.80–82
DNA vaccines are cost-effective, stimulate effective cellular and humoral immune responses, and are considered an ideal alternative to traditional vaccines. MNs enable the efficient delivery of DNA vaccines and offer a simple method that enhances patient compliance, particularly during pandemics.83 Interestingly, studies have shown that DNA vaccine delivery to the skin via MNs outperforms traditional intramuscular injection in terms of inducing conjugated antibodies and recalling antibody secretion responses. This superior outcome may be attributed to the direct delivery of vaccine DNA to numerous antigen-presenting cells in the skin through MNs, resulting in improved protection against lethal infections.84,85 However, the immunogenicity of DNA vaccines is relatively weak compared to inactivated vaccines, necessitating further improvements in MN technology to recruit immune cells, such as antigen-presenting cells, and enhance the potency of DNA vaccines. In Table 3, MNs for delivering cells, exosomes, and nucleic acids are presented.
| Seeds | Type | MN design | Application | Advantages | Drawbacks | |
|---|---|---|---|---|---|---|
| Materials | Height/diameter | |||||
| Melanocyte63 | Hollow MN | Silicon | 400–700 μm/75–150 μm | Delivery of melanocytes to the skin for vitiligo | Can achieve precise delivery of cells and precise control of cell volume | A high concentration of cells may produce blockage; silicon MN has the risk of fracture |
| Mesenchymal stromal cell66 | Hydrogel MN | Elastin-like polypeptide gel/HA | 600 μm/300 μm | Delivery of mesenchymal stromal cell for myocardial infarction | Basal lysis can be achieved without suturing and disassembly | Requires open-heart surgery and other instruments for delivery, difficult to translate clinically |
| Cardiac stromal cells67 | Hydrogel MN | PVA | 600 μm/300 μm | Delivery of cardiac stromal cells for myocardial infarction | MN generates channels that allow the exchange of material between cells and the host myocardium | Requires open-heart surgery, difficult to translate clinically |
| CAR-T cells64 | Solid MN | PLGA | 1500 μm/500 μm | CAR-T treatment | Etching increases CAT-T cell infiltration | Cellular action is limited by the rate of PLGA degradation |
| MSC70 | Hydrogel MN | Gelma | 300 μm/300 μm | Spinal cord injury repair | In situ, the production and release of exosomes can be achieved | The substrate and tip are the same material, substrate separation is not achieved and the needle can be easily dislodged due to external influence |
| Exosome86 | Hydrogel MN | Methacrylamide HA | 860 μm/360 μm | Promotes diabetic wound healing | The ability of MSC NVs to express therapeutic cytokines can be significantly enhanced when treated with iron nanoparticles | The synthesis step is more complicated |
| Exosome71 | Soluble MN | Carboxymethylcellulose/alginate | 700 μm/210 μm | Enables skin-targeted delivery of curcumin | Addresses the low solubility, bioavailability, and in vivo stability of curcumin | Passive uptake of exosomes efficiency needs to be improved |
| Exosome72 | Hydrogel MN | Gelatin | 500 μm/270 μm | Prevents cardiac fibrosis after myocardial infarction | Addresses poor miRNA stability | Passive uptake of exosomes efficiency needs to be improved |
| Exosome73 | Hydrogel MN | Gelma/HA/PVA | 800 μm/250 μm | Promotes Achilles tendinopathy healing | Enables the active release of exosomes | Complex synthesis; environment-dependent efficiency of action |
| SiRNA81 | Hydrogel MN | HA | 800 μm/300 μm | Delivery of siRNA | Can resist partial nucleic acid degradation | Uncontrollable release rate, too fast in the initial phase |
| DNA82 | Soluble MN | Poly(vinylpyrrolidone) (PVP) | 600 μm/300 μm | Delivery of DNA | Helps nucleic acids to undergo endosomal escape | Cannot accurately determine the amount of DNA delivered from the MN patch to the skin |
| DNA84 | Soluble MN | HA | 750 μm/200 μm | Delivery of DNA influenza vaccine | Superior to conventional intramuscular injection in terms of induction of conjugated antibodies, antibody-secreted recall responses, and interferon-secreted T cells | The relatively weak immunogenicity of the DNA vaccine is also not addressed |
4. Conclusions
Cells, exosomes, and nucleic acids are essential elements in biomedical engineering with significant clinical potential. However, their application is hindered by challenges such as cellular immunogenicity, instability of exosomes and nucleic acids, and difficulties in extraction and culture. Extensive studies have demonstrated that MNs provide an ideal solution for addressing seed extraction, identification, activity maintenance, and delivery issues. Each type of MN has its advantages and disadvantages, and constructing suitable MNs according to specific usage scenarios is a critical aspect of MN research.However, there are still many problems in the application and clinical translation of MNs. (1) MN is limited by its volume, and the amount of cells, exosomes, or nucleic acids obtained is small, which makes it difficult to be applied to clinical treatment. (2) Although Cryomicroneedles largely solve the problem of activity retention, they lose their proper mechanical properties in a few tens of seconds after being removed from the cryogenic environment and may cause some sensory discomfort. (3) The administration of MN deviates from the minimally invasive concept, for example, open-heart surgery is required to expose the heart and achieve myocardial delivery of MN. (4) Most MNs of the contained substance are passively released, and efficiency is difficult to ensure. (5) It is difficult to precisely quantify the amount of seeds delivered. There may be many similar problems, and our work gives some ideas, but more research is needed to demonstrate its feasibility.
In the future, the development of MN in biomedical engineering should primarily serve clinical diagnosis and treatment. This can be achieved by: (1) developing integrated disease indicator detection kits, which are highly valuable for early prevention and treatment of diseases. (2) Vigorous development of wearable and smart MN devices for real-time monitoring and integrated disease management. (3) Develop high-throughput MN extraction methods to apply seed components to clinical disease treatment. (4) Promote local delivery of MN to achieve intelligent, on-demand, responsive release, combined with biobatteries to actively release the contained components. (5) Precisely control the dose of MN delivery to facilitate standardized clinical translation. (6) Maintain the concept of minimally invasive surgery and utilize new structures, such as capsule MNs, to achieve minimally invasive clinical applications of MNs. It is foreseeable that MNs will bridge the gap between conservative treatment and surgery and become the backbone of minimally invasive therapies in clinical practice.
Author contributions
Shufei Zhang: Conceptualization, writing – original draft, visualization. Lian Yang: Resources, writing – review & editing. Jianfeng Liu: Resources, supervision. Hanyue Li: Investigation. Shasha Hong: Investigation. Li Hong: Writing-review & editing, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We would like to thank all the researchers who have contributed to the relevant studies covered in this paper. This work was financially supported by Hubei provincial Key Research and Development Program (2022BCA045); the National Key Research and Development Program of China (2021YFC2701300); the National Natural Science Foundation of China (81971364; 82001527), Second level fund of the second medical leading talents project of Hubei province (No. [2019]47) and Natural Science Foundation of Hubei Province (2022CFB124, 2021CFB113).References
- Y. Wang, Z. Li, F. Mo, T. J. Chen-Mayfield, A. Saini, A. M. LaMere and Q. Hu, Chem. Soc. Rev., 2023, 52(3), 1068–1102 RSC.
- A. Joorabloo and T. Liu, J. Controlled Release, 2023, 356, 463–480 CrossRef CAS PubMed.
- L. He, Y. Chen, S. Lin, R. Shen, H. Pan, Y. Zhou, Y. Wang, S. Chen and J. Ding, Aging Cell, 2023, 22(6), e13840 CrossRef CAS PubMed.
- M. Zhou, B. Li, C. Liu, M. Hu, J. Tang, J. Min, J. Cheng and L. Hong, Int. Immunopharmacol., 2021, 101(Pt B), 108223 CrossRef CAS PubMed.
- P. Makvandi, M. Kirkby, A. Hutton, M. Shabani, C. Yiu, Z. Baghbantaraghdari, R. Jamaledin, M. Carlotti, B. Mazzolai, V. Mattoli and R. F. Donnelly, Nano-Micro Lett., 2021, 13(1), 93 CrossRef CAS PubMed.
- S. Henry, D. V. McAllister, M. G. Allen and M. R. Prausnitz, J. Pharm. Sci., 1998, 87(8), 922–925 CrossRef CAS PubMed.
- A. Himawan, L. K. Vora, A. D. Permana, S. Sudir, A. R. Nurdin, R. Nislawati, R. Hasyim, C. J. Scott and R. F. Donnelly, Adv. Healthcare Mater., 2023, 12(5), e2202066 CrossRef PubMed.
- J. Yang, J. Yang, X. Gong, Y. Zheng, S. Yi, Y. Cheng, Y. Li, B. Liu, X. Xie, C. Yi and L. Jiang, Adv. Healthcare Mater., 2022, 11(10), e2102547 CrossRef PubMed.
- K. Aich, T. Singh and S. Dang, Drug Delivery Transl. Res., 2022, 12(7), 1556–1568 CrossRef CAS PubMed.
- D. V. McAllister, P. M. Wang, S. P. Davis, J. H. Park, P. J. Canatella, M. G. Allen and M. R. Prausnitz, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(24), 13755–13760 CrossRef CAS PubMed.
- N. Sargioti, T. J. Levingstone, E. D. O’Cearbhaill, H. O. McCarthy and N. J. Dunne, Bioengineering, 2022, 10(1), 24 CrossRef PubMed.
- I. Es, A. Kafadenk, M. B. Gormus and F. Inci, Small, 2023, e2206510 CrossRef PubMed.
- C. J. Martin, C. J. Allender, K. R. Brain, A. Morrissey and J. C. Birchall, J. Controlled Release, 2012, 158(1), 93–101 CrossRef CAS PubMed.
- C. S. Kolli and A. K. Banga, Pharm. Res., 2008, 25(1), 104–113 CrossRef CAS PubMed.
- D. D. Zhu, Q. L. Wang, X. B. Liu and X. D. Guo, Acta Biomater., 2016, 41, 312–319 CrossRef CAS PubMed.
- W. Yu, G. Jiang, Y. Zhang, D. Liu, B. Xu and J. Zhou, J. Mater. Chem. B, 2017, 5(48), 9507–9513 RSC.
- S. Kang, J. E. Song, S. H. Jun, S. G. Park and N. G. Kang, Pharmaceutics, 2022, 14(9), 1758 CrossRef CAS PubMed.
- R. Ingrole and H. S. Gill, J. Pharmacol. Exp. Ther., 2019, 370(3), 555–569 CrossRef CAS PubMed.
- U. Angkawinitwong, A. J. Courtenay, A. M. Rodgers, E. Larraneta, H. O. McCarthy, S. Brocchini, R. F. Donnelly and G. R. Williams, ACS Appl. Mater. Interfaces, 2020, 12(11), 12478–12488 CrossRef CAS PubMed.
- R. Haj-Ahmad, H. Khan, M. S. Arshad, M. Rasekh, A. Hussain, S. Walsh, X. Li, M. W. Chang and Z. Ahmad, Pharmaceutics, 2015, 7(4), 486–502 CrossRef CAS PubMed.
- B. Z. Chen, M. C. He, X. P. Zhang, W. M. Fei, Y. Cui and X. D. Guo, Drug Delivery Transl. Res., 2022, 12(11), 2730–2739 CrossRef CAS PubMed.
- X. Li, Q. Xu, J. Wang, P. Zhang, Y. Wang and J. Ji, J. Mater. Chem. B, 2021, 9(27), 5528–5536 RSC.
- F. J. Verbaan, S. M. Bal, D. J. van den Berg, J. A. Dijksman, M. van Hecke, H. Verpoorten, A. van den Berg, R. Luttge and J. A. Bouwstra, J. Controlled Release, 2008, 128(1), 80–88 CrossRef CAS PubMed.
- N. Nirmayanti, A. Alhidayah, J. T. Usman, J. F. Nur, M. N. Amir and A. D. Permana, AAPS PharmSciTech, 2022, 24(1), 5 CrossRef PubMed.
- P. Ananda, D. Elim, H. S. Zaman, W. Muslimin, M. Tunggeng and A. D. Permana, Int. J. Pharm., 2021, 609, 121204 CrossRef CAS PubMed.
- B. Chiang, Y. C. Kim, H. F. Edelhauser and M. R. Prausnitz, Exp. Eye Res., 2016, 145, 424–431 CrossRef CAS PubMed.
- C. D. Owens, W. J. Gasper, J. P. Walker, H. F. Alley, M. S. Conte and S. M. Grenon, J. Vasc. Surg., 2014, 59(4), 1016–1024 CrossRef PubMed.
- C. G. Li, C. Y. Lee, K. Lee and H. Jung, Biomed. Microdevices, 2013, 15(1), 17–25 CrossRef PubMed.
- X. Jiang and P. B. Lillehoj, Microsyst. Nanoeng., 2020, 6, 96 CrossRef CAS PubMed.
- X. Luo, Q. Yu, Y. Liu, W. Gai, L. Ye, L. Yang and Y. Cui, ACS Sens., 2022, 7(5), 1347–1360 CrossRef CAS PubMed.
- A. K. Shakya, C. H. Lee and H. S. Gill, J. Allergy Clin. Immunol., 2018, 142(6), 2007–2011 CrossRef CAS PubMed.
- R. Murty, A. Sankaranarayanan, I. I. Bowland, J. Mena-Lapaix and M. R. Prausnitz, Pharmaceutics, 2022, 14(2), 347 CrossRef CAS PubMed.
- A. K. Shakya, C. H. Lee and H. S. Gill, Mol. Pharm., 2020, 17(8), 3033–3042 CrossRef CAS PubMed.
- Y. Ito, E. Hagiwara, A. Saeki, N. Sugioka and K. Takada, Eur. J. Pharm. Sci., 2006, 29(1), 82–88 CrossRef CAS PubMed.
- Y. Zeng, C. Wang, K. Lei, C. Xiao, X. Jiang, W. Zhang, L. Wu, J. Huang and W. Li, Adv. Healthcare Mater., 2023, e2300250 CrossRef PubMed.
- Y. Meng, X. J. Li, Y. Li, T. Y. Zhang, D. Liu, Y. Q. Wu, F. F. Hou, L. Ye, C. J. Wu, X. D. Feng, X. J. Ju and L. Jiang, ACS Appl. Mater. Interfaces, 2023, 15, 13892–13906 CAS.
- E. Zhao, T. Xiao, Y. Tan, X. Zhou, Y. Li, X. Wang, K. Zhang, C. Ou, J. Zhang, Z. Li and H. Liu, ACS Appl. Mater. Interfaces, 2023, 15(6), 7725–7734 CrossRef CAS PubMed.
- Y. Li, X. J. Ju, H. Fu, C. H. Zhou, Y. Gao, J. Wang, R. Xie, W. Wang, Z. Liu and L. Y. Chu, ACS Appl. Mater. Interfaces, 2023, 15(1), 638–650 CrossRef CAS PubMed.
- M. Kim, B. Jung and J. H. Park, Biomaterials, 2012, 33(2), 668–678 CrossRef CAS PubMed.
- R. F. Donnelly, K. Mooney, M. T. McCrudden, E. M. Vicente-Perez, L. Belaid, P. Gonzalez-Vazquez, J. C. McElnay and A. D. Woolfson, J. Pharm. Sci., 2014, 103(5), 1478–1486 CrossRef CAS PubMed.
- Q. Yang, Y. Wang, T. Liu, C. Wu, J. Li, J. Cheng, W. Wei, F. Yang, L. Zhou, Y. Zhang, S. Yang and H. Dong, ACS Nano, 2022, 16(11), 18366–18375 CrossRef CAS PubMed.
- W. Park, S. W. Maeng, J. W. Mok, M. Choi, H. J. Cha, C. K. Joo and S. K. Hahn, Biomacromolecules, 2023, 24(3), 1445–1452 CrossRef CAS PubMed.
- J. Chi, X. Zhang, C. Chen, C. Shao, Y. Zhao and Y. Wang, Bioact. Mater., 2020, 5(2), 253–259 CrossRef PubMed.
- L. Fan, X. Zhang, X. Liu, B. Sun, L. Li and Y. Zhao, Adv. Healthcare Mater., 2021, 10(9), e2002249 CrossRef PubMed.
- J. Y. Li, Y. H. Feng, Y. T. He, L. F. Hu, L. Liang, Z. Q. Zhao, B. Z. Chen and X. D. Guo, Acta Biomater., 2022, 153, 308–319 CrossRef CAS PubMed.
- Z. Guo, H. Liu, Z. Shi, L. Lin, Y. Li, M. Wang, G. Pan, Y. Lei and L. Xue, J. Mater. Chem. B, 2022, 10(18), 3501–3511 RSC.
- X. Zhang, X. Fu, G. Chen, Y. Wang and Y. Zhao, Adv. Sci., 2021, 8(17), e2101210 CrossRef PubMed.
- Z. Chen, X. Han, X. Ouyang, J. Fang, X. Huang and H. Wei, Theranostics, 2019, 9(22), 6354–6368 CrossRef CAS PubMed.
- Y. Wang, C. N. Phillips, G. S. Herrera, C. E. Sims, J. J. Yeh and N. L. Allbritton, RSC Adv., 2013, 3(24), 9264–9272 RSC.
- T. S. Martins, M. Vaz and A. G. Henriques, Anal. Bioanal. Chem., 2023, 415(7), 1239–1263 CrossRef CAS PubMed.
- R. Paul, A. C. Saville, J. C. Hansel, Y. Ye, C. Ball, A. Williams, X. Chang, G. Chen, Z. Gu, J. B. Ristaino and Q. Wei, ACS Nano, 2019, 13(6), 6540–6549 CrossRef CAS PubMed.
- H. Li, J. Feng, Y. Wang, G. Liu, X. Chen and L. Fu, J. Agric. Food Chem., 2021, 69(24), 6879–6887 CrossRef CAS PubMed.
- Y. Qiao, J. Du, R. Ge, H. Lu, C. Wu, J. Li, S. Yang, S. Zada, H. Dong and X. Zhang, Anal. Chem., 2022, 94(14), 5538–5545 CrossRef CAS PubMed.
- B. Yang, J. Kong and X. Fang, Nat. Commun., 2022, 13(1), 3999 CrossRef CAS PubMed.
- P. R. Miller, R. M. Taylor, B. Q. Tran, G. Boyd, T. Glaros, V. H. Chavez, R. Krishnakumar, A. Sinha, K. Poorey, K. P. Williams, S. S. Branda, J. T. Baca and R. Polsky, Commun. Biol., 2018, 1, 173 CrossRef PubMed.
- K. Lee, Y. Xue, J. Lee, H. J. Kim, Y. Liu, P. Tebon, E. Sarikhani, W. Sun, S. Zhang, R. Haghniaz, B. Celebi-Saltik, X. Zhou, S. Ostrovidov, S. Ahadian, N. Ashammakhi, M. R. Dokmeci and A. Khademhosseini, Adv. Funct. Mater., 2020, 30(23), 2000086 CrossRef CAS PubMed.
- V. D. Bui, S. Son, W. Xavier, V. Q. Nguyen, J. M. Jung, J. Lee, S. Shin, W. Um, J. Y. An, C. H. Kim, Y. Song, Y. Li and J. H. Park, Biomaterials, 2022, 287, 121644 CrossRef CAS PubMed.
- H. Chang, S. Chew, M. Zheng, D. Lio, C. Wiraja, Y. Mei, X. Ning, M. Cui, A. Than, P. Shi, D. Wang, K. Pu, P. Chen, H. Liu and C. Xu, Nat. Biomed. Eng., 2021, 5(9), 1008–1018 CrossRef CAS PubMed.
- M. Cui, M. Zheng, C. Wiraja, S. Chew, A. Mishra, V. Mayandi, R. Lakshminarayanan and C. Xu, Adv. Sci., 2021, 8(21), e2102327 CrossRef PubMed.
- J. Yu, C. Kuwentrai, H. R. Gong, R. Li, B. Z. Zhang, X. Lin, X. Wang, J. D. Huang and C. Xu, Acta Biomater., 2022, 148, 133–141 CrossRef CAS PubMed.
- X. Zhang, X. Fu, G. Chen, Y. Wang and Y. Zhao, Adv. Sci., 2021, 8(17), e2101210 CrossRef PubMed.
- C. Farias, R. Lyman, C. Hemingway, H. Chau, A. Mahacek, E. Bouzos and M. Mobed-Miremadi, Bioengineering, 2018, 5(3), 59 CrossRef CAS PubMed.
- B. Gualeni, S. A. Coulman, D. Shah, P F. Eng, H. Ashraf, P. Vescovo, G. J. Blayney, L. D. Piveteau, O. J. Guy and J. C. Birchall, Br. J. Dermatol., 2018, 178(3), 731–739 CrossRef CAS PubMed.
- H. Li, Z. Wang, E. A. Ogunnaike, Q. Wu, G. Chen, Q. Hu, T. Ci, Z. Chen, J. Wang, D. Wen, H. Du, J. Jiang, J. Sun, X. Zhang, G. Dotti and Z. Gu, Natl. Sci. Rev., 2022, 9(3), b172 CrossRef PubMed.
- A. N. Kharlamov, H. J. Duckers, H. M. van Beusekom, P. C. Smits, E. C. Perin and P. W. Serruys, Int. J. Cardiol., 2013, 165(2), 217–221 CrossRef PubMed.
- S. Hu, D. Zhu, Z. Li and K. Cheng, ACS Nano, 2022, 16(10), 15935–15945 CrossRef CAS PubMed.
- J. Tang, J. Wang, K. Huang, Y. Ye, T. Su, L. Qiao, M. T. Hensley, T. G. Caranasos, J. Zhang, Z. Gu and K. Cheng, Sci. Adv., 2018, 4(11), t9365 CrossRef PubMed.
- M. R. Prausnitz, Y. Gomaa and W. Li, Nat. Med., 2019, 25(10), 1471–1472 CrossRef CAS PubMed.
- Y. Ju, Y. Hu, P. Yang, X. Xie and B. Fang, Mater. Today Bio, 2023, 18, 100522 CrossRef CAS PubMed.
- M. Han, H. Yang, X. Lu, Y. Li, Z. Liu, F. Li, Z. Shang, X. Wang, X. Li, J. Li, H. Liu and T. Xin, Nano Lett., 2022, 22(15), 6391–6401 CrossRef CAS PubMed.
- S. S. Yerneni, E. P. Yalcintas, J. D. Smith, S. Averick, P. G. Campbell and O. B. Ozdoganlar, Acta Biomater., 2022, 149, 198–212 CrossRef CAS PubMed.
- J. Yuan, H. Yang, C. Liu, L. Shao, H. Zhang, K. Lu, J. Wang, Y. Wang, Q. Yu, Y. Zhang, Y. Yu and Z. Shen, Adv. Healthcare Mater., 2023, e2202959 CrossRef PubMed.
- A. Liu, Q. Wang, Z. Zhao, R. Wu, M. Wang, J. Li, K. Sun, Z. Sun, Z. Lv, J. Xu, H. Jiang, M. Wan, D. Shi and C. Mao, ACS Nano, 2021, 15(8), 13339–13350 CrossRef CAS PubMed.
- C. Kondor-Koch, H. Riedel, K. Soderberg and H. Garoff, Proc. Natl. Acad. Sci. U. S. A., 1982, 79(15), 4525–4529 CrossRef CAS PubMed.
- F. Yamamoto, M. Furusawa, I. Furusawa and M. Obinata, Exp. Cell Res., 1982, 142(1), 79–84 CrossRef CAS PubMed.
- E. Gonzalez-Gonzalez, T. J. Speaker, R. P. Hickerson, R. Spitler, M. A. Flores, D. Leake, C. H. Contag and R. L. Kaspar, Mol. Ther., 2010, 18(9), 1667–1674 CrossRef CAS PubMed.
- L. Daugimont, N. Baron, G. Vandermeulen, N. Pavselj, D. Miklavcic, M. C. Jullien, G. Cabodevila, L. M. Mir and V. Preat, J. Membr. Biol., 2010, 236(1), 117–125 CrossRef CAS PubMed.
- K. Lee, J. D. Kim, C. Y. Lee, S. Her and H. Jung, Biomaterials, 2011, 32(30), 7705–7710 CrossRef CAS PubMed.
- S. O. Choi, Y. C. Kim, J. W. Lee, J. H. Park, M. R. Prausnitz and M. G. Allen, Small, 2012, 8(7), 1081–1091 CrossRef CAS PubMed.
- A. Kumar, P. Wonganan, M. A. Sandoval, X. Li, S. Zhu and Z. Cui, J. Controlled Release, 2012, 163(2), 230–239 CrossRef CAS PubMed.
- M. Wang, Y. Han, X. Yu, L. Liang, H. Chang, D. C. Yeo, C. Wiraja, M. L. Wee, L. Liu, X. Liu and C. Xu, Adv. Healthcare Mater., 2020, 9(2), e1900635 CrossRef PubMed.
- J. McCaffrey, C. M. McCrudden, A. A. Ali, A. S. Massey, J. W. McBride, M. T. McCrudden, E. M. Vicente-Perez, J. A. Coulter, T. Robson, R. F. Donnelly and H. O. McCarthy, J. Controlled Release, 2016, 226, 238–247 CrossRef CAS PubMed.
- J. W. Hooper, J. W. Golden, A. M. Ferro and A. D. King, Vaccine, 2007, 25(10), 1814–1823 CrossRef CAS PubMed.
- J. M. Song, Y. C. Kim, E. O, R. W. Compans, M. R. Prausnitz and S. M. Kang, Mol. Ther., 2012, 20(7), 1472–1480 CrossRef CAS PubMed.
- Y. C. Kim, J. M. Song, A. S. Lipatov, S. O. Choi, J. W. Lee, R. O. Donis, R. W. Compans, S. M. Kang and M. R. Prausnitz, Eur. J. Pharm. Biopharm., 2012, 81(2), 239–247 CrossRef CAS PubMed.
- W. Ma, X. Zhang, Y. Liu, L. Fan, J. Gan, W. Liu, Y. Zhao and L. Sun, Adv. Sci., 2022, 9(13), e2103317 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2023 |