Single-atom catalysts for thermal- and electro-catalytic hydrogenation reactions
Jingfang
Zhang
a,
Hongjuan
Zhang
a,
Yongmeng
Wu
b,
Cuibo
Liu
b,
Yi
Huang
 *bc,
Wei
Zhou
*bc,
Wei
Zhou
 *b and
Bin
Zhang
*b and
Bin
Zhang
 *b
*b
aDepartment of Chemistry, College of Science, Hebei Agricultural University, Baoding 071001, China
bSchool of Science, Institute of Molecular Plus, Tianjin University, Tianjin 300072, China. E-mail: weizhou@tju.edu.cn; bzhang@tju.edu.cn
cInstitute of Environmental & Applied Chemistry, College of Chemistry, Central China Normal University, Wuhan 430079, China. E-mail: yihuang@mail.ccnu.edu.cn
First published on 1st November 2021
Abstract
Hydrogenation reactions are among the most significant transformations in the chemical, energy, and environmental industries, which calls for a new generation of promising catalysts towards economic growth and environmental sustainability. Single-atom catalysts (SACs) have emerged as attractive candidates because of their maximum atom utilization, and high catalytic activity and selectivity. In this review, we introduce the unique properties of SACs including structural and electronic features, to guide the rational design of SACs and demonstrate a more detailed understanding of reaction mechanisms. Then, thermal catalytic hydrogenation of organic compounds and electrocatalytic hydrogenation with a particular focus on nitrate electroreduction reactions over SACs are further summarized, and the underlying mechanisms are discussed. Finally, the emerging issues and related perspectives for the future development of SACs in this field are proposed.
1. Introduction
Hydrogenation is one of the most important transformations used for manufacture and processing in the petrochemical, fine chemical, and environmental industries.1–5 Generally, the hydrogenation reaction refers to a reduction process, which results in the addition of hydrogen to unsaturated compounds. The compounds are not only limited to organic ones (such as alkynes, aldehydes, alkenes, ketones, nitroarenes, and so on), but also span a variety of inorganic ones (such as metal carbonates, nitrates, carbon dioxide, nitrogen, and so on).6–10 The thermal-catalytic hydrogenation reaction, in most cases, uses gaseous hydrogen (H2) as the hydrogen source to reduce unsaturated compounds with a suitable catalyst capable of dissociating hydrogen gas. For example, in the thermal-catalytic hydrogenation of alkynes under reaction conditions of 175 °C and 0.1 MPa H2, ethylene could be obtained at 94% conversion over the Au/SiO2 catalyst.11 However, hydrogenation by thermal catalysis is impractical when H2 is unavailable. Alternatively, electricity can be stored to supply reduction equivalents,12 and thus the electrochemical method is emerging as a promising approach for hydrogenation transformations. In hydrogenation reactions including both thermal- and electro-catalytic transformations, selectivity holds the key to the production of desired products. Selective hydrogenation reactions provide a great opportunity for improving the selectivity, which means that one of the functional groups is preferentially hydrogenated to a certain degree while others remain intact, when two or more unsaturated functional groups are present in one substrate, or different unsaturated substrates exist in the catalytic system.13 For example, unsaturated hydrocarbons such as alkynes and alkenes coexist in the raw materials of the petroleum industry, and it is expected that alkynes are selectively hydrogenated to alkenes while alkenes either produced or fed are not further saturated to alkanes. Therefore, selective hydrogenation transformations are of great importance for the efficient production of targeted chemicals in both bulk and fine chemical industries.The achievement of selective hydrogenation transformations is largely attributed to highly active and selective catalysts. Currently, catalysts can be classified as homogeneous and heterogeneous catalysts with metal centers as active sites. Homogeneous catalysts often exhibit high selectivity because of their well-defined single-site structure, however, the disadvantages of poor stability, hard separation, and difficulty to reuse make them unfavorable for industrial applications.14 In contrast, heterogeneous catalysts dominate large-scale industrial production in terms of reusability, easy separation, and good stability even under relatively harsh reaction conditions. The selectivity of heterogeneous catalysts in hydrogenation reactions relies on the absorption ability of reaction intermediates on the surface of catalysts, which is determined by the electronic and geometric structures of active sites.15 Multiple catalytic sites in common heterogeneous catalysts possess different coordination environments and electronic structures, leading to various adsorption modes of reaction intermediates and thus poor selectivity. Taking the semi-hydrogenation of acetylene over the Pd metal surface as an example, three possible adsorption modes of ethylene may occur in the reaction process according to the configuration of Pd atoms: ethylidyne mode and di–σ mode on continuous Pd active sites (3-fold sites and 2-fold sites, respectively), and π-bonded mode on isolated Pd single atoms.16,17 The strong absorption strength of ethylidyne mode or di–σ mode will result in dimerization or over-hydrogenation of acetylene into ethane, and the weak absorption strength of π-bonded mode is conducive to the desorption of ethylene from the Pd surface and thus prevents its further hydrogenation to ethane.18,19 Therefore, it is highly desirable to construct heterogeneous catalysts with well-defined and uniform active sites for achieving excellent selectivity in hydrogenation transformations.
Single-atom catalysts (SACs) with atomically dispersed metal atoms on supports have recently emerged as a new frontier to bridge the gap between homogeneous catalysts with well-defined structures and heterogeneous catalysts with structural varieties. The ultimate dispersion of SACs typically relies on strong interactions between single atoms and supports, where supports can be considered to serve the role of ligands in homogeneous catalysts.20 In turn, the strong interactions in SACs will afford them a unique electronic structure and coordination environment, which are directly related to their catalytic activity in thermal- and electro-catalytic reactions, including selective hydrogenation transformations.15,20–24 Moreover, the homogeneity of active sites in SACs provides great potential to achieve a high selectivity because of their uniform electronic and geometric structures. The well-defined and uniform structures of SACs provide a promising platform to study relationships between the structure and catalytic performance, and facilitate a comprehensive understanding of the reaction mechanism, achieving a rational design principle for SACs.
In this review, we summarize recent advances in SACs towards hydrogenation reactions via thermal- and electro-catalytic transformations. The unique geometric and electronic structures of SACs and the general design principle for constructing high-performance SACs are summarized. Then, we focus on their applications in hydrogenation reactions including thermal-catalytic hydrogenation of organic compounds and electro-catalytic nitrate reduction reactions, with an attempt to reveal the potential influencing factors on catalytic activity and selectivity and illustrate the underlying mechanisms at the molecular level. Lastly, the major challenges and opportunities for future research on the design of SACs for hydrogenation reactions are presented.
2. Unique properties and design principles of SACs
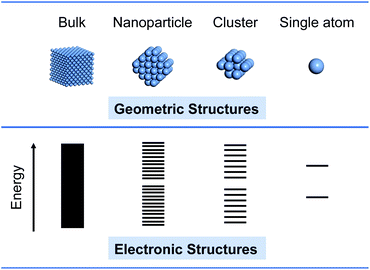
Supported metals are among the most important catalysts in many critical applications, for example, chemical transformation, energy conversion, and environmental remediation. In fact, a large proportion of metal atoms are not involved in the catalytic reaction, because catalysis mainly occurs on the surface of catalysts. Hence, smaller sized metals with high surface/volume ratios will possess more catalytically active sites, in favor of the promotion of catalytic performance. When the size of the metal is decreased from bulk to single atoms, its geometric and electronic structures will strongly change (Fig. 1). The size effects can be mainly featured in the following aspects: (1) More atoms with an unsaturated coordination environment are exposed on the surface.25,26 (2) Due to the quantum size effect, the confinement of electrons will lead to the shift from continuous energy bands to discrete atomic orbitals, increased energy levels and a distinctive highest occupied molecular orbital (HOMO) – lowest unoccupied molecular orbital (LUMO) gap.27–29 (3) The chemical bonding of metal and the support becomes stronger and charge transfer may occur between them.30,31 As a result, these size-dependent effects will cause distinct reactivities in bulk and counterparts.SACs with isolated metal single atoms on the support represent a new generation of catalysts owing to their dual advantages of homogeneous and heterogeneous catalysis. With maximum atom utilization, the development of SACs provides a promising approach to reasonable use of metal resources and achieving atomic economy, especially for noble-metal-based catalysts.32–36 In terms of reactivity, SACs with fully exposed atoms possess more active sites, and the unsaturated coordination environment and charge transfer between single metal atoms and the support will effectively improve the intrinsic activity of active sites.37,38 In this regard, a variety of synthetic methodologies have been developed for SACs. For example, mass-selected soft loading39 and atomic layer deposition40,41 are powerful methods to synthesize SACs. However, the two methods are not suitable for large-scale production due to the requirement of expensive equipment and low yield. Currently, wet-chemistry methods for fabricating SACs, such as defect engineering strategy,42–44 spatial confinement strategy,45–47 and coordination design strategy,48–50 have been widely implemented. Moreover, advanced characterization techniques have provided powerful support to confirm the structures of SACs. Scanning tunneling microscopy (STM) and aberration-corrected electron microscopy (ACEM) techniques can offer direct routes to observe single atoms.51,52 Synchrotron-radiated X-ray absorption fine structure (XAFS) can provide average information about the local atomic and electronic structure of single atoms and their neighboring atoms.53,54 Using CO as the probe molecule, Fourier transform infrared (FTIR) spectroscopy can provide additional information to determine the structure and dispersion of metal centers.55,56 By combining these characterization techniques with density functional theory (DFT) calculations, great progress will be made in design and synthesis, expansion of application fields, and further uncovering the activity origin of SACs.
Heterogeneous catalysis usually involves the adsorption of reactants on a catalyst, the surface reaction of adsorbed species, and the desorption of products.57 Generally, the initial absorption behavior determines the following reaction pathways, and is correlated with the catalytic activity and selectivity.58 In terms of the absorption behavior, the absorption manner and strength of a reactant in SACs are totally different from those in metal nanoparticles/clusters. Due to the atomic and homogeneous dispersion of metal atoms in SACs, the only absorption mode of the substrate atop the metal site is allowed, leading to excellent catalytic selectivity. In contrast, supported metal nanoparticles/clusters with continuous assemblies of metal atoms usually have multiple absorption modes with the tendency of 3-fold sites, followed by 2-fold and atop sites,16,17 and consequently the catalytic selectivity is poor. Because of the ultimate dispersion of active metals in SACs, the single metal atom is directly bonded to the functional species on the support and stabilized by the interactions between single metal atoms and the support. The unsaturated coordination configuration and the unique electronic structure of metal sites provided by the metal-support interactions in SACs are usually considered to be responsible for the optimized adsorption strength of the reactant and thus the extraordinary catalytic activity. For example, Zhang and co-workers synthesized Ru/NC SACs by pyrolysis of ruthenium acetylacetonate and C, N-containing precursors for the reductive amination of aldehydes/ketones.59 When the pyrolysis temperature is changed, both the Ru–N coordination structure (e.g., Ru1–N5, Ru1–N4, and Ru1–N3) and the electron density of the Ru single atoms are varied, leading to different catalytic performance. As a consequence, the resulting Ru1–N3 structure with the largest electron density exhibits moderate capability for hydrogen activation, which affords the highest activity and selectivity for the reductive amination of aldehydes/ketones to primary amines. In general, regulating the geometric and electronic structures of active metal centers via metal-support interactions can be exploited as a common design principle for more efficient SACs towards specific reaction pathways.20,60,61
3. Thermal- and electro-catalytic hydrogenation reactions over SACs
3.1 Thermal-catalytic hydrogenation reactions
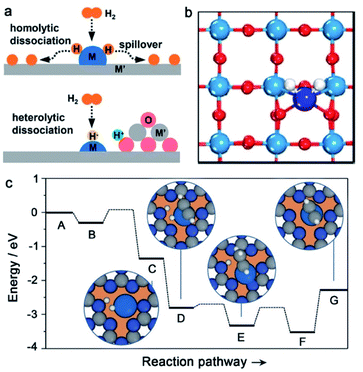 | ||
| Fig. 2 (a) Two representative H2 activation pathways: homolytic and heterolytic dissociation of H2. Reproduced with permission from ref. 15. Copyright 2021 American Chemical Society. (b) Adsorption of H2 on Pt/WOx(001) SACs. Reproduced with permission from ref. 65. Copyright 2020 Elsevier. (c) Energies and models for Pd SAC catalyzed hydrogenation of acetylene. Reproduced with permission from ref. 67. Copyright 2015 John Wiley and Sons. | ||
In the homolytic pathway, H2 is homolytically dissociated into H atoms to form two metal–Hδ− species. The homolytic dissociation of H2 always occurs on the metal surface with partially occupied d-orbitals (such as VIII group metals), which can accept the σ electrons of H2 and donate d-electrons to the σ* antibonding orbital of H2. Due to the donation behavior of d-electrons, it is more favorable for metal sites with a high electron density to proceed with the homolytic dissociation of H2. Moreover, the completion of the homolytic dissociation of H2 often requires multi-center ensembles of metal surfaces in a heterogeneous metal catalyst. Although the two hydrogen species formed in a homolytic pathway have indistinguishable reactivities, hydrogen spillover occurs easily on metal surfaces, for example, hydrogen species migrate from the metal surface to the support, accompanied by the electron transfer process and simultaneous reduction of the support surface.13 In this way, the H2 activation and hydrogenation sites are physically separated. When the operation conditions change (e.g., hydrogen partial pressure), hydrogen reverse spillover can also happen, which is thermodynamically favorable for late transition metals, except for Au.62–64 Interestingly, SACs (e.g., Pt1/WOx) have recently been reported to cleave H2 in a homolytic dissociation pathway (Fig. 2b).65 The Pt single atoms are well stabilized by the oxygen vacancies on the WOx(001) surface. Due to the electron transfer from oxygen vacancies to the Pt single atoms, Pt single atoms carry a negative charge. The DFT results prove that the negatively charged Pt single atoms can facilitate the homolytic dissociation of H2 to metal–Hδ− species, resembling that occurring in metal ensembles. However, the as-formed metal–H species prefer to transfer to its neighboring O2c on WOx, implying a hydrogen spillover process to form Brønsted acid sites. As a result, the H2 dissociation on the Pt1/WOx surface occurs in a heterolytic pathway, but it involves a combination of the homolytic cleavage and the further hydrogen spillover process.
In the heterolytic pathway, H2 is heterolytically dissociated into H+/H− pairs. Different from the homolytic dissociation pathway, H2 molecules can be readily dissociated on electron-deficient metal sites in a heterolytic manner. The heterolytic dissociation of H2 often requires the assistance of nucleophilic atoms from supports or surface organic modifiers, where H+ binds to a proton acceptor (e.g., O or N atom) and H− binds to a d-block metal atom.66 The produced H+/H− pairs prefer to reduce polar unsaturated groups, which can largely improve the selectivity of hydrogenation reactions. When the polar unsaturated groups are far away from metal sites, they can also be hydrogenated in the outer sphere because the H+/H− pairs can migrate from the catalyst to the substrates. The heterolytic activation pathway is well known in both homogeneous and heterogeneous catalysts. SACs as a new generation of catalysts have also been reported to activate H2 by the heterolytic pathway. For example, Pérez-Ramírez and co-workers synthesized Pd SACs by anchoring Pd atoms into the cavities of mesoporous polymeric graphitic carbon nitride (mpg-C3N4).67 The activation of H2 takes place through the heterolytic dissociation pathway on single-site Pd, where the cleaved H+ moves to a nearby N atom from mpg-C3N4 to yield N–Hδ+, and the cleaved H− binds to the Pd atom to form Pd–Hδ−. The step is almost barrier-free and exothermic by −1.34 eV compared with the zero-point energy of the gas-phase molecule. The favorable H2 activation step is beneficial to the following hydrogenation process. DFT calculations prove that the hydrogenation of acetylene is a stepwise process (Fig. 2c), which begins with Hδ− transfer from Pd to absorbed acetylene on the Pd site, and then Hδ+ from a nearby N–H group adds to the half-hydrogenated intermediate generated in the previous step. Following this pathway, the hydrogenated products (i.e., alkenes) are obtained.
3.1.2.1 Support effect. It has been well established that the H2 activation step is highly related to the geometric and electronic structures of catalytic metal centers.68 As for SACs, the single atoms are stabilized by the strong interactions with the support via metal–metal bonds or coordination with heteroatoms (e.g., O, S, N, etc.) in the support, which can, in turn, regulate the local chemical environment of catalytic metal sites. In this regard, the underlying supports play a critical role in determining the performance of SACs in thermal-catalytic hydrogenation transformations. Many important types of supports have been developed, such as metals,69 metal (oxyhydr)oxides,70–72 carbides,73 zeolites,74 carbon-based nanomaterials,75etc.
In SACs, most attention is paid to the binding sites with metal centers in the first coordination sphere (Fig. 3), analogous to the ligands of metalloenzymes in nature. The nature of binding sites provides a great possibility for the modulation of the electronic structure of metal centers and thus the catalytic performance. For example, Li and co-workers employed a template-sacrificial approach to construct Fe single atoms on P-doped carbon for hydrogenation of different unsaturated compounds (e.g., N-heterocycles, and functionalized nitroarenes).76 Their experimental and theoretical results proved that one Fe single atom was coordinated with four P atoms and one dioxygen molecule, forming a pyramidal O2–Fe–P4 structure. The O2–Fe–P4 sites can be reduced by H2 to transform into Fe–P4 sites during hydrogenation catalysis, which exhibited high activity and selectivity. However, the corresponding Fe–N–C catalyst with planar Fe–N4 active sites was almost inactive for these hydrogenation reactions under the same reaction conditions. The comparison of Fe–P4 sites and Fe–N4 sites in hydrogenation performance suggested the important role of P binding sites with Fe single atoms for the hydrogenation activity.
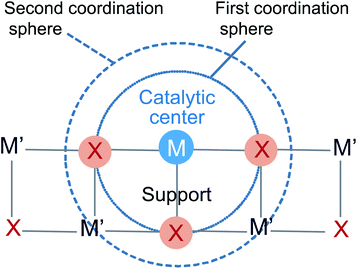 | ||
| Fig. 3 Schematic of the first and second coordination spheres in SACs. Reproduced from ref. 68, under terms of Creative Commons license, published 2018 Oxford University Press. | ||
When the binding atoms with metal centers remain the same, altering the type of binding species can also tune the catalytic hydrogenation performance. Li and co-workers synthesized a series of Cu single atoms on N-doped carbon and investigated the influence of N-doping types (pyrrolic-N and pyridinic-N) on catalytic hydrogenation performance of quinoline (Fig. 4a and b).75 The dominant pyrrolic-N in Cu SACs displayed two fold improved activity compared to pyridinic-N doped Cu SACs. Differential charge density analysis revealed that the electron density of Cu species with pyrrolic-N coordination was higher than that with pyridinic-N coordination, which was beneficial for the formation of the Cu–H bond and decreased the energy barrier of the hydrogenation pathway, thus leading to the enhanced hydrogenation performance. Similarly, the type of P species coordinated with catalytic metal centers also affects the hydrogenation activity.76 A series of single-atom Fe on P-doped carbon with different percentages of graphitic P group (Pgrap) were prepared by changing the carbonization temperature (denoted as Fe–Px–PCCs, where x stands for the carbonization temperature). It was found that the content of Pgrap was highly correlated with the conversion of quinoline catalyzed by Fe–Px–PCCs (Fig. 4c). As a result, the Fe–P900–PCC catalyst with the highest Pgrap content exhibited the best hydrogenation activity.
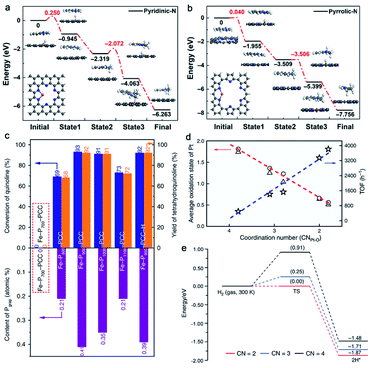 | ||
| Fig. 4 Quinoline hydrogenation process for the coordination of pyridinic-N (a) and pyrrolic-N (b). Reproduced from ref. 75, under the terms of Creative Commons license, published 2020 Springer Nature. (c) The relationships between hydrogenation activity of quinoline and the content of Pgrap. Reproduced from ref. 76, under the terms of Creative Commons license, published 2020 Springer Nature. (d) The correlation between the coordination number of Pt–O and the average oxidation state of Pt and hydrogenation activity of Pt SACs. (e) Energy barriers of H2 dissociation on Pt SACs with different coordination numbers of Pt–O. Reproduced from ref. 72, under the terms of Creative Commons license, published 2019 Springer Nature. | ||
In addition, changing the coordination number (CN) of metal centers, even with the same binding atom, often has an effect on thermal-catalytic hydrogenation performance. For example, Zhang and co-workers used ethanediamine to chelate Pt cations and then removed the ligand by rapid thermal treatment (RTT) to prepare Pt single atoms on the Fe2O3 support (Pt1/Fe2O3-T, T stands for the RTT temperature), which acted as the model catalyst to investigate the relationships between the coordination structure of the Pt single atom and hydrogenation activity.72 The CN of Pt–O and oxidation state of Pt can be finely tuned by adjusting the RTT temperature. As the RTT temperature increased from 500 to 600 °C, the CN of Pt–O in the first shell continuously decreased from 3.8 to 1.8, and the oxidation state of Pt became lower (Fig. 4d). Correspondingly, Pt1/Fe2O3-600 exhibited the best activity with the highest turnover frequency (TOF) of 3809 h−1 at 40 °C among all the Pt1/Fe2O3-T catalysts. As a result, the hydrogenation activity can be well optimized by modifying the coordination and electronic structures of the Pt single atoms. DFT calculations proved that H2 can dissociate spontaneously on two-coordinated Pt1/Fe2O3-600, while three-coordinated Pt1/Fe2O3-550 and four-coordinated Pt1/Fe2O3-500 need a barrier of 0.25 and 0.91 eV to dissociate H2, respectively (Fig. 4e). It can be concluded that the lower the Pt–O CN, the easier the H2 dissociation on Pt SACs, thus the higher the hydrogenation activity of Pt SACs. Such a structure–performance relationship with CN as a key descriptor is also established in SACs for various catalytic applications, such as Fe SACs for selective oxidation of the C–H bond,77 and Co SACs for CO2 electroreduction.78
Beyond the first coordination sphere, the metal cations in metal-based supports, or the functional groups of the carbon substrate (e.g., epoxy groups) in the second coordination sphere (Fig. 3) are readily involved in the catalytic process,79,80 and also have an important effect on hydrogenation performance. For example, Gates and co-workers studied the catalytic performance of ethene hydrogenation over supported single-site Ir complexes (Ir(C2H4)2(acac)).79 MgO and HY zeolite (DAY zeolite) were employed as the supports. The rational design of the two supported catalysts ensured the uniformity in the first coordination sphere, where each Ir atom was coordinated with two oxygen atoms from MgO or DAY zeolite supports and two C2H4 ligands. By evaluating the ethene hydrogenation activity, they found that the Ir complexes on DAY zeolite exhibited 20 times activity enhancement compared to that on MgO. Because of the electron-withdrawing effect of DAY zeolite, the electron density of Ir on DAY zeolite was much lower than that on MgO. The deficient electron structure of Ir on DAY zeolite allowed the simultaneous bonding of Ir with both H2 and C2H4, facilitating the hydrogenation process. In contrast, the electron enrichment of Ir on MgO suppressed the above bonding process, thus hindering the step of H2 activation and leading to a poor catalytic activity. The engineering of the second coordination sphere to optimize the catalytic performance was also demonstrated in electrocatalysis, such as the NiFe coordination polymer catalyst for water oxidation81 and Co SACs for acidic oxygen reduction reaction.80
In addition, the CN of metal centers in the first coordination sphere can be tuned by regulating the second coordination sphere, which is often accompanied by different catalytic performances. For example, Zheng and co-workers employed various supports (e.g., Cu2O, Al2O3, ZnO, and TiO2) to anchor atomically dispersed Pd catalysts for semihydrogenation of terminal alkynes.71 They demonstrated that atomically dispersed Pd on the Cu2O (Pd1/Cu2O) catalyst exhibited high activity and selectivity in the hydrogenation of alkynes, while Pd on other supports was inactive. The Pd1/Cu2O catalyst was prepared by the galvanic displacement reaction between Pd2+ and Cu+ of the Cu2O support. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image showed the atomic distribution of Pd (Fig. 5a). Extended X-ray absorption fine structure (EXAFS) spectroscopy revealed the existence of Pd–O and Pd–Cu bonds without the Pd–Pd bond in the Pd1/Cu2O catalyst (Fig. 5b). X-ray absorption near-edge structure (XANES) spectra were used to analyze the valence state of Pd (Fig. 5c). The valence of Pd in Pd1/Cu2O is +1 and two-coordinated, while Pd on other metal oxide supports is close to +2 and is four-coordinated. The lower valence and unsaturated coordination structure of Pd in Pd1/Cu2O favor the heterolytic activation pathway of H2 to yield O–Hδ+ and Pd–Hδ− species. DFT calculations confirm that the activation barrier was 0.33 eV, and the reaction energy was exothermic by 0.79 eV (Fig. 5d). In contrast, the valence state of +2 and the four-coordinated structure of Pd on other metal oxide supports make Pd inactive for H2 activation, leading to poor catalytic activities. Moreover, the desorption energy (0.26 eV) of styrene on H2-preadsorbed Pd(I) on Pd1/Cu2O is higher than the energy barriers (0.32 and 1.26 eV) required for the following addition of H atoms onto styrene, which effectively prevents the overhydrogenation of phenylacetylene and thus promotes the selectivity (Fig. 5e). More importantly, Pd1/Cu2O also exhibits wide applicability in the hydrogenation of many alkynes with high selectivity. This work provides a good demonstration of the tunability of activity and selectivity in hydrogenation catalysis by manipulating the local coordination environment of active sites via the second coordination sphere.
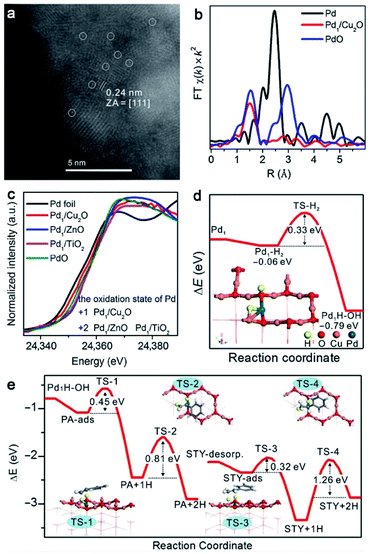 | ||
| Fig. 5 (a) HAADF-STEM image of Pd1/Cu2O. (b) EXAFS spectra at the Pd K-edge of Pd1/Cu2O, Pd foil, and PdO. (c) XANES spectra of Pd catalysts on various supports, Pd foil, and PdO. (d) Energies and model of Pd1/Cu2O in the heterolytic activation of H2. (e) Energies of intermediates and transition states for hydrogenation of phenylacetylene on Pd1/Cu2O. Reproduced from ref. 71, published in CCS Chemistry 2019; Cu2O-supported atomically dispersed Pd catalysts for semihydrogenation of terminal alkynes: critical role of oxide supports is available online (DOI: 10.31635/ccschem.019.20190008). | ||
Therefore, it is important to understand the structure–performance relationships of SACs at the molecular level, including the first and second coordination spheres, rather than individual metal centers.
3.1.2.2 The effect of dopants. In heterogeneous catalysis, it is well known that doping is an effective way to tune the electronic structure of catalytic metal centers and modify the adsorption behavior of reactants, achieving the optimized catalytic performance. Introducing dopants into the vicinal metal sites in SACs can also make a significant influence on catalytic reactivity.
The Group I alkali metals (e.g., Li, Na, and K) lie in the s-block of the periodic table, which readily donate the single s electron in their valence shell. Since the first discovery of the alkali doping effect in 1845,82 the roles of alkali additives have been extensively studied in thermal-catalytic hydrogenation reactions.83,84 Recently, the addition of alkali metal cations into SACs has been reported to promote the hydrogenation reactions via electronic and structural effects.85–87 Another important role of alkali metal cations is that they are able to stabilize the single metal atoms to prevent their aggregation in the presence of hydrogen.88–90 Taking the Pt SAC (with a Pt loading amount of 2.16%) catalyzed hydrogenation reaction of 3-nitrostyrene as an example, the obtained 3-aminostyrene in a high yield relies on the high chemoselectivity of the hydrogenation reaction. According to the previous reports, it is hard to achieve high selectivity over high-loading Pt catalysts due to the easy aggregation properties of single atoms to nanoparticles (with a diameter >1 nm) with high loadings.91 Zhang and co-workers reported that it is possible to transform the nonchemoselective high loading Pt/FeOx catalyst into a highly chemoselective one by the addition of the alkali metal (Fig. 6a).92 By addition of 5 wt% Na, the resulting 5% Na to 2.16% Pt/FeOx catalyst exhibited the best catalytic performance for the hydrogenation reaction of 3-nitrostyrene, with an enhanced chemoselectivity of 97.4% to 3-aminostyrene compared to that of 66.4% for the Na-free 2.16% Pt/FeOx catalyst. At the same time, no obvious change was observed in the catalytic activity of 5% Na to 2.16% Pt/FeOx and Na-free 2.16% Pt/FeOx catalysts. As shown in Fig. 6b and c, it seems that the average size of Pt particles increases after the introduction of Na ions. However, by examination of an individual Pt particle (Fig. 6d and e), one can see that the particles in the 5.03% Na to 2.16% Pt/FeOx catalyst are assembled by loosely and randomly stacked single atoms or clusters of only a few atoms, in contrast with the well-crystallized Pt particles in the 2.16% Pt/FeOx catalyst. In other words, the Na cations can serve the role of ‘interfacial chemical glue’ to stabilize the single Pt atoms and restrain the formation of the Pt nanocrystal. The XANES results of the Pt LIII edge displayed a gradual increase in the whiteline intensity with an increased Na content in these catalysts, indicating that the oxidation state of Pt became more positive. They attributed the positively charged Pt to the interactions between Pt and surface NaFeO2 species via a bridge linkage of Pt–O–Na–O–Fe. Further characterization indicated that the nitro group was more preferentially and strongly adsorbed on NaFeO2 compared with FeOx, leading to the high chemoselectivity of the nitro group hydrogenation over the 5.03% Na to 2.16% Pt/FeOx catalyst.
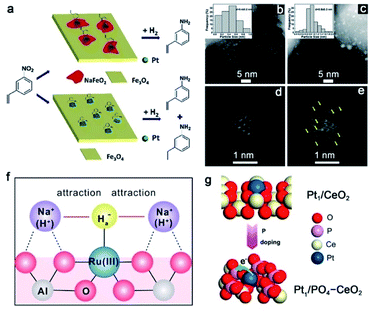 | ||
| Fig. 6 (a) The proposed mechanism for hydrogenation of 3-nitrostyrene over Na-containing and Na-free Pt/FeOx catalysts. HAADF-STEM images of (b and d) 2.16% Pt/FeOx and (c, e) 5.03% Na to 2.16% Pt/FeOx. Reproduced from ref. 92, under terms of Creative Commons license, Published 2017 by Royal Society of Chemistry. (f) Schematic diagram of the role of Na+ cations in stabilizing the Ru(III) species. Reproduced with permission from ref. 93. Copyright 2020, Springer Nature. (g) Schematic illustration of the formation of Pt1/PO4–CeO2 by P doping. Reproduced with permission from ref. 94. Copyright 2019, American Chemical Society. | ||
Zheng and co-workers also demonstrated the promotion effect of alkali cations in the hydrogenation of various substrates.93 They fabricated two Ru SACs on Al2O3 using NaOH and NH4OH as precipitators, denoted as Ru(Na) and Ru(H), respectively. Ru(Na) exhibited enhanced activity and stability in the hydrogenation catalysis compared with those of Ru(H), which can be explained by two important functions of Na+ cations. On one hand, H2 can be favorably dissociated in a heterolytic manner to form Ru–Hδ− and O–Hδ+. The presence of Na+ cations can stabilize hydride species owing to the stronger Na+⋯Hδ− attraction on Ru(Na) than H+⋯Hδ− attraction on Ru(H). Furthermore, the coulombic interactions also restrain the hydride species over Ru(III) from migrating to interfacial oxygen, thus suppressing the reduction and aggregation of Ru (Fig. 6f). On the other hand, the Na+ additives can promote the addition of activated hydrogen species to the substrates by stabilizing the negatively charged intermediates and transition states, experimentally proved by the H2/D2 isotopic study related to H2 activation as the rate-determining step (RDS). However, the presence of Na+ is ineffective in promoting the hydrogenation catalysis when the RDS is H2 activation. The obvious promotion effect of Na+ cations would be observed in the hydrogenation of inert unsaturated substrates with high-barrier hydrogen transfer as the RDS. As expected, Ru(Na) displayed enhanced activity in the hydrogenation of dimethyl oxalate and phenol compared with that of Ru(H).
In addition to vicinal alkali metal sites, doping non-metal elements (e.g., P) into SACs can also facilitate hydrogenation catalysis. For example, Lu and co-workers reported that, with the assistance of P doping, the resulting Pt1/PO4–CeO2 exhibited 10 times higher hydrogenation activity than Pt1/CeO2 for a broad range of substrates (e.g., styrene, cyclohexene, phenylacetylene, and nitrobenzene) (Fig. 6g).94 Structural characterization indicated that P was bonded to the oxygen atoms on Pt–O moieties to form P–O–Pt coordination. The P species serves as an electron acceptor and induces the charge transfer from Pt to P, endowing Pt single atoms with a more positive charge in Pt1/PO4–CeO2. Taking styrene as the probe molecule, they found that the strong adsorption ability of styrene and hydrogen spillover over Pt1/PO4–CeO2 resulted in remarkable activity enhancement.
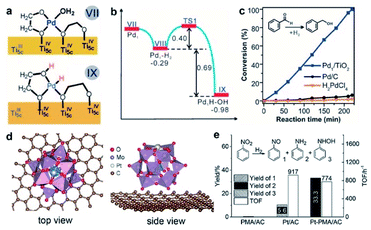
3.1.2.3 The effect of surface ligands. For homogeneous catalysts, the coordination environment of metal sites depends on the organic ligands, which is often correlated with the catalytic activity and selectivity. Apart from the role of binding sites in metal centers, the organic ligands also act as proton acceptors in hydrogenation catalysis.95,96 The great functions of a ligand have inspired the design of highly stable and active SACs. In the case of SACs, the organic ligands can be introduced onto the surface of supports, and the single metal atoms are coordinated to the functional groups (e.g., hydroxyl, thiol, amino, etc.) of organic ligands.97 DFT calculations proved that the type and concentration of oxygen-containing ligands on the carbon support played a critical role in the electronic structure, stability, and catalytic activity of Pt SACs.98 Recently, stable atomically dispersed Pd on ethylene glycolate (EG) ligands modified TiO2 was synthesized by a photochemical strategy.21 When EG-free TiO2 is used as the support, Pd nanoparticles instead of single atoms are formed. Therefore, it can be concluded that the surface EG ligands play a critical role in stabilizing atomically dispersed Pd during the preparation process. Using the hydrogenation of styrene as a probe reaction, Pd1/EG-TiO2 SACs exhibited excellent catalytic activity in the hydrogenation of styrene with a TOF of 8973 h−1 at room temperature. It is found that the EG ligands help to activate H2 in a heterolytic manner to produce H+/H− pairs. The activation barrier for this step is 0.40 eV, and the reaction energy is exothermic by 0.69 eV (Fig. 7a and b). The theoretical and experimental results suggested that the hydrogenation process of styrene began with the transfer of Hδ− from Pd to the terminal CH2 by overcoming a barrier of only 0.47 eV to form the half hydrogenated intermediate. Then, Hδ+ from the nearby OH group was added to the half hydrogenated intermediate with a barrier of 0.73 eV, yielding the product (i.e., ethylbenzene). As a comparison, the Pd1/TiO2 SACs without EG ligands displayed a significantly reduced activity with a TOF of only 1930 h−1. Therefore, the unique H2 activation pathway effectively promoted the hydrogenation reaction. As expected, Pd1/EG-TiO2 SACs also showed much better activity in the hydrogenation of polar unsaturated C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (Fig. 7c).
O bonds (Fig. 7c).
 | ||
| Fig. 7 (a) Models and (b) energies of Pd1/EG-TiO2 and intermediates in the heterolytic H2 activation. (c) Catalytic performances of different catalysts in hydrogenation of benzaldehyde. Reproduced with permission from ref. 21. Copyright 2016, American Association for the Advancement of Science. (d) Top view and side view of the optimized structure of Pt1 on PMA/graphene. (e) Catalytic performances of different catalysts in hydrogenation of nitrobenzene. Reproduced with permission from ref. 100. Copyright 2016 John Wiley and Sons. | ||
In addition to the organic groups, inorganic heteropolyacids have been considered as a class of good ligands for single metal species.99 Specifically, phosphomolybdic acid (H3PMo12O40, PMA) has a classical Keggin structure, which can offer a variety of coordination sites including the single corner site, bridge site (the Oc–Obr-bridge site), three-fold hollow site (3-H_Oc and 3-H_Obr), and four-fold hollow site (4-H). For example, Yan and co-workers synthesized a stable Pt1 SAC on PMA-modified active carbon.100 The DFT results revealed that the Pt atom preferred to bond with four bridging oxygen atoms in the four-fold hollow site with a lower adsorption energy of −5.72 eV compared with other possible coordination sites. The optimized structure of Pt1 on PMA/graphene is shown in Fig. 7d, which has the capacity of stabilizing high loading of Pt single atoms. The XANES and X-ray photoelectron spectroscopy (XPS) results suggested that the charge transfer occurred from Pt to PMA, which made Pt positively charged and thus favored the adsorption of H2. In the catalytic hydrogenation of nitrobenzene, Pt–PMA/AC exhibited outstanding activity and selectivity (Fig. 7e). Though the TOFs of Pt–PMA/AC (ca. 800 h−1) and Pt/AC (ca. 900 h−1) were comparable, the selectivity towards aniline showed enormous improvement from only 50% over Pt/AC to 100% over Pt–PMA/AC. The enhanced performance was ascribed to the favorable adsorption and polarization of the intermediates over positively charged Pt. Importantly, Pt–PMA/AC retained the original activity and selectivity over five cycles, implying the high stability of the catalysts.
3.2 Electrocatalytic hydrogenation
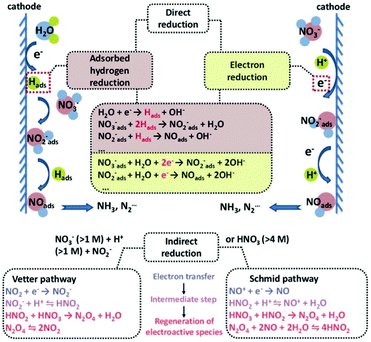
Electrocatalytic hydrogenation has emerged as an energy-efficient and environment-friendly method in catalytic transformations. The initial exploration of electrocatalytic hydrogenation can be dated back to the early 20th century, which pioneered the electrochemical reductions of unsaturated compounds in aqueous media.101–104 The electrocatalytic hydrogenation process involves the generation of absorbed hydrogen (Hads) on the cathode by electroreduction of H2O or H3O+, followed by the reaction of an absorbed substrate and Hads on the cathode, and the desorption of the hydrogenated product. Compared with thermal-catalytic hydrogenation, electrocatalytic hydrogenation has several advantages: (1) the Hads originates from the reduction of H2O via the Volmer reaction, and thus the supply and mass transport of poorly soluble H2 are bypassed. (2) The Hads is electrochemically generated in situ on the cathode surface under mild conditions, without overcoming a high dissociation barrier of H2.105 (3) The amount of Hads is controlled by changing the applied potential.106 Therefore, the electrocatalytic hydrogenation reaction can proceed in a greener, milder, and more controllable way. However, the electrocatalytic hydrogenation is always accompanied by the competing hydrogen evolution reaction (HER), leading to the consumption of Hads through Tafel or Heyrovsky reactions and lowering the faradaic efficiency of electrocatalytic hydrogenation.107 In this section, we take nitrate electroreduction and CO2 electroreduction reactions as typical examples to introduce the recent advances in electrocatalytic hydrogenation.3.2.1.1 Reaction pathways of nitrate electroreduction. As nitrogen (N) contains a wide range of oxidation valences from +5 to −3, nitrate can be electrocatalytically reduced to a variety of nitrogen-containing intermediates or products, such as NO2−, NO, N2O, N2, NH2OH, and NH3. Hence, the NO3RR is a complex multi-electron-involving process. In terms of the reaction pathways, the NO3RR can be classified as an indirect autocatalytic reduction pathway and direct electrocatalytic reduction pathway (Fig. 8).121 The indirect autocatalytic reduction pathway means that NO2 or NO+ instead of nitrate participates in the electron-transfer process, and it only proceeds under the highly acidic media conditions in the presence of nitrate (>1 M) and nitrite or highly concentrated nitric acid solutions (>4 M).122–124 Generally, the NO3RR at low concentrations (below 1 M) is carried out through a direct electrocatalytic reduction pathway, including absorbed hydrogen reduction and electron reduction routes (Fig. 9).125 The main products are N2 and NH3 because of their high thermodynamical stability. The specific reactions are shown as follows (eqn (1) and (2)):110
| 2NO3− + 12H+ + 10e− → N2 + 6H2O, E° = 1.17 V vs. SHE | (1) |
| NO3− + 9H+ + 8e− → NH3 + 3H2O, E° = −0.12 V vs. SHE | (2) |
 | ||
| Fig. 8 The proposed reaction pathways for the NO3RR. Reproduced with permission from ref. 121. Copyright 2021 The Royal Society of Chemistry. | ||
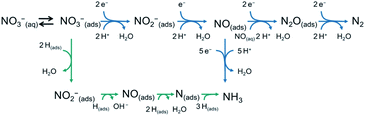 | ||
| Fig. 9 The electron and absorbed hydrogen mediated direct electrocatalytic routes for the NO3RR. Reproduced with permission from ref. 125. Copyright 2021 Elsevier. | ||
In the adsorbed hydrogen-mediated route, the generation of Hadsvia the reduction of H2O initiates the reaction. Due to strong reducing ability of Hads, the absorbed nitrate is reduced by the stepwise Hads addition via a variety of intermediates such as NO2−ads, NOads, Nads, NHads, and NH2ads. As a result, the dominant product is NH3 instead of N2, because the much lower migration barrier (0.10 eV) of Hads than that of Nads (0.75 eV) kinetically favors the formation of the N–H bond compared with the N–N bond.126
In the electron reduction route, the absorbed nitrate is first reduced to nitrite (NO2−ads), which is the rate-determining step. The reaction rate strongly depends on the concentration of nitrate. At low concentrations (<0.1 M), the reaction order of nitrate is positive. The main factor that influences the reaction rate is the competing co-adsorption of anions (e.g., sulfate) from the electrolyte.122 At high concentrations (>0.1 M), the reaction order is negative. This is most likely caused by the fact that the high coverage of nitrate impedes the absorption of other necessary species (e.g., water), highlighting the critical role of free surface sites in the reaction rate.122 The second step is the reduction of NO2−ads into NOads. The NOads is a key intermediate for the end products. When NOads is directly reduced, the end product is NH3via the intermediates of HNOads, H2NOads, and NH2OHads. When NOads reacts with NO dissolved in solution (NOaq), the end product is N2via the intermediate of N2Oads. In addition, N2 can also be produced via other intermediates from the reduction of NOads, such as Nads, or NH2ads.121
3.2.1.2 Mechanism of nitrate electroreduction over SACs. Selectively converting nitrate into environmentally friendly N2 or value-added NH3 through the electrocatalytic NO3RR has been a promising approach to mitigate environmental concerns and obtain chemical products. However, the NO3RR involves a multi-electron/-proton transfer process for the generation of N2 and NH3, leading to the slow reaction kinetics. Moreover, besides the competing NO3−-to-N2 reaction, the conversion of NO3− into NH3 is often accompanied by the generation of H2 through the HER, resulting in low faradaic efficiency.127–129 Therefore, it is desirable to explore efficient and selective NO3RR electrocatalysts to improve the reaction rate and obtain exclusive products.
Due to the unique electronic properties and structural homogeneity of active sites, SACs exhibit high catalytic activity and selectivity in many fields. As expected, SACs hold great promise as excellent electrocatalysts towards the NO3RR. Guo and co-workers used DFT calculations to predict the promising potential of SACs in the NO3RR.130 Using the adsorption energy of NO3− (ΔG*NO3) as a descriptor, they found that Ti/g-CN and Zr/g-CN stood out with low limiting potentials of −0.39 and −0.41 V vs. the reversible hydrogen electrode (RHE), respectively, among a variety of transition metal (from Ti to Au) SACs supported on graphitic carbon nitrides (g-CN). Ti/g-CN and Zr/g-CN SACs can also achieve high-selectivity for the NO3RR towards NH3, because large energy barriers impede the formation of byproducts (NO2, NO, N2O, and N2). Moreover, the adsorption strength of NO3− on Ti/g-CN and Zr/g-CN is stronger than that of the H proton, confirming the NO3RR as favorable compared to the competing HER. This work provides theoretical validation for SACs as promising NO3RR electrocatalysts with high activity and selectivity.
Wang and co-workers synthesized Fe SACs with the Fe–N coordination configuration on the carbon matrix by a pyrolysis-etching-pyrolysis strategy.131 The Fe SACs exhibited high-efficiency NO3RR activity with a high faradaic efficiency of about 75% and a remarkable yield rate of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg h−1 mgcat.−1 at −0.85 V, which is much superior to those of the Fe nanoparticle catalyst (Fig. 10a and b). In addition, the catalyst showed long-term durability after 20 consecutive cycles. DFT calculations revealed Fe–N4 centers in Fe SACs as highly active sites exhibiting much lower thermodynamic barriers, which are responsible for the outstanding NO3RR performance. A similar enhancement effect of Fe–N centers for the NO3RR is also reported in the FeNx site/Fe nanoparticle system.132 Liu and co-workers reported atomically dispersed Fe atoms anchored on S and N-codoped carbon for efficient NO3RR.133 Theoretical and experimental investigations suggested that S-doping created more defects on the N-doped carbon basal plane, which is beneficial to the stabilization of single Fe atoms. Moreover, S-modification can regulate the coordination environments of single Fe atoms, forming a FeN4S2 coordination structure as the dominating active site of the Fe SAC, and improve the basal conductivity. Benefiting from these advantages, the S-modified Fe SACs exhibited high-efficiency NO3RR activity, achieving a maximum nitrate removal capacity of 7822 mg N g−1 Fe and a high faradaic efficiency of 78.4% at a low potential of −0.57 V vs. RHE, for N2 production with 100% selectivity. In addition, the catalyst had good cycling stability with ignorable change after 100 cycles. Yu and co-workers reported a polymer-hydrogel strategy to synthesize N-coordinated single Fe sites on carbon for efficient NO3RR.127 The catalyst exhibited a maximum NH3 yield rate of 2.75 mg h−1 cm−2 with nearly 100% faradaic efficiency. They used surface interrogation scanning electrochemical microscopy (SI-SECM) to investigate the site density of Fe moieties with a dynamic oxidation state by recording the passed charge amounts (Fig. 10c). The transformations of Fe(III/II) in the potential range of 0.8–0.9 V and Fe(II/0) in the potential range of −0.1–0 V are observed for the Fe SAC (Fig. 10d). The potential ranges for the redox behaviors of the Fe–Nx moiety in Fe SACs are more positive and narrower than those in Fe nanoparticles, highlighting the unique thermodynamic and kinetic properties of Fe SACs. They attributed the excellent NO3RR activity and selectivity of Fe SACs to the preoccupied mechanism of NO3− at the Fe(II) site prior to the formation of Fe(0), impeding the competitive HER that usually occurred in bulk catalysts (Fig. 10d). As a result, Fe SACs exhibited a remarkable TOF of 0.7 s−1 at 0.7 V in contrast with that of Fe nanoparticles (0.06 s−1) (Fig. 10e).
000 μg h−1 mgcat.−1 at −0.85 V, which is much superior to those of the Fe nanoparticle catalyst (Fig. 10a and b). In addition, the catalyst showed long-term durability after 20 consecutive cycles. DFT calculations revealed Fe–N4 centers in Fe SACs as highly active sites exhibiting much lower thermodynamic barriers, which are responsible for the outstanding NO3RR performance. A similar enhancement effect of Fe–N centers for the NO3RR is also reported in the FeNx site/Fe nanoparticle system.132 Liu and co-workers reported atomically dispersed Fe atoms anchored on S and N-codoped carbon for efficient NO3RR.133 Theoretical and experimental investigations suggested that S-doping created more defects on the N-doped carbon basal plane, which is beneficial to the stabilization of single Fe atoms. Moreover, S-modification can regulate the coordination environments of single Fe atoms, forming a FeN4S2 coordination structure as the dominating active site of the Fe SAC, and improve the basal conductivity. Benefiting from these advantages, the S-modified Fe SACs exhibited high-efficiency NO3RR activity, achieving a maximum nitrate removal capacity of 7822 mg N g−1 Fe and a high faradaic efficiency of 78.4% at a low potential of −0.57 V vs. RHE, for N2 production with 100% selectivity. In addition, the catalyst had good cycling stability with ignorable change after 100 cycles. Yu and co-workers reported a polymer-hydrogel strategy to synthesize N-coordinated single Fe sites on carbon for efficient NO3RR.127 The catalyst exhibited a maximum NH3 yield rate of 2.75 mg h−1 cm−2 with nearly 100% faradaic efficiency. They used surface interrogation scanning electrochemical microscopy (SI-SECM) to investigate the site density of Fe moieties with a dynamic oxidation state by recording the passed charge amounts (Fig. 10c). The transformations of Fe(III/II) in the potential range of 0.8–0.9 V and Fe(II/0) in the potential range of −0.1–0 V are observed for the Fe SAC (Fig. 10d). The potential ranges for the redox behaviors of the Fe–Nx moiety in Fe SACs are more positive and narrower than those in Fe nanoparticles, highlighting the unique thermodynamic and kinetic properties of Fe SACs. They attributed the excellent NO3RR activity and selectivity of Fe SACs to the preoccupied mechanism of NO3− at the Fe(II) site prior to the formation of Fe(0), impeding the competitive HER that usually occurred in bulk catalysts (Fig. 10d). As a result, Fe SACs exhibited a remarkable TOF of 0.7 s−1 at 0.7 V in contrast with that of Fe nanoparticles (0.06 s−1) (Fig. 10e).
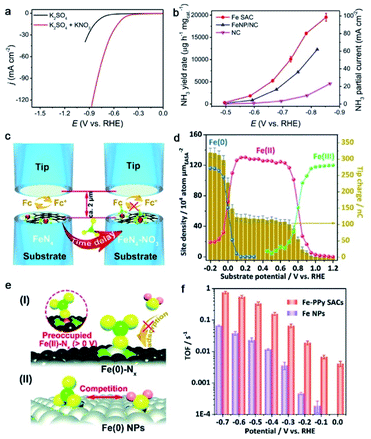 | ||
| Fig. 10 (a) Polarization curves of the Fe SAC in K2SO4 electrolyte with and without KNO3. (b) NH3 yield rate and partial current density of the Fe SAC and its counterparts. Reproduced from ref. 131, under the terms of Creative Commons license, published 2021 Springer Nature. (c) The schematic illustration of the SI-SECM setup for identifying Fe sites. (d) The active site density and corresponding titration charges of Fe SACs at different potentials. (e) The proposed NO3RR mechanism on the Fe SAC and bulk surface. (f) TOFs of Fe SACs and Fe nanoparticles for the NO3RR. Reproduced with permission from ref. 127. Copyright 2021 The Royal Society of Chemistry. | ||
Besides atomically dispersed Fe sites as active centers for the NO3RR, single Cu sites have been reported to catalyze the NO3RR. Feng and co-workers reported a highly active and selective NO3RR catalyst composed of single Cu atoms anchored on nitrogenated carbon nanosheets (Cu–N–C) by pyrolysis of a Cu-based metal–organic framework.134 AC-STEM and XAFS analysis confirmed the successful synthesis of the Cu–N–C catalyst with atomically dispersed Cu–N2 and Cu–N4 mixed coordination structures. Compared to the Cu plate, Cu–N–C exhibited better activity and stability towards the NO3RR. Apart from NO3−, the absorption of NO2− originating from the reduction of NO3− also played a significant role in facilitating the NO3RR. DFT calculations demonstrated that the adsorption of NO3− and NO2− on Cu–Nx active sites (particularly Cu–N2) in Cu–N–C is more favorable than that on the Cu(111) plane in the Cu nanoparticles, suppressing the release of nitrite into the solution, which is confirmed by kinetic measurements. In addition, the Cu–N–C catalysts showed outstanding stability after 20 consecutive cycles.
4. Summary and perspectives
Thermal- and electro-catalytic hydrogenation reactions are important transformations in the chemical, energy and environmental industries. The development of advanced catalysts is of significance to achieve high activity and selectivity for both thermal- and electro-catalytic hydrogenation transformations. SACs represent the maximum utilization efficiency of metal atoms, providing a promising opportunity for constructing cost-effective catalysts, in particular noble metal catalysts. With 100% exposed active sites and unique electronic structures, SACs exhibit remarkable catalytic activity and selectivity. Furthermore, structural simplicity and homogeneity of SACs can offer ultimate facileness for identifying the structure of catalytically active sites and facilitate a comprehensive understanding of the catalytic process and reaction mechanism, and in turn guides the rational design of high-efficiency catalysts. In this study, SACs are shown to act as promising catalysts in hydrogenation transformations.Although great progress has been made in the SAC-catalyzed hydrogenation reaction via both thermal- and electro-catalytic routes, some challenges and promising opportunities should be considered in terms of fundamental research and practical applications:
(1) A large number of SACs have been successfully applied to the thermal-catalytic hydrogenation of unsaturated groups, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and N
N, and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. However, limited SACs are developed for electrocatalytic hydrogenation, especially for the NO3RR, though specific SACs with high-efficiency performance have already been predicted theoretically.130 Therefore, the exploration of smart synthetic methods for development of SACs with a controlled coordination environment of metal atoms is highly desirable. This is also highly expected to achieve large-scale synthesis to meet the demand of practical applications.
O bonds. However, limited SACs are developed for electrocatalytic hydrogenation, especially for the NO3RR, though specific SACs with high-efficiency performance have already been predicted theoretically.130 Therefore, the exploration of smart synthetic methods for development of SACs with a controlled coordination environment of metal atoms is highly desirable. This is also highly expected to achieve large-scale synthesis to meet the demand of practical applications.
(2) The fabrication of SACs with both excellent activity and stability is highly desired in the field of catalysis. Increased single-atom metal-support interaction (SAMSI) is beneficial for the stabilization of SACs, however, excessively strong SAMSI is often accompanied by a certain reduction in reaction activity.145,146 Therefore, more efforts should be made to break the activity–stability seesaw effect of SACs. For example, the exploration of single atom alloy or trimer catalysts with synergistic functions of different sites is an urgent need to achieve high activity without compromising the stability for thermal-/electro-catalytic hydrogenations.69,147
(3) It has been well accepted that the regulation of electronic structures of metal sites in SACs predominates catalytic performance. It should be noted that the catalytic process not only involves the participation of metal centers, but also is assisted by potential active centers, or other functional atoms through structural effects in the first and second coordination spheres. However, current studies mostly pay little attention to the regulation of the second coordination spheres and its effects on catalytic kinetics, especially for electrocatalytic hydrogenation (e.g., NO3RR). Therefore, one needs to design and synthesize SACs at the molecular level through second coordination sphere engineering to further optimize the hydrogenation performance.
(4) A comprehensive understanding of the structure–property relationship at the molecular level is the foundation for the rational design of SACs and the further enhancement of catalytic performance. The big challenge lies in advancing the investigation of actual active sites, including the kinetic evolution under reaction conditions, and the associated reaction paths. Therefore, promoting the development of theoretical computation and in situ characterization technologies, such as in situ electron microscopy, in situ XAFS, and in situ electrochemical characterization techniques is in great demand. In addition, kinetics measurements should be conducted for gaining a deep insight into the reaction mechanism.127,148
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21805069 and 22071173), Natural Science Foundation of Hebei Province (B2019204269), and Foundation of Hebei Agricultural University (ZD201716 and PT2018001).References
- P. Grange, Catal. Rev.: Sci. Eng., 1980, 135–181 CrossRef CAS.
- T. C. Ho, Catal. Rev.: Sci. Eng., 1988, 30, 117–160 CrossRef CAS.
- X. Jiang, X. Nie, X. Guo, C. Song and J. G. Chen, Chem. Rev., 2020, 120, 7984–8034 CrossRef CAS.
- W. Wang, S. Wang, X. Ma and J. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- L. Alig, M. Fritz and S. Schneider, Chem. Rev., 2019, 119, 2681–2751 CrossRef CAS PubMed.
- Y. Zhai, Z. Zhu, C. Zhu, K. Chen, X. Zhang, J. Tang and J. Chen, Mater. Today, 2020, 38, 99–113 CrossRef CAS.
- R. Jia, Y. Wang, C. Wang, Y. Ling, Y. Yu and B. Zhang, ACS Catal., 2020, 10, 3533–3540 CrossRef CAS.
- X. Yan, D. Liu, H. Cao, F. Hou, J. Liang and S. X. Dou, Small Methods, 2019, 3, 1800501 CrossRef.
- S. Lux, G. Baldauf-Sommerbauer and M. Siebenhofer, ChemSusChem, 2018, 11, 3357–3375 CrossRef CAS PubMed.
- P. Sharma, J. Sebastian, S. Ghosh, D. Creaser and L. Olsson, Catal. Sci. Technol., 2021, 11, 1665–1697 RSC.
- X. Liu, C.-Y. Mou, S. Lee, Y. Li, J. Secrest and B. W. L. Jang, J. Catal., 2012, 285, 152–159 CrossRef CAS.
- N. Singh, Y. Song, O. Y. Gutiérrez, D. M. Camaioni, C. T. Campbell and J. A. Lercher, ACS Catal., 2016, 6, 7466–7470 CrossRef CAS.
- L. Zhang, M. Zhou, A. Wang and T. Zhang, Chem. Rev., 2019, 120, 683–733 CrossRef PubMed.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- K. Liu, R. Qin and N. Zheng, J. Am. Chem. Soc., 2021, 143, 4483–4499 CrossRef CAS.
- G. Hamm, T. Schmidt, J. Breitbach, D. Franke, C. Becker and K. Wandelt, Z. Phys. Chem., 2009, 223, 209–232 CrossRef CAS.
- M. Li and J. Shen, Thermochim. Acta, 2001, 379, 45–50 CrossRef CAS.
- H. Zhou, X. Yang, L. Li, X. Liu, Y. Huang, X. Pan, A. Wang, J. Li and T. Zhang, ACS Catal., 2016, 6, 1054–1061 CrossRef CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sørensen, C. H. Christensen and J. K. Nørskov, Science, 2008, 320, 1320–1322 CrossRef CAS PubMed.
- J. Zhang, C. Liu and B. Zhang, Small Methods, 2019, 3, 1800481 CrossRef.
- P. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu and N. Zheng, Science, 2016, 352, 797–801 CrossRef CAS PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- Y. Peng, B. Lu and S. Chen, Adv. Mater., 2018, 30, 1801995 CrossRef.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS.
- R. Vanharde and F. Hartog, Surf. Sci., 1969, 15, 189–230 CrossRef.
- N. Lopez, T. V. W. Janssens, B. S. Clausen, Y. Xu, M. Mavrikakis, T. Bligaard and J. K. Nørskov, J. Catal., 2004, 223, 232–235 CrossRef CAS.
- X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- M. Valden, X. Lai and D. W. Goodman, Science, 1998, 281, 1647–1650 CrossRef CAS.
- E. Roduner, Chem. Soc. Rev., 2006, 35, 583–592 RSC.
- B. Yoon, H. Häkkinen, U. Landman, A. S. Wörz, J.-M. Antonietti, S. Abbet, K. Judai and U. Heiz, Science, 2005, 307, 403–407 CrossRef CAS.
- C. T. Campbell, Nat. Chem., 2012, 4, 597–598 CrossRef CAS.
- B. Qiao, J. Liu, Y.-G. Wang, Q. Lin, X. Liu, A. Wang, J. Li, T. Zhang and J. Liu, ACS Catal., 2015, 5, 6249–6254 CrossRef CAS.
- X. Chen, M. Peng, X. Cai, Y. Chen, Z. Jia, Y. Deng, B. Mei, Z. Jiang, D. Xiao, X. Wen, N. Wang, H. Liu and D. Ma, Nat. Commun., 2021, 12, 2664 CrossRef CAS PubMed.
- P. Li, M. Wang, X. Duan, L. Zheng, X. Cheng, Y. Zhang, Y. Kuang, Y. Li, Q. Ma, Z. Feng, W. Liu and X. Sun, Nat. Commun., 2019, 10, 1711 CrossRef PubMed.
- Z. Luo, Y. Ouyang, H. Zhang, M. Xiao, J. Ge, Z. Jiang, J. Wang, D. Tang, X. Cao, C. Liu and W. Xing, Nat. Commun., 2018, 9, 2120 CrossRef.
- S. Li, Y. Xu, Y. Chen, W. Li, L. Lin, M. Li, Y. Deng, X. Wang, B. Ge, C. Yang, S. Yao, J. Xie, Y. Li, X. Liu and D. Ma, Angew. Chem., Int. Ed., 2017, 56, 10761–10765 CrossRef CAS PubMed.
- Y. Chen, S. Ji, C. Chen, Q. Peng, D. Wang and Y. Li, Joule, 2018, 2, 1242–1264 CrossRef CAS.
- L. Jiao and H.-L. Jiang, Chem, 2019, 5, 786–804 CAS.
- S. Abbet, A. Sanchez, U. Heiz, W.-D. Schneider, A. M. Ferrari, G. Pacchioni and N. Rösch, J. Am. Chem. Soc., 2000, 122, 3453–3457 CrossRef CAS.
- H. Yan, H. Cheng, H. Yi, Y. Lin, T. Yao, C. Wang, J. Li, S. Wei and J. Lu, J. Am. Chem. Soc., 2015, 137, 10484–10487 CrossRef CAS PubMed.
- B. J. O'Neill, D. H. K. Jackson, J. Lee, C. Canlas, P. C. Stair, C. L. Marshall, J. W. Elam, T. F. Kuech, J. A. Dumesic and G. W. Huber, ACS Catal., 2015, 5, 1804–1825 CrossRef.
- G. Liu, A. W. Robertson, M. M. Li, W. C. H. Kuo, M. T. Darby, M. H. Muhieddine, Y. C. Lin, K. Suenaga, M. Stamatakis, J. H. Warner and S. C. E. Tsang, Nat. Chem., 2017, 9, 810–816 CrossRef CAS.
- J. Zhang, X. Wu, W.-C. Cheong, W. Chen, R. Lin, J. Li, L. Zheng, W. Yan, L. Gu, C. Chen, Q. Peng, D. Wang and Y. Li, Nat. Commun., 2018, 9, 1002 CrossRef.
- J. Wan, W. Chen, C. Jia, L. Zheng, J. Dong, X. Zheng, Y. Wang, W. Yan, C. Chen, Q. Peng, D. Wang and Y. Li, Adv. Mater., 2018, 30, 1705369 CrossRef.
- Y. Chen, S. Ji, Y. Wang, J. Dong, W. Chen, Z. Li, R. Shen, L. Zheng, Z. Zhuang, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 6937–6941 CrossRef CAS.
- X. Wang, W. Chen, L. Zhang, T. Yao, W. Liu, Y. Lin, H. Ju, J. Dong, L. Zheng, W. Yan, X. Zheng, Z. Li, X. Wang, J. Yang, D. He, Y. Wang, Z. Deng, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 9419–9422 CrossRef CAS PubMed.
- J. D. Kistler, N. Chotigkrai, P. Xu, B. Enderle, P. Praserthdam, C. Y. Chen, N. D. Browning and B. C. Gates, Angew. Chem., Int. Ed., 2014, 53, 8904–8907 CrossRef CAS.
- H. Wu, H. Li, X. Zhao, Q. Liu, J. Wang, J. Xiao, S. Xie, R. Si, F. Yang, S. Miao, X. Guo, G. Wang and X. Bao, Energy Environ. Sci., 2016, 9, 3736–3745 RSC.
- H. Fei, J. Dong, M. J. Arellano-Jimenez, G. Ye, N. Dong Kim, E. L. Samuel, Z. Peng, Z. Zhu, F. Qin, J. Bao, M. J. Yacaman, P. M. Ajayan, D. Chen and J. M. Tour, Nat. Commun., 2015, 6, 8668 CrossRef CAS PubMed.
- H. Fei, J. Dong, Y. Feng, C. S. Allen, C. Wan, B. Volosskiy, M. Li, Z. Zhao, Y. Wang, H. Sun, P. An, W. Chen, Z. Guo, C. Lee, D. Chen, I. Shakir, M. Liu, T. Hu, Y. Li, A. I. Kirkland, X. Duan and Y. Huang, Nat. Catal., 2018, 1, 63–72 CrossRef CAS.
- V. Madhavan, W. Chen, T. Jamneala, M. F. Crommie and N. S. Wingreen, Science, 1998, 280, 567–569 CrossRef CAS PubMed.
- D. Deng, X. Chen, L. Yu, X. Wu, Q. Liu, Y. Liu, H. Yang, H. Tian, Y. Hu, P. Du, R. Si, J. Wang, X. Cui, H. Li, J. Xiao, T. Xu, J. Deng, F. Yang, P. N. Duchesne, P. Zhang, J. Zhou, L. Sun, J. Li, X. Pan and X. Bao, Sci. Adv., 2015, 1, e1500462 CrossRef PubMed.
- Z. Miao, X. Wang, M. C. Tsai, Q. Jin, J. Liang, F. Ma, T. Wang, S. Zheng, B. J. Hwang, Y. Huang, S. Guo and Q. Li, Adv. Energy Mater., 2018, 8, 1801226 CrossRef.
- M. Zhang, Y. G. Wang, W. Chen, J. Dong, L. Zheng, J. Luo, J. Wan, S. Tian, W. C. Cheong, D. Wang and Y. Li, J. Am. Chem. Soc., 2017, 139, 10976–10979 CrossRef CAS PubMed.
- A. S. Hoffman, C.-Y. Fang and B. C. Gates, J. Phys. Chem. Lett., 2016, 7, 3854–3860 CrossRef CAS.
- J. Liu, F. R. Lucci, M. Yang, S. Lee, M. D. Marcinkowski, A. J. Therrien, C. T. Williams, E. C. Sykes and M. Flytzani-Stephanopoulos, J. Am. Chem. Soc., 2016, 138, 6396–6399 CrossRef CAS PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef.
- K. Brandt, M. E. Chiu, D. J. Watson, M. S. Tikhov and R. M. Lambert, J. Phys. Chem. C, 2012, 116, 4605–4611 CrossRef CAS.
- H. Qi, J. Yang, F. Liu, L. Zhang, J. Yang, X. Liu, L. Li, Y. Su, Y. Liu, R. Hao, A. Wang and T. Zhang, Nat. Commun., 2021, 12, 3295 CrossRef CAS PubMed.
- K. Jiang, S. Back, A. J. Akey, C. Xia, Y. Hu, W. Liang, D. Schaak, E. Stavitski, J. K. Nørskov, S. Siahrostami and H. Wang, Nat. Commun., 2019, 10, 3997 CrossRef.
- S. Yang, Y. J. Tak, J. Kim, A. Soon and H. Lee, ACS Catal., 2017, 7, 1301–1307 CrossRef CAS.
- H.-Y. T. Chen, S. Tosoni and G. Pacchioni, ACS Catal., 2015, 5, 5486–5495 CrossRef CAS.
- E. A. I. Shor, V. A. Nasluzov, A. M. Shor, G. N. Vayssilov and N. Rösch, J. Phys. Chem. C, 2007, 111, 12340–12351 CrossRef CAS.
- J. A. Santana and N. Rösch, Phys. Chem. Chem. Phys., 2012, 14, 16062–16069 RSC.
- M. Zhou, M. Yang, X. Yang, X. Zhao, L. Sun, W. Deng, A. Wang, J. Li and T. Zhang, Chin. J. Catal., 2020, 41, 524–532 CrossRef CAS.
- O. Eisenstein and R. H. Crabtree, New J. Chem., 2013, 37, 21–27 RSC.
- G. Vile, D. Albani, M. Nachtegaal, Z. Chen, D. Dontsova, M. Antonietti, N. López and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2015, 54, 11265–11269 CrossRef CAS PubMed.
- P. Liu and N. Zheng, Natl. Sci. Rev., 2018, 5, 636–638 CrossRef CAS.
- G. Kyriakou, M. B. Boucher, A. D. Jewell, E. A. Lewis, T. J. Lawton, A. E. Baber, H. L. Tierney, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Science, 2012, 335, 1209–1212 CrossRef CAS.
- Y. Xu, M. Chu, F. Liu, X. Wang, Y. Liu, M. Cao, J. Gong, J. Luo, H. Lin, Y. Li and Q. Zhang, Nano Lett., 2020, 20, 6865–6872 CrossRef CAS PubMed.
- K. Liu, R. Qin, L. Zhou, P. Liu, Q. Zhang, W. Jing, P. Ruan, L. Gu, G. Fu and N. Zheng, CCS Chem., 2019, 1, 207–214 CAS.
- Y. Ren, Y. Tang, L. Zhang, X. Liu, L. Li, S. Miao, D. Sheng Su, A. Wang, J. Li and T. Zhang, Nat. Commun., 2019, 10, 4500 CrossRef.
- Y. Ma, Y. Ren, Y. Zhou, W. Liu, W. Baaziz, O. Ersen, C. Pham-Huu, M. Greiner, W. Chu, A. Wang, T. Zhang and Y. Liu, Angew. Chem., Int. Ed., 2020, 59, 21613–21619 CrossRef CAS.
- Y. Cao, J. Guerrero-Sańchez, I. Lee, X. Zhou, N. Takeuchi and F. Zaera, ACS Catal., 2020, 10, 3431–3443 CrossRef CAS.
- J. Zhang, C. Zheng, M. Zhang, Y. Qiu, Q. Xu, W.-C. Cheong, W. Chen, L. Zheng, L. Gu, Z. Hu, D. Wang and Y. Li, Nano Res., 2020, 13, 3082–3087 CrossRef.
- X. Long, Z. Li, G. Gao, P. Sun, J. Wang, B. Zhang, J. Zhong, Z. Jiang and F. Li, Nat. Commun., 2020, 11, 4074 CrossRef CAS.
- W. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, J. Am. Chem. Soc., 2017, 139, 10790–10798 CrossRef CAS PubMed.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 1944–1948 CrossRef CAS.
- J. Lu, P. Serna, C. Aydin, N. D. Browning and B. C. Gates, J. Am. Chem. Soc., 2011, 133, 16186–16195 CrossRef CAS PubMed.
- C. Tang, L. Chen, H. Li, L. Li, Y. Jiao, Y. Zheng, H. Xu, K. Davey and S. Z. Qiao, J. Am. Chem. Soc., 2021, 143, 7819–7827 CrossRef CAS.
- W. Li, F. Li, H. Yang, X. Wu, P. Zhang, Y. Shan and L. Sun, Nat. Commun., 2019, 10, 5074 CrossRef CAS PubMed.
- J. Doebereiner, Ann. Phys., 1845, 140, 94–96 CrossRef.
- F. J. Williams, A. Palermo, S. Tracey, M. S. Tikhov and R. M. Lambert, J. Phys. Chem. B, 2002, 106, 5668–5672 CrossRef CAS.
- N. Mahata, K. V. Raghavan and V. Vishwanathan, Appl. Catal., A, 1999, 182, 183–187 CrossRef CAS.
- W. D. Mross, Catal. Rev.: Sci. Eng., 1983, 25, 591–637 CrossRef CAS.
- S. R. d. Miguel, A. A. Castro, O. A. Scelza and J. Soria, Catal. Lett., 1995, 32, 281–291 CrossRef.
- Y. Chai, G. Wu, X. Liu, Y. Ren, W. Dai, C. Wang, Z. Xie, N. Guan and L. Li, J. Am. Chem. Soc., 2019, 141, 9920–9927 CrossRef CAS PubMed.
- M. Yang, J. Liu, S. Lee, B. Zugic, J. Huang, L. F. Allard and M. Flytzani-Stephanopoulos, J. Am. Chem. Soc., 2015, 137, 3470–3473 CrossRef CAS.
- M. Yang, S. Li, Y. Wang, J. A. Herron, Y. Xu, L. F. Allard, S. Lee, J. Huang, M. Mavrikakis and M. Flytzani-Stephanopoulos, Science, 2014, 346, 1498–1501 CrossRef CAS PubMed.
- J. Lin, A. Wang, B. Qiao, X. Liu, X. Yang, X. Wang, J. Liang, J. Li, J. Liu and T. Zhang, J. Am. Chem. Soc., 2013, 135, 15314–15317 CrossRef CAS.
- H. Wei, X. Liu, A. Wang, L. Zhang, B. Qiao, X. Yang, Y. Huang, S. Miao, J. Liu and T. Zhang, Nat. Commun., 2014, 5, 5634 CrossRef CAS PubMed.
- H. Wei, Y. Ren, A. Wang, X. Liu, X. Liu, L. Zhang, S. Miao, L. Li, J. Liu, J. Wang, G. Wang, D. Su and T. Zhang, Chem. Sci., 2017, 8, 5126–5131 RSC.
- R. Qin, L. Zhou, P. Liu, Y. Gong, K. Liu, C. Xu, Y. Zhao, L. Gu, G. Fu and N. Zheng, Nat. Catal., 2020, 3, 703–709 CrossRef CAS.
- Y. Ma, B. Chi, W. Liu, L. Cao, Y. Lin, X. Zhang, X. Ye, S. Wei and J. Lu, ACS Catal., 2019, 9, 8404–8412 CrossRef CAS.
- W. W. N. O and R. H. Morris, ACS Catal., 2013, 3, 32–40 CrossRef CAS.
- N. M. Rezayee, D. C. Samblanet and M. S. Sanford, ACS Catal., 2016, 6, 6377–6383 CrossRef CAS.
- Y. T. Kim, T. Uruga and T. Mitani, Adv. Mater., 2006, 18, 2634–2638 CrossRef CAS.
- M. Mahmoodinia, P.-O. Åstrand and D. Chen, J. Phys. Chem. C, 2017, 121, 20802–20812 CrossRef CAS.
- P. He, B. Xu, X. Xu, L. Song and X. Wang, Chem. Sci., 2016, 7, 1011–1015 RSC.
- B. Zhang, H. Asakura, J. Zhang, J. Zhang, S. De and N. Yan, Angew. Chem., Int. Ed., 2016, 55, 8319–8323 CrossRef CAS.
- S. Fokin, Z. Elektrochem., 1906, 12, 749–762 CrossRef.
- F. Fichter and R. Stocker, Chem. Ber., 1914, 47, 2015 Search PubMed.
- U. Pomilio, Z. Elektrochem., 1915, 21, 444 CAS.
- W. M. Leslie and J. A. V. Butler, Trans. Faraday Soc., 1936, 32, 989–998 RSC.
- J. Lessard, in Organic Electrochemistry, ed. O. Hammerich and B. Speiser, CRC Press, 5th edn, 2015, pp. 1658–1664 Search PubMed.
- J. M. Chapuzet, A. Lasia and J. Lessard, in Electrocatalysis, ed. J. Lipkowski and P. N. Ross, Wiley-VCH, 1998, pp. 155–196 Search PubMed.
- X. H. Chadderdon, D. J. Chadderdon, J. E. Matthiesen, Y. Qiu, J. M. Carraher, J.-P. Tessonnier and W. Li, J. Am. Chem. Soc., 2017, 139, 14120–14128 CrossRef CAS PubMed.
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Science, 2018, 360, eaar6611 CrossRef.
- J. Martínez, A. Ortiz and I. Ortiz, Appl. Catal., B, 2017, 207, 42–59 CrossRef.
- S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski and P. Westerhoff, Appl. Catal., B, 2018, 236, 546–568 CrossRef CAS.
- N. Lehnert, H. T. Dong, J. B. Harland, A. P. Hunt and C. J. White, Nat. Rev. Chem., 2018, 2, 278–289 CrossRef CAS.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. D. Schamphelaire, T. H. Pham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41, 3354–3360 CrossRef CAS.
- A. D. Fonseca, J. G. Crespo, J. S. Almeida and M. A. Reis, Environ. Sci. Technol., 2000, 34, 1557–1562 CrossRef CAS.
- R. Epsztein, O. Nir, O. Lahav and M. Green, Chem. Eng. J., 2015, 279, 372–378 CrossRef CAS.
- N. Barrabés and J. Sá, Appl. Catal., B, 2011, 104, 1–5 CrossRef.
- Y. H. Liou, S. L. Lo, C. J. Lin, C. Y. Hu, W. H. Kuan and S. C. Weng, Environ. Sci. Technol., 2005, 39, 9643–9648 CrossRef CAS.
- F. Yao, Q. Yang, Y. Zhong, X. Shu, F. Chen, J. Sun, Y. Ma, Z. Fu, D. Wang and X. Li, Water Res., 2019, 157, 191–200 CrossRef CAS PubMed.
- H. Xu, J. Wu, W. Luo, Q. Li, W. Zhang and J. Yang, Small, 2020, 16, 2001775 CrossRef CAS.
- C. Chen, K. Li, C. Li, T. Sun and J. Jia, Environ. Sci. Technol., 2019, 53, 13868–13877 CrossRef CAS.
- Q. Hu, Y. Qin, X. Wang, Z. Wang, X. Huang, H. Zheng, K. Gao, H. Yang, P. Zhang, M. Shao and C. He, Energy Environ. Sci., 2021, 14, 4989–4997 RSC.
- Y. Wang, C. Wang, M. Li, Y. Yu and B. Zhang, Chem. Soc. Rev., 2021, 50, 6720–6733 RSC.
- M. T. de Groot and M. T. M. Koper, J. Electroanal. Chem., 2004, 562, 81–94 CrossRef CAS.
- K. J. Vetter, Z. Elektrochem., 1959, 63, 1189–1191 CAS.
- G. Schmid, Z. Elektrochem., 1961, 65, 531–534 CAS.
- X. Zhang, Y. Wang, C. Liu, Y. Yu, S. Lu and B. Zhang, Chem. Eng. J., 2021, 403, 126269 CrossRef CAS.
- H. Shin, S. Jung, S. Bae, W. Lee and H. Kim, Environ. Sci. Technol., 2014, 48, 12768–12774 CrossRef CAS PubMed.
- P. Li, Z. Jin, Z. Fang and G. Yu, Energy Environ. Sci., 2021, 14, 3522–3531 RSC.
- G.-F. Chen, Y. Yuan, H. Jiang, S.-Y. Ren, L.-X. Ding, L. Ma, T. Wu, J. Lu and H. Wang, Nat. Energy, 2020, 5, 605–613 CrossRef CAS.
- E. Pérez-Gallent, M. C. Figueiredo, I. Katsounaros and M. T. M. Koper, Electrochim. Acta, 2017, 227, 77–84 CrossRef.
- H. Niu, Z. Zhang, X. Wang, X. Wan, C. Shao and Y. Guo, Adv. Funct. Mater., 2020, 31, 2008533 CrossRef.
- Z. Y. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F. Y. Chen, J. Y. T. Kim, Y. Xia, K. Heck, Y. Hu, M. S. Wong, Q. Li, I. Gates, S. Siahrostami and H. Wang, Nat. Commun., 2021, 12, 2870 CrossRef CAS.
- W. Teng, J. Fan and W. X. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 28091–28099 CrossRef CAS.
- J. Li, M. Li, N. An, S. Zhang, Q. Song, Y. Yang and X. Liu, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2105628118 CrossRef CAS.
- T. Zhu, Q. Chen, P. Liao, W. Duan, S. Liang, Z. Yan and C. Feng, Small, 2020, 16, e2004526 CrossRef.
- J. Liu, Y. Cai, R. Song, S. Ding, Z. Lyu, Y.-C. Chang, H. Tian, X. Zhang, D. Du, W. Zhu, Y. Zhou and Y. Lin, Mater. Today, 2021, 48, 95–114 CrossRef CAS.
- R. Sun, Y. Liao, S.-T. Bai, M. Zheng, C. Zhou, T. Zhang and B. F. Sels, Energy Environ. Sci., 2021, 14, 1247–1285 RSC.
- Y. Pan, R. Lin, Y. Chen, S. Liu, W. Zhu, X. Cao, W. Chen, K. Wu, W.-C. Cheong, Y. Wang, L. Zheng, J. Luo, Y. Lin, Y. Liu, C. Liu, J. Li, Q. Lu, X. Chen, D. Wang, Q. Peng, C. Chen and Y. Li, J. Am. Chem. Soc., 2018, 140, 4218–4221 CrossRef CAS.
- H. Shang, T. Wang, J. Pei, Z. Jiang, D. Zhou, Y. Wang, H. Li, J. Dong, Z. Zhuang, W. Chen, D. Wang, J. Zhang and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 22465–22469 CrossRef CAS PubMed.
- L. Han, S. Song, M. Liu, S. Yao, Z. Liang, H. Cheng, Z. Ren, W. Liu, R. Lin, G. Qi, X. Liu, Q. Wu, J. Luo and H. L. Xin, J. Am. Chem. Soc., 2020, 142, 12563–12567 CrossRef CAS.
- A. Guan, Z. Chen, Y. Quan, C. Peng, Z. Wang, T.-K. Sham, C. Yang, Y. Ji, L. Qian, X. Xu and G. Zheng, ACS Energy Lett., 2020, 5, 1044–1053 CrossRef CAS.
- D. Gao, T. Liu, G. Wang and X. Bao, ACS Energy Lett., 2021, 6, 713–727 CrossRef CAS.
- Y. Wang, Y. Liu, W. Liu, J. Wu, Q. Li, Q. Feng, Z. Chen, X. Xiong, D. Wang and Y. Lei, Energy Environ. Sci., 2020, 13, 4609–4624 RSC.
- F. Franco, C. Rettenmaier, H. S. Jeon and B. Roldan Cuenya, Chem. Soc. Rev., 2020, 49, 6884–6946 RSC.
- M. Li, H. Wang, W. Luo, P. C. Sherrell, J. Chen and J. Yang, Adv. Mater., 2020, 32, 2001848 CrossRef CAS.
- R. Lang, X. Du, Y. Huang, X. Jiang, Q. Zhang, Y. Guo, K. Liu, B. Qiao, A. Wang and T. Zhang, Chem. Rev., 2020, 120, 11986–12043 CrossRef CAS PubMed.
- J. Jones, H. Xiong, A. T. DeLaRiva, E. J. Peterson, H. Pham, S. R. Challa, G. Qi, S. Oh, M. H. Wiebenga, X. I. P. Hernández, Y. Wang and A. K. Datye, Science, 2016, 353, 150–154 CrossRef CAS PubMed.
- J. Gu, M. Jian, L. Huang, Z. Sun, A. Li, Y. Pan, J. Yang, W. Wen, W. Zhou, Y. Lin, H. J. Wang, X. Liu, L. Wang, X. Shi, X. Huang, L. Cao, S. Chen, X. Zheng, H. Pan, J. Zhu, S. Wei, W. X. Li and J. Lu, Nat. Nanotechnol., 2021, 16, 1141–1149 CrossRef CAS PubMed.
- C. Paolucci, I. Khurana, A. A. Parekh, S. Li, A. J. Shih, H. Li, J. R. Di Iorio, J. D. Albarracin-Caballero, A. Yezerets, J. T. Miller, W. N. Delgass, F. H. Ribeiro, W. F. Schneider and R. Gounder, Science, 2017, 357, 898–903 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |