Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal–organic framework based systems for CO2 sensing
Andreea
Gheorghe
 ,
Olivier
Lugier
,
Bohui
Ye
and
Stefania
Tanase
,
Olivier
Lugier
,
Bohui
Ye
and
Stefania
Tanase
 *
*
Van’t Hoff Institute of Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH, The Netherlands. E-mail: s.grecea@uva.nl
First published on 18th August 2021
Abstract
Monitoring CO2 levels in the atmosphere as well as in work place environments is strictly regulated. Commercial sensors based on polymeric materials have low operating temperature, yet exhibit low selectivity. Molecular systems such as metal–organic frameworks (MOFs) are promising materials that can be used for CO2 sensing applications. They are formed through strong interactions between metal ions or clusters with easy-to-modify organic linkers and have exceptionally high surface areas and well-defined accessible pores. The host–guest interactions in MOFs and their responsiveness to physical and chemical stimuli can be exploited to address the critical issues in chemical sensing applications, such as fast response, sensitivity and specificity. This review provides an overview of the techniques that can be used to detect CO2 through the use of MOFs, highlighting the most promising MOF materials that exhibit CO2 sensing properties. The potential of MOFs in the development of CO2 sensors is also discussed.
Introduction
Global warming is primarily caused by emissions of CO2 and other harmful gases (NOx, SOx, and volatile organic compounds) into the atmosphere, and it mostly comes from human activities through industries and vehicles.1 The research station of the National Oceanic and Atmospheric Administration (NOAA) of the U.S. Department of Commerce started measuring CO2 levels in the late 1950s when the atmospheric CO2 concentration was at around 315 ppm.2 On the 8th of April 2021, the daily average of CO2 levels was 421 ppm, the first time in human history that the number has been so high.3 Whereas rising carbon dioxide levels in the atmosphere cause great concern worldwide, most of us pay little to no attention to risks posed by CO2 changes indoors. Recent studies show that decision-making and other cognitive abilities decline at elevated indoor CO2 levels due to inadequate ventilation in buildings.4 Despite the fact that we breath in and out carbon dioxide, the maximum safe level of carbon dioxide is at 5000 ppm. Above the safe CO2 level, it can cause symptoms such as fatigue, headache, anxiety, loss of energy, jelly legs and lung ventilation problems.5 Therefore, it is of utmost importance to have reliable CO2 sensors which can be used in health and safety applications, including surveillance and air quality (low CO2 levels) as well as by industries that produce CO2 (high CO2 levels) in order to protect their workers from the harmful effects of augmented CO2 levels (Fig. 1).6Commercial CO2 sensors are classified in two major types: nondispersive infrared (NDIR) optical and chemical sensors. A NDIR sensor uses an infrared beam that does not scatter upon interaction with guest molecules. The NDIR CO2 sensor measures the abundance or concentration of CO2 through the partially absorbed light by CO2 when CO2 is between the IR light source and detector. Since the concentration of gases is proportional to the transmission of the IR beam, the change of the IR beam intensity is small when the concentration of CO2 is low and therefore, it can fall below the detection capacity of the detector. This results in high detection limit in NDIR sensors. A typical NDIR CO2 sensor has a detection limit of 0.2 ppm with an accuracy of ±30 ppm.7 NDIR sensors usually operate between 1 and 10 μm,8 a range in which the absorption of water vapour occurs. Since CO2 gas detection is often carried out in conditions where water vapour is present, water vapour interference is one of the biggest challenge when NDIR sensors are used to detect CO2.9 Absorption interference caused by water vapor or other molecules can be (partially) solved using optical filters and interference correction. However, by using optical filters, the spectral absorption of CO2 is drastically reduced due to refractive property of the optical filter. Additionally, using optical filter increases the price of the NDIR sensors.10
Commercial chemical CO2 sensors consist of a sensing layer made up by polymers that operate at room temperature. Polymers used for CO2 detection often contain amine groups. The sensing principle of these amine-based polymers is based on an acid–base reaction.11,12 The interaction between CO2 and amine groups is reversible and it involves the formation of ionic species. This interaction is an acid base equilibrium, and the sensing mechanism of polymer-based CO2 sensors is based on the pH change upon the reaction of amines with CO2 to form carbamates and cationic species of ammonia or amine. During this process, carbamic acids are formed as intermediates. Carbamates formed during the acid–base reaction are thermal unstable molecules and CO2 can be released upon heating, allowing to re-use the active sites of the polymer-based sensors.11
Advantages of polymer-based sensors include high sensitivity, short response time, low power consumption and small size, allowing for portable use.13 Nevertheless, the main drawbacks are the poor selectivity and short and long-term sensor drift that lead to inaccurate measurements over time. Therefore, polymer-based sensors are calibrated prior the shipment, overtime and the zero point needs to be recalibrated to maintain long-term stability.14 Metal oxide sensing layers have emerged as a promising choice in the gas sensor industry.15 Their working principle is based on the redox reactions between the target gas and the oxygen species bound on the surface of the metal oxide.16 Reaction of these oxygen species with reducing gases or a competitive adsorption and replacement of the adsorbed oxygen by other molecules lead to an increased conductivity. The oxygen species are believed to be dominant at operating temperature of 300–450 °C which is the working temperature for most metal oxide gas sensors.15 The limitations resulting from such high temperatures include poor selectivity (cross-sensitivity) and baseline drift.17,18
A comprehensive account of commercial sensing materials for CO2 sensor devices, including their properties and relative advantages has been reported in literature.19 Emerging sensing materials include carbon nanotubes (CNTs),20 graphene,21 MoS2,22 zeolites23 and metal–organic frameworks (MOFs).24 Among them, MOFs are the preferred choice because their structure can be tailored to enhance the selectivity towards a target analyte, and to tune the interplay between structure and properties.25–27
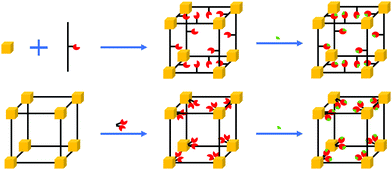
MOFs are porous materials composed of metal ion nodes and organic linkers (Fig. 2).28 Their modular nature allows for great synthetic tunability, affording both fine chemical and structural control. They have unique characteristics, being able to take-up, hold and release molecules from their pores in a selective manner. Their high surface area to volume ratio is especially beneficial for sensing applications as the high surface area to volume ratio enhances the chance of interaction between the sensing materials and analytes, which leads to high sensitivity. The properties of MOFs beyond those of traditional sensing materials include adsorption properties tailored to specific analytes,26 tunable dielectric properties,25 intrinsic porosity that allows through-solid mass and ion transport, mechanical properties in between polymers and inorganics29 and electronic conductance tailored from practically zero to over a hundred S cm−2.30
The potential of MOFs for CO2 capture and storage has been studied in great detail.31,32 The selective adsorption of CO2 molecules in the pores of MOFs is achieved by using different synthetic strategies for the design of MOFs. They involve the choice of specific organic linkers, use of open metal sites and the incorporation of functional groups inside the framework (Fig. 2).33 Therefore, the pores of MOFs can act as molecular sieves.34 By varying the metal ions and organic linkers, the window and pore size can be tuned to achieve selective adsorption of CO2, leaving out undesired interactions inside the framework.35 Fine tuning of the selective adsorption of MOFs allows for developing MOFs as sensing layers for accurate detection of CO2 levels as well as their integration in chemical sensors.36,37 This review discusses the working principle of MOF-based sensors used for CO2 detections, highlighting the pros and cons of MOF materials. Since the integration of MOFs as thin-films in sensor devices requires to prepare micro- or/and nano-scaled MOF crystals which have controlled size, shape, and morphology, we also present the common fabrication techniques for MOF thin-films on electrode surfaces and assess their practical applicability.
CO2 sensing using MOFs
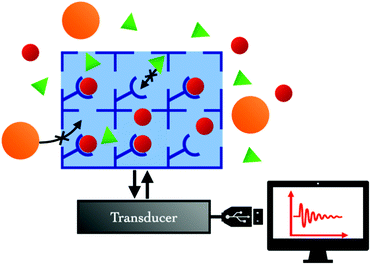
Sensor devices require two main constituents: a sensing layer and a transducer (Fig. 3). The sensing layer interacts selectively with the target analytes, so that various changes in the physico-chemical properties of the system (e.g., capacitance, mass, conductivity, optical properties, etc.) are detected. The transducer translates the changes in measurable signals.The advantage of MOFs over traditional sensing materials comes from their capacity to concentrate the target analyte within their pores, acting like a sponge with astonishing surface to volume ratio, and their potential selectivity for specific analytes that can be adjusted by tuning the structure and/or chemical composition of the MOF. In such MOF-based sensing devices, the transducer component usually provides information related to variations in mass, electronic properties or optical properties.38 In the following sections, we will discuss the transducer types which have been studied so far for CO2 detection using MOFs as sensing layers (Table 1).
| MOF | Transduction approach | Range tested LoD | Response/recovery time | CO2 selective | Water stability | Potential for other analytes | Ref. |
|---|---|---|---|---|---|---|---|
| LoD* = estimated or extrapolated LoD; — = information not provided by the authors. | |||||||
| ZIF-8 | Fabry–Perrot cavities | 0–100 vol% | <0.15 s/<0.30 s (≈3 s for 1 mL min−1 flow rate) | Yes (size selective) | High | Yes | 39 |
| LoD: 0.019 vol% (1 Hz low-pass filtering) | |||||||
| ZIF-8 | SEIRA | 0–100% vol% | —/— | Yes (size selective) | High | Yes | 43 |
| LoD*: 52 ppm | |||||||
| ZIF-8 | SERS | — to — | 8 min/— | Yes (size selective) | High | Yes | 44 |
| LoD: 5 × 10−8 M | |||||||
| HKUST-1 | IR absorption (optical fibres) | 20 ppm to 100 vol% | 10 s/106 s (100 vol%) | No (H2O, N2) | Low | Yes | 49 |
| LoD: 20 ppm | 12 s/530 s (30 ppm) | ||||||
| Mg-MOF-74 (chemically tuned) | Permittivity | 200 to 5000 ppm | Minutes/— | No | Low | Yes | 26 |
| LoD: 200 ppm | |||||||
| Co-MOF-74 (chemically tuned) | Electrical conductivity | 100% (30 mL min−1) | 200 s/— | No (CH4, N2, H2O) | Low | Yes | 54 |
| LoD: — | |||||||
| Cu3(hexaimino benzene)2 | Electrical conductivity | 400–2500 ppm | 7–8 min (0% RH) | — | High | Yes | 55 |
| LoD*: 67 ppm | 10–11 min (80% RH) | ||||||
| Al-MIL-53 + conductive carbon | Electrical conductivity | 75 vol% (in CH4) | 30 s | Yes (over CH4) | High | Yes | 25 |
| 100 vol% | |||||||
| LoD: — | |||||||
| CDMOF-2 | Electrochemical impedance spectroscopy | 0–100 vol% | —/— | Yes | High | Yes | 62 |
| LoD: <10 vol% | |||||||
| Zn-MOF-74 | Impedance | 0–2000 ppm | — | Low | Medium | Yes | 57 |
| LoD: 500 ppm | |||||||
| NH2-M-MOF-74 | Kelvin probe | 400–4000 ppm | — | — (H2O sensitive) | Medium | Yes | 68 |
| LoD: — | |||||||
| MAF-34 | Luminescence | — | —/— | — | High | Yes (benzene, nitrobenzene, ethanol) | 70 |
| — | |||||||
| UiO-66-ONa (tuned UiO-66) | Luminescence (colorimetry) | — to — | ∼67 s/— | Yes | High | Expected | 71 |
| LoD: 3.5 × 10−7 M | |||||||
| ZIF-8 | Bimodal waveguide interferometry | 1–100 vol% | <10 s | Yes (over H2O, CH4) | High | Yes | 72 |
| LoD: 3130 ppm (RT) | |||||||
| 774 ppm (278 K) | |||||||
| Cu2(nbdc)2(dabco) | Mass (QCM) | 10–100 vol% | Seconds/seconds | Yes (over O2, N2, H2, CH4) | Medium | Expected | 73 |
| LoD: 10 vol% (56% RH) | |||||||
| HKUST-1 | LSPR spectroscopy | 0–100 vol% | Few seconds (10 vol%) | — | Low | Yes (tested for SF6) | 74 |
| LoD: 10 vol% | |||||||
| LoD*: ≪10 vol% | |||||||
Refractive index (RI) sensing
Although the variations of CO2 concentrations only trigger negligible change in refractive index multiple techniques involving MOFs have been developed to take advantage of this phenomenon. Kim et al.39 reported a CO2 sensor with a dynamic range of 0 to 100 vol% of CO2 and a resolution of 0.019 vol%. Their sensor consists of two fibre-optic terminated with Fabry–Perrot (FP) cavities40 made of thin films of ZIF-8, or [Zn(melm)2] where melm = 2-methylimidazolate, of different thickness for each fibre-optic. The reflection spectrum of each FP cavity depends on their dimension (i.e., the thickness of the MOF thin film) and respective refractive index, which depends on the concentration of CO2 molecules present in the films of ZIF-8. By combining two precisely tailored FP cavities, the authors were able to obtain a differential response to the changes in the refractive index of the system which enables low limit of detection (LoD) and a high resolution. This sensor shows multiple advantages such as its small size and straightforward fabrication method (dip-coating) that allows batch production. Furthermore, this method can potentially be adapted to detect any gas by replacing ZIF-8 with different types of MOF displaying affinities for other analytes.Other studies focused on combining MOFs and surface plasmon resonance spectroscopy to monitor small changes in CO2 concentrations. Surface plasmon resonance (SPR) is the coherent oscillation of conduction band electrons at the interface of a metal and a dielectric material upon light exposure. The resonance frequency of surface plasmons is closely related to the refractive index of the system. Hence, the phase and intensity values of the light reflected at the aforementioned interface can be used to obtain information on the RI, and thus, on the composition of the dielectric material.41,42
Chong et al.43 proposed a complex sensor in which surface plasmon polaritons (SPP) are created by shining light onto a suspended silicon nitride (SiN4) window on top of which, specially designed gold nanoarrays were patterned and covered by a film of ZIF-8. By tuning the properties of the SiN4 window, the gold nanoarrays and the MOF layer, the authors were able to match the resonance frequency of the SPPs to a specific CO2 infrared absorption band resulting in a calculated enhancement factor of ca. 1800×. Despite the lower enhancement performances of their prototype compared to the model, due to imperfect testing conditions, the authors estimated its LoD at 52 ppm. The intricate design and working principle of this sensor could become a liability for a successful transitioning from numerical models or laboratory prototypes to mass-produced devices. Nevertheless, these results prove that surface-enhanced infrared absorption (SEIRA) can be used to fabricate highly sensitive CO2 detectors. Furthermore, like the above-mentioned sensor based on double Fabry–Perrot ZIF-8 cavities, this device could detect other gases provided that the resonance frequency of the SPPs can be tuned to overlap with a characteristic infrared absorption band of the target analyte.
Huang et al.44 proposed a similar method that combines the CO2 absorption capacity of ZIF-8 films with localised surface plasmon (LSPP) resonance from gold nanoparticles on silver nanowire to develop a surface enhanced Raman scattering (SERS) based sensor. The authors could mould the silver nanowires together to form free-standing films of the CO2 sensing material. Again, the LSPP acts as signal enhancer while extra thin films (1 nm to 3 nm) of ZIF-8 are used to selectively concentrate CO2 molecules. A promising LoD of 5 × 10−8 M was obtained with a response time of eight minutes. Although only demonstrated as a proof of concept, such SERS-based sensor could virtually detect any analyte at low concentration, provided that it has distinctive Raman bands and displays affinity for the MOF used. Yet, the restrictions imposed by the thickness of the MOF layer (3 nm maximum) are expected to limit the number of compatible MOFs. Indeed, the current synthesis methods used to fabricate MOF thin films require improvements to gain precise control over the main properties of the material. Nevertheless, the versatility of Raman spectroscopy and the relative ease of fabrication (when using ZIF-8) of the proposed prototype are two enticing arguments in favour of such sensor.
Spectral near infrared sensing
Infrared (IR) sensors are commonly used to detect and identify gases. Most of the analytes absorb IR radiations at specific wavelengths in direct relation with their chemical composition (i.e., their chemical bonds). The subsequent absorption bands can be used to detect and quantify the presence of the target analyte in a specific surrounding.45,46 Although most commercially available mid-IR sensors are too large for portable use, optical fibres offer relatively cheap and convenient alternative for the fabrication of portable IR gas detectors.47 The challenge of using optical fibre near infrared (NIR) sensors is that the gases do not have fundamental vibration bands at NIR regions. The detection must arise from the overtones of the fundamental vibration bands.48 A fundamental vibration is excited by absorbing the (vibrational) quantum energy by a molecule in its ground state. When two of such quantum energies are absorbed, the first overtone is excited, and so on to higher overtones. Overtones from the fundamental vibrational bond that absorbs at the mid-IR frequency are generally weak. Thus, NIR sensors have low detection sensitivity.Enhanced absorption can be achieved by increasing the optical path via optic fibres of several meters length or by using reflecting mirrors.50 Alternatively, the cladding of the optical fibre can be removed by etching to allow coating with a sensitive layer to extend the light path and make use of evanescent wave absorption in order to achieve high reasonable detection.48,51,52 For example, Chong et al.49 have developed an ultra-short NIR fibre-optic CO2 sensor by depositing a thin layer of HKUST-1, or [Cu3(btc)2(H2O)3] where btc = benzentricarboxylate, on the core of an optical fibre. The MOF thin film was grown on the 5 cm-long etched single mode fibre through a layer-by-layer (LbL) method. When CO2 selectively interacts with the HKUST-1 layer via unsaturated open Cu2+ metal sites, the refractive index of the sensing layer draws closer to the refractive index of the fibre and a larger portion of light propagates into the film, which is in direct correlation with CO2 concentration. Fig. 4 shows the absorption spectrum obtained by Chong et al.49 The reference CO2 spectrum exhibited both vibrational bands and rotational fine lines. The missing rotational fine lines of the absorption spectrum of the CO2 within HKUST-1 was attributed to tightly confined CO2 molecules with no freedom of rotation.49 Using their HKUST-1 coated single-mode optical fibre, the authors were able to develop a CO2 sensor with a detection limit down to 20 ppm. The main drawbacks of such sensor at low CO2 concentrations originate from its low adsorption and desorption kinetics. This was attributed to the adsorption mechanism of the gas within the MOF framework. At low concentrations, the gas chemisorbs onto the unsaturated metal sites, as opposed to a physisorption when detecting high CO2 concentrations.49
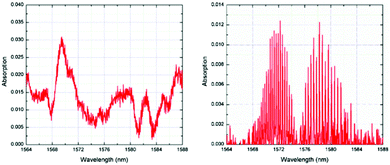 | ||
| Fig. 4 Absorption spectrum of CO2 confined within HKUST-1 (left) and of reference CO2 gas inside quartz gas cell (right).49 | ||
Chemiresistive and chemicapacitive-based sensing
Physicochemical systems such as MOFs possess energy storage and dissipation properties. Electrochemical sensing examines these MOF properties translated as electric capacity and resistance.53 Capacitance is described by the electrical charge storage at a certain potential. In chemical capacitive sensors, the dielectric constant or permittivity of the MOF as a function of time is monitored upon adsorption of CO2.Zhao et al.26 reported the on-chip solvothermal growth of MOF films onto Pt interdigitated electrodes. The sensor was used for the detection of benzene and CO2 at room temperature. LoD for CO2 of both pristine and ethylene diamine modified Mg-MOF-74, also known as [Mg2(dobdc)(H2O)2] where dobdc = 2,5-dihydroxy-1,4-benzenedicarboxylate, was 200 ppm. An enhanced capacitive response by 25% was reported for the ethylenediamine functionalized Mg-MOF-74 material which corelates to a higher amount of gas adsorbed due to the basicity of the amine groups coordinated to the open metal sites. It was shown that the sensor is still stable after 3 weeks storage and has a reversible behaviour upon exposure to inert gas. Overall, this study demonstrates that custom-tailored amine–CO2 interactions can be used as an effective strategy to enhance the sensitivity toward CO2.
In the case of chemiresistive gas sensing, changes in the electronic resistance of the MOF sensing layer are monitored. Yet, the major bottleneck in the design of MOF-based chemiresistive gas sensors is the synthetic challenges associated with the organic linkers commonly used for electrically conductive or semiconductive MOFs. To overcome this, Caro et al.54 prepared MOF-74 composites through gas-phase incorporation of organic semiconducting molecules, such as 7,7,8,8-tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF). To assess the CO2 sensing of these composites, the electrical current was measured as a function of the applied voltage under CO2 atmosphere. An increased electrical conductivity was observed for the Co-MOF-74 containing TTF as compared to the bare Co-MOF-74 and the TCNQ loaded Co-MOF-74.54 The response time was within ca. 200 s. This behaviour might be related to the interaction between TTF and Co-MOF-74, proposed to be based on through-bond conduction, but the electron conduction may also take place through the π–π stacking network.54 The phenomenon reported and characterised in this study is of high relevance for CO2 sensing.
Dinca et al.55 have demonstrated the sensing of CO2 at ambient conditions by using a 2D framework with a high density of NH moieties, namely Cu3(hexaiminobenzene)2, in a broad relative humidity (RH) range (i.e. 10% to 80% RH). The sensor was prepared by simple drop-casting of as-synthesized polycrystalline material onto interdigitated electrodes. Its stable response independent of the humidity was attributed to the acid–base adducts and bicarbonate salt formation at the organic–inorganic node of the MOF upon exposure to CO2. The sensor device has a robust performance and good repeatability (ca. 7 days) at levels of CO2 ranging from outdoor atmospheric level (400 ppm) up to limits typically found indoors (2500 ppm). Although the sensor sensitivity has drifted over time, as is generally observed for other chemiresistors, it still remained RH-independent after three months. Despite these promising results which reveal the potential of 2D MOFs in tuning and optimising chemiresistive responses, the synthesis of conductive 2D MOFs remains synthetically challenging and limited to conjugated benzene rings and porphyrin rings.56
To circumvent the low conductivity of MOFs, composite materials have also been synthesised.25,57 Kaskel et al. prepared conductive carbon composites films comprising Al-MIL-53, also known as [Al(OH)(bdc)]x where bdc = 1,4-benzenedicarboxylate.25 The sensor device includes a dried paste containing carbon black or single walled carbon nanotubes and polytetrafluoroethylene (PTFE) which are placed as a film on glass slides using silver conductive paint. The sensing principle used is based on the change of the conductivity near the percolation threshold of the composite material as a result of the well- known breathing effect of Al-MIL-53 framework. Upon CO2 adsorption, MOF crystals expand at a specific pressure. Consequently, the percolating network created by the conductive additive, that allows electrons to flow through the composite, is disrupted. This causes the specific resistance of the material to increase which translates in a sudden decrease in the electrical conductivity (Fig. 5).25 The response time of Al-MIL-53/conductive carbon sensors was below 30 s at partial pressure of CO2 up to 6 bar. It was shown that using different carbon materials affects the response time, i.e. small isotropic particles of carbon black led to shorter response time as compared with highly anisotropic carbon nanotubes.25
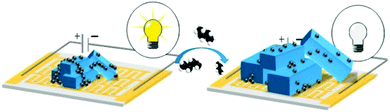 | ||
| Fig. 5 Switchable MIL-53-carbon additive composite for CO2 sensing.25 | ||
Impedance-based sensing
The existence of electrically conductive and semi-conductive MOFs has been the primary reason behind exploiting MOFs for sensing of gases and/or vapours.58 Using impedance spectroscopy, the requirement of the sensing material to possess high electrical conductivity is circumvented because the electrical impedance of a MOF-based sensing layer can be measured over a range of frequencies of an applied electrical current. Furthermore, impedance-based sensors bring also advantages in terms of their low-cost, small size and highly interchangeable features.It is well known that MOFs containing hydroxyl functional groups in their frameworks release protons into their nanopores or nanochannels with relatively low activation energies and thereby exhibit proton conduction.59,60 This property can be exploited in CO2 sensing since the adsorption of CO2 can alter the concentration of protons which leads to changes in the proton conduction properties.61
Gassensmith et al.57 reported a Rb-based MOF, containing γ-cyclodextrin as organic linker, to be an effective CO2 sensing material due to the change of ionic conductivity upon its reaction with CO2. This MOF, namely CDMOF-2 or [(C48H80O40)(RbOH)2 (CH2Cl2)0.5]n, covalently binds CO2 to the non-coordinated free primary hydroxyl groups of its linkers, forming alkyl carbonic acid functional groups on the glucose unit (Fig. 6). Because carbonic acids are more acidic than primary alcohols, one would expect that the CO2-bound CD-MOF-2 will show higher conductivity than pristine CD-MOF-2. Such behaviour would be expected due to easier release of the proton on alkyl groups as compared to alcohols. Surprisingly, the conductivity of CDMOF-2 decreased by a factor of ∼550 after CO2 sorption as a result of increased steric hindrances of the primary alcohol groups with the carbonates.62 This sensor has a detection range from 0 vol% to 100 vol%. It was also shown that the chemical reaction is reversible over multiple cycles without affecting the sensor performances (Fig. 7).
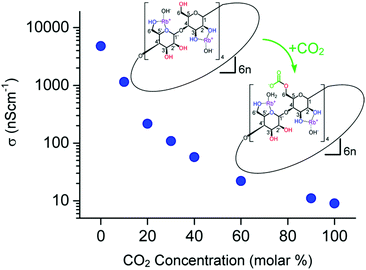 | ||
| Fig. 6 Variations in the conductivity of CDMOF-2 upon CO2 absorption/desorption cycles.62 | ||
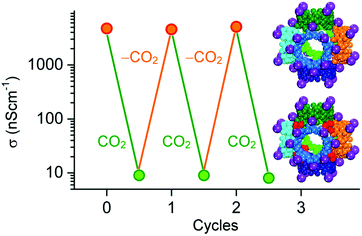 | ||
| Fig. 7 Graph of the average conductivity values of CDMOF-2 as a function of the CO2 concentration in N2 atmosphere. The structural change of the MOF's γ-cyclodextrin (γ-CD) linker upon reaction with CO2 is also shown.62 | ||
Impedance CO2 sensors were also fabricated using MOFs known for their selective CO2 adsorption and high proton conductivity properties, namely Zn-MOF-74 and NdMo-MOF, or [Nd(mpca)2Nd(H2O)6Mo(CN)8]·nH2O where mpca = 5-methyl-2-pyrazinecarboxylate.61,63,64 The sensors were fabricates by mixing MOF powders with polyvinylpyrrolidone (PVP) and deposited as thin films on Pt sensors via drop casting. Both sensors gave a response upon changing the CO2 concentration. Notably, the Zn-MOF-74 sensor showed low cross-sensitivity when changing the CO2 concentration from 0 to 2000 ppm at different RH: 40%, 50% and 60%.61 Same sensor also exhibits a fast response when the CO2 concentration changes from 0 to 500 ppm at 50% RH. Although the exposure to synthetic air led to sensor deactivation cause by the presence of oxygen, the process is reversible upon exposure to inert conditions.61
Field effect transistors and Kelvin probe
Sensors based on field effect transistors (FET) measure the electrical signal variations of the sensing material due to changes in its work function induced by the interaction with the target analyte.65 The work function of a system represents the energy required to release electrons from a material's surface. Changes in a material's work function can be transduced using Kelvin probes.66 These vibrating capacitor devices measure the contact potential difference between the sensing material and a reference material as electrons flow from the material with the lowest work function to the other.67Pentyala et al.68 studied the CO2 sensing properties of various M-MOF-74 (M = Mg, Co, Zn or Ni) materials by using a Kelvin probe to detect the variations in the work function of the system upon interaction with CO2. The materials tested share identical crystalline structures comprising pores of a relatively large diameters and, more importantly, free coordination sites on their metal centres. Once in the pores, the CO2 molecules coordinate to the metal cations effectively and alter the electronic properties of the material, such as its work function. By monitoring these fluctuations, one can extrapolate information on the CO2 concentration in the environment of the device. Successful CO2 detection was obtained with each M-MOF-74 tested, although sensing was heavily influenced by humidity levels. The authors further investigated the potential of MOF-74, by functionalizing the pores of the Mg-based analogue with ethylene diamine via post-synthetic procedures. In this MOF analogue, referred to as NH2-Mg-MOF-74, one of the amine groups of the ethylene diamine coordinates to the metal centre, while the other is oriented towards the inside of the pore. The free amine moiety can react with adsorbed CO2 molecules through reversible acid–base reactions which also leads to changes in the work function (Fig. 8a). NH2-Mg-MOF-74 displays significantly better sensing performances than Mg-MOF-74, particularly under humid conditions as the selectivity of the amine for CO2 exceeds that of the metal centres. Interestingly, both the NH2-Mg-MOF-74 and M-MOF-74 materials exhibit reversible behaviour.68 This work successfully proves the potential of MOFs for CO2 sensing by using work function measurements. The current LoD remains uncertain and, as mentioned by the authors, the unmodified MOFs have shown poor stability under humid conditions as was to be expected based on the hydrophilic nature of their metal centres and tendency to hydrolyse. Nevertheless, by taking advantage of the chemical tuneability of MOFs, the authors were able to redesign their sensing material to increase both the stability and performances of the sensors.
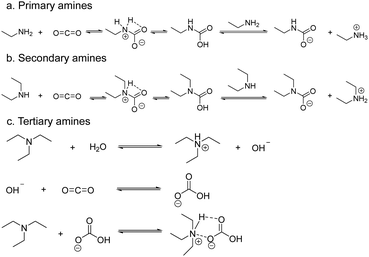 | ||
| Fig. 8 The formation of carbamates upon interaction with CO2 of primary (a), secondary (b), and tertiary amines (c).11 | ||
Luminescence-based sensing
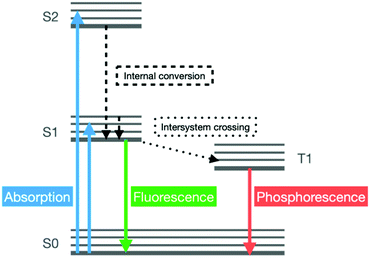
Luminescence describes the emission of light that occurs in certain systems following the cold excitation of their electrons, triggered by the absorption of an incident photon of a specific energy, to higher electronic states (Fig. 9).Due to their hybrid nature, MOFs often display two photon-emitting components, namely their organic linkers and metal ions. Moreover, additional emitting components can be added to the framework by loading its pores with luminescent guests. The interactions between the metal centres or linkers of the MOF and the guest molecules within its pores influence the luminescence properties. Such variation in the luminescence of the material can be measured and it provides detailed information on the concentration of specific guest molecules in the surrounding environment.69
Qi et al.70 designed a sensor that relies on the variations in the luminescence properties of a Zn(II) based MOF, namely MAF-34 or [Zn7(ip)12](OH)2 where ip = 1H-imidazo[4,5-f][1,10]phenanthroline, upon interaction with CO2 molecules. The crystalline structure of this flexible MOF is strongly affected by the presence of guest molecules in the pores. When loaded with guest molecules, the strain applied on the crystal structure of the framework prevents linker–linker interactions, effectively shifting the λmax of its fluorescent emission. Thus, low-pressure CO2 adsorption shifts the emission color from strong cyan photoluminescence (λmax = 487 nm) to yellowish orange (λmax = 540 nm).70 The sensing response was observed for three repeated cycles. MAF-34 also displayed sensing capability for vapours of various solvents (e.g., benzene, nitrobenzene, ethanol) and further testing showed that thin films of the MOF can be grown on substrates while retaining their fluorescence, a significant advantage for device-integration.70
Tank et al.71 used post-synthetic modification techniques to tune the chemical composition of UiO-66, also known as Zr6O4(OH)4(bdc)6 where bdc = 1,4-benzenedicarboxylate, by effectively integrating sodium phenolic functional groups within the pores of the MOF. The modified UiO-66, known as UiO-66-ONa, displays excellent sensitivity for CO2 as the sodium phenolic moieties are transformed into OH groups upon reacting with CO2 molecules. The respective fluorescence bands of UiO-66-ONa and UiO-66-OH are shifted by 50 nm. Therefore, by measuring the fluorescence spectra of a suspension of the MOF powder, one can probe the CO2 concentration in the surrounding atmosphere. Using this method, it was obtained a LoD of 3.5 × 10−7 M and a response time of ca. 65 seconds. The advantages of this material include strong chemical selectivity towards CO2, a well-studied and relatively easy synthesis procedure and excellent stability. On the other hand, using a suspension is unpractical for device integration and the recycling protocol, involving exposure to NaOH to convert UiO-66-OH back to UiO-66-ONa, suggests that the MOF probe is pH sensitive which could limit its effectiveness. Nevertheless, further development could overcome the aforementioned limitations and the concept of a CO2 sensor built on a well-known and robust MOF, which can be made using easy synthesis methods and with significant potential for chemical tuneability, is very promising. Furthermore, such method could virtually be used to probe any analyte provided that several requirements are fulfilled: the effective diameter of the analyte is small enough for it to access the pores, the functional group shows selective chemical reactivity with the analyte and the luminescence properties of the MOF before and after the reaction is significant enough to be measured accurately.
Challenges associated with the integration of MOFs in devices
The integration of MOFs into devices remains one of the major hindrances to the development of mass fabricated and every-day use MOF-based sensors. The problem partly originates from the poor performances of many of these hybrid materials in open atmosphere due to water interference (detection of water molecules instead of analyte) or their low water stability as they tend to hydrolyse under humid conditions.75,76Chocarro-Ruiz et al.72 provide a good example of how to tackle the humidity problem by making the deliberate decision to prioritise stability over sensitivity for increased durability. They used ZIF-8 nanoparticles, which are known to be water stable, as sensing component in a bimodal waveguide interferometric sensor that detects small fluctuations of the refractive index due to the interaction between an analyte and a sensing material. The sensor displays a linear response to CO2 concentrations varying from 1 vol% to 100 vol%, CO2 selectivity over water and CH4 and good stability as the sensor remained efficient after being stored 1 month in air (or 1 day at 80% RH). The LoD of 3130 ppm at room temperature (down to 774 ppm at 278 K), although relatively high compared to other MOF-based sensors, is suitable for multiple real-life applications. Moreover, the sensor showed constant performances through 50 consecutive measurements and reproducible performances from batch to batch.
While Chocarro-Ruiz et al.72 demonstrated the value of choosing a stable MOF as sensing component despite of higher LoD, tailoring the properties of MOFs to improve their stability remains a powerful approach for designing sensing materials with potential for real-life applications. This is supported by the studies of Kim et al.73 (discussed below) and Pentyala et al.24 (discussed above). Nevertheless, other hurdles regarding synthesis often remain for water-stable MOFs (e.g., ZIF-8, UiO-66, lanthanide-based MOFs)72,77 or MOFs species rendered water-stable through structural and/or chemical tuning (e.g., DMOF-1, MOF-74),73,78 especially when grown as thin films on substrates. These limitations stem from our poor understanding of the influence that both intrinsic properties of the MOF specimen and synthesis conditions have over the properties (e.g., thickness, crystalline orientation, roughness, homogeneity) of the subsequent thin film. Thus, to be able to grow these so-called surface-mounted metal–organic frameworks (SURMOFs) with high level of control over their aforementioned properties, extensive optimisation procedures are required for every MOF specimen. In addition to nucleation and growth mechanisms, the interaction between a SURMOF and its substrate is another crucial – yet misunderstood – phenomenon for an effective synthesis, long-term stability and overall efficiency of the device that rely on that SURMOF to operate. Thus, the incorporation of MOFs as full-fledged electronic components in devices still lags as many prototypes rely on loose single crystals or powders while other applications remain currently unrealistic. These considerations advocate for more experimental and theoretical investigations to unravel the mechanisms of nucleation, growth and general synthesis of MOFs and SURMOFs.
One of the few studies tackling the complexity of MOFs’ growth on solid surfaces was conducted by Kim et al.73 and it is focused on a series of pillar-layer SURMOFs based on Cu2(bdc)2(dabco) (bdc = 1,4-benzenedicarboxylic acid; dabco = 1,4-diazabicyclo[2.2.2]octane), also known as DMOF-1. Various analogues of DMOF-1 with different functionalised versions of its bdc linker were grown directly on hydroxy double salt (HDS) surfaces composed of copper-doped and aluminium-doped ZnO. It was shown that aside from the type of linker used, the growth conditions, i.e., reaction time and linker ratio, also affect the properties of the films (e.g., thickness, crystalline orientation, roughness). Although, as other studies showed, the exact influence of the synthesis parameters on their properties is not well understood, the authors successfully developed a synthetic procedure that yields homogeneous thin films of Cu2(bdc)2(dabco) and its analogues at ambient conditions and on cm-scale substrates. It was also examined the potential of Cu2(nbdc)2(dabco), where nbdc = 1,4-napthalenedicarboxyic acid, as a CO2 sensing material for quartz crystal microbalance (QCM) and optical fibre techniques. This MOF displayed promising CO2 sensing properties using both techniques, being able to detect up to 10 vol% of CO2, even at 56% RH, with strong CO2 selectivity (as compared to O2, H2, CH4 and N2 gases).
The CO2 (and SF6) sensor based on localized surface plasmon resonance (LSPR) spectroscopy and developed by Kreno et al.74 follows relatively easy fabrication protocols. It consists of an extended pattern of Ag nanoarray coated with a thin film of HKUST-1 as sensing component. The arrays of Ag nanoparticles are fabricated using nanosphere lithography79 whilst the film of HKUST-1 is obtained using a LbL liquid phase epitaxy (LPE) method,80 both well-known and relatively easy techniques with potential for high-throughput fabrication. Due to the enhancement factor provided by the presence of LSPs, the system can detect small variations in the refractive index of the thin film of HKUST-1 upon its interaction with CO2 molecules. The sensor showed successful CO2 detection within a few seconds for a concentration of 10 vol% CO2. Nevertheless, it is important to note that the reported data suggest that much lower LoD could be reached. While similar concepts with better sensing capabilities were discussed, the simplicity of the fabrication method and the room for improvement, in particular the sensing performances of the MOF, make this sensor attractive for inexpensive and large-scale manufacturing.
Lastly, as the aforementioned work from Chocarro-Ruiz et al.72 already suggested, researchers should keep their designs and fabrication methods simple as much as possible to enable reproduceable and scalable manufacturing. Yet, the importance of this argument should be nuanced in the case of devices showing exceptional sensing performances or unique capabilities or functionalities. Moreover, as the field of MOF-based sensing continuous to grow, overlooked properties such as the size of the sensor device, its power consumption, and electronics overheads will require increasing attention.81
Conclusions
Rigorous safety regulations for both indoor air quality and environmental exhaust from industrial processes have led to increased research focused on the development of effective gas sensors. All current technologies for CO2 sensing have their own set of advantages and limitations that make them relevant for specific sensing applications. For instance, devices based on optical detection have high sensitivity although they suffer from water interferences, polymer-based chemical sensors are produced relatively cheaply yet have shorter operating life and conductometric metal-oxide sensors are limited by their high operating temperatures despite that they enable the fabrication of portable instruments.MOFs are promising sensing layers for selective and sensitive CO2 detection as a result of their extremely high surface area, relatively wide range of operating temperatures (relevant for practical applications) as well as their structural and chemical tunability. Especially, the possibility to tailor the properties of MOFs enables synthetic chemists to gain control over the MOF–CO2 interactions by fine-tuning the pore sizes and/or active sites of these materials through meticulous selection of organic and inorganic parts and advanced MOF functionalization. From this perspective, we have discussed the different transducers used by researchers to design prototypes or develop proofs of concept for MOF-based sensors for CO2 detection. The challenges associated with the integration of MOFs as central components in sensing devices are also discussed. These challenges, mostly linked to the low hydrolytic stability and the general lack of understanding of the growth mechanisms of MOFs as thin layers, hold back the transition of MOF-based CO2 sensing devices from laboratory prototypes to off-the-shelf items.
We strongly believe that the potential of MOFs for chemical sensing applications justifies the research efforts to solve the aforementioned hurdles (and others like toxicity, low electrical conductivity, poor mechanical stability, etc.) or to optimize the existing prototypes. Thus, we advocate for further studies on these materials in relation not only to CO2 sensing, but also in relation to other relevant chemical sensing applications (such as H2S, NH3, N2O, volatile organic compounds and explosive sensing), as any insight would be beneficial for the development of MOF-based technologies.
Author contributions
Dr Andreea Gheorghe and Olivier Lugier contributed equally to this work in terms of logic, content construction and writing. Bohui Ye contributed to the literature search and writing parts of the article. Dr Stefania Tanase guided the writing of the article in terms of the topic, content structure and core components and contributed to the writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is also part of the Research Priority Area Sustainable Chemistry of the University of Amsterdam, http://suschem.uva.nl.Notes and references
- J. Bongaarts, Popul. Dev. Rev., 1992, 18, 299–319 CrossRef.
- J. C. Pales and C. D. Keeling, J. Geophys. Res., 1965, 70, 6053–6076 CrossRef CAS.
- M. McGee, CO2 Records, https://www.co2.earth/co2-records, (accessed May 7, 2021).
- K. Huttunen, in Clinical Handbook of Air Pollution-Related Diseases, ed. F. Capello and A. V. Gaddi, Springer International Publishing, Cham, 2018, pp. 107–114 Search PubMed.
- Industrial Scientific, The Gas Detection People, Carbon Dioxide Gas Detectors, http://www.indsci.com/products/carbon-dioxide/, (accessed April 2, 2018).
- W. J. Fisk, D. Faulkner and D. P. Sullivan, Accuracy of CO2 sensors in commercial buildings: a pilotstudy, Ernest Orlando Lawrence Berkeley NationalLaboratory, Berkeley, CA (US), 2006 Search PubMed.
- Edaphic, http://www.edaphic.com.au/knowledge-base/articles/gas-articles/ndir-explained/, (accessed July 3, 2018).
- Y.-W. Sun, Y. Zeng, W.-Q. Liu, P.-H. Xie, K.-L. Chan, X.-X. Li, S.-M. Wang and S.-H. Huang, Chin. Phys. B, 2012, 21, 090701 CrossRef.
- C. Mitra, Infrared Spectroscopy – Principles, Advances, and Applications, IntechOpen, 2018, pp. 5–12 Search PubMed.
- T.-V. Dinh, I.-Y. Choi, Y.-S. Son and J.-C. Kim, Sens. Actuators, B, 2016, 231, 529–538 CrossRef CAS.
- C. Chen, J. Kim and W.-S. Ahn, Korean J. Chem. Eng., 2014, 31, 1919–1934 CrossRef CAS.
- S. Lin and P. Theato, Macromol. Rapid Commun., 2013, 34, 1118–1133 CrossRef CAS PubMed.
- S. Srinives, T. Sarkar, R. Hernandez and A. Mulchandani, Anal. Chim. Acta, 2015, 874, 54–58 CrossRef CAS PubMed.
- X. Liu, S. Cheng, H. Liu, S. Hu, D. Zhang and H. Ning, Sensors, 2012, 12, 9635–9665 CrossRef PubMed.
- G. F. Fine, L. M. Cavanagh, A. Afonja and R. Binions, Sensors, 2010, 10, 5469–5502 CrossRef CAS PubMed.
- S. M. Kanan, O. M. El-Kadri, I. A. Abu-Yousef and M. C. Kanan, Sensors, 2009, 9, 8158–8196 CrossRef CAS PubMed.
- J. Zhang, Z. Qin, D. Zeng and C. Xie, Phys. Chem. Chem. Phys., 2017, 19, 6313–6329 RSC.
- A. Tricoli, M. Righettoni and A. Teleki, Angew. Chem., Int. Ed., 2010, 49, 7632–7659 CrossRef CAS PubMed.
- G. Korotcenkov, Handbook of Gas Sensor Materials, Springer, New York, NY, 2013 Search PubMed.
- Z. Ahmad, S. Manzoor, M. Talib, S. S. Islam and P. Mishra, Mater. Sci. Eng., B, 2020, 255, 114528 CrossRef CAS.
- E. Singh, M. Meyyappan and H. S. Nalwa, ACS Appl. Mater. Interfaces, 2017, 9, 34544–34586 CrossRef CAS PubMed.
- M. Schleicher and M. Fyta, ACS Appl. Electron. Mater., 2020, 2, 74–83 CrossRef CAS.
- I. G. Giannakopoulos, D. Kouzoudis, C. A. Grimes and V. Nikolakis, Adv. Funct. Mater., 2005, 15, 1165–1170 CrossRef CAS.
- V. Pentyala, P. Davydovskaya, R. Pohle, G. Urban and O. Yurchenko, Procedia Eng., 2014, 87, 1071–1074 CrossRef CAS.
- P. Freund, L. Mielewczyk, M. Rauche, I. Senkovska, S. Ehrling, E. Brunner and S. Kaskel, ACS Sustainable Chem. Eng., 2019, 7, 4012–4018 CrossRef CAS.
- H. Yuan, J. Tao, N. Li, A. Karmakar, C. Tang, H. Cai, S. J. Pennycook, N. Singh and D. Zhao, Angew. Chem., Int. Ed., 2019, 58, 14089–14094 CrossRef CAS PubMed.
- N. Wang, A. Mundstock, Y. Liu, A. Huang and J. Caro, Chem. Eng. Sci., 2015, 124, 27–36 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- N. C. Burtch, J. Heinen, T. D. Bennett, D. Dubbeldam and M. D. Allendorf, Adv. Mater., 2018, 30, 1704124 CrossRef PubMed.
- S. Jung, L. Huelsenbeck, Q. Hu, S. Robinson and G. Giri, ACS Appl. Mater. Interfaces, 2021, 13, 10202–10209 CrossRef CAS PubMed.
- Y. Lin, C. Kong, Q. Zhang and L. Chen, Adv. Energy Mater., 2017, 7, 1601296 CrossRef.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- R. Krishna and J. M. van Baten, Sep. Purif. Technol., 2012, 87, 120–126 CrossRef CAS.
- M. Dinca and J. R. Long, J. Am. Chem. Soc., 2005, 127, 9376–9377 CrossRef CAS PubMed.
- W. Zhuang, D. Yuan, D. Liu, C. Zhong, J.-R. Li and H.-C. Zhou, Chem. Mater., 2012, 24, 18–25 CrossRef CAS.
- K. K. Gangu, S. Maddila, S. B. Mukkamala and S. B. Jonnalagadda, Inorg. Chim. Acta, 2016, 446, 61–74 CrossRef CAS.
- M. D. Allendorf, A. Schwartzberg, V. Stavila and A. A. Talin, Chem. – Eur. J., 2011, 17, 11372–11388 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- H.-T. Kim, W. Hwang, Y. Liu and M. Yu, Opt. Express, 2020, 28, 29937 CrossRef CAS PubMed.
- R. Islam, M. M. Ali, M.-H. Lai, K.-S. Lim and H. Ahmad, Sensors, 2014, 14(4), 7451–7488 CrossRef PubMed.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef CAS.
- X. Chong, Y. Zhang, E. Li, K.-J. Kim, P. R. Ohodnicki, C. Chang and A. X. Wang, ACS Sens., 2018, 3, 230–238 CrossRef CAS PubMed.
- K. Huang, S. Gong, L. Zhang, H. Zhang, S. Li, G. Ye and F. Huang, Chem. Commun., 2021, 57, 2144–2147 RSC.
- Chemistry Libre Texts Library, Infrared spectroscopy, https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Spectroscopy/Vibrational_Spectroscopy/Infrared_Spectroscopy, (accessed April 4, 2018).
- International sensor technology, Infrared gas sensor, http://www.intlsensor.com/pdf/infrared.pdf, (accessed April 5, 2018).
- B. Lee, Opt. Fiber Technol., 2003, 9, 57–79 CrossRef CAS.
- X. Chong, K.-J. Kim, P. R. Ohodnicki, E. Li, C.-H. Chang and A. X. Wang, IEEE Sens. J., 2015, 15, 5327–5332 CAS.
- X. Chong, K.-J. Kim, E. Li, Y. Zhang, P. R. Ohodnicki, C.-H. Chang and A. X. Wang, Sens. Actuators, B, 2016, 232, 43–51 CrossRef CAS.
- X. Wang and O. S. Wolfbeis, Anal. Chem., 2020, 92, 397–430 CrossRef CAS PubMed.
- K. Wysokiński, M. Napierała, T. Stańczyk, S. Lipiński and T. Nasiłowski, Sensors, 2015, 15, 31888–31903 CrossRef PubMed.
- J. Hodgkinson and R. P. Tatam, Meas. Sci. Technol., 2012, 24, 012004 CrossRef.
- Novocontrol, Dielectric Spectroscopy, Conductivity Spectroscopy, and Electrochemical Impedance Spectroscopy, http://www.novocontrol.de/php/intro_overview.php, (accessed April 19, 2018).
- I. Strauss, A. Mundstock, M. Treger, K. Lange, S. Hwang, C. Chmelik, P. Rusch, N. C. Bigall, T. Pichler, H. Shiozawa and J. Caro, ACS Appl. Mater. Interfaces, 2019, 11, 14175–14181 CrossRef CAS PubMed.
- I. Stassen, J.-H. Dou, C. Hendon and M. Dincă, ACS Cent. Sci., 2019, 5, 1425–1431 CrossRef CAS PubMed.
- C. Liu, Y. Gu, C. Liu, S. Liu, X. Li, J. Ma and M. Ding, ACS Sens., 2021, 6, 429–438 CrossRef CAS PubMed.
- M. E. DMello, N. G. Sundaram and S. B. Kalidindi, Chem. – Eur. J., 2018, 24, 9220–9223 CrossRef CAS PubMed.
- L. Liu, Y. Zhou, S. Liu and M. Xu, ChemElectroChem, 2018, 5, 6–19 CrossRef CAS.
- N. C. Jeong, B. Samanta, C. Y. Lee, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 51–54 CrossRef CAS PubMed.
- A. Shigematsu, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 2034–2036 CrossRef CAS PubMed.
- B. Ye, A. Gheorghe, R. van Hal, M. Zevenbergen and S. Tanase, Mol. Syst. Des. Eng., 2020, 5, 1071–1076 RSC.
- J. J. Gassensmith, J. Y. Kim, J. M. Holcroft, O. K. Farha, J. F. Stoddart, J. T. Hupp and N. C. Jeong, J. Am. Chem. Soc., 2014, 136, 8277–8282 CrossRef CAS PubMed.
- S. Tanase, M. C. Mittelmeijer-Hazeleger, G. Rothenberg, C. Mathonière, V. Jubera, J. M. M. Smits and R. de Gelder, J. Mater. Chem., 2011, 21, 15544–15551 RSC.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- A. Lv, Y. Pan and L. Chi, Sensors, 2017, 17, 213 CrossRef PubMed.
- Kelvin Probe, http://www.kelvinprobe.info/technique-theory.htm, (accessed April 12, 2018).
- N. A. Surplice and R. J. D’Arcy, J. Phys. E: Sci. Instrum., 1970, 3, 477–482 CrossRef.
- V. Pentyala, P. Davydovskaya, M. Ade, R. Pohle and G. Urban, Sens. Actuators, B, 2016, 225, 363–368 CrossRef CAS.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- X.-L. Qi, R.-B. Lin, Q. Chen, J.-B. Lin, J.-P. Zhang and X.-M. Chen, Chem. Sci., 2011, 2, 2214–2218 RSC.
- Y. Tang, J. Chen, H. Wu, J. Yu, J. Jia, W. Xu, Y. Fu, Q. He, H. Cao and J. Cheng, Dyes Pigm., 2020, 172, 107798 CrossRef CAS.
- B. Chocarro-Ruiz, J. Pérez-Carvajal, C. Avci, O. Calvo-Lozano, M. I. Alonso, D. Maspoch and L. M. Lechuga, J. Mater. Chem. A, 2018, 6, 13171–13177 RSC.
- K.-J. Kim, J. E. Ellis, B. H. Howard and P. R. Ohodnicki, ACS Appl. Mater. Interfaces, 2021, 13, 2062–2071 CrossRef CAS PubMed.
- L. E. Kreno, J. T. Hupp and R. P. Van Duyne, Anal. Chem., 2010, 82, 8042–8046 CrossRef CAS PubMed.
- N. Al-Janabi, P. Hill, L. Torrente-Murciano, A. Garforth, P. Gorgojo, F. Siperstein and X. Fan, Chem. Eng. J., 2015, 281, 669–677 CrossRef CAS.
- H. Jasuja, N. C. Burtch, Y. Huang, Y. Cai and K. S. Walton, Langmuir, 2013, 29, 633–642 CrossRef CAS PubMed.
- X. Liu, N. K. Demir, Z. Wu and K. Li, J. Am. Chem. Soc., 2015, 137, 6999–7002 CrossRef CAS PubMed.
- S. Zuluaga, E. M. A. Fuentes-Fernandez, K. Tan, F. Xu, J. Li, Y. J. Chabal and T. Thonhauser, J. Mater. Chem. A, 2016, 4, 5176–5183 RSC.
- C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2001, 105, 5599–5611 CrossRef CAS.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS PubMed.
- D. M. Wilson, S. Hoyt, J. Janata, K. Booksh and L. Obando, IEEE Sens. J., 2001, 1, 256–274 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |