Superoxide dismutase nanozymes: an emerging star for anti-oxidation
Hanqing
Zhao†
ab,
Ruofei
Zhang†
ab,
Xiyun
Yan
*abc and
Kelong
Fan
 *abc
*abc
aCAS Engineering Laboratory for Nanozyme, Key Laboratory of Protein and Peptide Pharmaceutical, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China. E-mail: fankelong@ibp.ac.cn; yanxy@ibp.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 101408, China
cNanozyme Medical Center, School of Basic Medical Sciences, Zhengzhou University, Zhengzhou 450052, Henan, China
First published on 28th May 2021
Abstract
Superoxide dismutases (SODs) are a group of metalloenzymes that catalyze the dismutation of superoxide radicals (O2˙−) into hydrogen peroxide (H2O2) and oxygen (O2). As the first line of defense against reactive oxygen species (ROS)-mediated damage, SODs are expected to play an important role in the treatment of oxidative stress-related diseases. However, the clinical applications of SODs have been severely limited by their structural instability and high cost. Compared with natural enzymes, nanozymes, nanomaterials with enzyme-like activity, are more stable, and economical, can be easily modified and their activities can be adjusted. Due to their excellent characteristics, nanozymes have attracted widespread attention in recent years and are expected to become effective substitutes for natural enzymes in many application fields. Importantly, some nanozymes with SOD-like activity have been developed and proved to have a mitigating effect on diseases caused by oxidative stress. These studies on SOD-like nanozymes provide a feasible strategy for breaking through the dilemma of SOD clinical applications. However, at present, the specific catalytic mechanism of SOD-like nanozymes is still unclear, and many important issues need to be resolved. Although there are many comprehensive reviews to introduce the overall situation of the nanozyme field, the research on SOD-like nanozymes still lacks a systematic review. From the structure and mechanism of natural SOD enzymes to the structure and regulation of SOD-like nanozymes, and then to the measurement and application of nanozymes, this review systematically summarizes the recent progress in SOD-like nanozymes. The existing shortcomings and possible future research hotspots in the development of SOD-like nanozymes are summarized and prospected. We hope that this review would provide ideas and inspirations for further research on the catalytic mechanism and rational design of SOD-like nanozymes.
1. Introduction
Reactive oxygen species (ROS) are formed from incomplete reduction of oxygen molecules. As shown in Fig. 1, excessive production of ROS leads to oxidative stress, a harmful process that acts as an important mediator of cellular structural damage, especially lipid peroxidation and membrane damage, and protein and DNA oxidative damage.15 Oxidative damage is a double-edged sword. On the one hand, it damages normal tissues and cells as mentioned above, while on the other hand, oxidative damage is able to induce cell senescence and apoptosis, which may be used for anti-tumor and antibacterial applications. Therefore, reasonable regulation of ROS is a promising strategy to treat a variety of redox related diseases. There are three main forms of ROS: superoxide radical (O2˙−), hydrogen peroxide (H2O2), and hydroxyl radical (˙OH). Among them, O2˙−, the addition of an electron to dioxygen, is the principal one.19 O2˙− is produced in the mitochondria of cells mainly through metabolic processes or physical irradiation to “activate” oxygen, which further interacts with other molecules to produce “secondary” ROS.20 O2˙− is converted to H2O2 by superoxide dismutase (SOD), and then some transition metals (such as Fe2+, Cu+, etc.) may decompose H2O2 into highly toxic ˙OH (Fenton reactions).21 Therefore, regulating the amount of O2˙−in vivo is very important for the treatment of ROS and oxidative stress-related diseases.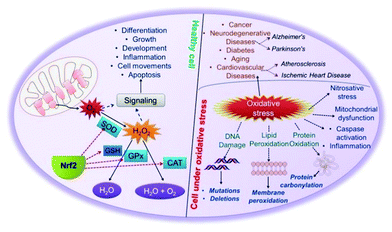 | ||
| Fig. 1 Cellular antioxidant machinery and oxidative stress.8 Reproduced with permission. Copyright 2009, The Royal Society of Chemistry. | ||
SOD has the ability to scavenge O2˙−,23 which catalyzes the dismutation of O2˙− to produce O2 and H2O2 as shown in eqn (1)–(3):
| M(n+1)+ + O2˙− → Mn+ + O2 | (1) |
| Mn+ + O2˙− (+ 2H+) → M(n+1)+ + H2O2 | (2) |
| Overall: O2˙− + O2˙− + 2H+ → O2 + H2O2 | (3) |
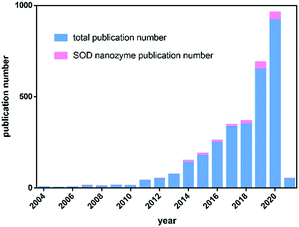
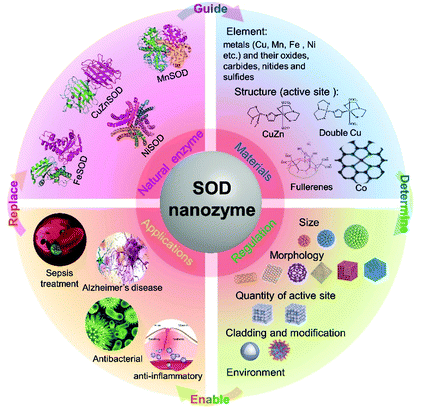
Although natural enzymes have high catalytic efficiency, there are some limitations to their applications, including the high cost of preparation and purification, harsh reaction conditions, poor stability and so on. Based on this, a series of artificial enzymes have been developed to replace natural enzymes. Among them, nanozymes, a new generation of artificial enzymes, are currently a hot research topic. Nanozymes are a type of nanomaterial with intrinsic enzyme-like activity, which have unique characteristics compared with natural enzymes, such as low cost, high stability, easy production and preparation, easy tuning, etc.Table 1 compares the advantages and disadvantages of natural enzymes and nanozymes in detail. Fe3O4 magnetic nanoparticles were reported to have peroxidase-like activity in 2007, which was the first reported nanozyme.26 Since then, the field of nanozymes has developed rapidly. Based on our statistical analysis of nanozyme-related publications from the Web of Science database, by January 2021, more than 1000 kinds of nanozymes have been reported, the composition of which involves 51 elements. There are more than 300 scientific researchers from 31 countries exploring this field. Most nanozymes exhibited redox-related enzyme-like activities, mainly including the activities of oxidase, peroxidase, catalase and SOD. Among them, SOD-like nanozymes are the earliest discovered, but the least studied. There were more than 3000 nanozyme-related studies reported totally by January 2021, while there were only less than 200 studies related to SOD-like activity (Fig. 2). It is evident that, compared with other nanozymes, the development of SOD-like nanozymes was deficient. In addition, although there are some reviews that systematically summarize nanozymes, almost no specific review of SOD-like nanozymes has been reported. Considering the important prospects and practicality of SOD-like nanozymes in biomedical applications, it is necessary to make a comprehensive summary of its research to clarify its development and challenges. Thus, we summarize the advances of SOD-like nanozymes in the following sections. First, the structure and activity mechanism of natural SODs is briefly introduced. Then, based on the research of natural SODs, the catalytic mechanism and regulatory factors of the SOD-like activity of nanozymes are expounded. After that, the measurement methods and applications of SOD-like nanozymes in various fields are summarized. Finally, the challenges and prospects in the current development are put forward. We hope to deepen the understanding of the SOD-like activity of nanozymes and promote future research through this review (Scheme 1).
| Natural enzymes | Nanozymes | |
|---|---|---|
| Stability | Limited | High |
| Cost | High | Low |
| Production | Complex | Easy |
| Storage | Short term | Long term |
| Recyclable | No | Yes |
| Environments | Only mild | Could be harsh |
| Catalytic activity | Very high | High |
| Biocompatibility | Good | Limited |
| Selectivity | High | Limited |
2. Structure and catalytic mechanism of natural SOD enzymes
Natural SODs, which are generally composed of proteins and metal cofactors, are considered to be necessary for aerobic cells. Natural SODs exist in most prokaryotic cells and some eukaryotic cells, and are widely present in various organelles. Based on different cofactors, natural SOD enzymes are divided into four types: copper-zinc SOD (CuZnSOD), manganese SOD (MnSOD), iron SOD (FeSOD), and nickel SOD (NiSOD). The structure and mechanism of these natural SOD enzymes are briefly introduced below.2.1 Copper-zinc superoxide dismutase (CuZnSOD)
As shown in Fig. 3, the structure of natural CuZnSOD is composed of two subunits linked by histidine, each of which has an active center composed of metal atoms, Cu and Zn. In the active center, Cu acts as the catalytic site, while Zn maintains the stability of the enzyme.27 During the catalytic reaction, Cu is alternatively reduced and oxidized in continuous encounters with O2˙−, changing between +2 and +1 valence states. As shown in eqn (4) and (5), Cu2+Zn2+SOD first adsorbs and binds O2˙− and then mediates its oxidation. In this process, Cu2+Zn2+SOD turns into Cu+Zn2+SOD with slight changes in the structure while oxidizing O2˙− to O2. Then, Cu+Zn2+SOD combines with another molecule O2˙−, reducing it to H2O2 and returning to the original form of Cu2+Zn2+SOD.28 In this process, copper must be exposed to the solvent outside the enzyme so that it can bind with the O2˙− to initiate the reaction.29–31| Cu2+Zn2+SOD + O2˙− → Cu+Zn2+SOD + O2 | (4) |
| Cu+Zn2+SOD + O2˙− (+2H+) → Cu2+Zn2+SOD + H2O2 | (5) |
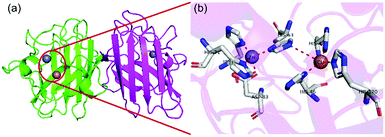 | ||
| Fig. 3 Schematic illustration of CuZnSOD. (a) The structure and (b) active sites of human CuZnSOD (pdb ref: 1pu0). Picture created with PyMOL. | ||
The rate of catalytic reaction is related to the electrostatic attraction of negatively charged O2˙− into the positively charged active center. Positively charged arginine near the active site greatly enhances electrostatic guidance. Once this arginine is mutated to uncharged isoleucine or negatively charged glutamic acid or aspartic acid, the catalytic efficiency (kcat) of CuZnSOD decreases.32 Another study showed that site-specific mutants that increase local positive charge such as changing negatively charged glutamic acid to glutamine near the active site produced more efficient enzymes than wild-type enzymes.33 The role of zinc-bridging imidazole in CuZnSOD is to maintain the geometry of the copper coordination sphere, allow the rapid dissociation of the combined peroxide, and make CuZnSOD pH-independent.34 Once the Zn is removed, the second reduction reaction (eqn (5)) in the dismutation reaction of Cu2+apoSOD becomes pH-dependent.
2.2 Manganese superoxide dismutase (MnSOD)
Despite catalyzing a similar reaction, the active center structure of MnSOD is different from that of CuZnSOD. MnSOD has both dimer and tetramer structures. For prokaryotes (such as bacteria), MnSOD is in the dimer form, while the MnSOD of eukaryotic cells is usually in the tetramer form. Take the human MnSOD structure as an example (Fig. 4). There is one manganese active site in each subunit, and each manganese is coordinated with four protein ligands (three histidine residues and one aspartic acid residue) and a single-oxygen-containing molecule (H2O or OH−). Fig. 4(b) shows the inner sphere of the active center that interacts directly with manganese. The outer shell of the active center includes His30, Tyr34, Phe77, Trp78, Trp123, Gln143, Trp161, and Glu162, which are key residues to the highly efficient enzymatic activity.35 The substrate is diffused to the active site through His30 and Tyr34 and binds with manganese ions. Phe77, Trp78, Trp123 and Trp161 form a hydrophobic cage to promote the interaction between the substrate and manganese ions. As shown in Fig. 5, manganese is caved in the lumen of the active site and forms a hydrogen bond network with the surrounding side chain residues such as Tyr34 and Gln143 and with two single-oxygen-containing molecules at the opening of the cavity.36 The role of the hydrogen bond network is probably to promote proton transfer in the process of O2˙− reduction to H2O2, and maintain the stability and catalytic activity of MnSOD. Like other SODs, the catalytic mechanism of MnSOD involves a cycle between Mn3+ and Mn2+. However, due to the complexity of the structure, the catalytic mechanism of MnSOD involves more steps than other SODs and involves a series of proton transfers (eqn (6)–(9)).24,36| Mn3+SOD(OH−) + O2˙− (+ H+) → Mn2+SOD(H2O) + O2 | (6) |
| Mn2+SOD(H2O) + O2˙− (+ H+) → Mn3+SOD(OH−) + H2O2 | (7) |
| Mn2+SOD(H2O) + O2˙− → Mn3+SOD(H2O) − O22− | (8) |
| Mn3+SOD(H2O) − O22− (+ H+) → Mn3+SOD(OH−) + H2O2 | (9) |
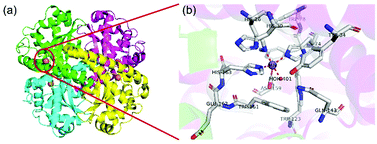 | ||
| Fig. 4 Schematic illustration of MnSOD. (a) The structure and (b) active sites of human MnSOD (pdb ref: 1n0j). Picture created with PyMOL. | ||
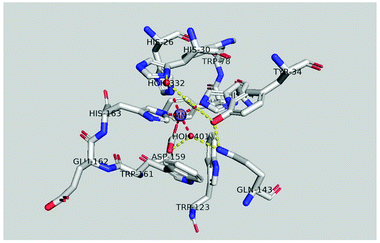 | ||
| Fig. 5 Hydrogen bond network and superoxide-dependent proton transfer mechanism (pdb ref: 1n0j). Picture created with PyMOL. | ||
2.3 Iron superoxide dismutase (FeSOD)
FeSOD was first discovered in prokaryotes, of which the structure is similar to that of MnSOD. As shown in Fig. 6, FeSOD is a dimer and each monomer has an iron-centered active site that contains a single iron binding three histidines, an aspartic acid and a water molecule. Although the active sites of FeSOD and MnSOD are similar in structure, the substitution of their metal ions with each other results in reduced activity.37 The overall catalytic mechanism of FeSOD also relies on the conversion between Fe2+ and Fe3+ in a ping-pong manner.24,37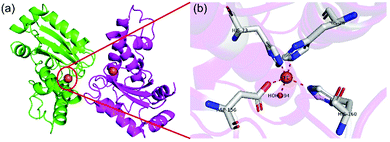 | ||
| Fig. 6 Schematic illustration of FeSOD. (a) The structure and (b) active sites of E. coli FeSOD (pdb ref: 1isa). Picture created with PyMOL. | ||
2.4 Nickel superoxide dismutase (NiSOD)
NiSOD was discovered in recent years. The structure of NiSOD is a homohexamer, with a Ni binding hook formed by the combination of Ni and some amino acid residues in the center of each monomer (Fig. 7). Unlike other SODs, in addition to histidine and aspartic acid, NiSOD has a large number of cysteine residues linked to the active center Ni via S–Ni bonds. However, the metal-bound cysteines are highly oxidation-sensitive and susceptible to damage. Studies have shown that all Ni related redox enzymes contain cysteine or redox non-harmless ligands. How NiSOD avoids the oxidative damage of S–Ni bonds and the catalytic mechanism of NiSOD are still being explored. However, it is certain that the mechanism of catalytic disproportionation is related to the transformation between Ni2+ and Ni3+.38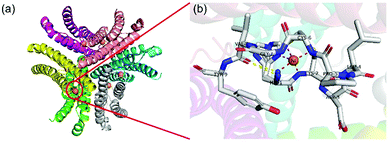 | ||
| Fig. 7 Schematic illustration of NiSOD. (a) The structure and (b) active sites of Streptomyces coelicolor NiSOD (pdb ref: 1t6u). Picture created with PyMOL. | ||
In summary, this section briefly introduces the active center of natural SODs and their catalytic mechanism. The active centers of the above four common natural SODs have similar structures, that is, usually containing histidine and metal ions that are always coordinated with the nitrogen in the amino acid residues in the catalytic microenvironment. Besides, these natural SODs all have their own unique structures, which are mainly reflected in the use of hydrogen bond network, hydrophobic, and electric charge interactions, coordination with special amino acids and so on. These special structures make natural enzymes different in their substrate affinity, proton transfer efficiency, and redox reactivity, which ultimately lead to their different catalytic efficiencies. We hope that the summary of the structure and mechanism of natural SOD enzymes can provide references and inspirations for future research, so that SOD-like nanozymes will more closely simulate the natural enzymes, so as to exhibit a more potent catalytic performance.
3. The development and mechanism of SOD nanozymes
On the basis of the research on the structure and mechanism of natural SODs, various types of nanozymes have been gradually developed.3.1 Classification of SOD-like nanozymes
In 1985, Kroto et al. reported that C60 exhibited superoxide radical scavenging activity, which is an early proof that materials may simulate SOD-like activity, but the concept of nanozymes had not yet been proposed.39 Since then, various SOD-like nanomaterials have been gradually developed. Up to now, about 100 kinds of nanozymes have been found to exhibit SOD-like activity, most of which consist of transition metals (e.g. Fe, Co, etc.) and elements such as carbon, nitrogen, oxygen, sulfur, etc. The following is a brief introduction of the most-studied nanozymes with SOD-like activity and their catalytic mechanism.The synthesis methods of cerium oxide nanozymes include the oxidation of Ce(NO3)3·6H2O with an oxidizing agent, such as hydrogen peroxide, ammonium hydroxide or NaOH.42–44 Another method is to induce a reaction between (NH4)2Ce(NO3)6 and CH3COONa.45
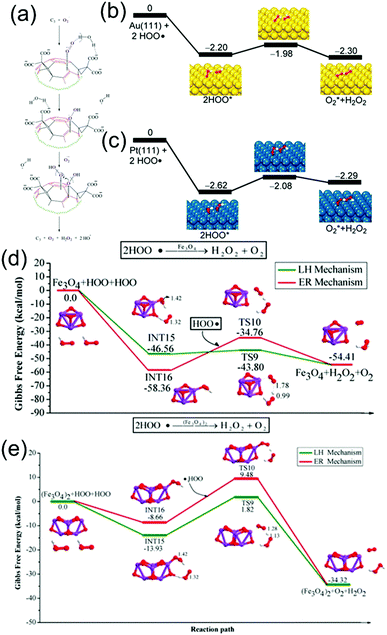 | ||
| Fig. 8 Different kinds of nanozymes and their mechanism exploration. (a) Schematic representation of the catalytic interaction of C3 and O2˙−. Chemical bonds colored in red are associated with electron-deficient areas. The incoming superoxide ions and the oxygen atoms derived from them are colored in purple. Broken lines represent the hydrogen bonding between oxygen and hydrogen atoms and hyphenated lines are used to represent the electrostatic attraction between negatively charged oxygen atoms and electron-deficient areas on the C60 moiety. In the proposed mechanism, C3 is suggested to electrostatically drive superoxide anions toward electron-deficient areas on its surface until a second O2˙− arrives to undergo dismutation with the help of protons from carboxyl groups and/or surrounding water molecules.4 Reproduced with permission. Copyright 2004, Elsevier. (b and c) Potential energy profiles for rearrangements of two HO2˙ groups on the (111) facets of (b) Au and (c) Pt.5 Reproduced with permission. Copyright 2015, American Chemical Society. (d and e) Calculated reaction energy profiles corresponding to the SOD-like activity of (d) Fe3O4 and (e) (Fe3O4)2.14 Reproduced with permission. Copyright 2019, American Chemical Society. | ||
Fullerenes used in studies are mostly obtained through purchase and are usually modified by various chemical methods. The synthesis of other carbon materials involves more diverse and complex chemical reactions and involves more steps. For example, in a study a pyrolysis method was used to mix melamine and formalin/phenol together using a Pluronic F127 soft template as the nitrogen and carbon sources, respectively, to prepare N-doped porous carbon nanospheres.48 In another study, aniline and colloidal silica were mixed together, then the aniline was polymerized and carbonized, and the silica was removed to synthesize N-doped sponge-like carbon spheres.49
At the same time, some materials composed of two or more metals such as Au and Pt or Fe and Cu,59,60 some polymers such as PLGA,61 PEG,62 and hydrogels63 and materials with different shapes such as graphitic carbon nitride nanosheets43 and NiO nanoflowers54 have been developed. However, the current research on the catalytic mechanism is not in-depth. Several new methods for exploring the mechanisms are being developed. For example, density functional theory (DFT) and microkinetic modeling are used to determine the reaction route. The team of Guo used this method to evaluate whether the catalytic mechanism of a range of nanomaterials resembles Langmuir–Hinshelwood (LH) or Eley–Rideal (ER) processes.14 Taking (Fe3O4)n as an example, they listed various intermediate transition states in two reaction routes, LH and ER, respectively, and calculated the reaction energy profiles of the reaction generated in each transition state (Fig. 8(d) and (e)). Then, they evaluated the heat release of the potential energy surface of the two mechanisms and finally proved that both Fe3O4 and (Fe3O4)2 reacted through the LH route. In addition, they also evaluated a series of kinetic parameters in the reaction process by means of microkinetic modeling, which further proved that both Fe3O4 and (Fe3O4)2 reacted through the LH pathway.14 In a similar way, their team also evaluated the SOD mechanism of (Co3O4)n nanozymes and ConFe3−nO4 (n = 1–2) nanozymes. The results showed that, unlike (Fe3O4)n, (Co3O4)n was catalyzed through the ER pathway and that CoFe2O4 exhibited a better SOD-like activity than Co2FeO4.64,65
There are many metal nanozymes, and their synthesis methods are also diversified. Some metallic elemental nanoparticles are usually prepared by reduction methods. For example, in a study, ethanol was used as the reducing agent to prepare nano-Pt by reducing H2PtCl6, and PVP as the protective reagent to control the size of Pt nanoparticles.50 Some metal oxides are usually prepared by the oxidation or reduction of metal-containing salts. For example, in a study Co3O4 was formed through the oxidation of Co (NO3)2·6H2O by ammonia and hydrogen peroxide.55 In another study, Mn3O4 was prepared by adding oleic acid into KMnO4 and then calcining the mixture in air.66 The hydrothermal method is also used in the synthesis of Mn3O4 by adding Mn(OAc)2·4H2O and anhydrous ethanol together.67
3.2 The regulation of the SOD-like activity of nanozymes
There are many factors affecting the SOD-like activity of nanozymes, including the physical and chemical properties of nanomaterials such as size, shape, composition, external modification of nanomaterials, and different reaction systems. This section briefly describes the factors and regulation methods of SOD-like activity.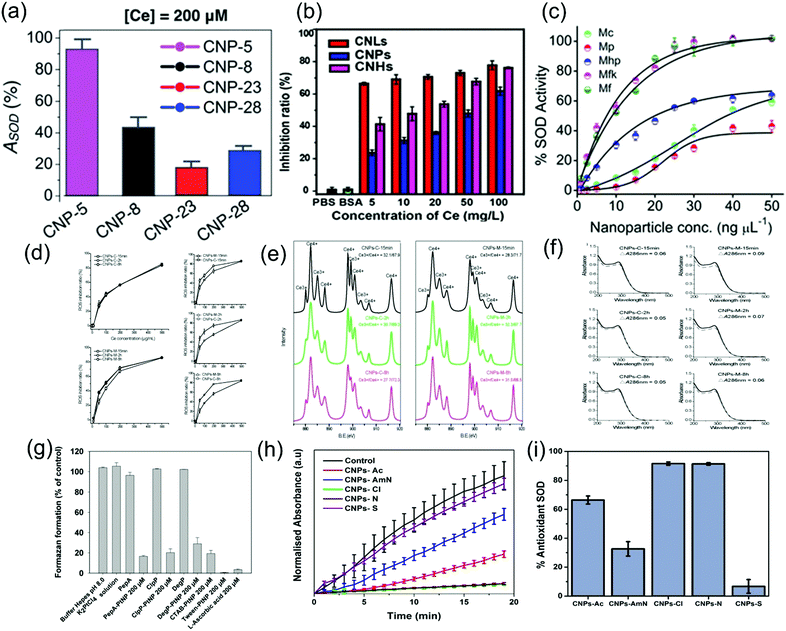 | ||
| Fig. 9 Effects of different regulation methods on the SOD activity of nanozymes. (a) Effect of size on the enzyme activity of cerium oxide nanoparticles with sizes of 4.5 nm, 7.8 nm, 23 nm, and 28 nm.9 Reproduced with permission. Copyright 2009, The Royal Society of Chemistry. (b) Effect of morphology on the enzyme activity of ceria nanochains (CNHs), ceria nanoclusters (CNLs) and ceria nanoparticles (CNPs).11 Reproduced with permission. Copyright 2017, American Chemical Society. (c) Effect of morphology on the enzyme activity of Mn3O4 nano-cubes, nano-polyhedrons, nano-hexagonal plates, nanoflakes and nanoflowers.12 Reproduced with permission. Copyright 2018, John Wiley and Sons. (d) ROS scavenging efficiency of CNPS-M and CNPS-C. (e) The Ce3+/Ce4+ ratio of the material was characterized by XPS. (f) The change of absorbance before and after oxidation.13 Reproduced with permission. Copyright 2017, Elsevier. (g) Effects of different coated proteins on the superoxide anion quenching ability and the SOD-like activity of PtNPs.17 Reproduced with permission. Copyright 1991, The Royal Society of Chemistry. (h) SOD-like activity of CNPs synthesized using different precursors, cerium(III) acetate (CNPs-Ac), cerium(IV) ammonium nitrate (CNPs-AmN), cerium(III) chloride (CNPs-Cl), cerium(III) nitrate (CNPs-N), and cerium(III) sulfate (CNPs-S). Absorbance of WST-1 formazan dye at 440 nm in a 96-well plate plotted against the time course of the reaction. (i) The percent of antioxidant capacity of CNPs correlates with the SOD-like activity of CNPs.18 Reproduced with permission. Copyright 2017, American Chemical Society. | ||
Besides the cladding component, some modified components also affect the SOD-like activity of nanozymes. A study modified cerium oxide nanoparticles with different anions of the precursor salt counterions. The results in Fig. 9(h) and (i) showed that cerium(III) nitrate (CNPs-N) and cerium(III) chloride (CNPs-Cl) exhibited a higher SOD-like activity, followed by cerium(III) acetate (CNPs-Ac), while cerium(III) sulfate (CNPs-S) and cerium(IV) ammonium nitrate (CNPs-AmN) did not show significant SOD-like activity because of their low dispersion stability. The surface of CNPS-N presents more Ce3+, resulting in a higher SOD-like activity. Although the Ce3+ concentration on the surface of CNPS-Cl is low, the presence of Cl− changes the catalytic antioxidant performance of CNPS-Cl because it indirectly alters the surface chemistry, which decreases the oxidation potential. The transformation of Ce3+ to Ce4+ is more likely to occur in the presence of Cl– ions, thus improving SOD-like activity.18 In addition, studies have shown that silicon dioxide NPs modified by imidazole, carboxyl and phenol exhibited SOD-like activity.71 Modification of cerium oxide nanoparticles with some ligands such as triethyl phosphite (TEP) mediated redox reactions and regulated SOD-like activity by increasing the ratio of Ce3+ and Ce4+ on the material surface.72
In summary, a series of SOD-like nanozymes have been developed on the basis of the natural enzyme elements and the mechanism of active sites. In addition, the activity mechanism and regulation mode of these SOD-like nanozymes have been gradually explored. However, the research on the mechanism of SOD-like nanozymes is still relatively shallow. Some new exploratory techniques and methods also urgently need to be developed. We hope that the summary of this section can inspire further mechanism research, so as to develop SOD-like nanozymes with a higher activity, more explicit active sites and a better coordination between the active sites and framework structure.
4. Methods for measuring the SOD-like activity of nanozymes
Compared with peroxidase, oxidase and catalase, the characterization method for the SOD-like activity of nanozymes was not standardized yet. At present, the method for measuring the SOD-like activity of nanozymes is limited to qualitatively comparing the activities between nanozymes, and there are few quantitative studies on the specific activity and kinetic parameters. In fact, compared with the other enzyme activities that can be directly measured, the determination of SOD is relatively difficult. This may be due to the fact that the catalytic substrate of SOD, O2˙−, is a transient product and extremely unstable. Thus, the SOD-like activity is mostly determined by an indirect method. This section summarizes the current commonly used methods for the determination of SOD-like activity, hoping to provide some references for the development of more suitable methods for the determination of SOD-like activity in the future.At present, most of the commonly used methods for the determination of the SOD-like activity of nanozymes are modified from the natural SOD activity assays. The key reagents in these methods are usually composed of two parts: the first part is used to produce O2˙− and the second part is used to detect O2˙−. In most cases the principle is as follows. First, O2˙− are generated through the first part of the reagent. These O2˙− cause color or fluorescence changes in detection reagents such as chromogenic agents, and the amount of total O2˙− produced in the system can be detected by these changes. When the nanozyme with SOD-like activity is added to the detection system, the production of O2˙− is reduced, thereby inhibiting the changes in the detection reagent. By comparing the color/fluorescence change of the detection reagent before and after adding the nanozyme, the SOD-like activity of the nanozyme can be calculated.
The first method used to measure the SOD-like activity of the nanozyme was the detection system containing hypoxanthine, xanthine oxidase and cytochrome c. This method was first proposed by Korsvik et al. to detect the SOD-like activity of two different cerium oxide nanoparticles at different concentrations.10 Singh et al. also used this method to determine the SOD-like activity of cerium oxide nanoparticles at different pH values.74 Briefly, the mechanism is that xanthine oxidase catalyzes the hypoxanthine to produce O2˙−, and then O2˙− oxidizes cytochrome c, resulting in the production of reduced cytochrome c with ultraviolet absorption at 550 nm. When a SOD-like nanozyme is added, it competes with cytochrome c for O2˙−, thus inhibiting the reduction of cytochrome c and reducing the change of absorbance. By measuring the changes of absorbance at 550 nm before and after nanozyme addition within a certain period of time, the SOD-like activity of different types and concentrations of material were determined. Using this method, the SOD-like activity of cerium oxide nanoparticles at different concentrations was compared (Fig. 10(a) and (b)). In some cases, excess catalase is added to the system to remove the product H2O2. Xanthine is also used in some methods instead of hypoxanthine.76
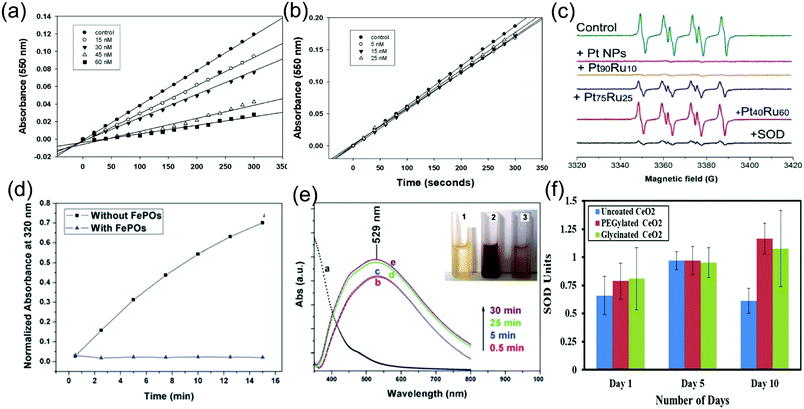 | ||
| Fig. 10 Different methods to characterize the SOD activity of nanozymes. (a and b) The SOD-like activity of two different cerium oxide nanoparticles with different Ce3+/Ce4+ ratios at different concentrations determined using hypoxanthine, xanthine oxidase and cytochrome c system.10 Reproduced with permission. Copyright 1996, The Royal Society of Chemistry. (c) ESR spectra of BMPO/˙OOH generated from the control sample, PtRu NP nanozyme at different ratios of Pt and Ru, and natural SOD-like enzyme.16 Reproduced with permission. Copyright 2009, The Royal Society of Chemistry. (d) FePOs had inhibitory effects on the autoxidation of pyrogallol. (e) The degree of autoxidation of pyrogallol changed with time: (1) only FePOs microflowers; (2) FePOs microflowers and pyrogallol; and (3) only pyrogallol.22 Reproduced with permission. Copyright 1996, The Royal Society of Chemistry. (f) The SOD-like activity of CeO2 nanoparticles in various layer materials represented by a specific activity: U.25 Reproduced with permission. Copyright 2019, American Chemical Society. | ||
Except for using xanthine and xanthine oxidase, there are other methods for O2˙− production. Examples include the use of riboflavin under light illumination,11 riboflavin and methionine,77 and the direct production of O2˙−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 from KO2, which is rarely used due to its extreme instability and toxicity.
78 from KO2, which is rarely used due to its extreme instability and toxicity.
With the development of the above methods, cytochrome c was found to be not sensitive and stable enough, and some other colorimetric reagents were used to replace cytochrome c, such as nitro blue tetrazolium (NBT),25 water soluble tetrazolium salts (WST),9etc. Using these methods, a number of kits for the determination of SOD-like activity have also been developed.79 In addition to colorimetric reagents, some other reagents have been used to detect O2˙−, such as lucigenin, which is detected by chemiluminescence,80 and hydroethidine, an O2˙− fluorescent probe.81 In addition, electron paramagnetic resonance (EPR) or electron spin resonance (ESR) is also used to detect the amount of O2˙−. The ESR spectrum in Fig. 10(c) shows the enzyme activity of natural SODs and the PtRu nanozyme at different ratios of Pt and Ru.16
Some other methods are also used to measure SOD-like activity, and some reagents are able to change color while producing O2˙−, such as the pyrogallol autoxidation method. Pyrogallol produces O2˙− by autoxidation under alkaline conditions and further produces colored products such as o-hydroxy-o-benzoquinone with an ultraviolet absorption peak at 325 nm. In the presence of SOD, the colored products formed by pyrogallol reacting with O2˙− are inhibited, and the SOD-like activity is determined by the inhibition rate.82 By controlling the presence of FePOs and the presence of pyrogallol, it is proved that the autoxidation of pyrogallol is able to measure the SOD-like activity of FePOs (Fig. 10(d) and (e)).22 Similarly, the epinephrine autoxidation method uses epinephrine autoxidation to produce O2˙− and then produces adrenochrome with an ultraviolet absorption peak at 480 nm, which is inhibited in the presence of SOD-like nanozymes.83
In addition to the qualitative comparison of the activity of nanozymes, there are also a few studies using specific activity and enzyme reaction kinetic parameters and other methods to quantify the SOD-like activity. For example, Damle et al. reported that, in the xanthine, xanthine oxidase and NBT systems, the production rate of O2˙− was regulated by adjusting the concentration of xanthine oxidase to fix the absorbance change value at 550 nm of NBT to 0.025 per minute. SOD-like activity is detected as the percentage of absorbance increase inhibited at 550 nm, also known as the inhibition rate. The unit of enzyme activity is defined as the amount of enzyme that inhibits the change of NBT by 50% per minute, namely IC50. The corresponding specific activity is measured by normalizing the amount of nanozyme. As shown in Fig. 10(f), they used this method to measure the SOD-like activity of cerium oxide nanoparticles in various layer materials.25 Moreover, Dashtestani et al. used the pyrogallol autoxidation method to explore the kinetic parameters of enzyme reactions, such as IC50 and kcat, of NACu-Cys, which consists of the Cu-Cys complex and nano-albumin (NA), through the Michaelis–Menten equation.82
Some common methods for the determination of SOD-like activity of nanozymes is shown in Table 2. In present, the SOD-like nanozyme activity determination method still faces some challenges. The most important one is that the enzyme activities of different materials in different studies could not be compared due to the inconsistency of the substrate and sensitivity of different determination methods. Liu et al. compared various determination methods and evaluated the advantages and disadvantages of each method. They used the xanthine/xanthine oxidase method and the irradiation of riboflavin method to produce O2˙−, and employed hydroethidine (HE), NBT, iodonitrotetrazolium chloride (INT), WST, cytochrome c, and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (EPR) to detect O2˙−. Finally, they came to several conclusions: (1) the HE probe may be disturbed by the oxidizing nanozymes; (2) the NBT probe has limited sensitivity due to the poor water solubility of its products; (3) the sensitivity of WST is higher than those of NBT and INT; (4) the method of producing O2˙− by riboflavin irradiation may be disturbed by the electron accepting ability of nanozymes; (5) EPR is sensitive and both the quality of DMPO and incubation time are important factors for the EPR measurement.84 In addition, there is another problem that deserves attention. Most of the current measurement methods have been derived from the method of determination of natural SOD enzyme activity, which might not be very suitable for inorganic nanocomposites. Other components in the system may cause disturbance to the measured results, for example, various kinds of buffer may result in gathering or breakdown of the nanozyme, and the Ethylene Diamine Tetraacetic Acid (EDTA) in the system may complex the metal ion components which determines the SOD-like activity, and so on. Therefore, better assay methods for detecting the SOD-like activity of nanozymes need to be established.
| Nanozymes | Reagent/enzyme activity determination methods | Representative methods | Ref. |
|---|---|---|---|
| a GdYVO4:Eu3+ NPs: Gd(0.7)Y(0.2)Eu(0.1)VO4; Mn-MPSA-PCC: melamine-phytic acid-super molecule-aggregation modified on the surface of PCC through the assembly of amino groups, phosphate groups and oxygen-containing groups on PCC; Ce-MGBs: mesoporous bioactive glasses (MBGs) modified with cerium ions. | |||
| C60-PLGA | Hypoxanthine, xanthine oxidase enzyme, DMPO | EPR | 85 |
| C60, C70 | Xanthine, xanthine oxidase, cytochrome c | IC50 | 86 |
| CeO2−x NPs and GdYVO4:Eu3+ NPsa | Epinephrine autoxidation | Inhibition ratio | 87 |
| Cerium oxide nanoparticles | SOD assay kit | UV absorbance | 88 |
| Mn-NPs | Riboflavin, methionine, NBT | Inhibition ratio | 89 |
| MnO NPs | Xanthine, xanthine oxidase, cytochrome c | Inhibition ratio, kSOD | 90 |
| Cerium oxide | Hypoxanthine, xanthine oxidase, cytochrome c | UV absorbance; specific activity: U | 91 |
| Biogenic magnetic nanoparticles | Pyrogallol autoxidation | Specific activity: U | 92 |
| Mn-MPSA-PCCa | KO2 | Cyclic voltammetry | 93 |
| MnOx | Xanthine, xanthine oxidase, BMPO | ESR | 94 |
| Gd@C82 | Xanthine, xanthine oxidase, lucigenin | Chemiluminescence | 95 |
| CuTA | Xanthine, xanthine oxidase, INT | UV absorbance | 96 |
| Ce-MGBsa | WST assay kit | UV absorbance | 97 |
| NiO nanoflowers | Pyrogallol autoxidation; KO2 | UV absorbance; IC50 | 54 |
5. Antioxidant applications of SOD-like nanozymes
5.1 As antioxidants to protect cells
Utilizing the ROS scavenging ability, SOD-like nanozymes have been widely used to reduce the oxidative stress of the cell. For example, Bhushan et al. delivered cerium oxide nanoparticles into cells by coating with albumin, improving biocompatibility without reducing the antioxidant activity of cerium oxide nanoparticle nanozymes. Semi-quantitative RT-PCR analysis confirmed that albumin modified SOD-like nanozymes successfully defended against free radicals and alleviated oxidative damage to cells, while biocompatible albumin-encapsulated cerium oxide nanoparticles protected the antioxidant defense system of cells and protected cells from oxidative stress-mediated apoptosis.45 Kamada et al. demonstrated that UV-induced DNA damage was inhibited by Ce-doped titanate nanosheets (CE-TNS).98 It has also been found that cerium oxide nanoparticles eliminated ROS and prevented cell apoptosis by reducing the level of Caspase 3/7 and the loss of mitochondrial membrane potential. In addition to reducing ultraviolet A ray-induced fibroblast death, cerium oxide nanoparticles also promoted cell migration and proliferation.99However, the use of SOD-like activity alone is not sufficient to eliminate all kinds of ROS. Therefore, nanozymes with the advantage of multi-enzyme activities are designed to further exert their antioxidant effects through cascade reactions. In the study of Singh et al., a Mn3O4 nanozyme with the activities of catalase, glutathione peroxidase (GPx) and SOD was constructed. The multi-enzyme activities of these materials depend on many factors, such as morphology, specific surface area, pore size and redox properties. The cascade reactions could occur between these enzymatic activities (Fig. 11(a)). In detail, the Mn3O4 nanozyme captured O2˙− and produced oxygen and H2O2 through SOD-like activity. Then, through catalase and GPx activities, the generated H2O2 was further converted into water and oxygen. As shown in Fig. 11(b) and (c), the Mn3O4 nanozyme greatly reduced the level of ROS in mitochondria and cells. Thus, SOD-like nanozymes provide significant protection against ROS mediated protein oxidation, lipid peroxidation and DNA damage.8
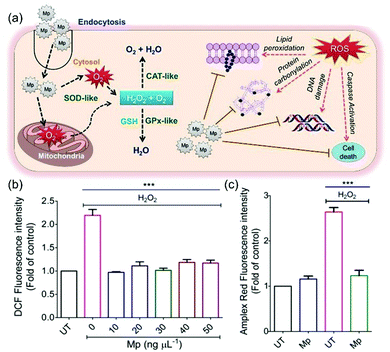 | ||
| Fig. 11 Mn3O4 nanozyme exerted antioxidant effects in cells through cascading reactions. (a) Multi-enzyme mimetic Mn3O4 nanoflowers (Mp) modulated the redox state of mammalian cells without altering the cellular antioxidant machinery under oxidative stress conditions. (b and c) ROS scavenging activity of Mp was evaluated in mammalian cells. (b) Relative mean DCFDA fluorescence intensity of cells left untreated (UT) or pretreated with variable concentrations of Mp prior to H2O2 treatment. (c) The H2O2 scavenging ability of Mp was confirmed using a H2O2 specific dye, Amplex Red.8 Reproduced with permission. Copyright 2009, The Royal Society of Chemistry. | ||
In all, SOD-like nanozymes have the ability to eliminate ROS and therefore protect cells from oxidative stress such as DNA damage, protein oxidation and lipid peroxidation. Through the cascade of SOD-like activity and other activities such as catalase and glutathione peroxidase, the antioxidant function of nanozymes could be further promoted.
5.2 Treatment of oxidative stress related diseases
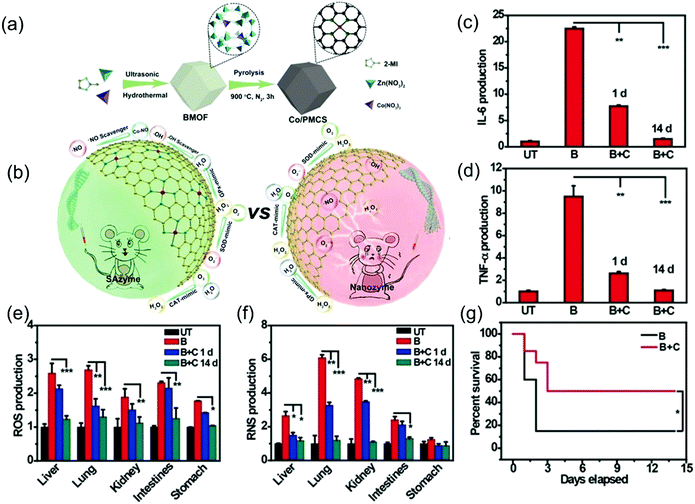 | ||
| Fig. 12 Co/PMCS nanozyme was used to treat sepsis. (a) Illustration of the formation of Co/PMCS. (b) The use of multi-antioxidant SAzymes for sepsis management that defeats nanozymes. (c and d) Detection of (c) IL-6 and (d) TNF-α in vivo in different groups. (e and f) Determination of (e) ROS and (f) RNS in vivo in different tissues. UT: untreated, B: LPS, C: Co/PMCS. (g) Survival curves of the two groups observed for 14 days.3 Reproduced with permission. Copyright 2020, John Wiley and Sons. | ||
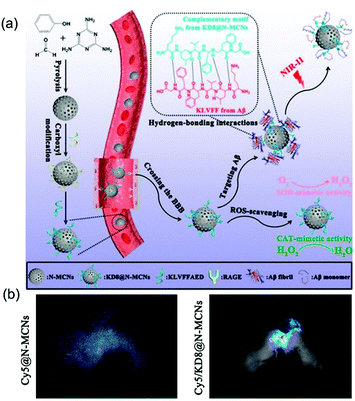 | ||
| Fig. 13 KD8@N-MCNs nanozyme was used to treat Alzheimer's disease. (a) Schematic diagram of KD8@N-MCNs synthesis and the mechanism of action of KD8@N-MCNs. (b) In vivo imaging of 3xTg-AD mice administered with Cy5@N-MCNs or Cy5/KD8@N-MCNs.6 Reproduced with permission. Copyright 2020, American Chemical Society. | ||
In all, SOD-like nanozymes are used in the treatment of a large series of antioxidant diseases such as sepsis, Alzheimer's disease and almost all kinds of inflammation based on their ability to eliminate ROS. SOD-like nanozymes would achieve a better therapeutic effect by coating, modifying or co-loading other drugs.
5.3 Other applications
In addition to the treatment of diseases, SOD-like nanozymes have been widely used in other fields. Li et al. developed a method for in vivo encapsulation of MnO2 nanozyme shells on the surface of living yeast cells. The nanozyme shell not only enhanced the tolerance of cells to severe physical stressors, including dehydration and lyase, but also kept the cells alive after prolonged exposure to high levels of toxic chemicals. In addition, these coated cells were fully restored to growth and function by simple biomolecular stimulation to remove their shells. This strategy has the potential to be applied to a wide range of living cells.108In the process of winemaking, with the accumulation of alcohol and changes in pH, temperature and osmotic pressure, yeast may produce a large amount of ROS, thus reducing the vitality and fermentation efficiency of yeast. Zhou et al. used FeCo-PBA NPs to scavenge ROS, thus greatly improving the tolerance of yeast to ethanol and reactive oxygen species, improving the metabolic activity of yeast, and increasing alcohol production.81 ROS is an important cause of cell and organism senescence. Kim et al. constructed nano-Pt and treated nematodes. The results showed that nano-Pt significantly reduced the accumulation of lipofuscin and ROS induced by paraquat, and the lifespan of nematodes was significantly enhanced after treatment, proving that the Pt nanozyme has an anti-aging effect.50 In addition, some nanozymes are also used as sperm cryopreserved protectants to combat the oxidative stress caused by ROS production during sperm cryopreservation, thus reducing apoptotic necrosis and enhancing the activity.109 Some other applications such as the detection of O2˙− also use the SOD-like activity of nanozymes.110
Although the SOD-like activity of nanozymes is mainly used for antioxidant therapy, due to the multi-enzyme activities of nanozymes, they sometimes also cascade with peroxidase activity or Fenton regents to promote the generation of hydroxyl free radicals, thereby being used to treat tumors by promoting oxidation. By modifying the small-molecule inhibitor 3-amino-1,2,4-triazole (3-AT) and PEG on zeolitic imidazole framework-67 (ZIF-67) nanoparticles, PZIF67-AT was prepared by Sang et al. (Fig. 14(a)). As shown in Fig. 14(b), the SOD-like activity of ZIF-67 was used to convert O2˙− into H2O2, and then chemodynamic therapy (CDT) was used to convert H2O2 into more ˙OH through Fenton reaction. In addition, ZIF-67 could also deplete glutathione to inhibit the conversion of H2O2 into a non-toxic form. Moreover, 3-AT inhibited the enzymatic activity of catalase, thus inhibiting the decomposition of H2O2. Based on this, the PZIF67-AT nanozyme enhanced the production of ˙OH to destroy the tumor. After treatment with the PZIF67-AT nanozyme, the size, weight and volume of the tumor were significantly reduced (Fig. 14(c)–(e)). Moreover, the level of apoptosis in tumor tissues also increased after PZIF67-AT nanozyme treatment as shown by the TUNEL staining in Fig. 14(f).111 In another way, as shown in Fig. 15, Kang et al. prepared speckled RuTe hollow nanorods (RuTeNRs) which exhibited the multiple activities of peroxidase/SOD/catalase. Through the cascade of these activities, oxygen and ROS (˙OH) were abundantly produced, which on the one hand alleviated the hypoxic environment of the tumor and on the other hand were combined with photodynamic and photothermal therapy to destroy the tumor.2
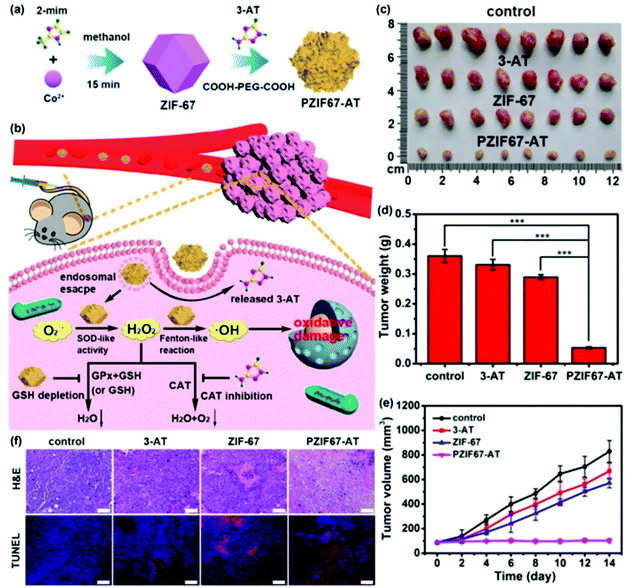 | ||
| Fig. 14 Antitumor study of zeolitic imidazole framework-67 (ZIF-67) nanozyme as a H2O2 homeostasis disruptor for intensive chemodynamic therapy. (a) Preparation of PZIF-67-AT nanoparticles. (b) Intensive chemodynamic therapy induced by PZIF-67-AT nanoparticles. (c) Photographs of the dissected tumors treated with four samples for 14 days. (d) Weights of the tumors. (e) Changes in tumor volumes. (f) Tumor tissue staining by H&E and TUNEL.7 Reproduced with permission. Copyright 2021, Elsevier. | ||
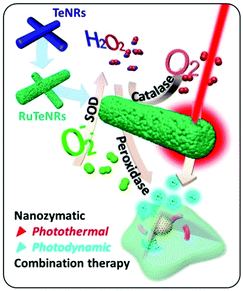 | ||
| Fig. 15 Schematic illustration of RuTeNR synthesis and their nonrecurring circuit nanozymatic enhancement for hypoxic cancer phototherapeutic application.2 Reproduced with permission. Copyright 2020, American Chemical Society. | ||
Table 3 gives some more examples of the application of SOD-like nanozymes. This covers almost all the applications of SOD-like nanozymes to date. We hope that the summary of various applications of SOD-like nanozymes can provide some inspiration for future research and applications. We also hope that SOD-like nanozymes can be applied to more and more suitable fields.
| Nanozyme (drugs used together) | Types of enzyme activity | Application/disease | Ref. |
|---|---|---|---|
| a Pt@PCN222-Mn: Mn(III)porphyrin and Pt NP within a nanoscale Zr-based MOF, PCN222; ZIF-67/Cu0.76Co2.24O4 NSs: “raisin pudding”-type ZIF-67/Cu0.76Co2.24O4 nanospheres; Cu5.4O USNPs: ultrasmall Cu5.4O nanoparticles; MnTPP/ERGO/GCE: manganese(III) tetraphenyl porphine/electrochemical reduced graphene oxide/glassy carbon electrode; CTMDs: Cu-TCPP MOF nanodots; PGNPNF: cerium oxide nanoparticle functionalised polycaprolactone (PCL)-gelatin nanofiber. | |||
| Pt@PCN222-Mna | SOD/catalase | Inflammatory bowel disease | 112 |
| ZIF-67/Cu0.76Co2.24O4 NSsa | Peroxidase/SOD/GPx/laccase-like activities | Real-time detection of living neurotransmitters | 113 |
| Cu5.4O USNPsa | SOD/catalase/GPx | Acute kidney/liver injury, wound healing | 114 |
| CeNPs (PTEN plasmid) | SOD | Prostate cancer | 115 |
| MnTPP/ERGO/GCEa | SOD | Real-time monitoring of O2˙− from breast cancer cells | 116 |
| Single-atom Pt/CeO2 | SOD/catalase/peroxidase/GPx | Bandage for brain trauma and reducing neuroinflammation | 117 |
| CTMDsa | SOD/GPx-like | Alleviating endotoxemia | 118 |
| Cerium oxide nanoparticles | SOD | Restraining cerulein induced acute pancreatitis | 119 |
| MoO3−x nanodots | Catalase/SOD | Treatment of amyloid induced neurotoxicity | 120 |
| CuxO nanoparticle clusters | Peroxidase/SOD/catalase/GPx | Ameliorating Parkinson's disease | 121 |
| PGNPNFa | SOD | Wound healing applications | 122 |
| Mn3O4 nanozymes | SOD/catalase | Protecting ear-inflammation | 67 |
| FePO4 | SOD | Real-time detection of O2˙− | 123 |
| Pt-NPs | SOD/catalase | Alleviating apoptosis | 124 |
| Pt NPs | SOD | Treating vascular diseases such as atherosclerosis | 125 |
6. Conclusion and prospects
As a new generation of artificial enzymes, SOD-like nanozymes have great potential to replace natural SODs in removing ROS, protecting cells from oxidative damage, reducing tissue inflammation and anti-aging, and effectively solving the limitations of natural SODs.In the development process of this field, the exploration of mechanism is the most important and difficult process. Therefore, this paper reviews the structure and activity mechanism of several natural SODs, in order to summarize the mechanism of SOD-like nanozymes and provide a direction for their design and regulation.
Due to the development of different kinds of nanozymes with high enzyme activity, methods for the characterization of enzyme activity have been gradually developed. At the same time, the abundant species and high enzyme activity make the application of SOD-like nanozymes gradually diversified. Based on their ability to remove O2˙− in ROS, the SOD-like activity of nanozymes is mainly used in antioxidant therapy. Due to the unique multi-enzyme activity characteristics of nanozymes, the cascade of multi-enzyme activities helps nanozymes to remove more kinds of ROS, thus greatly improving the antioxidant treatment efficiency of nanozymes. In addition, nanozymes modified with other ligands, such as some proteins, plasmids, inhibitors, etc., can achieve more functions.
Although the activity of SOD-like nanozymes has developed rapidly in recent years and the number of publications has increased rapidly, there still exist many problems. First, the catalytic mechanism of nanozymes to exert SOD-like activity is still unclear. At present, the development of SOD-like nanozymes mostly relies on random synthesis and screening, and few high-activity nanozymes are directly synthesized through rational design. Therefore, it is necessary to develop new methods and techniques to further explore the mechanism and active sites of nanozymes. Second, the activity of current SOD-like nanozymes is not satisfactory. To date, there are almost no nanozymes that can match the activity of natural SODs. Natural SOD enzymes enhance their substrate affinity through their fine structural regulation, so as to have high catalytic efficiency. However, although the structure of some nanozymes imitates the active sites of natural enzymes, their activity cannot exceed that of natural enzymes, and their structure–activity relationship is still not very thorough. Through in-depth exploration of the mechanism, nanozymes with a higher enzyme activity are expected to be developed. Third, the SOD-like activity determination method is not perfect. So far, the methods of SOD-like activity measurement are not uniform, and there is no uniform quantitative standard. Only qualitative comparison of several materials in the same study can be realized, and the activity of materials in different studies cannot be directly compared. Due to the different chemical nature of natural enzymes and nanozymes, some methods are not completely applicable to nanozymes although they are applicable to natural SODs. Therefore, new SOD-like activity determination methods and uniform measurement units for SOD-like nanozymes are expected to be established. In addition, due to the unknown active sites of nanozymes, most of the current methods are based on the weights of the materials, and the catalytic efficiency of each active site cannot be measured. Fourth, the application of SOD-like nanozymes is limited. To date, the application of SOD-like nanozymes mainly focuses on the treatment of diseases in the antioxidant field, and some new suitable applications are expected to be explored. Fifth, some common problems of nanozymes remain to be solved, such as the specificity of enzyme activity, toxicity, metabolic problems and so on. At present, the most commonly used methods for the study of the biocompatibility and biosafety of nanozymes include histopathological staining, blood routine and blood biochemical testing to evaluate the potential impact of nanoparticles on body tissues. The results of animal experiments showed that cerium oxide,61 carbon materials48 and other nanozymes126 exhibit good biocompatibility. In addition, a number of ultra-small nanozymes that can be cleared by the kidney have been designed.114 However, the biocompatibility of most nanozymes is still a problem to be studied in depth. Most studies have not effectively evaluated the metabolic pathways of nanozymes. The potential effects of nanozymes on body tissues mainly include the following: firstly, after entering the organism, nanozymes may interact with biological components in the blood or cell fluid to form protein crowns, which may affect the catalytic activity of nanozymes on the one hand, and may cause biological safety issues on the other hand. Secondly, many nanozymes exhibit toxic peroxidase-like activity under acidic conditions, so some seemingly safe nanozyme materials may become toxic after entering the acid vesicles of cells. Thirdly, nanozymes may also interact with intracellular components such as proteins, nucleic acids and small biological molecules to disrupt their normal physiological functions. At present, there are few comprehensive studies on the biocompatibility mechanism of nanozymes, and further development of indicators for measuring their biocompatibility is needed.
In addition to the existing strategies for improving various existing nanozymes, it is a very promising strategy to directly create high-activity SOD-like nanozymes by imitating the active sites of natural enzymes. At present, there have been some studies on nanozymes imitating the natural enzyme active sites. For example, Patriarca et al. inserted a series of metal-center complexes into different kinds of mesoporous silica junctions, as shown in Fig. 16. For the 1 and 2 imidazolate complexes, the silica channels limited the geometry of the μ-imidazolate-Cu(II)2 core and changed the relative orientations of the two copper coordination planes, resulting in a higher enzyme activity. Unlike imidazole bridging compounds, however, the insertion of 3 in mesoporous silica caused the hybrid material to become less stable, showing partial release into aqueous solution and dissociation, where the enzymatic activity of 3 is low. This was determined by the positional relationship between the active centers. This result was demonstrated by encapsulation with two different silica structures.1,127 Another experiment combined different metal active center complexes into boehmite nanoparticles (BNPs). It was found that binuclear Cu2+ complexes showed a significant increase in SOD-like activity. This is because BNPs have positive ζ-potential, which helps drive O2˙− to the active centers, consistent with the active sites of natural enzymes.128 However, there are few studies on the use of natural enzyme active centers to guide the activity of nanozymes, and we look forward to the review of this article to provide more ideas for future. For example, the active centers of SOD are mostly composed of metal ions coordinated by C, N, O and other elements of surrounding amino acids. According to these coordination principles, nanozymes with SOD-like active center structures can be designed, such as some single atom nanozymes synthesized with MOFs and other materials as precursors. Some features of the catalytic microenvironment near the SOD active site may also be used to optimize nanozymes, such as cave shape, hydrophilicity, hydrophobicity, and charge properties. Moreover, the catalytic performance of SOD-like nanozymes may also be improved based on the simulation of the flexible metal-free frame architecture of the SOD active site.
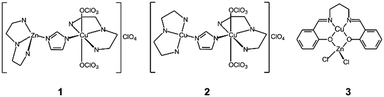 | ||
| Fig. 16 Different metal-centre complexes were developed.1 Reproduced with permission. Copyright 2020, Elsevier. | ||
Although there are still some problems in the development of SOD-like nanozymes, their advantages in replacing natural enzymes are nonnegligible. Nanozymes with SOD-like activity have wide application prospects. This review briefly introduces the development of SOD-like nanozymes, summarizes their mechanism, enzymatic activity and applications, and discusses their deficiencies in the current development, hoping to serve as a reference for future research. We expect that more SOD-like nanozymes with high activity will be developed and applied in a wider range of aspects.
Author contributions
Hanqing Zhao: writing – original draft preparation. Ruofei Zhang: writing – review and editing. Xiyun Yan: supervision. Kelong Fan: conceptualization, supervision, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31900981), the Strategic Priority Research Program of CAS (XDB29040101), CAS Interdisciplinary Innovation Team (JCTD-2020-08), the Key Research Program of Frontier Sciences, CAS (Grant No. QYZDY-SSW-SMC013), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2019093).References
- M. Patriarca, V. Daier, G. Cami, E. Riviere, C. Hureau and S. Signorella, J. Inorg. Biochem., 2020, 207, 111050 CrossRef CAS PubMed.
- S. Kang, Y. G. Gil, D. H. Min and H. Jang, ACS Nano, 2020, 14, 4383–4394 CrossRef CAS PubMed.
- F. F. Cao, L. Zhang, Y. W. You, L. R. Zheng, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2020, 59, 5108–5115 CrossRef CAS PubMed.
- S. S. Ali, J. I. Hardt, K. L. Quick, J. S. Kim-Han, B. F. Erlanger, T. T. Huang, C. J. Epstein and L. L. Dugan, Free Radical Biol. Med., 2004, 37, 1191–1202 CrossRef CAS PubMed.
- X. M. Shen, W. Q. Liu, X. J. Gao, Z. H. Lu, X. C. Wu and X. F. Gao, J. Am. Chem. Soc., 2015, 137, 15882–15891 CrossRef CAS PubMed.
- M. Ma, N. Gao, X. Li, Z. Liu, Z. Pi, X. Du, J. Ren and X. Qu, ACS Nano, 2020, 14, 9894–9903 CrossRef CAS PubMed.
- X. Zhang, S. Lin, S. Liu, X. Tan, Y. Dai and F. Xia, Coord. Chem. Rev., 2021, 429, 213652 CrossRef CAS.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Nanoscale, 2019, 11, 3855–3863 RSC.
- V. Baldim, F. Bedioui, N. Mignet, I. Margaill and J. F. Berret, Nanoscale, 2018, 10, 6971–6980 RSC.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058, 10.1039/b615134e.
- Z. Y. Yang, S. L. Luo, Y. P. Zeng, C. M. Shi and R. Li, ACS Appl. Mater. Interfaces, 2017, 9, 6839–6848 CrossRef CAS PubMed.
- N. Singh, M. Geethika, S. M. Eswarappa and G. Mugesh, Chem. – Eur. J., 2018, 24, 8393–8403 CrossRef CAS PubMed.
- J. He, L. Zhou, J. Liu, L. Yang, L. Zou, J. Y. Xiang, S. W. Dong and X. C. Yang, Appl. Surf. Sci., 2017, 402, 469–477 CrossRef CAS.
- S. B. Guo and L. Guo, J. Phys. Chem. C, 2019, 123, 30318–30334 CrossRef CAS.
- L. Baud and R. Ardaillou, Am. J. Physiol., 1986, 251, F765–F776 CAS.
- C. Liu, Y. Y. Yan, X. W. Zhang, Y. Y. Mao, X. Q. Ren, C. Y. Hu, W. W. He and J. J. Yin, Nanoscale, 2020, 12, 3068–3075 RSC.
- B. H. San, S. H. Moh and K. K. Kim, J. Mater. Chem., 2012, 22, 1774–1780 RSC.
- S. Barkam, J. Ortiz, S. Saraf, N. Eliason, R. Mccormack, S. Das, A. Gupta, C. Neal, A. Petrovic, C. Hansor, M. D. Sevilla, A. Adhikary and S. Seal, J. Phys. Chem. C, 2017, 121, 20039–20050 CrossRef CAS.
- D. M. Miller, G. R. Buettner and S. D. Aust, Free Radical Biol. Med., 1990, 8, 95–108 CrossRef CAS PubMed.
- M. Valko, H. Morris and M. T. D. Cronin, Curr. Med. Chem., 2005, 12, 1161–1208 CrossRef CAS PubMed.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- W. Wang, X. P. Jiang and K. Z. Chen, Chem. Commun., 2012, 48, 7289–7291 RSC.
- I. Fridovich, Photochem. Photobiol., 1978, 28, 733–741 CrossRef CAS PubMed.
- I. A. Abreu and D. E. Cabelli, Biochim. Biophys. Acta, Proteins Proteomics, 2010, 1804, 263–274 CrossRef CAS PubMed.
- M. A. Damle, A. P. Jakhade and R. C. Chikate, ACS Omega, 2019, 4, 3761–3771 CrossRef CAS.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- K. M. Beem, W. E. Rich and K. V. Rajagopalan, J. Biol. Chem., 1974, 249, 7298–7305 CrossRef CAS.
- M. A. Hough and S. S. Hasnain, Structure, 2003, 11, 937–946 CrossRef CAS PubMed.
- J. S. Richardson, K. A. Thomas, B. H. Rubin and D. C. Richardson, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 1349–1353 CrossRef CAS PubMed.
- N. Boden, M. C. Holmes and P. F. Knowles, Biochem. Biophys. Res. Commun., 1974, 57, 845–848 CrossRef CAS PubMed.
- I. Fridovich, Annu. Rev. Biochem., 1975, 44, 147–159 CrossRef CAS PubMed.
- C. L. Fisher, D. E. Cabelli, J. A. Tainer, R. A. Hallewell and E. D. Getzoff, Proteins: Struct., Funct., Genet., 1994, 19, 24–34 CrossRef CAS PubMed.
- E. D. Getzoff, D. E. Cabelli, C. L. Fisher, H. E. Parge, M. S. Viezzoli, L. Banci and R. A. Hallewell, Nature, 1992, 358, 347–351 CrossRef CAS PubMed.
- L. M. Ellerby, D. E. Cabelli, J. A. Graden and J. S. Valentine, J. Am. Chem. Soc., 1996, 118, 6556–6561 CrossRef CAS.
- J. Azadmanesh, S. R. Trickel and G. E. O. Borgstahl, J. Struct. Biol., 2017, 199, 68–75 CrossRef CAS PubMed.
- J. Azadmanesh and G. E. O. Borgstahl, Antioxidants, 2018, 7, 16 CrossRef PubMed.
- M. S. Lah, M. M. Dixon, K. A. Pattridge, W. C. Stallings, J. A. Fee and M. L. Ludwig, Biochemistry, 1995, 34, 1646–1660 CrossRef CAS PubMed.
- J. Shearer, Acc. Chem. Res., 2014, 47, 2332–2341 CrossRef CAS PubMed.
- H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- S. Torbruegge, M. Reichling, A. Ishiyama, S. Morita and O. Custance, Phys. Rev. Lett., 2007, 99, 056101 CrossRef PubMed.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- A. Vincent, S. Babu, E. Heckert, J. Dowding, S. M. Hirst, T. M. Inerbaev, W. T. Self, C. M. Reilly, A. E. Masunov, T. S. Rahman and S. Seal, ACS Nano, 2009, 3, 1203–1211 CrossRef CAS PubMed.
- X. L. Ren, X. W. Meng, J. Ren and F. Q. Tang, RSC Adv., 2016, 6, 92839–92844 RSC.
- Y. S. Yang, Z. Mao, W. J. Huang, L. H. Liu, J. L. Li and Q. Z. Wu, Sci. Rep., 2016, 6, 7 CrossRef PubMed.
- B. Bhushan and P. Gopinath, J. Mater. Chem. B, 2015, 3, 4843–4852 RSC.
- L. L. Dugan, J. K. Gabrielsen, S. P. Yu, T. S. Lin and D. W. Choi, Neurobiol. Dis., 1996, 3, 129–135 CrossRef CAS PubMed.
- S. S. Ali, J. I. Hardt and L. L. Dugan, Nanomedicine, 2008, 4, 283–294 CrossRef CAS PubMed.
- K. L. Fan, J. Q. Xi, L. Fan, P. X. Wang, C. H. Zhu, Y. Tang, X. D. Xu, M. M. Liang, B. Jiang, X. Y. Yan and L. Z. Gao, Nat. Commun., 2018, 9, 11 CrossRef PubMed.
- J. Q. Xi, G. Wei, Q. W. Wu, Z. L. Xu, Y. W. Liu, J. Han, L. Fan and L. Z. Gao, Biomater. Sci., 2019, 7, 4131–4141 RSC.
- J. Kim, M. Takahashi, T. Shimizu, T. Shirasawa, M. Kajita, A. Kanayama and Y. Miyamoto, Mech. Ageing Dev., 2008, 129, 322–331 CrossRef CAS PubMed.
- W. W. He, Y. T. Zhou, W. G. Warner, X. N. Hu, X. C. Wu, Z. Zheng, M. D. Boudreau and J. J. Yin, Biomaterials, 2013, 34, 765–773 CrossRef CAS PubMed.
- K. Korschelt, R. Ragg, C. S. Metzger, M. Kluenker, M. Oster, B. Barton, M. Panthofer, D. Strand, U. Kolb, M. Mondeshki, S. Strand, J. Brieger, M. N. Tahir and W. Tremel, Nanoscale, 2017, 9, 3952–3960 RSC.
- D. Lieb, I. Kenkell, J. L. Miljkovic, D. Moldenhauer, N. Weber, M. R. Filipovic, F. Grohn and I. Ivanovic-Burmazovic, Inorg. Chem., 2014, 53, 1009–1020 CrossRef CAS PubMed.
- J. S. Mu, X. Zhao, J. Li, E. C. Yang and X. J. Zhao, J. Mater. Chem. B, 2016, 4, 5217–5221 RSC.
- J. L. Dong, L. N. Song, J. J. Yin, W. W. He, Y. H. Wu, N. Gu and Y. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 1959–1970 CrossRef CAS PubMed.
- T. M. Chen, H. Zou, X. J. Wu, C. C. Liu, B. Situ, L. Zheng and G. W. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 12453–12462 CrossRef CAS PubMed.
- Z. H. Miao, S. S. Jiang, M. L. Ding, S. Y. Sun, Y. Ma, M. R. Younis, G. He, J. G. Wang, J. Lin, Z. Cao, P. Huang and Z. B. Zha, Nano Lett., 2020, 20, 3079–3089 CrossRef CAS PubMed.
- S. Sahar, A. Zeb, C. Ling, A. Raja, G. Wang, N. Ullah, X. M. Lin and A. W. Xu, ACS Nano, 2020, 14, 3017–3031 CrossRef CAS.
- M. Kajita, K. Hikosaka, M. Iitsuka, A. Kanayama, N. Toshima and Y. Miyamoto, Free Radicals Res., 2007, 41, 615–626 CrossRef CAS.
- Z. Moradi-Shoeili, React. Kinet., Mech. Catal., 2017, 120, 323–332 CrossRef CAS.
- V. Singh, S. Singh, S. Das, A. Kumar, W. T. Self and S. Seal, Nanoscale, 2012, 4, 2597–2605 RSC.
- E. L. G. Samuel, D. C. Marcano, V. Berka, B. R. Bitner, G. Wu, A. Potter, R. H. Fabian, R. G. Pautler, T. A. Kent, A. L. Tsai and J. M. Tour, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 2343–2348 CrossRef CAS PubMed.
- J. D. Weaver and C. L. Stabler, Acta Biomater., 2015, 16, 136–144 CrossRef CAS PubMed.
- S. B. Guo, Y. Han and L. Guo, Catal. Surv. Asia, 2020, 24, 70–85 CrossRef CAS.
- Y. Han, Z. J. Zhang and L. Guo, Catal. Surv. Asia, 2020, 24, 166–177 CrossRef CAS.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Angew. Chem., Int. Ed., 2017, 56, 14267–14271 CrossRef CAS PubMed.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. C. Lin, X. Y. Wang, J. J. X. Wu, S. R. Li and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- A. Gupta, S. Das, C. J. Neal and S. Seal, J. Mater. Chem. B, 2016, 4, 3195–3202 RSC.
- V. Baldim, N. Yadav, N. Bia, A. Graillot, C. Loubat, S. Singh, A. S. Karakoti and J.-F. Berret, ACS Appl. Mater. Interfaces, 2020, 12, 42056–42066 CrossRef CAS PubMed.
- V. Jain, S. Bhagat, M. Singh, V. Bansal and S. Singh, RSC Adv., 2019, 9, 33195–33206 RSC.
- X. H. Chen, W. Jiang, Q. R. Cheng, Z. Q. Pan and H. Zhou, Micro Nano Lett., 2014, 9, 157–161 CrossRef CAS.
- V. Patel, M. Singh, E. L. H. Mayes, A. Martinez, V. Shutthanandan, V. Bansal, S. Singh and A. S. Karakoti, Chem. Commun., 2018, 54, 13973–13976 RSC.
- Y. Liu, H. H. Wu, M. Li, J. J. Yin and Z. H. Nie, Nanoscale, 2014, 6, 11904–11910 RSC.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753 CrossRef CAS PubMed.
- U. Carmona, L. B. Zhang, L. Li, W. Munchgesang, E. Pippel and M. Knez, Chem. Commun., 2014, 50, 701–703 RSC.
- X. L. Ren, M. Q. Wang, X. He, Z. Li, J. Zhang, W. Zhang, X. D. Chen, H. Ren and X. W. Meng, Chin. Chem. Lett., 2018, 29, 1865–1868 CrossRef CAS.
- L. Huang, Y. S. Niu, R. G. Li, H. Z. Liu, Y. Wang, G. F. Xu, Y. Li and Y. H. Xu, Anal. Chem., 2019, 91, 5753–5761 CrossRef CAS PubMed.
- G. Wu, V. Berka, P. J. Derry, K. Mendoza, E. Kakadiaris, T. Roy, T. A. Kent, J. M. Tour and A. L. Tsai, ACS Nano, 2019, 13, 11203–11213 CrossRef CAS PubMed.
- Z. Liu, L. N. Xie, K. Q. Qiu, X. X. Liao, T. W. Rees, Z. Z. Zhao, L. N. Ji and H. Chao, ACS Appl. Mater. Interfaces, 2020, 12, 31205–31216 CrossRef CAS PubMed.
- M. M. Sozarukova, M. A. Shestakova, M. A. Teplonogova, D. Y. Izmailov, E. V. Proskurnina and V. K. Ivanov, Russ. J. Inorg. Chem., 2020, 65, 597–605 CrossRef CAS.
- R. W. Zhou, P. Y. Wang, Y. R. Guo, X. F. Dai, S. Q. Xiao, Z. Fang, R. Speight, E. W. W. Thompson, P. J. Cullen and K. Ostrikov, Nanoscale, 2019, 11, 19497–19505 RSC.
- F. Dashtestani, H. Ghourchian and A. Najafi, Bioorg. Chem., 2018, 80, 621–630 CrossRef CAS.
- D. R. Bohn, F. O. Lobato, A. S. Thill, L. Steffens, M. Raabe, B. Donida, C. R. Vargas, D. J. Moura, F. Bernardi and F. Poletto, J. Mater. Chem. B, 2018, 6, 4920–4928 RSC.
- Y. Liu, Y. Zhang, Q. Liu, Q. Wang, A. Lin, J. Luo, Y. Du, Y.-W. Lin and H. Wei, Analyst, 2021, 146, 1872–1879 RSC.
- N. Higashi, T. Shosu, T. Koga, M. Niwa and T. Tanigawa, J. Colloid Interface Sci., 2006, 298, 118–123 CrossRef CAS.
- P. Witte, F. Beuerle, U. Hartnagel, R. Lebovitz, A. Savouchkina, S. Sali, D. Guldi, N. Chronakis and A. Hirsch, Org. Biomol. Chem., 2007, 5, 3599–3613 RSC.
- V. K. Klochkov, A. V. Grigorova, O. O. Sedyh and Y. V. Malyukin, Colloids Surf., A, 2012, 409, 176–182 CrossRef CAS.
- R. N. McCormack, P. Mendez, S. Barkam, C. J. Neal, S. Das and S. Seal, J. Phys. Chem. C, 2014, 118, 18992–19006 CrossRef CAS.
- M. Yang, W. Jiang, Z. Q. Pan and H. Zhou, J. Inorg. Organomet. Polym., 2015, 25, 1289–1297 CrossRef CAS.
- R. Ragg, A. M. Schilmann, K. Korschelt, C. Wieseotte, M. Kluenker, M. Viel, L. Volker, S. Preiss, J. Herzberger, H. Frey, K. Heinze, P. Blumler, M. N. Tahir, F. Natalio and W. Tremel, J. Mater. Chem. B, 2016, 4, 7423–7428 RSC.
- T. Naganuma, Nano Res., 2017, 10, 199–217 CrossRef CAS.
- Y. Pan, Y. M. Wang, X. Y. Fan, W. B. Wang, X. M. Yang, D. Z. Cui and M. Zhao, Environ. Microbiol. Rep., 2019, 11, 140–146 CrossRef CAS PubMed.
- X. Cai, Q. M. Gao, S. H. Zuo, H. L. Zhao and M. B. Lan, Electroanalysis, 2020, 32, 598–605 CrossRef CAS.
- X. M. Jiang, P. Gray, M. Patel, J. W. Zheng and J. J. Yin, J. Mater. Chem. B, 2020, 8, 1191–1201 RSC.
- I. V. Mikheev, M. M. Sozarukova, E. V. Proskurnina, I. E. Kareev and M. A. Proskurnin, Molecules, 2020, 25, 2506 CrossRef CAS PubMed.
- S. C. Lin, Y. Cheng, H. Zhang, X. Y. Wang, Y. Y. Zhang, Y. J. Zhang, L. Y. Miao, X. Z. Zhao and H. Wei, Small, 2020, 15, 1902123 CrossRef PubMed.
- V. Nicolini, G. Malavasi, G. Lusvardi, A. Zambon, F. Benedetti, G. Cerrato, S. Valeri and P. Luches, Ceram. Int., 2019, 45, 20910–20920 CrossRef CAS.
- K. Kamada and N. Soh, J. Phys. Chem. B, 2015, 119, 5309–5314 CrossRef CAS PubMed.
- F. M. Ribeiro, M. M. de Oliveira, S. Singh, T. S. Sakthivel, C. J. Neal, S. Seal, T. Ueda-Nakamura, S. d. O. Silva Lautenschlager and C. V. Nakamura, Front. Bioeng. Biotechnol., 2020, 8, 577557 CrossRef PubMed.
- C. X. Ren, D. D. Li, Q. X. Zhou and X. G. Hu, Biomaterials, 2020, 232, 119752 CrossRef CAS PubMed.
- D. Q. Yu, M. M. Ma, Z. W. Liu, Z. F. Pi, X. B. Du, J. S. Ren and X. G. Qu, Biomaterials, 2020, 255, 10 CrossRef PubMed.
- Y. H. Gu, Y. X. Huang, Z. Y. Qiu, Z. B. Xu, D. D. Li, L. Chen, J. Jiang and L. Z. Gao, Sci. China: Life Sci., 2020, 63, 68–79 CrossRef CAS PubMed.
- H. Li, J. Yan, D. Meng, R. Cai, X. Gao, Y. Ji, L. Wang, C. Chen and X. Wu, ACS Nano, 2020, 14, 12854–12865 CrossRef PubMed.
- P. Allawadhi, A. Khurana, S. Allwadhi, K. Joshi, G. Packirisamy and K. K. Bharani, Nano Today, 2020, 35, 100982 CrossRef CAS PubMed.
- Y. Chen, J. B. Tan, Q. Zhang, T. Xin, Y. L. Yu, Y. Nie and S. Y. Zhang, Nano Lett., 2020, 20, 6548–6555 CrossRef CAS PubMed.
- J. L. Zhao, W. Gao, X. J. Cai, J. J. Xu, D. W. Zou, Z. S. Li, B. Hu and Y. Y. Zheng, Theranostics, 2019, 9, 2843–2855 CrossRef CAS PubMed.
- L. Y. Wu, G. Y. Liu, W. Y. Wang, R. B. Liu, L. Y. Liao, N. Cheng, W. T. Li, W. F. Zhang and D. J. Ding, Int. J. Nanomed., 2020, 15, 2515–2527 CrossRef CAS PubMed.
- W. Li, Z. Liu, C. Q. Liu, Y. J. Guan, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2017, 56, 13661–13665 CrossRef CAS PubMed.
- F. Dashtestani, H. Ghourchian and A. Najafi, Mater. Sci. Eng., C, 2019, 94, 831–840 CrossRef CAS PubMed.
- X. Cai, L. B. Shi, W. Q. Sun, H. L. Zhao, H. Li, H. Y. He and M. B. Lan, Biosens. Bioelectron., 2018, 102, 171–178 CrossRef CAS.
- Y. J. Sang, F. F. Cao, W. Li, L. Zhang, Y. W. You, Q. Q. Deng, K. Dong, J. S. Ren and X. G. Qu, J. Am. Chem. Soc., 2020, 142, 5177–5183 CrossRef CAS.
- Y. F. Liu, Y. Cheng, H. Zhang, M. Zhou, Y. J. Yu, S. C. Lin, B. Jiang, X. Z. Zhao, L. Y. Miao, C. W. Wei, Q. Y. Liu, Y. W. Lin, Y. Du, C. J. Butch and H. Wei, Sci. Adv., 2020, 6, 10 Search PubMed.
- J. Liu, W. Zhang, M. H. Peng, G. Y. Ren, L. H. Guan, K. Li and Y. Q. Lin, ACS Appl. Mater. Interfaces, 2020, 12, 29631–29640 CAS.
- T. F. Liu, B. W. Xiao, F. Xiang, J. L. Tan, Z. Chen, X. R. Zhang, C. Z. Wu, Z. W. Mao, G. X. Luo, X. Y. Chen and J. Deng, Nat. Commun., 2020, 11, 16 CrossRef PubMed.
- S. Singh, R. Asal and S. Bhagat, J. Biomed. Mater. Res., Part A, 2018, 106, 3152–3164 CrossRef CAS PubMed.
- M. Cui, J. J. Ren, X. F. Wen, N. Li, Y. F. Xing, C. Zhang, Y. Y. Han and X. P. Ji, Chem. Res. Chin. Univ., 2020, 36, 774–780 CrossRef CAS.
- R. J. Yan, S. Sun, J. Yang, W. Long, J. Y. Wang, X. Y. Mu, Q. F. Li, W. T. Hao, S. F. Zhang, H. L. Liu, Y. L. Gao, L. F. Ouyang, J. C. Chen, S. J. Liu, X. D. Zhang and D. Ming, ACS Nano, 2019, 13, 11552–11560 CrossRef CAS PubMed.
- L. Zhang, Y. Zhang, Z. Z. Wang, F. F. Cao, Y. J. Sang, K. Dong, F. Pu, J. S. Ren and X. G. Qu, Mater. Horiz., 2019, 6, 1682–1687 RSC.
- A. Khurana, P. Anchi, P. Allawadhi, V. Kumar, N. Sayed, G. Packirisamy and C. Godugu, Nanomedicine, 2019, 14, 1805–1825 CrossRef CAS.
- Q. S. Han, X. H. Wang, X. L. Liu, Y. F. Zhang, S. F. Cai, C. Qi, C. Wang and R. Yang, J. Colloid Interface Sci., 2019, 539, 575–584 CrossRef CAS.
- C. L. Hao, A. H. Qu, L. G. Xu, M. Z. Sun, H. Y. Zhang, C. L. Xu and H. Kuang, J. Am. Chem. Soc., 2019, 141, 1091–1099 CrossRef CAS PubMed.
- H. A. Rather, R. Thakore, R. Singh, D. Jhala, S. Singh and R. Vasita, Bioact. Mater., 2018, 3, 201–211 CrossRef PubMed.
- Y. Wang, M. Q. Wang, L. L. Lei, Z. Y. Chen, Y. S. Liu and S. J. Bao, Microchim. Acta, 2018, 185, 140 CrossRef PubMed.
- P. Jawaid, M. U. Rehman, Q. L. Zhao, K. Takeda, K. Ishikawa, M. Hori, T. Shimizu and T. Kondo, J. Cell. Mol. Med., 2016, 20, 1737–1748 CrossRef CAS PubMed.
- W. F. Zheng, B. Jiang, Y. Hao, Y. Y. Zhao, W. Zhang and X. Y. Jiang, Biofabrication, 2014, 6, 045004 CrossRef CAS PubMed.
- Q. X. Zhang, H. Tao, Y. Y. Lin, Y. Hu, H. J. An, D. L. Zhang, S. B. Feng, H. Y. Hu, R. B. Wang, X. H. Li and J. X. Zhang, Biomaterials, 2016, 105, 206–221 CrossRef CAS.
- M. Patriarca, V. Daier, G. Cami, N. Pellegri, E. Riviere, C. Hureau and S. Signorell, Microporous Mesoporous Mater., 2019, 279, 133–141 CrossRef CAS.
- A. Martinez-Camarena, J. M. Linares, A. Domenech-Carbo, J. Alarcon and E. Garcia-Espana, RSC Adv., 2019, 9, 41549–41560 RSC.
Footnote |
| † Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |