Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of some new distyrylbenzene derivatives using immobilized Pd on an NHC-functionalized MIL-101(Cr) catalyst: photophysical property evaluation, DFT and TD-DFT calculations†
Esmaeil Niknama,
Ali Mahmoodib,
Farhad Panahi *a,
Maryam Heydari Dokoohakia,
Amin Reza Zolghadra and
Ali Khalafi-Nezhad*a
*a,
Maryam Heydari Dokoohakia,
Amin Reza Zolghadra and
Ali Khalafi-Nezhad*a
aDepartment of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran. E-mail: panahi@shirazu.ac.ir; panahichem@ymail.com
bDepartment of Polymer Engineering and Color Technology, Amirkabir University of Technology, Tehran, Iran
First published on 29th March 2021
Abstract
In this study the catalytic application of a heterogeneous Pd-catalyst system based on metal organic framework [Pd–NHC–MIL-101(Cr)] was investigated in the synthesis of distyrylbenzene derivatives using the Heck reaction. The Pd–NHC–MIL-101(Cr) catalyst showed high efficiency in the synthesis of these π-conjugated materials and products were obtained in high yields with low Pd-contamination based on ICP analysis. The photophysical behaviors for some of the synthesized distyrylbenzene derivatives were evaluated. The DFT and TD-DFT methods were employed to determine the optimized molecular geometry, band gap energy, and the electronic absorption and emission wavelengths of the new synthesized donor–π–acceptor (D–π–A) molecules in the gas phase and in various solvents using the chemical model B3LYP/6-31+G(d,p) level of theory.
Introduction
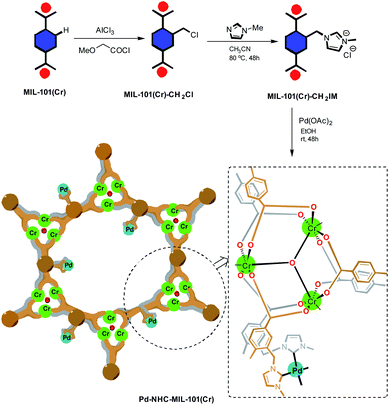
The synthesis of fluorescent compounds to be used in organic light emitting diodes (OLEDs),1–5 solar cells,6,7 organic field effect transistors (OFETs),8,9 sensing,10–12 and fluorescent probes13–16 is highly considered. Stilbene compounds are a significant class of fluorescent organic π-conjugated compounds, which are widely used in the above mentioned applications.17–26 Due to the systematic relationship between the fluorescence properties of the fluorescent materials and their chemical structures, stilbenes are an interesting class of compounds which permit us to simply fine tune the photophysical properties via available chemical modifications.27–31 To synthesize stilbenes, different organic methodologies such as Wittig reaction,32–34 Horner–Wadsworth–Emmons reaction,35 catalytic aldehyde olefinations,36 and Mizoroki–Heck reaction37,38 have been developed. Palladium-catalyzed coupling reactions are key tools in stilbene synthesis because they consist of a family of cross coupling reactions, allowing diversity oriented synthesize of stilbenes.39–42 Thus, Mizoroki–Heck reaction has been extensively used in the synthesis of stilbene compounds.43,44In this work, in continuation of our program on the synthesis of stilbene derivatives,45–50 a highly efficient heterogeneous catalyst system [Pd–NHC–MIL-101(Cr)]51 was introduced to be applied in the synthesis of stilbene derivatives using Mizoroki–Heck coupling reaction.52–57 The Pd–NHC–MIL-101(Cr) catalyst system showed remarkable catalytic activity in the Heck reaction51 and in order to further show its utility in organic synthesis we investigate its applicability in the synthesis of distyrylbenzenes (DSBs). The synthetic pathway toward synthesis of [Pd–NHC–MIL-101(Cr)] catalyst system is shown in Scheme 1.
Results and discussion
Catalytic activity evaluation of Pd–NHC–MIL-101(Cr) catalyst in the synthesis of distyrylbenzenes (DSBs)
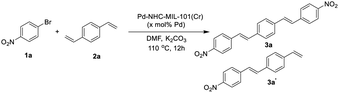
In order to show the catalytic applicability of [Pd–NHC–MIL-101(Cr)] catalyst in the synthesis of DSBs and stilbenes, a model reaction was selected and different conditions were checked to obtain high yields of desired products (Table 1).| Entry | x | 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a ratio 2a ratio |
Yield 3ab | Yield 3a′b |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2a (based on the ratio), Pd–NHC–MIL-101(Cr) (x mol%), DMF (5 mL), K2CO3 (2.5 mmol), 110 °C, 12 h.b NMR yield.c The Pd/C was used as catalyst. The yields in parentheses related to isolated yields. | ||||
| 1 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6 | 85 (79) |
| 2 | 1.5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 20 |
| 3 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
5 | 88 (81) |
| 4 | 1.5 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 (77) | 2 |
| 5 | 1.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
6 | 78 (71) |
| 6 | 1.25 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 8 |
| 7 | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
4 | 87 (78) |
| 8 | 2.0 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 | 20 |
| 9 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
20 | 58c |
| 10 | 1.5 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 | 18c |
As shown in Table 1, using different ratios of starting materials in the presence of Pd–NHC–MIL-101(Cr) catalyst, it is possible to obtain both compounds 3a and 3a′ in high yields. In order to synthesize DSBs in high yield, the ratio of aryl halide to 1,4-distylbenzene was selected 2.2 to 1 (Table 1, entry 4). Also, the best yield for mono-substituted product was achieved using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 ratios for 1a
2.2 ratios for 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a (Table 1, entry 3). No improvement in the reaction yield was observed by increasing the catalyst loading more than 1.5 mol% (Table 1, entries 5–8).58 Using Pd/C as a traditional catalyst,59–62 3a′ was obtained in lower yield of 58% (same conditions and stoichiometry), demonstrating important role of MOF structure in homoselectivity63 to obtain 3a′ in high yield (Table 1, entries 9 & 10). Also, the ICP analysis of the product using Pd–NHC–MIL-101(Cr) catalyst showed less than 2 ppm of Pd while the amount of Pd-content for the product obtained using Pd/C catalyst was around 16 ppm. This experiment showed that the efficacy of this Pd MOF-based catalyst in the synthesis of this class of π-conjugated materials with low Pd-contamination which is very important in their applications.
2a (Table 1, entry 3). No improvement in the reaction yield was observed by increasing the catalyst loading more than 1.5 mol% (Table 1, entries 5–8).58 Using Pd/C as a traditional catalyst,59–62 3a′ was obtained in lower yield of 58% (same conditions and stoichiometry), demonstrating important role of MOF structure in homoselectivity63 to obtain 3a′ in high yield (Table 1, entries 9 & 10). Also, the ICP analysis of the product using Pd–NHC–MIL-101(Cr) catalyst showed less than 2 ppm of Pd while the amount of Pd-content for the product obtained using Pd/C catalyst was around 16 ppm. This experiment showed that the efficacy of this Pd MOF-based catalyst in the synthesis of this class of π-conjugated materials with low Pd-contamination which is very important in their applications.
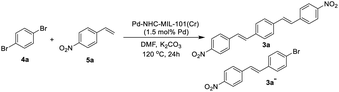
Next we checked the synthesis of DSB derivatives using the reaction of 1,4-dibromobenzene and styrene (Table 2). The Pd–NHC–MIL-101(Cr) catalyst can effectively catalyze this coupling reaction and it is possible to control the reaction to obtain both 3a and 3a′′ in high yields. The synthesis of 3a′′ is important because it can be used for the synthesis of unsymmetrical DSB incorporating two different functional groups in the ends of pi-conjugated system.50 Using 1.5 mol% of Pd–NHC–MIL-101(Cr) catalyst and ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 for 4a
2.2 for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a, DSB 3a was obtained in 84% isolated yield (Table 2, entry 3). Employing the same catalyst loading and reveres ratio of 4a
5a, DSB 3a was obtained in 84% isolated yield (Table 2, entry 3). Employing the same catalyst loading and reveres ratio of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (2.2
5a (2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), compound 3a′′ was obtained in 86% (Table 2, entry 4). Again, in order to check the homoselectivity of the Pd–NHC–MIL-101(Cr) catalyst in mono-functionalization using Heck chemistry the reaction was checked using a Pd/C catalyst. Using this catalyst system compound 3a′′ was obtained in lower yield of 63% (same conditions and stoichiometry). This experiment also represents the key role of MOF structure in homoselectivity (Table 2, entries 5 & 6). The Pd content of the products in this reaction was also evaluated using ICP analysis and it was observed that the obtained product using Pd–NHC–MIL-101(Cr) catalyst has only 3.1 ppm of Pd, while for the product obtained in the presence of homogeneous is around 22 ppm. Accordingly, this heterogeneous Pd catalyst system based on MOF is efficient in the synthesis of DSBs with low Pd-contamination.
1), compound 3a′′ was obtained in 86% (Table 2, entry 4). Again, in order to check the homoselectivity of the Pd–NHC–MIL-101(Cr) catalyst in mono-functionalization using Heck chemistry the reaction was checked using a Pd/C catalyst. Using this catalyst system compound 3a′′ was obtained in lower yield of 63% (same conditions and stoichiometry). This experiment also represents the key role of MOF structure in homoselectivity (Table 2, entries 5 & 6). The Pd content of the products in this reaction was also evaluated using ICP analysis and it was observed that the obtained product using Pd–NHC–MIL-101(Cr) catalyst has only 3.1 ppm of Pd, while for the product obtained in the presence of homogeneous is around 22 ppm. Accordingly, this heterogeneous Pd catalyst system based on MOF is efficient in the synthesis of DSBs with low Pd-contamination.
| Entry | 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a ratio 5a ratio |
Yield 3ab | Yield 3a′′b |
|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2a (based on the ratio), Pd–NHC–MIL-101(Cr) (1.5 mol%), DMF (5 mL), K2CO3 (2.5 mmol), 110 °C, 12 h.b NMR yield.c The Pd/C was used as catalyst. The yields in parentheses related to isolated yields. | |||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
82 (76) | 12 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 80 (73) |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
84 (77) | 8 |
| 4 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 86 (79) |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2 |
69 | 19c |
| 6 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21 | 63c |
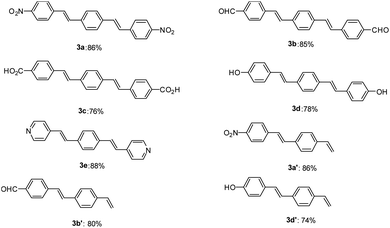
After optimization of the reaction conditions, in order to show the applicability of this catalyst system in synthesis of stilbene and DSBs, some different derivatives were synthesized and results are depicted in Fig. 1.
As shown in Fig. 1, both electron-withdrawing and electron-donating groups on aryl rings worked well with this methodology. The synthesis of these DSBs is important. For example, compound 3b derivatives were used as an amine-sensitive dye for detection of proteins.64 These stilbene derivatives were also used for the preparation of polycyclic aromatic hydrocarbons (PAHs) and nanographene.65 Synthesis of hydroxylated stilbenes is important in biological application point of view and using this catalyst system, compounds 3d and 3d′ was successfully synthesized in high yields.66 Pyridine-based stilbenes are important in the preparation of porous coordination polymers.67
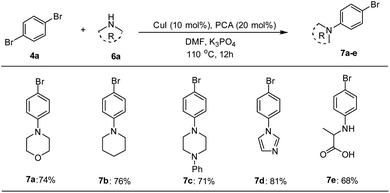
The catalytic applicability of Pd–NHC–MIL-101(Cr) catalyst system was also investigated in the synthesis of unsymmetrical DSBs under optimized conditions. First, some amine-functionalized aryl bromides were synthesized using a Cu-catalyzed N-arylation reaction based on a known procedure in the literature (Scheme 2).68
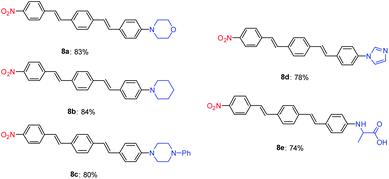
The Mizoroki–Heck coupling reaction between synthetic amine-functionalized aryl halides (7a–e) and compound 3b′ in the presence of Pd–NHC–MIL-101(Cr) catalyst afforded D–π–A systems in high isolated yields (Fig. 2).
Photophysical properties investigation of compounds 8a–e
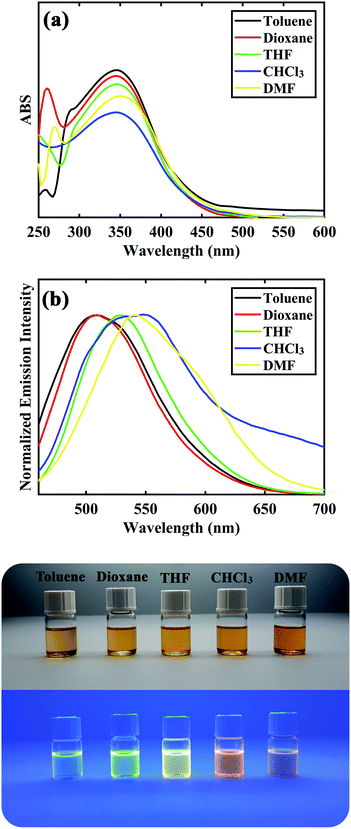
After synthesizing and characterization of D–π–A DSBs, their photophysical properties were investigated and results are depicted in Table 3 and Fig. 3. All of the distyrylbenzene derivatives showed a broad absorption band between 328–355 nm corresponding to the intramolecular charge transfer (ICT) transfer between donor and acceptor moieties in the molecules. Solvent polarity had minimal effect on the absorption band of all compounds showing their low dipole moment at the ground state. The compounds were found to be fluorescence in all solvents with an emission maximum between 496–550 nm. The large stokes shifts with values between 8445–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 cm−1 for the samples suggest that the fluorescence could be due to intramolecular charge transfer (ICT). The emission spectra experienced a red shift from 507, 501, 508, 496, and 507 in toluene (least polarity) to 540, 532, 532, 533, and 541 in DMF (most polarity) for compounds 8a to 8e, respectively. As shown in Fig. 3, compound 8c showed a blue-green fluorescence under UV lamp in toluene and its fluorescence changed to green, yellow, and orange hue upon increasing solvent polarity. This trend was also observed for other distyrylbenzene derivatives suggesting a strong positive solvatochromic effect for the compounds (see ESI†).
349 cm−1 for the samples suggest that the fluorescence could be due to intramolecular charge transfer (ICT). The emission spectra experienced a red shift from 507, 501, 508, 496, and 507 in toluene (least polarity) to 540, 532, 532, 533, and 541 in DMF (most polarity) for compounds 8a to 8e, respectively. As shown in Fig. 3, compound 8c showed a blue-green fluorescence under UV lamp in toluene and its fluorescence changed to green, yellow, and orange hue upon increasing solvent polarity. This trend was also observed for other distyrylbenzene derivatives suggesting a strong positive solvatochromic effect for the compounds (see ESI†).
| Solvent | λab (nm) | λem (nm) | Stock shifts (cm−1) | ε (L mol−1 cm−1) | E (eV) |
|---|---|---|---|---|---|
8a![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) M M |
|||||
| DMF | 355 | 540 | 9650 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 |
3.49 |
| CHCl3 | 355 | 552 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053 053 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757 757 |
3.49 |
| THF | 355 | 529 | 9265 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 671 671 |
3.49 |
| Dioxane | 355 | 509 | 8522 | 122904 | 3.49 |
| Toluene | 355 | 507 | 8445 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 301 301 |
3.49 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
8b![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P P |
|||||
| DMF | 355 | 532 | 9372 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 756 756 |
3.49 |
| CHCl3 | 345 | 535 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 293 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302 |
3.59 |
| THF | 352 | 524 | 9325 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 162 |
3.52 |
| Dioxane | 352 | 506 | 8646 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 334 334 |
3.52 |
| Toluene | 350 | 501 | 8611 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
3.54 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
8c![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Z Z |
|||||
| DMF | 342 | 532 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442 442 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
3.62 |
| CHCl3 | 340 | 545 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 063 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654 |
3.65 |
| THF | 340 | 527 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 436 436 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 341 |
3.65 |
| Dioxane | 340 | 511 | 9842 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
3.65 |
| Toluene | 340 | 508 | 9726 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 293 |
3.65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
8d![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) I I |
|||||
| DMF | 330 | 533 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 541 541 |
97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 190 |
3.75 |
| CHCl3 | 330 | 557 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 349 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 833 |
3.75 |
| THF | 330 | 530 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435 435 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 939 |
3.75 |
| Dioxane | 328 | 504 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 646 646 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910 |
3.78 |
| Toluene | 330 | 496 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141 141 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 460 |
3.75 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
8e![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) A A |
|||||
| DMF | 350 | 541 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 087 087 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461 461 |
3.54 |
| CHCl3 | 345 | 550 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803 803 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 392 392 |
3.59 |
| THF | 345 | 529 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 081 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 873 |
3.59 |
| Dioxane | 345 | 509 | 9339 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 013 |
3.59 |
| Toluene | 345 | 507 | 9261 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 189 189 |
3.59 |
The stabilization of the excited state by more polar solvents was the reason for the observed solvatochromism. It should be noted that the more solvent dependency of emission spectra compared to that of absorption spectra for all compounds could be attributed to more ICT characteristic of the samples in their excited state than that of their ground states.69–71
The pH sensitivity of D–π–A DSBs were also evaluated and results are summarized in Table 4. As detailed in this table, upon decreasing the pH from 7 to 3, no meaningful change was observed in emission band of the samples. With further decreasing of the pH from 3 to 1, a weak blue shift with values between 15 to 25 nm was observed for the fluorescent compounds. The observed blue shift could be assigned to diminishing of intramolecular charge transfer (ICT) when the chromophores were protonated by TFA. Surprisingly, a strong red shift with values between 55–83 nm was detected for the compound 8c in strong acidic condition.
| Comp. | λem (nm) pH = 7 | λem (nm) pH = 6 | λem (nm) pH = 5 | λem (nm) pH = 4 | λem (nm) pH = 3 | λem (nm) pH = 2 | λem (nm) pH = 1 |
|---|---|---|---|---|---|---|---|
| 8a | 552 | 552 | 552 | 552 | 552 | 552 | 552 |
| 534 | 533 | 530 | |||||
| 8b | 535 | 535 | 535 | 535 | 535 | 535 | 535 |
| 519 | 518 | 514 | |||||
| 8c | 545 | 545 | 545 | 545 | 545 | 545 | 545 |
| 530 | 529 | 524 | |||||
| 8d | 557 | 557 | 557 | 557 | 557 | 557 | 557 |
| 537 | 537 | 533 | |||||
| 8e | 550 | 550 | 550 | 550 | 550 | 528 | 526 |
| 531 | 605 | 633 |
DFT and TD-DFT calculations
In order to further clarify the experimental results, the optimized molecular structures of compounds 8a–e DSBs are illustrated in Fig. 4 using density functional theory (DFT) at the B3LYP level. In this work, the B3LYP-D3 and ωB97XD functional methods which include empirical dispersions were also employed in calculations and their computed maximum absorption wavelengths were found to be more deviated from experimental results.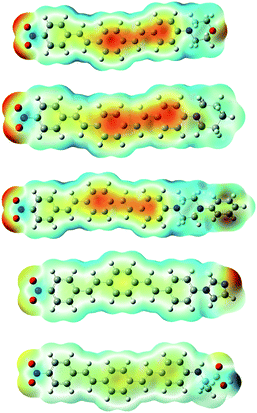 | ||
| Fig. 4 Optimized structures of 8a–e molecules at the B3LYP/6-31+G(d,p) level in the gas phase and electrostatic potential maps. ESP contours are color-coded from red (negative) to blue (positive). | ||
These DSBs are D–π–A molecules consisting of the same electron withdrawing nitrobenzene moiety as well as different electron donating centers (morpholine, piperidine, piperazine, imidazole, and alanine), which are connected by π-conjugation in the middle. To illustrate the electronic distribution around molecular surface and also to probe the sites of electrophilic attack (negative potential) and nucleophilic reaction (positive potential) for investigated molecular systems, molecular electrostatic potential (MEP) surfaces were obtained. It is clearly seen in Fig. 4, in the MEP surface for the 8a–e derivatives, oxygen atoms of nitro groups and the center conjugated moieties through the π-bridge illustrate regions of negative electrostatic potential (electron-rich) while the hydrogen atoms carry the most positive potentials.
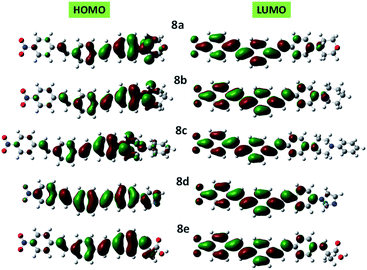
Clear elucidation of electron density distribution on the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the compounds 8a–e configurations were plotted in Fig. 5. The HOMO of the compounds 8a–e is mainly located to the donor segments whereas the LUMO is concentrated to the terminal nitro substituent which further verified that the charge distribution on such molecules is extremely influenced by NO2.
Table 5 signifies the difference between theoretical values of HOMO–LUMO band gap energy for 8a–e DSBs in gas phase and solvent media. The calculated electrochemical band gap energies of the 8a–e derivatives in gas phase are found in the range of 2.41–2.85 eV. The band gap energies were estimated to be in the order of 8b < 8e < 8a < 8c < 8d in gas phase. Overall, the band gap of DSBs decreases in selected solvents of varying polarities. As the electric permittivity of the solvents declines along the series DMF (ε = 37.22), THF (ε = 7.43), CHCl3 (ε = 4.71), toluene (2.37), and dioxane (ε = 2.21), the band gap energies of all DSBs increase, respectively. The results clearly reveal that the electron-donating ability of donor moieties in these compounds leads to the changing of band gap energy.
| Compound | 8a | 8b | 8c | 8d | 8e |
|---|---|---|---|---|---|
| Gas | 2.48 | 2.41 | 2.46 | 2.85 | 2.42 |
| DMF | 2.11 | 2.05 | 2.11 | 2.52 | 2.06 |
| CHCl3 | 2.20 | 2.13 | 2.20 | 2.62 | 2.15 |
| THF | 2.16 | 2.10 | 2.17 | 2.58 | 2.12 |
| Dioxane | 2.30 | 2.23 | 2.31 | 2.72 | 2.25 |
| Toluene | 2.30 | 2.22 | 2.30 | 2.70 | 2.23 |
The absorption (λab) and emission (λem) wavelengths, the oscillator strength, and main assignments of 8a–e molecules in a variety of solvents were predicted from TD-DFT calculations and listed in Table 6. For instance, the electronic absorption band with the highest wavelength of 8c compound has been determined at 395.1 nm in DMF, 394.5 nm in CHCl3, 394.6 nm in THF, 392.3 nm in dioxane, and 393.3 nm in toluene solvent. In line with experimental UV-Vis spectra, the λab of 8d compound is less than others. The electronic absorption of 8a–e derivatives essentially originates from HOMO − 1 → LUMO transition. As obtained for 8a–e series, the experimental and calculated maximum absorption values follow a similar trend while some deviations (∼9–11%) from the experimental values are observed. This deviation could be expected from the bulk solvent effects in experimental conditions while the calculated data are obtained by considering implicit solvent models.
| Solvent | λab (nm) | Osi. stren. | Major contributions | λem (nm) |
|---|---|---|---|---|
| 8a | ||||
| DMF | 397.6 | 0.957 | H − 1 → LUMO (84%) | 514.1 |
| CHCl3 | 394.6 | 1.042 | H − 1 → LUMO (78%) | 486.8 |
| THF | 396.7 | 1.009 | H − 1 → LUMO (81%) | 502.1 |
| Dioxane | 393.5 | 1.134 | H − 1 → LUMO (68%) | 463.1 |
| Toluene | 394.5 | 1.118 | H − 1 → LUMO (70%) | 465.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 8b | ||||
| DMF | 398.1 | 0.985 | H − 1 → LUMO (85%) | 502.7 |
| CHCl3 | 396.3 | 1.066 | H − 1 → LUMO (79%) | 495.9 |
| THF | 396.4 | 1.036 | H − 1 → LUMO (82%) | 505.6 |
| Dioxane | 392.2 | 1.156 | H − 1 → LUMO (71%) | 470.8 |
| Toluene | 394.3 | 1.140 | H − 1 → LUMO (72%) | 474.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 8c | ||||
| DMF | 395.1 | 0.505 | H − 1 → LUMO (86%) | 519.5 |
| CHCl3 | 394.5 | 0.670 | H − 1 → LUMO (75%) | 490.5 |
| THF | 394.6 | 0.780 | H − 1 → LUMO (85%) | 501.5 |
| Dioxane | 392.3 | 0.519 | H − 1 → LUMO (64%) | 479.5 |
| Toluene | 393.3 | 0.628 | H − 1 → LUMO (74%) | 482.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 8d | ||||
| DMF | 361.6 | 0.891 | H − 1 → LUMO (55%) | 511.3 |
| CHCl3 | 359.6 | 0.506 | H − 1 → LUMO (52%) | 487.2 |
| THF | 360.2 | 0.521 | H − 1 → LUMO (44%) | 508.2 |
| Dioxane | 357.4 | 0.585 | H − 1 → LUMO (69%) | 442.6 |
| Toluene | 358.0 | 0.589 | H − 1 → LUMO (70%) | 473.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 8e | ||||
| DMF | 396.2 | 1.098 | H − 1 → LUMO (77%) | 503.5 |
| CHCl3 | 394.8 | 1.040 | H − 1 → LUMO (81%) | 491.2 |
| THF | 395.1 | 1.012 | H − 1 → LUMO (85%) | 501.3 |
| Dioxane | 391.9 | 1.030 | H − 1 → LUMO (62%) | 456.3 |
| Toluene | 393.0 | 1.029 | H − 1 → LUMO (72%) | 467.8 |
Conclusions
In conclusion we have developed an efficient palladium catalyst system based on MOFs in the synthesis of a very important class of fluorescence compounds, DSBs, using Heck chemistry. Using this synthetic methodology it is possible to synthesize different DSB derivatives in good to excellent yields. It seems that the MOF structure is effectively facilitate the Heck reaction between bis-alkenes or aryl halides homoselectivity in order to have mono-functionalized products in good yields. Mono-functionalized products in the both forms of vinyl- and halogen-functionalized stilbenes are important in the synthesis of unsymmetrical DSB derivatives which open our hands to have D–π–A systems. Using Pd–NHC–MIL-101(Cr) catalyst it is possible to synthesis both symmetrical and unsymmetrical DSBs in high yields. Some new D–π–A DSBs which are containing different amino groups (D group) and nitro group (A group) were synthesized successfully using this new synthetic methodology in high isolated yields. The photophysical properties of these fluorescence compounds were investigated and DFT calculations were accomplished to investigate the optimized molecular geometry, band gap energy, and the electronic absorption and emission wavelengths.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the research councils of Shiraz University is gratefully acknowledged.Notes and references
- Y. Wang, W. Liu, S. Ye, Q. Zhang, Y. Duan, R. Guo and L. Wang, J. Mater. Chem. C, 2020, 8, 9678–9687 RSC
.
- Y. Xu, X. Liang, Y. Liang, X. Guo, M. Hanif, J. Zhou, X. Zhou, C. Wang, J. Yao, R. Zhao, D. Hu, X. Qiao, D. Ma and Y. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 31139–31146 CrossRef CAS PubMed
.
- C. H. Lee, S. H. Choi, S. J. Oh, J. H. Lee, J. W. Shim, C. Adachi and S. Y. Lee, RSC Adv., 2020, 10, 42897–42902 RSC
.
- S. K. Pathak, Y. Xiang, M. Huang, T. Huang, X. Cao, H. Liu, G. Xie and C. Yang, RSC Adv., 2020, 10, 15523–15529 RSC
.
- C. Kok, C. Doyranli, B. Canımkurbey, S. P. Mucur and S. Koyuncu, RSC Adv., 2020, 10, 18639–18647 RSC
.
- C. Yao, B. Liu, Y. Zhu, L. Hong, J. Miao, J. Hou, F. He and H. Meng, J. Mater. Chem. A, 2019, 7, 10212–10216 RSC
.
- X. Du, Y. Yuan, L. Zhou, H. Lin, C. Zheng, J. Luo, Z. Chen, S. Tao and L.-S. Liao, Adv. Funct. Mater., 2020, 30, 1909837 CrossRef CAS
.
- P. Tisovský, A. Gáplovský, K. Gmucová, M. Novota, M. Pavúk and M. Weis, Org. Electron., 2019, 68, 121–128 CrossRef
.
- M. R. Koli, A. Labiod, S. Chakraborty, M. Kumar, P. Lévêque, G. Ulrich, N. Leclerc, D. Jacquemin and S. Mula, ChemPhotoChem, 2020, 4, 729–741 CAS
.
- N. De Acha, C. Elosúa, J. M. Corres and F. J. Arregui, Sensors, 2019, 19, 599 CrossRef PubMed
.
- J. Ma, Y. Wang, G. Liu, N. Xu and X. Wang, RSC Adv., 2020, 10, 44712–44718 RSC
.
- S. Tsumura, K. Ohira, K. Imato and Y. Ooyama, RSC Adv., 2020, 10, 33836–33843 RSC
.
- D. Aydin, Talanta, 2020, 210, 120615 CrossRef CAS PubMed
.
- L. Long, Y. Han, X. Yuan, S. Cao, W. Liu, Q. Chen, K. Wang and Z. Han, Food Chem., 2020, 331, 127359 CrossRef CAS PubMed
.
- H.-Y. Kwon, X. Liu, E. G. Choi, J. Y. Lee, S.-Y. Choi, J.-Y. Kim, L. Wang, S.-J. Park, B. Kim, Y.-A. Lee, J.-J. Kim, N. Y. Kang and Y.-T. Chang, Angew. Chem., Int. Ed., 2019, 58, 8426–8431 CrossRef CAS PubMed
.
- W. Chen, T. Matsunaga, D. L. Neill, C. Yang, T. Akaike and M. Xian, Angew. Chem., 2019, 131, 16213–16216 CrossRef
.
- S. Mishra, P. Awasthi, J. Singh, R. K. Gupta, V. Singh, R. Kant, R. Jeet, D. Goswami and A. Goel, J. Org. Chem., 2018, 83, 3669–3678 CrossRef CAS PubMed
.
- H. Y. Chung, J. Oh, J.-H. Park, I. Cho, W. S. Yoon, J. E. Kwon, D. Kim and S. Y. Park, J. Phys. Chem. C, 2020, 124, 18502–18512 CrossRef CAS
.
- I.-H. Park, L. Chu, K. Leng, Y. F. Choy, W. Liu, I. Abdelwahab, Z. Zhu, Z. Ma, W. Chen, Q.-H. Xu, G. Eda and K. P. Loh, Adv. Funct. Mater., 2019, 29, 1904810 CrossRef
.
- A. Granados and A. Vallribera, Dyes Pigm., 2019, 170, 107597 CrossRef CAS
.
- A. Gopinath, K. Ramamurthy, M. Subaraja, C. Selvaraju and A. S. Nasar, New J. Chem., 2018, 42, 10243–10253 RSC
.
- E. Zamani, H. Yahyaei, A. Khosravi, M. Mohseni and H. Shaki, J. Macromol. Sci., Part B: Phys., 2019, 58, 772–781 CrossRef CAS
.
- M. Pilehkouhi, H. Shaki, A. Khosravi, M. Khorasani and E. Zamani, J. Macromol. Sci., Part B: Phys., 2018, 57, 151–167 CrossRef CAS
.
- E. Zamani, H. Shaki, M. Rafizadeh, A. Khosravi and M. Pilehkouhi, Fibers Polym., 2017, 18, 1431–1437 CrossRef CAS
.
- Z. Li, B. Huang, Y. Wang, W. Yuan, Y. Wu, R. Yu, G. Xing, T. Zou and Y. Tao, RSC Adv., 2020, 11, 160–163 RSC
.
- W. M. Pazin, A. K. A. Almeida, V. Manzoni, J. M. M. Dias, A. C. F. de Abreu, M. Navarro, A. S. Ito, A. S. Ribeiro and I. N. de Oliveira, RSC Adv., 2020, 10, 28484–28491 RSC
.
- G. Ji, N. Wang, X. Yin and P. Chen, Org. Lett., 2020, 22, 5758–5762 CrossRef CAS PubMed
.
- F. Gao, L. Yang, L. Yang, H. Li and S. Zhang, J. Fluoresc., 2010, 20, 353–364 CrossRef CAS PubMed
.
- S. Mukherjee, P. Pal, D. Maity and S. Baitalik, J. Photochem. Photobiol., A, 2019, 378, 94–104 CrossRef CAS
.
- B. Łukasik, J. Milczarek, R. Pawlowska, R. Żurawiński and A. Chworos, New J. Chem., 2017, 41, 6977–6980 RSC
.
- J. Shi, M. A. Izquierdo, S. Oh, S. Y. Park, B. Milián-Medina, D. Roca-Sanjuán and J. Gierschner, Org. Chem. Front., 2019, 6, 1948–1954 RSC
.
- D. C. Harrowven, I. L. Guy, M. Howell and G. Packham, Synlett, 2006, 2006, 2977–2980 CrossRef
.
- Z. A. Khan, A. Iqbal and S. A. Shahzad, Mol. Diversity, 2017, 21, 483–509 CrossRef PubMed
.
- H. Meier, S. Kim and A. Oehlhof, Synthesis, 2009, 2009, 848–852 CrossRef
.
- A. Szukalski, K. Parafiniuk, K. Haupa, W. Goldeman, B. Sahraoui, F. Kajzar and J. Mysliwiec, Dyes Pigm., 2017, 142, 507–515 CrossRef CAS
.
- V. Tyagi and R. Fasan, Angew. Chem., 2016, 128, 2558–2562 CrossRef
.
- A. Karbach, T. Stemler, C. Kopp and W. E. Trommer, Synthesis, 2014, 46, 3103–3109 CrossRef CAS
.
- M. Singh and N. P. Argade, Synthesis, 2012, 44, 2895–2902 CrossRef CAS
.
- H. H. Rau and N. S. Werner, Bioorg. Med. Chem. Lett., 2018, 28, 2693–2696 CrossRef CAS PubMed
.
- N. Rameau, B. Russo, S. Mangematin, C. Pinel and L. Djakovitch, Appl. Catal., A, 2018, 560, 132–143 CrossRef CAS
.
- C. I. Traficante, C. Fagundez, G. L. Serra, E. G. Mata and C. M. L. Delpiccolo, ACS Comb. Sci., 2016, 18, 225–229 CrossRef CAS PubMed
.
- T. R. Girase and A. R. Kapdi, Chem.–Asian J., 2019, 14, 2611–2619 CrossRef CAS
.
- F. C. Demidoff, F. P. de Souza and C. D. Netto, Synthesis, 2017, 49, 5217–5223 CrossRef CAS
.
- A. Skhiri, R. B. Salem, J.-F. Soulé and H. Doucet, Synthesis, 2016, 48, 3097–3106 CrossRef CAS
.
- A. Mahmoodi, F. Panahi, F. Eshghi and E. Kimiaei, J. Lumin., 2018, 199, 165–173 CrossRef CAS
.
- F. S. Miri, S. Gorji Kandi and F. Panahi, J. Fluoresc., 2020, 30, 917–926 CrossRef CAS PubMed
.
- H. Karimi-Alavijeh, F. Panahi and A. Gharavi, J. Appl. Phys., 2014, 115, 093706 CrossRef
.
- M. T. Sharbati, F. Panahi, A.-R. Nekoei, F. Emami and K. Niknam, J. Photonics Energy, 2014, 4, 043599 CrossRef CAS
.
- M. T. Sharbati, F. Panahi and A. Gharavi, IEEE Photonics Technol. Lett., 2010, 22, 1695–1697 CAS
.
- F. Panahi, A. Mahmoodi, S. Ghodrati and F. Eshghi, RSC Adv., 2020, 11, 168–176 RSC
.
- E. Niknam, F. Panahi and A. Khalafi-Nezhad, Appl. Organomet. Chem., 2020, 34, e5470 CrossRef CAS
.
- K. R. Balinge and P. R. Bhagat, C. R. Chim., 2017, 20, 773 CrossRef CAS
.
- Y.-Q. Tang, J.-M. Lu and L.-X. Shao, J. Organomet. Chem., 2011, 696, 3741 CrossRef CAS
.
- A. V. Astakhov, O. V. Khazipov, A. Y. Chernenko, D. V. Pasyukov, A. S. Kashin, E. G. Gordeev, V. N. Khrustalev, V. M. Chernyshev and V. P. Ananikov, Organometallics, 2017, 36, 1981 CrossRef CAS
.
- M. Sreenivasulu, K. S. Kumar, P. R. Kumar, K. B. Chandrasekhar and M. Pal, Org. Biomol. Chem., 2012, 10, 1670 RSC
.
- A. L. Gottumukkal, J. G. de Vries and A. J. Minnaard, Chem.–Eur. J., 2011, 17, 3091 CrossRef PubMed
.
- M. A. Taige, A. Zeller, S. Ahrens, S. Goutal, E. Herdtweck and T. Strassner, J. Organomet. Chem., 2007, 692, 1519 CrossRef CAS
.
- The ICP analysis of Pd–NHC–MIL-101(Cr) catalyst shows that it contains 1.3 mmol g−1 of Pd.
- S. Bhavania, M. A. Ashfaq, D. Rambabu, M. V. B. Rao and M. Pal, Arabian J. Chem., 2019, 12, 3836 CrossRef
.
- A. Perosa, P. Tundo, M. Selva, S. Zinovyeva and A. Testa, Org. Biomol. Chem., 2004, 2, 2249 RSC
.
- K. Khler, R. G. Heidenreich, J. G. E. Krauter and J. Pietsch, Chem.–Eur. J., 2002, 8, 622 CrossRef
.
- X.-Y. Zhou, X. Chen and L.-G. Wang, Synthesis, 2017, 49, 5364 CrossRef CAS
.
- M. A. Zolfigol, K. Amani, A. Ghorbani-Choghamarani, M. Hajjami, R. Ayazi-Nasrabadi and S. Jafari, Catal. Commun., 2008, 9, 1739 CrossRef CAS
.
- J. Kumpf, J. Freudenberga and U. H. F. Bunz, Analyst, 2015, 140, 3136 RSC
.
- Z. A. Kasun, H. Sato, J. Nie, Y. Mori, J. A. Bender, S. T. Roberts and M. J. Krische, Chem. Sci., 2018, 9, 7866 RSC
.
- J. Zhang, A. Konsmo, A. Sandberg, X. Wu, S. Nyström, U. Obermüller, B. M. Wegenast-Braun, P. Konradsson, M. Lindgren and P. Hammarström, J. Med. Chem., 2019, 62, 2038–2048 CrossRef CAS PubMed
.
- I.-H. Park, K. Sasaki, H. S. Quah, E. Lee, M. Ohba, S. S. Lee and J. J. Vittal, Cryst. Growth Des., 2019, 19, 1996–2000 CrossRef CAS
.
- M. Suen, L. Hang, W. Lee, A. S. C. Chan and F. Y. Kwong, Tetrahedron Lett., 2008, 49, 6192–6194 CrossRef
.
- A. Paul, A. Biswas, S. Sinha, S. S. Shah, M. Bera, M. Mandal and N. D. P. Singh, Org. Lett., 2019, 21, 2968–2972 CrossRef CAS PubMed
.
- A. Gopinath, K. Ramamurthy, M. Subaraja, C. Selvaraju and A. S. Nasar, New J. Chem., 2018, 42, 10243–10253 RSC
.
- S. Mishra, P. Awasthi, J. Singh, R. K. Gupta, V. Singh, R. Kant, R. Jeet, D. Goswami and A. Goel, J. Org. Chem., 2018, 83, 3669–3678 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data, copy of 1H NMR and 13C NMR of synthesized compounds, and some photophysical data of fluorescence compounds. See DOI: 10.1039/d1ra00457c |
| This journal is © The Royal Society of Chemistry 2021 |