Carbon-incorporated Fe3O4 nanoflakes: high-performance faradaic materials for hybrid capacitive deionization and supercapacitors†
Lei
Chen
a,
Xingtao
Xu
*b,
Lijia
Wan
a,
Guang
Zhu
 c,
Yanjiang
Li
c,
Ting
Lu
a,
Munirah D.
Albaqami
d,
Likun
Pan
c,
Yanjiang
Li
c,
Ting
Lu
a,
Munirah D.
Albaqami
d,
Likun
Pan
 *a and
Yusuke
Yamauchi
*a and
Yusuke
Yamauchi
 *be
*be
aShanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai, 200062, P. R. China. E-mail: lkpan@phy.ecnu.edu.cn
bJST-ERATO Yamauchi Materials Space-Tectonics Project and International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: XU.Xingtao@nims.go.jp
cKey Laboratory of Spin Electron and Nanomaterials of Anhui Higher Education Institutes, Suzhou University, Suzhou 234000, P. R. China
dChemistry Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
eAustralian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, QLD 4072, Australia. E-mail: y.yamauchi@uq.edu.au
First published on 22nd February 2021
Abstract
Here, we introduce a new strategy using urea for the synthesis of carbon-incorporated 2D Fe3O4 (2D-Fe3O4/C) nanoflakes under solvothermal conditions with the following pyrolysis process under an inert atmosphere. Thanks to the structural advantages of 2D-Fe3O4/C, including 2D flakes providing a larger accessible surface area and exposing more active sites, as well as carbon incorporation promoting electrical conductivity for faster charge transfer, the 2D-Fe3O4/C displays a high specific capacitance of 386 F g−1 at 1 A g−1 in a three-electrode system. More importantly, when further assembled into a hybrid supercapacitor with pre-synthesized NiCo-layered double hydroxides as positive electrodes, the assembled supercapacitor device delivers a high-energy density of 32.5 W h kg−1 at 400 W kg−1 and little capacitance loss with bending angles ranging from 0° to 180°. As another capacitive application in desalination, 2D-Fe3O4/C also shows a high desalination capacity of 28.5 mg g−1 over 7.5 min, which suggests a very high mean desalination rate of 3.8 mg g−1 min−1. Our results not only highlight the significance of 2D metal oxide nanosheets/nanoflakes, but also hold great potential for high-performance capacitive applications in supercapacitors and desalination.
Introduction
Transition metal oxides (TMOs) are one of the most widely studied materials in various fields. Among the reported TMOs, Fe3O4 has particularly attracted attention due to its simple structure, environmental friendliness, ease of synthesis, and feasibility of commercial production.1,2 As a result, a series of Fe3O4-based materials have been explored in electrochemical applications, including electrocatalysis, batteries, supercapacitors, capacitive deionization (CDI), etc.3–7 Generally, a simple chemical co-precipitation reaction of a Fe2+/Fe3+ mixture in a basic medium can easily achieve the mass production of Fe3O4 materials,8 but it usually produces bulk Fe3O4 with medium performance. Obstacles to the high electrochemical performance of Fe3O4 materials must be overcome, including poor electrical conductivity, aggregation tende ncy, and slow ion diffusion characteristics;9,10 however, in reality, there are still many challenges.It is advantageous to incorporate carbon into Fe3O4 materials to improve their electrochemical performance. Incorporating carbon is expected to effectively improve the electrical conductivity and prevent Fe3O4 particles from agglomerating.11 For many years, many efforts have been made to generate carbon/Fe3O4 hybrids through various methods, resulting in a variety of species, including graphene/Fe3O4,12 carbon nanotubes/Fe3O4,13 and Fe3O4@carbon.14 Unfortunately, these strategies usually involve multi-step synthesis and expensive synthetic carbon additives, and the process is too complicated and time consuming to be applied on a large scale. Furthermore, the morphological control of the carbon/Fe3O4 hybrid is also very instructive for improving the electrochemical performance. The designed nanostructures, especially two-dimensional (2D) nanosheets/nanoflakes, usually exhibit unusual characteristics beyond ordinary structures, including increased accessible surface area, shortened ion diffusion pathway, and more exposed electrochemically active sites.15,16 However, the construction of the 2D Fe3O4 architecture is only realized in some studies with complex synthesis steps and high production costs,17 and it is still in its infancy and extremely challenging.
To address the issues, herein, we report a facile chemical approach to preparing carbon-incorporated Fe3O4 nanoflakes (denoted as 2D-Fe3O4/C) that possess superior potential for hybrid capacitive applications in supercapacitors and desalination. It is well known that urea chemistry, that is, the urea-mediated soft-chemical strategy, has recently been developed to prepare transition metal-based nanostructures on a large scale.18–20 Under the urea-mediated coordination process, a 2D iron-containing complex nanoflake can be successfully obtained in this work, which subsequently can serve as a precursor for 2D-Fe3O4/C via pyrolysis. Consequently, 2D-Fe3O4/C exhibits several advantages, such as more exposed electrochemically active sites and a shortened ion diffusion pathway from the engineered 2D nanostructure, as well as improved electrical conductivity from carbon incorporation, which thereby endows 2D-Fe3O4/C with a superior faradaic capacitive characteristic. As a proof of concept, the obtained 2D-Fe3O4/C is subsequently investigated in hybrid supercapacitors and hybrid CDI, revealing that 2D-Fe3O4/C delivers a high specific capacitance of 386 F g−1 at 1 A g−1 (in 2 M KOH), superior desalination performance of 28.5 mg g−1 at 1.2 V, and good cycling stability. What's more, 2D-Fe3O4/C also exhibits high energy and power density (32.5 W h kg−1 at 400 W kg−1) when coupled with NiCo-layered double hydroxide (NiCo-LDH) positive electrodes. All of these results show the great potential of 2D-Fe3O4/C for hybrid supercapacitors and hybrid CDI.
Results and discussion
The iron-containing complex was first fabricated with ferric chloride and urea under solvothermal conditions in ethylene glycol. The detailed synthetic process is described in the experimental section of the ESI.†Fig. 1a and b show field emission scanning electron microscopy (FESEM) images of the as-prepared iron-containing complex precursor, which clearly displays a flake-like morphology with a smooth surface and size ranging from 100 nm to hundreds of nanometers. After that, the iron-containing complex was carbonized in a nitrogen atmosphere at 400, 450, and 500 °C, producing the 2D-Fe3O4/C materials named as 2D-Fe3O4/C-400, 2D-Fe3O4/C-450, and 2D-Fe3O4/C-500, respectively. FESEM images of the prepared 2D-Fe3O4/C are shown in Fig. 1c, d and Fig. S1 (ESI†), exhibiting that the flake-like morphology with a rough surface has been well retained after pyrolysis. Such a unique structure is expected to favor electrolyte penetration and electron transfer during the charging process. Moreover, elemental mapping images of the representative sample, 2D-Fe3O4/C-450 (Fig. S2, ESI†), indicate that the Fe, O, and C elements are homogeneously distributed in the 2D-Fe3O4/C nanoflake, suggesting the uniform distribution of Fe3O4 within the carbon.The phase and structure information of the as-prepared 2D-Fe3O4/C samples was investigated through X-ray diffraction (XRD) and Raman measurements (Fig. 2a and b). The XRD patterns in Fig. 2a indicate that all 2D-Fe3O4/C samples exhibit typical diffraction peaks at around 30.1°, 35.5°, 43.1°, 57.2°, and 62.8°, indexed to the (220), (311), (400), (511), and (440) planes of Fe3O4 (JCPDS PDF#19–0629), respectively. This suggests the generation of Fe3O4 at a low pyrolysis temperature. The Raman spectra presented in Fig. 2b indicate the scattering peaks of Fe3O4 at 400–800 cm−1, among which the scattering peaks at around 490 and 670 cm−1 refer to the T2g and A1g vibration modes of Fe3O4, respectively.21,22 The two broad peaks referring to D- and G-bands in the carbon materials are located at around 1360 and 1580 cm−1, respectively. Remarkably, the relative intensity ratios of the D- to G-band (ID/IG) for 2D-Fe3O4/C-400, 2D-Fe3O4/C-450, and 2D-Fe3O4/C-500 are 0.83, 0.83, and 0.85, respectively, which confirms the generation of tremendous defects and disordered structures within the 2D-Fe3O4/C samples.23
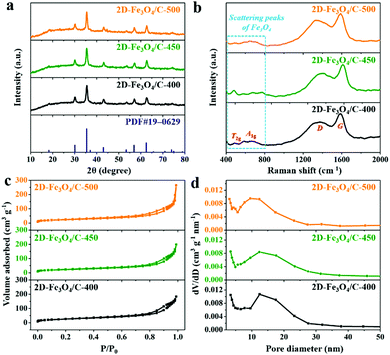 | ||
| Fig. 2 (a) XRD patterns, (b) Raman spectra, (c) N2 adsorption/desorption isotherms, and (d) pore size distributions of 2D-Fe3O4/C. | ||
N2 adsorption/desorption isotherms were further used to identify the specific surface area (SSA) and pore size distribution of the 2D-Fe3O4/C samples (Fig. 2c and d). The N2 adsorption/desorption isotherms of all 2D-Fe3O4/C samples in Fig. 2c evidently exhibit type IV curves with small hysteresis loops. The pore size distributions (Fig. 2d) reveal the presence of abundant mesopores (2–50 nm) in all 2D-Fe3O4/C samples. Moreover, the 2D-Fe3O4/C-450 sample possesses the highest SSA and pore volume of 90.8 m2 g−1 and 0.34 cm3 g−1, respectively, which will positively provide substantial ion accommodation sites and favor the enhancement of the electrochemical performance.
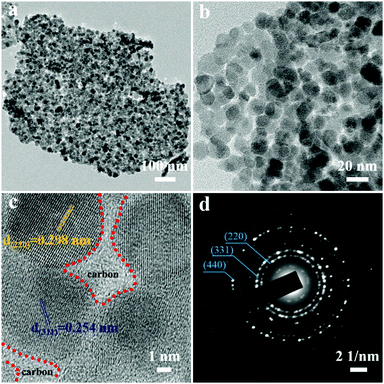
To further identify structural details of 2D-Fe3O4/C, the 2D-Fe3O4/C-450 was further chosen as the representative example and then investigated by transmission electron microscopy (TEM). As revealed in Fig. 3a and b, 2D-Fe3O4/C nanoflakes are composed of assembled nanoparticles that have a size of 10–20 nm. An additional high-resolution TEM (HRTEM) image in Fig. 3c clearly shows that the amorphous carbon is filled within the gap among Fe3O4 nanoparticles, which is expected to prevent the agglomeration of assembled Fe3O4 nanoparticles and improve the electrical conductivity of the nanoflakes for efficient electron transfer. The lattice fringes of the nanoparticles show interplanar spacings of 0.254 and 0.298 nm, corresponding to the (311) and (220) planes of Fe3O4, respectively.24 The selected area electron diffraction (SAED) pattern image of 2D-Fe3O4/C-450 (Fig. 3d) indicates a polycrystalline nature, and the diffraction rings are well indexed to the (220), (311), and (400) planes of Fe3O4 (JCPDS PDF#19–0629).
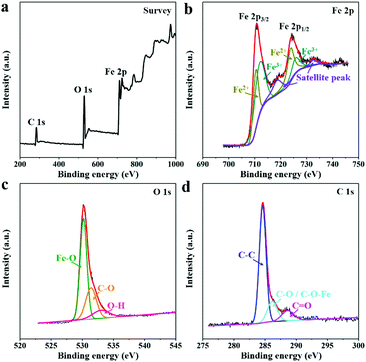
The elemental composition and valence states of the as-synthesized 2D-Fe3O4/C-450 were analyzed by X-ray photoelectron spectroscopy (XPS) measurement. The XPS survey spectrum (Fig. 4a) of 2D-Fe3O4/C-450 indicates the presence of coexistent Fe, O, and C elements. The high-resolution XPS spectrum of Fe 2p (Fig. 4b) is fitted with two peaks assigned to electron orbitals of Fe 2p1/2, Fe 2p3/2, and shakeup satellites. The spin orbits of Fe 2p1/2 and Fe 2p3/2 can be further divided into two peaks, implying the presence of Fe2+ and Fe3+.25 The high-resolution O 1s spectrum (Fig. 4c) can be fitted into three peaks corresponding to the Fe–O bond at 530.2 eV, the C–O bond at 531.4 eV, and the O–H bond at 533.0 eV.26 The high-resolution C 1s spectrum shown in Fig. 4d can be deconvoluted into peaks at 284.6, 286.2, and 288.6 eV, corresponding to C–C, C–O (C–O–Fe), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.26 The detailed carbon contents within the 2D-Fe3O4/C samples are verified by thermogravimetric analysis (Fig. S3, ESI†), revealing values of approximately 13.5, 10.5, and 6.82 wt% for 2D-Fe3O4/C-400, 2D-Fe3O4/C-450, and 2D-Fe3O4/C-500, respectively.
O, respectively.26 The detailed carbon contents within the 2D-Fe3O4/C samples are verified by thermogravimetric analysis (Fig. S3, ESI†), revealing values of approximately 13.5, 10.5, and 6.82 wt% for 2D-Fe3O4/C-400, 2D-Fe3O4/C-450, and 2D-Fe3O4/C-500, respectively.
Rapid growth of the commercial electrical device market has evoked high demand for a new energy storage system that could supply higher energy and power than traditional systems.23,27–29 As compared with traditional dielectric capacitors, supercapacitors that could provide higher energy density while maintaining a high power output have been attracting significant interest in recent years.30–33 Although supercapacitors usually have extremely long cycling lives, their limited energy-to-power ratio is unfavorable for practical application on an industry scale.34,35 This is because in common symmetrical supercapacitor devices, carbon materials are commonly employed as active materials for electrodes; however, limited by their working principles based on electrical double layer (EDL) theory, the fabricated supercapacitors usually suffer from comparatively inferior energy density and capacitance, although their cycling stability is very good.36,37 In contrast, TMOs rely on a pseudocapacitive mechanism through a fast redox process to store more charges and, therefore, can achieve higher specific capacitance, although the cycling stability of TMOs is still very poor. By combining TMOs with carbon materials, the resultant TMO/C composites can achieve both excellent cycling stability and high capacitance, which have been widely studied in hybrid supercapacitors that have higher energy/power ratio as compared to common symmetric supercapacitors.38 Currently, the pursuit of new designs and the exploration of suitable electrode materials with excellent electrochemical activity and unique architecture to promote capacitive performance is highly desirable and of great significance.39,40 As for our 2D-Fe3O4/C nanoflakes, the unique 2D architecture can supply more exposed electrochemically active sites and shorten the ion diffusion pathway, and the filled carbon content within the gap among neighbored Fe3O4 nanoparticles prevents the aggregation of Fe3O4 nanoparticles and improves the electrical conductivity for faster electron transfer. Therefore, the engineered 2D-Fe3O4/C are expected to have a superior faradaic capacitive characteristic as compared to other present materials.
To evaluate the potential of 2D-Fe3O4/C for hybrid supercapacitors, cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) measurements were first carried out to study the capacitance of 2D-Fe3O4/C in a three-electrode configuration with 2 M KOH aqueous solution as the electrolyte. Fig. S4a (ESI†) shows the CV curves of all 2D-Fe3O4/C electrodes at 5 mV s−1 from −1.2 to 0 V. It is noted that a pair of redox peaks can be clearly observed in all samples, indicating the pseudocapacitive behavior. Furthermore, the CV curves of all of the 2D-Fe3O4/C electrodes at scan rates varying from 2 to 50 mV s−1 in 2 M KOH electrolytes are shown in Fig. S4b–d (ESI†), which displays the redox peaks at any scan rate. Based on further electrochemical kinetic analysis (Fig. S5, ESI†), the total capacitances of all 2D-Fe3O4/C electrodes are ascribed to the surface capacitive effects and diffusion-controlled process. The EIS measurements were then carried out, and the resulting Nyquist plots are shown in Fig. S6 (ESI†). All curves consist of a small semicircle at high frequency and a steep straight line at low frequency. Evidently, 2D-Fe3O4/C-450 exhibits much lower Rct (0.20 Ω) than those of 2D-Fe3O4/C-400 (0.96 Ω) and 2D-Fe3O4/C-500 (0.71 Ω), suggesting that the 2D-Fe3O4/C-450 electrode displays the lowest charge transfer resistance, which will promote the electrochemical properties.
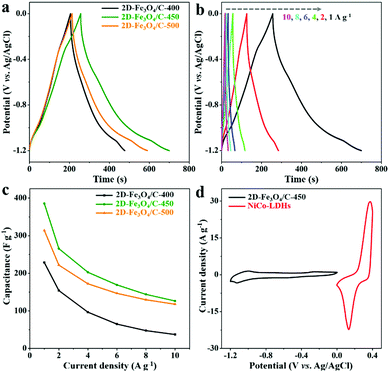
The GCD curves of all 2D-Fe3O4/C electrodes at 1 A g−1 in Fig. 5a, representing distorted triangular shapes, further suggest the redox behavior of 2D-Fe3O4/C materials, and the highest capacitance of 386 F g−1 is achieved by 2D-Fe3O4/C-450. Fig. 5b shows the GCD curves of 2D-Fe3O4/C-450 at 1, 2, 4, 6, 8, and 10 A g−1, displaying all curves as distorted triangular shapes, due to the redox mechanism of 2D-Fe3O4/C-450. The corresponding capacitance values of 2D-Fe3O4/C-450 versus the other two 2D-Fe3O4/C samples are shown in Fig. 5c. As clearly seen, at any current density, the capacitance of 2D-Fe3O4/C-450 is the highest, suggesting the best capacitive performance.
Therefore, 2D-Fe3O4/C-450 was selected as the negative electrode for constructing a hybrid supercapacitor, with NiCo-LDHs serving as the positive electrode. The charge balance between 2D-Fe3O4/C-450 and NiCo-LDHs electrodes is optimized on the basis of the CV curves shown in Fig. 5d, and the mass loading ratio of 2D-Fe3O4/C-450 to NiCo-LDHs within the hybrid supercapacitors is determined to be 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The subsequent two-electrode electrochemical analysis in 2 M KOH aqueous solution by varying the operation potential window indicates that the potential window for 2D-Fe3O4/C-450//NiCo-LDHs can be extended to a high value of 1.6 V with excellent reversibility (Fig. S7 ESI†).
1. The subsequent two-electrode electrochemical analysis in 2 M KOH aqueous solution by varying the operation potential window indicates that the potential window for 2D-Fe3O4/C-450//NiCo-LDHs can be extended to a high value of 1.6 V with excellent reversibility (Fig. S7 ESI†).
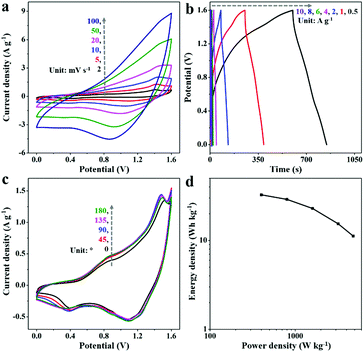
A hybrid supercapacitor composed of 2D-Fe3O4/C-450 as the negative electrode, NiCo-LDHs as the positive electrode, and KOH-gel as the electrolyte was then constructed. Fig. 6a displays the CV curves of the hybrid supercapacitor at varying scan rates from 2 to 100 mV s−1 between 0 and 1.6 V. The shape of the CV curves does not change much, even at higher scan rates, indicating good reversibility and high rate capability. Fig. 6b shows the distorted GCD curves of the hybrid supercapacitors at varying current densities from 0.5 to 10 A g−1, suggesting the redox behavior. What's more, the hybrid supercapacitor achieves a capacitance retention of 71.5% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles (Fig. S8, ESI†), revealing good cycling stability.
000 charge/discharge cycles (Fig. S8, ESI†), revealing good cycling stability.
The feasibility of the hybrid supercapacitor was further investigated by CV curves with diverse bending angles from 0° to 180° (Fig. 6c). Little capacitance loss could be observed after varying the bending angles, suggesting the good potential of our materials for flexible supercapacitor applications. The Ragone plots of the hybrid supercapacitor are illustrated in Fig. 6d. With an energy density of 32.5 W h kg−1 at a power density of 400 W kg−1, our hybrid supercapacitor exhibits performances comparable to those of previous Fe3O4-based hybrid supercapacitors, such as Ni(OH)2//F2RF-150 (4.1 W h kg−1, 661.5 W kg−1),41 MnHCF//Fe3O4/rGO (27.9 W h kg−1, 2183.5 W kg−1),42 CPY//C-G/AFC (18.3 W h kg−1, 351 W kg−1),12 and Fe3O4@Fe2O3//Fe3O4@MnO2 (26.6 W h kg−1, 500 W kg−1).43
With the worsening of the energy and environmental issues,44–47 the exploration of new-class water treatments that save energy and are environmentally friendly has taken on new urgency. In this regard, CDI has been developed based on electrical double layer theory and has received increasing interest over the past decade.48–55 Porous carbons are the most widely used materials for CDI electrodes, including several subfamilies such as graphene,56,57 activated/templated carbon,58–62 and metal–organic framework-derived carbons.63–66 However, the low desalination capacity of carbon materials largely limits the further application of CDI on an industrial scale.67 To address this issue, hybrid CDI that uses a redox electrode to replace one of the two carbon electrodes has been developed.68–70 Generally, TMOs are good candidates for electrode materials for HCDI,71–74 and, recently, Fe3O4 materials have received increasing interest in the HCDI field due to their high desalination performance and great abundance.75 In this section, we plan to evaluate the potential of our 2D-Fe3O4/C for HCDI applications.
Before desalination tests, the capacitive performance of the 2D-Fe3O4/C-450 electrode was first studied by CV curves in 1 M NaCl solution at 10–100 mV s−1 to evaluate its desalination ability (Fig. 7a). The polarization of the electrode is inapparent, which indicates fast charge transfer kinetics, possibly due to a novel two-dimensional flake structure. In addition, the distorted rectangular curves reveal the redox behavior of 2D-Fe3O4/C-450 in NaCl solution. We further conducted electrochemical kinetic analysis, as shown in Fig. S9 (ESI†). The inset of Fig. S9a (ESI†) exhibits the profile of log(i) versus log(v), revealing b values between 0.5 and 1 (Fig. S9a, ESI†), which suggests the combined electrochemical behaviors of the surface-controlled charging and diffusion-controlled Na ion-insertion mechanisms. Therefore, the current response at a fixed potential could be expressed by the following equation:76–78
| i (V) = k1v + k2v1/2, | (1) |
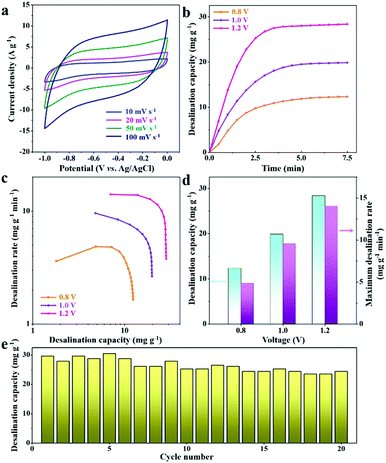
Furthermore, hybrid CDI systems composed of 2D-Fe3O4/C-450 and activated carbon electrodes were constructed. Fig. 7b depicts the profiles of the desalination capacity variations for the 2D-Fe3O4/C-450 electrode over 7.5 min in a 500 mg L−1 NaCl solution at varying operation voltages from 0.8 to 1.2 V. It is observed that once the voltage is applied between the opposite electrodes, the desalination capacity rapidly increases, reaching the maximum value at 7.5 min, which suggests desalination equilibrium. The corresponding CDI Ragone plots (Fig. 7c) depict that, with the increase of operation voltage, the plots tend to shift toward the region corresponding to higher desalination capacity and rate. The detailed values of desalination capacity and maximum desalination rate at varying voltages are displayed in Fig. 7d, revealing that the desalination capacity of the 2D-Fe3O4/C-450 electrode at 1.2 V reaches 28.5 mg g−1 with a maximum desalination rate of 14.0 mg g−1 min−1. For the total desalination process over 7.5 min, the mean desalination rate is 3.8 mg g−1 min−1. As compared with previous reports of TMOs (Table S1, ESI†), these values are still the state of the art, possibly due to the novel 2D nanoflake structure, which provides a large accessible surface area for ion accommodation and a short ion diffusion pathway for mass transport, as well as the significant improvement of the electrical conductivity of the Fe3O4 by the introduced carbons. Finally, the cycling stability of the hybrid CDI cell was studied in NaCl solution with a concentration of 500 mg L−1 (Fig. 7e), which shows that a good desalination capacity retention of 83% remains after 20 cycles, suggesting good cyclability.
Conclusions
In this work, we have successfully synthesized 2D-Fe3O4/C by the simple carbonization of an iron-containing complex for hybrid capacitive applications of supercapacitors and CDI. The obtained 2D-Fe3O4/C composed of carbon-decorated Fe3O4 nanoflakes possesses several advantageous features for high electrochemical performance, including 2D nanoflakes that provide more exposed active sites, larger accessible surface areas, and shortened electron diffusion distances, as well as the decorated carbon significantly improving electrical conductivity and preventing the aggregation of Fe3O4 nanoparticles. Consequently, 2D-Fe3O4/C achieves a maximum specific capacitance of 386 F g−1 at 1 A g−1 in 2 M KOH, and a high-energy density of 32.5 W h kg−1 at 400 W kg−1 when coupled with NiCo-LDHs for constructing a hybrid supercapacitor. Besides the great potential for hybrid supercapacitors, 2D-Fe3O4/C also shows a high desalination capacity of 28.5 mg g−1 with a very high mean desalination rate of 3.8 mg g−1 min−1. Overall, we not only provide a new synthetic approach of 2D Fe3O4 nanostructures, but also demonstrate the potential of the obtained 2D Fe3O4 nanostructures for applications in hybrid supercapacitors and CDI.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (21771064 and 51909066), the Innovative Research Team of Anhui Provincial Education Department (2016SCXPTTD), Primary Research and Development Program of Anhui Province (201904a05020087) and the Key Discipline of Materials Science and Engineering of Suzhou University (2017XJZDXK3) is gratefully acknowledged. This work was partially funded by the Researchers Supporting Project (RSP-2020/267), King Saud University, Riyadh, Saudi Arabia. Dr Xu acknowledges the support from the JSPS Postdoctoral Fellowship for Overseas Researchers (20F20338). This work was partially performed in part at the Queensland node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia’s researchers.Notes and references
- S. Sheng, W. Liu, K. Zhu, K. Cheng, K. Ye, G. Wang, D. Cao and J. Yan, Fe3O4 nanospheres in situ decorated graphene as high-performance anode for asymmetric supercapacitor with impressive energy density, J. Colloid Interface Sci., 2019, 536, 235–244 CrossRef CAS.
- Y. S. Lim, C. W. Lai and S. B. Abd, Hamid, Porous 3D carbon decorated Fe3O4 nanocomposite electrode for highly symmetrical supercapacitor performance, RSC Adv., 2017, 7, 23030–23040 RSC.
- H.-M. Zhang, Y. Zhao, Y. Zhang, M. Zhang, M. Cheng, J. Yu, H. Liu, M. Ji, C. Zhu and J. Xu, Fe3O4 encapsulated in porous carbon nanobowls as efficient oxygen reduction reaction catalyst for Zn-air batteries, Chem. Eng. J., 2019, 375, 122058 CrossRef CAS.
- J. Mao, D. Niu, N. Zheng, G. Jiang, W. Zhao, J. Shi and Y. Li, Fe3O4-embedded and N-doped hierarchically porous carbon nanospheres as high-performance lithium ion battery anodes, ACS Sustainable Chem. Eng., 2019, 7, 3424–3433 CrossRef CAS.
- X. Zeng, L. Zhu, B. Yang and R. Yu, Necklace-like Fe3O4 nanoparticle beads on carbon nanotube threads for microwave absorption and supercapacitors, Mater. Des., 2020, 189, 108517 CrossRef CAS.
- H. Li, Z. Y. Leong, W. Shi, J. Zhang, T. Chen and H. Y. Yang, Hydrothermally synthesized graphene and Fe3O4 nanocomposites for high performance capacitive deionization, RSC Adv., 2016, 6, 11967–11972 RSC.
- R. Chen, K. Zhu, Q. Gan, Y. Yu, T. Zhang, X. Liu, M. Ye and Y. Yin, Interfacial solar heating by self-assembled Fe3O4@C film for steam generation, Mater. Chem. Front., 2017, 1, 2620–2626 RSC.
- S. Wu, A. Sun, F. Zhai, J. Wang, W. Xu, Q. Zhang and A. A. Volinsky, Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation, Mater. Lett., 2011, 65, 1882–1884 CrossRef CAS.
- L. Li, P. Gao, S. Gai, F. He, Y. Chen, M. Zhang and P. Yang, Ultra small and highly dispersed Fe3O4 nanoparticles anchored on reduced graphene for supercapacitor application, Electrochim. Acta, 2016, 190, 566–573 CrossRef CAS.
- X. Zhao, Y. Jia and Z. H. Liu, GO-graphene ink-derived hierarchical 3D-graphene architecture supported Fe3O4 nanodots as high-performance electrodes for lithium/sodium storage and supercapacitors, J. Colloid Interface Sci., 2019, 536, 463–473 CrossRef CAS.
- T. Kou, B. Yao, T. Liu and Y. Li, Recent advances in chemical methods for activating carbon and metal oxide based electrodes for supercapacitors, J. Mater. Chem. A, 2017, 5, 17151–17173 RSC.
- H. Fan, R. Niu, J. Duan, W. Liu and W. Shen, Fe3O4@carbon nanosheets for all-solid-state supercapacitor electrodes, ACS Appl. Mater. Interfaces, 2016, 8, 19475–19483 CrossRef CAS PubMed.
- A. Kumar, D. Sarkar, S. Mukherjee, S. Patil, D. Sarma and A. Shukla, Realizing an asymmetric supercapacitor employing carbon nanotubes anchored to Mn3O4 cathode and Fe3O4 anode, ACS Appl. Mater. Interfaces, 2018, 10, 42484–42493 CrossRef CAS PubMed.
- C. Han, L. Xu, H. Li, R. Shi, T. Zhang, J. Li, C.-P. Wong, F. Kang, Z. Lin and B. Li, Biopolymer-assisted synthesis of 3D interconnected Fe3O4@carbon core@shell as anode for asymmetric lithium ion capacitors, Carbon, 2018, 140, 296–305 CrossRef CAS.
- W. Eom, A. Kim, H. Park, H. Kim and T. H. Han, Graphene-Mimicking 2D Porous Co3O4 Nanofoils for Lithium Battery Applications, Adv. Funct. Mater., 2016, 26, 7605–7613 CrossRef CAS.
- G. Yang, M. Wu and C. Wang, Ultrathin Zn2(OH)3VO3 nanosheets: First synthesis, excellent lithium-storage properties, and investigation of electrochemical mechanism, ACS Appl. Mater. Interfaces, 2016, 8, 23746–23754 CrossRef CAS.
- C. Yin, C. Gong, J. Chu, X. Wang, C. Yan, S. Qian, Y. Wang, G. Rao, H. Wang, Y. Liu, X. Wang, J. Wang, W. Hu, C. Li and J. Xiong, Ultrabroadband Photodetectors up to 10.6 μm Based on 2D Fe3O4 Nanosheets, Adv. Mater., 2020, 32, 2002237 CrossRef CAS PubMed.
- T. Jin, X. Sang, R. R. Unocic, R. T. Kinch, X. Liu, J. Hu, H. Liu and S. Dai, Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy, Adv. Mater., 2018, 30, 1707512 CrossRef PubMed.
- F. Qu, Y. Yuan and M. Yang, Programmed synthesis of Sn3N4 nanoparticles via a soft chemistry approach with urea: application for ethanol vapor sensing, Chem. Mater., 2017, 29, 969–974 CrossRef CAS.
- C. Schliehe, J. Yuan, S. Glatzel, K. Siemensmeyer, K. Kiefer and C. Giordano, Iron nitride and carbide: from crystalline nanoparticles to stable aqueous dispersions, Chem. Mater., 2012, 24, 2716–2721 CrossRef CAS.
- G. Zhou, D.-W. Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L. Wen, G. Q. Lu and H.-M. Cheng, Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS.
- Y. Dong, K. Md, Y.-S. Chui, Y. Xia, C. Cao, J.-M. Lee and J. A. Zapien, Synthesis of CNT@Fe3O4-C hybrid nanocables as anode materials with enhanced electrochemical performance for lithium ion batteries, Electrochim. Acta, 2015, 176, 1332–1337 CrossRef CAS.
- L. Wan, D. Yan, X. Xu, J. Li, T. Lu, Y. Gao, Y. Yao and L. Pan, Self-assembled 3D flower-like Fe3O4/C architecture with superior lithium ion storage performance, J. Mater. Chem. A, 2018, 6, 24940–24948 RSC.
- D. Yoon, J. Hwang, W. Chang and J. Kim, Uniform one-pot anchoring of Fe3O4 to defective reduced graphene oxide for enhanced lithium storage, Chem. Eng. J., 2017, 317, 890–900 CrossRef CAS.
- Q. Ai, Z. Yuan, R. Huang, C. Yang, G. Jiang, J. Xiong, Z. Huang and S. Yuan, One-pot co-precipitation synthesis of Fe3O4 nanoparticles embedded in 3D carbonaceous matrix as anode for lithium ion batteries, J. Mater. Sci., 2019, 54, 4212–4224 CrossRef CAS.
- Y. Liu, N. Wu, Z. Wang, H. Cao and J. Liu, Fe3O4 nanoparticles encapsulated in multi-walled carbon nanotubes possess superior lithium storage capability, New J. Chem., 2017, 41, 6241–6250 RSC.
- X. Liu, G. Feng, Z. Wu, Z. Yang, S. Yang, X. Guo, S. Zhang, X. Xu, B. Zhong and Y. Yamauchi, Enhanced sodium storage property of sodium vanadium phosphate via simultaneous carbon coating and Nb5+ doping, Chem. Eng. J., 2020, 386, 123953 CrossRef CAS.
- J. Wang, Y. Cui and D. Wang, Design of hollow nanostructures for energy storage, conversion and production, Adv. Mater., 2019, 31, 1801993 CrossRef.
- L. Wan, Y. Tang, L. Chen, K. Wang, J. Zhang, Y. Gao, J. Y. Lee, T. Lu, X. Xu, J. Li, Y. Zheng and L. Pan, In-situ construction of g-C3N4/Mo2CTx hybrid for superior lithium storage with significantly improved Coulombic efficiency and cycling stability, Chem. Eng. J., 2021, 410, 128349 CrossRef CAS.
- X. Xu, M. Wang, Y. Liu, Y. Li, T. Lu and L. Pan, In situ construction of carbon nanotubes/nitrogen-doped carbon polyhedra hybrids for supercapacitors, Energy Storage Mater., 2016, 5, 132–138 CrossRef.
- X. Xu, Y. Liu, M. Wang, C. Zhu, T. Lu, R. Zhao and L. Pan, Hierarchical hybrids with microporous carbon spheres decorated three-dimensional graphene frameworks for capacitive applications in supercapacitor and deionization, Electrochim. Acta, 2016, 193, 88–95 CrossRef CAS.
- X. Tang, H. Liu, X. Guo, S. Wang, W. Wu, A. K. Mondal, C. Wang and G. Wang, A novel lithium-ion hybrid capacitor based on an aerogel-like MXene wrapped Fe2O3 nanosphere anode and a 3D nitrogen sulphur dual-doped porous carbon cathode, Mater. Chem. Front., 2018, 2, 1811–1821 RSC.
- J. Wang, H. Tang, H. Ren, R. Yu, J. Qi, D. Mao, H. Zhao and D. Wang, pH-regulated synthesis of multi-shelled manganese oxide hollow microspheres as supercapacitor electrodes using carbonaceous microspheres as templates, Adv. Sci., 2014, 1, 1400011 CrossRef.
- A. Kumar, D. Sarkar, S. Mukherjee, S. Patil, D. D. Sarma and A. Shukla, Realizing an asymmetric supercapacitor employing carbon nanotubes anchored to Mn3O4 cathode and Fe3O4 anode, ACS Appl. Mater. Interfaces, 2018, 10, 42484–42493 CrossRef CAS.
- K. R. Shrestha, S. Kandula, G. Rajeshkhanna, M. Srivastava, N. H. Kim and J. H. Lee, An advanced sandwich-type architecture of MnCo2O4@N–C@MnO2 as an efficient electrode material for a high-energy density hybrid asymmetric solid-state supercapacitor, J. Mater. Chem. A, 2018, 6, 24509–24522 RSC.
- X. Deng, J. Li, L. Ma, J. Sha and N. Zhao, Three-dimensional porous carbon materials and their composites as electrodes for electrochemical energy storage systems, Mater. Chem. Front., 2019, 3, 2221–2245 RSC.
- L. Xie, F. Su, L. Xie, X. Guo, Z. Wang, Q. Kong, G. Sun, A. Ahmad, X. Li, Z. Yi and C. Chen, Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors, Mater. Chem. Front., 2020, 4, 2610–2634 RSC.
- S. Hou, X. Xu, M. Wang, Y. Xu, T. Lu, Y. Yao and L. Pan, Carbon-incorporated Janus-type Ni2P/Ni hollow spheres for high performance hybrid supercapacitors, J. Mater. Chem. A, 2017, 5, 19054–19061 RSC.
- N. Jabeen, A. Hussain, Q. Xia, S. Sun, J. Zhu and H. Xia, High-performance 2.6 V aqueous asymmetric supercapacitors based on in situ formed Na0.5MnO2 nanosheet assembled nanowall arrays, Adv. Mater., 2017, 29, 1700804 CrossRef.
- L. Wang, Y. Ouyang, X. Jiao, X. Xia, W. Lei and Q. Hao, Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density, Chem. Eng. J., 2018, 334, 1–9 CrossRef CAS.
- C. Zhao, X. Shao, Y. Zhang and X. Qian, Fe2O3/reduced graphene oxide/Fe3O4 composite in situ grown on Fe foil for high-performance supercapacitors, ACS Appl. Mater. Interfaces, 2016, 8, 30133–30142 CrossRef CAS.
- K. Lu, D. Li, X. Gao, H. Dai, N. Wang and H. Ma, An advanced aqueous sodium-ion supercapacitor with a manganous hexacyanoferrate cathode and a Fe3O4/rGO anode, J. Mater. Chem. A, 2015, 3, 16013–16019 RSC.
- X. Tang, R. Jia, T. Zhai and H. Xia, Hierarchical Fe3O4@Fe2O3 core-shell nanorod arrays as high-performance anodes for asymmetric supercapacitors, ACS Appl. Mater. Interfaces, 2015, 7, 27518–27525 CrossRef CAS PubMed.
- Z. Zhuge, X. Liu, T. Chen, Y. Gong, C. Li, L. Niu, S. Xu, X. Xu, Z. A. Alothman, C. Q. Sun, J. G. Shapter and Y. Yamauchi, Highly efficient photocatalytic degradation of different hazardous contaminants by CaIn2S4-Ti3C2Tx Schottky heterojunction: An experimental and mechanism study, Chem. Eng. J., 2020 DOI:10.1016/j.cej.2020.127838.
- S. Zhang, W. Xia, Q. Yang, Y. Valentino Kaneti, X. Xu, S. M. Alshehri, T. Ahamad, M. S. A. Hossain, J. Na, J. Tang and Y. Yamauchi, Core-shell motif construction: Highly graphitic nitrogen-doped porous carbon electrocatalysts using MOF-derived carbon@COF heterostructures as sacrificial templates, Chem. Eng. J., 2020, 396, 125154 CrossRef CAS.
- H. Huang, M. Yan, C. Yang, H. He, Q. Jiang, L. Yang, Z. Lu, Z. Sun, X. Xu, Y. Bando and Y. Yamauchi, Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction, Adv. Mater., 2019, 31, 1903415 CrossRef CAS.
- H. Tan, Y. Li, J. Kim, T. Takei, Z. Wang, X. Xu, J. Wang, Y. Bando, Y.-M. Kang, J. Tang and Y. Yamauchi, Sub-50 nm iron–nitrogen-doped hollow carbon sphere-encapsulated iron carbide nanoparticles as efficient oxygen reduction Catalysts, Adv. Sci., 2018, 5, 1800120 CrossRef.
- Z. Wang, X. Xu, J. Kim, V. Malgras, R. Mo, C. Li, Y. Lin, H. Tan, J. Tang, L. Pan, Y. Bando, T. Yang and Y. Yamauchi, Nanoarchitectured metal–organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization, Mater. Horiz., 2019, 6, 1433–1437 RSC.
- X. Xu, C. Li, C. Wang, L. Ji, Y. V. Kaneti, H. Huang, T. Yang, K. Wu and Y. Yamauchi, Three-dimensional nanoarchitecture of carbon nanotubes-interwoven metal–organic frameworks for capacitive deionization of saline water, ACS Sustainable Chem. Eng., 2019, 7, 13949–13954 CrossRef CAS.
- X. Xu, M. Wang, Y. Liu, T. Lu and L. Pan, Ultrahigh desalinization performance of asymmetric flow-electrode capacitive deionization device with an improved operation voltage of 1.8 V, ACS Sustainable Chem. Eng., 2017, 5, 189–195 CrossRef CAS.
- Z. Yue, Y. Ma, J. Zhang and H. Li, Pseudo-capacitive behavior induced dual-ion hybrid deionization system based on Ag@rGO‖Na1.1V3O7.9@rGO, J. Mater. Chem. A, 2019, 7, 16892–16901 RSC.
- Z. Yue, T. Gao and H. Li, Robust synthesis of carbon@Na4Ti9O20 core-shell nanotubes for hybrid capacitive deionization with enhanced performance, Desalination, 2019, 449, 69–77 CrossRef CAS.
- F. Zhou, T. Gao, M. Luo and H. Li, Heterostructured graphene@Na4Ti9O20 nanotubes for asymmetrical capacitive deionization with ultrahigh desalination capacity, Chem. Eng. J., 2018, 343, 8–15 CrossRef CAS.
- W. Xing, J. Liang, W. Tang, D. He, M. Yan, X. Wang, Y. Luo, N. Tang and M. Huang, Versatile applications of capacitive deionization (CDI)-based technologies, Desalination, 2020, 482, 114390 CrossRef CAS.
- S. Hou, X. Xu, M. Wang, T. Lu, C. Q. Sun and L. Pan, Synergistic conversion and removal of total Cr from aqueous solution by photocatalysis and capacitive deionization, Chem. Eng. J., 2018, 337, 398–404 CrossRef CAS.
- W. Dianbudiyanto and S.-H. Liu, Outstanding performance of capacitive deionization by a hierarchically porous 3D architectural graphene, Desalination, 2019, 468, 114069 CrossRef CAS.
- Y. Zhu, G. Zhang, C. Xu and L. Wang, Interconnected graphene hollow shells for high-performance capacitive deionization, ACS Appl. Mater. Interfaces, 2020, 12, 29706–29716 CAS.
- X. Xu, H. Tan, Z. Wang, C. Wang, L. Pan, Y. V. Kaneti, T. Yang and Y. Yamauchi, Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination, Environ. Sci. Nano, 2019, 6, 981–989 RSC.
- X. Xu, A. Enaiet Allah, C. Wang, H. Tan, A. A. Farghali, M. Hamdy Khedr, V. Malgras, T. Yang and Y. Yamauchi, Capacitive deionization using nitrogen-doped mesostructured carbons for highly efficient brackish water desalination, Chem. Eng. J., 2019, 362, 887–896 CrossRef CAS.
- S. Tian, J. Wu, X. Zhang, K. K. Ostrikov and Z. Zhang, Capacitive deionization with nitrogen-doped highly ordered mesoporous carbon electrodes, Chem. Eng. J., 2020, 380, 122514 CrossRef CAS.
- T. Lu, Y. Liu, X. Xu, L. Pan, A. A. Alothman, J. Shapter, Y. Wang and Y. Yamauchi, Highly efficient water desalination by capacitive deionization on biomass-derived porous carbon nanoflakes, Sep. Purif. Technol., 2021, 256, 117771 CrossRef CAS.
- Y. Li, Y. Liu, M. Wang, X. Xu, T. Lu, C. Q. Sun and L. Pan, Phosphorus-doped 3D carbon nanofiber aerogels derived from bacterial-cellulose for highly-efficient capacitive deionization, Carbon, 2018, 130, 377–383 CrossRef CAS.
- X. Xu, J. Tang, Y. Kaneti, H. Tan, T. Chen, L. Pan, T. Yang, Y. Bando and Y. Yamauchi, Unprecedented capacitive deionization performance of interconnected iron-nitrogen-doped carbon tubes in oxygenated saline water, Mater. Horiz., 2020, 7, 1404–1412 RSC.
- X. Xu, T. Yang, Q. Zhang, W. Xia, Z. Ding, K. Eid, A. M. Abdullah, M. Shahriar, A. Hossain, S. Zhang, J. Tang, L. Pan and Y. Yamauchi, Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes, Chem. Eng. J., 2020, 390, 124493 CrossRef CAS.
- J. Shen, Y. Li, C. Wang, R. Luo, J. Li, X. Sun, J. Shen, W. Han and L. Wang, Hollow ZIFs-derived nanoporous carbon for efficient capacitive deionization, Electrochim. Acta, 2018, 273, 34–42 CrossRef CAS.
- W. Zhang, A. Arramel, P. K. J. Wong, L. Zhang, J. Zheng, W. Zhang, H. Zhang, X. Yan, J. Qi and J. Li, Core–shell hybrid zeolitic imidazolate framework-derived hierarchical carbon for capacitive deionization, J. Mater. Chem. A, 2020, 8, 14653–14660 RSC.
- Y. Liu, C. Nie, X. Liu, X. Xu, Z. Sun and L. Pan, Review on carbon-based composite materials for capacitive deionization, RSC Adv., 2015, 5, 15205–15225 RSC.
- W. Shi, X. Liu, T. Deng, S. Huang, M. Ding, X. Miao, C. Zhu, Y. Zhu, W. Liu and F. Wu, Enabling superior sodium capture for efficient water desalination by a tubular polyaniline decorated with Prussian blue nanocrystals, Adv. Mater., 2020, 32, 1907404 CrossRef CAS.
- Z. Chen, X. Xu, Z. Ding, K. Wang, X. Sun, T. Lu, M. Konarova, M. Eguchi, J. G. Shapter, L. Pan and Y. Yamauchi, Ti3C2 MXenes-derived NaTi2(PO4)3/MXene nanohybrid for fast and efficient hybrid capacitive deionization performance, Chem. Eng. J., 2021, 407, 127148 CrossRef CAS.
- Z. Ding, X. Xu, Y. Li, K. Wang, T. Lu and L. Pan, Significantly improved stability of hybrid capacitive deionization using nickel hexacyanoferrate/reduced graphene oxide cathode at low voltage operation, Desalination, 2019, 468, 114078 CrossRef CAS.
- Z. Xie, X. Shang, J. Yang, B. Hu, P. Nie, W. Jiang and J. Liu, 3D interconnected boron-and nitrogen-codoped carbon nanosheets decorated with manganese oxides for high-performance capacitive deionization, Carbon, 2020, 158, 184–192 CrossRef CAS.
- Y. Liu, X. Gao, L. Zhang, X. Shen, X. Du, X. Dou and X. Yuan, Mn2O3 nanoflower decorated electrospun carbon nanofibers for efficient hybrid capacitive deionization, Desalination, 2020, 494, 114665 CrossRef CAS.
- A. S. Yasin, A. Y. Mohamed, I. M. Mohamed, D.-Y. Cho, C. H. Park and C. S. Kim, Theoretical insight into the structure-property relationship of mixed transition metal oxides nanofibers doped in activated carbon and 3D graphene for capacitive deionization, Chem. Eng. J., 2019, 371, 166–181 CrossRef CAS.
- T. Wu, G. Wang, S. Wang, F. Zhan, Y. Fu, H. Qiao and J. Qiu, Highly stable hybrid capacitive deionization with a MnO2 anode and a positively charged cathode, Environ. Sci. Technol. Lett., 2018, 5, 98–102 CrossRef CAS.
- N. T. Trinh, S. Chung, J. K. Lee and J. Lee, Development of high quality Fe3O4/rGO composited electrode for low energy water treatment, J. Energy Chem., 2016, 25, 354–360 CrossRef.
- S. Ardizzone, G. Fregonara and S. Trasatti, “Inner” and “outer” active surface of RuO2 electrodes, Electrochim. Acta, 1990, 35, 263–267 CrossRef CAS.
- W. Yan, T. Ayvazian, J. Kim, Y. Liu, K. C. Donavan, W. Xing, Y. Yang, J. C. Hemminger and R. M. Penner, Mesoporous manganese oxide nanowires for high-capacity, high-rate, hybrid electrical energy storage, ACS Nano, 2011, 5, 8275–8287 CrossRef CAS.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors, J. Am. Chem. Soc., 2009, 131, 1802–1809 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm00946f |
| This journal is © the Partner Organisations 2021 |