Catalytic conversion of ferrous carbonate to higher hydrocarbons under mild conditions and its application in transformation of CO2 to liquid fuels†
Yulv
Yu‡
,
Jin
Huang‡
and
Yuan
Wang
 *
*
Beijing National Laboratory for Molecular Sciences, State Key Lab. for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: wangy@pku.edu.cn
First published on 17th October 2019
Abstract
The generation of liquid fuels of higher hydrocarbons under mild conditions by consuming CO2 would bring about many economic and environmental benefits. Here we report a finding that Pt and Ru nanoparticles deposited on FeCO3 could catalyze the conversion of carbonate ions in FeCO3 and H2 to higher hydrocarbons under mild conditions. Coupling this new reaction route with the carbonation of formed surface ferrous species resulted in the conversion of CO2 and H2 to higher hydrocarbons (C2–C26) at low or even ambient temperatures due to the low activation-energy for CHx coupling in the process. At 40 °C, the selectivity for C5–C26 hydrocarbons and C2+ compounds reached 49.6% and 77.7%, respectively. This finding sheds a new light on regenerating multi-carbon energy carriers in a sustainable manner.
Introduction
Ferrous carbonate is a main component of siderite which is a worldwide distributed iron mineral with large deposits. So far, ferrous carbonate has been utilized as a fertilizer, feed additive, and raw material for steel production.1,2 Conversion of CO32− in ferrous carbonate to organic products such as liquid fuels or feed-stocks in the chemical industry under mild conditions is an attractive new subject. On the other hand, the increasing demand for fossil energy and resources has caused a continuous increase of CO2 concentration in the atmosphere, resulting in serious environmental problems such as the greenhouse effect and ocean acidification. The catalytic conversion of CO2 to value-added products has attracted increasing attention.3–6Higher hydrocarbons (CxHy, x ≥ 2) with carbon numbers of 2–4, 5–12, and 13–22 are used as feed-stocks for manufacture of polymers, gasoline, and diesel, respectively. Currently, these products are predominantly produced by petroleum refining or in coal chemical industry based on Fischer–Tropsch (F–T) synthesis at high temperatures.7,8
Conversion of ferrous carbonate or CO2 and hydrogen to higher hydrocarbons at low or ambient temperatures may create many energy and ecological benefits, because it will make it possible to produce high-value products in an environmentally friendly manner using H2 derived from renewable energy, reduce energy consumption, decrease the dependence on fossil resources, and avoid over emission of CO2 in the conversion process.
We have been engaging in the development of iron oxide supported platinum group metal nanocluster catalysts for a long time.9–11 Herein, we report a new finding that Ru and Pt nanoparticles deposited on ferrous carbonate could catalyze the conversion of carbonate ions in FeCO3 and hydrogen to higher hydrocarbons at low temperatures, and the coupling of this new reaction (CTHC) with carbonation of the formed ferrous species by CO2 could establish a new route (C-CTHC) to realize the conversion of CO2 and H2 to hydrocarbons (C1–C26) at low or even ambient temperatures (40–130 °C).
Results and discussion
Ferrous carbonate supported Ru and Pt nanoparticles (Ru–Pt/FeCO3) were prepared by depositing Pt nanoclusters stabilized with ethylene glycol and simple ions12 and Ru oxide nanoparticles on iron oxide,13,14 consecutively, followed by treating the obtained solid intermediate with hydrogen and CO2 in a mixture of cyclohexane and water at 160 °C (see the ESI†). The Pt and Ru contents of Ru–Pt/FeCO3 were measured using an inductively coupled plasma atomic emission spectrometer (ICP-AES) to be 1.6% and 2.1%, respectively.The X-ray diffraction (XRD) pattern of Ru–Pt/FeCO3 exhibited the diffraction signals of crystal planes of FeCO3, while the diffraction peaks of the supported metal nanoparticles were too weak to analyze due to the strong diffraction signals of the support (Fig. S1, ESI†).
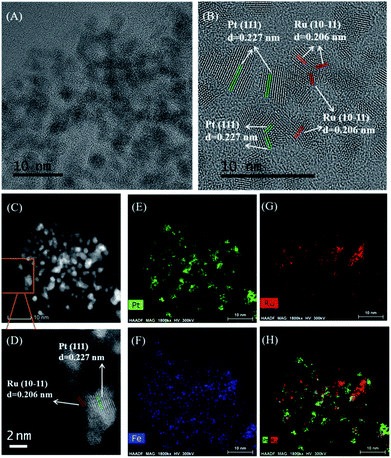
As shown in the transmission electron microscope (TEM) image of Ru–Pt/FeCO3 (Fig. 1A), metal nanoparticles dispersed on FeCO3 had an average diameter of 1.8 nm, with a standard deviation of 0.4 nm (Fig. S2, ESI†). Pt and Ru nanoparticles with the inter-fringe distances of 0.227 and 0.206 nm were observed in the high resolution TEM image (Fig. 1B), respectively, indicating that Ru–Pt/FeCO3 was composed of Ru and Pt nanoparticles deposited on FeCO3. EDX (energy dispersive X-ray spectroscopy) mapping measurements revealed that many Pt nanoparticles were in contact with Ru nanoparticles in Ru–Pt/FeCO3 (Fig. 1H).
X-ray photoelectron spectroscopy (XPS) measurements on Ru–Pt/FeCO3 revealed that the electron binding energies of Ru 3d5/2 and Pt 4f7/2 levels had values of 280.0 and 71.3 eV, respectively, indicating that Ru and Pt were in metallic states.15,16 The electron binding energies of O 1s and Fe 2p3/2 levels were measured to be 531.9 and 710.0 eV, respectively, which were consistent with those in FeCO3 (Fig. S3, ESI†).17
We found that heating Ru–Pt/FeCO3 and 3.75 MPa of H2 (25 °C) at 130 °C for 24 h in a mixture of cyclohexane and water could produce C1–C26 hydrocarbons as the main products, with a selectivity for C2+ hydrocarbons of 26.2% and a time-yield of hydrocarbons (based on moles of carbon) of 181.0 mmol molmetal−1 h−1 at a FeCO3 conversion of 15.3% (Table 1), indicating that Pt and Ru nanoparticles on FeCO3 could catalyze a reaction between carbonate ions and H2 to form higher hydrocarbons (CTHC). The carbon balance, i.e. the ratio of carbon in the product to that consumed in CTHC, was measured to be 90.1% based on the experimental data (see the ESI†).
| T (°C) | P CO2 (MPa) | Selectivityb | Time-yieldb | |||
|---|---|---|---|---|---|---|
| CH4 | C2+c | C5+c | ROHc | |||
| a Reaction conditions: Ru–Pt/FeCO3, 0.3 g; water, 15 ml; cyclohexane, 15 ml; H2, 3.75 MPa; time, 24 h. b Selectivity (%) and time-yield (mmol molmetal−1 h−1) were calculated based on moles of carbon, where metal denotes platinum group metal. c C2+ and C5+ denote hydrocarbons with carbon numbers larger than 2 and 5. ROH denotes C1–C6 alcohols. d Reaction time was 8 hours. | ||||||
| 130 | — | 70.7 | 26.2 | 16.0 | 3.1 | 186.8 |
| 130 | 1.25 | 51.8 | 42.1 | 26.1 | 6.1 | 919.5d |
| 80 | — | 58.7 | 33.7 | 13.5 | 7.6 | 67.2 |
| 80 | 1.25 | 32.5 | 55.9 | 32.3 | 11.6 | 114.9 |
To confirm the new chemical conversion pathway, we prepared Ru–Pt/Fe13CO3 using 13C labeled carbon dioxide (13CO2) by the same method and performed the reaction of Ru–Pt/Fe13CO3 with H2 under the same conditions. The produced higher hydrocarbons were collected and analyzed by gas chromatography-mass spectrometry (GC-MS). In the mass spectra (MS) of pentane, a representative of the higher hydrocarbons, the molecular ion peak of [13C5H12]˙+ with a m/z value of 77 could be observed clearly, whereas that of [12C5H12]˙+ with a m/z value of 72 did not appear (Fig. 2), proving that carbon in the higher hydrocarbons was derived from carbonate ions in FeCO3.
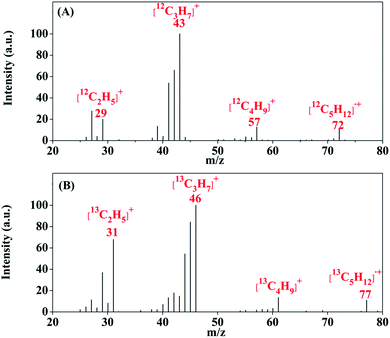 | ||
| Fig. 2 Mass spectrum of pentane produced in the reaction of H2 with Ru–Pt/Fe12CO3 (A) and Ru–Pt/Fe13CO3 (B) at 130 °C, respectively. | ||
Furthermore, the aforementioned CTHC was conducted under an atmosphere of 13CO2 (0.75 MPa, 25 °C) in cyclohexane at 130 °C. In the MS of the produced pentane, the enhancement in the signals for the 13C containing species could not be observed (Fig. S4, ESI†), demonstrating that higher hydrocarbons could be produced by the hydrogenation of FeCO3, without CO2 participation in the reaction.
After treating Ru–Pt/FeCO3 with hydrogen in a mixture of water and cyclohexane at 130 °C, the solid residue was separated for XPS measurements. The XPS band of Fe 2p3/2 in the sample, with a maximum at 710.5 eV and a shoulder band at 710.0 eV, revealed that a part of ferrous carbonate was transformed into iron oxide18 in the reaction. After the recovered sample was treated with a mixture of CO2 and H2 at 130 °C, the binding energy of Fe 2p3/2 in the collected sample changed back to 710.0 eV of FeCO3 (Fig. S5, ESI†), revealing the possibility of coupling CTHC with the carbonation reaction of surface ferrous species in the presence of CO2.
When CTHC was performed under an atmosphere of 13CO2 (0.75 MPa, 25 °C) at 130 °C in a mixture of water and cyclohexane, the relative intensities of signals for [C2H5]+, [C3H7]+, [C4H9]+, and [C5H12]˙+ containing 13C, with m/z values of 30–31, 44–46, 58–61, and 73–77, respectively, significantly enhanced in the MS of pentane (Fig. 3A), indicating that the CTHC process could couple with the carbonation reaction of formed ferrous species in the presence of water to form a new catalytic reaction route (C-CTHC) to synthesize higher hydrocarbons from CO2 and H2 under mild conditions as shown in Fig. 3B.
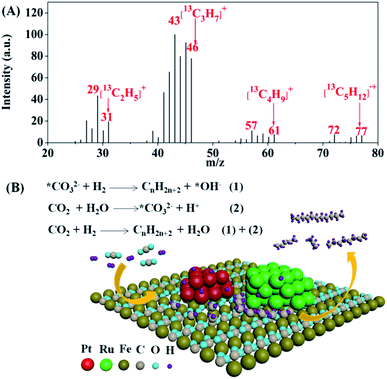 | ||
| Fig. 3 Mass spectrum of pentane produced in C-CTHC using 13CO2 in a mixture of water and cyclohexane (A), and a schematic of the proposed catalytic mechanism for C-CTHC (B). | ||
C-CTHC could produce C1–C26 hydrocarbons at 130 °C in a mixture of water and cyclohexane with a time-yield of hydrocarbons of 863.4 mmol molmetal−1 h−1 and a selectivity for C2+ hydrocarbons of 42.1% (Table 1), which were much higher than those of CTHC at the same temperature (Fig. S6, ESI†).
It was found that at 80 °C in the mixture of cyclohexane and water, CTHC could produce C1–C13 hydrocarbons with a selectivity for C2+ hydrocarbons of 33.7% and a time-yield of hydrocarbons of 62.1 mmol molmetal−1 h−1, while C-CTHC could produce C1–C26 hydrocarbons with a selectivity for C2+ hydrocarbons of 55.9% and a time-yield of hydrocarbons of 101.6 mmol molmetal−1 h−1 (Table 1). These experimental results implied that in the present conversion process of CO32− to hydrocarbons, the introduced CO2 is important for producing hydrocarbons with longer carbon chains and increasing the reaction rate at low temperatures, which may be due to the increase of CHx species concentration around the Pt nanoparticles (see below).
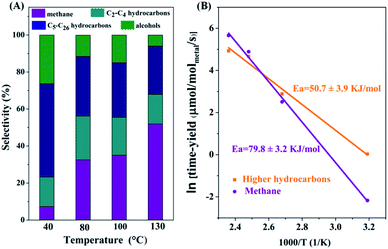
The product distributions for C-CTHC in a mixture of cyclohexane and water at different temperatures are shown in Fig. 4A. It is seen that at 40 °C, the selectivity for C5–C26 hydrocarbons and C2+ compounds reached 49.6% and 77.7%, respectively, while that for methane was only 7.3%. With the reaction temperature increasing, the time-yield of hydrocarbons increased obviously, and the selectivity for higher hydrocarbons decreased. At 130 °C, the selectivities for C5–C26 hydrocarbons and C2+ compounds were 26.1% and 45.8%, respectively, with a conversion of carbon in the substrates (including FeCO3 and CO2) of 5.1% in 8 hours. The distribution of the formed hydrocarbon products basically followed ASF (Anderson–Schulz–Flory) statistics with a chain growth factor (α) of 0.77 (Fig. S7, ESI†), which was consistent with the production of hydrocarbons with long carbon chains. CO, which was usually produced in the catalytic hydrogenation of CO2, was not detected by GC (detection limit: 60 ppm) in the products of the present catalytic system.
It was found that decreasing the pressure of H2 could increase the selectivity for C2+ hydrocarbons, with a declined time-yield. When H2 pressure varied from 3.75 MPa (25 °C) to 2.50 MPa (25 °C), the selectivity for C2+ hydrocarbons increased from 42.1% to 55.6%, while the time-yield of C2+ hydrocarbons declined from 387.1 to 292.6 mmol molmetal−1 h−1 as measured after the reaction was conducted for 8 hours at 130 °C. Upon decreasing H2 pressure to 1.25 MPa (25 °C), the selectivity for C2+ hydrocarbons reached 68.1%, with a time-yield of 109.2 mmol molmetal−1 h−1 under the same conditions (Table S1, ESI†).
Based on the time-yield of methane and C2+ hydrocarbons in C-CTHC at different temperatures (313, 373, 403, and 423 K), the apparent activation energy for the formation of methane was calculated to be 79.8 ± 3.2 kJ mol−1, while that for C2+ hydrocarbons was 50.7 ± 3.9 kJ mol−1 (Fig. 4B), implying that the activation energy for hydrogenation of CHx to form methane is much higher than that for the coupling of CHx (x = 2, 3) to form higher hydrocarbons in the present process, accounting for the increase in the selectivity for higher hydrocarbons with decreasing the reaction temperature.
It should be mentioned that over the previously reported catalytic systems, the synthesis of hydrocarbons with carbon numbers up to 15 through the CO2-FT process needed a high reaction temperature (∼290 °C) due to the high activation energy for the carbon–carbon coupling reaction,19,20 demonstrating the advantage of C-CTHC in the synthesis of multi-carbon compounds at low temperatures over CO2-FT.
To further study the roles of Ru and Pt nanoparticles in C-CTHC, Ru/FeCO3 and Pt/FeCO3 were prepared by the method described in this work in the absence of Pt or Ru, respectively. The diffraction peaks of ferrous carbonate are clearly observed in the XRD patterns of the prepared Ru/FeCO3 and Pt/FeCO3 (Fig. S8, ESI†). The average diameters of Ru and Pt nanoparticles in Ru/FeCO3 and Pt/FeCO3 were measured to be 1.7 ± 0.4 and 1.9 ± 0.3 nm, respectively, which were similar to those of metal nanoparticles in Ru–Pt/FeCO3. C-CTHC over Pt/FeCO3, Ru/FeCO3, and Ru–Pt/FeCO3 was conducted in a mixture of CO2 (1.25 MPa, 25 °C) and H2 (3.75 MPa, 25 °C) at 130 °C, respectively (Table S2, ESI†). The results revealed that Ru/FeCO3 could catalyze the reaction of CO32− with H2 to produce hydrocarbons (C1–C10) with a time-yield of 766 mmolCH2 molmetal−1 h−1, and the selectivity for methane was as high as 91.6%, implying that Ru nanoparticles deposited on FeCO3 could efficiently catalyze the hydrogenation of CO32− to produce 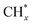 (* means adsorbed species), and
(* means adsorbed species), and 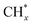 tended to couple with H* to form methane over Ru/FeCO3. On the other hand, Pt nanoparticles deposited on FeCO3 could catalyze the hydrogenation conversion of CO32− to hydrocarbons (C1–C14) with a selectivity for methane and C2+ hydrocarbon of 26.1 and 49.6%, respectively, and a time-yield of 175 mmolCH2 molmetal−1 h−1, implying that Pt nanoparticles deposited on FeCO3 were not as efficient as Ru/FeCO3 in catalyzing the hydrogenation of CO32− to form
tended to couple with H* to form methane over Ru/FeCO3. On the other hand, Pt nanoparticles deposited on FeCO3 could catalyze the hydrogenation conversion of CO32− to hydrocarbons (C1–C14) with a selectivity for methane and C2+ hydrocarbon of 26.1 and 49.6%, respectively, and a time-yield of 175 mmolCH2 molmetal−1 h−1, implying that Pt nanoparticles deposited on FeCO3 were not as efficient as Ru/FeCO3 in catalyzing the hydrogenation of CO32− to form 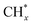 , but the formed
, but the formed 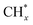 tended to couple with each other to form higher hydrocarbons over Pt/FeCO3. Ru and Pt nanoparticles deposited on FeCO3 could catalyze the hydrogen conversion of CO32− to produce C1–C26 hydrocarbons with a selectivity for C2+ hydrocarbons of 42.1% and a time-yield of 863 mmolCH2 molmetal−1 h−1. Based on these control experimental results, it can be concluded that in Ru–Pt/FeCO3, Ru nanoparticles were mainly responsible for the reduction of CO32− to form
tended to couple with each other to form higher hydrocarbons over Pt/FeCO3. Ru and Pt nanoparticles deposited on FeCO3 could catalyze the hydrogen conversion of CO32− to produce C1–C26 hydrocarbons with a selectivity for C2+ hydrocarbons of 42.1% and a time-yield of 863 mmolCH2 molmetal−1 h−1. Based on these control experimental results, it can be concluded that in Ru–Pt/FeCO3, Ru nanoparticles were mainly responsible for the reduction of CO32− to form 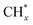 species, while Pt nanoparticles played an important role in catalyzing the coupling of
species, while Pt nanoparticles played an important role in catalyzing the coupling of 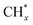 produced on themselves, or migrated from the attached or adjacent Ru nanoparticles, to form higher hydrocarbons. The synergistic effect between Pt and Ru nanoparticles supported on FeCO3 is responsible for the formation of hydrocarbons with very long carbon chains over Ru–Pt/FeCO3 at low temperatures.
produced on themselves, or migrated from the attached or adjacent Ru nanoparticles, to form higher hydrocarbons. The synergistic effect between Pt and Ru nanoparticles supported on FeCO3 is responsible for the formation of hydrocarbons with very long carbon chains over Ru–Pt/FeCO3 at low temperatures.
A FeCO3 supported PtRu alloy nanocluster catalyst (PtRu/FeCO3, Ru/Pt atomic ratio = 2.5) was prepared with the described method by replacing the Pt or Ru nanoclusters with PtRu alloy nanoclusters21 and used as a catalyst for C-CTHC. It was found that the time-yield of hydrocarbons over PtRu/FeCO3 (80 mmolCH2 molmetal−1 h−1) was much lower than that over Ru–Pt/FeCO3, and the carbon chain of higher hydrocarbons generated over PtRu/FeCO3 (C2–C11) was much shorter than that generated over Ru–Pt/FeCO3 (C2–C26), implying that the FeCO3 supported PtRu alloy nanocluster is not as efficient as Ru–Pt/FeCO3 in catalyzing C-CTHC (Table S2, ESI†).
A control catalyst composed of Ru and Pt nanoclusters deposited on TiO2 (Ru–Pt/TiO2) was prepared (see the ESI†) and used for the hydrogenation of CO2. Ru–Pt/TiO2 could catalyze the reaction of CO2 with H2 in a mixture of water and cyclohexane at 130 °C to produce C1–C4 hydrocarbons and C1–C2 alcohols, with selectivities of CH4 and higher hydrocarbons of 97.9% and 1.2%, respectively, demonstrating the important role of FeCO3 as a functional support in the conversion of CO2 and H2 to long-chain hydrocarbons under mild conditions (Table S3, ESI†).
Fe3O4 supported Ru and Pt nanoparticles (Ru–Pt/Fe3O4) were also prepared for comparison. It was found that Ru–Pt/Fe3O4 could catalyze the conversion of CO2 and H2 to higher hydrocarbons (C2–C26) with a selectivity of 11.3%, which supported the proposed mechanism of carbonation reaction of CO2 with surface iron species in C-CTHC.
Cyclohexane in the reaction system played an important role in generating long-chain hydrocarbons in C-CTHC. When the reaction was conducted in water at 130 °C, hydrocarbons with more than 7 carbon atoms were not detected in the products (Table S4, ESI†). As a hydrophobic solvent, cyclohexane could dissolve the formed long-chain hydrocarbons, promoting its desorption from the catalytic sites. Furthermore, cyclohexane covered on the catalyst might efficiently decrease the probability of carbon-chain termination with OH or H.
The stability of Ru–Pt/FeCO3 in C-CTHC was tested at 130 °C in a mixture of 1.25 MPa (25 °C) of CO2 and 3.75 MPa (25 °C) of H2 (see the ESI†). The conversion of carbon in the substrates over the catalyst in 8 hours maintained at 4.8 ± 0.3% in the three cycles, and the selectivity for C2+ hydrocarbons and C5+ hydrocarbons maintained at 40.0 ± 2.0% and 24.0 ± 2.0%, respectively, implying that the catalyst possessed good stability under the reaction conditions (Fig. S9, ESI†).
Conclusions
This work establishes a new route for the synthesis of higher hydrocarbons (C2–C26), which is characterized by a reaction of ferrous carbonate and hydrogen to form the hydrocarbons, coupled with carbonation of formed ferrous species by carbon dioxide. The low activation energy for the carbon–carbon coupling reaction in C-CTHC led to the formation of the higher hydrocarbons at low or even ambient temperatures, exhibiting advantages of C-CTHC in synthesis of multi-carbon compounds from CO2 under mild conditions. The synergistic effect between Pt and Ru nanoparticles on FeCO3 played an important role in generating higher hydrocarbons.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their thanks for the support from the NSFC (grants 21573010 and 21821004) and the Chinese Ministry of Science and Technology (2016YFE0118700).Notes and references
- Handbook of Mineralogy, ed. J. W. Anthony, R. A. Bideaux, K. W. Bladh and M. C. Nichols, Mineralogical Society of America, Chantilly, VA 20151-1110, USA, 2003 Search PubMed.
- I. S. Alcala, F. Bellon, M. C. del Campillo, V. Barron and J. Torrent, Sci. Hortic., 2012, 138, 17–23 CrossRef.
- J. J. Wang, G. N. Li, Z. L. Li, C. Z. Tang, Z. C. Feng, H. Y. An, H. L. Liu, T. F. Liu and C. Li, Sci. Adv., 2017, 3, e1701290 CrossRef PubMed.
- O. Martin, A. J. Martin, C. Mondelli, S. Mitchell, T. F. Segawa, R. Hauert, C. Drouilly, D. Curulla-Ferre and J. Perez-Ramirez, Angew. Chem., Int. Ed., 2016, 55, 6261–6265 CrossRef CAS PubMed.
- Z. H. He, Q. L. Qian, J. Ma, Q. L. Meng, H. C. Zhou, J. L. Song, Z. M. Liu and B. X. Han, Angew. Chem., Int. Ed., 2016, 55, 737–741 CrossRef CAS PubMed.
- Y. Wang, Z. Z. Zhang, L. N. Zhang, Z. B. Luo, J. N. Shen, H. X. Lin, J. L. Long, J. S. Wu, X. Z. Fu, X. X. Wang and C. Li, J. Am. Chem. Soc., 2018, 140, 14595–14598 CrossRef CAS PubMed.
- J. Li, Y. L. He, L. Tan, P. P. Zhang, X. B. Peng, A. Oruganti, G. H. Yang, H. Abe, Y. Wang and N. Tsubaki, Nat. Catal., 2018, 1, 787–793 CrossRef CAS.
- F. Jiao, J. J. Li, X. L. Pan, J. P. Xiao, H. B. Li, H. Ma, M. M. Wei, Y. Pan, Z. Y. Zhou, M. R. Li, S. Miao, J. Li, Y. F. Zhu, D. Xiao, T. He, J. H. Yang, F. Qi, Q. Fu and X. H. Bao, Science, 2016, 351, 1065–1067 CrossRef CAS PubMed.
- Y. L. Yu, J. Huang and Y. Wang, ChemCatChem, 2018, 10, 4863–4867 CAS.
- J. L. Zhang, Y. Wang, H. Ji, Y. G. Wei, N. Z. Wu, B. J. Zuo and Q. L. Wang, J. Catal., 2005, 229, 114–118 CrossRef CAS.
- M. H. Liang, X. D. Wang, H. Q. Liu, H. C. Liu and Y. Wang, J. Catal., 2008, 255, 335–342 CrossRef CAS.
- Y. Wang, J. W. Ren, K. Deng, L. L. Gui and Y. Q. Tang, Chem. Mater., 2000, 12, 1622–1627 CrossRef CAS.
- C. Lian, Y. L. Yu, K. Zhang, A. Gao and Y. Wang, Catal. Sci. Technol., 2018, 8, 1528–1534 RSC.
- L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- Z. Y. Sun, Z. M. Liu, B. X. Han, Y. Wang, J. M. Du, Z. L. Xie and G. J. Han, Adv. Mater., 2005, 17, 929–932 Search PubMed.
- C. Lian, K. Zhang and Y. Wang, Acta Phys.-Chim. Sin., 2017, 33, 984–992 CAS.
- J. K. Heuer and J. K. Stubbins, Corros. Sci., 1999, 41, 1231–1243 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- U. Rodemerck, M. Holena, E. Wagner, Q. Smejkal, A. Barkschat and M. Baerns, ChemCatChem, 2013, 5, 1948–1955 CrossRef CAS.
- M. D. Porosoff, B. H. Yan and J. G. Chen, Energy Environ. Sci., 2016, 9, 62–73 RSC.
- L. W. Zhang, A. Gao, Y. Liu, Y. Wang and J. T. Ma, Electrochim. Acta, 2014, 132, 416–422 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9se00612e |
| ‡ Equally contributed. |
| This journal is © The Royal Society of Chemistry 2020 |