Open Access Article
Open Access ArticleCarbon nanotubes: functionalisation and their application in chemical sensors
Mohd Nurazzi Norizana,
Muhammad Harussani Moklisa,
Siti Zulaikha Ngah Demona,
Norhana Abdul Halima,
Alinda Samsuria,
Imran Syakir Mohamadb,
Victor Feizal Knight c and
Norli Abdullah*a
c and
Norli Abdullah*a
aDepartment of Chemistry and Biology, Centre for Defence Foundation Studies, Universiti Pertahanan Nasional Malaysia, Kem Perdana Sungai Besi, 57000 Kuala Lumpur, Malaysia. E-mail: norli.abdullah@upnm.edu.my
bFaculty of Mechanical Engineering, Universiti Teknikal Malaysia Melaka, Hang Tuah Jaya, 76100 Durian Tunggal, Melaka, Malaysia
cResearch Centre for Chemical Defence, Universiti Pertahanan Nasional Malaysia, Kem Perdana Sungai Besi, 57000 Kuala Lumpur, Malaysia
First published on 9th December 2020
Abstract
Carbon nanotubes (CNTs) have been recognised as a promising material in a wide range of applications, from safety to energy-related devices. However, poor solubility in aqueous and organic solvents has hindered the utilisation and applications of carbon nanotubes. As studies progressed, the methodology for CNTs dispersion was established. The current state of research in CNTs either single wall or multiwall/polymer nanocomposites has been reviewed in context with the various types of functionalisation presently employed. Functionalised CNTs have been playing an increasingly central role in the research, development, and application of carbon nanotube-based nanomaterials and systems. The extremely high surface-to-volume ratio, geometry, and hollow structure of nanomaterials are ideal for the adsorption of gas molecules. This offers great potential applications, such as in gas sensor devices working at room temperature. Particularly, the advent of CNTs has fuelled the invention of CNT-based gas sensors which are very sensitive to the surrounding environment. The presence of O2, NH3, NO2 gases and many other chemicals and molecules can either donate or accept electrons, resulting in an alteration of the overall conductivity. Such properties make CNTs ideal for nano-scale gas-sensing materials. Conductive-based devices have already been demonstrated as gas sensors. However, CNTs still have certain limitations for gas sensor application, such as a long recovery time, limited gas detection, and weakness to humidity and other gases. Therefore, the nanocomposites of interest consisting of polymer and CNTs have received a great deal of attention for gas-sensing application due to higher sensitivity over a wide range of gas concentrations at room temperature compared to only using CNTs and the polymer of interest separately.
1. Introduction
The discovery of CNTs by Iijima in 1991 (ref. 1) has driven global scientific research and technological attention with the hope to revolutionise various frontiers in the field of nanotechnology. CNTs can be described as a graphite sheet rolled up into a nanoscale-tube (which are single-wall carbon nanotubes (SWCNTs)), or with additional graphene tubes around the core of an SWCNT (which are multi-wall CNTs (MWCNTs)). The diameters are in the range between fractions of nanometres and tens of nanometres and lengths are up to several centimetres with both their ends normally capped by fullerene-like structures.2 The fascinating properties associated with CNTs were believed to open new paths in the material world, especially in the field of conductive polymer and CNT-based composites. Since then, various techniques to incorporate CNTs in polymer matrices have been designed with a desire to fabricate new advanced materials with multifunctional properties. Some of these properties were directed towards transferring the unique electrical properties associated with CNTs to rather insulating polymer matrices with the aim of obtaining conducting polymer composites.The exceptional mechanical properties associated with CNTs reported vary in different literatures. Theoretical and experimental results on CNTs have shown extraordinary high elastic modulus, greater than 1 TPa (the elastic modulus of diamond is 1.2 TPa) and reported strengths of 10 to 100 times higher than the strongest steel at a fraction of the weight.3 CNTs also possess superior thermal and electrical properties: thermally stable up to 2800 °C in vacuum, electrical conductivity of about 103 S cm−1 with electric-current-carrying capacity 1000 times higher, thermal conductivity of about 1900 W m−1 K−1, which is about twice as high as diamond.4,5
With these promising strengths, CNTs have tremendous potential for applications in many scientific and technological fields, especially for use as composite fillers in polymers to improve the mechanical, thermal, and electrical properties of resulting composites. There have been indications of a number of potential applications for CNTs, including microwave absorption,6,7 corrosion protection,8,9 reinforced materials in natural fibre composites,10,11 electromagnetic interference shielding (EMI),12,13 batteries,14,15 solar cells,16–19 chemical sensor,20–23 hydrogen storage,24,25 and field-emission materials.26,27 Other than CNTs, Chen and Shao (2016) had listed other types of carbonaceous materials, such as amorphous porous carbon, graphene, carbon black, carbon nanofibre, and graphite.28,29 Due to their superior and unique electronic properties, inert properties of carbonaceous materials are widely used as counter electrodes (CE).30–34 Their advantage and limitations on the structural and electronic properties of the listed carbonaceous materials are summarised in Table 1.
| Carbon materials | Advantages | Limitations | Structural | Electrical | Ref. |
|---|---|---|---|---|---|
| Amorphous porous carbon | High surface area, advanced porous system, abundant defective sites, superior chemical inertness | Relative low conductivity, poor adhesion with FTO | Consists of an outer spherical shell with porous interior structure, a covalent random network composed of sp3 and sp2 hybridised carbons without grain boundaries, non-crystalline | High electronic conductivity and high surface area, electronic conductivity and ionic conductivity, with specific capacities of 212 mA h g−1 and 162 mA h g−1 at 0.5C and 1C, respectively | 35 |
| Graphene | Excellent conductivity, fast charged carrier mobility, good mechanical strength, high optical transparency, good mechanical inertness | Low surface area arising from the easy aggregation, low quantities of defective sites | Crystalline carbon materials, monolayers of carbon atoms arranged in a honeycomb network, giant aromatic macromolecule | Conducts both electricity and heat, thermal conductivity and mechanical stiffness (3000 W m−1 K−1 and 1060 GPa, respectively) | 36 |
| Graphite | Good conductivity, corrosion resistance, excellent thermal stability | Poor porous system, low surface area | Stacks of graphene layers, weak interactions that hold the graphene sheets together | High electrical and thermal conductivity, thermal conductivity 25 to 470 W m−1 K−1, electrical resistivity 5 × 10−4 to 30 × 10−4 Ω cm | 37 and 38 |
| Carbon black | Plentiful defective sites, good chemical inertness | Low surface area, inappropriate pore size, inadequate conductivity | Typical particle sizes range from around 8 to 100 nm for furnace blacks | Highly structured carbon blacks provide higher viscosity, greater electrical conductivity and easier dispersion for specialty carbon blacks, electrical volume resistivity between 1 to 106 Ω cm | 39 |
| Carbon nanofibre | Excellent mechanical strength, high thermal conductivity, good chemical inertness | Insufficient conductivity, low surface area, inferior porous system | Cylindrical nanostructures with graphene layers arranged as stacked cones, cups or plates, diameters from 50 to 200 nm | High electrical conductivity, and high thermal conductivity, intrinsic conductivity, at room temperature to be 5 × 10−5 Ω cm | 40 and 41 |
| Carbon nanotube | Large surface area, high electrical conductivity, good chemical inertness | Low quantities of defective sites | Crystalline carbon materials, most of the physical properties of carbon nanotubes derive from graphene, carbon atoms are densely organised in a regular sp2-bonded atomic-scale honeycomb (hexagonal) pattern, sp2 hybridization of carbon builds a layered construction with weak out-of-plane bonding of the van der Waals form and strong in-plane bounds | High electrical conductivity, high thermal conductivity, resistivity of the SWCNT is 10−4 Ω cm at 27 °C, the SWCNT ropes able to sustain much higher stable current densities, as high as 1013 A cm−2 | 41 and 42 |
The physical and catalytic properties of CNTs make it ideal to be used in chemical sensors. Most notably, the high degree of organisation and high aspect ratio of CNTs has pronounced excellent chemical, thermal, and mechanical properties in terms of stiffness, Young's modulus, flexibility, and electrical conductivity.43–46 The remarkable properties of CNTs are useful for constructing nanoscale devices and developing multifunctional composite materials.47–49 The two main types of CNTs are single-walled CNTs (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) (Fig. 1). SWCNTs are sp2 hybridised carbon in a hexagonal honeycomb structure that is rolled into a hollow tube morphology, while MWCNTs are multiple concentric tubes encircling one another.50 Recently, CNTs have started to attract ever increasing interest as a conductive filler and reinforcement for polymeric composites. Apart from high electrical conductivity, CNTs also have unique electronic and optical properties for development of organoelectronic devices.51,52 The possibility to achieve reasonably high conductivity at very low CNT concentration, between 0.0025 and 4 wt%, is owed to its high aspect ratio (L/D, where L is length of CNT and D is diameter of CNT) from 100 to 1000, which makes them an ideal candidate to be harnessed for several potential applications especially in electronic devices and structural components. Such high aspect ratio facilitates CNT to form a ‘network-like’ structure in the composites at a particular low concentration termed as percolation.53 In other words, increase in CNT concentration/loading usually results in transition from a poor-conducting state to a good conducting state at a certain threshold volume fraction of the conductive filler. The volume fraction of CNTs, necessary for building a continuous conductive path inside the polymer matrix, is referred to as the percolation threshold. It seems promising to prepare the materials with small content and ratio which preserve the desired mechanical properties of a polymer along with a higher electrical conductivity.54 Fig. 2(c) shows the transmission electron microscopy (TEM) of purified MWCNTs with long intertwined CNTs. As observed, the obtained CNTs with diameter of about 20 nm were clean, and almost all impurities have been removed without destroying the basic structure of the nanotubes.55
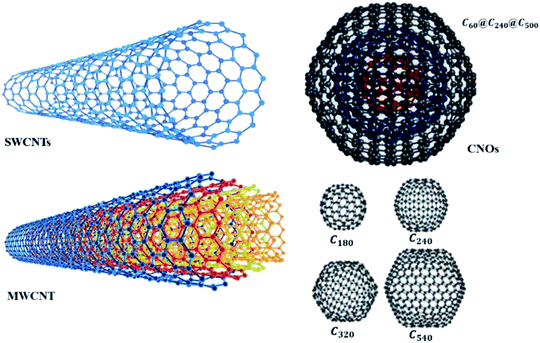 | ||
| Fig. 1 Nanostructures of SWCNTs and MWCNTs, fullerenes and a carbon nano-onion (CNO). Reproduced with permission from ref. 60, copyright 2008, RSC. | ||
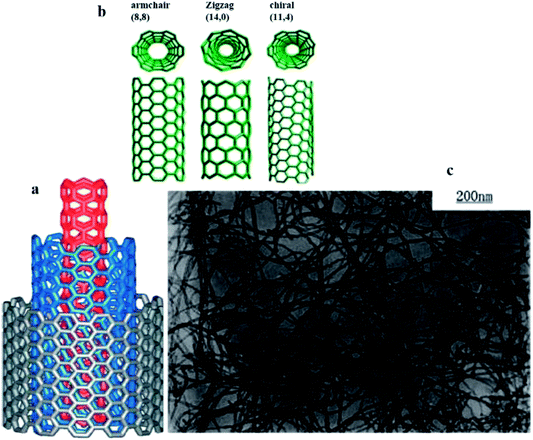 | ||
| Fig. 2 (a) Structure of a multi-walled carbon nanotube made up of three shells of hexagonal lattice sheet of different chirality, (b) roll-up of a graphene sheet that leads to three different types of CNTs and (c) image of purified MWNTs with carboxylation functionalisation under TEM. Reproduced with permission from ref. 55, copyright 2011, Composites Part A: Applied Science and Manufacturing. | ||
Numerous other CNT forms, such as double-walled CNTs,56 bamboo,57 and herringbone58 have also been synthesised and discovered. The nanostructure of CNTs has been reviewed by Delgado et al. (2007), where fullerenes and CNTs are only the tip of the iceberg and, more recently, a wide variety of new carbon nanostructures, such as endohedral fullerenes, cup-stacked nanotubes, nanohorns, nanotori, nanobuds, and nanoonions, have emerged as new and fascinating forms of carbon. The chemical and physical properties of these new forms of carbon are currently being unravelled.59 SWCNTs can be classified as either semi-conducting or metallic allotropes, depending on the chirality. The distinction of semi-conducting or metallic is important for their use in different sensors, but the physical separation of allotropes has proven to be one of the more difficult challenges to overcome. In MWCNTs, a single metallic layer results in the entire nanotube displaying metallic behaviour. Fig. 2(a) shows the structure of MWCNTs made up of three shells of different chirality, while Fig. 2(b) shows the formation of SWCNTs through the roll-up of graphene with respect to the hexagonal lattice sheet leading to the three different types of CNTs.
The recent discovery on CNTs also has attracted considerable attention due to their dimensions and structure-sensitive properties. The high electrical conductivity of these nanostructures allows the utilisation of CNTs as electrode material, and in combination with its strong electrocatalytic activity offers the ability to mediate electron transfer reactions.61,62 The facility of electron transfer between the electroactive species and the electrode offers great promise especially for fabricating chemical sensors.63 Referring to Jacobs et al. (2010), the uniqueness of CNTs leads to enhancement of electronic properties, a large edge plane/basal plane ratio, and rapid electrode kinetics. Therefore, CNT-based sensors generally have higher sensitivity, lower limits of detection (LOD), and faster electron transfer kinetics than traditional carbon electrodes.64
Besides the aforementioned superior properties of CNTs, owing to the rigidity, chemical inertness, and strong π–π interactions of nanotubes, pure CNTs cannot be processed, as they are difficult to dissolve or disperse in common volatile organic solvents (VOS) or polymeric matrices. Due to van der Waals forces of CNTs, the nanotubes tend to agglomerate with each other which results in difficulty of dispersion. In addition to the size effect of CNTs, the physical nature of particles also plays an important role in dispersing them into a polymer matrix. As produced CNTs are held together in bundles or entanglements consisting of 50 to a few hundred individual CNTs by van der Waals force.65 It has been proven that these bundles and agglomerates result in diminished mechanical and electrical properties of composites as compared with theoretical predictions related to individual CNTs.66,67 The challenge is on how to incorporate individual CNTs, or at least relatively thin CNT bundles or disentangled CNTs, inside a polymer interest. In other words, dispersion of CNTs is not only a geometrical problem, which is dealing with the length and size of the CNTs, but also relates to a method on how to separate individual CNTs from CNT agglomerates and stabilise them in a polymer matrix to avoid secondary agglomeration.
Therefore, the side walls of CNTs must be chemically modified to improve their dispersion or solubility in solvents or polymers, and improve the interaction and reactivity with the polymer by hydrogen bonding interaction.68 Besides that, altering the surface of nanotubes strongly affects solubility properties, which can affect the ease of fabrication of CNT sensors.64 As reviewed by Zhao and Stoddart (2009), the non-covalent functionalisation by small molecules, grafting to or wrapping nanotubes with polymers can also alter the electrochemical properties of the material itself.69 In mechanical properties enhancement, the incorporation of chemical functionalised CNTs into such polymer enables chemical covalent bonding between the SWCNTs/MWCNTs and the material of interest. Examples of such covalent linkages achieved through chemical functionalisation have been utilised in SWCNT reinforced polymer composites.70,71 In addition, chemical functionalisation is used to enhance the nanotube–polymer interface. Increasing the interfacial bonding between SWCNTs and the polymer will improve the interfacial strength and thus improves load transfer mechanism to the SWCNTs, with the goal of improving the macro and microscopic mechanical properties of the composite system.72
Several methods of functionalisation involve chemical,73 electrochemical,74 mechano-chemical,75 and plasma treatment.76 The functionalisation of CNTs may be treated to functionalise their surfaces and side chain. In chemical functionalisation, the most common treatment with strong acids removes the end caps and also shorten the length of the CNTs. Acid treatment also adds oxide groups, primarily carboxylic acids, carbonyl, and hydroxyl groups to the tube ends and defect sites of CNTs (Fig. 3).77 Further chemical reactions can be performed on these oxide groups to functionalise with groups such as amides, thiols, or other groups.78–80 Balasubramanian and Burghard also had clustered the method of covalent functionalisation of SWCNTs that was accomplished through three different approaches, namely, thermally activated chemistry, electrochemical modification, and photochemical functionalisation.81 Fig. 4 shows the different methods of functionalisation of CNTs.82,83
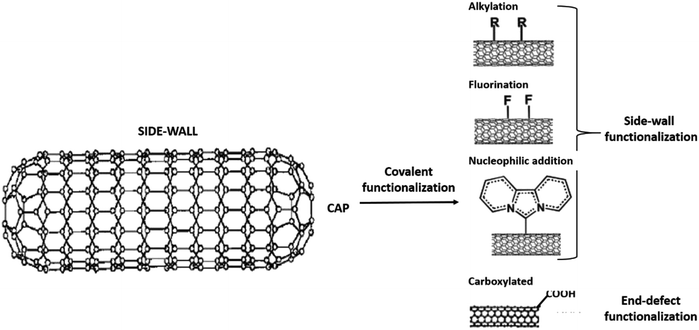 | ||
| Fig. 3 The covalent functionalisation phenomena at the side and end-cap of CNT structure. Adapted from ref. 77, copyright 2015, Elsevier. | ||
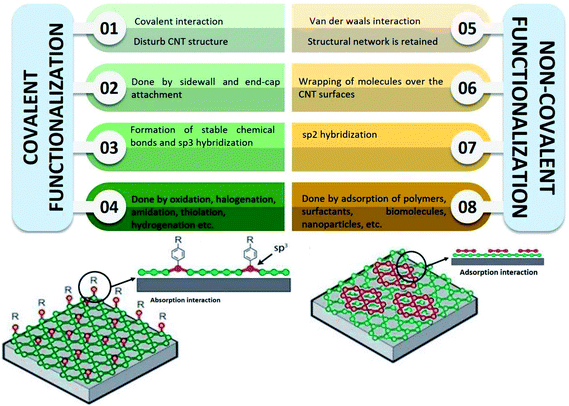 | ||
| Fig. 4 Comparison between covalent and non-covalent functionalisation of CNTs. Adapted from ref. 36, copyright 2015, Elsevier. And adapted from ref. 37, copyright 2015, Elsevier. | ||
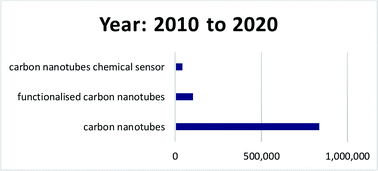
Here, a review of recent research towards the development of functionalised CNT-based sensors for the detection of chemical analytes is reviewed. Because of the breadth of research that has been published in this area, this review is only limited to recent publications within the past ten years, 2010 to 2020 (Fig. 5). For that past ten years, about 837![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publications related to the keyword “carbon nanotubes”, and about 103
000 publications related to the keyword “carbon nanotubes”, and about 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publications related to the keyword “functionalised carbon nanotubes” were found in Google Scholar (6th November 2020). In addition, about 43
000 publications related to the keyword “functionalised carbon nanotubes” were found in Google Scholar (6th November 2020). In addition, about 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 publications related to “carbon nanotubes chemical sensor” were found. In Malaysia, about 121 publications related to the keyword “carbon nanotubes chemical sensor” were found (data extracted from http://www.lens.org on 6th November 2020). These findings indicate that CNTs is an interesting subject to be studied, and many more explorations can be done that could lead to huge impacts to the nation. This paper elaborates on the fundamental of CNTs, the covalent and non-covalent functionalisation involved for CNTs, types of analytes, applications of CNTs in sensors, and future perspectives for functionalised CNTs in chemical sensors application.
700 publications related to “carbon nanotubes chemical sensor” were found. In Malaysia, about 121 publications related to the keyword “carbon nanotubes chemical sensor” were found (data extracted from http://www.lens.org on 6th November 2020). These findings indicate that CNTs is an interesting subject to be studied, and many more explorations can be done that could lead to huge impacts to the nation. This paper elaborates on the fundamental of CNTs, the covalent and non-covalent functionalisation involved for CNTs, types of analytes, applications of CNTs in sensors, and future perspectives for functionalised CNTs in chemical sensors application.
2. Covalent functionalisation of CNTs
Covalent functionalisation of CNTs can be achieved by either direct addition reactions of functional groups or the “active agents” to the sidewalls of nanotubes or modification of appropriate surface-bound functional groups on the nanotubes end.84,85 The most common starting functionalisation method engaged to functionalise CNTs with covalent functionalisation is by oxidation process, which results in the formation of carboxyl groups (–COOH) on the surface of nanotubes. Oxidation has become a must in functionalisation because it oxidatively introduces carboxyl groups which is useful for further modifications. Carboxyl groups enable covalent coupling of molecules through the creation of amide and ester bonds.81,86 There are two common acid treatments used; one refluxes the nanotubes with a solution of nitric acid87 and the other exposes the sample to a mixture of sulphuric acid/nitric acid (HNO3/H2SO4) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by volume) under high power sonication for a maximum of 6 hours.88 Fig. 6 shows the covalent functionalisation of nanotubes through oxidation process using H2SO4/HNO3.89 As a result, the functionalisation provides stable dispersions of CNTs in a range of polar solvents, including with water.90 Besides that, the covalent attachments of functional groups modify the stacking and layering properties of CNTs by altering the hydrogen bonding through reduction of van der Waals interactions between the CNTs and strongly facilitates the separation of nanotube bundles into individual tubes.91
3 by volume) under high power sonication for a maximum of 6 hours.88 Fig. 6 shows the covalent functionalisation of nanotubes through oxidation process using H2SO4/HNO3.89 As a result, the functionalisation provides stable dispersions of CNTs in a range of polar solvents, including with water.90 Besides that, the covalent attachments of functional groups modify the stacking and layering properties of CNTs by altering the hydrogen bonding through reduction of van der Waals interactions between the CNTs and strongly facilitates the separation of nanotube bundles into individual tubes.91
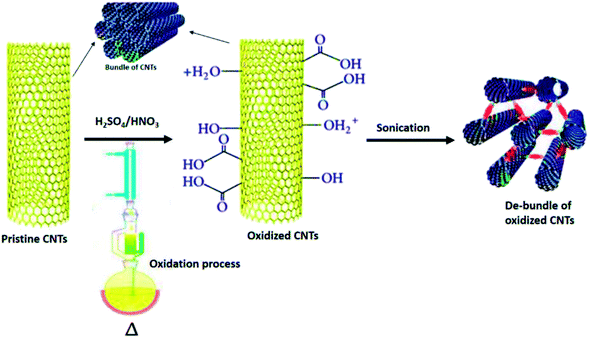 | ||
| Fig. 6 Functionalisation of CNT through the oxidation process using H2SO4/HNO3. Adapted from ref. 43, copyright 2015, Elsevier. | ||
Despite the fact that the first oxidations were developed to open and filling CNTs, the carboxyl, hydroxyl, and carboxylate groups generated all through surface oxidation of these materials have demonstrated useful moieties to bond new reactive chains that improve solubility, processing, and compatibility with other materials, thus allow scientists to take advantage of CNTs properties. Due to the relevance of this type of functionalisation, next sections shall deal with the different studies related to chemical functionalisation, after oxidation, by using the OH pertained to –COOH groups generated during the oxidation process. The organic carboxyl groups formed on a nanotube surface localised at the defects in functionalised single-walled and multi-walled nanotubes and suitable reactive organic groups from other chemical chains are prone to react in this zone. However, in some cases, deterioration due to employment of concentrated inorganic acids combined with high power sonication are responsible for creating a large number of defects on the CNTs sidewalls, and in some extreme cases, CNTs are fragmented into smaller pieces. These damages result in severe deterioration of mechanical, electrical, and thermal properties of CNTs.65 This route greatly enhances the solubility of CNTs in common solvents and helps in dispersing CNTs in many polymer matrices. In addition to assisting in de-bundling, these treatments also significantly improve phase adhesion with the host matrix, thus help in processing of the polymer/CNT interface.
Goyanes et al. (2006) studied the effect of acid treatment and ultrasonication on MWCNTs using nitric acid with a mixture of HNO3 and H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by volume).92 The sample was ultrasonicated using ultrasonic bath (Selecta Ultrasons-H model, which has a nominal frequency of 40 kHz with power of 950 W). The preset time of sonication varied from 2, 4, and 6 hours. The result showed that the treatments with HNO3 and mixture of HNO3 and H2SO4 (1
3 by volume).92 The sample was ultrasonicated using ultrasonic bath (Selecta Ultrasons-H model, which has a nominal frequency of 40 kHz with power of 950 W). The preset time of sonication varied from 2, 4, and 6 hours. The result showed that the treatments with HNO3 and mixture of HNO3 and H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by volume), which were applied for a short period of time, did not show significant effect on the side walls of MWCNTs. An additional peak was observed at 1200 cm−1 in the FTIR spectrum when the MWCNTs were treated with the acid mixture under ultrasonic vibration for 2 h, but their UV/Vis spectrum did not change significantly. These results indicate that new C–O groups appeared in the open ends of the nanotubes without modifying the structure of the sidewalls. Longer reaction time with the acid mixture treatment began to destroy the sidewalls of the nanotubes, as showed by the UV/Vis spectra. Finally, a longer treatment time of 6 hours completed the destruction of the nanotube sidewalls and shorter nanotubes were observed under atomic force microscopy (AFM), as shown in Fig. 7(a–e). The UV/Vis spectra of all samples are shown in Fig. 7(f).
3 by volume), which were applied for a short period of time, did not show significant effect on the side walls of MWCNTs. An additional peak was observed at 1200 cm−1 in the FTIR spectrum when the MWCNTs were treated with the acid mixture under ultrasonic vibration for 2 h, but their UV/Vis spectrum did not change significantly. These results indicate that new C–O groups appeared in the open ends of the nanotubes without modifying the structure of the sidewalls. Longer reaction time with the acid mixture treatment began to destroy the sidewalls of the nanotubes, as showed by the UV/Vis spectra. Finally, a longer treatment time of 6 hours completed the destruction of the nanotube sidewalls and shorter nanotubes were observed under atomic force microscopy (AFM), as shown in Fig. 7(a–e). The UV/Vis spectra of all samples are shown in Fig. 7(f).
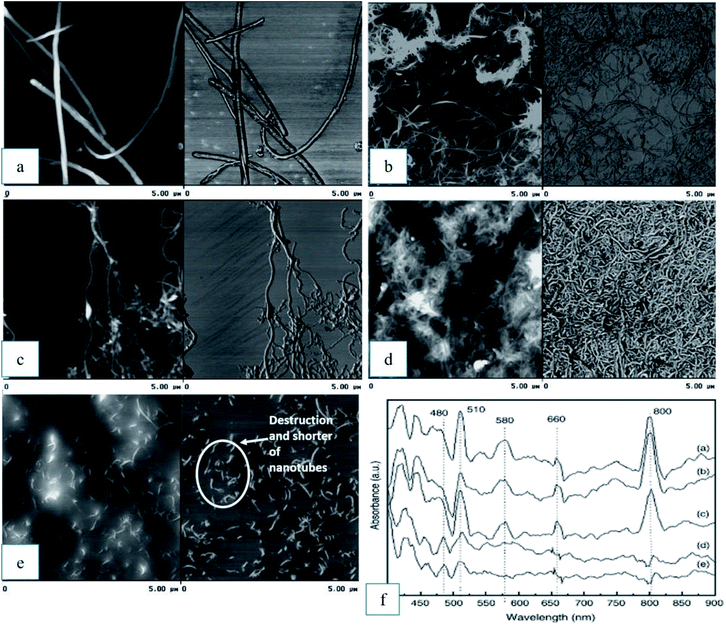 | ||
| Fig. 7 AFM image (a) as-received MWCNTs and the effect of the oxidation process after: (b) HNO3 treatment; (c) HNO3/H2SO4 2 hour, (d) HNO3/H2SO4 4 hour, (e) HNO3/H2SO4 6 hour, and (f) UV/Vis spectra for sample (a) to (e). Reproduced with permission from ref. 92, copyright 2011, Diamond and Related Materials. | ||
Chen et al. (1998) showed that the oxidation process for SWCNTs involved extensive ultrasonic treatment in a mixture of HNO3/H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by volume) that led to the opening of the nanotube caps and the formation of holes in the sidewalls. The final products were fragments of nanotube with lengths of 100 nm to 300 nm, while the ends and sidewalls were decorated with a high density of various oxygen containing groups (mainly carboxyl groups). However, under less vigorous conditions, such as refluxing in nitric acid, the shortening of the nanotubes can be minimised. Chemical modification was limited mostly to the opening of the nanotube caps and to the formation of functional groups at defect sites along the sidewalls.93 Therefore, nanotubes functionalised in this manner basically retain their pristine electronic and mechanical properties.94 As a result of the presence of fullerene-like end-caps which are sensitive to oxidation, the oxidation of CNTs may be readily performed to create oxidised CNTs.95,96
3 by volume) that led to the opening of the nanotube caps and the formation of holes in the sidewalls. The final products were fragments of nanotube with lengths of 100 nm to 300 nm, while the ends and sidewalls were decorated with a high density of various oxygen containing groups (mainly carboxyl groups). However, under less vigorous conditions, such as refluxing in nitric acid, the shortening of the nanotubes can be minimised. Chemical modification was limited mostly to the opening of the nanotube caps and to the formation of functional groups at defect sites along the sidewalls.93 Therefore, nanotubes functionalised in this manner basically retain their pristine electronic and mechanical properties.94 As a result of the presence of fullerene-like end-caps which are sensitive to oxidation, the oxidation of CNTs may be readily performed to create oxidised CNTs.95,96
High chemical reactivity, such as fluorination of CNTs, has become popular for initial investigation of covalent functionalisation because the CNTs sidewalls were expected to be inert.97,98 The fluorinated CNTs have C–F bonds that are weaker than those in alkyl fluorides,99 thus providing substitution sites for additional functionalisation.100 Besides sidewall fluorination of CNTs, other similar methods including cycloaddition, such as Diels–Alder reaction, carbene and nitrene addition,101,102 chlorination,103 bromination,104 hydrogenation,105 azomethine ylides,106 have also been successfully functionalised and employed. All these methods can be regarded as derivatives of sidewall functionalisation of CNTs.
Furthermore, Ma et al. (2010) classified the functionalised CNT through carboxylation as defect functionalisation (Fig. 8).65 This type of functionalisation has been discussed in Section 2. Referring to Fig. 8B, this process takes advantage of chemical transformation of defect sites on CNTs. Defect sites can be the open ends and/or holes in the sidewalls, pentagon or heptagon irregularities in the hexagon graphene framework. Oxygenated sites can also be considered as defects. Defects can be created on the sidewalls and at the open ends of CNTs by an oxidative process with strong acids such as HNO3, H2SO4, or a mixture of them,107 or with strong oxidants such as KMnO4,108 ozonolysis,109 and reactive plasma.110 The defects on CNTs created by oxidants are stabilised by bonding with carboxylic acid (–COOH) or hydroxyl (–OH) groups. Next, these functional groups have rich chemistry, thus the CNTs can be used as precursors for further chemical reactions, such as silanation,111,112 polymer grafting,113 esterification,114 thiolation,115 alkylation and arylation,116,117 and even can be tagged with metal coordination compounds.118 CNTs functionalised in this way are soluble in many organic solvents because the nature of CNTs is changed from hydrophobic to hydrophilic due to the attachment of polar groups. Chan et al. functionalised SWCNT with zwitterionic functional groups.119 The functionalisation comprised of two steps. In the first step, SWCNT–COOH was reacted with thionyl chloride (SOCl2) to produce acetylated SWCNT. In the second step, the acetylated SWCNT underwent esterification using 3-dimethylamino-1-propanol, (CH3)2–N–CC3H6–OH. As a result, the functionalised SWCNT has attached positive (tertiary amine group) and negative charges (carboxylated group).
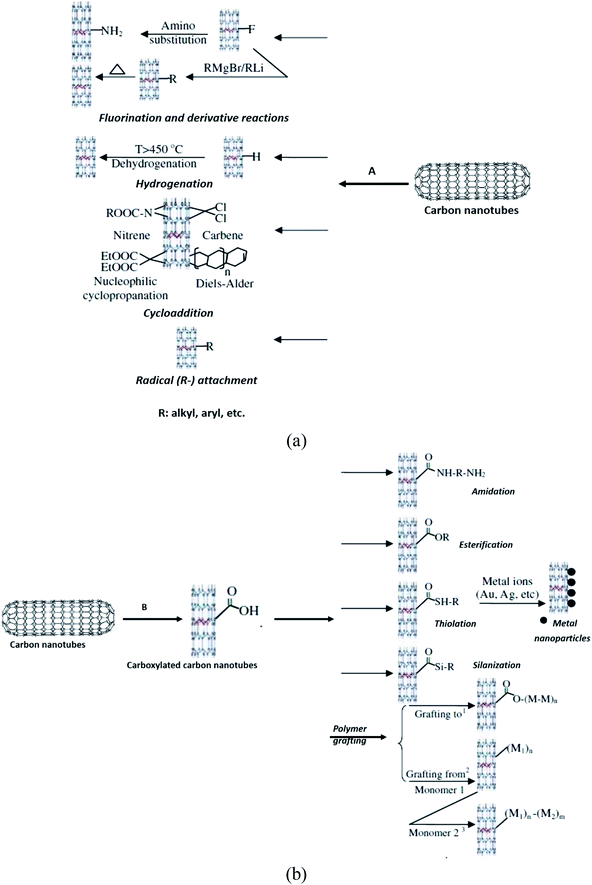 | ||
| Fig. 8 The route of (a) covalent functionalisation of CNTs (A: direct sidewall functionalisation) and (b) (B: defect functionalisation). Reproduced with permission from ref. 65, copyright 2011, Diamond and Related Materials. | ||
Besides the attachment of carboxylic, hydroxyl, polymer, and other hydrophilic groups, attachment of inorganic compounds such as TiO2 and Fe3O4 on CNTs have been reported for electromagnetic and microwave absorption purposes.120,121 Vatanpour et al. (2012) prepared MWCNT–TiO2 via precipitation of TiCl4 precursor on acid-treated MWCNT.122 The acid treated MWCNT was functionalised with monohydrate citric acid. The synthesis of MWCNT–poly citric was aimed to get a better deposition of TiO2 on the CNT. Next, TiO2 nanoparticles were coated on MWCNT–poly citric acid by dispersing both functionalised CNT and TiCl4 (as TiO2 precursor) into a 1 M HCl solution at ambient temperature. Poly citric acid was then removed and the TiO2 deposit was produced as the end-product. Based on Wang et al. (2016), MWCNT/Fe3O4 was prepared using a hydro-thermal method.123 Prior to Fe3O4 attachment, the CNT was acid-treated with an acid mixture of H2SO4/HNO3 (3/1) for 4 h at 65 °C. The acid treated MWCNT was then mixed with FeCl3 and FeSO4 (molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and then ultrasonicated. The mixture was then stirred at 50 °C for 0.5 h and further stirred at 65 °C for 1 h at pH 12. The MWCNT/Fe3O4 was obtained as precipitate and the hybrids improved the hydrophilicity of membrane surface, applied in trapping pollutants in membrane filtration.
1), and then ultrasonicated. The mixture was then stirred at 50 °C for 0.5 h and further stirred at 65 °C for 1 h at pH 12. The MWCNT/Fe3O4 was obtained as precipitate and the hybrids improved the hydrophilicity of membrane surface, applied in trapping pollutants in membrane filtration.
The covalent functionalisation was widely used in CNTs application via acid treatment, carboxylation and fluorination methods. Based on the aforementioned studies, the covalent modification towards CNT composites produced high stability functionalisation. This mechanism led to efficient load transfer from the polymer/CNT matrix through covalent bonding. In addition, the covalent functionalisation severely altered the inherent properties of the CNTs; changed the electrical conductivity and thermal properties, and in some cases, shortened the length of nanotubes. Some CNT applications are incompatible with covalent modification, thus non-covalent functionalisation is preferred.
3. Non-covalent functionalisation of CNTs
Non-covalent functionalisation is a synonym interaction between CNTs and the interest conductive polymer. Referring to Bose et al. (2010), non-covalent functionalisation is an efficient alternative to tailor the CNT and polymer interface and yet preserving the reliability of the tubes.53 This route is particularly attractive because of the possibility of adsorbing various groups of ordered architectures on the CNT surface without disturbing the extended p-conjugation of the nanotubes. In the last few years, non-covalent surface treatment has received lots of attention and various strategies have been proposed for the debundling of CNTs in the presence of a modifier. These approaches have been addressed in connection with surface coating/wrapping of low molecular weight surfactants (anionic/cationic),124,125 polymers,126 liquid crystalline p-conjugated oligomers,127 and amphiphilic cationic polymer molecules.128Non-covalent functionalisation is realised via enthalpy-driven interactions, such as π–π, CH–π, and NH–π, between the CNT surface and the dispersants and/or entropy-driven interaction; i.e. hydrophobic interaction using surfactants.129 In the case of the surfactant dispersion, sodium dodecyl sulfate (SDS),130 sodium dodecyl benzene sulfonate (SDBS),131 sodium cholate (SC),132 cethyltrimethylammonium bromide (CTAB),133 Brij, Tween, and Triton X134 have typically been used due to their availability and low cost.135 The non-covalently surrounded polymers remained even after the washing process, such as filtration, to provide ‘polymer-wrapped CNTs’. In some applications, such wrapped dispersants act as a contaminant but in some cases, the wrapped CNTs synergistically improve the performance of the CNTs if the polymers are strategically designed and positioned.
From numerous analytical models, it is quite evident that the percolation threshold has an intimate relationship with L/D of CNTs. Therefore, it can be stated that any types of pre-treatments that affect L/D of CNT would eventually influence the percolation thresholds in the composites. The ‘effective L/D’ (of disentangled CNT) seems to be important in governing the percolation thresholds in the composites and the can be manipulated by various pre-treatments. The chemical functionalisation of CNTs adversely affects effective L/D due to the involvement of severe chemical conditions. In contrast, non-covalent routes enable significant exfoliation of CNTs mediated by the local environment of various modifiers and increase the effective L/D. Different pre-treatments involve different levels of interactions between molecules and the matrix, and greatly influence charge transport mechanisms in the composites.53 Fig. 9 shows a schematic diagram of the non-covalent functionalisation routes of polymer towards CNTs.129,136
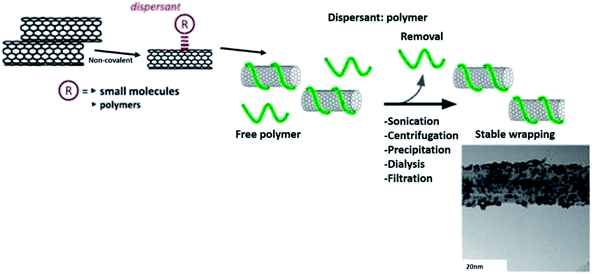 | ||
| Fig. 9 Non-covalent functionalisation routes. Adapted from ref. 129, copyright 2015, Elsevier. And adapted from ref. 136, copyright 2015, Elsevier. | ||
Due to the tailorable design of the polymers and the improvement of composites inherent properties, the concept of non-covalent functionalisation has recently been widely utilised and renowned. Compared to covalent functionalisation, this modification produces lower stability of functionalisation and ineffective polymer reinforcement due to the absence of covalent bonding between CNTs and polymer. Yet, non-covalent modification does not destroy the conjugated system of the CNTs sidewalls and end-cap. Therefore, it does not affect the final structural properties of the material. Next, conjugated polymers wrapped meritoriously via non-covalent functionalisation of CNTs are due to π–π stacking and van der Waals interactions between the polymer chains comprising of aromatic rings and the exteriors of CNTs. Non-covalent functionalisation is an alternative method of improving the interfacial properties of nanotubes.
4. Fabrication method of CNTs/nanocomposite
A variety of synthesisation methods have been reported in order to incorporate CNTs into various polymeric interests. The main motive is to prevent the agglomeration of CNTs and realise their uniformity dispersion inside polymer matrix. There is no single method to achieve perfect dispersion of different CNTs in different types of polymer matrices, which is universally applicable to all situations. Many factors need to be considered when selecting a proper technique for CNT dispersion, such as physical states of the polymer (solid or liquid) and chemical (thermoplastic or thermoset), dimensions and content of CNTs to be introduced, availability of techniques and fabrication processes, ease of its synthesis from suitable monomer, desired performance indices of composites and cost constraints, and the parameter of the solvent used. This section briefly describes the important processing methods for synthesis of CNT-based polymer nanocomposites.4.1 Solution mixing
Solution mixing is the most common method for the fabrication of CNT/polymer nanocomposites because it is amenable to small sample sizes, and is one of the simplest fabrication methods for surface coating or making thin films.137 Typically, solution mixing involves three major steps: dispersion of CNTs in a suitable solvent by mechanical mixing, magnetic agitation or sonication. The solvent can also dissolve polymer resins. Subsequently, the dispersed CNTs are mixed with polymer matrix at room or elevated temperatures. The nanocomposite is finally obtained by precipitating or casting the mixture. However, an often encountered limitation in solution mixing is the slow evaporation of solvent that provides sufficient time for CNTs re-agglomeration and differential settling, resulting in homogenous CNTs dispersion in matrix (e.g. CNTs content is lowest at the casted film/sheet's surface, and shows a uniform/random gradient across the thickness and maximum at both surfaces due to the extensive tube settling) and observation of non-uniform and inferior properties.138 The solvent evaporation rate-related limitations can be resolved by gently pouring CNT/polymer nanocomposite dispersion on a rotating substrate (spin coating)139 or over a heated substrate (drop-casting).140 However, use of spin coating is limited only to thin films (few nanometres thick) which cannot be peeled off from the substrate, whereas drop casting has issues in terms of uniform drying across thickness and high possibility of void formation. Another versatile method exploits coagulation141 of CNT/polymer dispersion by pouring into an excess of non-solvent, thereby achieving rapid precipitation of polymer chains which immediately entrap CNTs (without providing sufficient time for CNTs diffusion and settling). Nevertheless, solution processing is still widely used and is one of the important steps in the processing of thermosetting matrices-based nanocomposites.4.2 Melt blending
Melt blending is another commonly used method to fabricate CNT/thermoplastic polymer nanocomposites. Thermoplastic polymers, such as polyethylene,142 polypropylene,143 polystyrene,144 and other thermoplastic polymer can be processed as matrix materials in this method. The major advantage of this method is that no solvent is employed to disperse CNTs. Melt blending uses high temperature and high shear force to disperse CNTs in a polymer matrix and is most compatible with current industrial practices. Special equipment, such as mini-extruder and injection machine, which are capable of being operated at an elevated temperature and generate high shear forces, are employed to disperse CNTs. Melt blending or variants of this technique are frequently used to produce CNT/polymer composite fibres. Compared with the solution mixing methods, this technique is generally considered less effective to disperse CNTs in polymers, and its application is also limited to low filler concentrations in thermoplastic matrices.1454.3 In situ polymerisation
In situ polymerisation is an efficient method to obtain uniform dispersion of CNTs in a thermosetting polymer (Fig. 10). In this method, CNTs are mixed with monomers, either in the presence or absence of a solvent, and then these monomers are polymerised via addition or condensation reactions with a hardener or curing agents at an elevated temperature. One of the major advantages of this method is that covalent bonding can be formed between the functionalised CNTs and polymer matrix, resulting in much improved mechanical properties of composites through strong interfacial bonds. This method often gives better filler dispersion, especially at higher filler contents than melt mixing, and in the case of in situ polymerisation, it is possible to separate aggregated nanotubes with low energy input in a solvent with low viscosity like toluene in pre-treatment.146 This approach uses a wet chemical online-filled method to prepare ferrite-filled MWCNTs and in situ chemical synthesis of chitosan-decorated ferrite-filled MWCNTs/polythiophene composites. The decoration of chitosan onto the surface of the ferrite-filled MWCNTs improves the dispersion of the ferrite-filled MWCNTs in the matrix of polythiophene and reduces the agglomeration of the ferrite-filled MWCNTs. Furthermore, this method is applicable for the preparation of other MWCNT–magnetic composites such as surfactant-decorated MxFe2−xO4-filled MWCNTs/conductive polymer composites for use in the electromagnetic devices.147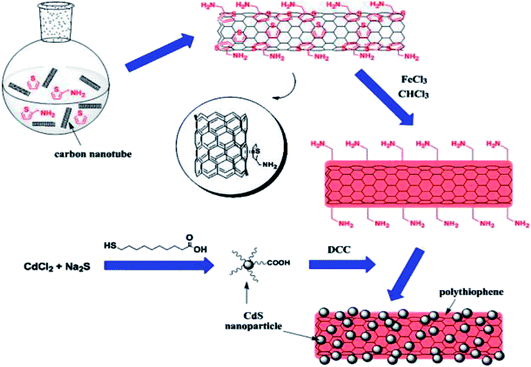 | ||
| Fig. 10 Schematic diagram of decorating MWCNTs with CdS NPs with in situ polymerised PTh acting as an inter-linker. Reproduced with permission from ref. 148, copyright 2015, Elsevier. | ||
4.4 Latex technology
A relatively new approach to incorporate CNTs into a polymer matrix is based on the use of latex technology. Latex is a colloidal dispersion of discrete polymer particles, usually in an aqueous medium. By using this technology, it is possible to disperse single and multi-walled CNTs in most polymers that are produced by emulsion polymerisation, or that can be brought into the form of an emulsion. Contrary to the in situ polymerisation system, the addition of CNTs in this technique takes place after the polymer has been synthesised. The first step of the process consists of exfoliation (for SWCNT bundles) or dispersion/stabilisation (for MWCNT entanglements) of CNTs in an aqueous surfactant solution. This is followed by mixing the stable dispersion of surfactant-treated CNTs with polymer latex. After freeze-drying and subsequent melt-processing, a nanocomposite consisting of dispersed CNTs in a polymer matrix is obtained. The advantages of this technique are obvious; the whole process is easy (because it basically consists of a simple mixing of two aqueous components), versatile, reproducible, and reliable, and allows incorporation of individual CNTs into a highly viscous polymer matrix. The solvent used for CNT dispersion is water, thus the process is safe, environmentally friendly, and low-cost. Nowadays, polymer latex is industrially produced in a large scale. Fig. 11 shows the preparation of MWCNTs efficiently dispersed in natural rubber (NR)-latex with the aid of hyper branched tri-chain sulphosuccinate anionic surfactants, specifically sodium 1,4-bis(neopentyloxy)-3-(neopentyloxycarbonyl)-1,4-dioxobutane-2-sulphonate (TC14). The results revealed that the introduction of a third chain with terminal methyl groups on the surfactant chains profoundly influences the homogenisation of MWCNTs in NR-latex matrices. Interestingly, the results are consistent with the results of surface tension studies,149 where the introduction of a third chain with a highly methylated group lowers the surface energy, resulting in efficient partitioning at the MWCNTs/NR-latex interface.150 Table 2 tabulates the summary of the fabrication methods of CNT/nanocomposites.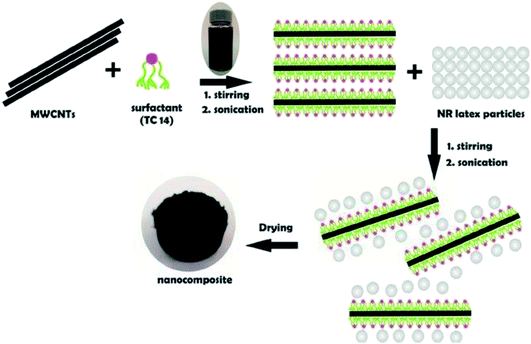 | ||
| Fig. 11 Schematic diagram of the latex technology of MWCNTs dispersed in NR latex. Reproduced with permission from ref. 150, copyright 2015, Elsevier. | ||
| Specification | Solution mixing | Melt binding | In situ polymerisation | Latex technology |
|---|---|---|---|---|
| Mechanism | (1) Dispersion of CNTs in solvent (magnetic stirring, reflux, and ultrasonication), (2) mixing with polymer, (3) recovery of the nanocomposites (casting a film) | Involves the melting of polymer pellets into viscous liquid with high shear forces application | Involves mixing of nanofiller with monomers in a solvent, followed by in situ polymerisation | The CNT dispersion is mixed with a given polymer latex to form homogenous CNT/latex dispersion |
| Advantages | (1) Wider applicability, (2) better dispersion, (3) rigorous mixing in solvent | (4) Wide applicability, (5) good dispersion, (6) low cost, (7) simplicity to facilitate large scale production | (8) Widest applicability, (9) best dispersion, (10) enables grafting of polymer macromolecules onto the wall of CNTs, (11) allows preparation of nanocomposites with high CNT loading, (12) very good miscibility with polymer matrix | (14) Possible in disperse CNTs in polymers produced by emulsion polymerisation, (15) facile process, (16) reproducible, and reliable, (17) allows incorporation of individual CNTs into a highly viscous polymer matrix |
| Disadvantages | Slow evaporation lead to CNTs aggregation, (1) inapplicable for industrial scale processes, (2) low stability, (3) Residual solution | (4) High shear force and temperature can deteriorate nanocomposite and polymer intrinsic properties, (5) poor dispersion, (6) large residual stress, (7) low interfacial bonding strength | (9) Involve complex procedures and processing steps, (10) requires expensive reactants, (11) residual monomer, (12) large residual stress, (13) matrix strength decline | (14) Mechanical properties of the material were not significantly improved |
4.5 Other methods
To obtain CNT/polymer nanocomposites with very high CNT content or for some specific applications, new methods have been developed in recent years. The new methods include densification, sol–gel method, spinning of coagulant, layer-by-layer deposition, and pulverisation.5. Functionalised CNTs in chemical sensor
Chemical sensors are attracting tremendous interest because of their widespread applications in industry, environmental monitoring, space exploration, biomedicine, and pharmaceuticals. Gas sensors with high sensitivity and selectivity are required for leakage detections of explosive gases such as hydrogen, and for real-time detections of toxic or pathogenic gases in industries. There is also a strong demand for the ability to monitor and control the environment, especially with the increasing concerns on global warming.151–153 There are several types of sensors intended for various applications that include electrochemical sensors, biosensors, surface acoustic wave sensors, immunosensors, and chemiresistive sensors. However, a leading candidate is the chemiresistor, which is chemical sensor based on the simple change in resistance in response to the binding of analytes. Advantages of chemiresistors include low power consumption and the ease of high precision resistance measurements.154This review section discusses the application of functionalised CNTs in chemiresistive sensors. Chemiresistive sensor is one of the best transduction units, attributing to its simplicity, rapid response, and low-cost procurement. These are also the reasons why most commercialised gas sensors are fabricated into chemiresistive sensors.155 A chemiresistive sensor translates chemical information via the changing in two-point contact electrical resistance.156 Electrical resistance is a simple electrical signal to be analysed and requires only minimum supportive electronics for building deployable, compact, and self-contained systems. For the case of conducting polymer, this operation mode is greatly alterable upon nano-structuring. The resulting sensors exploit composition and structure dependent charge transport as well as adsorption, in order to fine-tune the gas sensor performance.157 In chemiresistive sensors, an active layer is usually deposited over an array of electrodes to measure the electrical resistance change in the presence of target analytes. The primary charge carrier and type of gas interacting with the active layer induce the change of sensor's resistance upon gas exposure.158,159
Several materials have been utilised and added as fillers in gas sensors, including metal oxides, organic semiconductors, and carbon nanotubes.160 Metal oxides are the most widely used materials for chemiresistors.161 Even with their sensitivity, the applications of these materials have been limited by high power consumption and poor selectivity. Organic semiconductors, especially conjugated polymers, have long been considered as chemiresistor materials.162 The integration of molecular recognition into their structures is attractive. However, these materials are limited by electrostatic/dielectric interferences and fragile organic–metal interfaces. CNT field effect transistors have been studied as chemical and biological sensors.163–165 These devices are sensitive because their resistance can change drastically in the presence of analytes via charge transfer (doping), carrier pinning, and/or modification of the Schottky barrier at the nanotube/metal contact. Among existing sensor materials of metal oxides, organic semiconductors, and CNTs, the latter are particularly intriguing because of its unique properties such as large surface area, good environment stability, and excellent mechanical properties.166,167
Theoretically, the carbon atoms in a carbon nanotube are surface atoms, which makes them optimally suited for components of chemical sensors. Hence, it is not surprising that gas sensors made from individual nanotubes show good sensitivity and exhibit fast response and substantially higher sensitivity than that of existing solid-state sensors at room temperature upon the exposure to gaseous molecules such as NO2 or NH3,168,169 in comparison to commercially available classical semiconductor sensors, which in general operate above 200 °C.170 However, a necessary prerequisite is that the molecules to be detected must have a distinct electron donating or accepting ability, for example, NH3 as a donor and NO2 as an acceptor.
It has been reported that CNTs are very sensitive to the surrounding environment. The presence of O2, NH3, NO2 gases and many other molecules can either donate or accept electrons, resulting in an alteration of the overall conductivity.171,172 This is because the adsorption of these molecules on the nanotubes is associated with a partial charge transfer, which alters the charge-carrier concentration, or alternatively, the adsorbed molecules may affect the potential barriers present at the tube-electrode contacts. In any circumstance, the resulting change in the electrical resistance of the individual nanotube is utilised as a sensor signal. However, for the detection of molecules that are only weakly adsorbed (e.g., CO and H), the change in resistance is often too small. A possible method to overcome this drawback is by the functionalisation of the side wall of CNT with a conductive polymer.
Since the most common gas sensing principle is the adsorption and desorption of gas molecules on sensing materials, it is quite understandable that by increasing the contact interfaces between the analytes and sensing materials, the sensitivity can be significantly enhanced. Recent development in nanotechnology has created huge potential to build highly sensitive, low cost, portable sensors with low power consumption. The extremely high surface-to-volume ratio and hollow structure of nanomaterials is ideal for adsorption and storage of gas molecules. Therefore, gas sensors based on nanomaterials with conductive polymer, such as CNTs, nanowires, nanofibers, and nanoparticles, have been investigated widely.173–175 Fig. 12 shows the schematic diagram of chemically conductive polymer-functionalised MWCNTs, which contain COOH groups attached along the sidewall of the MWCNTs and (b) the proposed mechanism for ethanol (alcohol) vapour detection using MWCNTs–OOH sensors, where the COOH groups tend to react with the ethanol molecules at room temperature.176,177
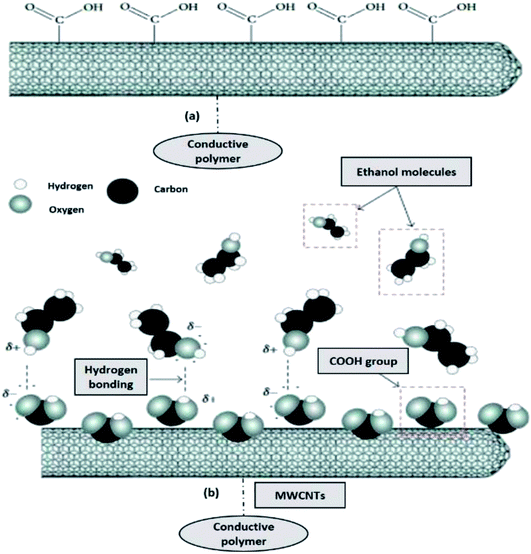 | ||
| Fig. 12 (a) Schematic diagram of carboxylated functionalised of MWCNTs, and (b) proposed mechanism for ethanol (alcohol) vapour detection using conductive polymer–MWCNTs–OOH sensors. Adapted from ref. 176, copyright 2015, Elsevier. | ||
Many different types of organic materials have been used for gas sensing. The simplest organic compounds that can be electrically conductive are polymers, based on carbon and hydrogen.178 Organic conducting polymers including polypyrrole (PPy),179 polyaniline (PANI),180 polythiophene (PTh),181 poly(3,4-ethylenedioxythiophene) (PEDOT),182 and polyacetylene (PA)183 are examples of materials for fabricating gas sensors. Organic polymers are one of the principal materials applied in gas sensing systems. Some conducting polymers can behave like semiconductors due to their heterocyclic compounds which display physicochemical characteristics. As a result, reversible changes in the sensing layer's conductivity can be detected upon polar chemicals' adsorption on the surfaces at room temperature.184 This effect is believed to be caused by the charge transfer between gas molecules and the polymer or the polymer film's swelling.185 This sensing response has intensively increased motivation to develop high sensitive and selective chemical sensors by tailoring the compounds of different organic polymers with functionalised CNTs.
Due to adsorption of interested analytes, there are volumetric changes of the matrix polymer. This leads to a distinct change in percolation-type conductivity around a critical composition of the material, which is known as “percolation threshold”. Generally, the percolation threshold is dependent on the shape of the conducting particle. Conductive polymer consisting of particles with higher aspect ratio shows lower threshold and higher sensitivity.186 CNTs, with almost one-dimensional thread-like structure and good conductivity, are ideal as the dispersed particles in this conducting particles–insulating matrix composition for gas sensing systems. Therefore, CNTs/conductive polymer have been intensively studied for gas sensors.187–190
The nanocomposite of PPy and carboxylated multi-walled carbon nanotubes (MWCNT–COOH) was synthesised by in situ chemical oxidative polymerisation method using HCl as a dopant and ammonium persulphate (APS) as an oxidant for the detection of NH3.191 The synergistic effects of the PPy-coated MWCNTs showed that the most sensitive PPy/MWCNT nanocomposites sensor towards NH3 gas was obtained at 4 wt% MWCNT content and found to be stable in operation against the variation in operating temperature and humidity (Fig. 13). The increase in sensitivity with increase in MWCNT content is attributed to the increase in surface area of the composite material, providing more active sites for adsorption of NH3 gas molecules. Thus increase in sensitivity and further increase in MWCNT content leads the nanocomposite electrically shorted, thereby increasing the percolation effect by highly conductive carbon nanotubes. Besides, increased sensitivity from 3.07% to 17.11% over 200 to 2000 ppm of NH3 concentration is because the sensitivity of sensor depends on the removal of adsorbed oxygen molecules by reaction of target analyte and generation of electrons. For smaller gas concentration exposed on the fixed surface area of sensor, lower surface reaction occurred due to lower exposure of analyte. On the other hand, an increase in gas concentration raises the surface reaction due to larger surface exposure. Fig. 14 shows the comparison of gas sensing sensitivity of PPy/MWCNT (4 wt%) sensor towards 2000 ppm NH3 with other analytes.
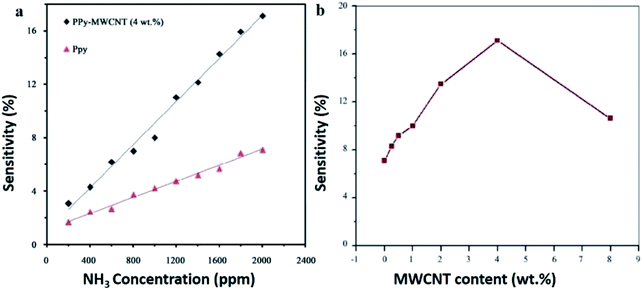 | ||
| Fig. 13 Sensitivity of PPy/MWCNT with (a) different NH3 concentration and (b) different MWCNT loadings towards the NH3 at 2000 ppm. Reproduced from ref. 191, copyright 2015, Elsevier. | ||
 | ||
| Fig. 14 Gas sensing sensitivity of PPy/MWCNT (4 wt%) sensor towards 2000 ppm of NH3. Reproduced from ref. 191, copyright 2015, Elsevier. | ||
Low-cost, conductive Pap@CNT–NH2@PPy (conductive paper strip) composites were prepared through sonochemical polymerisation of pyrrole in the presence of oxidising agent and tosylate co-dopant (TS) on cellulosic paper strips decorated with aminophenyl-modified (MWCNT–NH2).175 The Pap@CNT–NH2@PPy end materials served as chemiresistive NH3 sensors exhibited an outstanding response of 525% to 0.1 ppm level of NH3 at room temperature with good stability for a long period of time and remained the same at different times, resulting in highly reproducible sensing characteristics. Comparative gas sensing properties analysis of the nanocomposite-based gas sensors synthesised with different ratio molar CNT/amine revealed excellent sensor performance for Pap@CNT–N1/1@PPy nanocomposite in the concentration range of 0.005 to 0.05 ppm of NH3 with high sensitivity and low LOD of 0.04 ppb. An increase in the electrical resistance of CNT@PPy sensor was observed when it was exposed to NH3 gas. This attributed to the charge transfer mechanism between NH3 and CNT@PPy surface. As a matter of fact, upon the interaction of NH3 with PPy, the polymer loses its electron and this electron transfer between PPy positive holes and NH3 causes a depression in the charge-carrier concentration resulting in decreased overall conductivity. However, in air, the reverse reaction that NH4+ ion decomposes into ammonia takes place and changes the conductivity of PPy to higher values. The mechanism of the PPy–NH3 interaction is as follow:
| PPyH + NH3 → PPy + NH4+ |
Philip et al. (2003) fabricated a nanocomposite thin film of polymethylmethacrylate (PMMA) with MWCNTs and oxidation-modified MWCNTs (f-MCNTs) for gas sensing purposes.192 The resistance changes of both nanocomposites were evaluated upon exposure to dichloromethane, chloroform, and acetone. Both the CNT/PMMA and the f-CNT/PMMA nanocomposites showed increasing resistance upon exposure to these vapours at room temperature. Table 3 indicates that the different nanocomposites have different responsiveness (S) or sensitivity towards the applied vapours. This behaviour was explained on the basis of volume expansion and polar interaction of the CNT surface with vapour molecules. The f-CNT/PMMA showed significant improvement on the sensor's behaviour including in sensitivity and the response time and recovery, as shown in Fig. 15. This can be explained by the effects of oxidation on the electronic properties of CNTs. In non-functionalised CNT/PMMA composites, the dispersion of CNTs in the polymer matrix is not uniform. This means that there is only a small volume through which the nanotubes form a conducting path through the polymer matrix so that the increase in resistance due to swelling of the PMMA matrix is less. The responsiveness of the nanocomposites can be determined from equation
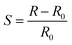 | (1) |
| Vapours | Responsiveness (S) | |
|---|---|---|
| CNT/PMMA | f-CNT/PMMA | |
| DCM | 9.94 | 809 |
| Chloroform | 7.57 | 407 |
| Acetone | 6.35 | 84 |
| Methanol | 4.29 | 45 |
| Ethyl acetate | 2.23 | 30 |
| Toluene | 2.24 | 1.04 |
 | ||
| Fig. 15 (a) Response of the CNT/PMMA composite to analytes and sensor response, and (b) response of the f-CNT/PMMA composite to analytes and sensor response towards dichloromethane, chloroform and acetone vapours. Reproduced from ref. 194, copyright 2015, Elsevier. | ||
The oxidation functionalisation of nanotubes built COOH and OH groups along the sidewall and the caps of the CNTs.193 The created functional groups can physically interact through the PMMA, resulting in strong interfacial adhesion and better dispersion of f-CNTs in the polymer matrix. The volume of the conducting channel through the PMMA matrix is large so that a small swelling of the matrix can induce a large increase in resistance. This explains the large increase in response for f-CNT composite when exposed to dichloromethane, chloroform, and acetone, which can swell the polymer matrix to a large extent. The polar groups on the nanotube surface also increased by adsorption of solvent molecules and give a better response. This is proved by the fact that polar solvents like methanol showed an improved response to f-CNT/PMMA composites even though they are not good solvents for PMMA.
Inspired by the enhanced gas-sensing performance of the one-dimensional hierarchical structure, one-dimensional hierarchical PANi/MWCNT were prepared.195 The p-type PANI/MWCNT (p-PANI/MWCNT) and n-type PANI/MWCNT (n-PANI/MWCNT) showed higher sensitivity towards NO2 and NH3, respectively, where the response times of p-PANI/CNT and n-PANI/CNT to 50 ppm of NO2 and NH3 were only 5.2 and 1.8 s, respectively. The estimated limit of detections (LOD) for NO2 and NH3 was as low as 16.7 and 6.4 ppb, respectively. After three months, the responses of p-PANI/CNT and n-PANI/CNT decreased by 19.1% and 11.3%, respectively. It was found that the one-dimensional hierarchical structures and deeper charge depletion layer enhanced by structural changes of PANI contributed to the sensitive and fast responses to NO2 and NH3. This work also looks forward to the development prospects of cost effective and high-performance PANI/MWCNT-based sensors in the potentials of interface engineering in improving gas-sensing performance.
In addition, Zhang et al.195 (2020) suggested that the high and fast response of the nanocomposites should be attributed to the following three factors; (1) for the hierarchical p-PANI/MWCNT, a large number of micropores exist in the conductive networks of hierarchical p-PANI/MWCNT arranged in disorder and the hierarchical structure provides a large number of passageways, which provide sufficient and fast channels for the diffusion. It is well known that gas-sensing process occurs mainly on the surface of sensing materials. Therefore, the effective exposure of sensing materials to the gas molecules largely determines the sensor's sensing performance. The high permeability of hierarchical p-PANI/MWCNT allows target gas molecules to rapidly contact with PANI fibres by rapid diffusion through the channels, hence shortening the response and recovery time, and enhancing the sensitivity. (2) Compared with PANI, MWCNTs demonstrate higher carrier mobility. Therefore, the carrier mobilities of PANI can be reinforced by constructing core–shell PANI/MWCNTs composites. The conjugated interfaces between PANI and MWCNTs provide the percolation path with higher carrier mobility. Furthermore, when the target gas (NO2) is in contact with the p-PANI/MWCNTs, the process of charge transfers between the target gas and the hierarchical p-PANI/MWCNTs is accelerated due to the enhanced carrier mobility, i.e., the response and recovery time are also shortened. (3) Due to electron-rich amino groups of PANI. For electron-deficient NO2, these electron-rich amino groups of PANI act as baits to induce the oxidisation of NO2 to be adsorbed on the surface of PANI, enhancing the sensitivity. Due to the uniform core–shell structure of n-PANI/MWCNT, many p–n heterojunctions are formed at the interface between n-type PANI and p-type MWCNTs. Therefore, the n-PANI/MWCNT not only have the above advantages of p-PANI/MWCNT, but also have a unique p–n heterojunction structure. The combination of electrons in n-type PANI and holes in p-type MWCNTs at the interface result in lower carrier concentration, enhancing the sensitivity of n-PANI/MWCNT.196,197
Another study on NH3 detection was done by Xue et al. (2017) using PANI/MWCNT.198 The nanocomposites showed high sensitivity towards NH3 from 200 ppb to 50 ppm, fast response and recovery time with 85 s and 20 s, respectively at room-temperature operation without external aid, reliable flexibility and excellent selectivity to NH3 compared to other volatile organic compounds (Fig. 16). The excellent sensing performance is probably ascribed to the synergetic effects of PANI and MWCNT, the high surface area (54.187 m2 g−1) of nanocomposite films, and effective network sensing channels. The film with simple preparation, high sensitivity, small size, and robust flexibility, could be implanted into electronic devices for potentially monitoring NH3 in anaerobic digestion in real-time for the high efficiency and better stability of anaerobic digestion for renewable energy sources. In short, electrons provided from the adsorbed NH3 molecules onto PANI transfer easily from PANI to MWCNT because of the lower conductivity of PANI and lower energy barrier between PANI and carbon nanomaterials, about 101 meV. Then, the electrons effectively transfer from MWCNT to the PANI fibre due to lower energy barrier and finally to the electrodes. Therefore, the sensing response was effectively improved in PANI/MWCNT nanocomposite network.
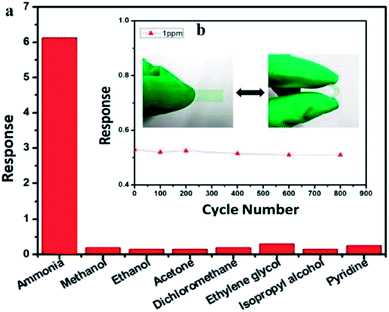 | ||
| Fig. 16 (a) Selectively sensing response to 10 ppm of NH3 and other analytes for the PANI/MWCNT nanocomposite film and (b) gas sensing response of the PANI/MWCNT nanocomposite film in bending and extending states to 1 ppm of NH3. Reproduced from ref. 198, copyright 2017, Elsevier. | ||
PANI nanocomposites doped with carboxylic acid functionalised multi-walled carbon nanotube (c-MWCNT) were synthesised by in situ chemical oxidation polymerisation of aniline monomer using ammonium persulfate in the presence of c-MWCNT.199 The nanocomposites showed better response to chloroform (CHCl3) vapour as compared to pure PANI. Fig. 17 shows the suggested mechanism of CHCl3 molecules with PANI/c-MWCNT nanocomposite. For nanocomposite sensors, the sensing performances in terms of sensor response, response time, and reproducibility increased with increasing c-MWCNT concentration, up to 3 wt% (Fig. 18), while the response of the 2 wt% pristine MWCNT in the nanocomposite (S = 3.4) was found to be ten times lower than that of the PANI/c-MWCNT nanocomposite (PC3) (S = 32.8) at chloroform concentration of 250 ppm. This indicates good selectivity and response of PANI/c-MWCNT towards chloroform rather than pure PANI/MWCNT. Next, the decrease in DC electrical resistance of c-MWCNT-doped PANI nanocomposite upon exposure to chloromethane vapour indicates significant interactions between vapour molecules and conjugated PANI chains. Among the studied nanocomposite sensors, the highest sensing capability was observed for the sensor containing 3 wt% c-MWCNTs. The sensor response exhibited a good linear relationship with c-MWCNT concentration at all studied CHCl3 concentrations. The difference in response of the PANI/c-MWCNT sensor for different chlorinated methane vapours could be utilised for selective detection of the vapours.
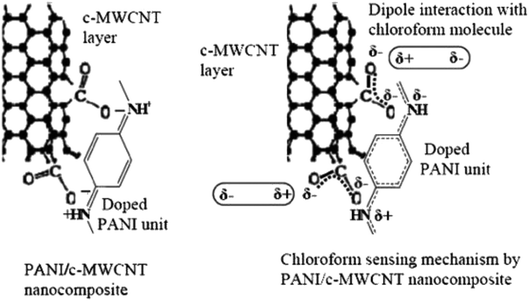 | ||
| Fig. 17 Suggested mechanism of CHCl3 molecules with PANI/c-MWCNT nanocomposite. Reproduced from ref. 199, copyright 2015, Elsevier. | ||
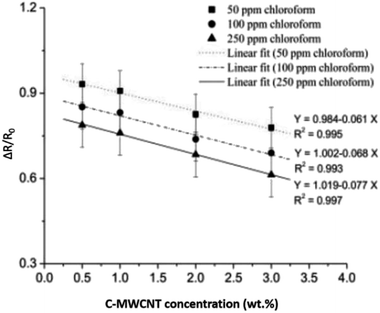 | ||
| Fig. 18 Variation in sensor response toward CHCl3 vapour as a function of c-MWCNT concentration in PANI/c-MWCNT nanocomposite. Reproduced from ref. 199, copyright 2015, Elsevier. | ||
Zhang et al. (2006) demonstrated a facile fabrication method to make chemical gas sensors using carboxylated SWCNT (SWCNT–COOH) with 80 to 90% purity and were electrochemically functionalised with PANI.200 The potential advantage of the proposed method is to enable targeted functionalisation with different materials to allow for creation of high-density individually addressable nano sensor arrays. The PANI–SWNT–COOH network-based sensors were tested for on-line monitoring of ammonia gas. The results showed superior sensitivity of 2.44% response of NH3 (which is 60 times higher than intrinsic SWNT based sensors), a detection limit as low as 50 ppbv, and good reproducibility upon repeated exposure to 10 ppmv NH3. Higher sensitivities were observed at lower temperatures. These results indicate that electrochemical functionalisation of SWNTs provides a promising new method of creating highly advanced nano sensors with improved sensitivity, detection limit, and reproducibility. Fig. 19 shows the schematic diagram of PANI coated with SWCNT–COOH on interdigitated electrodes on the Si substrate. Fig. 20 shows SEM images of a bare SWCNT before and after electropolymerisation, and their NH3 sensitivity (PANI was coated on SWCNT–COOH using a two-electrode configuration at 0.8 V for 5 minutes).
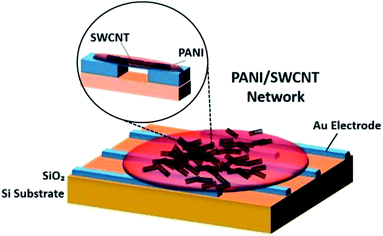 | ||
| Fig. 19 Schematic diagram of PANI coated with SWCNT–COOH on interdigitated electrodes. Adapted from ref. 200, copyright 2015, Elsevier. | ||
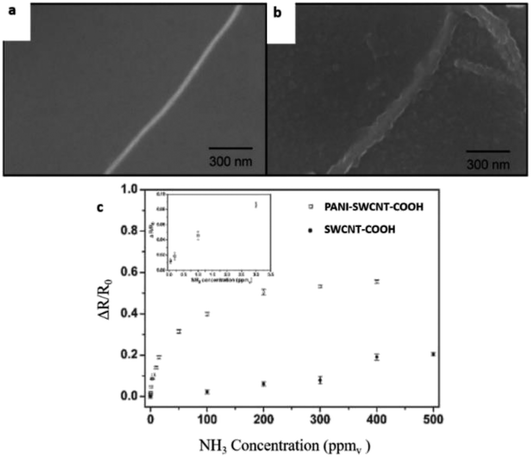 | ||
| Fig. 20 (a) SEM images of a bare SWCNT before electropolymerisation, (b) SEM image of coated SWCNT–COOH after electropolymerisation, and (c) NH3 sensitivity of PANI–SWCNTs–COOH and unfunctionalised SWCNTs (SWCNT–COOH, without PANI). Reproduced from ref. 200, copyright 2015, Elsevier. | ||
An interesting study of PTh-based/SWCNT on the detection of n-methylphenethylamine (NMPEA) vapour, one of the most widespread, harmful and addictive illegal drugs in the world, was conducted by Zhang et al. (2018).201 The regioregular poly[3-(6-carboxyhexyl)thiophene-2,5-diyl] (P3CT) and regioregular poly(3-octylthiophene-2,5-diyl) (P3OT) (Fig. 21) was used in the study and was non-covalently functionalised with SWCNTs. NMPEA was detected at concentrations of as low as 4 ppb exposure. Results found that the acid–base interaction between the amine compounds and the carboxylic acid groups in the polymer contributed to extraordinary sensitivity of the P3CT/CNT sensor towards amine compounds, where the carboxylic acid group in the P3CT acted as a receptor of amine compounds and enhanced the interaction between the amine compounds and the sensor materials. The sensors were also able to distinguish NMPEA from two other amine compounds, various volatile chemical compounds (VOCs), and water vapour by observing the recoverability of the sensor's signal after exposure even when the vapour concentrations of amines were several orders of magnitude lower than those of the VOCs.
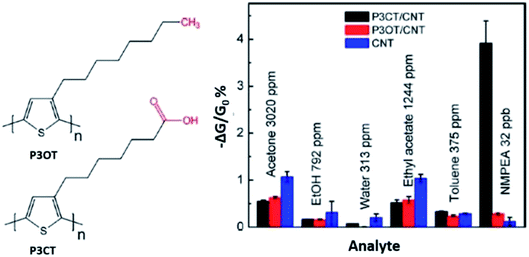 | ||
| Fig. 21 The chemical structure of P3OT and P3CT, and their responses of the P3CT/SCNT, P3OT/CNT sensor, and the non-functionalised CNT sensor to 20 s vapour exposures of various compounds (1% of saturated vapour) and 32 ppb of NMPEA. Reproduced from ref. 201, copyright 2018, Elsevier. | ||
Another study was done by Hussain et al. (2020) on the electrical conductivity and NH3 sensing for PTh/MWCNTs nanocomposites.202 The performance of PTh/MWCNTs nanocomposites showed significantly enhanced electrical conductivity, where PTh/MWCNTs-3 (PTh/MWCNTs nanocomposite containing 15% MWCNTs to the weight of monomers) was found to be an ultra-sensitive (detection limit of 0.1 ppm), completely reversible, highly selective, and stable ammonia sensor at room temperature. The sensing response of PTh/MWCNTs-3 at 2000, 1500, 1000, 500, 400, 200, 100, 50, 1, and 0.1 ppm of NH3 was found to be 88.70%, 73.27%, 62.04%, 51.65%, 46.96%, 42.97%, 38.86%, 35.18%, 32.93%, and 27.66%, respectively. The results showed that relative humidity only had a small effect on NH3 sensing properties of PTh/MWCNTs-3. Fig. 22 shows the proposed mechanism of the interaction of NH3 with PTh/MWCNTs nanocomposite. Table 4 shows the summary of sensing performance of certain conductive polymer/CNTs towards different analytes.
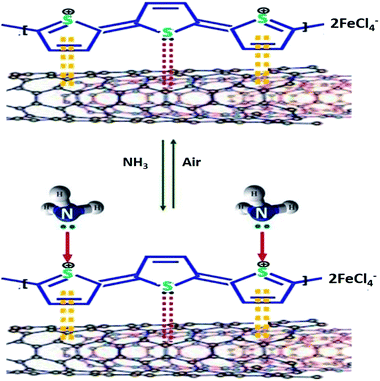 | ||
| Fig. 22 Shows the proposed mechanism of the interaction of NH3 with PTh/MWCNTs nanocomposite. Reproduced from ref. 202, copyright 2020, Elsevier. | ||
| Analyte | Conductive polymer | CNTs composition limit | Operating temperature | Detection range | Performance | Ref. |
|---|---|---|---|---|---|---|
| Chemical warfare stimulant | ||||||
| DMMP | PANI (SWNT) | — | Room temperature | 1 to 10 ppm | Response: 27.1%, response time: 5.5 s, gas concentration: 10 ppm | 203 |
| PANI (MWCNT) | 1 wt% | 50 °C | 332 to 800 ppm | Response: 1%, gas concentration: 332 ppm | 204 | |
| PPy (Co3O4@Au/MWCNT) | 6.5 wt% | Room temperature | 20 to 120 ppm | Response: 98%, response time: 60 s, recovery time: 439 s, gas concentration: 120 ppm | 188 | |
| Malathion | PANI (SWNT) | — | Room temperature | 2.0 × 10−7 M to 14.0 × 10−7 M | Recovery: 100.002% | 205 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Toxic gases | ||||||
| NO2 | PTh (SWCNT) | — | Room temperature | 0.01 to 10 ppm | Response: 28%, response time: 330 s, gas concentration: 10 ppm | 206 |
| DCM | PANI (MWCNT) | 1 wt% | 50 °C | 481 to 1442 ppm | Response time: 200 s, gas concentration: 1442 ppm | 204 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Volatile organic compounds (VOC) | ||||||
| Ammonia | PANI | — | Room temperature | 4 to 30 ppm | Response: 418%, gas concentration: 4 ppm, response time: 167 s, recovery time: 379 s | 207 |
| PANI (MWCNT) | — | Room temperature | 2 to 10 ppm | Response time: 6 s, recovery time: 6 s | 173 | |
| PANI (MWCNT) | 5 mg | Room temperature | 0.2 to 15 ppm | Response time: 67 s, gas concentration: 12 ppm | 208 | |
| PANI (SWCNT) | 1 mg mL−1 in 0.5 mL aqueous solution | Room temperature | 0 to 500 ppm | Response: 2.44% | 200 | |
| Nitrotoluene | Poly(TPP) (SWCNT) | 0.2 μL drop – until desired resistance achieved | Room temperature | 50 to 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
Response time: 8 min, gas concentration: 9 ppm | 209 |
| Acetone | Poly(TPP) (SWCNT) | 0.2 μL drop – until desired resistance achieved | Room temperature | 50 to 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
Response: 0.25%, gas concentration: 9 ppm | 209 |
| Formaldehyde | Polyethyleneimine (SWCNT) | — | Room temperature | 0 to 0.80 ppm | Gas concentration: 20 ppb, response time: 18 s, response: 95% | 210 |
| H2 | PANI (MWCNT) | 4 wt% | Room temperature | — | Response > 20% | 211 |
6. Conclusions and future outlook
CNT has prominent mechanical and electrical properties as well as good electron transport properties which prove that the development of CNT/polymer nanocomposites could contribute in expanding many areas of applications. The deficiencies from these nanocomposites need to be solved, such as lack of stability, solubility, selectivity, and poor interfacial adhesion between CNT and polymers. From recent studies and journals, a lot of strategies and efforts have been discussed to functionalise the paramount discovery of interaction between CNT and polymers. Still, the application of CNT in real products are still in early stage of realisation but has a bright future for a new industrialisation era. It was introduced that either pristine or functionalised CNT may change the properties of conductive polymer matrices. Some factors, such as the shape, size, type, aspect ratio, dispersion, and alignment of CNT within the nanocomposite affect the properties of nanocomposites. Here, covalent functionalisation of CNTs modified the stacking and layering properties of CNT mostly by changing the hydrogen bonding by reduction of van der Waals interactions between the CNTs and led to debundling of the tubes which contribute to the well dispersion of CNTs and good sensitivity towards analytes. In general, the attachment of carboxylic, hydroxyl, polymer, and other hydrophilic groups help in improving the chemical structure of CNTs by filling in the defect sites, thus improving the conductivity and charge transfer of the nanocomposites. In addition, the main advantages of CNT functionalisation with polymer are to ensure uniform dispersion of the nanocomposites and prevent the accumulation and agglomeration of CNTs which happens through attraction-depletion of the CNT itself. The nanocomposites are synthesised through chemical oxidative polymerisation, electrochemical oxidative polymerisation, and direct solution-mixing methods. Therefore, in situ polymerisation is one of the efficient methods to obtain uniform dispersion of CNTs in a thermosetting polymer. The aforementioned method involves the addition or condensation reactions of the monomers with CNTs. Melt blending, latex technology, and simpler solution mixing are other ways to fabricate CNT/polymers. More facile and efficient methods need to be developed for a better future.One of the nanocomposites applications highlighted is chemical sensor. The infamous one is the CNT-based chemiresistive gas sensor. In the light of this approach, CNT/conductive polymers nanocomposites synergise the construction of better transducer, acquired the simplicity, rapid response, and low-cost process. Generally, the sensor translates chemical interaction between the receptors and analytes into changing electrical resistance, a simple electric signal to be analysed. The detection performance is exploited by the chemical composition and structure, conductivity and charge transport, along with its adsorption ability. At this juncture, the parameters need to be considered for sensor performance are sensitivity, analyte concentration, operation temperature, limit of detection, recovery time, response time, response, and selectivity. Even if the behaviour and composition of CNTs and the properties of CNT/conductive polymer are well understood, still, there are many hitherto fundamental arguments about nanocomposites that need to be discovered, such as techniques in fabrication of gas sensor, modifications and functionalisation of CNTs, the charge transfer in nanocomposites, processing cost as well as introduction of graphene in gas sensing. Further studies are paramount to discover each property and interaction on CNT-based nanocomposites.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Newton fund's Program and Malaysia Partnership and Alliances in Research (MyPAiR) for ISIS-NEWTON/2019/SG/01 and Chemical Defence Research Centre (CHEMDEF) for a research grant (UPNM/2018/CHEMDEFF/ST/3) are gratefully acknowledged.References
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 1991, 354, 56–58 CrossRef CAS.
- B. De, S. Banerjee, K. D. Verma, T. Pal, P. K. Manna and K. K. Kar, Carbon nanotube as electrode materials for supercapacitors, Handbook of Nanocomposite Supercapacitor Materials II, Springer, 2020, pp. 229–43 Search PubMed.
- M. Ahmadi, O. Zabihi, M. Masoomi and M. Naebe, Synergistic effect of MWCNTs functionalization on interfacial and mechanical properties of multi-scale UHMWPE fibre reinforced epoxy composites, Compos. Sci. Technol., 2016, 134, 1–11 CrossRef CAS.
- B. Maruyama and K. Alam, Carbon nanotubes and nanofibers in composite materials, SAMPE J., 2002, 38, 59–70 CAS.
- P. G. Collins and P. Avouris, Nanotubes for Electronics – Scientific American, Nature Publishing Group, San Francisco, 2000, vol. 283, pp. 62–69 Search PubMed.
- Z. Wu, Z. Yang, K. Pei, X. Qian, C. Jin and R. Che, Dandelion-like carbon nanotube assembly embedded with closely separated Co nanoparticles for high-performance microwave absorption materials, Nanoscale, 2020, 12, 10149–10157 RSC.
- Z. Mo, R. Yang, D. Lu, L. Yang, Q. Hu and H. Li, et al., Lightweight, three-dimensional carbon Nanotube@TiO2 sponge with enhanced microwave absorption performance, Carbon, 2019, 144, 433–439 CrossRef CAS.
- L. F. C. Souto and B. G. Soares, Polyaniline/carbon nanotube hybrids modified with ionic liquids as anticorrosive additive in epoxy coatings, Prog. Org. Coat., 2020, 143, 105598 CrossRef CAS.
- A. G. Hassan, M. A. M. Yajid, S. N. Saud, T. A. A. Bakar, A. Arshad and N. Mazlan, Effects of varying electrodeposition voltages on surface morphology and corrosion behavior of multi-walled carbon nanotube coated on porous Ti-30 at%-Ta shape memory alloys, Surf. Coat. Technol., 2020, 401, 126257 CrossRef CAS.
- R. O. Medupin, O. K. Abubakre, A. S. Abdulkareem, R. A. Muriana and A. S. Abdulrahman, Carbon Nanotube Reinforced Natural Rubber Nanocomposite for Anthropomorphic Prosthetic Foot Purpose, Sci. Rep., 2019, 9, 1–11 CrossRef.
- M. S. Z. Abidin, T. Herceg, E. S. Greenhalgh, M. Shaffer and A. Bismarck, Enhanced fracture toughness of hierarchical carbon nanotube reinforced carbon fibre epoxy composites with engineered matrix microstructure, Compos. Sci. Technol., 2019, 170, 85–92 CrossRef.
- D. Feng, D. Xu, Q. Wang and P. Liu, Highly stretchable electromagnetic interference (EMI) shielding segregated polyurethane/carbon nanotube composites fabricated by microwave selective sintering, J. Mater. Chem. C, 2019, 7, 7938–7946 RSC.
- E. Zhou, J. Xi, Y. Guo, Y. Liu, Z. Xu and L. Peng, et al., Synergistic effect of graphene and carbon nanotube for high-performance electromagnetic interference shielding films, Carbon, 2018, 133, 316–322 CrossRef CAS.
- M. Chen, Q. S. Jing, H. B. Sun, J. Q. Xu, Z. Y. Yuan and J. T. Ren, et al., Engineering the Core–Shell-Structured NCNTs-Ni2Si@Porous Si Composite with Robust Ni–Si Interfacial Bonding for High-Performance Li-Ion Batteries, Langmuir, 2019, 35, 6321–6332 CrossRef CAS.
- F. Guo, T. Kang, Z. Liu, B. Tong, L. Guo and Y. Wang, et al., Advanced Lithium Metal–Carbon Nanotube Composite Anode for High-Performance Lithium–Oxygen Batteries, Nano Lett., 2019, 19, 6377–6384 CrossRef CAS.
- M. Chen, G. C. Wang, W. Q. Yang, Z. Y. Yuan, X. Qian and J. Q. Xu, et al., Enhanced synergetic catalytic effect of Mo2C/NCNTs@Co heterostructures in dye-sensitized solar cells: fine-tuned energy level alignment and efficient charge transfer behavior, ACS Appl. Mater. Interfaces, 2019, 11, 42156–42171 CrossRef CAS.
- M. Chen, G. C. Wang, L. L. Shao, Z. Y. Yuan, X. Qian and Q. S. Jing, et al., Strategic Design of Vacancy-Enriched Fe1−xS Nanoparticles Anchored on Fe3C-Encapsulated and N-Doped Carbon Nanotube Hybrids for High-Efficiency Triiodide Reduction in Dye-Sensitized Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10, 31208–31224 CrossRef CAS.
- M. Chen, G. Zhao, L. L. Shao, Z. Y. Yuan, Q. S. Jing and K. J. Huang, et al., Controlled synthesis of nickel encapsulated into nitrogen-doped carbon nanotubes with covalent bonded interfaces: the structural and electronic modulation strategy for an efficient electrocatalyst in dye-sensitized solar cells, Chem. Mater., 2017, 29, 9680–9694 CrossRef CAS.
- M. Chen, L. L. Shao, X. W. Lv, G. C. Wang, W. Q. Yang and Z. Y. Yuan, et al., In situ growth of Ni-encapsulated and N-doped carbon nanotubes on N-doped ordered mesoporous carbon for high-efficiency triiodide reduction in dye-sensitized solar cells, Chem. Eng. J., 2020, 124633 CrossRef CAS.
- N. Janudin, N. Abdullah, F. M. Yasin, M. H. Yaacob, M. Z. Ahmad and L. C. Abdullah, et al., Low cost and room temperature methane detection using multi walled-carbon nanotubes functionalized with octadecanol, ZULFAQAR Journal of Defence Science, Engineering & Technology, 2018, 1 Search PubMed.
- N. Janudin, N. Abdullah, W. M. Z. Wan Yunus, F. M. Yasin, M. H. Yaacob and N. Mohamad Saidi, et al., Effect of functionalized carbon nanotubes in the detection of benzene at room temperature, J. Nanotechnol., 2018, 2018, 1–7 CrossRef.
- D. Maity, K. Rajavel and R. T. R. Kumar, Polyvinyl alcohol wrapped multiwall carbon nanotube (MWCNTs) network on fabrics for wearable room temperature ethanol sensor, Sens. Actuators, B, 2018, 261, 297–306 CrossRef CAS.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Carbon nanotube chemical sensors, Chem. Rev., 2018, 119, 599–663 CrossRef.
- M. S. Yahya and M. Ismail, Improvement of hydrogen storage properties of MgH2 catalyzed by K2NbF7 and multiwall carbon nanotube, J. Phys. Chem. C, 2018, 122, 11222–11233 CrossRef CAS.
- M. Mananghaya, D. Yu, G. N. Santos and E. Rodulfo, Scandium and titanium containing single-walled carbon nanotubes for hydrogen storage: a thermodynamic and first principle calculation, Sci. Rep., 2016, 6, 27370 CrossRef CAS.
- S. Park, A. P. Gupta, S. J. Yeo, J. Jung, S. H. Paik and M. Mativenga, et al., Carbon nanotube field emitters synthesized on metal alloy substrate by PECVD for customized compact field emission devices to be used in X-ray source applications, Nanomaterials, 2018, 8, 378 CrossRef.
- Y. Song, J. Li, Q. Wu, C. Yi, H. Wu and Z. Chen, et al., Study of film thickness effect on carbon nanotube based field emission devices, J. Alloys Compd., 2020, 816, 152648 CrossRef CAS.
- M. Chen and L. L. Shao, Review on the recent progress of carbon counter electrodes for dye-sensitized solar cells, Chem. Eng. J., 2016, 304, 629–645 CrossRef CAS.
- S. Z. N. Demon, A. I. Kamisan, N. Abdullah, S. A. M. Noor, O. K. Khim and N. A. M. Kasim, et al., Graphene-based Materials in Gas Sensor Applications: A Review, Sens. Mater., 2020, 32, 759–777 Search PubMed.
- M. Chen, R. H. Zha, Z. Y. Yuan, Q. S. Jing, Z. Y. Huang and X. K. Yang, et al., Boron and phosphorus co-doped carbon counter electrode for efficient hole-conductor-free perovskite solar cell, Chem. Eng. J., 2017, 313, 791–800 CrossRef CAS.
- M. Chen, L. L. Shao, Y. X. Guo and X. Q. Cao, Nitrogen and phosphorus co-doped carbon nanosheets as efficient counter electrodes of dye-sensitized solar cells, Chem. Eng. J., 2016, 304, 303–312 CrossRef CAS.
- M. Chen, L. L. Shao, Y. Xia, Z. Y. Huang, D. L. Xu and Z. W. Zhang, et al., Construction of highly catalytic porous TiOPC nanocomposite counter electrodes for dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2016, 8, 26030–26040 CrossRef CAS.
- M. Chen, L. L. Shao, Z. Y. Yuan, Q. S. Jing, K. J. Huang and Z. Y. Huang, et al., General strategy for controlled synthesis of NixPy/carbon and its evaluation as a counter electrode material in dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2017, 9, 17949–17960 CrossRef CAS.
- M. Chen, L. L. Shao, X. Qian, T. Z. Ren and Z. Y. Yuan, Direct synthesis of cobalt nanoparticle-imbedded mesoporous carbons for high-performance dye-sensitized solar cell counter electrodes, J. Mater. Chem. C, 2014, 2, 10312–10321 RSC.
- W. Yang, S. Mao, J. Yang, T. Shang, H. Song and J. Mabon, et al., Large-deformation and high-strength amorphous porous carbon nanospheres, Sci. Rep., 2016, 6, 24187 CrossRef CAS.
- B. Alemour, M. H. Yaacob, H. N. Lim and M. R. Hassan, Review of Electrical Properties of Graphene Conductive Composites, Int. J. Nanoelectron. Mater., 2018, 11, 371–398 Search PubMed.
- AZoM, Graphite (C) - Classifications, Properties & Applications, Azo Materials, 2002, available from: https://www.azom.com Search PubMed.
- L. F. Castañeda, F. C. Walsh, J. L. Nava and C. P. de Leon, Graphite felt as a versatile electrode material: properties, reaction environment, performance and applications, Electrochim. Acta, 2017, 258, 1115–1139 CrossRef.
- M. E. Spahr, S. Zürcher, M. Rodlert-bacilieri, F. Mornaghini and T. M. Gruenberger, High-conductive carbon black with low viscosity, US Pat., US20180155552A1, 2018.
- W. Lu, T. He, B. Xu, X. He, H. Adidharma and M. Radosz, et al., Progress in catalytic synthesis of advanced carbon nanofibers, J. Mater. Chem. A, 2017, 5, 13863–13881 RSC.
- D. Burton, P. Lake and A. Palmer, AIP Conf. Proc., 2020, 2068(1), 020061 Search PubMed.
- M. Kavitha and A. M. Kalpana, Carbon Nanotubes: Properties and Applications-A Brief Review, i-manager's Journal on Electronics Engineering, 2017, 7, 1 Search PubMed.
- K. R. Reddy, B. C. Sin, C. H. Yoo, W. Park, K. S. Ryu and J. S. Lee, et al., A new one-step synthesis method for coating multi-walled carbon nanotubes with cuprous oxide nanoparticles, Scr. Mater., 2008, 58, 1010–1013 CrossRef CAS.
- H. J. Choi, K. Zhang and J. Y. Lim, Multi-Walled Carbon Nanotube/Polystyrene Composites Prepared by in situ Bulk Sonochemical Polymerization, J. Nanosci. Nanotechnol., 2007, 7, 3400–3403 CrossRef CAS.
- A. Cwirzen, K. Habermehl-Cwirzen and V. Penttala, Surface decoration of carbon nanotubes and mechanical properties of cement/carbon nanotube composites, Adv. Cem. Res., 2008, 20, 65–73 CrossRef CAS.
- H. H. So, J. W. Cho and N. G. Sahoo, Effect of carbon nanotubes on mechanical and electrical properties of polyimide/carbon nanotubes nanocomposites, Eur. Polym. J., 2007, 43, 3750–3756 CrossRef CAS.
- I. A. Kinloch, J. Suhr, J. Lou, R. J. Young and P. M. Ajayan, Composites with carbon nanotubes and graphene: an outlook, Science, 2018, 362, 547–553 CrossRef CAS.
- X. Zhang, W. Lu, G. Zhou and Q. Li, Understanding the mechanical and conductive properties of carbon nanotube fibers for smart electronics, Adv. Mater., 2020, 32, 1902028 CrossRef CAS.
- X. Yao, X. Gao, J. Jiang, C. Xu, C. Deng and J. Wang, Comparison of carbon nanotubes and graphene oxide coated carbon fiber for improving the interfacial properties of carbon fiber/epoxy composites, Composites, Part B, 2018, 132, 170–177 CrossRef CAS.
- H. Wei, Y. Wang, J. Guo, B. Qiu, D. Ding, S. Wei, et al., Synthesis of Multifunctional Carbon Nanostructures, Handbook of Carbon Nano Materials (Volumes 7–8), 2015, vol. 7, p. 89 Search PubMed.
- M. A. Morsi, A. Rajeh and A. A. Al-Muntaser, Reinforcement of the optical, thermal and electrical properties of PEO based on MWCNTs/Au hybrid fillers: nanodielectric materials for organoelectronic devices, Composites, Part B, 2019, 173, 106957 CrossRef CAS.
- N. S. N. Sa'aya, S. Z. N. Demon, N. Abdullah, V. F. K. E. Abd Shatar and N. A. Halim, Optical and Morphological Studies of Multiwalled Carbon Nanotube-incorporated Poly(3-hexylthiophene-2, 5-diyl) Nanocomposites, Sens. Mater., 2019, 31, 2997–3006 Search PubMed.
- S. Bose, R. A. Khare and P. Moldenaers, Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: a critical review, Polymer, 2010, 51, 975–993 CrossRef CAS.
- H. Li, D. Yuan, P. Li and C. He, High conductive and mechanical robust carbon nanotubes/waterborne polyurethane composite films for efficient electromagnetic interference shielding, Composites, Part A, 2019, 121, 411–417 CrossRef CAS.
- L. Chen, X. J. Pang, M. Z. Qu, Q. T. Zhang, B. Wang and B. L. Zhang, et al., Fabrication and characterization of polycarbonate/carbon nanotubes composites, Composites, Part A, 2006, 37, 1485–1489 CrossRef.
- Y. Wang, X. Zhang, S. You and Y. Hu, One-step electrosynthesis of visible light responsive double-walled alloy titanium dioxide nanotube arrays for use in photocatalytic degradation of dibutyl phthalate, RSC Adv., 2020, 10, 21238–21247 RSC.
- Y. Cheng, J. Cao, H. Lv, H. Zhao, Y. Zhao and G. Ji, In situ regulating aspect ratio of bamboo-like CNTs via CoxNi1−x-catalyzed growth to pursue superior microwave attenuation in X-band, Inorg. Chem. Front., 2019, 6, 309–316 RSC.
- X. Wei, X. Cao, Y. Wang, G. Zheng, K. Dai and C. Liu, et al., Conductive herringbone structure carbon nanotube/thermoplastic polyurethane porous foam tuned by epoxy for high performance flexible piezoresistive sensor, Compos. Sci. Technol., 2017, 149, 166–177 CrossRef CAS.
- J. L. Delgado, M. Á. Herranz and N. Martin, The nano-forms of carbon, J. Mater. Chem., 2008, 18, 1417–1426 RSC.
- J. L. Delgado, M. A. Herranz and N. Martin, The nano-forms of carbon, J. Mater. Chem., 2008, 18, 1417–1426 RSC.
- Y. Zhang, L. Liu, B. Van der Bruggen and F. Yang, Nanocarbon based composite electrodes and their application in microbial fuel cells, J. Mater. Chem. A, 2017, 5, 12673–12698 RSC.
- Y. Liu, J. Zhang, Y. Cheng and S. P. Jiang, Effect of carbon nanotubes on direct electron transfer and electrocatalytic activity of immobilized glucose oxidase, ACS Omega, 2018, 3, 667–676 CrossRef CAS.
- J. Wang, Carbon-nanotube based electrochemical biosensors: a review, Electroanalysis, 2005, 17, 7–14 CrossRef CAS.
- C. B. Jacobs, M. J. Peairs and B. J. Venton, Carbon nanotube based electrochemical sensors for biomolecules, Anal. Chim. Acta, 2010, 662, 105–127 CrossRef CAS.
- P. C. Ma, N. A. Siddiqui, G. Marom and J. K. Kim, Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: a review, Composites, Part A, 2010, 41, 1345–1367 CrossRef.
- R. I. Rubel, M. H. Ali, M. A. Jafor and M. M. Alam, Carbon nanotubes agglomeration in reinforced composites: a review, AIMS Mater. Sci., 2019, 6, 756 Search PubMed.
- S. M. Sabet, H. Mahfuz, A. C. Terentis, M. Nezakat and J. Hashemi, Effects of POSS functionalization of carbon nanotubes on microstructure and thermomechanical behavior of carbon nanotube/polymer nanocomposites, J. Mater. Sci., 2018, 53, 8963–8977 CrossRef CAS.
- G. J. Bahun, C. Wang and A. Adronov, Solubilizing single-walled carbon nanotubes with pyrene-functionalized block copolymers, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1941–1951 CrossRef CAS.
- Y. L. Zhao and J. F. Stoddart, Noncovalent functionalization of single-walled carbon nanotubes, Acc. Chem. Res., 2009, 42, 1161–1171 CrossRef CAS.
- R. Konnola and K. Joseph, Effect of side-wall functionalisation of multi-walled carbon nanotubes on the thermo-mechanical properties of epoxy composites, RSC Adv., 2016, 6, 23887–23899 RSC.
- Y. Zhang and S. Huang, Significant improvements in the mechanical properties of chitosan functionalized carbon nanotubes/epoxy composites, RSC Adv., 2016, 6, 26210–26215 RSC.
- J. Chen, L. Yan, W. Song and D. Xu, Interfacial characteristics of carbon nanotube-polymer composites: a review, Composites, Part A, 2018, 114, 149–169 CrossRef CAS.
- K. P. Maity, A. Patra and V. Prasad, Influence of the chemical functionalization of carbon nanotubes on low temperature ac conductivity with polyaniline composites, J. Phys. D: Appl. Phys., 2020, 53, 125303 CrossRef CAS.
- A. F. Quintero-Jaime, D. Cazorla-Amorós and E. Morallón, Electrochemical functionalization of single wall carbon nanotubes with phosphorus and nitrogen species, Electrochim. Acta, 2020, 340, 135935 CrossRef CAS.
- O. I. Nakonechna, N. N. Belyavina, M. M. Dashevskyi, K. O. Ivanenko and S. L. Revo, Novel Ti2CuCx and Ti3Cu2Cx Carbides Obtained by Sintering of Products of Mechanochemical Synthesis of Ti, Cu and Carbon Nanotubes, Phys. Chem. Org. Solid State, 2018, 19, 179–185 CrossRef CAS.
- M. G. Trulli, E. Sardella, F. Palumbo, G. Palazzo, L. C. Giannossa and A. Mangone, et al., Towards highly stable aqueous dispersions of multi-walled carbon nanotubes: the effect of oxygen plasma functionalization, J. Colloid Interface Sci., 2017, 491, 255–264 CrossRef.
- B. Singh, S. Lohan, P. S. Sandhu, A. Jain and S. K. Mehta, Functionalized carbon nanotubes and their promising applications in therapeutics and diagnostics, Nanobiomater. Med. Imaging, 2016, 455–478 CAS.
- S. Ajori, R. Ansari and M. Darvizeh, Vibration characteristics of single-and double-walled carbon nanotubes functionalized with amide and amine groups, Phys. B, 2015, 462, 8–14 CrossRef CAS.
- R. Afrin and N. A. Shah, Room temperature gas sensors based on carboxyl and thiol functionalized carbon nanotubes buckypapers, Diamond Relat. Mater., 2015, 60, 42–49 CrossRef CAS.
- N. Janudin, L. C. Abdullah, N. Abdullah, F. M. Yasin, N. M. Saidi and N. A. M. Kasim, Characterization of Amide and Ester Functionalized Multiwalled Carbon Nanotubes, Asian J. Chem., 2018, 30, 1613–1616 CrossRef CAS.
- K. Balasubramanian and M. Burghard, Chemically functionalized carbon nanotubes, Small, 2005, 1, 180–192 CrossRef CAS.
- S. Kumar, R. Rani, N. Dilbaghi, K. Tankeshwar and K. H. Kim, Carbon nanotubes: a novel material for multifaceted applications in human healthcare, Chem. Soc. Rev., 2017, 46, 158–196 RSC.
- J. Azadmanjiri, V. K. Srivastava, P. Kumar, M. Nikzad, J. Wang and A. Yu, Two-and three-dimensional graphene-based hybrid composites for advanced energy storage and conversion devices, J. Mater. Chem. A, 2018, 6, 702–734 RSC.
- B. Ribeiro, E. C. Botelho, M. L. Costa and C. F. Bandeira, Carbon nanotube buckypaper reinforced polymer composites: a review, Polímeros, 2017, 27, 247–255 CrossRef.
- S. Mallakpour and S. Soltanian, Surface functionalization of carbon nanotubes: fabrication and applications, RSC Adv., 2016, 6, 109916–109935 RSC.
- N. Janudin, N. Abdullah, W. M. Z. W. Yunus, F. M. Yasin, M. H. Yaacob and N. Kasim, et al., Carbon nanofibers functionalized with amide group for ammonia gas detection, AIP Conf. Proc., 2019, 2068, 020061 CrossRef.
- S. A. Shamsuddin, M. N. Derman, U. Hashim, M. Kashif, T. Adam and N. H. A. Halim, et al., Nitric acid treated multi-walled carbon nanotubes optimized by Taguchi method, AIP Conf. Proc., 2016, 1756, 090002 CrossRef.
- P. I. Gonzalez-Chi, O. Rodríguez-Uicab, C. Martin-Barrera, J. Uribe-Calderon, G. Canché-Escamilla and M. Yazdani-Pedram, et al., Influence of aramid fiber treatment and carbon nanotubes on the interfacial strength of polypropylene hierarchical composites, Composites, Part B, 2017, 122, 16–22 CrossRef CAS.
- A. Zeino, A. Abulkibash, M. Khaled and M. Atieh, Bromate removal from water using doped iron nanoparticles on multiwalled carbon nanotubes (CNTS), J. Nanomater., 2014, 2014, 1–9 CrossRef.
- T. Zhang, K. Xi, M. Gu and Z. S. Jiang, Phosphoryl choline-grafted water-soluble carbon nanotube, Chin. Chem. Lett., 2008, 19, 105–109 CrossRef CAS.
- C. A. Dyke and J. M. Tour, Covalent Functionalization of Single-Walled Carbon Nanotubes for Materials Applications, J. Phys. Chem. A, 2004, 108, 11151–11159 CrossRef CAS.
- S. Goyanes, G. R. Rubiolo, A. Salazar, A. Jimeno, M. A. Corcuera and I. Mondragon, Carboxylation treatment of multiwalled carbon nanotubes monitored by infrared and ultraviolet spectroscopies and scanning probe microscopy, Diamond Relat. Mater., 2007, 16, 412–417 CrossRef CAS.
- J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao and P. C. Eklund, et al., Solution properties of single-walled carbon nanotubes, Science, 1998, 282, 95–98 CrossRef CAS.
- J. Zhang, H. Zou, Q. Qing, Y. Yang, Q. Li and Z. Liu, et al., Effect of chemical oxidation on the structure of single-walled carbon nanotubes, J. Phys. Chem. B, 2003, 107, 3712–3718 CrossRef CAS.
- E. Malikov, O. H. Akperov, M. B. Muradov, G. Eyvazova, A. M. Maharramov and A. Kukovecz, et al., Oxidation of multiwalled carbon nanotubes using different oxidation agents like nitric acid and potassium permanganate, News Baku Univ., 2014, 4, 49–59 Search PubMed.
- H. Korri-Youssoufi, B. Zribi, A. Miodek and A. M. Haghiri-Gosnet, Carbon-Based Nanomaterials for Electrochemical DNA Sensing, Nanotechnology and Biosensors, Elsevier, 2018, pp. 113–50 Search PubMed.
- M. Adamska and U. Narkiewicz, Fluorination of carbon nanotubes – a review, J. Fluorine Chem., 2017, 200, 179–189 CrossRef CAS.
- J. H. Kim, E. Jeong and Y. S. Lee, Characteristics of fluorinated CNTs added carbon foams, Appl. Surf. Sci., 2016, 360, 1009–1015 CrossRef CAS.
- H. F. Bettinger, Experimental and Computational Investigations of the Properties of Fluorinated Single-Walled Carbon Nanotubes, ChemPhysChem, 2003, 4, 1283–1289 CrossRef CAS.
- H. Touhara, A. Yonemoto, K. Yamamoto, S. Komiyama, S. Kawasaki and F. Okino, et al., Fluorination of cup-stacked carbon nanotubes, structures and properties, MRS Online Proc. Libr., 2004, 858, HH12.3 CrossRef.
- A. Setaro, M. Adeli, M. Glaeske, D. Przyrembel, T. Bisswanger and G. Gordeev, et al., Preserving π-conjugation in covalently functionalized carbon nanotubes for optoelectronic applications, Nat. Commun., 2017, 8, 1–7 CrossRef.
- Z. Hu, Q. Shao, X. Xu, D. Zhang and Y. Huang, Surface initiated grafting of polymer chains on carbon nanotubes via one-step cycloaddition of diarylcarbene, Compos. Sci. Technol., 2017, 142, 294–301 CrossRef CAS.
- V. K. Abdelkader, S. Scelfo, C. García-Gallarín, M. L. Godino-Salido, M. Domingo-García and F. J. López-Garzón, et al., Carbon tetrachloride cold plasma for extensive chlorination of carbon nanotubes, J. Phys. Chem. C, 2013, 117, 16677–16685 CrossRef CAS.
- S. Zdanowska, M. Pyzalska, J. Drabowicz, D. Kulawik, V. Pavlyuk and T. Girek, et al., Carbon nanotubes functionalized by salts containing stereogenic heteroatoms as electrodes in their battery cells, Pol. J. Chem. Technol., 2016, 18, 22–26 CAS.
- Z. Zhou, E. K. Orcutt, H. C. Anderson and K. J. Stowers, Hydrogen surface modification of a carbon nanotube catalyst for the improvement of ethane oxidative dehydrogenation, Carbon, 2019, 152, 924–931 CrossRef CAS.
- C. Ménard-Moyon, N. Izard, E. Doris and C. Mioskowski, Separation of semiconducting from metallic carbon nanotubes by selective functionalization with azomethine ylides, J. Am. Chem. Soc., 2006, 128, 6552–6553 CrossRef.
- O. Olugbenga, D. Michael and I. Suny, Investigation on purification potential of multiwalled carbon nanotubes using organic-mineral acid mixture, Nanosci. Nanoeng., 2018, 4, 1–8 Search PubMed.
- T. J. Aitchison, M. Ginic-Markovic, J. G. Matisons, G. P. Simon and P. M. Fredericks, Purification, cutting, and sidewall functionalization of multiwalled carbon nanotubes using potassium permanganate solutions, J. Phys. Chem. C, 2007, 111, 2440–2446 CrossRef CAS.
- M. L. Sham and J. K. Kim, Surface functionalities of multi-wall carbon nanotubes after UV/ozone and TETA treatments, Carbon, 2006, 44, 768–777 CrossRef CAS.
- R. Malik, C. McConnell, N. T. Alvarez, M. Haase, S. Gbordzoe and V. Shanov, Rapid, in situ plasma functionalization of carbon nanotubes for improved CNT/epoxy composites, RSC Adv., 2016, 6, 108840–108850 RSC.
- P. C. Ma, J. K. Kim and B. Z. Tang, Functionalization of carbon nanotubes using a silane coupling agent, Carbon, 2006, 44, 3232–3238 CrossRef CAS.
- J. Wang, H. Jia, L. Ding, L. Zhu, X. Dai and X. Fei, et al., Utilization of silane functionalized carbon nanotubes-silica hybrids as novel reinforcing fillers for solution styrene butadiene rubber, Polym. Compos., 2013, 34, 690–696 CrossRef CAS.
- Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani and M. Salami-Kalajahi, Functionalization of carbon nanotubes by combination of controlled radical polymerization and “grafting to” method, Adv. Colloid Interface Sci., 2020, 102126 CrossRef CAS.
- F. A. Abuilaiwi, T. Laoui, M. Al-Harthi and M. A. Atieh, Modification and functionalization of multiwalled carbon nanotube (MWCNT) via fischer esterification, Arabian J. Sci. Eng., 2010, 35, 37–48 CAS.
- Z. Su, Y. Cheng, C. Li, Y. Xiong, L. Xiao and S. Chen, et al., Dispersing gold nanoparticles on thiolated polyaniline-multiwalled carbon nanotubes for development of an indole-3-acetic acid amperometric immunosensor, Nanoscale Adv., 2019, 1, 3607–3613 RSC.
- Y. Maeda, S. Minami, Y. Takehana, J. S. Dang, S. Aota and K. Matsuda, et al., Tuning of the photoluminescence and up-conversion photoluminescence properties of single-walled carbon nanotubes by chemical functionalization, Nanoscale, 2016, 8, 16916–16921 RSC.
- K. Adachi and Y. Tsukahara, Surface modification of carbon nanotubes by anionic approach, Curr. Opin. Chem. Eng., 2016, 11, 106–113 CrossRef.
- G. Ostojic and M. C. Hersam, Functionalization of carbon nanotubes with metallic moieties, US Pat., US8562905B2, 2019.
- W. F. Chan, E. Marand and S. M. Martin, Novel zwitterion functionalized carbon nanotube nanocomposite membranes for improved RO performance and surface anti-biofouling resistance, J. Membr. Sci., 2016, 509, 125–137 CrossRef CAS.
- J. Liu, R. Che, H. Chen, F. Zhang, F. Xia and Q. Wu, et al., Microwave absorption enhancement of multifunctional composite microspheres with spinel Fe3O4 cores and anatase TiO2 shells, Small, 2012, 8, 1214–1221 CrossRef CAS.
- C. L. Zhu, M. L. Zhang, Y. J. Qiao, G. Xiao, F. Zhang and Y. J. Chen, Fe3O4/TiO2 core/shell nanotubes: synthesis and magnetic and electromagnetic wave absorption characteristics, J. Phys. Chem. C, 2010, 114, 16229–16235 CrossRef CAS.
- V. Vatanpour, S. S. Madaeni, R. Moradian, S. Zinadini and B. Astinchap, Novel antibifouling nanofiltration polyethersulfone membrane fabricated from embedding TiO2 coated multiwalled carbon nanotubes, Sep. Purif. Technol., 2012, 90, 69–82 CrossRef CAS.
- C. Wang, H. Wu, F. Qu, H. Liang, X. Niu and G. Li, Preparation and properties of polyvinyl chloride ultrafiltration membranes blended with functionalized multi-walled carbon nanotubes and MWCNTs/Fe3O4 hybrids, J. Appl. Polym. Sci., 2016, 133, 1–8 Search PubMed.
- T. Hertel, A. Hagen, V. Talalaev, K. Arnold, F. Hennrich and M. Kappes, et al., Spectroscopy of single-and double-wall carbon nanotubes in different environments, Nano Lett., 2005, 5, 511–514 CrossRef CAS.
- L. Vaisman, H. D. Wagner and G. Marom, The role of surfactants in dispersion of carbon nanotubes, Adv. Colloid Interface Sci., 2006, 128, 37–46 CrossRef.
- P. Bilalis, D. Katsigiannopoulos, A. Avgeropoulos and G. Sakellariou, Non-covalent functionalization of carbon nanotubes with polymers, RSC Adv., 2014, 4, 2911–2934 RSC.
- X. Wang, L. Bai, S. Kong, Y. Song and F. Meng, Star-shaped supramolecular ionic liquid crystals based on pyridinium salts, Liq. Cryst., 2019, 46, 512–522 CrossRef CAS.
- J. Dai, R. M. F. Fernandes, O. Regev, E. F. Marques and I. Furó, Dispersing carbon nanotubes in water with amphiphiles: dispersant adsorption, kinetics, and bundle size distribution as defining factors, J. Phys. Chem. C, 2018, 122, 24386–24393 CrossRef CAS.
- T. Fujigaya and N. Nakashima, Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants, Sci. Technol. Adv. Mater., 2015, 16, 024802 CrossRef.
- S. M. Ghoreishi, M. Behpour, S. Mousavi, A. Khoobi and F. S. Ghoreishi, Simultaneous electrochemical determination of dopamine, ascorbic acid and uric acid in the presence of sodium dodecyl sulphate using a multi-walled carbon nanotube modified carbon paste electrode, RSC Adv., 2014, 4, 37979–37984 RSC.
- B. I. Kharisov, O. V. Kharissova and A. V. Dimas, The dispersion, solubilization and stabilization in “solution” of single-walled carbon nanotubes, RSC Adv., 2016, 6, 68760–68787 RSC.
- X. Zeng, D. Yang, H. Liu, N. Zhou, Y. Wang and W. Zhou, et al., Detecting and Tuning the Interactions between Surfactants and Carbon Nanotubes for Their High-Efficiency Structure Separation, Adv. Mater. Interfaces, 2018, 5, 1700727 CrossRef.
- M. V. Manilo, N. Lebovka and S. Barany, Combined effect of cetyltrimethylammonium bromide and laponite platelets on colloidal stability of carbon nanotubes in aqueous suspensions, J. Mol. Liq., 2017, 235, 104–110 CrossRef CAS.
- M. Park, J. Park, J. Lee and S. Y. Ju, Scaling of binding affinities and cooperativities of surfactants on carbon nanotubes, Carbon, 2018, 139, 427–436 CrossRef CAS.
- A. Ishibashi and N. Nakashima, Strong chemical structure dependence for individual dissolution of single-walled carbon nanotubes in aqueous micelles of biosurfactants, Bull. Chem. Soc. Jpn., 2006, 79, 357–359 CrossRef CAS.
- T. Fujigaya, M. Okamoto and N. Nakashima, Design of an assembly of pyridine-containing polybenzimidazole, carbon nanotubes and Pt nanoparticles for a fuel cell electrocatalyst with a high electrochemically active surface area, Carbon, 2009, 47, 3227–3232 CrossRef CAS.
- Y. C. Jung, H. H. Kim, Y. A. Kim, J. H. Kim, J. W. Cho and M. Endo, et al., Optically active multi-walled carbon nanotubes for transparent, conductive memory-shape polyurethane film, Macromolecules, 2010, 43, 6106–6112 CrossRef CAS.
- W. Khan, R. Sharma and P. Saini, Carbon nanotube-based polymer composites: synthesis, properties and applications, Carbon Nanotubes: Curr. Prog. Their Polym. Compos., 2016, 1–46 CAS.
- C. Backes, A. M. Abdelkader, C. Alonso, A. Andrieux-Ledier, R. Arenal and J. Azpeitia, et al., Production and processing of graphene and related materials, 2D Materials, 2020, 7, 022001 CrossRef CAS.
- J. M. Benoit, B. Corraze, S. Lefrant, W. J. Blau and P. Bemier, Transport properties of PMMA-carbon nanotubes composites, Synth. Met., 2001, 121, 1215–1216 CrossRef CAS.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS.
- Q. Wang, T. Wang, J. Wang, W. Guo, Z. Qian and T. Wei, Preparation of antistatic high-density polyethylene composites based on synergistic effect of graphene nanoplatelets and multi-walled carbon nanotubes, Polym. Adv. Technol., 2018, 29, 407–416 CrossRef CAS.
- A. Chafidz, F. H. Latief, U. A. Samad, A. Ajbar and W. Al-Masry, Nanoindentation creep, nano-impact, and thermal properties of multiwall carbon nanotubes–polypropylene nanocomposites prepared via melt blending, Polym.-Plast. Technol. Eng., 2016, 55, 1373–1385 CrossRef CAS.
- J. P. S. da Silva, B. G. Soares, S. Livi and G. M. O. Barra, Phosphonium-based ionic liquid as dispersing agent for MWCNT in melt-mixing polystyrene blends: rheology, electrical properties and EMI shielding effectiveness, Mater. Chem. Phys., 2017, 189, 162–168 CrossRef.
- P. Sen, K. Suresh, R. V. Kumar, M. Kumar and G. Pugazhenthi, A simple solvent blending coupled sonication technique for synthesis of polystyrene (PS)/multi-walled carbon nanotube (MWCNT) nanocomposites: effect of modified MWCNT content, J. Sci., 2016, 1, 311–323 Search PubMed.
- A. Funck and W. Kaminsky, Polypropylene carbon nanotube composites by in situ polymerization, Compos. Sci. Technol., 2007, 67, 906–915 CrossRef CAS.
- Y. Xie, J. Zhao, Z. Le, M. Li, J. Chen and Y. Gao, et al., Preparation and electromagnetic properties of chitosan-decorated ferrite-filled multi-walled carbon nanotubes/polythiophene composites, Compos. Sci. Technol., 2014, 99, 141–146 CrossRef CAS.
- M. Feng, R. Sun, H. Zhan and Y. Chen, Decoration of carbon nanotubes with CdS nanoparticles by polythiophene interlinking for optical limiting enhancement, Carbon, 2010, 48, 1177–1185 CrossRef CAS.
- A. Mohamed, K. Trickett, S. Y. Chin, S. Cummings, M. Sagisaka and L. Hudson, et al., Universal surfactant for water, oils, and CO2, Langmuir, 2010, 26, 13861–13866 CrossRef CAS.
- A. Mohamed, A. K. Anas, S. A. Bakar, A. A. Aziz, M. Sagisaka and P. Brown, et al., Preparation of multiwall carbon nanotubes (MWCNTs) stabilised by highly branched hydrocarbon surfactants and dispersed in natural rubber latex nanocomposites, Colloid Polym. Sci., 2014, 292, 3013–3023 CrossRef CAS.
- N. H. K. Azman, M. M. Ramli and S. S. M. Isa, A Review of hybridization of carbon nanotube into graphene for gas sensor application, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 1–8 Search PubMed.
- S. T. Navale, D. K. Bandgar, M. A. Chougule and V. B. Patil, Facile method of preparation of PbS films for NO2 detection, RSC Adv., 2015, 5, 6518–6527 RSC.
- S. J. Choi, D. M. Lee, H. Yu, J. S. Jang, M. H. Kim and J. Y. Kang, et al., All-carbon fiber-based chemical sensor: improved reversible NO2 reaction kinetics, Sens. Actuators, B, 2019, 290, 293–301 CrossRef CAS.
- R. Tang, Y. Shi, Z. Hou and L. Wei, Carbon nanotube-based chemiresistive sensors, Sensors, 2017, 17, 882 CrossRef.
- A. A. Baharuddin, B. C. Ang, A. S. M. A. Haseeb, Y. C. Wong and Y. H. Wong, Advances in chemiresistive sensors for acetone gas detection, Mater. Sci. Semicond. Process., 2019, 103, 104616 CrossRef CAS.
- O. Lupan, V. Cretu, V. Postica, N. Ababii, O. Polonskyi and V. Kaidas, et al., Enhanced ethanol vapour sensing performances of copper oxide nanocrystals with mixed phases, Sens. Actuators, B, 2016, 224, 434–448 CrossRef.
- Y. C. Wong, B. C. Ang, A. S. M. A. Haseeb, A. A. Baharuddin and Y. H. Wong, Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review, J. Electrochem. Soc., 2019, 167, 037503 CrossRef.
- D. R. Miller, S. A. Akbar and P. A. Morris, Nanoscale metal oxide-based heterojunctions for gas sensing: a review, Sens. Actuators, B, 2014, 204, 250–272 CrossRef CAS.
- I. V. Zaporotskova, N. P. Boroznina, Y. N. Parkhomenko and L. V. Kozhitov, Carbon nanotubes: Sensor properties. A review, Mod. Electron. Mater., 2016, 2, 95–105 CrossRef.
- L. Camilli and M. Passacantando, Advances on sensors based on carbon nanotubes, Chemosensors, 2018, 6, 62 CrossRef CAS.
- J. W. Yoon and J. H. Lee, Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: recent progress and future perspectives, Lab Chip, 2017, 17, 3537–3557 RSC.
- J. V. Yakhmi, V. Saxena and D. K. Aswal, Conducting polymer sensors, actuators and field-effect transistors, Funct. Mater., 2012, 61–110 CAS.
- M. Ghodrati, A. Mir and A. Farmani, Carbon nanotube field effect transistors-based gas sensors, Nanosensors for Smart Cities, Elsevier, 2020, pp. 171–83 Search PubMed.
- M. Ghodrati, A. Farmani and A. Mir, Nanoscale sensor-based tunneling carbon nanotube transistor for toxic gases detection: a first-principle study, IEEE Sens. J., 2019, 19, 7373–7377 CAS.
- N. Janudin, F. M. Yasin, N. Abdullah, M. H. Yaacob, M. Z. Ahmad and R. N. I. R. Othman, et al., Carbon nanotubes-based gas sensor in detection of methane gas at room temperature, ZULFAQAR Journal of Defence Science, Engineering & Technology, 2018, 1 Search PubMed.
- G. Neri, First fifty years of chemoresistive gas sensors, Chemosensors, 2015, 3, 1–20 CrossRef CAS.
- R. Abdel-Karim, Y. Reda and A. Abdel-Fattah, Nanostructured Materials-Based Nanosensors, J. Electrochem. Soc., 2020, 167, 037554 CrossRef CAS.
- L. A. Panes-Ruiz, M. Shaygan, Y. Fu, Y. Liu, V. Khavrus and S. Oswald, et al., Toward highly sensitive and energy efficient ammonia gas detection with modified single-walled carbon nanotubes at room temperature, ACS Sens., 2018, 3, 79–86 CrossRef CAS.
- C. Piloto, Carbon nanomaterials for room temperature gas sensing, Queensland University of Technology, 2016 Search PubMed.
- C. Paoletti, M. He, P. Salvo, B. Melai, N. Calisi and M. Mannini, et al., Room temperature amine sensors enabled by sidewall functionalization of single-walled carbon nanotubes, RSC Adv., 2018, 8, 5578–5585 RSC.
- A. H. Al-Husseini, W. R. Saleh and A. Al-Sammarraie, A Specific NH3 Gas Sensor of a Thick MWCNTs-OH Network for Detection at Room Temperature, J. Nano Res., 2019, 98–108 CAS.
- Y. Li, M. Hodak, W. Lu and J. Bernholc, Mechanisms of NH3 and NO2 detection in carbon-nanotube-based sensors: an ab initio investigation, Carbon, 2016, 101, 177–183 CrossRef CAS.
- S. Abdulla, T. L. Mathew and B. Pullithadathil, Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection, Sens. Actuators, B, 2015, 221, 1523–1534 CrossRef CAS.
- A. Husain, S. Ahmad, M. U. Shariq and M. M. A. Khan, Ultra-Sensitive, Highly Selective and Completely Reversible Ammonia Sensor Based on Polythiophene/SWCNT Nanocomposite, Materialia, 2020, 100704 CrossRef CAS.
- O. Hamouma, N. Kaur, D. Oukil, A. Mahajan and M. M. Chehimi, Paper strips coated with polypyrrole-wrapped carbon nanotube composites for chemi-resistive gas sensing, Synth. Met., 2019, 258, 116223 CrossRef CAS.
- M. L. Y. Sin, G. C. T. Chow, G. M. K. Wong, W. J. Li, P. H. W. Leong and K. W. Wong, Ultralow-power alcohol vapor sensors using chemically functionalized multiwalled carbon nanotubes, IEEE Trans. Nanotechnol., 2007, 6, 571–577 Search PubMed.
- Y. Wang and J. T. W. Yeow, A review of carbon nanotubes-based gas sensors, J. Sens., 2009, 2009, 1–24 CrossRef.
- M. C. Petty, T. Nagase, H. Suzuki and H. Naito, Molecular Electronics, Springer Handbook of Electronic and Photonic Materials, Springer, 2017, p. 1 Search PubMed.
- B. Liu, X. Liu, Z. Yuan, Y. Jiang, Y. Su and J. Ma, et al., A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature, Sens. Actuators, B, 2019, 295, 86–92 CrossRef CAS.
- D. Zhang, X. Fan, X. Hao and G. Dong, Facile fabrication of polyaniline nanocapsule modified zinc oxide hexagonal microdiscs for H2S gas sensing applications, Ind. Eng. Chem. Res., 2019, 58, 1906–1913 CrossRef CAS.
- A. Husain, S. Ahmad and F. Mohammad, Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite, Mater. Chem. Phys., 2020, 239, 122324 CrossRef CAS.
- J. Ram, R. G. Singh, F. Singh, V. Kumar, V. Chauhan and R. Gupta, et al., Development of WO 3-PEDOT:PSS hybrid nanocomposites based devices for liquefied petroleum gas (LPG) sensor, J. Mater. Sci.: Mater. Electron., 2019, 30, 13593–13603 CrossRef CAS.
- A. Sáaedi, P. Shabani and R. Yousefi, High performance of methanol gas sensing of ZnO/PAni nanocomposites synthesized under different magnetic field, J. Alloys Compd., 2019, 802, 335–344 CrossRef.
- A. H. Shah, Applications of carbon nanotubes and their polymer nanocomposites for gas sensors, Carbon Nanotubes: Curr. Prog. Their Polym. Compos., 2016, 459–494 CAS.
- G. W. Hunter, S. Akbar, S. Bhansali, M. Daniele, P. D. Erb and K. Johnson, et al., Editors' choice—critical review—a critical review of solid state gas sensors, J. Electrochem. Soc., 2020, 167, 037570 CrossRef CAS.
- A. Mora, F. Han and G. Lubineau, Estimating and understanding the efficiency of nanoparticles in enhancing the conductivity of carbon nanotube/polymer composites, Results Phys., 2018, 10, 81–90 CrossRef.
- C. Liu, H. Tai, P. Zhang, Z. Yuan, X. Du and G. Xie, et al., A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature, Sens. Actuators, B, 2018, 261, 587–597 CrossRef CAS.
- S. Ramesh, Y. J. Lee, S. Msolli, J. G. Kim, H. S. Kim and J. H. Kim, Synthesis of a Co3O4@gold/MWCNT/polypyrrole hybrid composite for DMMP detection in chemical sensors, RSC Adv., 2017, 7, 50912–50919 RSC.
- J. Wan, Y. Si, C. Li and K. Zhang, Bisphenol a electrochemical sensor based on multi-walled carbon nanotubes/polythiophene/Pt nanocomposites modified electrode, Anal. Methods, 2016, 8, 3333–3338 RSC.
- J. N. Gavgani, H. S. Dehsari, A. Hasani, M. Mahyari, E. K. Shalamzari and A. Salehi, et al., A room temperature volatile organic compound sensor with enhanced performance, fast response and recovery based on N-doped graphene quantum dots and poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) nanocomposite, RSC Adv., 2015, 5, 57559–57567 RSC.
- S. G. Bachhav and D. R. Patil, Study of polypyrrole-coated MWCNT nanocomposites for ammonia sensing at room temperature, J. Mater. Sci. Chem. Eng., 2015, 3, 30 CAS.
- B. Philip, J. K. Abraham, A. Chandrasekhar and V. K. Varadan, Carbon nanotube/PMMA composite thin films for gas-sensing applications, Smart Mater. Struct., 2003, 12, 935 CrossRef CAS.
- Y. Fan, M. Burghard and K. Kern, Chemical defect decoration of carbon nanotubes, Adv. Mater., 2002, 14, 130–133 CrossRef CAS.
- K. H. An, S. Y. Jeong, H. R. Hwang and Y. H. Lee, Enhanced sensitivity of a gas sensor incorporating single-walled carbon nanotube–polypyrrole nanocomposites, Adv. Mater., 2004, 16, 1005–1009 CrossRef CAS.
- W. Zhang, S. Cao, Z. Wu, M. Zhang, Y. Cao and J. Guo, et al., High-Performance Gas Sensor of Polyaniline/Carbon Nanotube Composites Promoted by Interface Engineering, Sensors, 2020, 20, 149 CrossRef CAS.
- N. R. Tanguy, M. Thompson and N. Yan, A review on advances in application of polyaniline for ammonia detection, Sens. Actuators, B, 2018, 257, 1044–1064 CrossRef CAS.
- S. Bai, Y. Tian, M. Cui, J. Sun, Y. Tian and R. Luo, et al., Polyaniline@SnO2 heterojunction loading on flexible PET thin film for detection of NH3 at room temperature, Sens. Actuators, B, 2016, 226, 540–547 CrossRef CAS.
- L. Xue, W. Wang, Y. Guo, G. Liu and P. Wan, Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors, Sens. Actuators, B, 2017, 244, 47–53 CrossRef CAS.
- P. Kar and A. Choudhury, Carboxylic acid functionalized multi-walled carbon nanotube doped polyaniline for chloroform sensors, Sens. Actuators, B, 2013, 183, 25–33 CrossRef CAS.
- T. Zhang, M. B. Nix, B. Y. Yoo, M. A. Deshusses and N. V. Myung, Electrochemically functionalized single-walled carbon nanotube gas sensor, Electroanalysis, 2006, 18, 1153–1158 CrossRef CAS.
- Y. Zhang, B. R. Bunes, N. Wu, A. Ansari, S. Rajabali and L. Zang, Sensing methamphetamine with chemiresistive sensors based on polythiophene-blended single-walled carbon nanotubes, Sens. Actuators, B, 2018, 255, 1814–1818 CrossRef CAS.
- A. Husain, S. Ahmad and F. Mohammad, Electrical Conductivity and Ammonia Sensing Studies on Polythiophene/MWCNTs Nanocomposites, Materialia, 2020, 100868 CrossRef CAS.
- R. Yoo, J. Kim, M. J. Song, W. Lee and J. S. Noh, Nano-composite sensors composed of single-walled carbon nanotubes and polyaniline for the detection of a nerve agent simulant gas, Sens. Actuators, B, 2015, 209, 444–448 CrossRef CAS.
- C. P. Chang and C. L. Yuan, The fabrication of a MWNTs–polymer composite chemoresistive sensor array to discriminate between chemical toxic agents, J. Mater. Sci., 2009, 44, 5485–5493 CrossRef CAS.
- S. Ebrahim, R. El-Raey, A. Hefnawy, H. Ibrahim, M. Soliman and T. M. Abdel-Fattah, Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion, Synth. Met., 2014, 190, 13–19 CrossRef CAS.
- S. Gaikwad, G. Bodkhe, M. Deshmukh, H. Patil, A. Rushi and M. D. Shirsat, et al., Chemiresistive sensor based on polythiophene-modified single-walled carbon nanotubes for detection of NO2, Mod. Phys. Lett. B, 2015, 29, 1540046 CrossRef CAS.
- M. Eising, C. E. Cava, R. V. Salvatierra, A. J. G. Zarbin and L. S. Roman, Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor, Sens. Actuators, B, 2017, 245, 25–33 CrossRef CAS.
- L. He, Y. Jia, F. Meng, M. Li and J. Liu, Gas sensors for ammonia detection based on polyaniline-coated multi-wall carbon nanotubes, Mater. Sci. Eng., B, 2009, 163, 76–81 CrossRef CAS.
- T. Sarkar, S. Srinives, S. Sarkar, R. C. Haddon and A. Mulchandani, Single-walled carbon nanotube–poly(porphyrin) hybrid for volatile organic compounds detection, J. Phys. Chem. C, 2014, 118, 1602–1610 CrossRef CAS.
- Y. Lu, M. Meyyappan and J. Li, A carbon-nanotube-based sensor array for formaldehyde detection, Nanotechnology, 2010, 22, 055502 CrossRef.
- S. Srivastava, S. S. Sharma, S. Kumar, S. Agrawal, M. Singh and Y. K. Vijay, Characterization of gas sensing behavior of multi walled carbon nanotube polyaniline composite films, Int. J. Hydrogen Energy, 2009, 34, 8444–8450 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |