Open Access Article
Open Access ArticleCerium oxide nanoparticles: properties, biosynthesis and biomedical application
Kshitij RB Singh†
 ,
Vanya Nayak†
,
Vanya Nayak†
 ,
Tanushri Sarkar
,
Tanushri Sarkar
 and
Ravindra Pratap Singh
and
Ravindra Pratap Singh
 *
*
Department of Biotechnology, Faculty of Science, Indira Gandhi National Tribal University, Amarkantak, Madhya Pradesh 484887, India. E-mail: rpsnpl69@gmail.com; ravindra.singh@igntu.ac.in; Tel: +91-91-0934-6565
First published on 21st July 2020
Abstract
Nanotechnology is the branch of science which deals with particles ranging between 1–100 nm. These particles are called nanoparticles, and they exhibit unique electronic, optical, magnetic, and mechanical properties, which make them different from the bulk material. These properties of nanomaterials help them to find a variety of applications in the biomedical, agricultural, and environmental domains. Cerium oxide nanoparticles have gained a lot of attention as a potential future candidate for ending various kinds of problems by exhibiting redox activity, free radical scavenging property, biofilm inhibition, etc. Synthesis of these nanoparticles can be performed very easily by utilizing chemical or biological methods. But in this review, the focus is laid on the biosynthesis of these nanoparticles; as the biosynthesis method makes the cerium oxide nanoparticle less toxic and compatible with the living tissues, which helps them to find their path as an anticancer, anti-inflammatory and antibacterial agents. The pre-existing reviews have only focused on details relating to properties/applications/synthesis; whereas this review draws attention towards all the aspects in single review covering all the details in depth such as biosynthesis methods and its effect on the living tissues, along with properties, biomedical applications (diagnostic and therapeutic) and future outlook of the cerium oxide nanoparticle.
1 Introduction
Nanotechnology in the past decade has shown a momentous growth and revolutionized the biomedical, industrial, environmental, and material sciences domains.1–7 It is the study of extremely small things and its applications in various fields. Particles of size ranging from 1 to 100 nm are considered nanoparticles, and they exhibit higher surface to volume ratio.8 Nanoparticles exhibit unique properties like electronic, optical, magnetic, and mechanical properties due to their size, which makes them different from the bulk material. There are various types of nanoparticles: carbon nanotubes (multiwalled & single-walled), fullerenes, metals (Au, Ag, etc.), metal oxides (zinc oxide (ZnO), cerium oxide (CeO2), titanium oxide (TiO), etc.), liposome-bound, dendrimer-bound, albumin-bound, polymeric, quantum dots (CdSe, CdTe), magnetic nanoparticles, etc.9–12 Further, these nanoparticles play a very crucial role in pollution reduction from the environment, as these nanoparticles have a large surface area, which also enables them to be used in wastewater treatment.13Cerium is a member of the lanthanide group and is the most abundant rare metal, with an atomic number of 58; it shows 3.19 eV wide-bandgap along with high excitation energy.2 It exhibits catalytic properties due to shielding of 5p and 4d electrons in the 4f orbital.14 Cerium oxide in the bulk state exists in both +3 and +4 state, which helps them to form CeO2 (Fig. 1) and CeO2−x and therefore exhibits antioxidant properties.15,16 Free radicals are produced in a very minute amount during normal metabolism and contain an electron in the outermost shell. They are produced within a cell and incorporates: superoxide (O2+), hydrogen radicals, lipid hydroperoxides, etc.17 Normal oxygen metabolism yields reactive oxygen species (ROS) as a by-product, and plays a major role in inflammation which affects normal cellular function, and further leads to pathogenicity by damaging cell membranes, protein, and DNA; thus triggering apoptosis. Further, from the investigation, it is well known that cerium oxide nanoparticle act as a catalyst, which mimics the feature of antioxidant enzyme superoxide dismutase (SOD) and scavenges ROS or free radicals.15,18 Due to the high reactive surface area provided by the fluorite crystalline lattice structure of cerium oxide, it helps them in the neutralization of the free radicals.19 This nanoparticle is utilized for making solar cells,20 as a catalyst for fuel oxidation,21 chemical–mechanical polarization,22 and corrosion protection;23 they also exhibit biorelevant activities and are considered as potential pharmacological agents.24 Further, the lattice structure of cerium oxide nanoparticles forms oxygen vacancies, which make them act as a scavenger of free radicals in the physiological conditions.25,26
Prior reviews27–30 have laid focus on single aspects, namely properties/synthesis/applications of cerium oxide nanoparticles. But this review presents the updated and detailed development of cerium oxide (metal oxide) nanoparticle applications in the biomedical domain by considering the diagnostic and therapeutic aspect of this nanoparticle; these nanoparticles have gained a lot of attention in the biomedical field in the recent years and is still developing at a faster pace. Apart from the biomedical application of cerium oxide nanoparticles, this review also highlights some glimpse of cerium oxide nanoparticles application in the environmental and agricultural fields and further elaborates its physicobiochemical properties, various biological methods and protocols for synthesis (biosynthesis). Biosynthesized nanoparticles have gained a lot of attention in recent years for application in diagnosis and therapeutics, as they are simple, efficient, and cost-effective.6 But comprehensive review based on the biosynthesis of cerium nanoparticles is not yet present. Thus, this review emphasizes the biosynthesis of cerium oxide nanoparticles in detail. Hence, this review analyzes the current status of the cerium oxide nanoparticles in the biomedical domain along with its prospects.
2 Physicobiochemical properties
2.1 Physicochemical properties
Cerium is the most abundant rare earth alkali element which is listed in the F block of the periodic table, and they are found in minerals, namely synchysite, hydroxyl bastnasite, monazite, zircon, rhabdophane, sallanite, and bastnasite. Cerium exhibits exceptional character of cycling between the two ionic states, which is Ce3+ and Ce4+, and this is possible due to the presence of ground-state electron in the 4f (Xe 4f15d16s2) orbital which enables it to exhibit redox properties. Further, the cerium oxide nanoparticle (Ce4O8) is a face-centered cubic (fcc) fluorite lattice comprising of eight oxygen atoms bonded to the cerium atom (Fig. 2), and the complete unit cell (Ce4O8) measures 5.1 Å on an edge.31 The building blocks of nanoparticles are the crystallite nature of the particle, and in the cerium oxide nanoparticle, polycrystallinity is more common. Generally, the crystallite unit depends on the synthesis method, and the crystallites are analyzed through the X-ray diffraction technique.32 Moreover, hierarchal assembly of the unit's cells into crystallites and crystallites to particles can be done by self-assembly of particles into sheets, rods, hollow variants, etc. which are larger structures.33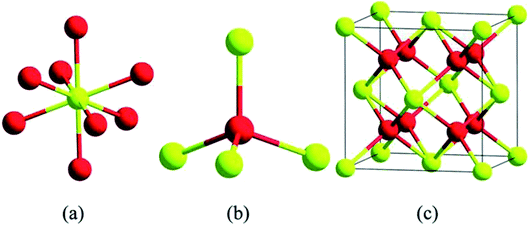 | ||
| Fig. 2 Structurally analyzed ceria crystals Ce4O8 (unit cell); in (a) and (b) yellow color represents eight-folds of cerium atoms and red represents four-fold oxygen atoms in ceria crystal structure; (c) is the basic fcc fluorite lattice structure of Ce4O8 (reproduced from K. Reed et al., Environ. Sci.: Nano, The Royal Society of Chemistry, 2014 (ref. 22)). | ||
Density and molar mass of cerium is 6.770 gm cm−3 (approximately) and 140.12 g mol−1 respectively; it is malleable and at room temperature oxidizes very readily. It also shows excellent thermal properties with melting and boiling temperature of 798 °C and 3424 °C respectively.34 Cerium in its oxide form represents the cubic fluorite structure, and at the nanoscale range, it maintains the same structure along with oxygen deficiencies, which provide it with redox reaction sites. Further, the cubic fluorite structure shows three low-index planes (100), (110), and (111), and the dipole moments perpendicular to the surface shows charged plane, neutral, and none respectively. Interactions between the adsorbed molecules with the cerium's surface are dependent on the crystal surfaces and plane properties exhibited by the cerium nanoparticles. The structure also enhances the catalytic property. Unlike the (100) and (111), (110) does not present the o-terminal endings, rather it has a Ce center with O-ions (C1, C2). The ability of cerium oxide nanoparticles to exists in +3 and +4 valence states helps them to exhibit two oxidation states, which are Ce3+ and Ce4+.35,36 Cerium oxide is highly unsaturated, which contributes to the instability and promotes restructuring of the surface. Further, this also affects the microstructure and physicochemical environment, which affects their chemical reactivity. They can also switch between two oxidation states that are from trivalent +3 to tetravalent +4, giving them the capability to show redox reactions.37–40 The elimination of the oxygen ions by cerium oxide leads to the non-stoichiometric and reduced metal oxide, which clears the presence of certain binding energy between Ce3+ and oxygen atoms.41
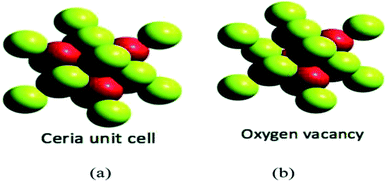 | ||
| Fig. 3 (a) Represents the unit cell structure of cerium oxide nanoparticles and (b) represents single oxygen vacancy of cerium oxide unit cell, where absence of one oxygen atom in left side (uppermost) and forward octant position can be seen (reproduced from K. Reed et al., Environ. Sci.: Nano, The Royal Society of Chemistry, 2014 (ref. 22)). | ||
Measurement of the Ce3+/Ce4+ ratio can be useful to understand the concentration of oxygen vacancy. Oxygen vacancy can construct itself, and they can also be quantified by a numeral called oxygen storage capacity (OSC). This numeral can be expressed as oxygen micromoles released per-gram of starting material. The OSC value of cerium dioxide in the gas phase is 1452.47 μmol per O2 per g and can be explained from the below-mentioned equilibrium reaction:
| CeO2 ↔ CeO2−x + x/2O2 for x = 0.5 then CeO1.5 (Ce2O3) | (1) |
Further, commonly used cerium oxide is prepared as micro- or nano-scale crystals but not as a gas phase molecule. The entirely reduced commercial cerium oxide can be just a fraction of the OSC value calculated theoretically in eqn (1). OSC equilibrium equation signifies a reversible reaction and it justifies that this material can act as a catalyst, as it’s an idea that is fundamental. Indeed, solid cerium oxide particles can be assumed as oxygen buffer;31 this provides/removes oxygen from the surrounding environment by reacting to lack/excess of oxygen in the existing environment. This ability to extract oxygen atoms reversibly from the lattice can be utilized for catalytic oxidation of various materials, namely CO and other exhaust gases which are partially oxidized (Fig. 4).
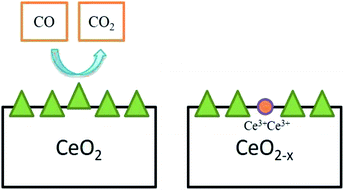 | ||
| Fig. 4 Oxygen vacancy created by CeO2 particle while oxidizing CO to CO2, and two Ce4+ atoms were reduced simultaneously (adapted from K. Reed et al., Environ. Sci.: Nano, The Royal Society of Chemistry, 2014 (ref. 22)). | ||
There are two different thoughts for the mechanism of cerium oxide on the SOD-mimetic and hydrogen peroxide catalase. The initial thought is that the Ce3+/Ce4+ ions, interact directly to neutralize superoxide and destroy peroxide, and this is known as the ionic mechanism. The other thought is that SOD-mimetic and hydrogen peroxide catalase reactions proceed by annihilation and oxygen vacancy creation with the cerium ionic states by interchanging between +3 and +4 to support the oxygen vacancy state. Further, referring to the ionic mechanism, the SOD mimetic component is favored by an increase in the Ce3+/Ce4+ ratio,47 and on the other hand, catalase reaction is favored by a decrease in this ratio.48 Apart from focusing on ratios of ions, let's explore the thermodynamic aspect of the Ce3+/Ce4+ in detail by investigative dynamic reaction chemistry. The unique and complete balanced reaction is embodied in the eqn (2) and (3), which represents the SOD-catalytic like dismutation reaction.
| 3Ce4+ + 3O2− → 3Ce3+ + 3O2 (Ce4+ reduction) | (2) |
| Ce3+ + O2− + 2H+ → Ce4+ + H2O2 (Ce3+ oxidation) | (3) |
Considering a point that which type of cations can be doped in cerium lattice, for the same, it can be assumed that cations with low ionic radii than cerium ion can be the ideal candidate for doping, but this is not always true. The thermodynamic aspect specially enthalpy of incorporated dopant must be taken into consideration, in this regard modern computational quantum mechanical methods can be useful. Further, K. Reed et al.22 investigated and found that substitution of the small iron atom of 78 pm to 97 pm cerium was endothermic by 4.3 eV per Fe2O3 unit, but in the case of 116 pm lanthanum, the substitution was exothermic by 3.3 eV per La2O3 unit. Hence, from the studies, its well understood that approx. 2–4% of iron is doped into cerium lattice in low-temperature conditions, and the iron associated was in amorphous form.
Many investigators65–70 have studied the optical characteristic of cerium oxide thin films by utilizing UV-vis transmittance measurements. The deposition of material on the substrate to form thin film generates interference effects and creates oscillations, which determine the spectra.71 Hence, the amplitude of the oscillations provides a refractive index. Higher the amplitude, higher is the refractive index of the film; an increase in the thickness of the film, higher is the number of oscillations.72 Films of cerium oxide show excellent optical properties thus offering them applications as electro-optical and optoelectronic devices.65,66,73–77 These films have a high refractive index, dc permittivity, and transparency in visible and IR (near- and mid-) region. In every study, the values of the refractive index were different and fall in the range of 1.6–2.4.76,78–80 Further, the direct band-gap falls in the range of 3.2–3.6 eV, while the indirect band-gap is ranged between 2.9–3.3 eV. Cerium oxide nanoparticles prepared by hydroxide mediated method was having a particle size of 6.4 nm and further, studies related to optical property was performed by utilizing UV-visible absorption and fluorescence spectroscopy, which revealed that the prepared cerium oxide nanoparticle recorded the absorbance peak at 349 nm, and band-gap of 3.1 eV; photoluminescence spectra (PL) showed violet emission peak at 477 nm, due to interface traps at boundaries of grain, and PL has shown slight emission peak at 508 nm which might have resulted due to surface defect or oxygen defects.81
2.2 Biological properties
 | ||
| Fig. 5 Schematic representation of SOD reaction mechanism by cerium oxide nanoparticle (reproduced from I. Celardo et al., Nanoscale, The Royal Society of Chemistry, 2011 (ref. 24)). | ||
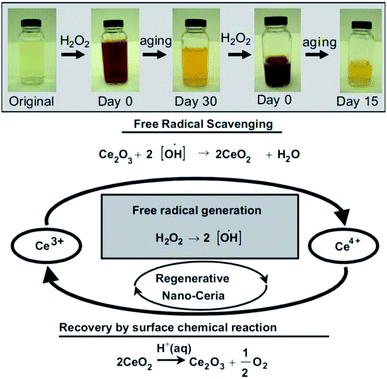 | ||
| Fig. 6 Top: Observation of change in color when, cerium oxide nanoparticle coated with dextran was treated with H2O2 at different time intervals. Bottom: Schema of chemical reaction showing auto-regenerative properties cerium oxide nanoparticles and possible mode of mechanism data of the cerium oxide nanoparticle autocatalytic behavior and free-radical scavenging property (reproduced from M. Das et al., Biomaterials, Elsevier, 2007 (ref. 105)). | ||
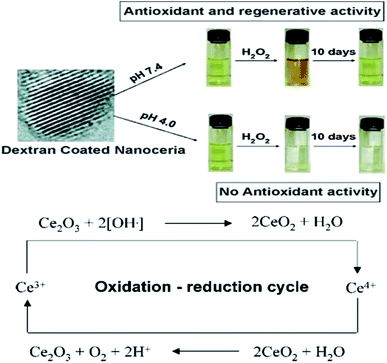 | ||
| Fig. 7 Top: Color change in solutions of cerium oxide nanoparticle coated with dextran at basic and acidic pH environment on addition of H2O2. Bottom: Detailed probable revised mode of mechanism data cerium oxide nanoparticle auto-regenerative attribute and free-radical scavenging property (reproduced from J. M. Perez et al., Small, John Wiley and Sons, 2008 (ref. 106)). | ||
Cerium oxide is capable of scavenging gaseous free radicals that is nitric oxide, which is found in the living cells and these nanoparticles have the ability to interchange between the Ce3+ and Ce4+ redox states which is provided by the substantial oxygen storage capacity in their structure.107 The scavenging of the reactive nitrogen species (RNS) is important as they cause damage to the biomolecules like DNA, RNA, etc. by forming toxic products that cause mutations in them. However, the RNS helps in cell signaling, vasodilation, and immune response.108 Cerium oxide nanoparticles interact with peroxynitrite (ONOO−) which are formed due to the interaction of the superoxide radicals with the nitric oxides.22 The cerium oxide nanoparticles +3 and +4 ratios are inversely proportional to the scavenging property of the nitric oxide radicals; that means if the ratio is low the scavenging action will be high and vice versa.109,110
3 Biosynthesis
The various method of synthesis of nanoparticles determines its physiochemical properties (morphology, varying size, etc.). To obtain physiochemical properties which are beneficial; synthesis parameters are carefully administered.15 There are mainly two approaches applied in the synthesis of nanoparticles which are demonstrated in the Fig. 8. Further, explaining these approaches of nanoparticle synthesis, the first one is top-down, in this approach large molecules are decomposed into smaller form known as nanoparticles; this method is also known as destructive approach. Second approach of nanoparticle synthesis is bottom-up, in this process the nanoparticles are prepared from elementary substances (atoms); this approach is also called as building-up.109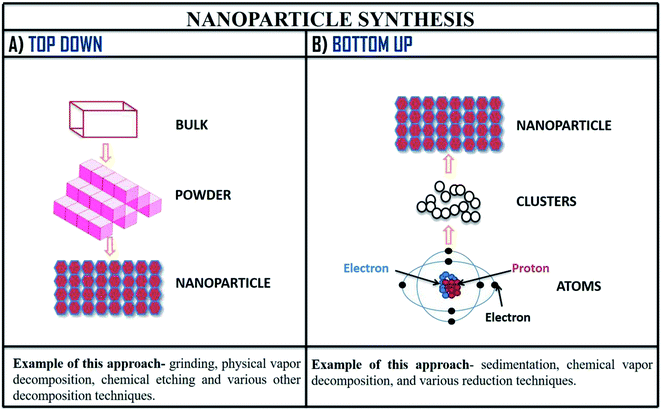 | ||
| Fig. 8 Method of nanoparticle synthesis; (A) top-down approach and (B) bottom-up approach of nanoparticle synthesis with examples. | ||
Numerous preparation techniques (Table 1) have been implied to produce cerium oxide nanoparticles, involving chemical and biological synthesis.111–115 Nowadays, researchers are adapting the fundamentals of green chemistry for the synthesis of nanoparticles as they are nontoxic and environment friendly, and this eco-friendly process is termed as biosynthesis; it is cost-effective and simpler alternative to chemical method of synthesis. Synthesis of cerium oxide nanoparticle using natural matrices as stabilizing agents decreases the bio-compatibility concerns. Cerium oxide nanoparticles biosynthesis method is mediated by polymers, plants and nutrient. Synthesis of cerium oxide nanoparticle is generally preferred by chemical methods because it helps to determine size and shape of these nanoparticles. But the problem still persist with the chemical method of synthesis that it has low biocompatibility and for synthesis using this method they require higher energy consumption to maintain high pressure and temperature; due to these problems the interest in minimizing wastage of energy resources, implementation of sustainable process is the need of present, and for the same adapting the fundamentals of green chemistry is increasing. Table 2 emphasize biosynthesis along with the laboratory protocol for synthesis of cerium oxide nanoparticle.110,116–125
| (C) | Fungal mediated synthesis | ||
|---|---|---|---|
| S. no. | Fungus name | Synthesis procedure | Reference |
| 1 | Humicola sp. |  |
125 |
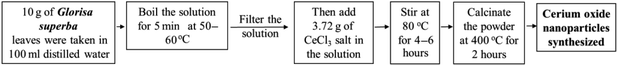
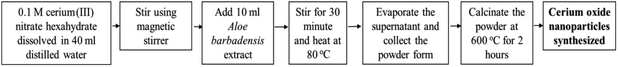
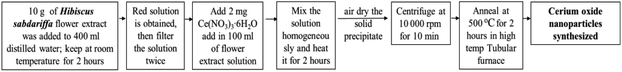
Plant mediated metal oxide synthesis is a part of biosynthesis method, in this method extract of plants act as an agent for capping and stabilizing; thus, resulting in relatively higher yield of nanoparticles when compared to chemical synthesis method.126–130 From Acalypha indica leaf extract in the aqueous form, pure and stable cerium oxide nanoparticles can be synthesized and this method was found to be simple, cost effective and efficient.110,131 Another approach for biosynthesis of stable and well-dispersed cerium oxide nanoparticle was successfully accomplished by the plant extract of Petroselinum crispum, as they were capable of reducing the cerium ammonium nitrate [(NH4)2Ce(NO3)6] salt.116 Gloriosa superba plant extract were able to reduce the cerium salt and synthesized 5 nm spherical shaped small crystals of cerium oxide nanoparticles with a higher surface area.117 Aloe barbadensis miller plant, commonly known as Aloe vera along with the cerium(III) nitrate hexahydrate was used for the biosynthesis of spherical cerium oxide nanoparticles with a mean diameter of 63.3 nm.118 Olea europaea leaf extract act as a reducing agent by chelating cerium nitrate and thus produces the 24 nm pure single-face cubic structure of cerium oxide nanoparticles, which can be effectively used for biomedical applications.119 By using Hibiscus sabdariffa flower extract, the first entire green synthesis protocol was designed to synthesize pure cerium nanoparticles without the use of any additional standard components (reduction or oxidization agent); the synthesized cerium oxide nanoparticles exhibited an average particle size of ∼3.9 nm and were spherical in shape.120 Another plant-based approach for the synthesis of cerium oxide nanoparticles was from Azadirachta indica, which is also known as neem (a native plant of India); extract of this plant was able to reduce cerium nitrate [Ce(NO3)2·6H2O], resulting into cerium oxide nanoparticles with higher quality yield and cubic crystalline structure.121
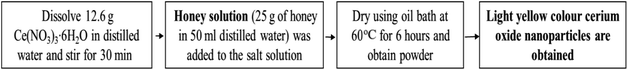
Natural polymers like starch which act as stabilizers can be utilized for synthesis of cerium oxide nanoparticles; the synthesized particle from this method are smaller in size. Cerium oxide nanoparticle can be synthesized by sol–gel method mediated by starch in aqueous solution from cerium nitrate salt; the resulting nanoparticles will be cubic fluoride in shape with a mean diameter of 6 nm.122 A simple, cost-effective method to synthesize cerium oxide nanoparticles is by using honey which can act as the stabilizing agent when dissolved with the cerium(III) nitrate hexahydrate [Ce(NO3)3·6H2O].123 Nutrient mediated synthesis is extremely cost effective and by utilizing egg white (nutrient substrate), as a stabilizing agent result in the controlled isotropic synthesis of cerium oxide nanoparticles.132 Pectin a non-toxic biopolymer which can be obtained by extracting Indian red Citrus maxima peels, can be utilized for biosynthesis of cerium oxide nanoparticles and the resulting nanoparticles exhibited size of ≤40 nm (average); were cubic fluorite structure and spherical in shape.124,133 Synthesis of these nanoparticle can also be achieved by the help of nontoxic and renewable degraded agarose, as it has capping capabilities.134 The cerium oxide nanoparticle synthesized from different method will have varying particle size, crystallization temperature, thermal stability, morphology, fluorescence properties, luminescence, etc. and these attributes offer the synthesized material applications in various domains; in Table 3, few most commonly synthesized nanoparticles from different method are demonstrated with striking difference in its properties (physicobiochemical). Hence, the above discussed methods give an overview of biosynthesis methods for the synthesis of the cerium oxide nanoparticles giving a reference of well-characterized properties.
| S. no. | Source | Particle size | Crystallization temperature | Morphology | Others | Ref. |
|---|---|---|---|---|---|---|
| 1 | Acalypha indica | 25–30 nm | 800 °C | Spherical-fcc (face centered cubic) shaped structure. | Thermal stability temperature was 998 °C | 110 |
| 2 | Gloriosa superba | 5 nm | 400 °C | Spherical-fcc structure | Showed bioluminescence at 486 nm, and exhibits antibacterial activity | 117 |
| 3 | Olea europa | 24 nm | ∼500 °C | Spherical and homogenous | Thermal stability at 50–600 °C, and exhibits antibacterial and antifungal activity | 119 |
| 4 | Azadirachta indica | 10–15 nm | ∼240–250 °C | Fcc-structure | It showed thermal decomposition between 329–434 °C | 121 |
| 5 | Starch | ∼6 nm | 200–400 °C | Spherical-fcc structure | Showed low toxicity to N2a cell lines | 122 |
| 6 | Food-mediated (honey) | ∼23 nm | 200–800 °C | Uniform spherical structure | Thermal stability at 400–800 °C | 123 |
| 7 | Pectin | 5–40 nm | ∼400 °C | Spherical | Showed antioxidant, antibacterial, and bio–luminescence activity | 124 |
| 8 | Fungal-mediated | 12–20 nm | 300 °C | Spherical | Showed bioluminescence activity | 125 |
| 9 | Egg-white | 25 nm | 200–800 °C | Fluorite cubic structure | Thermal stability at 20–1000 °C, and showed non-toxic effect on periodontal fibroblast cells | 132 |
| 10 | Degraded agarose | ∼10 nm | 200–800 °C | Fcc-structure | Thermal stability at ∼20–1000 °C | 134 |
4 Biomedical applications
One of the most potential metal oxide nanoparticles after the silver oxide nanoparticles, which gained attention in past few decades are cerium oxide nanoparticles; they exhibit enormous range of applications in different domains like agriculture, environmental and biomedical. Application of cerium oxide nanoparticle in the biomedical domain are enormous. From an investigation135 performed by scientists it is found that cerium oxide nanoparticles should have either pro-oxidant or anti-oxidant properties to be nontoxic to humans; as cerium oxide nanoparticles are not found in humans and till date no clearing mechanism is known, which will lead to systemic toxicity in humans. From the above investigation it can be highlighted that nanoparticle interaction with microenvironment should be considered while designing effective nanocarriers. At present, use of these nanoparticles have good grip in industrial applications, but biomedical applications are still developing. Till date lot of biomedical study related with diagnosis and treatment of life-threatening diseases by utilizing cerium oxide nanoparticles are performed and result obtained from these studies have shown good sign. Cerium oxide nanoparticles have enormous application in this field which are elaborated below and in Fig. 9.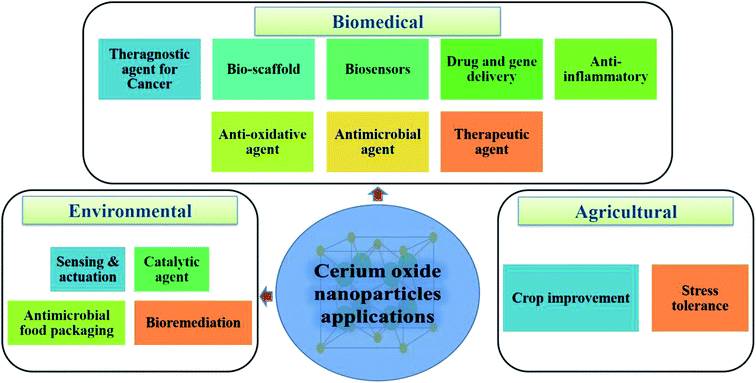 | ||
| Fig. 9 Potential applications of cerium oxide nanoparticles in the biomedical, environmental, and agricultural domains. | ||
4.1 Anti-cancer
Cancer is the leading cause of death worldwide and cerium oxide nanoparticles exhibit cytoprotectant property, this make them induce ROS formation in the cancer cells which make these nanoparticles a good anticancer agent; this is due to deregulation of antioxidant enzyme expression and acidic environment in the cancer cells which develops ROS, further leading to the generation of RNS interfering with intracellular activities.106,136 The anti-invasive property of cerium oxide nanoparticle leads to regulation of antioxidant enzymes by inducing radio-sensations; this regulates the quantity of ROS and provides radioprotection to normal cells. Thus, this can be used as the potential radiation sensitizers for cancer therapy by protecting normal cells surrounded by radiation damage.137–140 As these nanoparticle exhibit redox-activity due to which it can become new prototype for the treatment of cancer.141 Recent investigations have revealed that cerium oxide nanoparticles are nontoxic to normal cell lines and can be successfully used for the treatment of cancer; but this nanoparticle can't be used for prostate cancer care as analyzed by the MTT colorimetric assay.142,143 In in vivo and in vitro study by L. Alili et al.,144 they demonstrated effect of cerium oxide nanoparticles coated with polymer in human melanoma cells, and result of their study suggest, that these nanoparticle which were non-toxic to stromal cells showed cytotoxic, pro-apoptotic, and anti-invasive activity to melanoma cells; further this study was first to confirm that cerium oxide nanoparticle exhibit tumor suppressing properties in vivo and opens avenue for future cancer therapeutic development. In another study, the investigators prepared cerium oxide nanoparticles for different concentrations by utilizing two different methods that are hydrolysis (for +3 oxidation state) and hydrothermal (for +4 oxidation state); when these prepared nanoparticles by different method where tested on the human lung cancer cells in time dependent manner for 24, 48 and 72 hours. The cancer cells at nanoparticle concentration between 3.5–23.3 μg showed dose- and time-dependent cytotoxicity by inducing ROS, and it also demonstrated that the different oxidation states lead to the different cytotoxic levels, as the hydrothermal-cerium oxide nanoparticle showed more cytotoxicity when compared to the hydrolysis-cerium oxide nanoparticles, due to their higher cellular uptake.145 Further, on colon cancer cells of human these nanoparticles exhibit cytotoxicity that resulted in production of ROS, which causes depolarization of mitochondrial membrane leading to apoptosis.146 An in vivo study,147,148 on ovarian cancer cells was performed to check the inhibitory effect of cerium oxide nanoparticles for metastasis and angiogenesis; results suggest that they are better candidate for ovarian cancer treatment as it shows significant decrease in viability of cancer cells and increased cancer cell death. In other study, related with anti-cancer effect of cerium oxide nanoparticle in fibrosarcoma (cancer cells) cell lines, these nanoparticles generate ROS and as a result of ROS generation it lead to apoptosis.149 Doxorubicin loaded cerium oxide nanoparticles avert tumor cell-released growth factor (GF)-dependent modulation of stromal cells, namely neoangiogenesis and transdifferentiation. Thus, mediate their protection from apoptotic cell death initiated by doxorubicin. Beside this, in in vivo cerium oxide nanoparticles also decreases tumor invasion and tumor growth. In contrast, cerium oxide nanoparticles generate ROS, which causes cell death and doxorubicin loaded with this nanoparticle (combinational approach of treatment) help to enhance rate of apoptosis in the desired tumor cells (Fig. 10).150 Hence, it can be concluded that the cerium oxide nanoparticle are best anti-cancer agent as it has excellent tumor suppressing properties in vitro, as well as in in vivo for various type of cancers such as lung, colon, ovarian, etc.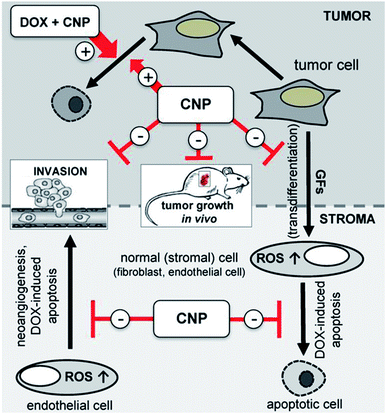 | ||
| Fig. 10 Schematic illustration of cerium oxide nanoparticles loaded doxorubicin mode of mechanism interaction data of stromal-tumor for cancer treatment by combinational approach (reproduced from P. Brenneisen and A. Reichert, Antioxidants, MDPI, 2018 (ref. 150)). | ||
4.2 Antibacterial
Cerium oxide nanoparticles when interact with bacterial cell by electrostatic attraction it generates ROS, which leads to bacterial cell death; this determines its antibacterial efficiency.151–153 The mechanism behind a decrease in the membrane permeability and bacterial cell death by cerium oxide nanoparticle may be due to the release of some ions, which particularly react with the thiol (SH) group found in the proteins of the bacterial cell membrane. These nanoparticles in bacterial cells cause a disturbance in intracellular functions (DNA replications, cell division, and cellular respiration), which induces ROS.154 Further, cerium oxide nanoparticle synthesized by the green method was utilized to study antibacterial activity by using Gram-negative bacteria Escherichia coli and Gram-positive bacteria Staphylococcus aureus, and the result of this study suggest Gram-positive bacteria were more susceptible to this nanoparticles as compared to the Gram-negative bacteria.117 The concentration of cerium oxide nanoparticle also determines the antibacterial efficiency, to prove this an experiment was designed to determine the antibacterial efficiency at different concentrations of nanoparticles, and this revealed that for the binding of metal oxide with the bacterial cell wall, electrostatic forces are required which will result into bacterial growth inhibition. Thus, a high concentration of these nanoparticles will exhibit excellent antibacterial efficacy.155 On the contrary, differences in membrane surface, surface charge density, and the metabolic processes are also responsible for the variation in the inhibitory effect of cerium oxide nanoparticles on Gram-negative and Gram-positive bacteria.156 Moreover, when E. coli bacterial cells are exposed to cerium oxide nanoparticles, these nanoparticles are directly taken up by the bacterial cell surface, which causes oxidative stress and leads to bacterial cell death.156,157 In cyanobacteria, oxygenic photosynthesis induces ROS production, and cerium oxide nanoparticle's Ce3+ site reacts with the produced ROS, which results in an oxidative reaction. This reaction further produces anions and radicals, which impairs membrane integrity and causes bacterial cell death.158 Besides cerium oxide nanoparticles, hybrid chitosan-cerium oxide nanoparticles also exhibit extraordinary antibacterial properties by disrupting bacterial cell membranes, which causes cell death; but it is only possible at a high concentration of this hybrid nanoparticles.159 The major problem with polysaccharide encapsulated bacteria is that it prevents direct contact of nanoparticles with the cell wall, which will lead to hindering antibacterial activity, and to overcome this problem indirect interaction or contact mechanism can be used. For this, ROS is produced outside the cell and then transferred to the cell via cell membrane; this results in the degradation of bacterial protein and nucleic acids, which finally causes cell death.1604.3 Anti-oxidant potential
An imbalance between ROS, production of nitrogen species, and anti-oxidant level cause oxygen stress, as nitrogen species and ROS, is a potent oxidizing and nitrating agent.161 These nanoparticles show remarkable antioxidant properties by scavenging the free radical's; thus, offering them potential medical application. Further, when cerium oxide nanoparticles were exposed with brain tissue of rat to analyze the antioxidant activity, it was observed that these nanoparticles increase thiol content and initiate caspase-3 activity, which declines the oxidative DNA damage and lipid peroxidation; resulting in enhanced antioxidant activity and also act as a neuroprotective agent.162 From another study, it was well established that these nanoparticles have free radical scavenging properties and radioprotective effects, which will protect it from induced oxidative damage, thus providing it anti-oxidative potential.139 Later it was determined that the Levan polysaccharide coated cerium oxide nanoparticles showed synergistic anti-oxidation property against H2O2 in the NIH3T3 cells. Thus, Levan polysaccharide coating offers stability and water solubility to the cerium oxide nanoparticles; as Levan and its derivatives show anti-oxidants, anti-inflammatory, and anti-tumor properties.163 In the human epithelial cell line (BEAS-2B), these nanoparticles exhibit oxidative stress against KBrO3.164 And further in epithelial cells, these nanoparticles hinder H2O2 and stops overproduction of ROS, which leads to a decrease in cell death and helps prevent cardiovascular diseases.165 Hence, these nanoparticles have the potential anti-oxidant ability and exhibit enormous biomedical applications.4.4 Anti-inflammation
Cerium oxide nanoparticles as mentioned earlier in this review has radical scavenging and auto regeneration mechanisms, which offer them the unique potential to be considered as an anti-inflammatory agent.166 Pro-inflammatory enzymes like iNOS expression cause rapid generation of free radicals in the body; protein in the iNOS gets activated by ROS, and macrophages produce NO. ROS production should not be stopped, and its level should also be not declined, but it can be reduced. As ROS depletion can result in pathological consequences, and they are essential for normal cellular functions. When the cerium oxide nanoparticles were tested on J774A.1 cell, it suppresses iNOS and ROS production; thus, acting as an anti-inflammatory agent.18 Further, cerium oxide nanoparticles when injected in the murine model of cardiomyopathy it suppresses pro-inflammatory markers like monocytes chemo-attractant protein-1 (MCP-1), interleukin-6 and also decreased oxidative stress. Thus, these nanoparticles in mice improved cardiac dysfunction and can play a key role as an agent for the treatment of ischemic heart disease.1674.5 Drug delivery
Cerium oxide nanoparticles are well known for their cytotoxic properties towards cancer cells, thus offering them anticancer activity. Further, these nanoparticles can also be utilized as a vector to deliver drugs.168 A study was conducted by a group of investigators to develop drugs for targeted photodynamic therapy of drug-resistant human breast cancer; they synthesized multifunctional nanocomposite chlorin e6 (Ce6)–folic acid (FA)–polyethylenimine–PEGylation cerium nanoparticles (PPCNPs) [Ce6–FA–PPCNPs], and these nanocarriers promoted cellular uptake. Hence, the result of this study suggests that these nanocomposites formed by cerium oxide nanoparticles are multifaceted and effective drug delivery systems.169 Further, carboxybenzenesulfonamide (an hCAII (human carbonic anhydrase) enzyme inhibitor) and carboxyfluorescein (a fluorophore for tracking nanoparticle in vivo and in vitro), when attached with cerium oxide nanoparticles via intermediate linker epichlorohydrin, can be used as a potential drug delivery system for the treatment of glaucoma.170 Moreover, from other investigations, it was suggested that cerium oxide nanoparticles due to their redox properties can be utilized to develop a very responsive drug delivery system.171Cerium oxide nanoparticles coated with polyacrylic acid can be used as a vector to carry drugs; it was synthesized and loaded with drug combination (doxorubicin and ganetespib) for effective treatment of NSCLC (non-small-cell lung cancer). The result of this study (Fig. 11) signifies that cerium oxide-based multifunctional nanoparticles can be used for targeted drug delivery treatment for this type of lung cancer by utilizing combination therapy.172 In the case of ovarian cancer cells, cerium oxide nanoparticles loaded with drug doxorubicin (CeO2–Dox) showed increased cell proliferation, cellular uptake, and apoptosis. Further, considering the drug release mechanism by carrier nanoparticle, it is released when it gets an acidic environment.173 Cerium oxide nanoparticles coated with dextran loaded curcumin as a drug was utilized for the treatment of childhood neuroblastoma, and the result signifies that cerium oxide nanoparticles coated with drug dextran loaded curcumin, induced cytotoxicity in the neuroblastoma cells. On the contrary, it was not harming the normal cells.174 Hence, it can be emphasized that multifunctional nanoparticles based on cerium oxide nanoparticles are best suitable to be utilized as a vector for synergistic and targeted drug delivery.175
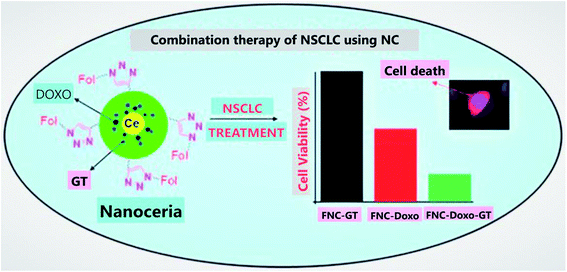 | ||
| Fig. 11 Combination therapy for non-small-cell lung cancer (NSCLC) by using NC (cerium oxide nanoparticles), which results as an excellent tool for targeted drug delivery system. In this illustration, FNC is folate decorated cerium oxide nanoparticles, GT is ganetespib, Dox is doxorubicin, and nanoceria is cerium oxide nanoparticles (reproduced from S. Sulthana et al., Mol. Pharm., American Chemical Society, 2017 (ref. 172)). | ||
4.6 Gene-delivery
Cerium oxide nanoparticles in 2016 were reported as a carrier for gene delivery, as the investigators of the study have synthesized hybrid cerium oxide–dimethyldioctadecylammonium bromide (DODAB) multifunctional nanoparticles, which replaced viral vector and was used to transfect plasma DNA (pEGFPN1) in different cell lines. Further, the transfection efficiency of nanovector (CeO2/DODAB) was analyzed in vivo by injecting it into the muscle of tibialis mice, and result signifies that it shows 3.5 folds higher fluorescence intensity then naked DNA treatment and it also didn't exhibit any toxic effect to the cells, but this nanovector showed approx. 17% less transfection efficiency than commercially available in vivo-jetPEI transfection reagents. Hence, it can be concluded that cerium oxide nanoparticles can be efficiently utilized as a transporter in the gene delivery method and can be the best alternative for viral vector gene delivery methods.1764.7 Bioscaffolds
Cerium oxide nanoparticle exhibits excellent pharmacological potential, which offers them applicability as a scaffold and makes them an extraordinary therapeutic agent. Porous bioactive glass scaffolds with cerium oxide nanoparticles were used for regeneration, and the result suggests that cerium oxide nanoparticles were nontoxic to cells, and they enhanced osteoblastic differentiation without the use of osteogenic supplements, namely dexamethasone, ascorbic acid, etc. They also found enhanced collagen production by bone-marrow-derived human mesenchymal stem cells (HMSCs). Thus, this study suggests that cerium oxide nanoparticles due to their oxygen buffering properties can be used as excellent bioscaffolds.177 Further, C. Mandoli et al.178 suggested that cerium oxide and poly(D,L-lactic-co-glycolic acid) powders when mixed at specific concentrations by solvent castings on pre-patterned molds; they can be used as a 2-dimensional polymeric ceramic bio-supports for in vitro stem cell culturing (Fig. 12). The findings of this study highlight that hybrid polymer ceramic bioactive scaffolds of cerium oxide nanoparticles can have potentiality as a tool for tissue engineering applications. Hence, these studies suggest that cerium oxide nanoparticle can be effectively used as a bioscaffold due to its unique anti-oxidant property; it also has virtuous hold to be a possible material for tissue engineering.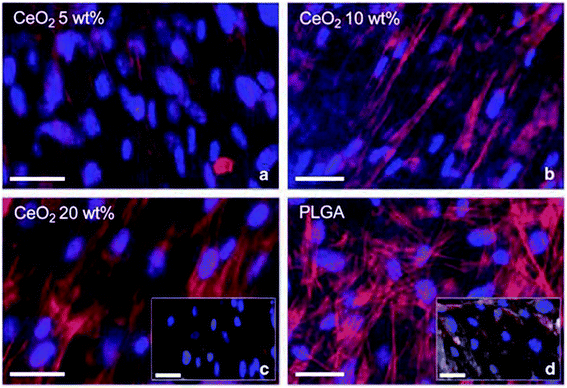 | ||
| Fig. 12 Immunofluorescence monitoring of in vitro culture of adult murine resident cardiac stem cell incubated by different concentration of cerium oxide nanoparticle-poly(D,L-lactic-co-glycolic acid) composite material for 6 days: (a) 5, (b) 10, and (c) 20% wt%, together with (d) poly(D,L-lactic-co-glycolic acid) (PLGA) as a control (reproduced from C. Mandoli et al., Adv. Funct. Mater., John Wiley and Sons, 2010 (ref. 178)). | ||
4.8 Treatment of diseases
The unique pharmacological properties to treat diseases shown by the cerium have been used for more than a century ago.179 The root causes of neurodegenerative diseases (Parkinson's disease, ischemic strokes, etc.) are proved to be due to an increase in oxidative stress and free radical production.180 Cerium oxide nanoparticles show neutron shielding effects by damaging the formation of free radicals, and it also affects the signal transduction pathways, which causes neuronal cell death; hence, these nanoparticles are considered as a potential element in treating the neurodegenerative diseases particularly Alzheimer's disease. These nanoparticles because of their antioxidant property, influence the signal transduction pathway when they are exposed to antibody-treated cells, and can be used as therapeutic material in the treatment of neurological diseases.17,105,181–184 The third common cause of death on the list globally is the ischemic stroke/cerebral stroke, in which clot/hemorrhage is formed in the blood due to the lack of blood flow in the brain, and cerium oxide nanoparticles encapsulated with phospholipid-polyethylene glycol can play a very important role to protect from the ischemic stroke.185 The insufficient secretion of endogenous insulin caused by the increase in oxidative stress leads to a metabolic disorder known as diabetes mellitus and for the decrease in the low blood glucose levels the cerium oxide nanoparticle when combined with the sodium selenium, leads to a decrease in the ROS levels and increases the secretion of insulin. Hence, cerium oxide nanoparticles can also be used for the treatment of a lifestyle-related major disorder that is diabetes.186–188Further, cerium oxide nanoparticles have been studied for the treatment of the retinal diseases, as ROS damages the sensitive cells of the retina, which leads to retinal diseases (retinal degeneration, diabetic retinopathy, etc.) which can cause partial/total loss of vision.189 Moreover, cerium oxide nanoparticles when studied in in vitro cell culture and in vivo for the treatment of retinal diseases, it was found that these nanoparticles induce intracellular peroxides, which prevents retinal degeneration. Further, it can be concluded from this study, that cerium oxide nanoparticles decrease the ROS, upregulates the expression of neuroprotection-associated genes, and downregulates apoptosis signaling pathway (it slows photoreceptor degeneration).190–192 In another study, it was found that these nanoparticles can induce the regression of pre-existing pathologic retinal neovasculature and can also inhibit the rise in ROS.193 Hence, these properties and mode of mechanism data of cerium oxide nanoparticles suggest their potential role as a therapeutic agent for the treatment of life-threatening diseases.
4.9 Cerium oxide based biosensors
Biosensors are an analytical device that recognizes, analyses, and converts the biological/physical/chemical signals into measurable signals (electrochemical, an optical signal, etc.). These analytical devices consist of sensing elements, transducers, and signal processors; its working mechanism is as follows: sensing element detects an analyte and is passed to the transducer to produce a signal, which is amplified by the signal processor.194–198 From all the metal oxide nanoparticles, cerium oxide nanoparticle is mostly considered to be used for the development of biosensor as they exhibit distinctive properties, namely high mechanical strength, oxygen ion conductivity, biocompatibility, high storage capacity, large surface area, and are nontoxic to the living cells.Biosensor based on sol–gel derived cerium oxide nanoparticles was developed for the detection of cholesterol; this is a cost-effective approach for clinical diagnosis of coronary diseases. As sol–gel derived cerium oxide nanoparticles film was considered and it resulted in many advantages like optical transparency, thermal stability, and low processing temperature. In this experiment by using electrophoretic deposition, cerium oxide nanoparticle was deposited on the indium-tin-oxide (ITO) coated glass substrate to form a film, and further cholesterol oxidase (ChOx) was immobilized for the detection of cholesterol. Hence, the result of this study showed higher sensitivity, selectivity and suggested that these nanoparticles can be a potential material, which can be used in the form of a film for fabricating efficient biosensor, as they are highly chemically stable which helps them to immobilize biomolecules.199 Another biosensor was fabricated for the estimation of hydrogen peroxide (H2O2) based on cerium oxide nanoparticle film deposited on indium-tin-oxide (ITO) glass substrate immobilized with horseradish peroxidise (HRP). Their result signifies that biosensors based on cerium oxide nanoparticles show excellent detection selectivity and sensitivity.200 Further, for blood glucose level monitoring, a sol–gel derived cerium oxide nanoparticle, glucose biosensor was fabricated by depositing this nanoparticle on the gold electrode, showing a high affinity for glucose with enhanced sensitivity.201
The lactate imbalance causes diseases like hypoxia/acute heart disorders, so for the easy detection of lactate in blood, an electrochemical biosensor was developed using hydroxide mediated cerium oxide nanoparticles, fabricated on glassy carbon electrode (GCE) and the cerium oxide nanoparticles act as an interface to immobilizes nicotinamide adenine dinucleotide (NADH) and lactate dehydrogenase (LDH) on the GCE surface.202 Hence, it can be concluded that cerium oxide nanoparticles can be considered as a promising material for the development of the biosensor, but further investigations are required to reach the stage of commercialization for this type of futuristic technology.
5 Conclusion and prospects
Nanotechnologies have revolutionized all the fields of science and technology such as chemistry, biology, physics, material sciences, etc. and it deals with the study and application of extremely small things at the nanoscale (1–100 nm) range. Cerium is an abundant rare earth metal that exhibits many defects on the surface, mainly the oxygen vacancies, which leads to the co-existence of two oxidation states: cerium(III) and cerium(IV), enabling them to exhibit redox catalytic activity. Biosynthesis of nanoparticles has gained a lot of attention due to its environment-friendly procedure, as it utilizes plant extracts, microbes, nutrients, etc. for the synthesis of nanoparticles. Thus, the biosynthesized cerium oxide nanoparticles are non-toxic and bio-compatible to the living cells and tissues. In this review, focus is laid on cerium oxide nanoparticles physicobiochemical properties, their biomedical applications, and prospects. This review also puts the reader's main focus on the several biosynthesis methods of the cerium oxide nanoparticle highlighting the major green sources for synthesis such as plant extracts, nutrient and natural mediated synthesis.Due to an increase in the prevalence of lifestyle diseases,203 development in the medical field is the need of the current scenario, and the urge to provide treatment for life-threatening diseases like cancer, HIV, etc. increases. The cerium oxide nanoparticle has been recognized in the field of biomedicines due to their unique redox properties, and are widely being used for the treatment of cancer, as an antimicrobial agent, bio-scaffold, and also for the fabrication of biosensor devices. Apart from the biomedical fields, these nanoparticles are also used in solar cells,20 fuel oxidation catalysis,21 chemical mechanical polarization,22 and corrosion protection.23 These nanoparticles have enormous properties, thus offering them vast applications in the biomedical field as well as in agriculture and environmental domains.
Currently, we are experiencing the global viral (COVID-19) pandemic, which is threatening to the human civilization, and cerium oxide nanoparticle can be utilized for the production and commercialization of antimicrobial PPE (personal protective equipment), surgery suits, sanitizers, etc. because of their great antimicrobial efficiency, about which we have embellished in this review. Further, there has been no evidence of cerium present in the human body naturally, and to date, there is no known mode of mechanism data pertaining to the clearing mechanism of these nanoparticles in the human body, which can lead to systemic toxicity. Thus, the focus can be laid on exploring the nanotoxicological data of the cerium oxide nanoparticles. The multifunctional cerium oxide nanoparticles based on natural biomaterials have shown promising applications in the biomedical field and in the review, it's highlighted in detail. Hence, from the detailed study of these nanoparticles, we can suggest that researchers should lay their focus on engineering the cerium oxide nanoparticles by functionalizing them with synthetic materials (peptide nucleic acids, xeno-nucleic acids, threose nucleic acid, morpholino, etc.)204,205 for more beneficial applications. In addition to this, the cerium oxide nanoparticles could have potential application potentialities towards agriculture and environmental domain, data are still lacking or in very infancy phase and need to be explored.
Funding
This review did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.Conflicts of interest
Author's declare no conflict of interest for this work.Acknowledgements
The authors would like to extend their gratitude of thanks to Vice-chancellor, Indira Gandhi National Tribal University, for providing facilities to prepare this review and we are also thankful to all the faculty members of Department of Biotechnology, Indira Gandhi National Tribal University, Amarkantak, M.P., India for their support. Special thanks to Dr Parikipandla Sridevi, Department of Biotechnology, IGNTU, Amarkantak for her unconditional support throughout this work.References
- C. M. Rico, S. Majumdar, M. Duarte-Gardea, J. R. Peralta-Videa and J. L. Gardea-Torresdey, J. Agric. Food Chem., 2011, 59, 3485–3498, DOI:10.1021/jf104517j
.
- S. Razzaque, S. Z. Hussain, I. Hussain and B. Tan, Polymers, 2016, 8, 156, DOI:10.3390/polym8040156
.
- R. P. Singh, Int. J. Electrochem., 2011, 2011, 1–30, DOI:10.4061/2011/125487
.
- R. P. Singh, B. K. Oh, K. K. Koo, J. Y. Jyoung, S. Jeong and J. W. Choi, BioChip J., 2009, 2, 223–234 Search PubMed
.
- R. P. Singh, J. Bioanal. Biomed., 2016, 8, e143, DOI:10.4172/1948-593x.1000e143
.
- R. P. Singh, J.-W. Choi, A. Tiwari and A. C. Pandey, in Biomedical Materials and Diagnostic Devices, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2012, pp. 215–262, DOI:10.1002/9781118523025.ch7
.
- R. P. Singh, in Food Safety and Human Health, Elsevier, 2019, pp. 285–318, DOI:10.1016/b978-0-12-816333-7.00011-4
.
- J. K. Fard, S. Jafari and M. A. Eghbal, Adv. Pharm. Bull., 2015, 5, 447–454, DOI:10.15171/apb.2015.061
.
- E. C. Wang and A. Z. Wang, Integr. Biol., 2014, 6, 9–26, 10.1039/c3ib40165k
.
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396–414, DOI:10.1016/j.scitotenv.2008.06.042
.
- G. A. Husseini and W. G. Pitt, Adv. Drug Delivery Rev., 2008, 60, 1137–1152, DOI:10.1016/j.addr.2008.03.008
.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244, DOI:10.1002/anie.200602866
.
- D. K. Tiwari, J. Behari and P. Sen, World Appl. Sci. J., 2008, 3, 417–433 Search PubMed
.
- C. Bouzigues, T. Gacoin and A. Alexandrou, ACS Nano, 2011, 5, 8488–8505, DOI:10.1021/nn202378b
.
- A. Dhall and W. Self, Antioxidants, 2018, 7, 97, DOI:10.3390/antiox7080097
.
- J. T. Dahle and Y. Arai, Int. J. Environ. Res. Public Health, 2015, 12, 1253–1278, DOI:10.3390/ijerph120201253
.
- B. A. Rzigalinski, K. Meehan, R. M. Davis, Y. Xu, W. C. Miles and C. A. Cohen, Nanomedicine, 2006, 1, 399–412, DOI:10.2217/17435889.1.4.399
.
- S. M. Hirst, A. S. Karakoti, R. D. Tyler, N. Sriranganathan, S. Seal and C. M. Reilly, Small, 2009, 5, 2848–2856, DOI:10.1002/smll.200901048
.
- A. Y. Estevez and J. S. Erlichman, Nanomedicine, 2014, 9, 1437–1440, DOI:10.2217/nnm.14.87
.
- A. Corma, P. Atienzar, H. García and J.-Y. Chane-Ching, Nat. Mater., 2004, 3, 394–397, DOI:10.1038/nmat1129
.
- H. Jung, D. B. Kittelson and M. R. Zachariah, Combust. Flame, 2005, 142, 276–288, DOI:10.1016/j.combustflame.2004.11.015
.
- K. Reed, A. Cormack, A. Kulkarni, M. Mayton, D. Sayle, F. Klaessig and B. Stadler, Environ. Sci.: Nano, 2014, 1, 390–405, 10.1039/c4en00079j
.
- V. K. Ivanov, A. B. Shcherbakov and A. V. Usatenko, Russ. Chem. Rev., 2009, 78, 855–871, DOI:10.1070/rc2009v078n09abeh004058
.
- I. Celardo, J. Z. Pedersen, E. Traversa and L. Ghibelli, Nanoscale, 2011, 3, 1411–1420, 10.1039/c0nr00875c
.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058, 10.1039/b615134e
.
- S. M. Hirst, A. Karakoti, S. Singh, W. Self, R. Tyler, S. Seal and C. M. Reilly, Environ. Toxicol., 2013, 28, 107–118, DOI:10.1002/tox.20704
.
- S. Kargozar, F. Baino, S. J. Hoseini, S. Hamzehlou, M. Darroudi, J. Verdi, L. Hasanzadeh, H.-W. Kim and M. Mozafari, Nanomedicine, 2018, 13, 3051–3069, DOI:10.2217/nnm-2018-0189
.
- S. Seal, A. Jeyaranjan, C. J. Neal, U. Kumar, T. S. Sakthivel and D. C. Sayle, Nanoscale, 2020, 12, 6879–6899, 10.1039/d0nr01203c
.
- F. Gao, Q. Lu and S. Komarneni, J. Nanosci. Nanotechnol., 2006, 6, 3812–3819, DOI:10.1166/jnn.2006.609
.
- C. Walkey, S. Das, S. Seal, J. Erlichman, K. Heckman, L. Ghibelli, E. Traversa, J. F. McGinnis and W. T. Self, Environ. Sci.: Nano, 2015, 2, 33–53, 10.1039/c4en00138a
.
- A. Trovarelli, Catalysis by Ceria and Related Materials, published by imperial college press and distributed by World Scientific Publishing Co., 2002, vol. 2, p. 528, DOI:10.1142/p249
.
- A. L. Patterson, Phys. Rev., 1939, 56, 978–982, DOI:10.1103/physrev.56.978
.
- D. C. Sayle, S. Seal, Z. Wang, B. C. Mangili, D. W. Price, A. S. Karakoti, S. V. T. N. Kuchibhatla, Q. Hao, G. Möbus, X. Xu and T. X. T. Sayle, ACS Nano, 2008, 2, 1237–1251, DOI:10.1021/nn800065g
.
- T. X. T. Sayle, M. Molinari, S. Das, U. M. Bhatta, G. Möbus, S. C. Parker, S. Seal and D. C. Sayle, Nanoscale, 2013, 5, 6063–6073, 10.1039/c3nr00917c
.
- D. C. Sayle, X. Feng, Y. Ding, Z. L. Wang and T. X. T. Sayle, J. Am. Chem. Soc., 2007, 129, 7924–7935, DOI:10.1021/ja070893w
.
- A. S. Karakoti, S. V. N. T. Kuchibhatla, D. R. Baer, S. Thevuthasan, D. C. Sayle and S. Seal, Small, 2008, 4, 1210–1216, DOI:10.1002/smll.200800219
.
- J. Conesa, Surf. Sci., 1995, 339, 337–352, DOI:10.1016/0039-6028(95)00595-1
.
- G. S. Herman, Surf. Sci., 1999, 437, 207–214, DOI:10.1016/s0039-6028(99)00723-2
.
- T. Suzuki, I. Kosacki, H. U. Anderson and P. Colomban, J. Am. Ceram. Soc., 2004, 84, 2007–2014, DOI:10.1111/j.1151-2916.2001.tb00950.x
.
- F. Esch, S. Fabris, L. Zhou, T. Montini, C. Africh, P. Fornasiero, G. Comelli and R. Rosei, Science, 2005, 309, 752–755, DOI:10.1126/science.1111568
.
- K. Reinhardt and H. Winkler, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, DOI:10.1002/14356007.a06_139
.
- C. T. Campbell, Science, 2005, 309, 713–714, DOI:10.1126/science.1113955
.
- J. Kullgren, K. Hermansson and C. Castleton, J. Chem. Phys., 2012, 137, 044705, DOI:10.1063/1.4723867
.
- P. Dutta, S. Pal, M. S. Seehra, Y. Shi, E. M. Eyring and R. D. Ernst, Chem. Mater., 2006, 18, 5144–5146, DOI:10.1021/cm061580n
.
- S. Deshpande, S. Patil, S. V. N. T. Kuchibhatla and S. Seal, Appl. Phys. Lett., 2005, 87, 133113, DOI:10.1063/1.2061873
.
- J.-D. Cafun, K. O. Kvashnina, E. Casals, V. F. Puntes and P. Glatzel, ACS Nano, 2013, 7, 10726–10732, DOI:10.1021/nn403542p
.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709, DOI:10.1016/j.biomaterials.2008.03.014
.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. S. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738, 10.1039/b922024k
.
- R. K. Hailstone, A. G. DiFrancesco, J. G. Leong, T. D. Allston and K. J. Reed, J. Phys. Chem. C, 2009, 113, 15155–15159, DOI:10.1021/jp903468m
.
- A. Migani, G. N. Vayssilov, S. T. Bromley, F. Illas and K. M. Neyman, J. Mater. Chem., 2010, 20, 10535–10546, 10.1039/c0jm01908a
.
- J. Paier, C. Penschke and J. Sauer, Chem. Rev., 2013, 113, 3949–3985, DOI:10.1021/cr3004949
.
- S. Babu, R. Thanneeru, T. Inerbaev, R. Day, A. E. Masunov, A. Schulte and S. Seal, Nanotechnology, 2009, 20, 085713, DOI:10.1088/0957-4484/20/8/085713
.
- P. Nicholls, Arch. Biochem. Biophys., 2012, 525, 95–101, DOI:10.1016/j.abb.2012.01.015
.
- J. D. Lambeth, Nat. Rev. Immunol., 2004, 4, 181–189, DOI:10.1038/nri1312
.
- M. D. Brand, Exp. Gerontol., 2010, 45, 466–472, DOI:10.1016/j.exger.2010.01.003
.
- S. Baer and G. Stein, J. Chem. Soc., 1953, 10, 3176–3179, 10.1039/jr9530003176
.
- P. B. Sigler and B. J. Masters, J. Am. Chem. Soc., 1957, 79, 6353–6357, 10.1039/jr9530003176
.
- B. H. J. Bielski and E. Saito, J. Phys. Chem., 1962, 66, 2266–2268, DOI:10.1021/ja01581a003
.
- E. Saito and B. H. J. Bielski, J. Am. Chem. Soc., 1961, 83, 4467–4468, DOI:10.1021/ja01482a039
.
- V. Baldim, F. Bedioui, N. Mignet, I. Margaill and J.-F. Berret, Nanoscale, 2018, 10, 6971–6980, 10.1039/c8nr00325d
.
- A. Wolcott, D. Gerion, M. Visconte, J. Sun, A. Schwartzberg, S. Chen and J. Z. Zhang, J. Phys. Chem. B, 2006, 110, 5779–5789, DOI:10.1021/jp057435z
.
- A. M. Schwartzberg, T. Y. Olson, C. E. Talley and J. Z. Zhang, J. Phys. Chem. B, 2006, 110, 19935–19944, DOI:10.1021/jp062136a
.
- N. Sivakumar, A. Narayanasamy, C. N. Chinnasamy and B. Jeyadevan, J. Phys.: Condens. Matter, 2007, 19, 386201, DOI:10.1088/0953-8984/19/38/386201
.
- A. L. Gurgel, J. M. Soares, D. S. Chaves, D. S. Chaves, M. M. Xavier, M. A. Morales and E. M. Baggio-Saitovitch, J. Appl. Phys., 2010, 107, 09A746, DOI:10.1063/1.3339784
.
- G. Atanassov, R. Thielsch and D. Popov, Thin Solid Films, 1993, 223, 288–292, DOI:10.1016/0040-6090(93)90534-v
.
- M. G. Krishna, A. Hartridge and A. K. Bhattacharya, Mater. Sci. Eng., B, 1998, 55, 14–20, DOI:10.1016/s0921-5107(98)00203-7
.
- D. P. Norton, J. D. Budai and M. F. Chisholm, Appl. Phys. Lett., 2000, 76, 1677–1679, DOI:10.1063/1.126133
.
- S. Guo, H. Arwin, S. N. Jacobsen, K. Järrendahl and U. Helmersson, J. Appl. Phys., 1995, 77, 5369–5376, DOI:10.1063/1.359225
.
- L. Méchin, A. Chabli, F. Bertin, M. Burdin, G. Rolland, C. Vannuffel and J.-C. Villégier, J. Appl. Phys., 1998, 84, 4935–4940, DOI:10.1063/1.368738
.
- A. H. Morshed, M. E. Moussa, S. M. Bedair, R. Leonard, S. X. Liu and N. El-Masry, Appl. Phys. Lett., 1997, 70, 1647–1649, DOI:10.1063/1.118658
.
- R. Swanepoel, J. Phys. E: Sci. Instrum., 1983, 16, 1214–1222, DOI:10.1088/0022-3735/16/12/023
.
- C. Mansilla, Solid State Sci., 2009, 11, 1456–1464, DOI:10.1016/j.solidstatesciences.2009.05.001
.
- T. Ami and M. Suzuki, Mater. Sci. Eng., B, 1998, 54, 84–91, DOI:10.1016/s0921-5107(98)00133-0
.
- S. Logothetidis, P. Patsalas, E. K. Evangelou, N. Konofaos, I. Tsiaoussis and N. Frangis, Mater. Sci. Eng., B, 2004, 109, 69–73, DOI:10.1016/j.mseb.2003.10.048
.
- F. Marabelli and P. Wachter, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 1238–1243, DOI:10.1103/physrevb.36.1238
.
- P. Patsalas, S. Logothetidis and C. Metaxa, Appl. Phys. Lett., 2002, 81, 466–468, DOI:10.1063/1.1494458
.
- T. Inoue, Y. Yamamoto, S. Koyama, S. Suzuki and Y. Ueda, Appl. Phys. Lett., 1990, 56, 1332–1333, DOI:10.1063/1.103202
.
- B. P. Gorman, V. Petrovsky, H. U. Anderson and T. Petrovsky, J. Mater. Res., 2004, 19, 573–578, DOI:10.1557/jmr.2004.0070
.
- R. M. Bueno, J. M. Martinez-Duart, M. Hernandez-Velez and L. Vazquez, J. Mater. Sci., 1997, 32, 1861–1865, DOI:10.1023/a:1018509007844
.
- P. Patsalas, S. Logothetidis, L. Sygellou and S. Kennou, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 035104, DOI:10.1103/physrevb.68.035104
.
- M. M. Ali, H. S. Mahdi, A. Parveen and A. Azam, AIP Conf. Proc., 2018, 1953, 030044, DOI:10.1063/1.5032379
.
- X. Guo, C. Chen, Y. Zhang, Y. Xu and H. Pang, Energy Storage Mater., 2019, 23, 439–465, DOI:10.1016/j.ensm.2019.04.017
.
- F. Zhou, X. Zhao, H. Xu and C. Yuan, J. Phys. Chem. C, 2007, 111, 1651–1657, DOI:10.1021/jp0660435
.
- N. Maheswari and G. Muralidharan, Energy Fuels, 2015, 29, 8246–8253, DOI:10.1021/acs.energyfuels.5b02144
.
- A. Sundaresan, R. Bhargavi, N. Rangarajan, U. Siddesh and C. N. R. Rao, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 161306, DOI:10.1103/physrevb.74.161306
.
- J. Luňáček, O. Životský, P. Janoš, M. Došek, A. Chrobak, M. Maryško, J. Buršík and Y. Jirásková, J. Alloys Compd., 2018, 753, 167–175, DOI:10.1016/j.jallcom.2018.04.115
.
- P. Cohen, Nat. Cell Biol., 2002, 4, E127–E130, DOI:10.1038/ncb0502-e127
.
- J. Rawlings, W. W. Cleland and A. C. Hengge, J. Inorg. Biochem., 2003, 93, 61–65, DOI:10.1016/S0162-0134(02)00435-x
.
- S. J. Franklin, Curr. Opin. Chem. Biol., 2001, 5, 201–208, DOI:10.1016/s1367-5931(00)00191-5
.
- M. E. Branum and L. Que, J. Biol. Inorg Chem., 1999, 4, 593–600, DOI:10.1007/s007750050382
.
- M. E. Branum, A. K. Tipton, S. Zhu and L. Que, J. Am. Chem. Soc., 2001, 123, 1898–1904, DOI:10.1021/ja0010103
.
- H. Katada, H. Seino, Y. Mizobe, J. Sumaoka and M. Komiyama, J. Biol. Inorg Chem., 2008, 13, 249–255, DOI:10.1007/s00775-007-0315-x
.
- S. E. Bunn, C. T. Liu, Z.-L. Lu, A. A. Neverov and R. S. Brown, J. Am. Chem. Soc., 2007, 129, 16238–16248, DOI:10.1021/ja076847d
.
- A. G. Cassano, V. E. Anderson and M. E. Harris, Biopolymers, 2004, 73, 110–129, DOI:10.1002/bip.10517
.
- M. Livieri, F. Mancin, G. Saielli, J. Chin and U. Tonellato, Chem.–Eur. J., 2007, 13, 2246–2256, DOI:10.1002/chem.200600672
.
- M. H. Kuchma, C. B. Komanski, J. Colon, A. Teblum, A. E. Masunov, B. Alvarado, S. Babu, S. Seal, J. Summy and C. H. Baker, Nanomedicine, 2010, 6, 738–744, DOI:10.1016/j.nano.2010.05.004
.
- S. Singh, T. Dosani, A. S. Karakoti, A. Kumar, S. Seal and W. T. Self, Biomaterials, 2011, 32, 6745–6753, DOI:10.1016/j.biomaterials.2011.05.073
.
- Y. Xue, Y. Zhai, K. Zhou, L. Wang, H. Tan, Q. Luan and X. Yao, Chem.–Eur. J., 2012, 18, 11115–11122, DOI:10.1002/chem.201200983
.
- A. Dhall, A. Burns, J. Dowding, S. Das, S. Seal and W. Self, Environ. Sci.: Nano, 2017, 4, 1742–1749, 10.1039/c7en00394c
.
- B. Lipinski, Oxid. Med. Cell. Longevity, 2011, 2011, 1–9, DOI:10.1155/2011/809696
.
- J. P. Giraldo, M. P. Landry, S. M. Faltermeier, T. P. McNicholas, N. M. Iverson, A. A. Boghossian, N. F. Reuel, A. J. Hilmer, F. Sen, J. A. Brew and M. S. Strano, Nat. Mater., 2014, 13, 400–408, DOI:10.1038/nmat3890
.
- H. Wu, N. Tito and J. P. Giraldo, ACS Nano, 2017, 11, 11283–11297, DOI:10.1021/acsnano.7b05723
.
- Y. Xue, Q. Luan, D. Yang, X. Yao and K. Zhou, J. Phys. Chem. C, 2011, 115, 4433–4438, DOI:10.1021/jp109819u
.
- B. Drew and C. Leeuwenburgh, Ann. N. Y. Acad. Sci., 2002, 959, 66–81, DOI:10.1111/j.1749-6632.2002.tb02084.x
.
- M. Das, S. Patil, N. Bhargava, J.-F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Biomaterials, 2007, 28, 1918–1925, DOI:10.1016/j.biomaterials.2006.11.036
.
- J. M. Perez, A. Asati, S. Nath and C. Kaittanis, Small, 2008, 4, 552–556, DOI:10.1002/smll.200700824
.
- J. M. Dowding, S. Seal and W. T. Self, Drug Delivery Transl. Res., 2013, 3, 375–379, DOI:10.1007/s13346-013-0136-0
.
- J. M. Dowding, T. Dosani, A. Kumar, S. Seal and W. T. Self, Chem. Commun., 2012, 48, 4896–4898, 10.1039/c2cc30485f
.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908–931, DOI:10.1016/j.arabjc.2017.05.011
.
- S. K. Kannan and M. Sundrarajan, Int. J. Nanosci., 2014, 13, 1450018, DOI:10.1142/s0219581x14500185
.
- H.-W. He, X.-Q. Wu, W. Ren, P. Shi, X. Yao and Z.-T. Song, Ceram. Int., 2012, 38, S501–S504, DOI:10.1016/j.ceramint.2011.05.063
.
- J.-D. Hu, Y.-X. Li, X.-Z. Zhou and M.-X. Cai, Mater. Lett., 2007, 61, 4989–4992, DOI:10.1016/j.matlet.2007.03.097
.
- J. C. Yu, L. Zhang and J. Lin, J. Colloid Interface Sci., 2003, 260, 240–243, DOI:10.1016/s0021-9797(02)00168-6
.
- H. Z. Song, H. B. Wang, S. W. Zha, D. K. Peng and G. Y. Meng, Solid State Ionics, 2003, 156, 249–254, DOI:10.1016/S0167-2738(02)00688-4
.
- M. J. Godinho, R. F. Gonçalves, L. P. S Santos, J. A. Varela, E. Longo and E. R. Leite, Mater. Lett., 2007, 61, 1904–1907, DOI:10.1016/j.matlet.2006.07.152
.
- A. M. Korotkova, P. O. Borisovna, G. I. Aleksandrovna, K. D. Bagdasarovna, B. D. Vladimirovich, K. D. Vladimirovich, F. A. Alexandrovich, K. M. Yurievna, B. E. Nikolaevna, K. D. Aleksandrovich, C. M. Yurievich and L. S. Valerievich, Curr. Nanomater., 2019, 4, 176–190, DOI:10.2174/2405461504666190911155421
.
- A. Arumugam, C. Karthikeyan, A. S. Haja Hameed, K. Gopinath, S. Gowri and V. Karthika, Mater. Sci. Eng., C, 2015, 49, 408–415, DOI:10.1016/j.msec.2015.01.042
.
- G. Sai Priya, A. Kanneganti, K. Anil Kumar, K. Venkateswara Rao and S. Bykkam, International Journal of Scientific and Research Publication, 2014, 4, 1–4 Search PubMed
.
- Q. Maqbool, M. Nazar, S. Naz, T. Hussain, N. Jabeen, R. Kausar, S. Anwaar, F. Abbas and T. Jan, Int. J. Nanomed., 2016, 11, 5015–5025, DOI:10.2147/ijn.s113508
.
- N. Thovhogi, A. Diallo, A. Gurib-Fakim and M. Maaza, J. Alloys Compd., 2015, 647, 392–396, DOI:10.1016/j.jallcom.2015.06.076
.
- J. K. Sharma, P. Srivastava, S. Ameen, M. S. Akhtar, S. K. Sengupta and G. Singh, Mater. Res. Bull., 2017, 91, 98–107, DOI:10.1016/j.materresbull.2017.03.034
.
- M. Darroudi, M. Sarani, R. Kazemi Oskuee, A. Khorsand Zak, H. A. Hosseini and L. Gholami, Ceram. Int., 2014, 40, 2041–2045, DOI:10.1016/j.ceramint.2013.07.116
.
- M. Darroudi, S. J. Hoseini, R. Kazemi Oskuee, H. A. Hosseini, L. Gholami and S. Gerayli, Ceram. Int., 2014, 40, 7425–7430, DOI:10.1016/j.ceramint.2013.12.089
.
- S. N. Patil, J. S. Paradeshi, P. B. Chaudhari, S. J. Mishra and B. L. Chaudhari, Appl. Biochem. Biotechnol., 2016, 180, 638–654, DOI:10.1007/s12010-016-2121-9
.
- S. A. Khan and A. Ahmad, Mater. Res. Bull., 2013, 48, 4134–4138, DOI:10.1016/j.materresbull.2013.06.038
.
- R. P. Singh, V. K. Shukla, R. S. Yadav, P. K. Sharma, P. K. Singh and A. C. Pandey, Adv. Mater. Lett., 2011, 2, 313–317, DOI:10.5185/amlett.indias.204
.
- V. K. Shukla, R. P. Singh and A. C. Pandey, J. Alloys Compd., 2010, 507, L13–L16, DOI:10.1016/j.jallcom.2010.07.156
.
- R. P. Singh, in Plant Nanobionics, ed. R. Prasad, Springer, Cham, 2019, pp. 77–113, DOI:10.1007/978-3-030-16379-2_4
.
- R. P. Singh, in Plant Nanobionics. Nanotechnology in the Life Sciences, ed. R. Prasad, Springer, Cham, 2019, pp. 115–176, DOI:10.1007/978-3-030-16379-2_5
.
- R. P. Singh, K. Kumar, R. Rai, A. Tiwari, J. W. Choi and A. C. Pandey, in Synthesis, characterization and application of Smart material, ed. R. Rai, Nova Science Publishers, Inc, USA, 2012, pp. 225–238 Search PubMed
.
- J. Gagnon and K. M. Fromm, Eur. J. Inorg. Chem., 2015, 2015, 4510–4517, DOI:10.1002/ejic.201500643
.
- H. Kargar, H. Ghazavi and M. Darroudi, Ceram. Int., 2015, 41, 4123–4128, DOI:10.1016/j.ceramint.2014.11.108
.
- P. Mohanpuria, N. K. Rana and S. K. Yadav, J. Nanopart. Res., 2008, 10, 507–517, DOI:10.1007/s11051-007-9275-x
.
- H. Kargar, F. Ghasemi and M. Darroudi, Ceram. Int., 2015, 41, 1589–1594, DOI:10.1016/j.ceramint.2014.09.095
.
- C. Xu and X. Qu, NPG Asia Mater., 2014, 6, e90, DOI:10.1038/am.2013.88
.
- G. Waris and H. Ahsan, J. Carcinog., 2006, 5, 1–8, DOI:10.1186/1477-3163-5-14
.
- L. Alili, M. Sack, A. S. Karakoti, S. Teuber, K. Puschmann, S. M. Hirst, C. M. Reilly, K. Zanger, W. Stahl, S. Das, S. Seal and P. Brenneisen, Biomaterials, 2011, 32, 2918–2929, DOI:10.1016/j.biomaterials.2010.12.056
.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569, DOI:10.1016/j.nano.2012.10.010
.
- R. A. Madero-Visbal, B. E. Alvarado, J. F. Colon, C. H. Baker, M. S. Wason, B. Isley, S. Seal, C. M. Lee, S. Das and R. Mañon, Nanomedicine, 2012, 8, 1223–1231, DOI:10.1016/j.nano.2011.12.011
.
- J. Colon, N. Hsieh, A. Ferguson, P. Kupelian, S. Seal, D. W. Jenkins and C. H. Baker, Nanomedicine, 2010, 6, 698–705, DOI:10.1016/j.nano.2010.01.010
.
- T. Sahu, S. S. Bisht, K. R. Das and S. Kerkar, Curr. Nanosci., 2013, 9, 588–593, DOI:10.2174/15734137113099990084
.
- M. Pešić, A. Podolski-Renić, S. Stojković, B. Matović, D. Zmejkoski, V. Kojić, G. Bogdanović, A. Pavićević, M. Mojović, A. Savić, I. Milenković, A. Kalauzi and K. Radotić, Chem.-Biol. Interact., 2015, 232, 85–93, DOI:10.1016/j.cbi.2015.03.013
.
- G. Renu, V. V. D. Rani, S. V. Nair, K. R. V. Subramanian and V.-K. Lakshmanan, Adv. Sci. Lett., 2012, 6, 17–25, DOI:10.1166/asl.2012.3312
.
- L. Alili, M. Sack, C. von Montfort, S. Giri, S. Das, K. S. Carroll, K. Zanger, S. Seal and P. Brenneisen, Antioxid. Redox Signaling, 2013, 19, 765–778, DOI:10.1089/ars.2012.4831
.
- W. Lin, Y. W. Huang, X.-D. Zhou and Y. Ma, Int. J. Toxicol., 2006, 25, 451–457, DOI:10.1080/10915810600959543
.
- S. K. Jana, P. Banerjee, S. Das, S. Seal and K. Chaudhury, J. Nanopart. Res., 2014, 16, 2441, DOI:10.1007/s11051-014-2441-z
.
- S. Giri, A. Karakoti, R. P. Graham, J. L. Maguire, C. M. Reilly, S. Seal, R. Rattan and V. Shridhar, PLoS One, 2013, 8, e54578, DOI:10.1371/journal.pone.0054578
.
- M. Hijaz, S. Das, I. Mert, A. Gupta, Z. Al-Wahab, C. Tebbe, S. Dar, J. Chhina, S. Giri, A. Munkarah, S. Seal and R. Rattan, BMC Cancer, 2016, 16, 220, DOI:10.1186/s12885-016-2206-4
.
- E. Nourmohammadi, H. Khoshdel-sarkarizi, R. Nedaeinia, H. R. Sadeghnia, L. Hasanzadeh, M. Darroudi and R. Kazemi oskuee, J. Cell. Physiol., 2019, 234, 4987–4996, DOI:10.1002/jcp.27303
.
- P. Brenneisen and A. Reichert, Antioxidants, 2018, 7, 31, DOI:10.3390/antiox7020031
.
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. I. Yeh, M. R. Wiesner and A. E. Nel, Nano Lett., 2006, 6, 1794–1807, DOI:10.1021/nl061025k
.
- T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134, DOI:10.1021/nn800511k
.
- E. Burello and A. P. Worth, Nanotoxicology, 2011, 5, 228–235, DOI:10.3109/17435390.2010.502980
.
- G.-X. Tong, F.-F. Du, Y. Liang, Q. Hu, R.-N. Wu, J.-G. Guan and X. Hu, J. Mater. Chem. B, 2013, 1, 454–463, 10.1039/c2tb00132b
.
- K. Gopinath, V. Karthika, C. Sundaravadivelan, S. Gowri and A. Arumugam, J. Nanostruct. Chem., 2015, 5, 295–303, DOI:10.1007/s40097-015-0161-2
.
- D. A. Pelletier, A. K. Suresh, G. A. Holton, C. K. McKeown, W. Wang, B. Gu, N. P. Mortensen, D. P. Allison, D. C. Joy, M. R. Allison, S. D. Brown, T. J. Phelps and M. J. Doktycz, Appl. Environ. Microbiol., 2010, 76, 7981–7989, DOI:10.1128/aem.00650-10
.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156, DOI:10.1021/es060999b
.
- I. Rodea-Palomares, S. Gonzalo, J. Santiago-Morales, F. Leganés, E. García-Calvo, R. Rosal and F. Fernández-Piñas, Aquat. Toxicol., 2012, 122–123, 133–143, DOI:10.1016/j.aquatox.2012.06.005
.
- R. P. Senthilkumar, V. Bhuvaneshwari, R. Ranjithkumar, S. Sathiyavimal, V. Malayaman and B. Chandarshekar, Int. J. Biol. Macromol., 2017, 104, 1746–1752, DOI:10.1016/j.ijbiomac.2017.03.139
.
- N. M. Zholobak, V. K. Ivanov and A. B. Shcherbakov, in Nanobiomaterials in Antimicrobial Therapy, Elsevier, 2016, pp. 419–450, DOI:10.1016/b978-0-323-42864-4.00012-9
.
- B. A. Rzigalinski, Technol. Cancer Res. Treat., 2005, 4, 651–659, DOI:10.1177/153303460500400609
.
- A. Ranjbar, S. Soleimani Asl, F. Firozian, H. Heidary Dartoti, S. Seyedabadi, M. Taheri Azandariani and M. Ganji, J. Mol. Neurosci., 2018, 66, 420–427, DOI:10.1007/s12031-018-1191-2
.
- S.-J. Kim and B. H. Chung, Carbohydr. Polym., 2016, 150, 400–407, DOI:10.1016/j.carbpol.2016.05.021
.
- L. Rubio, B. Annangi, L. Vila, A. Hernández and R. Marcos, Arch. Toxicol., 2016, 90, 269–278, DOI:10.1007/s00204-015-1468-y
.
- S. Chen, Y. Hou, G. Cheng, C. Zhang, S. Wang and J. Zhang, Biol. Trace Elem. Res., 2013, 154, 156–166, DOI:10.1007/s12011-013-9678-8
.
- A. Gojova, J.-T. Lee, H. S. Jung, B. Guo, A. I. Barakat and I. M. Kennedy, Inhalation Toxicol., 2009, 21, 123–130, DOI:10.1080/08958370902942582
.
- J. Niu, A. Azfer, L. M. Rogers, X. Wang and P. E. Kolattukudy, Cardiovasc. Res., 2007, 73, 549–559, DOI:10.1016/j.cardiores.2006.11.031
.
- N. Thakur, P. Manna and J. Das, J. Nanobiotechnol., 2019, 17, 84, DOI:10.1186/s12951-019-0516-9
.
- H. Li, C. Liu, Y.-P. Zeng, Y.-H. Hao, J.-W. Huang, Z.-Y. Yang and R. Li, ACS Appl. Mater. Interfaces, 2016, 8, 31510–31523, DOI:10.1021/acsami.6b07338
.
- S. Patil, S. Reshetnikov, M. K. Haldar, S. Seal and S. Mallik, J. Phys. Chem. C, 2007, 111, 8437–8442, DOI:10.1021/jp067666l
.
- F. Muhammad, A. Wang, W. Qi, S. Zhang and G. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 19424–19433, DOI:10.1021/am5055367
.
- S. Sulthana, T. Banerjee, J. Kallu, S. R. Vuppala, B. Heckert, S. Naz, T. Shelby, O. Yambem and S. Santra, Mol. Pharm., 2017, 14, 875–884, DOI:10.1021/acs.molpharmaceut.6b01076
.
- J. Das, Y.-J. Choi, J. W. Han, A. M. M. T. Reza and J.-H. Kim, Sci. Rep., 2017, 7, 9513, DOI:10.1038/s41598-017-09876-w
.
- I. Kalashnikova, J. Mazar, C. J. Neal, A. L. Rosado, S. Das, T. J. Westmoreland and S. Seal, Nanoscale, 2017, 9, 10375–10387, 10.1039/c7nr02770b
.
- Y. Zhang, X. Wu, C. Hou, K. Shang, K. Yang, Z. Tian, Z. Pei, Y. Qu and Y. Pei, Int. J. Nanomed., 2018, 13, 2161–2173, DOI:10.2147/ijn.s152002
.
- J. Das, J. W. Han, Y.-J. Choi, H. Song, S.-G. Cho, C. Park, H. G. Seo and J.-H. Kim, Sci. Rep., 2016, 6, 29197, DOI:10.1038/srep29197
.
- A. S. Karakoti, O. Tsigkou, S. Yue, P. D. Lee, M. M. Stevens, J. R. Jones and S. Seal, J. Mater. Chem., 2010, 20, 8912, 10.1039/c0jm01072c
.
- C. Mandoli, F. Pagliari, S. Pagliari, G. Forte, P. Di Nardo, S. Licoccia and E. Traversa, Adv. Funct. Mater., 2010, 20, 1617–1624, DOI:10.1002/adfm.200902363
.
- K. Apel and H. Hirt, Annu. Rev. Plant Biol., 2004, 55, 373–399, DOI:10.1146/annurev.arplant.55.031903.141701
.
- J. Emerit, M. Edeas and F. Bricaire, Biomed. Pharmacother., 2004, 58, 39–46, DOI:10.1016/j.biopha.2003.11.004
.
- D. Schubert, R. Dargusch, J. Raitano and S.-W. Chan, Biochem. Biophys. Res. Commun., 2006, 342, 86–91, DOI:10.1016/j.bbrc.2006.01.129
.
- B. D'Angelo, S. Santucci, E. Benedetti, S. Di Loreto, R. Phani, S. Falone, F. Amicarelli, M. P. Ceru and A. Cimini, Curr. Nanosci., 2009, 5, 167–176, DOI:10.2174/157341309788185523
.
- J. Geng, M. Li, J. Ren, E. Wang and X. Qu, Angew. Chem., Int. Ed., 2011, 50, 4184–4188, DOI:10.1002/anie.201007067
.
- S. Varadarajan, S. Yatin, M. Aksenova and D. A. Butterfield, J. Struct. Biol., 2000, 130, 184–208, DOI:10.1006/jsbi.2000.4274
.
- C. K. Kim, T. Kim, I.-Y. Choi, M. Soh, D. Kim, Y.-J. Kim, H. Jang, H.-S. Yang, J. Y. Kim, H.-K. Park, S. P. Park, S. Park, T. Yu, B.-W. Yoon, S.-H. Lee and T. Hyeon, Angew. Chem., Int. Ed., 2012, 51, 11039–11043, DOI:10.1002/anie.201203780
.
- A. C. Maritim, R. A. Sanders and J. B. Watkins, J. Biochem. Mol. Toxicol., 2003, 17, 24–38, DOI:10.1002/jbt.10058
.
- N. Pourkhalili, A. Hosseini, A. Nili-Ahmadabadi, S. Hassani, M. Pakzad, M. Baeeri, A. Mohammadirad and M. Abdollahi, World J. Diabetes, 2011, 2, 204–210, DOI:10.4239/wjd.v2.i11.204
.
- N. Pourkhalili, A. Hosseini, A. Nili-Ahmadabadi, M. Rahimifard, M. Navaei-Nigjeh, S. Hassani, M. Baeeri and M. Abdollahi, Toxicol. Mech. Methods, 2012, 22, 476–482, DOI:10.3109/15376516.2012.673093
.
- N. Sanvicens, V. Gómez-Vicente, I. Masip, A. Messeguer and T. G. Cotter, J. Biol. Chem., 2004, 279, 39268–39278, DOI:10.1074/jbc.m402202200
.
- J. Chen, S. Patil, S. Seal and J. F. McGinnis, Nat. Nanotechnol., 2006, 1, 142–150, DOI:10.1038/nnano.2006.91
.
- L. Kong, X. Cai, X. Zhou, L. L. Wong, A. S. Karakoti, S. Seal and J. F. McGinnis, Neurobiol. Dis., 2011, 42, 514–523, DOI:10.1016/j.nbd.2011.03.004
.
- X. Cai, S. A. Sezate, S. Seal and J. F. McGinnis, Biomaterials, 2012, 33, 8771–8781, DOI:10.1016/j.biomaterials.2012.08.030
.
- X. Zhou, L. L. Wong, A. S. Karakoti, S. Seal and J. F. McGinnis, PLoS One, 2011, 6, e16733, DOI:10.1371/journal.pone.0016733
.
- N. J. Ronkainen, H. B. Halsall and W. R. Heineman, Chem. Soc. Rev., 2010, 39, 1747–1763, 10.1039/b714449k
.
- D. R. Thévenot, K. Toth, R. A. Durst and G. S. Wilson, Anal. Lett., 2001, 34, 635–659, DOI:10.1081/al-100103209
.
- R. P. Singh, D. Y. Kang and J. W. Choi, Adv. Mater. Lett., 2010, 1, 48–54, DOI:10.5185/amlett.2010.3106
.
- R. P. Singh and A. C. Pandey, Anal. Methods, 2011, 3, 586–592, 10.1039/c0ay00502a
.
- R. P. Singh, in Nanotechnology, ed. R. Prasad, M. Kumar and V. Kumar, Springer Singapore, Singapore, 2017, pp. 293–303, DOI:10.1007/978-981-10-4573-8_14
.
- A. A. Ansari, A. Kaushik, P. R. Solanki and B. D. Malhotra, Electrochem. Commun., 2008, 10, 1246–1249, DOI:10.1016/j.elecom.2008.06.003
.
- A. A. Ansari, P. R. Solanki and B. D. Malhotra, J. Biotechnol., 2009, 142, 179–184, DOI:10.1016/j.jbiotec.2009.04.005
.
- A. A. Ansari, P. R. Solanki and B. D. Malhotra, Appl. Phys. Lett., 2008, 92, 263901, DOI:10.1063/1.2953686
.
- N. Nesakumar, S. Sethuraman, U. M. Krishnan and J. B. B. Rayappan, J. Colloid Interface Sci., 2013, 410, 158–164, DOI:10.1016/j.jcis.2013.08.009
.
- K. R. B. Singh, M. Fernandes, T. Sarkar and P. Sridevi, Infect. Non Infect. Dis., 2019, 4, 1–7, DOI:10.24966/inid-8654/100027
.
- K. R. B. Singh, P. Sridevi and R. P. Singh, 2019, Authorea, preprint, DOI:10.22541/au.157773086.64212371.
- R. P. Singh, B.-K. Oh and J.-W. Choi, Bioelectrochemistry, 2010, 79, 153–161, DOI:10.1016/j.bioelechem.2010.02.004
.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |





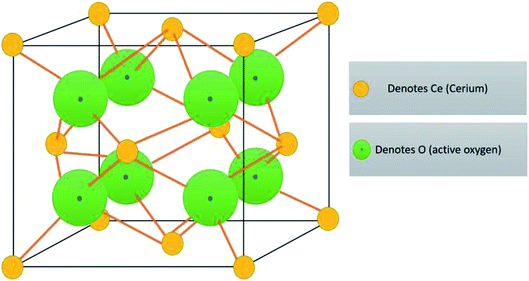
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)