Open Access Article
Open Access ArticleEnantioselective Michael reaction of anthrone catalyzed by chiral tetraoxacalix[2]arene[2]triazine derivatives†
Hayriye Nevin Genc *
*
Department of Science Education, A. K. Education Faculty, Necmettin Erbakan University, Konya 42090, Turkey. E-mail: hngenc@erbakan.edu.tr; Fax: +90 332 3238225; Tel: +90 332 3238220/5534
First published on 5th July 2019
Abstract
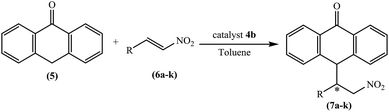
A highly enantioselective Michael addition reaction of anthrone with nitroalkenes by chiral tetraoxacalix[2]arene[2]triazine catalysts was investigated as a novel topic. The stereoselective conversion progressed smoothly by employing 10 mol% of the catalyst and afforded the corresponding Michael adducts with acceptable to high enantioselectivities (up to 97% ee) and very high yields (up to 96%).
1. Introduction
The chemical properties of anthrone and compounds containing the anthrone skeleton are significant in organic chemistry. Anthrones and their enol tautomers, i.e., 9-anthrols form a vital part of anthracenes since the oxidation of the central rings yields 9,10-anthraquinones, while their reduction affords anthracenes, which are useful intermediates.1,2 However, naturally occurring compounds bearing the anthrone platform are isolated either as O- or C-glycosides or in a free form from a broad diversity of plants and shrubs such as rhubarb, cassia, and cascara sagrada.3,4 Several such substances have noteworthy biological characteristics and are utilized as antimicrobial, emetic, antipsoriatic or androgen receptors and telomerase blockers.5,6 Recent studies have demonstrated that some anthrone- or anthraquinone-based naturally occurring compounds show strong and distinctive antitumor behaviours.7–11The Michael reaction of carbon-centered nucleophiles with different Michael acceptors provides a straightforward and robust technique for the formation of C–C bonds and has received prevalent preference in the production of organic materials. As a result, substantial works have been carried out for the development of the enantioselective forms of this conversion.12–14 Although remarkable developments have emerged in the catalytic asymmetric Michael reaction, developing a new Michael reaction for the effective production of different novel materials remains a significant target for studies conducted in both academic and industrial contexts. In this field of study, similar to the case of a Michael donor, various carbon-centered nucleophiles such as aldehydes and ketones,15–19 malonate esters,20–22 ketoesters,23 and 1,3-diketones24–26 have been comprehensively studied; in contrast, not much development has taken place in the improvement of the usage of anthrone as a nucleophile for the Michael addition reaction.27–33
Calixarenes and macromolecules bearing one or more calixarene platforms are known as efficient supramolecular materials. Heteroatom-bridged calixaromatics, also called heteroaromatic calixarenes, are a novel group of macrocyclic host compounds in supramolecular chemistry.34,35 Despite their exceptional physical and bonding characteristics that come from the electronic and steric influences of heteroatom bridges, heteroaromatic calixarenes are far rarer in usage.36,37 The self-tuning and fine-tuning cavities of their electronic characteristics make heteroaromatic calixarenes strong macrocyclic hosts regarding their interactions with neutral organic guests38,39 and with positively40 and negatively charged compounds.41–43 Oxygen- and nitrogen-bridged calix[2]arene[2]triazines are noteworthy heterocalixaromatics.44–47 Based on the characteristics of the heteroatoms in the bridging units, calix[2]arene[2]triazines use adaptable conformational constructs and may provide a diverse set of cavity sizes. Not limited to interactions of inclusion,48 these materials may also show π–π interactions of aromatic rings and hydrogen bonding interactions on the triazine nitrogen atoms as chiral host compounds.
In the past decade, we have reported the synthesis and applications of lower-rim-substituted calix[4]arene-based macromolecules with different functional groups as multiple H-bond donor chiral catalysts for stereoselective conversions.49–51 Likewise, we have recently reported substituted tetraoxacalix[2]arene[2]triazine derivatives with different purposes as chiral catalysts in the stereoselective Michael additions of isobutyraldehyde using different substituted and unsubstituted aromatic trans-β-nitrostyrenes.52 Here, we described an efficient one-pot process to synthesize optically active tetraoxacalix[2]arene[2]triazine derivatives and their possible applications as organic catalysts in the Michael addition of anthrone to β-nitroolefins under mild conditions. As far as we know, this study is the first implementation of tetraoxacalix[2]arene[2]triazine catalysts in the enantioselective reactions of anthrone.
2. Experimental
2.1. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the desired products as crystalline solids. The products were characterized by a combination of 1H NMR, 13C NMR, FTIR,55,56 and elemental analysis.
1, v/v) to afford the desired products as crystalline solids. The products were characterized by a combination of 1H NMR, 13C NMR, FTIR,55,56 and elemental analysis.
Compound 4a. Crystalline solid; 75% yield; α25D = −205.00 (c 1, CHCl3); mp 220–222 °C; IR (cm−1): 1358, 1479, 1563, 1708, 3261; 1H NMR (400 MHz, CDCl3): δ = 1.21–1.40 (m, 8H), 2.98–3.17 (m, 4H), 4.72 (d, 2H, J = 6.7 Hz), 5.04 (d, J = 6.8 Hz, 2H), 6.18 (s, 2H), 7.16–7.38 (m, 32H), 7.82–7.87 (m, 6H), NH-signals not found; 13C NMR (100 MHz, CDCl3): δ = 23.34, 29.47, 46.29, 51.17, 58.64, 65.80, 124.74, 127.33, 127.63, 128.00, 128.23, 128.43, 128.60, 128.73, 129.05, 129.68, 130.00, 138.20, 140.53, 140.68, 158.20, 162.20, 166.00, 169.10; anal. calcd. for C68H58N10O6 (1111.25): C, 73.49; H, 5.26; N, 12.60%; found: C, 73.51; H, 5.31; N, 12.48%.
Compound 4b. Crystalline solid; 78% yield; α25D = +218.00 (c 1, CHCl3); mp 326–328 °C; IR (cm−1): 1365, 1483, 1569, 1705, 3281; 1H NMR (400 MHz, CDCl3): δ = 1.19–1.35 (m, 8H), 1.82–2.12 (m, 16H), 2.60–2.75 (m, 10H), 2.85–2.90 (m, 8H), 4.13 (q, J = 2.7 Hz, 2H), 7.01–7.15 (m, 20H), 7.20 (t, J = 8.4, 0.5 Hz, 2H), 7.25–7.30 (m, 4H), 7.37–7.42 (m, 2H), NH-signals not found; 13C NMR (100 MHz, CDCl3): δ = 14.16, 21.03, 27.51, 32.04, 32.50, 34.14, 57.00, 60.47, 62.33, 102.86, 116.90, 128.41, 128.90, 128.96, 130.28, 140.40, 157.07, 158.21, 166.00; anal. calcd. for C66H74N10O4 (1071.35): C, 73.99; H, 6.96; N, 13.07%; found: C, 74.15; H, 7.12; N, 12.98%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to furnish the corresponding products. The ee% values of the Michael reaction products were determined by chiral HPLC analysis using Daicel Chiralpak OD-H or AS-H columns. The HPLC conditions for products 7a–7k are shown in Table 1; the 1H NMR, 13C NMR and FTIR spectroscopy values of products 7a–7k are shown in Table 2.
1) to furnish the corresponding products. The ee% values of the Michael reaction products were determined by chiral HPLC analysis using Daicel Chiralpak OD-H or AS-H columns. The HPLC conditions for products 7a–7k are shown in Table 1; the 1H NMR, 13C NMR and FTIR spectroscopy values of products 7a–7k are shown in Table 2.
| Product | Column | Hexane/2-propanol | Flow rate | tR (minor) | tR (major) |
|---|---|---|---|---|---|
| 7a | AS-H | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.7 mL min−1 | 25.96 min | 23.25 min |
| 7b | OD-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 15.94 min | 12.85 min |
| 7c | OD-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 11.82 min | 10.06 min |
| 7d | AS-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 12.85 min | 10.15 min |
| 7e | OD-H | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1.0 mL min−1 | 18.14 min | 15.32 min |
| 7f | AS-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 13.25 min | 10.42 min |
| 7g | AS-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 17.43 min | 14.65 min |
| 7h | AS-H | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1.0 mL min−1 | 18.19 min | 16.02 min |
| 7i | OD-H | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.0 mL min−1 | 19.62 min | 26.21 min |
| 7j | AS-H | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1.0 mL min−1 | 26.11 min | 21.93 min |
| 7k | AS-H | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1.0 mL min−1 | 12.35 min | 10.19 min |
| 7a | Mp | 147–148 °C |
| IR (cm−1) | 928, 1310, 1548, 1600, 1671 | |
| 1H NMR (400 MHz, CDCl3) | 4.03–4.06 (m, 1H), 4.53 (d, J = 3.7 Hz, 1H), 4.60 (dd, J = 13.3, 7.0 Hz, 1H), 4.88–4.91 (dd, J = 13.3, 9.1 Hz, 1H), 6.07 (d, J = 7.6 Hz, 2H), 6.91 (t, J = 7.8 Hz, 2H), 7.14–7.17 (m, 1H), 7.39–7.43 (m, 2H), 7.50 (d, J = 8.5 Hz, 2H), 7.60–7.68 (m, 2H), 7.94 (d, J = 7.8 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 45.9, 52.8, 76.4, 126.1, 126.9, 127.8, 128.0, 128.1, 128.2, 128.3, 128.7, 131.9, 132.2, 132.9, 133.4, 134.6, 139.8, 142.3, 183.1 | |
| 7b | Mp | 65–67 °C |
| IR (cm−1) | 929, 1317, 1552, 1598, 1671 | |
| 1H NMR (400 MHz, CDCl3) | 4.31–4.39 (m, 2H), 4.59–4.69 (m, 2H), 6.18 (d, J = 8.5 Hz, 1H), 6.80 (d, J = 6.7 Hz, 1H), 7.00 (d, J = 7.1 Hz, 1H), 7.40–7.61 (m, 4H), 7.63 (s, 2H), 8.13 (t, J = 7.8 Hz, 2H) | |
| 13C NMR (100 MHz, CDCl3) | 45.0, 48.0, 74.0, 126.3, 127.1, 127.3, 127.9, 128.3, 128.5, 128.6, 129.8, 130.3, 131.7, 132.5, 133.0, 133.3, 133.6, 134.9, 136.0, 138.9, 140.2, 183.4 | |
| 7c | Mp | 141–143 °C |
| IR (cm−1) | 935, 1322, 1551, 1600, 1671 | |
| 1H NMR (400 MHz, CDCl3) | 4.18–4.24 (m, 1H), 4.37 (dd, J = 13.3, 8.4 Hz, 1H), 4.50 (dd, J = 13.6, 6.9 Hz, 1H), 4.68 (d, J = 3.6 Hz, 1H), 5.57 (d, J = 3.3 Hz, 1H), 6.20–6.22 (m, 1H), 7.00 (d, J = 7.5 Hz, 1H), 7.19 (d, J = 1.1 Hz, 1H), 7.49–7.54 (m, 4H), 7.60–7.63 (m, 1H), 8.20 (t, J = 6.1 Hz, 2H) | |
| 13C NMR (100 MHz, CDCl3) | 44.8, 46.9, 73.9, 109.3, 110.1, 126.0, 127.3, 127.8, 128.2, 128.3, 128.5, 133.0, 133.2, 133.5, 133.7, 139.9, 140.5, 142.7, 148.4, 183.2 | |
| 7d | Mp | 63–65 °C |
| IR (cm−1) | 938, 1320, 1555, 1601, 1659 | |
| 1H NMR (400 MHz, CDCl3) | 4.00–4.03 (m, 1H), 4.81 (d, J = 5.4 Hz, 1H), 4.89–5.01 (m, 1H), 5.12 (dd, J = 13.9, 5.5 Hz, 1H), 6.60–6.62 (m, 1H), 7.10–7.13 (m, 2H), 7.29–7.36 (m, 2H), 7.51–7.65 (m, 5H), 7.96 (d, J = 7.6 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 45.0, 49.9, 77.1, 126.0, 126.1, 126.9, 127.8, 128.2, 129.0, 129.4, 130.0, 131.9, 132.2, 132.4, 133.1, 135.0, 139.8, 140.3, 181.3 | |
| 7e | Mp | 69–72 °C |
| IR (cm−1) | 936, 1313, 1529, 1551, 1604, 1670 | |
| 1H NMR (400 MHz, CDCl3) | 4.69–4.74 (m, 1H), 4.83 (d, J = 5.2 Hz, 1H), 5.09 (dd, J = 13.6, 10.3 Hz, 1H), 5.30 (dd, J = 13.9, 5.5 Hz, 1H), 6.69–6.72 (m, 1H), 7.29–7.38 (m, 3H), 7.48–7.62 (m, 6H), 7.90 (dd, J = 13.9, 7.6 Hz, 2H) | |
| 13C NMR (100 MHz, CDCl3) | 44.9, 45.5, 76.8, 123.9, 125.8, 126.1, 127.6, 127.8, 128,2, 128.9, 129.1, 129.2, 129.5, 131.9, 132.0, 132.5, 132.9, 134.0, 140.0, 140.9, 150.0, 183.1 | |
| 7f | Mp | 129–131 °C |
| IR (cm−1) | 929, 1320, 1509, 1555, 1600, 1661 | |
| 1H NMR (400 MHz, CDCl3) | 3.19 (s, 1H), 4.39–4.43 (m, 1H), 4.69 (d, J = 3.9 Hz, 1H), 4.79 (dd, J = 13.1, 10.1 Hz, 1H), 5.09 (dd, J = 13.4, 5.9 Hz, 1H), 6.03 (d, J = 6.5 Hz, 1H), 6.50 (t, J = 7.4 Hz, 1H), 6.70 (d, J = 8.0 Hz, 1H), 7.10 (t, J = 7.3 Hz, 1H), 7.39–7.50 (m, 4H), 7.59–7.62 (t, J = 7.4 Hz, 2H), 7.82 (d, J = 7.4 Hz, 1H), 7.89 (m, J = 7.9 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 44.9, 45.0, 54.8, 76.7, 109.9, 120.4, 121.3, 124.6, 125.8, 126.2, 127.5, 128.0, 128.4, 129.0, 129.4, 131.9, 132.9, 141.0, 142.6, 156.9, 183.0 | |
| 7g | Mp | 115–117 °C |
| IR (cm−1) | 934, 1313, 1549, 1600, 1661 | |
| 1H NMR (400 MHz, CDCl3) | 4.01–4.06 (m, 1H), 4.56–4.62 (m, 2H), 4.89 (dd, J = 13.3, 8.8 Hz, 1H), 5.99 (d, J = 7.3 Hz, 1H), 6.17 (s, 1H), 6.86 (t, J = 7.9 Hz, 1H), 7.32 (d, J = 9.1 Hz, 1H), 7.42–7.59 (m, 4H), 7.65–7.71 (m, 2H), 8.05 (d, J = 7.6 Hz, 1H), 8.15 (d, J = 7.7 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 45.9, 53.0, 76.1, 121.9, 126.6, 127.2, 127.6, 128.3, 128.4, 128.5, 128.7, 129.6, 131.6, 131.9, 132.7, 133.0, 133.5, 134.6, 135.7, 139.0, 141.6, 183.0 | |
| 7h | Mp | 156–158 °C |
| IR (cm−1) | 929, 1315, 1548, 1600, 1659 | |
| 1H NMR (400 MHz, CDCl3) | 2.09 (s, 3H), 4.00–4.04 (m, 1H), 4.49 (d, J = 3.5 Hz, 1H), 4.54 (dd, J = 13.1, 7.1 Hz, 1H), 4.82 (dd, J = 13.1, 8.9 Hz, 1H), 5.99 (d, J = 8.0 Hz, 2H), 6.74 (d, J = 7.7 Hz, 2H), 7.35–7.45 (m, 2H), 7.49 (d, J = 7.4 Hz, 2H), 7.56–7.61 (m, 2H), 8.03 (d, J = 7.7 Hz, 1H), 8.10 (d, J = 7.3 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 20.9, 45.9, 53.1, 75.8, 126.4, 126.9, 127.4, 127.9, 128.3, 128.5, 128.6, 129.9, 132.4, 132.8, 133.4, 134.3, 138.1, 139.3, 142.0, 182.9 | |
| 7i | Mp | 119–121 °C |
| IR (cm−1) | 929, 1312, 1511, 1548, 1599, 1671 | |
| 1H NMR (400 MHz, CDCl3) | 3.71 (s, 3H), 3.99–4.01 (m, 1H), 4.49 (d, J = 3.8 Hz, 1H), 4.57 (dd, J = 13.3, 7.4 Hz, 1H), 4.82 (dd, J = 13.3, 9.1 Hz, 1H), 6.00 (d, J = 8.8 Hz, 2H), 6.50 (d, J = 8.8 Hz, 2H), 7.40–7.54 (m, 4H), 7.60–7.67 (m, 2H), 8.02 (d, J = 7.9 Hz, 1H), 8.10 (d, J = 7.3 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 45.9, 52.4, 54.9, 76.9, 113.2, 125.0, 127.1, 127.5, 127.8, 128.0, 128.2, 128.3, 129.4, 132.6, 132.8, 133.9, 134.8, 139.0, 141.9, 160.0, 182.9 | |
| 7j | Mp | 168–171 °C |
| IR (cm−1) | 929, 1321, 1549, 1600, 1659 | |
| 1H NMR (400 MHz, CDCl3) | 4.01–4.04 (m, 1H), 4.49 (d, J = 3.4 Hz, 1H), 4.60 (dd, J = 13.3, 7.6 Hz, 1H), 4.87 (dd, J = 13.3, 8.5 Hz, 1H), 5.99 (d, J = 8.5 Hz, 2H), 6.92 (d, J = 8.5 Hz, 2H), 7.40–7.55 (m, 4H), 7.61–7.67 (m, 2H), 8.03 (d, J = 7.9 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 45.8, 52.5, 76.4, 126.8, 127.0, 127.2, 127.6, 127.9, 128.1, 128.2, 128.4, 130.0, 131.9, 132.2, 132.9, 133.1, 134.0, 134.5, 138.9, 141.7, 182.9 | |
| 7k | Mp | 171–172 °C |
| IR (cm−1) | 929, 1323, 1511, 1548, 1605, 1658 | |
| 1H NMR (400 MHz, CDCl3) | 4.01–4.06 (m, 1H), 4.55 (d, J = 3.7 Hz, 1H), 4.60 (dd, J = 13.3, 7.3 Hz, 1H), 4.90 (dd, J = 13.3, 9.1 Hz, 1H), 5.99 (dd, J = 8.8, 5.2 Hz, 2H), 6.67 (t, J = 8.8 Hz, 2H), 7.45–7.58 (m, 4H), 7.64–7.68 (m, 2H), 7.98 (d, J = 7.3 Hz, 1H), 8.07 (d, J = 7.9 Hz, 1H) | |
| 13C NMR (100 MHz, CDCl3) | 46.3, 52.8, 76.6, 114.8, 115.2, 127.1, 127.6, 127.9, 128.0, 128.2, 128.5, 128.9, 129.5, 130.8, 131.9, 132.8, 133.6, 134.5, 138.9, 142.1, 161.9, 182.9 |
3. Results and discussion
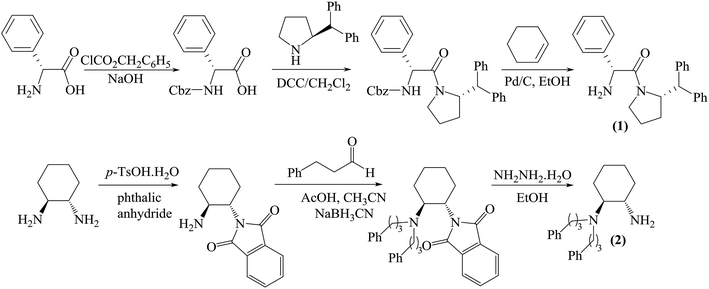
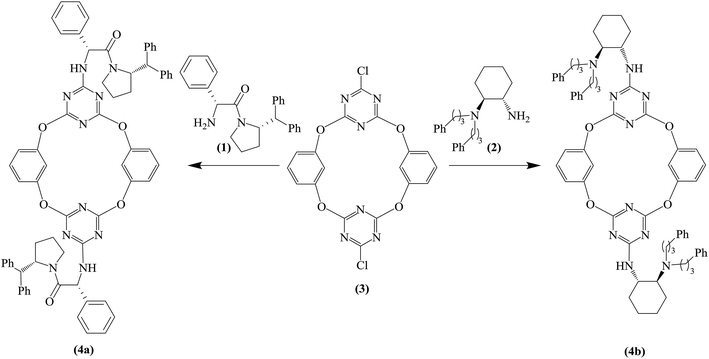
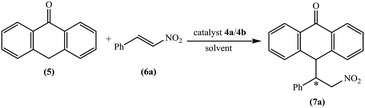
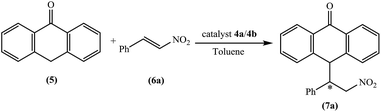
The chiral tetraoxacalix[2]arene[2]triazine derivatives 4a and 4b, which were chosen as chiral catalysts in the model asymmetric Michael reaction, were synthesized in four steps starting from (R)-phenylglycine and (1S,2S)-(+)-1,2-diaminocyclohexane, respectively. The chiral subunits (R)-2-amino-1-((S)-2-benzhydrylpyrrolidin-1-yl)-2-phenylethanone 1 and (1S,2S)–N,N-bis(3-phenylpropyl) cyclohexane-1,2-diamine 2, which were synthesized in three steps, were prepared according to previously reported procedures,57,58 as illustrated in Scheme 1. Subsequently, the chiral tetraoxacalix[2]arene[2]triazines 4a and 4b were synthesized from 1 and 2, respectively, in overall good yields (up to 75–78%), as illustrated in Scheme 2.In the first review of the conditions, having selected the Michael addition of anthrone 5 and trans-β-nitrostyrene 6a as the enantioselective reaction, we found that the tetraoxacalix[2]arene[2]triazine catalysts catalyzed the process effectively, affording the expected adduct 7a in an optically active form. The reaction progressed successfully in nonpolar solvents including hexane, CHCl3, CH2Cl2 or toluene (Table 3, entries 1–8); however, when we used a polar solvent, such as ethyl acetate, diethyl ether or acetone, significant decrease was observed in chemical yield and enantioselectivity (Table 3, entries 15–20). This may be due to the fact that the polar solvents interacted with the organocatalysts through hydrogen bonding to weaken the activation ability of 4a and 4b towards the reaction. As projected by our tentative hypothesis, the best yield (96%) and enantioselectivity (97% ee) were obtained using the less polar solvent, i.e., toluene.
| Entrya | Catalyst | Solvent | Time (h) | Yieldb (%) | eec,d (%) |
|---|---|---|---|---|---|
| a Conditions: anthrone (0.48 mmol), trans-β-nitrostyrene (0.40 mmol) and 4a/4b (10 mol%) in solvents (4.0 mL).b Isolated yield after flash chromatograpy.c Determined by HPLC.d Determined by comparing reported data. | |||||
| 1 | 4a | Hexane | 72 | 88 | 93 |
| 2 | 4b | Hexane | 72 | 90 | 95 |
| 3 | 4a | CHCl3 | 48 | 85 | 90 |
| 4 | 4b | CHCl3 | 48 | 89 | 91 |
| 5 | 4a | CH2Cl2 | 48 | 81 | 92 |
| 6 | 4b | CH2Cl2 | 48 | 83 | 93 |
| 7 | 4a | Toluene | 48 | 95 | 96 |
| 8 | 4b | Toluene | 48 | 96 | 97 |
| 9 | 4a | CH3CN | 72 | 92 | 80 |
| 10 | 4b | CH3CN | 72 | 95 | 81 |
| 11 | 4a | Xylene | 72 | 87 | 91 |
| 12 | 4b | Xylene | 72 | 90 | 95 |
| 13 | 4a | THF | 72 | 82 | 84 |
| 14 | 4b | THF | 72 | 85 | 87 |
| 15 | 4a | EtOAc | 48 | 87 | 80 |
| 16 | 4b | EtOAc | 48 | 91 | 85 |
| 17 | 4a | Et2O | 72 | 83 | 88 |
| 18 | 4b | Et2O | 72 | 86 | 89 |
| 19 | 4a | Acetone | 48 | 85 | 82 |
| 20 | 4b | Acetone | 48 | 88 | 85 |
Other aspects of this reaction such as temperature, use of the recycled catalyst, and catalyst loading were investigated (Table 4). When the same reaction was performed at 0 °C with 10 mol% of 4a and 4b as catalysts, the desired adduct 7a was obtained with low to 88–89% ee in 83–85% yield, with further extension of the reaction time (72 h, Table 4, entries 3 and 4). In addition, similar to the reaction conducted at 0 °C, the reaction carried out at −20 °C showed less desired yields and enantioselectivities than the reaction at room temperature (Table 4, entries 1 and 2). Additionally, we conducted recycling analysis of the chiral catalysts 4a and 4b in the Michael reaction of 5 and 6a. Thus, the compounds 4a and 4b could be easily recycled by flash chromatography alongside Michael adducts. However, by prolonging the reaction time, low enantioselectivity and yield values were found at room temperature, as seen in Table 4 (entries 7 and 8). In the presence of 10 mol% catalysts, 4a and 4b showed similar enantioselectivities, but 4b showed slightly higher catalytic activity than 4a (Table 4, entries 5 and 6). When the loading of the catalysts 4a and 4b went up to 15 mol%, trans-β-nitrostyrene gave Michael products in low yields with 93–95% and 93–95% enantiomeric excess, respectively (Table 4, entries 9 and 10). These results were better than that for the use of 5 mol% of catalyst, in which case the yields of the Michael products were 88% for catalyst 4a and 90% for catalyst 4b and the enantiomeric excesses of the Michael products were 89% for catalyst 4a and 90% for catalyst 4b (Table 4, entries 11 and 12). These findings led us to choose the reaction conditions using toluene as a solvent at room temperature in the presence of 10 mol% of 4b to probe the scope of nitroolefins.
| Entrya | Catalyst | Temp. (°C) | Time (h) | Yieldb (%) | eec,d (%) |
|---|---|---|---|---|---|
| a Conditions: anthrone (0.48 mmol), trans-β-nitrostyrene (0.40 mmol), and 4a/4b (10 mol%) in toluene (4.0 mL).b Isolated yield.c Determined by HPLC.d Determined by comparing reported data.e Reaction was performed with recycled catalyst.f 15 mol% of catalyst was used.g 5 mol% of catalyst was used. | |||||
| 1 | 4a | −20 | 72 | 80 | 85 |
| 2 | 4b | −20 | 72 | 81 | 86 |
| 3 | 4a | 0 | 72 | 83 | 88 |
| 4 | 4b | 0 | 72 | 85 | 89 |
| 5 | 4a | r.t. | 48 | 95 | 96 |
| 6 | 4b | r.t. | 48 | 96 | 97 |
| 7e | 4a | r.t. | 72 | 75 | 86 |
| 8e | 4b | r.t. | 72 | 78 | 90 |
| 9f | 4a | r.t. | 48 | 93 | 93 |
| 10f | 4b | r.t. | 48 | 95 | 95 |
| 11g | 4a | r.t. | 48 | 88 | 89 |
| 12g | 4b | r.t. | 48 | 90 | 90 |
As we enhanced the reaction details for the Michael addition of anthrone 5 to trans-β-nitrostyrene 6a (catalyst 4b 10 mol% in toluene at room temperature), a set of different nitrostyrenes with various substituent groups were analyzed, and the results are summarized in Table 5. These nitrostyrenes reacted with anthrone to afford the corresponding adducts 7a–7k in moderate to excellent yields with excellent enantioselectivities (Table 5, entries 1–11). As demonstrated in Table 5, anthrone reacts smoothly with a wide range of ortho-, meta- or para-substituted nitrostyrenes with electron-releasing or electron-withdrawing groups and the corresponding Michael adducts in good to excellent yields (86–96%) and enantioselectivities (81–97%) are obtained. To our satisfaction, trans-β-nitrostyrene and 4-Me-nitrostyrene as Michael acceptors gave good yields and enantioselectivities (Table 5, entries 1 and 8).
| Entrya | Ar | Time (h) | Product | Yieldb (%) | eec,d (%) |
|---|---|---|---|---|---|
| a Conditions: anthrone (0.48 mmol), trans-β-nitrostyrene (0.40 mmol), and 4b (10 mol%) in toluene (4.0 mL).b Isolated yield after flash chromatograpy.c Determined by HPLC.d The configurations were determined by comparing reported data. | |||||
| 1 | C6H5 | 48 | 7a | 96 | 97 |
| 2 | 2,4-Cl2–C6H4 | 36 | 7b | 88 | 82 |
| 3 | 2-Furyl-C6H4 | 48 | 7c | 94 | 88 |
| 4 | 2-Br–C6H4 | 36 | 7d | 94 | 81 |
| 5 | 2-NO2–C6H4 | 48 | 7e | 91 | 89 |
| 6 | 2-OMe–C6H4 | 48 | 7f | 86 | 95 |
| 7 | 3-Br–C6H4 | 36 | 7g | 93 | 96 |
| 8 | 4-Me–C6H4 | 48 | 7h | 95 | 97 |
| 9 | 4-OMe–C6H4 | 48 | 7i | 95 | 91 |
| 10 | 4-Cl–C6H4 | 36 | 7j | 91 | 82 |
| 11 | 4-F–C6H4 | 36 | 7k | 91 | 92 |
In conclusion, we developed highly efficient asymmetric Michael addition of anthrone to nitroalkenes catalyzed by tetraoxa-bridged calix[2]arene[2]triazine organocatalysts. The results of our study were similar to the literatures which were used thiourea-tertiary amine,32 cinchona alkaloids,33 cinchona-based chiral polyesters,59 and Ar-BINMOLs60 catalysts for the reaction of anthrone to a series of nitroalkenes. The steric bulkiness and carbonyl groups were crucial in this reaction to give the corresponding adducts in lower ee than that for the catalyst without carbonyl groups. Efforts to elucidate the mechanistic details of this catalytic system and to further extend the scope and limitations of these kinds of organocatalysts are currently in progress.
4. Conclusions
In conclusion, in this work, a new class of chiral tetraoxa-bridged calix[2]arene[2]triazine derivatives described as effective organocatalysts for the Michael reaction of anthrone to various nitrostyrenes was reported for the first time. The addition reactions were carried out smoothly in toluene at room temperature by utilizing 10 mol% of 4a and 4b to give Michael products with high yields (up to 96%) and ee values (up to 97%).Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Srinivas, K. Yesudas, K. Bhanuprakash, J. V. Rao and L. A. Giribabu, J. Phys. Chem. C, 2009, 113, 20117–20126 CrossRef CAS.
- R. Iwaura, M. Ohnishi-Kameyama and T. Lizawa, Chem.–Eur. J., 2009, 15, 3729–3735 CrossRef CAS.
- L. Krenn, R. Pradhan, A. Presser, G. Reznicek and B. Kopp, Chem. Pharm. Bull., 2004, 52, 391–393 CrossRef CAS.
- F. Diaz, H.-B. Chai, Q. Mi, B.-N. Su, J. S. Vigo, J. G. Graham, F. Cabieses, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, S. M. Swanson and A. D. Kinghorn, J. Nat. Prod., 2004, 67, 352–356 CrossRef CAS PubMed.
- D. W. Cameron and C. E. Skene, Aust. J. Chem., 1996, 49, 617–624 CrossRef CAS.
- H.-S. Huang, J.-M. Hwang, Y.-M. Jen, J.-J. Lin, K.-Y. Lee, C.-H. Shi and H.-C. Hsu, Chem. Pharm. Bull., 2001, 49, 969–973 CrossRef CAS.
- V. Kren and T. Rezanka, FEMS Microbiol. Rev., 2008, 32, 858–889 CrossRef CAS PubMed.
- K. Müuller, Appl. Microbiol. Biotechnol., 2001, 56, 9–16 CrossRef.
- T. Pecere, M. V. Gazzola, C. Mucignat, C. Parolin, F. D. Vecchia, A. Cavaggini, G. Basso, A. Diaspro, B. Salvato, M. Carli and G. Pulci, Cancer Res., 2000, 60, 2800–2804 CAS.
- Y. Shiono, N. Shino, S. Seo, S. Oka and Y. Z. Yamazaki, Naturforscher, 2002, 57c, 923–929 Search PubMed.
- A. Zuse, D. Schmidt, S. Baasner, K. J. Büohm, K. Müuller, M. Gerlach, E. G. Güunther, E. Unger and H. Prinz, J. Med. Chem., 2007, 50, 6059–6066 CrossRef CAS PubMed.
- B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS PubMed.
- O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877–1894 CrossRef CAS.
- D. Almasi, D. A. Alonso and C. Najera, Tetrahedron: Asymmetry, 2007, 18, 299–365 CrossRef CAS.
- H. Huang and E. N. Jacobsen, J. Am. Chem. Soc., 2006, 128, 7170–7171 CrossRef CAS PubMed.
- M. P. Lalonde, Y. Chen and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 6366–6370 CrossRef CAS PubMed.
- W. Wang, J. Wang and H. Li, Angew. Chem., Int. Ed., 2005, 43, 1369–1371 CrossRef PubMed.
- Y. Hayashi, T. Gotoh, T. Hayasji and M. Shoji, Angew. Chem., Int. Ed., 2005, 44, 4212–4215 CrossRef CAS PubMed.
- T. Ishii, S. Fiujioka, Y. Sekiguchi and H. Kotsuki, J. Am. Chem. Soc., 2004, 126, 9558–9559 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Tang and L. Deng, J. Am. Chem. Soc., 2004, 126, 9906–9907 CrossRef PubMed.
- T. Okino, Y. Hoashi, T. Furukawa, X. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119–125 CrossRef CAS PubMed.
- M. Terada, H. Ube and Y. Yaguchi, J. Am. Chem. Soc., 2006, 128, 1454–1455 CrossRef CAS PubMed.
- H. Li, Y. Wang, L. Tang, F. Wu, X. Liu, C. Guo, B. M. Foxman and L. Deng, Angew. Chem., Int. Ed., 2005, 44, 105–108 CrossRef CAS PubMed.
- J. Wang, H. Li, W. Duan, L. Zu and W. Wang, Org. Lett., 2005, 7, 4713–4716 CrossRef CAS PubMed.
- F.-Z. Peng, Z.-H. Shao, B.-M. Fan, H. Song, G.-P. Li and H.-B. Zhang, J. Org. Chem., 2008, 73, 5202–5205 CrossRef CAS PubMed.
- P. Gao, C. Wang, Y. Wu, Z. Zhou and C. Tang, Eur. J. Org. Chem., 2008, 4563–4566 CrossRef CAS.
- J. Shen, T. T. Nguyen, Y.-P. Goh, W. Ye, X. Fu, J. Xu and C.-H. Tan, J. Am. Chem. Soc., 2006, 128, 13692–13693 CrossRef CAS PubMed.
- A.-N. Alba, N. Bravo, A. Moyano and R. Rios, Tetrahedron Lett., 2009, 50, 3067–3069 CrossRef CAS.
- D. Akalay, G. Durner and M. W. Gobel, Eur. J. Org. Chem., 2008, 2365–2368 CrossRef CAS.
- C. Wu, W. Li, J. Yang, X. Liang and J. Ye, Org. Biomol. Chem., 2010, 8, 3244–3250 RSC.
- A. Zea, G. Valero, A. N. R. Alba, A. Moyano and R. Rios, Adv. Synth. Catal., 2010, 352, 1102–1106 CrossRef CAS.
- Y.-H. Liao, H. Zhang, Z.-J. Wu, L.-F. Cun, X.-M. Zhang and W.-C. Yuan, Tetrahedron: Asymmetry, 2009, 20, 2397–2402 CrossRef CAS.
- M. Shi, Z.-Y. Lei, M.-X. Zhao and J.-W. Shi, Tetrahedron Lett., 2007, 48, 5743–5746 CrossRef CAS.
- M. M. Naseer, D. X. Wang, L. Zhao, Z. T. Huang and M. X. Wang, J. Org. Chem., 2011, 76, 1804–1813 CrossRef CAS PubMed.
- S. Li, D. X. Wang and M. X. Wang, Tetrahedron Lett., 2012, 53, 6426–6429 CrossRef CAS.
- A. I. Vicente, J. M. Caio, J. Sardinha, C. Oiteiro, R. Delgado and V. Felix, Tetrahedron, 2012, 68, 670–680 CrossRef CAS.
- L. X. Wang, L. Zhao and D. X. Wang, Chem. Commun., 2011, 9690–9692 RSC.
- M. H. Duker, H. Schafer, M. Zeller and V. A. Azov, J. Org. Chem., 2013, 78, 4905–4912 CrossRef PubMed.
- W. Zhao, K. Hu, C. Wang, S. Liang, B. Niu, L. He, K. Lu, B. Ye and S. Zhang, J. Chromatogr. A, 2012, 1223, 72–78 CrossRef CAS PubMed.
- H. B. Yang, D. X. Wang, Q. Q. Wang and M. X. Wang, J. Org. Chem., 2007, 72, 3757–3763 CrossRef CAS PubMed.
- D. X. Wang, Q. Y. Zheng, Q. Q. Wang and M. X. Wang, Angew. Chem., Int. Ed., 2008, 47, 7485–7488 CrossRef CAS PubMed.
- D. X. Wang, S. X. Fa, Y. Liu, B. Y. Hou and M. X. Wang, Chem. Commun., 2012, 11458–11460 RSC.
- S. Pan, D. X. Wang, L. Zhao and M. X. Wang, Tetrahedron, 2012, 68, 9464–9477 CrossRef CAS.
- M.-X. Wang, Acc. Chem. Res., 2012, 45, 182–195 CrossRef CAS PubMed.
- W. Maes and W. Dehaen, Chem. Soc. Rev., 2008, 37, 2393–2402 RSC.
- H. Tsue, K. Ishibashi and R. Tamura, Top. Heterocycl. Chem., 2008, 17, 73–96 CAS.
- X. D. Wang, D. X. Wang, Z. T. Huang and M. X. Wang, Supramol. Chem., 2014, 26, 601–606 CrossRef CAS.
- W. Zhao, W. Wang, H. Chang, S. Cui, K. Hu, L. He, K. Lu, J. Liu, W. Wu, J. Qian and S. Zhang, J. Chromatogr. A, 2012, 1251, 74–81 CrossRef CAS PubMed.
- H. N. Naziroglu and A. Sirit, Tetrahedron: Asymmetry, 2016, 27, 201–207 CrossRef.
- H. N. Genc and A. Sirit, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 39–49 CrossRef CAS.
- H. N. Naziroglu, M. Durmaz, S. Bozkurt, A. S. Demir and A. Sirit, Tetrahedron: Asymmetry, 2012, 23, 164–169 CrossRef CAS.
- H. N. Genc, U. Ozgun and A. Sirit, Chirality, 2019, 31, 293–300 CrossRef CAS PubMed.
- M.-X. Wang and H.-B. Yang, J. Am. Chem. Soc., 2004, 126, 15412–15422 CrossRef CAS PubMed.
- S. Bozkurt and M. B. Türkmen, Tetrahedron: Asymmetry, 2016, 27, 443–447 CrossRef CAS.
- Y. Huangfu, K. Ruan, H. Qiu, Y. Lu, C. Liang, J. Kong and J. Gu, Composites, Part A, 2019, 121, 265–272 CrossRef CAS.
- C. Liang, H. Qiu, Y. Han, H. Gu, P. Song, L. Wang, J. Kong, D. Cao and J. Gu, J. Mater. Chem. C, 2019, 7, 2725–2733 RSC.
- H. N. Naziroglu and A. Sirit, Turk. J. Chem., 2012, 36, 659–670 CAS.
- T. Bui, S. Syed and C. F. Barbas III, J. Am. Chem. Soc., 2009, 131, 8758–8759 CrossRef CAS PubMed.
- B. T. Kumpuga and S. Itsuno, J. Catal., 2018, 361, 398–406 CrossRef CAS.
- G. Gao, X.-F. Bai, H.-M. Yang, J.-X. Jiang, G.-Q. Lai and L.-W. Xu, Eur. J. Org. Chem., 2011, 5039–5046 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03029h |
| This journal is © The Royal Society of Chemistry 2019 |