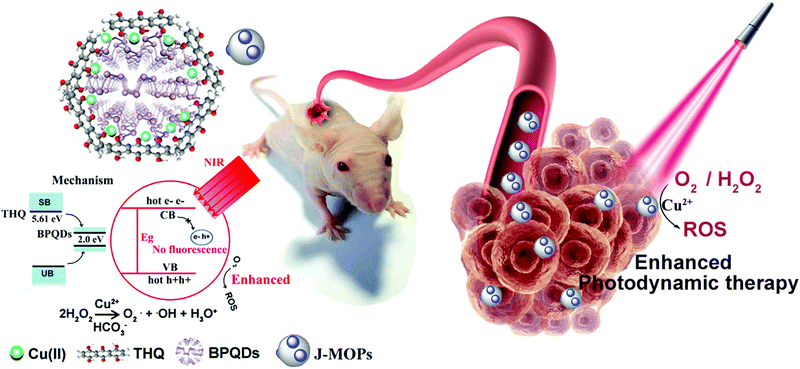
The design of Janus black phosphorus quantum dots@metal–organic nanoparticles for simultaneously enhancing environmental stability and photodynamic therapy efficiency†
Da
Zhang‡
 abc,
Ziguo
Lin‡
acd,
Shanyou
Lan
acd,
Haiyan
Sun
*e,
Yongyi
Zeng
abc,
Ziguo
Lin‡
acd,
Shanyou
Lan
acd,
Haiyan
Sun
*e,
Yongyi
Zeng
 *abcd and
Xiaolong
Liu
*abcd and
Xiaolong
Liu
 *abc
*abc
aThe United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, P. R. China. E-mail: xiaoloong.liu@gmail.com
bMengchao Med-X Center, Fuzhou University, Fuzhou 350116, P. R. China
cThe Liver Center of Fujian Province, Fujian Medical University, Fuzhou 350025, P. R. China
dLiver Disease Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, P. R. China. E-mail: lamp1973@medmail.com.cn
eDepartment of Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, P. R. China. E-mail: 2254103187@qq.com
First published on 14th February 2019
Abstract
Although newly-developed black phosphorus quantum dots (BPQDs) with unique photocatalytic properties have attracted much attention for biomedical applications, especially phototherapy, the environmental instability and weak photocatalytic activity of BPQDs remains a considerable challenge preventing their clinical application. Herein, we have designed new Janus nanoparticles (J-MOPs) based on BPQDs and tetrahydroxyanthraquinone (THQ)–Cu metal–organic particles (MOPs) to simultaneously improve the environmental stability and photocatalytic activity for the PDT treatment of cancers. The J-MOPs were simply assembled from BPQDs and THQ via Cu-mediated P–Cu–THQ “host–metal–guest” coordination effects. The encapsulation of BPQDs inside J-MOPs and P–Cu bonds effectively isolated BPQDs from water–air and occupied the P lone-pair electrons, respectively, to improve the stability of BPQDs. Furthermore, the dramatic electron–hole separation and migration abilities of J-MOPs increased the reactivity toward O2, which enhanced singlet oxygen (1O2) generation against cancers compared with BPQDs alone when exposed to 670 nm laser irradiation. Intriguingly, tumor acid-triggered J-MOPs degradation resulted in the release of Cu2+ ions that further served as a Fenton-like agent to generate ˙OH from H2O2, which enhanced the antitumor effects of the J-MOPs. Therefore, we have highlighted the potential of J-MOPs as a photocatalyst for photodynamic cancer therapy.
Introduction
Black phosphorus nanosheets (BPNs)/quantum dots (BPQDs) are two-dimensional (2D) materials that have attracted extensive interests in recent years owing to their unique anisotropic layered structure, high electron mobility, proper band gap (spanning from 0.3 eV (bulk value) to ∼2.1 eV (monolayer value)) and excellent photocatalytic properties.1–5 Especially in biomedical fields, the unique photocatalytic activity of BPNs/BPQDs with smaller nanosizes (<10 nm) could be used as safety photosensitizer agents for cancer photodynamic therapy owing to their extremely high biosafety and minimized side effects. These include effective elimination of BPNs/BPQDs by the renal and liver systems6–11 and biodegradation into phosphorus to serve as a nutrient in the body. However, the inherent instability of BPNs/BPQDs under water–air conditions seriously hinders their biomedical applications. Furthermore, the weak absorption of BPNs/BPQDs in the optical windows of biological tissues and low photocatalysis activity in hypoxic microenvironment (TME) significantly limit their applications in cancer treatment.12–15 Therefore, the development of an effective strategy to simultaneously improve the environmental stability and maximize the photocatalytic activity of BPNs/BPQDs is urgently needed to further explore its applications in biosystems.Some strategies to enhance the environmental stability of BPNs/BPQDs have been developed in recent years. One strategy involved occupying the phosphorus lone-pair electrons in BPNs/BPQDs by dropping metal ions, such as Ti and lanthanides (Ln = Gd, Tb, Eu, Nd, etc.), onto them.16–20 Another strategy involved adsorbing organic materials/inorganic molecules onto the surface of BPNs/BPQDs, including peptides, dyes, and polydopamine biomaterials.21–25 The third approach is forming self-assembled or cooperated nanostructures, such as PLGA, to isolate BPQDs.26,27 Furthermore, to enhance the photocatalysis activity of BPNs/BPQDs, the electromagnetic near-field enhancement mechanism of plasmonic metals has been used to boost singlet oxygen generation, while another strategy is increasing the NIR absorption of BPNs/BPQDs or enhancing their local-field plasmonic effect using gold nanoparticles through the localized surface plasmon resonance (LSPR) effect.28,29 Furthermore, the fabrication of nanohybrids with electron–hole separation and migration abilities enhanced the reactivity with H2O and O2 to generate 1O2 through the effect of photogenerated electrons and holes.2,30–32 Recently, so-called Janus nanoparticles (JNPs) have simultaneously presented two different sets of material and chemical properties in an “all-in-one” platform, which might be an excellent and effective strategy for designing nanohybrids for cancer therapy.33–41 To date, the construction of JNPs from metal–organic nanoparticles and BPQDs to simultaneously improve the environmental stability and enhance 1O2 generation ability for PDT treatment has yet to be reported.
In this study, we designed new Janus nanoparticles (J-MOPs) based on BPQDs and THQ–Cu MOPs to simultaneously improve the environmental stability and ROS generation for enhancing PDT efficiency (Fig. 1). In our design, the J-MOPs were simply self-assembled from THQ and BPQDs by Cu2+-mediated coordination through Cu–P and THQ–Cu bonds. The advanced nanostructure exhibited several features, as follows: (i) excellent water–air stability of the encapsulated BPQDs due to Cu–P bonds occupying the phosphorus lone-pair electrons and isolating the BPQDs from air and water; (ii) significantly enhanced ROS generation owing to the electron–hole separation and migration ability of the J-MOPs nanostructure; and (iii) effective oxidative stress injury to tumor cells and excellent PDT antitumor effects both in vitro and in vivo.
Results and discussion
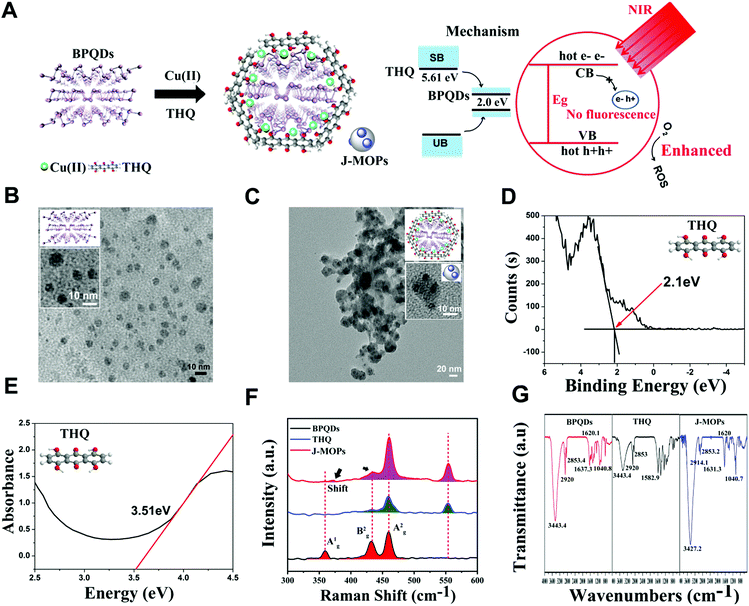
The J-MOPs were designed and synthesized as shown in Fig. 2A. Briefly, the prefabricated BPQDs, THQ organic dye, and Cu2+ ions were first mixed in aqueous solution (pH 7.4) and then vigorously stirred for 1 h. After centrifugation, the J-MOPs were obtained. The size and morphology of the prepared BPQDs and J-MOPs were characterized by transmission electron microscopy (TEM). As shown in Fig. 2B and C, the BPQDs showed a narrow size distribution of around 6 ± 2.8 nm with a typical dot morphology similar to those reported previously.1–8 The J-MOPs showed an average size of 25 ± 6.9 nm in diameter, with the BPQDs embedded inside the THQ–Cu MOPs framework corresponding to the enlarged TEM image. The energy band (LUMO) and band gap (Eg) of THQ were calculated from the X-ray photoelectron spectroscopy and absorption spectra, respectively (Fig. 2D and E). The HOMO of THQ was calculated to be 5.61 eV using the following equation: LUMO = HOMO − Eg. Raman spectra suggested that J-MOPs consisted of BPQDs with three specific prominent peaks, including one out-of-plane phonon mode (Ag1) at 361.2 cm−1 and two in-plane modes, Bg2 and Ag2, at 439.2 cm−1 and 466.5 cm−1, respectively.1–8 Furthermore, THQ showed two prominent peaks at 466.5 cm−1 and 557 nm−1 (Fig. 2F). Notably, the THQ signals from J-MOPs at 439.2 cm−1, 466.5 cm−1, and 557.4 cm−1 were higher than those of THQ alone, which might be due to surface-enhanced Raman scattering. Interestingly, the Ag1 mode of J-MOPs at 361.2 cm−1 was right-shifted to 370.4 cm−1 and much lower than that of the BPQDs, which might be due to the Cu–P and Cu–THQ coordination bond interactions. To confirm this result, X-ray photoelectron spectroscopy (XPS) was performed. As shown in Fig. S1 (ESI†), the XPS spectrum of J-MOPs showed a sub-band at P2p 130.1 eV, which was attributed to Cu-bonded P. The strong peak at 532 eV of O1s was attributed to hydride adsorption by THQ. XPS also showed typical Cu2p3/2 and Cu2p1/2 peaks with measured binding energies of 935.1 and 955.0 eV, which corresponded to Cu2+ on the J-MOPs. Furthermore, the J-MOPs were mainly composed of P, Cu, O, and C elements, and Cu was successfully coordinated into the BPQDs. These results were further confirmed by energy-dispersive X-ray spectroscopy (EDS), suggesting interactions between Cu–P and Cu–THQ in the J-MOPs (Fig. S2, ESI†). To further confirm the compositions of the J-MOPs, Fourier-transform infrared spectroscopy (FTIR) was performed. As shown in Fig. 2G, absorption bands at 3427.2, 2914.1, 1631.3, and 1040.7 cm−1 corresponded to stretching vibrations of –OH, –CH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O groups, respectively indicating the existence of THQ and BPQDs in the J-MOPs. Furthermore, the weaker absorption of J-MOPs compared with that of THQ at 1620 cm−1 was due to the stretching vibration of the C
C, and C–O groups, respectively indicating the existence of THQ and BPQDs in the J-MOPs. Furthermore, the weaker absorption of J-MOPs compared with that of THQ at 1620 cm−1 was due to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in conjugated ketones resonating between C–O/C
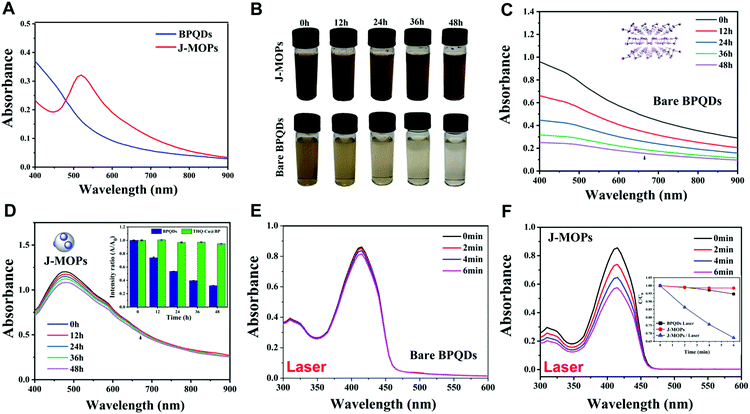
O groups in conjugated ketones resonating between C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Cu2+, indicating the presence of Cu(II) in these J-MOPs.12,42 Furthermore, the optical absorption properties of the J-MOPs and BPQDs were analyzed to confirm the existence of Cu2+ ions. As shown in Fig. 3A, the J-MOPs exhibited a new absorption peak at 520 nm, which was attributed to Cu2+ ions from the J-MOPs.12 The J-MOPs also showed a broad absorption from 450 nm to 850 nm, which was much higher than that of the bare BPQDs, owing to the surface enhancement of THQ–Cu complexes in the π-conjugation system.
O and Cu2+, indicating the presence of Cu(II) in these J-MOPs.12,42 Furthermore, the optical absorption properties of the J-MOPs and BPQDs were analyzed to confirm the existence of Cu2+ ions. As shown in Fig. 3A, the J-MOPs exhibited a new absorption peak at 520 nm, which was attributed to Cu2+ ions from the J-MOPs.12 The J-MOPs also showed a broad absorption from 450 nm to 850 nm, which was much higher than that of the bare BPQDs, owing to the surface enhancement of THQ–Cu complexes in the π-conjugation system.
Next, the water–air and physiological stabilities of the prepared J-MOPs were investigated, respectively. As shown in Fig. 3B, the color of the J-MOPs remained brown after exposing to water–air conditions for 48 h, while the color of the bare BPQD solution faded to transparent after 48 h under the same conditions. Furthermore, ultraviolet-visible-near infrared (UV-vis-NIR) spectra were recorded, and the results clearly suggested that the absorbance of the prepared J-MOPs was very stable owing to the Cu–P bonds and the effective encapsulation of the BPQDs to isolate them from water and air. Meanwhile, the absorbance intensity of the bare BPQDs significantly decreased with increasing storage time (Fig. 3C and D). Furthermore, the J-MOPs were very stable in PBS buffer solution (pH 7.4) and medium (containing 10% FBS), compared with naked BPQDs (Fig. S3, ESI†). Next, we further evaluated the reactive oxygen species (ROS) generation ability of our J-MOPs using an ROS indicator (1,3-diphenylisobenzofuran, DPBF) during 670 nm laser irradiation, using the bare BPQDs as a control.16–25 As shown in Fig. 3E and F, and Fig. S4 (ESI†), the J-MOP-encapsulated BPQDs within PBS buffer exhibited a sharp decline in DPBF absorbance in the range of 350 nm to 450 nm when exposed to 670 nm lasers with a power intensity of 0.1 W cm−2. However, the bare BPQDs within PBS buffer only showed a slight decline in DPBF absorbance in the same environment and under the same laser power conditions owing to the instability and self-degradation of BPQDs under water–air conditions. Furthermore, by normalizing the absorbance at 415 nm, the absorbance of the J-MOPs decreased 6.2-fold compared with that of the BPQDs after irradiation for 6 min (Fig. 3F, inset picture). These results indicated that the ROS generation ability of our designed J-MOPs was significantly enhanced comparing with bare BPQDs, because the BPQDs could constantly donate electrons and accept holes from THQ through Cu2+ ions as a bridge, which led to a reduction in electron–hole recombination (Fig. S5, ESI†).29–32 These results suggested that our J-MOPs with dramatic electron–hole separation and migration abilities could enhance the reactivity with O2 to produce ROS. Altogether, our designed J-MOPs not only improved the stability of BPQDs, but also enhanced their ROS generation ability.
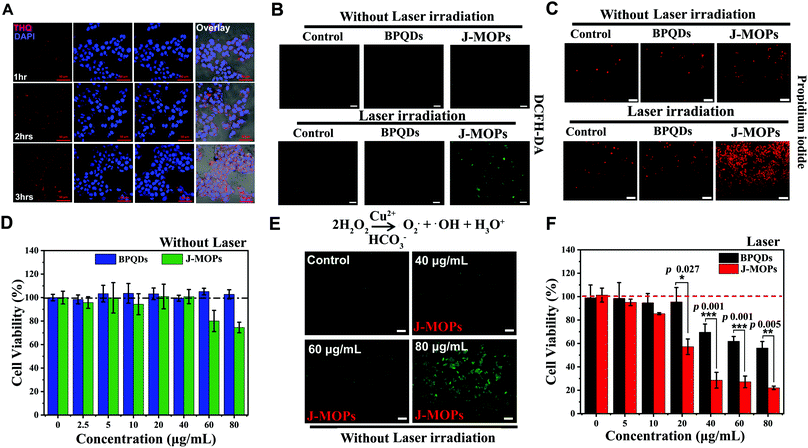
Before therapeutic applications, the cellular uptake of J-MOPs by hepatocellular carcinoma (HepG2) cells was evaluated using confocal scanning microscopy (CLSM). As shown in Fig. 4A, the THQ organic dye with red fluorescent signals (561 nm laser excitation) from J-MOPs was almost invisible in the cytoplasm of HepG2 cells after incubation for 1 h. With prolonged incubation, the red fluorescence intensity of THQ from J-MOPs was significantly enhanced in the cytoplasm of cells at 37 °C owing to the tumor acid-triggered degradation of J-MOPs, which was consistent with the characteristics of MOPs,12,42,44 suggesting the effective uptake of J-MOPs by cancer cells. Next, we investigated the intracellular ROS generation of J-MOPs using intracellular ROS fluorescence indicator DCFH-DA after 670 nm laser irradiation. As shown in Fig. 4B, HepG2 cells alone with or without laser irradiation showed background fluorescence (green). Meanwhile, HepG2 cells treated with either bare BPQDs or the J-MOPs without laser irradiation showed no fluorescence intensity enhancements. In contrast, the green fluorescence intensity was significantly increased in J-MOPs-treated cells after 670 nm laser irradiation for 5 min (0.1 W cm−2). Notably, HepG2 cells treated with BPQDs at the same concentration showed relatively weak fluorescence owing to the degradation and instability of BPQDs during incubation. These results indicated that our prepared J-MOPs could act as an effective photosensitizing agent for enhancing intracellular ROS generation upon 670 nm laser irradiation. Next, the antitumor effect of J-MOPs was investigated. After HepG2 cells were incubated with J-MOPs and exposed to 670 nm laser irradiation, the cells were stained with PI to directly visualize dead cells according to our previously reported protocol.12,28,44,45 As shown in Fig. 4C, only background red fluorescence was observed in the cells treated with PBS alone or BPQDs (20 μg mL−1) or J-MOPs (BPQDs, 20 μg mL−1) without laser irradiation. Similarly, the cells treated with PBS alone upon 670 nm laser irradiation showed only background levels of red fluorescence. However, significantly more dead cells were observed in J-MOPs-treated HepG2 cells after 670 nm laser irradiation compared with the BPQDs-treated cells subjected to the same laser irradiation conditions or any other groups. To further quantitatively analyze the antitumor effect of our J-MOPs, CCK8 assays were conducted. The cytotoxicity of J-MOPs without laser irradiation was evaluated first. As shown in Fig. 4D and Fig. S6 (ESI†), after incubation with different concentrations of J-MOPs (calculated by BPQDs) for 24 h, the HepG2 cell viabilities of J-MOPs-treated cells remained higher than 95%, even up to 40 μg mL−1, which was comparable with that of bare BPQDs-treated cells. The results were similar for the toxicity of J-MOPs-treated normal liver cells (LO2). However, when the J-MOPs concentration was increased above 60 μg mL−1, our J-MOPs showed certain cytotoxicity, even without laser irradiation (for example, 25.5% of cell growth inhibition at 80 μg mL−1 in HepG2; and 11.5% of cell growth inhibition in LO2), which might be due to acid-triggered cleavage of the coordination bonds between P–Cu and Cu–THQ, followed by Cu2+ release as a Fenton-like agent for ROS generation from endogenous H2O2.42–44 To prove this assumption, Cu2+ release from J-MOPs under tumor-specific acidic conditions (pH 6.5) was investigated. As shown in Fig. S7 (ESI†), 20.6% of Cu2+ was released from the J-MOPs after 48 h incubation at pH 6.5, indicating that the J-MOPs could slowly release Cu2+ into tumor acidic environments. J-MOPs acting as Fenton-like agents for ROS generation in endogenous H2O2 was further confirmed by examining intracellular ROS generation under dark conditions. As shown in Fig. 4E, the green fluorescence intensity of DCFH-DA in J-MOPs-treated cells increased in a concentration-dependent manner, suggesting that the Cu2+ ions from degraded J-MOPs could induce severe oxidative stress inside cells. Furthermore, the cell viability of J-MOPs-treated HepG2 cells under 670 nm laser irradiation (0.1 W cm−2, 5 min) was significantly decreased to 22% at 80 μg mL−1, while the bare BPQDs-treated cells retained 56.1% cell viability under the same laser irradiation conditions, suggesting that our J-MOPs had a dramatically enhanced ROS generation ability and PDT efficiency in vitro (Fig. 4F).
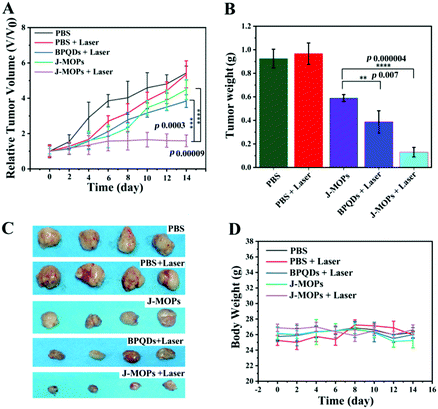
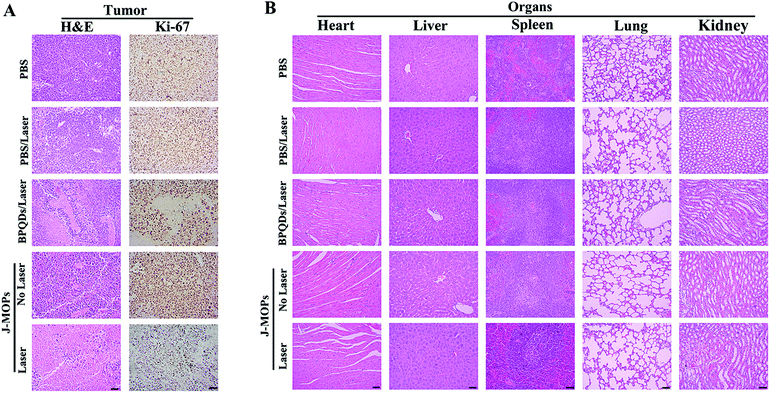
To further investigate the antitumor tumor effects of our J-MOPs in vivo, HepG2 tumor-bearing nude mice received different treatments, as mentioned in the Experimental section (ESI†), and the tumor volumes were measured using a Vernier caliper every 2 days. As shown in Fig. 5A, the mice treated with PBS either with or without laser irradiation experienced rapid tumor growth, indicating that NIR laser irradiation under these power conditions alone had no influence on tumor growth. Although mice treated with BPQDs showed some tumor growth delay when irradiated with a 670 nm laser, the tumors still showed relatively rapid growth. Notably, the mice treated with J-MOPs in the dark (without laser irradiation) showed certain tumor growth delay, due to Cu2+ released from J-MOPs as a Fenton-like agent against tumor growth. More obviously, mouse tumors treated with J-MOPs under 670 nm laser irradiation showed the strongest tumor growth inhibition due to an enhanced PDT efficiency and synergy with the Fenton-like activity of Cu2+ against tumors. To further confirm these results in vivo, ex vivo fresh tumors were crushed, centrifuged, and incubated with J-MOPs with or without 670 nm laser irradiation (0.1 W cm−2, 5 min). The ˙OH from the tumor microenvironment H2O2 and total ROS from J-MOPs upon 670 nm laser irradiation were determined using TMB and DCFH-DA probes, respectively.46 As shown in Fig. S8 (ESI†), the TMB absorbance of J-MOPs under dark conditions was much higher than that of the control under the same conditions, which was due to Cu2+ released from J-MOPs as Fenton-like agents to generate ˙OH from H2O2. In contrast, the DCFH-DA fluorescence intensity of J-MOPs with laser irradiation was obviously higher than that of J-MOPs in the dark and the control groups without laser irradiation, suggesting that the total ROS generation (singlet oxygen (1O2) and ˙OH) was resulting from J-MOPs upon 670 nm laser irradiation. The additional DCFH-DA fluorescence of J-MOPs in the dark was derived from ˙OH. The therapeutic efficacy was also confirmed by calculating the ex vivo tumor weight (Fig. 5B and C), and investigating hematoxylin–eosin (H&E) and immunohistochemical (IHC) (Ki67) staining after 48 h of indicated treatments. As shown in Fig. 6A, the tumors of J-MOPs-treated mice after 670 nm laser irradiation showed typical cytoskeleton collapse and nucleus dissociation. These phenomena were much more serious than BPQDs-treated mice under the same laser irradiation conditions. Additionally, the Ki67 signal in the tumors of J-MOPs-treated mice after laser irradiation was much weaker than that of any other groups, further demonstrating the excellent therapeutic efficacy of our J-MOPs. Finally, the body weight was investigated to roughly evaluate the toxicity of J-MOPs. As shown in Fig. 5D, no significant body-weight loss was observed in mice from any group, suggesting the excellent biocompatibility of our designed J-MOPs. This was further confirmed by H&E staining of the major organs (heat, liver, spleen, lung, and kidney) after the indicated treatments (Fig. 6B). In summary, these results clearly demonstrated that our prepared J-MOPs could serve as an effective photosensitizing agent against tumors with excellent biosafety.
Conclusions
We have reported a novel and simple strategy to fabricate Janus nanoparticles based on BPQDs and THQ–Cu MOPs to simultaneously improve the environmental stability and ROS generation ability of black phosphorus materials for PDT treatment. In this novel nanostructure, the J-MOPs not only protected against BPQDs degradation through effective encapsulation and Cu–P bonds, but also endowed BQPDs with dramatic electron/hole separation and migration abilities to enhance the ROS generation during PDT treatment. Interestingly, the tumor microenvironment-triggered degradation of J-MOPs could release Cu2+ ions to serve as Fenton-like agents to further improve the antitumor efficacy by synergizing with PDT. Therefore, we have highlighted the potential of our designed J-MOPs to acts as a promising cancer phototherapy agent for possible clinical applications.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
D. Z. and Z. G. L. contributed equally to this work. This work was supported by the Natural Science Foundation of China (Grant No. 61805041, U1505221, 61727823, 61875141, and 61575044); the Natural Science Foundation of Fujian Province (Grant No. 2016J01174); the Medical Innovation Project of Fujian province (Grant No. 2018-CX-49); and the Wu Jie-Ping Medical Foundation (Grant No. LDWJPMF-102-17007).References
- L. Bai, X. Wang, S. Tang, Y. Kang, J. Wang, Y. Yu, Z. K. Zhou, C. Ma, X. Zhang, J. Jiang, P. K. Chu and X. F. Yu, Adv. Mater., 2018, 30, 3641 Search PubMed
.
- M. Wen, J. Wang, R. Tong, D. Liu, H. Huang, Y. Yu, Z.-K. Zhou, P. K. Chu and X.-F. Yu, Adv. Sci., 2018, 1801321 Search PubMed
.
- X. Zhang, H. Xie, Z. Liu, C. Tan, Z. Luo, H. Li, J. Lin, L. Sun, W. Chen, Z. Xu, L. Xie, W. Huang and H. Zhang, Angew. Chem., 2015, 54, 3653 CrossRef CAS PubMed
.
- R. Gui, H. Jin, Z. Wang and J. Li, Chem. Soc. Rev., 2018, 47, 6795 RSC
.
- S. C. Dhanabalan, J. S. Ponraj, Z. Guo, S. Li, Q. Bao and H. Zhang, Adv. Sci., 2017, 4, 1600305 CrossRef PubMed
.
- L. Chan, P. Gao, W. Zhou, C. Mei, Y. Huang, X. F. Yu, P. K. Chu and T. Chen, ACS Nano, 2018, 12, 12401–12415 CrossRef CAS PubMed
.
- W. Zhou, T. Pan, H. Cui, Z. Zhao, P. K. Chu and X. F. Yu, Angew. Chem., 2018, 3, 779 Search PubMed
.
- W. Zhou, H. Cui, L. Ying and X. F. Yu, Angew. Chem., 2018, 57, 10268 CrossRef CAS PubMed
.
- J. Shao, C. Ruan, H. Xie, Z. Li, H. Wang, P. K. Chu and X. F. Yu, Adv. Sci., 2018, 5, 1700848 CrossRef PubMed
.
- W. Chen, J. Ouyang, H. Liu, M. Chen, K. Zeng, J. Sheng, Z. Liu, Y. Han, L. Wang, J. Li, L. Deng, Y. N. Liu and S. Guo, Adv. Mater., 2017, 29, 1603864 CrossRef PubMed
.
- W. Tao, X. Zhu, X. Yu, X. Zeng, Q. Xiao, X. Zhang, X. Ji, X. Wang, J. Shi, H. Zhang and L. Mei, Adv. Mater., 2017, 29, 1802061 Search PubMed
.
- D. Zhang, M. Wu, Z. Cai, N. Liao, K. Ke, H. Liu, M. Li, G. Liu, H. Yang, X. Liu and J. Liu, Adv. Sci., 2018, 5, 1700648 CrossRef PubMed
.
- M. Liu, L. Wang, X. Zheng, S. Liu and Z. Xie, ACS Appl. Mater. Interfaces, 2018, 10, 24638 CrossRef CAS PubMed
.
- D. Zhang, A. Zheng, J. Li, M. Wu, L. Wu, Z. Wei, N. Liao, X. Zhang, Z. Cai, H. Yang, G. Liu, X. Liu and J. Liu, Theranostics, 2017, 7, 164 CrossRef CAS PubMed
.
- L. Wang, M. Huo, Y. Chen and J. Shi, Adv. Healthcare Mater., 2018, 7, 1701156 CrossRef PubMed
.
- Z. Guo, S. Chen, Z. Wang, Z. Yang, F. Liu, Y. Xu, J. Wang, Y. Yi, H. Zhang, L. Liao, P. K. Chu and X. F. Yu, Adv. Mater., 2017, 29, 1703811 CrossRef PubMed
.
- Y. Zhao, L. Tong, Z. Li, N. Yang, H. Fu, L. Wu, H. Cui, W. Zhou, J. Wang, H. Wang, P. K. Chu and X.-F. Yu, Chem. Mater., 2017, 29, 7131–7139 CrossRef CAS
.
- Z. Sun, Y. Zhao, Z. Li, H. Cui, Y. Zhou, W. Li, W. Tao, H. Zhang, H. Wang, P. K. Chu and X.-F. Yu, Small, 2017, 13, 1602896 CrossRef PubMed
.
- L. Wu, J. Wang, J. Lu, D. Liu, N. Yang, H. Huang, P. K. Chu and X. Yu, Small, 2018, 29, 1801405 CrossRef PubMed
.
- Y. Zhao, H. Wang, H. Huang, Q. Xiao, Y. Xu, Z. Guo, H. Xie, J. Shao, Z. Sun, W. Han, X. F. Yu, P. Li and P. K. Chu, Angew. Chem., 2016, 55, 5003 CrossRef CAS PubMed
.
- X. Zeng, M. Luo, G. Liu, X. Wang, W. Tao, Y. Lin, X. Ji, L. Nie and L. Mei, Adv. Sci., 2018, 5, 1800510 CrossRef PubMed
.
- H. Wang, K. Hu, Z. Li, C. Wang, M. Yu, Z. Li and Z. Li, Small, 2018, 14, 1801701 CrossRef PubMed
.
- R. Gusmao, Z. Sofer and M. Pumera, ACS Nano, 2018, 12, 5666 CrossRef CAS PubMed
.
- S. T. Han, L. Hu, X. Wang, Y. Zhou, Y. J. Zeng, S. Ruan, C. Pan and Z. Peng, Adv. Sci., 2017, 4, 1600435 CrossRef PubMed
.
- W. Chen, J. Ouyang, X. Yi, Y. Xu, C. Niu, W. Zhang, L. Wang, J. Sheng, L. Deng, Y. N. Liu and S. Guo, Adv. Mater., 2018, 30, 1703458 CrossRef PubMed
.
- M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang, X. Chen, D. K. Sang, C. Xing, Z. Li, B. Dong, F. Xing, D. Fan, S. Bao, H. Zhang and Y. Cao, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 501 CrossRef CAS PubMed
.
- J. Shao, H. Xie, H. Huang, Z. Li, Z. Sun, Y. Xu, Q. Xiao, X. F. Yu, Y. Zhao, H. Zhang, H. Wang and P. K. Chu, Nat. Commun., 2016, 7, 12967 CrossRef CAS PubMed
.
- D. Zhang, X. Lin, S. Lan, H. Sun, X. Wang, X. Liu, Y. Zhang and Y. Zeng, Part. Part. Syst. Charact., 2018, 35, 1800010 CrossRef
.
- H. Zhang, H. Li, H. Fan, J. Yan, D. Meng, S. Hou, Y. Ji and X. Wu, Nano Res., 2018, 11, 1456 CrossRef CAS
.
- F. He, G. Chen, Y. Yu, S. Hao, Y. Zhou and Y. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 7171 CrossRef CAS PubMed
.
- J. Chen, C. L. Dong, D. Zhao, Y. C. Huang, X. Wang, L. Samad, L. Dang, M. Shearer, S. Shen and L. Guo, Adv. Mater., 2017, 29, 1606198 CrossRef PubMed
.
- W. He, H. K. Kim, W. G. Wamer, D. Melka, J. H. Callahan and J. J. Yin, J. Am. Chem. Soc., 2013, 136, 750–757 CrossRef PubMed
.
- G. Song, M. Chen, Y. Zhang, L. Cui, H. Qu, X. Zheng, M. Wintermark, Z. Liu and J. Rao, Nano Lett., 2018, 18, 182 CrossRef CAS PubMed
.
- Y. Ju, H. Zhang, J. Yu, S. Tong, N. Tian, Z. Wang, X. Wang, X. Wang, X. Su, X. Chu, J. Lin, Y. Ding, G. Li, F. Sheng and Y. Hou, ACS Nano, 2017, 11, 9239 CrossRef CAS PubMed
.
- J. Song, B. Wu, Z. Zhou, G. Zhu, Y. Liu, Z. Yang, L. Lin, G. Yu, F. Zhang, G. Zhang, H. Duan, G. Stucky and X. Chen, Angew. Chem., 2017, 129, 8222 CrossRef
.
- Y. H. Zhu, X. Yang, D. Bao, X. F. Bie, T. Sun, S. Wang, Y. S. Jiang, X. B. Zhang, J.-M. Yan and Q. Jiang, Joule, 2018, 2, 736–746 CrossRef CAS
.
- Q. Zhang, H. Zhong, F. Meng, D. Bao, X. Zhang and X. Wei, Nano Res., 2018, 11, 1294–1300 CrossRef CAS
.
- S. Yuan, S. Wang, L. Li, Y. H. Zhu, X. B. Zhang and J. M. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 9178–9184 CrossRef CAS PubMed
.
- M. M. Shi, D. Bao, B. R. Wulan, Y. H. Li, Y. F. Zhang, J. M. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1606550 CrossRef PubMed
.
- X. Chen, N. Li, Z. Kong, W.-J. Ong and X. Zhao, Mater. Horiz., 2018, 5, 9–27 RSC
.
- D. Bao, Q. Zhang, F. L. Meng, H. X. Zhong, M. M. Shi, Y. Zhang, J. M. Yan, Q. Jiang and X. B. Zhang, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed
.
- D. Zhang, H. Xu, X. Zhang, Y. Liu, M. Wu, J. Li, H. Yang, G. Liu, X. Liu, J. Liu and Z. Yuan, ACS Appl. Mater. Interfaces, 2018, 10, 25203 CrossRef CAS PubMed
.
- M. F. Poyton, A. M. Sendecki, X. Cong and P. S. Cremer, Cu2+ Binds to Phosphatidylethanolamine and Increases Oxidation in Lipid Membranes, J. Am. Chem. Soc., 2016, 138, 1584 CrossRef CAS PubMed
.
- C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079 CrossRef CAS PubMed
.
- D. Zhang, Z. Cai, N. Liao, S. Lan, M. Wu, H. Sun, Z. Wei, J. Li and X. Liu, Chem. Sci., 2018, 9, 7390 RSC
.
- A. Üzer, S. Durmazel, E. Erçağ and R. Apak, Sens. Actuators, B, 2017, 247, 98 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00623g |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |