Biodegradable metallic bone implants
Cijun
Shuai
abc,
Sheng
Li
a,
Shuping
Peng
 bc,
Pei
Feng
a,
Yuxiao
Lai
d and
Chengde
Gao
bc,
Pei
Feng
a,
Yuxiao
Lai
d and
Chengde
Gao
 *a
*a
aState Key Laboratory of High Performance Complex Manufacturing, College of Mechanical and Electrical Engineering, Central South University, Changsha 410083, China. E-mail: gaochengde@csu.edu.cn; Fax: +86-731-8887-9044; Tel: +86-731-8480-5412
bJiangxi University of Science and Technology, Ganzhou 341000, China
cKey Laboratory of Organ Injury, Aging and Regenerative Medicine of Hunan Province, Changsha 410008, China
dShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, 518055, China
First published on 30th January 2019
Abstract
Biodegradable metals, such as Mg and Mg alloys, Fe and Fe alloys, and Zn and Zn alloys, are drawing increased attention as bone implant materials owing to their biodegradability. Among them, Mg and Mg alloys have similar densities and elastic moduli as compared to those of natural bone, but they degrade too quickly in human physiological environments, resulting in excessive release of hydrogen and premature loss of strength. Fe and Fe alloys are known for their outstanding mechanical properties, while their degradation rates are too slow to meet the requirements of bone repair. In comparison, Zn and Zn alloys have suitable degradation rates when compared with the growth rates of natural bone. However, their poor strength and ductility constrain their applications in bone repair. This review summarizes the current status of research on the use of biodegradable metals in bone implants. Their biodegradability, mechanical properties, and biocompatibility are systematically reviewed. On the basis of presentation, efforts made to improve the deficiencies of these biodegradable metals, such as alloying and heat treatment, are summarized. The problems and further directions are also put forward for biodegradable metallic bone implants.
Introduction
Bone fractures and defects are becoming increasingly common with the rapid increase in aging population, traffic accidents, sport injuries, and illnesses.1,2 Although autologous and allogeneic bones are considered to be the ideal candidates for bone repair, they are restricted by either limited sources or immunologic rejection.3 In view of this, numerous research efforts have been devoted toward developing implants as bone substitutes. A desired bone implant is supposed to uniformly degrade in the human body and be progressively replaced by the growing tissue until the bone repair process is complete.4,5 The implant's mechanical properties such as strength, elastic modulus, and hardness should be comparable with or slightly higher than those of the natural bone for sufficient load-bearing capacity without looseness or displacement.6 Moreover, it should not cause toxicity or inflammation in the human body,7,8 but it should promote bone regeneration by osteoinduction and osteogenesis.9–11Till date, various kinds of bone implant materials such as biopolymers, bioceramics, and biomedical metals have been developed.12,13 Biopolymers are a type of material with good plasticity and biocompatibility. Nevertheless, low strength, poor hydrophilicity, and aseptic inflammation risk restrict their applications in bone repair.14–16 Bioceramics that combine excellent activity and compatibility are usually limited by their disadvantage of brittleness. When compared with biopolymers and bioceramics, biomedical metals have integrated mechanical properties, becoming more suitable for load-bearing applications. Commonly used metals in clinical applications include titanium alloys, stainless steels, and cobalt–chromium alloys. Although these metals can serve as partial functional substitutes for natural bones, they cannot degrade and get retained as permanent implants.17–19 As a result, a second surgery is always needed to remove such implants after bone healing. Repeated surgery inevitably incurs additional costs and pain to patients.20,21 Therefore, researchers are investigating biomedical metals that can progressively degrade in the human body and completely dissolve after bone repair without leaving any residue.22
In such a situation, biodegradable metals such Mg and Mg alloys (Mg-based alloys), Fe and Fe alloys (Fe-based alloys), and Zn and Zn alloys (Zn-based alloys) have been proposed for use as bone implants.23 Mg is one of the most abundant cations in the human body and exists primarily in the bone,24,25 positively influencing the metabolism of enzymes and RNA and DNA structures. As a Group IIA element, Mg has high chemical activity and easily corrodes to form porous oxidation films on its surface.4,26 Thereafter, the oxidation film is likely to fall off, particularly in solutions containing chlorine ions, thereby accelerating the degradation process. Fe is one of the essential trace elements in the human body and plays a major role in oxygen transport and many enzymatic reactions. Moreover, Fe is susceptible to oxidation in humid conditions, which is regarded as a degradable property.27,28 As another essential element, Zn plays a key role in physiological functions such as bone metabolism, gene expression, and synthesis of various transcription factors.29,30 In recent years, it has also been recognized as a biodegradable material, thereby exhibiting potential applications for bone repair. On account of both biosafety and biodegradability, three types of metals are garnering research focus in the field of bone repair.4,31,32
This review systematically introduces the characteristics of biodegradable metals. Further, their potential uses as bone implants are evaluated with regard to biodegradability, mechanical properties, and biocompatibility. Moreover, attempts to regulate the biodegradability and mechanical properties are summarized and discussed. The future development trends for biodegradable metallic bone implants are also suggested.
1. Types of biodegradable metals
A series of biodegradable metals such as Mg, Fe, and Zn have been investigated. Mg-Based alloys, known as revolutionary metals in biomedical applications, have been the prime targets for studies on biodegradable metallic implants in the past decade.3,33 As bone implants, they have inherent advantages owing to the fact that their density and elastic modulus are similar to those of natural bones, but their applications are severely restricted by high degradation rates and inadequate mechanical properties. Till now, a series of Mg-based alloys such as Mg–Al,4,34 Mg–Ca,35,36 Mg–Zn,37–40 Mg–Sr,33,41,42 Mg–RE (RE: rare earth),27,43–45 Mg–Mn,46,47 Mg–Cu,48 Mg–Ag,49 and Mg–Si50 have been developed, aiming to resolve the above deficiencies. Fe is usually alloyed with other elements to form materials that can accelerate the biodegradation process.51–53 Further, the alloying elements should be carefully selected to alleviate biosafety concerns. Thus far, Fe-based alloys usable for bone repair can be classified into binary and ternary alloys, such as Fe–Mn,54–57 Fe–W,51,52 Fe–Al,52 Fe–C,52,58 Fe–Ag,58 Fe–Mn–C,59–61 Fe–Mn–Pd,55 and Fe–Mn–Si.62 Zn, lately reported as a new kind of biodegradable metal, is receiving increasing attention. Commonly used Zn-based alloys in the industry usually contain large quantities of toxic elements: for example, ZA series alloys contain up to 40 wt% toxic Al element,34,43,63,64 which inevitability raises biosafety issues. On the account of biosafety, current studies on Zn-based alloys for bone repair have mainly concentrated upon a few newly developed alloys: for instance, Zn–Mg,63,65,66 Zn–Ca,63 Zn–Cu,67,68 and Zn–Sr,63 which have shown acceptable biosafety and appropriate mechanical properties for use as bone implants.692. Biodegradability
It is expected that once biodegradable metals are implanted in the human body, they could gradually degrade at a suitable rate that matches the recovery rate of bone tissues.70,71 Therefore, it is necessary to investigate the degradation mechanisms, degradation behavior, and corresponding regulation methods of biodegradable metals.2.1 Degradable mechanisms
Many degradation phenomena of metals are driven by electrochemical reactions.65,72 Therefore, electrochemical principles are commonly used to describe the degradation mechanisms of metallic implants.| Anodic reaction Mg → Mg2+ + 2e− | (1) |
| Cathodic reaction 2H2O + 2e → H2↑ + 2OH− | (2) |
| Overall reaction Mg + 2H2O → Mg(OH)2↓ + H2↑ | (3) |
At the same time, porous Mg(OH)2 on the surface of Mg-based alloys is easily eroded by solutions containing chlorine ions owing to the following reaction:
| Mg(OH)2 + 2Cl− → MgCl2 + 2OH− | (4) |
Thus, the Mg(OH)2 layers cannot protect the Mg matrix against further corrosion, which considerably accelerates the degradation process.34 The degradation types of Mg-based alloys strongly depend on the composition and structural states of the alloys.76–79 Further, pitting corrosion is usually undesirable due to the possible stress concentration and significant reduction in the mechanical properties of Mg-based alloys.35,80–83
| Anodic reaction Fe → Fe2+ + 2e− | (5) |
| Cathodic reaction O2 + 2H2O + 2e− → 4OH− | (6) |
| Overall reaction 2Fe + 2H2O + O2 → 2Fe(OH)2↓ | (7) |
Fe2+ may be transformed into Fe3+ under alkaline and oxygen environments, and new products form as follows:
| Fe2+ → Fe3+ + e− | (8) |
| Fe3+ + 3OH− → Fe(OH)3↓ | (9) |
| Fe(OH)2 + 2FO(OH) → Fe3O4↓ + H2O | (10) |
At an early stage, Fe-based alloys gradually degrade according to the reactions (5)–(10), and the degradation products mainly consist of Fe(OH)2, Fe(OH)3, and Fe3O4. These degradation products are compact and have a worthwhile protective effect on the Fe matrix.28,85 As a result, further degradation is deterred, leading to a slow degradation rate for bone repair. When compared with Mg-based alloys, no gas is produced during the degradation process of Fe-based alloys.28 Meanwhile, Fe always undergoes a uniform degradation process, while Fe-based alloys might show local corrosion owing to inhomogeneous chemical compositions, nonuniform stress distributions, etc.85,86
| Anodic reaction Zn → Zn2+ + 2e− | (11) |
| Cathodic reaction 2H2O + O2 + 4e− → 4OH− | (12) |
| Overall reaction 2Zn + 2H2O + O2 → 2Zn(OH)2↓ | (13) |
As a result, insoluble Zn(OH)2 is formed on the surface and acts as a passive layer. However, Zn(OH)2 is converted into a soluble salt due to the presence of chlorine ions on the basis of the following reaction:87
| 6Zn(OH)2↓ + Zn2+ + 2Cl− → 6Zn(OH)2·ZnCl2 | (14) |
To conclude, these three kinds of alloys generally degrade because of electrochemical corrosion, as shown in Fig. 1. In solution, biodegradable metals release electrons and get oxidized into metal ions (Mn+) according to the anodic reactions. The electrons participate in the cathodic reactions (i.e., water reduction for Mg-based alloys and oxygen reduction for both Fe- and Zn-based alloys) and result in local alkalization. The organic molecules in solution may absorb on the metal surface, affecting the anodic reactions, as shown in Fig. 1(a). Subsequently, the degradation layers of M(OH)n form on the metal surface, accompanied by the continuous invasion of media and Mn+ release, as shown in Fig. 1(b). It is worth noting that the degradation layers of M(OH)n may be eroded by the solution containing chloride ions (Fig. 1(c)), particularly for Mg-based alloys. At the same time, Ca2+ and PO43− in the solution can induce apatite formation on the degradation layers because of local alkalization, which is beneficial for cell adhesion. With increasing time, the cells gradually proliferate and new tissues are formed on the metal surface. As the degradation proceeds, more degradation products form on the surface and the metal matrix continues to dissolve, which may lead to the partial fall-off of the matrix, as shown in Fig. 1(d). As a result, fresh matrix is exposed to the media and undergoes a new round of degradation.27
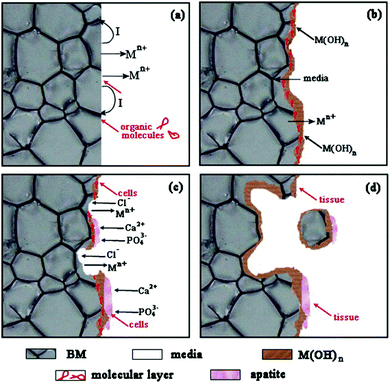 | ||
| Fig. 1 Degradation schematic diagram of biodegradable metals. Reproduced with permission from ref. 27. Copyright 2014 Elsevier. | ||
2.2 Degradation behavior
In electrochemical tests, the current transient response is digitally collected and the inherent corrosion properties, namely, corrosion potential, corrosion current density, and impedance, can be determined. Thereafter, the corrosion potential and current density are utilized to calculate the degradation rates according to the following equation:94,95
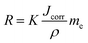 | (15) |
In comparison, immersion tests can be used to simulate relatively long-lasting degradation behavior of metallic implants. In this method, the degradation rates of biodegradable metals are determined by mass loss based on the following equation:28
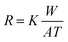 | (16) |
The mass loss can be determined by means of hydrogen evolution, direct weighing, or ion concentration. For Mg-based alloys, the H2 released during immersion is collected to estimate the mass loss according to eqn (3). In particular, every mol of H2 that is generated by the degradation of 1 mol Mg corresponds to 24 g loss of Mg. This method can facilitate the acquisition of numerous and detailed degradation data by real-time observations of the volume of hydrogen evolution.4,61 Another method to determine mass loss is by directly weighing the mass before and after immersion and then calculating the difference. To obtain accurate mass data after immersion, the degradation products need to be primarily removed with chromic acid and distilled water.96 This method has been extensively applied to Mg-, Fe-, and Zn-based alloys. Furthermore, mass loss can be determined from the ion concentration. In particular, the samples are removed from the immersion solution, and the degradation products are separated. Thereafter, the degradation products and an appropriate amount of acid (nitric acid) are added together into the immersion solution. The concentrations of ions, such as Zn2+, Mg2+, Fe2+, and Fe3+, are analyzed by using inductively coupled plasma atomic emission spectrometry and then used to determine the mass loss based on the following equation:29,35,74
| W = CV | (17) |
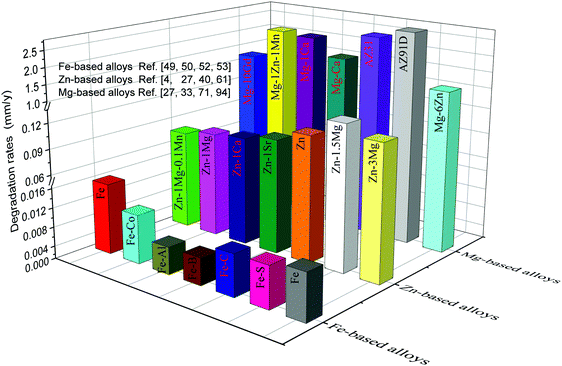
The degradation rates of metallic implants should be maintained within a reasonable range in order to ensure they maintain their functions during the service period.99,100 Extremely slow degradation can hinder the growth of new bone, while extremely rapid degradation cannot provide structural support to the defect site. The size and shape of the implant depend on the size and shape of the defect bone, resulting in different repair periods. For bone repair, it has been reported that the degradation rates of bone implants should be lower than 0.5 and higher than 0.2 mm y−1 to match bone healing.3,101 The degradation rates of typical metallic implants are shown in Fig. 2. Evidently, Mg-based alloys exhibit the highest degradation rates, approximately ranging from 0.8 to 2.7 mm y−1, which is above the tolerable degradation rates of bone implants. The degradation rates of Zn-based alloys are mainly between 0.1 and 0.3 mm y−1, appearing as prospective alternatives. In contrast, the slowest degradation rates, less than 0.1 mm y−1, are observed for Fe-based alloys. Hence, the degradation rates of these alloys, particularly Mg- and Fe-based alloys, need to be regulated to meet the clinical requirements of bone implants.4,29,42,51,52,63,102–104
Biodegradable Mg was first employed as an internal fixation plate to fix bone fracture in 1907.109 This attempt failed because the Mg plate degraded extremely rapidly and generated a lot of hydrogen in vivo. Subsequently, Troitskii and Znamenski used Mg alloys as bone repair materials to treat bone fracture, which did not achieve satisfactory results because of subcutaneous bubbles and severe loss of mechanical strength caused by rapid degradation.110,111 These studies have shown that Mg-based alloys degrade too quickly to achieve effective repair of damaged bones, which restricts their applications in bone repair.
In recent years, the corrosion resistance of Mg-based alloys has been significantly improved by smelting and surface treatment technologies.112,113 Accordingly, Mg-based alloys have again aroused worldwide attention. The in vivo degradation behaviors of WE43 Mg alloy implants were studied by Torroni et al.114 in sheep cranial bones. They found that lymph-node Mg accumulation showed no differences between the studied sheep and control (no implants) groups after implantation for 6 weeks, indicating good biocompatibility. Moreover, there was no hydrogen bubble in the degradation process and new bones surrounding the implants were found, suggesting osteogenesis-promoting properties. They further investigated their histomorphologic characteristics of artificially aged WE43 Mg alloys on the calvarial bone of sheep.115 The histomorphological analysis of the bone-implant after 6 weeks showed increased interfacial stability between the bone and WE43 Mg alloy implants, indicating that the alloy seemed to be suitable for use as a biodegradable bone implant. Witte et al.116 studied the degradation behaviors of LAE442 Mg alloy in the medial femur condyle of rabbits. They did not find subcutaneous gas cavities at 2, 4, 6, and 12 weeks postoperatively. Meanwhile, these implants were observed to be in direct bone contact and no fibrous capsule was visible, which indicated an acceptable host response. Duygulu et al.64 studied the in vivo degradation of AZ31 Mg alloy used as bone implants. They implanted this alloy into sheep hip bone. Three months later, it was found that alloy degradation could guide osteoblast growth toward the implant site. Mg alloy (AZ31, AZ91, WE43, and LAE442) rods with a diameter of 1.5 mm and length of 20.0 mm were implanted into the femora of guinea pigs to investigate the mechanical properties of bone-implant interactions after degradation and degradable self-reinforced poly-96L/4D-lactide rods were also implanted as the control.34 Fluoroscopic images of the bone-implant area (Fig. 3(A1)) revealed that the Mg alloy rods degraded nonuniformly as compared to the polymer rods. Moreover, the mineral apposition rate around the Mg alloy rods, particularly WE43 Mg alloy, was higher than that around the polymer rods (Fig. 3(A2)). In vivo mechanical properties and bone-implant interfacial strength of AZ31 Mg alloy after degradation were also studied by Tan et al.117 They implanted AZ31 Mg alloy with Si-containing coating, AZ31 Mg alloy, and poly-L-lactide (PLLA) into rabbit femur and then assessed the interfacial strength after degradation by extraction torque (Fig. 3(B1)). It could be observed that the extraction torque of the as-coated AZ31 Mg alloy at week 21 was much higher than those of AZ31 Mg alloy and PLLA (Fig. 3(B2)), indicating stronger osteosynthesis during the degradation process. This could be attributed to the moderate degradation rate of the as-coated AZ31 Mg alloy. Moreover, one-point bending load tests (Fig. 3(C1)) revealed that the PLLA implant broke down at week 4 owing to bulk degradation and AZ31 implants lost 26.4% of their original bending strength at week 4, 35.8% at week 12, and 42.4% at week 21 (Fig. 3(C2)) due to rapid degradation. In contrast, the as-coated AZ31 implants showed significantly higher mechanical stability with a loss of 13.4% at week 4, 17.1% at week 12, and 29.9% at week 21 due to improved degradation resistance, which facilitated the osteosynthesis process as evidenced by a higher Ca/P atomic ratio than those in the other implants (Fig. 3(D1) and Fig. 3(D2)).
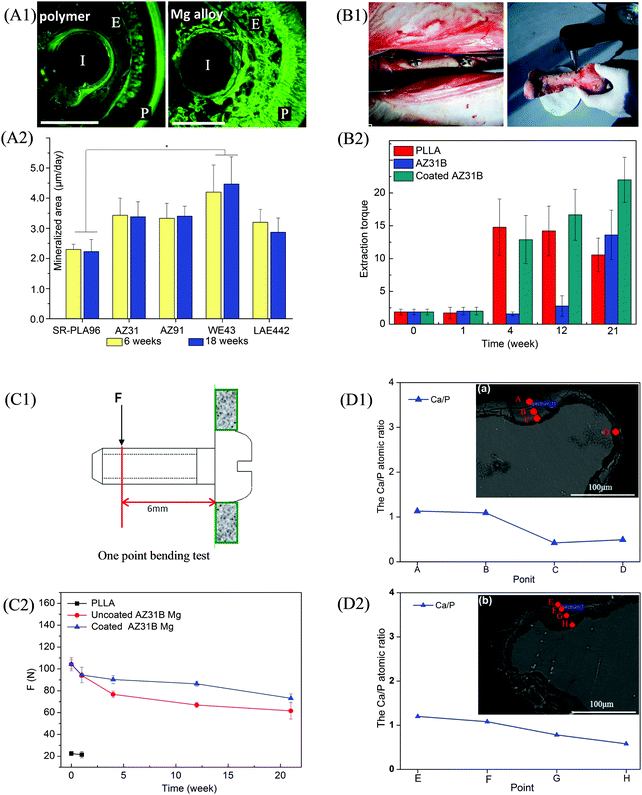 | ||
| Fig. 3 Implantation trials of Mg-based alloys. (A1) Fluoroscopic images of Mg alloy rods at week 18 (I, E, and P represent implant residual, endosteal bone formation, and periosteal bone formation, respectively) and (A2) mineralized area of AZ31, AZ91, WE43, and LAE442 Mg alloy rods at weeks 6 and 18 post-implantation in guinea pigs; a polymer rod served as the control. (B1) Representative digital images of AZ31B Mg alloy and coated AZ31B Mg alloy implanted in rabbit femur, and subsequent extraction torque test; (B2) extraction torque of AZ31B and coated AZ31B Mg alloy implants at weeks 0, 1, 4, 12, and 21; PLA implants served as the control. (C1) Schematic diagram of one-point bending test and (C2) bending strengths of AZ31B and coated AZ31B at weeks 0, 1, 4, 12, and 21 post-implantation in rabbit femur; PLA served as the control. The cross-section morphology and elemental composition of the bone-implant interface after implantation of (D1) AZ31B Mg alloy and (D2) coated AZ31B Mg alloy in rabbit femur at week 21. Reproduced with permission from ref. 34,117. Copyright 2005 2014 Elsevier. | ||
Fe-Based implants have also attracted increased attention with regard to bone repair. Fe, Fe–5 wt% TCP (Fe–TCP), Fe-5 wt% hydroxyapatite (Fe–HA), and Fe–5 wt% (40% TCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60% HA) (Fe–BCP) implants were fabricated by using powder metallurgy and implanted into sheep femurs (Fig. 4(A)).118 It was observed that black degradation products were formed on the Fe-based implants, as indicated by the white arrows. Surface appearance and roughness of these implants retrieved at day 70 (Fig. 4(B)) showed that Fe–HA, Fe–TCP, and Fe–BCP had rougher surfaces than Fe, indicating higher degradation rates after the incorporation of bioceramics. Meanwhile, the degradation layers of Fe–HA, Fe–TCP, and Fe–BCP were thicker than that of Fe (Fig. 4(C)), further indicating faster degradation rates occurred on the former list of implants, particularly Fe–BCP. A Fe–Mn–Si alloy with length of 25.3 mm, width of 2.45 mm, and thickness of 0.45 mm was implanted in a rabbit bone for 30 days.119 It was found that the implant weight was reduced from the initial 0.2284 g to 0.2277 g due to degradation. The SEM images of the implant shown in Fig. 5(A) and 5(B) revealed a corroded surface with different types of compounds. On the implant surface, the distributions of Fe, Mn, and Si elements before and after sonication cleaning are shown in Fig. 5(C) and (D), respectively, where a large number of oxides were observed after degradation. Moreover, the Mn elemental content was lower than that of Si, indicating more degradation of Mn compounds than Si compounds in the Fe-based alloy.
60% HA) (Fe–BCP) implants were fabricated by using powder metallurgy and implanted into sheep femurs (Fig. 4(A)).118 It was observed that black degradation products were formed on the Fe-based implants, as indicated by the white arrows. Surface appearance and roughness of these implants retrieved at day 70 (Fig. 4(B)) showed that Fe–HA, Fe–TCP, and Fe–BCP had rougher surfaces than Fe, indicating higher degradation rates after the incorporation of bioceramics. Meanwhile, the degradation layers of Fe–HA, Fe–TCP, and Fe–BCP were thicker than that of Fe (Fig. 4(C)), further indicating faster degradation rates occurred on the former list of implants, particularly Fe–BCP. A Fe–Mn–Si alloy with length of 25.3 mm, width of 2.45 mm, and thickness of 0.45 mm was implanted in a rabbit bone for 30 days.119 It was found that the implant weight was reduced from the initial 0.2284 g to 0.2277 g due to degradation. The SEM images of the implant shown in Fig. 5(A) and 5(B) revealed a corroded surface with different types of compounds. On the implant surface, the distributions of Fe, Mn, and Si elements before and after sonication cleaning are shown in Fig. 5(C) and (D), respectively, where a large number of oxides were observed after degradation. Moreover, the Mn elemental content was lower than that of Si, indicating more degradation of Mn compounds than Si compounds in the Fe-based alloy.
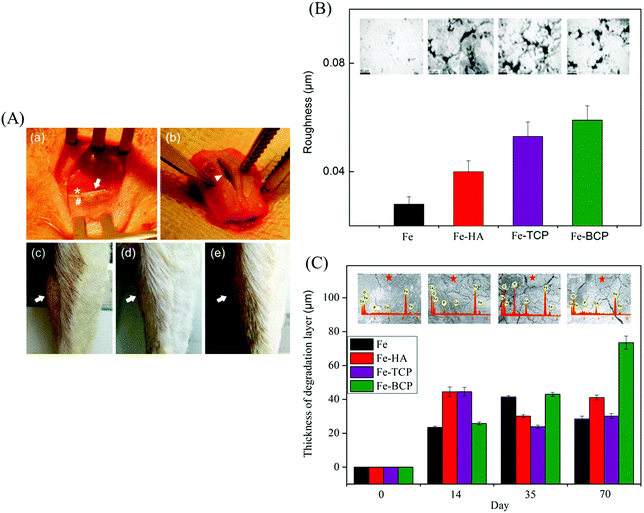 | ||
| Fig. 4 Implantation trials of Fe-based alloys. (A) Implantation in sheep leg bones: (a) implantation insertion showing bone (*), periosteum (#), and implant (arrow); (b) implant extraction (arrow head); (c–e) swelling observation (arrow) after 9, 14, and 35 days, respectively. (B) Surface morphologies and corresponding roughness values of Fe, Fe–HA, Fe–TCP, and Fe–BCP implants after 70 days. (C) Surface morphology, elemental composition, and degradation layer thickness after 0, 14, 35, and 70 days. Reproduced with permission from ref. 118. Copyright 2014 John Wiley and Sons. | ||
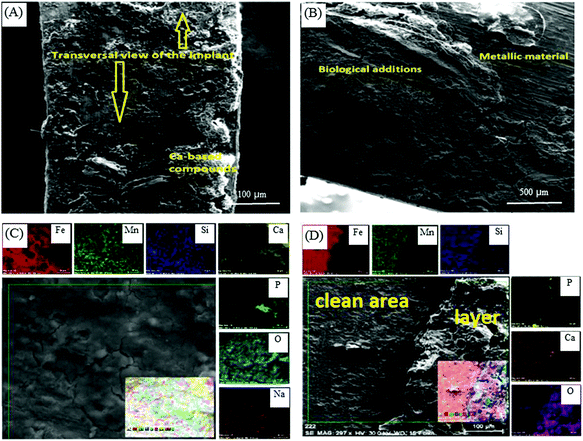 | ||
| Fig. 5 SEM images and EDS analysis showing the micrographs and distributions of Fe, Mn, and Si elements on the surface of Fe–Mn–Si alloy after implantation in rabbit for 30 days: implant surface (A) at the macroscale, (B) at the microscale, (C) before sonication cleaning, and (D) after sonication cleaning. Reproduced with permission from ref. 119. Copyright 2017 IOP org. | ||
Zn-Based alloys have been considered as promising biodegradable bone implants in the recent years. In 2011, Zn-based alloys were first proposed by Vojtech et al.120,121 for potential bone repair owing to their satisfactory biodegradability. Thereafter, Zn–1Sr, Zn–1Ca, and Zn–1Mg alloys were implanted into mouse femurs by Li et al.63 Cross-section histology at week 8 revealed that the periosteum and cortical bone around Zn–1Sr, Zn–1Ca, and Zn–1Mg implants, particularly Zn–1Sr implant, were more than that of the sham control group (Fig. 6(A); green fluorescence represented new bone). In addition, the micro-CT images of Zn–1Sr, Zn–1Ca, and Zn–1Mg implants at week 8 showed higher bone volume (Fig. 6(B)). Moreover, the in vivo degradation rates of Zn–1Sr, Zn–1Ca, and Zn–1Mg implants were 0.22, 0.19, and 0.17 mm y−1, respectively, which were similar to the bone healing rate. In another study, Zn and Zn–0.05Mg (wt%) alloy were implanted into the rabbit femur to investigate their degradability rates. The histology of the bone-implant interface at weeks 12 and 24 post-degradation are shown in Fig. 6(C) (implant indicated with white arrow; cortical bone indicated with blue arrow).120 At week 12, a new bone formed (red arrow) around the implants; at week 24, bone trabecula (with green arrow) formed as a junction between the new bone and cortical bone. Further, it was determined that a large amount of Ca element (red area) was enriched near the bone-implant interface, indicating that the degradations of both Zn and Zn–0.05Mg alloy could induce new bone formation.
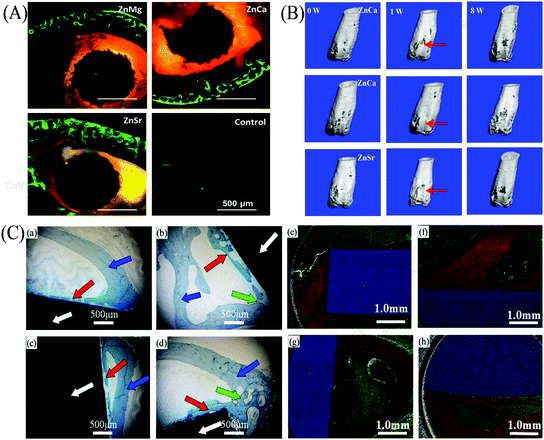 | ||
| Fig. 6 Implantation trials of Zn-based alloys. (A) Bone-implant interface histology of Zn–Mg, Zn–Ca, and Zn–Sr alloys at week 8; the sham served as the control. (B) Micro-CT 3D images of Zn–Mg, Zn–Ca, and Zn–Sr implants at weeks 0, 1, and 8. (C) Bone-implant interface histology of (a) Zn at week 12, (b) Zn at week 24, (c) Zn–0.05Mg alloy at week 12, and (d) Zn–0.05Mg alloy at week 24 (implant is indicated with white arrow, cortical bone is indicated with blue arrow, new bone is indicated with red arrow, and bone trabecula is indicated with green arrow). Elemental distributions at the bone-implant interface of (e) Zn at week 12, (f) Zn at week 24, (g) Zn–0.05Mg alloy at week 12 and (h) Zn–0.05Mg alloy at week 24 (Ca element is indicated in red, C element in green, and Zn element in blue). Reproduced with permission from ref. 63. Copyright 2015 Nature Publishing Group; Reproduced with permission from ref. 120. Copyright 2018 Elsevier. | ||
Taking into account the influence of oxygen, cells, proteins, phosphates, amino acids, and other molecules, the in vivo degradation behavior of biodegradable metallic bone implants are complex, and therefore, still need to be comprehensively investigated.122–124
2.3 Regulation of degradation behaviors
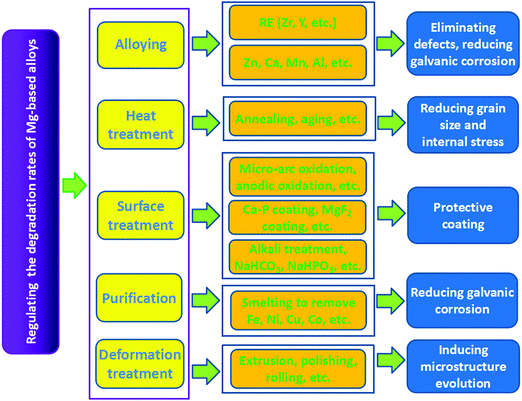
Ideal bone implants should have appropriate degradation rates to match the restoration process of the new bone after implantation. Hence, several studies have been carried out in the recent years to regulate the degradation rates of biodegradable metals.125–127Surface treatment mainly refers to the use of physical or chemical methods to generate a surface film or a passivation layer on the Mg matrix, which improves the corrosion resistance of Mg-based alloys at the initial stage of implantation.106,136,137 The widely used methods include microarc oxidation, alkali heat treatment, electrochemical treatment, phosphate layers, fluoridation treatment, etc. Zhang et al.138 coated a ceramic layer on AZ91 Mg alloy by microarc oxidation. The corrosion potential of the alloy rose from −1.5786 to −0.43019 V after the treatment. Immersion tests in Hank's solution showed that the average weight loss rate of the treated AZ91 Mg alloy was 15 times lower than that of the untreated alloy, which indicated a significant effect of microarc oxidation in improving corrosion resistance. To investigate the effect of alkaline heat treatment on corrosion resistance, Mg–Ca alloys were immersed in three different alkaline solutions (NaHCO3, Na2CO3, and Na2HPO4) for 24 h and then treated at 773 K for 12 h.36 It was found that MgO layers with thicknesses of approximately 26, 9, and 13 μm were formed on the surface of alloys after the treatment. In vitro degradation tests verified that the corrosion resistance of Mg–Ca alloys could be effectively improved as a result of these MgO layers in the following order: heated Na2CO3 > heated Na2HPO4 > heated NaHCO3. Wang et al.139 investigated Mg–Zn–Ca alloy coated with HA by the pulse electrodeposition method. They discovered that the current density of the alloy decreased from 110 to 25 μA cm−2 and the potential increased from −1645 to −1414 mV, indicating improved corrosion resistance of the Mg alloy by HA coating. Hiromoto et al.140 investigated Mg alloy coated with octacalcium phosphate by chemical solution deposition. They found that the degradation rate was restricted by approximately one-half with a suppressed Mg-ion release. Further, nearly uniform corrosion occurred. Xu et al.141 coated Mg alloy with a calcium phosphate (Ca–P) layer through a phosphating process. The Ca–P layer on the alloy surface was composed of netlike CaHPO4·2H2O, which reduced the corrosion of the Mg alloy. Fluoridation coating, which was mainly composed of MgF2 and MgO, was prepared on the Mg alloy by Yan et al.142 through chemical reaction using hydrofluoric acid. Electrochemical impedance spectroscopy tests demonstrated significantly increased corrosion resistance of the Mg alloy. The corrosion inhibition of Mg alloys by fluoride treatments were also confirmed by Pereda et al.143 They found that the protective characteristics of the MgF2 layer ensured the gradual degradation process of Mg alloys. Moreover, the influences of the MgF2 layer on the corrosion rate of LAE442 Mg alloy was studied in vivo.116 These results showed that the MgF2 layer could effectively reduce the degradation rate during the first 6 weeks of implantation without generating subcutaneous bubbles and elevated fluoride content in the adjacent bones. Moreover, purification to reduce galvanic corrosion between the Mg matrix and the impurity and deformation treatment to induce microstructure evolution are other effective ways to decelerate the degradation process of Mg-based alloys.144,145 In summary, the strategies, techniques, and corresponding mechanisms for regulating the degradation rates of Mg-based alloys are shown in Fig. 7.
3. Mechanical properties
As bone implants, mechanical supports are required for efficient load bearing. It is proposed that the yield strength (YS), ultimate tensile strength (UTS), and elongation rate (E) are the major indicators of mechanical properties.35,154,155 To regulate these parameters, numerous studies have been carried out to understand the mechanical properties of Mg-, Fe-, and Zn-based alloys.32,156,1573.1 Mg-Based alloys
Mg-Based alloys possess Young's modulus (35–45 GPa) that is close to that of natural bone (3–20 GPa), which prevents the stress-shielding effect. In addition, Mg-based alloys are also known for their high specific strength and high specific stiffness.158,159 However, Mg-based alloys generally possess hexagonal close-packed (HCP) structure with only a few slip planes,160 which results in relatively low ductility for bone implant applications. For example, the E of Mg is less than 6%. Moreover, the UTS of Mg is poor, about 50 MPa. Hence, various methods such as grain refinement, deformation, and heat treatment have been proposed to improve the mechanical properties.Grain refinement can enhance the mechanical properties of Mg-based alloys mainly through the blockage of dislocation motion and dispersion of internal stress. Grain refinement is commonly achieved by adding refiners such as RE (Ce, Y, etc.), Ca, Zr, Sr, and Zn.35,37,41,161,162 It has been found that the second phase in AZ91D Mg alloy can be transformed from a thick mesh into a fine granular shape after adding 0.6% Ce and 0.3% Y (wt%), which can improve the UTS of the alloy from 124 to 213 MPa.161 Brar et al.163 also confirmed that the grain size of the matrix decreased with the addition of Sr, improving the mechanical properties of Mg. Moreover, deformation (extrusion, forging, rolling, etc.) and heat treatment (solid solution, annealing, aging, etc.) are important ways to regulate the microstructure and reduce the internal stress within Mg-based alloys, thereby improving the mechanical properties. The mechanical properties of the extruded EW75M Mg alloy were studied under different extrusion conditions.164 At an extrusion temperature of 400 °C, extrusion speed of 12 m min−1, and extrusion ratio of 20, the UTS, YS, and E values of the alloy reached up to 335 MPa, 240 MPa, and 16.5%, respectively, owing to the refined microstructure. Zhang et al.164 investigated the effects of hot extrusion and aging on the mechanical properties of the Mg–3Nd–0.2Zn–0.4Zr alloy. The alloy was first extruded at the extrusion ratio of 25 to induce grain recrystallization and then aged at 200 °C for 10 h to reduce the residual stress generated in the extrusion process, during which grain refinement and second-phase precipitation occurred. As a result, the YS, UTS, and E values of the alloy were improved to 137 MPa, 236 MPa, and 17%, respectively.
3.2 Fe-Based alloys
Fe-Based alloys have better mechanical properties such as strength and plasticity as compared to Mg- and Zn-based alloys, basically meeting the requirements for use as bone implants. Studies on the mechanical properties are fewer than those on the degradation rates of Fe-based alloys. Several methods such as new preparation technologies, heat treatment, and alloying have been attempted using Fe-based alloys to investigate the effect on their mechanical properties. Moravej et al.149 reported that a grain size of 4 μm in electroformed Fe resulted in high YS (360 MPa) and UTS (423 MPa). After annealing at 550 °C, the ductility improved by 18% because of recrystallization and stress relief. Fe and Mn powders were sintered and rolled to fabricate Fe–Mn alloys by Čapek et al.82 Their results revealed that the YS value (235 MPa) of the fabricated alloys was higher than that of as-cast Fe–Mn alloys, and the UTS was comparable to that of SS316L. Meanwhile, the maximum E value reached was 31%, which is about 60% that of the as-cast Fe–Mn alloys (50%). Fe–Mn alloys (0, 0.5, 2.7, and 6.9 wt% Mn) with height of 250 mm and diameter of 60 mm were prepared by sand casting and then forged at 1050 °C.165 All these alloys exhibited worthwhile mechanical properties as compared to Mg-based alloys: for example, Fe–2.5Mn alloy had UTS of 495 MPa, YS of 295 MPa, and E of 28%. Moreover, the mechanical properties of Fe–Mn alloys with other Mn contents such as Fe20Mn,53,57 Fe25Mn,57 and Fe35Mn54,57 are also systematically evaluated, which further confirmed the positive effect of alloying Mn on the mechanical properties of Fe-based alloys.3.3 Zn-Based alloys
With regard to Zn-based alloys, the low strength and poor plasticity significantly restricted their applications as bone implants. It is known that Zn has a HCP crystal structure, which induces brittleness. Zn is also soft with low mechanical strength; for instance, the tensile strength of as-cast Zn is only about 30 MPa.166 Furthermore, Zn-based alloys would undergo a recrystallization process under stress, resulting in lower resistance to creep. Therefore, the mechanical properties of Zn-based alloys have been the focus in the study for use as bone implants.3,167Similar to Mg-based alloys, alloying, deformation, and heat treatment are also the commonly used methods to improve the mechanical properties of Zn-based alloys. Because of the high solubility of Mg in Zn, Kubasek et al.65 prepared Zn–Mg alloys with various Mg contents (0.5–3 wt%) through hot extrusion. It was found that Zn–Mg alloys consisted of fine grains of 12 μm. The strength and hardness were improved with increasing Mg content, from 120 MPa and 44 HV for Zn to 380 MPa and 97 HV for Zn–1.6Mg alloy, respectively; however, Zn–1.6Mg alloy showed no plasticity owing to the excess brittleness of the Mg2Zn11 phase. In comparison, the Zn–0.8Mg alloy exhibited a worthwhile combination of strength and plasticity (YS of 203 MPa, UTS of 301 MPa, and E of 15%) due to the absence of the brittle Mg2Zn11 phase. The effects of alloying and deformation on the mechanical properties of as-cast Zn–Mg alloys were studied by Liu et al.29 YS and UTS were improved to 114 MPa and 131 MPa, respectively, as compared to those of Zn, owing to the increased volume fraction of the eutectic mixture. Moreover, the coarse dendrites of the primary grains were mostly transformed into strip grains after hot rolling. Consequently, the YS, UTS, and E values of Zn–Mg alloy were further enhanced to 195 MPa, 299 MPa, and 26%, respectively. Alloying Ag was also found to obviously reduce the grain size of the Zn matrix,168,169 and the relationship between the grain size and Ag content is shown in Fig. 8(A). It is effectively established that grain refinement can enhance the mechanical properties of Zn-based alloys. Jasinska et al.170 prepared Zn–xAg (x from 2.5 to 7.0 wt%) alloys to investigate the relationship between the mechanical properties and Ag contents (Fig. 8(B)). YS and UTS showed an upward trend with an increase in the Ag contents. Due to the grain refinement of the Zn matrix and increased volume fraction of the second phase of AgZn3, the maximum YS (236 MPa) and UTS (367 MPa) values for Zn–7Ag alloy could be achieved. Nevertheless, the addition of Ag to Zn also reduced elongation from 60% for Zn down to 32–36% for Zn–xAg alloys. In spite of the above efforts, it is challenging to improve the mechanical properties of Zn-based alloys for applications in bone implants.171
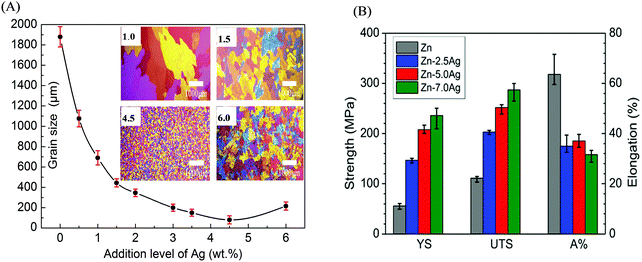 | ||
| Fig. 8 Effects of alloying Ag on the microstructure and mechanical properties of Zn-based alloys. (A) Microstructural evolution and grain size of Zn matrix with increasing Ag content. (B) Mechanical properties of Zn–Ag alloys with different Ag contents. Reproduced with permission from ref. 169. Copyright 2014 Elsevier. | ||
In general, the mechanical properties of biodegradable alloys have been improved by novel preparation methods (rapid solidification170 and electroforming149), alloying, processing deformation (extrusion, rolling, and forging), and heat treatment (solution treatment, annealing, and aging) to meet the requirements of bone implants.3,172 The representative enhancing strategies and resulting mechanical properties of biodegradable metals are summarized in Table 1.
| Compositions (wt%) | Preparation methods | Post treatments | YS (MPa) | UTS (MPa) | E (%) | Ref. |
|---|---|---|---|---|---|---|
| Mg–3Al | Casting | Solid solution treatment | 150 | 255 | 3 | 54 |
| Mg–2Ca | Rapid solidification | — | — | 380 | 7.3 | 35 |
| Mg–Zn–0.8Sr | Zone solidification | Heat treatment | 117 | 210 | 12 | 37 |
| LAE442 | Casting | — | 148 | 247 | 18 | 173 |
| AZ31 | Casting | — | — | 260 | 15 | 35 |
| Mg–2Sr | Casting | Hot rolling | 147 | 213.3 | — | 41 |
| Mg–6Zn | Casting | Heat treatment & extrusion | 280 | 170 | 19 | 39 |
| Mg–Y–RE–Zr | Powder metallurgy | — | 250 | 275 | 10 | 44 |
| Mg–5.0Y–7.0Gd–1.3Nd–0.5Zr | Casting | Extrusion | 162 | 234 | 26 | 164 |
| Mg–Y | Casting | — | 156 | 257 | 14 | 41 |
| Mg–5.0Y–7.0Gd–1.3Nd–0.5Zr | Casting | Extrusion & aging | 189 | 243 | 21 | 164 |
| Mg–3Sn–0.5Mn | Casting | Extrusion | 150 | 240 | 13 | 29 |
| ZW21 | Casting | Squeezing | 200 | 270 | 17 | 161 |
| WE43 | Casting | Heat treatment | 170 | 220 | 44 | 64 |
| WE43 | Casting | Extrusion & heat treatment | 195 | 280 | 10 | 20 |
| Mg–1Zn | Casting | — | 89 | 187 | 11 | 37 |
| Mg–Zn–Mn | Zone solidification | Extrusion | 246 | 280 | 22 | 29 |
| Mg–Y–Zn | Casting | Extrusion | — | 250–270 | 17–20 | 174 |
| Mg–Zn–1Sr | Zone solidification | Heat treatment | 130 | 249 | 13 | 37 |
| AZ31 | Casting | — | 110–180 | 255–290 | 15–21 | 4 |
| WE43A | Casting | Heat treatment | 162 | 250 | — | 173 |
| Fe–30Mn–C | Casting | Hot rolling | 373 | 1010 | 88 | 175 |
| Fe–30Mn–C | Casting | Forging | — | 205 | 16 | 176 |
| Fe–30Mn–6Si | Casting | Solution treatment | 180 | 450 | 16 | 62 |
| Fe–30Mn | Casting | Forging | 299 | 372 | 94 | 82 |
| Fe–30Mn | Casting | Rolling | — | 530 | 15 | 14 |
| Fe–C | Casting | Rolling | 440 | 600 | 7 | 52 |
| Fe–35Mn | Casting | Annealing | 230 | 430 | 30 | 54 |
| Fe–10Mn–Pd | Casting | Heat treatment | 850–950 | 1450–1550 | 2–8 | 55 |
| Fe | Casting | Rolling | — | 310 | 15 | 14 |
| Fe | Electroforming | Annealing | 270 | 290 | 18 | 149 |
| Zn–3Cu–0.5Fe | Casting | — | 232 | 284 | 33 | 68 |
| Zn–0.8Mg | Casting | Extrusion | 203 | 301 | 15 | 68 |
| Zn–4Cu | Casting | Extrusion | 250 | 270 | 51 | 67 |
| Zn–Mg–Mn | Casting | Rolling | 195 | 300 | 26 | 29 |
| Zn–1Ca | Casting | Rolling | 7 | 253 | 13 | 63 |
| Zn–1Mg | Casting | Rolling | — | 300 | 16 | 177 |
| Zn–1Mg | Casting | Rolling | 190 | 237 | 12 | 63 |
| Zn–0.05Mg | Casting | Extrusion | 160 | 225 | 26 | 120 |
| Zn–1Sr | Casting | Rolling | 188 | 229 | 20 | 63 |
| Zn–1Mg–1Ca | Casting | Extrusion | 138 | 198 | 9 | 177 |
| Zn–5Mg–1Fe | Casting | Extruded-draw tube | 150–187 | 180–230 | 5–26 | 65 |
| Zn–1Mg–1Sr | Casting | Extrusion | 140 | 201 | 10 | 177 |
4. Biocompatibility
Biocompatibility is a vital property of metallic implants for bone repair. It is generally known that Mg, Fe, and Zn are essential elements in the human body.178–180 Although this can be regarded as the evidence of good biocompatibility, metallic implants directly release ions into the human body, affecting the surrounding cells, tissues, and blood.181,182 Therefore, cytocompatibility, histocompatibility, and hemocompatibility have been the primary evaluation indicators of the biocompatibility of biodegradable metallic implants.183,1844.1 Cytocompatibility
As metallic implants degrade in the human body, metal ions are released and impact the cells. Cytocompatibility can be evaluated in terms of cytotoxicity by the extracts of biodegradable metals.67,185–187 It was reported that the extracts of Mg–1Sn, Mg–1Zn, and Mg–1Al alloys reduced the viability of fibroblasts and osteoblasts, but the extracts of Mg–1Zn and Mg–1Al alloys did not show negative effects on cell viability.188 Marrow cells in touch with alkali- and heat-treated Mg exhibited the evidence of common morphology, and cellular lysis and inhibitory effect on cell growth were not detected.189 It is known that Mg-based alloys may exhibit a certain level of cytotoxicity because of the influences of ion concentrations and pH values of the extracts.190,191 Even so, Mg–2Sr alloy showed Grade I cytotoxicity, which was acceptable for bone implants.191 Li et al.37 revealed that murine fibroblast viability decreased with an increase in the extract concentration of Mg–Zn–Sr alloy on day 1, while became basically equivalent in all the extracts on day 3, indicating the normal proliferation of murine fibroblasts in the extracts of Mg–Zn–Sr alloy. With regard to Mg–Zn alloy, the extracts showed marginal impact on the morphology and proliferation of murine fibroblasts with cytotoxicity in the range of Grade 0–1, which indicated the favorable cytocompatibility of Mg–Zn alloy.39 Chen et al.48 incubated preosteoblasts in the extracts of Mg and Mg–Cu alloys for 12 h. The cell cytoskeleton in Fig. 9(A) showed that the cells had normal morphology in all the extracts, implying marginal cytotoxicities of Mg and Mg–Cu alloys. Therefore, it can be concluded that most Mg-based alloys have acceptable cytotoxicity as bone implants.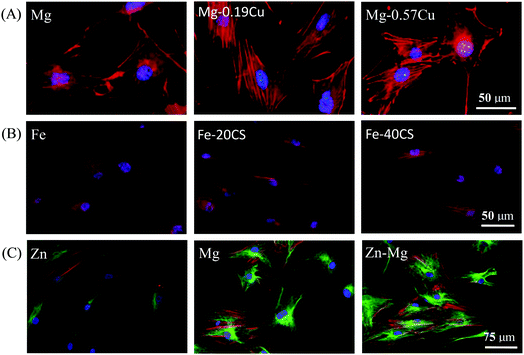 | ||
| Fig. 9 Cytoskeletal morphology showing the cytocompatibility of biodegradable metals. (A) Preosteoblasts cultured in Mg and Mg–Cu alloy extracts for 12 h (nuclei indicated in blue and actin in red).48 (B) Human bone mesenchymal stem cells exposed to Fe, Fe–20CS, and Fe–40CS alloys for 24 h (nuclei indicated in blue and actin in red).192 (C) Human osteoblasts exposed to Zn, Mg, and Zn–3Mg alloy for 7 days (nuclei indicated in blue, actin in red, and microtubule in green).194 Reproduced with permission from ref. 48. Copyright 2016 Nature Publishing Group; reproduced with permission from ref. 192. Copyright 2017 Elsevier; reproduced with permission from ref. 194. Copyright 2015 Elsevier, respectively. | ||
With regard to Fe-based alloys, although they exhibit a slow release of ions during degradation, Liu et al.52 found that the vitality of smooth muscle cells was reduced in Fe extracts after alloying S, Co, Sn, C, B, or Al. Moreover, Fe–W51 extracts also showed mild cytotoxicity to smooth muscle cells in terms of cell viability. Another research150 showed that the proliferation of smooth muscle cells decreased owing to the degradation of Fe. In vitro cytotoxicity of Fe30Mn alloys to murine fibroblasts was studied by Hermawan et al.56,57 Owing to the presence of Mn, the extracts of the alloys were slightly more toxic than that of Fe; however, metabolic activity of the cells was still above the tolerable 70% limit. Huang et al.148 found that after culturing the extracts of Fe–Au or Fe–Ag alloys, the viability of smooth muscle cells was evidently suppressed, while the viability of murine fibroblasts was always maintained around 100% during incubation. Hermawan et al.53 assessed the viability of mouse fibroblasts in terms of metabolic activities by incubation in the medium with different Fe concentrations. The assay showed the cell viability remained up to 95% along the tested concentrations, which resembled the control group results. Metabolic activity assay of smooth muscle cells was also performed for 24, 48, and 72 h to study the cytotoxicity of electroformed Fe.150 The results revealed that the electroformed Fe induced no obvious inhibition effect on the metabolic activity as compared to 316L SS owing to its inert property. The cytocompatibility of Fe, Fe–20CS (wt%, calcium silicate), and Fe–40CS were evaluated by Wang et al.,192 and the results are shown in Fig. 9(B). Evidently, the cytoskeletons of human bone mesenchymal stem cells were well defined and did not exhibit significant differences with regard to distribution or morphology. It seems that the endurance capacity of cells for cytotoxicity differs with the types of Fe-based alloys. The contents of alloyed elements need to be carefully considered according to biomedical science (for instance, the daily intake value of elements and ion concentrations in the human body) and material science (for instance, the Fe phase diagram to obtain suitable mechanical properties for bone implants).
The cytotoxicity of Zn-based alloys as bone implants has also attracted intense attention. Kubasek et al.66 verified that the maximum concentrations of Zn2+ were 80 μmol L−1 and 120 μmol L−1 for human osteosarcoma cells and murine fibroblasts, respectively. Gong et al.153 reported that fibroblast viability was not obviously influenced after incubation in Zn–1Mg extracts for 24 h and 48 h when compared with that of the control group, which indicated Zn–Mg exhibited nontoxicity toward the cells. Li et al.63 investigated the effects of alloying Mg, Ca, or Sr elements into Zn on human umbilical endothelial cells, smooth muscle cells, and human osteoblast cells. It was found that the viabilities of human umbilical endothelial cells and human osteoblasts significantly increased, whereas the viability of smooth muscle cells did not show a prominent increase. The cytotoxicity of Zn alloy was also evaluated by culturing murine fibroblasts in the extracts for l, 3, and 5 days.193 It was found that the proliferation rate of murine fibroblasts showed marginal statistical significance when compared with the control. Murni et al.194 incubated human osteoblasts in the extracts of Zn, Mg, and Zn–3Mg alloy for 7 days, and the cytoskeleton morphologies revealed that Zn–3Mg alloy had lower toxicity as compared to Zn and Mg, as shown in Fig. 9(C). In general, different cell types have different responses toward metal ions, and the activity of the cells decreases with an increase in the metal ion concentrations.195 Here, the physiology, total amount in human body, ion concentration limits, and daily allowance of metals are summarized in Table 2.
| Elements | Physiology | Total amount | Ion concentration limits or blood serum level | Daily allowance | Ref. |
|---|---|---|---|---|---|
| Mg | Maintaining nucleic acid structure stability, regular cell proliferation stabilizer of RNA, DNA | 25 g | 0.73–1.06 mmol L−1 | 400 mg | 27, 173 and 176 |
| Fe | Transfer of oxygen by blood, essential for metabolism | 5 g | 5.0–17.6 g L−1 | 10 mg | 173 and 176 |
| Zn | Co-factor for enzyme reaction, bone, and muscle, essential for immune system | 2 g | 12.4–17.4 μmol L−1 | 6.5–40 mg | 54, 144 and 196 |
| Al | Factor of Alzheimer's disease, excess causing neurotoxicity | 300 mg | 2.1–4.8 μg L−1 | 60 mg | 173 and 197 |
| Mn | Activation of enzyme systems, involving in metabolism, influencing immune system, blood clotting, bone growth | 12 mg | 0.8 μg L−1 | 5 mg | 27, 82 and 173 |
| Si | Promoting growth of bone and connective tissue | — | — | 20–50 mg | 82 and 175 |
| Sr | Osteogenesis effect | 0.3 g | — | 4 mg | 33 and 173 |
| Ca | Main skeletal element abundant mineral in bone | 1–1.1 kg | 2.1–4.8 μg L−1 | 1000–1500 g | 173 and 198 |
| RE (Y, Gd, Ce, etc.) | Y, Nd, Gd, etc., trace nontoxic element, anticancerogenic properties | 247 μg | — | — | 17, 173 and 197 |
| Zr | High concentration in liver and gallbladder; | 250 mg | — | 3.5 mg | 198 |
| Li | Overdose reducing kidney function and nervous system disorders, possible teratogenic effects | — | 2–4 ng g−1 | 0.2–0.6 mg | 175 and 198 |
| Ni | Genotoxicity, carcinogenicity | — | 0.05–0.23 μg L−1 | — | 173 |
| Be | Carcinogenicity | — | 2 μg m−3 | — | 173 |
| Cu | Involving in synthesis of enzymes, antiseptic effects | — | 74–131 μmol L−1 | — | 198 |
4.2 Histocompatibility and hemocompatibility
Histocompatibility and hemocompatibility are also considered as sensitive indicators of the biocompatibility of bone implants.21,199 Ideal bone implants should neither cause an inflammatory response in tissues, nor cause lotting, hemolysis, and destruction of platelets in blood.200 The histocompatibility and hemocompatibility of biodegradable metals are usually evaluated in terms of biomarkers, hemolysis rate, and platelet adhesion.201Porous AZ91D Mg alloys were implanted into rabbit femur by Med et al.196 and the results showed that porous AZ91D Mg alloy implants promoted bone remodeling with no harmful effects on the surrounding tissues. WE43 Mg alloys were randomly implanted into femur of 72 rats for 4, 12, and 24 weeks by Castellani et al.202 No relevant inflammatory responses in consequence of WE43 Mg alloy implantation were detected in the blood samples of these rats. A similar method was followed by Peng et al.,203 who implanted Mg–Y–Nd–RE alloy in rats for the same time periods and did not observe any inflammatory responses either, indicating good hemocompatibility. For Fe-based alloys, hemocompatibility studies were carried out on Fe–W, Fe–CNT, Fe–Pd, Fe–Pt, Fe–Au, Fe–Ag, and Fe–Fe2O3. It was found that all the hemolysis rates were lower than 5%, and the platelets adhering to the alloy surfaces showed no significant differences in both shape and number as compared to those of the control.51,148,204,205 Ulum et al.118 measured the swelling thickness caused by tissue response in sheep femur. It was found that Fe had the lowest degree of tissue response as compared to those of Fe–TCP and SS316. Meanwhile, the amounts of hemoglobin, white blood cells, and red blood cells were within the normal levels during the entire experimental period, indicating good histocompatibility. In other words, a majority of biodegradable Fe-based alloys have satisfactory histocompatibility and hemocompatibility with hemolysis rates less than 5%, appropriate clotting time, and no signs of thrombogenicity or obvious inflammatory response.52,82,176 As to Zn-based alloys, no inflammation was observed by radiographs analysis after the implantation of Zn–Mg, Zn–Ca, and Zn–Sr alloys into mouse femur for 8 weeks.63 Similar results were obtained by Xiao et al.,120 who implanted Zn–0.05Mg alloy into rabbit bone and did not find any inflammation at 4, 12, and 24 weeks, demonstrating good histocompatibility. Jin et al.193 reported that fibrous tissues were detected at the interface of the Zn–Ag alloy and bone tissue, validating the capability of bone formation. Liu et al.29 studied the hemolysis rates of Zn–Mg–Mn alloys. Their results showed that hot-rolled Zn–Mg–Mn alloy exhibited a hemolysis rate of 1.10%, which is much lower than that of Zn (4.10%). In vitro degradation tests showed no harmful effect of the alloy on erythrocytes, and platelets on the alloy surface maintained a round shape with no signs of thrombogenicity. Generally, Zn-based alloys do not induce obvious adverse reactions when they come into contact with tissue and blood, exhibiting a worthwhile combination of histocompatibility and hemocompatibility.
5. Conclusive remarks
Biodegradable metals are emerging as promising candidates for bone implants. At present, there is a fair amount of research on biomedical Mg- and Fe-based alloys. In the light of these studies, Mg-based alloys are believed to possess worthwhile elastic modulus and good biocompatibility. However, they degrade too quickly as compared to the growth of new bone. Fe-Based alloys have a good combination of mechanical properties and biocompatibility, while their main drawback is extremely slow degradation rates. Zn-Based alloys are characterized by homogeneous corrosion process and moderate degradation rates, but they are limited by insufficient strength and ductility.In recent years, considerable efforts have been devoted toward developing metallic implants with a combination of matching biodegradability, sufficient mechanical properties, and favorable biocompatibility for bone repair. For example, a series of commercial biodegradable Mg-based alloys, such as WE43, ZEK100, and LAE442 Mg alloys, have been attempted and they have showed improved corrosion resistance. Moreover, many in vivo studies have also demonstrated favorable degradation behavior and osteogenesis capacity for bone repair applications. Nevertheless, additional studies are needed, focusing on the following aspects. (1) The impact of the microstructure on degradation behavior because of the complex human environment. (2) Evolution mechanisms of the mechanical properties after alloying. (3) Fatigue performance in human physiological environment to avoid early failure of implants. (4) The influence of alloying elements on biocompatibility of metallic implants serving for a long term. (5) Composition analysis and safety evaluation of degradation products. (6) The establishment of more comprehensive in vitro evaluation criteria to provide accurate results.
To conclude, biodegradable metals—as a new generation of metallic biomaterials—have shown promising potential in implants for bone repair and are getting increased attention around the world. Due to the complexity of the human physiological environment, research on biodegradable metallic bone implants is a long-term process. With further development, they are expected to partially replace traditional metallic bone implants used in clinics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the following funds: (1) the Natural Science Foundation of China (51705540, 51575537, 81572577); (2) Hunan Provincial Natural Science Foundation of China (2018JJ3671, 2016JJ1027); (3) the Open-End Fund for the Valuable and Precision Instruments of Central South University; (4) the Project of Hunan Provincial Science and Technology Plan (2017RS3008); (5) National Postdoctoral Program for Innovative Talents (BX201700291); (6) the Project of State Key Laboratory of High Performance Complex Manufacturing, Central South University.References
- F. Feyerabend, J. Fischer, J. Holtz, F. Witte, R. Willumeit, H. Drücker, C. Vogt and N. Hort, Acta Biomater., 2010, 6, 1834–1842 CrossRef CAS PubMed.
- P. Tian and X. Liu, Regener. Biomater., 2015, 2, 135–151 CrossRef CAS PubMed.
- M. Erinc, W. H. Sillekens, R. Mannens and R. J. Werkhoven, Acta Metall., 2009, 209–214 CAS.
- L. Tan, X. Yu, P. Wan and K. Yang, J. Mater. Sci. Technol., 2013, 29, 503–513 CrossRef CAS.
- G. Levy and E. Aghion, Acta Biomater., 2013, 9, 8624–8630 CrossRef CAS PubMed.
- X. Liang, Y. Qi, Z. Pan, Y. He, X. Liu, S. Cui and J. Ding, Mater. Chem. Front., 2018, 2, 1539–1553 RSC.
- S. S. Elrahman, Pharmacol. Res., 2003, 47, 189–194 CrossRef CAS.
- Y. Nakamura, Y. Tsumura, Y. Tonogai and T. Shibata, J. Health Sci., 2008, 45, P15–P15 Search PubMed.
- Q. Xu, M. Hashimoto, T. T. Dang, T. Hoare, D. S. Kohane, G. M. Whitesides, R. Langer and D. G. Anderson, Small, 2009, 5, 1575 CrossRef CAS PubMed.
- E. Sikora, Recent Patents on Corrosion Science, 2012, vol. 2, pp. 81–97(17) Search PubMed.
- D. Mushahary, R. Sravanthi, Y. Li, M. J. Kumar, N. Harishankar, P. D. Hodgson, C. Wen and G. Pande, Int. J. Nanomed., 2013, 2013, 2887–2902 Search PubMed.
- E. N. Zare, M. M. Lakouraj and M. Mohseni, Synth. Met., 2014, 187, 9–16 CrossRef.
- A. Chaya, S. Yoshizawa, K. Verdelis, N. Myers, B. J. Costello, D. T. Chou, S. Pal, S. Maiti, P. N. Kumta and C. Sfeir, Acta Biomater., 2015, 18, 262–269 CrossRef CAS PubMed.
- B. Wegener, B. Sievers, S. Utzschneider, P. Müller, V. Jansson, S. Rößler, B. Nies, G. Stephani, B. Kieback and P. Quadbeck, Mater. Sci. Eng., B, 2011, 176, 1789–1796 CrossRef CAS.
- S. Han, Y. Liu, X. Nie, Q. Xu, F. Jiao, W. Li, Y. Zhao, Y. Wu and C. Chen, Small, 2012, 8, 1596–1606 CrossRef CAS PubMed.
- A. K. Mohanty, M. Misra and G. Hinrichsen, Macromol. Mater. Eng., 2000, 276-277, 1–24 CrossRef.
- H. Tapiero and K. D. Tew, Biomed. Pharmacother., 2003, 57, 399 CrossRef CAS.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546–554 CrossRef CAS PubMed.
- M. Montani, A. G. Demir, E. Mostaed, M. Vedani and B. Previtali, Rapid Prototyp. J., 2017, 23, 514–523 CrossRef.
- C. Gao, P. Feng, S. Peng and C. Shuai, Acta Biomater., 2017, 61, 1 CrossRef CAS PubMed.
- C. Gao, S. Peng, P. Feng and C. Shuai, Bone Res., 2017, 5, 17059 CrossRef CAS PubMed.
- G. Z. Yin, X. Yang, Z. Zhou and Q. Li, Mater. Chem. Front., 2018, 2, 544–553 RSC.
- A. Oriňák, R. Oriňáková, Z. O. Králová, A. M. Turoňová, M. Kupková, M. Hrubovčáková, J. Radoňák and R. Džunda, J. Porous Mater., 2014, 21, 131–140 CrossRef.
- C. M. Serre, M. Papillard, P. Chavassieux, J. C. Voegel and G. Boivin, J. Biomed. Mater. Res., 1998, 42, 626–633 CrossRef CAS PubMed.
- N. E. Saris, E. Mervaala, H. Karppanen, J. A. Khawaja and A. Lewenstam, Clin. Chim. Acta, 2000, 294, 1–26 CrossRef CAS.
- C. Seal, K. Vince and M. A. Hodgson, IOP Conf. Ser.: Mater. Sci. Eng., 2009, 012011 Search PubMed.
- Y. F. Zheng, X. N. Gu and F. Witte, Mater. Sci. Eng., R, 2014, 77, 1–34 CrossRef.
- E. Zhang, H. Chen and F. Shen, J. Mater. Sci.: Mater. Med., 2010, 21, 2151–2163 CrossRef CAS PubMed.
- X. Liu, J. Sun, F. Zhou, Y. Yang, R. Chang, K. Qiu, Z. Pu, L. Li and Y. Zheng, Mater. Des., 2016, 94, 95–104 CrossRef CAS.
- L. Rink and L. Rink, Zinc in Human Health, 2011, vol. 11, pp. 63–87 Search PubMed.
- T. Jurgeleit, E. Quandt and C. Zamponi, Adv. Mater. Sci. Eng., 2015, 2015, 1–9 CrossRef.
- A. Zomorodian, M. P. Garcia, E. S. T. Moura, J. C. Fernandes, M. H. Fernandes and M. F. Montemor, Acta Biomater., 2013, 9, 8660–8670 CrossRef CAS PubMed.
- D. Tie, R. Guan, H. Liu, A. Cipriano, Y. Liu, Q. Wang, Y. Huang and N. Hort, Acta Biomater., 2015, 29, 455–467 CrossRef PubMed.
- F. Witte, V. Kaese, H. Haferkamp, E. Switzer, A. Meyerlindenberg, C. J. Wirth and H. Windhagen, Biomaterials, 2005, 26, 3557–3563 CrossRef CAS PubMed.
- Z. Li, X. Gu, S. Lou and Y. Zheng, Biomaterials, 2008, 29, 1329–1344 CrossRef CAS PubMed.
- X. N. Gu and W. Y. Zheng, Acta Biomater., 2009, 5, 2790–2799 CrossRef CAS PubMed.
- H. Li, Q. Peng, X. Li, K. Li, Z. Han and D. Fang, Mater. Des., 2014, 58, 43–51 CrossRef CAS.
- Z. S. Seyedraoufi and M. Sh, J. Mech. Behav. Biomed. Mater., 2013, 21, 1–8 CrossRef CAS PubMed.
- S. Zhang, X. Zhang, C. Zhao, J. Li, S. Yang, C. Xie, H. Tao, Z. Yan, Y. He and J. Yao, Acta Biomater., 2010, 6, 626 CrossRef CAS PubMed.
- J. N. Li, P. Cao, X. N. Zhang, S. X. Zhang and Y. H. He, J. Mater. Sci., 2010, 45, 6038–6045 CrossRef CAS.
- X. N. Gu, X. H. Xie, N. Li, Y. F. Zheng and L. Qin, Acta Biomater., 2012, 8, 2360–2374 CrossRef CAS PubMed.
- M. Bornapour, N. Muja, D. Shumtim, M. Cerruti and M. Pekguleryuz, Acta Biomater., 2013, 9, 5319 CrossRef CAS PubMed.
- A. C. Hãnzi, P. Gunde, M. Schinhammer and P. J. Uggowitzer, Acta Biomater., 2009, 5, 162–171 CrossRef PubMed.
- H. Windhagen, K. Radtke, A. Weizbauer, J. Diekmann, Y. Noll, U. Kreimeyer, R. Schavan, C. Stukenborgcolsman and H. Waizy, Biomed. Eng. Online, 2013, 12, 62 CrossRef PubMed.
- M. Vlček, F. Lukáč, H. Kudrnová, B. Smola, I. Stulíková, M. Luczak, G. Szakács, N. Hort and R. Willumeitrömer, Materials, 2017, 10, 55 CrossRef PubMed.
- L. Yang and E. Zhang, Mater. Sci. Eng., C, 2009, 29, 1691–1696 CrossRef CAS.
- M. A. Ze-Qun, F. U. Guang-Yan and X. L. Zhang, J. Shenyang Inst. Chem. Technol., 2017, 1, 35–41 Search PubMed.
- L. Chen, X. Fu, H. Pan, W. Peng, W. Lei, L. Tan, K. Wang, Z. Ying, Y. Ke and P. K. Chu, Sci. Rep., 2016, 6, 27374 CrossRef PubMed.
- D. Tie, F. Feyerabend, W. D. Müller, R. Schade, K. Liefeith, K. U. Kainer and R. Willumeit, Eur. Cells Mater., 2013, 25, 284 CrossRef CAS PubMed.
- E. Zhang, L. Yang, J. Xu and H. Chen, Acta Biomater., 2010, 6, 1756–1762 CrossRef CAS PubMed.
- J. Cheng and Y. F. Zheng, J. Biomed. Mater. Res., Part B, 2013, 101, 485–497 CrossRef CAS PubMed.
- B. Liu and Y. F. Zheng, Acta Biomater., 2011, 7, 1407–1420 CrossRef CAS PubMed.
- H. Hermawan, A. Purnama, D. Dube, J. Couet and D. Mantovani, Acta Biomater., 2010, 6, 1852–1860 CrossRef CAS PubMed.
- H. Hermawan, D. Dubã and D. Mantovani, Adv. Mater. Res., 2006, 15–17, 107–112 Search PubMed.
- M. Schinhammer, A. C. Hänzi, J. F. Löffler and P. J. Uggowitzer, Acta Biomater., 2010, 6, 1705 CrossRef CAS PubMed.
- H. Hermawan, H. Alamdari, D. Mantovani and D. Dubã, Powder Metall., 2008, 51, 38–45 CrossRef CAS.
- H. Hermawan, D. Dubé and D. Mantovani, J. Biomed. Mater. Res., Part A, 2010, 93A, 1–11 CAS.
- J. Čapek, K. Stehlíková, A. Michalcová, Š. Msallamová and D. Vojtěch, Mater. Chem. Phys., 2016, 181, 501–511 CrossRef.
- W. Xu, X. Lu, L. Tan and K. Yang, Acta Metall. Sin., 2011, 47, 1342–1347 CAS.
- M. Schinhammer, C. M. Pecnik, F. Rechberger, A. C. Hänzi, J. F. Löffler and P. J. Uggowitzer, Acta Mater., 2012, 60, 2746–2756 CrossRef CAS.
- E. Mouzou, C. Paternoster, R. Tolouei, P. Chevallier, C. A. Biffi, A. Tuissi and D. Mantovani, Mater. Lett., 2016, 181, 362–366 CrossRef CAS.
- B. Liu, Y. F. Zheng and L. Ruan, Mater. Lett., 2011, 65, 540–543 CrossRef CAS.
- H. F. Li, X. H. Xie, Y. F. Zheng, Y. Cong, F. Y. Zhou, K. J. Qiu, X. Wang, S. H. Chen, L. Huang and L. Tian, Sci. Rep., 2015, 5, 10719 CrossRef CAS PubMed.
- O. Duygulu, R. A. Kaya, G. Oktay and A. A. Kaya, Mater. Sci. Forum, 2007, 546, 421–424 Search PubMed.
- J. Kubasek, I. Pospíšilová, D. Vojtěch, E. Jablonska and T. Ruml, Mater. Technol., 2014, 48, 623–629 CAS.
- J. Kubásek, D. Vojtěch, E. Jablonská, I. Pospíšilová, J. Lipov and T. Ruml, Mater. Sci. Eng., C, 2016, 58, 24 CrossRef PubMed.
- J. Niu, Z. Tang, H. Huang, J. Pei, H. Zhang, G. Yuan and W. Ding, Mater. Sci. Eng., C, 2016, 69, 407–413 CrossRef CAS PubMed.
- R. Yue, H. Huang, G. Ke, H. Zhang, J. Pei, G. Xue and G. Yuan, Mater. Charact., 2017, 134, 114–122 CrossRef CAS.
- Y. Liu, L. Wang, L. Cao, C. Shang, Z. Wang, H. Wang, L. He, J. Yang, H. Cheng and J. Li, Mater. Chem. Front., 2017, 1, 2495–2510 RSC.
- I. S. Berglund, H. S. Brar, N. Dolgova, A. P. Acharya, B. G. Keselowsky, M. Sarntinoranont and M. V. Manuel, J. Biomed. Mater. Res., Part B, 2012, 100B, 1524–1534 CrossRef CAS PubMed.
- C. Shen, X. Liu, B. Fan, P. Lan, F. Zhou, X. Li, H. Wang, X. Xiao, L. Li and S. Zhao, RSC Adv., 2016, 6, 86410–86419 RSC.
- P. K. Bowen, J. Drelich and J. Goldman, Adv. Mater., 2013, 25, 2577–2582 CrossRef CAS PubMed.
- X. Wang, S. Xu, S. Zhou, W. Xu, M. Leary, P. Choong, M. Qian, M. Brandt and Y. M. Xie, Biomaterials, 2016, 83, 127–141 CrossRef CAS PubMed.
- Z. Zhen, X. I. Ting-Fei and Y. F. Zheng, Acta Metall. Sin., 2013, 23, 2283–2293 CAS.
- M. Pogorielov, E. Husak, A. Solodivnik and S. Zhdanov, Interven. Med. Appl. Sci., 2017, 9, 27 CrossRef PubMed.
- J. Zhang, C. Xu, Y. Jing, S. Lv, S. Liu, D. Fang, J. Zhuang, M. Zhang and R. Wu, Sci. Rep., 2015, 5, 13933 CrossRef PubMed.
- J. Hofstetter, E. Martinelli, S. Pogatscher, P. Schmutz, E. Povoden-Karadeniz, A. M. Weinberg, P. J. Uggowitzer and J. F. Löffler, Acta Biomater., 2015, 23, 347–353 CrossRef CAS PubMed.
- Q. Peng, X. Li, M. Ning, R. Liu and H. Zhang, Mater. Sci. Eng., C, 2012, 10, 128–137 CAS.
- Y. Song, E. H. Han, D. Shan, D. Y. Chang and B. S. You, Corros. Sci., 2012, 65, 322–330 CrossRef CAS.
- Y. Zheng and W. U. Yuanhao, Acta Metall. Sin., 2017, 53, 257–297 CAS.
- S. Agarwal, J. Curtin, B. Duffy and S. Jaiswal, Mater. Sci. Eng., C, 2016, 68, 948 CrossRef CAS PubMed.
- J. Čapek, J. Kubãsek, D. Vojtäch, E. Jablonskã, J. Lipov and T. Ruml, Mater. Sci. Eng., C, 2016, 58, 900–908 CrossRef PubMed.
- G. Song and S. Song, A Possible Biodegradable Magnesium Implant Material, 2007 Search PubMed.
- P. Bajger, J. M. Ashbourn, V. Manhas, Y. Guyot, K. Lietaert and L. Geris, Biomech. Model. Mechanobiol., 2016, 1–12 Search PubMed.
- Y. Zheng, Y. Li, J. Chen and Z. Zou, Mater. Des., 2014, 24, 547–553 CAS.
- D. Hong, D. T. Chou, O. I. Velikokhatnyi, A. Roy, B. Lee, I. Swink, I. Issaev, H. A. Kuhn and P. N. Kumta, Acta Biomater., 2016, 45, 375–386 CrossRef CAS PubMed.
- X. Liu, J. Sun, K. Qiu, Y. Yang, Z. Pu, L. Li and Y. Zheng, J. Alloys Compd., 2016, 664, 444–452 CrossRef CAS.
- L. Zhao, Z. Zhang, Y. Song, S. Liu, Y. Qi, X. Wang, Q. Wang and C. Cui, Mater. Des., 2016, 108, 136–144 CrossRef CAS.
- E. Mostaed, M. Sikora-Jasinska, A. Mostaed, S. Loffredo, A. G. Demir, B. Previtali, D. Mantovani, R. Beanland and M. Vedani, J. Mech. Behav. Biomed. Mater., 2016, 60, 581–602 CrossRef CAS PubMed.
- W. R. Osório, J. E. Spinelli, N. Cheung and A. Garcia, Mater. Sci. Eng., A, 2006, 420, 179–186 CrossRef.
- C. Wang, H. T. Yang, X. Li and Y. F. Zheng, J. Mater. Sci. Technol., 2016, 32, 909–918 CrossRef.
- D. Hong, P. Saha, D. T. Chou, B. Lee, B. E. Collins, Z. Tan, Z. Dong and P. N. Kumta, Acta Biomater., 2013, 9, 8534–8547 CrossRef CAS PubMed.
- W. D. Mueller, M. F. L. D. Mele, M. L. Nascimento and M. Zeddies, J. Biomed. Mater. Res., Part A, 2009, 90A, 487–495 CrossRef CAS PubMed.
- Y. Chen, Z. Xu, C. Smith and J. Sankar, Acta Biomater., 2014, 10, 4561–4573 CrossRef CAS PubMed.
- D. T. Chou, D. Hong, P. Saha, J. Ferrero, B. Lee, Z. Tan, Z. Dong and P. N. Kumta, Acta Biomater., 2013, 9, 8518–8533 CrossRef CAS PubMed.
- J. Hofstetter, E. Martinelli, S. Pogatscher, P. Schmutz, E. Povodenkaradeniz, A. M. Weinberg, P. J. Uggowitzer and J. F. Löffler, Acta Biomater., 2015, 23, 347 CrossRef CAS PubMed.
- F. Witte, J. Fischer, J. Nellesen, C. Vogt, J. Vogt, T. Donath and F. Beckmann, Acta Biomater., 2010, 6, 1792–1799 CrossRef CAS PubMed.
- M. Niinomi, M. Nakai and J. Hieda, Acta Biomater., 2012, 8, 3888 CrossRef CAS PubMed.
- M. Jamesh, G. Wu, Y. Zhao and P. K. Chu, Corros. Sci., 2013, 69, 158–163 CrossRef CAS.
- K. W. K. Yeung and K. H. M. Wong, Biodegradable metallic materials for orthopaedic implantations: A review, IOS Press, 2012 Search PubMed.
- S. Y. Cho, S. W. Chae, K. W. Choi, H. K. Seok, Y. C. Kim, J. Y. Jung, S. J. Yang, G. J. Kwon, J. T. Kim and M. Assad, J. Biomed. Mater. Res., Part B, 2013, 101B, 201–212 CrossRef CAS PubMed.
- X. Li, C. Chu and P. K. Chu, Bioact. Mater., 2016, 1, 77–84 CrossRef PubMed.
- N. T. Kirkland, N. Birbilis and M. P. Staiger, Acta Biomater., 2012, 8, 925–936 CrossRef CAS PubMed.
- G. U. Xue-Nan and Y. F. Zheng, Front. Mater. Sci., 2010, 4, 111–115 CrossRef.
- L. Csaderova, E. Martines, K. Seunarine, N. Gadegaard, C. D. Wilkinson and M. O. Riehle, Small, 2010, 6, 2755 CrossRef CAS PubMed.
- A. C. Hänzi, I. Gerber, M. Schinhammer, J. F. Löffler and P. J. Uggowitzer, Acta Biomater., 2010, 6, 1824–1833 CrossRef PubMed.
- M. S. Dambatta, S. Izman, H. Hermawan and D. Kurniawan, Adv. Mater. Rech., 2014, 845, 7–11 Search PubMed.
- E. Zhang, L. Xu, G. Yu, F. Pan and K. Yang, J. Biomed. Mater. Res., Part A, 2009, 90, 882 CrossRef PubMed.
- C. Wang, L. Wang, S. Yu, F. Huang, H. Liu and Z. Yu, Guangdong Chem. Ind., 2016, 3, 51–60 Search PubMed.
- J. Nagels, M. Stokdijk and P. M. Rozing, J. Shoulder Elbow Surg., 2003, 12, 35–39 CrossRef PubMed.
- F. Y. Hung, T. S. Lui, L. H. Chen, H. W. Chang and Z. F. Chen, J. Mater. Sci., 2007, 42, 5020–5028 CrossRef CAS.
- H. S. Brar, M. O. Platt, M. Sarntinoranont, P. I. Martin and M. V. Manuel, JOM, 2009, 61, 31–34 CrossRef CAS.
- J. Jiang, F. Zhang, A. Ma, D. Song, J. Chen, H. Liu and M. Qiang, Metals, 2015, 6, 3 CrossRef.
- A. Torroni, C. Xiang, L. Witek, E. D. Rodriguez, P. G. Coelho and N. Gupta, J. Craniomaxillofac. Surg., 2017, S1010518217303256 Search PubMed.
- A. Torroni, C. Xiang, L. Witek, E. D. Rodriguez, R. L. Flores, N. Gupta and P. G. Coelho, J. Craniomaxillofac. Surg., 2018, 46, 473–478 CrossRef PubMed.
- F. Witte, J. Fischer, J. Nellesen, C. Vogt, J. Vogt, T. Donath and F. Beckmann, Acta Biomater., 2010, 6, 1792–1799 CrossRef CAS PubMed.
- T. Lili, W. Qiang, L. Xiao, W. Peng, Z. Guangdao, Z. Qiang and Y. Ke, Acta Biomater., 2014, 10, 2333–2340 CrossRef PubMed.
- M. F. Ulum, A. K. Nasution, A. H. Yusop, A. Arafat, M. R. A. Kadir, V. Juniantito, D. Noviana and H. Hermawan, J. Biomed. Mater. Res., Part B, 2015, 103, 1354 CrossRef CAS PubMed.
- F. Săndulache, S. Stanciu, N. Cimpoeşu, T. Stanciu, R. Cimpoeşu, A. Enache and R. Baciu, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 209, 012049 Search PubMed.
- C. Xiao, L. Wang, Y. Ren, S. Sun, E. Zhang, C. Yan, Q. Liu, X. Sun, F. Shou and J. Duan, J. Mater. Sci. Technol., 2018, 34, 1618–1627 CrossRef.
- D. Vojtěch, J. Kubásek, J. Serák and P. Novák, Acta Biomater., 2011, 7, 3515–3522 CrossRef PubMed.
- K. C. Hribar, R. B. Metter, J. L. Ifkovits, T. Troxler and J. A. Burdick, Small, 2009, 5, 1830–1834 CrossRef CAS PubMed.
- X. Zhao, L. L. Shi and J. Xu, J. Mater. Sci. Technol., 2013, 29, 781–787 CrossRef CAS.
- K. Tomantschger, G. Palumbo and D. Facchini, US Pat., US9119906B2, 2015 Search PubMed.
- F. Moszner, A. S. Sologubenko, M. Schinhammer, C. Lerchbacher, A. C. Hänzi, H. Leitner, P. J. Uggowitzer and J. F. Löffler, Acta Mater., 2011, 59, 981–991 CrossRef CAS.
- M. Sikora-Jasinska, C. Paternoster, E. Mostaed, R. Tolouei, R. Casati, M. Vedani and D. Mantovani, Mater. Sci. Eng., C, 2017, 81, 511–521 CrossRef CAS PubMed.
- E. Mostaed, M. Sikora-Jasinska, A. Mostaed, S. Loffredo, A. G. Demir, B. Previtali, D. Mantovani, R. Beanland and M. Vedani, J. Mech. Behav. Biomed. Mater., 2016, 60, 581 CrossRef CAS PubMed.
- F. Witte, J. Fischer, J. Nellesen, H. Crostack, V. Kaese, A. Pisch, F. Beckmann and H. Windhagen, Biomaterials, 2006, 27, 1013–1018 CrossRef CAS PubMed.
- X. Liu, Z. Yue, T. Romeo, J. Weber, T. Scheuermann, S. Moulton and G. Wallace, Acta Biomater., 2013, 9, 8671–8677 CrossRef CAS PubMed.
- D. H. Cho, H. N. Ji, B. W. Lee, K. M. Cho and I. M. Park, J. Alloys Compd., 2016, 676, 461–468 CrossRef CAS.
- L. I. Guanqun, Mater. Rev., 2005, 11, 23–34 Search PubMed.
- X. Wang, G. Qi, Q. Cai and B. Wei, Dev. Appl. Mater., 2002, 5, 18–31 Search PubMed.
- S. Tekumalla, S. Seetharaman, A. Almajid and M. Gupta, Metals, 2014, 5, 1–39 CrossRef.
- C. Liu, Y. Xin, G. Tang and P. K. Chu, Mater. Sci. Eng., A, 2007, 456, 350–357 CrossRef.
- H. Kuwahara, N. Mazaki, M. Mabuchi, C. Wein and T. Aizawa, Mater. Sci., 2003, 1, 1007–1012 Search PubMed.
- K. Chiu, M. Wong, F. Cheng and H. Man, Surf. Coat. Technol., 2007, 202, 590–598 CrossRef CAS.
- P. Salunke, V. Shanov and F. Witte, Mater. Sci. Eng., B, 2011, 176, 1711–1717 CrossRef CAS.
- X. P. Zhang, Z. P. Zhao, F. M. Wu, Y. L. Wang and J. Wu, J. Mater. Sci., 2007, 42, 8523–8528 CrossRef CAS.
- H. X. Wang, S. K. Guan, X. Wang, C. X. Ren and L. G. Wang, Acta Biomater., 2010, 6, 1743 CrossRef CAS PubMed.
- S. Hiromoto, M. Inoue, T. Taguchi, M. Yamane and N. Ohtsu, Acta Biomater., 2015, 11, 520–530 CrossRef CAS PubMed.
- L. Xu, F. Pan, G. Yu, L. Yang, E. Zhang and K. Yang, Biomaterials, 2009, 30, 1512–1523 CrossRef CAS PubMed.
- T. Yan, L. Tan, B. Zhang and K. Yang, J. Mater. Sci. Technol., 2014, 30, 666–674 CrossRef CAS.
- M. D. Pereda, C. Alonso, L. Burgos-Asperilla, J. A. D. Valle, O. A. Ruano, P. Perez and M. A. F. L. D. Mele, Acta Biomater., 2010, 6, 1772–1782 CrossRef CAS PubMed.
- H. Wang, Y. Estrin and Z. Zúberová, Mater. Lett., 2008, 62, 2476–2479 CrossRef CAS.
- S. Yang, S. Zhang, J. Li, C. Zhao and X. Zhang, Acta Biomater., 2010, 6, 1736 CrossRef PubMed.
- C. S. Obayi, R. Tolouei, C. Paternoster, S. Turgeon, B. A. Okorie, D. O. Obikwelu, G. Cassar, J. Buhagiar and D. Mantovani, Acta Biomater., 2015, 17, 68–77 CrossRef CAS PubMed.
- W. Q. Wang, L. U. Shan and Q. I. Min, J. Funct. Biomater., 2013, 44, 468–470 CAS.
- T. Huang, J. Cheng, D. Bian and Y. Zheng, J. Biomed. Mater. Res., Part B, 2016, 104, 225 CrossRef CAS PubMed.
- M. Moravej, F. Prima, M. Fiset and D. Mantovani, Acta Biomater., 2010, 6, 1726–1735 CrossRef CAS PubMed.
- M. Moravej, A. Purnama, M. Fiset, J. Couet and D. Mantovani, Acta Biomater., 2010, 6, 1843 CrossRef CAS PubMed.
- X. Liu, J. Sun, Y. Yang, F. Zhou, Z. Pu, L. Li and Y. Zheng, Mater. Lett., 2016, 162, 242–245 CrossRef CAS.
- G. Lin, R. Hu, W. C. Law, C. K. Chen, Y. Wang, C. H. Li, Q. T. Nguyen, C. K. Lai, H. S. Yoon and X. Wang, Small, 2013, 9, 2757–2763 CrossRef CAS PubMed.
- H. Gong, K. Wang, R. Strich and J. G. Zhou, J. Biomed. Mater. Res., Part B, 2015, 103, 1632–1640 CrossRef CAS PubMed.
- B. Dong, W. Zhou, L. Yang, L. Nan, Y. Zheng and Z. Sun, Acta Biomater., 2016, 41, 351 CrossRef PubMed.
- M. M. Saleh, A. H. Touny, M. A. Al-Omair and M. M. Saleh, Biomed. Mater. Eng., 2016, 27, 87–99 CAS.
- H. Hermawan, Springerbriefs in Mater., 2012, pp. 1–11 Search PubMed.
- K. Bobe, E. Willbold, I. Morgenthal, O. Andersen, T. Studnitzky, J. Nellesen, W. Tillmann, C. Vogt, K. Vano and F. Witte, Acta Biomater., 2013, 9, 8611–8623 CrossRef CAS PubMed.
- M. Staiger, A. Pietak, J. Huadmai and G. Dias, Biomaterials, 2006, 27, 1728–1734 CrossRef CAS PubMed.
- Z. Gui, Z. Kang and Y. Li, J. Alloys Compd., 2016, 685, 222–230 CrossRef CAS.
- J. Yang, G. L. Koons, G. Cheng, L. Zhao, A. G. Mikos and F. Z. Cui, Biomed. Mater., 2017, 13, 2–27 Search PubMed.
- M. X. Wang, H. Zhou, L. Wang, W. Li and Y. Zhao, J. Mater. Eng., 2007, 37, 1–5 Search PubMed.
- A. Drynda, N. Deinet, N. Braun and M. Peuster, J. Biomed. Mater. Res., Part A, 2009, 91A, 360–369 CrossRef CAS PubMed.
- H. S. Brar, J. Wong and M. V. Manuel, J. Mech. Behav. Biomed. Mater., 2012, 7, 87–95 CrossRef PubMed.
- L. I. Yong-Jun, K. Zhang, L. I. Xing-Gang, M. A. Ming-Long, H. Z. Wang and H. E. Lan-Qiang, Chin. J. Nonferrous Met., 2010, 20, 1692–1697 Search PubMed.
- A. Drynda, T. Hassel, F. W. Bach and M. Peuster, J. Biomed. Mater. Res., Part B, 2015, 103, 649 CrossRef PubMed.
- H. Li, Y. Zheng and L. Qin, Prog. Nat. Sci.: Mater. Int., 2014, 24, 414–422 CrossRef CAS.
- A. G. Demir, L. Monguzzi and B. Previtali, Addit. Manuf., 2017, 15, 20–28 CrossRef CAS.
- Z. Liu, F. Wang, D. Qiu, J. A. Taylor and M. Zhang, Metall. Mater. Trans. E, 2013, 44, 4025–4030 CrossRef CAS.
- Z. Liu, D. Qiu, F. Wang, J. A. Taylor and M. Zhang, Acta Mater., 2014, 79, 315–326 CrossRef CAS.
- M. Sikora-Jasinska, E. Mostaed, A. Mostaed, R. Beanland, D. Mantovani and M. Vedani, Mater. Sci. Eng., C, 2017, 77, 1170 CrossRef CAS PubMed.
- L. Wang, B. Ji, Y. Hu, R. Liu and W. Sun, Chemosphere, 2017, 184, 594–600 CrossRef CAS PubMed.
- J. Cheng, B. Liu, Y. H. Wu and Y. F. Zheng, J. Mater. Sci. Technol., 2013, 29, 619–627 CrossRef CAS.
- F. Witte, N. Hort, C. Vogt, S. Cohen, K. U. Kainer, R. Willumeit and F. Feyerabend, Curr. Opin. Solid State Mater. Sci., 2008, 12, 63–72 CrossRef CAS.
- A. C. Hänzi, F. H. D. Torre, A. S. Sologubenko, P. Gunde, R. Schmid-Fetzer, M. Kuehlein, J. F. Löffler and P. J. Uggowitzer, Philos. Mag. Lett., 2009, 89, 377–390 CrossRef.
- C. Wang, Y. Cui, H. Liu, Y. U. Sen, Y. Zhang and Y. U. Zhentao, Mater. Rev., 2015, 1, 21–32 Search PubMed.
- D. Vojtěch, J. Kubásek and J. Čapek, Mater. Technol., 2015, 49, 877–882 Search PubMed.
- H. Li, H. Yang, Y. Zheng, F. Zhou, K. Qiu and X. Wang, Mater. Des., 2015, 83, 95–102 CrossRef CAS.
- A. Yamamoto, R. Honma and M. Sumita, J. Biomed. Mater. Res., Part A, 1998, 39, 331–340 CrossRef CAS.
- H. Hermawan, D. Ramdan and J. R. P. Djuansjah, Met. Biomed. Appli., InTech, 2011 Search PubMed.
- M. Schinhammer, I. Gerber, A. C. Hänzi and P. J. Uggowitzer, Mater. Sci. Eng., C, 2013, 33, 782–789 CrossRef CAS PubMed.
- S. Loher, O. D. Schneider, T. Maienfisch, S. Bokorny and W. J. Stark, Small, 2008, 4, 824–832 CrossRef CAS PubMed.
- N. M. Daud, N. B. Sing, A. H. Yusop, F. A. A. Majid and H. Hermawan, J. Orthop. Translat., 2014, 2, 177–184 CrossRef.
- J. You, R. Shao, X. Wei, S. Gupta and C. Li, Small, 2010, 6, 1022–1031 CrossRef CAS PubMed.
- D. Dziuba, A. Meyer-Lindenberg, J. M. Seitz, H. Waizy, N. Angrisani and J. Reifenrath, Acta Biomater., 2013, 9, 8548–8560 CrossRef CAS PubMed.
- F. Witte, Acta Biomater., 2010, 6, 1680 CrossRef CAS PubMed.
- S. Zhang, J. Li, Y. Song, C. Zhao, X. Zhang, C. Xie, Y. Zhang, H. Tao, Y. He and Y. Jiang, Mater. Sci. Eng., C, 2009, 29, 1907–1912 CrossRef CAS.
- N. Ostrowski, B. Lee, N. Enick, B. Carlson, S. Kunjukunju, A. Roy and P. N. Kumta, Acta Biomater., 2013, 9, 8704 CrossRef CAS PubMed.
- X. Gu, Y. Zheng, Y. Cheng, S. Zhong and T. Xi, Biomaterials, 2009, 30, 484–498 CrossRef CAS PubMed.
- L. Li, J. Gao and Y. Wang, Surf. Coat. Technol., 2004, 185, 92–98 CrossRef CAS.
- M. F. Ulum, D. Paramitha, S. Estuningsih, D. Noviana and H. Hermawan, Eur. Cells Mater., 2013, 2, 59–71 Search PubMed.
- Y. H. Yun, Z. Dong, D. Yang, M. J. Schulz, V. N. Shanov, S. Yarmolenko, Z. Xu, P. Kumta and C. Sfeir, Mater. Sci. Eng., C, 2009, 29, 1814–1821 CrossRef CAS.
- S. Wang, Y. Xu, J. Zhou, H. Li, J. Chang and Z. Huan, Bioact. Mater., 2016, 2, 10–18 CrossRef PubMed.
- G. Jin, H. Qin, H. Cao, S. Qian, Y. Zhao, X. Peng, X. Zhang, X. Liu and P. K. Chu, Biomaterials, 2014, 35, 7699–7713 CrossRef CAS PubMed.
- N. S. Murni, M. S. Dambatta, S. K. Yeap, G. R. A. Froemming and H. Hermawan, Mater. Sci. Eng., C, 2015, 49, 560–566 CrossRef CAS PubMed.
- J. Ma, N. Zhao and D. Zhu, ACS Biomater. Sci. Eng., 2015, 1, 1174–1182 CrossRef CAS PubMed.
- F. W. Med, J. Reifenrath, P. P. Müller, H. A. Crostack, J. Nellesen, F. W. Bach, D. Bormann and M. Rudert, Materialwiss. Werkstofftech., 2006, 37, 504–508 CrossRef.
- J. C. Gao, D. Hu and C. J. Song, J. Funct. Mater., 2012, 43, 2577–2583 CAS.
- Y. Zheng, G. U. Xuenan, L. I. Nan and W. Zhou, Materials, 2011, 4, 34–46 Search PubMed.
- N. M. Di, C. Carlostella, M. Magni, M. Milanesi, P. D. Longoni, P. Matteucci, S. Grisanti and A. M. Gianni, Blood, 2016, 99, 3838 Search PubMed.
- J. Wang, J. Xu, W. Liu, Y. Li and L. Qin, Sci. Rep., 2016, 6, 26341 CrossRef CAS PubMed.
- L. Molly, H. Vandromme, M. Quirynen, E. Schepers, J. L. Adams and D. Van Steenberghe, J. Periodontol., 2008, 79, 1108–1115 CrossRef PubMed.
- C. Castellani, R. A. Lindtner, P. Hausbrandt, E. Tschegg, S. E. Stanzl-Tschegg, G. Zanoni, S. Beck and A. M. Weinberg, Acta Biomater., 2011, 7, 432–440 CrossRef CAS PubMed.
- Q. Peng, Y. Huang, L. Zhou, N. Hort and K. U. Kainer, Biomaterials, 2010, 31, 398–403 CrossRef CAS PubMed.
- T. Huang, J. Cheng and Y. F. Zheng, Mater. Sci. Eng., C, 2014, 35, 43–53 CrossRef CAS PubMed.
- J. Cheng, T. Huang and Y. F. Zheng, J. Biomed. Mater. Res., Part A, 2014, 102, 2277–2287 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2019 |