Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A decanuclear [DyIII6ZnII4] cluster: a {ZnII4} rectangle surrounding an octahedral {DyIII6} single molecule magnet†
Nikoleta
Stavgianoudaki
a,
Milosz
Siczek
 b,
Tadeusz
Lis
b,
Giulia
Lorusso
b,
Tadeusz
Lis
b,
Giulia
Lorusso
 c,
Marco
Evangelisti
c,
Marco
Evangelisti
 c and
Constantinos J.
Milios
c and
Constantinos J.
Milios
 *a
*a
aDepartment of Chemistry, The University of Crete, Voutes 71003, Herakleion, Greece. E-mail: komil@uoc.gr
bFaculty of Chemistry, University of Wroclaw, Joliot-Curie 14, Wroclaw 50-383, Poland
cInstituto de Ciencia de Materiales de Aragón (ICMA), CSIC – Universidad de Zaragoza, 50009 Zaragoza, Spain
First published on 18th February 2019
Abstract
An octahedral {DyIII6} cage within a diamagnetic {ZnII4} rectangle is reported, with magnetic relaxation studies revealing single-molecule magnet behaviour for the complex under zero external dc field with Ueff = 43 K and τo = 1 × 10−5 s.
The field of lanthanide-based molecular magnetic materials has witnessed a vast growth over the last few years, due to the deeper understanding of the fundamental parameters that govern the magnetic behaviour of the 4f centres. Especially for single molecule magnets (SMMs) or single-ion magnets (SIMs), i.e. molecules that are able to retain their magnetism once magnetized at low temperatures upon removal of the external magnetic field,1 employment of 4f centres has led to exceptional magnetic properties, due to both the magnitude of the spin, as well as the spin–orbit coupling based magnetic anisotropy that the 4f species possess.2 Furthermore, for Dy-based SMMs/SIMs the uniaxial magnetic anisotropy of the Dy atoms plays a crucial role in enhancing the energy barrier, Ueff, needed for the re-orientation of the magnetisation, and thus molecular nanomagnets with Ueff values over 1000 K, and blocking temperatures as high as 60 K have been reported,3 with the best SMM/SIM being the recently reported organometallic complex [(CpiPr5)Dy(Cp*)]+ (CpiPr5 = penta-iso-propylcyclopentadienyl) with an Ueff value of ∼2210 K and a blocking temperature of ∼80 K.4 On the other hand, regarding mixed-metal 3d–4f heterometallic complexes as SMM candidates, the situation seems to differ; despite the ongoing research for the construction and characterization of 3d–4f SMMs, the energy barrier for such species remains rather low compared to 4f species, with [Co2Dy(LBr)2(H2O)]NO3 (LBr = 2,2′,2′′-(((nitrilotris(ethane-2,1diyl))tris(azanediyl))tris(methylene))tris-(4-bromophenol)) holding the record of Ueff = 600 K,5i.e. ∼1/4 of the highest Ueff reported so far for the best lanthanide SIM/SMM. Such a discrepancy may be attributed to either weak (and most commonly antiferromagnetic) interactions between the 3d and 4f metal centres, leading to low lying split sublevels, or to the perturbation of the 4f local magnetic field due to random transversal secondary magnetic fields created by the nearby paramagnetic metal ions, resulting in quantum tunnelling of the magnetization and, thus, faster magnetic relaxation.6
Recently we embarked on a project of constructing heterometallic ZnII–LnIII clusters and investigating their magnetic properties, as a means of: (i) protecting the 4f-centres from the influence of 3d paramagnetic centres and (ii) employing a diamagnetic 3d metal atom for structural stability and diversity of the products.7 Herein we report our latest finding regarding a decanuclear [ZnII4DyIII6] complex upon employment of the Schiff-base ligand 2-(β-naphthalideneamino)-2-hydroxymethyl-1-propanol, H3L (Scheme 1). The reaction of Zn(OAc)2·2H2O, Dy(NO3)3·5H2O and H3L in MeOH in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio under solvothermal conditions, and in the presence of base NEt3, gave complex [Dy6Zn4O2(L)2(HL)2(OAc)8(CH3O)4(H2O)2]·4MeOH (1·4MeOH) in good yield, which was characterized by means of X-ray single crystal crystallography.‡
1 ratio under solvothermal conditions, and in the presence of base NEt3, gave complex [Dy6Zn4O2(L)2(HL)2(OAc)8(CH3O)4(H2O)2]·4MeOH (1·4MeOH) in good yield, which was characterized by means of X-ray single crystal crystallography.‡
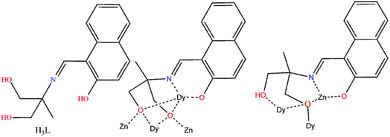
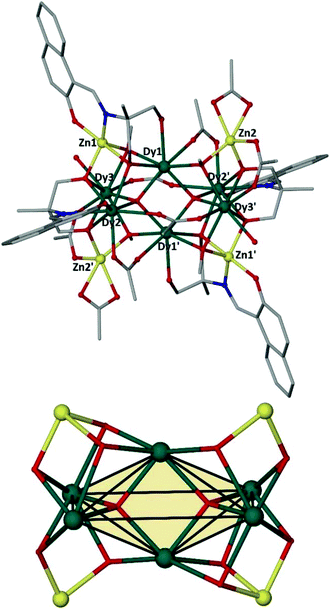
Complex 1 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. 1, top). Its metallic core consists of a diamagnetic {ZnII4} rectangular unit of ∼6.12 × 6.42 Å dimensions, surrounding a central magnetic {DyIII6} slightly “squeezed” octahedron (Fig. 1, bottom). The basal DyIII ions (Dy2, Dy2′, Dy3, Dy3′) are located ∼3.49 and ∼5.64 Å apart, while the axial DyIII centres (Dy1, Dy1′) are found ∼1.73 Å above and below the basal plane.
space group (Fig. 1, top). Its metallic core consists of a diamagnetic {ZnII4} rectangular unit of ∼6.12 × 6.42 Å dimensions, surrounding a central magnetic {DyIII6} slightly “squeezed” octahedron (Fig. 1, bottom). The basal DyIII ions (Dy2, Dy2′, Dy3, Dy3′) are located ∼3.49 and ∼5.64 Å apart, while the axial DyIII centres (Dy1, Dy1′) are found ∼1.73 Å above and below the basal plane.
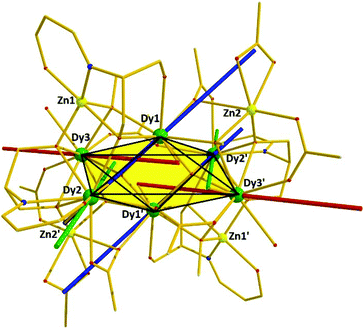
The six metallic centres of the octahedron are held by a combination of two central μ4-O2− bridges, four monoatomic methoxide bridges, four μ–κ1O:κ1O′ acetates and four ligands adopting two coordination modes; two of them are found in the doubly deprotonated form, HL2−, with a μ3–κ3O:κ1N:κ1O′:κ1O′′ coordination mode, while the remaining two are fully deprotonated, L3−, adopting a μ4–κ3O:κ3O′:κ1N:κ1O′′ coordination fashion. The linkage of the two metallic sub-units occurs via (i) the deprotonated ligands found in the molecule, (ii) the four methoxide groups, and (iii) two μ–κ1O:κ1O′ acetate groups. Finally, two chelate acetates and two terminal water molecules fill the coordination environment of the metallic centres. All Zn centres are five-coordinate adopting square pyramidal geometry (for Zn2/Zn2′, τ = 0.160), and severely twisted square pyramidal/trigonal bipyramidal geometry (for Zn1/Zn1′, τ = 0.546). Regarding the 4f centers, their ideal geometries were found upon performing SHAPE analysis:8 Dy2 and Dy3 are eight-coordinate with square antiprismatic (D4d) geometry (SAPR-8: S(δi,θi) = 0.958 and S(δi,θi) = 1.056, for Dy2 and Dy3, respectively), while Dy1 is seven-coordinate with distorted capped trigonal prismatic geometry (C2ν) (S(δi,θi) = 11.476 for CTP) (Fig. S1 and S2†). Finally, the {DyIII6} octahedron deviates slightly from the ideal octahedral geometry (CShM = 10.649). In the crystal lattice, the molecules pack forming layers stabilized mainly by intermolecular H-bonds between the decanuclear species and the solvate methanol molecules (Fig. 2).
 | ||
| Fig. 2 Crystal packing of 1, highlighting the intermolecular H-bonds (black bold dotted lines). Each colour corresponds to an individual molecule of 1. | ||
DC magnetic susceptibility measurements were performed on 1 in the 2–300 K temperature range under an applied magnetic field of 0.1 T, and the results are plotted as χMT vs. T in Fig. 3, with the isothermal magnetisation (M vs. H) curves shown in the inset. The room-temperature χMT value of 81.2 cm3 mol−1 K is slightly smaller than the theoretical value of 85.0 cm3 mol−1 K expected for six DyIII ions (S = 5/2, L = 5, J = 15/2, 6H15/2, gJ = 4/3). Upon cooling, the χMT product remains practically unchanged until ∼150 K, below which steadily decreases reaching 41.05 cm3 mol−1 K at 2 K, possibly suggesting the presence of weak antiferromagnetic interactions within the cluster (a Curie–Weiss analysis gave θ = −2.9 K) and/or depopulation of the DyIII Stark sub-levels. The isothermal magnetization versus B plots increase rapidly, reaching values of 29.2 and 28.5NμB at 5 T, for T = 2 and 5 K, respectively, significantly lower than the theoretical value of ∼60NμB for a hexanuclear [DyIII6] complex; this is mainly attributed to the presence of magnetic anisotropy and to the depopulation of the Stark sublevels.9
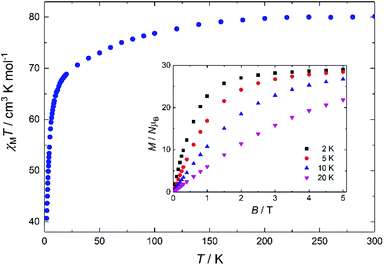 | ||
| Fig. 3 Plot of χMT vs. T for 1 in the 2–300 K temperature range, under an applied field of 0.1 T. Inset: M vs. B for 1 in the 0–5 T and 2.0–20.0 K field and temperature ranges. | ||
Given: (i) the large remaining magnetic moment, even at 2 K, and (ii) the square antiprismatic geometry of the Dy2 and Dy3 centres (and their symmetry related) that promotes suitable charge distribution with axial anisotropy on the DyIII centres,10 we investigated the AC dynamic magnetic properties of 1. The measurements show temperature and frequency (f) dependent fully-formed in-phase, χ′M, and out-of-phase, χ′′M, signals, under zero-applied DC field and a 3.5 AC field oscillating at various frequencies, in the temperature range between ca. 3 and 15 K (Fig. 4). This behaviour seems to indicate slow relaxation of the magnetization, which is typical for a SMM.
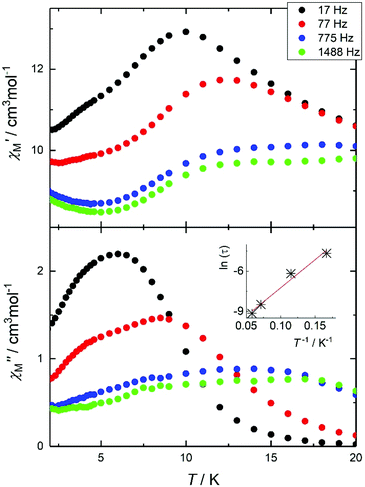 | ||
| Fig. 4 (Top) In-phase, χ′M, and out-of-phase (bottom), χ′′M, signals for 1 at various frequencies in the 17–1488 Hz range. Inset: Arrhenius fit of the effective time constants. | ||
The main feature is a cusp in the in-phase component that occurs at approximately 10.0 K for f = 17 Hz, accompanied by a cusp in the out-of-phase component at somewhat lower temperature (Fig. 4). Effective time constants can be obtained from the reciprocal angular frequency at maximum absorption. As typical of a thermal activation process, the zero-field relaxation times so obtained can be approximated by the Arrhenius law τ = τo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ueff/kBT), where τ = (2πf)−1, τo is an attempt frequency and Ueff an effective energy barrier. The results are presented in the inset of Fig. 4, affording Ueff = 43 K and τo = 1 × 10−5 s. The AC susceptibility reveals that the magnetic relaxation is particularly complex in 1, since at least another (secondary) relaxation pathway can be spotted at somewhat lower temperature than that of the main feature. In order to visualize the orientation of the anisotropy axis for each DyIII ion present in 1, we employed the electrostatic model reported by Chilton et al., which is based on electrostatic energy minimization for the prediction of the ground state magnetic anisotropy axis,11 assuming that in the absence of high symmetry the ground-state of DyIII ions is a doublet along the anisotropy axis with mJ = ± 15/2.12 Following this approach, the ground state magnetic anisotropy axes in 1 were found to form three pairs from the symmetry-related Dy centres (Fig. 5); for Dy1(Dy1′) the axis is tilted towards the μ4-O1 oxide atom, and towards O2 belonging to a monoatomic methoxide bridge and O2F belonging to bridging acetate group. For Dy2(Dy2′) the axis is pointing towards the μ4-O1 oxide atom and towards O2B(O2B′) from a deprotonated aromatic hydroxyl group, while for Dy3(Dy3′) the axis is tilted towards the μ4-O1′oxide atom and the O2D′ atom of a bridging acetate group.
exp(Ueff/kBT), where τ = (2πf)−1, τo is an attempt frequency and Ueff an effective energy barrier. The results are presented in the inset of Fig. 4, affording Ueff = 43 K and τo = 1 × 10−5 s. The AC susceptibility reveals that the magnetic relaxation is particularly complex in 1, since at least another (secondary) relaxation pathway can be spotted at somewhat lower temperature than that of the main feature. In order to visualize the orientation of the anisotropy axis for each DyIII ion present in 1, we employed the electrostatic model reported by Chilton et al., which is based on electrostatic energy minimization for the prediction of the ground state magnetic anisotropy axis,11 assuming that in the absence of high symmetry the ground-state of DyIII ions is a doublet along the anisotropy axis with mJ = ± 15/2.12 Following this approach, the ground state magnetic anisotropy axes in 1 were found to form three pairs from the symmetry-related Dy centres (Fig. 5); for Dy1(Dy1′) the axis is tilted towards the μ4-O1 oxide atom, and towards O2 belonging to a monoatomic methoxide bridge and O2F belonging to bridging acetate group. For Dy2(Dy2′) the axis is pointing towards the μ4-O1 oxide atom and towards O2B(O2B′) from a deprotonated aromatic hydroxyl group, while for Dy3(Dy3′) the axis is tilted towards the μ4-O1′oxide atom and the O2D′ atom of a bridging acetate group.
Conclusions
In conclusion, in this work we present the synthesis and characterisation of a novel decanuclear [DyIII6ZnII4] cluster, upon employment of the Schiff-base ligand 2-(β-naphthalideneamino)-2-hydroxymethyl-1-propanol, H3L, with the cluster's topology describing a {ZnII4} rectangle surrounding a {DyIII6} octahedron. Furthermore, investigation of the magnetic properties, revealed possible SMM behaviour with Ueff = 43 K and τo = 1 × 10−5 s. To the best of our knowledge, complex 1 represents the first example of a decanuclear [DyIII6Zn4] single molecule magnet.Synthetic efforts are currently underway in order to isolate more Zn-4f clusters, as a means of investigating the effect of the diamagnetic ions on the magnetic behaviour of the 4f centres.
Conflicts of interest
The authors wish to declare no conflicts of interest.Acknowledgements
GL and ME thank MINECO for funding (MAT2015-68204-R).Notes and references
- For representative reviews on SMMs see: G. Aromi and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef CAS; R. Bircher, G. Chaboussant, C. Dobe, H. U. Güdel, S. T. Ochsenbein, A. Sieber and O. Waldman, Adv. Funct. Mater., 2006, 16, 209 CrossRef; D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef PubMed; G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66 CrossRef; C. J. Milios and R. E. P. Winpenny, Struct. Bonding, 2015, 164, 1 Search PubMed; L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092 RSC; X.-Y. Wang, C. Avendañoa and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213 RSC; L. R. Piquer and E. C. Sañudo, Dalton Trans., 2015, 44, 8771 RSC; R. A. Layfield, Organometallics, 2014, 33, 1084 CrossRef; L. K. Thompson and L. N. Dawe, Coord. Chem. Rev., 2015, 289, 13 CrossRef; S. Demir, I.-R. Jeon, J. R. Long and T. D. Harris, Coord. Chem. Rev., 2015, 289, 149 CrossRef; P. Happ, C. Plenk and E. Rentschler, Coord. Chem. Rev., 2015, 289, 238 CrossRef; G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135 RSC.
- See for example: R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS; D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef; J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC; Y.-N. Guo, G.-F. Xu, Y. Guo and J. Tang, Dalton Trans., 2011, 40, 9953 RSC; P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef; Y.-N. Guo, G.-F. Xu, P. Gamez, L. Zhao, S.-Y. Lin, R. Deng, J. Tang and H.-J. Zhang, J. Am. Chem. Soc., 2010, 132, 8538 CrossRef PubMed; Y.-N. Guo, G.-F. Xu, W. Wernsdorfer, L. Ungur, Y. Guo, J. Tang, H.-J. Zhang, L. F. Chibotaru and A. K. Powell, J. Am. Chem. Soc., 2011, 133, 11948 CrossRef PubMed; B. M. Day, F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880 CrossRef PubMed; S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655 RSC; A. F. R. Kilpatrick, F.-S. Guo, B. M. Day, A. Mansikkamäki, R. A. Layfield and F. G. N. Cloke, Chem. Commun., 2018, 54, 7085 RSC; M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574 CrossRef PubMed.
- Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071 CrossRef CAS PubMed; D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Buechner and A. A. Popov, Chem. Commun., 2017, 53, 7901 RSC; Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829 CrossRef PubMed; S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Sci., 2016, 7, 5181 RSC; Y.-S. Meng, L. Xu, J. Xiong, Q. Yuan, T. Liu, B.-W. Wang and S. Gao, Angew. Chem., Int. Ed., 2018, 57, 1 CrossRef; A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2018, 54, 8273 RSC; F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamaeki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445 CrossRef PubMed; C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS PubMed.
- J.-L. Liu, J.-Y. Wu, G.-Z. Huang, Y.-C. Chen, J.-H. Jia, L. Ungur, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, Sci. Rep., 2015, 5, 16621 CrossRef CAS PubMed.
- M. A. Palacios, S. Titos-Padilla, J. Ruiz, J. M. Herrera, S. J. A. Pope, E. K. Brechin and E. Colacio, Inorg. Chem., 2014, 53, 1465 CrossRef CAS PubMed; A. Bhunia, M. T. Gamer, L. Ungur, L. F. Chibotaru, A. K. Powell, Y. Lan, P. W. Roesky, F. Menges, C. Riehn and G. Niedner-Schatteburg, Inorg. Chem., 2012, 51, 9589 CrossRef PubMed.
- N. Stavgiannoudaki, M. Siczek, T. Lis, R. Inglis and C. J. Milios, Chem. Commun., 2016, 52, 343 RSC.
- M. Llunell, D. Casanova, J. Girera, P. Alemany and S. Alvarez, SHAPE, version 2.0, Barcelona, Spain, 2010 Search PubMed.
- J. K. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729 CrossRef CAS PubMed; S. Osa, T. Kido, N. Matsumoto, N. Re, A. Pochaba and J. Mrozinski, J. Am. Chem. Soc., 2004, 126, 420 CrossRef PubMed.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed; M. A. AlDamen, J. M. Clemente-Juan, E. Coronado, C. Mart-Gastaldo and A. Gaita-Arino, J. Am. Chem. Soc., 2008, 130, 8874 CrossRef PubMed; G. Zhou, T. Han, Y.-S. Ding, N. F. Chilton and Y.-Z. Zheng, Chem. – Eur. J., 2017, 23, 1 CrossRef.
- N. F. Chilton, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and A. Soncini, Nat. Commun., 2013, 4, 2551 CrossRef PubMed.
- See for example: S. K. Langley, N. F. Chilton, L. Ungur, B. Moubaraki, L. F. Chibotaru and K. S. Murray, Inorg. Chem., 2012, 51, 11873 CrossRef CAS PubMed; G. Cucinotta, M. Perfetti, J. Luzon, M. Etienne, P.-E. Car, A. Caneschi, G. Calvez, K. Bernot and R. Sessoli, Angew. Chem., Int. Ed., 2012, 51, 1606 CrossRef PubMed; Y.-N. Guo, G.-F. Xu, W. Wernsdorfer, L. Ungur, Y. Guo, J. Tang, H.-J. Zhang, L. F. Chibotaru and A. K. Powell, J. Am. Chem. Soc., 2011, 133, 11948 CrossRef PubMed; N. F. Chilton, S. K. Langley, B. Moubaraki, A. Soncini, S. R. Batten and K. S. Murray, Chem. Sci., 2013, 4, 1719 RSC; F. Tuna, C. A. Smith, M. Bodensteiner, L. Ungur, L. F. Chibotaru, E. J. L. McInnes, R. E. P. Winpenny, D. Collison and R. A. Layfield, Angew. Chem., Int. Ed., 2012, 51, 6976 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full details of the experimental microanalyses and crystallographic data. CCDC 1892887. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9dt00440h |
‡ Crystal data for 1·4MeOH: C83H112Dy6N4O40Zn4, M = 3042.24, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.591 (3) Å, b = 13.807 (3) Å, c = 14.560 (3) Å, α = 80.22 (2)°, β = 69.83 (2)°, γ = 77.36 (2)°, V = 2489.2 (10) Å3, Z = 1, T = 80 K, R1 (I > 2σ) = 0.037 and wR2 (all data) = 0.074 for 37 , a = 13.591 (3) Å, b = 13.807 (3) Å, c = 14.560 (3) Å, α = 80.22 (2)°, β = 69.83 (2)°, γ = 77.36 (2)°, V = 2489.2 (10) Å3, Z = 1, T = 80 K, R1 (I > 2σ) = 0.037 and wR2 (all data) = 0.074 for 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 reflections collected, 10 200 reflections collected, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607 observed reflections (I > 2σ(I)) of 14 607 observed reflections (I > 2σ(I)) of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 033 (Rint = 0.039) unique reflections and 634 parameters, GOF = 1.02. CCDC reference number: 1892887.† 033 (Rint = 0.039) unique reflections and 634 parameters, GOF = 1.02. CCDC reference number: 1892887.† |
| This journal is © The Royal Society of Chemistry 2019 |