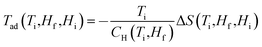Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gaudefroyite: a mineral with excellent magnetocaloric effect suitable for liquefying hydrogen
Rukang
Li
ab,
Guangjing
Li
c and
Colin
Greaves
 *c
*c
aBeijing Centre for Crystal Research and Development, Key Laboratory of Functional Crystals and Laser Technology, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: rkli@mail.ipc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cSchool of Chemistry, University of Birmingham, Birmingham B15 2TT, UK. E-mail: c.greaves@bham.ac.uk
First published on 13th September 2017
Abstract
Gaudefroyite contains chains of edge-linked MnO6 octahedra on a Kagomé lattice which results in magnetic frustration between the chains and overall spin-liquid behaviour below 9 K. Magnetization and specific heat measurements indicate very large entropy increases for temperatures of 10–30 K on removal of applied magnetic fields as low as 2 T. This is attributed to the degenerate magnetic ground states originating from the frustrated Kagomé lattice. Direct temperature change measurements confirm that the mineral has excellent low-field magnetocaloric properties suitable for refrigeration to produce liquid hydrogen.
Magnetocaloric (MC) refrigeration is both energy-efficient and environmentally friendly:1,2 energy efficiencies > 50% are theoretically achievable (cf. 35% for compressor-based systems) with no risk from greenhouse gases. Although materials with enhanced MC effect (MCE) are still needed for more efficient domestic refrigeration3,4 and to achieve extremely low temperatures, e.g. for space exploration,5 their advantages have more recently been considered for producing liquid hydrogen (LH2, b.p. 20 K)6,7 for fuel storage in a clean hydrogen economy.8 Unfortunately, known MC materials do not meet the requirements of efficiency and chemical stability while operating at low magnetic fields (2 T), and no other methods, e.g. thermoelectric cooling, are efficient in this temperature range. The mineral gaudefroyite has magnetic features which have signposted a possible solution to these problems, and here we report results which support its unique properties.
The MCE results from the increase in magnetic entropy of a magnetic material when an external magnetic field is removed. Under adiabatic conditions, the temperature falls to maintain a constant overall entropy. Efficient MC refrigeration requires a large change in magnetic entropy, which has traditionally been achieved with paramagnetic ions with very large effective moments such as Gd3+ (S = J = 7/2). However, a serious problem for producing LH2 in this way is the reduction in MCE at temperatures of ∼20 K for such materials (e.g. gadolinium gallium garnet (GGG), GdPO4) or the more recently proposed molecular magnets.9,10 The metallic ferromagnetic (FM) and the “giant” MC intermetallic materials11,12 are also unsuitable because of long term degradation in contact with LH2.6 Although Dy-doped GGG (or the aluminium analogue (GAG)) show useful characteristics, their need for a superconducting magnet to generate magnetic fields of 6 T restricts their applicability.6,13 Various proposals to increase the MCE at fields that can be generated by permanent magnets (∼2 T) have been reported, in particular the use of FM intermetallics that have a first order structural transition and display giant MCE behavior.3,4,14 However, these materials are limited by their small operating temperature range and energy inefficiency. Reducing the magnetic interaction to one-dimension (1D)15 or even zero-dimension (0D)16 and employing magnetic frustration have also been proposed. With respect to frustration, theoretical predictions suggested enhanced MCE in geometrically frustrated spin systems with Kagomé, garnet and pyrochlore lattices or even molecular systems because of the large entropy provided by the highly degenerate magnetic ground states in zero field.17,18 Importantly, the MCE was predicted to give very rapid cooling for fields providing near magnetic saturation, which was subsequently confirmed for the pyrochlore structure.19
Our previous study of the mineral gaudefroyite, Ca4Mn3O3(BO3)3CO3, and its synthetic analogue YCa3Mn3O3(BO3)4, revealed interesting magnetic features that suggested the possibility for enhanced MC properties. Both materials contain 1D chains of Mn3+ ions20,21 and, at low temperatures, the Mn magnetic moments in any given chain display FM order. Extended linear clusters of FM-coupled moments can simulate a system with “giant” spins. However, the chains are located on a Kagomé lattice that inhibits 3D magnetic order. MCE enhancement is expected from both the giant spins and also from the frustration preventing interchain order. The co-operative effects could therefore provide very rapid cooling rate with high MCE for a stable material free from expensive rare earth elements.
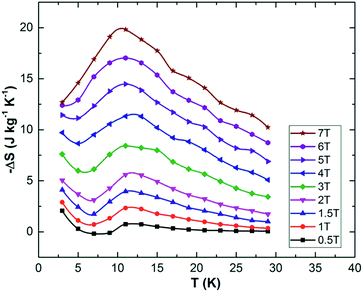
Gaudefroyite is found in hydrothermal manganese deposits and good crystals (Fig. 1(a) and (b)) can be found in South Africa.22 The hexagonal structure23,24 has 1D chains of edge-shared, Jahn–Teller distorted MnO6 octahedra aligned along [001] and linked by BO3 groups (Fig. 1(c)); in the ab plane, the chains define a 2D Kagomé net. The hexagonal channel of the net hosts Ca2+ ions and CO3 groups; additional Ca2+ ions sit in the smaller trigonal channels. The FM MnO6 chains experience antiferromagnetic exchange within the ab plane but the inherent frustration gives different magnetic structures for the mineral and YCa3Mn3O3(BO3)4. In the latter, the moments order below 7.5 K into a q = 0 Kagomé type magnetic structure (Fig. 1(d)) but in gaudefroyite itself, the moments freeze to a spin-liquid state. The difference is an example of order by disorder – the disordered Y/Ca sublattice in YCa3Mn3O3(BO3)4 promotes magnetic order. The spin-liquid state with large density of unfrozen entropy stimulated us to study the MCE of natural gaudefroyite.
 | ||
| Fig. 1 (a) Crystal of gaudefroyite as obtained and (b) cut/polished with the size of 4.6 × 2.5 × 6 mm3 (a × b × c). (c) Crystal structure viewed approximately along [001] and showing the unit cell: the chains of MnO6 octahedra are blue, Ca green, C black, B blue and O red. (d) The magnetic Kagomé lattice of the chains of Mn3+ ions (blue spheres) and the magnetic order in YCa3Mn3O3(BO3)4.21 | ||
The mineral samples used in our study were from Wessels Mine, Kalahari Manganese Field, Northern Cape Province, South Africa, with hexagonal prism shaped crystals of typical size 6 × 7 × 21 mm3 (Fig. 1(a)). The crystals were cut to provide 3 × 1 × 4 mm3, 4.6 × 2.5 × 6 mm3 (Fig. 1(b)) and 2.4 × 4.1 × 5.8 mm3 crystals for different measurements. X-ray fluorescence analyses showed that the raw crystal contained only minor impurities of Cl (1.2%), Si (0.26%), Fe (0.21%) and S (0.15%).
GdCa3Mn3O3(BO3)4 was synthesised using the method previously described: pure Gd2O3, CaCO3, MnO2 and H3BO3 were heated in air initially at 600 °C for 10 h and subsequently for three periods of 24 h at 1050 °C.21 Magnetic and specific heat data were collected from a Quantum Design PPMS magnetometer using the oriented crystals with size of 3 × 1 × 4 mm3 for gaudefroyite and a sintered pellet for GdCa3Mn3O3(BO3)4.
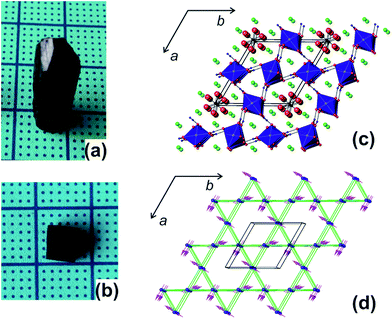
Field dependent magnetisations in gaudefroyite at temperatures below 15 K with the field applied perpendicular to [001] revealed a sharp increase in magnetisation to >60% of its saturated value with a field of only 2 T (Fig. 2(a)), indicative of a large MCE effect at small field. The entropy changes calculated from the magnetisation curves,25Fig. 2(b), show a maximum value of |ΔS| ≈ 7 J kg−1 K−1 at 11 K for a field of 2 T and agree with those from integration of the specific heats25 at 0 and 2 T, Fig. 2(c). Fig. 2(b) also compares ΔS data with those at the same field for Gd(HCOO)3.9 Whereas ΔS for gaudefroyite remains > 3 J kg−1 K−1 for temperatures of 6–24 K, i.e. suitable for LH2 production, the paramagnetic Gd salt is excellent at very low temperatures but significantly inferior above ∼10 K. Fig. 3(a) shows that for a field of 7 T, the entropy change increases to 15 J kg−1 K−1. Importantly, these entropy changes are already very close to those for giant MCE behavior in some intermetallic FM materials,11,12 and for the best Dy- or Fe-doped gadolinium garnets – we stress that gaudefroyite contains no rare earth ions. It should be noted that despite the anisotropy, Fig. 2(b), the properties of a powder-averaged, sintered sample would also be excellent.
 | ||
| Fig. 2 (a) Low temperature isothermal magnetization of gaudefroyite within the easy plane, ab. (b) Entropy changes of gaudefroyite for a field of 2 T obtained from the magnetisation (m in legend) and specific heat (Cp in legend) measurements; the data are compared with those for Gd(HCOO)3.9 (c) Specific heat of gaudefroyite under zero field and a magnetic field of 2 T (in the ab plane). | ||
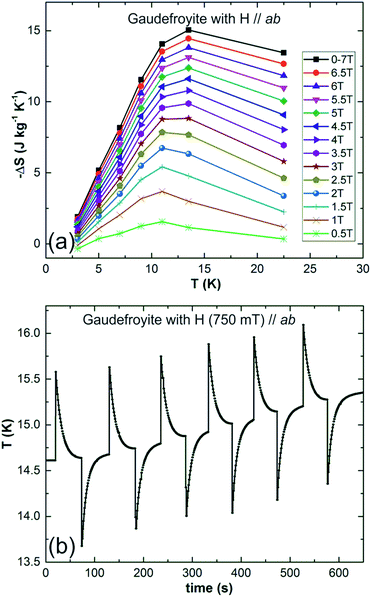 | ||
| Fig. 3 (a) Entropy changes as a function of temperature for fields of 0.5 to 7 T for gaudefroyite and (b) temperature change of gaudefroyite on applying and removing a field of 750 mT. | ||
Direct temperature change was recorded with a thermocouple glued on one side of the gaudefroyite crystal of size 2.4 × 4.1 × 5.8 mm3 and with the other side glued on a 2 mm thick copper plate attached to the base of a cryostat (Quantum Design with optic option and a magnet of maximum field 760 mT). Data collected at ∼15 K, Fig. 3(b), show that ΔT = −0.96 K for a field of 750 mT. The expected adiabatic temperature change, Tad can be estimated from the crude approximation:26
For the practical application of MC refrigeration, a more important parameter, the relative cooling power (RCP) defined as the product of maximum entropy change (ΔS) and full width at half maximum of the ΔSM − T curve, is often used to evaluate cooling power of different MC materials. For gaudefroyite, we found RCP > 100 J kg−1 for the field change of 0–2 T, which is the highest among similar compounds at this field and temperature range.27 This strong enhancement of RCP, due to high ΔSM being spread over a large temperature range, was also observed in the spin glass systems of Gd2NiSi3 and Er2NiSi3 and was attributed to the spin frustration.27
In an actual MC refrigerator, especially with active magnetic regeneration (AMR) cycles, several pre-requisites are demanded for the MC materials:2,28 (1) a large entropy change at the relevant temperature for a field less than 2 T; (2) a large thermal conductivity for efficient heat exchange between the MCE material and cooling media; (3) low magnetic or structural hysteresis and high electronic resistance for reducing AC operation loss29 and (4) high chemical stability in contact with the cooling media (e.g. LH2).
Thermal conductivity (κ) measurements (steady heat flow method30 on the oriented crystal 4.6 × 2.5 × 6 mm3) are shown in Fig. 4(a). Along c, κ is about 10 W m−1 K−1 for most of the temperature range examined, with a maximum of 14 W m−1 K−1 at 42 K; it drops to 3 W m−1 K−1 at 5 K. Although the thermal conductivity is an order of magnitude smaller than GGG single crystals or high purity metals, it is similar to sintered ceramics or pressed pellets,30 which are generally used in MC refrigerators. Gaudefroyite shows no magnetic order down to 2 K, but a spin-liquid forms below 9 K and gives a small hysteresis of 180 Oe at 1.8 K, Fig. 4(b).20 The hysteresis disappears above 9 K, which is excellent for producing LH2 at 20 K. We expect that for this application, gaudefroyite will show high stability as do other oxide MC materials.
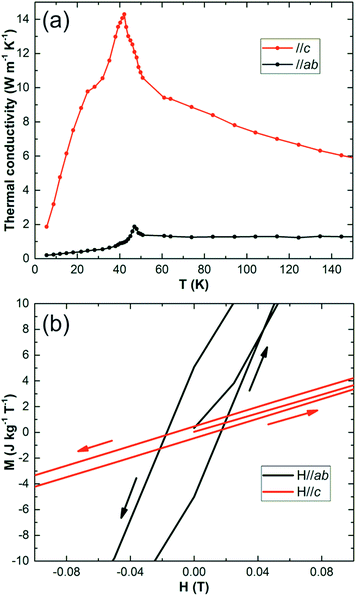 | ||
| Fig. 4 (a) Variation of thermal conductivity with temperature for gaudefroyite along c (upper plot) and in the ab plane (lower plot). (b) Magnetic hysteresis of gaudefroyite at 1.8 K. | ||
Gaudefroyite therefore has excellent, low temperature MC properties and is, in fact, unique in simultaneously displaying:
(1) Strong, low field MCE (H < 2 T), due to the giant spins on the MnO6 chains;
(2) Very fast cooling rate due to the magnetic frustration between the chains.
Nevertheless, since previous work21 showed that rare earth substituted analogues of gaudefroyite can be synthesised, we have examined the effect of increasing the total magnetic spin number by synthesizing GdCa3Mn3O3(BO3)4. For this material, even larger entropy changes are observed, especially for large applied fields and lower temperatures: Fig. 5 indicates a change of 20 J kg−1 K−1 for a ceramic sample of GdCa3Mn3O3(BO3)4 for a field of 7 T. Even for polycrystalline materials, and at smaller field, the entropy change is larger than the powder average of the gaudefroyite crystal. This behavior means that the magnetic spins of Gd3+ and Mn3+ are decoupled and each FM MnO6 chain responds as a giant spin to enhance the total internal field experienced by the paramagnetic Gd3+ spins. Although the incorporation of Gd results in a higher entropy change in high fields, the major change observed is the remarkable enhanced entropy change at lower temperatures. In fact we believe that GdCa3Mn3O3(BO3)4 is unique in providing an entropy change that is greater than 10 J kg−1 K−1 for all temperatures below 20 K for a field of 5 T.
In conclusion, gaudefroyite provides excellent MC properties between 10 and 20 K. If even better performance is needed at lower temperatures, the introduction of some Gd can achieve this. We have examined only Mn3+ ions and anticipate that the synthesis of materials with similar FM chains could lead to even further enhancements in the MC characteristics of materials with related structures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the National Instrumentation Program (No. 2012YQ120048) of P. R. China and the UK Engineering and Physical Sciences Research Council (EPSRC GR/S2672/01).Notes and references
- O. Sari and M. Balli, Int. J. Refrig., 2014, 37, 8–15 CrossRef.
- A. Kitanovski, J. Tušek, U. Tomc, U. Plaznik, M. Ožbolt and A. Poredoš, in Magnetocaloric Energy Conversion: From Theory to Applications, Springer International Publishing, Switzerland, 2015 Search PubMed.
- B. F. Yu, M. Liu, P. W. Egolf and A. Kitanovski, Int. J. Refrig., 2010, 33, 1029–1060 CrossRef CAS.
- J. Liu, T. Gottschall, K. P. Skokov, J. D. Moore and O. Gutfleisch, Nat. Mater., 2012, 11, 620–626 CrossRef CAS PubMed.
- I. D. Hepburn, I. Davenport and A. Smith, Space Sci. Rev., 1995, 74, 215–223 CrossRef.
- T. Numazawa, K. Kamiya, T. Utaki and K. Matsumoto, Cryogenics, 2014, 62, 185–192 CrossRef CAS.
- I. M. Park and S. K. Jeong, IOP Conf. Series: Materials Science and Engineering, 2015, vol. 101, p. 012106 Search PubMed.
- K. Sakata, E. Mizutani and K. Fukuda, J. Power Sources, 2016, 159, 100–106 CrossRef.
- G. Lorusso, J. W. Sharples, E. Palacios, O. Roubeau, E. K. Brechin, R. Sessoli, A. Rossin, F. Tuna, E. J. L. McInnes, D. Collison and M. Evangelisti, Adv. Mater., 2013, 25, 4653–4656 CrossRef CAS PubMed.
- Y. C. Chen, J. Prokleska, W. J. Xu, J. L. Liu, J. Liu, W. X. Zhang, J. H. Jia, V. Sechovsky and M. L. Tong, J. Mater. Chem. C, 2015, 3, 12206–12211 RSC.
- T. Samanta, I. Das and S. Banerjee, Appl. Phys. Lett., 2007, 91, 152506 CrossRef.
- Z. J. Mo, J. Shen, L. Q. Yan, C. C. Tang, J. Lin, J. F. Wu, J. R. Sun, L. C. Wang, X. Q. Zheng and B.-G. Shen, Appl. Phys. Lett., 2013, 103, 052409 CrossRef.
- T. Numazawa, K. Kamiya, T. Okano and K. Matsumoto, Phys. B, 2003, 329–333, 1656–1657 CrossRef CAS.
- V. K. Pecharsky and K. A. Gschneidner Jr, Phys. Rev. Lett., 1997, 78, 4494–4497 CrossRef CAS.
- D. Serantes, V. Vega, W. O. Rosa, V. M. Prida, B. Hernando, M. Pereiro and D. Baldomir, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 104431 CrossRef.
- J. W. Sharples, D. Collison, E. J. L. McInnes, J. Schnack, E. Palacios and M. Evangelisti, Nat. Commun., 2014, 5, 5321 CrossRef CAS PubMed.
- M. E. Zhitomirsky, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 104421 CrossRef.
- J. Schnack, R. Schmidt and J. Richter, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 054413 CrossRef.
- S. S. Sosin, L. A. Prozorova, A. I. Smirnov, A. I. Golov, I. B. Berkutov, O. A. Petrenko, G. Balakrishnan and M. E. Zhitomirsky, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 094413 CrossRef.
- R. K. Li and C. Greaves, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 132411 CrossRef.
- R. K. Li and C. Greaves, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 172403 CrossRef.
- J. Gutzmer and N. J. Beukes, Ore Geol. Rev., 1996, 11, 405–428 CrossRef.
- M. M. Granger and J. Protas, C. R. Acad. Sci., 1965, 260, 4553 CAS.
- C. Hoffmann, T. Armbruster and M. Kunz, Eur. J. Mineral., 1997, 9, 7–19 CrossRef CAS.
- V. K. Pecharsky and K. A. Gschneidner Jr, J. Appl. Phys., 1999, 86, 565–575 CrossRef CAS.
- A. Smith, C. R. H. Bahl, R. Bjørk, K. Engelbrecht, K. K. Nielsen and N. Pryds, Adv. Energy Mater., 2012, 2, 1288–1318 CrossRef CAS.
- S. Pakhira, C. Mazumdar, R. Ranganathan, S. Giri and M. Avdeev, Phys. Rev. B, 2016, 94, 104414 CrossRef.
- P. Wikus, E. Canavan, S. T. Heine, K. Matsumoto and T. Numazawa, Cryogenics, 2014, 62, 150–152 CrossRef CAS.
- A. M. Aliev, A. B. Batdalov, L. N. Khanov, V. V. Koledov, V. G. Shavrov, I. S. Tereshina and S. V. Taskaev, J. Alloys Compd., 2016, 676, 601–605 CrossRef CAS.
- T. Numazawa, O. Arai, Q. Hu and T. Noda, Meas. Sci. Technol., 2001, 12, 2089–2094 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |