Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The ameliorating effect of the combined extract from Greek Thymus vulgaris and bee's honey on the hydrocortisone-induced osteoporosis in rat bone cells via modulating the bone turnover, oxidative stress, and inflammation
Marwa M. Abu-Seriea and
Noha H. Habashy *b
*b
aDepartment of Medical Biotechnology, Genetic Engineering, Biotechnology Research Institute, City for Scientific Research and Technology Applications (SRTA-City), New Borg EL-Arab 21934, Alexandria, Egypt
bBiochemistry Department, Faculty of Science, Alexandria University, Alexandria 21511, Egypt. E-mail: noha.Habashi@alexu.edu.eg; nohahhm@gmail.com; Fax: +20-2(03) 3911794; Tel: +20 1273431731
First published on 7th August 2018
Abstract
Many of the functional foods are designed to decrease the risk of chronic diseases like osteoporosis (OP) which is the most common bone disorder affecting millions of people. For the first time, the present study evaluated the effect of the combination between the Greek Thymus vulgaris water extract (TVE) and bee's honey (BH) against hydrocortisone (HC)-induced OP in vitro. The characterization of TVE, BH, and their combined extract (TV–BH) was examined. In addition, the current work assessed the bone turnover, oxidative stress, and inflammatory markers in bone cells. The results revealed the presence of considerable amounts of phenolics, flavonoids, anthocyanins, and flavonols in TVE and BH as well as important minerals and vitamins for the bone health. The TV–BH showed a synergistic (combination index <1) attenuation effect for the HC-induced bone cell damage through significant (p < 0.05) up-regulation of the hydroxyapatite, osteocalcin, phosphorous, and collagen contents. In addition, it significantly (p < 0.05) suppressed the tartrate-resistant acid phosphatase activity and hydroxyproline level as well as the oxidative and inflammatory stress. Data also observed the more potent anti-osteoporotic effect of the combined extract than the commonly used bisphosphonate drug (alendronate). In conclusion, the administration of TV–BH improved the glucocorticoid-induced bone damage, inflammation, and oxidative stress and as a result, it might be a promising therapeutic option for the OP disorder.
Introduction
Osteoporosis (OP) is the most prevalent socioeconomic musculoskeletal disease affecting mainly postmenopausal women and older people with significant morbidity and mortality. This disease causes severe pain, disability, and affects females more than males. It is a systematic multifactorial skeletal disorder characterized by deteriorating bone tissues, reduced bone mass, and increased fracture risk. Bone is a supporting framework of the body consisting mainly of the matrix and four cell types, osteoblast, osteoclast, osteocyte and osteoprogenitor.1 Osteoblasts synthesize and secrete bone matrix components, mainly collagen, osteocalcin, and alkaline phosphatase (ALP) while osteoclasts are important for bone resorption.2 The alteration in the activity or a number of osteoblasts and osteoclasts will disrupt bone homeostasis which is the main cause of OP.1 OP is related to many risk factors such as smoking, hormonal factors, diabetes, low calcium and vitamin D intake as well as glucocorticoids.2The long-term treatment with glucocorticoids drugs largely induces OP (secondary OP). These drugs, like hydrocortisone (HC), are most potent anti-inflammatory and immunosuppressive compounds that are widely used in the treatment of various diseases such as autoimmune disorders and rheumatoid arthritis. But they have serious adverse effects on multiple organ systems including bone cells. Because these drugs are able to induce oxidative stress and gene expression of nuclear factor-kappa (NF-κ)B, cyclooxygenase (COX)-2 and tumor necrosis factor (TNF)-α on bone cells. These effects decrease the bone matrix deposition by osteoblasts and increase bone resorption by osteoclasts.3 Several medications are used to treat glucocorticoid-induced OP such as calcium and active vitamin D intake, hormone replacement therapy, and bisphosphonates (BPs). The most often treatments are the BPs which are analog for the inorganic pyrophosphate.4 Alendronate (ALD), etidronate, clodronate, tiludronate, and pamidronate are the most commonly recommended BP drugs.5 These drugs able to attach to the bone surface and inhibit the osteoclasts function by different mechanisms.4 However, they are associated with certain side effects such as flu-like symptoms, jaw osteonecrosis, ulcers in the gastrointestinal tract, and they are not safe for patients with severe renal impairment.4,5 Therefore, searching for new natural alternative therapies with fewer or no side effects has become important to treat OP.
Thyme (Thymus vulgaris, TV) is one of the human nutritional plants belonging to the Lamiaceae family. It was cultivated in many countries such as Iran, Spain, France, Greece, Portugal, and Italy.6 Its fresh or dried leaves can be used as a spice and its essential oils used in cosmetics and food additives. TV has ancient traditional uses in the treatment of coughs, headaches, diarrhea, worms, constipation, kidney malfunction, and cancer. In addition, it has various valuable effects, such as antiseptics, antispasmodic, antioxidants, bactericide, and antihelmintic properties. These effects are related to its constituents such as carvacrol, flavonoids, eugenol, thymol, phenols, and luteolin.7
Bee's honey (BH) is a natural food known since ancient times by the ancient Egyptians, Greeks, and Chinese and it has been mentioned in the Islamic Hadith (saying of the prophet Mohamed). It has potential uses in medicine due to its anti-microbial, acute and chronic wound healing,8 antioxidant, anti-inflammatory and anti-gastritis effects. The flowers used by bees are responsible for the nutritional values and the chemical constituents of BH, which mainly (95–97%) are sugars. In addition, it contains a mixture of different constituents such as proteins, enzymes, vitamins, polyphenols, minerals, organic and amino acids.9
The excessive intake of vegetables and fruits has been correlated with a decrease in the risk of various diseases like OP.10 Various studies were conducted to study the anti-osteoporotic effects of the polyphenol-rich foods.11–13 However, few of them were examined the effect of BH or TV (not the Greek type) on this bone disorder.14 Hence there are no previous studies investigating the anti-osteoporotic effect of the combination between TV and BH (TV–BH), the present work evaluated this effect on the HC-induced OP in vitro. The in vitro antioxidant and anti-inflammatory potentials of TVE were proved recently by the authors15 and these two activities were reported for BH.9 Therefore, TV–BH may be a promising therapy for OP disease, which is highly associated with the inflammatory and oxidative stress.3 In addition, the present study examined certain constituents in TVE and BH useful for bone formation and health to explain the probable anti-osteoporotic role better.
Materials and methods
Materials
Folin–ciocalteau reagent, 4-hydroxycinnamic acid (4-HCA), catechin, quercetin (QR), butylated hydroxytoluene (BHT), 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS), 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe, HC, thiobarbituric acid (TBA), reduced glutathione (GSH), propidium iodide (PI), collagenase type I, 3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT), and ALD drug were obtained from Sigma-Aldrich (St. Louis, MO, USA). Roswell Park Memorial Institute (RPMI)-1640 medium, Dulbecco's Modified Eagle Medium (DMEM), α-Minimum Essential Medium Eagle (MEM), trypsin and fetal bovine serum (FBS) were purchased from Lonza, USA. Gene JET RNA purification kit, cDNA synthesis kit, 2X SYBR green master mix kit and protease inhibitor cocktails (PIC) were obtained from ThermoScientific, USA. Osteocalcin ELISA Kit was supplied from Nordic Bioscience Diagnostics (N-MID, Denmark). ALP colorimetric kit was purchased from Biosystems (Barcelona, Spain). The phosphorous colorimetric kit was obtained from Chema Diagnostica (Campania, Italy). TNF-α ELISA kit was purchased from RayBiotech, USA and collagen type IV (COL 4) ELISA kit was supplied from Kamiya Biomedical, US. Primers for NF-κB and COX-2 were purchased from Bioneer, Korea. Other chemicals were obtained with a high grade.Extract preparation
The dried TV plant (NCBI:txid49992) was imported from Greece and BH was purchased from a local market in Egypt, then both were authenticated by expert botanist before their use to prepare TVE and TV–BH. The BH produced by Apis mellifera bees (NCBI:txid7460) from the citrus fruits nectar.The TVE was prepared as described previously.15 In brief; 50 g of the dried TV plant was powdered, mixed for 10 min with 250 ml of distilled water using an electric blender and soaked for 24 h at 25 °C. Then the solution was filtered and the residues were extracted again. The filtrates were combined, freeze-dried by the lyophilizer (Telstar, Terrassa, Spain), and the powder (TVE, the yield of 9%) was kept in a dark bottle at −20 °C until used.
For preparing the combined extract (TV–BH), 4 g of BH was mixed with 1 g of TV powder and left at 4 °C for 1 week prior to use. The studied extracts were dissolved in distilled water and filtered using syringe filters (0.22 μm) before using in the analyses.
Extracts characterization
For mineral analysis, 1 g of TVE or BH was digested in nitric acid (55%) and perchloric acid (70%) for 1 h at 100 °C. The digested solution was cooled, filtered, and diluted to 10 ml, and then an aliquot of this solution was analyzed using Atomic Absorption Spectrophotometer (Perkin Elmer model 2380, Norwalk, CT, USA).
Proline and vitamin content
Proline content was determined colorimetrically by the ninhydrin-based method.20 It was extracted from TVE or BH using 300 μL of pure ethanol and the solution was heated at 95 °C then mixed with an acid ninhydrin solution (1% ninhydrin, 60% ethanol, 20% acetic acid). The absorbance was recorded at 520 nm after 40 min incubation at 95 °C. The proline content was calculated from the calibration curve of standard proline.The concentration of vitamin C and K was estimated in TVE or BH by colorimetric methods using 2,4 dinitrophenylhydrazine (2,4 DNPH) and standard vitamins.21,22 For vitamin C determination, the deproteinized extract or standard was mixed and incubated with a mixture of 2,4-DNPH (3%), thiourea (0.4%), CuSO4 (0.05%) and H2SO4 (65%) at 37 °C for 1.5 h. At the end of the incubation period, H2SO4 was added and the absorbance of the colored solution was read at 520 nm after 30 min. Vitamin K was quantified by mixing and incubating 1 ml of each extract or standard with 0.25% 2,4-DNPH and ethanol (70%) at 75 °C for 15 min with vigorous shaking. Then alcoholic ammonia was added and the absorbance of the produced color was recorded after 15 min at 635 nm.
In vitro antioxidant activities
The antioxidant activities of TVE, BH, or TV–BH were evaluated in vitro using different assays, including the total antioxidant capacity (TAC), β-carotene-linoleate bleaching, and ABTS+ radical cation-decolorization assays.The TAC of the single and combined extracts was determined using a mixture of 28 mM sodium phosphate, 0.6 M H2SO4, and 4 mM ammonium molybdate. This mixture was incubated with each of the single or combined extract, standard antioxidant (BHT) or water (blank) at 95 °C for 90 min; then the absorbance was measured at 695 nm.23
The β-carotene-linoleate bleaching assay examines the anti-lipid peroxidation activity of the samples using an emulsion from β-carotene, linoleic acid and Tween-80.24 The ability of the extract to inhibit the radicals that produced from linoleic acid oxidation as indicated by the decrease in the bleaching rate of β-carotene was measured. The absorbance (a, b) was read at 490 nm immediately and after 180 min (t), respectively. Then the degradation rate (DR) of the extract, standard (BHT), and control (water instead of the extract) was calculated from the equation: [DR = ln(a/b) × (1/t)]. The antioxidant activity was calculated as % of inhibition using the formula: antioxidant activity (%) = (DRcontrol − DRextract/DRcontrol) × 100. The extract concentration that made inhibition of the β-carotene bleaching by 50% (IC50 value) was calculated.
The ABTS+ radical cation-decolorization assay assesses the loss of the blue-green color of ABTS+ radical upon its reduction by the antioxidants to ABTS.25 ABTS+ radical was prepared before mixing with the single or combined extract by incubating ABTS (7 mM) with potassium persulphate (140 mM) for 16 h at 25 °C in the dark. The absorbance was read at 734 nm and the % of ABTS+ radical inhibition was calculated for IC50 value determination and the results were compared with BHT.
Bone cell isolation and cultivation
The bone cells were isolated from the sterilized femur and tibia of three albino male rats (71–73 g) following the method of Wong et al.26 with some modifications. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Alexandria University and City for Scientific Research and Technology Applications (SRTA-City). All animal procedures were approved by the Animal Ethics Committee of the National Health and Medical Research Council policies and the Ministry of Health and Population, Egypt.Bones were dissected into 1 mm pieces and washed well to remove blood, marrow cells, and any soft tissues. Bone pieces were placed in DMEM containing 100 U ml−1 penicillin/streptomycin for 20 min in a CO2 incubator (New Brunswick Scientific, Netherlands) at 37 °C, 5% CO2 and 90% relative humidity. Bone cells were successfully isolated by digestion with 100 U ml−1 collagenase type I and 25% trypsin. Cells were cultured with α-MEM containing 20% FBS in 6-well culture plate in a CO2 incubator at the same previous conditions. After 24 h, the medium was aspirated and replaced daily thereafter until they reached confluence (90%) within 3 weeks. Then trypsinization was done for cells using trypsin (25%) containing EDTA (0.02%). After washing, cells were tested for the viability and counted using 0.5% trypan blue stain.
Cytotoxicity assay
About 1 × 104 bone cells were seeded per well in 96-well plate and 100 μL of serial dilutions of each single or combined extract or ALD were added and incubated in 5% CO2 incubator. After 72 h, 20 μL of MTT (5 mg ml−1 in phosphate buffered saline, PBS) was added per well and incubated in a CO2 incubator for further 3 h. Then 100 μL of DMSO was added to each pellet (formazan crystals, MTT byproduct) for solubilization and the absorbance was read at 570 nm using an ELISA reader (BMG LabTech, Germany). Cell viability was determined and a relation between it and each of the studied extract or standard drug concentrations was plotted for calculating the safe concentrations (EC100, 100% cell viability).Development of OP in vitro model and treatment protocol
Cells from bone were incubated with serial concentrations of HC (1.25, 2.5, 5, 10, 20 mg ml−1). After 48 h, the % of bone cell damage and bone cell turnover (formation and resorption) markers were assessed at each HC concentration. Then the IC50 values were calculated for the % cell damage and from the % inhibition or depletion values of each bone turnover marker. The best concentration of HC that was selected for the establishment of the OP in vitro model was calculated as the mean value for these IC50 values. After induction, cells were treated with the safe concentration (EC100) of each single or combined extract or the standard drug. Then cells were morphologically observed using a phase contrast inverted microscope (Olympus, Tokyo, Japan) after 72 h incubation in a CO2 incubator. The % of bone cell damage, bone cell turnover markers, inflammatory mediators, and oxidative stress parameters were evaluated as follows.Evaluation of bone cell damage by PI-staining assay
PI stain was used to determine the percentage of bone cell damage due to its ability to stain the chromatin of dead cells only. About (1 × 104) of the untreated and HC-, TVE-, BH-, (TV–BH)-, and ALD-treated cells in 96-well plate were washed twice and then suspended in 100 μL PBS containing 10 μg ml−1 PI. After 30 min, plates were read using a fluorescence plate reader (BMG LabTech, Germany) at 520 nm emission and 490 nm excitation.Biochemical markers of bone cell turnover
The medium of the untreated and HC-, TVE-, BH-, (TV–BH)-, or ALD-treated bone cells were removed and used in the quantification of osteocalcin level. The bone cell lysate was prepared by washing cells twice with PBS containing PIC then cells were treated with trypsin, washed twice, and suspended. Cells were lysed in PBS using a sonicator (2 cycles, each of 10 s duration) then cell lysate was centrifuged at 600g for 5 min, suspended in PBS, and used for the biochemical assays.Bone cell formation markers
The markers of bone cell formation were assessed, including mineralization, osteocalcin, phosphorous, ALP, and collagen content.The production of extracellular matrix calcium deposits by osteoblasts (mineralization, bone nodule formation) was detected by alizarin red staining.27 Bone cell monolayers in a 6-well plate were fixed in 10% formaldehyde for 15 min at 25 °C and stained with 40 mM alizarin red (pH 4.1). The stained cells were visualized and photographed with the phase contrast microscope. Then the absorbance was read at 405 nm and the concentration was determined using the hydroxyapatite (calcium phosphate tribasic, Ca10[PO4]6[OH]2) calibration curve. The osteocalcin concentration was determined by specific ELISA Kit using two highly specific capture and detection monoclonal antibodies following the manual instructions. Phosphorous was assessed in bone cell lysates using the Chema Diagnostica colorimetric kit following the manufacturer's protocol. The method based on the formation of the colorless phosphomolybdate complex in an acidic medium which can be measured at 340 nm. The intracellular ALP was determined in the bone cell extract that was prepared by mixing 100 μL of 0.1% sodium dodecyl sulfate (SDS) with the cell lysates at 4 °C for 40 min. ALP activity was measured colorimetrically using 6 mM 4-nitrophenyl phosphate (4-NPP) as a substrate and the procedure was continued according to the ALP colorimetric kit manual. The increase in absorbance was measured per min for 3 min at 405 nm. One unit of ALP activity is defined as the amount of enzyme which produced 1 μmol of 4-NP/min and the specific activity is expressed as units of enzyme activity per mg of protein. Collagen content was determined in the cell lysates using the specific ELISA kit following the manual protocol.
Bone cell resorption markers
Tartrate-resistant acid phosphatase (TRAP) activity and hydroxyproline content were determined as markers of the bone cell resorption.The TRAP activity was determined in the cell extract (prepared as described in the ALP assay) according to Burstone method.28 The solution of 4-NPP (2.2 mg ml−1) in 0.5 M sodium tartrate solution containing 0.5 M sodium acetate and adjusted to pH 6.1 was used as a substrate. The absorbance was read at 405 nm and the enzyme activity was determined using 4-NP calibration curve. Then the specific activity of the enzyme that was defined as the amount of enzyme required to hydrolyze 1 μmol of 4-NPP per min per mg protein at 37 °C was calculated. Hydroxyproline content in cell lysates was measured using chloramines T and dimethylaminoborane solutions.29 The absorbance of the produced color was read spectrophotometrically at 560 nm and the concentration was determined from the hydroxyproline calibration curve.
Determination of the oxidative stress parameters
The intracellular reactive oxygen species (ROS) level, lipid peroxidation, GSH, and the antioxidant enzymes were investigated in the untreated control, HC-, TVE-, BH-, (TV–BH)-, and ALD-treated bone cells.The ROS level was assessed using DCFH-DA fluorescent probe method.30 This method is extremely sensitive to the change in the cellular redox state. Bone cells were preloaded with 5 μM of DCFH-DA at 37 °C for 30 min. The cellular esterases cleave DCFH-DA producing a H2DCF non-fluorescent product, which was oxidized by ROS to DCF fluorescent molecules. The intensity of the produced fluorescence was examined by the flow cytometry (FACS Calibur, Becton Dickinson, USA) at an excitation (488 nm) and an emission (530 nm) wavelength. Results were analyzed using the CELLQuest program (Becton Dickinson).
The medium of 1 × 106 bone cells was used in determining the lipid peroxidation and bone cell lysate was used in the assay of the antioxidant indices and total protein levels. The degree of lipid peroxidation was assessed by determination of the TBA reactive substances (TBARS) level.31 GSH level was measured using Ellman's reagent (5,5′-dithiobis2-nitrobenzoic acid) for production of a yellow-colored product with a maximum absorbance at 412 nm.32 Glutathione peroxidase (GPX) activity was determined colorimetrically using GSH and cumene hydroperoxide as substrates.33 The activity of Cu/Zn superoxide dismutase (SOD) was assessed using the pyrogallol autoxidation method,34 in which the change in absorbance during 2 min was measured at 420 nm. The unit of activity is defined as the amount of enzyme that inhibits the rate of autoxidation of 20 mM pyrogallol by 50% under standard conditions.
Determination of the inflammatory mediators
About 1 × 106 of the untreated bone cells and cells that treated with HC, TVE, BH, (TV–BH), or ALD were centrifuged at 600g for 10 min. The culture medium per well was collected and used for quantification of the TNF-α protein following the manufacturer's protocol of the ELISA kit. Also, NO level was determined in the culture medium by the Griess reaction, which produced colored azo dye with a maximum absorbance at 490 nm.35 While cells were detached with trypsin–EDTA; then washed twice with PBS and used for total RNA extraction following the manual protocol of Gene JET RNA Purification Kit. The cDNA was synthesized from 1 μg of each RNA sample by reverse transcriptase-PCR using the cDNA Synthesis Kit. Then the NF-κB and COX-2 gene expression were determined by real-time quantitative polymerase chain reaction (qRT-PCR, Qiagen, Germany). Levels of the gene expressions of target genes and reference gene (β-actin) were quantified using the gene-specific forward and reverse primers. The following primers were used: NF-κB, forward 5′-CCTCGGGGTCCTACTCAAGA-3′, reverse: 5′-AGAGGTGTCGTCCCATCGTA-3′; COX-2, forward: 5′-CCCATGTCAAAACCGTGGTG-3′, reverse: 5′-CTTGTCAGGAATCTCGGCGT-3′; and β-actin, forward: 5′-CCACCATGTACCCAGGCATT-3′, reverse: 5′-CCTAGAAGCATTTGCGGTGC-3′.The reaction mixture enclosed 0.3 μl of each forward and reverse primer (10 μM), 12.5 μL of the 2X SYBR green master mix, and 50 ng cDNA templates. Then, the total volume was completed to 25 μl with nuclease-free water. The qPCR program was applied as one cycle of enzyme activation for 15 min at 95 °C followed by 40 cycles of denaturation for 15 s at 95 °C, annealing for 1 min at 60 °C and extension for 30 s at 72 °C. The expression of target genes was calculated using the comparative Ct method (threshold cycle number at cross-point between amplification plot and threshold). The CT values of each target gene were normalized to that of β-actin according to manufacturer's manual and the change in the gene expression (2−ΔΔCT) was calculated.
Total protein content
The total protein content of the cell lysate and cell extract (SDS-treated cell lysate) was determined using Bradford Coomassie brilliant blue assay.36 The produced blue colored complex was read at 595 nm. The concentration of the protein was determined from the bovine serum albumin standard curve.Impact evaluation of TV and BH combination
The combination of food extracts may give higher (synergistic), lower (antagonistic), or no new (additive) effect compared to the effect of the single extracts. Evaluation of this new effect can be done through determination of the combination index (CI) value. Here the CompuSyn software was used to calculate the CI values for the in vitro antioxidant activities and for other studied parameters, these values were calculated by dividing the predictable value on the practical value. The predictable value between extract 1 and extract 2 is determined as [(practical value for extract 1)/(control value)] × [(practical value for extract 2)/(control value)] × (control value).15 The CI value may be equal to 1 (additive effect), <1 (synergistic effect) or >1 (antagonistic effect).37,38Data analysis
The results are presented as mean ± SE and the comparison between the mean values of different treatments was done using one-way ANOVA via Duncan's test. The significance was determined at p < 0.05 and the analysis was performed for three measurements using SPSS software version 16. GraphPad PRISM software version 6 with appropriate 95% confidence interval was used for determination of the IC50 and EC100 values. The CI value and the CI plots for the in vitro antioxidant assays were achieved by the CompuSyn software (ComboSyn, Inc, Paramus, NJ).Results and discussion
Extracts constituents
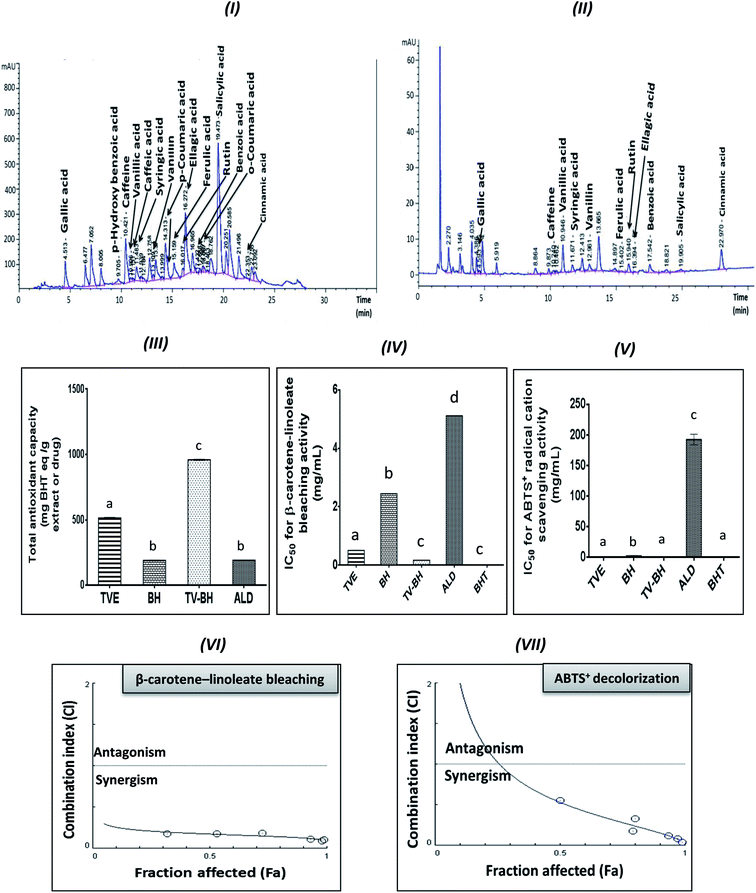
Table 1 represented the chemical constituents of TVE and BH. The results revealed the presence of considerable amounts of proline, phenolics, flavonoids, anthocyanins, flavonols, and important minerals and vitamins in TVE. BH contains these compounds except flavonoids. In addition, the HPLC analysis detected fifteen phenolic compounds in TVE (Fig. 1I) and twelve ones in BH (Fig. 1II) using identified phenolic standards (Table 1) by comparing their particular retention times. These constituents are necessary for bone health and strength.| BH | TVE | Constituent |
|---|---|---|
| a Results are presented as mean ± SE (n = 3). Different letters in the same row are significantly different at p < 0.05. BH, bee's honey; Cy-3-glc, cyanidin-3-glucoside; 4-HCA, 4-hydroxycinnamic acid; QR, quercetin; TVE, Greek Thymus vulgaris water extract; ND, not detected. | ||
| Amino acids (μmol g−1 extract) | ||
| 0.812 ± 0.101b | 1.859 ± 0.159a | Proline |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Phytochemicals | ||
| 6.561 ± 0.056b | 627.194 ± 0.111a | Phenolics (mg 4-HCA eq. g−1 extract) |
| ND | 135.674 ± 0.000 | Flavonoids (mg catechin eq. g−1 extract) |
| ND | 1.076 ± 0.165 | Anthocyanins (mg Cy-3-glc eq. g−1 extract) |
| ND | 41.830 ± 0.000 | Flavonols (mg QR eq. g−1 extract) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Phenolics (μg g−1 extract) | ||
| 11.7 ± 0.009b | 214.041 ± 0.990a | Gallic acid |
| ND | 302.360 ± 1.800 | p-Hydroxybenzoic acid |
| 10.300 ± 0.543b | 589.863 ± 0.876a | Caffeine |
| 86.200 ± 0.200b | 45.166 ± 0.166a | Vanillic acid |
| 18.400 ± 0.400b | 11.922 ± 0.800a | Caffeic acid |
| 23.300 ± 0.622b | 147.263 ± 0.655a | Syringic acid |
| 23.300 ± 0.511b | 37.711 ± 2.333a | Vanillin |
| ND | 182.656 ± 3.000 | p-Coumaric acid |
| 2.700 ± 0.004b | 184.660 ± 0.233a | Ferulic acid |
| 90.600 ± 1.003b | 241.111 ± 2.544a | Rutin |
| 4.623 ± 0.788b | 1646.741 ± 13.456a | Ellagic acid |
| 406.005 ± 4.773b | 37.777 ± 0.444a | Benzoic acid |
| ND | 21.768 ± 0.099 | o-Coumaric acid |
| 36.893 ± 1.223b | 4179.990 ± 3.655a | Salicylic acid |
| 27.643 ± 3.754b | 1.045 ± 0.001a | Cinnamic acid |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Vitamins (mg g−1 extract) | ||
| 0.711 ± 0.236b | 3.242 ± 0.361a | C |
| 11.005 ± 0.344b | 146.875 ± 9.375a | K |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Minerals (μg g−1 extract) | ||
| 66.600 ± 0.012b | 11.940 ± 0.001a | Cu |
| 11.600 ± 0.003b | 284.000 ± 0.000a | Zn |
| 2.998 ± 0.010b | 254.000 ± 0.002a | Se |
| 22.000 ± 0.004b | 3000.000 ± 0.001a | Ca |
| 20.000 ± 0.059b | 7.016 ± 0.877a | P |
In vitro antioxidant activity
The graphs III–V in Fig. 1 clearly showed the potent antioxidant activities of TVE and BH more than ALD. The figure also demonstrated that the potency of TVE was more than BH. In comparison with the standard antioxidant (BHT), TVE exhibited similar ABTS+ radical cation scavenging activity, but BH demonstrated less activity. Regarding the potency of the β-carotene-linoleate bleaching, TVE and BH had activities lower than BHT. These results confirm our previously published data that revealed the anti-radical and anti-lipid peroxidation potentials of TVE.15 In addition, the antioxidant activity of Egyptian39 and Indian40 TV and BH41 were previously confirmed.On the other hand, TV–BH showed the synergistic bleaching ability to the β-carotene-linoleate and synergistic scavenging activity for the ABTS+ radical with higher potency compared to ALD. Using Chou–Talalay analysis, the CI graph was plotted (Fig. 1VI and VII) and all the CI values were less than 1 (synergistic effect).
The antioxidant effect of TVE and BH may be related to their constituents, including phenolic compounds and vitamin C (Table 1). The highest content of these compounds in TVE gave it the superior antioxidant activities. Phenolic compounds are the most important and potent antioxidants in plants and other foods that protect from the oxidative stress damage and decrease the risk of chronic diseases. The anthocyanins and flavonols content of TVE are important functional food constituents with potent antioxidant activity.42 In addition, the identified phenolic acids in both single extracts (Table 1) were reported previously as potent ROS scavengers.43 Also, the presence of vitamin C with the phenolic compounds in the studied extracts can synergistically amplify their antioxidant potential.42,44 This action helps in more scavenging and detoxification of the ROS and decreases its level.
The combination of food extracts may help in the elevation of the antioxidant power of the mixture more than the single extracts.15,45 This may be related to specific kinds of interactions occurred between the constituents of the single food extracts.46 Therefore, the interaction between TV and BH constituents after combination enhanced the antioxidant capability of the combined extract (TV–BH). However, the exact interaction to which this effect owed to need further investigations.
Bone cell viability and cytotoxic effect of the studied single and combined extracts
The results showed that TVE, BH, and TV–BH had the same safety effect on the bone cells and they were safer than ALD. The values of the EC100 were 4.656 ± 0.141, 4.508 ± 0.277, 5.059 ± 0.066, and 1.007 ± 0.007 mg ml−1, respectively. The values of IC50 as obtained from the MTT assay were 1.500 ± 0.026, 0.749 ± 0.003, 1.459 ± 0.003, and 0.026 ± 0.018 mg ml−1, respectively. These values have significantly (p < 0.05) differed from each other and mean that the studied single and combined extracts are less cytotoxic than ALD on the bone cells. These properties increase the nutritional value of the studied extracts and gave them great public health importance.Improvement of the HC-induced bone turnover disturbance by the studied single and combined extracts
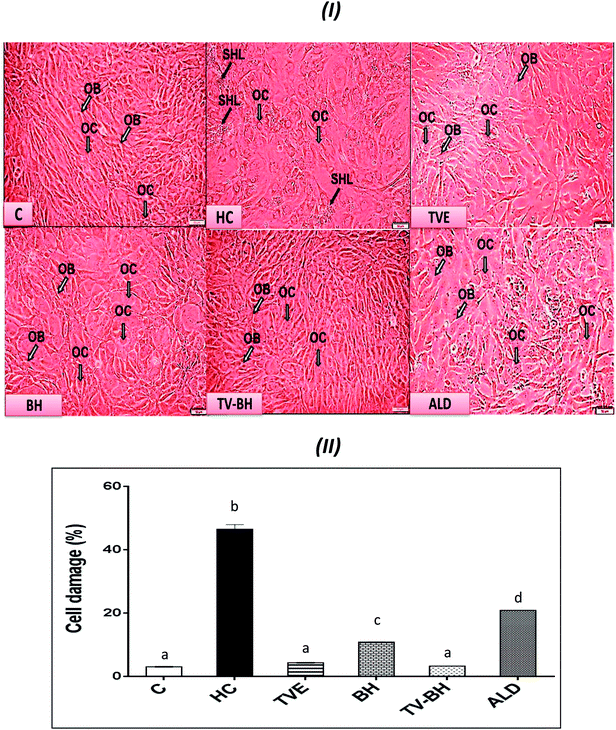
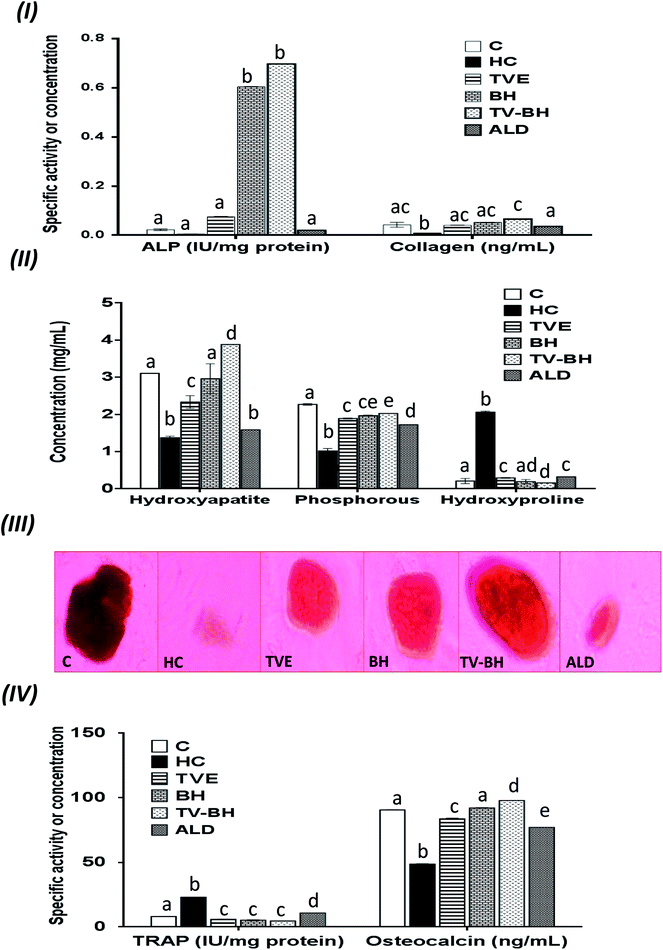
In the current study, HC was able to induce OP in the bone cells at the concentration of 9.167 ± 2.123 mg ml−1 by disrupting the bone homeostasis. This concentration represents the average value of the IC50 values for all the studied bone markers at different HC concentrations (Table 2). The results revealed that the order of the bone markers inhibition or depletion by HC was the collagen > osteocalcin > ALP and hydroxyproline > P, hydroxyapatite, and TRAP. Fig. 2 showed the degree of the bone cell damage after their exposure to the HC and the studied treatments. The morphology of the bone cells (Fig. 2I) after HC treatment showed a sharp decrease in the osteoblasts and increase in the osteoclasts. In addition, the degree of the bone cell damage as indicated by PI staining (Fig. 2II) was highly significantly (p < 0.05) elevated over the untreated control cells. Fig. 3 showed that HC significantly (p < 0.05) down-regulated all the tested bone formation markers and up-regulated all the tested bone resorption markers. These results indicated that the exposure of the bone cells to HC induced disturbance in the bone homeostasis and increased cell damage. This means that HC at the concentration of about 9 mg ml−1 able to induce OP in the bone cells. The results are in line with the previous study of Xie et al.47| Hydroxyproline | TRAP | Collagen | ALP | P | Osteocalcin | Hydroxyapatite | Cell damage | Bone marker |
|---|---|---|---|---|---|---|---|---|
| a Results are expressed as mean ± SE (n = 3). Different letters for the bone markers are significantly different at p < 0.05. ALP, alkaline phosphatase; P, phosphorous; TRAP, tartrate-resistant acid phosphatase; IC50, hydrocortisone concentration that caused 50% cell damage or (inhibition or depletion) for the bone marker. | ||||||||
| 9.332 ± 0.017c | 17.175 ± 0.190d | 1.176 ± 0.153a | 7.117 ± 1.081c | 14.606 ± 0.265d | 4.040 ± 0.299b | 15.562 ± 0.612d | 4.330 ± 1.528 | IC50 (mg ml−1) |
The treatment of these damaged cells with TVE or TV–BH relieved the bone cells damage through decrease the osteoclasts and increase the osteoblasts numbers (Fig. 2I and II). Results also showed that BH had less improving effect for the bone cell damage. However, the healing effects of all the studied extracts were significantly (p < 0.05) superior to ALD. Also, the treatment with either extract significantly (p < 0.05) able to increase the bone formation markers and decrease the bone resorption markers as compared to the HC-treated cells (Fig. 3). The enhancing potency of both extracts for the bone turnover parameters was more than ALD. On the other hand, the treatment with TV–BH extract exhibited a synergistic improving effect for the bone turnover parameters (CI < 1, Table 3) except for ALP (CI > 1, antagonistic effect). These results agree with the previous study that was conducted on the combined extract from Egyptian TV with other herbs.14
| Effect | (CI)a | Parameters |
|---|---|---|
| a CI (combination index) value of <1 means synergistic effect; >1 means antagonistic effect; = 1 means additive effect. ALP; alkaline phosphatase, TRAP; tartrate-resistant acid phosphatase, NO; nitric oxide, NF-κB; nuclear factor kappa B, TNF- α; tumor necrosis factor-α, SOD; superoxide dismutase, GPX; glutathione peroxidase. | ||
| Bone turnover markers | ||
| Synergistic | 0.572 | Hydroxyapatite (mg ml−1) |
| Synergistic | 0.870 | Osteocalcin (ng ml−1) |
| Synergistic | 0.807 | Phosphorous (mg ml−1) |
| Antagonistic | 2.931 | ALP activity (IU mg−1 protein) |
| Synergistic | 0.767 | Collagen (ng ml−1) |
| Synergistic | 0.817 | TRAP activity (IU mg−1 protein) |
| Synergistic | 0.922 | Hydroxyproline (mg ml−1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Inflammatory mediators | ||
| Synergistic | 0.114 | NO (nmol ml−1) |
| Synergistic | 0.000 | COX-2 (expression fold) |
| Synergistic | 0.000 | NF-κB (expression fold) |
| Synergistic | 0.313 | TNF-α (ng ml−1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Oxidative stress parameters | ||
| Synergistic | 0.278 | Lipid peroxidation (nmoL ml−1) |
| Synergistic | 0.421 | GSH (nmoL ml−1) |
| Synergistic | 0.945 | SOD (IU mg−1 protein) |
| Synergistic | 0.381 | GPX (IU mg−1 protein) |
Excess glucocorticoids induce an imbalance between the osteoblasts and osteoclasts by decrease osteoblasts proliferation and lifespan and increase the osteoclasts lifespan and formation. This will increase the bone resorption and suppress the bone matrix formation. Therefore, the synthesis of osteocalcin, ALP, and collagen (Fig. 3I and IV) by osteoblasts was decreased after exposure the bone cells to the HC. This may cause depletion of the hydroxyapatite and phosphorus content (Fig. 3II and III) due to the importance of these proteins in bone mineralization. Since ALP is an essential enzyme promotes the initial phase of bone mineralization by decreasing the pyrophosphate concentration and increasing the inorganic phosphate concentration. However, osteocalcin can bind strongly to the hydroxyapatite and deposited it in a matrix using collagen fibrils as a template.2 In addition, the elevation in the osteoclasts numbers caused secretion of the high amount of TRAP. This enzyme is elevated in OP and helps in the generation of ROS to destroy collagen and other bone matrix proteins. Also, it promotes the attachment of the integrin receptors on the membranes of osteoclasts with the bone matrix for digestion and resulted in the formation of the saucer-shaped Howship's lacunae “SHL” (Fig. 2I). Digestion of bone matrix collagen protein leads to the depletion of its content in bone cells and elevation of the hydroxyproline level.2,48
The presence of many anti-osteoporotic agents in TVE and BH such as vitamin C and K, proline, minerals, and phytochemicals (Table 1) enable them to improve the bone damage that induced by HC. Vitamin C plays vital roles in collagen synthesis, osteoblasts regeneration, and inhibits osteoclasts formation.28 As a result, the synthesis of the bone extracellular matrix components by osteoblasts such as ALP, collagen, and osteocalcin was increased in TVE- and BH-treated cells. While the digestion of collagen and other bone matrix proteins, hydroxyproline level, and the synthesis of TRAP by osteoclasts were decreased. This means that the treatment with either TVE or BH returns the bone cells homeostasis. Also, the presence of considerable amounts of proline in TVE and BH helps in the synthesis of new collagen protein and improved its content in bone cells. The Ca and P content in TVE and BH (Table 1) is necessary to the hydroxyapatite crystals formation and bone calcification.7 Therefore, the treatment of the damaged bone cells with these extracts led to the elevation of P and hydroxyapatite levels as compared with the HC-treated cells. These findings are in accordance with the previous studies that were examined the Egyptian TV49 and BH.50 Zn is another essential element in the studied extracts; its anti-osteoporotic effect can be supported by an elevation in the bone mineralization and reduce the osteoclast resorption activities.51 TVE and BH also contain vitamin K which is a crucial vitamin for bone mineralization2 and synthesis of bone matrix proteins.28 Furthermore, the presence of anthocyanins, flavonols, rutin, and phenolic acids in the studied extracts (Table 1) enable them to protect the bone cells from the HC-induced bone resorption.10–13,52
Regarding the combination between TVE and BH, such combination maximized (synergistic action) the increase in the bone cell formation and the reduction in the bone cell resorption markers (Table 3). This may be owed to the interaction between the different constituents in both extracts including phenolic compounds, minerals, vitamins, and others.
Reduction of the HC-induced oxidative stress in bone cells by the studied single and combined extracts
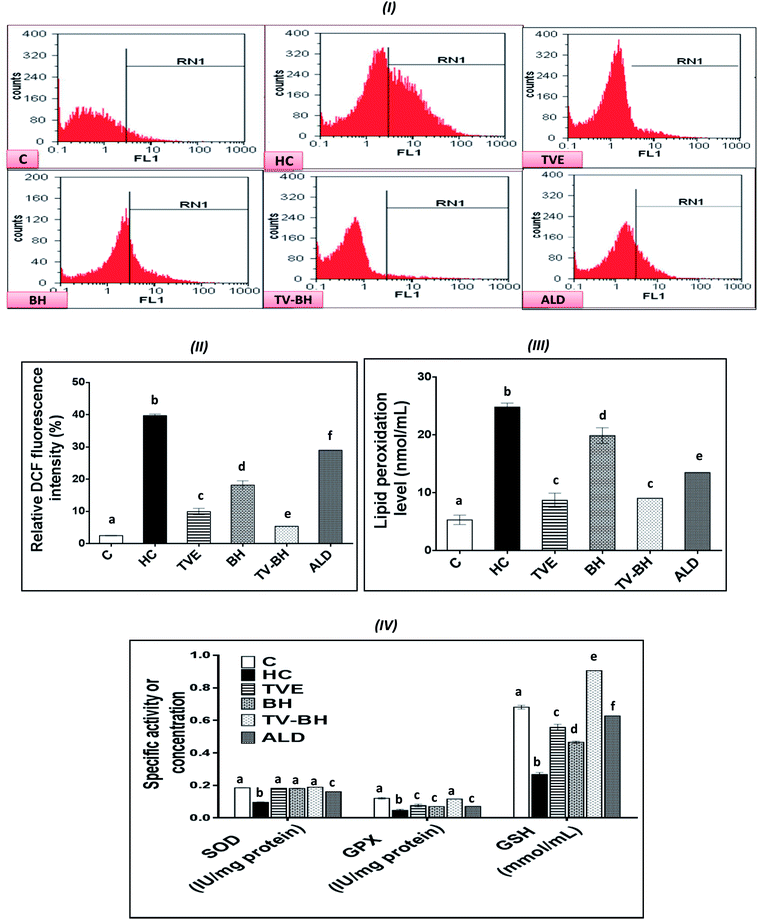
The graphs in Fig. 4 showed that HC induced significant (p < 0.05) overproduction of ROS (Fig. 4I and II) and lipid peroxidation (Fig. 4III) as compared to the control. Also, it induced a significant (p < 0.05) reduction in the level of GSH and the activities of both SOD and GPX (Fig. 4IV). These results indicated that HC induced oxidative stress in bone cells. The balance between ROS and the redox system in bone cells was efficiently preserved after the treatment with TVE and BH. This occurred through significant (p < 0.05) depletion in the intracellular ROS and lipid peroxidation levels and significant (p < 0.05) elevation in the antioxidant enzymes activities. The data revealed the higher antioxidant potency of TVE than BH while both extracts showed higher or similar antioxidant power as compared to the ALD. These results confirmed our previous data which elucidated the ability of TVE to prevent the LPS-induced oxidative stress in the white blood cells.15 Also, the ability of BH to suppress the oxidative stress in vivo was reported previously by Halawa et al.53 Moreover, the present study demonstrated the synergistic (CI < 1) combination of TVE and BH in the reduction of the oxidative stress in bone cells (Table 3). Hence, TV–BH reduced the ROS and lipid peroxidation levels that seemed much narrower than the untreated control group and normalized SOD and GPX activities with potency significantly (p < 0.05) greater than ALD.Glucocorticoids were reported to induce oxidative stress in collagen-producing tissues, such as skin, bone, and tendons in a time and dose-dependent manner and this will involve in their toxicity. This damage occurred by increasing the mitochondrial oxidation and production of the high amount of superoxide radicals. The flow cytometric analysis of the intracellular ROS in bone cells confirms the excessive ROS production after HC treatment (Fig. 4I and II) which produced extreme cellular damage. When the ROS level overwhelms the cellular scavenging ability, cells may undergo necrosis or apoptosis to severe the mitochondrial damage as observed in Fig. 2II. Overproduction of ROS may also result in consumption and depletion of the GSH level in the bone cells.44 In addition, SOD, the first line of defense in the cellular antioxidant defense system,34 can be inhibited and exhausted by ROS (Fig. 4IV). Further reduction in the SOD activity can be happened due to the overproduction of the hydrogen peroxide as a result of the HC-induced inhibition of the GPX biosynthesis.54 GPX activity can also be decreased due to the GSH depletion (Fig. 4IV) and the elevation of NO production (Fig. 5I) in bone cells. The decrease in SOD, GPX, and GSH levels with ROS overproduction in HC-treated cells will affect the cellular biomolecules especially lipids causing lipid peroxidation (Fig. 4III). This will induce disturbance and alteration in the biomembranes fluidity, permeability, and integrity, leading finally to their functional loss.44 Oxidative stress plays a role in the initiation and progression of OP by inducing the imbalance between the osteoblastic and osteoclastic activities.3
The treatment with TVE and BH significantly (p < 0.05) decreased the intracellular ROS level, which revealed the potent scavenging ability of these extracts and confirms their in vitro TAC and antiradical activities (Fig. 1III–V). This led to increase in the redox state of the cell and protect the cellular biomolecules from the ROS damage effects. As a result, the lipid peroxidation level was reduced as well as the antioxidant enzymes and GSH were preserved in the TVE- and BH-treated bone cells. TVE showed higher antioxidant potency than BH which might be owed to its ingredients. These healing effects of the studied extracts may be owed to their constituents such as phenolics44 and minerals (Table 1).55,56 The prevention of the oxidative stress and enhancing the antioxidant defense system in bone cells may lead to inhibition of the bone resorption. Thus, the anti-osteoporotic effect of the studied extracts and their abilities to suppress the HC-induced excessive bone damage could be owed to their antioxidant activities.
The synergistic activity of the TV–BH may be owed to its synergistic TAC and antiradical activities (Fig. 1III–V). This effect probably related to the interaction or the co-existence of the single extracts ingredients and was in line with our recently published results.15
Suppression of the HC-induced inflammation by the studied single and combined extracts
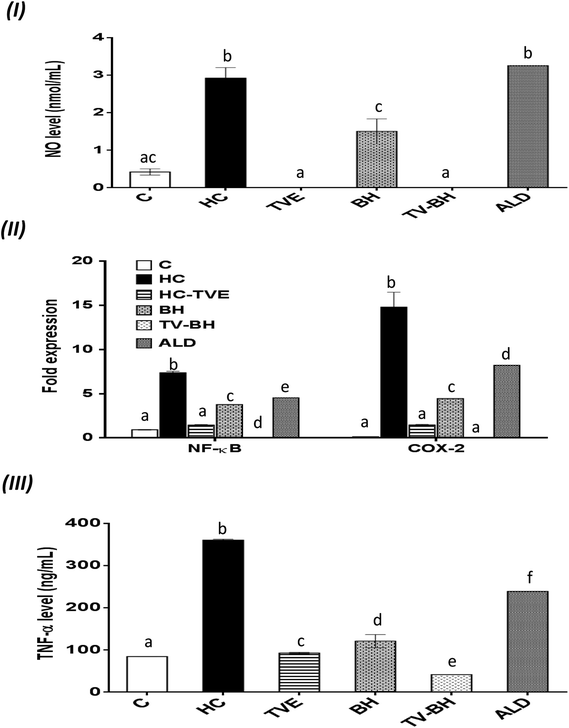
The oxidative stress and the overproduction of ROS in bone cells will signify the bone damage by activation of the inflammatory genes in the cells. The results in Fig. 5 showed that HC induced significant (p < 0.05) up-regulation of the NF-κB and COX-2 gene expression and the NO and TNF-α protein levels. The treatment with TVE or BH suppressed the production of these inflammatory mediators. Hence, BH significantly (p < 0.05) depleted the level of NO, and the expression of NF-κB, and COX-2 while TVE normalized them (Fig. 5I and II). Also, TNF-α protein level was significantly (p < 0.05) decreased after the treatment with these tested extracts (Fig. 5III). However, ALD significantly (p < 0.05) suppressed these mediators except the NO level with potency less than TVE or BH. Regarding the combined extract (TV–BH), it exerted synergistic anti-inflammatory activities of all the studied inflammatory mediators (CI < 1, Table 3). The potency of this combined extract was significantly (p < 0.05) more than ALD.The improved level of ROS due to the oxidative stress state that provided in the bone cells by HC may be the reason for the induction of NF-κB gene expression. This pathway amplifies the inflammatory response through enhancing the gene expression of other inflammatory mediators such as iNOS, COX-2, and TNF-α. These inflammatory molecules stimulate more oxidative stress within the cell.57 The secretion of inflammatory mediators by bone cells and induction of inflammation are common in OP disease. These mediators act as another cause for bone resorption through activation of the osteoclastogenesis and decrease bone formation.2
The potent antioxidant effect of the TVE or BH helps in the reduction of the inflammatory mediators in the bone cells. This may be attributed to their pivotal roles in the scavenging and reduction of the ROS (Fig. 4I and II), the major inducer of the NF-κB pathway. In addition, the phenolic52 and minerals56 content (Table 1) of these tested extracts may enhance their anti-inflammatory activities. The ability of TVE and BH to control the NF-κB pathway and its related inflammatory mediators gives them a powerful therapeutic potential for the OP and other chronic inflammatory diseases. The superior anti-inflammatory role of the TVE to the BH may be related to its higher antioxidant activities (Fig. 1III–V). In agreement with these findings, our recently published data demonstrated the ability of TVE to decrease the LPS-induced inflammation in the white blood cells.15 Moreover, the anti-inflammatory effect of the Indian TV40 and BH41 was studied previously.
Although this highly anti-inflammatory efficiency of the studied single extracts, the combination of them maximized their actions (synergistic effect). This may be owed to the effect of their components while the exact mechanisms that involved in this synergism need for further investigations.
Conclusion
This work elucidates that TVE more than BH had an anti-osteoporotic effect in the HC-induced bone damage. The combination of both extracts (TV–BH) maximizes this role and therefore it represents a promising therapeutic alternative to avoid the glucocorticoid-induced OP. This potential effect seems to be mediated by the potent antioxidant and anti-inflammatory activities as well as the direct effect of this combined extract on the bone cell homeostasis. In addition, TV–BH can be used instead of BP drugs due to its safe and most effective role in the bone cells.Conflicts of interest
Authors have no conflicts of interest to declare.Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.Abbreviations
| ABTS | 2,2-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid |
| ALD | Alendronate |
| ALP | Alkaline phosphatase |
| BH | Bee's honey |
| BHT | Butylated hydroxytoluene |
| BPs | Bisphosphonates |
| CI | Combination index |
| COL 4 | Collagen type IV |
| COX-2 | Cyclooxygenase-2 |
| Cy-3-glc | Cyanidin-3-glucoside |
| DCFH-DA | 2,7-Dichlorofluorescin diacetate |
| DMEM | Dulbecco's modified Eagle medium |
| 2,4 DNPH | 2,4 Dinitrophenylhydrazine |
| DR | Degradation rate |
| FBS | Fetal bovine serum |
| GPX | Glutathione peroxidase |
| GSH | Reduced glutathione |
| HC | Hydrocortisone |
| 4-HCA | 4-Hydroxycinnamic acid |
| MEM | α-Minimum essential medium Eagle |
| MTT | 3-(4,5-Dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide |
| NF-κB | Nuclear factor-kappa B |
| 4-NPP | 4-Nitrophenyl phosphate |
| OP | Osteoporosis |
| PI | Propidium iodide |
| PIC | Protease inhibitor cocktail |
| QR | Quercetin |
| ROS | Reactive oxygen species |
| RPMI-1640 | Roswell Park Memorial Institute |
| SDS | Sodium dodecyl sulfate |
| SHL | Saucer-shaped Howship's lacunae |
| SOD | Superoxide dismutase |
| TBA | Thiobarbituric acid |
| TBARS | TBA reactive substances |
| TNF-α | Tumor necrosis factor |
| TRAP | Tartrate-resistant acid phosphatase |
| TV | Thymus vulgaris |
References
- M. T. Valenti, L. D. Carbonare and M. Mottes, Int. J. Mol. Sci., 2017, 18, 1–9 CrossRef PubMed.
- U. Kini and B. N. Nandeesh, in Radionuclide and Hybrid Bone Imaging, 2012, vol. 9783642024009, pp. 29–57 Search PubMed.
- M. Abdollahi, B. Larijani, R. Rahimi and P. Salari, Therapy, 2005, 2, 787–796 CrossRef.
- C. McGreevy and D. Williams, Ther. Adv. Drug Saf., 2011, 2, 159–172 CrossRef PubMed.
- N. S. Carvalho, M. M. Silva, R. O. Silva, L. A. D. Nicolau, T. S. L. Araujo, D. S. Costa, N. A. Sousa, L. K. M. Souza, P. M. G. Soares and J. V. R. Medeiros, Dig. Dis. Sci., 2015, 61, 400–409 CrossRef PubMed.
- A. Zarezadeh, M. Jannati, M. Mirza and I. S. Ashourabadi, World Appl. Sci. J., 2013, 27, 1427–1430 Search PubMed.
- H. Javed, S. Erum, S. Tabassum and F. Ameen, Journal of Asian Scientific Research, 2013, 3, 974–982 Search PubMed.
- S. K. Saikaly and A. Khachemoune, Am. J. Clin. Dermatol., 2017, 18, 237–251 CrossRef PubMed.
- C. E. Manyi-Loh, A. M. Clarke and R. N. Ndip, Afr. J. Microbiol. Res., 2011, 5, 844–852 Search PubMed.
- L. G. Rao, N. Kang and A. V. Rao, Phytochemicals - A Global Perspective of Their Role in Nutrition and Health, 2012, vol. 52, pp. 467–476 Search PubMed.
- X. Zeng, J. Tian, L. Cui, Y. Wang, Y. Su, X. Zhou and X. He, Molecules, 2014, 19, 263–278 CrossRef PubMed.
- B. T. T. Luyen, B. H. Tai, N. P. Thao, S. H. Lee, H. D. Jang, Y. M. Lee and Y. H. Kim, J. Korean Soc. Appl. Biol. Chem., 2014, 57, 573–579 CrossRef.
- J. R. Chen, O. P. Lazarenko, J. Zhang, M. L. Blackburn, M. J. J. Ronis and T. M. Badger, J. Bone Miner. Res., 2014, 29, 1043–1053 CrossRef PubMed.
- M. M. Elkomy and F. G. Elsaid, J. Basic Appl. Zool., 2015, 72, 81–88 CrossRef.
- N. H. Habashy, M. M. Abu Serie, W. E. Attia and S. A. M. Abdelgaleil, J. Funct. Foods, 2018, 40, 317–328 CrossRef.
- M. S. Taga, E. E. Miller and D. E. Pratt, J. Am. Oil Chem. Soc., 1984, 61, 928–931 CrossRef.
- J. Zhishen, T. Mengcheng and W. Jianming, Food Chem., 1999, 64, 555–559 CrossRef.
- M. Mónica Giusti and R. E. Wrolstad, in Handbook of Food Analytical Chemistry, 2005, vol. 2–2, pp. 19–31 Search PubMed.
- A. Kumaran and R. J. Karunakaran, LWT--Food Sci. Technol., 2007, 40, 344–352 CrossRef.
- A. Pitzschke, A. Fraundorfer, M. Guggemos and N. Fuchs, J. Food Sci. Nutr., 2015, 3, 242–251 CrossRef PubMed.
- S. T. Omaye, J. David Turnbull and H. E. Sauberlich, Methods Enzymol., 1979, 62, 3–11 Search PubMed.
- M. D. Rockville, United States Pharmacopeia Convention, USP United States Pharmacop., 2003, p. 1445 Search PubMed.
- S. N. Tyagi, A. Saxena and B. D. Patel, Am.-Eurasian J. Sci. Res., 2010, 5, 201–206 Search PubMed.
- J. C. M. Barreira, I. C. F. R. Ferreira, M. B. P. P. Oliveira and J. A. Pereira, Food Chem., 2008, 107, 1106–1113 CrossRef.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 CrossRef PubMed.
- M. M. Wong, L. G. Rao, H. Ly, L. Hamilton, S. Ish-Shalom, W. Sturtridge, J. Tong, R. McBroom, R. G. Josse and T. M. Murray, Osteoporosis Int., 1994, 4, 21–31 CrossRef.
- C. A. Gregory, W. G. Gunn, A. Peister and D. J. Prockop, Anal. Biochem., 2004, 329, 77–84 CrossRef PubMed.
- M. S. Burstone, J. Natl. Cancer Inst., 1958, 21, 523–538 Search PubMed.
- G. K. Reddy and C. S. Enwemeka, Clin. Biochem., 1996, 29, 225–229 CrossRef PubMed.
- S. Simizu, M. Imoto, N. Masuda, M. Takada and K. Umezawa, Cancer Res., 1996, 56, 4978–4982 Search PubMed.
- H. Ohkawa, N. Ohishi and K. Yagi, Anal. Biochem., 1979, 95, 351–358 CrossRef PubMed.
- G. L. Ellman, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef PubMed.
- J. T. Rotruck, A. L. Pope, H. E. Ganther, A. B. Swanson, D. G. Hafeman and W. G. Hoekstra, Science, 1973, 179, 588–590 CrossRef PubMed.
- S. Marklund and G. Marklund, Eur. J. Biochem., 1974, 47, 469–474 CrossRef PubMed.
- L. Marcocci, J. J. Maguire, M. T. Droylefaix and L. Packer, Biochem. Biophys. Res. Commun., 1994, 201, 748–755 CrossRef PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef PubMed.
- J. R. Zhou, L. Li and W. Pan, Am. J. Clin. Nutr., 2007, 86, 882S–888S CrossRef PubMed.
- T.-C. Chou, Pharmacol. Rev., 2006, 58, 621–681 CrossRef PubMed.
- M. A. Hamzawy, E. S. M. El-Denshary, N. S. Hassan, F. Manaa and M. A. Abdel-Wahhab, Global J. Pharmacol., 2012, 6, 106–117 Search PubMed.
- M. N. Beidokhti and H. S. Prakash, Int. J. Pharm. Pharm. Sci., 2013, 5, 100–104 Search PubMed.
- N. G. Vallianou, P. Gounari, A. Skourtis, J. Panagos and C. Kazazis, Gen. Med.: Open Access, 2014, 2, 132 Search PubMed.
- D. M. Kasote, S. S. Katyare, M. V. Hegde and H. Bae, Int. J. Biol. Sci., 2015, 11, 982–991 CrossRef PubMed.
- F. Shahidi and P. Ambigaipalan, J. Funct. Foods, 2015, 18, 820–897 CrossRef.
- P. Sharma, A. B. Jha, R. S. Dubey and M. Pessarakli, J. Bot., 2012, 2012, 1–26 CrossRef.
- M. M. Abu Serie, N. H. Habashy and W. E. Attia, BMC Complementary Altern. Med., 2018, 18, 154–167 CrossRef PubMed.
- S. Wang, K. A. Meckling, M. F. Marcone, Y. Kakuda and R. Tsao, J. Agric. Food Chem., 2011, 59, 960–968 CrossRef PubMed.
- H. Xie, T. Wu, L. Huang and C. Li, Zhongguo Zhongyao Zazhi, 1997, 22, 238–240 Search PubMed.
- A. Hayman, Autoimmunity, 2008, 41, 218–223 CrossRef PubMed.
- A. Salem, Int. J. Nutr. Food Sci., 2015, 4, 509–517 CrossRef.
- S. S. M. Zaid, S. A. Sulaiman, K. N. M. Sirajudeen and N. H. Othman, BMC Complementary Altern. Med., 2012, 10, 82 CrossRef PubMed.
- K. B. Hadley, S. M. Newman and J. R. Hunt, J. Nutr. Biochem., 2010, 21, 297–303 CrossRef PubMed.
- C. L. Shen, V. von Bergen, M. C. Chyu, M. R. Jenkins, H. Mo, C. H. Chen and I. S. Kwun, Nutr. Res., 2012, 32, 897–910 CrossRef PubMed.
- H. Halawa, N. El-Nefiaw, N. A. Makhlouf and A. A. Mady, J. Arab Soc. Med. Res., 2009, 4, 197–208 Search PubMed.
- A. Orzechowski, P. Ostaszewski, A. Brodnicka, J. Wilczak, M. Jank, B. Balasińska, K. Grzelkowska, T. Ploszaj, J. Olczak and A. Mrówczyńska, Horm. Metab. Res., 2000, 32, 174–180 CrossRef PubMed.
- Q. Zhang, L. Chen, K. Guo, L. Zheng, B. Liu, W. Yu, C. Guo, Z. Liu, Y. Chen and Z. Tang, Biol. Trace Elem. Res., 2013, 154, 255–261 CrossRef PubMed.
- S. A. E. M. Bashandy, E. A. A. Omara, H. Ebaid, M. M. Amin and M. S. Soliman, Asian Pac. J. Trop. Biomed., 2016, 6, 1056–1064 CrossRef.
- M. J. Morgan and Z. Liu, Cell Res., 2011, 21, 103–115 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |