Open Access Article
Open Access ArticleNeutral two-dimensional organometallic–organic hybrid polymers based on pentaphosphaferrocene, bipyridyl linkers and CuCl†
M.
Elsayed Moussa
a,
B.
Attenberger
a,
E. V.
Peresypkina
 bc and
M.
Scheer
bc and
M.
Scheer
 *a
*a
aInstitut für Anorganische Chemie der Universität Regensburg, 93040 Regensburg, Germany. E-mail: manfred.scheer@chemie.uni-r.de
bNikolaev Institute of Inorganic Chemistry, Siberian Division of RAS, Acad. Lavrentyev prosp. 3, 630090 Novosibirsk, Russia
cNovosibirsk State University, ul. Pirogova, 2, 630090 Novosibirsk, Russia
First published on 8th December 2017
Abstract
The reaction of the Pn ligand complex [Cp*Fe(η5-P5)] (1: Cp* = η5-C5Me5) with CuCl in the presence of 4,4′-bipyridine or 1,2-di(4-pyridyl)ethylene leads to the formation of three unprecedented neutral 2D organometallic–organic hybrid networks, the constitutional isomers [Cu2Cl2{Cp*Fe(μ3,η5:1:1-P5)}(μ,η1:1-C10H8N2)]n (2 and 3) and the coordination polymer [(CuCl)2{Cp*Fe(μ3,η5:1:1-P5)}(μ,η1:1-C12H10N2)]n (4) with isomeric square (2 and 3) and honeycomb (4) layer topologies.
In recent years, coordination polymers (CPs) have received great attention due to their high structural diversity associated with a wide variety of chemical and physical properties.1 These compounds are generally assembled via the coordination of multitopic organic linkers usually bearing N, O or S donor atoms to metal ions or clusters.2a–d Due to the lack of using organometallic building blocks in this field,2e–g our group developed an alternative concept by using organometallic polyphosphorus and polyarsenic ligand complexes with flexible coordination modes as organometallic connectors between metal ions allowing the formation of 1D, 2D and 3D CPs.3 Moreover, one of those polyphosphorus ligand complexes, the tetrahedrane complex [Cp2Mo2(CO)4(η2-P2)] (Cp = C5H5) (A) was reacted with AgI and CuI salts of the weakly coordinating anions (WCA) Al{OC(CF3)3}4−, and FAl{OC(C6F5)(C6F10)}3− and the anion BF4−, in the presence of bipyridyl linkers to give unprecedented organometallic–organic hybrid CPs.4 However, the cavities formed in the polycationic chains of these networks are tightly occupied by anions, making them rather unattractive candidates for possible applications as for instance gas storage purposes.
Otherwise, the pentaphosphaferrocene [Cp*Fe(η5-P5)] (1: Cp* = η5-C5Me5)5 reacts with CuI halides depending on the reaction conditions to different 1D and 2D polymers,3e,h,i nano-sized fullerene-like spherical aggregates6 and a huge organometallic-based capsule.7 We therefore became interested in exploring the possibility to establish a novel class of organometallic–organic CPs using the cyclo-P5 ligand complex 1 in combination with metal cations and organic linkers. Moreover, we targeted the synthesis of neutral hybrid networks instead of the already developed synthesis of cationic frameworks from the P2 ligand complex A. Obviously, the AgI or CuI salts of WCA are no suitable candidates for such purposes as the voids in the resulting structures can be occupied by the counterions. Therefore, the use of coordinating anions such as Cl− could be useful, as they can coordinate much more strongly to the metal centers leading to targeted networks with substantial (non-occupied) porosity. Herein, we report on the synthesis of the first pentaphosphaferrocene-based 2D neutral polymeric compounds. These unprecedented hybrid CPs are obtained from a three-component reaction of the pentaphosphaferrocene 1 in combination with the bipyridyl linkers 4,4′-bipyridine or 1,2-di(4-pyridyl)ethylene and CuCl. To the best of our knowledge, no other neutral organometallic–organic hybrid CPs has been yet reported.
In a first approach, the cyclo-P5 ligand complex 1 was reacted with CuCl and 4,4′-bipyridine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. This reaction afforded the products 2 or 3 as brown crystals in excellent yields (84% and 75%, respectively, see the ESI†), depending on the used solvent mixture. Compound 2 is obtained when a CH2Cl2 solution of 1 is slowly added to a CH3CN
1 stoichiometry. This reaction afforded the products 2 or 3 as brown crystals in excellent yields (84% and 75%, respectively, see the ESI†), depending on the used solvent mixture. Compound 2 is obtained when a CH2Cl2 solution of 1 is slowly added to a CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (1
CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution mixture of CuCl and 4,4′-bipyridine, while compound 3 is isolated when a toluene solution of 1 is added to a CH3CN
1) solution mixture of CuCl and 4,4′-bipyridine, while compound 3 is isolated when a toluene solution of 1 is added to a CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (1
CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
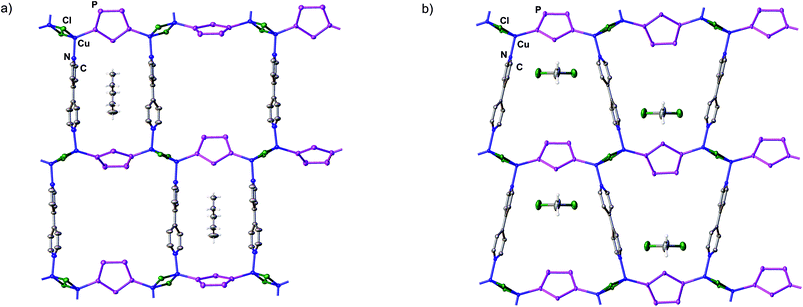
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution mixture of CuCl and 4,4′-bipyridine. The compounds 2 and 3, respectively, crystallize at room temperature from n-pentane (2) or toluene (3) diffusion into the solutions of the crude reaction mixtures, and their structures were examined by X-ray crystallography (see the ESI†). Their crystal structures reveal that the assemblies 2 and 3 represent unprecedented neutral 2D organometallic–organic hybrid polymers with the general formula [Cu2Cl2{Cp*Fe(μ3,η5:1:1-P5)}(μ,η1:1-C10H8N2)]n (Fig. 1).
1) solution mixture of CuCl and 4,4′-bipyridine. The compounds 2 and 3, respectively, crystallize at room temperature from n-pentane (2) or toluene (3) diffusion into the solutions of the crude reaction mixtures, and their structures were examined by X-ray crystallography (see the ESI†). Their crystal structures reveal that the assemblies 2 and 3 represent unprecedented neutral 2D organometallic–organic hybrid polymers with the general formula [Cu2Cl2{Cp*Fe(μ3,η5:1:1-P5)}(μ,η1:1-C10H8N2)]n (Fig. 1).
Interestingly, the polymers 2 and 3 are constitutional isomers with a planar layered constitution providing different types of rings in the 2D polymeric arrangements (Fig. 1). Small (with a maximum dimension of 1.14 nm)8 and large (with a maximum dimension of 1.51 nm)8 rectangular rings in 2 and one-type trapezoidal rings (with a maximum dimension 1.34 nm)8 in 3 appear, depending on the coordination of the 4,4′-bipyridine linkers to one or the other Cu ion of the Cu2Cl2 dimer. In both kinds of these isomeric square (sql) layers 2 and 3, two [Cu2Cl2] four-membered rings are bridged by a pentaphosphaferrocene molecule with the cyclo-P5 ligand adopting the 1,3-bridging mode and forming infinite chains. These chains are in turn connected via the 4,4′-bipyridine linkers coordinated to the copper atoms to give the final 2D structures. Consequently, each copper ion in 2 and 3 possesses a distorted tetrahedral coordination geometry (2 Cl + 1 P + 1 N). The 4,4′-bipyridine linkers in the polymers 2 and 3 are well separated from each other (from ∼7.9 Å in 2 up to ∼11.8 Å in 3) and therefore do not show any intramolecular π–π stacking interactions. The P–P bond lengths in 2 and 3 are in the range between 2.101(3) and 2.120(5) Å, which is essentially consistent with those of the non-coordinated complex 1 (2.117 Å).9 The Cu–P bond lengths in 2 and 3 are in the range between 2.173(5) and 2.184(5) Å.
In the crystal structure of network 2, one n-pentane molecule is embedded in each of the small cavities of the rings and only partially occupies these cavities. These guest molecules are stabilized by CH⋯π interactions between the methylene hydrogens of the n-pentane molecules and the π clouds of the pyridyl moieties of the 4,4′-bipyridine linker (CH⋯π (pyridyl centroid) distance is 3.097(9) Å). Similarly, one CH2Cl2 molecule is partially embedded in each of the cavities of the rings of 3. These CH2Cl2 molecules are stabilized by the Cl⋯π interactions between the chlorine atoms of the CH2Cl2 molecules and the π clouds of the pyridyl moieties of the 4,4′-bipyridine linker (Cl⋯π (pyridyl centroid) distances are 3.318(4)–3.498(2) Å).
These results motivated us to expand these investigations by using the slightly longer linker 1,2-di(4-pyridyl)ethylene. The reaction of 1 with CuCl and 1,2-di(4-pyridyl)ethylene in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry leads to the formation of compound 4 as brown blocks in moderate yield (40%, see the ESI†) suitable for single crystal X-ray structure analysis. Compound 4 is a novel neutral 2D organometallic–organic hybrid polymer of the formula [(CuCl)2{Cp*Fe(μ3,η5:1:1-P5)}2(μ,η1:1-C12H10N2)]n. The stoichiometry of the building blocks in the resulting polymer 4 is different from that of the used starting materials. Polymer 4 is also obtained in a similar yield upon using a 2
1 stoichiometry leads to the formation of compound 4 as brown blocks in moderate yield (40%, see the ESI†) suitable for single crystal X-ray structure analysis. Compound 4 is a novel neutral 2D organometallic–organic hybrid polymer of the formula [(CuCl)2{Cp*Fe(μ3,η5:1:1-P5)}2(μ,η1:1-C12H10N2)]n. The stoichiometry of the building blocks in the resulting polymer 4 is different from that of the used starting materials. Polymer 4 is also obtained in a similar yield upon using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of 1, CuCl and 1,2-di(4-pyridyl)ethylene. In 4, the [CuCl] units are connected by the organic linkers in contrast to the [Cu2Cl2] rings in 2 and 3 and therefore give honeycomb layers (hcb topology) (Fig. 2). Owing to the doubled stoichiometric amount of pentaphosphaferrocene spacers in 4 as compared to 2 and 3, larger rings are formed with a maximum dimension of 2.24 nm, which are left empty in this solvent-free structure.8 As a result, π–π stacking occurs between the almost parallel Cp* ligands of 1 and the planar 1,2-di(4-pyridyl)ethylene linkers with interplanar distances of 3.47–3.54 Å (cf. ESI†), in contrast to the polymers 2 and 3 where no such π–π stacking is observed.
1 stoichiometric ratio of 1, CuCl and 1,2-di(4-pyridyl)ethylene. In 4, the [CuCl] units are connected by the organic linkers in contrast to the [Cu2Cl2] rings in 2 and 3 and therefore give honeycomb layers (hcb topology) (Fig. 2). Owing to the doubled stoichiometric amount of pentaphosphaferrocene spacers in 4 as compared to 2 and 3, larger rings are formed with a maximum dimension of 2.24 nm, which are left empty in this solvent-free structure.8 As a result, π–π stacking occurs between the almost parallel Cp* ligands of 1 and the planar 1,2-di(4-pyridyl)ethylene linkers with interplanar distances of 3.47–3.54 Å (cf. ESI†), in contrast to the polymers 2 and 3 where no such π–π stacking is observed.
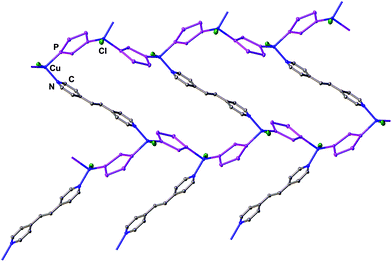 | ||
| Fig. 2 Fragment of a single layer in the neutral 2D polymeric network 4; Cp*FeP5 are represented as cyclo-P5 fragments; H atoms are omitted for clarity. | ||
The P–P bond lengths in 4 are in the range between 2.105(2) and 2.115(8) Å, comparable to those of the non-coordinated ligand 1 and the polymers 2 and 3. The Cu–P bond in 4 (2.210(9) Å) is slightly elongated compared to those in 2 and 3.
The compounds 2–4 are only very slightly soluble in donor solvents such as CH3CN but completely insoluble in other common organic solvents such as CH2Cl2, THF and n-pentane. Their room temperature 31P{1H} NMR spectra in CD3CN exhibit single signals at ca. 151 ppm, which are very similar to that of the free ligand complex 1 (152.2 ppm)3d revealing a degradation of the polymeric structure in solution.
Conclusions
In conclusion, we have shown the possibility of using the pentaphosphaferrocene ligand complex (1) in a three- component reaction with CuCl and the bipyridyl linkers 4,4′-bipyridine or 1,2-di(4-pyridyl)ethylene to synthesize a new class of hybrid CPs. This new reaction allows the synthesis of three unique neutral 2D organometallic–organic hybrid CPs with different layer topologies. These results show the importance of this novel method using Pn ligand complexes for the synthesis of a new class of hybrid CPs. For the first time, the use of CuCl allows the approach to unprecedented neutral organometallic–organic hybrid CPs in which the meshes are only occupied by solvent molecules, in contrast to previously obtained cationic hybrid CPs with the anions in the voids. This finding opens up a new chapter in this chemistry. Current investigations involve the use of multitopic pyridine-based linkers to synthesize neutral 3D organometallic–organic hybrid networks as an analogy to the very well-studied class of neutral 3D metal organic frameworks (MOFs).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was comprehensively supported by the European Research Council by Grant ERC-2013-AdG 339072. The COST action CM 1302 (SIPs) is gratefully acknowledged.Notes and references
- (a) A. Winter and U. S. Schubert, Chem. Soc. Rev., 2016, 45, 5311 RSC; (b) X. Zhang, W. Wang, Z. Hu, G. Wang and K. Uvdal, Coord. Chem. Rev., 2015, 284, 206 CrossRef CAS; (c) C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079 CrossRef CAS PubMed; (d) L. Carlucci, G. Ciani, D. M. Proserpio, T. G. Mitina and V. A. Blatov, Chem. Rev., 2014, 114, 7557 CrossRef CAS PubMed; (e) R. Haldar and T. K. Maji, Angew. Chem., Int. Ed., 2014, 44, 11772 CrossRef PubMed; (f) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed; (g) W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS PubMed.
- (a) E. Lee, H. Ju, S. Kim, K.-M. Park and S. S. Lee, Cryst. Growth Des., 2015, 15, 5427 CrossRef CAS; (b) E. Lee, K.-M. Park, M. Ikeda, S. Kuwahara, Y. Habata and S. S. Lee, Inorg. Chem., 2015, 54, 5372 CrossRef CAS PubMed; (c) R. Charkabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef PubMed; (d) F. A. Cotton, E. V. Dikarev and M. A. Petrukhina, Angew. Chem., Int. Ed., 2001, 40, 1521 CrossRef CAS; (e) P. J. Stang, B. Olenyuk, J. Fan and A. M. Arif, Organometallics, 1996, 15, 904 CrossRef CAS; (f) K. Škoch, I. Císařová and P. Štěpnička, Inorg. Chem., 2014, 53, 568 CrossRef PubMed; (g) K. Škoch, I. Císařová, J. Schulz, U. Siemeling and P. Štěpnička, Dalton Trans., 2017, 46, 10339 RSC.
- Selected publications: (a) M. E. Moussa, M. Fleishmann, E. V. Peresypkina, L. Dütsch, M. Seidl, G. Balázs and M. Scheer, Eur. J. Inorg. Chem., 2017, 25, 3222 CrossRef PubMed; (b) C. Heindl, A. Kuntz, E. V. Peresypkina, A. V. Virovets, M. Zabel, D. Lüdeker, G. Brunklaus and M. Scheer, Dalton Trans., 2015, 44, 6502 RSC; (c) C. Heindl, E. V. Peresypkina, A. V. Virovets, V. Y. Komarov and M. Scheer, Dalton Trans., 2015, 44, 10245 RSC; (d) M. Fleishmann, S. Welsch, E. V. Peresypkina, A. V. Virovets and M. Scheer, Chem. – Eur. J., 2015, 21, 14332 CrossRef PubMed; (e) F. Dielmann, C. Heindl, F. Hastreiter, E. V. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Angew. Chem., Int. Ed., 2014, 53, 13605 CrossRef CAS PubMed; (f) E.-M. Rummel, M. Eckhardt, M. Bodensteiner, E. V. Peresypkina, W. Kremer, C. Gröger and M. Scheer, Eur. J. Inorg. Chem., 2014, 10, 1625 CrossRef; (g) H. Krauss, G. Balázs, M. Bodensteiner and M. Scheer, Chem. Sci., 2010, 1, 337 RSC; (h) M. Scheer, L. J. Gregoriades, A. V. Virovets, W. Kunz, R. Neueder and I. Krossing, Angew. Chem., Int. Ed., 2006, 45, 5689 CrossRef CAS PubMed; (i) J. Bai, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 1737 CrossRef CAS; (j) J. Bai, E. Leiner and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 783 CrossRef CAS.
- (a) M. E. Moussa, M. Seidl, G. Balazs, A. V. Virovets, B. Attenberger, A. Schreiner and M. Scheer, Chem. – Eur. J., 2017, 16199 CrossRef CAS PubMed; (b) M. E. Moussa, B. Attenberger, M. Fleischmann, A. Schreiner and M. Scheer, Eur. J. Inorg. Chem., 2016, 28, 4538 CrossRef PubMed; (c) M. Elsayed Moussa, B. Attenberger, E. V. Peresypkina, M. Fleischmann, G. Balázs and M. Scheer, Chem. Commun., 2016, 52, 10004 RSC; (d) B. Attenberger, E. V. Peresypkina and M. Scheer, Inorg. Chem., 2015, 54, 7021 CrossRef CAS PubMed; (e) B. Attenberger, S. Welsch, M. Zabel, E. Peresypkina and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 11516 CrossRef CAS PubMed.
- (a) O. J. Scherer and T. Brück, Angew. Chem., Int. Ed. Engl., 1987, 26, 59 Search PubMed; (b) O. J. Scherer, T. Brück and G. Wolmershäuser, Chem. Ber., 1988, 121, 935 CrossRef CAS.
- Selected articles: (a) E. V. Peresypkina, C. Heindl, A. V. Virovets and M. Scheer, Struct. Bonding, 2016, 174, 321 CrossRef; (b) C. Heindl, S. Reisinger, C. Schwarzmaier, L. Rummel, A. V. Virovets, E. V. Peresypkina and M. Scheer, Eur. J. Inorg. Chem., 2016, 5, 743 CrossRef PubMed; (c) C. Heindl, E. V. Peresypkina, D. Lüdeker, G. Brunklaus, A. V. Virovets and M. Scheer, Chem. – Eur. J., 2016, 22, 2599 CrossRef CAS PubMed; (d) C. Heindl, E. V. Peresypkina, A. V. Virovets, W. Kremer and M. Scheer, J. Am. Chem. Soc., 2015, 137, 10938 CrossRef CAS PubMed; (e) F. Dielmann, C. Heindl, F. Hastreiter, E. V. Peresypkina, A. V. Virovets, R. M. Gschwind and M. Scheer, Angew. Chem., 2014, 126, 13823 ( Angew. Chem. Int. Ed. , 2014 , 53 , 13605 ) CrossRef; (f) M. Scheer, A. Schindler, R. Merkle, B. P. Johnson, M. Linseis, R. Winter, C. E. Anson and A. V. Virovets, J. Am. Chem. Soc., 2007, 129, 13386 CrossRef CAS PubMed; (g) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781 CrossRef CAS PubMed; (h) C. Heindl, E. Peresypkina, A. V. Virovets, I. S. Bushmarinov, M. G. Medvedev, B. Krämer, B. Dittrich and M. Scheer, Angew. Chem., Int. Ed., 2017, 43, 13237–13243 CrossRef PubMed.
- S. Welsch, C. Groeger, M. Sierka and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 1435 CrossRef CAS PubMed.
- Calculated from the largest distance between the Cu+ ions minus the doubled ionic radius of Cu+ for the coordination number 4 (0.74 Å).
- R. Blom, T. Brück and O. J. Scherer, Acta Chem. Scand., 1989, 43, 458 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of syntheses, characterization and X-ray crystallography. CCDC 1583104–1583106. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt04286h |
| This journal is © The Royal Society of Chemistry 2018 |