Copper-catalyzed highly selective approach to 2-boroallylic silanes from allenylsilanes†
Liu
Song
a,
Weiming
Yuan
b and
Shengming
Ma
 *ac
*ac
aDepartment of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China. E-mail: masm@sioc.ac.cn
bDepartment of Chemistry, East China Normal University, 3663 North Zhongshan Lu, Shanghai 200062, P. R. China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China
First published on 21st March 2017
Abstract
A highly chemo- and regioselective copper-catalyzed borylcupration of 1,2-allenylsilanes affords 2-borylallylsilanes by applying the ligand effect. Many synthetically attractive functional groups are well tolerated. As demonstrated, such 2-borylallylsilanes are very useful dimetallic reagents in organic synthesis. A rationale for the regioselectivity switch is provided.
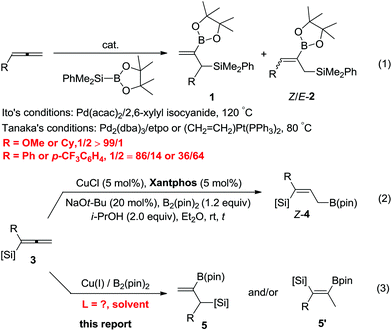
The dimetallic reagents containing C–B bonds and C–Si bonds are very important synthetic intermediates in organic synthesis due to their relatively high stability, difference in reactivities, low toxicity and broad functional group compatibility.1 Among them, 2-borylallylic silanes, which contain both vinylboronate and allylsilane units, would be very useful in various organic transformations since allylic silanes2 are well-known for their wide applications in Hiyama couplings,3 dihydroxylation,4 aminohydroxylation,5 epoxidation,6 cyclopropanation,7 and Lewis acid-mediated [2 + 2] and [3 + 2] annulation with electron-deficient olefins8 or free radical additions.9 While the vinylboronate moiety is very useful in Suzuki cross-coupling reactions.10
Due to the significance of such dimetallic reagents, highly selective approaches to such entities are of great importance. However, there are only a few examples reported and the related synthetic methods are rather limited:11 for example the palladium-catalyzed silaboration of allenes was developed independently by Ito and Tanaka in 1999, which affords a mixture of regioisomeric allylic silanes 1 with a terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the regioisomer Z/E-2. In a very recent study,12 we also disclosed a copper-catalyzed borylcupration of allenylsilanes affording 3-silylallylboronates Z-4 exclusively (eqn (2)).13 We envisioned whether such 1,2-allenylsilanes may be borylcuprated with B2(pin)2 for the highly selective preparation of 2-borylallylsilanes 5 by applying a different catalyst (eqn (3)). Herein, we report the realization of such a concept with an excellent regioselectivity by applying the ligand effect (Scheme 1).
C bond and the regioisomer Z/E-2. In a very recent study,12 we also disclosed a copper-catalyzed borylcupration of allenylsilanes affording 3-silylallylboronates Z-4 exclusively (eqn (2)).13 We envisioned whether such 1,2-allenylsilanes may be borylcuprated with B2(pin)2 for the highly selective preparation of 2-borylallylsilanes 5 by applying a different catalyst (eqn (3)). Herein, we report the realization of such a concept with an excellent regioselectivity by applying the ligand effect (Scheme 1).
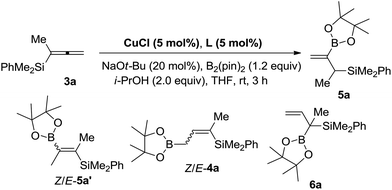
Initially, when we conducted the reaction of 3a with 1.2 equiv. of B2(pin)2 and 2.0 equiv. of i-PrOH in THF at room temperature with DPPE as the ligand, to our delight, a different product, 2-borylallylic silane 5a with a terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was indeed afforded, albeit only in 30% NMR yield with 59% recovery of 3a. The formation of alkenyl boronate Z/E-5a′ and regioisomeric allylic boronates Z/E-4a
C bond was indeed afforded, albeit only in 30% NMR yield with 59% recovery of 3a. The formation of alkenyl boronate Z/E-5a′ and regioisomeric allylic boronates Z/E-4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and 6a was NOT detected (Table 1, entry 1), indicating the excellent switch of regioselectivity compared to the reported results.12 Inspired by this result, other bidentate ligands were further screened. The reaction with rac-BINAP as the ligand afforded 5a in a much higher yield of 96% (entry 2). Finally, we found that BIPHEP is the most efficient for this transformation yielding 5a in 98% yield (entry 3). Subsequently, a brief screening of the solvent did not lead to better reaction media (entries 4 and 5). Compared with other copper catalysts such as CuBr and CuI, CuCl is the most efficient (entries 6 and 7). Control experiments showed that the ligand and base are both essential to the reaction (entries 8 and 9). Thus, we defined 5 mol% CuCl with 5 mol% BIPHEP as the catalyst, 20 mol% NaOt-Bu as the base, and 2.0 equiv. of i-PrOH in THF at room temperature as the standard reaction conditions for the further study.
13 and 6a was NOT detected (Table 1, entry 1), indicating the excellent switch of regioselectivity compared to the reported results.12 Inspired by this result, other bidentate ligands were further screened. The reaction with rac-BINAP as the ligand afforded 5a in a much higher yield of 96% (entry 2). Finally, we found that BIPHEP is the most efficient for this transformation yielding 5a in 98% yield (entry 3). Subsequently, a brief screening of the solvent did not lead to better reaction media (entries 4 and 5). Compared with other copper catalysts such as CuBr and CuI, CuCl is the most efficient (entries 6 and 7). Control experiments showed that the ligand and base are both essential to the reaction (entries 8 and 9). Thus, we defined 5 mol% CuCl with 5 mol% BIPHEP as the catalyst, 20 mol% NaOt-Bu as the base, and 2.0 equiv. of i-PrOH in THF at room temperature as the standard reaction conditions for the further study.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Ligand | NMR yield of 5a (%) | Recovery of 3a (%) |
|---|---|---|---|
| a Reaction conditions: 0.2 mmol of 3a, 5 mol% CuCl, 5 mol% ligand, 20 mol% NaOt-Bu, 0.24 mmol of B2(pin)2, 0.4 mmol of i-PrOH in 3 mL of THF at rt for 3 h under an argon atmosphere. b PhCH3 was used instead of THF. c Et2O was used instead of THF. d CuBr was instead of CuCl. e CuI was instead of CuCl. f NaOt-Bu was not added. | |||
| 1 | DPPE | 30 | 59 |
| 2 | rac-BINAP | 96 | — |
| 3 | BIPHEP | 98 | — |
| 4b | BIPHEP | 89 | 4 |
| 5c | BIPHEP | 68 | 24 |
| 6d | BIPHEP | 92 | — |
| 7e | BIPHEP | 21 | 75 |
| 8 | — | 0 | 71 |
| 9f | BIPHEP | 0 | 77 |
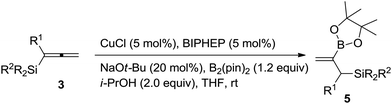
With the optimized conditions in hand, we started to examine the substrate scope: with CH3 and SiMe2Ph substituents, the reaction proceeded smoothly to afforded the desired product 5a in 90% yield (Table 2, entry 1); gratifyingly, the reaction could be conducted on a one-gram scale in an excellent yield with the same selectivity (entry 2); it is known that the substituents on silicon have serious influences on the properties of 1,2-allylsilanes,2g thus, we examine the substituents on silicon – the reaction could proceed smoothly in excellent yields irrespective of whether the Si-substituents are MePh2, Ph3 or t-BuMe2 (entries 3–5); the reaction of the allenylsilane with R1 = H could also proceed smoothly in 76% yield (entry 6); R1 may be an alkyl group beyond Me such as Et, n-C3H7 or n-C8H17 (entries 7–9); other alkyl groups such as i-Bu, Bn or CH2CH2Ph may also work under the standard conditions (entries 10–12); R1 may also be a phenyl group by conducting the reaction at 45 °C (entry 13). Furthermore, 1,2-allenylsilanes with synthetically attractive functional groups such as ester, cyano, ketone, or TBS ether are also compatible to yield the corresponding products 5m–5q in decent yields (entries 14–18).
| Entry | R1 | R | R2 | Time (h) | Yield of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1.0 mmol of 3, 0.05 mmol of CuCl, 0.05 mmol of BIPHEP, 0.2 mmol of NaOt-Bu, 1.2 mmol of B2(pin)2, 2.0 mmol of i-PrOH in 3 mL of THF at rt. b Isolated yield. c The reaction is carried out in a one-gram scale. d Conditions: 0.4 mmol of NaOt-Bu and 1.4 mmol of B2(pin)2 at 45 °C. e Conditions: 0.35 mmol of NaOt-Bu and 1.4 mmol of B2(pin)2 in 10 mL of THF. | |||||
| 1 | Me | Me | Ph (3a) | 3 | 90 (5a) |
| 2c | Me | Me | Ph (3a) | 2.5 | 90 (5a) |
| 3 | Me | Ph | Me (3b) | 3 | 88 (5b) |
| 4 | Me | Ph | Ph (3c) | 3 | 90 (5c) |
| 5 | Me | Me | tBu (3d) | 3 | 76 (5d) |
| 6 | H | Me | Ph (3e) | 3 | 76 (5e) |
| 7 | Et | Me | Ph (3f) | 2 | 89 (5f) |
| 8 | n-C3H7 | Me | Ph (3g) | 3 | 87 (5g) |
| 9 | n-C8H17 | Me | Me (3h) | 3 | 79 (5h) |
| 10 | i-Bu | Me | Me (3i) | 3 | 87 (5i) |
| 11 | Bn | Me | Me (3j) | 3 | 83 (5j) |
| 12 | CH2CH2Ph | Me | Me (3k) | 3 | 85 (5k) |
| 13d | Ph | Me | Ph (3l) | 17 | 72 (5l) |
| 14 | CH2COOMe | Me | Ph (3m) | 4 | 85 (5m) |
| 15e | CH2CH2CN | Me | Me (3n) | 10 | 80 (5n) |
| 16 | CH2COPh | Me | Me (3o) | 13 | 62 (5o) |
| 17 | (CH2)2OTBS | Me | Me (3p) | 3 | 72 (5p) |
| 18 | (CH2)3OTBS | Me | Me (3q) | 3 | 71 (5q) |
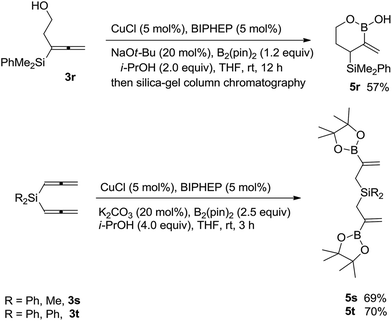
Interestingly, the substrate with an unprotected hydroxyl group 3r afforded the cyclic boronic acid product 5r in 57% yield after column chromatography (Scheme 2). Furthermore, the reaction of bis(propadienyl) silanes 3s and 3t afforded highly functionalized bis(2-borylallyl)silane products 5s and 5t in decent yields (Scheme 2).
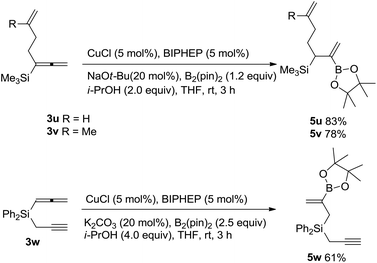
It is also observed that the reactivity of such a silyl-substituted allene unit is much higher than those of C–C double bonds and C–C triple bonds: no reaction occurred in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in 3u and 3v,14 the reaction of 1,2-allenyl propargyl silane 3w afforded allylic silane 5w with borylation occurring in the allene moiety exclusively (Scheme 3).15
C bond in 3u and 3v,14 the reaction of 1,2-allenyl propargyl silane 3w afforded allylic silane 5w with borylation occurring in the allene moiety exclusively (Scheme 3).15
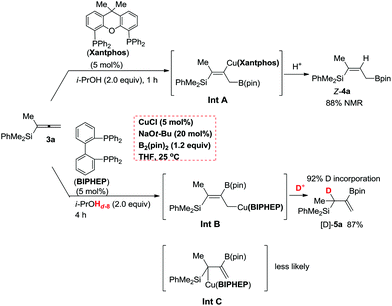
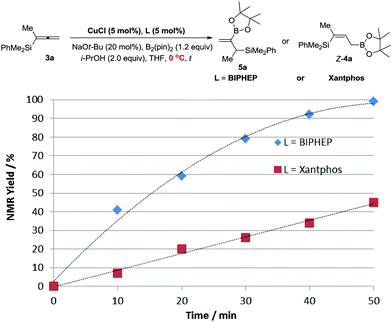
According to the DFT study performed by Ito et al. for such a borylcupration with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in alkenes by applying Xantphos as the ligand, the steric effect of the (pin)B unit dictates the regioselectivity.16 Thus, we reasoned that with Xantphos, the corresponding borylcupration reaction produces Int A with the B(pin) connected to the terminal carbon atom (Scheme 4).12 Due to the more sterically bulky bidentate nature of Xantphos17 we speculated that the reactivity of (pin)B-Cu(Xantphos) towards the allene unit should be much lower than that of (pin)B-Cu(BIPHEP). This was confirmed by the collection of data from the control reactions of 3a in the same solvent, i.e., THF, at a lower temperature of 0 °C with Xantphos or BIPHEP, affording Z-4a and 5a, respectively (Fig. 1).
C bond in alkenes by applying Xantphos as the ligand, the steric effect of the (pin)B unit dictates the regioselectivity.16 Thus, we reasoned that with Xantphos, the corresponding borylcupration reaction produces Int A with the B(pin) connected to the terminal carbon atom (Scheme 4).12 Due to the more sterically bulky bidentate nature of Xantphos17 we speculated that the reactivity of (pin)B-Cu(Xantphos) towards the allene unit should be much lower than that of (pin)B-Cu(BIPHEP). This was confirmed by the collection of data from the control reactions of 3a in the same solvent, i.e., THF, at a lower temperature of 0 °C with Xantphos or BIPHEP, affording Z-4a and 5a, respectively (Fig. 1).
Furthermore, the reaction of 3a with BIPHEP in the presence of i-PrOHd-8 (99% D) formed the η1-allylic Cu intermediate Int B, which was subsequently protonated regiospecifically at the γ-position to afford the observed product [D]-5a with 92% deuterium incorporation at the non-terminal allylic position exclusively in 87% yield (Scheme 4). Of course, the formation of Int C where the C–Cu bond is in the alpha position of the silyl group may NOT be completely excluded although it is NOT sterically favored. It should be noted that further study is required to unveil the interesting ligand effect.
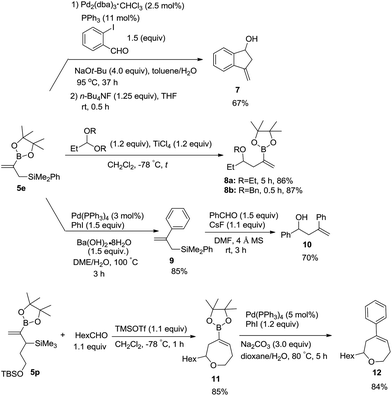
To demonstrate the utility of these products, Suzuki–Miyaura coupling of 5e with 2-iodobenzaldehyde was conducted, which was followed by intramolecular allylation promoted with n-Bu4NF to afford 3-methyleneindan-1-ol 7 in a combined yield of 67% (Scheme 5).18 Nucleophilic allylation of acetals with 5e in the presence of TiCl4 led to 2-boryl-substituted homoallyl ethers 8a and 8b in high yields.19 The Suzuki coupling product of 5e with iodobenzene 9 was treated with benzaldehyde to produce homoallyl alcohol 10 in 70% yield.11b,20 Under the catalysis of TMSOTf, the product 5p bearing a siloxyalkyl group may react with an aldehyde to undergo allylation and in situ etherification producing cyclic alkenyl boronate 11 in 85% yield, which may further be coupled with iodobenzene to afford 7-membered cyclic ether 12 in 84% yield.19
In summary, we have developed the highly regioselective borylcupration of 1,2-allenylsilanes, which provides an efficient method for the preparation of 2-borylallylic silanes with a terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond from readily available 1,2-allenylsilanes21 in good to excellent yields with an excellent regioselectivity under mild conditions by applying the ligand effect. The synthetic potential has been demonstrated. Further studies including the interesting ligand effect are underway in this laboratory.
C bond from readily available 1,2-allenylsilanes21 in good to excellent yields with an excellent regioselectivity under mild conditions by applying the ligand effect. The synthetic potential has been demonstrated. Further studies including the interesting ligand effect are underway in this laboratory.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21232006) is greatly appreciated. We thank Mr Pengbin Li in this group for reproducing the results of 5f and 5u presented in this study.Notes and references
- For reviews on organodimetallic chemistry, see: (a) P. Knochel and R. D. Singer, Chem. Rev., 1993, 93, 2117 CrossRef CAS; (b) I. Marek and J. F. Normant, Chem. Rev., 1996, 96, 3241 CrossRef CAS PubMed; (c) I. Beletskaya and C. Moberg, Chem. Rev., 1999, 99, 3435 CrossRef CAS PubMed; (d) M. Suginome and Y. Ito, Chem. Rev., 2000, 100, 3221 CrossRef CAS PubMed; (e) I. Beletskaya and C. Moberg, Chem. Rev., 2006, 106, 2320 CrossRef CAS PubMed.
- (a) M. A. Brook, Silicon in Organic, Organometallic and Polymer Chemistry, Wiley Interscience, New York, 2000 Search PubMed; (b) T. Kawamura and J. K. Kochi, J. Am. Chem. Soc., 1972, 94, 648 CrossRef CAS; (c) E. Langkopf and D. Schinzer, Chem. Rev., 1995, 95, 1375 CrossRef CAS; (d) J. Burfeindt, M. Patz, M. Müller and H. Mayr, J. Am. Chem. Soc., 1998, 120, 3629 CrossRef CAS; (e) J. B. Lambert, Y. Zhao, R. W. Emblidge, L. A. Salvador, X. Liu, J.-H. So and E. C. Chelius, Acc. Chem. Res., 1999, 32, 183 CrossRef CAS; (f) M. Sugawara and J. I. Yoshida, J. Org. Chem., 2000, 65, 3135 CrossRef CAS PubMed; (g) L. Chabaud, P. James and Y. Landais, Eur. J. Org. Chem., 2004, 3173 CrossRef CAS.
- (a) Y. Hatanaka, Y. Ebina and T. Hiyama, J. Am. Chem. Soc., 1991, 113, 7075 CrossRef CAS; (b) S. E. Denmark and N. S. Werner, J. Am. Chem. Soc., 2008, 130, 16382 CrossRef CAS PubMed.
- (a) J. S. Panek and J. Zhang, J. Org. Chem., 1993, 58, 294 CrossRef CAS; (b) Y. Landais and S. S. Surange, Tetrahedron Lett., 2001, 42, 581 CrossRef CAS; (c) Y. Landais, C. Mahieux, K. Schenk and S. S. Surange, J. Org. Chem., 2003, 68, 2779 CrossRef CAS PubMed.
- (a) R. Angelaud and Y. Landais, Tetrahedron Lett., 1997, 38, 1407 CrossRef CAS; (b) R. Angelaud, O. Babot, T. Charvat and Y. Landais, J. Org. Chem., 1999, 64, 9613 CrossRef CAS; (c) Y. Landais and E. Zekri, Eur. J. Org. Chem., 2002, 4037 CrossRef CAS.
- (a) J. S. Panek, R. M. Garbaccio and N. F. Jain, Tetrahedron Lett., 1994, 35, 6453 CrossRef CAS; (b) Y. Landais and L. Parra-Rapado, Tetrahedron Lett., 1996, 37, 1205 CrossRef CAS.
- (a) P. Mohr, Tetrahedron Lett., 1995, 36, 722 Search PubMed; (b) Y. L. Lin and E. Turos, J. Organomet. Chem., 2001, 630, 57 CrossRef CAS; (c) F. Allais, R. Angelaud, B. Camuzat-Dedenis, K. Julienne and Y. Landais, Eur. J. Org. Chem., 2003, 1069 CrossRef CAS.
- (a) H. J. Knölker, N. Foitzik and O. Schmitt, Tetrahedron Lett., 1999, 40, 3557 CrossRef; (b) S. Giese, L. Kastrup, D. Stiens and F. G. West, Angew. Chem., Int. Ed., 2000, 39, 1970 CrossRef CAS.
- (a) H. Sakurai, A. Hosomi and M. Kumada, J. Org. Chem., 1969, 34, 1764 CrossRef CAS; (b) N. A. Porter, G. Zhang and A. D. Reed, J. Org. Chem., 1997, 62, 6702 CrossRef CAS; (c) K. Miura, H. Saito, T. Nakagawa, T. Hondo, J.-I. Tateiwa, M. Sonoda and A. Hosomi, J. Org. Chem., 1998, 63, 5740 CrossRef CAS PubMed; (d) L. Chabaud, Y. Landais and P. Renaud, Org. Lett., 2002, 4, 4257 CrossRef CAS PubMed.
- For reviews, see: (a) D. S. Matteson, Chem. Rev., 1989, 89, 1535 CrossRef CAS; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (c) A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS; (d) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (e) M. Tobisu and N. Chatani, Angew. Chem., Int. Ed., 2009, 48, 3565 CrossRef CAS PubMed.
- For Pd-catalyzed silaboration of allenes, see: (a) M. Suginome, Y. Ohmori and Y. Ito, Synlett, 1999, 1567 CrossRef; (b) S. Onozawa, Y. Hatanaka and M. Tanaka, Chem. Commun., 1999, 1863 RSC; (c) M. Suginome, Y. Ohmori and Y. Ito, J. Organomet. Chem., 2000, 611, 403 CrossRef CAS; (d) M. Suginome, T. Ohmura, Y. Miyake, S. Mitani, Y. Ito and M. Murakami, J. Am. Chem. Soc., 2003, 125, 11174 CrossRef CAS PubMed; (e) T. Ohmura, H. Taniguchi and M. Suginome, J. Am. Chem. Soc., 2006, 128, 13682 CrossRef CAS PubMed; (f) T. Ohmura and M. Suginome, Org. Lett., 2006, 8, 2503 CrossRef CAS PubMed; (g) T. Ohmura and M. Suginome, Bull. Chem. Soc. Jpn., 2009, 82, 29 CrossRef CAS.
- W. Yuan, L. Song and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 3140 CrossRef CAS PubMed.
- (a) W. Yuan and S. Ma, Adv. Synth. Catal., 2012, 354, 1867 CrossRef CAS; (b) F. Meng, B. Jung, F. Haeffner and A. H. Hoveyda, Org. Lett., 2013, 15, 1414 CrossRef CAS PubMed; (c) K. Semba, M. Shinomiya, T. Fujihara, J. Terao and Y. Tsuji, Chem. – Eur. J., 2013, 19, 7125 CrossRef CAS PubMed; (d) W. Yuan, X. Zhang, Y. Yu and S. Ma, Chem. – Eur. J., 2013, 19, 7193 CrossRef CAS PubMed; (e) T. S. N. Zhao, Y. Yang, T. Lessing and K. J. Szabó, J. Am. Chem. Soc., 2014, 136, 7563 CrossRef CAS PubMed; (f) H. Jang, B. Jung and A. H. Hoveyda, Org. Lett., 2014, 16, 4658 CrossRef CAS PubMed; (g) K. Semba, T. Fujihara, J. Terao and Y. Tsuji, Tetrahedron, 2015, 71, 2183 CrossRef CAS; (h) K. Yeung, R. E. Ruscoe, J. Rae, A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 11912 CrossRef CAS PubMed; (i) R. Y. Liu, Y. Yang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 14077 CrossRef CAS PubMed; (j) W. Zhao and J. Montgomery, J. Am. Chem. Soc., 2016, 138, 9763 CrossRef CAS PubMed; (k) F. Meng, X. Li, S. Torker, Y. Shi, X. Shen and A. H. Hoveyda, Nature, 2016, 537, 387 CrossRef CAS PubMed; (l) T. Fujihara, A. Sawada, T. Yamaguchi, Y. Tani, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2017, 56, 1539 CrossRef CAS PubMed.
- For selected examples of the copper-catalyzed hydroboration of alkenes, see: (a) Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 3160 CrossRef CAS PubMed; (b) Y. Sasaki, C. Zhong, M. Sawamura and H. Ito, J. Am. Chem. Soc., 2010, 132, 1226 CrossRef CAS PubMed; (c) Y. Sasaki, Y. Horita, C. Zhong, M. Sawamura and H. Ito, Angew. Chem., Int. Ed., 2011, 50, 2778 CrossRef CAS PubMed; (d) F. Meng, H. Jang and A. H. Hoveyda, Chem. – Eur. J., 2013, 19, 3204 CrossRef CAS PubMed; (e) K. Kubota, E. Yamamoto and H. Ito, Adv. Synth. Catal., 2013, 355, 3527 CrossRef CAS; (f) A. Parra, L. Amenos, M. Guisan-Ceinos, A. Lopez, J. L. G. Ruano and M. Tortosa, J. Am. Chem. Soc., 2014, 136, 15833 CrossRef CAS PubMed; (g) H. Iwamoto, K. Kubota and H. Ito, Chem. Commun., 2016, 52, 591 Search PubMed.
- For selected examples of the copper-catalyzed hydroboration of terminal alkynes, see: (a) Y. Lee, H. Jang and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 18234 CrossRef CAS PubMed; (b) H. Jang, A. R. Zhugralin, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7859 CrossRef CAS PubMed; (c) A. L. Moure, P. Mauleón, R. G. Arrayás and J. C. Carretero, Org. Lett., 2013, 15, 2054 CrossRef CAS PubMed; (d) Z.-J. Yao, S. Hong, W. Zhang, M. Liu and W. Deng, Tetrahedron Lett., 2016, 57, 910 CrossRef CAS.
- K. Kubota, E. Yamamoto and H. Ito, J. Am. Chem. Soc., 2013, 135, 2635 CrossRef CAS PubMed.
- J. Huang, J. Chan, Y. Chen, C. J. Borths, K. D. Baucom, R. D. Larsen and M. M. Faul, J. Am. Chem. Soc., 2010, 132, 3674 CrossRef CAS PubMed.
- (a) J. Cvengroš, J. Schütte, N. Schlörer, J. Neudörfl and H.-G. Schmalz, Angew. Chem., Int. Ed., 2009, 48, 6148 CrossRef PubMed; (b) E. D. D. Calder and A. Sutherland, Org. Lett., 2015, 17, 2514 CrossRef CAS PubMed.
- M. Suginome, Y. Ohmori and Y. Ito, J. Am. Chem. Soc., 2001, 123, 4601 CrossRef CAS PubMed.
- P. Stephane and H. Jack, Tetrahedron Lett., 1989, 30, 3419 CrossRef.
- D. A. Evans, Z. K. Sweeney, T. Rovis and J. S. Tedrow, J. Am. Chem. Soc., 2001, 123, 12095 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation and characterisation data as well as 1H and 13C NMR spectra of all compounds. See DOI: 10.1039/c7qo00135e |
| This journal is © the Partner Organisations 2017 |