β-Carboline-directed decarboxylative acylation of ortho-C(sp2)–H of the aryl ring of aryl(β-carbolin-1-yl)methanones with α-ketoacids under palladium catalysis†‡
Shivalinga Kollea and
Sanjay Batra*ab
aMedicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Sector 10, Jankipuram Extension, Sitapur Road, Lucknow-226031, UP, India. E-mail: batra_san@yahoo.co.uk; s_batra@cdri.res.in
bAcademy for Scientific and Innovative Research, New Delhi-110025, India
First published on 18th May 2016
Abstract
A palladium-catalysed β-carboline directed decarboxylative acylation of ortho-C(sp2)–H of the aryl ring of aryl(β-carbolin-1-yl)methanones using α-oxocarboxylic acid as the acyl ion equivalent to form (2-aroylaryl)(β-carbolin-2-yl)methanones is described. The utility of these products for preparing β-carboline-tethered phthalazine systems is also demonstrated.
Introduction
Transition-metal-catalysed decarboxylative cross-coupling reactions have become an important tool for the formation of C–C bonds.1 A variety of carboxylic acids and their salts are used as coupling partners in such decarboxylative reactions. In particular, α-oxocarboxylic acids have received considerable attention as they affect acylation of the inactivated C–H bond under mild conditions. Goossen et al. first reported the Cu/Pd-catalysed decarboxylative cross-coupling reaction between aryl bromides and α-oxocarboxylates to form biaryl ketones.2 Later Ge et al. reported the Pd-catalysed decarboxylative acylation of acetanilides and 2-aryl pyridines at the ortho-position of the phenyl ring with α-oxocarboxylic acids via ligand-assisted ortho-C(sp2)–H activation in the presence of an oxidant.3 Subsequently different directing groups including azobenzenes,4 azoxybenzenes,5 carboxylic acids,6 cyclic enamides,7 O-methyl ketoximes,8 O-phenyl carbamates,9 phenylacetamides,10 2-aryloxypyridines,11 pyridine-N-oxides,12 indolines,13 thioethers,14 N-nitroso anilines,15 tetrahydroquinolines16 and indoles17 have been used for performing the acylation via ligand-assisted activation of the ortho-C(sp2)–H bond leading to formation of biaryl ketones with high regioselectivities under mild reaction conditions.β-Carboline, a privileged scaffold, is core unit of several natural alkaloids and bioactive compounds endowed with diverse pharmacological properties.18 The first example of β-carboline-directed ruthenium-catalyzed arylation was reported by Gandhi et al.19 Subsequently we reported Pd(OAc)2-catalysed regioselective alkoxylation (C–O bond formation) of aryl(β-carbolin-1-yl)methanones employing β-carboline directed ortho-C(sp2)–H activation of an aryl ring under oxidative conditions.20 Continuing with our efforts to study the scope of β-carboline directed C(sp2)–H activation for installing various functional groups, we now disclose a Pd-catalysed β-carboline directed decarboxylative acylation using α-oxocarboxylic acid as the acyl ion source to form (2-aroylaryl)(β-carbolin-2-yl)methanones.
Results and discussion
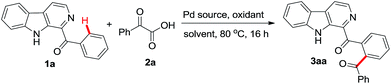
Our study began by heating phenyl(β-carbolin-2-yl)methanone 1a (0.1 g, 0.367 mmol) with 1.5 equiv. of phenyl oxocarboxylic acid 2a, 10 mol% of Pd(OAc)2 and 1 equiv. of (NH4)2S2O8 as the oxidant in dichloroethane (DCE) as the solvent at 80 °C in air. After 16 h assessment of TLC indicated the formation of major product which upon column chromatographic purification gave a solid compound in 72% yield which was identified to be the required 3aa. In order to improve the yield of 3aa, an optimization study with respect to solvent, oxidant, Pd-catalyst and reaction temperature was carried out and the results are summarized in Table 1. Screening of different solvents like dioxane, THF and diglyme revealed that the yield of 3aa was superior in DCE (compare entry 1 with entries 2–4). Investigating reaction with different oxidants including Na2S2O8, K2S2O8, PhI(OAc)2 and Cu(OAc)2 revealed that the yield of 3aa was superior with K2S2O8 (compare entry 6 with entries 5, 7–8). Notably increasing the amount of K2S2O8 from 1 equiv. to 2 equiv. enhanced the yield of 3aa to 82% (entry 9) but further increase did not affect the output (entry 10). Lowering the catalyst loading from 10 mol% to 5 mol% had detrimental effect on the formation of 3aa (entry 11). In the absence of a catalyst no product formation was observed (entry 12). Reaction in the presence of other Pd-catalysts like PdCl2 gave lower yield of 3aa whereas Pd(TFA)2 afforded the product in comparable yield as obtained with Pd(OAc)2 (entries 13–14). Varying the reaction temperature revealed that better yield of product was isolated when the reaction was performed at 80 °C as compared to 60 °C or 100 °C (compare entry 9 with entries 15–16). Therefore, the standardized conditions which suited the formation of 3aa in our hands were heating 1a (1.0 equiv.) with α-oxocarboxylic acid (1.5 equiv.) in the presence of 10 mol% of Pd(OAc)2 and K2S2O8 (2 equiv.) in DCE as the medium at 80 °C for 16 h.| Entry | Pd source (mol%) | Oxidant (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a All reactions were performed using 1a (0.1 g, 0.367 mmol) and 2a (0.55 mmol) in a solvent (2 mL).b Isolated yield after column chromatography.c Reaction at 60 °C.d Reaction at 100 °C. | ||||
| 1 | Pd(OAc)2 (10) | (NH4)2S2O8 (1) | DCE | 72 |
| 2 | Pd(OAc)2 (10) | (NH4)2S2O8 (1) | Dioxane | 46 |
| 3 | Pd(OAc)2 (10) | (NH4)2S2O8 (1) | THF | 42 |
| 4 | Pd(OAc)2 (10) | (NH4)2S2O8 (1) | Diglyme | 54 |
| 5 | Pd(OAc)2 (10) | Na2S2O8 (1) | DCE | 73 |
| 6 | Pd(OAc)2 (10) | K2S2O8 (1) | DCE | 74 |
| 7 | Pd(OAc)2 (10) | PhI(OAc)2 (1) | DCE | 68 |
| 8 | Pd(OAc)2 (10) | Cu(OAc)2 (1) | DCE | 61 |
| 9 | Pd(OAc)2 (10) | K2S2O8 (2) | DCE | 82 |
| 10 | Pd(OAc)2 (10) | K2S2O8 (3) | DCE | 83 |
| 11 | Pd(OAc)2 (5) | K2S2O8 (2) | DCE | 55 |
| 12 | — | K2S2O8 (2) | DCE | 0 |
| 13 | PdCl2 (10) | K2S2O8 (2) | DCE | 52 |
| 14 | Pd(TFA)2 (10) | K2S2O8 (2) | DCE | 80 |
| 15c | Pd(OAc)2 (10) | K2S2O8 (2) | DCE | 71 |
| 16d | Pd(OAc)2 (10) | K2S2O8 (2) | DCE | 81 |
With the optimized conditions in hands, we assessed the scope of the protocol with different α-oxocarboxylic acids and β-carbolines. Initially a variety of α-oxocarboxylic acids (2b–i) were investigated for their reactions with phenyl(β-carbolin-1-yl)methanone (1a) (Scheme 1). α-Oxocarboxylic acids (2b–f) bearing phenyl ring with electron donating or electron withdrawing groups reacted with 1a affording the corresponding products 3ab–3af in good to excellent yields. Treating α-oxocarboxylic acid bearing thiophene moiety (2g) with 1a gave the product 3ag in 77% yield. However, aliphatic α-oxocarboxylic acids (2h, i) failed to react with 1a to yield the respective products 3ah, 3ai.
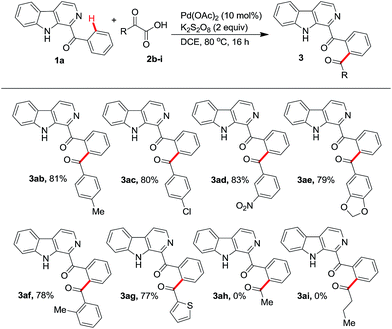 | ||
| Scheme 1 Scope of the protocol with different α-oxocarboxylic acids. All reactions were performed using 1a (0.5 mmol) and 2b–i (0.75 mmol) in 3 mL of solvent. | ||
Next the reaction of 2a was investigated with different starting aryl(β-carbolin-1-yl)methanones (1b–p) (Scheme 2). It was observed that the position of substituents on the aryl ring has direct bearing on the formation of the product. When the aryl ring was 3- or 4-substituted phenyl (1b–h), the products 3ba–3ha were isolated in 76–87% yields. Interestingly, for the 3-substituted phenyl ring carrying substrate (1g, h) the reaction was found to be regioselective to yield 3ga, 3ha. However, substrates 1i, j containing phenyl ring with ortho-substitution failed to give the required products 3ia, 3ja. Perhaps, this failure is attributed to the steric hindrance due to the presence of substituent at the ortho-position in the phenyl ring. Further, the failure of reaction with 1j inferred that the possibility of diacylation is ruled out. Even the heteroaryl(β-carbolin-1-yl)methanones 1k and 1l bearing thiophene and benzofuran units were found to undergo decarboxylative acylation with 2a affording the corresponding products 3ka and 3la in 72% and 74% yields, respectively. Substrates 1m, 1n, 1o, and 1p bearing bromo and chloro substitutions at 6-position and methyl ester at 3-position of the β-carboline unit, were also investigated to obtain respective products 3ma, 3na, 3oa and 3pa in excellent yields. Finally the scope of reaction was evaluated with phenyl(pyridin-2-yl)methanone 1q and it too reacted with 2a to give acylated product 3qa in 79% yield.
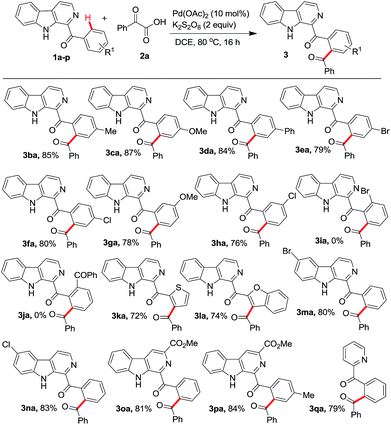 | ||
| Scheme 2 Scope of the protocol with different aryl(β-carbolin-2-yl)methanones. All reactions were performed using 1b–n (0.5 mmol) and 2a (0.75 mmol) in 3 mL of solvent. | ||
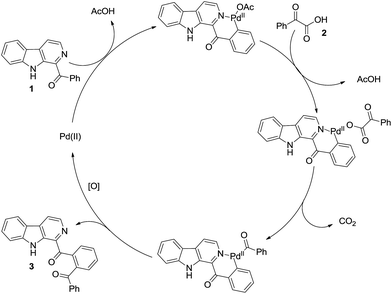
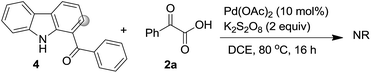
As already reported, mechanism wise the pyridine ring of the β-carboline unit undergo chelation with the Pd-catalyst followed by coupling reaction as delineated in Scheme 3. Here too, for negating the role of carbonyl for the C–H functionalization,21 we carried out reaction of (9H-carbazol-1-yl)(phenyl)methanone 4 under the optimized conditions (Scheme 4). It was found that the reaction was unsuccessful and 4 was recovered unreacted. This ascertained that the pyridine ring was involved in chelation with palladium.
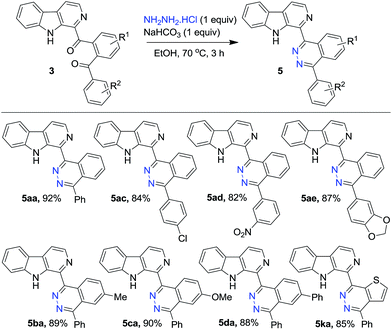
Finally, to demonstrate the utility of the isolated diaroyl-products belonging to 3, a randomly selected set of substrates were treated with hydrazine hydrochloride in the presence of NaHCO3 in ethanol as medium to prepare β-carboline tethered phthalazine derivatives 5 in 82–92% yields (Scheme 5). It may be noted the phthalazine pendant at the 3-position of β-carboline was prepared via Suzuki coupling between 3-chloro-β-carboline and phthalazine-1-boronic acid.22
Conclusions
In conclusion, we have demonstrated a Pd-catalysed decarboxylative acylation of aryl ring of aryl (β-carbolin-2-yl)methanones using α-oxocarboxylic acid as the acyl ion source via β-carboline directed C(sp2)–H activation under oxidative conditions. The products (2-aroylaryl)(β-carbolin-2-yl)methanones which were efficiently prepared could be readily transformed into β-carboline tethered phthalazine by treating them with hydrazine hydrochloride.Experimental
General experimental procedure for the acylation
To a round bottom flask containing DCE (3 mL) were added aryl(β-carbolin-1-yl)methanone 1 (0.5 mmol), α-oxocarboxylic acid 2 (0.75 mmol), Pd(OAc)2 (0.05 mmol) and K2S2O8 (1.0 mmol) in air. The reaction mixture was heated at 80 °C for 16 h. On completion of reaction (as assessed by TLC), the reaction mixture was diluted with water (25 mL) and extracted with methylene chloride (25 mL × 2). The organic layers were pooled, dried over anhydrous Na2SO4 and evaporated to obtain the crude product, which was purified by column chromatography over silica gel using hexanes/EtOAc (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) as eluent to obtain corresponding product 3.
10, v/v) as eluent to obtain corresponding product 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 769, 1168, 1220, 1455, 1580, 1651, 3022, 3431 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22 (t, J = 7.7 Hz, 1H), 7.29 (t, J = 7.7 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 7.46–7.56 (m, 4H), 7.61–7.64 (m, 1H), 7.77 (d, J = 7.5 Hz, 2H), 7.86–7.88 (m, 2H), 7.99 (d, J = 7.9 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 10.22 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.7, 120.8, 121.9, 128.2, 129.2, 129.4, 130.1, 130.3, 131.1, 131.6, 132.6, 135.8, 136.4, 137.5, 138.1, 139.5, 140.9, 141.3, 196.6, 198.1. MS (ESI+) m/z = 377.1. ESI-HRMS calculated for C25H16N2O2 [MH]+: 377.1290, found: 377.1292.
2, v/v). IR (KBr) νmax: 769, 1168, 1220, 1455, 1580, 1651, 3022, 3431 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22 (t, J = 7.7 Hz, 1H), 7.29 (t, J = 7.7 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 7.46–7.56 (m, 4H), 7.61–7.64 (m, 1H), 7.77 (d, J = 7.5 Hz, 2H), 7.86–7.88 (m, 2H), 7.99 (d, J = 7.9 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 10.22 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.7, 120.8, 121.9, 128.2, 129.2, 129.4, 130.1, 130.3, 131.1, 131.6, 132.6, 135.8, 136.4, 137.5, 138.1, 139.5, 140.9, 141.3, 196.6, 198.1. MS (ESI+) m/z = 377.1. ESI-HRMS calculated for C25H16N2O2 [MH]+: 377.1290, found: 377.1292.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 758, 1185, 1258, 1458, 1586, 1661, 3058, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.25 (s, 3H), 7.08 (d, J = 7.9 Hz, 2H), 7.22 (t, J = 7.0 Hz, 1H), 7.45–7.49 (m, 2H), 7.54 (t, J = 6.9 Hz, 2H), 7.59–7.62 (m, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.87 (d, J = 5.5 Hz, 2H), 8.00 (d, J = 7.9 Hz, 1H), 8.09 (d, J = 4.9 Hz, 1H), 10.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.7, 112.0, 118.6, 120.8, 121.8, 128.9, 129.1, 129.3, 130.1, 130.3, 130.4, 131.9, 131.5, 134.9, 135.8, 136.4, 138.1, 139.4, 141.2, 143.4, 196.3, 198.1. MS (ESI+) m/z = 391.2. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1450.
2, v/v). IR (KBr) νmax: 758, 1185, 1258, 1458, 1586, 1661, 3058, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.25 (s, 3H), 7.08 (d, J = 7.9 Hz, 2H), 7.22 (t, J = 7.0 Hz, 1H), 7.45–7.49 (m, 2H), 7.54 (t, J = 6.9 Hz, 2H), 7.59–7.62 (m, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.87 (d, J = 5.5 Hz, 2H), 8.00 (d, J = 7.9 Hz, 1H), 8.09 (d, J = 4.9 Hz, 1H), 10.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.7, 112.0, 118.6, 120.8, 121.8, 128.9, 129.1, 129.3, 130.1, 130.3, 130.4, 131.9, 131.5, 134.9, 135.8, 136.4, 138.1, 139.4, 141.2, 143.4, 196.3, 198.1. MS (ESI+) m/z = 391.2. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1450.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 772, 1185, 1225, 1456, 1591, 1659, 3013, 3433 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.21–7.25 (m, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.47–7.56 (m, 4H), 7.62–7.65 (m, 1H), 7.73 (d, J = 8.4 Hz, 2H), 7.87–7.89 (m, 2H), 7.99–8.04 (m, 2H), 10.24 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.8, 120.8, 120.9, 121.9, 128.6, 128.8, 129.4, 130.2, 130.5, 131.3, 131.7, 135.6, 135.8, 136.5, 138.1, 139.1, 139.3, 140.7, 141.3, 195.4, 197.8. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.
2, v/v). IR (KBr) νmax: 772, 1185, 1225, 1456, 1591, 1659, 3013, 3433 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.21–7.25 (m, 1H), 7.29 (d, J = 8.4 Hz, 2H), 7.47–7.56 (m, 4H), 7.62–7.65 (m, 1H), 7.73 (d, J = 8.4 Hz, 2H), 7.87–7.89 (m, 2H), 7.99–8.04 (m, 2H), 10.24 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.8, 120.8, 120.9, 121.9, 128.6, 128.8, 129.4, 130.2, 130.5, 131.3, 131.7, 135.6, 135.8, 136.5, 138.1, 139.1, 139.3, 140.7, 141.3, 195.4, 197.8. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 756, 1125, 1285, 1425, 1587, 1659, 3052, 3435 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.49–7.54 (m, 4H), 7.59 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.5 Hz, 1H), 7.88 (d, J = 4.9 Hz, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.99–8.03 (m, 2H), 8.10 (d, J = 7.7 Hz, 1H), 8.26 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.64 (s, 1H), 10.23 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 113.5, 119.6, 120.2, 120.8, 122.3, 124.4, 127.6, 128.8, 129.6, 130.7, 130.8, 131.5, 132.1, 135.4, 135.6, 136.1, 137.5, 138.5, 139.5, 139.8, 142.3, 148.0, 194.5, 196.4. MS (ESI+) m/z = 422.1. ESI-HRMS calculated for C25H15N3O4 [MH]+: 422.1141, found: 422.1144.
2, v/v). IR (KBr) νmax: 756, 1125, 1285, 1425, 1587, 1659, 3052, 3435 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.49–7.54 (m, 4H), 7.59 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.5 Hz, 1H), 7.88 (d, J = 4.9 Hz, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.99–8.03 (m, 2H), 8.10 (d, J = 7.7 Hz, 1H), 8.26 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.64 (s, 1H), 10.23 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 113.5, 119.6, 120.2, 120.8, 122.3, 124.4, 127.6, 128.8, 129.6, 130.7, 130.8, 131.5, 132.1, 135.4, 135.6, 136.1, 137.5, 138.5, 139.5, 139.8, 142.3, 148.0, 194.5, 196.4. MS (ESI+) m/z = 422.1. ESI-HRMS calculated for C25H15N3O4 [MH]+: 422.1141, found: 422.1144.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 726, 1025, 1155, 1224, 1455, 1581, 1649, 3026, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 5.78 (s, 2H), 6.67 (d, J = 8.1 Hz, 1H), 7.15 (d, J = 1.3 Hz, 1H), 7.22–7.25 (m, 1H), 7.31 (dd, J1 = 8.1 Hz, J2 = 1.4 Hz, 1H), 7.47–7.64 (m, 5H), 7.88 (d, J = 7.5 Hz, 1H), 7.92 (d, J = 4.9 Hz, 1H), 8.03 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 4.9 Hz, 1H), 10.18 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 101.8, 107.5, 109.4, 112.1, 118.7, 120.8, 120.8, 121.9, 127.0, 129.0, 129.3, 130.3, 130.4, 130.8, 131.5, 132.3, 135.9, 136.4, 138.2, 139.3, 141.1, 141.2, 147.8, 151.5, 194.9, 197.9. MS (ESI+) m/z = 421.1. ESI-HRMS calculated for C26H16N2O4 [MH]+: 421.1188, found: 421.1191.
2, v/v). IR (KBr) νmax: 726, 1025, 1155, 1224, 1455, 1581, 1649, 3026, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 5.78 (s, 2H), 6.67 (d, J = 8.1 Hz, 1H), 7.15 (d, J = 1.3 Hz, 1H), 7.22–7.25 (m, 1H), 7.31 (dd, J1 = 8.1 Hz, J2 = 1.4 Hz, 1H), 7.47–7.64 (m, 5H), 7.88 (d, J = 7.5 Hz, 1H), 7.92 (d, J = 4.9 Hz, 1H), 8.03 (d, J = 7.8 Hz, 1H), 8.15 (d, J = 4.9 Hz, 1H), 10.18 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 101.8, 107.5, 109.4, 112.1, 118.7, 120.8, 120.8, 121.9, 127.0, 129.0, 129.3, 130.3, 130.4, 130.8, 131.5, 132.3, 135.9, 136.4, 138.2, 139.3, 141.1, 141.2, 147.8, 151.5, 194.9, 197.9. MS (ESI+) m/z = 421.1. ESI-HRMS calculated for C26H16N2O4 [MH]+: 421.1188, found: 421.1191.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 725, 1185, 1221, 1432, 1592, 1655, 3021, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.27 (s, 3H), 7.09–7.15 (m, 2H), 7.22–7.26 (m, 2H), 7.47 (dd, J1 = 6.6 Hz, J2 = 1.3 Hz, 2H), 7.52 (d, J = 3.6 Hz, 2H), 7.59–7.65 (m, 2H), 7.71 (d, J = 7.1 Hz, 1H), 7.93 (d, J = 4.9 Hz, 1H), 8.04 (d, J = 7.8 Hz, 1H), 8.23 (d, J = 4.9 Hz, 1H), 10.29 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.13, 118.6, 120.8, 120.9, 121.9, 125.1, 129.3, 129.4, 129.8, 130.3, 130.8, 131.1, 131.2, 131.6, 132.0, 136.1, 136.3, 137.5, 138.3, 138.6, 140.5, 141.1, 141.3, 198.2, 199.5. MS (ESI+) m/z = 391.1. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1450.
2, v/v). IR (KBr) νmax: 725, 1185, 1221, 1432, 1592, 1655, 3021, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.27 (s, 3H), 7.09–7.15 (m, 2H), 7.22–7.26 (m, 2H), 7.47 (dd, J1 = 6.6 Hz, J2 = 1.3 Hz, 2H), 7.52 (d, J = 3.6 Hz, 2H), 7.59–7.65 (m, 2H), 7.71 (d, J = 7.1 Hz, 1H), 7.93 (d, J = 4.9 Hz, 1H), 8.04 (d, J = 7.8 Hz, 1H), 8.23 (d, J = 4.9 Hz, 1H), 10.29 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.13, 118.6, 120.8, 120.9, 121.9, 125.1, 129.3, 129.4, 129.8, 130.3, 130.8, 131.1, 131.2, 131.6, 132.0, 136.1, 136.3, 137.5, 138.3, 138.6, 140.5, 141.1, 141.3, 198.2, 199.5. MS (ESI+) m/z = 391.1. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1450.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 758, 1058, 1152, 1282, 1425, 1596, 1658, 3025, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.99 (d, J = 4.0 Hz, 1H), 7.23 (t, J = 6.9 Hz, 1H), 7.49–7.63 (m, 6H), 7.74–7.77 (m, 1H), 7.88 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 4.7 Hz, 1H), 8.02 (d, J = 7.6 Hz, 1H), 8.11 (d, J = 4.6 Hz, 1H), 10.25 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 118.8, 120.9, 121.9, 127.9, 128.7, 129.4, 130.5, 130.6, 131.1, 131.7, 133.6, 134.2, 134.9, 135.7, 136.5, 138.1, 139.1, 140.7, 141.3, 144.2, 188.3, 197.8. MS (ESI+) m/z = 383.1. ESI-HRMS calculated for C23H14N2O2S [MH]+: 383.0854, found: 383.0852.
2, v/v). IR (KBr) νmax: 758, 1058, 1152, 1282, 1425, 1596, 1658, 3025, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.99 (d, J = 4.0 Hz, 1H), 7.23 (t, J = 6.9 Hz, 1H), 7.49–7.63 (m, 6H), 7.74–7.77 (m, 1H), 7.88 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 4.7 Hz, 1H), 8.02 (d, J = 7.6 Hz, 1H), 8.11 (d, J = 4.6 Hz, 1H), 10.25 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 118.8, 120.9, 121.9, 127.9, 128.7, 129.4, 130.5, 130.6, 131.1, 131.7, 133.6, 134.2, 134.9, 135.7, 136.5, 138.1, 139.1, 140.7, 141.3, 144.2, 188.3, 197.8. MS (ESI+) m/z = 383.1. ESI-HRMS calculated for C23H14N2O2S [MH]+: 383.0854, found: 383.0852.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 778, 1159, 1222, 1412, 1591, 1652, 3015, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.41 (s, 3H), 7.20–7.29 (m, 3H), 7.36–7.52 (m, 5H), 7.74 (d, J = 1.4 Hz, 1H), 7.76 (d, J = 1.2 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.88 (dd, J1 = 4.9 Hz, J2 = 0.5 Hz, 1H), 7.99–8.02 (m, 1H), 8.11 (d, J = 4.9 Hz, 1H), 10.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.6, 112.0, 118.6, 120.8, 121.8, 128.2, 129.3, 129.7, 130.1, 130.7, 131.5, 132.5, 135.9, 136.4, 136.5, 137.6, 138.0, 140.9, 141.2, 141.2, 196.8, 197.6. MS (ESI+) m/z = 391.1. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1445.
2, v/v). IR (KBr) νmax: 778, 1159, 1222, 1412, 1591, 1652, 3015, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.41 (s, 3H), 7.20–7.29 (m, 3H), 7.36–7.52 (m, 5H), 7.74 (d, J = 1.4 Hz, 1H), 7.76 (d, J = 1.2 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.88 (dd, J1 = 4.9 Hz, J2 = 0.5 Hz, 1H), 7.99–8.02 (m, 1H), 8.11 (d, J = 4.9 Hz, 1H), 10.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.6, 112.0, 118.6, 120.8, 121.8, 128.2, 129.3, 129.7, 130.1, 130.7, 131.5, 132.5, 135.9, 136.4, 136.5, 137.6, 138.0, 140.9, 141.2, 141.2, 196.8, 197.6. MS (ESI+) m/z = 391.1. ESI-HRMS calculated for C26H18N2O2 [MH]+: 391.1447, found: 391.1445.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 758, 1187, 1281, 1421, 1589, 1660, 3033, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.84 (s, 3H), 7.04 (d, J = 2.5 Hz, 1H), 7.10 (dd, J1 = 8.6 Hz, J2 = 2.6 Hz, 1H), 7.16–7.28 (m, 3H), 7.34–7.38 (m, 1H), 7.43–7.51 (m, 2H), 7.75 (d, J = 1.4 Hz, 1H), 7.77 (d, J = 0.8 Hz, 1H), 7.89 (d, J = 4.9 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 4.9 Hz, 1H), 10.15 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 56.3, 113.5, 114.7, 115.3, 119.2, 120.3, 120.7, 122.2, 128.8, 129.4, 130.0, 131.2, 131.3, 133.4, 133.8, 135.6, 136.3, 137.1, 137.4, 142.2, 143.5, 161.3, 194.7, 195.9. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C26H18N2O3 [MH]+: 407.1396, found: 407.1395.
2, v/v). IR (KBr) νmax: 758, 1187, 1281, 1421, 1589, 1660, 3033, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.84 (s, 3H), 7.04 (d, J = 2.5 Hz, 1H), 7.10 (dd, J1 = 8.6 Hz, J2 = 2.6 Hz, 1H), 7.16–7.28 (m, 3H), 7.34–7.38 (m, 1H), 7.43–7.51 (m, 2H), 7.75 (d, J = 1.4 Hz, 1H), 7.77 (d, J = 0.8 Hz, 1H), 7.89 (d, J = 4.9 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 4.9 Hz, 1H), 10.15 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ (ppm) = 56.3, 113.5, 114.7, 115.3, 119.2, 120.3, 120.7, 122.2, 128.8, 129.4, 130.0, 131.2, 131.3, 133.4, 133.8, 135.6, 136.3, 137.1, 137.4, 142.2, 143.5, 161.3, 194.7, 195.9. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C26H18N2O3 [MH]+: 407.1396, found: 407.1395.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 660, 773, 1155, 1234, 1442, 1575, 1653, 3052, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.29–7.35 (m, 3H), 7.38–7.42 (m, 3H), 7.47–7.52 (m, 2H), 7.56 (s, 1H), 7.58 (d, J = 1.3 Hz, 1H), 7.75 (d, J = 1.6 Hz, 1H), 7.80 (d, J = 1.3 Hz, 1H), 7.82–7.85 (m, 2H), 7.89 (d, J = 4.9 Hz, 1H), 7.99–8.03 (m, 2H), 8.11 (d, J = 4.9 Hz, 1H), 10.29 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.7, 120.8, 120.9, 121.9, 127.5, 127.8, 128.3, 128.4, 129.2, 129.4, 129.4, 130.3, 131.2, 131.6, 132.7, 135.8, 136.5, 137.4, 137.9, 138.1, 139.7, 141.2, 141.8, 143.4, 196.6, 197.5. MS (ESI+) m/z = 453.2. ESI-HRMS calculated for C31H20N2O2 [MH]+: 453.1603, found: 453.1605.
2, v/v). IR (KBr) νmax: 660, 773, 1155, 1234, 1442, 1575, 1653, 3052, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.29–7.35 (m, 3H), 7.38–7.42 (m, 3H), 7.47–7.52 (m, 2H), 7.56 (s, 1H), 7.58 (d, J = 1.3 Hz, 1H), 7.75 (d, J = 1.6 Hz, 1H), 7.80 (d, J = 1.3 Hz, 1H), 7.82–7.85 (m, 2H), 7.89 (d, J = 4.9 Hz, 1H), 7.99–8.03 (m, 2H), 8.11 (d, J = 4.9 Hz, 1H), 10.29 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.7, 120.8, 120.9, 121.9, 127.5, 127.8, 128.3, 128.4, 129.2, 129.4, 129.4, 130.3, 131.2, 131.6, 132.7, 135.8, 136.5, 137.4, 137.9, 138.1, 139.7, 141.2, 141.8, 143.4, 196.6, 197.5. MS (ESI+) m/z = 453.2. ESI-HRMS calculated for C31H20N2O2 [MH]+: 453.1603, found: 453.1605.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 755, 1147, 1287, 1467, 1588, 1649, 3032, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.31–7.35 (m, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.51–7.54 (m, 1H), 7.56–7.66 (m, 3H), 7.77 (d, J = 1.6 Hz, 1H), 7.84–7.90 (m, 3H), 7.99 (d, J = 8.0 Hz, 1H), 8.11 (d, J = 7.9 Hz, 1H), 8.17 (d, J = 4.9 Hz, 1H), 10.27 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.8, 120.9, 121.9, 124.9, 128.4, 129.5, 130.2, 131.8, 132.1, 132.6, 133.0, 133.9, 135.4, 136.5, 136.8, 137.9, 138.1, 141.3, 142.8, 195.0, 196.7. MS (ESI+) m/z = 455.0. ESI-HRMS calculated for C25H15BrN2O2 [MH]+: 455.0395, found: 455.0392.
2, v/v). IR (KBr) νmax: 755, 1147, 1287, 1467, 1588, 1649, 3032, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.31–7.35 (m, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.51–7.54 (m, 1H), 7.56–7.66 (m, 3H), 7.77 (d, J = 1.6 Hz, 1H), 7.84–7.90 (m, 3H), 7.99 (d, J = 8.0 Hz, 1H), 8.11 (d, J = 7.9 Hz, 1H), 8.17 (d, J = 4.9 Hz, 1H), 10.27 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.8, 120.9, 121.9, 124.9, 128.4, 129.5, 130.2, 131.8, 132.1, 132.6, 133.0, 133.9, 135.4, 136.5, 136.8, 137.9, 138.1, 141.3, 142.8, 195.0, 196.7. MS (ESI+) m/z = 455.0. ESI-HRMS calculated for C25H15BrN2O2 [MH]+: 455.0395, found: 455.0392.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 669, 771, 1157, 1216, 1403, 1599, 1654, 3019, 3435 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.32 (t, J = 7.8 Hz, 2H), 7.43 (t, J = 7.5 Hz, 1H), 7.46–7.52 (m, 3H), 7.59 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.86–7.89 (m, 2H), 8.01 (d, J = 7.8 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 10.17 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.8, 120.9, 121.9, 128.4, 128.9, 129.5, 130.2, 130.9, 131.7, 131.9, 133.0, 135.4, 136.5, 136.7, 136.9, 137.5, 138.1, 141.2, 142.7, 195.2, 196.6. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.
2, v/v). IR (KBr) νmax: 669, 771, 1157, 1216, 1403, 1599, 1654, 3019, 3435 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.32 (t, J = 7.8 Hz, 2H), 7.43 (t, J = 7.5 Hz, 1H), 7.46–7.52 (m, 3H), 7.59 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.86–7.89 (m, 2H), 8.01 (d, J = 7.8 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 10.17 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.8, 120.9, 121.9, 128.4, 128.9, 129.5, 130.2, 130.9, 131.7, 131.9, 133.0, 135.4, 136.5, 136.7, 136.9, 137.5, 138.1, 141.2, 142.7, 195.2, 196.6. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 799, 1185, 1225, 1324, 1434, 1579, 1664, 3032, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.87 (s, 3H), 6.99 (dd, J1 = 8.6 Hz, J2 = 2.6 Hz, 1H), 7.21–7.25 (m, 1H), 7.27–7.31 (m, 3H), 7.39 (t, J = 7.3 Hz, 1H), 7.50–7.55 (m, 3H), 7.71 (d, J = 7.2 Hz, 2H), 7.88 (d, J = 4.9 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.11 (d, J = 4.9 Hz, 1H), 10.25 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 55.8, 112.1, 114.8, 115.3, 118.6, 120.8, 120.8, 121.8, 128.1, 129.3, 130.1, 131.6, 131.9, 132.2, 132.9, 135.9, 136.2, 137.9, 138.2, 141.3, 142.4, 162.2, 195.7, 198.6. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C26H18N2O3 [MH]+: 407.1396, found: 407.1399.
2, v/v). IR (KBr) νmax: 799, 1185, 1225, 1324, 1434, 1579, 1664, 3032, 3434 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.87 (s, 3H), 6.99 (dd, J1 = 8.6 Hz, J2 = 2.6 Hz, 1H), 7.21–7.25 (m, 1H), 7.27–7.31 (m, 3H), 7.39 (t, J = 7.3 Hz, 1H), 7.50–7.55 (m, 3H), 7.71 (d, J = 7.2 Hz, 2H), 7.88 (d, J = 4.9 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.11 (d, J = 4.9 Hz, 1H), 10.25 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 55.8, 112.1, 114.8, 115.3, 118.6, 120.8, 120.8, 121.8, 128.1, 129.3, 130.1, 131.6, 131.9, 132.2, 132.9, 135.9, 136.2, 137.9, 138.2, 141.3, 142.4, 162.2, 195.7, 198.6. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C26H18N2O3 [MH]+: 407.1396, found: 407.1399.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 745, 1057, 1285, 1423, 1586, 1650, 3045, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22 (t, J = 7.7 Hz, 1H), 7.28–7.34 (m, 2H), 7.42 (t, J = 7.2 Hz, 1H), 7.48–7.54 (m, 4H), 7.74 (d, J = 7.6 Hz, 2H), 7.82 (s, 1H), 7.88 (d, J = 4.8 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.06 (d, J = 4.9 Hz, 1H), 10.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.7, 120.9, 121.9, 128.3, 129.5, 129.9, 130.2, 130.4, 130.5, 131.7, 132.8, 135.2, 136.4, 137.2, 137.6, 138.2, 139.2, 141.2, 141.3, 195.6, 196.6. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0901.
2, v/v). IR (KBr) νmax: 745, 1057, 1285, 1423, 1586, 1650, 3045, 3436 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22 (t, J = 7.7 Hz, 1H), 7.28–7.34 (m, 2H), 7.42 (t, J = 7.2 Hz, 1H), 7.48–7.54 (m, 4H), 7.74 (d, J = 7.6 Hz, 2H), 7.82 (s, 1H), 7.88 (d, J = 4.8 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.06 (d, J = 4.9 Hz, 1H), 10.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 118.9, 120.7, 120.9, 121.9, 128.3, 129.5, 129.9, 130.2, 130.4, 130.5, 131.7, 132.8, 135.2, 136.4, 137.2, 137.6, 138.2, 139.2, 141.2, 141.3, 195.6, 196.6. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0901.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 758, 1024, 1185, 1258, 1425, 1587, 1645, 3054, 3438 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.14 (d, J = 5. Hz, 1H), 7.23 (t, J = 7.2 Hz, 1H), 7.34–7.40 (m, 3H), 7.47 (dd, J1 = 16.2 Hz, J2 = 7.8 Hz, 2H), 7.78 (d, J = 5.0 Hz, 1H), 7.85 (d, J = 1.3 Hz, 1H), 7.87 (s, 1H), 8.03–8.07 (m, 2H), 8.46 (d, J = 4.9 Hz, 1H), 10.14 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 119.2, 120.7, 120.9, 121.9, 127.2, 128.7, 129.4, 129.5, 131.8, 133.3, 134.7, 135.6, 136.6, 137.1, 137.3, 137.5, 141.1, 148.6, 184.7, 195.0. MS (ESI+) m/z = 383.1. ESI-HRMS calculated for C23H14N2O2S [MH]+: 383.0854, found: 383.0856.
2, v/v). IR (KBr) νmax: 758, 1024, 1185, 1258, 1425, 1587, 1645, 3054, 3438 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.14 (d, J = 5. Hz, 1H), 7.23 (t, J = 7.2 Hz, 1H), 7.34–7.40 (m, 3H), 7.47 (dd, J1 = 16.2 Hz, J2 = 7.8 Hz, 2H), 7.78 (d, J = 5.0 Hz, 1H), 7.85 (d, J = 1.3 Hz, 1H), 7.87 (s, 1H), 8.03–8.07 (m, 2H), 8.46 (d, J = 4.9 Hz, 1H), 10.14 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.1, 119.2, 120.7, 120.9, 121.9, 127.2, 128.7, 129.4, 129.5, 131.8, 133.3, 134.7, 135.6, 136.6, 137.1, 137.3, 137.5, 141.1, 148.6, 184.7, 195.0. MS (ESI+) m/z = 383.1. ESI-HRMS calculated for C23H14N2O2S [MH]+: 383.0854, found: 383.0856.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 852, 1042, 1167, 1258, 1402, 1592, 1661, 3052, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.23–7.29 (m, 4H), 7.34 (t, J = 7.1 Hz, 1H), 7.47 (t, J = 8.2 Hz, 1H), 7.52–7.53 (m, 3H), 7.67 (d, J = 8.4 Hz, 1H), 7.87–7.89 (m, 4H), 8.0 (d, J = 7.8 Hz, 1H), 10.18 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.3, 112.6, 119.5, 120.7, 121.1, 121.9, 123.0, 124.5, 127.5, 128.4, 128.5, 128.7, 129.3, 129.6, 131.8, 132.9, 134.3, 136.5, 137.0, 138.5, 141.2, 148.2, 154.7, 183.7, 189.9. MS (ESI+) m/z = 417.1. ESI-HRMS calculated for C27H16N2O3 [MH]+: 417.1239, found: 417.1240.
2, v/v). IR (KBr) νmax: 852, 1042, 1167, 1258, 1402, 1592, 1661, 3052, 3432 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.23–7.29 (m, 4H), 7.34 (t, J = 7.1 Hz, 1H), 7.47 (t, J = 8.2 Hz, 1H), 7.52–7.53 (m, 3H), 7.67 (d, J = 8.4 Hz, 1H), 7.87–7.89 (m, 4H), 8.0 (d, J = 7.8 Hz, 1H), 10.18 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.3, 112.6, 119.5, 120.7, 121.1, 121.9, 123.0, 124.5, 127.5, 128.4, 128.5, 128.7, 129.3, 129.6, 131.8, 132.9, 134.3, 136.5, 137.0, 138.5, 141.2, 148.2, 154.7, 183.7, 189.9. MS (ESI+) m/z = 417.1. ESI-HRMS calculated for C27H16N2O3 [MH]+: 417.1239, found: 417.1240.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 712, 1157, 1254, 1401, 1597, 1671, 3020, 3431 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.30–7.35 (m, 3H), 7.43 (t, J = 7.5 Hz, 1H), 7.54–7.58 (m, 3H), 7.62–7.66 (m, 1H), 7.76–7.81 (m, 3H), 7.85 (d, J = 7.7 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 8.10 (d, J = 1.3 Hz, 1H), 10.27 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 113.5, 113.6, 118.7, 122.5, 124.5, 128.3, 129.3, 130.2, 130.3, 130.4, 131.3, 132.0, 132.7, 136.1, 136.4, 137.4, 138.3, 139.4, 139.7, 140.9, 196.7, 198.0. MS (ESI+) m/z = 455.0. ESI-HRMS calculated for C25H15BrN2O2 [MH]+: 455.0395, found: 455.0397.
2, v/v). IR (KBr) νmax: 712, 1157, 1254, 1401, 1597, 1671, 3020, 3431 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.30–7.35 (m, 3H), 7.43 (t, J = 7.5 Hz, 1H), 7.54–7.58 (m, 3H), 7.62–7.66 (m, 1H), 7.76–7.81 (m, 3H), 7.85 (d, J = 7.7 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 8.10 (d, J = 1.3 Hz, 1H), 10.27 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 113.5, 113.6, 118.7, 122.5, 124.5, 128.3, 129.3, 130.2, 130.3, 130.4, 131.3, 132.0, 132.7, 136.1, 136.4, 137.4, 138.3, 139.4, 139.7, 140.9, 196.7, 198.0. MS (ESI+) m/z = 455.0. ESI-HRMS calculated for C25H15BrN2O2 [MH]+: 455.0395, found: 455.0397.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 728, 1186, 1231, 1445, 1597, 1658, 3028, 3438 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.29–7.35 (m, 3H), 7.39–7.42 (m, 2H), 7.52–7.54 (m, 2H), 7.60–7.64 (m, 1H), 7.76–7.78 (m, 3H), 7.84 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 1.6 Hz, 1H), 8.04 (d, J = 4.9 Hz, 1H), 10.28 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 113.2, 118.7, 121.5, 121.9, 126.3, 128.3, 129.3, 129.5, 130.2, 130.3, 130.3, 130.6, 131.2, 132.7, 136.1, 136.6, 137.4, 138.3, 139.4, 139.5, 140.9, 196.6, 198.0. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.
2, v/v). IR (KBr) νmax: 728, 1186, 1231, 1445, 1597, 1658, 3028, 3438 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.29–7.35 (m, 3H), 7.39–7.42 (m, 2H), 7.52–7.54 (m, 2H), 7.60–7.64 (m, 1H), 7.76–7.78 (m, 3H), 7.84 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 1.6 Hz, 1H), 8.04 (d, J = 4.9 Hz, 1H), 10.28 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 113.2, 118.7, 121.5, 121.9, 126.3, 128.3, 129.3, 129.5, 130.2, 130.3, 130.3, 130.6, 131.2, 132.7, 136.1, 136.6, 137.4, 138.3, 139.4, 139.5, 140.9, 196.6, 198.0. MS (ESI+) m/z = 411.1. ESI-HRMS calculated for C25H15ClN2O2 [MH]+: 411.0900, found: 411.0904.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 798, 1054, 1263, 1404, 1575, 1668, 1716 3017, 3437 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.83 (s, 3H), 7.27–7.33 (m, 3H), 7.42 (t, J = 7.4 Hz, 1H), 7.49–7.56 (m, 4H), 7.64–7.68 (m, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.98 (d, J = 7.6 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 8.74 (s, 1H), 10.50 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 52.4, 112.5, 120.9, 121.1, 121.6, 121.9, 128.1, 129.8, 130.2, 130.6, 130.8, 131.6, 131.9, 132.8, 135.6, 136.7, 136.9, 137.3, 139.6, 140.6, 141.5, 165.8, 196.7, 198.0. MS (ESI+) m/z = 435.1. ESI-HRMS calculated for C27H18N2O4 [MH]+: 435.1345, found: 435.1348.
2, v/v). IR (KBr) νmax: 798, 1054, 1263, 1404, 1575, 1668, 1716 3017, 3437 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.83 (s, 3H), 7.27–7.33 (m, 3H), 7.42 (t, J = 7.4 Hz, 1H), 7.49–7.56 (m, 4H), 7.64–7.68 (m, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.98 (d, J = 7.6 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 8.74 (s, 1H), 10.50 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 52.4, 112.5, 120.9, 121.1, 121.6, 121.9, 128.1, 129.8, 130.2, 130.6, 130.8, 131.6, 131.9, 132.8, 135.6, 136.7, 136.9, 137.3, 139.6, 140.6, 141.5, 165.8, 196.7, 198.0. MS (ESI+) m/z = 435.1. ESI-HRMS calculated for C27H18N2O4 [MH]+: 435.1345, found: 435.1348.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 789, 1059, 1272, 1409, 1571, 1662, 1717 3011, 3439 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.41 (s, 3H), 3.86 (s, 3H), 7.27–7.47 (m, 6H), 7.49–7.54 (m, 2H), 7.77 (d, J = 7.6 Hz, 2H), 8.01–8.07 (m, 2H), 8.76 (s, 1H), 10.47 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.7, 52.5, 112.5, 120.8, 121.1, 121.6, 122.0, 128.1, 128.6, 129.8, 130.2, 130.4, 131.3, 131.9, 132.7, 133.7, 135.8, 136.5, 136.6, 137.1, 137.4, 141.2, 141.5, 165.9, 196.9, 197.3. MS (ESI+) m/z = 449.1. ESI-HRMS calculated for C28H20N2O4 [MH]+: 449.1501, found: 449.1505.
2, v/v). IR (KBr) νmax: 789, 1059, 1272, 1409, 1571, 1662, 1717 3011, 3439 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.41 (s, 3H), 3.86 (s, 3H), 7.27–7.47 (m, 6H), 7.49–7.54 (m, 2H), 7.77 (d, J = 7.6 Hz, 2H), 8.01–8.07 (m, 2H), 8.76 (s, 1H), 10.47 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.7, 52.5, 112.5, 120.8, 121.1, 121.6, 122.0, 128.1, 128.6, 129.8, 130.2, 130.4, 131.3, 131.9, 132.7, 133.7, 135.8, 136.5, 136.6, 137.1, 137.4, 141.2, 141.5, 165.9, 196.9, 197.3. MS (ESI+) m/z = 449.1. ESI-HRMS calculated for C28H20N2O4 [MH]+: 449.1501, found: 449.1505.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 1430, 1467, 1598, 1662, 2925 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.18–7.21 (m, 1H), 7.32 (t, J = 7.7 Hz, 2H), 7.45 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 4.1 Hz, 2H), 7.57–7.61 (m, 1H), 7.66–7.75 (m, 4H), 7.99 (d, J = 7.9 Hz, 1H), 8.22–8.23 (m, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 123.4, 126.6, 128.3, 129.3, 130.2, 130.3, 130.4, 131.2, 132.7, 137.0, 137.4, 139.3, 140.6, 148.6, 153.7, 195.7, 196.6. MS (ESI+) m/z = 288.1. ESI-HRMS calculated for C19H13NO2 [MH]+: 288.1025, found: 288.1030.
2, v/v). IR (KBr) νmax: 1430, 1467, 1598, 1662, 2925 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.18–7.21 (m, 1H), 7.32 (t, J = 7.7 Hz, 2H), 7.45 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 4.1 Hz, 2H), 7.57–7.61 (m, 1H), 7.66–7.75 (m, 4H), 7.99 (d, J = 7.9 Hz, 1H), 8.22–8.23 (m, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 123.4, 126.6, 128.3, 129.3, 130.2, 130.3, 130.4, 131.2, 132.7, 137.0, 137.4, 139.3, 140.6, 148.6, 153.7, 195.7, 196.6. MS (ESI+) m/z = 288.1. ESI-HRMS calculated for C19H13NO2 [MH]+: 288.1025, found: 288.1030.General experimental procedure for the synthesis of compound 5
To a round bottom flask containing ethanol (3 mL) were added (2-aroylaryl)(β-carbolin-2-yl)methanone 3 (0.25 mmol), hydrazine hydrochloride (0.25 mmol) and NaHCO3 (0.25 mmol). The reaction mixture was heated at 70 °C for 3 h. On completion of reaction (as monitored by TLC), the solvent was evaporated to get a residue, which was purified by column chromatography over silica gel using hexanes/EtOAc (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) as eluent to obtain appropriate product 5.
10, v/v) as eluent to obtain appropriate product 5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 658, 781, 1045, 1285, 1485, 3085, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.19–7.25 (m, 1H), 7.48 (d, J = 3.6 Hz, 2H), 7.51–7.54 (m, 4H), 7.75–7.78 (m, 2H), 7.91 (t, J = 7.3 Hz, 1H), 8.01 (d, J = 5.0 Hz, 1H), 8.05–8.10 (m, 2H), 8.61 (d, J = 5.0 Hz, 1H), 10.03 (d, J = 8.4 Hz, 1H), 11.16 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.9, 120.2, 121.7, 126.6, 126.7, 128.8, 128.9, 129.6, 129.9, 130.3, 130.9, 132.0, 132.6, 133.0, 134.3, 136.2, 136.5, 137.8, 138.7, 140.9, 155.4, 159.6. MS (ESI+) m/z = 373.1. ESI-HRMS calculated for C25H16N4 [MH]+: 373.1453, found: 373.1455.
2, v/v). IR (KBr) νmax: 658, 781, 1045, 1285, 1485, 3085, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.19–7.25 (m, 1H), 7.48 (d, J = 3.6 Hz, 2H), 7.51–7.54 (m, 4H), 7.75–7.78 (m, 2H), 7.91 (t, J = 7.3 Hz, 1H), 8.01 (d, J = 5.0 Hz, 1H), 8.05–8.10 (m, 2H), 8.61 (d, J = 5.0 Hz, 1H), 10.03 (d, J = 8.4 Hz, 1H), 11.16 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.9, 120.2, 121.7, 126.6, 126.7, 128.8, 128.9, 129.6, 129.9, 130.3, 130.9, 132.0, 132.6, 133.0, 134.3, 136.2, 136.5, 137.8, 138.7, 140.9, 155.4, 159.6. MS (ESI+) m/z = 373.1. ESI-HRMS calculated for C25H16N4 [MH]+: 373.1453, found: 373.1455.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 667, 768, 1022, 1242, 1461, 3041, 3392 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.23–7.28 (m, 1H), 7.51–7.55 (m, 4H), 7.73 (d, J = 8.2 Hz, 2H), 7.83 (t, J = 7.2 Hz, 1H), 7.96 (t, J = 7.2 Hz, 1H), 8.03–8.07 (m, 2H), 8.13 (d, J = 7.8 Hz, 1H), 8.65 (d, J = 5.0 Hz, 1H), 10.07 (d, J = 8.5 Hz, 1H), 11.11 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 116.0, 120.3, 121.5, 121.7, 126.2, 126.6, 128.9, 129.1, 129.2, 131.0, 131.6, 132.3, 132.8, 134.9, 136.0, 136.2, 137.9, 138.5, 140.9, 155.7, 158.6. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C25H15ClN4 [MH]+: 407.1063, found: 407.1065.
2, v/v). IR (KBr) νmax: 667, 768, 1022, 1242, 1461, 3041, 3392 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.23–7.28 (m, 1H), 7.51–7.55 (m, 4H), 7.73 (d, J = 8.2 Hz, 2H), 7.83 (t, J = 7.2 Hz, 1H), 7.96 (t, J = 7.2 Hz, 1H), 8.03–8.07 (m, 2H), 8.13 (d, J = 7.8 Hz, 1H), 8.65 (d, J = 5.0 Hz, 1H), 10.07 (d, J = 8.5 Hz, 1H), 11.11 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 116.0, 120.3, 121.5, 121.7, 126.2, 126.6, 128.9, 129.1, 129.2, 131.0, 131.6, 132.3, 132.8, 134.9, 136.0, 136.2, 137.9, 138.5, 140.9, 155.7, 158.6. MS (ESI+) m/z = 407.1. ESI-HRMS calculated for C25H15ClN4 [MH]+: 407.1063, found: 407.1065.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 663, 768, 1012, 1289, 1412, 3082, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.49 (d, J = 3.6 Hz, 2H), 7.73 (t, J = 7.9 Hz, 1H), 7.85 (t, J = 7.6 Hz, 1H), 7.97 (t, J = 8.7 Hz, 2H), 8.05 (d, J = 5.0 Hz, 1H), 8.12 (d, J = 7.7 Hz, 2H), 8.39 (d, J = 8.2 Hz, 1H), 8.63–8.66 (m, 2H), 10.09 (d, J = 8.5 Hz, 1H), 11.07 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 116.2, 120.4, 121.5, 121.8, 124.5, 125.3, 125.5, 126.3, 126.6, 128.9, 129.6, 129.9, 131.2, 132.7, 133.2, 136.2, 136.3, 138.0, 138.2, 140.9, 148.6, 156.2, 157.4. MS (ESI+) m/z = 418.1. ESI-HRMS calculated for C25H15N5O2 [MH]+: 418.1304, found: 418.1308.
2, v/v). IR (KBr) νmax: 663, 768, 1012, 1289, 1412, 3082, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.22–7.26 (m, 1H), 7.49 (d, J = 3.6 Hz, 2H), 7.73 (t, J = 7.9 Hz, 1H), 7.85 (t, J = 7.6 Hz, 1H), 7.97 (t, J = 8.7 Hz, 2H), 8.05 (d, J = 5.0 Hz, 1H), 8.12 (d, J = 7.7 Hz, 2H), 8.39 (d, J = 8.2 Hz, 1H), 8.63–8.66 (m, 2H), 10.09 (d, J = 8.5 Hz, 1H), 11.07 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 116.2, 120.4, 121.5, 121.8, 124.5, 125.3, 125.5, 126.3, 126.6, 128.9, 129.6, 129.9, 131.2, 132.7, 133.2, 136.2, 136.3, 138.0, 138.2, 140.9, 148.6, 156.2, 157.4. MS (ESI+) m/z = 418.1. ESI-HRMS calculated for C25H15N5O2 [MH]+: 418.1304, found: 418.1308.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 666, 762, 1068, 1211, 1412, 3015, 3398 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.01 (s, 2H), 6.94 (d, J = 8.3 Hz, 1H), 7.17–7.24 (m, 3H), 7.47 (d, J = 3.8 Hz, 2H), 7.77 (t, J = 7.9 Hz, 1H), 7.86–7.90 (m, 1H), 7.99 (d, J = 5.0 Hz, 1H), 8.08 (dd, J1 = 7.8 Hz, J2 = 4.3 Hz, 2H), 8.59 (d, J = 5.0 Hz, 1H), 9.97 (d, J = 8.4 Hz, 1H), 11.13 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 101.7, 108.6, 110.6, 112.2, 115.8, 120.2, 121.5, 121.7, 124.7, 126.5, 126.6, 128.7, 128.9, 130.3, 130.9, 131.9, 132.6, 136.1, 137.8, 138.7, 140.9, 148.1, 148.9, 155.2, 159.0. MS (ESI+) m/z = 417.1. ESI-HRMS calculated for C26H16N4O2 [MH]+: 417.1352, found: 417.1355.
2, v/v). IR (KBr) νmax: 666, 762, 1068, 1211, 1412, 3015, 3398 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.01 (s, 2H), 6.94 (d, J = 8.3 Hz, 1H), 7.17–7.24 (m, 3H), 7.47 (d, J = 3.8 Hz, 2H), 7.77 (t, J = 7.9 Hz, 1H), 7.86–7.90 (m, 1H), 7.99 (d, J = 5.0 Hz, 1H), 8.08 (dd, J1 = 7.8 Hz, J2 = 4.3 Hz, 2H), 8.59 (d, J = 5.0 Hz, 1H), 9.97 (d, J = 8.4 Hz, 1H), 11.13 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 101.7, 108.6, 110.6, 112.2, 115.8, 120.2, 121.5, 121.7, 124.7, 126.5, 126.6, 128.7, 128.9, 130.3, 130.9, 131.9, 132.6, 136.1, 137.8, 138.7, 140.9, 148.1, 148.9, 155.2, 159.0. MS (ESI+) m/z = 417.1. ESI-HRMS calculated for C26H16N4O2 [MH]+: 417.1352, found: 417.1355.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 668, 768, 1063, 1218, 1409, 3019, 3397 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.50 (s, 3H), 7.22–7.26 (m, 1H), 7.51–7.58 (m, 5H), 7.75–7.78 (m, 3H), 7.84 (s, 1H), 8.05 (d, J = 5.1 Hz, 1H), 8.13 (d, J = 7.8 Hz, 1H), 8.64 (d, J = 5.0 Hz, 1H), 9.95 (d, J = 8.7 Hz, 1H), 11.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.8, 120.2, 121.6, 121.7, 124.9, 125.5, 127.0, 128.8, 128.8, 129.5, 130.3, 130.9, 134.6, 136.2, 136.8, 137.8, 138.9, 140.9, 142.8, 155.4, 159.4. MS (ESI+) m/z = 387.2. ESI-HRMS calculated for C26H18N4 [MH]+: 387.1610, found: 387.1615.
2, v/v). IR (KBr) νmax: 668, 768, 1063, 1218, 1409, 3019, 3397 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.50 (s, 3H), 7.22–7.26 (m, 1H), 7.51–7.58 (m, 5H), 7.75–7.78 (m, 3H), 7.84 (s, 1H), 8.05 (d, J = 5.1 Hz, 1H), 8.13 (d, J = 7.8 Hz, 1H), 8.64 (d, J = 5.0 Hz, 1H), 9.95 (d, J = 8.7 Hz, 1H), 11.19 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.8, 120.2, 121.6, 121.7, 124.9, 125.5, 127.0, 128.8, 128.8, 129.5, 130.3, 130.9, 134.6, 136.2, 136.8, 137.8, 138.9, 140.9, 142.8, 155.4, 159.4. MS (ESI+) m/z = 387.2. ESI-HRMS calculated for C26H18N4 [MH]+: 387.1610, found: 387.1615.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 664, 768, 1068, 1214, 1408, 3017, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.76 (s, 3H), 7.17–7.21 (m, 1H), 7.29 (d, J = 2.4 Hz, 1H), 7.45–7.53 (m, 6H), 7.75–7.77 (m, 2H), 7.97 (d, J = 5.0 Hz, 1H), 8.07 (d, J = 7.8 Hz, 1H), 8.57 (d, J = 5.0 Hz, 1H), 9.97 (d, J = 9.4 Hz, 1H), 11.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 55.7, 112.2, 115.8, 120.2, 121.5, 121.7, 121.9, 123.9, 128.8, 128.8, 129.0, 129.6, 129.7, 130.0, 130.9, 131.2, 136.2, 136.9, 137.8, 138.9, 140.9, 154.9, 159.0. MS (ESI+) m/z = 403.2. ESI-HRMS calculated for C26H18N4O [MH]+: 403.1559, found: 403.1562.
2, v/v). IR (KBr) νmax: 664, 768, 1068, 1214, 1408, 3017, 3395 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.76 (s, 3H), 7.17–7.21 (m, 1H), 7.29 (d, J = 2.4 Hz, 1H), 7.45–7.53 (m, 6H), 7.75–7.77 (m, 2H), 7.97 (d, J = 5.0 Hz, 1H), 8.07 (d, J = 7.8 Hz, 1H), 8.57 (d, J = 5.0 Hz, 1H), 9.97 (d, J = 9.4 Hz, 1H), 11.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 55.7, 112.2, 115.8, 120.2, 121.5, 121.7, 121.9, 123.9, 128.8, 128.8, 129.0, 129.6, 129.7, 130.0, 130.9, 131.2, 136.2, 136.9, 137.8, 138.9, 140.9, 154.9, 159.0. MS (ESI+) m/z = 403.2. ESI-HRMS calculated for C26H18N4O [MH]+: 403.1559, found: 403.1562.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 651, 712, 1012, 1281, 1415, 3084, 3397 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.25 (s, 1H), 7.36–7.42 (m, 3H), 7.52–7.58 (m, 7H), 7.83 (d, J = 5.7 Hz, 2H), 8.06 (d, J = 3.8 Hz, 1H), 8.16 (dd, J1 = 19.3 Hz, J2 = 7.6 Hz, 2H), 8.25 (s, 1H), 8.66 (d, J = 4.3 Hz, 1H), 10.14 (d, J = 8.7 Hz, 1H), 11.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.9, 120.2, 121.5, 121.7, 124.0, 125.6, 127.2, 127.7, 128.7, 128.8, 128.9, 129.3, 129.6, 129.7, 130.3, 130.9, 131.9, 136.2, 136.6, 137.9, 139.6, 140.9, 144.6, 155.3, 159.8. MS (ESI+) m/z = 449.2. ESI-HRMS calculated for C31H20N4 [MH]+: 449.1766, found: 449.1765.
2, v/v). IR (KBr) νmax: 651, 712, 1012, 1281, 1415, 3084, 3397 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.25 (s, 1H), 7.36–7.42 (m, 3H), 7.52–7.58 (m, 7H), 7.83 (d, J = 5.7 Hz, 2H), 8.06 (d, J = 3.8 Hz, 1H), 8.16 (dd, J1 = 19.3 Hz, J2 = 7.6 Hz, 2H), 8.25 (s, 1H), 8.66 (d, J = 4.3 Hz, 1H), 10.14 (d, J = 8.7 Hz, 1H), 11.21 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.2, 115.9, 120.2, 121.5, 121.7, 124.0, 125.6, 127.2, 127.7, 128.7, 128.8, 128.9, 129.3, 129.6, 129.7, 130.3, 130.9, 131.9, 136.2, 136.6, 137.9, 139.6, 140.9, 144.6, 155.3, 159.8. MS (ESI+) m/z = 449.2. ESI-HRMS calculated for C31H20N4 [MH]+: 449.1766, found: 449.1765.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 8
EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). IR (KBr) νmax: 684, 781, 1015, 1242, 1412, 3042, 3398 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.21–7.28 (m, 1H), 7.52–7.59 (m, 5H), 7.69 (d, J = 5.5 Hz, 1H), 7.95–7.99 (m, 3H), 8.14 (d, J = 7.9 Hz, 1H), 8.66 (d, J = 5.0 Hz, 1H), 10.14 (s, 1H), 11.41 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.3, 116.1, 120.3, 121.3, 121.8, 122.5, 128.9, 129.0, 129.6, 129.8, 130.6, 135.1, 135.7, 136.8, 137.2, 137.2, 137.7, 128.3, 141.2, 154.3, 155.2. MS (ESI+) m/z = 379.1. ESI-HRMS calculated for C23H14N4S [MH]+: 379.1017, found: 379.1015.
2, v/v). IR (KBr) νmax: 684, 781, 1015, 1242, 1412, 3042, 3398 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.21–7.28 (m, 1H), 7.52–7.59 (m, 5H), 7.69 (d, J = 5.5 Hz, 1H), 7.95–7.99 (m, 3H), 8.14 (d, J = 7.9 Hz, 1H), 8.66 (d, J = 5.0 Hz, 1H), 10.14 (s, 1H), 11.41 (s, 1H). 13C NMR (100 MHz, CDCl3): δ (ppm) = 112.3, 116.1, 120.3, 121.3, 121.8, 122.5, 128.9, 129.0, 129.6, 129.8, 130.6, 135.1, 135.7, 136.8, 137.2, 137.2, 137.7, 128.3, 141.2, 154.3, 155.2. MS (ESI+) m/z = 379.1. ESI-HRMS calculated for C23H14N4S [MH]+: 379.1017, found: 379.1015.Acknowledgements
One of the authors (SK) acknowledges the University Grant Commission, New Delhi for the fellowships. The authors acknowledge SAIF division of CSIR-CDRI for providing spectroscopic data. This work was supported by MOES grant to SB.References
- For some reviews on decarboxylative coupling reactions, see: (a) L. J. Goossen, N. Rodriguez and K. Goossen, Angew. Chem., Int. Ed., 2008, 47, 3100 CrossRef CAS PubMed; (b) L. J. Goossen, F. Collet and K. Goossen, Isr. J. Chem., 2010, 50, 617 CrossRef CAS; (c) N. Rodriguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030 RSC; (d) J. Cornella and I. Larrosa, Synthesis, 2012, 5, 653 Search PubMed; (e) K. Park and S. Lee, RSC Adv., 2013, 3, 14165 RSC; (f) C. Shen, P. Zhang, Q. Sun, S. Bai, T. S. A. Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC.
- L. J. Goossen, F. Rudolphi, C. Oppel and N. Rodriguez, Angew. Chem., Int. Ed., 2008, 47, 3043 CrossRef CAS PubMed.
- (a) P. Fang, M. Li and H. Ge, J. Am. Chem. Soc., 2010, 132, 11898 CrossRef CAS PubMed; (b) M. Li and H. Ge, Org. Lett., 2010, 12, 3464 CrossRef CAS PubMed.
- H. Li, P. Li, H. Tan and L. Wang, Chem.–Eur. J., 2013, 19, 14432 CrossRef CAS PubMed.
- N. Xu, P. Li, Z. Xie and L. Wang, Chem.–Eur. J., 2016, 22, 2236 CrossRef CAS PubMed.
- J. Miao and H. Ge, Org. Lett., 2013, 15, 2930 CrossRef CAS PubMed.
- H. Wang, L. Guo and X. H. Duan, Org. Lett., 2012, 14, 4358 CrossRef CAS PubMed.
- M. Kim, J. Park, S. Sharma, A. Kim, E. Park, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 925 RSC.
- S. Sharma, A. Kim, E. Park, J. Park, M. Kim, J. H. Kwak, S. H. Lee, Y. H. Jung and I. S. Kim, Adv. Synth. Catal., 2013, 4, 667 CrossRef.
- J. Park, M. Kim, S. Sharma, E. Park, A. Kim, S. H. Lee, J. H. Kwak, Y. H. Jung and I. S. Kim, Chem. Commun., 2013, 49, 1654 RSC.
- J. Yao, R. Feng, Z. Wu, Z. Liu and Y. Zhanga, Adv. Synth. Catal., 2013, 355, 1517 CrossRef CAS.
- R. Suresh, R. S. Kumaran, V. Senthilkumar and S. Muthusubramanian, RSC Adv., 2014, 4, 31685 RSC.
- M. Kim, N. K. Mishra, J. Park, S. Han, Y. Shin, S. Sharma, Y. Lee, E. K. Lee, J. H. Kwaka and I. S. Kim, Chem. Commun., 2014, 50, 14249 RSC.
- B. Xu, W. Liu and C. Kuang, Eur. J. Org. Chem., 2014, 2576 CrossRef CAS.
- Y. Wu, L. Sun, Y. Chen, Q. Zhou, J. W. Huang, H. Miao and H. B. Luo, J. Org. Chem., 2016, 81, 1244 CrossRef CAS PubMed.
- L. Han, Y. Wang, H. Song, H. Han, L. Wang, W. Chu and Z. Sun, RSC Adv., 2016, 6, 20637 RSC.
- (a) C. Pan, H. Jin, X. Liu, Y. Cheng and C. Zhu, Chem. Commun., 2013, 49, 2933 RSC; (b) L. Yu, P. Li and L. Wang, Chem. Commun., 2013, 49, 2368 RSC; (c) C. Wang, S. Wang, H. Li, J. Yan, H. Chi, X. Chen and Z. Zhang, Org. Biomol. Chem., 2014, 12, 1721 RSC.
- (a) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS PubMed; (b) A. Teichert, J. Schmidt, A. Porzel, N. Arnold and L. Wessjohann, J. Nat. Prod., 2007, 70, 1529 CrossRef CAS PubMed; (c) W. Wang, S. J. Nam, B. C. Lee and H. Kang, J. Nat. Prod., 2008, 71, 163 CrossRef CAS PubMed; (d) Y. F. Chen, P. C. Kuo, H. H. Chan, I. J. Kuo, F. W. Lin, C. R. Su, M. L. Yang, D. T. Li and T. S. Wu, J. Nat. Prod., 2010, 73, 1993 CrossRef CAS PubMed; (e) H. Huang, Y. Yao, Z. He, T. Yang, J. Ma, X. Tian, Y. Li, C. Huang, X. Chen, W. Li, S. Zhang, C. Zhang and J. Ju, J. Nat. Prod., 2011, 74, 2122 CrossRef CAS PubMed; (f) H. D. H. Showalter, J. Nat. Prod., 2013, 76, 455 CrossRef CAS PubMed; (g) H. Jin, P. Zhang, K. Bijian, S. Ren, S. Wan, M. A. A. Jamali and T. Jiang, Mar. Drugs, 2013, 11, 1427 CrossRef CAS PubMed; (h) W. R. Sawadogo, M. Schumacher, M. H. Teiten, C. Cerella, M. Dicato and M. Diederich, Molecules, 2013, 18, 3641 CrossRef CAS PubMed; (i) G. Hamilton, Mar. Drugs, 2014, 12, 1377 CrossRef PubMed; (j) S. U. Dighe, S. Khan, I. Soni, P. Jain, S. Shukla, R. Yadav, P. Sen, S. M. Meeran and S. Batra, J. Med. Chem., 2015, 58, 3485 CrossRef CAS PubMed; (k) Y. Ling, C. Xu, L. Luo, J. Cao, J. Feng, Y. Xue, Q. Zhu, C. Ju, F. Li, Y. Zhang, Y. Zhang and X. Ling, J. Med. Chem., 2015, 58, 9214 CrossRef CAS PubMed; (l) R. Otto, R. Penzis, F. Gaube, O. Adolph, K. J. Fohr, P. Warncke, D. Robaa, D. Appenroth, C. Fleck, C. Enzensperger, J. Lehmann and T. Winckler, J. Med. Chem., 2015, 58, 6710 CrossRef CAS PubMed.
- S. Rajkumar, S. Karthik and T. Gandhi, J. Org. Chem., 2015, 80, 5532 CrossRef CAS PubMed.
- S. Kolle and S. Batra, Org. Biomol. Chem., 2015, 13, 10376 CAS.
- (a) S. Ueno, N. Chatani and F. Kakiuchi, J. Org. Chem., 2007, 72, 3600 CrossRef CAS PubMed; (b) G. Shan, X. Yang, L. Ma and Y. Rao, Angew. Chem., Int. Ed., 2012, 51, 13070 CrossRef CAS PubMed; (c) V. S. Thirunavukkarasu and L. Ackermann, Org. Lett., 2012, 14, 6209 Search PubMed; (d) F. Mo, L. J. Trzepkowski and G. Dong, Angew. Chem., Int. Ed., 2012, 51, 13075 CrossRef CAS PubMed; (e) G. Shan, X. Han, Y. Lin, S. Yua and Y. Rao, Org. Biomol. Chem., 2013, 11, 2318 RSC; (f) P. Y. Choy and F. Y. Kwong, Org. Lett., 2013, 15, 270 CrossRef CAS PubMed; (g) Z. Huang, H. N. Lim, F. Mo, M. C. Young and G. Dong, Chem. Soc. Rev., 2015, 44, 7764 RSC.
- S. Y. Hyun, Korean Pat., KR 2014145355, 2014Chem. Abstr., 162, 170198.
Footnotes |
| † CDRI communication no. 9334. |
| ‡ Electronic supplementary information (ESI) available: Copies of 1H and 13C-spectra of all compounds are provided. See DOI: 10.1039/c6ra11811a |
| This journal is © The Royal Society of Chemistry 2016 |