Open Access Article
Open Access ArticleUnusual seeding effect in the liquid-assisted high-pressure polymorphism of chlorpropamide†
B. A.
Zakharov
ab,
S. V.
Goryainov
c and
E. V.
Boldyreva
*a
aInstitute of Solid State Chemistry and Mechanochemistry, Siberian Branch of Russian Academy of Sciences, ul. Kutateladze, 18, Novosibirsk 630128, Russia. E-mail: b.zakharov@yahoo.com; eboldyreva@yahoo.com
bNovosibirsk State University, ul. Pirogova, 2, Novosibirsk 630090, Russia
cV.S. Sobolev Institute of Geology and Mineralogy, Siberian Branch of Russian Academy of Sciences, pr. Acad. Koptyug 3, Novosibirsk 630090, Russia. E-mail: svg@igm.nsc.ru
First published on 6th June 2016
Abstract
When crystals of four polymorphs – a stable α-form and three metastable ones (β-, γ-, δ-forms) – are simultaneously present in a pressure cell, immersed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pentane–isopentane mixture, the least stable β-form recrystallises exclusively into the γ-form (if no δ-form seed is present) or concomitantly into the γ- and δ-forms (if a seed of the δ-form is present). However, the β-form never recrystallises to the stable α-form, even if the latter is also present as a potential seed.
1 pentane–isopentane mixture, the least stable β-form recrystallises exclusively into the γ-form (if no δ-form seed is present) or concomitantly into the γ- and δ-forms (if a seed of the δ-form is present). However, the β-form never recrystallises to the stable α-form, even if the latter is also present as a potential seed.
Studies of polymorphic molecular crystals are important in many fields of chemistry, including, among others, theory of intermolecular interactions, supramolecular chemistry, crystal engineering, crystallisation theory, chemical engineering, pharmaceutical chemistry, and high-pressure science.1–11 For a compound prone to polymorphism, it is not possible to predict which phase will be formed at a selected (T, P) point based solely on a thermodynamic phase diagram. Instead, there exists a complex interplay of nucleation and growth kinetics, alongside thermodynamics, that leads to these unpredictable results. This is reflected in phenomena which were termed “concomitant polymorphism” (several phases co-exist under the same conditions, often in the same batch),12 “disappearing polymorphs” (a fast-growing metastable polymorph obtained once can no longer be crystallised after a seed of the stable form has been obtained),13,14 “pre-nucleation” (molecular clusters in solution pre-determine the structure of a crystalline nucleus formed at later stages),15 “seeding-triggered crystallisation” (a seed of a phase triggers the crystallisation of the same phase),16 and “template crystallisation” (molecules or molecular clusters in solution influence crystallisation of a certain crystalline form, even if not included in the final crystal structure themselves).17,18 High pressure has been recently proposed as a tool to obtain new polymorphs of pharmaceuticals, which, if preserved on flash-decompression, can be used as seeds for mass crystallisation under ambient conditions.19–23 However, the control of high-pressure polymorphism is not a trivial task.19,24–28 The role of kinetic factors known to influence crystallisation under ambient conditions becomes enormously significant at high pressure, when molecular motions are even more restricted, the sample is located in a confined space of a gasket hole, and the pressure-transmitting fluid is not mixed and is often viscous. Thus diffusion and convection processes are hindered. As a result of the kinetic control of nucleation and nuclei growth,28 different phases can form, depending on the choice of the starting polymorph,29–31 the hydrostatic medium,32–37 or the compression/decompression protocol.26,38–42
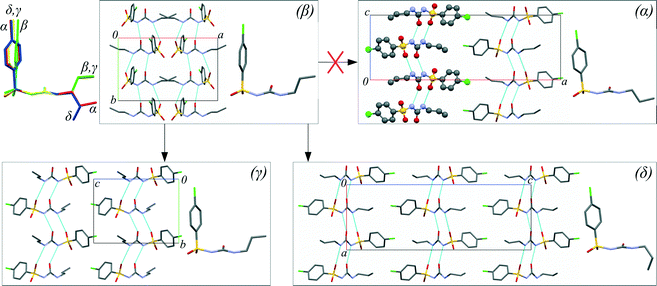
An antidiabetic drug, chlorpropamide (4-chloro-N-(propylaminocarbonyl)benzenesulfonamide, C10H13ClN2O3S), is particularly prone to polymorphism.43,44 In addition to the commercially available α-polymorph, which is the thermodynamically stable form under ambient conditions,45 four other polymorphs (β-, γ-, δ-, ε-) can be preserved for an indefinitely long period time under ambient conditions. These four additional phases can be obtained either from solution crystallisation in different solvents (β-, γ-, δ-) or at high temperature (ε-).46–48 All known polymorphs have similar hydrogen-bonded chains involving the urea-like core, but differ in the conformation adopted by the phenyl ring and alkyl tails, and the relative orientation of the hydrogen-bonded chains (Fig. 1 and Table S1 in the ESI†). Chlorpropamide is therefore an ideal model system with which the roles of molecular flexibility and intermolecular interactions in forming and rearranging the crystal structures of organic crystals can be studied. The five polymorphs respond differently to cooling49–51 and heating,48 with each ambient-condition polymorph transforming into a unique non-ambient-temperature form. One could expect similarly high versatility in behavior on hydrostatic compression.
To date, only the effect of pressure on the α-polymorph, which is the stable form at ambient pressure,45 has been reported.34–37 The α-polymorph undergoes at least one phase transition with increasing pressure in saturated ethanol solution (recrystallisation is possible).37 The same phase transition seems to also take place in dry powder samples without adding any pressure-transmitting fluids,34 though the transition in this case was reported to be kinetically hindered, and thus believed to be solvent-assisted.34 In the present work, we aimed to monitor the pressure-induced structural response of four polymorphs in a fluid that does not dissolve any of the polymorphs at ambient pressure. However, our experiments have shown that one of the metastable polymorphs, the β-form, starts recrystallising as the pressure increases. Interestingly, the other polymorphs do not dissolve in the same fluid, even at high pressures. Even more interestingly, a crystal of the most stable polymorph (the α-form) present in the pressure cell simultaneously with the β-polymorph does not act as a seed, whereas another metastable polymorph (the δ-form) does. We report these observations and rationalise them in the present paper.
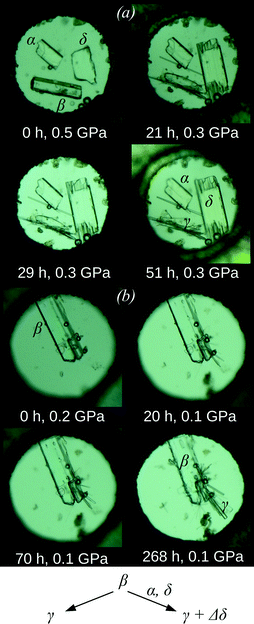
Crystals of the α-, β-, and δ-polymorphs were simultaneously loaded into a diamond anvil cell (DAC) to ensure identical compression conditions for a better comparison of their response to increasing pressure. Samples were kept at ambient temperature in all experiments. A pentane–isopentane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was selected as the pressure-transmitting medium, which does not visibly dissolve any of the three chlorpropamide polymorphs under ambient conditions and has a high hydrostatic limit of 7 GPa.52 The pressure was increased directly to 0.5 GPa and held for two hours. Following this period, the β-polymorph had dissolved very slightly (the crystal edges had become slightly rounded), whereas the α- and δ-polymorphs had not changed (Fig. 2a and S1†). The following day, the pressure in the DAC had spontaneously decreased to 0.3 GPa, and small, thin needles had appeared. Additionally, the crystal of the δ-polymorph had increased in size and the crystal of the β-polymorph had begun dissolving, whereas the crystal of the α-form had remained visually unchanged (Fig. 2a and S1†).
1) was selected as the pressure-transmitting medium, which does not visibly dissolve any of the three chlorpropamide polymorphs under ambient conditions and has a high hydrostatic limit of 7 GPa.52 The pressure was increased directly to 0.5 GPa and held for two hours. Following this period, the β-polymorph had dissolved very slightly (the crystal edges had become slightly rounded), whereas the α- and δ-polymorphs had not changed (Fig. 2a and S1†). The following day, the pressure in the DAC had spontaneously decreased to 0.3 GPa, and small, thin needles had appeared. Additionally, the crystal of the δ-polymorph had increased in size and the crystal of the β-polymorph had begun dissolving, whereas the crystal of the α-form had remained visually unchanged (Fig. 2a and S1†).
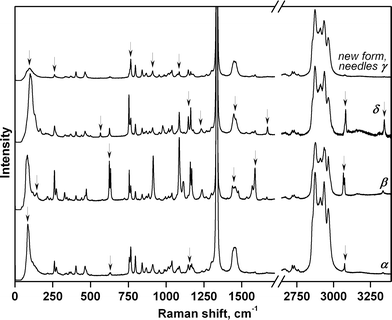
High-pressure vibrational spectra were measured in situ for each individual polymorph crystal by using a Raman confocal microscope. The Raman spectra of the newly formed needle-shaped crystals did not match those of any of the polymorphs originally loaded into the DAC (α-, β-, or δ-forms). However, the Raman data were of insufficient quality to reliably identify the new phase (Fig. 3). To identify the new needle-shaped phase unambiguously, a single crystal of β-chlorpropamide was placed alone in the DAC, again in a pentane–isopentane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Partial dissolution of the crystal and the growth of multiple tiny needles (with similar appearance to those described above) were observed (Fig. 2b). An attempt to characterize this phase in situ by single-crystal X-ray diffraction, even using synchrotron radiation, was unsuccessful; the sample contained too many crystallites for a successful single-crystal diffraction approach, and too few with a similar orientation for a powder diffraction experiment. In order to study the structure of a selected crystal of the new phase, the DAC was thus decompressed and opened. The hydrostatic fluid evaporated immediately upon opening the cell, and the needles of the high-pressure phase did not dissolve or recrystallize. An individual needle (of approximate size 0.15 × 0.01 × 0.01 mm3) was selected and single-crystal diffraction showed that the needles corresponded to γ-chlorpropamide.47 This was particularly surprising as it is known to be less dense under ambient pressure (1.416 g cm−3) than the α- and δ-polymorphs (1.450 and 1.455 g cm−3, respectively48,49). An additional experiment was carried out to confirm that the γ-polymorph in pentane–isopentane does not undergo any phase transitions in the pressure range of these experiments (below 1 GPa), either on compression or on decompression. Thus, in addition to optical microscopy observations, X-ray diffraction completely excludes the possibility that the γ-polymorph might be formed during decompression of another high-pressure phase or upon subsequent opening of the DAC.
1). Partial dissolution of the crystal and the growth of multiple tiny needles (with similar appearance to those described above) were observed (Fig. 2b). An attempt to characterize this phase in situ by single-crystal X-ray diffraction, even using synchrotron radiation, was unsuccessful; the sample contained too many crystallites for a successful single-crystal diffraction approach, and too few with a similar orientation for a powder diffraction experiment. In order to study the structure of a selected crystal of the new phase, the DAC was thus decompressed and opened. The hydrostatic fluid evaporated immediately upon opening the cell, and the needles of the high-pressure phase did not dissolve or recrystallize. An individual needle (of approximate size 0.15 × 0.01 × 0.01 mm3) was selected and single-crystal diffraction showed that the needles corresponded to γ-chlorpropamide.47 This was particularly surprising as it is known to be less dense under ambient pressure (1.416 g cm−3) than the α- and δ-polymorphs (1.450 and 1.455 g cm−3, respectively48,49). An additional experiment was carried out to confirm that the γ-polymorph in pentane–isopentane does not undergo any phase transitions in the pressure range of these experiments (below 1 GPa), either on compression or on decompression. Thus, in addition to optical microscopy observations, X-ray diffraction completely excludes the possibility that the γ-polymorph might be formed during decompression of another high-pressure phase or upon subsequent opening of the DAC.
These experiments proved to be highly reproducible. Invariably, in the absence of δ-form seeds, needles of the γ-form recrystallised from the β-polymorph. Alternatively, in the presence of a δ-form seed, simultaneous slow recrystallisation of the β-crystal into both the γ-form (new crystals) and the δ-form (an increase in the size of the seed crystals) was observed. It therefore appears that the high-pressure transformations of β-chlorpropamide below 0.5 GPa are not solid-state phase transitions, but result from recrystallisation. Interestingly, the α-form – the most stable polymorph under ambient conditions, though not the densest one45 – neither acted as a seed in the recrystallisation of the β- or γ-polymorphs nor recrystallised into the denser δ-form.
It would not be expected that the relative densities of the polymorphs could change in the absence of any phase transitions before 0.5 GPa. To verify this assumption, the unit cell volumes of the α-, γ-, and δ- polymorphs were measured at high pressure using single-crystal X-ray diffraction. All three crystals were loaded into the same DAC simultaneously, ensuring identical conditions for each polymorph. Paraffin was used as the pressure-transmitting medium in lieu of pentane–isopentane to exclude any recrystallisation events and allow hydrostatic conditions up to 5 GPa.53 The relative densities were calculated from unit cell parameters measured at 0.35 and 0.50 GPa (see the ESI† for cell parameters). The densities at 0.35 and 0.50 GPa were as follows: α-polymorph – 1.528 and 1.546 g cm−3; γ-polymorph – 1.522 and 1.549 g cm−3; δ-polymorph – 1.534 and 1.554 g cm−3, respectively.
Based on the relative densities, one might expect three high-pressure recrystallisation pathways for β-chlorpropamide: β → α, β → γ, or β → δ. However, only the two latter processes are observed. It is rather common that the presence of a stable polymorph suppresses the crystallisation of a metastable form.54 In contrast, the high-pressure behavior of chlorpropamide, when a seed of the most stable (α-) polymorph does not grow, but instead, one metastable polymorph (γ-) grows as new crystals even without seeding, and another metastable form present as a seed (δ-) increases in size, is highly unusual. It therefore becomes important to consider the relative rates of the required molecular re-organisation that are sufficient to yield critical nucleation clusters, sufficient only to grow an existing crystal, or so energetically unfavourable that neither nucleation nor growth occurs.
One can rationalise the observed phenomena having compared the molecular conformations and molecular packing in the polymorphs (Fig. 1), and assuming that both the conformations of individual molecules and molecular clusters can be preserved on dissolution. Neither the β → γ nor the β → δ transformation requires a basic change in the packing of molecular H-bonded chains, whereas the β → α transformation would require that every second band should be turned “upside down”. The β → γ transformation requires some rotation of the phenyl ring, but no major change in the position of the alkyl tail, whereas for both the β → δ and β → α transformations, the molecular conformation must change (Fig. 1). Thus, this suggests that the γ-form has the lowest nucleation barrier. The newly formed crystals of the γ-polymorph were located near the source of chlorpropamide molecules: the β-form crystal. This is consistent with the hypothesis that the molecular conformation and the fragments of the original crystal are preserved in solution for some time after dissolution, and the nuclei of the γ-phase are formed from these fragments. The presence of a seed of the denser δ-polymorph, with a different molecular conformation, favors molecular reconfiguration/reorientation as these molecules can now not form new crystals, but can instead be built into the denser phase where the molecules are already in the necessary conformation. It is important to note that the β → δ transformation does not require a change in the relative orientation of the hydrogen-bonded chains, in stark contrast to the β → α transition. The latter can therefore not occur (Fig. 1).
The ability of a molecule to preserve its conformation on dissolution from a crystal has been discussed in the literature previously,55–57 though it has only been experimentally proven for a few cases to date.58 The “crystalline conformation” can be stabilized both by interactions in clusters that remain on dissolution and, in some special cases, by interactions with the solvent. This dissolution process, which does not occur “molecule by molecule”, but by dissolution of molecular clusters or entire “double layers”, has also been discussed, modeled and, in some cases, even observed experimentally.59 One can expect the conditions within the confined space of a diamond anvil cell at high pressure to be particularly favorable for preserving molecular clusters on dissolution, especially if a hydrostatic fluid does not readily separate the molecules from each other on dissolution. Pentane and isopentane solute molecules are in fact not likely to break strong hydrogen bonds formed by the “urea core” of chlorpropamide chain clusters.
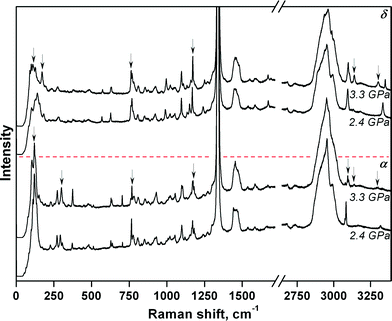
The complex mutual effect of chlorpropamide polymorphs at relatively low (0.5 GPa) pressures is not the only manifestation of the kinetic control of phase transitions in this system. As crystals of the β-polymorph recrystallised into other polymorphs slowly at 0.5 GPa, the parent crystals of each polymorph could be partially preserved. As such, the four polymorphs – α-, β-, γ-, and δ-forms – could be compressed further and their response to pressure could be monitored by Raman spectroscopy. For the α-, β- and δ-polymorphs, the structural responses were monitored up to 6.9 GPa and on subsequent decompression to 0.4 GPa. The needle-shaped crystals of the γ-form were not stable under the laser beam and were destroyed during data collection at 3.3 GPa (Fig. 4 and S2†).
The largest changes in the Raman spectra were observed upon increasing the pressure from 2.4 to 3.3 GPa (α- and δ-forms) and upon increasing the pressure from 1.2 to 2.4 GPa (β-form) (Fig. S2†). For the α-polymorph, this range corresponds to that over which the α → α′ phase transition was previously observed when using saturated ethanol solution as the pressure-transmitting fluid37 (Table S1†). Despite the fact that major changes in the α- and δ-chlorpropamide Raman spectra occur across the same pressure range, it is clear from the spectra (Fig. 4) that the δ-form does not transform into the α′-form, and is likely to form another phase at 3.3 GPa. It is therefore clear that the pressure-induced solid-state phase transition for each of the α-, β- and δ-chlorpropamide polymorphs is unique, giving different high-pressure phases. The different routes followed by each polymorph correspond to the minimum barrier required for the solid-state rearrangement, not to the formation of the thermodynamically most stable polymorph as the final product. In this respect, chlorpropamide behaves similarly to polymorphs of glycine.29–31
Conclusions
The example of chlorpropamide adds a new dimension to studying the complexities of the polymorphism of organic crystals in general, and of high-pressure polymorphism, in particular. Kinetic barriers for the structural rearrangement in the solid state and crystallisation at high pressure from solution can be significant and result in the formation of metastable materials. The resulting products may simply be those with the lowest kinetic barrier for nucleation.60 This barrier can obviously be changed in the presence of a seed, but not every polymorph can act as a seed (even being denser and even being more stable; these two criteria do not always coincide). Further, even in the presence of the most stable phase as a seed, other crystallisation routes to relatively less stable forms remain possible. The solid-state transformations can also give not the thermodynamically most stable phase, but the polymorph which can be formed more easily from the starting crystal structure.61 Transformations in a diamond anvil pressure cell can combine the restrictions of confined crystallisation in a small volume with the limitations of convection, the diffusion of molecules through a viscous medium and the difficulties of structural rearrangement of molecules and molecular packing in the solid state.Experimental
Single crystals of α- and δ-chlorpropamide were grown using techniques described in ref. 45. β-Chlorpropamide was grown from saturated ethanol solution.Pressure was generated using a Boehler-Almax62 diamond anvil cell (DAC) with a stainless steel gasket (hole diameter 300 μm). The ruby fluorescence line was used for pressure calibration with an accuracy of ±0.05 GPa.63 A pentane–isopentane mixture was used as the pressure-transmitting medium.52 Paraffin was used as the pressure-transmitting medium53 in the high-pressure X-ray diffraction experiment aimed at determining the relative densities of the α-, γ-, and δ-polymorphs of chlorpropamide. An Oxford Diffraction Gemini R Ultra diffractometer (Mo Kα) and CrysAlisPro64 software were used to determine the cell parameters.
Optical microscopy experiments were carried out using a Nikon AZ100 microscope.
A Horiba Jobin Yvon LabRam HR800 spectrometer was used for Raman spectroscopy. The spectra were obtained with the 532 nm line of a 40 mW neodymium solid-state laser (Nd:YAG neodymium-doped yttrium aluminium garnet Nd:Y3Al5O12 with a frequency-doubled line). The spectra were recorded on a 1024-channel LN/CCD detector from −10 to 3800 cm−1 with 2 cm−1 spectral resolution. The power of the laser beam on the sample inside the DAC was below 10 mW. Raman spectra were collected in back-scattering geometry using an Olympus BX41 microscope. This microscope used an Olympus 50× objective lens with a long working distance of LWD = 11 mm. Using a 0.5 numerical aperture produced a focal spot diameter of ~2 μm. An edge filter almost completely suppressed the low-frequency Raman spectrum below 60 cm−1 and attenuated that over the range 60–100 cm−1. The laser light that remained after scattering and collection in the spectrometer, subsequently suppressed by the edge filter to the residual low-intensity line at 0 cm−1, was used for additional calibration correction of each recorded spectrum.
Single-crystal synchrotron X-ray diffraction data were collected at the Swiss-Norwegian Beamline BM01A at the European Synchrotron Radiation Facility (ESRF, Grenoble, France, experiment CH-4526 (PILATUS 2M hybrid pixel detector, wavelength 0.81984 Å)). The data were converted and integrated using the SNBL toolbox65 and CrysAlisPro64 software packages. The crystal structure was refined with SHELXL.66 Complete structural data were deposited in the CSD67 with refcode CCDC 1437552.
Acknowledgements
The study was supported by a grant from RSF 14-13-00834. Assistance of Dr. A. Mikheykin with the single-crystal diffraction experiment at the BM01 beamline at the ESRF is acknowledged. We thank Dr. N. Chukanov and Mr. A. Marchuk for crystallisation of the α- and δ-chlorpropamide polymorphs (respectively), and Mr. A. Michalchuk for language polishing and useful comments. We are grateful to Prof. G. Romanenko and Mr. G. Letyagin from the International Tomography Center SB RAS for their help with the additional diffraction experiment to measure the cell parameters of the three polymorphs at high pressures.References
- A. J. Cruz-Cabeza, S. M. Reutzel-Edens and J. Bernstein, Chem. Soc. Rev., 2015, 44, 8619 RSC.
- M. A. Neumann, F. J. J. Leusen and J. Kendrick, Angew. Chem., Int. Ed., 2008, 47, 2427 CrossRef CAS PubMed.
- X.-Z. Li, M. Li, Z. Li, J.-Z. Hou, X.-C. Huang and D. Li, Angew. Chem., Int. Ed., 2008, 47, 6371 CrossRef CAS PubMed.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., Int. Ed., 2009, 48, 7087 CrossRef CAS PubMed.
- E. Patyk, J. Skumiel, M. Podsiadło and A. Katrusiak, Angew. Chem., Int. Ed., 2012, 51, 2146 CrossRef CAS PubMed.
- N. Marom, R. A. DiStasio, V. Atalla, S. Levchenko, A. M. Reilly, J. R. Chelikowsky, L. Leiserowitz and A. Tkatchenko, Angew. Chem., Int. Ed., 2013, 52, 6629 CrossRef CAS PubMed.
- A. Lemmerer, J. Bernstein, U. J. Griesser, V. Kahlenberg, D. M. Többens, S. H. Lapidus, P. W. Stephens and C. Esterhuysen, Chem. – Eur. J., 2011, 17, 13445 CrossRef CAS PubMed.
- J. Bernstein, Cryst. Growth Des., 2011, 11, 632 CAS.
- D. Singhal and W. Curatolo, Adv. Drug Delivery Rev., 2004, 56, 335 CrossRef CAS PubMed.
- R. J. Davey, S. L. M. Schroeder and J. H. ter Horst, Angew. Chem., Int. Ed., 2013, 52, 2167 CrossRef PubMed.
- A. Gavezzotti, J. Pharm. Sci., 2007, 96, 2232 CrossRef CAS PubMed.
- J. Bernstein, R. J. Davey and J.-O. Henck, Angew. Chem., Int. Ed., 1999, 38, 3440 CrossRef.
- D.-K. Bučar, R. W. Lancaster and J. Bernstein, Angew. Chem., Int. Ed., 2015, 54, 6972 CrossRef PubMed.
- J. D. Dunitz and J. Bernstein, Acc. Chem. Res., 1995, 28, 193 CrossRef CAS.
- C. Shahar, S. Dutta, H. Weissman, L. J. W. Shimon, H. Ott and B. Rybtchinski, Angew. Chem., Int. Ed., 2016, 55, 179 CrossRef CAS PubMed.
- T. Seki, K. Sakurada and H. Ito, Angew. Chem., Int. Ed., 2013, 52, 12828 CrossRef CAS PubMed.
- M. J. Prakash, P. Raghavaiah, Y. S. R. Krishna and T. P. Radhakrishnan, Angew. Chem., Int. Ed., 2008, 120(21), 3969 CrossRef.
- I. Weissbuch, V. Y. Torbeev, L. Leiserowitz and M. Lahav, Angew. Chem., Int. Ed., 2005, 44, 3226 CrossRef CAS PubMed.
- M. A. Neumann, J. van de Streek, F. P. A. Fabbiani, P. Hidber and O. Grassmann, Nat. Commun., 2015, 6, 7793 CrossRef CAS PubMed.
- F. P. A. Fabbiani, G. Buth, D. C. Levendis and A. J. Cruz-Cabeza, Chem. Commun., 2014, 50, 1817 RSC.
- F. P. A. Fabbiani, D. C. Levendis, G. Buth, W. F. Kuhs, N. Shankland and H. Sowa, CrystEngComm, 2010, 12, 2354 RSC.
- F. P. A. Fabbiani, NATO Sci. Peace Secur. Ser. B Phys. Biophys., 2010, 545 CrossRef.
- I. D. H. Oswald, I. Chataigner, S. Elphick, F. P. A. Fabbiani, A. R. Lennie, J. Maddaluno, W. G. Marshall, T. J. Prior, C. R. Pulham and R. I. Smith, CrystEngComm, 2009, 11, 359 RSC.
- E. Patyk, M. Podsiadło and A. Katrusiak, Cryst. Growth Des., 2015, 15, 5670 CAS.
- M. Anioła and A. Katrusiak, Cryst. Growth Des., 2015, 15, 764 Search PubMed.
- R. Gajda, A. Katrusiak and J. Crassous, CrystEngComm, 2009, 11, 2668 RSC.
- R. Lee, J. A. K. Howard, M. R. Probert and J. W. Steed, Chem. Soc. Rev., 2014, 43, 4300 RSC.
- E. Boldyreva, Cryst. Growth Des., 2007, 7, 1662 CAS.
- E. V. Boldyreva, S. N. Ivashevskaya and H. Sowa, Z. Kristallogr., 2005, 220, 50 CAS.
- S. V. Goryainov, E. N. Kolesnik and E. V. Boldyreva, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 357, 340 CrossRef CAS.
- E. V. Boldyreva, S. N. Ivashevskaya, H. Sowa, H. Ahsbahs and H. P. Weber, Dokl. Akad. Nauk, 2004, 396, 358 Search PubMed.
- E. Eikeland, M. K. Thomsen, S. R. Madsen, J. Overgaard, M. A. Spackman and B. B. Iversen, Chem. – Eur. J., 2016, 22(12), 4061–4069 CrossRef CAS PubMed.
- F. P. A. Fabbiani, D. R. Allan, W. I. F. David, A. J. Davidson, A. R. Lennie, S. Parsons, C. R. Pulham and J. E. Warren, Cryst. Growth Des., 2007, 7, 1115 CAS.
- E. V. Boldyreva, V. Dmitriev and B. C. Hancock, Int. J. Pharm., 2006, 327, 51 CrossRef CAS PubMed.
- J. Wąsicki, D. P. Kozlenko, S. E. Pankov, P. Bilski, A. Pajzderska, B. C. Hancock, A. Medek, W. Nawrocik and B. N. Savenko, J. Pharm. Sci., 2009, 98, 1426 CrossRef PubMed.
- S. E. Kichanov, D. P. Kozlenko, J. Wąsicki, W. Nawrocik, L. S. Dubrovinsky, H.-P. Liermann, W. Morgenroth and B. N. Savenko, J. Pharm. Sci., 2015, 104, 81 CrossRef CAS PubMed.
- Y. V. Seryotkin, T. N. Drebushchak and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 77 CAS.
- J. Ridout, L. S. Price, J. A. K. Howard and M. R. Probert, Cryst. Growth Des., 2014, 14, 3384 CAS.
- B. A. Zakharov, N. A. Tumanov and E. V. Boldyreva, CrystEngComm, 2015, 17, 2074 RSC.
- M. Fisch, A. Lanza, E. Boldyreva, P. Macchi and N. Casati, J. Phys. Chem. C, 2015, 119, 18611 CAS.
- E. V. Boldyreva, T. P. Shakhtshneider and H. Ahsbahs, J. Therm. Anal. Calorim., 2002, 68, 437 CrossRef CAS.
- N. A. Tumanov, E. V. Boldyreva, B. A. Kolesov, A. V. Kurnosov and R. Quesada Cabrera, Acta Crystallogr., Sect. B: Struct. Sci., 2010, 66, 458 CAS.
- A. P. Ayala, M. W. C. Caetano, S. B. Honorato, J. Mendes Filho, H. W. Siesler, S. N. Faudone, S. L. Cuffini, F. T. Martins, C. C. P. da Silva and J. Ellena, J. Raman Spectrosc., 2012, 43, 263 CrossRef CAS.
- A. J. Cruz-Cabeza and J. Bernstein, Chem. Rev., 2014, 114, 2170 CrossRef CAS PubMed.
- V. A. Drebushchak, T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, J. Therm. Anal. Calorim., 2008, 93, 343 CrossRef CAS.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, o4393 CAS.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2007, 63, o355 CAS.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2008, 64, o623 CAS.
- T. N. Drebushchak, Y. A. Chesalov and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 770 CAS.
- T. N. Drebushchak, V. A. Drebushchak and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., 2011, 67, 163 CAS.
- T. N. Drebushchak, A. A. Ogienko and E. V. Boldyreva, CrystEngComm, 2011, 13, 4405 RSC.
- G. J. Piermarini, J. Appl. Phys., 1973, 44, 5377 CrossRef CAS.
- J. W. Otto, J. K. Vassiliou and G. Frommeyer, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 3253 CrossRef CAS.
- J. R. Cox, L. A. Ferris and V. R. Thalladi, Angew. Chem., Int. Ed., 2007, 46, 4333 CrossRef CAS PubMed.
- A. Gavezzotti, Molecular Aggregation, International Union of Crystallography Monographs on Crystallography, Oxford University Press, 2006, p. 442 Search PubMed.
- A. Gavezzotti, Cryst. Res. Technol., 2013, 48, 793 CrossRef CAS.
- N. B. Leonidov, Russ. Chem. J., 1997, 41, 5 Search PubMed.
- N. B. Leonidov, P. M. Zorky and A. E. Masunov, Acta Crystallogr., Sect. A: Found. Crystallogr., 1993, 49, c185 Search PubMed.
- D. Gidalevitz, R. Feidenhans'l, S. Matlis, D.-M. Smilgies, M. J. Christensen and L. Leiserowitz, Angew. Chem., Int. Ed. Engl., 1997, 36, 955 CrossRef CAS.
- W. Ostwald, Z. Phys. Chem., 1897, 22, 289 CAS.
- Reactivity of Molecular Solids, Molecular Solid State Series, ed. E. V. Boldyreva and V. V. Boldyrev, Wiley, Chichester, 1999 Search PubMed.
- R. Boehler, Rev. Sci. Instrum., 2006, 77, 115103 CrossRef.
- G. J. Piermarini, S. Block, J. D. Barnett and R. A. Forman, J. Appl. Phys., 1975, 46, 2774 CrossRef CAS.
- CrysAlisPro, Agilent Technologies UK, Yarnton, Oxfordshire, UK, 2013 Search PubMed.
- V. Dyadkin, SNBL Tool box, Swiss Norwegian Beamline at ESRF, Grenoble, France, 2013 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 CrossRef PubMed.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Summary of chlorpropamide polymorphs' crystal data (Table S1), cell parameters of chlorpropamide polymorphs at different pressures (Table S2), and photographs and Raman spectra of chlorpropamide (Fig. S1 and S2). CCDC 1437552. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce00711b |
| This journal is © The Royal Society of Chemistry 2016 |