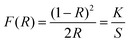DOI:
10.1039/C4QI00243A
(Research Article)
Inorg. Chem. Front., 2015,
2, 361-368
Macroscopic polarity control with alkali metal cation size and coordination environment in a series of tin iodates†
Received
31st December 2014
, Accepted 28th January 2015
First published on 29th January 2015
Abstract
A series of stoichiometrically similar tin(IV) iodates, A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6 have been hydrothermally synthesized. X-ray diffraction was used to determine the crystal structures of the reported materials. All six materials reveal zero-dimensional molecular structures that consist of SnO6 octahedra and IO3 polyhedra. However, the size and coordination environment of cations significantly influence the macroscopic centricities of the materials. While Li2Sn(IO3)6 and Na2Sn(IO3)6 crystallize in the noncentrosymmetric (NCS) polar space group, P63, the K, Rb, Cs, and Sn phases crystallize in the centrosymmetric (CS) nonpolar space group, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . Infrared and UV-vis spectroscopy, thermal analyses, and local dipole moment calculations are reported. With NCS polar materials, powder second-harmonic generation (SHG) properties, polarization, and piezoelectric measurements are also presented. The NCS properties are mainly attributable to the parallel alignment of the lone pairs in I5+ cations.
. Infrared and UV-vis spectroscopy, thermal analyses, and local dipole moment calculations are reported. With NCS polar materials, powder second-harmonic generation (SHG) properties, polarization, and piezoelectric measurements are also presented. The NCS properties are mainly attributable to the parallel alignment of the lone pairs in I5+ cations.
Introduction
Molecular materials have been of broad and current interest attributable to their wide range of important applications in photonics, information processing, data storage, and nanotechnology.1 Synthetic chemists have made continuous efforts to tune the characteristics of the materials by controlling the orientation and alignment of the molecules. Among the many functional compounds, materials crystallizing in noncentrosymmetric (NCS) structures have drawn enormous attention because of their technologically advanced properties, such as second-order nonlinear optical (NLO), piezoelectric, and multiferroic behaviors.2 Especially, polar materials exhibiting permanent dipole moments along a specifically defined direction are of great interest attributed to their two unique properties, i.e., pyroelectricity and ferroelectricity.3–5 Although the two interesting properties are found in polar materials, ferroelectricity is only observed when a switchable polarization exists within the crystal. In other words, a ferroelectric material is a pyroelectric material that has reversible polarization. Two successful approaches to develop superior performing polar materials are the crystallographically ordered d0 transition metal oxyfluorides6–10 and oxides containing second-order Jahn–Teller (SOJT) distortive cations.11–22 With the former, changes in the bond network notably influence different crystal polarities. With the latter, incorporating locally polar polyhedra, such as octahedrally coordinated d0 transition metal cations and lone pair cations, into an extended backbone plays an important role in creating a macroscopic polar structure. In addition, a number of NCS polar materials have been observed from d10 transition metal cations revealing polar displacement as well as compounds composed of borate groups exhibiting unsymmetrical π-orbital systems.23–27 However, these local asymmetric building units frequently fall into line in an antiparallel manner and result in centrosymmetric (CS) nonpolar structures. Therefore, it is more important to understand the key factors deciding the crystallographic centricity through close structural examinations. A series of alkali metal titanium iodate materials, A2Ti(IO3)6 (A = Li, Na, K, Rb, Cs, Ag), with zero-dimensional structures have been recently reported, in which the size of alkali metal cations affects significantly the overall polarity of the materials.28–30 Other reported elements controlling the macroscopic polarity include framework flexibility and the hydrogen bonding effect.31–36 Here we report hydrothermal syntheses and thorough characterization of six new stoichiometrically similar molecular tin iodates, A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6. We will demonstrate how the cation size and coordination environment influence the polarity of the materials. With the polar iodates, Li2Sn(IO3)6 and Na2Sn(IO3)6, detailed NCS functional properties, such as second-harmonic generation (SHG), piezoelectricity, and polarization measurements will also be reported.
Experimental
Syntheses
Li2CO3 (Hayashi, 98.0%), Na2CO3 (Hayashi, 99.5%), K2CO3 (Jin Chemical, 99.5%), Rb2CO3 (Alfa Aesar, 99.8%), Cs2CO3 (Aldrich, 99.0%), SnCl2 (Alfa Aesar, 98%), and HIO3 (Alfa Aesar, 99.0%) were used as received. Single crystals of the reported compounds have been obtained through hydrothermal reactions. For Li2Sn(IO3)6, 0.185 g (2.50 × 10−3 mol) of Li2CO3, 0.119 g (6.25 × 10−4 mol) of SnCl2, 5.940 g (3.38 × 10−2 mol) of HIO3, and 10 mL of deionized water were combined. For Na2Sn(IO3)6, 0.265 g (2.50 × 10−3 mol) of Na2CO3, 0.119 g (6.25 × 10−4 mol) of SnCl2, 5.940 g (3.38 × 10−2 mol) of HIO3, and 10 mL of deionized water were combined. For K2Sn(IO3)6, 0.173 g (1.25 × 10−3 mol) of K2CO3, 0.119 g (6.25 × 10−4 mol) of SnCl2, 1.320 g (7.50 × 10−2 mol) of HIO3, and 5 mL of deionized water were combined. For Rb2Sn(IO3)6, 0.289 g (1.25 × 10−3 mol) of Rb2CO3, 0.119 g (6.25 × 10−4 mol) of SnCl2, 1.100 g (6.25 × 10−3 mol) of HIO3, and 7.5 mL of deionized water were combined. For Cs2Sn(IO3)6, 0.407 g (1.25 × 10−3 mol) of Cs2CO3, 0.237 g (1.25 × 10−3 mol) of SnCl2, 1.650 g (9.38 × 10−3 mol) of HIO3, and 7.5 mL of deionized water were combined. For Sn2+Sn4+(IO3)6, 0.053 g (5.00 × 10−4 mol) of Na2CO3, 0.017 g (1.25 × 10−4 mol) of SnO, 1.056 g (6.00 × 10−3 mol) of HIO3, and 2 mL of deionized water were combined. The respective mixtures were transferred into 23 mL Teflon-lined stainless steel autoclaves. After sealing, the autoclaves were gradually heated to 230 °C for 4 days, before being cooled to room temperature at a rate of 6 °C h−1. After cooling, the autoclaves were opened and the products were recovered by filtration and washed with water. Colorless crystals of Li2Sn(IO3)6, Na2Sn(IO3)6, K2Sn(IO3)6, Rb2Sn(IO3)6, and Cs2Sn(IO3)6 were obtained in 54%, 46%, 78%, 72%, and 85% yields, respectively, based on SnCl2. Although several attempts have been made, we were not able to synthesize a pure phase of Sn2+Sn4+(IO3)6. A few colorless cube crystals of Sn2+Sn4+(IO3)6 were manually extracted from black plate crystals of SnO for single crystal X-ray diffraction. Thus, with Sn2+Sn4+(IO3)6, only the crystal structure will be given here. Powder X-ray diffraction patterns on the bulk samples for other five compounds revealed that the synthesized materials are pure and in very good agreement with the generated patterns from the single-crystal data (see the ESI†).
Single-crystal X-ray diffraction
The crystal structures of the reported materials were determined by a standard crystallographic method. A colorless rod (0.018 × 0.020 × 0.073 mm3) for Li2Sn(IO3)6, a colorless rod (0.020 × 0.020 × 0.100 mm3) for Na2Sn(IO3)6, a colorless cube (0.036 × 0.038 × 0.038 mm3) for K2Sn(IO3)6, a colorless cube (0.030 × 0.031 × 0.031 mm3) for Rb2Sn(IO3)6, a colorless cube (0.031 × 0.031 × 0.032 mm3) for Cs2Sn(IO3)6, and a colorless cube (0.031 × 0.031 × 0.032 mm3) for Sn2+Sn4+(IO3)6 were used for single crystal X-ray diffraction analyses. Diffraction data were collected at room temperature using a Bruker SMART BREEZE diffractometer equipped with a 1k CCD area detector using graphite monochromated Mo Kα radiation. A narrow-frame method was used with an exposure time of 10 s per frame, and scan widths of 0.30° in omega to collect a hemisphere of data. The maximum correction applied to the intensities was <1%. The data were integrated using the SAINT program,37 with the intensities corrected for polarization, Lorentz factor, air absorption, and absorption attributed to the variation in the path length through the detector faceplate. The data were solved with SHELXS-9738 and refined using SHELXL-97.39 All calculations were performed using the WinGX-98 crystallographic software package.40 Crystallographic data and selected bond distances for the reported materials are summarized in Tables 1 and 2, respectively.
Table 1 Crystallographic data for A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6
|
R(F) = ∑||Fo| − |Fc||/∑|Fo|.
R
w(F2) = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2.
|
| Formula |
Li2Sn(IO3)6 |
Na2Sn(IO3)6 |
K2Sn(IO3)6 |
Rb2Sn(IO3)6 |
Cs2Sn(IO3)6 |
Sn2(IO3)6 |
| Formula weight |
1181.99 |
1214.09 |
1246.31 |
1339.05 |
1433.93 |
1286.82 |
| Crystal system |
Hexagonal |
Hexagonal |
Trigonal |
Trigonal |
Trigonal |
Trigonal |
| Space group |
P63 (no. 173) |
P63 (no. 173) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) (no. 148) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) (no. 148) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) (no. 148) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148) (no. 148) |
|
a = b/Å |
9.4532(10) |
9.7154(10) |
11.3300(10) |
11.5049(10) |
11.7196(10) |
11.3779(10) |
|
c/Å |
5.1680(3) |
5.2239(10) |
11.3841(10) |
11.4933(10) |
11.7232(10) |
11.2743(2) |
|
V/Å3 |
399.96(13) |
427.02(14) |
1265.6(3) |
1318.2(3) |
1394.5(3) |
1264.0(3) |
|
Z
|
1 |
1 |
3 |
3 |
3 |
3 |
|
T/K |
298.0(2) |
298.0(2) |
298.0(2) |
298.0(2) |
298.0(2) |
298.0(2) |
|
λ/Å |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
|
R(F)a |
0.0405 |
0.0593 |
0.0129 |
0.0189 |
0.0138 |
0.0279 |
|
R
w(Fo2)b |
0.1024 |
0.1454 |
0.0295 |
0.0454 |
0.0248 |
0.0677 |
| Flack parameter |
0.01(4) |
0.08(3) |
N/A |
N/A |
N/A |
N/A |
Table 2 Selected bond distances (Å) for A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6
| Bond |
Li2Sn(IO3)6 |
Na2Sn(IO3)6 |
| Sn(1)–O(1) × 3 |
2.070(19) |
2.10(3) |
| Sn(1)–O(1) × 3 |
2.115(18) |
2.14(3) |
| I(1)–O(1) |
1.875(19) |
1.87(3) |
| I(1)–O(2) |
1.798(16) |
1.77(3) |
| I(1)–O(3) |
1.791(17) |
1.80(2) |
| A(1)–O(2) × 3 |
2.11(3) |
2.28(2) |
| A(1)–O(3) × 3 |
2.25(3) |
2.42(3) |
| Bond |
K2Sn(IO3)6 |
Rb2Sn(IO3)6 |
Cs2Sn(IO3)6 |
SnSn(IO3)6 |
| Sn(1)–O(1) × 6 |
2.0434(19) |
2.038(3) |
2.037(2) |
2.034(6) |
| I(1)–O(1) |
1.8653(19) |
1.864(3) |
1.853(2) |
1.856(6) |
| I(1)–O(2) |
1.799(2) |
1.804(3) |
1.796(2) |
1.806(5) |
| I(1)–O(3) |
1.7964(19) |
1.788(3) |
1.782(2) |
1.787(5) |
| A(1)–O(1) × 3 |
2.934(2) |
3.016(3) |
3.117(2) |
3.142(6) |
| A(1)–O(2) × 3 |
2.868(2) |
2.988(3) |
3.142(2) |
2.878(5) |
| A(1)–O(3) × 3 |
2.921(2) |
3.004(3) |
3.149(2) |
2.827(6) |
Powder X-ray diffraction (PXRD)
PXRD data were obtained using a Bruker D8-Advance diffractometer (Cu Kα radiation). The diffraction data were collected at room temperature with 40 kV and 40 mA. The ground samples were mounted on sample holders and scanned in the 2θ range of 5–70° with a step size of 0.02° and a step time of 0.2 s.
Infrared (IR) spectroscopy
IR spectra were recorded using a Thermo Scientific Nicolet 6700 FT-IR spectrometer in the spectral range of 400–4000 cm−1. The samples were embedded in KBr matrixes for the measurements.
UV-vis spectroscopy
UV-vis diffuse reflectance spectra were recorded using a Varian Cary 500 scan UV-vis-NIR spectrophotometer equipped with a double-beam photomultiplier tube in the range of 200–1500 nm at room temperature.
Thermogravimetric analysis (TGA)
TGA was performed using a Setaram LABSYS TG-DTA thermogravimetric analyser. The samples were contained in alumina crucibles and heated to 1000 °C at a rate of 10 °C min−1 under flowing argon.
Scanning electron microscopy (SEM)/energy-dispersive analysis by X-ray (EDAX)
SEM/EDAX analyses were conducted by using Hitachi S-3400N/Horiba energy EX-250 instruments. EDAX for A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) reveal A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ratios of approximately N/A
I ratios of approximately N/A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6, 1.9
5.6, 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9, 1.8
5.9, 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2, 1.7
6.2, 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2, and 2.0
6.2, and 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.8, respectively.
5.8, respectively.
Powder second-harmonic generation (SHG) measurements
Powder SHG measurements on polar NCS compounds, Li2Sn(IO3)6 and Na2Sn(IO3)6 were performed using a modified Kurtz NLO system41 with 1064 nm radiation using a DAWA Q-switched Nd:YAG laser operating at 20 Hz. SHG efficiencies for polycrystalline samples have been known to depend on particle size; thus, powder samples were graded into distinct ranges of particle size. To compare suitably with the known SHG materials, crystalline α-SiO2 and LiNbO3 were also sieved into the same particle size ranges. Samples with a particle size range of 45–63 μm were used to compare SHG efficiencies. Polycrystalline samples with different particle size ranges were placed in individual capillary tubes. The frequency-doubled SHG light, 532 nm green, was gathered and detected by a photomultiplier tube (PMT, Hamamatsu). A narrow-pass interference filter (532 nm) was attached to the PMT to detect only the SHG light. To monitor the SHG signal, a digital oscilloscope (Tektronix TDS1032) was connected. A detailed description of the equipment and the methodology has been published.42
Piezoelectric measurements
Converse piezoelectric measurements were performed using a Radiant Technologies RT66A piezoelectric test system with a TREK (model 609E-6) high-voltage amplifier, Precision Materials Analyzer, Precision High-Voltage Interface, and MTI 2000 Fotonic Sensor. NCS Na2Sn(IO3)6 was pressed into a 12 mm diameter and ∼0.8 mm thick pellets and sintered at 300 °C for 6 h. A conducting solver paste was applied on both sides of the pellet surfaces for electrodes. A maximum voltage of 500 V was applied to the sample.
Polarization measurements
Polarization measurements were carried out using a Radiant Technologies RT66A ferroelectric test system with a TREK high-voltage amplifier between room temperature and 200 °C in a Delta 9023 environmental test chamber. The unclamped pyroelectric coefficient, dP/dT, was determined by measuring polarization (P) as a function of temperature (T). A detailed statement of the methodology used has been reported.42 The same pellet of Na2Sn(IO3)6 used for piezoelectric measurements was utilized for polarization measurements. To determine the ferroelectric behaviour, polarization measurements were carried out at room temperature under a static electric field of 15.0 kV cm−1 at frequencies ranging from 100 to 1000 Hz. For pyroelectric measurements, polarization was measured statically from room temperature to 200 °C in 20 °C increments, with an electric field of 15.0 kV cm−1. The temperature was allowed to stabilize before polarization was measured.
Results and discussion
Crystal structures
All six stoichiometrically similar tin iodates exhibit zero-dimensional structures that are composed of SnO6 octahedra and IO3 groups (see Fig. 1).
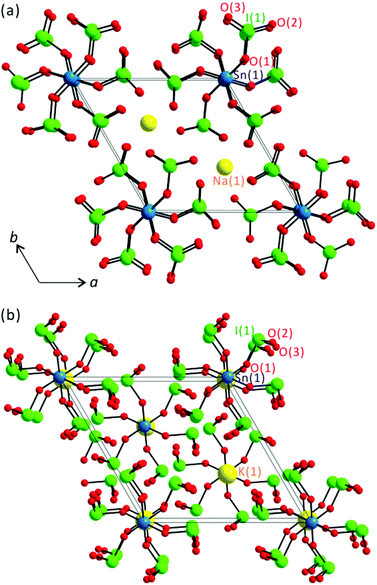 |
| | Fig. 1 Ball-and-stick models for (a) NCS polar Na2Sn(IO3)6 and (b) CS nonpolar K2Sn(IO3)6 in the ab-plane. | |
As seen in Fig. 1, the large molecular groups of SnO6 and IO3 polyhedra are kept apart by alkali metal or Sn2+ cations. Thus, the reported materials share a general connectivity term of {[SnO6/2]2− 6[IO1/2O2/1]0}2−, with charge balance retained by two alkali metal cations or an Sn2+ cation. Interestingly, however, while Li2Sn(IO3)6 and Na2Sn(IO3)6 crystallize in the NCS polar space group, P63 (no. 173), A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6 crystallize in the CS nonpolar space group, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148). The Sn4+ cations in NCS Li2Sn(IO3)6 and Na2Sn(IO3)6 reveal C3-type distortions and result in three short and three long Sn–O bonds. Here the Sn4+ cation is disordered over two sites with 50% occupancy on each site, which makes the materials effectively 0-dimensional molecular structures. Similar disordered structures have been observed from polar titanium iodates.28,29 The observed Sn–O bond lengths for Li2Sn(IO3)6 and Na2Sn(IO3)6 are 2.070(19)–2.115(18) Å and 2.10(3)–2.14(3) Å, respectively. The I5+ cations in Li2Sn(IO3)6 and Na2Sn(IO3)6 are in an asymmetric trigonal pyramidal environment attributed to their stereoactive lone pairs. The I–O bond distances for Li2Sn(IO3)6 and Na2Sn(IO3)6 range from 1.791(17) to 1.875(19) Å and from 1.77(3) to 1.87(3) Å, respectively. The Li+ and Na+ cations are in pseudo-octahedral coordination moieties with six oxygen ligands with bond lengths in the range of 2.11(3)–2.42(3) Å. With CS A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6, the Sn4+ cations are in the center of their normal octahedra with one unique Sn–O bond length of approximately 2.04 Å. Similar to those of Li2Sn(IO3)6 and Na2Sn(IO3)6, each I5+ cation in CS A2Sn(IO3)6 and Sn2+Sn4+(IO3)6 is linked to three oxygen atoms in a trigonal pyramidal environment with the I–O bond lengths in the approximate range of 1.78–1.87 Å (see Table 2). The K+, Rb+, Cs+, and Sn2+ cations in CS tin iodates are in ninefold coordination environments with contact distances in the range of 2.827(6)–3.149(2) Å. Similar coordination moieties for CS tin iodates strongly indicate that the materials are isostructural to each other and the lone pair on Sn2+ is not stereoactive but inert. A similar inertness of the lone pair cations has been observed previously in other materials.29,43,44 Bond valence sum calculations45,46 for the alkali-metal cations, Sn2+, Sn4+, and I5+ on the reported tin iodates resulted in values of 0.90–1.26, 2.05, 3.70–4.15, and 4.93–5.03, respectively.
(no. 148). The Sn4+ cations in NCS Li2Sn(IO3)6 and Na2Sn(IO3)6 reveal C3-type distortions and result in three short and three long Sn–O bonds. Here the Sn4+ cation is disordered over two sites with 50% occupancy on each site, which makes the materials effectively 0-dimensional molecular structures. Similar disordered structures have been observed from polar titanium iodates.28,29 The observed Sn–O bond lengths for Li2Sn(IO3)6 and Na2Sn(IO3)6 are 2.070(19)–2.115(18) Å and 2.10(3)–2.14(3) Å, respectively. The I5+ cations in Li2Sn(IO3)6 and Na2Sn(IO3)6 are in an asymmetric trigonal pyramidal environment attributed to their stereoactive lone pairs. The I–O bond distances for Li2Sn(IO3)6 and Na2Sn(IO3)6 range from 1.791(17) to 1.875(19) Å and from 1.77(3) to 1.87(3) Å, respectively. The Li+ and Na+ cations are in pseudo-octahedral coordination moieties with six oxygen ligands with bond lengths in the range of 2.11(3)–2.42(3) Å. With CS A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6, the Sn4+ cations are in the center of their normal octahedra with one unique Sn–O bond length of approximately 2.04 Å. Similar to those of Li2Sn(IO3)6 and Na2Sn(IO3)6, each I5+ cation in CS A2Sn(IO3)6 and Sn2+Sn4+(IO3)6 is linked to three oxygen atoms in a trigonal pyramidal environment with the I–O bond lengths in the approximate range of 1.78–1.87 Å (see Table 2). The K+, Rb+, Cs+, and Sn2+ cations in CS tin iodates are in ninefold coordination environments with contact distances in the range of 2.827(6)–3.149(2) Å. Similar coordination moieties for CS tin iodates strongly indicate that the materials are isostructural to each other and the lone pair on Sn2+ is not stereoactive but inert. A similar inertness of the lone pair cations has been observed previously in other materials.29,43,44 Bond valence sum calculations45,46 for the alkali-metal cations, Sn2+, Sn4+, and I5+ on the reported tin iodates resulted in values of 0.90–1.26, 2.05, 3.70–4.15, and 4.93–5.03, respectively.
As can be seen in Fig. 2, all of the lone pairs on the iodates are aligned in a parallel manner in Li2Sn(IO3)6 and Na2Sn(IO3)6, in which a produced macroscopic dipole moment makes the materials crystallographically polar. However, in A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6, the lone pairs on the iodate polyhedra positioned trans to each other are oriented in opposite directions (see Fig. 3), which results in a complete cancellation of the polarization generated from the local dipole moments. Thus, the materials crystallize in the CS nonpolar space group.
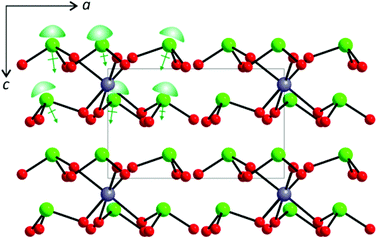 |
| | Fig. 2 Ball-and-stick representation of Na2Sn(IO3)6 in the ac-plane. Note that the lone pairs on the iodates are aligned in a parallel manner and a net moment is produced along the [001] direction. Lone pairs on the I5+ cations are drawn schematically and are not the result of the electron localization function (ELF) calculations. | |
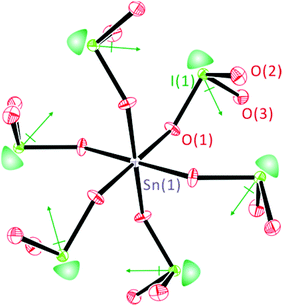 |
| | Fig. 3 ORTEP (50% probability ellipsoids) drawing of Cs2Sn(IO3)6. Note that the lone pairs on iodate polyhedra positioned trans to each other are oriented in opposite directions, which results in a complete cancellation of the polarization and macroscopic CS. Lone pairs on the I5+ cations are drawn schematically and are not the result of the electron localization function (ELF) calculations. | |
IR spectroscopy
The reported tin iodate compounds reveal Sn–O and I–O vibrations in the IR spectra. The Sn–O vibrations are found at ca. 660–663 cm−1. I–O stretching and bending are also observed around 709–824 and 426–497 cm−1, respectively. The assignments are consistent with those previously published.47–49 The IR spectra are given in the ESI.†
UV-vis diffuse reflectance spectroscopy
UV-vis diffuse reflectance spectra were recorded for the reported tin iodates. Absorption (K/S) data were calculated from the Kubelka–Munk function:50,51
in which S is the scattering, K is the absorption, and R is the reflectance. In the K/S vs. E plots, extrapolating the linear part of the rising curve to zero yielded the onset of absorption at 3.9, 4.0, 4.0, 4.1, and 4.1 eV for Li2Sn(IO3)6, Na2Sn(IO3)6, K2Sn(IO3)6, Rb2Sn(IO3)6, and Cs2Sn(IO3)6, respectively (see the ESI†). The band gaps for the reported compounds are attributed to the interaction of the Sn–O and I–O bonds and the distortions arising from IO3 groups.
Thermogravimetric analysis (TGA)
The thermal behaviors of the reported materials were investigated using TGA. TGA measurements showed that the materials decompose above 400 °C. Powder XRD for the thermally decomposed products revealed SnO2 and alkali metal tin oxides. The TGA diagrams are found in the ESI.†
Noncentrosymmetric (NCS) properties
Li2Sn(IO3)6 and Na2Sn(IO3)6, crystallize in the NCS polar space group, P63; thus, their SHG properties have been investigated. The SHG efficiencies of NCS tin iodates are very strong; both Li2Sn(IO3)6 and Na2Sn(IO3)6 exhibit SHG efficiencies ∼400 times that of α-SiO2, which compares well to BaTiO3 (400 times that of α-SiO2).41 The SHG efficiencies for alkali metal titanium iodates, Li2Ti(IO3)6 and Na2Ti(IO3)6, are ∼500 and ∼400 times that of α-SiO2, respectively.28,29 The larger SHG efficiency of Li2Ti(IO3)6 compared to that of Li2Sn(IO3)6 may be attributed to the constructive addition of polarizations from both TiO6 and IO3 polyhedra. Overall, the similar efficiencies strongly indicate that the SHG for iodates is mainly attributable to the alignment of the dipole moments in the IO3 groups (see Fig. 2 and the Dipole moment calculations section). In addition, we were able to determine the type I phase-matching capabilities for the NCS polar materials by sieving them into various particle sizes and measuring the SHG as a function of particle size. As seen in Fig. 4, both Li2Sn(IO3)6 and Na2Sn(IO3)6 are phase-matchable and can be classified as the class A category of SHG materials, as defined by Kurtz and Perry.41
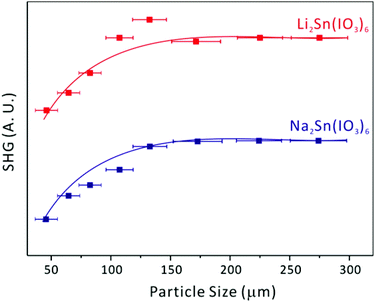 |
| | Fig. 4 Phase matching curve (Type I) for Li2Sn(IO3)6 and Na2Sn(IO3)6. The curves are to guide the eye and are not fit to the data. | |
Converse piezoelectric measurements on bulk Na2Sn(IO3)6 revealed a d33 charge constant of 29.2 pm V−1 (see the ESI†). The value compares well to that for other iodates, i.e., KIO3 (d33 = 39 pm V−1) and Na2Ti(IO3)6 (d33 = 60 pm V−1).29,52 Ferroelectric and pyroelectric measurements for Na2Sn(IO3)6 were also performed. Although the material is polar, Na2Sn(IO3)6 is not ferroelectric as can be seen in the ferroelectric measurements (see the ESI†). Polarization measurements using 15 kV cm−1 indicated an induced maximum polarization of only 0.07 μC cm−2. In order to be ferroelectric, the dipole moment needs to be reversible. In Na2Sn(IO3)6, the distortion associated with Sn4+ is negligible, as we will discuss in more detail later (see the Dipole moment calculations section). With I5+ in the IO3 polyhedron in an extended solid-state structure, macroscopic polarization cannot be switched in the presence of an external electric field, because polarization reversal for the lone pair cation is extremely unfavorable. The energetically very unfavorable polarization reversal of the IO3 polyhedra has been demonstrated before through calculations.29 Although Na2Sn(IO3)6 is not ferroelectric, the compound is pyroelectric. Thus, the pyroelectric coefficient for Na2Sn(IO3)6 has been determined by measuring the polarization between room temperature and 200 °C, with an external electric field of 15.0 kV cm−1. The obtained pyroelectric coefficient is approximately 0.5 μC m−2 K−1, which is similar to that of sodium titanium iodate, Na2Ti(IO3)6 (0.8 μC m−2 K−1).29
Dipole moment calculations
The reported stoichiometrically similar compounds contain asymmetric polyhedra, i.e., IO3 groups attributed to the lone pairs. Thus, the asymmetric environment of the IO3 polyhedra has been quantified through local dipole moment calculations using the method described before.8,9,53 The distortion of SnO6 octahedra has been also calculated for comparison. We found that the local dipole moments for IO3 and SnO6 groups in A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) are calculated to be about 13.1–13.9 and 0–0.4 D (Debyes), respectively, which are very similar values to those of the previously reported iodate materials.28,29,49 As we described earlier, while the polarization arising from the distortion of SnO6 octahedra is negligible, the polarization associated with IO3 polyhedra is significant. Thus, the functional properties for NCS polar materials are attributed to a net moment originated from the alignment of IO3 groups. The local dipole moments for the IO3 and SnO6 groups are summarized in Table 3.
Table 3 Calculation of dipole moments for IO3 and SnO6 polyhedra in A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs). D = Debyes
| Compound |
Species |
Dipole moment (D) |
| Li2Sn(IO3)6 |
I(1)O3 |
13.2 |
| Sn(1)O6 |
0.4 |
| Na2Sn(IO3)6 |
I(1)O3 |
13.1 |
| Sn(1)O6 |
0.3 |
| K2Sn(IO3)6 |
I(1)O3 |
13.5 |
| Sn(1)O6 |
0 |
| Rb2Sn(IO3)6 |
I(1)O3 |
13.2 |
| Sn(1)O6 |
0 |
| Cs2Sn(IO3)6 |
I(1)O3 |
13.9 |
| Sn(1)O6 |
0 |
NCS polar vs. CS nonpolar structures
Although all six stoichiometrically similar materials, A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6, share a molecular structure consisting of SnO6 octahedra and IO3 polyhedra, an interesting change in macroscopic polarity occurs from the NCS polar structure for the compounds containing smaller cations, Li+ and Na+, to the CS nonpolar structure for those with larger cations, K+, Rb+, Cs+, and Sn2+. With Li2Sn(IO3)6 and Na2Sn(IO3)6, the alkali metal cations, Li+ and Na+ are in a six-coordinate pseudo-octahedral coordination environment attributed to their smaller cation size. To maintain this octahedral coordination mode around the Li+ and Na+ cations, the IO3 groups should be aligned in a parallel manner around the Sn4+ cation, which subsequently results in an NCS polar structure (see Fig. 5a). It should be noted, however, that this parallel alignment in a crowded environment is only possible when the ionic radii of the cations are small. However, the cations, K+, Rb+, Cs+, and Sn2+ in CS nonpolar structures maintain nine-coordinate environments with oxide ligands attributable to the larger ionic radii (see Fig. 5b). As seen in Fig. 5b, the larger A+ cations can have nine oxide contacts by rotating the IO3 polyhedra with respect to the Sn4+ cation. Thus, the local dipole moments for the IO3 polyhedra point in opposite directions equally and render the material CS nonpolar. A similar cation size effect on the coordination environments and macroscopic centricity has been observed before.29,54,55
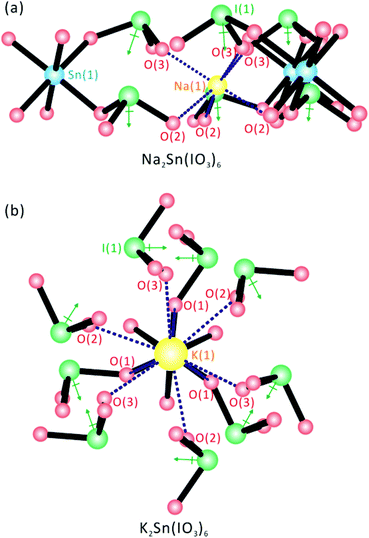 |
| | Fig. 5 Ball-and-stick representations for (a) NCS Na2Sn(IO3)6 and (b) CS K2Sn(IO3)6, with the alkali metal coordination environments emphasized. Note that the IO3 groups in Na2Sn(IO3)6 are aligned in a parallel manner attributed to the six-coordinate Na+ cation, whereas the IO3 polyhedra in K2Sn(IO3)6 rotate with respect to Sn4+ due to the nine-coordinate K+ cation. | |
Conclusions
Six new tin iodates, A2Sn(IO3)6 (A = Li, Na, K, Rb, Cs) and Sn2+Sn4+(IO3)6, have been synthesized hydrothermally and their structures were determined through single crystal X-ray diffraction. All six stoichiometrically similar tin iodates exhibit molecular structures that are composed of SnO6 octahedra and IO3 groups. While the compounds containing smaller cations, Li+ and Na+, are NCS polar, those with the larger cations, K+, Rb+, Cs+, and Sn2+, are CS nonpolar. The smaller alkali metal cations, Li+ and Na+, are in a six-coordinate pseudo-octahedral coordination moiety and contact with oxide ligands on six different IO3 groups. The compact interactions demand the lone pairs in the IO3 groups to align in a parallel manner and let Li2Sn(IO3)6 and Na2Sn(IO3)6 crystallize in the NCS polar space group. The larger cations, K+, Rb+, Cs+, and Sn2+, interact with oxide ligands on the IO3 and SnO6 groups in nine-coordinate environments. To maintain these contacts, the IO3 polyhedra should rotate with respect to the SnO6 octahedron, in which the rotation aligns the polarizations from the IO3 groups in opposite directions. Thus, all tin iodates with larger cations, K2Sn(IO3)6, Rb2Sn(IO3)6, Cs2Sn(IO3)6, and Sn2+Sn4+(IO3)6 crystallize in the CS nonpolar space group. The NCS functional properties such as SHG, piezoelectricity, and pyroelectricity are attributable to the parallel alignment of the lone pairs on the I5+ cations.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIP) (grants: 2013R1A2A2A01007170 and 2014M3A9B8023478). TTT and PSH thank the Welch Foundation (E-1457) for support.
Notes and references
-
D. W. Bruce, D. O'Hare and R. I. Walton, Molecular Materials, John Wiley & Son, Ltd, West Sussex, UK, 2010 Search PubMed.
- P. S. Halasyamani and K. R. Poeppelmeier, Inorg. Chem., 2008, 47, 8427 CrossRef CAS PubMed.
-
M. E. Lines and A. M. Glass, Principles and Applications of Ferroelectrics and Related Materials, Oxford University Press, Oxford, UK, 1991 Search PubMed.
-
S. B. Lang and D. K. Das-Gupta, Handbook of Advanced Electronic and Photonic Materials and Devices, Academic Press, San Francisco, USA, 2001 Search PubMed.
-
T. Hahn, International Tables for Crystallography, Volume A: Space Group Symmetry, Kluwer Academic, Dordrecht, The Netherlands, 2006 Search PubMed.
- P. A. Maggard, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2001, 123, 7742 CrossRef CAS.
- M. E. Welk, A. J. Norquist, F. P. Arnold, C. L. Stern and K. R. Poeppelmeier, Inorg. Chem., 2002, 41, 5119 CrossRef CAS PubMed.
- P. A. Maggard, T. S. Nault, C. L. Stern and K. R. Poeppelmeier, J. Solid State Chem., 2003, 175, 27 CrossRef CAS.
- H. K. Izumi, J. E. Kirsch, C. L. Stern and K. R. Poeppelmeier, Inorg. Chem., 2005, 44, 884 CrossRef CAS.
- M. R. Marvel, J. Lesage, J. Baek, P. S. Halasyamani, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2007, 129, 13963 CrossRef CAS PubMed.
- U. Opik and M. H. L. Pryce, Proc. R. Soc. London, Ser. A, 1957, 238, 425 CrossRef CAS.
- R. F. W. Bader, Mol. Phys., 1960, 3, 137 CrossRef CAS.
- R. F. W. Bader, Can. J. Chem., 1962, 40, 1164 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1969, 91, 4947 CrossRef CAS.
- R. G. Pearson, J. Mol. Struct. (THEOCHEM), 1983, 103, 25 CrossRef.
- R. A. Wheeler, M.-H. Whangbo, T. Hughbanks, R. Hoffmann, J. K. Burdett and T. A. Albright, J. Am. Chem. Soc., 1986, 108, 2222 CrossRef CAS PubMed.
- J. B. Goodenough, Annu. Rev. Mater. Sci., 1998, 28, 1 CrossRef CAS.
- H.-S. Ra, K. M. Ok and P. S. Halasyamani, J. Am. Chem. Soc., 2003, 125, 7764 CrossRef CAS PubMed.
- E. O. Chi, K. M. Ok, Y. Porter and P. S. Halasyamani, Chem. Mater., 2006, 18, 2070 CrossRef CAS.
- H. Jiang, S. Huang, Y. Fan, J.-G. Mao and W. Cheng, Chem. – Eur. J., 2008, 14, 1972 CrossRef CAS PubMed.
- C.-F. Sun, C.-L. Hu, X. Xu, J.-B. Ling, T. Hu, F. Kong, X.-F. Long and J.-G. Mao, J. Am. Chem. Soc., 2009, 131, 9486 CrossRef CAS PubMed.
- C. Huang, C.-L. Hu, X. Xu, B.-P. Yang and J.-G. Mao, Dalton Trans., 2013, 42, 7051 RSC.
- S. Pan, J. P. Smit, B. Watkins, M. R. Marvel, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2006, 128, 11631 CrossRef CAS PubMed.
- Y. Inaguma, M. Yoshida and T. Katsumata, J. Am. Chem. Soc., 2008, 130, 6704 CrossRef CAS PubMed.
- H. Wu, H. Yu, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215 CrossRef CAS PubMed.
- H. Yu, S. Pan, H. Wu, Z. Yang, L. Dong, X. Su, B. Zhang and H. Li, Cryst. Growth Des., 2013, 13, 3514 CAS.
- X. Lin, F. Zhang, S. Pan, H. Yu, F. Zhang, X. Dong, S. Han, L. Dong, C. Bai and Z. Wang, J. Mater. Chem. C, 2014, 2, 4257 RSC.
- H.-Y. Chang, S.-H. Kim, P. S. Halasyamani and K. M. Ok, J. Am. Chem. Soc., 2009, 131, 2426 CrossRef CAS PubMed.
- H.-Y. Chang, S.-H. Kim, K. M. Ok and P. S. Halasyamani, J. Am. Chem. Soc., 2009, 131, 6865 CrossRef CAS PubMed.
- C.-F. Sun, G.-L. Hu, F. Kong, B.-P. Yang and J.-G. Mao, Dalton Trans., 2010, 39, 1473 RSC.
- R. E. Sykora, K. M. Ok, P. S. Halasyamani and T. E. Albrecht-Schmitt, J. Am. Chem. Soc., 2002, 124, 1951 CrossRef CAS PubMed.
- J. Goodey, K. M. Ok, J. Broussard, C. Hofmann, F. V. Escobedo and P. S. Halasyamani, J. Solid State Chem., 2003, 175, 3 CrossRef CAS.
- K. M. Ok, J. Baek, P. S. Halasyamani and D. O'Hare, Inorg. Chem., 2006, 45, 10207 CrossRef CAS PubMed.
- M.-H. Choi, S.-H. Kim, H. Y. Chang, P. S. Halasyamani and K. M. Ok, Inorg. Chem., 2009, 48, 8376 CrossRef CAS PubMed.
- D. W. Lee, S. J. Oh, P. S. Halasyamani and K. M. Ok, Inorg. Chem., 2012, 50, 4473 CrossRef PubMed.
- Y. H. Kim, D. W. Lee and K. M. Ok, Inorg. Chem., 2014, 53, 1250 CrossRef CAS PubMed.
-
SAINT, Program for Area Detector Absorption Correction; version 4.05, Siemens Analytical X-ray Instruments, Madison, WI, USA, 1995 Search PubMed.
-
G. M. Sheldrick, SHELXS-97 - A program for automatic solution of crystal structures, University of Goettingen, Goettingen, Germany, 1997 Search PubMed.
-
G. M. Sheldrick, SHELXL-97 - A program for crystal structure refinement, University of Goettingen, Goettingen, Germany, 1997 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS PubMed.
- K. M. Ok, E. O. Chi and P. S. Halasyamani, Chem. Soc. Rev., 2006, 35, 710 RSC.
- A.-V. Mudring, Eur. J. Inorg. Chem., 2007, 882 CrossRef CAS.
- F. Rieger and A.-V. Mudring, Inorg. Chem., 2007, 46, 446 CrossRef CAS PubMed.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- M. C. F. Alves, S. C. Souza, M. R. S. Silva, E. C. Paris, S. J. G. Lima, R. M. Gomes, E. Longo, A. G. d. Souza and I. M. G. d. Santos, J. Therm. Anal. Calorim., 2009, 97, 179 CrossRef CAS.
- E. Moreira, J. M. Henriques, D. L. Azevedo, E. W. S. Caetano, V. N. Freire and E. L. Albuquerque, J. Solid State Chem., 2011, 184, 921 CrossRef CAS PubMed.
- D. W. Lee, S. B. Kim and K. M. Ok, Dalton Trans., 2012, 41, 8348 RSC.
- P. Kubelka and F. Munk, Z. Tech. Phys., 1931, 12, 593 Search PubMed.
- J. Tauc, Mater. Res. Bull., 1970, 5, 721 CrossRef CAS.
-
H. Landolt, Numerical Values and Functions from the Natural Sciences and Technology (New Series), Group 3: Crystal and Solid State Physics, Springer Verlag, Berlin, Germany, 1979 Search PubMed.
- J. Galy and G. Meunier, J. Solid State Chem., 1975, 13, 142 CrossRef CAS.
- S.-J. Oh, D. W. Lee and K. M. Ok, Inorg. Chem., 2012, 51, 5393 CrossRef CAS PubMed.
- S.-e. Bang, D. W. Lee and K. M. Ok, Inorg. Chem., 2014, 53, 4756 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic file in CIF format, calculated and observed X-ray diffraction patterns, TGA diagrams, IR spectra, UV-vis diffuse reflectance spectra, piezoelectric, polarization, and pyroelectric measurement data. CCDC 1041408–1041413. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00243a |
|
| This journal is © the Partner Organisations 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . Infrared and UV-vis spectroscopy, thermal analyses, and local dipole moment calculations are reported. With NCS polar materials, powder second-harmonic generation (SHG) properties, polarization, and piezoelectric measurements are also presented. The NCS properties are mainly attributable to the parallel alignment of the lone pairs in I5+ cations.
. Infrared and UV-vis spectroscopy, thermal analyses, and local dipole moment calculations are reported. With NCS polar materials, powder second-harmonic generation (SHG) properties, polarization, and piezoelectric measurements are also presented. The NCS properties are mainly attributable to the parallel alignment of the lone pairs in I5+ cations.![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148)![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148)![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148)![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148)
(no. 148)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ratios of approximately N/A
I ratios of approximately N/A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6, 1.9
5.6, 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9, 1.8
5.9, 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2, 1.7
6.2, 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2, and 2.0
6.2, and 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.8, respectively.
5.8, respectively.

![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148). The Sn4+ cations in NCS Li2Sn(IO3)6 and Na2Sn(IO3)6 reveal C3-type distortions and result in three short and three long Sn–O bonds. Here the Sn4+ cation is disordered over two sites with 50% occupancy on each site, which makes the materials effectively 0-dimensional molecular structures. Similar disordered structures have been observed from polar titanium iodates.28,29 The observed Sn–O bond lengths for Li2Sn(IO3)6 and Na2Sn(IO3)6 are 2.070(19)–2.115(18) Å and 2.10(3)–2.14(3) Å, respectively. The I5+ cations in Li2Sn(IO3)6 and Na2Sn(IO3)6 are in an asymmetric trigonal pyramidal environment attributed to their stereoactive lone pairs. The I–O bond distances for Li2Sn(IO3)6 and Na2Sn(IO3)6 range from 1.791(17) to 1.875(19) Å and from 1.77(3) to 1.87(3) Å, respectively. The Li+ and Na+ cations are in pseudo-octahedral coordination moieties with six oxygen ligands with bond lengths in the range of 2.11(3)–2.42(3) Å. With CS A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6, the Sn4+ cations are in the center of their normal octahedra with one unique Sn–O bond length of approximately 2.04 Å. Similar to those of Li2Sn(IO3)6 and Na2Sn(IO3)6, each I5+ cation in CS A2Sn(IO3)6 and Sn2+Sn4+(IO3)6 is linked to three oxygen atoms in a trigonal pyramidal environment with the I–O bond lengths in the approximate range of 1.78–1.87 Å (see Table 2). The K+, Rb+, Cs+, and Sn2+ cations in CS tin iodates are in ninefold coordination environments with contact distances in the range of 2.827(6)–3.149(2) Å. Similar coordination moieties for CS tin iodates strongly indicate that the materials are isostructural to each other and the lone pair on Sn2+ is not stereoactive but inert. A similar inertness of the lone pair cations has been observed previously in other materials.29,43,44 Bond valence sum calculations45,46 for the alkali-metal cations, Sn2+, Sn4+, and I5+ on the reported tin iodates resulted in values of 0.90–1.26, 2.05, 3.70–4.15, and 4.93–5.03, respectively.
(no. 148). The Sn4+ cations in NCS Li2Sn(IO3)6 and Na2Sn(IO3)6 reveal C3-type distortions and result in three short and three long Sn–O bonds. Here the Sn4+ cation is disordered over two sites with 50% occupancy on each site, which makes the materials effectively 0-dimensional molecular structures. Similar disordered structures have been observed from polar titanium iodates.28,29 The observed Sn–O bond lengths for Li2Sn(IO3)6 and Na2Sn(IO3)6 are 2.070(19)–2.115(18) Å and 2.10(3)–2.14(3) Å, respectively. The I5+ cations in Li2Sn(IO3)6 and Na2Sn(IO3)6 are in an asymmetric trigonal pyramidal environment attributed to their stereoactive lone pairs. The I–O bond distances for Li2Sn(IO3)6 and Na2Sn(IO3)6 range from 1.791(17) to 1.875(19) Å and from 1.77(3) to 1.87(3) Å, respectively. The Li+ and Na+ cations are in pseudo-octahedral coordination moieties with six oxygen ligands with bond lengths in the range of 2.11(3)–2.42(3) Å. With CS A2Sn(IO3)6 (A = K, Rb, Cs) and Sn2+Sn4+(IO3)6, the Sn4+ cations are in the center of their normal octahedra with one unique Sn–O bond length of approximately 2.04 Å. Similar to those of Li2Sn(IO3)6 and Na2Sn(IO3)6, each I5+ cation in CS A2Sn(IO3)6 and Sn2+Sn4+(IO3)6 is linked to three oxygen atoms in a trigonal pyramidal environment with the I–O bond lengths in the approximate range of 1.78–1.87 Å (see Table 2). The K+, Rb+, Cs+, and Sn2+ cations in CS tin iodates are in ninefold coordination environments with contact distances in the range of 2.827(6)–3.149(2) Å. Similar coordination moieties for CS tin iodates strongly indicate that the materials are isostructural to each other and the lone pair on Sn2+ is not stereoactive but inert. A similar inertness of the lone pair cations has been observed previously in other materials.29,43,44 Bond valence sum calculations45,46 for the alkali-metal cations, Sn2+, Sn4+, and I5+ on the reported tin iodates resulted in values of 0.90–1.26, 2.05, 3.70–4.15, and 4.93–5.03, respectively.