Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Probing disease-related proteins with fluorogenic composite materials
Xiao-Peng
He
a,
Yi
Zang
b,
Tony D.
James
c,
Jia
Li
*b and
Guo-Rong
Chen
*a
aKey Laboratory for Advanced Materials & Institute of Fine Chemicals, East China University of Science and Technology (ECUST), 130 Meilong Rd., Shanghai 200237, PR China. E-mail: mrs_guorongchen@ecust.edu.cn
bNational Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences (CAS), 189 Guo Shoujing Rd., Shanghai 201203, PR China. E-mail: jli@simm.ac.cn
cDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK
First published on 4th December 2014
Abstract
Construction of composite materials based on the self-assembly of fluorescently labeled biomolecules with a variety of micro- or nano-quenching materials (by the Förster Resonance Energy Transfer mechanism) for the fluorogenic recognition of disease-related proteins has become a dynamic research topic in the field of fluorescence recognition. Here we summarize the recent progress on the composition of fluorescence dye-labeled biomolecules including sugars, peptides and nucleotides with organic (graphene and carbon nanotubes) and inorganic (gold nanoparticles) materials. Their application in the fluorescence detection of proteins and enzymes on both the molecular and cellular levels is discussed. Perspectives are proposed with respect to the future directions of employing these composite materials in the recognition of pathological proteins.
Yi Zang received her PhD in Pharmacology from SIMM (CAS). Following a postdoctoral fellowship at M. D. Anderson cancer center in the US, she returned to SIMM in 2010 as an associate professor, and was promoted to professor in 2014. Zang focuses her research on the interactome and identification of novel physiological roles of AMPK, the mechanistic study of cell fate regulation using chemical biology tools, and detection of protein–carbohydrate interactions for disease diagnosis and targeted therapy. |
Jia Li received his PhD from SIMM in 2000 and was promoted to professor in 2005. He stayed at the University of Cambridge (UK, February 2003–August 2003) and Garvan Institute of Medical Research (Australia, August 2004–February 2005) as a visiting scholar. From year 2013 he has been appointed as a deputy director of SIMM. Li's research interests are centered on the investigation of mechanisms of metabolic diseases for identifying key genetic and biochemical events, chemical biology and high-throughput drug screening. He received the China National Fund for Distinguished Young Scientists in 2011. |
Guo-Rong Chen received her BS in Organic Chemical Engineering (1975) from ECUST. She conducted her research at the Glycochemistry Lab of University Lyon 1 from 1989–1991; she revisited the lab in years 1996, 1998 and 2002. Then she spent two years at Shanghai Institute of Materia Medica (CAS) as a visiting scholar. She was appointed as a professor at ECUST in 2001, and her research interests involve glycochemistry, medicinal glycochemistry, chemical glycobiology and green fine chemicals. |
Key learning points(1) Principles of constructing fluorogenic composite materials (FCMs).(2) Use of graphene, carbon nanotubes and gold nanoparticles as quenching materials. (3) Scope of using FCMs for fluorescence detection of disease-related proteins. (4) Future improvement and extension of FCMs towards disease theranostics. |
Introduction
Receptor-type proteins and enzymes, which distribute on the cell membrane or in certain cell organelles, play a pivotal role in numerous physiological processes. For example, the G-protein coupled receptors (GPCRs) found in eukaryotes can recognize and bind to small molecules, peptides and proteins, activating myriads of important signaling pathways within cells. However, over-expression or malfunction of these protein receptors may cause fatal human diseases. It is estimated that around 40% of all commercial drugs target a GPCR.1 Protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) modulate reversibly the phosphorylation of the tyrosine residue of biologically important proteins and peptides.2 Many small-molecules that quench the catalytic activity of disease-related PTKs have been approved as anticancer drugs. Meanwhile, inhibition of malfunctional PTPs is becoming a promising strategy towards the treatment of a number of human diseases.3Given this evidence, ingenious detection and revelation of the recognition between biomolecules and their pairing disease-related proteins may facilitate not only disease diagnoses but also modern drug research & development. Current laboratory and clinical protocols to probe these sensory events largely depend on the immunofluorescence technique (such as the enzyme-linked immunosorbent assay – ELISA). Despite its modularity, reproducibility and high sensitivity, this technique generally requires onerous and elaborate manipulation procedures, long detection time (repeated incubation, blocking and rinsing steps) and high detection cost (use of expensive antibodies).
As a consequence, alternative methods for probing disease-related proteins with simplified detection procedures have been actively developed in recent years. These including surface plasmon resonance, quartz crystal microbalance, electric field effect and electrochemistry techniques have made possible the detection of label-free proteins in a sensitive manner. Nevertheless, these techniques still need derivatization before detection and generally employ costly facilities. In contrast, the fluorescence technique, owing to its high sensitivity and simplicity in manipulation, has extensively been used in the interpretation of chemo- as well as bio-recognition events. It is also a mainstream technique employed for the signal readout of fluorescently labeled proteins in conventional biochemical antibody-based sandwich assays.
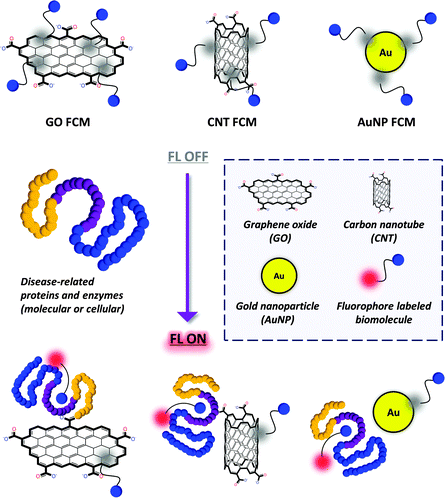
Recently, composite materials that consist of fluorescence (FL) dye labeled biomolecules assembled with a quenching material (such as graphene, carbon nanotube and gold nanoparticle) have been developed for the simple fluorogenic detection of nucleotides as well as proteins and enzymes (Fig. 1). Compared with the conventional methods, these materials are of substantial merit in terms of the following factors: (1) the fluorescently labeled biomolecules can spontaneously adhere to the surface of a material to generate a fluorogenic composite material (FCM) using the Förster Resonance Energy Transfer (FRET) mechanism;4 (2) these composite materials can be used to detect label-free proteins with short detection time and easy-to-manipulate detection procedures; (3) clustering of biomolecules at a material interface can enhance their binding avidity with proteins, which overcomes the inherent low binding affinity between small molecules and a protein; (4) since most of these detection strategies are solution-based, live cells and pathogens that express a pathological protein can be captured sensitively in a label-free manner, and even in vivo.
Here we summarise the recent progress made with respect to the ingenious detection of disease-related proteins using FCMs. This simple approach may provide promising tools for the advancement of disease theranostics.
General principle for the construction of FCMs
The general principle for the construction of FCMs depends on the self-assembly of fluorophore labeled biomolecules on the surface (which does not need derivatization prior to functionalization) of quenching material including carbon nanotubes (CNTs), gold nanoparticles (AuNPs), and particularly graphene (Fig. 1). We note that graphene oxide (GO) is commonly used due to its satisfactory water solubility and better biocompatibility. The assembly process is based on non-covalent contacts such as π-stacking, electrostatic interactions and van der waal forces, producing the composite materials in a spontaneous manner.The FCMs when formed exhibit a quenched FL (OFF) because of the FRET effect between the dye-labeled biomolecule (FRET donor) and quenching material (FRET acceptor). The broad adsorption band of the quenching materials can effectively overlap the emission band of the fluorescent dyes used. Meanwhile, the quantum yields of the materials are so low that no FL emission from the materials is observed upon excitation of the dyes.5
When a target that selectively recognizes the biomolecule is added, the FL can be recovered (ON). This is due to the fact that the target may competitively form a new complex with (such as a protein receptor) or cleave (such as an enzyme) the dye-labeled biomolecule, resulting in decomposition of the FCMs. Nucleotides and oligopeptides are such widely used biomolecules that can strongly bind to the surface of the materials due to their structural complexity. In contrast, small-molecule ligands such as sugars have insufficient ‘binding groups’. Therefore, the covalent attachment of a fluorescent dye may provide not only the detection signal but also a ‘binder’ to the surface of the materials.
Graphene oxide (GO)-based FCMs
The ideal graphene is a pure atomic thin flake (nano- to micro-meters in lateral size) with exceptionally high mechanistic strength and good heat and electric conductivity, as first discovered by the Geim and Novoselov lab.5 Because of its broad absorbance spectrum, graphene has been proven to be a universal quencher of nearly all organic dyes. Its oxidized form, GO, has enhanced water solubility and good biocompatibility compared to the pristine graphene. As a result, GO has received much interest for the development of biomaterials and probes for drug delivery and, especially FL detection of biomolecules. In these systems, GO serves as both the quenching reagent and a platform to cluster the probe molecules, facilitating the sensitivity of the fluorogenic recognition.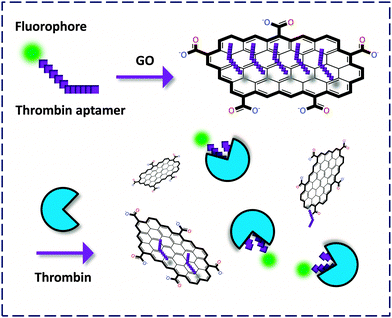 | ||
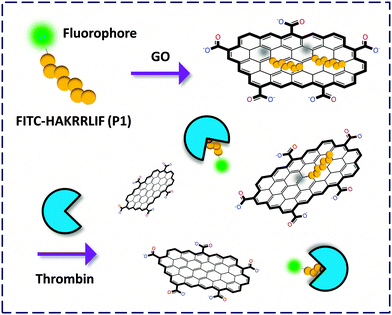
| Fig. 2 Fluorogenic composite materials based on aptamers covalently labeled with a fluorophore for detection of thrombin. | ||
Yang and co-workers6 first described an aptasensor fabricated by stacking of a specific human thrombin aptamer labeled with 6-carboxyl fluorescein (FAM) with GO. On the basis of the strong binding affinity between the aptamer and the protein, the GO-FCM could sensitively and selectively detect thrombin with a limit of detection (LOD) of 2 nM. Then Li and co-workers7 reported a similar aptamer based GO-FCM that achieved picomolar detection of thrombin in phosphate buffered saline (PBS), goat serum and bovine serum. Both studies proved that using GO as a quenching platform is superior in terms of sensitivity to using CNT. This could be possibly reasoned by the broader contact surface of GO (two-dimensional) than CNT (one-dimensional) for binding dye-labeled biomolecules, thereby enhancing the aptamer–protein interaction. The use of aptamers as precursors to form composites with GO for bio-recognition opens up an effective way for the fluorescence detection of proteins.
Bi and co-workers8 presented a strategy employing the chemiluminescence resonance energy transfer (CRET) between a luminol–H2O2–horseradish peroxidase complex and the FAM dye. Upon thrombin–aptamer binding, the FL could be recovered with a satisfactory LOD of 0.1 pM. This strategy takes advantage of the activation of a luminescent substrate without the requirement of external light excitation. And the reaction-based detection protocol provides the most sensitive GO-FCM so far for thrombin detection. Hah and co-workers9 demonstrated the applicability of peptide nucleic acids (PNAs) as aptamers in the construction of GO-FCM for thrombin. Ye and co-workers10 developed a molecular beacon-like FCM consisting of three ss-DNAs tagged with different FL dyes for the simultaneous sensing of thrombin, sequence-specific DNA and heavy metal ions. This study proves that GO can be used as a versatile central platform for the concomitant binding and recognition of multiple targets.
Subsequently, two independent groups revealed new criteria for developing GO-FCM based thrombin probes. On the one hand, an aggregation-induced-emission (AIE)-based11 GO-FCM aptasensor was developed by Tian and co-workers.12 In this system the quenched FL of a free AIE dye indicator originally stacked on GO could be released upon intercalation into the quadruplex aptamer–thrombin complex (Fig. 3). Notably, the detection does not require the labeling of either the analyte or the bio-ligand, facilitating a ‘double-label-free’ detection criterion for thrombin as well as for other proteins. Indeed, the AIE mechanism, being opposite to the conventional aggregation-caused-quenching, has emerged as a promising tool for the design of fluorogenic biosensors. AIE-based sensors produce a specific FL ‘OFF–ON’ signal upon aggregation with the analyte, which does not require extensive synthetic efforts of the sensors.
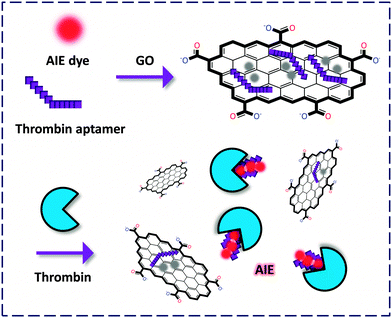 | ||
| Fig. 3 Fluorogenic composite materials based on the composition of a label-free aptamer, an aggregation-induced-emission (AIE) fluorophore and GO for detection of thrombin. | ||
On the other hand, an interesting solid-support detection of thrombin was devised by Furukawa and co-workers.13 They conjugated the FAM-labeled aptamer to pyrene that is a strong ‘binder’ to the surface of GO, and the GO-FCM was further deposited onto a silicon surface. Upon recognition, the FL of FAM was recovered without detaching from the surface (due to the presence of the strong pyrene binder), as visualized using an atomic force microscope (AFM). This system was successfully applied in the microchannel detection of thrombin, paving the way for future solid-phase high-throughput monitoring of proteins (Fig. 4).
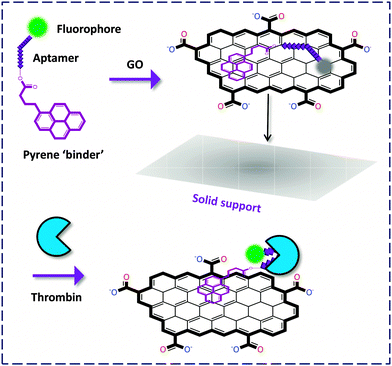 | ||
| Fig. 4 Thrombin detection on a solid support without detachment of the fluorophore-labeled biomolecule from the GO surface. | ||
Detection of other disease-related proteins using the versatile GO-FCMs was also achieved. Yang and co-workers14 developed a general pyrene-conjugated peptide-GO FCM for the detection of the antibody of a glycoprotein gp120 that is pivotal for HIV-1 invasion. An aptasensor was developed by Li and co-workers15 for the detection of an oncoprotein, platelet derived growth factor-BB (PDGF-BB), which regulates growth and division of tumor cells. This FCM could selectively discriminate between the highly homogenous PDGF-BB and -AA.
Qu and co-workers16 constructed a GO-FCM that consists of a fluorescein isothiocyanate (FITC)-labeled peptide ligand for sensing a prognostic indicator of early-stage cancer, cyclin A2 (Fig. 5). This platform was manifested to be 1200-fold more sensitive than a well-known Tb3+ chelating macrocycle-based probe and 10-fold better than a CNT-based probe. Alzheimer's disease (AD) is currently an incurable and irreversible disease affecting millions of people every year. The same researchers reported the construction of a GO-FCM composed of thioflavin-S (ThS) that is a clinical staining dye of the amyloid β (Aβ) fibrils toxic to neuronal cells of AD patients.17 Complexation of Aβ with the material activated the FL of ThS. Subsequently, near infrared (NIR) irradiation quenched the FL by dissociating the Aβ fibrils because of the strong NIR absorption ability of nano-GO, producing a localised temperature increase. The FCM was demonstrated to be useful in complex cerebrospinal fluid. This study exemplifies the theranostic potential of GO-FCMs for real biological samples.
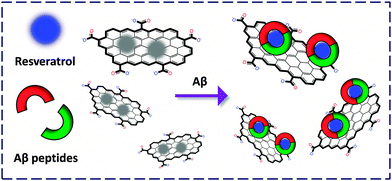
We reported the fabrication of a resveratrol (a natural product abundantly found in red wine to bind selectively with Aβ) confined GO-FCM for the sensitive detection of both Aβ monomers and fibrils (Fig. 6).18 The composite material was used to stain senile plaques in mice brain sections. Compared to the conventional immunofluorescence antibody staining, the cost of the former was lowered by more than 1000-fold and the detection time was lowered by more than 20-fold. We also fabricated an FCM formed between boronate receptors and GO for the selective detection of fructose, offering a new technique for probing disease-related glycoproteins.19
Sugars found on the cell membrane are significant signaling molecules modulating a number of biological as well as pathological events through interactions with their pairing receptors (lectins). Ingenious detection of sugar–lectin interactions at the cellular level may offer invaluable tools for deciphering glycomics.20,21
Liu and co-workers22 customised a tetrameric mannoside tagged with a neutral benzothiadiazole conjugated polymer, FBT. The glycopolymer itself responded to a lectin with increased FL probably due to a change in the micro-environment from hydrophilic to hydrophobic. In contrast, composition of which with GO led to full suppression of the strong background FL of the compound and, notably, improvement of the sensitivity. More importantly, the FCM could detect an E. Coli (a gram-negative bacterium) strain that expresses a mannose receptor (MG1655) in a fluorogenic manner while showing slight response to that without expression of the receptor (Top 10). This paves the way for FCM-based detection of microbes (Fig. 7).
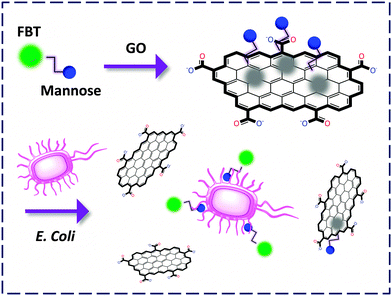 | ||
| Fig. 7 Capturing E. coli expressing mannose receptors by a GO-FCM confined with mannoside labelled with an FBT polymer. | ||
We described the preparation of an N-acetyl-galactosyl rhodamine B (GalNAc-RB) confined FCM for the fluorogenic probing of the asialoglycoprotein receptors (galactoside-selective) expressed on the membrane of a hepatoma cell, Hep-G2.23 The RB dye moiety acts not only as a reporter unit but also as a binder facilitating the clustering of the non-aromatic GalNAc to the GO surface probably by, π-stacking and electrostatic interactions. We then observed that the change in the sugar to N-acetyl-glucoside (GlcNAc) as well as knockdown of the receptor caused an obvious decrease of the FL signal. This unravels the promise of using these materials for the selective analysis of sugar–lectin interactions at the cellular level. Notably, conventional techniques are unable to sense live cells since the cells need lysis prior to detection. This drawback has been overcome using the GO-FCMs due to their ability to spontaneously produce the detection signal upon recognition (Fig. 8).
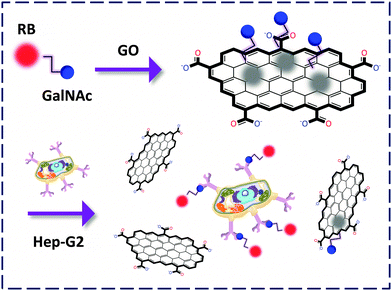 | ||
| Fig. 8 Capturing Hep-G2 expressing the asialoglycoprotein receptors by a GO-FCM confined with GalNAc-rhodamine B (RB). | ||
Peptide-based FCMs were also constructed to probe receptor-type biomarkers on the surface of cancer cells. Chen and co-workers24 prepared a pyrene-tagged RGD cyclic peptide confined FCM that showed sharp fluorogenic response to MDA-MB-435, a cancer cell line that over-expresses integrin which binds to the peptide. Negligible response was observed to MCF-7 cells with a low integrin expression level. FCM confined with FITC-tagged octreotide was developed by Huang and co-workers25 to target the somatostatin receptor subtype 2 over-expressed on AR42J cancer cells. In contrast, the FCM showed no FL response to a control CHO cell line due to the lack of the cancer-specific receptor. These studies demonstrate that the fluorogenic detection is dependent on the selective ligand–receptor interactions, even with live cells.
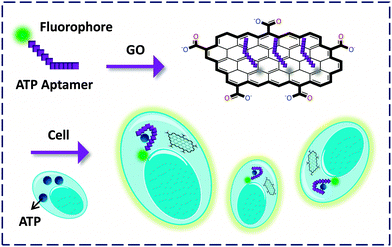
In addition to the transmembrane proteins, Lin and co-workers26 prepared an FCM confined with ATP aptamers for probing ATP within cells. They demonstrated that GO in this system could serve as an efficient cargo to deliver the aptamer into mice epithelial cells. The FL of FAM was then observed by binding to the intracellular ATP. Also, GO was shown to protect the aptamer from being cleaved during the process of entering the cell, providing a new tool for the tracking of endogenous species expressed by pathological cells (Fig. 9).
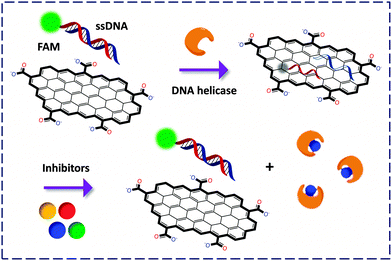
While the general detection principle relies on the cleavage/release of the fluorophore-labeled portion of an enzyme substrate from GO, some pioneering work is noteworthy. The first GO-FCM based enzymatic activity assay was developed by Min and co-workers28 for the detection of helicase using GO mixed with FAM-labeled dsDNA. Once unwound by the enzyme, the resulting ssDNAs with or without FAM labelling could adsorb onto the GO surface, quenching the FL. The authors then exploited this ‘ON–OFF’ mechanism to screen inhibitors of helicase in a fluorogenic manner (since competitive binding of the inhibitor to the enzyme weakened the unwinding of the fluorescent dsDNA, Fig. 10). When compared with the conventional 32P based helicase assay, the use of the GO-FCM is advantageous for its conciseness in manipulation, low cost, high shelf-stability and potential to be used in high-throughput drug screening.
Then the same group reported an extended study on the construction of a GO-FCM tethered with two ssDNAs labeled with different fluorophores for the simultaneous detection of two helicases of hepatitis C virus.29 With this platform concurrent fluorogenic screening of drug candidates of the two pathological enzymes was realised. Ye and co-workers30 demonstrated the first non-ssDNA based GO-FCM confined with a peptide. Once cleaved by thrombin the FL was released with nanomolar LOD.
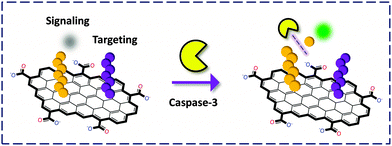
Chu and co-workers31 developed a covalent tactic by linking two different peptides to the surface or edge carboxylic acids of GO through the amide bond (Fig. 11). One FAM-labeled peptide served as a substrate for caspase-3, a central mediator for initiation and propagation of apoptosis. The other served as a targeting reagent for cancer cells. The authors observed that the covalent GO–peptide conjugate could fluorescently image cancer cells treated with an apoptosis inducer (to over-express caspase-3). Interestingly, a non-covalent complex of the GO–peptide did not produce any FL, implying that the peptide without coupling to GO was not stable enough to be carried into the cells in this case.
Prostate-specific antigen (PSA) exists in the body fluid as a biomarker for prostate cancer. Ma and co-workers32 stacked an FITC-labelled peptide that can be degraded by proteolytically active PSA with GO. Thus, a GO-FCM was produced for quantification of a urine sample spiked with PSA. This provides a more rapid and economic tool for the simple clinical determination of a cancer biomarker than the conventional ELISA-based methods. Exonuclease III (Exo III)-aided signal amplification for detection of lysozyme (Lys), an enzyme associated with a range of diseases including monocytic leukemia, was developed by Yu and co-workers.33 In this strategy, Exo III served as a cleaver for the exposed single-stranded tail sequence of a hairpin probe aptamer–Lys complex. Recyclable aptamer–Lys fluorogenic interactions were thus made possible to improve the sensitivity.
Protein tyrosine phosphatases (PTPs) participate in the pathogenesis of various fatal human diseases. Li and co-workers34 stacked an FITC-labelled phospho-peptide with GO for evaluation of the catalytic activity of PTP1B (the first identified PTP member related to type II diabetes and breast cancer). In the absence of PTP1B the peptide on the GO surface could not be degraded by chymotrypsin due to the existence of the phosphate ‘blocker’. However, its presence led to substrate dephosphorylation, and thus cleavage of the peptide releasing the fluorophore. This research provides a new chemical tool for probing the PTP pathology and facilitating PTP-based drug discovery.3
Carbon nanotube (CNT)-based FCMs
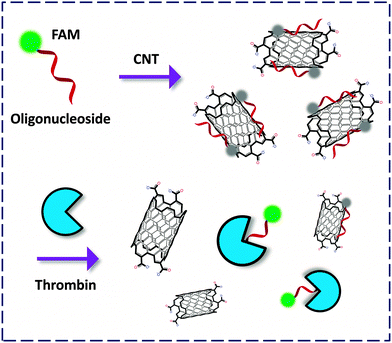
Carbon nanotubes can be viewed morphologically as a curled graphene flake. These single-walled (SW) or multi-walled cylindrical nano-carbon allotropes possess unique properties suited for use in nanotechnology.35 Photophysical investigations reveal that SWNTs are general quenching materials for FL dyes. Despite this, CNT-FCMs have been developed for the analyses of DNA and RNA, whereas relatively less examples were available for detection of disease-related proteins.36,37The pioneering work by Tan and co-workers38 described an FCM formed between an FAM-thrombin aptamer and SWNT. The fluorescent oligonucleotide-based aptamer could twine against the wall of the NT to quench the FL, whereas binding of thrombin to the aptamer recovered the FL (Fig. 12). Xu and co-workers devised SWNT-based dahlia-like single-walled carbon nanohorns (SWCNHs) as a quenching platform to produce CNT-FCMs. This unique FCM composed of FAM-aptamers has been used to detect thrombin based on either cleavage-39 or binding-induced40 FL recovery with pM LOD. The satisfactorily low LOD could be a result of the exceptional morphological effect of the SWCNHs. These studies also highlight the importance of their structural effect on the activity of carbon material-based FCMs.
Liang and co-workers41 developed a multicolor CNT-based nanoprobe for the simultaneous detection of three cancer-related proteases. The peptides labeled separately with three different organic dyes were covalently linked to the CNTs by amidation, with quenched FL. The probe showed excellent sensitivity with low interference from competing analytes towards the multicolor detection of the enzymes (Fig. 13). This study reveals that, similar to GO, CNTs can also serve as a versatile platform to bind multiple biomolecules for multi-target biosensing.
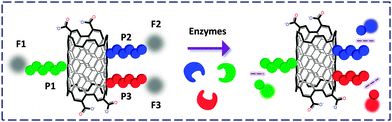 | ||
| Fig. 13 SWNT FCM functionalized covalently with three peptides (P) labelled with different fluorophores (F). | ||
Gold nanoparticle (AuNP)-based FCMs
Gold nanoparticles (AuNPs) are a class of inorganic nanomaterials broadly employed in the construction of chemo- and bio-probes. The simplest detection criterion employing the AuNPs is the ‘naked-eye’ colorimetric approach based on the analyte-modulated dispersion and aggregation of the modified particles. Localized surface plasmon resonance is another widely exploited property of AuNPs for ultrasensitive molecular sensing.42 Given that they are good quenchers of organic dyes, AuNP-FCMs were developed for FL analysis of proteins and enzymes.Jiang and co-workers43 took advantage of the electrostatic interaction between RB and citrate-coated AuNP to produce a RB-coated FCM for the detection of acetylcholinesterase (AChE) in the cerebrospinal fluid of AD transgenic mice. AChE could cleave acetylthiocholine to generate positively charged thiocholine that replaces the RB attached on the particle surface, releasing the FL. This fluorogenic detection was also accompanied by a sharp colour change (Fig. 14). This study provides a dual-signal detection criterion using the unique optical properties of AuNPs.
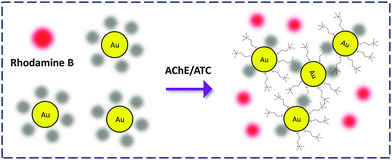 | ||
| Fig. 14 Rhodamine B-attached AuNP FCM for the detection of acetylcholinesterase (AChE) that deacetylates acetylthiocholine (ATC). | ||
A similar replacement-based immunoassay was developed by Zhao and co-workers.44 Replacement of an FTIC-labeled antigen by the analyte antigen in a serum sample led to fluorogenic determination of immunoglobulin M (IgM) with picomolar LOD. Liang and co-workers45 presented a strategy by functionalising a single AuNP with three different antibodies tagged with different organic dyes. The FCM was able to generate multicolor FL signals in both solution and human serum samples by competitive surface binding of the analyte peptides against that of dye-labeled peptides.
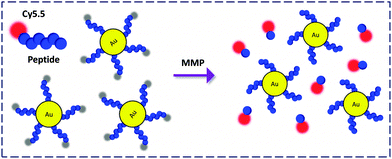
AuNPs covalently functionalised with a protease substrate labeled via a near infrared (NIR) dye, Cy5.5, was prepared by Ahn and co-workers (Fig. 15).46 This material was used to detect the enzymatic activity of matrix metalloprotease (MMP) which plays a key role in promoting cancer progression. Notably, due to the NIR emission, the FCM was further applied for the sensitive monitoring of MMP activity in a tumor-bearing mice model with or without treatment with an inhibitor. This suggests the applicability and promise of AuNP-FCMs for in vivo theranostics.
Human telomerase in cancer cells cannot be regularly shortened, leading to indefinite division of cells. In metastatic cancer tissues, over-expression of the enzyme is detected. Ju and co-workers47 designed a molecular beacon containing a nick dividing the beacon into a shorter telomerase primer sequence pairing partially with a Cy5-labelled longer sequence that forms a loop on AuNPs. In the presence of telomerase the primer was elongated from the 3′ end producing a repeated sequence complementary to the 3′ end stem of the longer sequence. This caused substantial hybridization which unfolds the loop, and turns on the FL.
Summary and perspective
The rapid development of modern nanotechnology has made possible the fruitful production of organic and inorganic nano-material-based devices and probes for addressing biological systems. This review summarises recent progression of the use of fluorogenic composite materials (FCMs) for probing disease-related proteins. The three general quenching materials for organic dyes, i.e. gold nano-particles (AuNPs), carbon nanotubes (CNTs), and graphene oxide (GO, ranging from nano- to micro-size), have all been shown to be suitable building blocks for the construction of FCM probes. The materials serve both as a quencher and as a platform that clusters dye-labelled biomolecules (or in some cases provides a cell-permeable carrier) for the detection of an analyte protein with high sensitivity and selectivity. Because of the solution-based detection rationale, proteins expressed on the cell surface, within cells as well as in living animals, can be captured by these FCMs.Despite the promise of the FCMs, some important problems still need to be overcome in future systems. Firstly, the particle size and the structural effect on the activity of FCMs are always neglected, unfortunately. Huang and co-workers48 revealed in a recent study that GO of different nano-sizes impacted significantly on the quenching of fluorescent biomolecules as well as on the sensing performance of the resulting FCMs for a metal ion. This interesting phenomenon was also observed during our recent research regarding GO-FCMs for detection of sugar–lectin interactions on both the molecular and cellular levels (unpublished results). As a consequence, we propose that the size and structural effects should be investigated and optimized in future studies where FCMs are developed. Likewise, the relationship between the oxygen content of the carbon materials and their sensing performance for an analyte should be elaborated.
Secondly, a number of previous studies have suggested that the two-dimensional GO has better sensitivity than the one-dimensional CNTs in bio-sensing, and even superior to the zero-dimensional AuNPs.49 However, the fundamental rationale behind these observations, other than the intuitive morphological distinction, should be delineated. Furthermore, unlike oligo-peptides and -nucleosides, small-molecule ligands such as monosaccharides and oligosaccharides can hardly self-assemble to the surface of the carbon materials due to the absence of sufficient ‘binding’ groups (such as aromatic groups). In these specific cases, the choice and the coupling manner of a dye with the ligand should be carefully designed, since both factors may impact the self-assembling efficiency. In addition to boronate-based receptors,19,50 we also envisage the use of sugar aptamers in the future development of FCMs for probing glycomes in the body fluid and on the surface of cells and tissues.
Last but not the least, the majority of the currently developed GO and CNT-based FCMs dissemble upon recognition to produce the fluorogenic signal, especially in the detection of receptor–ligand associations. This fact is a result of the fragile non-covalent attachment of the dye-labelled biomolecules with the materials via non-specific absorptions. Although this composition manner is simple, one cannot imagine its stability and hence suitability with complex biological systems (such as in cells and in vivo). Covalent functionalization of fluorescent bio-ligands with the carbon materials is certainly a good solution, but will increase the fabrication cost and complexity of the probe. Alternatively, mimicking the solid gold–thiol bonding in fabricating AuNP-FCMs and introduction of an additional ‘binder’ that strongly immobilizes the ligands to the surface of GO or CNT could prove a promising strategy. Pyrene owing to its planarity and aromaticity might be a good choice as exemplified by Furukawa and co-workers.13
Although there remain a number of problems to be resolved for the real application of these next-generation materials, we are confident that the first commercial FCM for clinical disease diagnosis or for industrial high-throughput drug discovery will be achieved in the near future.
Acknowledgements
This research is supported by the 973 project (2013CB733700), the National Science Fund for Distinguished Young Scholars (81125023), the National Natural Science Foundation of China (21176076, 21202045), the Program of Shanghai Subject Chief Scientist (13XD1404300), the Key Project of Shanghai Science and Technology Commission (13NM1400900) and the Fundamental Research Funds for Central Universities. The Catalysis And Sensing for our Environment (CASE) network is thanked for research exchange opportunities.Notes and references
- J. P. Overington, B. Al-Lazikani and A. L. Hopkins, Nat. Rev. Drug Discovery, 2006, 5, 993–996 CrossRef CAS PubMed.
- A. J. Barr, E. Ugochukwu, W. H. Lee, O. N. F. King, P Filippakopoulos, I. Alfano, P. Savitsky, N. A. Burgess-Brown, S. Müller and S. Knapp, Cell, 2009, 136, 352–363 CrossRef CAS PubMed.
- For a recent review, see: X.-P. He, J. Xie, Y. Tang, J. Li and G.-R. Chen, Curr. Med. Chem., 2012, 19, 2399–2405 CrossRef CAS.
- E. Morales-Narváez and A. Merkoçi, Adv. Mater., 2012, 24, 3298–3308 CrossRef PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- C.-H. Lu, H.-H. Yang, C.-L. Zhu, X. Chen and G.-N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS PubMed.
- H. Chang, L. Tang, Y. Wang, J. Jiang and J. Li, Anal. Chem., 2010, 82, 2341–2346 CrossRef CAS PubMed.
- S. Bi, T. Zhao and B. Luo, Chem. Commun., 2012, 48, 106–108 RSC.
- E. J. Lee, H. K. Lim, Y. S. Cho and S. S. Hah, RSC Adv., 2013, 3, 5828–5831 RSC.
- M. Zhang, B.-C. Yin, W. Tan and B.-C. Ye, Biosens. Bioelectron., 2011, 26, 3260–3265 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- M. Li, K. Ma, S. Zhu, S. Yao, Z. Liu, B. Xu, B. Yang and W. Tian, Anal. Chem., 2014, 86, 298–303 CrossRef PubMed.
- K. Furukawa, Y. Ueno, E. Tamechika and H. Hibino, J. Mater. Chem. B, 2013, 1, 1119–1124 RSC.
- C.-H. Yang, J. Li, X.-L. Zhang, A.-X. Zheng, H.-H. Yang, X. Chen and G.-N. Chen, Anal. Chem., 2011, 83, 7276–7282 CrossRef PubMed.
- J. Liang, R. Wei, S. He, Y. Liu, L. Guo and L. Li, Analyst, 2013, 138, 1726–1732 RSC.
- X. Wang, C. Wang, K. Qu, Y. Song, J. Ren, D. Miyoshi, N. Sugimoto and X. Qu, Adv. Mater., 2010, 20, 3967–3971 CAS.
- M. Li, X. Yang, J. Ren, K. Qu and X. Qu, Adv. Mater., 2012, 24, 1722–1728 CrossRef CAS PubMed.
- X.-P. He, Q. Deng, L. Cai, C.-Z. Wang, Y. Zang, J. Li, G.-R. Chen and H. Tian, ACS Appl. Mater. Interfaces, 2014, 6, 5379–5382 CAS.
- X. Sun, B. Zhu, D.-K. Ji, Q. Chen, X.-P. He, G.-R. Chen and T. D. James, ACS Appl. Mater. Interfaces, 2014, 6, 10078 CAS.
- X.-P. He, X.-W. Wang, X.-P. Jin, H. Zhou, X.-X. Shi, G.-R. Chen and Y.-T. Long, J. Am. Chem. Soc., 2011, 133, 3649–3657 CrossRef CAS PubMed.
- Z. Li, S.-S. Deng, Y. Zang, Z. Gu, X.-P. He, G.-R. Chen, K. Chen, T. D. James, J. Li and Y.-T. Long, Sci. Rep., 2013, 3, 2293 Search PubMed.
- L. Wang, K.-Y. Pu, J. Li, X. Qi, H. Li, H. Zhang, C. Fan and B. Liu, Adv. Mater., 2011, 23, 4386–4391 CrossRef CAS PubMed.
- H.-L. Zhang, X.-L. Wei, Y. Zang, J.-Y. Cao, S. Liu, X.-P. He, Q. Chen, Y.-T. Long, J. Li, G.-R. Chen and K. Chen, Adv. Mater., 2013, 24, 1722–1728 CrossRef.
- Z. Wang, P. Huang, A. Bhirde, A. Jin, Y. Ma, G. Niu, N. Neamati and X. Chen, Chem. Commun., 2012, 48, 9768–9770 RSC.
- B. Feng, L. Guo, L. Wang, L. Fan, J. Lu, J. Gao, C. Fan and Q. Huang, Anal. Chem., 2013, 85, 7732–7737 CrossRef CAS PubMed.
- Y. Wang, Z. Li, D. Hu, C.-T. Lin, J. Li and Y. Lin, J. Am. Chem. Soc., 2010, 132, 9274–9276 CrossRef CAS PubMed.
- H. Jang, J. Lee and D.-H. Min, J. Mater. Chem. B, 2014, 2, 2452–2460 RSC.
- H. Jang, Y.-K. Kim, H.-M. Kwon, W.-S. Yeo, D.-E. Kim and D.-H. Min, Angew. Chem., Int. Ed., 2010, 49, 5703–5707 CrossRef CAS PubMed.
- H. Jang, S.-R. Ryoo, Y.-K. Kim, S. Yoon, H. Kim, S. W. Han, B.-S. Choi, D.-E. Kim and D.-H. Min, Angew. Chem., Int. Ed., 2013, 52, 2340–2344 CrossRef CAS PubMed.
- M. Zhang, B.-C. Yin, X.-F. Wang and B.-C. Ye, Chem. Commun., 2011, 47, 2399–2401 RSC.
- H. Wang, Q. Zhang, X. Chu, T. Chen, J. Ge and R. Yu, Angew. Chem., Int. Ed., 2011, 50, 7065–7069 CrossRef CAS PubMed.
- T. Feng, D. Feng, W. Shi, X. Li and H. Ma, Mol. BioSyst., 2012, 8, 1441–1445 RSC.
- C. Chen, J. Zhao, J. Jiang and R. Yu, Talanta, 2012, 101, 357–361 CrossRef CAS PubMed.
- F. Wang, C. Liu, Y. Fan, Y. Wang and Z. Li, Chem. Commun., 2014, 50, 8161–8163 RSC.
- S. J. Tans, M. H. Devoret, H. Dai, A. Thess, R. E. Smalley, L. J. Geerligs and C. Dekker, Nature, 1997, 386, 474–477 CrossRef CAS.
- Z. Zhu, R. Yang, M. You, X. Zhang, Y. Wu and W. Tan, Anal. Bioanal. Chem., 2010, 396, 73–83 CrossRef CAS PubMed.
- C. Li and G. Shi, J. Photochem. Photobiol., C, 2014, 19, 20–34 CrossRef CAS PubMed.
- R. Yang, Z. Tang, J. Yan, H. Kang, Y. Kim, Z. Zhu and W. Tan, Anal. Chem., 2008, 80, 7408–7413 CrossRef CAS PubMed.
- S. Zhu, Z. Liu, L. Hu, Y. Yuan and G. Xu, Chem. – Eur. J., 2012, 18, 16556–16561 CrossRef CAS PubMed.
- S. Zhu, S. Han, L. Zhang, S. Parveen and G. Xu, Nanoscale, 2011, 3, 4589–4592 RSC.
- Y. Huang, M. Shi, K. Hu, S. Zhao, X. Lu, Z.-F. Chen, J. Chen and H. Liang, J. Mater. Chem. B, 2013, 1, 3470–3476 RSC.
- For a recent review, see: Y. Zhang, Y. Guo, Y. Xianyu, W. Chen, Y. Zhao and X. Jiang, Adv. Mater., 2013, 25, 3802–3819 CrossRef CAS PubMed.
- D. Liu, W. Chen, Y. Tian, S. He, W. Zheng, J. Sun, Z. Wang and X. Jiang, Adv. Healthcare Mater., 2012, 1, 90–95 CrossRef CAS PubMed.
- J. Chen, Y. Huang, S. Zhao, X. Lu and J. Tian, Analyst, 2012, 137, 5885–5890 RSC.
- M. Shi, J. Chen, Y. Huang, K. Hu, S. Zhao, Z.-F. Chen and H. Liang, RSC Adv., 2013, 3, 13884–13890 RSC.
- S. Lee, E.-J. Cha, K. Park, S.-Y. Lee, J.-K. Hong, I.-C. Sun, S. Y. Kim, K. Choi, I. C. Kwon, K. Kim and C.-H. Ahn, Angew. Chem., Int. Ed., 2008, 47, 2804–2807 CrossRef CAS PubMed.
- R. Qian, L. Ding, L. Yan, M. Lin and H. Ju, J. Am. Chem. Soc., 2014, 136, 8205–8208 CrossRef CAS PubMed.
- H. Zhang, S. Jia, M. Lv, J. Shi, X. Zuo, S. Su, L. Wang, W. Huang, C. Fan and Q. Huang, Anal. Chem., 2014, 86, 4047–4051 CrossRef CAS PubMed.
- F. Li, H. Pei, L. Wang, J. Lu, J. Gao, B. Jiang, X. Zhao and C. Fan, Adv. Funct. Mater., 2013, 23, 4140–4148 CrossRef CAS.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |