A new family of carbon materials: synthesis of MOF-derived nanoporous carbons and their promising applications
Watcharop
Chaikittisilp
a,
Katsuhiko
Ariga
*ab and
Yusuke
Yamauchi
*abc
aWorld Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: Ariga.Katsuhiko@nims.go.jp; Yamauchi.Yusuke@nims.go.jp
bPrecursory Research for Embryonic Science and Technology (PRESTO) & Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), 1-1 Namiki, Tsukuba 305-0044, Japan
cFaculty of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo 169-8555, Japan
First published on 12th November 2012
Abstract
Nanoporous carbons possessing high surface area and narrow pore size distribution are among the most important classes of porous materials that are practically utilized in industries. Recently, several metal-organic frameworks (MOFs) or porous coordination polymers (PCPs) have been demonstrated as promising precursors to create functional nanoporous carbons. In this highlight article, we briefly review the recent progress in preparation of these novel MOF-derived nanoporous carbons. Some promising applications in energy and environment-related areas and future outlook are also discussed.
 Watcharop Chaikittisilp | Watcharop Chaikittisilp obtained his bachelor's degree with a First Class Honors and Gold Medal in 2003 from Chulalongkorn University (Thailand). Then, he received a Monbukagakusho Scholarship from MEXT and joined The University of Tokyo (Japan) where he obtained his master's (2006) and PhD (2010) degrees under the supervision of Prof. Tatsuya Okubo. After working as a postdoctoral fellow with Prof. Christopher W. Jones at Georgia Institute of Technology (USA), he returned to Japan and joined the National Institute for Materials Science (NIMS) in 2012 as a MANA research associate. His current research interests include designed synthesis of functional nanoporous materials and their applications toward sustainable society. |
 Katsuhiko Ariga | Katsuhiko Ariga received his PhD degree from the Tokyo Institute of Technology (TIT). He worked as an Assistant Professor at TIT, a postdoctoral fellow at the University of Texas at Austin, USA, a group leader at the Japan Science and Technology agency (JST), an Associate Professor at the Nara Institute of Science and Technology. In 2004, he moved to the National Institute for Materials Science (NIMS) where he is currently the Director of Supermolecules Group and Principal Investigator of World Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA). His major interests are the fabrication of novel functional nanostructures based on molecular recognition and self-assembly. |
 Yusuke Yamauchi | Yusuke Yamauchi received his bachelor's degree in 2003, master's degree in 2004, and PhD in 2007 from Waseda University. After receiving his PhD, he joined the National Institute for Materials Science (NIMS) as a permanent staff. In 2008, he was promoted as a head of “Inorganic nanoporous materials laboratory” at NIMS. Currently, he concurrently serves as PRESTO researcher of Japan Science and Technology Agency (JST), Visiting Associate Professor at Waseda University in Japan, and Visiting Professor at Tianjin University in China. He has authored and co-authored over 200 refereed journal publications. His present research interest is rational synthesis of novel nanoporous materials. |
Introduction
Increasing needs for functional materials associated with molecules, ions, and clusters in the nanometer length scale have stimulated the creation of nanomaterials with precise control over molecular, nanoscopic, and mesoscopic structures.1,2 Unique interactions with such nanoobjects within confined nanospatial networks make nanoporous materials of particular interest for a wide variety of applications including adsorption, catalysis, electronics, and drug delivery.3–5Among many porous materials, nanoporous carbons have been the most important and traditionally employed so far due to their good thermal and chemical stability.6 They have routinely been prepared by pyrolysis followed by physical or chemical activation of organic precursors. Although the resultant activated porous carbons possess high surface area, their structures are disordered with broad pore size distribution and thereby limiting their utilization dealing with molecular discrimination. Efforts to construct nanoporous carbons featuring ordered porous structures and/or narrow pore size distributions involve the templating approaches. Ordered nanoporous carbons can be achieved by carbonization or chemical vapor deposition of carbon sources within the ordered hard-templates (i.e., nanocast or hard-templated carbon)7–9 or carbonization of ordered polymeric carbon gels prepared by an organic–organic soft-templating approach from resols and block copolymers (soft-templated carbon).10–12 Nanoporous carbons prepared by the latter method have diverse structures and morphologies; however, they can be obtained only from a few suitable combinations of thermally stable carbon gels and less stable organic templates that can be thermally decomposed before completion of the carbonization process. In contrast, the former nanocasting approach offers nanoporous carbons with ordered micro-, meso-, and macropores depending on the original hard-templates (i.e., zeolites, mesoporous silicas, and colloidal crystals, respectively), although it is slightly complex and unfavorable for large-scale production.
Currently, metal-organic frameworks (MOFs) or porous coordination polymers (PCPs) have gained particular attention in recent years as a novel class of nanoporous materials mainly because of their designable framework structures modularly built from transition-metal clusters as nodes and organic ligands as struts. Their diverse structures and functions can thus, in principle, be tailored for “on demand” applications.13–15 Inspired by their diverse structures, high surface area, and large pore volume, MOFs (or PCPs) have been considered as an alternative precursor to construct nanoporous carbons, as they may broaden the library of nanoporous carbons with novel structures and properties. Thus far, several MOFs, such as MOF-5,16 Al-PCP,17 and ZIF-8,18 have been demonstrated as promising precursors, yielding highly nanoporous carbons showing excellent properties in gas adsorption, electrochemical capacitance, sensing, and catalysis. In this highlight, we describe several methods to synthesize MOF-derived nanoporous carbons. In the following section, utilization of such MOF-derived carbons in energy and environment-related areas is briefly summarized and finally the future direction of these materials is discussed.
Utilization of MOFs as sacrificial templates
Representative MOFs used to create nanoporous carbons are shown in Fig. 1. They are microporous materials with crystallographic pore apertures of 7.8 × 7.8 Å2 (MOF-5), 7.7 × 7.7 Å2 and 3.0 × 3.0 Å2 (Al-PCP), and 3.4 × 3.4 Å2 (ZIF-8). As illustrated in Fig. 2, a primary carbon precursor, typically furfuryl alcohol (FA), having the molecular dimensions of 8.4 × 6.4 × 4.3 Å3,19 is impregnated and subsequently polymerized inside the micropores of MOFs. During thermal carbonization in an inert atmosphere, the formation of porous carbon networks and the decomposition of MOFs occur simultaneously; therefore, MOFs function as both a sacrificial template and a secondary carbon precursor.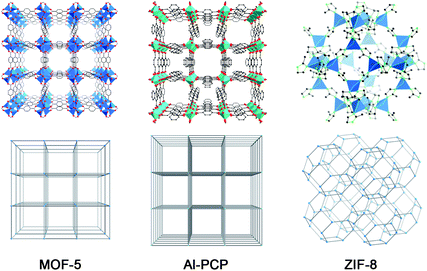 | ||
| Fig. 1 Crystal structures (top) and simplified framework structures (bottom) of MOF-5 (Zn4O(1,4-benzenedicarboxylate)3; left), Al-PCP (Al(OH)(1,4-naphthalenedicarboxylate); middle), and ZIF-8 (Zn(2-methylimidazolate)2; right). | ||
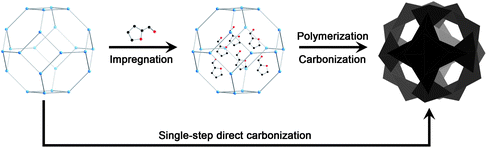 | ||
| Fig. 2 Schematic representation of construction of nanoporous carbons from MOFs with furfuryl alcohol as a carbon source (upper panel) and direct carbonization of MOFs (lower panel). | ||
Xu et al. demonstrated the application of MOFs as a sacrificial template for synthesis of nanoporous carbons, for the first time.20 They introduced FA into the micropores of MOF-5 by a vapor phase protocol. After carbonization at 1000 °C, nanoporous carbon with a very high specific Brunauer–Emmett–Teller (BET) surface area of 2872 m2 g−1 was obtained. However, at a lower temperature of 800 °C, the resultant carbon exhibited a much lower BET surface area of 417 m2 g−1. The material obtained at the decomposition temperature of MOF-5 (530 °C) possessed even a lower BET surface area of 217 m2 g−1. In the following paper,21 they modified the synthesis protocol by using a wet impregnation method to infiltrate FA into the pores of MOF-5, yielding the nanoporous with improved surface areas, particularly at low carbonization temperatures, ranging from 1141 to 3040 m2 g−1. Besides FA, glycerol, carbon tetrachloride and ethylenediamine, and phenolic resin have been used as carbon sources with MOF-5.22,23
Although the early attempts in this research area were successfully made by using MOF-5, control over porous structures and particle morphologies was rarely presented. Yamauchi et al. showed that porous carbon nanofibers can be prepared through carbonization of FA-infiltrated fiber-shaped Al-PCP.24 After carbonization, nanoporous carbon fibers were obtained with the embedded γ-alumina particles. Further treatment with an HF aqueous solution could remove γ-alumina and thereby resulting in pure porous carbons with a BET surface area of 513 m2 g−1.
Functionalization of MOFs-derived nanoporous carbon
In general, MOF structures are somewhat collapsed during the carbonization process due to their inferior thermal stability. As a result, no ordered nanoporous carbons were sometimes obtained. Using a reinforced framework strategy by surface coating, partially ordered microporous carbons could be prepared from Cr-MIL-101.25 Before impregnation of carbon precursors (resol resins), the internal and external surfaces of Cr-MIL-101 were functionalized with aminosilanes coordinated to unsaturated chromium sites to reinforce the MOFs during the carbonization process.Further improvement of textural properties of MOF-derived nanoporous carbons was achieved via mild chemical activation with KOH.23,26 Significant increases in porosity (up to 240%) after KOH activation at relatively low temperature were confirmed.26 Mokaya et al. suggested that the activation process mainly enhanced the existing porosity, especially the pores of 15–25 Å, rather than generating new larger pores.
Dramatic increases in surface areas of MOF-derived nanoporous carbon were reported by Xu and his co-workers.27 Instead of MOF-5, they used the in-house synthesized ZIF-8 as the starting material. Nanoporous carbons obtained after carbonization of FA-infiltrated ZIF-8 at 1000 °C exhibited a BET surface area of 3405 m2 g−1 and a total pore volume of 2.58 cm3 g−1. Again, at a lower temperature of 800 °C, the carbon had a lower BET surface area of 2169 m2 g−1. As shown in Fig. 1, ZIF-8 possesses a cage-type pore structure with large pore cavities of 11.6 Å connected by its small pore apertures of 3.4 Å, which is smaller than the molecular dimensions of FA. Although the authors suggested that FA molecules were able to access into the ZIF-8 cavity under their synthesis conditions, it is still difficult to understand how the larger FA molecules can pass through the smaller pore windows. As is widely known, MOFs are considered as soft-crystals;14 their framework structures are somewhat flexible and thus their crystallographic pore dimensions may be a little different from the real pores, especially at high temperatures where molecules have high mobility. Obviously, careful investigation is needed to give more insights into the infiltration process. Surprisingly, nanoporous carbons prepared under similar conditions but using the commercial ZIF-8 showed significantly lower BET surface areas (e.g., 1131 m2 g−1 at the carbonization temperature of 1000 °C).26 This substantial difference suggests that, in addition to the preparation procedures, the qualities and properties of the parent MOFs may largely affect the textural properties of the resulting nanoporous carbons.
Introduction of heteroatoms, such as boron, nitrogen, and sulphur, into the carbon frameworks can considerably affect the physical properties. Especially, nitrogen-doped nanoporous carbons are useful as base catalysts, CO2 adsorbents, H2 storage media, and supercapacitor electrodes.12,28–32 In general, the use of MOFs constructed from nitrogen-containing ligands, for instance, zeolitic imidazolate frameworks (ZIFs) as the starting precursors would afford nitrogen-doped carbons. During carbonization, however, carbon–nitrogen bonds are substantially broken, resulting in carbons with a low content of doped nitrogen. Increase in nitrogen contents can be reached by using nitrogen-containing molecules such as ethylenediamine and acetonitrile as a carbon source.23,31 As a specific case, semiconducting nanoporous graphitic carbon nitrides (g–C3N4) can be prepared by using cyandiamide, dicyandiamide or melamine as a carbon source.33,34 Recently, Yamauchi et al. reported N-doped nanoporous carbon with fibrous morphology prepared from Al-PCP and dicyandiamide.35 However, due to the presence of carbon atoms in Al-PCP, the obtained material had the C/N molar ratio slightly higher than the theoretical value of carbon nitride.
Direct carbonization of MOFs
Because of the large carbon content in MOFs, one can expect that highly nanoporous carbon can also be achieved by direct carbonization of MOFs without the need for any additional carbon precursors (Fig. 2). This method has a privilege over the previous pathway described above, because it is a facile and single-step procedure. This approach was, for the first time, mentioned for MOF-5, but no detailed investigation was presented.23 The first comprehensive investigation of this approach was reported by Yamauchi et al.36 After direct carbonization of Al-PCP at 800 °C, the nanoporous carbon possessing an extremely high surface area over 5000 m2 g−1 and a large pore volume of 4.3 cm3 g−1 was obtained. It was also demonstrated that the appropriate carbonization temperature was very crucial to realize such high surface area and large pore volume.Nanoporous carbons displaying hierarchical pore structures could be formed by direct carbonization of MOF-5 and its isoreticular MOFs (IRMOFs).37 This work suggested that the pore characteristics of MOF-derived carbons could simply be tuned by selecting suitable MOFs with isoreticular structures. However, the MOF-derived carbon materials reported so far generally possessed broad pore size distributions and contained large cracks and/or voids due to insufficient amounts of carbon sources and anisotropic shrinkage of MOF structures. Very recently, we reported nanoporous carbons having high surface areas (up to 1110 m2 g−1), and narrow and sharp pore size distributions, prepared by direct carbonization of commercial ZIF-8.38 Although the carbon materials were disordered porous structures, their uniform pore sizes were very close to that of the parent ZIF-8. Moreover, no mesopores, macropores, or large cracks were present. This was mainly due to the cubic sodalite structure of ZIF-8.
Very recently, Kim and colleagues systematically investigated the direct carbonization of a series of well-known zinc-containing MOFs.39 Interestingly, they found that even non-porous MOFs could result in highly nanoporous carbons. Also, they suggested that there is likely a linear relationship between the Zn/C atomic ratios of the MOF precursors and the BET surface areas of the resulting nanoporous carbons. This finding may allow us to control the surface areas of MOF-derived carbon materials by simply selecting the starting MOFs with suitable Zn/C ratios.
Energy and environment-related utilizations
The continuous consumption of fossil fuel resources is a major cause of the global climate change. Technologies pertinent to renewable energy conversion and storage to replace the fossil fuel have rapidly been developed in recent years.40–42 Carbon-based nanoporous materials also play critical roles in this rapid and important field. MOF-derived nanoporous carbons and carbon hybrids have been proved to be efficient in many applications including H2 storage, toxic aromatic compounds sensing, supercapacitance, and electrocatalysis.As is well known, carbon materials such as activated charcoal have been used as adsorbents for centuries.6 Due to high surface areas and the presence of micropores in MOF-derived carbons, they were studied as H2 storage materials.20,26,27,37 At 77 K and 1 atm, the carbon material prepared by direct carbonization of MOF-5 showed the highest uptake of 3.25 wt% because it contained a substantial amount of ultramicropores.37 High pressure H2 adsorption measurements further suggested that H2 uptakes in the MOF-derived carbons were comparable or superior to other relevant carbon materials.26 For example, ZIF-8-derived carbons exhibited H2 uptake up to 6.2 wt% at 77 K and 20 bar. In comparison with the parent MOFs, exceptional increases in H2 storage capacities were observed at 298 K and up to 100 bar.37 MOF-5-derived carbons showed increases in capacities by a factor of 5 at 1 bar and by a factor of 2 at 100 bar.
Encouraged by the remarkably high surface area of carbons obtained by direct carbonization of Al-PCP, they were examined as a sensing material for toxic aromatic compounds.36 The fast response and high uptakes toward aromatic compounds such as benzene and toluene were due to the presence of graphitic sp2-hybridized carbons. Uptake of benzene vapor over the Al-PCP-derived carbon was about 4 times higher than the commercial active carbon.
Nanoporous carbon materials can store electricity as charge at the electrode–electrolyte interfaces through reversible ion adsorption on the surface of carbons. MOF-derived nanoporous carbons were evaluated as potential electrodes for electrical double layer capacitors (EDLCs).20–23,27,38 Their capacitances along with the measurement conditions are summarized in Table 1. The representative nanoporous carbons with very high capacitances in both acidic and alkali electrolytes are also presented for comparison.43,44 MOF-derived nanoporous carbons had the capacitances comparable to the highest values reported for carbon EDLC electrodes likely because of high surface areas and optimal micropore diameters. In addition to gravimetric capacitances (in F g−1), interfacial (in F cm−2) and volumetric (in F cm−3) capacitances are also important toward miniaturization of devices, especially portable electronic devices. Of particular interest, Z-900, a nanoporous carbon prepared by direct carbonization of ZIF-8 at 900 °C, had very high interfacial and volumetric capacitances (Table 1).38 These results also indicated that the surface area and pore volume of Z-900 were efficiently utilized to store charge for EDLCs.
| Material | Electrolyte | Potential range/V | Scan rate/mV s−1 | Particle densitya/g cm−3 | Capacitanceb | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| F g−1 | μF cm−2 | F cm−3 | ||||||
| a Particle density = [total pore volume + 1/ρcarbon]−1, where ρcarbon is the true density of carbon (2 g cm−3).38 b Gravimetric capacitances in F g−1 were taken from the references; interfacial capacitances in μF cm−2 were normalized to the BET surface area; volumetric capacitances in F cm−3 were calculated from the estimated particle density of carbon materials. | ||||||||
| NPC | 1 M H2SO4 | −0.5 to 0.5 | 5 | 0.39 | 204 | 7 | 80 | 20 |
| NPC650 | 1 M H2SO4 | −0.5 to 0.5 | 5 | 0.51 | 167 | 11 | 84 | 21 |
| MC-A | 6 M KOH | −1.0 to 0 | 2 | 0.55 | 208 | 12 | 114 | 23 |
| MPC-A | 6 M KOH | −1.0 to 0 | 2 | 0.41 | 196 | 15 | 81 | 23 |
| MAC-A | 6 M KOH | −1.0 to 0 | 2 | 0.61 | 271 | 12 | 165 | 23 |
| C800 | 1 M H2SO4 | −0.5 to 0.5 | 5 | 0.50 | 188 | 9 | 94 | 27 |
| C1000 | 1 M H2SO4 | −0.5 to 0.5 | 5 | 0.32 | 161 | 5 | 52 | 27 |
| Z-900 | 0.5 M H2SO4 | −0.2 to 1.0 | 5 | 0.93 | 214 | 20 | 200 | 38 |
| Cf. Zeolite-templated N-doped nanoporous carbon by nanocasting in an acidic electrolyte | ||||||||
| Y-AN | 1 M H2SO4 | 0 to 0.6 | 2 | 0.74 | 340 | 20 | 250 | 43 |
| Cf. Carbon fiber-based material in an alkali electrolyte | ||||||||
| ACF4 | 6 M KOH | 0 to 1.0 | 1 | 0.38 | 371 | 11 | 139 | 44 |
One of the advantages of using MOFs for preparation of nanoporous carbons is that carbon–metal/metal oxide hybrids can simply be achieved after carbonization.24,25 The catalytic oxygen reduction reaction (ORR) at the cathode is a critical process for fuel cells and metal–air batteries.45,46 In fuel cells, the cathode ORR is six or more orders of magnitude lower than the anode hydrogen oxidation reaction and thus limits the overall performance of fuel cells.45 Development of non-precious metal catalysts for ORR becomes very important to realize low-cost, high-performance fuel cells, particularly for fuel-cell vehicles. The research group at Argonne National Laboratory prepared ORR catalysts by thermal activation of MOFs built from cobalt ions and nitrogen-containing ligands.47,48 They suggested that thermal treatment of a cobalt imidazolate framework, Co(imidazolate)2, resulted in a porous framework with Co–N4 sites that have been thought to be an active site for ORR.48 The Dodelet research group has developed a series of ORR catalysts bearing Fe–N4 active sites.49,50 The latest ORR catalyst was prepared from iron acetate, phenanthroline, and ZIF-8.50 Before thermal treatment, iron acetate, phenanthroline, and ZIF-8 were well mixed in an ethanol/water solution, followed by mechanochemical treatment by ballmilling. The resulting ORR catalysts showed an incredible improvement in performances under pure oxygen for non-precious metal catalysts. However, the role of ZIF-8 in the formation of such efficient ORR catalysts is still unclear.
Summary and future outlook
As described in this highlight, we can clearly see the rapid progress in research on MOF-derived nanoporous carbon materials. Two distinguished methods shown in Fig. 2 have been employed to prepare highly nanoporous carbons. Considering thousands of known MOFs in the literature, there is plenty of space to improve the physico-chemical properties of MOF-derived carbons. The previous results suggest that selection of appropriate MOFs, right combination of MOFs and carbon sources, and thermal treatment conditions are all critical to realize MOFs with desired properties and functions.Control over structural orders of this class of carbons is rare and remains a challenge. Development of nanoporous carbons with uniform pores at different length scales (e.g., hierarchical pores) is still needed for many applications dealing with the diffusion of molecules and ions. Incorporation of heteroatoms into the carbon networks should be more focused. In particular, nanoporous carbons with highly doped nitrogen are of importance for CO2 capture and photocatalysis. Preparation of carbon–metal/metal oxide hybrid materials with high surface areas is also interesting as they can be used as catalysts and pseudo-capacitance electrodes. Enhancement of electrical conductivity of MOF-derived carbons is also important for many applications. Carbonization of MOFs containing metals that are able to catalyze the formation of carbon nanotubes (e.g., iron and cobalt) could be one of the possible routes to increase the fraction of graphitic sp2-hybridized carbons and thereby conductivity of materials.
In addition to the reported applications, many other potential uses of carbon materials are not yet examined for MOF-derived nanoporous carbons. These include adsorbents for removal of heavy metals and toxic species from drinking water, magnetically-recoverable supported catalysts, drug delivery carriers, electrolyte/membrane materials for fuel cells, cathode materials and sulfur hosts for lithium–sulfur batteries, and so on. MOF-derived nanoporous carbons and carbon hybrids have high potential for such applications and thus should be more explored.
Overall, research on preparation of carbons from MOFs has opened up an exciting avenue toward functional carbon-based nanoporous materials. This new approach would provide us a simpler and more designable way to achieve functional carbon materials, particularly, for energy and environment-related utilization. We strongly believe that the aforementioned interesting and important research topics will be realized in the near future.
References
- M. Ramanathan, S. M. Kilbey, II, Q. Ji, J. P. Hill and K. Ariga, J. Mater. Chem., 2012, 22, 10389 RSC.
- K. Ariga, T. Mori and J. P. Hill, Adv. Mater., 2012, 24, 158 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- K. Ariga, A. Vinu, Y. Yamauchi, Q. Ji and J. P. Hill, Bull. Chem. Soc. Jpn., 2012, 85, 1 CrossRef CAS.
- Y. Yamauchi, N. Suzuki, L. Radhakrishnan and L. Wang, Chem. Rec., 2009, 9, 321 CrossRef CAS.
- (a) H. Jankowska, A. Swiatkowski and J. Choma, Active Carbon, Ellis Horwood, 1991 Search PubMed; (b) B. Fang, M. Kim, S. Q. Fan, J. H. Kim, D. P. Wilkinson, J. Ko and J. S. Yu, J. Mater. Chem., 2011, 21, 8742 RSC; (c) B. Fang, S. Q. Fan, J. H. Kim, M. S. Kim, M. Kim, N. K. Chaudhari, J. Ko and J. S. Yu, Langmuir, 2010, 26, 11238 CrossRef CAS; (d) J. H. Kim and J. S. Yu, Phys. Chem. Chem. Phys., 2010, 12, 15301 RSC.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- T. Kyotani, T. Nagai, S. Inoue and A. Tomita, Chem. Mater., 1997, 9, 609 CrossRef CAS.
- H. Yang and D. Zhao, J. Mater. Chem., 2005, 15, 1217 CAS.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang, Z. Li, C. Yu, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053 CrossRef CAS.
- D. Feng, Y. Lv, Z. Wu, Y. Dou, L. Han, Z. Sun, Y. Xia, G. Zheng and D. Zhao, J. Am. Chem. Soc., 2011, 133, 15148 CrossRef CAS.
- Z. Wu, P. A. Webley and D. Zhao, J. Mater. Chem., 2012, 22, 11379 RSC.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef CAS.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695 CrossRef CAS.
- J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS.
- A. Comotti, S. Bracco, P. Sozzani, S. Horike, R. Matsuda, J. Chen, M. Takata, Y. Kubota and S. Kitagawa, J. Am. Chem. Soc., 2008, 130, 13664 CrossRef CAS.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 CrossRef CAS.
- H. Wang, L. Huang, B. A. Holmberg and Y. Yan, Chem. Commun., 2002, 1708 RSC.
- B. Liu, H. Shioyama, T. Akita and Q. Xu, J. Am. Chem. Soc., 2008, 130, 5390 CrossRef CAS.
- B. Liu, H. Shioyama, H. Jiang, X. Zhang and Q. Xu, Carbon, 2010, 48, 456 CrossRef CAS.
- D. Yuan, J. Chen, S. Tan, N. Xia and Y. Liu, Electrochem. Commun., 2009, 11, 1191 CrossRef CAS.
- J. Hu, H. Wang, Q. Gao and H. Guo, Carbon, 2010, 48, 3599 CrossRef CAS.
- L. Radhakrishnan, J. Reboul, S. Furukawa, P. Srinivasu, S. Kitagawa and Y. Yamauchi, Chem. Mater., 2011, 23, 1225 CrossRef CAS.
- Y. Meng, G.-H. Wang, S. Bernt, N. Stock and A.-H. Lu, Chem. Commun., 2011, 47, 10479 RSC.
- A. Almasoudi and R. Mokaya, J. Mater. Chem., 2012, 22, 146 RSC.
- H.-L. Jiang, B. Liu, Y.-Q. Lan, K. Kuratani, T. Akita, H. Shioyama, F. Zong and Q. Xu, J. Am. Chem. Soc., 2011, 133, 11854 CrossRef CAS.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884 CrossRef CAS.
- G.-P. Hao, W.-C. Li, D. Qian, G.-H. Wang, W.-P. Zhang, T. Zhang, A.-Q. Wang, F. Schüth, H.-J. Bongard and A.-H. Lu, J. Am. Chem. Soc., 2011, 133, 11378 CrossRef CAS.
- R. Babarao, S. Dai and D. Jiang, J. Phys. Chem. C, 2012, 116, 7106 CAS.
- Y. Xia, G. S. Walker, D. M. Grant and R. Mokaya, J. Am. Chem. Soc., 2009, 131, 16493 CrossRef CAS.
- K. K. R. Datta, V. V. Balasubramanian, K. Ariga, T. Mori and A. Vinu, Chem.–Eur. J., 2011, 17, 3390 CrossRef CAS.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- Y. Fukasawa, K. Takanabe, A. Shimojima, M. Antonietti, K. Domen and T. Okubo, Chem.–Asian J., 2011, 6, 103 CrossRef CAS.
- M. Hu, J. Reboul, S. Furukawa, L. Radhakrishnan, Y. Zhang, P. Srinivasu, H. Iwai, H. Wang, Y. Nemoto, N. Suzuki, S. Kitagawa and Y. Yamauchi, Chem. Commun., 2011, 47, 8124 RSC.
- M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, J. Am. Chem. Soc., 2012, 134, 2864 CrossRef CAS.
- S. J. Yang, T. Kim, J. H. Im, Y. S. Kim, K. Lee, H. Jung and C. R. Park, Chem. Mater., 2012, 24, 464 CrossRef CAS.
- W. Chaikittisilp, M. Hu, H. Wang, H.-S. Huang, T. Fujita, K. C.-W. Wu, L.-C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259 RSC.
- S. Lim, K. Suh, Y. Kim, M. Yoon, H. Park, D. N. Dybtsev and K. Kim, Chem. Commun., 2012, 48, 7447 RSC.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384 RSC.
- A. J. Jacobson, Chem. Mater., 2010, 22, 660 CrossRef CAS.
- (a) G. A. Olah, G. K. S. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881 CrossRef CAS; (b) P. Bollini, S. A. Didas and C. W. Jones, J. Mater. Chem., 2011, 21, 15100 RSC.
- C. O. Ania, V. Khomenko, E. Raymundo-Piñero, J. B. Parra and F. Béguin, Adv. Funct. Mater., 2007, 17, 1828 CrossRef CAS.
- B. Xu, F. Wu, R. Chen, G. Cao, S. Chen, Z. Zhou and Y. Yang, Electrochem. Commun., 2008, 10, 795 CrossRef CAS.
- M. K. Debe, Nature, 2012, 486, 43 CrossRef CAS.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wamg, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517 CrossRef CAS.
- G. Goenaga, S. Ma, S. Yuan and D.-J. Liu, ECS Trans., 2010, 33, 579 CrossRef CAS.
- S. Ma, G. A. Goenaga, A. V. Call and D.-J. Liu, Chem.–Eur. J., 2011, 17, 2063 CrossRef CAS.
- M. Lefèvre, E. Proietti, F. Jaouen and J.-P. Dodelet, Science, 2009, 324, 71 CrossRef.
- E. Proietti, F. Jaouen, M. Lefèvre, N. Larouche, J. Tian, J. Herranz and J.-P. Dodelet, Nat. Commun., 2011, 2, 416 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
