Flower-like CuO microspheres with enhanced catalytic performance for dimethyldichlorosilane synthesis†
Zailei
Zhang
ab,
Hongwei
Che
a,
Yingli
Wang
a,
Jiajian
Gao
a,
Xilin
She
b,
Jin
Sun
b,
Ziyi
Zhong
c and
Fabing
Su
*a
aState Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, China 100190. E-mail: fbsu@mail.ipe.ac.cn
bCollege of Chemical and Environmental Engineering, Qingdao University, Qingdao, China 266071
cInstitute of Chemical Engineering and Sciences, A*star, 1 Pesek Road, Jurong Island, Singapore 627833
First published on 8th February 2012
Abstract
Flower-like CuO microspheres synthesized by a facile hydrothermal method were found to be an effective catalyst for the Rochow reaction with a higher dimethyldichlorosilane selectivity and Si conversion because of the enhanced formation of an active CuxSi phase and mass transport.
Metal oxide nanostructures are of great interest because of their unique physical and chemical properties, which may lead to significantly important applications in industry. As a result of these diverse structures and properties, such metal oxides are considered as good candidates for catalysts or catalyst supports.1–5 Moreover, their metal components often exhibit multiple oxidation states, which is of high potential for catalysis. Since the 1940s, metallic copper6 and copper compounds such as Cu2O,7 CuO,7 CuCl,8 Cu3Si,9 and Cu–Cu2O–CuO composite10 mixed with Zn promoter additives11 have been reported to be active for the Rochow reaction.12 Until today, this reaction is still regarded as the most economical route in the direct synthesis of methylchlorosilane (MCS) in the organosilane industry, in which solid silicon directly reacts with chloromethane (MeCl) over copper catalysts. The production of dimethyldichlorosilane from this reaction is most desirable for organosilane materials. Since this reaction involves complicated gas–solid–solid phase catalysis and generates many by-products, its catalytic mechanism has not been fully understood yet13,14 and there is still plenty of room for improvement of the copper catalysts. Generally, the copper-based catalysts employed both in the organosilane industry15 and fundamental studies16,17 are densely packed, irregular in morphology at the micrometre size level and investigations using copper catalysts with specific morphology for the above reaction have not been found in the literature.
As an interesting transition metal oxide, CuO has been widely applied in catalysis.18–20 In recent years, CuO materials with various nano/microstructures such as nanowires,21 nanoribbons,22 pricky microspheres,23 urchins,24 flowers,25,26 dumbbells,27 honeycomb,28 plates,29 hollow,30 and core–shell structures31 have been successfully synthesized. These offer numerous opportunities to explore the morphology effect of the CuO materials as catalysts in the Rochow reaction.
In this work, flower-like CuO microspheres are synthesized by a hydrothermal method (for details, see the ESI†) and applied in the catalytic production of dimethyldichlorosilane. Compared with the commercial irregular CuO particles, these flower-like CuO microspheres show much higher dimethyldichlorosilane selectivity and Si conversion (see Formula S1†), demonstrating the significance of the morphological structure of the copper catalysts on catalytic properties.
Fig. 1a shows the XRD pattern of the flower-like CuO microspheres (sample C3 in Table S1†), indicating the formation of monoclinic symmetry CuO (JCPDS No.089-5896). Fig. 1b shows their SEM image. It is seen that the CuO microspheres with a size of 2–6 μm are assembled from nanoplates with tens of nanometres in size (inset of Fig. 1b). Fig. 1c shows a TEM image of the C3 sample with a dense internal structure. Fig. 1d presents a TEM image of the CuO nanoplates assembling the flower-like microspheres. The inset of Fig. 1d shows the HRTEM image of the nanoplate edges, showing a lattice plane distance of 0.25 nm, which is in agreement with the (−111) plane distance of monoclinic CuO (0.2516 nm tabulated from XRD).
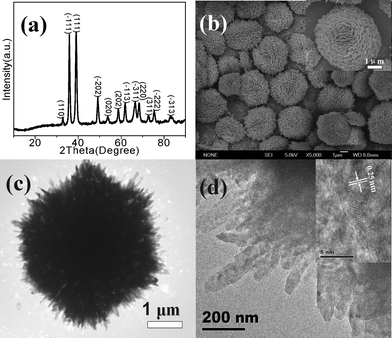 | ||
| Fig. 1 XRD pattern (a), SEM (b) (inset of SEM is its high-magnification), and TEM images (c) and on the edge (d) of flower-like CuO microspheres (C3) (inset of Fig. 1d is the HRTEM image). | ||
The effect of the synthesis conditions on the morphology of the CuO samples was extensively investigated, including the reaction temperature (Fig. S1†), time (Fig. S2†), the copper nitrate amount (Fig. S3†), ammonia water amount (Fig. S4†), sodium hydroxide amount (Fig. S5†), sodium nitrate amount (Fig. S6†), the solvent volume ratios of water/alcohol (Fig. S7†), copper precursors (Fig. S8†), and mineralizers (Fig. S9†). Based on the above experiments, an optimized set of reaction conditions for synthesizing the flower-like CuO microspheres was determined, which includes using a reaction temperature of 130 °C, a reaction time of 18 h, 1.0 g of copper nitrate, 30 ml of ammonia water, 10 ml of sodium hydroxide, 5.0 g of sodium nitrate, and 50 ml of alcohol. The reactions involved in the formation of flower-like CuO microspheres are shown in eqn (1)–(3).
| Cu2+ + 4NH3·H2O → (Cu(NH3)4)2+ + 4H2O | (1) |
| (Cu(NH3)4)2+ + 2OH− → Cu(OH)2↓ + 4NH3↑ | (2) |
| Cu(OH)2 → CuO↓ + H2O | (3) |
(Cu(NH3)4)2+ is firstly created from copper nitrate and ammonia water.24 The (Cu(NH3)4)2+ species then react with OH− at a certain temperature to form intermediate Cu(OH)2.23 Finally, CuO is formed via the dehydration of Cu(OH)2 according to the chemical reaction given in eqn (3).25 Also, the formation process of the flower-like CuO microspheres is proposed in Scheme 1. In the first stage, small CuO nanoplates are formed via the aggregation of CuO nanoparticles, which are generated by the hydrolysis of copper nitrate in the presence of sodium hydroxide and ammonia water. Thereafter, upon a prolonged heat treatment, the rate of nucleation and ripening becomes faster, and the formed nanoparticles are aggregated via crystal grain growth. These small CuO nanoplates self-assemble into small flower-like CuO. In the subsequent second stage, the initially formed small flower-like CuO particles grow into large flower-like CuO composed of large thin CuO nanoplates. In the third stage, as the diameter of the precipitates increases, the resultant material deposits on CuO nanoplates causing them to grow into flower-like CuO through Ostwald ripening,32 and these flower-like CuO microspheres composed of thin CuO nanoplates further grow into flower-like CuO composed of thicker CuO nanoplates. NaNO3 as a mineralizer plays a crucial role for the formation of CuO microspheres with a narrower size distribution (Fig. S9†).
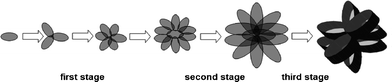 | ||
| Scheme 1 Illustration of the formation process of flower-like CuO microspheres. | ||
As we know, the product in the Rochow reaction mainly contains methyltrichlorosilane (CH3SiCl3, M1), dimethyldichlorosilane ((CH3)2SiCl2, M2), and trimethylchlorosilane ((CH3)3SiCl, M3) (see Formula S1†), in which M2 is most highly demanded as the organosilane monomer for organosilane materials in industry.33,34 Thus, a high M2 selectivity is the top priority in the Rochow reaction. Table 1 shows the catalytic performance of various flower-like CuO microspheres and commercial CuO particles. It can be seen that the flower-like CuO microspheres obtained in this work exhibit much higher selectivity (SM2 > 81%) and Si conversion (CSi > 25%) than those obtained by the commercial CuO irregular particles (Fig. S10†) (SM2 = 51.9% and CSi = 15.0%). Compared with commercial copper catalysts Cu–Cu2O–CuO, whose M2 selectivity is more than 80%,35 flower-like CuO microspheres show higher M2 selectivity and a little lower Si conversion. N2 adsorption isotherms and XRD patterns of these CuO samples can be found in Fig. S11.† The surface areas and average crystal sizes are compiled in Table S2.†
It has been known that, in the Rochow reaction, alloyed CuxSi species such as Cu3Si36 are believed to be the key catalytic active species,37 on which M2 is generated.38 Cu3Si is formed between the copper catalyst and the Si interface,39 and is an indicator of activity for a copper catalyst. Contact mass is the solid mixture comprised of the Si and copper catalyst (here CuO). In order to reveal the reason behind the enhanced catalytic performance demonstrated by the flower-like CuO microspheres, XRD patterns for the contact masses containing various CuO catalysts before and after the reaction are measured. Before the reaction, there is no difference observed among the XRD patterns of the fresh contact masses (Fig. S12†). After the reaction, the Cu phase in the reacted contact masses appeared with no other obvious difference among these samples (Fig. 2A). An enlarged view in the 2θ angle range of 40–50° (Fig. 2B) shows the presence of Cu3Si and Cu6.69Si species, suggesting the formation of alloyed CuxSi active components. The formation of Cu originates from reaction of chlorosilane with the lattice oxygen in the CuO catalysts.7 When copper and silicon are brought together at elevated temperatures, the Cu3Si (η phase) forms.40 CuO is continuously reacting and activated with chlorosilane. Then copper diffuses away from the CuO interface. The flux of Cu is toward the Si interface, at which new Cu3Si forms.39 It has been reported that methylchlorosilanes can be produced from MeCl monolayers that have been generated on Cu3Si surfaces containing excess silicon.9 The amount of Cu3Si can influence the Si conversion and M2 selectivity.41 Although the Cu peaks for all the reacted contact masses are very similar, a much higher intensity of CuxSi for the flower-like CuO microspheres than commercial CuO is observed, suggesting that the flower-like CuO microspheres are more active in generating CuxSi than the commercial CuO. This may be because the hierarchical structures of the flower-like CuO microspheres have larger surface areas and smaller crystal size (Table S2†), which cause the facile reduction of CuO to Cu (Fig. S13†) and the generation of a large contact interface between the solid catalyst and the solid silicon. This thus leads to the formation of more active CuxSi alloyed phases, and can explain why the flower-like CuO microspheres have an enhanced catalytic activity for M2 production. On the other hand, the developed hierarchical structure is able to enhance gas transportation, which may be another reason that results in the higher Si conversion as compared with the irregular and dense commercial CuO. Moreover, compared with the commercial copper catalysts Cu–Cu2O–CuO,10 the flower-like CuO microspheres also show better M2 selectivity although their Si conversion is a little less. This is because the commercial copper catalysts contain other copper components such as Cu and Cu2O, together with other promoters such as Sn and P,11 which may increase the Si conversion. The structure–catalytic performance relationship revealed in this study will be helpful in developing good catalysts for dimethylchlorosilane synthesis.
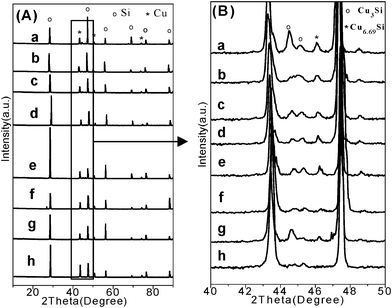 | ||
| Fig. 2 (A) XRD patterns of contact masses after reaction, and (B) enlarged view in the 2θ angle range of 40–50° (a: C1+Si, b: C2+Si, c: C3+Si, d: C4+Si, e: C14+Si, f: C16+Si, g: C25+Si, and h: commercial CuO+Si). | ||
In conclusion, we have synthesized flower-like CuO microspheres by a hydrothermal method and demonstrated the importance of morphological structure of CuO materials as copper catalysts in the Rochow catalytic reaction. Compared with the irregular commercial CuO particles, the flower-like CuO microspheres show a higher M2 selectivity and Si conversion because the developed hierarchical structure within the CuO microspheres enables generation of more active alloyed CuxSi species and enhances the transportation of the gaseous feed within the catalyst. This work provides insight into the design of copper catalysts for the organosilane industry.
Acknowledgements
Financial support of this work was from the CAS-Locality Cooperation Program (No. DBNJ-2011-058), the State Key Laboratory of Multiphase Complex Systems (No. MPCS-2011-D-14), the National Natural Science Foundation of China (No. 21031005), and the China Postdoctoral Science Foundation (No. 20110490597).References
- M. Zhu and G. Diao, J. Phys. Chem. C, 2011, 115, 18923 CAS.
- L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397 CrossRef CAS.
- M. Zhu and G. Diao, Catal. Sci. Technol., 2012, 2, 82 CAS.
- M. Zhu and G. Diao, Nanoscale, 2011, 3, 2748 RSC.
- M. Zhu and G. Diao, J. Phys. Chem. C, 2011, 115, 24743 CAS.
- A. Jörg and B. Klaus, J. Organomet. Chem., 2008, 693, 2483 CrossRef.
- L. Lewis, W. Ligon and J. Carnahan, Silicon Chem., 2002, 1, 23 CrossRef CAS.
- R. Voorhoeve, B. Geertsem and J. C. Vlugter, J. Catal., 1965, 4, 43 CrossRef CAS.
- D. Sun and B. E. Bent, Catal. Lett., 1997, 46, 127 CrossRef CAS.
- M. Barr, T. Murphy and M. Williams, USP-20050176981, 2005 Search PubMed.
- A. Gordon, B. Hinch and D. Strongin, Catal. Lett., 2009, 133, 14 CrossRef CAS.
- E. G. Rochow, J. Am. Chem. Soc., 1945, 67, 963 CrossRef CAS.
- R. J. Voorhoeve, J. A. Lips and J. C. Vlugter, J. Catal., 1964, 3, 414 CrossRef CAS.
- N. Floquet, S. Yilmaz and J. L. Falconer, J. Catal., 1994, 148, 348 CrossRef CAS.
- C. P. H. Lyons and G. S. Roussillon, USP-4661613, 1987 Search PubMed.
- J. M. Bablin, L. N. Lewis, P. Bui and M. Gardner, Ind. Eng. Chem. Res., 2003, 42, 3532 CrossRef CAS.
- W. Luo, G. Wang and J. Wang, Ind. Eng. Chem. Res., 2006, 45, 129 CrossRef CAS.
- Y. Feng and X. Zheng, Nano Lett., 2010, 10, 4762 CrossRef CAS.
- A. Gervasini, P. Carniti, S. Bennici and C. Messi, Chem. Mater., 2007, 19, 1319 CrossRef CAS.
- C. K. Chen, Y. W. Chen, C. H. Lin, H. P. Lin and C. F. Lee, Chem. Commun., 2010, 46, 282 RSC.
- X. Jiang, T. Herricks and Y. Xia, Nano Lett., 2002, 2, 1333 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 8124 CrossRef CAS.
- Y. Xu, D. Chen and X. Jiao, J. Phys. Chem. B, 2005, 109, 13561 CrossRef CAS.
- L. Xu, S. Sithambaram, Y. Zhang, C. H. Chen, L. Jin, R. Joesten and S. L. Suib, Chem. Mater., 2009, 21, 1253 CrossRef CAS.
- M. Vaseem, A. Umar, S. H. Kim and Y. B. Hahn, J. Phys. Chem. C, 2008, 112, 5729 CAS.
- D. Gao, G. Yang, J. Li, J. Zhang, J. Zhang and D. Xue, J. Phys. Chem. C, 2010, 114, 18347 CAS.
- H. Wang, Q. Shen, X. Li and F. Liu, Langmuir, 2009, 25, 3152 CrossRef CAS.
- Y. Liu, Y. Chu, Y. Zhuo, M. Li, L. Li and L. Dong, Cryst. Growth Des., 2007, 7, 467 CAS.
- M. Yang, J. He, X. Hu, C. Yan and Z. Cheng, Environ. Sci. Technol., 2011, 45, 6088 CrossRef CAS.
- S. Y. Gao, S. X. Yang, J. Shu, S. X. Zhang, Z. D. Li and K. Jiang, J. Phys. Chem. C, 2008, 112, 19324 CAS.
- J. Li, S. Xiong, J. Pan and Y. Qian, J. Phys. Chem. C, 2010, 114, 9645 CAS.
- L. A. Gao and B. P. Ha, J. Cryst. Growth, 2007, 303, 616 CrossRef.
- Q. Zheng, H. G. Hu, W. W. Lin, C. R. Chen and X. L. Tao, Chem. J. Chin. Univ., 2005, 26, 592 Search PubMed.
- M. Yoshida, M. Goto and F. Nakanishi, Inorg. Chem. Commun., 2000, 3, 59 CrossRef CAS.
- K. M. Lewis and J. S. Ritscher, USP-7153991, 2006 Search PubMed.
- Z. Zhang, L. M. Wong, H. G. Ong, X. J. Wang, J. L. Wang, S. J. Wang, H. Chen and T. Wu, Nano Lett., 2008, 8, 3205 CrossRef CAS.
- L. Levin, Z. Atzmon, A. Katsman and T. Werber, Mater. Chem. Phys., 1995, 40, 56 CrossRef CAS.
- T. C. Frank, K. B. Kester and J. L. Falconer, J. Catal., 1985, 91, 44 CrossRef CAS.
- W. F. Banholzer, N. Lewis and W. Ward, J. Catal., 1986, 101, 405 CrossRef CAS.
- L. Stolt, H. D and F. M. D'Heurle, Thin Solid Films, 1990, 189, 269 CrossRef CAS.
- L. N. Lewis and W. J. Ward, Ind. Eng. Chem. Res., 2002, 41, 397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization. See DOI: 10.1039/c2ra00923d |
| This journal is © The Royal Society of Chemistry 2012 |
