Unravelling the electrochemical mechanisms for nitrogen fixation on single transition metal atoms embedded in defective graphitic carbon nitride†
Abstract
Electrochemical reduction of nitrogen (N2), in which the conversion of N2 to ammonia (NH3) takes place under mild conditions, is of timely significance for paving a way toward technological applications in agriculture and the chemical industry. In this work, various single transition metal atoms anchored on graphitic carbon nitride (g-C3N4) with nitrogen vacancies (TM@NVs-g-C3N4), acting as electrocatalysts for N2 reduction, were systematically investigated by means of density functional theory (DFT) calculations. Most of the isolated metal atoms (Ti, V, Co, Ni, Zr, Mo, Ru and Pt) can be fixed by the nitrogen vacancies stably after performing the molecular dynamics simulation. For hexagonal close-packed and body centered cubic metal atoms, their N2 chemisorption activity decreases as the coordination number of the single atom rises. Nevertheless, the anchored cubic close-packed metal atom does not serve as a good site for N2 adsorption and activation even with a low-coordination number. Among all studied TM single atoms, the single Ti atom is found to be the most promising catalyst for its excellent N2 reduction performance with a potential-limiting step of 0.51 eV and a rate-determining barrier of 0.57 eV. Atomic level insights are provided to elucidate the electrochemical mechanisms for N2 reduction. The coordination number of the 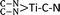 active center is accountable for the robust N2 reduction activity with high stability. Overall, this work exemplifies the in-depth investigations of different single TM atoms, including the coordination number and binding mode, which are essential to lay the groundwork for the advancement of single atom catalysis toward practical implementation.
active center is accountable for the robust N2 reduction activity with high stability. Overall, this work exemplifies the in-depth investigations of different single TM atoms, including the coordination number and binding mode, which are essential to lay the groundwork for the advancement of single atom catalysis toward practical implementation.

- This article is part of the themed collections: International Year of the Periodic Table : Single Atoms as Active Catalysts and Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...