Open Access Article
Open Access ArticleTailoring the extraction process and properties of polysaccharides from the lichen Evernia prunastri using natural eutectic solvents following a biorefinery approach†
Julie
Queffelec
*ab,
Maria Dolores
Torres Pérez
 *a,
Herminia
Domínguez
a,
Giulia
Ischia
b and
Svitlana
Filonenko
*a,
Herminia
Domínguez
a,
Giulia
Ischia
b and
Svitlana
Filonenko
 b
b
aCINBIO, Universidade de Vigo, Department of Chemical Engineering, Faculty of Sciences, Campus Ourense, Edificio Politécnico, As Lagoas, 32004, Ourense, Spain. E-mail: matorres@uvigo.gal
bMax Planck Institute of Colloids and Interfaces, Department of Colloids Chemistry, Am Mühlenberg 1, 14476 Potsdam, Germany
First published on 16th May 2025
Abstract
Natural eutectic solvents (NaESs) for the valorization of biomass have been gaining interest in recent years. However, almost no study is available on their impact on the physicochemical and mechanical properties of polysaccharides and their plasticizing power during extraction. In this work, microwave-assisted extraction was combined with hydrophilic NaESs to intensify the extraction process of polysaccharides from the lichen Evernia prunastri, modifying their mechanical properties to obtain viscoelastic materials. With this integrated extraction/formulation process, the energy consumption and production time were reduced by 35% and 23%, respectively. Four solvent systems, namely, sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine, lactic acid
betaine, lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine, glycerol
betaine, glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine and oxalic acid
betaine and oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline, were tested. The maximum polysaccharide extraction yield of 44.31% was obtained with oxalic acid
proline, were tested. The maximum polysaccharide extraction yield of 44.31% was obtained with oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline at 100 °C for 5 min. For lactic acid
proline at 100 °C for 5 min. For lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine and glycerol
betaine and glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine, the highest yields were reached at 120 °C for 15 min (35.93% and 32.93%, respectively). The type of eutectic solvent chosen had a significant impact on the physicochemical and mechanical properties of the extracted polysaccharides. With glycerol
betaine, the highest yields were reached at 120 °C for 15 min (35.93% and 32.93%, respectively). The type of eutectic solvent chosen had a significant impact on the physicochemical and mechanical properties of the extracted polysaccharides. With glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine, polysaccharides with higher molecular weights were obtained. Directly drying after the extraction, a flexible material was obtained, exhibiting elastic behavior under deformation, with a Young's modulus of 20.6 MPa and a maximum deformation of 94%. With lactic acid
betaine, polysaccharides with higher molecular weights were obtained. Directly drying after the extraction, a flexible material was obtained, exhibiting elastic behavior under deformation, with a Young's modulus of 20.6 MPa and a maximum deformation of 94%. With lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine, a material exhibiting a plastic deformation of up to 245% and less resistance (Young's modulus of 12.1 MPa) was obtained. With oxalic acid
betaine, a material exhibiting a plastic deformation of up to 245% and less resistance (Young's modulus of 12.1 MPa) was obtained. With oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline, the material exhibited elastic deformation of up to 24% and a Young's modulus of only 9.9 MPa. A particular interest was also given to the valorization of the residual solid obtained from the extraction into high-value-added artificial humic substances.
proline, the material exhibited elastic deformation of up to 24% and a Young's modulus of only 9.9 MPa. A particular interest was also given to the valorization of the residual solid obtained from the extraction into high-value-added artificial humic substances.
Green foundation1. This work sets a precedent for the intensification of natural polysaccharide extraction and formulation, combining them into one step using natural eutectic solvents. A particular effort was also made to follow a biorefinery approach.2. Using NaDESs, polysaccharides can be extracted and formulated into plasticized materials, reducing the energy and time consumption by 35% and 23%, respectively, compared with traditional polysaccharide formulation and with mechanical properties varying with the eutectic used. The formulated polymers can be used as coatings or bioplastic materials. 3. This work describes a combined approach to polysaccharide isolation and formulation for the first time. The work encourages further investigation into adjusting the process for biorefinery schemes, adapting it to other natural biomasses, and optimization of the coating applications. Studying the recycling of the eutectics after use would also be highly valuable for improving the process. |
1. Introduction
Traditionally used in folk medicine or as dyes, the lichen Evernia prunastri has gained considerable interest since decades, notably for its large set of unique metabolites, which display antibacterial, antioxidant, antitumoral or photoprotective properties.1 These secondary metabolites, called lichenic acids, are investigated for their potential drug use and extracted for the fragrance industry as “oak moss absolute” to give a woody smell and fix perfumes. However, after the extraction of these secondary metabolites, polysaccharides remain in the residual solid, with potential as bio-based materials. Indeed, the main compounds of the cell walls and extracellular matrices of Evernia prunastri are glucans and galactomannans, which are known for their gel and film-forming properties, respectively, with good biocompatibility, safety and biodegradability.2–5 They can thus be found in nutraceuticals, cosmetics, and food products as texturizing agents, stabilizers, edible coatings, films and drug delivery systems.6,7 The valorization of all lichen fractions could be addressed following a biorefinery concept, giving added value to the wastes from lichenic acid extraction. Conventional polysaccharide extraction with alkaline or acidic hot water may present the drawbacks of using aggressive chemicals and, in some cases, of being time and energy consuming and causing polysaccharide degradation. The desire to reduce the use of contaminant solutions and to increase energy efficiency has led to the emergence of new extraction techniques, based on using greener solvents, such as ionic liquids or natural deep eutectic solvents (NaDESs), and intensification strategies, such as ultrasounds or microwaves. Microwaves are electromagnetic waves with a frequency ranging from 0.3 to 300 GHz, usually 2450 MHz. These nonionizing radiations heat samples through two mechanisms: the dipolar rotation and the ionic conduction. Contrary to the conventional heating techniques via conduction and convection, the heat transfer with microwaves occurs from the inner part of the sample to the exterior, leading to much faster heating. The increase in pressure in the raw material cells due to water evaporation can also lead to their disruption, more easily releasing metabolites and increasing the extraction yields.8–10 The reduction in heating time, even at low power, and the high extraction yields make it a sustainable technology. However, the efficiency of microwave-assisted extraction depends on the choice of the solvent used. It should be able to absorb microwaves (high dielectric constant) but also dissipate energy into heat (high dielectric loss factor).11 Water is considered as a good solvent for microwave heating. However, in the past few decades, it has been observed that eutectic solvents can be even better, reaching higher temperatures in a much shorter time than water.12,13 NaDESs are solvents made of two or three primary or secondary plant metabolites, mainly sugars, polyols, amino acids and organic acids. Mixed, they establish hydrogen bonds that lower the overall melting point of the system, making them liquid at a much lower temperature than each compound independently. They are called “deep” when their eutectic point temperature is lower than that of the ideal liquid mixture.14 In function of their chemical composition, they can be classified into 5 different categories.15,16 They are usually prepared by heating and stirring, or freeze-dried after dissolution, but microwave and ultrasounds have been more recently used to reduce the preparation time.17–19 These solvents raised an increasing interest in the last decade for the expected low toxicity, relatively low cost, easy and rapid preparation, low melting point, high boiling point, selectivity and high dissolving capacity.20–23 They are considered to be a safer, less expensive and biodegradable alternative to ionic liquids. Originally hydrophilic, some hydrophobic ones have now been produced, as well as, more recently, supramolecular DESs, which are DESs integrated into macrocyclic molecules such as cyclodextrins.16 They have found a wide range of applications, from functionalized material synthesis, energy (DES as a precursor of graphene nanocomposites, or as an electrocatalyst or electrolyte for battery applications), environmental remediation (DES synthesis of nitrogen-doped carbon or porous nanomaterials for gas and wastewater contaminant adsorption), biomedicine (gel sensors, drug solubilization and delivery systems), biodiesel and chemical production to extraction and fractionation.16,24 They have been indeed studied for the extraction and fractionation of cellulose, hemicellulose and lignin from biomass, with high yields.25–28 Moreover, their low toxicity and beneficial properties such as plasticizers could make them interesting to keep in final formulations after the extraction.29,30 Some studies also highlighted the use of eutectic solvents for the acetylation or cationization of sugars and cellulose and as plasticizers for starch.15,31–33 However, there is a lack of studies on the impact of extraction with eutectics on the mechanical properties and formulation of polysaccharides. Usually, polysaccharides are extracted from biomass, washed and dried to obtain a powder that can be afterward formulated into films, by dissolving them in water, adding a plasticizer and drying it. As presented in a previous work where menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid was used in biphase with water to extract lichen's polysaccharides, the energy consumption of the formulation of film was estimated to be 16.5 kW h (ref. 13) (5 h of freeze-drying at 1300 W, heating and stirring at 70 °C for 2 h, 500 W to dissolve the polysaccharides and drying the films in an oven at 40 °C for 24 h, 1500 W with a duty cycle of 25%). Current challenges regarding energy and resource consumption lead us to redesign our production processes to make them more efficient. Process intensification may be a way forward. As some NaDESs are good solvents for biomass and exhibit plasticizing effects, it has been hypothesized that new polysaccharide-based materials with enhanced mechanical properties might be obtained directly from the extraction. This combined effect of eutectics as solvents and plasticizers at the same time has never been studied yet. The elimination of some of the typical steps for polysaccharide formulation would thus improve the energy and time efficiency of film production. To follow a biorefinery approach and upgrade all fractions of the lichen, a particular focus was also placed on valorizing the residual solid after the eutectic extraction by studying the production of artificial humic substances (AHSs). AHSs are complex heterogeneous, high-molecular-weight compounds synthesized from biomass through an innovative hydrothermal process called hydrothermal humification.34,35 The AHS conventionally consists of three fractions: humin (the solid fraction), humic acid (the solid recovered from the base extract via acidic precipitation), and fulvic acid (the liquid phase). AHS mimics the natural counterpart contained in soil. Their research is in its early stages, and there is an increasing interest in their potential means of tackling soil-related issues in agriculture.35 Indeed, thanks to their functional groups that can complex and chelate minerals, they play a key role in micronutrient transport within the soil and increase their availability to plants.36
lauric acid was used in biphase with water to extract lichen's polysaccharides, the energy consumption of the formulation of film was estimated to be 16.5 kW h (ref. 13) (5 h of freeze-drying at 1300 W, heating and stirring at 70 °C for 2 h, 500 W to dissolve the polysaccharides and drying the films in an oven at 40 °C for 24 h, 1500 W with a duty cycle of 25%). Current challenges regarding energy and resource consumption lead us to redesign our production processes to make them more efficient. Process intensification may be a way forward. As some NaDESs are good solvents for biomass and exhibit plasticizing effects, it has been hypothesized that new polysaccharide-based materials with enhanced mechanical properties might be obtained directly from the extraction. This combined effect of eutectics as solvents and plasticizers at the same time has never been studied yet. The elimination of some of the typical steps for polysaccharide formulation would thus improve the energy and time efficiency of film production. To follow a biorefinery approach and upgrade all fractions of the lichen, a particular focus was also placed on valorizing the residual solid after the eutectic extraction by studying the production of artificial humic substances (AHSs). AHSs are complex heterogeneous, high-molecular-weight compounds synthesized from biomass through an innovative hydrothermal process called hydrothermal humification.34,35 The AHS conventionally consists of three fractions: humin (the solid fraction), humic acid (the solid recovered from the base extract via acidic precipitation), and fulvic acid (the liquid phase). AHS mimics the natural counterpart contained in soil. Their research is in its early stages, and there is an increasing interest in their potential means of tackling soil-related issues in agriculture.35 Indeed, thanks to their functional groups that can complex and chelate minerals, they play a key role in micronutrient transport within the soil and increase their availability to plants.36
The aim of this work was to evaluate the performance and plasticizing properties of different eutectics for the microwave-assisted extraction of cell-wall polysaccharides from the lichen Evernia prunastri, how their composition influences the structure of polysaccharides and to what extent, they can change or even improve their mechanical performance compared to those extracted by hydrothermal microwave extraction. The valorization of the residual solid after the eutectic extraction through the production of high-value-added AHS was also explored. The authors are not aware that similar works using eutectics as extraction solvents and plasticizers to obtain ready-to-use polysaccharides, with different mechanical properties in function of the eutectic used, have been explored.
2. Results and discussion
2.1. Eutectic solvent selection and characterization
The selection of eight eutectic solvents was done based on the literature according to the following criteria. The first point was the price of the eutectic solvents. To design the most cost-effective process, the least expensive compounds were favored whenever possible. The toxicity of the compounds used was also taken into account seriously, not to exclude some potential applications, such as topical use. Particularly, choline chloride is currently prohibited for cosmetic uses (EU Cosmetic Regulation No. 1223/2009). It has also been extensively studied in the context of eutectic solvents, and thus, was excluded from this study. As the objective of this work was to extract as much polysaccharides as possible and study how the plasticizing properties of the eutectic solvents behaved on the polysaccharides, eutectics with good extraction properties such as acidic ones (with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet and OA
Bet and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro) were selected, as they were leading to interesting extraction yields of polysaccharides from lignocellulosic materials,25–27 as well as compounds known for their plasticizing properties such as glycerol and sorbitol.37 Lastly, particular interest was paid to betaine. Indeed, as a zwitterion, it could be thought that the ionic interaction between betaine molecules would be much stronger than the hydrogen bonds responsible for eutectic solvent formation. However, the shielding of the positive charge by methyl groups makes it an excellent hydrogen bond acceptor, which is worth studying.38 Betaine is moreover a safe compound, produced on an industrial scale from renewable sources (by-product of sugar production), already used in healthcare product formulations. However, OA
Pro) were selected, as they were leading to interesting extraction yields of polysaccharides from lignocellulosic materials,25–27 as well as compounds known for their plasticizing properties such as glycerol and sorbitol.37 Lastly, particular interest was paid to betaine. Indeed, as a zwitterion, it could be thought that the ionic interaction between betaine molecules would be much stronger than the hydrogen bonds responsible for eutectic solvent formation. However, the shielding of the positive charge by methyl groups makes it an excellent hydrogen bond acceptor, which is worth studying.38 Betaine is moreover a safe compound, produced on an industrial scale from renewable sources (by-product of sugar production), already used in healthcare product formulations. However, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet, FA
Bet, FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet and CA
Bet and CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet (after any kind of mechanical stress) turned into solids. In addition, a LA
Bet (after any kind of mechanical stress) turned into solids. In addition, a LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro mixture get colored even if no heat was applied. It was, then, decided to continue working on OA
Pro mixture get colored even if no heat was applied. It was, then, decided to continue working on OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro, Sorb
Pro, Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet, LA
Bet, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet and Gly
Bet and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet. The visual aspect of the solvents is displayed in Fig. 1.
Bet. The visual aspect of the solvents is displayed in Fig. 1.
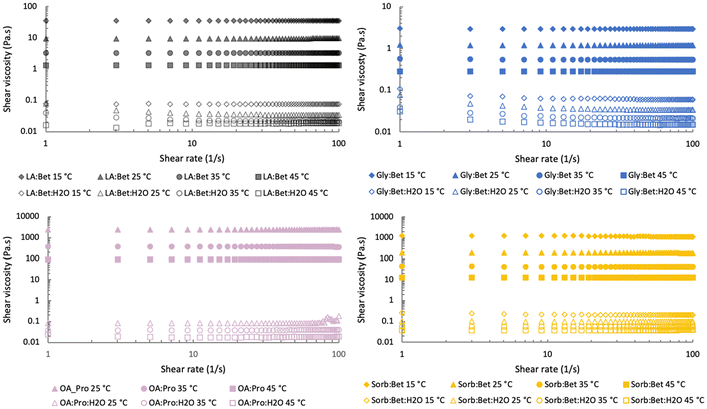
The usually high viscosity of eutectic solvents can make them difficult to manipulate. Indeed, the NaDES presented high resistance to flow, especially OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro and Sorb
Pro and Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet with shear viscosities at 25 °C higher than 1000 Pa s and 100 Pa s, respectively (Fig. 2). For this reason, water (in a molar ratio of 5) was required to be added to the aforementioned systems. The molar ratios of 0.8, 2, 8 and 9.2 were also tested, but 5 molar was the lowest water amount for which an acceptable viscosity was reached for all solvents. A strong impact of temperature on the viscosity typical for ionic solvents was observed. The addition of water lowered the influence of temperature on the viscosity and caused its decrease up to 0.03 Pa s for LA
Bet with shear viscosities at 25 °C higher than 1000 Pa s and 100 Pa s, respectively (Fig. 2). For this reason, water (in a molar ratio of 5) was required to be added to the aforementioned systems. The molar ratios of 0.8, 2, 8 and 9.2 were also tested, but 5 molar was the lowest water amount for which an acceptable viscosity was reached for all solvents. A strong impact of temperature on the viscosity typical for ionic solvents was observed. The addition of water lowered the influence of temperature on the viscosity and caused its decrease up to 0.03 Pa s for LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet and Gly
Bet and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet and up to 0.1 Pa s for OA
Bet and up to 0.1 Pa s for OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro and Sorb
Pro and Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet (Fig. 2). Although the addition of water in eutectic solvents is widely used to solve the viscosity issue, a too high amount of water can lead to obtaining a solution instead of a tertiary system. DSC and FTIR spectra were performed to study whether a tertiary eutectic system or a dilution was obtained after the addition of water.
Bet (Fig. 2). Although the addition of water in eutectic solvents is widely used to solve the viscosity issue, a too high amount of water can lead to obtaining a solution instead of a tertiary system. DSC and FTIR spectra were performed to study whether a tertiary eutectic system or a dilution was obtained after the addition of water.
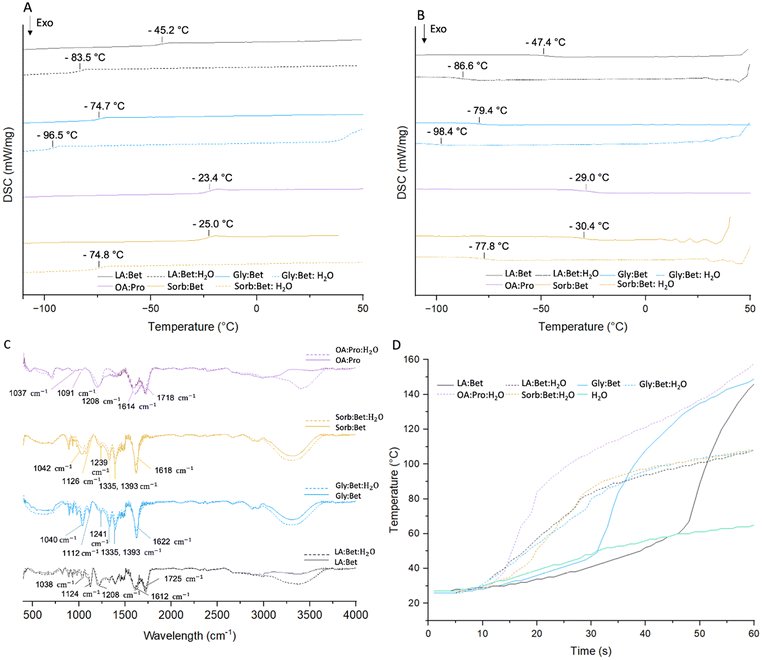
DSC heating and cooling thermograms of the eutectics with (dash) and without (continuous) water are displayed in Fig. 3A and B. (Note that OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O was not homogeneous at less than 35 °C, so only the DSC analysis of OA
H2O was not homogeneous at less than 35 °C, so only the DSC analysis of OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro was performed. Hence, the results are not demonstrated for this solvent.) All systems exhibit the typical glass transition specific to eutectic solvents, characterized by a change in the heat capacity, at −45.2 °C, −74.7 °C, −23.4 °C, and −25 °C, respectively, for LA
Pro was performed. Hence, the results are not demonstrated for this solvent.) All systems exhibit the typical glass transition specific to eutectic solvents, characterized by a change in the heat capacity, at −45.2 °C, −74.7 °C, −23.4 °C, and −25 °C, respectively, for LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet, Gly
Bet, Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet, OA
Bet, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro and Sorb
Pro and Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet. As observed by ref. 39 and 40, these glass transition temperatures shifted to lower temperatures (−83.5 °C, −96.5 °C and −74.8 °C, respectively) when water was incorporated into the binary NaDES. The drop in phase transition temperature of these eutectic solvents with the addition of water can be explained by the plasticizing effect of water.41 Water hydroxyl groups interact with those of the solvent, destabilizing its supramolecular structure and increasing the mobility of the compounds, resulting in a decrease in the glass transition temperature.42 In addition, no thermal effect from separate components was observed, meaning that tertiary eutectic solvents were indeed obtained by the addition of water.39,43
Bet. As observed by ref. 39 and 40, these glass transition temperatures shifted to lower temperatures (−83.5 °C, −96.5 °C and −74.8 °C, respectively) when water was incorporated into the binary NaDES. The drop in phase transition temperature of these eutectic solvents with the addition of water can be explained by the plasticizing effect of water.41 Water hydroxyl groups interact with those of the solvent, destabilizing its supramolecular structure and increasing the mobility of the compounds, resulting in a decrease in the glass transition temperature.42 In addition, no thermal effect from separate components was observed, meaning that tertiary eutectic solvents were indeed obtained by the addition of water.39,43
The FTIR spectra of the different solvents with (dash) and without (continuous) water are shown in Fig. 3C. Regardless of the presence of water, all solvents have the same characteristic peaks. For lactic acid and betaine, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the lactic acid carboxyl group at 1725 cm−1 and COO− asymmetric stretching of betaine at 1612 cm−1, C–N stretching from the quaternary ammonium group of betaine at 1124 cm−1 and C–O stretching at 1035 and 1208 cm−1 of the hydroxyl and carboxyl groups of lactic acid, respectively, were described by Tang Weiwei 2019. For glycerol and betaine, the peak at 1040 cm−1 may be due to the C–O stretching vibration of the hydroxyl group of glycerol, the C–N stretching vibration at 1112 cm−1, C–O stretching at 1241 cm−1 and COO− asymmetric stretching at 1622 cm−1 and symmetric stretching at 1335 and 1393 cm−1 for betaine.44 The C–O stretching vibration of sorbitol hydroxyls is observed at 1042 cm−1, and C–N, C–O and COO− stretching at 1126, 1239 and 1618 cm−1 for betaine. Regarding oxalic acid and proline, C
O stretching of the lactic acid carboxyl group at 1725 cm−1 and COO− asymmetric stretching of betaine at 1612 cm−1, C–N stretching from the quaternary ammonium group of betaine at 1124 cm−1 and C–O stretching at 1035 and 1208 cm−1 of the hydroxyl and carboxyl groups of lactic acid, respectively, were described by Tang Weiwei 2019. For glycerol and betaine, the peak at 1040 cm−1 may be due to the C–O stretching vibration of the hydroxyl group of glycerol, the C–N stretching vibration at 1112 cm−1, C–O stretching at 1241 cm−1 and COO− asymmetric stretching at 1622 cm−1 and symmetric stretching at 1335 and 1393 cm−1 for betaine.44 The C–O stretching vibration of sorbitol hydroxyls is observed at 1042 cm−1, and C–N, C–O and COO− stretching at 1126, 1239 and 1618 cm−1 for betaine. Regarding oxalic acid and proline, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carboxyl group of oxalic acid and proline can be seen at 1718 and 1614 cm−1, C–N stretching of amine from proline at 1037 and 1091 cm−1 and C–O stretching of the carboxyl group of oxalic acid at 1208 cm−1. The addition of water broadens the peak at 3000–3500 cm−1, the region for the OH group vibration. Some shifts in characteristic peaks are also observed after the addition of water. The most obvious ones being shifts from 1208 to 1217 cm−1 and from 1208 to 1220 cm−1 of the C–O stretching vibration of oxalic acid and lactic acid probably due to changes in hydrogen bonds associated with the addition of water.45 The microwave heating profiles (40 W, 1 min) of the different eutectic solvents (0.8 g) with (dash) and without (continuous) water are displayed in Fig. 3D. As ref. 43 and 46 also observed, there is a decrease in the microwave heating efficiency with the addition of water in the eutectic solvents. All eutectics (binary and tertiary) exhibit a much higher overall heating rate than water only, making them efficient solvents for microwave heating. Even though eutectics without water reach a much higher final temperature than those with water, two strongly different trends can be observed on their heating profiles. At the beginning, all the eutectics with water have a higher heating rate. They reach 80 °C in less than 30 seconds, whereas binary NaDES reach only 35–45 °C. However, tertiary eutectics face a drastic decrease in their heating rate and only reach 100 °C after 1 min, when binary solvents face an increase in their heating rate and can reach up to 140 °C. Exception is made for OA
O stretching of the carboxyl group of oxalic acid and proline can be seen at 1718 and 1614 cm−1, C–N stretching of amine from proline at 1037 and 1091 cm−1 and C–O stretching of the carboxyl group of oxalic acid at 1208 cm−1. The addition of water broadens the peak at 3000–3500 cm−1, the region for the OH group vibration. Some shifts in characteristic peaks are also observed after the addition of water. The most obvious ones being shifts from 1208 to 1217 cm−1 and from 1208 to 1220 cm−1 of the C–O stretching vibration of oxalic acid and lactic acid probably due to changes in hydrogen bonds associated with the addition of water.45 The microwave heating profiles (40 W, 1 min) of the different eutectic solvents (0.8 g) with (dash) and without (continuous) water are displayed in Fig. 3D. As ref. 43 and 46 also observed, there is a decrease in the microwave heating efficiency with the addition of water in the eutectic solvents. All eutectics (binary and tertiary) exhibit a much higher overall heating rate than water only, making them efficient solvents for microwave heating. Even though eutectics without water reach a much higher final temperature than those with water, two strongly different trends can be observed on their heating profiles. At the beginning, all the eutectics with water have a higher heating rate. They reach 80 °C in less than 30 seconds, whereas binary NaDES reach only 35–45 °C. However, tertiary eutectics face a drastic decrease in their heating rate and only reach 100 °C after 1 min, when binary solvents face an increase in their heating rate and can reach up to 140 °C. Exception is made for OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O that, even facing a decrease in its heating rate, still reaches 160 °C. This behavior may come from the high value of the Kamlet–Taft dipolarity/polarizability (π*) parameter presented in Table 1. It means that the electron distribution can be easily distorted by an external electric field, leading to an increase in the dipolar rotation mechanism. González-Rivera et al. (2021)12 also studied the microwave absorption behaviour of a eutectic made with oxalic acid (ChCl
H2O that, even facing a decrease in its heating rate, still reaches 160 °C. This behavior may come from the high value of the Kamlet–Taft dipolarity/polarizability (π*) parameter presented in Table 1. It means that the electron distribution can be easily distorted by an external electric field, leading to an increase in the dipolar rotation mechanism. González-Rivera et al. (2021)12 also studied the microwave absorption behaviour of a eutectic made with oxalic acid (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA), as they observed particularly good extraction properties for this solvent. They showed that the microwave response of the eutectic was increasing with the increase in the microwave power, indicating its good microwave absorption properties. This high absorption power was also associated with the high dipolar/polarization of the solvent, correlated with a high π*. Second, the potential formation of oxalate anions may increase the ionic conduction, resulting in a higher heat generation. The low heating rate of the binary solvents at the beginning may be explained by their very high viscosity (Fig. 1). A too high viscosity may result in slower heating, as molecular movements and heat dissipation are more difficult than in systems with lower viscosity. However, as shown in Fig. 2, the shear viscosity of binary eutectics drastically decreases with the increase in temperature, which may explain the increase in their heating rate after reaching 50–60 °C. However, many factors can positively or negatively affect the ability of solvents to absorb microwaves (characterized by the dielectric constant) and dissipate the energy as heat (characterized by the dielectric loss). The large difference in the solvent heating rate with and without water after 30 s is probably due to a combination of these factors. Indeed, the addition of water is likely to increase the specific heat capacity of the system, resulting in a slower heating rate. Moreover, water may disrupt the hydrogen bonds established between the binary solvents, reducing their dielectric properties.46,47 Although the presence of water decreases the overall heating rate of the solvents, it is required to improve their usability.
OA), as they observed particularly good extraction properties for this solvent. They showed that the microwave response of the eutectic was increasing with the increase in the microwave power, indicating its good microwave absorption properties. This high absorption power was also associated with the high dipolar/polarization of the solvent, correlated with a high π*. Second, the potential formation of oxalate anions may increase the ionic conduction, resulting in a higher heat generation. The low heating rate of the binary solvents at the beginning may be explained by their very high viscosity (Fig. 1). A too high viscosity may result in slower heating, as molecular movements and heat dissipation are more difficult than in systems with lower viscosity. However, as shown in Fig. 2, the shear viscosity of binary eutectics drastically decreases with the increase in temperature, which may explain the increase in their heating rate after reaching 50–60 °C. However, many factors can positively or negatively affect the ability of solvents to absorb microwaves (characterized by the dielectric constant) and dissipate the energy as heat (characterized by the dielectric loss). The large difference in the solvent heating rate with and without water after 30 s is probably due to a combination of these factors. Indeed, the addition of water is likely to increase the specific heat capacity of the system, resulting in a slower heating rate. Moreover, water may disrupt the hydrogen bonds established between the binary solvents, reducing their dielectric properties.46,47 Although the presence of water decreases the overall heating rate of the solvents, it is required to improve their usability.
| Solvent | E NR | π* | α | β |
|---|---|---|---|---|
| Water | 48.62 | 1.100 | 1.140 | 0.475 |
LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BET BET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
49.29 | 1.142 | 0.967 | 0.550 |
GLY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BET BET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
48.87 | 1.184 | 1.032 | 0.575 |
SORB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BET BET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
48.96 | 1.057 | 1.094 | 0.718 |
OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PRO PRO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
44.67 | 1.472 | 1.762 | 0.228 |
2.2. Influence of the eutectic solvents on the polysaccharide extraction yield, composition and molecular weights
The different average molecular weights and the polydispersity index (PDI) can give interesting information on the size and heterogenicity of the extracted polysaccharides. Indeed, the calculation of the number average molecular weight (Mn) is more influenced by low-molecular-weight polysaccharides, while the weight average molecular weight (Mw) gives more importance to high-molecular-weight ones. Moreover, the PDI indicates how homogeneous are the polysaccharide molecular weights; a PDI = 1 meaning monodisperse. In Fig. 4, a different trend in Mn (Fig. 4A), Mw (Fig. 4B) and PDI (Fig. 4C) as a function of the solvent used can be observed. Polysaccharides extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O have statistically a significantly higher Mn (5.25 × 104–6.82 × 104) and Mw (1.85 × 105–2.33 × 105) than those extracted with the other eutectics (p-value of one-way ANOVA and Turkey tests <5%) and a homogeneous high polydispersity index (3.34–3.99). Polysaccharides with a wide range of globally high molecular weights were then extracted. On the contrary, Mn and Mw of the polysaccharides extracted with OA
H2O have statistically a significantly higher Mn (5.25 × 104–6.82 × 104) and Mw (1.85 × 105–2.33 × 105) than those extracted with the other eutectics (p-value of one-way ANOVA and Turkey tests <5%) and a homogeneous high polydispersity index (3.34–3.99). Polysaccharides with a wide range of globally high molecular weights were then extracted. On the contrary, Mn and Mw of the polysaccharides extracted with OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and LA
H2O and LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O are globally lower, respectively, between 2.40 × 104 and 4.18 × 104 and 2.71 × 104 and 5.23 × 104 g mol−1 for Mn and between 7.87 × 104 and 1.89 × 105 and 9.19 × 104 and 1.86 × 105 g mol−1 for Mw (no statistical difference between the two solvents). The use of acid eutectic solvents may enhance the hydrolysis of the glycosidic bonds, leading to lower molecular weight fractions.48–50 These molecular weights are coherent with the molecular weight of galactomannans and glucans extracted from different types of biomasses.51–53 Regarding the PDI, no statistical difference was observed between the three solvents but the values were much more dispersed for LA
H2O are globally lower, respectively, between 2.40 × 104 and 4.18 × 104 and 2.71 × 104 and 5.23 × 104 g mol−1 for Mn and between 7.87 × 104 and 1.89 × 105 and 9.19 × 104 and 1.86 × 105 g mol−1 for Mw (no statistical difference between the two solvents). The use of acid eutectic solvents may enhance the hydrolysis of the glycosidic bonds, leading to lower molecular weight fractions.48–50 These molecular weights are coherent with the molecular weight of galactomannans and glucans extracted from different types of biomasses.51–53 Regarding the PDI, no statistical difference was observed between the three solvents but the values were much more dispersed for LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O than for Gly
H2O than for Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, which has a relatively constant PDI with the extraction temperature and time. To compare, water seems to extract high-molecular-weight polysaccharides with less heterogenicity (for 120 °C, 25 min, Mn = 5.58 × 104, Mw = 1.48 × 105 and PDI = 2.66). Focusing on the maximum extraction yield of each solvents, it can be observed that the use of glycerol
H2O, which has a relatively constant PDI with the extraction temperature and time. To compare, water seems to extract high-molecular-weight polysaccharides with less heterogenicity (for 120 °C, 25 min, Mn = 5.58 × 104, Mw = 1.48 × 105 and PDI = 2.66). Focusing on the maximum extraction yield of each solvents, it can be observed that the use of glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine
betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (120 °C, 15 min) yields relatively high-molecular-weight polysaccharides with a high polydispersity index (Mn = 6.18 × 104, Mw = 2.23 × 105, PDI = 3.61), LA
H2O (120 °C, 15 min) yields relatively high-molecular-weight polysaccharides with a high polydispersity index (Mn = 6.18 × 104, Mw = 2.23 × 105, PDI = 3.61), LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (120 °C, 15 min) yields relatively low-molecular-weight polysaccharides with high polydispersity index (Mn = 2.91 × 104, Mw = 1.093 × 105, PDI = 3.74), and OA
H2O (120 °C, 15 min) yields relatively low-molecular-weight polysaccharides with high polydispersity index (Mn = 2.91 × 104, Mw = 1.093 × 105, PDI = 3.74), and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (100 °C, 5 min) yields low-molecular-weight polysaccharides with intermediate polydispersity index (Mn = 3.21 × 104, Mw = 7.87 × 104, PDI = 3.12).
H2O (100 °C, 5 min) yields low-molecular-weight polysaccharides with intermediate polydispersity index (Mn = 3.21 × 104, Mw = 7.87 × 104, PDI = 3.12).
Fig. 5 shows the evolution of the poly/oligosaccharide yield and the composition of the different eutectic phases before the ethanolic precipitation (A–D) and the yield and composition of the precipitated polysaccharides (E, F) as a function of the solvents and the extraction conditions. For Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O after the addition of ethanol, the whole eutectic extract formed a soft solid, which explains why no precipitated polysaccharide yield is indicated. It is also important to note that, even after washing the polysaccharides, there is an amount of eutectic remaining, which confers unique mechanical properties to the polysaccharides, but which also enters the gravimetric extraction yield. It means that these yields give an idea of the best extracting conditions but cannot be used for comparisons among solvents. Data in Fig. 5A confirmed that Sorb
H2O after the addition of ethanol, the whole eutectic extract formed a soft solid, which explains why no precipitated polysaccharide yield is indicated. It is also important to note that, even after washing the polysaccharides, there is an amount of eutectic remaining, which confers unique mechanical properties to the polysaccharides, but which also enters the gravimetric extraction yield. It means that these yields give an idea of the best extracting conditions but cannot be used for comparisons among solvents. Data in Fig. 5A confirmed that Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet has the lowest polysaccharide and oligosaccharide extraction yields, barely reaching 20% under the harshest condition. These low yields coincide with the high amount of residual solid recovered. The yields of polysaccharides and oligosaccharides in the liquid phase (A–D) and precipitated form (E, F) for the three other eutectics exhibit similar behaviors. LA
Bet has the lowest polysaccharide and oligosaccharide extraction yields, barely reaching 20% under the harshest condition. These low yields coincide with the high amount of residual solid recovered. The yields of polysaccharides and oligosaccharides in the liquid phase (A–D) and precipitated form (E, F) for the three other eutectics exhibit similar behaviors. LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Gly
H2O and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O exhibit similar trends with the increase in the extraction yield with time and temperature, reaching maximum polysaccharide and oligosaccharide yields in the liquid phase of, respectively, 48.88 ± 0.59 g per 100 g lichen and 35.26 ± 0.26 g per 100 g lichen at 120 °C during 25 min, and a maximum precipitation of, respectively, 35.93 and 32.93% at 120 °C and 15 min. Interestingly, OA
H2O exhibit similar trends with the increase in the extraction yield with time and temperature, reaching maximum polysaccharide and oligosaccharide yields in the liquid phase of, respectively, 48.88 ± 0.59 g per 100 g lichen and 35.26 ± 0.26 g per 100 g lichen at 120 °C during 25 min, and a maximum precipitation of, respectively, 35.93 and 32.93% at 120 °C and 15 min. Interestingly, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O has a very different behavior, reaching its maximum polysaccharide and oligosaccharide yields in the liquid phase at 80 °C and 25 min (51.06 ± 1.23 g per 100 g lichen) and 100 °C and 5 min (50.49 ± 2.32 g per 100 g lichen) and its best precipitated polysaccharide yield (44.31%) at 100 °C, 5 min. These yields are in the same range as those obtained by hydrothermal microwave-assisted extraction at 180–200 °C during 5 min.54 The use of these eutectic solvents allows for an increased extraction yield of polysaccharides at low temperatures, better preventing them from degradation. It is also interesting to observe the evolution of the glucan/galactomannan percentage in the eutectic phases. Although the extraction conditions do not appear to impact the glucan content, which remains relatively constant, galactomannans are more challenging to extract. They are indeed more effectively extracted at higher temperatures and longer extraction times for Sorb
H2O has a very different behavior, reaching its maximum polysaccharide and oligosaccharide yields in the liquid phase at 80 °C and 25 min (51.06 ± 1.23 g per 100 g lichen) and 100 °C and 5 min (50.49 ± 2.32 g per 100 g lichen) and its best precipitated polysaccharide yield (44.31%) at 100 °C, 5 min. These yields are in the same range as those obtained by hydrothermal microwave-assisted extraction at 180–200 °C during 5 min.54 The use of these eutectic solvents allows for an increased extraction yield of polysaccharides at low temperatures, better preventing them from degradation. It is also interesting to observe the evolution of the glucan/galactomannan percentage in the eutectic phases. Although the extraction conditions do not appear to impact the glucan content, which remains relatively constant, galactomannans are more challenging to extract. They are indeed more effectively extracted at higher temperatures and longer extraction times for Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, LA
H2O, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Gly
H2O and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. Once again, the use of OA
H2O. Once again, the use of OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O reduces the temperature and extraction time needed to extract galactomannan. The very low yield of precipitated polysaccharides at 120 °C, 15–25 min with OA
H2O reduces the temperature and extraction time needed to extract galactomannan. The very low yield of precipitated polysaccharides at 120 °C, 15–25 min with OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O is adequate to the analysis of the liquid phases, where almost no polysaccharides and oligosaccharides were identified, whereas formic acid, acetic acid, levulinic acid and hydroxymethyl furfural were detected. This means that during this experiment, glucose, galactose and mannose are degraded into hydroxymethyl furfural and then into levulinic acid and formic acid.55 Note that formic acid can also be produced by the degradation of oxalic acid, which can explain its particularly high amount at 120 °C. This also demonstrates the high hydrolyzing power of the eutectic OA
H2O is adequate to the analysis of the liquid phases, where almost no polysaccharides and oligosaccharides were identified, whereas formic acid, acetic acid, levulinic acid and hydroxymethyl furfural were detected. This means that during this experiment, glucose, galactose and mannose are degraded into hydroxymethyl furfural and then into levulinic acid and formic acid.55 Note that formic acid can also be produced by the degradation of oxalic acid, which can explain its particularly high amount at 120 °C. This also demonstrates the high hydrolyzing power of the eutectic OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, which makes it possible to extract a high amount of saccharides at low temperatures but lead to significant degradation under harsher conditions. Polymers with a maximum yield were selected for further characterizations. First, their monosaccharide composition was determined by performing an acid hydrolysis followed by an HPLC analysis. The results are displayed in Fig. 5F. All polysaccharides are mainly composed of glucans and galactomannans (mannose backbone with galactose ramifications, whose M/G ratio varies), the main cell-wall polysaccharides of Evernia prunastri. However, the proportion of glucose, galactose and mannose varies as a function of the solvent use. Polysaccharides extracted with water and Gly
H2O, which makes it possible to extract a high amount of saccharides at low temperatures but lead to significant degradation under harsher conditions. Polymers with a maximum yield were selected for further characterizations. First, their monosaccharide composition was determined by performing an acid hydrolysis followed by an HPLC analysis. The results are displayed in Fig. 5F. All polysaccharides are mainly composed of glucans and galactomannans (mannose backbone with galactose ramifications, whose M/G ratio varies), the main cell-wall polysaccharides of Evernia prunastri. However, the proportion of glucose, galactose and mannose varies as a function of the solvent use. Polysaccharides extracted with water and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O have the highest glucose content and an almost similar galactose and mannose content, which would mean that the galactomannans have an M/G ratio of almost 1. On the contrary, for polysaccharides extracted with LA
H2O have the highest glucose content and an almost similar galactose and mannose content, which would mean that the galactomannans have an M/G ratio of almost 1. On the contrary, for polysaccharides extracted with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, glucose and galactose are detected in lower amounts than mannose. The high M/G ratio may be due to the degradation of galactose units due to a stronger action of these two solvents. This can also explain why the polysaccharides extracted with LA
H2O, glucose and galactose are detected in lower amounts than mannose. The high M/G ratio may be due to the degradation of galactose units due to a stronger action of these two solvents. This can also explain why the polysaccharides extracted with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O have a lower molecular weight than those extracted with Gly
H2O have a lower molecular weight than those extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. The M/G ratio affects many properties of the galactomannans, such as their solubility and their rheological properties. Modulating this ratio can thus be interesting. Since glucans are good at forming gels and galactomannans better at forming films, their different proportions can also influence the final mechanical properties of the dried material.
H2O. The M/G ratio affects many properties of the galactomannans, such as their solubility and their rheological properties. Modulating this ratio can thus be interesting. Since glucans are good at forming gels and galactomannans better at forming films, their different proportions can also influence the final mechanical properties of the dried material.
2.3. Influence of the eutectic solvents on the polysaccharides’ mechanical properties
As the mechanical properties of polysaccharides are highly dependent on their molecular weight and polydispersity, the viscoelastic properties (frequency sweep rheological test) of the precipitated polysaccharides, washed and centrifuged, before drying, were measured. Usually, higher molecular weights mean more chain entanglement, and thus higher viscosity, tensile strength and elastic modulus,56 while a high polydispersity index means higher flexibility and mobility of the chains. This can be observed in Fig. 6. Water and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, which give the highest molecular weights and galactose ramifications, also exhibit the highest elastic modulus (higher than 10
H2O, which give the highest molecular weights and galactose ramifications, also exhibit the highest elastic modulus (higher than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa). They both exhibit gel viscoelastic properties with an elastic modulus (G′) much higher than the viscous one (G′′) invariant with the frequency. On the contrary, OA
000 Pa). They both exhibit gel viscoelastic properties with an elastic modulus (G′) much higher than the viscous one (G′′) invariant with the frequency. On the contrary, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and LA
H2O and LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O exhibit weaker elastic properties with a closed modulus, respectively, around 5000 Pa and 1000 Pa, increasing with the frequency.
H2O exhibit weaker elastic properties with a closed modulus, respectively, around 5000 Pa and 1000 Pa, increasing with the frequency.
After drying in an oven at 50 °C, the polysaccharides had very different visual aspects and mechanical properties. The one extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O being still flexible and elastic, followed by the one extracted with LA
H2O being still flexible and elastic, followed by the one extracted with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, still flexible but more viscous and stickier. However, dry polysaccharides extracted with OA
H2O, still flexible but more viscous and stickier. However, dry polysaccharides extracted with OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O were hard breakable solids. To further study the discrepancies in the mechanical properties of the polysaccharides, a tensile test was performed. The sample preparation for this test was a bit complicated as low quantities were used and as all polysaccharides were very different. A tensile tester used was not optimal for our small samples but can give an interesting first overview of the mechanical properties of each material. As observed in Fig. 7, polysaccharides extracted with both Gly
H2O were hard breakable solids. To further study the discrepancies in the mechanical properties of the polysaccharides, a tensile test was performed. The sample preparation for this test was a bit complicated as low quantities were used and as all polysaccharides were very different. A tensile tester used was not optimal for our small samples but can give an interesting first overview of the mechanical properties of each material. As observed in Fig. 7, polysaccharides extracted with both Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O present an almost elastic deformation of, respectively, 94% and 24%, an ultimate tensile strength of 16.7 and 5.7 MPa and a Young modulus of 20.6 and 12.1 MPa. However, the material obtained with LA
H2O present an almost elastic deformation of, respectively, 94% and 24%, an ultimate tensile strength of 16.7 and 5.7 MPa and a Young modulus of 20.6 and 12.1 MPa. However, the material obtained with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O presents a wide uniform plastic deformation of 245% but is much less resistant, with an ultimate tensile strength of 3.7 MPa and a Young modulus of 9.9 MPa. These results show that the mechanical properties of the dried materials are strongly impacted by the choice of the solvent. It could therefore be envisioned to tailor these properties by choosing the eutectic mix as a function of the application desired for the material. The plasticizing power of some eutectic solvents due to their ability to form hydrogen bonds with polysaccharides has been already observed.37,57 However, the authors are not aware that such a strong combined effect of eutectics as solvents and plasticizers on the mechanical properties of polysaccharides, directly during the extraction, has been already demonstrated. Many works study eutectic solvents for the extraction of hemicellulose, or polysaccharides, but obtaining dried powder that still has to be formulated.58–60 The advantage of this process is the elimination of the drying and formulation steps usually required after the extraction of polysaccharides with water. Since drying is an energy-consuming operation, the sustainability of the processes using eutectic solvents should be emphasized. These flexible materials were obtained after only one drying step at 50 °C for 24 h (1500 W, estimated duty cycle of 30%). The total energy consumption to obtain this material was then 10.8 kW h, in 24 h, with no additional use of water.
H2O presents a wide uniform plastic deformation of 245% but is much less resistant, with an ultimate tensile strength of 3.7 MPa and a Young modulus of 9.9 MPa. These results show that the mechanical properties of the dried materials are strongly impacted by the choice of the solvent. It could therefore be envisioned to tailor these properties by choosing the eutectic mix as a function of the application desired for the material. The plasticizing power of some eutectic solvents due to their ability to form hydrogen bonds with polysaccharides has been already observed.37,57 However, the authors are not aware that such a strong combined effect of eutectics as solvents and plasticizers on the mechanical properties of polysaccharides, directly during the extraction, has been already demonstrated. Many works study eutectic solvents for the extraction of hemicellulose, or polysaccharides, but obtaining dried powder that still has to be formulated.58–60 The advantage of this process is the elimination of the drying and formulation steps usually required after the extraction of polysaccharides with water. Since drying is an energy-consuming operation, the sustainability of the processes using eutectic solvents should be emphasized. These flexible materials were obtained after only one drying step at 50 °C for 24 h (1500 W, estimated duty cycle of 30%). The total energy consumption to obtain this material was then 10.8 kW h, in 24 h, with no additional use of water.
2.4. Eutectic solvent discrepancies in extraction efficiency
To better understand the effect of the different eutectic solvents on the raw material, a SEM analysis was performed on the raw material and on the different residual solids (Fig. 8). Even if the lichen structure has been studied since the second half of the 20th century, their exact composition and the function of all their metabolites remain unknown. It is however well established that they are composed of an outer layer called cortex (inferior or superior) made of tightly packed fungal filament cells (hyphae) embedded in an extracellular matrix of extracellular polymeric substances (EPS), an algal layer and the medulla, made of loosely interlaced hyphae.61–67 Although the cell-wall polysaccharides that composed the fungal hyphae are mainly glucans, galactomannans and chitin, the composition of the EPS is still unclear. Polyuronic acids, glucans, heteropolysaccharides, pigments, phenolic compounds, lipids and proteins, secreted by both mycobionts and photobionts, should compose it.68 The structural composition of the milled raw material can be seen in Fig. 8A. Hyphae from the medulla and fragments of the cortical hyphae embedded in the extracellular matrix can be identified. It is then interesting to compare the different residual solids to understand which compounds were extracted by the different eutectic solvents. First, it seems that free hyphae from the medulla are extracted with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, Gly
H2O, Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, OA
H2O, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and water but still remain in the residual solid of the Sorb
H2O and water but still remain in the residual solid of the Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O extraction. Moreover, it seems that LA
H2O extraction. Moreover, it seems that LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, Gly
H2O, Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and water extracted some part of the extracellular matrix, making the embedded hyphae visible. This phenomenon is even more visible with OA
H2O and water extracted some part of the extracellular matrix, making the embedded hyphae visible. This phenomenon is even more visible with OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, where almost no EPS seems to remain. This observation is reinforced by the previously discussed extraction yield and the weight of residual solid recovered, the larger amount being for Sorb
H2O, where almost no EPS seems to remain. This observation is reinforced by the previously discussed extraction yield and the weight of residual solid recovered, the larger amount being for Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, followed by water, Gly
H2O, followed by water, Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and LA
H2O and LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and then OA
H2O and then OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O.46 An increase in the extraction of lignocellulosic materials with eutectic solvents, especially acidic ones, is also observed. Indeed, the carboxylic acids may destroy the biomass cell wall interacting with carbohydrate's hydroxyl groups.
H2O.46 An increase in the extraction of lignocellulosic materials with eutectic solvents, especially acidic ones, is also observed. Indeed, the carboxylic acids may destroy the biomass cell wall interacting with carbohydrate's hydroxyl groups.
To understand the discrepancies in extraction yields and residual solid appearance between the different solvents used, the Nile Red polarity parameter ENR and Kamlet–Taft parameters, namely the dipolarity/polarizability (π*) and the hydrogen bonding acidity and basicity (α,β), were determined using a solvatochromic scale empirical technique. Nile Red, 4-nitroaniline and 4-nitroanisol dyes were used for this purpose. Reichardt's dye is more commonly used, but, as it lost its absorbance band when protonated in an acidic medium, Nile Red was chosen instead.69 π* gives indication on dispersion and electrostatic interactions between the solvent and the solute, while α and β indicate the solvent ability to, respectively, donate or accept a proton to form a hydrogen bond with a solute. These parameters are presented in Table 1. It is interesting to observe that OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O has Kamlet–Taft parameters particularly different from those of the other solvents. When π* varied from 1.057 to 1.100 for water, LA
H2O has Kamlet–Taft parameters particularly different from those of the other solvents. When π* varied from 1.057 to 1.100 for water, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O Gly
H2O Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Sorb
H2O and Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, it has a value of 1.472 for OA
H2O, it has a value of 1.472 for OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. The same trend is observed for α, which is the highest for OA
H2O. The same trend is observed for α, which is the highest for OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O with 1.762 and between 0.967 and 1.140 for the other solvents. On the contrary, OA
H2O with 1.762 and between 0.967 and 1.140 for the other solvents. On the contrary, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O exhibits the lowest β value, 0.228 against 0.475–0.718 for the other. The Kamlet–Taft parameters have been correlated with the extraction rate of lignocellulosic materials. Some works70–72 found that hydrogen bonds acidic solvents with high π* and α lead to a higher delignification rate due to the higher hydrogen bonds donating ability and the release of H+.73 This could also explain why polysaccharides are degraded at a higher extraction temperature and time with OA.46,74,75 The role of oxalic acid in the esterification of hydroxyl groups is also underlined. OA
H2O exhibits the lowest β value, 0.228 against 0.475–0.718 for the other. The Kamlet–Taft parameters have been correlated with the extraction rate of lignocellulosic materials. Some works70–72 found that hydrogen bonds acidic solvents with high π* and α lead to a higher delignification rate due to the higher hydrogen bonds donating ability and the release of H+.73 This could also explain why polysaccharides are degraded at a higher extraction temperature and time with OA.46,74,75 The role of oxalic acid in the esterification of hydroxyl groups is also underlined. OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and LA
H2O and LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O might form ester bonds with cell-wall polysaccharides, helping to break the lichen's structure and enhancing the polysaccharide extraction. On the FT-IR spectrum of the polysaccharides extracted with Gly
H2O might form ester bonds with cell-wall polysaccharides, helping to break the lichen's structure and enhancing the polysaccharide extraction. On the FT-IR spectrum of the polysaccharides extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, LA
H2O, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (ESI 1†), we can observe some characteristic peaks of the solvents, highlighting their presence in the polysaccharides. As all polysaccharides were washed with ethanol 70%, these peaks may mean that the eutectics bonded with the polysaccharides through hydrogen bonds or maybe ester bonds for LA
H2O (ESI 1†), we can observe some characteristic peaks of the solvents, highlighting their presence in the polysaccharides. As all polysaccharides were washed with ethanol 70%, these peaks may mean that the eutectics bonded with the polysaccharides through hydrogen bonds or maybe ester bonds for LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and OA
H2O and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O.
H2O.
2.5. Eutectics after microwave
Lastly, the potential chemical reactions or degradations that the different eutectics could undergo at the best extraction yield were briefly studied. To do so, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Gly
H2O and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O without lichen were heated at 120 °C for 15 min and OA
H2O without lichen were heated at 120 °C for 15 min and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at 100 °C for 5 min. 1H NMR and FTIR were then performed on the solvents (Fig. 9A.1–3 and B). No discrepancies in the FTIR and NRM spectra of LA
H2O at 100 °C for 5 min. 1H NMR and FTIR were then performed on the solvents (Fig. 9A.1–3 and B). No discrepancies in the FTIR and NRM spectra of LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Gly
H2O and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O are observed, but the FTIR spectra of OA
H2O are observed, but the FTIR spectra of OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O before and after microwave presented some differences in peak shapes and intensities, which could highlight bond network reorganization in the eutectic during heating. The reduction of the broad peak at 3400 cm−1 corresponding to OH group vibration seems to indicate that water evaporated.12 It was found that, for the ChCl
H2O before and after microwave presented some differences in peak shapes and intensities, which could highlight bond network reorganization in the eutectic during heating. The reduction of the broad peak at 3400 cm−1 corresponding to OH group vibration seems to indicate that water evaporated.12 It was found that, for the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA solvent, the step at which water was added (before or after the eutectic formation or using dehydrated OA) had a strong impact on the water evaporation peaks in TGA thermograms. The lowest temperature was obtained when water was added after the eutectic formation (which is the case here), probably meaning that weaker bonds were created. This could explain the water evaporation that occurred during the heating of this eutectic. New peaks also seem to appear on the OA
OA solvent, the step at which water was added (before or after the eutectic formation or using dehydrated OA) had a strong impact on the water evaporation peaks in TGA thermograms. The lowest temperature was obtained when water was added after the eutectic formation (which is the case here), probably meaning that weaker bonds were created. This could explain the water evaporation that occurred during the heating of this eutectic. New peaks also seem to appear on the OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O NMR spectra, which may mean that some reactions occurred, leading to the production of some reaction products. The smell and coloration of Gly
H2O NMR spectra, which may mean that some reactions occurred, leading to the production of some reaction products. The smell and coloration of Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O after heating is probably due to the apparition of some Maillard compounds. The smell being attributed to small nitrogen-containing molecules such as pyrazine or Amadori and Heyns compounds; the color, to melanoidins.76 In small quantities, these compounds are difficult to identify in NMR and FTIR spectroscopy, which can explain why it seems to have no difference in the spectrum before and after heating. However, the synthesis of a low quantity of melanoidins would not be necessarily a problem. Indeed, melanoidins have been found to exhibit antioxidant and antibacterial properties.77 If some of these compounds are caught in the eutectic and polysaccharides, they might confer some bioactivity to our material. This suggests that LA
H2O after heating is probably due to the apparition of some Maillard compounds. The smell being attributed to small nitrogen-containing molecules such as pyrazine or Amadori and Heyns compounds; the color, to melanoidins.76 In small quantities, these compounds are difficult to identify in NMR and FTIR spectroscopy, which can explain why it seems to have no difference in the spectrum before and after heating. However, the synthesis of a low quantity of melanoidins would not be necessarily a problem. Indeed, melanoidins have been found to exhibit antioxidant and antibacterial properties.77 If some of these compounds are caught in the eutectic and polysaccharides, they might confer some bioactivity to our material. This suggests that LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and Gly
H2O and Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O may be recycled for other extractions. However, a much more comprehensive recyclability study should be performed to investigate the purification of the eutectics after use, the recovery of the ethanol used to precipitate the polysaccharides, and the number of extractions the same solvent can be used for.
H2O may be recycled for other extractions. However, a much more comprehensive recyclability study should be performed to investigate the purification of the eutectics after use, the recovery of the ethanol used to precipitate the polysaccharides, and the number of extractions the same solvent can be used for.
 | ||
Fig. 9
1H NMR spectra of LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (A.1), Gly H2O (A.1), Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (A.2) and OA H2O (A.2) and OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (A.3) after microwave, and their FTIR spectra (B). H2O (A.3) after microwave, and their FTIR spectra (B). | ||
2.6. Valorisation of the residual solid into artificial humic substances (AHSs)
Since the polysaccharides extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O presented the best mechanical properties, the residual solid from this extraction (around 50% of the initial mass of lichen) was selected to explore its conversion into AHSs via hydrothermal humification (HTH). As a comparison, raw lichen was also used for humification. The three different fractions of the AHS were studied, referred to humin (HU), humic acid (HA) and fulvic acid (FA). Table 2 summarizes the compositions and mass yields of the recovered solid phases. After HTH, the HU yield is 16.5 and 4.9% for the raw lichen and the residual solid, respectively. This indicates that the residual solid has a higher tendency for solubilization than its parent substrate. This phenomenon can be attributed to eutectic extraction, which serves as a pre-treatment stage for the lichen, weakening the lignocellulosic intramolecular bonds and facilitating further solubilization in the hydrothermal environment. In contrast, the yield of HA shows slight variation between the two substrates, with values of 3.7% and 3.2% for the raw lichen and the residual solid phase, respectively. Therefore, despite the eutectic stage, the absolute number of precursors for the HA likely remains similar, and the residual solid has, then, a higher conversion efficiency towards the HA (since the previous extraction with eutectic removed part of the hemicellulose). The C content passes from 40.8–42.0% for the starting substrate to 57.0–57.6% for the resulting HA, indicating a carbon-densification mechanism. The subsequent reduction in both the H/C and O/C atomic ratios from the initial substrate to the HA (reaching 1.2–1.3 and 0.5, respectively) indicates that the substrates undergo a loss of hydrogen, i.e. dehydrogenation, likely accompanied by increased aromaticity. Indeed, during the conversion, the initial substrate (either the lichen or the residual solid) undergoes hydrolysis, with carbohydrates subsequently converted into carboxylic acids through retro-aldol splitting reactions, which neutralize the base and promote the formation of furan derivatives.34 Due to the pH drop, these furan derivatives subsequently repolymerize with carboxylic acids, leading to the formation of the humic acid. DTG curves (Fig. 10A) confirm the higher thermal stability of the HA, with the apparition of a decomposition peak shifting from 325 °C for the starting substrate to ∼435 °C. The improved thermal stability is accompanied by an increase in fixed carbon, which achieves 33.2–34.9%. Generally, the improved carbon-densification and thermal stability are indicators of recalcitrance (i.e., capacity of not being attacked by the microbial community in soil) and, thus, the potential for stability in soil. The presence of a decomposition peak at 280 and 295 °C, respectively, for HA L22 and HA RSL24 might be related to a remaining fraction of degraded cellulose, which has a lower thermal stability than the initial material. These trends are also observed for the HU, the most recalcitrant fraction in natural soil,78 with the increase in fixed carbon to 45.1–52.7% for H L22 and H RSL24 and a decrease in H/C ratio to 1.1–1.3, respectively. Similar DTG profiles to those of HA are observed, with the same peak at 435 °C, indicating an increase in thermal stability compared to the raw material.
H2O presented the best mechanical properties, the residual solid from this extraction (around 50% of the initial mass of lichen) was selected to explore its conversion into AHSs via hydrothermal humification (HTH). As a comparison, raw lichen was also used for humification. The three different fractions of the AHS were studied, referred to humin (HU), humic acid (HA) and fulvic acid (FA). Table 2 summarizes the compositions and mass yields of the recovered solid phases. After HTH, the HU yield is 16.5 and 4.9% for the raw lichen and the residual solid, respectively. This indicates that the residual solid has a higher tendency for solubilization than its parent substrate. This phenomenon can be attributed to eutectic extraction, which serves as a pre-treatment stage for the lichen, weakening the lignocellulosic intramolecular bonds and facilitating further solubilization in the hydrothermal environment. In contrast, the yield of HA shows slight variation between the two substrates, with values of 3.7% and 3.2% for the raw lichen and the residual solid phase, respectively. Therefore, despite the eutectic stage, the absolute number of precursors for the HA likely remains similar, and the residual solid has, then, a higher conversion efficiency towards the HA (since the previous extraction with eutectic removed part of the hemicellulose). The C content passes from 40.8–42.0% for the starting substrate to 57.0–57.6% for the resulting HA, indicating a carbon-densification mechanism. The subsequent reduction in both the H/C and O/C atomic ratios from the initial substrate to the HA (reaching 1.2–1.3 and 0.5, respectively) indicates that the substrates undergo a loss of hydrogen, i.e. dehydrogenation, likely accompanied by increased aromaticity. Indeed, during the conversion, the initial substrate (either the lichen or the residual solid) undergoes hydrolysis, with carbohydrates subsequently converted into carboxylic acids through retro-aldol splitting reactions, which neutralize the base and promote the formation of furan derivatives.34 Due to the pH drop, these furan derivatives subsequently repolymerize with carboxylic acids, leading to the formation of the humic acid. DTG curves (Fig. 10A) confirm the higher thermal stability of the HA, with the apparition of a decomposition peak shifting from 325 °C for the starting substrate to ∼435 °C. The improved thermal stability is accompanied by an increase in fixed carbon, which achieves 33.2–34.9%. Generally, the improved carbon-densification and thermal stability are indicators of recalcitrance (i.e., capacity of not being attacked by the microbial community in soil) and, thus, the potential for stability in soil. The presence of a decomposition peak at 280 and 295 °C, respectively, for HA L22 and HA RSL24 might be related to a remaining fraction of degraded cellulose, which has a lower thermal stability than the initial material. These trends are also observed for the HU, the most recalcitrant fraction in natural soil,78 with the increase in fixed carbon to 45.1–52.7% for H L22 and H RSL24 and a decrease in H/C ratio to 1.1–1.3, respectively. Similar DTG profiles to those of HA are observed, with the same peak at 435 °C, indicating an increase in thermal stability compared to the raw material.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at 120 °C for 15 min and of their corresponding humin and humic acid. Experiments were performed at least in duplicate
H2O at 120 °C for 15 min and of their corresponding humin and humic acid. Experiments were performed at least in duplicate
| C (%) | H (%) | N (%) | O (%) | FC + A (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|---|
| Raw lichen (L) | 40.8 ± 0.1 | 5.9 ± 0.1 | 1.6 ± 0.3 | 51.7 ± 0.5 | 1.7 | 1.0 | 26.4 | |
| Humin (L) | 62.3 ± 0.0 | 5.7 ± 0.0 | 2.0 ± 0.1 | 30.0 ± 0.1 | 1.1 | 0.4 | 45.1 | 16.5 ± 2.6 |
| Humic acid (L) | 57.6 ± 0.1 | 5.7 ± 0.1 | 1.7 ± 0.0 | 35.0 ± 0.2 | 1.2 | 0.5 | 34.9 | 3.7 ± 0.0 |
| Residual solid (RS) | 42.0 ± 0.3 | 6.7 ± 0.1 | 1.3 ± 0.1 | 50.0 ± 0.5 | 1.8 | 0.9 | 15.0 | |
| Humin (RS) | 37.2 ± 1.2 | 4.0 ± 0.2 | 1.5 ± 0.1 | 57.3 ± 1.5 | 1.3 | 1.2 | 52.7 | 4.9 ± 0.6 |
| Humic acid (RS) | 57.0 ± 0.0 | 6.2 ± 0.0 | 2.1 ± 0.0 | 34.7 ± 0.0 | 1.3 | 0.5 | 33.2 | 3.2 ± 0.5 |
The FTIR spectra of the raw lichen, H L22, HA L22, H RSL24 and HA RSL24 are displayed in Fig. 10B. All spectra presented the broad absorption band around 3400 cm−1 from the –OH stretching vibrations in alcohols, phenols and water.34,79–81 The signals at 2920 and 2850 cm−1, probably due to the symmetric and asymmetric CH3 and CH2 vibrations in aliphatic groups, are also observed in all spectra but with a higher intensity in HA RSL24, H RSL24 and H L22.34,79,80 A shoulder around 1670 cm−1 due to carboxylic acid carbonyl groups is much more pronounced for H and HA L22 than for H and HA RSL24. The signal at 1730 cm−1 which may be due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in hemicellulose is reduced in H/HA RSL24, which is coherent as hemicellulose was previously extracted with the eutectic solvent.79,80 A decrease in carbohydrate signals at 1150 cm−1 (C–O–C) and 1020 cm−1 (C–O stretching) in H/HA RSL24 compared to the raw lichen agrees with the removal of carbohydrates during the eutectic extraction. This peak is particularly reduced in both HA, which is coherent with the hydrolysis that occurred during the conversion process.79
O stretching vibration in hemicellulose is reduced in H/HA RSL24, which is coherent as hemicellulose was previously extracted with the eutectic solvent.79,80 A decrease in carbohydrate signals at 1150 cm−1 (C–O–C) and 1020 cm−1 (C–O stretching) in H/HA RSL24 compared to the raw lichen agrees with the removal of carbohydrates during the eutectic extraction. This peak is particularly reduced in both HA, which is coherent with the hydrolysis that occurred during the conversion process.79
FA is a mixture of lower molecular weight compounds compared to humic acid, which is rich in carboxyl groups, phenols and furans both in its synthetic and natural forms.34,82–84 FA has a broad unresolved 1H-NMR spectrum (Fig. 10C), as its precise formulation remains unknown. FA from raw lichen is dominated by organic acids, keto-acids and hydroxyl acids, region 2–3 ppm (40%), short aliphatic structures below 2 ppm (25%), phenolic and furfural derivatives between 6 and 8.5 ppm (20%), by oxygenated aliphatic protons from the dehydration intermediates of carbohydrates, region 3–4 ppm (38%), short-chain aliphatic structures (37%) and organic acids, keto-acids and hydroxyl acids (15%) for its residual solid. Peaks in similar regions are observed in the 1H NMR spectra of natural FA from sandstone debris.84 As phenolic compounds and hemicellulose were extracted during the previous treatment, it seems coherent that FA RSL24 has lower signals at 6–8.5 ppm, which comes from phenolics and furan derivatives. However, signals below 2 ppm, coming from the hydrothermal breakdown of primary biomass into low-molecular-weight acids and aromatics, may be increased in FA RSL24 as the biomass already suffer a treatment with the eutectic solvent, making it easier to break down. This observation agrees with the lower amount of HU recovered using the residual solid. The lower amount of small acids in FA RSL24 might be due to their recondensation with furans and phenolic compounds. Two-dimensional HSQC NMR was used to analyze the CH, CH2 and CH3 groups (ESI 2†). The analysis of these spectra was well developed in previous works.79,83–85 Both spectra are very similar, with various signals for methyl CH3 (δC/δH: 10–25/0.5–1.5 ppm) and methylene CH2 (δC/δH: 20–40/1–2 ppm), CH2 linked to a carbonyl group (δC/δH: 30–40/2–3 ppm) and CH3 esters, observed in lignin (δC/δH: 50–60/3.5–4 ppm). (δC/δH: 60–90/3–4 ppm) is the oxygenated aliphatic region, where signals from intermediate and cyclic carbohydrates can be seen. More signals seem to be observed in this region for HA RSL24, which is coherent with the high number of signals in the oxygenated aliphatic proton region in the 1H NMR. Lastly, the high number of signals observed in the region (δC/δH: 110–140/6.5–8 ppm) are related to protons from aromatic compounds (phenols, furans). Similar spectra were obtained for artificial FA from Poplar bark.79
Several technological applications of artificial humic substances have been developed by researchers. Their main one being in agriculture, as a fertilizer synergist that helps maintaining soil quality by retaining nutrients and water in soil and by enhancing microbial activity.86–89 They can also have interest in remediation, to remove heavy metals from wastewaters as they have high binding ability.90 The AHS usually present a simpler structure than natural ones, where the presence of microbial lipids or Amadori-like amino acid condensates are observed as examples.87,91 However, artificial ones may still have a positive impact on soil, justifying the increasing number of research on the subject.
3. Experimental
3.1. Materials
Evernia prunastri has been collected in Galicia, Spain, in December 2023, dried in an oven at 40 °C and milled (<0.5 mm). A voucher specimen, identified in 2023 by Prof. María Eugenia López de Silanes (University of Vigo, Spain), can be found at the Herbarium SANT (SANT-Lich 12461-A) (University of Santiago de Compostela, Spain). A previous ethanolic extraction in Soxhlet has been performed to remove the lichenic substances.3.2. Eutectic solvent formation
Lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (2
betaine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (LA
1) (LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet), glycerin
Bet), glycerin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (2
betaine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Gly
1) (Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet), D-sorbitol
Bet), D-sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (1
betaine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Sorb
1) (Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet) and oxalic acid
Bet) and oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline (1
proline (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (OA
1) (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro) were synthesized dissolving the two compounds in ultra-pure water and freeze-drying them. They were then diluted with water to reach the molar ratios (2
Pro) were synthesized dissolving the two compounds in ultra-pure water and freeze-drying them. They were then diluted with water to reach the molar ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for lactic acid
5) for lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (LA
betaine (LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) and glycerin
H2O) and glycerin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (Gly
betaine (Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) and (1
H2O) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for D-sorbitol
5) for D-sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (Sorb
betaine (Sorb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) and oxalic acid
H2O) and oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline (OA
proline (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). Other solvents were tested but were unstable or never formed a homogeneous mixture. It was the case for oxalic acid
H2O). Other solvents were tested but were unstable or never formed a homogeneous mixture. It was the case for oxalic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (1
betaine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (OA
1) (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet), lactic acid
Bet), lactic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) proline (2
proline (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (LA
1) (LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro), citric acid
Pro), citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (1
betaine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (CA
1) (CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet), and formic acid
Bet), and formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) betaine (2
betaine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (FO
1) (FO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet).
Bet).
3.3. Microwave-assisted extraction
Microwave-assisted extraction was performed in a CEM Discover SP microwave at 80, 100 and 120 °C during 5, 15, and 25 minutes at a maximum power of 30 W. Control extractions were performed with water at 80 and 100 °C during 25 min and at 120 °C during 15 and 25 min (maximum power = 90 W). The samples were centrifuged at 9000 rpm for 10 min, and two weights of ethanol absolute were added to the liquid phase. The samples were centrifuged at 9000 rpm for 5 min and kept at 4 °C overnight to recover the cell-wall polysaccharides. They were washed with ethanol at 70% (v/v) before drying in an oven at 50–55 °C. The residual solid was washed with water and ethanol (70% v/v), freeze-dried and stored for further analyses (Fig. 11).3.4. Synthesis of artificial humic substances (AHSs)
Raw lichen and washed residual solid after the eutectic extraction with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O were used to produce AHSs via the same alkaline hydrothermal process used by Volikov et al. (2024).79
H2O were used to produce AHSs via the same alkaline hydrothermal process used by Volikov et al. (2024).79
Around 1–2 g of milled lichenic material (<0.5 mm) was mixed with KOH in deionized water at a biomass-to-water ratio of 0.3. The reaction mixture was placed in 30 mL pressurized reactors with polytetrafluoroethylene cups (Parr, Moline, IL, USA). The reactors were then heated at 220 °C in an oven for 4 h of residence time (starting after the heating phase of 25 min). Different KOH concentrations (from 10 to 50%) were tested to find that leading to a neutral pH at the end of the process. The KOH concentration (%KOH), defined as the percentage of KOH relative to the initial substrates on a mass basis, was set at 22% for the residual solid and 24% for raw lichen. After cooling down, the mixture was diluted with deionized water and centrifuged at 9000 rpm for 10 min, and the solid residue (i.e., humin) was recovered after drying at 80 °C. Then, the pH of the liquid phase was adjusted to 1.5 using HCL to recover a further solid phase, called humic acid. After centrifugation (9000 rpm, 10 min), humic acid was recovered and dialyzed in 3.5 kDa cellulose membrane against deionized water for one week before being freeze-dried. Fulvic acid (FA) was recovered from the acidic solution with a Bond Elut PPL cartridge (Agilent, USA, 200 mg sorbent). The cartridge was activated with methanol and washed with deionized water before passing the acid solution. It was then dried under pressurized air stream. FA was recovered by passing methanol through the cartridge and then evaporating it in a rotary evaporator.
3.5. Analytical techniques
Thermogravimetric analysis was performed using a thermos-microbalance TG 209 F1 Libra (Netzch, Selb, Germany) under a N2 atmosphere. Then 10 mg of sample in a platinum crucible was heated from 25 to 900 °C, at a rate of 10 K min−1, under a constant nitrogen flow of 20 mL min−1.
ENR = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591/λmax 591/λmax | (1) |
The Kamlet–Taft parameters π* (dipolarity/polarizability), α (hydrogen bond donating ability) and β (hydrogen bond accepting ability) were calculated using the same formula as Liang et al. (2021)92 with a slightly modified protocol (2)–(4):
 | (2) |
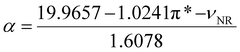 | (3) |
 | (4) |
 of, respectively, 4-Nitroanisole, 4-Nitroaniline and Nile Red in different solvents.
of, respectively, 4-Nitroanisole, 4-Nitroaniline and Nile Red in different solvents.
4. Conclusions
In this work, the impact of different natural eutectic solvents on the microwave-assisted extraction of cell-wall polysaccharides from Evernia prunastri was studied. It was found that the type of eutectic solvent used for the extraction had a strong impact on the chemical and mechanical properties of the recovered polysaccharides. After drying them, while those extracted with Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O exhibited particularly good mechanical properties (elastic deformation and the highest ultimate tensile strength), the material recovered with LA
H2O exhibited particularly good mechanical properties (elastic deformation and the highest ultimate tensile strength), the material recovered with LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bet
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O sustained a large plastic deformation with a much lower ultimate tensile strength. With OA
H2O sustained a large plastic deformation with a much lower ultimate tensile strength. With OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pro
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, a material slightly deformable with an intermediate ultimate tensile strength was obtained. This study provides an interesting initial insight into how eutectic solvents can be used to tailor the mechanical properties of polysaccharides. It means that, by more than just increasing the extraction yield, eutectic solvents can add value to the extracted polysaccharides. This process also eliminates the usual drying and formulation steps required when polysaccharides are extracted with conventional methods, reducing the energy consumption and production time by around 35% and 22%, respectively. This suggests that eutectic solvents may be promising for intensifying the extraction process of polysaccharides, conferring unique mechanical properties to the extracted fractions. Their use allows for potential applications in biomedical, pharmaceutical and cosmetics, where mechanical performance plays a secondary role while antimicrobial properties, such as those of some polysaccharides from lichens and melanoidins, would benefit formulations of wound dressings or topical gels, anti-inflammatory agents or moisturizers. Fortification of the extracted materials with natural fibers, cross linking or molding with biopolymers aimed to improve mechanical performance would extend the applications into bioplastics, especially for food and agricultural applications. This treatment also paves the way for its extension to the extraction/formulation of many other natural polysaccharides. It was also demonstrated that lichen residues after the extraction of polysaccharides can be valorized into high-value-added artificial humic substances, bringing us closer to the concept of biorefinery. Next investigation step would be to study carefully the recyclability of the eutectic solvents to increase even more the sustainability of this process. It would also be interesting to work on potential applications for the obtained materials, and to test the produced humic substances on soil.
H2O, a material slightly deformable with an intermediate ultimate tensile strength was obtained. This study provides an interesting initial insight into how eutectic solvents can be used to tailor the mechanical properties of polysaccharides. It means that, by more than just increasing the extraction yield, eutectic solvents can add value to the extracted polysaccharides. This process also eliminates the usual drying and formulation steps required when polysaccharides are extracted with conventional methods, reducing the energy consumption and production time by around 35% and 22%, respectively. This suggests that eutectic solvents may be promising for intensifying the extraction process of polysaccharides, conferring unique mechanical properties to the extracted fractions. Their use allows for potential applications in biomedical, pharmaceutical and cosmetics, where mechanical performance plays a secondary role while antimicrobial properties, such as those of some polysaccharides from lichens and melanoidins, would benefit formulations of wound dressings or topical gels, anti-inflammatory agents or moisturizers. Fortification of the extracted materials with natural fibers, cross linking or molding with biopolymers aimed to improve mechanical performance would extend the applications into bioplastics, especially for food and agricultural applications. This treatment also paves the way for its extension to the extraction/formulation of many other natural polysaccharides. It was also demonstrated that lichen residues after the extraction of polysaccharides can be valorized into high-value-added artificial humic substances, bringing us closer to the concept of biorefinery. Next investigation step would be to study carefully the recyclability of the eutectic solvents to increase even more the sustainability of this process. It would also be interesting to work on potential applications for the obtained materials, and to test the produced humic substances on soil.
Author contributions
Conceptualization: J. Q., M. D. T., S. F., and G. I.; methodology: J. Q., S. F., and G. I.; writing-review and editing: J. Q., M. D. T., H. D., S. F., and G. I.; supervision: S. F.Data availability
The data will be available from the authors of this work upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Queffelec thanks the University of Vigo and the DAAD Research Grants – Short-Term Grants, 2024 (57693450). G. Ischia thanks the Alexander Von Humboldt Foundation for the kind financial support. This work was financially supported by the Max Planck Society.References
- Y. Zhao, M. Wang and B. Xu, A Comprehensive Review on Secondary Metabolites and Health-Promoting Effects of Edible Lichen, J. Funct. Foods, 2021, 80, 104283, DOI:10.1016/J.JFF.2020.104283.
- F. Zhu, B. Du and B. Xu, A Critical Review on Production and Industrial Applications of Beta-Glucans, Food Hydrocolloids, 2016, 52, 275–288, DOI:10.1016/J.FOODHYD.2015.07.003.
- G. O. Coelho, M. J. A. Batista, A. F. Ávila, A. S. Franca and L. S. Oliveira, Development and Characterization of Biopolymeric Films of Galactomannans Recovered from Spent Coffee Grounds, J. Food Eng., 2021, 289, 110083, DOI:10.1016/J.JFOODENG.2020.110083.
- Y. Brummer, C. Defelice, Y. Wu, M. Kwong, P. J. Wood and S. M. Tosh, Textural and Rheological Properties of Oat Beta-Glucan Gels with Varying Molecular Weight Composition, J. Agric. Food Chem., 2014, 62(14), 3160–3167, DOI:10.1021/JF405131D/ASSET/IMAGES/LARGE/JF-2013-05131D_0007.JPEG.
- N. Yi, Z. Cheng, L. Yang, G. Edelman, C. Xue, Y. Ma, H. Zhu and H. Cheng, Fully Water-Soluble, High-Performance Transient Sensors on a Versatile Galactomannan Substrate Derived from the Endosperm, ACS Appl. Mater. Interfaces, 2018, 10(43), 36664–36674, DOI:10.1021/ACSAMI.8B11682.
- M. B. Santos and E. E. Garcia-Rojas, Recent Advances in the Encapsulation of Bioactive Ingredients Using Galactomannans-Based as Delivery Systems, Food Hydrocolloids, 2021, 118, 106815, DOI:10.1016/J.FOODHYD.2021.106815.
- G. C. G. Martínez-Avila, M. A. Aguilar-Gonzalez, M. L. Chávez-Gonzalez, D. K. Verma, H. Khan and C. N. Aguilar, Galactomannan: A Biodegradable Polymer Used for Bio-Based Edible Coating and Film Materials, in Appl. Biodegrad. Bio-Based Polym. Hum. Health Clean. Environ, 2021, pp 223–238. DOI:10.1201/9781003146360-11.
- N. Flórez, E. Conde and H. Domínguez, Microwave Assisted Water Extraction of Plant Compounds, J. Chem. Technol. Biotechnol., 2015, 90(4), 590–607, DOI:10.1002/jctb.4519.
- A. H. Nour, A. R. Oluwaseun, A. H. Nour, M. S. Omer, N. Ahmed, A. H. Nour, A. R. Oluwaseun, A. H. Nour, M. S. Omer and N. Ahmed, Microwave-Assisted Extraction of Bioactive Compounds (Review), in Microwave Heating - Electromagnetic Fields Causing Thermal and Non-Thermal Effects, IntechOpen, 2021. DOI:10.5772/intechopen.96092.
- Y. Mao, J. P. Robinson and E. R. Binner, Current Status of Microwave-Assisted Extraction of Pectin, Chem. Eng. J., 2023, 473, 145261, DOI:10.1016/j.cej.2023.145261.
- M. Omran, T. Fabritius, G. Chen and A. He, Microwave Absorption Properties of Steelmaking Dusts: Effects of Temperature on the Dielectric Constant (ε ′) and Loss Factor (ε ′′) at 1064 MHz and 2423 MHz, RSC Adv., 2019, 9(12), 6859–6870, 10.1039/C9RA00009G.
- J. González-Rivera, A. Mero, E. Husanu, A. Mezzetta, C. Ferrari, F. D'Andrea, E. Bramanti, C. S. Pomelli and L. Guazzelli, Combining Acid-Based Deep Eutectic Solvents and Microwave Irradiation for Improved Chestnut Shell Waste Valorization, Green Chem., 2021, 23(24), 10101–10115, 10.1039/D1GC03450B.
- J. Queffelec, W. Beraud, S. Ferron, J. Boustie, I. Rodríguez-González, B. Díaz-Reinoso, M. D. Torres and H. Domínguez, Alternatives for the Extraction of Bioactives and Biopolymers from Evernia Prunastri for the Formulation of Antimicrobial Bio-Based Films, Green Chem., 2024, 26(19), 10205–10224, 10.1039/D4GC02741H.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Basics and Properties of Deep Eutectic Solvents: A Review, Environ. Chem. Lett., 2021, 19(4), 3397–3408, DOI:10.1007/s10311-021-01225-8.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114(21), 11060–11082, DOI:10.1021/cr300162p.
- Y. Nie, Y. Zhou, Y. Zhang, D. Sun, D. Wu, L. Ban, S. Nanda, C. Xu and H. Zhang, Sustainable Synthesis of Functional Materials Assisted by Deep Eutectic Solvents for Biomedical, Environmental, and Energy Applications, Adv. Funct. Mater., 2025, 35, 2418957, DOI:10.1002/adfm.202418957.
- Y. Liu, Y. Zhang, S.-N. Chen, J. B. Friesen, D. Nikolić, M. P. Choules, J. B. McAlpine, D. C. Lankin, R. A. Gemeinhart and G. F. Pauli, The Influence of Natural Deep Eutectic Solvents on Bioactive Natural Products: Studying Interactions between a Hydrogel Model and Schisandra Chinensis Metabolites, Fitoterapia, 2018, 127, 212–219, DOI:10.1016/j.fitote.2018.02.024.
- B. M. Popovic, N. Micic, A. Potkonjak, B. Blagojevic, K. Pavlovic, D. Milanov and T. Juric, Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents – Ultrafast Microwave-Assisted NADES Preparation and Extraction, Food Chem., 2022, 366, 130562, DOI:10.1016/j.foodchem.2021.130562.
- C. Villa, D. Caviglia, F. S. R. d. Cuna, G. Zuccari and E. Russo, NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview, Gels, 2024, 10(2), 107, DOI:10.3390/GELS10020107.
- N. Mustafa, V. Spelbos, G.-J. Witkamp, R. Verpoorte and Y. Choi, Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations, Molecules, 2021, 26(9), 2645, DOI:10.3390/molecules26092645.
- L. Wils, S. Hilali and L. Boudesocque-Delaye, Biomass Valorization Using Natural Deep Eutectic Solvents: What's New in France?, Molecules, 2021, 26(21), 6556, DOI:10.3390/molecules26216556.
- C. Bakirtzi, K. Triantafyllidou and D. P. Makris, Novel Lactic Acid-Based Natural Deep Eutectic Solvents: Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Common Native Greek Medicinal Plants, J. Appl. Res. Med. Aromat. Plants, 2016, 3(3), 120–127, DOI:10.1016/j.jarmap.2016.03.003.
- B. D. Ribeiro, C. Florindo, L. Iff, M. A. Coelho and I. Marrucho, Novel Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents, ACS Sustainable Chem. Eng., 2015, 3, 150910100559005, DOI:10.1021/acssuschemeng.5b00532.
- L. He, L. Chen, B. Zheng, H. Zhou, H. Wang, H. Li, H. Zhang, C. C. Xu and S. Yang, Deep Eutectic Solvents for Catalytic Biodiesel Production from Liquid Biomass and Upgrading of Solid Biomass into 5-Hydroxymethylfurfural, Green Chem., 2023, 25(19), 7410–7440, 10.1039/D3GC02816J.
- A. Satlewal, R. Agrawal, S. Bhagia, J. Sangoro and A. J. Ragauskas, Natural Deep Eutectic Solvents for Lignocellulosic Biomass Pretreatment: Recent Developments, Challenges and Novel Opportunities, Biotechnol. Adv., 2018, 36(8), 2032–2050, DOI:10.1016/j.biotechadv.2018.08.009.
- M. Jablonský, A. Škulcová, A. Malvis and J. Šima, Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents, J. Biotechnol., 2018, 282, 46–66, DOI:10.1016/j.jbiotec.2018.06.349.
- B. S. Jacob Rani and S. Venkatachalam, Cleaner Approach for the Cascade Production of Nanocellulose, Nanohemicellulose and Nanolignin from Prosopis Juliflora, Carbohydr. Polym., 2022, 294, 119807, DOI:10.1016/j.carbpol.2022.119807.
- H. Schneider, N. Doskaliuk, E. Buchner, M. Antonietti and S. Filonenko, Reactive Eutectic Media for Lignocellulosic Biomass Fractionation, ChemSusChem, 2024, e202301780, DOI:10.1002/CSSC.202301780.
- S. X. Tan, A. Andriyana, S. Lim, H. C. Ong, Y. L. Pang and G. C. Ngoh, Natural Deep Eutectic Solvent (NADES) as Plasticizer for Bioplastic Film Fabrication. A Comparative Study, in AIJR Proc., 2022.
- A. S. B. d. Sousa, R. P. Lima, M. C. A. d. Silva, D. d. N. Moreira, M. M. E. Pintado and S. d. M. Silva, Natural Deep Eutectic Solvent of Choline Chloride with Oxalic or Ascorbic Acids as Efficient Starch-Based Film Plasticizers, Polymer, 2022, 259, 125314, DOI:10.1016/j.polymer.2022.125314.
- S. Ramesh, R. Shanti and E. Morris, Studies on the Plasticization Efficiency of Deep Eutectic Solvent in Suppressing the Crystallinity of Corn Starch Based Polymer Electrolytes, Carbohydr. Polym., 2012, 87(1), 701–706, DOI:10.1016/j.carbpol.2011.08.047.
- A. P. Abbott, A. D. Ballantyne, J. P. Conde, K. S. Ryder and W. R. Wise, Salt Modified Starch: Sustainable, Recyclable Plastics, Green Chem., 2012, 14(5), 1302–1307, 10.1039/C2GC16568F.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, O-Acetylation, of Cellulose and Monosaccharides Using a Zinc Based Ionic Liquid, Green Chem., 2005, 7(10), 705–707, 10.1039/B511691K.
- F. Yang, S. Zhang, K. Cheng and M. Antonietti, A Hydrothermal Process to Turn Waste Biomass into Artificial Fulvic and Humic Acids for Soil Remediation, Sci. Total Environ., 2019, 686, 1140–1151, DOI:10.1016/j.scitotenv.2019.06.045.
- F. Yang, Q. Fu and M. Antonietti, Anthropogenic, Carbon-Reinforced Soil as a Living Engineered Material, Chem. Rev., 2023, 123(5), 2420–2435, DOI:10.1021/acs.chemrev.2c00399.
- R. M. Boiteau, J. B. Shaw, L. Pasa-Tolic, D. W. Koppenaal and J. K. Jansson, Micronutrient Metal Speciation Is Controlled by Competitive Organic Chelation in Grassland Soils, Soil Biol. Biochem., 2018, 120, 283–291, DOI:10.1016/j.soilbio.2018.02.018.
- M. Zdanowicz, P. Staciwa, R. Jędrzejewski and T. Spychaj, Sugar Alcohol-Based Deep Eutectic Solvents as Potato Starch Plasticizers, Polymers, 2019, 11(9), 1385, DOI:10.3390/polym11091385.
- D. O. Abranches, L. P. Silva, M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor, ChemSusChem, 2020, 13(18), 4916–4921, DOI:10.1002/cssc.202001331.
- M. P. Heaney, L. Adhikari, A. L. Siegel, K. B. Pekar, J. B. Lefton, C. McGuire, P. Rungthanaphatsophon, J. R. Walensky, G. A. Baker and T. Runčevski, Deep Eutectic Solvents Comprising Creatine and Citric Acid and Their Hydrated Mixtures, Chem. Commun., 2022, 58(17), 2838–2841, 10.1039/D1CC06088K.
- H. Schneider, N. Merbouh, S. Keerthisinghe, M. Antonietti and S. Filonenko, Microwave-Irradiated Rapid Synthesis of Antimicrobial Pyrazine Derivatives in Reactive Eutectic Media, Green Chem., 2022, 24(24), 9745–9754, 10.1039/D2GC03122A.
- B. C. Hancock and G. Zografi, The Relationship Between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids, Pharm. Res., 1994, 11(4), 471–477, DOI:10.1023/A:1018941810744/METRICS.
- R. Craveiro, I. Aroso, V. Flammia, T. Carvalho, M. T. Viciosa, M. Dionísio, S. Barreiros, R. L. Reis, A. R. C. Duarte and A. Paiva, Properties and Thermal Behavior of Natural Deep Eutectic Solvents, J. Mol. Liq., 2016, 215, 534–540, DOI:10.1016/J.MOLLIQ.2016.01.038.
- H. Schneider, N. Merbouh, S. Keerthisinghe, M. Antonietti and S. Filonenko, Microwave-Irradiated Rapid Synthesis of Antimicrobial Pyrazine Derivatives in Reactive Eutectic Media, Green Chem., 2022, 24(24), 9745–9754, 10.1039/D2GC03122A.
- S. Zhu, H. Li, W. Zhu, W. Jiang, C. Wang, P. Wu, Q. Zhang and H. Li, Vibrational Analysis and Formation Mechanism of Typical Deep Eutectic Solvents: An Experimental and Theoretical Study, J. Mol. Graphics Modell., 2016, 68, 158–175, DOI:10.1016/J.JMGM.2016.05.003.
- I. V. Pires, Y. C. N. Sakurai, N. R. Ferreira, S. G. C. Moreira, A. M. da Cruz Rodrigues and L. H. M. da Silva, Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Pitaya, Molecules, 2022, 27(23), 8310, DOI:10.3390/molecules27238310.
- J. Wang, W. Jing, H. Tian, M. Liu, H. Yan, W. Bi and D. D. Y. Chen, Investigation of Deep Eutectic Solvent-Based Microwave-Assisted Extraction and Efficient Recovery of Natural Products, ACS Sustainable Chem. Eng., 2020, 8(32), 12080–12088, DOI:10.1021/acssuschemeng.0c03393.
- C. Gabriel, S. Gabriel, E. H. Grant, B. S. J. Halstead, D. Michael and P. Mingos, Dielectric Parameters Relevant to Microwave Dielectric Heating, Chem. Soc. Rev., 1998, 27, 41 RSC.
- I. Bodachivskyi, U. Kuzhiumparambil and D. Bradley, High Yielding Acid-Catalysed Hydrolysis of Cellulosic Polysaccharides and Native Biomass into Low Molecular Weight Sugars in Mixed Ionic Liquid Systems, ChemistryOpen, 2019, 8(10), 1316–1324, DOI:10.1002/OPEN.201900283.
- Y. Liu, L. Gao, L. Chen, W. Zhou, C. Wang and L. Ma, Exploring Carbohydrate Extraction from Biomass Using Deep Eutectic Solvents: Factors and Mechanisms, iScience, 2023, 26(9), 107671, DOI:10.1016/J.ISCI.2023.107671.
- W. Deng, H. Zhang, L. Xue, Q. Zhang and Y. Wang, Selective Activation of the C–O Bonds in Lignocellulosic Biomass for the Efficient Production of Chemicals, Chin. J. Catal., 2015, 36(9), 1440–1460, DOI:10.1016/S1872-2067(15)60923-8.
- M. F. Galrinho, L. M. Silva, G. R. Lopes, B. A. C. Ferreira, S. A. Valente, I. Ferreira, B. A. Pinheiro, A. S. Palma, D. V. Evtuguin, J. A. Lopes da Silva, M. Almeida, P. Ferreira, M. T. Cruz, M. A. Coimbra and C. P. Passos, The Study of Galactomannans with Different Molecular Weights and Their Ability to Form Microparticles Suitable for Pulmonary Delivery, Carbohydr. Polym., 2024, 339, 122268, DOI:10.1016/j.carbpol.2024.122268.
- M. V. Lesnichaya, G. P. Aleksandrova, B. G. Sukhov and A. V. Rokhin, Molecular-Weight Characteristics of Galactomannan and Carrageenan, Chem. Nat. Compd., 2013, 49(3), 405–410, DOI:10.1007/s10600-013-0625-x.
- B. Du, M. Meenu, H. Liu and B. Xu, A Concise Review on the Molecular Structure and Function Relationship of β-Glucan, Int. J. Mol. Sci., 2019, 20(16), 4032, DOI:10.3390/ijms20164032.
- J. Queffelec, N. Flórez-Fernández, M. D. Torres and H. Domínguez, Evernia Prunastri Lichen as a Source of Bioactive Glucans with Potential for Topical Applications, Int. J. Biol. Macromol., 2024, 258, 128859, DOI:10.1016/j.ijbiomac.2023.128859.
- L. Kupiainen, J. Ahola and J. Tanskanen, Kinetics of Glucose Decomposition in Formic Acid, Chem. Eng. Res. Des., 2011, 89(12), 2706–2713, DOI:10.1016/j.cherd.2011.06.005.
- R. Fijan, M. Basile, S. Šostar-Turk, E. Žagar, M. Žigon and R. Lapasin, A Study of Rheological and Molecular Weight Properties of Recycled Polysaccharides Used as Thickeners in Textile Printing, Carbohydr. Polym., 2009, 76(1), 8–16, DOI:10.1016/J.CARBPOL.2008.09.027.
- D. Skowrońska and K. Wilpiszewska, Deep Eutectic Solvents for Starch Treatment, Polymers, 2022, 14(2), 220, DOI:10.3390/polym14020220.
- Y. Wang, F. Xu, J. Cheng, X. Wu, J. Xu, C. Li, W. Li, N. Xie, Y. Wang and L. He, Natural Deep Eutectic Solvent-Assisted Extraction, Structural Characterization, and Immunomodulatory Activity of Polysaccharides from Paecilomyces Hepiali, Molecules, 2022, 27(22), 8020, DOI:10.3390/molecules27228020.
- R. Zhang, X. Yang, Y. Liu, J. Hu, K. Hu, Y. Liu, Q. Deng, S. Yang, F. Hao and X. Wen, Investigation of Natural Deep Eutectic Solvent for the Extraction of Crude Polysaccharide from Polygonatum Kingianum and Influence of Metal Elements on Its Immunomodulatory Effects, Talanta, 2024, 271, 125721, DOI:10.1016/j.talanta.2024.125721.
- N. Yahaya, A. H. Mohamed, M. Sajid, N. N. M. Zain, P.-C. Liao and K. W. Chew, Deep Eutectic Solvents as Sustainable Extraction Media for Extraction of Polysaccharides from Natural Sources: Status, Challenges and Prospects, Carbohydr. Polym., 2024, 338, 122199, DOI:10.1016/j.carbpol.2024.122199.
- U. Goodenough, Introduction to the Lichen Ultrastructure Series, Algal Res., 2020, 51, 102026, DOI:10.1016/J.ALGAL.2020.102026.
- U. Goodenough and R. Roth, Lichen 2. Constituents, Algal Res., 2021, 58, 102356, DOI:10.1016/J.ALGAL.2021.102356.
- R. Roth, R. Wagner and U. Goodenough, Lichen 3. Outer Layers, Algal Res., 2021, 56, 102332, DOI:10.1016/J.ALGAL.2021.102332.
- U. Goodenough, R. Wagner and R. Roth, Lichen 4. The Algal Layer, Algal Res., 2021, 58, 102355, DOI:10.1016/J.ALGAL.2021.102355.
- U. Goodenough and R. Roth, Lichen 5. Medullary and Bacterial Biofilm Layers, Algal Res., 2021, 58, 102333, DOI:10.1016/J.ALGAL.2021.102333.
- T. Spribille, Relative Symbiont Input and the Lichen Symbiotic Outcome, Curr. Opin. Plant Biol., 2018, 44, 57–63, DOI:10.1016/j.pbi.2018.02.007.
- T. Spribille, G. Tagirdzhanova, S. Goyette, V. Tuovinen, R. Case and W. F. Zandberg, 3D Biofilms: In Search of the Polysaccharides Holding Together Lichen Symbioses, FEMS Microbiol. Lett., 2020, 367(5), fnaa023, DOI:10.1093/femsle/fnaa023.
- R. Honegger and A. Haisch, Immunocytochemical Location of the (1 -> 3) (1 -> 4)-Beta-Glucan Lichenin in the Lichen-Forming Ascomycete Cetraria Islandica (Icelandic Moss), New Phytol., 2001, 150, 739–746, DOI:10.1046/j.1469-8137.2001.00122.x.
- J. Cao, J. Cao, H. Wang, L. Chen, F. Cao and E. Su, Solubility Improvement of Phytochemicals Using (Natural) Deep Eutectic Solvents and Their Bioactivity Evaluation, J. Mol. Liq., 2020, 318, 113997, DOI:10.1016/J.MOLLIQ.2020.113997.
- H. Ge, Y. Bai, R. Zhou, Y. Liu, J. Wei, S. Wang, B. Li and H. Xu, Explicable Machine Learning for Predicting High-Efficiency Lignocellulose Pretreatment Solvents Based on Kamlet-Taft and Polarity Parameters, ACS Sustainable Chem. Eng, 2024, 12(19), 7578–7590, DOI:10.1021/ACSSUSCHEMENG.4C01563/ASSET/IMAGES/LARGE/SC4C01563_0007.JPEG.
- J. Yu, X. Liu, S. Xu, P. Shao, J. Li, Z. Chen, X. Wang, Y. Lin and C. M. G. C. Renard, Advances in Green Solvents for Production of Polysaccharide-Based Packaging Films: Insights of Ionic Liquids and Deep Eutectic Solvents, Compr. Rev. Food Sci. Food Saf., 2023, 22(2), 1030–1057, DOI:10.1111/1541-4337.13099.
- X. Liu, W. Wei and S. Wu, Synergism of Organic Acid and Deep Eutectic Solvents Pretreatment for the Co-Production of Oligosaccharides and Enhancing Enzymatic Saccharification, Bioresour. Technol., 2019, 290, 121775, DOI:10.1016/J.BIORTECH.2019.121775.
- Y. Sun, X. Jia, R. Yang, X. Qin, X. Zhou, H. Zhang, W. Kong, J. Zhang and J. Wang, Deep Eutectic Solvents Boosting Solubilization and Se-Functionalization of Heteropolysaccharide: Multiple Hydrogen Bonds Modulation, Carbohydr. Polym., 2022, 284, 119159, DOI:10.1016/J.CARBPOL.2022.119159.
- Z. Ling, J. V. Edwards, Z. Guo, N. T. Prevost, S. Nam, Q. Wu, A. D. French and F. Xu, Structural Variations of Cotton Cellulose Nanocrystals from Deep Eutectic Solvent Treatment: Micro and Nano Scale, Cellulose, 2019, 26(2), 861–876, DOI:10.1007/S10570-018-2092-9/FIGURES/8.
- Y. Ma, Q. Xia, Y. Liu, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, Production of Nanocellulose Using Hydrated Deep Eutectic Solvent Combined with Ultrasonic Treatment, ACS Omega, 2019, 4(5), 8539–8547, DOI:10.1021/ACSOMEGA.9B00519/SUPPL_FILE/AO9B00519_SI_001.PDF.
- P. J. Andruszkiewicz, R. N. D'Souza, M. Corno and N. Kuhnert, Novel Amadori and Heyns Compounds Derived from Short Peptides Found in Dried Cocoa Beans, Food Res. Int., 2020, 133, 109164, DOI:10.1016/j.foodres.2020.109164.
- H.-Y. Wang, H. Qian and W.-R. Yao, Melanoidins Produced by the Maillard Reaction: Structure and Biological Activity, Food Chem., 2011, 128(3), 573–584, DOI:10.1016/j.foodchem.2011.03.075.
- J. Gamage, P. Voroney, A. W. Gillespie and J. Longstaffe, Chemical Composition of Soil Humin in an Organic Soil Profile, Appl. Geochem., 2024, 165, 105954, DOI:10.1016/j.apgeochem.2024.105954.
- A. Volikov, H. Schneider, N. V. Tarakina, N. Marzban, M. Antonietti and S. Filonenko, Artificial Humic Substances as Sustainable Carriers for Manganese: Development of a Novel Bio-Based Microfertilizer, Biofuel Res. J., 2024, 11(01), 2013–2024, DOI:10.18331/BRJ2024.11.1.3.
- W. M. Davis, C. L. Erickson, C. T. Johnston, J. J. Delfino and J. E. Porter, Quantitative Fourier Transform Infrared Spectroscopic Investigation Humic Substance Functional Group Composition, Chemosphere, 1999, 38(12), 2913–2928, DOI:10.1016/S0045-6535(98)00486-X.
- H. Niu, H. Yang, L. Tong, S. Zhong and Y. Liu, Spectral Study of Humic Substance Extract from Pressurized Oxidizing Slag of Carlin-Typed Gold Deposit, J. Phys.: Conf. Ser., 2019, 1347, 012027, DOI:10.1088/1742-6596/1347/1/012027.
- J. H. Weber and S. A. Wilson, The Isolation and Characterization of Fulvic Acid and Humic Acid from River Water, Water Res., 1975, 9(12), 1079–1084, DOI:10.1016/0043-1354(75)90105-0.
- L. B. Krivdin, Liquid-Phase NMR of Humic and Fulvic Acids, Magn. Reson. Chem., 2025, 63(2), 128–150, DOI:10.1002/mrc.5493.
- A. J. Simpson, J. Burdon, C. L. Graham, M. H. B. Hayes, N. Spencer and W. L. Kingery, Interpretation of Heteronuclear and Multidimensional NMR Spectroscopy of Humic Substances, Eur. J. Soil Sci., 2001, 52(3), 495–509, DOI:10.1046/j.1365-2389.2001.00402.x.
- V. Tkachenko, N. Marzban, S. Vogl, S. Filonenko and M. Antonietti, Chemical Insights into the Base-Tuned Hydrothermal Treatment of Side Stream Biomasses, Sustainable Energy Fuels, 2023, 7(3), 769–777, 10.1039/D2SE01513G.
- M. Wang, Y. Li, H. Peng, J. Wang, Q. Li, P. Li, J. Fan, S. Liu and G. Zheng, Review: Biotic and Abiotic Approaches to Artificial Humic Acids Production, Renewable Sustainable Energy Rev., 2023, 187, 113771, DOI:10.1016/j.rser.2023.113771.
- F. Yang and M. Antonietti, Artificial Humic Acids: Sustainable Materials against Climate Change, Adv. Sci., 2020, 7(5), 1902992, DOI:10.1002/advs.201902992.
- E. Efremenko, N. Stepanov, O. Senko, I. Lyagin, O. Maslova and A. Aslanli, Artificial Humic Substances as Biomimetics of Natural Analogues: Production, Characteristics and Preferences Regarding Their Use, Biomimetics, 2023, 8(8), 613, DOI:10.3390/biomimetics8080613.
- F. Yang, C. Tang and M. Antonietti, Natural and Artificial Humic Substances to Manage Minerals, Ions, Water, and Soil Microorganisms, Chem. Soc. Rev., 2021, 50(10), 6221–6239, 10.1039/D0CS01363C.
- A. Makan, Humic Substances, BoD – Books on Demand, 2021 Search PubMed.
- S. Pompe, M. Bubner, M. A. Denecke, T. Reich, A. Brachmann, G. Geipel, R. Nicolai, KH Heise and H. Nitsche, A Comparison of Natural Humic Acids with Synthetic Humic Acid Model Substances: Characterization and Interaction with Uranium(VI), Radiochim. Acta, 1996, 74(s1), 135–140, DOI:10.1524/ract.1996.74.special-issue.135.
- X. Liang, Y. Zhu, B. Qi, S. Li, J. Luo and Y. Wan, Structure-propertiy-performance relationships of lactic acid-based deep eutectic solvents with different hydrogen bond acceptors for corn stover pretreatment, Bioresour. Technol., 2021, 3336, 125312, DOI:10.1016/J.BIORTECH.2021.125312.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc01413a |
| This journal is © The Royal Society of Chemistry 2025 |