Chemiluminescence video assisted by chemometric modeling for forensic identification of blood at crime scenes†
Thomas F. F. T.
dos Santos
a,
José R.
S. Júnior
a,
Licarion
Pinto
 b,
Tadeu Morais
Cruz
c,
Jose Ailton M.
Nascimento
d,
Severino Carlos B.
Oliveira
d and
Vagner Bezerra
dos Santos
b,
Tadeu Morais
Cruz
c,
Jose Ailton M.
Nascimento
d,
Severino Carlos B.
Oliveira
d and
Vagner Bezerra
dos Santos
 *a
*a
aDepartment of Fundamental Chemistry, Federal University of Pernambuco, Av. Jornalista Aníbal Fernandes, Cidade Universitária, Recife, 50.740-560, Brazil. E-mail: vagner.bsantos@ufpe.br
bDepartment of Analytical Chemistry, State University of Rio de Janeiro, Rua São Francisco Xavier, Rio de Janeiro, Rio de Janeiro 20.550-900, Brazil
cSpecialized Forensic and Homicide Group, Department of Homicide and Personal Protection, Rua Dr João Lacerda, Cordeiro, Recife, 50.711-280, Brazil
dChemistry Departament, Federal Rural University of Pernambuco, Rua Manuel de Medeiros, Dois Irmãos, Recife, 52171-900, Brazil
First published on 24th June 2025
Abstract
The development of advanced chemiluminescent compounds and hematology methodologies has significant implications for forensic science, particularly for the detection of evidential residues at crime scenes. This study introduces a novel chemiluminescent (CL) method that utilizes a smartphone to produce digital videos of the chemiluminescent reaction between the luminol (5-amino-2,3-dihydrophthalazine-1,4-dione) reagent and blood. This innovative approach significantly reduces reagent consumption by 6 times, requiring less than 1 mL/0.01 g of sample/chemicals, which agrees with green chemistry principles. Blood samples used in this study were sourced from bovine liver and human subjects and were collected by the official forensic police at crime scenes. All samples were subsequently discarded by the criminal police. Frames from a 3-minutes video were processed using ImageJ software and the Color Grab app to generate RGB, HSV, and CMYK pattern recognition, combined with chemometric modeling. This enabled the differentiation of samples based on positive and negative patterns, effectively preventing false results. The pattern recognition models developed were able to distinguish bovine from human blood, even after dilution, which simulated attempts to hide traces at crime scenes through washing. The method demonstrated an accuracy of 90.30% with only four prediction errors and presented 100% sensitivity and specificity for the cotton + ceramics class, with 77.78% sensitivity and 93.10% specificity for both the wood and glass classes. Additionally, it was possible to estimate the age of the samples with a precision of 3.6 days. These results were obtained using a new data fusion strategy that facilitated the modeling of digital videos as a combination of frames to enhance model sensitivity and selectivity without increasing model complexity. These results indicate that the developed method is accurate, sensitive, and rapid. Supported by these results, this method represents a significant advancement in forensic science, offering a practical and efficient solution for crime scene investigations.
1. Introduction
In the field of forensic expertise, the criminal department plays a crucial role by collecting evidence at crime scenes.1 This evidence is highly important for elucidating a crime because it directs the line of investigation.2 In this context, some different materials are collected, and among them, semen, fingerprints, hair and blood are the most important.1,2 Blood constitutes approximately 8% of the human body. Cells, proteins, salts, water and iron make up the basic composition of blood, and approximately 55% of its total composition is plasma.2,3 The blood obtained by forensics can be fresh or old. Old blood is characterised as blood that has undergone multiple biological, chemical and physical aging processes.2 In this context, the heme group of hemoglobin may become oxidised, causing the oxidation of Fe2+ to Fe3+ and forming methaemoglobin with a brown hue that cannot effectively transport oxygen. This process is complex and can be influenced by environmental factors such as temperature, light, pH, and humidity.3 Moreover, water evaporation, platelet coagulation, and fibrinogen initiate a gelling process that makes blood identification difficult.4,5 On the other hand, blood is sometimes found diluted or contaminated with other substances, such as organic components, drugs, or cleaning products used to tamper with the scene of a crime, which makes the identification of blood a scientific challenge.5,6 Thus, forensic science has seen significant advancements in methods used to detect blood, blending traditional techniques with innovative technologies and more advanced methods, such as Raman spectroscopy and hyperspectral imaging, have emerged, offering non-destructive analysis capabilities that can identify blood.6–9 Additionally, DNA-based techniques, such as species-specific polymerase chain reaction (PCR) assays, have allowed forensic scientists to detect blood and confirm its human origin with remarkable precision.9 This integration of methods has improved the reliability of blood detection, thereby contributing to more accurate forensic investigations.9,10 However, these methods are typically performed at laboratories.7,8 Presumptive tests, such as luminol, are commonly employed owing to their ability to rapidly indicate the presence of blood through chemiluminescence.7,8 The luminol method (5-amino-2,3-dihydrophthalazine-1,4-dione) is the most commonly applied method for the in situ detection of blood owing to its chemiluminescent properties.7 The luminol reaction is better performed in a basic medium using a reagent, such as hydrogen peroxide, which is catalysed by the iron of hemoglobin.7,10 Under these reaction conditions, fast and intense blue light is emitted. However, this method presented some limitations, such as the short duration of the luminescence, and it is subject to interferences, such as the surface on which the blood is present, the presence of metallic compounds, oxidant agents and cleaning products.10,11 These limitations underscore the need for improved presumptive tests and confirmatory methods to ensure accurate blood identification.11–13 In this context, chemometric methods combined with spectrometry data have been reported in the literature.12–18 By analysing spectral data with statistical tools, Zhang et al. was able to distinguish blood from other substances and even the influence of temperature and humidity on the aging process of bloodstains.12 Cassidy et al. reported the use of digital images and an algorithm developed in MATLAB for the identification of bloodstains in forensic analyses.13 Winnepenninckx et al. described a study in which infrared light images were able to detect bloodstains in 71.7% of the tested materials. In addition, the authors conducted a time-influenced study of bloodstain deposition for 24 h, 1 week and 1 month.17 The authors described that time had a weak effect on the ability of infrared light and visual light to detect latent bloodstains.17 These were examples of the use of digital image-based (DIB) approaches together with chemometric tools aiming to obtain a simple, practical, low-cost and accessible methodology.13–18 However, these reported methods were not able to discriminate animals from human blood and estimate the age of the blood, which is very important for forensic investigation. Thus, in this context, we developed for the first time in the literature a chemiluminescence method using luminol reagent and data obtained from videos generated by smartphones for further assistance with chemometric modeling for an accurate identification of human bloodstains as well as an estimation of the age of human blood in crime scenes. It is important to highlight that bovine liver blood (BLB) samples were used in datasets 1, 2, and 3, while human blood samples were exclusively used in dataset 4.2. Materials and methods
2.1 Sample preparation, reagents and apparatus
Luminol (PubChem CID 10638) was obtained from Sigma Aldrich (SãoPaulo, Brazil) and was prepared by mixing the two most common formulations, Weber and Grodsky,19–22 using sodium carbonate (PubChem CID 10340) and hydrogen peroxide (PubChem CID 740). Animal blood samples were obtained from bovine liver purchased from a supermarket in Recife-Brazil. Using commercially available bovine liver, which naturally retains residual blood, we were able to initiate the experimental phase since this material is not considered live animal experimentation. The liver was crushed in a blender with about 10 to 20 mL of physiologic saline solution or ultra-pure water, Direct-Q 3 UV Water Purification System (MilliQ, Merck), resistivity >18.2 Mohm.cm, and afterward, it was filtered using a filter paper to remove the pieces of meat and the fatty layer. A physiological solution was used to maintain the pH and electrolyte conditions of the blood.2,3 Human blood samples were collected by forensic experts at crime scenes using vacuum blood collection tubes. All human blood samples were handled by the Official Forensic Police in crime scenes, Dr Tadeu Morais Cruz, Headman from the Specialized Group in Forensic Expertise and Homicide, Department of Homicide and Protection of Persons from the Scientific Police from Pernambuco-Brazil. After the chemiluminescent reaction with luminol and acquisition of the digital video were performed, the blood was subsequently discarded. Thus, the blood samples were not collected invasively, and the samples could not be traced back to specific individuals, confirming that none of these samples could be linked to individual identities. The blood samples were dropped on different surfaces, such as cotton, glass, wood and ceramics, to check the effect of the surfaces commonly found in crime scenes on the chemiluminescent results.12,13 The volume of blood was measured from 10 to 50 μL.12,13,17 This procedure was carried out to reduce the volume of human blood and was handled by the official forensic police at the crime scene. All concentrations of the solutions and samples used were 6 times reduced compared to other works reported in the literature to generate less residue and to reduce the consumption of luminol.20,21 To evaluate the effect of substrates during the DIB method, different surfaces were tested. After this, several potential interferent materials were tested for the identification of blood, such as lipstick, red ink and ketchup.13,17 Finally, the nature of the blood and the estimation of age were evaluated. For this, 4 different sample sets were performed: (1) 48 samples of bovine liver blood (BLB) were deposited over cotton, glass, wood and ceramics, and 3 samples were used for each substrate. This data set was used to investigate whether the time decay of the luminescence may provide sufficient selectivity to the chemometric model to check the effect of the substrate on the detection of blood. (2) 19 samples consisting of 13 positive fresh and old BLB samples and 6 negative samples were ketchup, paint and lipstick. All these 19 samples were analysed on wood, cotton, ceramics and glass substrates. (3) 126 samples of BLB on cotton, ceramics, wood and glass substrates were submitted to environmental action, such as rain, sun, and dust, to simulate a blood that was ageing in a real crime scene. Moreover, this sample set was used to include variability in the model blood samples and was compared with the human blood collected by the scientific police at crime scenes. In this study, all 126 samples were kept for 90 days in a place outside the laboratory but in a safe place to avoid loss of experiments. (4) Diluted, 12 samples, and not diluted human blood, 12 samples, were used to check if this sample set is different from the 3 sample sets. To build this dataset, 50 μL of human blood was diluted in 20 mL of ultrapure water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ratio). This 1
400 ratio). This 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ratio was considered satisfactory by forensic experts who performed the studies with human blood collected in the crime scene as a good volume to study possible adulteration action.23,24 The last dataset consisted of aged blood, which was used to build a model to estimate the time of blood collection at the scene of the crime. A total of 36 human blood samples in different substrates were aged for 0, 7, 14 and 21 days and used to build prediction models capable of estimating blood aging. For the undiluted samples, 50 μL of human blood was used. For all datasets, the first fifteen frames of the video from the chemiluminescence reaction were used for chemometric modelling.
400 ratio was considered satisfactory by forensic experts who performed the studies with human blood collected in the crime scene as a good volume to study possible adulteration action.23,24 The last dataset consisted of aged blood, which was used to build a model to estimate the time of blood collection at the scene of the crime. A total of 36 human blood samples in different substrates were aged for 0, 7, 14 and 21 days and used to build prediction models capable of estimating blood aging. For the undiluted samples, 50 μL of human blood was used. For all datasets, the first fifteen frames of the video from the chemiluminescence reaction were used for chemometric modelling.
2.2 DIB method and data collection
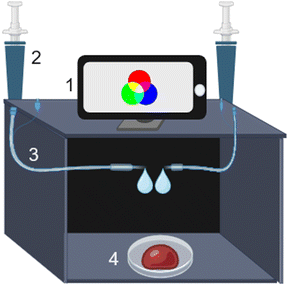
The videos from chemiluminescent reactions were recorded using a Galaxy Ultra S23 smartphone camera with a resolution of 8.3 MP that was coupled to a dark chamber made of cardboard containing 1 mL syringes and transparent polypropylene tubes 0.8 mm i.d to pump the reagents directly to the samples (Fig. 1). Moreover, because the luminol reagents containing hydrogen peroxide are unstable,25,26 this system allows the luminol reagent to be separated from the hydrogen peroxide using dedicated syringes that are simultaneously pressured only during the experiments. Moreover, the dark chamber isolates spurious radiation and collects the videos quickly, which is important for avoiding the loss of information. For this, the smartphone camera is coupled on top of the chamber with a small hole to record the luminescence of the samples. The videos were recorded in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 proportion at a 4K ultra-HD quality with 60 frames per second (fps) using 32 × 29 pixels for each frame. The effects of zoom and filters were not used. The chemiluminescent reaction was monitored for 3 min.
16 proportion at a 4K ultra-HD quality with 60 frames per second (fps) using 32 × 29 pixels for each frame. The effects of zoom and filters were not used. The chemiluminescent reaction was monitored for 3 min.
2.3 Data processing
After the sample was inserted into the DIB chamber (Fig. 1), the frames of the video were decomposed using ImageJ software and the Color Grab app. Thus, it is possible to obtain color values using different color systems, such as hue, saturation and value (HSV) and cyan, magenta, yellow, and key (CMYK). According to Fig. 2, it is possible to observe that the chemiluminescence of luminol in the presence of blood has a higher intensity in the blue channel, as expected.20 However, to obtain a high number of significant data points to generate better chemometric models, videos from the chemiluminescent reaction were recorded for 3 min, in which 35 frames were acquired for each experiment. These frames were decomposed using color models, and the data were used to build a more robust and reliable principal component analysis (PCA) model for identification.The tests were performed to create a bank of data for each sample under different real conditions to make the models robust and to discriminate accurately the blood in these conditions.
2.4 Potential interferents for blood sample analysis by DIB
Potential interferents for blood sample analysis was analyzed on different substrates: glass, cotton, ceramic, and wood surfaces (Fig. S1†). These substrates are some of the common materials where blood can be found in crime scenes.12,13,17 Moreover, other stains generated by ketchup and red ink are tested as potential interferers12,13,17 (Fig. S2 and S3†).2.5 Chemometric analysis
For the first dataset, RGB data were utilized to detect the presence of blood. The data were autoscaled, and a robust PCA model was applied to identify patterns within the samples that could help check the effect of the substrate on the chemiluminescence reaction. In the second dataset, fifteen frames were individually evaluated using robust PCA, with the autoscaled values of Red, Green, and Blue from the RGB model. Additionally, the Hue (H), Saturation (S), and Value (V) from the HSV model were tested, together with the cyan, magenta, yellow, and key (black) values from the CMYK model. Fifteen models were constructed to determine the effect of each substrate on blood identification.27–31 The third dataset involved recording more samples using the same approach as the second dataset. However, predictive models were developed only after a significant number of samples were analyzed, allowing the models to capture variance and build predictive accuracy. For this dataset, a low-variance filter was applied, indicating that the Y scale should be excluded owing to its lack of significant variation across samples. The remaining data were autoscaled and partitioned into training and test sets using the Kennard-Stone algorithm separately for each class.25–28 The training and test sets contained 70% and 30% of the samples, respectively, for each substrate (class). Following this, Partial Least Squares Discriminant Analysis (PLS-DA), Successive Projections Algorithm combined with Linear Discriminant Analysis (SPA-LDA) and Genetic Algorithm-Linear Discriminant Analysis (GA-LDA) were employed to construct the predictive models.27–31 Two approaches were used to build the predictive models. The first approach involves using all frames and color channels, allowing the algorithm to select the features that best distinguish the substrates. The second approach utilized only the frames and color channels identified as the most effective during the second dataset analysis. To evaluate the efficiency of all models, a contingency matrix was used to calculate classification metrics such as accuracy, sensitivity, and specificity.14 Finally, calibration prediction models were used to model aged human blood samples and to predict the time of blood collection. For this, PLS, and MLR (Multivariate Linear Regression) with SPA and GA external variable selection were used to build the prediction models. All chemometric analyses were performed using MATLAB and two chemometric web applications compiled in R version 4.3.1.14,27–313. Results and discussion
3.1 Chemometric analysis of the first dataset
The first dataset was used to investigate the presence of blood and differentiate it from false positives, such as lipstick, red ink and ketchup. Additionally, various substrates, including paper, ceramics, cotton, wood, and glass, were tested to determine whether the luminol chemiluminescent reaction contained sufficient information to distinguish blood regardless of the substrate used. For this initial analysis, no chemometric modeling was required, as the blue channel from the RGB system showed a significant difference between the true positive (BLB) and false positive samples, as illustrated in Fig. 3. A threshold value of 8 on the blue channel was established to separate the two classes. This value corresponds to the background signal in the absence of a chemiluminescent reaction, typically representing a spurious signal.32 As expected, all false positive samples exhibited this threshold value or background value.32 The threshold of 8 on the blue channel was calculated similarly to discriminant models, with the distinction that it was applied to only diluted BLB samples for true positives. This adjustment was necessary because the variation in true positive samples was significantly higher than in true negative samples, which showed practically no variation.33,34 As shown in Fig. 3, the most intense luminescent signals were observed for BLB on cotton and ceramic substrates. Cotton, being a microfiber, retains more BLB than wood, while ceramics, being more porous than glass, also retain more BLB on their surfaces. When comparing fresh with aged BLB (90 days), no significant difference in signal intensity was observed. The literature supports the idea that chemiluminescent methods based on luminol can detect blood even months or years after deposition.35–38 The catalytic role of Fe2+ or Fe3+ in the reaction can explain these results.35 Although the analysis of the single blue channel provided satisfactory results, further exploration of the full RGB data, along with other color models, such as CMYK and HSV, and more sophisticated chemometric models, was conducted in subsequent studies. The chemiluminescence reaction emitting blue radiation makes it obvious that this channel is important for further experiments. These efforts aim to extract additional information, such as discriminating between fresh and aged BLB, and obtaining other forensically relevant insights. According to the data, liver-residual blood (BLB) after simple filtration presented a uniform material that was useful for the objectives of the digital imaging-based approach. | ||
| Fig. 3 Two-dimensional plot of the blue channel of the RGB model versus the samples. The first six red circles represent the false positive samples and the thirteen blue circles represent the true positive samples. The subscripts “P” and “N” indicate positive and negative samples, respectively, and the numbers “1” and “2” indicate replicate samples under the same conditions. Substrates with no BLB and with interfering substances, such as ketchup, are labeled as negative (N), as they produce signals equal to or close to zero. Substrates with bovine blood (BLB) are labeled positive (P). Blue positive samples with luminol 1 or luminol 2 correspond to two different formulations of luminol found in the literature.26 “Diluted” refers to a sample of diluted BLB, and “Control” represents a sample containing only Fe3+ (0.0713 mol L−1) solution. “Fresh” and “Aged” refer to fresh and aged BLB samples, respectively, which are prepared for all substrates. | ||
3.2 Chemometric analysis of the second dataset
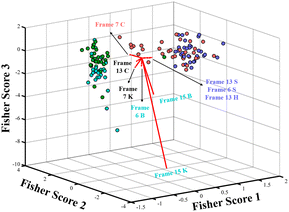
By visually inspecting Fig. 3, it was possible to identify samples containing blood; however, distinguishing between different substrates, such as cotton and ceramics, using only the blue channel was not possible using univariate approaches. Thus, the next step involved analyzing the data using a video-based approach, in which frames extracted from the video were processed using different color models, including CMYK and HSV, combined with chemometric modeling. This approach, which integrates digital video with chemometric modeling, was applied to enhance the selectivity of the luminol chemiluminescence analysis and to enable the simultaneous identification of blood for each substrate. In this experiment, blood from BLB was deposited onto cotton, ceramics, glass and wood to check if the substrate makes blood identification difficult. The reactions were conducted similarly to the first dataset; however, the reactions were carefully synchronized and recorded using digital video. It was observed that the highest intensity signal for the reaction occurred before frame number 15, as measured by the blue channel for all substrates, with data collected in triplicate. Additionally, data were acquired using the HSV and CMYK models. The results were organized into matrices, with samples arranged in rows and color channels in columns. Each frame's signals were organized into separate matrices for individual evaluation. A total of 15 frames were extracted from each 3-minute video.The 15 matrices were subjected to a low-variance filter, which indicated that the yellow channel from the CMYK model could be removed, as it did not exhibit significant variance useful for distinguishing the substrates. This result is expected because, according to Fig. 1, the yellow color is a combination of the red channel, and its color value is not significant.16,38 Subsequently, a robust principal component analysis (RobPCA) model was calculated for each frame to identify which frame provided the greatest differentiation among substrates. As expected, no single frame perfectly separated all substrates, even when using nine color channels from the RGB, HSV and CMYK models. This is evident in the score plots displayed for frames 6, 7, 11, 13, and 15, as shown in Fig. S4 in the ESI† and for all frames in Fig. 4. These five frames were selected because they demonstrated the highest degree of substrate separation among the 15 frames analyzed. The data from these frames were then merged into a single row-wise augmented data matrix by unfolding them, a strategy commonly employed in low-level data fusion.27–31 The combined dataset from the fused frames was autoscaled, and a RobPCA was performed. Utilizing the information from these five frames, it became possible to distinguish between the substrates, as illustrated in Fig. 4. Furthermore, as shown in Fig. 4, frames 13 and 15 were particularly crucial for differentiating substrates containing BLB. This approach highlights the effectiveness of combining multiple frames and color models to improve the accuracy and selectivity of luminol chemiluminescence-based forensic analysis.38 This result was interesting and useful for forensic police because it was demonstrated that it is possible to detect blood in different substrates, and the type of substance does not affect the detection of blood.17
As shown in Fig. 4, the B, V and K channels from the RGB, HSV and CMYK models, respectively, correlate with the color range from uncolor (K channel from CMYK model) to a bright blue color that depends on the B and the V channels (RGB and HSV channels)16,38 correlate with the chemiluminescence luminol reaction. Thus, these channels are shown to be the most significant for chemometric models.33,34 However, to check the usefulness of data from the RGB, HSV and CMYK, all were used for further chemometric approaches.
3.3 Chemometric analysis of the third dataset
Supported by the results of the second dataset analysis, it was possible to identify that digital videos present selective information that can be accessed by chemometric analysis to check the impact of the substrates on blood detection. Thus, the third dataset was designed to build predictive models that can be used to identify blood samples deposited in different substrates. Therefore, 126 samples were divided into 88 samples to build the model (training set) and 38 samples to test the model's efficiency (test set). Three algorithms were used to build the model; the Partial Least Squares Discriminant Analysis (PLS-DA) is an algorithm extensively used on classification models for many purposes.28–31 Two variable selection algorithms were evaluated, the genetic algorithm (GA) and the successive projection algorithm (SPA), whose selection was optimized to build a linear discriminant analysis (LDA) model. Three modelling strategies were evaluated: (1) all the color channels and frames were row-wise augmented into a matrix with 150 variables (15 frames and 10 color channels); (2) for all the fifteen frames, only the B, V and K color channels presented higher variation for the samples of the second dataset; and (3) the same matrix was used for the second dataset analysis composed of nine color channels row wise augmented with five frames into a matrix with 45 variables, which were selected by individual analysis of RobPCA made for each frame separately. The first approach does not result in efficient prediction models, which were already expected for PLS-DA owing to the high similarity between the substrate profiles. It was initially expected that SPA and GA would select the color channels and frames that resulted in models with high efficiency. However, overestimated models were built for training sets even limiting the maximum number of variables to be selected, indicating that both algorithms fail to optimize the variables selected for LDA models. Table 1 displays the contingency matrices that contain prediction results of the built models for both the first and second approaches. All the models from both approaches exhibit overestimation when comparing the prediction efficiency from the training and test sets, which indicates that all the six models can be used for predictions. However, the training set accuracy increases when it goes from the second to the third approach for all models; this enhancement was from 65% to 77%, from 75% to 78% and from 77% to 80%, for PLS-DA, SPA-LDA and GA-LDA models, respectively. This indicates that the frame selection using visual inspection of the RobPCA scores with the second dataset is efficient in enhancing the efficiency of all models. By inspection of Table 1 results, it is possible to see that cotton and ceramic samples present a high degree of similarity. For instance, LDA models with both variable selection methods tend to incorrectly classify ceramic samples as cotton on both the training and test sets. This indicates that the model is well adjusted, but there is a high degree of similarity owing to overlapping between these classes, which can be visually observed in the 3D Fisher's biplot for the GA-LDA model, as presented in Fig. 5.| First approach | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Train set | R/Pa | Cotton | Ceramics | Wood | Glass | Test set | Cotton | Ceramics | Wood | Glass |
| PLS | Cotton | 13 | 6 | 2 | 1 | PLS | 2 | 8 | 0 | 0 |
| Ceramics | 3 | 19 | 2 | 0 | 0 | 10 | 0 | 0 | ||
| Wood | 2 | 4 | 8 | 7 | 0 | 2 | 5 | 2 | ||
| Glass | 0 | 2 | 2 | 17 | 0 | 0 | 0 | 9 | ||
| SPA-LDA | Cotton | 14 | 4 | 2 | 2 | SPA-LDA | 2 | 8 | 0 | 0 |
| Ceramics | 4 | 17 | 3 | 0 | 1 | 9 | 0 | 0 | ||
| Wood | 3 | 2 | 12 | 4 | 0 | 1 | 8 | 0 | ||
| Glass | 1 | 2 | 6 | 12 | 0 | 0 | 0 | 9 | ||
| GA-LDA | Cotton | 13 | 4 | 3 | 2 | GA-LDA | 2 | 8 | 0 | 0 |
| Ceramics | 4 | 18 | 2 | 0 | 1 | 9 | 0 | 0 | ||
| Wood | 3 | 2 | 12 | 4 | 1 | 4 | 3 | 1 | ||
| Glass | 1 | 2 | 3 | 15 | 0 | 0 | 0 | 9 | ||
| Second approach | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Train set | R/Pa | Cotton | Ceramics | Wood | Glass | Test set | Cotton | Ceramics | Wood | Glass |
| PLS | Cotton | 19 | 3 | 0 | 0 | PLS | 7 | 3 | 0 | 0 |
| Ceramics | 4 | 20 | 0 | 0 | 6 | 4 | 0 | 0 | ||
| Wood | 1 | 0 | 14 | 6 | 0 | 0 | 6 | 3 | ||
| Glass | 0 | 0 | 6 | 15 | 0 | 0 | 3 | 6 | ||
| SPA-LDA | Cotton | 20 | 1 | 1 | 0 | SPA-LDA | 10 | 0 | 0 | 0 |
| Ceramics | 11 | 12 | 1 | 0 | 9 | 1 | 0 | 0 | ||
| Wood | 1 | 0 | 11 | 9 | 0 | 0 | 4 | 5 | ||
| Glass | 0 | 0 | 5 | 16 | 0 | 0 | 3 | 6 | ||
| GA-LDA | Cotton | 18 | 4 | 0 | 0 | GA-LDA | 9 | 1 | 0 | 0 |
| Ceramics | 10 | 14 | 0 | 0 | 8 | 2 | 0 | 0 | ||
| Wood | 0 | 1 | 14 | 6 | 0 | 0 | 9 | 0 | ||
| Glass | 0 | 0 | 6 | 15 | 0 | 0 | 2 | 7 | ||
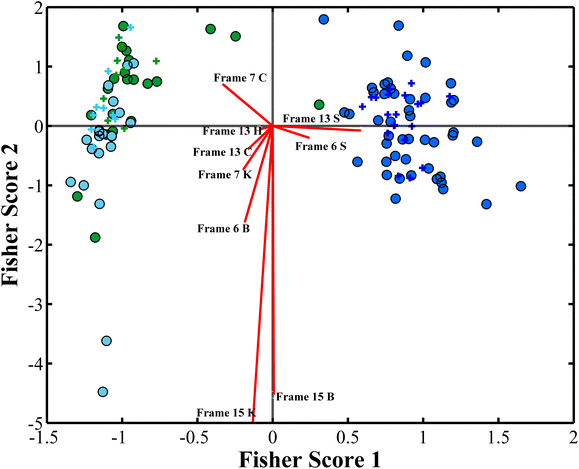
As illustrated in Fig. 5, the C channel (Cyan) from the CMYK model and H (Hue) and S (saturation) channels from HSV models presented useful importance variables together with the B channel, K channel and V channel previously discussed. Hue is the main color property used to distinguish between different colors, and saturation describes the intensity of a color in the HSV model. Thus, it makes sense that K, B and C are related to the color change from black (absence of signal) to blue color from the luminescence emission (Fig. 1), and H and S describe that the color and the intensity of this emission are important for the chemometric models.38 Wood and glass present similar signals; however, the LDA model identifies slight differences between them, especially for frame 15 on both blue (B) and black (K) color channels, as shown in Fig. 5. A slight difference between the wood and glass profiles can also be observed at the blue (B) channel at frame 6, as shown in Fig. 5. A slight difference in frame 7 C channel (Cyan) can be observed for ceramics that display a slightly higher signal when compared to cotton samples, as shown in Fig. 5. These results indicate that digital videos enable the differentiation of the substrate where blood samples were deposited. Frames 7, 13 and 15 were particularly important to distinguish the substrates. The GA-LDA model, which presents a higher accuracy rate for prediction (81%), does not efficiently distinguish cotton from ceramic samples using digital videos. Therefore, a new LDA model was built using the same optimized variables of the GA-LDA model but grouping ceramics and cotton as a single class. As a new LDA model was built, slight differences were obtained for the prediction once the threshold limit between classes was estimated again. The results of this new model can be observed in the contingency matrices for the training and test sets, as illustrated in Table 1. The biplot graph analysis remains the same; however, for three classes, it is now a two-dimensional plot, as shown in Fig. 6. The new GA-LDA model presented an accuracy of 90.30%, committing only 4 prediction errors, which was an outstanding classification for this study, resulting in 100% of sensitivity and specificity for class cotton + ceramics, and 77.78% of sensitivity and 93.10% of specificity for both wood and glass classes, respectively.
Fig. 6 shows that cotton + ceramics, wood and glass were in blue, green and cyan circles, respectively. The red lines indicate the loadings for the variables used in the model. These results show that the described method does not present significant differences between the cotton and ceramic substrates and that the differences between wood and glass were not so high. The method described in this study can be used as an alternative method in the forensic field, which significantly leads to the discrimination of bloodstains. The method presents an excellent accuracy of 90.30%, committing only 4 prediction errors, which is an outstanding classification for this study resulting in 100% of sensitivity and specificity for class cotton + ceramics, 77.78% of sensitivity and 93.10% of specificity for both wood and glass classes, respectively, which indicates that the method developed in this study is reliable and inexpensive and can perform quick analysis. Table 1 displays the model prediction of the first, second and third approaches. The results of this study demonstrate that the substrate in which blood is deposited does not significantly interfere with the identification of blood using the developed luminol chemiluminescence-based methodology. The analysis of digital video frames, combined with advanced chemometric modeling, consistently enabled the detection of blood across various substrates, including cotton, ceramics, wood, and glass. Although slight variations in signal intensity were observed between substrates, these differences were not substantial enough to hinder the accurate identification of blood.17 The robustness of the method is further highlighted by its ability to distinguish blood even after environmental stress and dilution, as well as its consistent performance across different substrates. The minimal differences observed between substrates, particularly in key frames and color channels (e.g., frames 7, 13, and 15), confirm that the chemiluminescent signal from blood remains detectable and distinguishable regardless of the surface.
All classification models were trained and tested using datasets that included all substrates. No substrate was excluded from any model, ensuring that sample variability was preserved across all classification approaches and that comparisons among them were fair and representative. This partition was performed using the Kennard-Stone algorithm for stratified partitioning (70% training, 30% test) per substrate class. What differed between the models, as also explained in the methodology was not the sample set but rather the feature selection strategies. Specifically, variable (i.e., frame and color channel) selection was performed differently across the models. In one approach, all frames and color channels were considered, allowing the algorithms to select the most informative features. In the second approach, only the features identified as most effective in the second dataset (used for exploratory analysis) were used to guide model construction. This strategy aimed to evaluate the consistency of the initial exploratory analysis observations, which demonstrate that the model uses the information previously highlighted as important and the methodology reproducibility, as presented in Table 1.
3.4 Chemometric analyses of the dataset containing human blood
The dilution of the fresh human blood was made using 50 μL of human blood and 20 mL of ultrapure water, which represents the proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
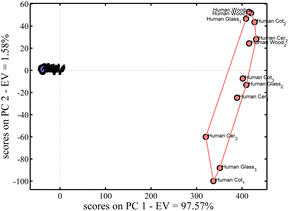
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400. Besides, these results show that even with a high dilution level,39–41 it is possible to detect and know that this blood is human. Relating that to a crime investigation, we can conclude that even when the suspect tries to wash or adulterate the crime scene, this method can detect blood traces in that crime scene.39–41 Then, these results highlight the simplicity and feasibility of implementing this method, which uses digital videos based on luminol chemiluminescent reaction for blood detection in crime scenes.38 With these results, it can be concluded that for human blood, the effect of the substrate is not important on blood identification; however, a visible difference can be observed in Fig. 7 between human (in red) and bovine (in blue-third dataset) blood. In bovine blood, the substrate could be distinguished, but this was not possible with human blood. Thus, the intensity of human blood covered the light difference between the signals of different substrates. Thus, this is a key and interesting finding: despite being deposited on different materials, human blood samples still form a cohesive group and remain clearly distinguishable from bovine blood. Thus, the variation is not enough to allow discrimination between substrates for human blood and is sufficient to differentiate it from animal blood, which are two important results of the present methodology.
400. Besides, these results show that even with a high dilution level,39–41 it is possible to detect and know that this blood is human. Relating that to a crime investigation, we can conclude that even when the suspect tries to wash or adulterate the crime scene, this method can detect blood traces in that crime scene.39–41 Then, these results highlight the simplicity and feasibility of implementing this method, which uses digital videos based on luminol chemiluminescent reaction for blood detection in crime scenes.38 With these results, it can be concluded that for human blood, the effect of the substrate is not important on blood identification; however, a visible difference can be observed in Fig. 7 between human (in red) and bovine (in blue-third dataset) blood. In bovine blood, the substrate could be distinguished, but this was not possible with human blood. Thus, the intensity of human blood covered the light difference between the signals of different substrates. Thus, this is a key and interesting finding: despite being deposited on different materials, human blood samples still form a cohesive group and remain clearly distinguishable from bovine blood. Thus, the variation is not enough to allow discrimination between substrates for human blood and is sufficient to differentiate it from animal blood, which are two important results of the present methodology.
Fig. 7 shows that in the PCA prediction, there is no clear differentiation between the blood and its substrates, but human blood has a distinct profile compared to animal blood. Fig. 8 shows the LDA model, which indicates that there is a significant difference between the human and bovine blood samples.
3.5 Chemometric analyses of human blood age
The ability to detect and analyze blood traces over time is critical for forensic investigations. In this study, 36 human blood samples in different substrates, such as cotton, ceramics, glass and wood, were subjected to luminol chemiluminescence DIB analysis across four-time intervals: 0 day (fresh blood), 7 days, 15 days, and 21 days. Chemometric tools, including PCA and PLS, were employed to evaluate the datasets, focusing on time-dependent changes and substrate variability. Fig. S5 in the ESI † displays the PCA analysis for the samples on 3 different day intervals categorized by days and substrates.For the analysis of Fig. S5 in the ESI material,† it is observed that the samples did not present substantial differences in signal for 0, 7 to 21 days. Therefore, regression supervised models were used to highlight the differences between these day intervals, aiming to develop a predictive model that can infer the number of days the samples were in contact with the substrate. It is important to highlight that when regression models were evaluated, the day zero (fresh blood) did not present any statistical difference from 7 days; therefore, the regression model was developed with the sample from 7 to 21 days. Thus, to avoid duplicate data without new information, which also prejudices the linear regression model owing to the lack of variance, day 0 data were not inserted into the model. This result is expected because the chemiluminescent signal continues at a very high intensity to detect the blood from fresh until the 7th day.40–43 By continuing the analysis, a full spectrum PLS (Partial Least Squares) regression and MLR (Multivariate Linear regression) models were evaluated: the latter one with SPA (Successive Projection Algorithm) and GA (Genetic Algorithm) external variable selection. The model that presented the lowest error was the MLR with GA external variable selection, which presented an RMSEP value of 3.6 and a regression coefficient (r) of 0.78, with a bias statistically equal to 0 confirmed by a t test. Fig. 9 displays the predicted versus the reference graph for the prediction of the samples with this model. It is possible to observe that with an error of 3.6 days, the difference between 7 and 15 days and from 15 to 21 days is not so pronounced, as presented by the error bars, which leads to an r value not so close to 1. The evaluation of the dataset and the reaction of the samples with luminol showed that cotton displayed signals for up to 21 days. However, wood showed no signal on day 15; wood, glass and the third ceramic samples exhibited no emission on the 21st day. To better understand why the signal with the samples with cotton remains until day 21 and the others do not, we examine their compositions and the effects of rain, temperature, humidity, and other factors.12,13,17 Moreover, cotton is composed of natural fibres with a highly porous structure allowing liquids to be absorbed and retained, which explains why the reaction is observed in 7, 15 and 21 days. Although also composed of cellulose, wood is denser and has a less porous structure, especially when treated or dried, which reduces its ability to retain liquids. Glass and ceramics are non-porous and hydrophobic materials, meaning that they do not absorb liquids. Any liquid in contact with these samples remains solely on the surface without being retained.44 By comparison with the literature, some authors have reported that it is possible to differentiate between fresh and older stains within a detection window of up to 30 days.45–48 According to the authors, the bloodstains aged up to 144 h (6 days) exhibited minimal changes in luminescence lifetime.46,47 Other authors successfully identified the age of the bloodstains using Multiple Linear Regression (MLR), Multiple Quadratic Regression (MQR), Support Vector Machine with radial kernel (SVMr), Support Vector Machine with polynomial kernel (SVMp), and Principal Components (PC), with SVMr showing the best result and an error of less than 3 days for 60% of the samples.47 All the experiments were conducted in a controlled environment, with no distinct experimental conditions, such as temperature, rainy days and humidity, which can alter the forensic analyses.47 In comparison with this result, our approach enables age estimation even when the bloodstain is older than 42 h, making it more accurate than the mentioned technique, and with higher robustness compared to other studies.46,47 Indeed, other authors reported that the time-influenced study of bloodstain until 1 month was not possible.17 Moreover, according to the forensic expert, a coauthor in this work (Tadeu Cruz), the detection window from 3 to 4 days for the identification and aging of the bloodstains is totally acceptable because in investigations involving violent crimes, a rapid analysis of evidence in the few moments is required, and this window found by our methodology aligns with the initial phase of an investigation because we can estimate the age of a blood with a precision of 3.6 days.45–47 Because of this, a wide interval of days was not used. Thus, our goal was to assess the feasibility of regression using simple, accessible digital tools, not to claim high-precision aging capabilities. Thus, the purpose of this regression model is to demonstrate that the chemiluminescent signal from luminol retains a measurable and systematic relationship with blood aging over time, even when using digital imaging. Additionally, other authors have reported studies addressing human vs. animal blood differentiation49 or human blood stain vs. substrates50 using spectroscopic techniques combined with chemometric tools. However, it is important to highlight that these studies employed hyperspectral imaging, which is a considerably more sophisticated and costly instrumentation compared to the smartphone-based methods proposed herein. The novelty of our study lies in demonstrating promising results for blood differentiation using digital images from a smartphone, which are far more accessible and practical for loco forensic applications, especially with limited resources owing to the low cost of the instrumentation used.
Supported by the results, we can confirm that the developed apparatus can perform in situ or in loco analyses directly on the substrate where blood is found, without extensive preparation and without the influence of the substrate on the detection capability of the analysis. By simply applying forensic reagents to the samples, it is possible to collect evidence and distinguish between human and bovine blood, as well as between positive and negative samples. Moreover, it is possible to estimate the age of the blood in a crime scene with a precision of 3.6 days. This highlights the efficiency of the new instrumentation, which, combined with innovative digital image video analysis and chemometric modeling, enables significantly faster analyses and real-time results51–55 Furthermore, the system allows continuous monitoring of the sample from the initial moment until forensic reagents are added, thereby preventing information loss during the process. All analytical models were developed following statistical modeling standards and machine learning principles and optimized using artificial intelligence tools to ensure accuracy and reliability.
It is important to highlight that based on the Brazilian Code of Criminal Procedure (article 159)56 and Law No. 12,830/2013,57 when a blood sample is collected at a crime scene, the activities carried out do not fall into the category of research involving human beings. Therefore, such activities do not require submission to a Research Ethics Committee. Furthermore, all blood samples were handled by the Official Forensic Police, Dr Tadeu Morais Cruz, Headman from the Specialized Group in Forensic Expertise and Homicide, Department of Homicide and Protection of Persons (co-author of this work) from Pernambuco-Brazil. Additionally, the entire collection procedure was carried out with great care, using personal protective equipment, as recommended by the practice guide for the forensic approach. Moreover, the blood samples were not collected invasively, and the samples could not be traced back to specific individuals, confirming that none of these samples could be linked to individual identities.
4. Conclusions
On the development of a new method to improve the identification of bloodstains by chemiluminescence, in this work, we introduce, for the first time, a new method based on the DIB method, called CDIB (chemiluminescence digital image-based method), using a dark chamber, a smartphone and low-level data fusion chemometric techniques to improve the presumptive method using luminol. The method demonstrated excellent accuracy, achieving 90.30% with only four prediction errors, making it an outstanding classification tool for this study. This resulted in 100% sensitivity and specificity for the cotton + ceramics class, and 77.78% sensitivity and 93.10% specificity for both the wood and glass classes. Thus, the method demonstrated robustness by showing no significant differentiation of blood based on the substrate, making it reliable for analysing bloodstains across various surfaces, such as cotton, ceramics, wood, and glass. Moreover, the method effectively distinguished human blood from animal blood, even when human blood was diluted to simulate attempts to conceal crime scenes through washing. Additionally, the model can estimate the age of bloodstains with a precision of 3.6 days, further enhancing forensic utility. Thus, these results indicate that the developed method is reliable and cost-effective and can deliver rapid analyses. Although our data results are preliminary, this method is sufficient for use as a forensic alternative in the initial stages of blood identification. Furthermore, this work highlights the possibility for forensic experts to have a trustworthy and reliable tool to analyse blood directly at a crime scene. This new method also aims to reduce the use of reagents by avoiding residues and generating less waste since a minimum amount of blood is required to make the detection.Data availability
The data supporting this article have been included as part of the ESI.† All data used for the chemometric models are available.Author contributions
Thomas F. F. T. dos Santos: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, and writing – review & editing. José R. S. Junior: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, and writing – review & editing. Licarion Pinto: conceptualization, data curation, investigation, methodology, software, supervision, validation, visualization, writing – original draft, and writing – review & editing. Tadeu Morais Cruz: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, and writing – review & editing. Jose Ailton M. Nascimento: data curation, investigation, methodology, validation, visualization, writing – original draft, and writing – review & editing. Severino Carlos B. Oliveira: data curation, investigation, methodology, validation, visualization, writing – original draft, and writing – review & editing. Vagner B. dos Santos: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources software, supervision, validation, visualization, writing – original draft, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE) (grants APQ-0942-1.06/22, APQ-0413-1.06/21, APQ-1050-1.06/24), the Conselho Nacional de Ciência e Tecnologia (CNPQ) (grants CNPQ 421147/2018-0, 441109/2023-3, 308422/2023-6), the CAPES scholarship 88887.483445/2020-00, and the Fundação de Amparo à Pesquisa no Rio de Janeiro (FAPERJ grant E26/200.204/2023) for the JCNE research scholarship. The authors also thank the Specialized Forensic and Homicide Group, Department of Homicide and Personal Protection, Recife, Brazil.References
-
R. Saferstein, Criminalistics: An Introduction to Forensic Science, Pearson Education, 13th edn, 2021 Search PubMed
.
-
J. E. Hall, Guyton and Hall Textbook of Medical Physiology, Elsevier, Philadelphia, 13th edn, 2016 Search PubMed
.
- S. Gariglio, M. C. David, A. Mattia, F. Consalvo, M. Scopetti, M. Padovano, S. D'Errico, D. Morena, P. Frati and A. Santurro, Toxics, 2024, 12, 670, DOI:10.3390/toxics12090670
.
- P. W. Buehler, F. D'Agnillo and D. J. Schaer, Trends Mol. Med., 2010, 16, 447–457, DOI:10.1016/j.molmed.2010.07.006
.
- C. Cuttaia, B. Di Stefano, C. S. Sorçaburu, R. Vetrini, C. Previderè and P. Fattorini, Separations, 2024, 11, 66, DOI:10.3390/separations11030066
.
- A. Kumar and J. Singh, Krishi Vigyan, 2020, 1, 5–9 Search PubMed
.
- F. Barni, S. W. Lewis, A. Berti, G. M. Miskelly and G. Lago, Talanta, 2007, 72, 896–913, DOI:10.1016/j.talanta.2006.12.045
.
- K. Virkler and I. K. Lednev, Forensic Sci. Int., 2009, 188, 1–17, DOI:10.1016/j.forsciint.2008.08.004
.
- Y. S. El-Sayed, O. I. Mohamed and K. M. Ashry, Forensic Sci. Med. Pathol., 2010, 6, 158–164, DOI:10.1007/s12024-009-9117-5
.
- M. Luedeke, E. Miller and J. E. Sprague, Forensic Sci. Int., 2016, 262, 156–159, DOI:10.1016/j.forsciint.2016.02.052
.
- M. N. Hochmeister, B. Budowle, R. Sparkes, O. Rudin, C. Gehrig, M. Thali, L. Schmidt, A. Cordier and R. Dirnhofer, J. Forensic Sci., 1999, 44, 597–602 CrossRef CAS PubMed
.
- R. Zhang, P. Wang, J. Chen, Y. Tian and J. Gao, Spectrochim. Acta Part A Mol. Spectrosc., 2023, 290, 122284 CrossRef CAS PubMed
.
- B. M. Cassidy, Z. Lu, J. P. Martin, S. K. Tazik, K. W. Kellogg, S. A. DeJong, E. O. Belliveau, K. E. Kilgore, S. M. Ervin, M. Meece- Rayle, A. M. Abraham, M. L. Myrick and S. L. Morgan, Forensic Sci. Int., 2017, 278, 396–403 CrossRef CAS PubMed
.
- L. P. da Silva, L. R. Brito, R. B. de Souza, C. F. P. M. Filho, V. B. dos Santos and L. Pinto, Forensic Chem., 2024, 35, 100563, DOI:10.1016/j.forc.2024.100563
.
- B. Li, X. L. Zhang, L. Y. Zhang, T. T. Wang, L. Li, C. G. Wang and Z. M. Su, Dyes Pigm., 2016, 134, 388–395, DOI:10.1016/j.dyepig.2016.07.014
.
- C. R. S. Oliveira, W. T. Suarez, N. Dos Anjos, R. Helena, F. J. N. Moreira, J. P. B. De Almeida and V. B. dos Santos, Rev. Virtual Quim., 2023, 15, e20230060, DOI:10.21577/1984-6835.20230060
.
- A. Winnepenninckx, E. Verhoeven, S. Vermeulen and B. Bekaert, Forensic Sci. Int., 2022, 331, 111167, DOI:10.1016/j.forsciint.2021.1111671
.
- M. de, O. K. Franco, W. J. Cardoso, C. B. Vilanculo, V. B. dos Santos, J. P. B. de Almeida, L. F. Capitán-Vallvey and W. T. Suarez, Anal. Methods, 2023, 15, 2896–2903, 10.1039/d3ay00311f
.
- S. Y. Lee, Y. I. Seo, B. S. Moon, J. P. Kim, J. M. Goh, N. K. Park and S. H. Shin, Forensic Sci. Int., 2020, 311, 110461, DOI:10.1016/j.forsciint.2020.110461
.
- K. Weber, Dtsch. Z. für Gesamte Gerichtl. Med., 1966, 57, 410–423, DOI:10.1007/BF00583303
.
- M. Grodsky, K. Wright, P. L. J. Kirk and J. Crim, Law, Criminol., 1951, 42, 95–103, DOI:10.2307/1140307
.
- I. Quinones, D. Sheppard, S. Harbison and D. Elliot, Can. Soc. Forensic Sci. J., 2007, 40, 1–14, DOI:10.1080/00085030.2007.10757151
.
- S. Hayashi, E. Kakizaki, A. Sonoda, N. Shinkawa, T. Shiragami and N. Yukawa, Forensic Sci. Int., 2019, 301, 195–200, DOI:10.1016/j.forsciint.2019.04.007
.
- V. Brenzini and R. Pathak, Forensic Sci. Int., 2018, 290, 178–185, DOI:10.1016/j.forsciint.2018.04.043
.
- C. He, Z. Luo, L. Zhang, Q. Zhang, C. He and X. Ren, Appl. Catal., A, 2024, 645, 119803, DOI:10.1016/j.apcata.2024.119803
.
- I. L. de Mattos, K. A. Shiraishi, A. D. Braz and J. R. Fernandes, Quim. Nova, 2003, 26, 373–380, DOI:10.1590/S0100-40422003000300015
.
- E. Acar, M. A. Rasmussen, F. Savorani, T. Næs and R. Bro, Chemom. Intell. Lab. Syst, 2013, 129, 53–63, DOI:10.1016/j.chemolab.2013.06.006
.
- E. Borràs, J. Ferré, R. Boqué, M. Mestres, L. Aceña and O. Busto, Anal. Chim. Acta, 2015, 891, 1–14, DOI:10.1016/j.aca.2015.04.042
.
- M. Hubert, P. J. Rousseeuw and K. Vanden Branden, Anal. Methods, 2017, 9, 2007–2013, 10.1039/C6AY03099H
.
- R. W. Kennard and L. A. Stone, Technometrics, 1969, 11, 137–148, DOI:10.1080/00401706.1969.10490666
.
- S. F. C. Soares, A. A. Gomes, M. C. U. de Araujo, A. R. Filho and R. K. H. Galvão, TrAC, Trends Anal. Chem., 2013, 42, 84–98, DOI:10.1016/j.trac.2012.09.006
.
-
M. Cocchi, A. Biancolillo and F. Marini, in Comprehensive Analytical Chemistry, Elsevier, 2018, vol. 82, pp, 265–299, DOI:10.1016/bs.coac.2018.08.006
.
- F. C. P. Ribeiro, A. S. Oliveira, A. Alisson, W. Marinho, M. P. Schneider, L. Pinto and A. A. Gomes, Microchem. J., 2019, 147, 622–627, DOI:10.1016/j.microc.2019.03.087
.
- B. C. Darzé, I. C. A. Lima, L. Pinto and A. S. Luna, Chemom. Intell. Lab. Syst., 2022, 231, 104696, DOI:10.1016/j.chemolab.2022.104696
.
- B. C. Darzé, I. C. A. Lima, L. Pinto and A. S. Luna, Chemom. Intell. Lab. Syst., 2023, 237, 104810, DOI:10.1016/j.chemolab.2023.104810
.
- E. K. N. da Silva, V. B. dos Santos, I. S. Resque, C. A. Neves, S. Moreira, G. C. Moreira, M. O. K. Franco and W. T. Suarez,
et al.
, Microchem. J., 2020, 158, 104986, DOI:10.1016/j.microc.2020.104986
.
- H. Z. W. Grotto, Rev. Bras. Hematol. Hemoter., 2010, 32, 1–10, DOI:10.1590/S1516-84842010005000050
.
- J. P. B. de Almeida, T. F. F. T. dos Santos, J. R. Sabino Júnior, E. V. F. do Amaral, C. R. S. Oliveira, M. V. Maia, W. T. Suarez, L. B. Ayres, C. D. Garcia and V. B. dos Santos, Anal. Methods, 2025, 17, 916–938, 10.1039/d4ay02097a
.
- I. Mustafa and T. A. Q. Hadwan, J. Lab. Physicians, 2020, 12, 244–249, DOI:10.1055/s-0040-1721156
.
- O. J. C. Soares, G. A. Silva, R. d. M. Macêdo, D. E. L. Lhama, V. Vescovi, R. J. A. do Nascimento, W. S. de Alencar, A. C. T. e Silva, J. A. S. de Sá, R. S. de Araújo and F. C. L. Ferreira, Res. Soc. Dev., 2022, 11, e38997, DOI:10.33448/rsd-v11i17.38997
.
- R. C. Douglas, Braz. J. Forensic Sci. Med. Law Bioeth., 2017, 6, 178, DOI:10.17063/bjfs6(2)y2017178
.
- M. Geraldo, P. Washington, R. Angela and S. Valéria, Rev. Bras. Crim, 2016, 5(1), 14–17, DOI:10.15260/rbc.v5i1.119
.
- A. V. da Silva, B. R. M. do Rêgo, M. F. S. Bezerra, R. L. Resque and M. R. F. Gomes, Braz. J. Dev., 2021, 7, 20368–20385, DOI:10.34117/bjdv7n2-603
.
- T. C. de Goede, A. M. Moqaddam, K. C. M. Limpens, S. A. Kooij, D. Derome, J. Carmeliet, N. Shahidzadeh and D. Bonn, Phys. Fluids, 2020, 32, 22101, DOI:10.1063/5.0037123
.
- J. Shin, S. Choi, J.-S. Yang, J. Song, J.-S. Choi and H.-I. Jung, Sens. Actuators, B, 2017, 243, 221–225, DOI:10.1016/j.snb.2016.11.142
.
- S. M. Shine, K. Suhling, A. Beavil, B. Daniel and N. Frascione, Anal. Methods, 2017, 9, 2007–2013, 10.1039/C6AY03099H
.
- A. Marrone, D. La Russa, A. Montesanto, V. Lagani, M. F. La Russa and D. Pellegrino, Molecules, 2021, 26, 6272, DOI:10.3390/molecules26206272
.
- B. Sobac and D. Brutin, Phys. Rev. E, 2011, 84, 011603, DOI:10.1103/PhysRevE.84.011603
.
- J. F. Q. Pereira, M. F. Pimentel, R. S. Honorato and R. Bro, Chemometr. Intell. Lab. Syst., 2021, 210, 104253, DOI:10.1016/j.chemolab.2021.104253
.
- G. J. Edelman, T. G. van Leeuwen and M. C. Aalders, J. Forensic Sci., 2015, 60, S188, DOI:10.1111/1556-4029.12591
.
- V. B. dos Santos, E. F. S. Campos, J. P. B. de Almeida, W. T. Suarez, C. R. S. Oliveira and S. C. B. de Oliveira, Microchem. J., 2023, 189, 108508, DOI:10.1016/j.microc.2023.108508
.
- L. P. S. Benedetti, V. B. dos Santos, T. A. Silva, E. Benedetti- Filho, V. L. Martins and O. Fatibello-Filho, Anal. Methods, 2015, 7, 4138, 10.1039/C5AY00529A
.
- J. P. B. de Almeida, V. B. dos Santos, G. A. do Nascimento, W. T. Suarez, W. M. de Azevedo, A. F. Ferreira and M. V. Maia, Anal. Methods, 2022, 14, 2631–2641, 10.1039/D2AY00678B
.
- N. Phuangsaijai, J. Jakmunee and S. Kittiwachana, J. Anal. Sci. Intell. Lab. Syst., 2023, 237, 104810 (
J. Anal. Sci. Technol.
, 2021
, 12
, 19
) CrossRef
.
- C. S. Silva, M. F. Pimentel, R. S. Honorato, C. Pasquini, J. M. Prats-Montalban and A. Ferrer, Analyst, 2014, 139, 5176–5184, 10.1039/C4AN00961D
.
- National health council from Brazil, https://www.gov.br/conselho-nacional-de-saude/pt-br/acesso-a-informacao/atos-normativos/resolucoes/2012/resolucao-no-466.pdf/view.
- Federal Government of Brazil, https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2013/lei/l12830.htm.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ay00633c |
| This journal is © The Royal Society of Chemistry 2025 |