Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in pharmacokinetics approach for herbal medicine
Kunming Zhanga,
Guangli Yana,
Aihua Zhanga,
Hui Suna and
Xijun Wang *ab
*ab
aSino-America Chinmedomics Technology Collaboration Center, National TCM Key Laboratory of Serum Pharmacochemistry, Chinmedomics Research Center of State Administration of TCM, Pharmacokinetics Laboratory, Laboratory of Metabolomics, Department of Pharmaceutical Analysis, Heilongjiang University of Chinese Medicine, Heping Road 24, Harbin 150040, China. E-mail: xijunwangls@126.com; Fax: +86-451-82110818; Tel: +86-451-82110818
bState Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Avenida Wai Long, Taipa, Macau, China
First published on 1st June 2017
Abstract
Traditional Chinese Medicine (TCM), an indispensable part of herbal medicine, has been used for treating many diseases and/or symptoms for thousands of years. As we know, the main active components of TCM can account for its therapeutic effects. However, rational use of TCM faces a series of obstacles due to a large diversity of species and inaccurate knowledge of the active components. In recent years, more and more applications of new technologies or methodologies for investigating the active components of TCM have provided us with much additional information on active substances. Pharmacokinetics is an effective tool which can be used to investigate the many components of TCM. A pharmacokinetics approach reveals the dynamic processes of active components in vivo, including their absorption, distribution, metabolism, and excretion which offer guidance for clinical rational uses of TCM. Therefore, the objective of this paper is to review the current status of TCM, application of pharmacokinetics in investigating TCM, and emerging trends.
1. Introduction
Traditional Chinese Medicine (TCM), an indispensable part of herbal medicine, can be defined as the utilization of herbs, animals, and minerals, for prevention and treatment of various diseases or symptoms under the guidance of TCM theory; thus, TCM has been gradually accepted and employed in the world. In 2015, Dr Tu, a Nobel Prize winner in physiology and medicine, attracted more attention of the world to TCM. Worldwide, TCM has been used for protecting the health of mankind for centuries. At present, TCM has effects on curing liver injury,1 chronic hepatitis B,2 experimental sepsis,3 cancer,4 periodontal pathogens,5 influenza A,6 gastric cancer cells,7 anti-NDV,8 gastrointestinal disorders,9 and diabetes mellitus10 in clinical practices. TCM efficacy relies on its bioactive constituents.11 However, the kinds of active components or effective fraction of TCM that act on therapeutic effects and dynamic processes of the active components of TCM in vivo are still unclear and ambiguous.Pharmacokinetics (PK), a new burgeoning technique, which is mainly used for investigating absorption,12–14 distribution,15 metabolism,16 and excretion17,18 of drugs in vivo, has been comprehensively applied to research the main active components of TCM. Currently, according to research data, PK coupled with other separation and identification techniques have important roles in screening the active components of TCM. Pharmacokinetic parameters, especially biological half time (T1/2),19–21 clearance (CL),22,23 area under concentration-time (AUC),24,25 etc., indicate the dynamic processes of active components of TCM in vivo. By comparing the pharmacokinetic parameters of the active components of TCM, we can know the characteristics of the active components in vivo. These basic findings will provide evidence for clinical rational and use of TCM.
By deeply investigating TCM, we have learned how active components contained in it exert their therapeutic effects. For example, artemisinin was used to protect against malaria.26 6,7-Dimethylesculetin, geniposide, and rhein were effective therapies against hepatic injury syndrome.27,28 Berberine was applied to treat nonbacterial prostatitis.29 In addition, we also understand the dynamic processes of active components of TCM in vivo from research using PK. For example, 5-hydroxymethyl-2-furoic acid, absorbed into blood from liu wei di huang wan, had rapid absorption and disposition processes, yet its elimination was slow in vivo.30 In order to enhance applications of PK with TCM, the goal of this article is to review the status of TCM, the application of PK on TCM, and to put forward PK application prospects with TCM.
2. The status of TCM
TCM, which possess a history of thousands years of application in clinical practice, is gaining more and more attention and respect in the world. With the development of TCM-based new drugs, treatment of complex diseases becomes more promising and realistic. However, a huge number of diseases have weakened human health. Fortunately, a large number of research publications in recent years indicate that the active components of TCM are becoming good choices for curing cancers and minimizing side reactions. For example, report results showed that Xiao-Ai-Tong inhibited pain and adverse reactions following morphine treatment for bone cancer pain.31 And bufalin, an effective component in Chansu, was considered as a potential anti-hepatocellular carcinoma therapeutic active component by means of inhibiting hepatoma cell proliferation, migration, invasion and adhesion.32 Subamolide A was used to treat human urothelial carcinoma in clinical practice.33 Also, Nakamura K. and his colleagues found that cordycepin, an active component of Cordyceps sinensis, has anticancer and anti-metastatic effects.34 Currently, breast cancer has become the secondary cause of cancer deaths among women, and approximately 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 women died of breast cancer in 2015.35 Fortunately, a lot of evidence shows that some active components of TCM will play an important role in treating breast cancer. Paclitaxel, a diterpenoid alkaloid, has been clinically used as therapy for breast cancer.36 Polygonatum odoratum extract has an effect on breast cancer by suppressing proliferation of breast cancer cells and inducing its apoptosis.37 According to the research of Shen K., cambogin, a bioactive component of Garcinia genus, also has a significant effect on breast cancer.38 At the same time, compound Kushen injection, as a candidate, was used to treat MCF-7 human breast cancer cells.39 Xue B., et al. found that anti-EV71 components can treat hand-foot-and-mouth disease which results from intestinal virus infection.40 Tetramethylpyrazine, an active component in Chuanxiong, might exert beneficial effects in primary open-angle glaucoma patients via regulating CXCR4 expression.41 Diammonium glycyrrhizinate liquor extract exerted a significant effect on hepatitis via its anti-inflammatory effects in clinical practice.42 And finally, Cortex Moutan showed important in vitro anti-diabetic effects via suppressing glucose uptake.43
450 women died of breast cancer in 2015.35 Fortunately, a lot of evidence shows that some active components of TCM will play an important role in treating breast cancer. Paclitaxel, a diterpenoid alkaloid, has been clinically used as therapy for breast cancer.36 Polygonatum odoratum extract has an effect on breast cancer by suppressing proliferation of breast cancer cells and inducing its apoptosis.37 According to the research of Shen K., cambogin, a bioactive component of Garcinia genus, also has a significant effect on breast cancer.38 At the same time, compound Kushen injection, as a candidate, was used to treat MCF-7 human breast cancer cells.39 Xue B., et al. found that anti-EV71 components can treat hand-foot-and-mouth disease which results from intestinal virus infection.40 Tetramethylpyrazine, an active component in Chuanxiong, might exert beneficial effects in primary open-angle glaucoma patients via regulating CXCR4 expression.41 Diammonium glycyrrhizinate liquor extract exerted a significant effect on hepatitis via its anti-inflammatory effects in clinical practice.42 And finally, Cortex Moutan showed important in vitro anti-diabetic effects via suppressing glucose uptake.43
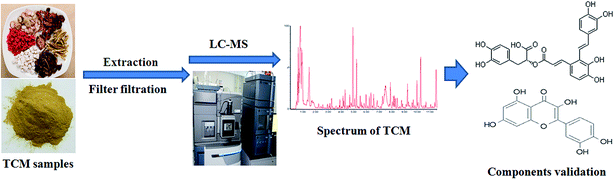
In recent years, the literature shows that identification of the components of TCM has made great progress, which should help to guide clinical rational drug use of TCM. Application of some analytical technologies makes an investigation of the components of TCM more rapid and accurate. Recent research results indicate that a rapid, sensitive, and effective method, high-performance liquid chromatography [HPLC] combined with mass spectrometry [MS], is useful for screening and identifying the components of a formula (Fig. 1). A total of 169 compounds were simultaneously identified and 11 of them were confirmed by high-performance liquid chromatography combined with mass spectrometry [HPLC-MS] in Xiang-Sha-Liu-Jun-Zi-Jia-Jian granules.44 Twenty active components, originating from Zhi-Zi-Da-Huang decoction, were quickly determined using efficient liquid chromatography integrated with mass spectrometry.45 In what was the first time to identify chemical constituents in a Shenqi Fuzheng injection, 81 major water-soluble ingredients were identified or accurately characterized by ultra-fast liquid chromatography combined with electrospray ionization quadrupole time-of-flight mass spectrometry.46 Twenty-eight components, found in Tianma-Gouteng-Yin, were identified by UHPLC/Q-TOF-MS and HPLC-ELSD methods and 20 of them were quantified.47 Ten effective compounds in Dachaihu Granule were identified by high-performance liquid chromatography combined with a diode array detector.48 Zhu F. X., et al. used HPLC-MS to analyze the major constituent in a Danmu injection and the findings indicated that 11 compounds were identified.49 At the same time, another method was used to identify the main components in a formula; for instance, the active ingredients of Shixiao San were screened by endothelial cells.50
An increasing amount of evidence indicated that another technique for identifying or screening ingredients of a single herb also is making good progress. For example, two main components of the volatile oils from Pogostemon cablin were detected using gas chromatography-flame ionization (GC-FID).51 Eighteen compounds in the herb medicine Siegesbeckia pubescens were acquired and identified by column chromatography on silica gel.52 In accordance with the investigation of Chen P. Y., we came to the conclusion that eight compounds contained in Cinnamon were identified by liquid chromatography combined with quadruple time-of-flight mass spectrometry and principal component analysis.53 Three main anthraquinones in Cassiae Semen were found by principal components analysis which can provide a good reference for quality evaluating of Cassiae Semen medicinal materials.54 Additionally, four bound ligands were identified and screened from Radix astragali extract;55 incidentally, that was the first time five active triterpenoids which were contained in Rosa davurica Pall56 were identified. Xie had isolated and identified five flavonoid glycosides and two derivatives from Scorzonera austriaca Wild.57 And 36 components were confirmed by HPLC with DAD and MS.58 Forty-two compounds, derived from Lamiophlomis rotata, were identified by LC/Q-TOF-MS.59 Technologies used to analyze the components of TCM are listed in Table 1.
| Major components | Analytic system | Injection volume | Flow rate | Mobile phase | Technologies | Stationary phase | Prescription | Reference |
|---|---|---|---|---|---|---|---|---|
| a HPLC: high performance liquid chromatography; MS: mass spectrometry; UHPLC: ultra-fast liquid chromatography; Q-TOF: quadrupole time-of-flight mass spectrometry; ELSD: evaporative light scattering detection; DAD: diode array detector; C-BC: cell-based screening; GC: gas chromatography; FID: flame ionization detection; NMR: nuclear magnetic resonance spectrometry; HSCCC: high speed countercurrent chromatography; ESI: electrospray ionization mass spectrometry; PCA: principal component analysis; SGCC: silica gel column chromatography. | ||||||||
| Flavonoids, alkaloids, triterpenic acids, triterpene saponins, lactones, etc. | LTQ-Orbitrap MS | 10 μl | 1.0 ml min−1 | 0.1% formic acid in water and acetonitrile | HPLC-MS | Diamonsil C18, column (250 × 4.6 mm, 5 μm) | XiangShaLiuJunZiJiaJian granules | 44 |
| Iridoid glycosides, flavonoids, anthraquinones, annins. | ESI-Q-MS | 10 μl | 0.8 ml min−1 | Acetonitrile and 0.1% formic acid in water | LC-MS | Phenomenex kinetex C18 column (150 × 4.6 mm, 2.6 μm) | ZhiZiDaHuang decoction | 45 |
| Organic acids, amino acids, oligosaccharides, alkaloids, nucleosides, phenylpropanoids, etc. | ESI-Q-TOF-MS/MS | 5 μl | 0.2 ml min−1 | Methanol-water containing 0.1% formic acid | UFLC-Q-TOF-MS/MS | C18 reversed-phase column (2.1 mm × 100 mm, 1.8 μm) | Shenqi Fuzheng injection | 46 |
| Non-saccharide small molecule components, fructose, glucose and sucrose. | Q-TOF-MS, ELSD | — | 0.4 ml min−1 | 0.1% formic acid in water and 0.1% formic acid in ACN | UHPLC-Q-TOF-MS, HPLC-ELSD | Waters acquity BEH C18 column (2.1 × 100 mm, 1.7 μm) | Tianma-Gouteng-Yin | 47 |
| Paeoniflorin, aloe-emodin, rhein, emodin, chrysophanol, physcion, naringin, etc. | DAD | 10 μl | 1.0 ml min−1 | Acetonitrile and 0.2% acetic acid | HPLC-DAD | Kromasil C18 column (250 × 4.6 mm, 5.0 μm) | Dachaihu granule | 48 |
| Phenolic acid and phenol glycoside, iridous glycoside and glycoalkaloid | ESI-MS, DAD | 20 μl | 1.0 ml min−1 | Acetonitrile and water containing 0.1% formic acid | LC-DAD-ESI-MSn | Welch material XB-C18 (4.6 mm × 250 mm, 5 μm) | Danmu injection | 49 |
| Quercetin-3-O-(2G-α-L-rhamnosyl)-rutinoside, quercetin-3-O-neohesperidoside, etc. | Q-TOF-MS | — | 0.2 ml min−1 | 0.01% formic acid in water and 0.01% formic acid in methanol | C-BC, UHPLC-Q-TOF-MS | Zorbax Eclipse plus C18 column (100 mm × 2.1 mm, 1.8 μm) | Shixiao San | 50 |
| Patchouli alcohol, pogostone | Chemometric techniques | 1.3 ml min−1 | High-purity (99.99%) nitrogen | GC-FID | HP-5 capillary column (30 m × 0.25 mm, 0.25 μm) | Pogostemon cablin | 51 | |
| 3,4′-Dimethoxy quercetin, 3,3′,4′-trimethoxy quercetin, 3,3′-dimethoxy quercetin, etc. | NMR | — | — | — | CC | — | Siegesbeckia pubescens | 52 |
| Coumarin, cinnamaldehyde, cinnamyl alcohol, cinnamic acid, 2-hydroxycinnamaldehyde, etc. | Q-TOF-MS, PCA | 5 μl | 0.3 ml min−1 | Water containing 0.1% formic acid and acetonitrile containing 0.1% formic acid | LC-Q-TOF-MS | Agilent Poroshell 120 SB-C18 column (4.6 × 150 mm, 2.7 μm) | Cinnamomum cassia | 53 |
| Aurantio obtusin, rhein, aloe emodin, emodin, chrysophanol and physcion | PCA | 20 μl | 0.8 ml min−1 | Acetonitrile and 0.1% phosphoric acid | HPLC | Kromasil C18, column (4.6 mm × 250 mm, 5 μm) | Cassiae Semen | 54 |
| Genistin, calycosin-7-O-β-D-glucoside, ononin, formononetin | ESI-Q-MS | — | 1.0 ml min−1 | Water containing 0.4% v/v acetic acid and acetonitrile containing 0.4% v/v acetic acid | HPLC-MS | Waters, SunFire C18 column (250 mm × 4.6 mm, 5 μm) | Radix astragali | 55 |
| Triterpenoids | — | — | 1.2 ml min−1 | 0.05% phosphoric acid aqueous solution and acetonitrile | HPLC | Merges C18 column (250 × 4.6 mm, 5 μm) | Rosa davurica Pall. | 56 |
| Flavonoid glycosides and derivatives | HR-ESI-MS, NMR | — | 3.0 ml min−1 | Acetonitrile | SGCC, HPLC, NMR | Gemini C18 110A column (250 mm × 10.00 mm, 5 μm) | Scorzonera austriaca Wild | 57 |
| Flavonoids, methylapigenin-O-pentoside isomers | DAD -ESI-MS | — | 0.2 ml min−1 | Methanol and 0.2% formic acid | HPLC -DAD-MS | Agilent Eclipse XDB C18 column (50 mm × 2.1 mm, 1.8 μm) | Egyptian Carob | 58 |
| Iridoids, flavonoids, phenylethanoid glycosides | Q-TOF-MS | — | — | — | LC-Q-TOF-MS | — | Lamiophlomis rotata | 59 |
| Essential oils | MS | 10 μl | 1.0 ml min−1 | Helium | GC-MS | Hewlett Packard HP-20 M polyethylene glycol column (50 m × 0.2 mm, 0.2 μm) | Origanum vulgare L | 108 |
| Volatile oils, alkaloids and flavonoids | — | — | — | — | GC-MS, HPLC | — | Fructus Aurantii Immaturus | 109 |
| Lignans, flavones, triterpenoidsaponins, phenolic acids, and other constituents | ESI-MS | — | 0.4 ml min−1 | Acetonitrile with 0.1% formic acid and water with 0.1% formic acid | UPLC-MS | Waters, ACQUITY BEH C18 column (2.1 mm × 100 mm, 1.7 μm). | Acanthopanax senticosus | 110 |
| Lawsone, 2-methoxy-1,4-naphthoquinone | DAD-EI-MS, NMR | 20 μl | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ml min−1 ml min−1 |
Acetonitrile-2.5% aqueous acetic acid | HSCCC, HPLC, EI-MS, NMR | ZORBAX XDB-C18 column (150 × 4.6 mm, 5 μm) | Impatiens balsamina L. | 111 |
3. The advantages of pharmacokinetics
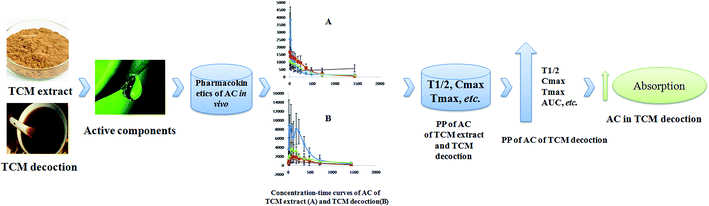
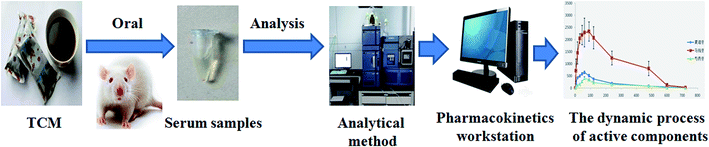
The application of PK on TCM has attracted more and more attention of researchers who are devoted to developing TCM. There are three advantages for applying PK on TCM. First, identifying and screening multi-components of TCM could clearly explain its effects. Wang X., et al. screened 9 compounds of Yin-Chen-Hao-Tang as the candidate components to explain Yin-Chen-Hao-Tang pharmacological effects via comparing the dynamic process of each composition in vivo.60 Ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rb3, and ginsenoside Rc, the potential bioactive components, contributed to the pharmacological effects of Nao Mai Tong formula from its pharmacokinetics behavior.61 Twenty-one primary compounds, the active fraction of the Xiao-Xu-Ming decoction, could exert anti-ischemic-stroke effects as determined by investigating their PK behaviors in plasma and brain.62 Second, clarifying and explaining the combination mechanism of active components in decoction (Fig. 2). The PK parameters of chrysophanol and physcion, the main effective compounds in Radixet Rhizoma Rhei and Dahuang Fuzi decoction, showed significant differences which were helpful to account for the combination mechanism of Dahuang Fuzi decoction.63 Haizao might increase the peak concentration of glycyrrhizic acid, which is contained in Gancao, by investigating PK profiles of different formulas of Haizao Yuhu decoction.64 By comparing the PK parameters of 10-deacetylbaccatin III, which is contained in taxane mixtures, Zhang X. et al. found that AUC0–U and concentrations of 10-deacetylbaccatin III were significantly increased and enhanced.65 Fan and his colleagues indicated that the absorption of liquiritigenin, isoliquiritigenin, glycyrrhizic acid, and glycyrrhetinic acid were enhanced after oral administration of Radix Glycyrrhizae and Ramulus Cinnamomi.66 The time of action of gastrodin could be prolonged in clinical studies by comparing its PK among different administered types of gastrodin.67 Third, showing and revealing the dynamic process of active components in vivo (Fig. 3). Some ingredients in Rhizoma chuanxiong and Radix puerariae remarkably influence plasma concentrations of ferulic acid and puerarin, which are the main effective ingredients in Nao-De-Sheng decoction (NDS). This result suggested that the ingredients in NDS enhanced the dissolution and absorption of ferulic acid and puerarin, delaying elimination.68 Three major bioactive components, typhaneoside, vanillic acid, and p-coumaric acid, had good absorption in vivo as discovered by investigating its C(max), T(max), T(1/2), and AUC(0∼t).69 Xiang-Fu-Si-Wu decoction essential oil/β-CD inclusion complex showed higher C(max), T(1/2), and larger AUC(0–24h) in vivo.70 Paclitaxe, the Taxus chinensis extract, might acquire higher blood concentration and its retention was remarkably improved.714. Current research of pharmacokinetics
4.1 Applications in single herb
With the development of PK, several literature references involved application of PK on components research of single herbs. In order to discover the reason that main components in single herb could cure diseases, comparing its PK parameters in vivo is a good choice of techniques. Wei B. et al. found that the absorption of six sedative and hypnotic lignans in an insomniac group were all incredibly higher than in a normal group by comparing the PK parameters of them.72 At the same time, that study also showed the six lignans were distributed mainly in the hypothalamus and a comparative study of the PK parameters of the six lignans indicated that the absorptions of them in the insomniac group were higher than in the normal group.73 After processing a single herb, the PK parameters of active components in TCM were changed. To compare PK parameters of ten alkaloids after oral administration of natural and wine-processed Rhizoma coptidis aqueous extracts, Qian XC drew the conclusion that the Cmax of coptisine and 8-oxocoptisine was enhanced as well as the AUC0–t of coptisine, palmatine, and 8-oxocoptisine; all were greatly increased after wine-processing.74 In addition, a huge number of research papers implied that the dynamic process of active components in a body will be exposed via its PK profile. In terms of the PK parameters of five active isoflavonoids, the active components of Radix Puerariae, Xiao B. X. and his colleague's research indicated that the isoflavonoids can quickly enter the brain and act on neuro-pharmacological activities.75 Columbianetin has rapid oral absorption, quick clearance, and good absolute bioavailability according to its PK properties.76 Nine of eleven alkaloids contained in Mahuang-Fuzi combination showed slower elimination by comparing their PKs in single-herb extracts.774.2 Applications for decoction
Decoctions (tang in Chinese) are frequently used as a basic herb–herb combination of Chinese formulas for achieving mutual reinforcement and decreasing adverse effects. Currently, many researchers found that investigating PK parameters of TCM could explain synergistic effects of herb medicine which was contained in a formula or recipe. According to the research of Liu R., berberine can prolong the elimination half-life of corynoline which was a component in Shuanghua Baihe tablets, and also increased its bioavailability.78 The Nao-De-Sheng decoction (NDS) could further improve ferulic acid and puerarin pharmacological potency in vivo by a PK study after oral administration of the monomer, medicinal substance aqueous extract, and NDS.79 Ganjiang may promote elimination of aconitine and hypaconitine and enhance the absorption of benzoylaconine, benzoylhypaconine, and benzoylmesaconine via comparing its PK after oral administration of Fuzi and Fuzi-Ganjiang aqueous extracts.80 The absorption of rhein was suppressed, and the time of rhein and emodin coming to their peak concentrations was delayed. Besides, the elimination of aloe-emodin and emodin was also found to be postponed in RPD via comparing the PKs of aloe-emodin, rhein, and emodin after oral administration of DaHuang-Mu-Dan-Tang (RPD) and rhubarb extracts.81 Additionally, some research results indicated that the active ingredients in decoction had no drug-interactions. For example, by comparing PK profiles of spinosin, mangiferin, and ferulic acid, which were the main active components in Suan-Zao-Ren decoction, it was seen that the PK parameters of ferulic acid were no different between these two groups.82 The applications of PK in TCM are listed in Table 2.| Name of plant | Model | Analytical method | Active components | Compartment model | Process | PK parameters | PK behavior | Reference |
|---|---|---|---|---|---|---|---|---|
| a T1/2: the half-time; Cmax: maximum plasma concentration; Tmax: time to reach the maximum concentrations; AUC0–t: area under concentration–time curve; C0: extrapolated plasma concentration at 0 min; Vdz/F: apparent volume of distribution; MRT: mean residence time. | ||||||||
| Schisandra chinensis | Insomnic | UFLC-MS/MS | Schisandrin, schisandrol B, schisantherin A, deoxyshisandrin, γ-schisandrin, gomisin N | Non-compartmental | DAS 2.1 | AUC, Cmax, T1/2, MRT, CLZ/F | The better absorption of the six analytes in model group | 72 |
| Rhizoma coptidis | Normal | UHPLC-ESI-MS/MS | Berberine, coptisine, palmatine, jatrorrhizine, epiberberine, magnoflorine, columbamine, noroxyhydrastinine, oxyberberine, 8-oxocoptisine | — | DAS 2.0 | T1/2, Cmax, Tmax, AUC0–t | Wine-processing did exert limited effects on the absorption of columbamine, noroxyhydrastinine, oxyberberine and 8-oxocoptisine | 74 |
| Pueraria lobata | Normal | UFLC-MS/MS | Puerarin, 3′-methoxypuerarin, 3′-hydroxypuerarin, daidzein, daidzein-8-C-apiosyl-(1-6)-glycoside | Non-compartmental | WinNonlin6.0 | Tmax, C0, AUC0–t, T1/2, etc. | Puerarin, 3′-methoxypuerarin, daidzein and daidzein-8-C-apiosyl-(1-6)-glycoside can quickly penetrate to the brain through the blood brain barrier | 75 |
| Angelica pubescens Maxim | Normal | HPLC | Columbianetin | Optimum compartment | DAS 1.0 | Cmax, V/F, T1/2 | Columbianetin has rapid oral absorption, quick clearance and good absolute bioavailability | 76 |
| Herba Ephedrae-Radix Aconiti Lateralis | Normal | UPLC-MS | Norephedrine, norpseudoephedrine, ephedrine, pseudoephedrine, methylephedrine, aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine and benzoylhypaconine | Non-compartmental | DAS 3.2 | Tmax, Cmax, AUC0–t, T1/2, etc. | Alkaloids (except methylephedrine, benzoylmesaconine and benzoylhypaconine) showed slower elimination | 77 |
| Corydalis bungeana Herba | Normal | LC-MS/MS | Corynoline | Non-compartmental | DAS 3.2 | Tmax, T1/2, MRT, AUC0–∞ | Shuanghua Baihe tablets prolonged the elimination half-life of corynoline and increased its bioavailability | 78 |
| Nao-De-Sheng decoction (NDS) | Normal | RP-HPLC | Ferulic acid, puerarin | Two-compartment | 3P97 | Tmax, Cmax, AUC0–t, AUC0–∞ | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Some ingredients in NDS may increase dissolution and absorption of ferulic Some ingredients in NDS may increase dissolution and absorption of ferulic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) andpuerarin, delay elimination, and subsequently enhance bioavailability of ferulic andpuerarin, delay elimination, and subsequently enhance bioavailability of ferulic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and and![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) puerarin puerarin |
79 |
| Radix Aconiti Lateralis | Normal | LC-MS/MS | Aconitine, hypaconitine, mesaconitine, benzoylaconine, benzoylhypaconine, benzoylmesaconine | — | 3P97 1.0 | T1/2, AUC0–t, Cmax, Tmax | Ganjiang could promote the elimination of aconitine and hypaconitine and enhance the absorption of benzoylaconine, benzoylhypaconine and benzoylmesaconine | 80 |
| Rhubarb peony decoction (RPD) | Normal | LC-MS | Aloe-emodin, rhein, emodin | — | — | T1/2, Cmax, Tmax | The absorption of rhein in rats was suppressed after oral administration RPD | 81 |
| Suan-Zao-Ren decoction (SZR) | Insomnic | UFLC-MS/MS | Spinosin, mangiferin, ferulic acid | Noncompartmental | DAS 2.1 | Tmax, Cmax, AUC0–t, T1/2 | The absorptions of spinosin and mangiferin in insomnic group were significantly lower than those in normal group | 82 |
Interestingly, one researcher used PK to study the components of formula on animal models. Results showed that the process of components of formula in vivo was significantly different by comparing PK parameters of the components of formula in normal and abnormal animal models. For example, the research of Liu Q. F. indicated that berberine as well as palmatine had higher uptake and slower elimination in rats with metabolic syndrome.83 Besides, plus–minus or absent–present herbs of formula could affect the dynamic process of components of formula in vivo by comparing PK parameters of these components in formula. For instance, in view of PK parameters of the main components after oral administration of a Gan-Sui-Ban-Xia Decoction plus–minus Gansui and Gancao anti-drug combination, the research of Zhang Y., et al. demonstrated that Gansui may lead to better absorption of glycyrrhizinic acid and liquiritin in Gancao, while Gansui and Gancao may lead to better and faster absorption of albiflorin, but worse absorption of paeoniflorin.84 By comparing the PK parameters of cisplatin in the absence or presence of zengmian yiliu granules (ZMYL), it was learned that ZMYL is a potential complementary and alternative medicine for cisplatin chemotherapy.85
In addition, the dynamic process of the main components of TCM in vivo would change via comparing its PK profile under the primary herb–herb combination or the formula. Meranzin hydrate and ferulic acid were absorbed and distributed rapidly in vivo by means of comparing PK parameters.86 Platycodonis radix could promote PK profiles of marker compounds which were contained in Shengxian decoction.87 Correspondingly, a Wuzhi capsule increased the mean plasma concentration of tacrolimus.88 In a Kushen–Gancao combination, the absorption of glycyrrhetinic acid was significantly lower than in the single herb.89 According to Hou and his team's research, the absorption of rhein was significantly enhanced both in herbal formulae and a single herbal extract over the pure compound in vivo.90 Radix Pueraria flavonoids were able to prolong the absorption of 1-deoxynojirimycin; however, it did not have an effect on the total amount of 1-deoxynojirimycin in vivo.91 In addition, the bioavailability of geniposide might be more enhanced and heightened in a single herbal extract and Gardenia herbal formulation than in its pure compound administration.92
5. Pharmacokinetics-pharmacodynamics
In early studies, the PK of TCM was just used to investigate absorption, distribution, metabolism, and excretion of active components contained in single or herb–herb combinations, as well as the interaction mechanism between components and formulas.93 Whereas the types of active components derived from single herb or complex formula were able to fully represent their pharmacological functions, this was still ambiguous and controversial. With the emerging pharmacokinetic-pharmacodynamic (PK-PD) model,94,95 we have a new tool to investigate TCM.Currently, the PK-PD model is comprehensively used for research with TCM; but it could further explain the compatibility mechanisms for formula and provide comprehensive information for clinical settings.96,97 What's more, increasing numbers of research reports have indicated that using the PK-PD model is a key to explain herb efficacy and herb–herb synergistic effects.98,99 A triptolide-loaded liposome hydrogel patch was able to treat rheumatoid arthritis based on its PK and pharmacodynamics study.100 The rhubarb–gardenia herb pair exerted enhanced hepatoprotective effects via a pharmacodynamic and PK study of five main chemical markers.101 Ren et al. revealed the mechanism of the anti-inflammatory activity of Huang-Lian-Jie-Du decoction via systematic PK and pharmacodynamic data of three major active constituents in Huang-Lian-Jie-Du decoction.102 Glycyrrhetinic acid combined with paeoniflorin, two primary active compounds in peony-liquorice decoctions, exerted a constant analgesic effect on dysmenorrhea.103 Zhan and his colleagues found that ginsenoside Rb1 coupled with schisandrin might delay the elimination of ginsenoside Rg1 and ginsenoside Rg1, Rb1, and schisandrin in a mixture displayed a synergistic effect on NO release.104 Rhein was able to influence the PK and pharmacodynamics of clozapine to reduce clozapine-induced constipation.105 And Yu and his team revealed the mechanism of borneol's ability to open the blood–brain barrier via its pharmacodynamics and PK research.106
6. Future perspectives
Rapid economic development and a rising focus on health in China has caused TCM to be noticed beyond that of Western countries.107 As we know, in order to guarantee the safety and effectiveness of TCM, research using pharmacokinetics is essential. Safety and effectiveness of TCM are key issues in investigating TCM. It is unrealistic that clinical rational use of TCM totally depends on pharmacokinetic parameters of active components that were derived from TCM. Usage and dosage of TCM must originate from a large exploration of clinical practices. Although the PK of TCM has solved some key problems with the application of TCM, it is still in an early stage of exploration. Currently, there are many issues with the PK of TCM and it still faces many new challenges. For example, the classic PK of TCM can't explain the overall concept of TCM. Therefore, the PK of TCM should be combined with other effective tools, such as network-pharmacology, network-pharmacy, and metabolic technology, to investigate TCM and provide effective and firsthand evidence for clinical rational use of it.7. Conclusion
TCM has a long history of protecting human health due to its effective constituents as well as satisfactory pharmacological activities. This review summarized the active components in a single herb or complex formula of TCM. We outlined applications of PK for screening and confirming the active fraction which exerts pharmacological effects of a single herb or a complex formula and explained the mechanisms and interactions of drug–drug or herb–herb via key active component's PK profiles. Additionally, this review also introduced PK and PD models in investigations of the interactions and pharmacological effects of active components contained in a single herb and in formula. We hope that this review will serve as useful guidance for further investigations of TCM.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81430093, 81373930, 81673586, 81302905), National Key Subject of Drug Innovation (Grant No. 2015ZX09101043-005, 2015ZX09101043-011), TCM State Administration Subject of Public Welfare of (Grant No. 2015468004), Specialized Research Fund for the Doctoral Program of Higher Education (20132327130001, 20122327120006), Application Technology and Development of Youth Talents Project in Harbin (2014RFQXJ116), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118).References
- X. Ma, J. H. Peng and Y. Y. Hu, Chinese Herbal Medicine-induced Liver Injury, J. Clin. Transl. Hepatol., 2014, 2(3), 170–175 Search PubMed.
- Z. Chen, X. Ma, Y. Zhao, J. Wang, Y. Zhang, Y. Zhu, L. Wang, C. Chen, S. Wei, Z. Yang, M. Gong, H. Shen, Z. Bai, Y. Guo, M. Niu and X. Xiao, Kushenin Combined with Nucleos(t)ide Analogues for Chronic Hepatitis B: A Systematic Review and Meta-Analysis, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 529636 Search PubMed.
- H. Sun, A. Zhang and X. Wang, Potential role of metabolomic approaches for Chinese medicine syndromes and herbal medicine, Phytother. Res., 2012, 26(10), 1466–1471 CAS.
- Y. H. Li, Y. B. Niu, Y. Sun, F. Zhang, C. X. Liu, L. Fan and Q. B. Mei, Role of phytochemicals in colorectal cancer prevention, World J. Gastroenterol., 2015, 21(31), 9262–9272 CrossRef CAS PubMed.
- S. Gunjal, A. V. Ankola and K. Bhat, In vitro antibacterial activity of ethanolic extract of Morus alba leaf against periodontal pathogens, Indian J. Dent. Res., 2015, 26(5), 533–536 CrossRef PubMed.
- J. H. Li, R. Q. Wang, W. J. Guo and J. S. Li, Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): A meta-analysis, J. Chin. Med. Assoc., 2016, 79(5), 281–291 CrossRef PubMed.
- J. Mu, T. Liu, L. Jiang, X. Wu, Y. Cao, M. Li, Q. Dong, Y. Liu and H. Xu, The Traditional Chinese Medicine Baicalein Potently Inhibits Gastric Cancer Cells, J. Cancer, 2016, 7(4), 453–461 CrossRef PubMed.
- Y. Jia, R. Xu, Y. Hu, T. Zhu, T. Ma, H. Wu and L. Hu, Anti-NDV activity of baicalin from a traditional Chinese medicine in vitro, J. Vet. Med. Sci., 2016, 78(5), 819–824 CrossRef CAS PubMed.
- R. Teschke, A. Wolff, C. Frenzel, A. Eickhoff and J. Schulze, Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders, World J. Gastroenterol., 2015, 21(15), 4466–4490 Search PubMed.
- L. He, H. Wang, C. Gu, X. He, L. Zhao and X. Tong, Administration of Traditional Chinese Blood Circulation Activating Drugs for Microvascular Complications in Patients with Type 2 Diabetes Mellitus, J. Diabetes Res., 2016, 2016, 1081657 Search PubMed.
- P. Liu, H. Yang, F. Long, H. P. Hao, X. Xu, Y. Liu, X. W. Shi, D. D. Zhang, H. C. Zheng, Q. Y. Wen, W. W. Li, H. Ji, X. J. Jiang, B. L. Zhang, L. W. Qi and P. Li, Bioactive equivalence of combinatorial components identified in screening of an herbal medicine, Pharm. Res., 2014, 31(7), 1788–1800 CrossRef CAS PubMed.
- H. Sun, F. Wu, A. Zhang, W. Wei, Y. Han and X. Wang, Pharmacokinetic study of schisandrin, schisandrol B, schisantherin A, deoxyschisandrin,and schisandrin Bin rat plasma after oral administration of Shengmaisan formula by UPLC-MS, J. Sep. Sci., 2013, 36(3), 485–491 CrossRef CAS PubMed.
- H. Sun, T. Dong, A. Zhang, J. Yang, G. Yan, T. Sakurai, X. Wu, Y. Han and X. Wang, Pharmacokinetics of hesperetin and naringenin in the Zhi Zhu Wan, a traditional Chinese medicinalformulae, and its pharmacodynamics study, Phytother. Res., 2013, 27(9), 1345–1351 CrossRef CAS PubMed.
- A. H. Zhang, H. Sun, Y. Han, G. L. Yan, Y. Yuan, G. C. Song, X. X. Yuan, N. Xie and X. J. Wang, Ultraperformance liquid chromatography-mass spectrometry based comprehensive metabolomics combined with pattern recognition and network analysis methods for characterization of metabolites and metabolic pathways from biological data sets, Anal. Chem., 2013, 85(15), 7606–7612 CrossRef CAS PubMed.
- Q. Yin, H. Sun, A. Zhang and X. Wang, Pharmacokinetics and tissue distribution study of scoparone in rats by ultraperformance liquid-chromatography with tandem high-definition mass spectrometry, Fitoterapia, 2012, 83(4), 795–800 CrossRef CAS PubMed.
- H. Chu, A. Zhang, Y. Han, S. Lu, L. Kong, J. Han, Z. Liu, H. Sun and X. Wang, Metabolomics approach to explore the effects of Kai-Xin-San on Alzheimer's disease using UPLC/ESI-Q-TOF mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1015–1016, 50–61 CrossRef CAS PubMed.
- H. Sun, W. Dong, A. Zhang, W. Wang and X. Wang, Ultra-performance liquid-chromatography with tandem mass spectrometry performing pharmacokinetic and biodistribution studies of croomine, neotuberostemonine and tuberostemonine alkaloids absorbed in the rat plasma after oral administration of Stemonae Radix, Fitoterapia, 2012, 83(8), 1699–1705 CrossRef CAS PubMed.
- H. Sun, Q. Yin, A. Zhang and X. Wang, UPLC-MS/MS performing pharmacokinetic and biodistribution studies of rhein, J. Sep. Sci., 2012, 35(16), 2063–2068 CrossRef CAS PubMed.
- M. H. Schultze-Mosgau, B. Schuett, F. T. Hafner, F. Zollmann, A. Kaiser, J. Hoechel and B. Rohde, Pharmacokinetics and safety of the selective progesterone receptor modulator vilaprisan in healthy postmenopausal women, Int. J. Clin. Pharmacol. Ther., 2017, 55(1), 16–24 CrossRef PubMed.
- S. R. Zhang, L. C. Zhu, Y. P. Jiang, J. Zhang, R. J. Xu, Y. S. Xu, B. Xia and S. L. Ma, Efficacy of afatinib, an irreversible ErbB family blocker, in the treatment of intracerebral metastases of non-small cell lung cancer in mice, Acta Pharmacol. Sin., 2017, 38(2), 233–240 CrossRef CAS PubMed.
- W. Dong, P. Wang, X. Meng, H. Sun, A. Zhang, W. Wang, H. Dong and X. Wang, Ultra-performance liquid chromatography-high-definition mass spectrometry analysis of constituents in the root of Radix Stemonae and those absorbed in blood after oral administration of the extract of the crude drug, Phytochem. Anal., 2012, 23(6), 657–667 CrossRef CAS PubMed.
- A. Zhang, H. Sun, X. Wang, G. Jiao, Y. Yuan and W. Sun, Simultaneous in vivo RP-HPLC-DAD quantification of multiple-component and drug–drug interaction by pharmacokinetics, using 6,7-dimethylesculetin, geniposide and rhein as examples, Biomed. Chromatogr., 2012, 26(7), 844–850 CrossRef CAS PubMed.
- S. Y. Byun, J. W. Jeong, J. H. Choi, K. P. Lee, H. Y. Youn, H. J. Maeng, K. H. Song, T. S. Koo and K. W. Seo, Pharmacokinetic study of meropenem in healthy beagle dogs receiving intermittent hemodialysis, J. Vet. Pharmacol. Ther., 2016, 39(6), 560–565 CrossRef CAS PubMed.
- S. Ren, R. Sechaud, Z. Su, H. C. Tillmann, H. Hara, X. Tan, J. Hou, R. Woessner and R. Zhao, Pharmacokinetics and safety of indacaterol and glycopyrronium (IND/GLY) following repeated once daily inhalation from a fixed-dose combination in healthy Chinese subjects, Int. J. Clin. Pharmacol. Ther., 2017, 55(2), 147–155 CrossRef PubMed.
- Q. Li, A. Zhang, H. Sun and X. Wang, Pharmacokinetics applications of traditional Chinese medicines, World Journal of Traditional Chinese Medicine, 2016, 2(1), 42–47 CrossRef.
- Y. Tu, The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine, Nat. Med., 2011, 17(10), 1217–1220 CrossRef CAS PubMed.
- A. Zhang, H. Sun, Y. Yuan, W. Sun, G. Jiao and X. Wang, An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents, Fitoterapia, 2011, 82(8), 1160–1168 CrossRef CAS PubMed.
- X. Wang, A. Zhang, P. Wang, H. Sun, G. Wu, W. Sun, H. Lv, G. Jiao, H. Xu, Y. Yuan, L. Liu, D. Zou, Z. Wu, Y. Han, G. Yan, W. Dong, F. Wu, T. Dong, Y. Yu, S. Zhang, X. Wu, X. Tong and X. Meng, Metabolomics coupled with proteomics advancing drug discovery toward more agile development of targeted combination therapies, Mol. Cell. Proteomics, 2013, 12(5), 1226–1238 CAS.
- H. Sun, H. Wang, A. Zhang, G. Yan, Y. Zhang, N. An and X. Wang, Berberine ameliorates nonbacterial prostatitis via multi-target metabolic network regulation, OMICS, 2015, 19(3), 186–195 CrossRef CAS PubMed.
- N. Zhang, L. Li, P. Wang, H. Sun, Z. Wu, C. Piao and X. Wang, Pharmacokinetics of the main compounds absorbed into blood after oral administration of Liu Wei Di Huang Wan, a typical combinatorial intervention of Chinese medical formula, J. Nat. Med., 2013, 67(1), 36–41 CrossRef CAS PubMed.
- Y. Cong, K. Sun, X. He, J. Li, Y. Dong, B. Zheng, X. Tan and X. J. Song, A Traditional Chinese Medicine Xiao-Ai-Tong Suppresses Pain through Modulation of Cytokines and Prevents Adverse Reactions of Morphine Treatment in Bone Cancer Pain Patients, Mediators Inflammation, 2015, 2015, 961635 Search PubMed.
- D. Z. Qiu, Z. J. Zhang, W. Z. Wu and Y. K. Yang, Bufalin, a component in Chansu, inhibits proliferation and invasion of hepatocellular carcinoma cells, BMC Complementary Altern. Med., 2013, 13, 185 CrossRef PubMed.
- C. H. Liu, C. Y. Chen, A. M. Huang and J. H. Li, Subamolide A, a component isolated from Cinnamomum subavenium, induces apoptosis mediated by mitochondria-dependent, p53 and ERK1/2 pathways in human urothelial carcinoma cell line NTUB1, J. Ethnopharmacol., 2011, 137(1), 503–511 CrossRef CAS PubMed.
- K. Nakamura, K. Shinozuka and N. Yoshikawa, Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis, J. Pharmacol. Sci., 2015, 127(1), 53–56 CrossRef CAS PubMed.
- Q. Lv, Z. Meng, Y. Yu, F. Jiang, D. Guan, C. Liang, J. Zhou, A. Lu and G. Zhang, Molecular Mechanisms and Translational Therapies for Human Epidermal Receptor 2 Positive Breast Cancer, Int. J. Mol. Sci., 2016, 17(12), pii: E2095 CrossRef PubMed.
- G. H. Grantham, A. D. S. T. Manhica and R. A. Armstrong, et al., Historical story on natural medicinal chemistry of Taxol, Chin. Tradit. Herb. Drugs, 2011, 42(10), 1878–1884 Search PubMed.
- Y. Tai, Y. M. Sun, X. Zou, Q. Pan, Y. D. Lan, Q. Huo, J. W. Zhu, F. Guo, C. Q. Zheng, C. Z. Wu and H. Liu, Effect of Polygonatum odoratum extract on human breast cancer MDA-MB-231 cell proliferation and apoptosis, Exp. Ther. Med., 2016, 12(4), 2681–2687 Search PubMed.
- K. Shen, F. Lu, J. Xie, M. Wu, B. Cai, Y. Liu, H. Zhang, H. Tan, Y. Pan and H. Xu, Cambogin exerts anti-proliferative and pro-apoptotic effects on breast adenocarcinoma through the induction of NADPH oxidase 1 and the alteration of mitochondrial morphology and dynamics, Oncotarget, 2016, 7(31), 50596–50611 Search PubMed.
- Z. Qu, J. Cui, Y. Harata-Lee, T. N. Aung, Q. Feng, J. M. Raison, R. D. Kortschak and D. L. Adelson, Identification of candidate anti-cancer molecular mechanisms of compound kushen injection using functional genomics, Oncotarget, 2016, 7(40), 66003–66019 Search PubMed.
- B. Xue, Z. Yao and R. Yu, Studies on anti-EV71 virus activity of traditional Chinese medicine and its clinical application in treatment of HFMD, Zhongguo Zhongyao Zazhi, 2011, 36(23), 3366–3370 Search PubMed.
- N. Yu, Z. Zhang, P. Chen, Y. Zhong, X. Cai, H. Hu, Y. Yang, J. Zhang, K. Li, J. Ge, K. Yu, X. Liu and J. Zhuang, Tetramethylpyrazine (TMP), an Active Ingredient of Chinese Herb Medicine Chuanxiong, Attenuates the Degeneration of Trabecular Meshwork through SDF-1/CXCR4 Axis, PLoS One, 2015, 10(8), e0133055 Search PubMed.
- C. Feng, H. Wang, C. Yao, J. Zhang and Z. Tian, Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan-Cao, prevents murine T-cell-mediated fulminant hepatitis in IL-10- and IL-6-dependent manners, Int. Immunopharmacol., 2007, 7(10), 1292–1298 CrossRef CAS PubMed.
- C. H. Lau, C. M. Chan, Y. W. Chan, K. M. Lau, T. W. Lau, F. C. Lam, W. T. Law, C. T. Che, P. C. Leung, K. P. Fung, Y. Y. Ho and C. B. Lau, Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol, Phytomedicine, 2007, 14(11), 778–784 CrossRef CAS PubMed.
- F. Wang, Q. Zhang, Z. Lu, Q. Wang, M. Wang, Y. Liu, S. Fu, X. Gao and X. Tang, Identification of chemical constituents in traditional Chinese medicine formula using HPLC coupled with linear ion trap-Orbitrap MS from high doses of medicinal materials to equivalent doses of formula: Study on Xiang-Sha-Liu-Jun-Zi-Jia-Jian granules, J. Sep. Sci., 2016, 39(9), 1619–1627 CrossRef CAS PubMed.
- Z. Tang, R. Yin, K. Bi, H. Zhu, F. Han, K. Chen and F. Wang, Simultaneous quantitative determination of 20 active components in the traditional Chinese medicine formula Zhi-Zi-Da-Huang decoction by liquid chromatography coupled with mass spectrometry: application to study the chemical composition variations in different combinations, Biomed. Chromatogr., 2015, 29(9), 1406–1414 CrossRef CAS PubMed.
- M. H. Liu, X. Tong, J. X. Wang, W. Zou, H. Cao and W. W. Su, Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry, J. Pharm. Biomed. Anal., 2013, 74, 141–155 CrossRef CAS PubMed.
- Y. Y. Huang, L. F. Liu, R. Q. Yue, J. Xu, A. Ho, M. Li and Q. B. Han, Full component analysis of Tianma-Gouteng-Yin, Chin. Med., 2016, 11, 44 CrossRef PubMed.
- Y. Hu, T. Lu, C. Mao, H. Wu, X. Zhang, J. Wang and J. Gu, Simultaneous determination of 10 components in traditional Chinese medicine Dachaihu Granule by reversed-phase-high-performance liquid chromatographic-diode array detector, Pharmacogn. Mag., 2013, 9(33), 33–38 CrossRef CAS PubMed.
- F. X. Zhu, J. J. Wang, X. F. Li, E. Sun and X. B. Jia, Qualitative and quantitative analysis of the major constituents in traditional Chinese medicine Danmu injection using LC-ESI-MS(n) and LC-DAD, Pharmacogn. Mag., 2014, 10(39), 254–264 CrossRef CAS PubMed.
- X. Wang, R. Zhang, L. Gu, Y. Zhang, X. Zhao, K. Bi and X. Chen, Cell-based screening identifies the active ingredients from Traditional Chinese Medicine formula Shixiao San as the inhibitors of atherosclerotic endothelial dysfunction, PLoS One, 2015, 10(2), e0116601 Search PubMed.
- Y. Yang, W. Kong, H. Feng, X. Dou, L. Zhao, Q. Xiao and M. Yang, Quantitative and fingerprinting analysis of Pogostemon cablin based on GC-FID combined with chemometrics, J. Pharm. Biomed. Anal., 2016, 121, 84–90 CrossRef CAS PubMed.
- R. Wang, Y. P. Shi, Q. Z. Wang and H. Cao, Chemical constituents from traditional Chinese medicine Siegesbeckia pubescens, Zhongguo Zhong Yao Za Zhi, 2014, 39(24), 4811–4815 CAS.
- P. Y. Chen, J. W. Yu, F. L. Lu, M. C. Lin and H. F. Cheng, Differentiating Parts of Cinnamomum cassia using LC-qTOF-MS in Conjunction with Principal Component Analysis, Biomed. Chromatogr., 2016, 30(9), 1449–1457 CrossRef CAS PubMed.
- L. J. Cao, J. Miao, J. X. Liu, W. Y. Gao and X. Li, Research on contents of anthraquinones in Cassiae Semen by principal component analysis, Zhongguo Zhong Yao Za Zhi, 2015, 40(13), 2589–2593 CAS.
- L. Liu, J. Leng, X. Yang, L. Liao, Y. Cen, A. Xiao and L. Ma, Rapid Screening and Identification of BSA Bound Ligands from Radix astragali Using BSA Immobilized Magnetic Nanoparticles Coupled with HPLC-MS, Molecules, 2016, 21(11), pii: E1471 CrossRef PubMed.
- Y. Huo, Y. Gao, J. Mi, X. Wang, H. Jiang and H. Zhang, Isolation and Simultaneous Quantification of Nine Triterpenoids from Rosa davurica Pall, J. Chromatogr. Sci., 2017, 55(2), 130–136 Search PubMed.
- Y. Xie, Q. S. Guo and G. S. Wang, Flavonoid Glycosides and Their Derivatives from the Herbs of Scorzonera austriaca Wild, Molecules, 2016, 21(6), pii: E803 CrossRef PubMed.
- A. I. Owis and el-M. B. El-Naggar, Identification and Quantification of the Major Constituents in Egyptian Carob Extract by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry, Pharmacogn. Mag., 2016, 12(1), S1–S6 CrossRef PubMed.
- L. Wu, L. Li, M. Wang, C. Shan, X. Cui, J. Wang, N. Ding, D. Yu and Y. Tang, Target and non-target identification of chemical components in Lamiophlomis rotata by liquid chromatography/quadrupole time-of-flight mass spectrometry using a three-step protocol, Rapid Commun. Mass Spectrom., 2016, 30(19), 2145–2154 CrossRef CAS PubMed.
- X. Wang, H. Sun, A. Zhang, G. Jiao, W. Sun and Y. Yuan, Pharmacokinetics screening for multi-components absorbed in the rat plasma after oral administration traditional Chinese medicine formula Yin-Chen-Hao-Tang by ultra performance liquid chromatography-electro spray ionization/quadrupole-time-of-flight mass spectrometry combined with pattern recognition methods, Analyst, 2011, 136(23), 5068–5076 RSC.
- C. Wu, L. Zhao, Y. Rong, G. Zhu, S. Liang and S. Wang, The pharmacokinetic screening of multiple components of the Nao Mai Tong formula in rat plasma by liquid chromatography tandem mass spectrometry combined with pattern recognition method and its application to comparative pharmacokinetics, J. Pharm. Biomed. Anal., 2016, 131, 345–354 CrossRef CAS PubMed.
- C. Wang, Z. Jia, Z. Wang, T. Hu, H. Qin, G. Du, C. Wu and J. Zhang, Pharmacokinetics of 21 active components in focal cerebral ischemic rats after oral administration of the active fraction of Xiao-Xu-Ming decoction, J. Pharm. Biomed. Anal., 2016, 122, 110–117 CrossRef CAS PubMed.
- X. Liu, H. Li, L. Wu, J. Xing, Y. Poh, H. Cai and B. C. Cai, Simultaneous quantification of chrysophanol and physcion in rat plasma by ultra fast liquid chromatography-tandem mass spectrometry and application of the technique to comparative pharmacokinetic studies of Radix et Rhei Rhizoma extract alone and Dahuang Fuzi Decoction, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 980, 88–93 CrossRef CAS PubMed.
- Y. Zhang, D. W. Qian, Y. Pan, Y. J. Zhai, X. P. Zhou, G. S. Zhong, Z. H. Zhu and J. A. Duan, Comparisons of pharmacokinetic profile of eleven bioactive components in Haizao Yuhu decoction modified with Haizao and Gancao anti-drug pair in normal rats, Zhongguo Zhong Yao Za Zhi, 2015, 40(23), 4672–4679 Search PubMed.
- X. Zhang, J. Lv, L. Wang and H. Shao, Comparison of Pharmacokinetics and Biodistribution of 10-Deacetylbaccatin III after Oral Administration as Pure Compound or in Taxus chinensis Extract: A Pilot Study, Planta Med., 2016, 82(3), 230–237 CrossRef CAS PubMed.
- Y. Fan, S. Man, H. Li, Y. Liu, Z. Liu and W. Gao, Analysis of bioactive components and pharmacokinetic study of herb–herb interactions in the traditional Chinese patent medicine Tongmai Yangxin Pill, J. Pharm. Biomed. Anal., 2016, 120, 364–373 CrossRef CAS PubMed.
- C. Tang, L. Wang, X. Liu, M. Cheng, Y. Qu and H. Xiao, Comparative pharmacokinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extract, J. Ethnopharmacol., 2015, 176, 49–54 CrossRef CAS PubMed.
- Z. Ouyang, M. Zhao, J. Tang and L. Pan, In vivo pharmacokinetic comparisons of ferulic acid and puerarin after oral administration of monomer, medicinal substance aqueous extract and Nao-De-Sheng to rats, Pharmacogn. Mag., 2012, 8(32), 256–262 CrossRef PubMed.
- H. Zeng, P. Xue, S. Su, X. Huang, E. Shang, J. Guo, D. Qian, Y. Tang and J. A. Duan, Comparative Pharmacokinetics of three major bioactive components in rats after oral administration of Typhae Pollen-Trogopterus Feces drug pair before and after compatibility, Daru, J. Pharm. Sci., 2016, 24, 2 CrossRef PubMed.
- J. Xi, D. Qian, L. P. Duan, Z. Zhu, J. Guo, Y. Zhang and Y. Pan, Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex, Molecules, 2015, 20(6), 10705–10720 CrossRef CAS PubMed.
- Z. Liu, X. Zheng, J. Lv, X. Zhou, Q. Wang, X. Wen, H. Liu, J. Jiang and L. Wang, Pharmacokinetic synergy from the taxane extract of Taxus chinensis improves the bioavailability of paclitaxel, Phytomedicine, 2015, 22(5), 573–578 CrossRef CAS PubMed.
- B. Wei, Q. Li, D. Su, R. Fan, L. Zhao, L. Geng, B. He, X. Chen, Y. Jia and K. Bi, Development of a UFLC-MS/MS method for simultaneous determination of six lignans of Schisandra chinensis (Turcz.) Baill. in rat plasma and its application to a comparative pharmacokinetic study in normal and insomnic rats, J. Pharm. Biomed. Anal., 2013, 77, 120–127 CrossRef CAS PubMed.
- B. Wei, Q. Li, R. Fan, D. Su, X. Ou, K. Chen, X. Chen, Y. Jia and K. Bi, UFLC-MS/MS method for simultaneous determination of six lignans of Schisandra chinensis (Turcz.) Baill. in normal and insomniac rats brain microdialysates and homogenate samples: towards an in-depth study for its sedative-hypnotic activity, J. Mass Spectrom., 2013, 48(4), 448–458 CrossRef CAS PubMed.
- X. C. Qian, L. Zhang, Y. Tao, P. Huang, J. S. Li, C. Chai, W. Li, L. Q. Di and B. C. Cai, Simultaneous determination of ten alkaloids of crude and wine-processed Rhizoma coptidis aqueous extracts in rat plasma by UHPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study, J. Pharm. Biomed. Anal., 2015, 105, 64–73 CrossRef CAS PubMed.
- B. X. Xiao, L. Feng, F. R. Cao, R. L. Pan, Y. H. Liao, X. M. Liu and Q. Chang, Pharmacokinetic profiles of the five isoflavonoids from Pueraria lobata roots in the CSF and plasma of rats, J. Ethnopharmacol., 2016, 184, 22–29 CrossRef CAS PubMed.
- Q. Luo, C. P. Wang, J. Li, W. F. Ma, Y. Bai, L. Ma, X. M. Gao, B. L. Zhang and Y. X. Chang, The pharmacokinetics and oral bioavailability studies of columbianetin in rats after oral and intravenous administration, J. Ethnopharmacol., 2013, 150(1), 175–180 CrossRef CAS PubMed.
- S. Song, Q. Tang, H. Huo, H. Li, X. Xing and J. Luo, Simultaneous quantification and pharmacokinetics of alkaloids in Herba Ephedrae-Radix Aconiti Lateralis extracts, J. Anal. Toxicol., 2015, 39(1), 58–68 CrossRef CAS PubMed.
- R. Liu, P. Gu, L. Wang, M. Cheng, Y. Wu, L. Zheng, Y. Liu and L. Ding, Study on the pharmacokinetic profiles of corynoline and its potential interaction intraditional Chinese medicine formula Shuanghua Baihe tablets in rats by LC-MS/MS, J. Pharm. Biomed. Anal., 2016, 117, 247–254 CrossRef CAS PubMed.
- Z. Ouyang, M. Zhao, J. Tang and L. Pan, In vivo pharmacokinetic comparisons of ferulic acid and puerarin after oral administration of monomer, medicinal substance aqueous extract and Nao-De-Sheng to rats, Pharmacogn. Mag., 2012, 8(32), 256–262 CrossRef PubMed.
- W. W. Peng, W. Li, J. S. Li, X. B. Cui, Y. X. Zhang, G. M. Yang, H. M. Wen and B. C. Cai, The effects of Rhizoma Zingiberis on pharmacokinetics of six Aconitum alkaloids in herb couple of Radix Aconiti Lateralis-Rhizoma Zingiberis, J. Ethnopharmacol., 2013, 148(2), 579–586 CrossRef CAS PubMed.
- Y. X. Zhang, J. S. Li, W. W. Peng, X. Liu, G. M. Yang, L. H. Chen and B. C. Cai, Comparative pharmacokinetics of aloe-emodin, rhein and emodin determined by liquid chromatography-mass spectrometry after oral administration of a rhubarb peony decoction and rhubarb extract to rats, Pharmazie, 2013, 68(5), 333–339 CAS.
- B. He, Q. Li, Y. Jia, L. Zhao, F. Xiao, C. Lv, H. Xu, X. Chen and K. Bi, A UFLC-MS/MS method for simultaneous quantitation of spinosin, mangiferin and ferulic acid in rat plasma: application to a comparative pharmacokinetics tudy in normal and insomnic rats, J. Mass Spectrom., 2012, 47(10), 1333–1340 CrossRef CAS PubMed.
- Q. F. Liu, X. J. Shi, Z. D. Li, M. K. Zhong, Z. Jiao and B. Wang, Pharmacokinetic comparisons of berberine and palmatine in normal and metabolic syndrome rats, J. Ethnopharmacol., 2014, 151(1), 287–291 CrossRef CAS PubMed.
- Y. Zhang, D. Qian, Y. Pan, Z. Zhu, J. Huang, J. Xi, J. Guo, X. Zhou, G. Zhong and J. Duan, Comparisons of the pharmacokinetic profile of four bioactive components after oral administration of gan-sui-ban-xia decoction plus–minus gansui and gancao drug combination in normal rats, Molecules, 2015, 20(5), 9295–9308 CrossRef CAS PubMed.
- Q. H. Zhang, C. Gong, H. Yang, H. Wei, W. B. Zhou, C. Qi and C. H. Wang, Pharmacokinetics of cisplatin in the absence or presence of zengmian yiliu granules (a traditional Chinese medicine compound) in rats determined via ICP-MS: an investigation on drug-herb interactions, Pharm. Biol., 2015, 53(2), 159–166 CrossRef CAS PubMed.
- X. J. Qiu, X. Huang, Z. Q. Chen, P. Ren, W. Huang, F. Qin, S. H. Hu, J. Huang, J. He, Z. Q. Liu and H. H. Zhou, Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of Chaihu-Shugan-San to patients with functional dyspepsia, J. Ethnopharmacol., 2011, 137(1), 205–213 CrossRef CAS PubMed.
- F. Zhang, Q. Zhan, S. Gao, X. Dong, B. Jiang, L. Sun, X. Tao and W. S. Chen, Chemical profile- and pharmacokinetics-based investigation of the synergistic property of platycodonis radix in traditional Chinese medicine formula Shengxian decoction, J. Ethnopharmacol., 2014, 152(3), 497–507 CrossRef CAS PubMed.
- H. Wei, X. Tao, P. Di, Y. Yang, J. Li, X. Qian, J. Feng and W. Chen, Effects of traditional chinese medicine Wuzhi capsule on pharmacokinetics of tacrolimus in rats, Drug Metab. Dispos., 2013, 41(7), 1398–1403 CrossRef CAS PubMed.
- L. Shi, X. Tang, X. Dang, Q. Wang, X. Wang, P. He, Q. Wang, L. Liu, X. Liu and Y. Zhang, Investigating herb-herb interactions: the potential attenuated toxicity mechanism of the combined use of Glycyrrhizae radix et rhizoma (Gancao) and Sophorae flavescentis radix (Kushen), J. Ethnopharmacol., 2015, 165, 243–250 CrossRef CAS PubMed.
- M. L. Hou, L. W. Chang, C. H. Lin, L. C. Lin and T. H. Tsai, Determination of bioactive components in Chinese herbal formulae and pharmacokinetics of rhein in rats by UPLC-MS/MS, Molecules, 2014, 19(4), 4058–4075 CrossRef PubMed.
- B. X. Xiao, Q. Wang, L. Q. Fan, L. T. Kong, S. R. Guo and Q. Chang, Pharmacokinetic mechanism of enhancement by Radix Pueraria flavonoids on the hyperglycemic effects of Cortex Mori extract in rats, J. Ethnopharmacol., 2014, 151(2), 846–851 CrossRef CAS PubMed.
- S. Cheng, L. C. Lin, C. H. Lin and T. H. Tsai, Comparative oral bioavailability of geniposide following oral administration of geniposide, Gardenia jasminoides Ellis fruits extracts and Gardenia herbal formulation in rats, J. Pharm. Pharmacol., 2014, 66(5), 705–712 CrossRef CAS PubMed.
- Y. Li, Y. Wang, W. Tai, L. Yang, Y. Chen, C. Chen and C. Liu, Challenges and Solutions of Pharmacokinetics for Efficacy and Safety of Traditional Chinese Medicine, Curr. Drug Metab., 2015, 16(9), 765–776 Search PubMed.
- B. Kamble, A. Gupta, I. Moothedath, L. Khatal, S. Janrao, A. Jadhav and B. Duraiswamy, Effects of Gymnema sylvestre extract on the pharmacokinetics and pharmacodynamics of glimepiride in streptozotocin induced diabetic rats, Chem.-Biol. Interact., 2016, 245, 30–38 CrossRef CAS PubMed.
- B. Ge, Z. Zhang and Z. Zuo, Radix Puerariae lobatae (Gegen) suppresses the anticoagulation effect of warfarin: a pharmacokinetic and pharmacodynamics study, Chin. Med., 2016, 11, 7 CrossRef PubMed.
- J. Song, J. Li, Y. Jin, H. Wang, S. Zheng and J. Gao, Pharmacokinetic-pharmacodynamic evaluation of the major componentastragaloside IV on the immunomodulatory effects of Yu-ping-feng prescription, Eur. J. Drug Metab. Pharmacokinet., 2014, 39(2), 103–110 CrossRef CAS PubMed.
- Z. Zhang, L. Qin, L. Peng, Q. Zhang, Q. Wang, Z. Lu, Y. Song and X. Gao, Pharmacokinetic-Pharmacodynamic Modeling to Study the Antipyretic Effect of Qingkailing Injection on Pyrexia Model Rats, Molecules, 2016, 21(3), 317 CrossRef PubMed.
- H. Li, Y. Zhang, Y. P. Yu, P. Wang, F. F. Li and X. L. Meng, Pharmacokinetic/pharmacodynamic modeling of antipyretic and reducing plasma concentration of NO effects of Rheum palmatum in rat, Zhongguo Zhong Yao Za Zhi, 2013, 38(8), 1231–1236 Search PubMed.
- H. X. Zhu, L. M. Pan, Q. C. Zhang, Y. P. Tang and L. W. Guo, Study on PK/PD model for traditional Chinese medicine biopharmaceutics based on principle of "correspondence of prescriptions and syndromes, Zhongguo Zhongyao Zazhi, 2013, 38(12), 2033–2038 Search PubMed.
- G. Chen, B. Hao, D. Ju, M. Liu, H. Zhao, Z. Du and J. Xia, Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis, Acta Pharm. Sin. B, 2015, 5(6), 569–576 CrossRef PubMed.
- L. C. Dong, Y. X. Fan, Q. Yu, J. Ma, X. Dong, P. Li and H. J. Li, Synergistic effects of rhubarb-gardenia herb pair in cholestatic rats at pharmacodynamic and pharmacokinetic levels, J. Ethnopharmacol., 2015, 175, 67–74 CrossRef CAS PubMed.
- W. Ren, R. Zuo, Y. N. Wang, H. J. Wang, J. Yang, S. K. Xin, L. Y. Han, H. Y. Zhao, S. Y. Han, B. Gao, H. Hu, Y. J. Hu, B. L. Bian and N. Si, Pharmacokinetic-Pharmacodynamic Analysis on Inflammation Rat Model after Oral Administration of Huang Lian Jie Du Decoction, PLoS One, 2016, 11(6), e0156256 Search PubMed.
- X. Ding, Y. Sun, Q. Wang, T. Pu, X. Li, Y. Pan and Y. Yang, Pharmacokinetics and pharmacodynamics of glycyrrhetinic acid with Paeoniflorin after transdermal administration in dysmenorrhea model mice, Phytomedicine, 2016, 23(8), 864–871 CrossRef CAS PubMed.
- S. Zhan, W. Guo, Q. Shao, X. Fan, Z. Li and Y. Cheng, A pharmacokinetic and pharmacodynamic study of drug–drug interaction between ginsenoside Rg1, ginsenoside Rb1 and schizandrin after intravenous administration to rats, J. Ethnopharmacol., 2014, 152(2), 333–339 CrossRef CAS PubMed.
- M. L. Hou, C. H. Lin, L. C. Lin and T. H. Tsai, The Drug–Drug Effects of Rhein on the Pharmacokinetics and Pharmacodynamics of Clozapine in Rat Brain Extracellular Fluid by In Vivo Microdialysis, J. Pharmacol. Exp. Ther., 2015, 355(1), 125–134 CrossRef CAS PubMed.
- B. Yu, M. Ruan, X. Dong, Y. Yu and H. Cheng, The mechanism of the opening of the blood–brain barrier by borneol: A pharmacodynamics and pharmacokinetics combination study, J. Ethnopharmacol., 2013, S0378–8741(13), 00735–00736 Search PubMed.
- F. Cheung, Modern TCM: Enter the clinic, Nature, 2011, 480, S94–S95 CrossRef CAS PubMed.
- F. Han, G. Q. Ma, M. Yang, L. Yan, W. Xiong, J. C. Shu, Z. D. Zhao and H. L. Xu, Chemical composition and antioxidant activities of essential oils from different parts of the oregano, J. Zhejiang Univ., Sci., B, 2017, 18(1), 79–84 CrossRef CAS PubMed.
- W. Tan, Y. Li, Y. Wang, Z. Zhang, T. Wang, Q. Zhou and X. Wang, Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions, Biomed. Pharmacother., 2017, 90, 244–252 CrossRef CAS PubMed.
- Y. Han, A. Zhang, H. Sun, Y. Zhang, X. Meng, G. Yan, L. Liu and X. Wang, High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms, J. Sep. Sci., 2017, 40(10), 2178–2187 CrossRef CAS PubMed.
- H. F. Jiang, Z. H. Zhuang, B. W. Hou, B. J. Shi, C. J. Shu, L. Chen, G. X. Shi and W. M. Zhang, Adverse Effects of Hydroalcoholic Extracts and the Major Components in the Stems of Impatiens balsamina L. on Caenorhabditis elegans, J. Evidence-Based Complementary Altern. Med., 2017, 2017, 4245830 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |