Highly efficient trans–cis isomerization of lycopene catalyzed by iodine-doped TiO2 nanoparticles†
Qingrui Sunad,
Cheng Yangb,
Jing Lib,
Waleed Aboshorab,
Husnain Razab and
Lianfu Zhang*abc
aState Key Laboratory of Food Science and Technology, Wuxi 214122, China. E-mail: lianfu@jiangnan.edu.cn; Fax: +86 510 85917081; Tel: +86 510 85917081
bSchool of Food Science and Technology, Jiangnan University, Wuxi 214122, China
cNational Engineering Research Center for Functional Food, Wuxi 214122, China
dSchool of Food Science and Technology, Bayi Agricultural University, Daqing 163319, China
First published on 21st December 2015
Abstract
Highly efficient trans–cis isomerization of lycopene was achieved in the presence of a novel iodine-doped titanium dioxide (I-TiO2) catalyst. The catalyst was prepared via a hydrothermal route and subsequent vacuum calcination and was characterized with thermogravimetric analysis, X-ray diffraction, nitrogen adsorption–desorption isotherms, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy techniques. Factors that affected the reaction parameters, such as the reaction temperature, the concentration of substrate, the weight of the catalyst and the reaction time, were investigated in detail. Application tests showed that the catalyst was highly active in the isomerization of lycopene in a heterogeneous reaction, and the conversion of all-trans-lycopene exceeded 80% after 2 h of reaction at 75 °C in ethyl acetate. Moreover, the catalyst displayed no significant loss of activity after five cycles of reuse.
1. Introduction
Lycopene, one of the most popular carotenoids in food, is mostly present in ripe tomatoes and tomato-based products. It is a polyisoprenoid compound with a molecular formula of C40H56 and 11 conjugated carbon–carbon double bonds. Because of steric hindrance effects, only 72 lycopene isomers are in fact structurally favourable.1 The most abundant isomers of lycopene (Scheme 1) in tomato-based products and in human serum and tissues are all-trans-lycopene and its mono-cis isomers, such as 5-cis-, 9-cis-, 13-cis- and 15-cis-lycopene.2–5Lycopene has attracted considerable attention in recent years because of its multiple bioactive effects associated with a reduced risk of chronic diseases, including some types of cancer, cardiovascular and cerebrovascular diseases, and myocardial infarction.6–11 In terms of geometric configuration, about 90% to 98% of native lycopene exists in the all-trans form, but more than 50% of the total lycopene found in human serum and tissues exists in a cis-isomeric form.12–14 It is therefore reasonable to deduce that cis-isomers might be more biologically active than the all-trans-isomer and that the intake of processed foods with a higher content of cis-isomers would offer health benefits. In fact, the conclusions of the tests both from cultured small intestinal cells and from ferrets strongly supported these suggestions.15,16 Moreover, the intake of tomato juice rich in cis-isomers of lycopene resulted in a great increase in plasma lycopene concentrations in humans compared to a sample that was abundant in the all-trans-isomer.17 Thus, there are potential health benefits from the enhancement of the cis-isomer content by applying processing interventions that induce the isomerization of lycopene.
Common methods for increasing the proportion of cis-lycopene using natural lycopene as a raw material include thermal isomerization and photoisomerization.18–22 However, these methods may be not realistic from an industrial point of view because of several drawbacks: the prolonged reaction time and lower conversion efficiency of cis-isomers by thermal isomerization; the requirement of a special reactor in photoisomerization, which is unsuitable for scale-up production, and the great difficulty in removing the catalyst from the product afterwards if inedible I2 is used in photoisomerization. These drawbacks highlight the importance of developing appropriate methods for trans–cis isomerization of lycopene for the food and pharmaceutical industries, especially for the manufacture of nutritional supplements and pharmaceutical ingredients.
Because the solid catalysts can be conveniently removed by simple filtration or centrifugation, enrichment of the lycopene in cis-isomers with the catalysts is not polluting or harmful to the food. However, to the best of our knowledge, a facile method for the synthesis of a solid catalyst for trans–cis isomerization of lycopene has not been demonstrated. Iodine-doped titanium dioxide (I-TiO2) is widely used as a photocatalyst for organic pollutant degradation in water, as reported by many researchers.23–26 Herein, the I-TiO2 solid catalyst prepared via a modified method was evaluated for the first time for trans–cis isomerization of lycopene without light irradiation.
2. Experimental
2.1. Chemicals and materials
Lycopene (97 wt% all-trans-lycopene) was obtained from North China Pharmaceutical Co., Ltd. (Shijiazhuang, China). Tetrabutyl titanate (≥98%) was obtained from Sinopharm Chemical Reagent Corporation (Shanghai, China). All the other materials were of either analytical or chromatographical grades and used without further purification.2.2. Catalyst preparation
The synthesis of iodine-doped TiO2 nanoparticles catalyst was performed with the hydrothermal method modified according to Shi and co-workers.24 Typically, solution A consisted of 5 mL of tetrabutyl titanate mixed with 1 mL of acetic acid, and solution B consisted of 242.9 mg KI dissolved in a mixture of 60 mL of aqueous solution and 10 mg of polyvinylpyrrolidone (PVP). Solution A was added dropwise into solution B with vigorous stirring. After being stirred continuously for 4 h at room temperature, the resulting colloidal solution was transferred into a 100 mL Teflon-lined autoclave and maintained at 100 °C for 24 h and was then cooled to room temperature. The precipitate was centrifuged, dried in a vacuum at 80 °C for 12 h and then calcined in a vacuum at 185 °C for 2 h. The calcined product was ground into uniform particles of powder. For comparison, an undoped TiO2 catalyst was prepared by the same procedure without the addition of KI.2.3. Catalyst characterization
Thermo gravimetric analyses (TGA) were carried out on a TGA/1 analyzer at a heating rate of 5 °C min−1 in N2 flow. The powder X-ray diffraction (XRD) patterns of the samples were determined with Bruker AXS D8 diffractometer using Cu Kα radiation. The accelerating voltage and emission current were 45 kV and 40 mA, respectively. Diffraction patterns in the 10–85° range were recorded at a rate of 2 degrees per minute. Specific surface areas of the samples were determined by measurement of the nitrogen adsorption–desorption isotherm at 77 K using the Brunauer–Emmett–Teller (BET) method with an ASAP2020 MP analyzer. The sample was pretreated at 160 °C for 3 h under vacuum prior to measurements. SEM images were collected on a field emission scanning electron microscope (Magellan 400) at 3 kV. Transmission electron microscopy (TEM) and the selected area electron diffraction (SAED) studies were carried out on an electron microscopy instrument (JEM-2010HT) at accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were performed on a XPS System (Thermo Fisher ESCALAB 250Xi) with a monochromatic Al Kα source and a charge neutralizer. High-resolution spectra were recorded at energy step size of 0.05 eV and at pass energy of 20 eV. All the binding energies were calibrated to the C1s peak at 284.6 eV of the surface adventitious carbon. Data was analyzed with XPSPEAK4.1 software (Raymund W.M.Kwork). FT-IR spectrum was recorded on a Thermo Nicolet iS10 (USA) instrument (operating conditions: resolution, 2 cm−1; scan, 36; operating temperature, 23–25 °C; and the frequency range, 4000–400 cm−1).2.4. Catalytic reaction
A certain amount of I-TiO2 catalyst and lycopene were charged into a 25 mL double-necked round bottom flask containing 20 mL of ethyl acetate as a solvent. The reactants were heated in a water bath with magnetic stirring (∼200 rpm). The reactions were conducted by applying a stream of N2 in the absence of light to inhibit oxidation of lycopene.2.5. Analyses of lycopene composition
The reaction mixtures were sampled, and the lycopene isomer profile was analyzed by HPLC (Agilent 1200) with an analytical polymeric C30-column (YMC Carotenoid S, 5 μm, 250 × 4.6 mm, China) and diode array detector. Column temperature was maintained at 30 °C. A solvent system of (A) methanol/acetonitrile (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, v/v) and solvent (B) methyltertbutyl ether was used as the mobile phase. The linear gradient elution was as follows: 0 to 20 min, the proportion of solvent A was decreased from 100% to 50%; and from 20 to 40 min, the proportion of solvent A was kept constant at 50%. The mobile phase flow rate was 1.0 mL min−1. The quantification of lycopene isomers in the samples was performed by peak area integration at 470 nm and showed a reliable approximation for the analysis of lycopene isomers.22,27 Isomers of lycopene were identified by comparison of the separated isomer retention time and spectrum with characteristics obtained from standard for all-trans-lycopene or identification data reported in the literature.27,28
75, v/v) and solvent (B) methyltertbutyl ether was used as the mobile phase. The linear gradient elution was as follows: 0 to 20 min, the proportion of solvent A was decreased from 100% to 50%; and from 20 to 40 min, the proportion of solvent A was kept constant at 50%. The mobile phase flow rate was 1.0 mL min−1. The quantification of lycopene isomers in the samples was performed by peak area integration at 470 nm and showed a reliable approximation for the analysis of lycopene isomers.22,27 Isomers of lycopene were identified by comparison of the separated isomer retention time and spectrum with characteristics obtained from standard for all-trans-lycopene or identification data reported in the literature.27,28
The selectivity to a particular lycopene isomer (Si) was calculated according to eqn (1).
 | (1) |
 | (2) |
3. Results and discussion
3.1. Activity of the I-TiO2 catalyst
Table 1 shows the activity of the I-TiO2 catalyst in the isomerization of lycopene in ethyl acetate solvent. I-TiO2 catalyst (entry 5) shows 83.4% all-trans-lycopene conversion with 23.1% selectivity of 5-cis-lycopene, 10.0% selectivity of 9-cis-lycopene and 27.4% selectivity of 13-cis-lycopene, respectively (see Fig. S1 and Table S1† for identification of lycopene isomer). Therefore, a total major lycopene isomer (5-cis, 9-cis and 13-cis) selectivity of 60.5% was achieved over the I-TiO2 catalyst after 2 h of reaction. For comparison, catalyst-free (entry 1) and undoped TiO2 (entry 3) experiments were carried out, and approximate 30% conversion of all-trans-lycopene were found, which is lower than that in the experiment with I-TiO2 catalyst. Although it was performed with a relatively longer reaction time (12 h), the conversion of all-trans-lycopene (approximate 38%) in the reactions (entry 2 and 4) was still lower than that in the 2 h of reaction with I-TiO2 catalyst. These experimental results clearly indicate that the I-TiO2 catalyst showed high catalytic activity for isomerization of lycopene in heterogeneous reaction and that the undoped TiO2 catalyst did not show activity for the isomerization of lycopene. Because the I-TiO2 catalyst was found to be the best-suited catalyst, we carried out full characterization of the I-TiO2 catalyst.| Entry | Catalyst | Time (h) | CEb (%) | Sic (%) | |||
|---|---|---|---|---|---|---|---|
| 5-cis | 9-cis | 13-cis | Others | ||||
| a Reaction conditions: solvent (ethyl acetate) 20 mL, substrate (lycopene) 20 mg, catalyst 10 mg, reaction temperature 75 °C.b CE: conversion of all-trans-lycopene.c Si: selectivity to a particular lycopene isomer. | |||||||
| 1 | No catalyst | 2 | 29.8 | 0.4 | 14.6 | 67.9 | 17.1 |
| 2 | No catalyst | 12 | 38.2 | 0.6 | 44.0 | 35.6 | 19.8 |
| 3 | Undoped TiO2 | 2 | 30.6 | 0.5 | 14.4 | 68.2 | 16.9 |
| 4 | Undoped TiO2 | 12 | 38.4 | 0.6 | 43.8 | 36.1 | 19.5 |
| 5 | I-TiO2 | 2 | 83.4 | 23.1 | 10.0 | 27.4 | 39.5 |
3.2. Catalyst characterization
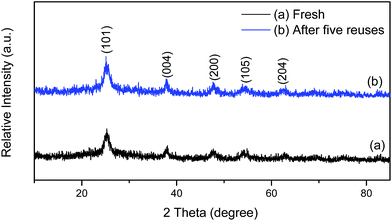
Fig. 1 shows the TG-DTG curves of I-TiO2 gel powders before vacuum calcination. The weight loss in the I-TiO2 was mainly divided into three stages. The first stage, which occurred between 60 °C and 103.6 °C, involved the loss of the physically absorbed water and organic small molecules in the sample, with an approximate weight loss of 0.41 wt%. The second step included a weight loss of approximately 2.83 wt% and occurred between 103.6 °C and 283.6 °C. This step might be attributable to the volatilization of iodine molecules originating from the oxidation of I− exposed on the surface and wrapped in the TiO2 nanoparticles,24 and thus indicates that a temperature of 185 °C, i.e., the boiling point of I2, could be chosen as the calcination temperature for preparation of the activated I-TiO2 catalyst. The third stage, which occurred between 283.6 °C and 400 °C could be characterized by the thermal decomposition of PVP.Fig. 2 shows the XRD patterns of the I-TiO2 sample. A series of characteristic peaks at 2θ value of 25.2°, 37.9°, 47.9°, 54.2°, and 62.6° were observed, matching the (101), (004), (200), (105) and (204) plans anatase titania (JCPDS file no. 21-1272).26 The average crystallite size calculated from the full width at half-maximum of the (101) peak based on the Debye–Scherrer equation was approximately 7.5 nm.
The nitrogen adsorption–desorption isotherms and the pore size distribution of the I-TiO2 samples are shown in Fig. 3. According to the IUPAC classification, the isotherm of the prepared sample exhibited a type IV isotherm with a type H2 hysteresis loop, which is characteristic of mesoporous solid materials. The curve of the pore size distribution indicates that the prepared sample had a narrow pore size distribution for the uniform pore size and well-ordered structure. The surface area and pore structure parameters of sample were calculated by BET equation and Barrett–Joyner–Halenda method. The average mesopore size was about 3.79 nm; the surface area was 181.29 m2 g−1 and the pore volume was 0.19 cm3 g−1.
Fig. 4a shows the morphology of I-TiO2 examined by SEM. Some regular grid structures were present on the particles of the I-TiO2 matrix. The grid structure was probably formed by PVP serving as a linking agent to bridge adjacent crystallites' TiO2, resulting in large quantities of narrow interstitial pores in the I-TiO2 matrix. Furthermore, the morphology of I-TiO2 catalyst was determined by TEM (Fig. 4b), which revealed that the dense particle consisted of well-dispersed irregular-shape nanoparticles. The pattern of representative selected-area electron diffraction, as shown in Fig. 4b (inset) also indicates that I-TiO2 nanoparticles had an anatase-phase lattice structure that was consistent with the XRD measurement.
 | ||
| Fig. 4 SEM (a) TEM and SAED (inset) images of fresh I-TiO2 (b) and TEM images of I-TiO2 after five reuses (c). | ||
Fig. 5a shows the presence of O, C, N, Ti, K and I elements in the I-TiO2 sample. The atomic percentages of elements determined from the XPS analysis for the catalyst sample showed O 53.03%, C 19.76%, N 1.54%, Ti 24.09%, K 1.16% and I 0.42%. It must be noted that XPS is a surface-sensitive characterisation and that the determined atomic contents cannot simply reflect the actual contents, whereas elemental analysis can realize this goal. The N element could be ascribed as the organic residues of PVP. The C element could come from burning organic residues.25 Such a high percentage of carbon in the I-TiO2 catalyst might be caused by vacuum calcination. The I3d XPS spectra exhibiting two prominent peaks for I-TiO2 sample is presented in Fig. 5b. The peak at 618.4 eV should be assigned to I3d5/2 photoelectrons and that at 629.9 eV to I3d3/2 ones, which suggests the existence of negatively charged iodine (I−).29 The inedible I2 was undetected, which is advantageous to the edible safety of isomerized lycopene product. In addition, the ionic radius of I− (0.216 nm) is much larger than that of O2− (0.124 nm) or Ti4+ (0.068 nm), and the substitution of Ti4+ or O2− ions in the lattice by I− would be theoretically impossible, as concluded by Siuzdak et al.23 Thus, I− species should be dispersed on the surface of the TiO2 particles or at the interstitial sites of the TiO2 lattice, as deduced by the researchers.23 Based upon the experimental results described in Table 1, we reasoned that the I− species might be active for catalytic trans–cis isomerization of lycopene. Fig. 5c shows the Ti2p XPS spectrum of the I-TiO2 sample. The two peaks at 458.4 eV and 464.1 eV that appear in Fig. 5c should be assigned to Ti2p3/2 and Ti2p1/2 photoelectrons of TiO2, respectively.23 Nano-TiO2 has high chemical and thermal stability and is allowed to come into contact with food; therefore, it has been widely used in the food packing materials industry. Thus, the use of nano-TiO2 as support of active iodine was further helpful to the edible safety of the isomerized lycopene product.
Fig. 6 shows FTIR spectrum of the catalyst. The bands observed at about 3415 cm−1 and 1637 cm−1 were ascribed to the stretching vibrations of the –OH groups present in the catalyst and the flexural vibrations of the H–O–H groups adsorbed onto the surface and channel of the TiO2 materials, respectively.25 The peaks at 1529 cm−1 and 1433 cm−1 were because of C–H flexure vibration and C–O stretching vibration, respectively, which could be a result of the precursor and solution used.25 The broad band below 700 cm−1 again confirmed the presence of Ti–O–Ti bonds.29 As for PVP, no signal in the FTIR spectrum was detected in the I-TiO2 sample, which might be due to the low PVP content.
3.3. Effect of reaction parameters
The effect of temperature on lycopene isomerization was investigated, and the results are illustrated in Fig. 7a. It was found that temperature had a prominent effect on all-trans-lycopene conversion. With an increase in temperature from 55 °C to 75 °C, the all-trans-lycopene conversion gradually increased from 50.02% to 83.35%, and the 5-cis-lycopene isomer selectivity gradually increased while the 13-cis-lycopene isomer selectivity gradually decreased. Moreover, increases in the temperature (Fig. 7a), catalyst weight (Fig. 7c) and time (Fig. 7d) indicated that conditions facilitated the formation of 5-cis-lycopene on the catalytic reaction. 5-cis-Lycopene was found to be the most stable and to have the greatest antioxidant property of the lycopene isomers.30,31 These findings will contribute to the development of specific lycopene isomers for practical applications. The observed decrease in all-trans-lycopene conversion (Fig. 7b) was seen because a high concentration of lycopene may not facilitate the isomerization reaction on the I-TiO2 catalyst surface. Moreover, the selectivity of the product changed a little (illustrated in Fig. 7b–d), which indicates that the I-TiO2 catalyst is a kind of non-selective catalyst. Fig. 7d shows the effect of reaction time on the isomerization of all-trans-lycopene. The results with the I-TiO2 catalyst reveal that the increase in reaction time from 2 h to 3 h slightly elevated the conversion of all-trans-lycopene. Therefore, the optimum catalytic reaction time was 2 h.With the thermal isomerisation of lycopene (lycopene concentration, 0.1 mg mL−1; temperature, 50 °C) using CHCl3 as a solvent reacted for 24 h, the conversion of all-trans-lycopene was 48.4%, as reported by Honda et al.,22 whereas a control experiment (catalyst, 2 mg; lycopene, 4 mg; chloroform, 40 mL) found that the conversion of all-trans-lycopene was 50.6% within only 2 h of reaction. In addition, the thermal isomerisation of lycopene (lycopene concentration, 0.5 mg mL−1) within 24 h of refluxing in ethyl acetate and the conversion of all-trans-lycopene reached 49.9%, as reported by Zhang et al.,32 whereas the conversion of all-trans-lycopene was 82.1% in a 2 h control experiment (catalyst, 10 mg; lycopene, 20 mg; ethyl acetate, 40 mL). Therefore, compared with previous studies, highly efficient trans–cis isomerization of lycopene can be achieved in the presence of this novel I-TiO2 catalyst. Moreover, the prominent advantage is that ethyl acetate is already approved for use in the manufacture of lycopene products from tomato extracts, and therefore the extraction and catalytic geometric isomerization can be achieved in a continuous production manner.
A plausible reaction mechanism to explain the role of the I-TiO2 catalyst in the isomerization of the all-trans-lycopene is depicted in Scheme 2 (taking the production of 5-cis and 9-cis as an example). Based on the Lewis acid-base theory, TiO2 is defined as an acid for its electron acceptor ability, whereas lycopene is a base owing to its electron-rich conjugated double-bond structure. In addition, according to Yeung et al.,33 a hydrid [H:(−)] in all-trans-lycopene could be removed by some acceptor to produce stable all-trans-lycopene allylic cations (for the cation, the order of stability: all-trans > cis). On the basis of these two theories, TiO2 might act as a hydrid [H:(−)] acceptor by associating with the double-bond moieties of all-trans-lycopene to produce allylic cations. At the same time, the carbenium ion could move along the carbon chain and be trapped by I−, and a favourable conformation intermediate to Z-double bonds could be formed. Upon hydrid [H:(−)] restoration and following a conjugated rearrangement, I− could leave an intermediate in a conformation favourable to the formation of cis-lycopene.
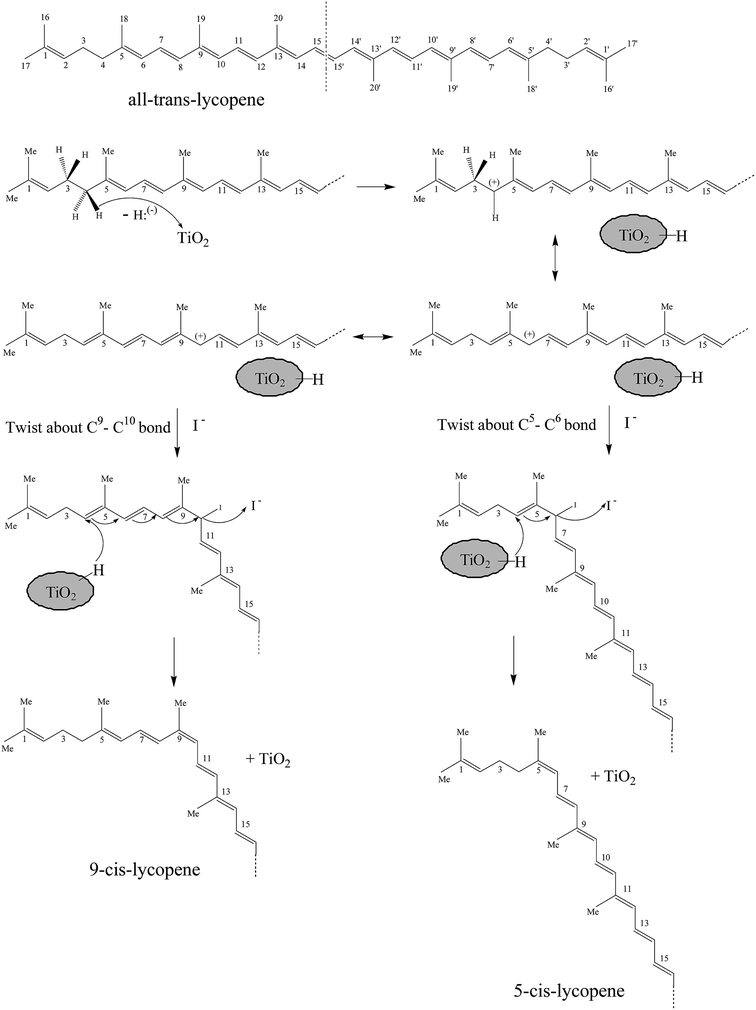 | ||
| Scheme 2 The plausible reaction mechanism for lycopene isomerization (taking the production of 5-cis and 9-cis as an example). | ||
3.4. Reusability of the catalyst
The reusability of the I-TiO2 catalyst was tested with respect to the isomerization of lycopene, and the results are shown in Fig. 8. The reaction was conducted for the first run under the conditions described in Fig. 8. After each recycle, the used catalyst was recovered by centrifugation and washed with 10 mL ethyl acetate and reused in the next reaction cycle. The results imply that the I-TiO2 catalyst could be used at least five times without significant loss of activity. In addition, as shown in Fig. 1b, the XRD pattern clearly demonstrated that the I-TiO2 catalyst remained a well-defined crystalline structure after five reuses and exhibited strong thermal stability of the catalyst. In contrast the TEM images of the spent catalyst (after five recycles) (Fig. 4c) revealed that the particle size of the I-TiO2 catalyst was nearly unchanged during catalysis. The leaching behavior of the catalyst was validated by comparing the filtration test and catalyst-free test. Using 10 mg of catalyst, the reaction (lycopene-free) was completed under the conditions described in Fig. 8. After cooling, the reactants were centrifuged to furnish a clear filtrate. A second batch of reactants (containing 20 mg of lycopene) was added to the filtrate, and the mixture was reacted again. In the case, the conversion of all-trans-lycopene was 33.6%, which was higher than the 29.8% conversion of all-trans-lycopene in the catalyst-free test (Table 1 [entry 1]), confirming that negligible leaching of catalytically active species occurred. Therefore, the slight decrease in the activity of the I-TiO2 catalyst was mainly caused by leaching of catalytically active species during the recyclability tests. | ||
| Fig. 8 Reusability of the I-TiO2 catalyst. Reaction conditions: solvent (ethyl acetate) 20 mL, substrate (all-trans-lycopene) 20 mg, catalyst 10 mg (first run), reaction temperature 75 °C, time 2 h. | ||
4. Conclusions
In summary, the I-TiO2 catalyst was successfully synthesized via a hydrothermal route and subsequent vacuum calcination. The as-synthesized I-TiO2 catalyst showed high catalytic activity for isomerization of lycopene in heterogeneous reaction and remained efficient after five reuses. Therefore, the present study provides an efficient and green catalytic route for the production of high content of cis-lycopene, which has potential applications in future.Acknowledgements
We thank financial support from the Jiangsu province “Collaborative Innovation Center for Food Safety and Quality Control” industry development program, the National Natural Science Foundation of China (31171724), the Fundamental Research Funds for the Central Universities (JUSRP51501) and Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP 20130093110008).References
- L. Zechmeister, A. L. LeRosen, W. A. Schroeder, A. Polgár and L. Pauling, J. Am. Chem. Soc., 1943, 65, 1940–1951 CrossRef CAS.
- W. Stahl, A. R. Sundquist, M. Hanusch, W. Schwarz and H. Sies, Clin. Chem., 1993, 39, 810–814 CAS.
- A. J. Melendez-Martinez, C. M. Stinco, C. Liu and X. D. Wang, Food Chem., 2013, 138, 1341–1350 CrossRef CAS PubMed.
- J. Schierle, W. Bretzel, I. Bühler, N. Faccin, D. Hess, K. Steiner and W. Schüep, Food Chem., 1997, 59, 459–465 CrossRef CAS.
- M. Richelle, B. Sanchez, I. Tavazzi, P. Lambelet, K. Bortlik and G. Williamson, Br. J. Nutr., 2010, 103, 1800–1807 CrossRef CAS PubMed.
- Z. Ke, L. Mucci, B. A. Rosner, S. K. Clinton, M. Loda, M. J. Stampfer and E. Giovannucci, J. Natl. Cancer Inst., 2014, 106, 1–10 Search PubMed.
- E. Giovannucci, E. B. Rimm, L. Yan, M. J. Stampfer and W. C. Willett, J. Natl. Cancer Inst., 2002, 94, 391 CrossRef CAS PubMed.
- D. J. Kim, N. Takasuka, H. Tsuda and H. Nishino, BioFactors, 2000, 13, 95 CrossRef CAS PubMed.
- P. H. Gann and F. Khachik, J. Natl. Cancer Inst., 2003, 95, 1563–1565 CrossRef CAS PubMed.
- J. McEneny, L. Wade, I. S. Young, L. Masson, G. Duthie, A. McGinty, C. McMaster and F. Thies, J. Nutr. Biochem., 2013, 24, 163–168 CrossRef CAS PubMed.
- R. Bala, D. Khanna, S. Mehan and S. Kalra, RSC Adv., 2015, 5, 72881–72892 RSC.
- R. B. van Breemen, X. Xu, M. A. Viana, L. Chen, M. Stacewicz-Sapuntzakis, C. Duncan, P. E. Bowen and R. Sharifi, J. Agric. Food Chem., 2002, 50, 2214–2219 CrossRef CAS PubMed.
- W. Stahl, W. Schwarz, A. R. Sundquist and H. Sies, Arch. Biochem. Biophys., 1992, 294, 173–177 CrossRef CAS PubMed.
- J. Shi, Y. Dai, Y. Kakuda, G. Mittal and S. J. Xue, Food Control, 2008, 19, 514–520 CrossRef CAS.
- A. C. Boileau, N. R. Merchen, K. Wasson, C. A. Atkinson and J. W. Erdman, J. Nutr., 1999, 129, 1176–1181 CAS.
- M. L. Failla, C. Chitchumroonchokchai and B. K. Ishida, J. Nutr., 2008, 138, 482–486 CAS.
- J. L. Cooperstone, R. A. Ralston, K. M. Riedl, T. C. Haufe, R. M. Schweiggert, S. A. King, C. D. Timmers, D. M. Francis, G. B. Lesinski, S. K. Clinton and S. J. Schwartz, Mol. Nutr. Food Res., 2015, 59, 658–669 CAS.
- H. Phan-Thi and Y. Waché, Food Chem., 2014, 156, 58–63 CrossRef CAS PubMed.
- S. Luterotti, D. Bicanic, K. Marković and M. Franko, Food Control, 2015, 48, 67–74 CrossRef CAS.
- I. J. P. Colle, L. Lemmens, G. N. Tolesa, S. van Buggenhout, K. de Vleeschouwer, A. M. van Loey and M. E. Hendrickx, J. Agric. Food Chem., 2010, 58, 12784–12789 CrossRef CAS PubMed.
- M. Honda, H. Igami, T. Kawana, K. Hayashi, M. Takehara, Y. Inoue and C. Kitamura, J. Agric. Food Chem., 2014, 62, 11353–11356 CrossRef CAS PubMed.
- M. Honda, N. Takahashi, T. Kuwa, M. Takehara, Y. Inoue and T. Kumagai, Food Chem., 2015, 171, 323–329 CrossRef CAS PubMed.
- K. Siuzdak, M. Szkoda, M. Sawczak, A. Lisowska-Oleksiak, J. Karczewski and J. Ryl, RSC Adv., 2015, 5, 50379–50391 RSC.
- Q. Shi, C. Jiang, Y. Wang, W. Yang and C. Yang, Appl. Surf. Sci., 2013, 273, 769–775 CrossRef CAS.
- Y. Wang, J. Ren, G. Liu and P. Peng, Mater. Chem. Phys., 2011, 130, 493–499 CrossRef CAS.
- Y. Ma, J.-W. Fu, X. Tao, X. Li and J.-F. Chen, Appl. Surf. Sci., 2011, 257, 5046–5051 CrossRef CAS.
- M. Takehara, M. Nishimura, T. Kuwa, Y. Inoue, C. Kitamura, T. Kumagai and M. Honda, J. Agric. Food Chem., 2014, 62, 264–269 CrossRef CAS PubMed.
- A. J. Melendez-Martinez, M. Paulino, C. M. Stinco, P. Mapelli-Brahm and X. D. Wang, J. Agric. Food Chem., 2014, 62, 12399–12406 CrossRef CAS PubMed.
- Y. Wang, F. Duo, S. Peng, F. Jia and C. Fan, J. Colloid Interface Sci., 2014, 430, 31–39 CrossRef CAS PubMed.
- G. A. Chasse, M. L. Mak, E. Deretey, I. Farkas, L. L. Torday, J. G. Papp, D. S. R. Sarma, A. Agarwal, S. Chakravarthi, S. Agarwal and A. V. Rao, J. Mol. Struct.: THEOCHEM, 2001, 571, 27–37 CrossRef CAS.
- L. Müller, P. Goupy, K. Fröhlich, O. Dangles, C. Caris-Veyrat and V. Böhm, J. Agric. Food Chem., 2011, 59, 4504–4511 CrossRef PubMed.
- L. Zhang, H. Zhang, K. H. Ndeurumi, K. L. Parkin and M. Venuste, Food Chem., 2012, 132, 2112–2117 CrossRef CAS.
- J. C. Y. Yeung, G. A. Chasse, E. J. Frondozo, L. L. Torday and J. G. Papp, J. Mol. Struct.: THEOCHEM, 2001, 546, 143–162 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24074c |
| This journal is © The Royal Society of Chemistry 2016 |