Oxidative decontamination of chemical warfare agent VX and its simulant using N,N-dichlorovaleramide
Pranav Kumar Gutch*a,
Avik Mazumderb and
Gundapu Ravirajua
aSynthetic Chemistry Division, Defence Research and Development Establishment, Jhansi Road, Gwalior-474002, India. E-mail: pkgutch@rediffmail.com; Fax: +91-751-2341148; Tel: +91-751-2390186
bVertox Laboratory, Defence Research and Development Establishment, Jhansi Road, Gwalior-474002, India. E-mail: avik@drde.drdo.in; Fax: +91-751-2341148; Tel: +91-751-2390183
First published on 22nd December 2015
Abstract
The optimized conditions have been reported for efficient, operationally simple and safe oxidative decontamination of chemical warfare agent O-ethyl-S-2-(N,N-diisopropylaminoethyl)methylphosphonothioate (VX) and its non-toxic simulant O,S-diethyl methylphosphonothioate (OSDEMP). A positive chlorine bearing reagent N,N-dichlorovaleramide (NCV) was tested successfully to effect decontamination of these compounds. These compounds were found to undergo instantaneous reaction with NCV in acetonitrile–water medium to form non-toxic products. The reaction was monitored by gas chromatography (GC) and the products were identified by gas chromatography coupled with mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy. The advantages of using this reagent are its low cost of production, ease of synthesis from commercially available raw materials and good shelf life (of more than one year).
1 Introduction
The massive and highly diverse family of organophosphorus compounds have a variety of uses. They are used for the synthesis of a variety of organic compounds having huge economic importance (viz. pesticides,1 herbicides,2 fungicides,3 insecticides,4 acaricides,5 fire retardants,6 metal extractants,7 surfactants,8 detergents,9 drugs10 and lubricant additives,11 etc.).Alongside these peaceful uses, they have also been used for the manufacture of highly toxic chemical warfare agents (CWAs).12 Owing to their ease of synthesis and toxic effects on life forms, these CWAs confer a great tactical/psychological advantage as weapons of mass destruction.13,14 In spite of the continued efforts of the world community to ostracize the CWAs, they continue to pose a constant threat to the civilized world.15 They have been repeatedly used during military conflicts16 and terror attacks.17 Among all chemical warfare agents reported to date,18 O-ethyl-S-2-(N,N-diisopropylaminoethyl)methylphosphonothioate (VX) and its analogues are the most toxic.19 According to the Acute Exposure Guideline Level three (AGEL-3) the lethal dose of VX is 0.0096 mg m−3 for a ten minute exposure to humans.20 Hence it is ten times more toxic than sarin21 (the chemical warfare agent used during attack on the Tokyo subway in 1995).22 Like other organophosphorus chemical warfare agents, VX rapidly inhibits acetylcholinesterase (AChE)23 enzyme by phosphorylating its active site. This leads to the accumulation of neurotransmitter acetylcholine, over-stimulation of cholinergic receptors and finally breakdown of neuromuscular function24 takes place. Hence, VX has been rightly classified as a nerve agent25 and it has been enlisted in Schedule 1.A.3 of the Chemical Weapons Convention (CWC).26
Hence, for the safe and effective management of accidental and intentional release of this chemical; efficient decontamination is a mandatory requirement. In order to achieve this goal, several physical, chemical and physico-chemical decontamination techniques have been developed. They make use of hydrolytic or solvolytic27–30 and oxidative reactions31,32 reactions.
Although pure VX is a persistent chemical at room temperature, a 95% pure sample of VX decomposes at a rate of 5% a month at 71 °C. The hydrolysis of VX is highly dependent on pH.32 It is quite water-soluble below its pKa of 7.9 owing to protonation of nitrogen. Hence above this pH it is less soluble in water. Its half-life is 100 days at pH 2 or 3 at 25 °C. It undergoes biphasic reaction and at pH 14 its half-life is 1.3 min.33 It is calculated that VX is approximately 1500 times slower in evaporating than nerve agent GB.34 Due to low volatility and high stability, VX can persist for months under average weather conditions.35,36 It is extremely toxic by absorption through skin and eye.37 VX has a tendency to rapidly penetrate skin without causing any injury.38 As compared to the G-agents, the vivo persistence of VX is substantially higher. This undermines the efficacy of treatment with oximes since the later have shorter persistence in comparison to the agent itself.39 Due to these reasons, immediate decontamination of the smallest drop of VX is very necessary.
The formation of up to about 10% of S-2-(N,N-diisopropylaminoethyl)methylphosphonothioic acid (also known as EA-2192) is another important aspect of basic hydrolysis of VX.32 Since this compound is equally toxic as VX,40 its formation is not desirable during the decontamination process. Although and enzymatic41 degradation of VX is fast and safe, the enzymes are expensive, difficult to produce and have poor shelf life.42 The micellar35 decontamination methods on the other hand are very sensitive to the reaction conditions.43
Many of the oxidative decontaminants utilize the oxidative power of positively charged chlorine species.32 These decontaminants include hypochlorites or their equivalents. Other prominent oxidative decontaminating reagents reported for the oxidation of to date include hydrogen peroxide,44 peroxymonosulfates,45 nanomaterials,46 self-decontaminating materials,47 inorganic oxidants,48 photocatalysts,49 iodosobenzoates,50 chloramides51 and household chemicals.52 We have reported the oxidative degradation of OSDEMP (a non-toxic simulant of VX) and sulfur mustard using positive chlorine bearing decontaminants.53–56 By far the oxidative methods are easy to implement as they instantly convert the sulfur containing CWAs to their corresponding innocuous oxidation products.
In the present work N,N-dichlorovaleramide (NCV) was first tested for the decontamination of OSDEMP, a non-toxic simulant of VX. Thereafter decontamination of highly toxic chemical warfare agent VX (Fig. 1) was attempted with this reagent. The extent of decontamination reactions was monitored using 31P{1H} NMR spectroscopy. Whereas, gas chromatograph coupled with flame photometric detector working in sulfur mode (GC-FPD(S)) is a more sensitive analytical technique, it was also used to ascertain complete disappearance of VX/OSDEMP. The GC-FPD(S) analysis provided information regarding progress of the reaction whereas rapid identification of the products in the reaction mixture was achieved by using GC-MS and phosphorus edited proton nuclear magnetic resonance spectroscopy (performed without and with standard addition of ethyl methylphosphonic acid (EMPA) to the NMR tube). The GCFPD(S) and GC-MS require aggressive experimental conditions and suffer from memory effects. Moreover, the non-volatile analytes had to be converted into their volatile derivatives prior to analysis by these two techniques. NMR spectroscopy was used as a complimentary technique for rapid analysis of the reaction mixtures with minimal sample preparation and memory effects. Being a soft analytical technique any further chemical reactions was avoided during analysis and the sample was recovered and reused for GC/GC-MS experiments. Nucleus specific observation of the analytes imparted selectivity to the NMR experiments.57–65 With the use of three analytical techniques in conjunction complete decontamination of VX/OSDEMP and concomitant formation of non-toxic products was ensured.
2 Experimental
2.1 Materials
The chemicals valeramide, 3-trimethylsilyl propionic acid-d4 (TSP), chloroform-d1, and acetonitrile-d3 were purchased from Sigma Aldrich Chemical Company, Milwaukee, USA. Acetonitrile and glacial acetic acid of AR grade were purchased from S.D. Fine-Chem Ltd, Mumbai, India. Sodium hypochlorite was prepared by passing chlorine gas at flow of 1 g min−1 in NaOH solution (15%, 100 mL) for 30 min. at 5 °C.2.2 Instruments used and conditions of analysis
The decontamination reaction was monitored using GC (Agilent 7890) coupled with flame photometric detector (FPD) working in sulfur mode. Whereas a GC (Agilent 7890) coupled with 5975C mass selective detector (MSD) working in EI mode (Agilent Technologies, San Jose, CA, USA) was used for identification of the products. Chromatographic separation was achieved using the following conditions: column HP-5 (30 m × 0.250 mm × 0.25μm) with a temperature program of 50 °C for 2 min followed by a linear gradient to 250 °C at 10 °C min−1, and hold at 250 °C for 5 min. The injector temperature was maintained at 250 °C while the transfer line was heated to 280 °C. The EI analysis was performed at 70 eV with ion source temperature at 230 °C and emission current of 400 mA. All NMR spectra were recorded on Bruker AV III 600 MHz spectrometer equipped with a 5 mm broadband observe probe head (BBFO) fitted with an actively shielded single axis Z-gradient and automated tuning and matching accessory. All NMR experiments66 were carried out at 25 °C after equilibrating the samples for 5 minutes at this temperature. The NMR spectra were acquired and processed using Topspin 3.2 software.2.3 Synthesis of O,S-diethyl methylphosphonothioate (OSDEMP), VX and N,N-dichloro valeramide (NCV)
The target compounds O,S-diethyl methyl phosphonothioate and VX and NCV were synthesized by as methods reported earlier.55,56 Structure and purity of all the compounds was confirmed by 1H, 31P{1H}, 31P-1H HMQC NMR spectroscopy and GC-MS. The active chlorine content of NCV was found to be 44%.67 Freshly synthesized NCV was stored in a stoppered glass vial at room temperature. In order to determine its shelf life, active chlorine was determined by iodometry once in a month for a period of one year. A marginal decrease of active chlorine was observed from 44.10 to 42.4%.2.4 Reaction of VX and OSDEMP with NCV
The reaction was carried out in NMR tube using CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O (5
D2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture to study the role of solvents and reaction time. The disappearance of the signal of VX (at 57.13 ppm) was observed. In order to ascertain the complete degradation of VX under the optimized conditions, NCV (2.0 equivalents) was added to a test-tube containing OSDEMP or VX (1.0 equivalent) dissolved in 6 mL CH3CN
1) solvent mixture to study the role of solvents and reaction time. The disappearance of the signal of VX (at 57.13 ppm) was observed. In order to ascertain the complete degradation of VX under the optimized conditions, NCV (2.0 equivalents) was added to a test-tube containing OSDEMP or VX (1.0 equivalent) dissolved in 6 mL CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (5
H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Samples were withdrawn at 5 minute time intervals to ascertain the complete degradation of VX. A 500 μL aliquot of the reaction mixture was extracted twice with 200 μL aliquots of hexane. The hexane layers were pooled, dried using anhydrous sodium sulfate and transferred to 5 mm NMR sample tubes. To this solution a 200 μL aliquot of CDCl3 was added and it was subjected to NMR. The samples were withdrawn and they were subjected to GC-FPD(S) and/or GC-MS analysis. In order to identify the degradation products formed during the reaction, a 600 μL aliquot was taken in a test tube were placed on a dry bath (50 °C) and concentrated near to dryness under a gentle purge of dry nitrogen (5 mL min−1). The residues were reconstituted in 600 μL CDCl3 and analyzed by NMR spectroscopy. Another 500 μL aliquot was cooled to 5 °C and freshly prepared ethereal solution of diazomethane was added slowly till a faint yellow color persisted. The solution was concentrated to 50 μL under a gentle stream of nitrogen (5 mL min−1) and injected into GC-FPD(S) and/or GC-MS (under the conditions specified in Section 2.2).
1). Samples were withdrawn at 5 minute time intervals to ascertain the complete degradation of VX. A 500 μL aliquot of the reaction mixture was extracted twice with 200 μL aliquots of hexane. The hexane layers were pooled, dried using anhydrous sodium sulfate and transferred to 5 mm NMR sample tubes. To this solution a 200 μL aliquot of CDCl3 was added and it was subjected to NMR. The samples were withdrawn and they were subjected to GC-FPD(S) and/or GC-MS analysis. In order to identify the degradation products formed during the reaction, a 600 μL aliquot was taken in a test tube were placed on a dry bath (50 °C) and concentrated near to dryness under a gentle purge of dry nitrogen (5 mL min−1). The residues were reconstituted in 600 μL CDCl3 and analyzed by NMR spectroscopy. Another 500 μL aliquot was cooled to 5 °C and freshly prepared ethereal solution of diazomethane was added slowly till a faint yellow color persisted. The solution was concentrated to 50 μL under a gentle stream of nitrogen (5 mL min−1) and injected into GC-FPD(S) and/or GC-MS (under the conditions specified in Section 2.2).
3 Results and discussion
The 31P{1H} NMR experiments on the reactions carried out in different solvents (viz. chloroform, dichloromethane, benzene, tetrahydrofuran, acetonitrile, water, ethyl acetate) and their admixtures. These experiments clearly showed that a mixture of CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (5
H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
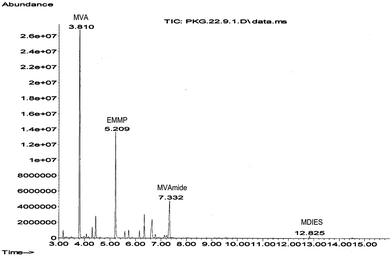
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was best suited for this reaction. A vigorous reaction ensued at room temperature with observable warming of the solution and concomitant disappearance of the 31P{1H} NMR signal of VX. Due to high specific capacity of water, the endogenous heat was handled efficiently by this solvent system. This solvent system also led to instantaneous reaction homogeneous reaction. This helped in efficient monitoring and optimizing the reaction conditions by GC/GC-MS and solution state NMR techniques. The complete absence of VX and EA2192 were confirmed by GC-FPD(S) and/or GC-MS analysis of the hexane extracts and diazomethane derivatives present in the reaction mixture (Fig. 2–4). The 31P{1H} NMR and GC-MS experiments also helped to ascertain the methyl derivatives of (2-(diisopropylamino)ethanesulfonate (i.e. MDIES) and ethyl methylphosphonate (i.e. EMMP) in the reaction mixture. The decontaminant NCV was converted to methyl derivatives of valeric acid (MVA) and valeramide (MVAmide). The extracted ion chromatograms were checked with an ion-command of m/z 114 on the GC-MS data recorded in full scan mode (m/z 40–400). No peaks corresponding to VX or the methyl ester of EA-2192 were observed in the extracted ion chromatograms. This not only indicated 100% decontamination of VX, it was also indicative of the absence of methyl ester of its toxic degradation product EA-2192.
1) was best suited for this reaction. A vigorous reaction ensued at room temperature with observable warming of the solution and concomitant disappearance of the 31P{1H} NMR signal of VX. Due to high specific capacity of water, the endogenous heat was handled efficiently by this solvent system. This solvent system also led to instantaneous reaction homogeneous reaction. This helped in efficient monitoring and optimizing the reaction conditions by GC/GC-MS and solution state NMR techniques. The complete absence of VX and EA2192 were confirmed by GC-FPD(S) and/or GC-MS analysis of the hexane extracts and diazomethane derivatives present in the reaction mixture (Fig. 2–4). The 31P{1H} NMR and GC-MS experiments also helped to ascertain the methyl derivatives of (2-(diisopropylamino)ethanesulfonate (i.e. MDIES) and ethyl methylphosphonate (i.e. EMMP) in the reaction mixture. The decontaminant NCV was converted to methyl derivatives of valeric acid (MVA) and valeramide (MVAmide). The extracted ion chromatograms were checked with an ion-command of m/z 114 on the GC-MS data recorded in full scan mode (m/z 40–400). No peaks corresponding to VX or the methyl ester of EA-2192 were observed in the extracted ion chromatograms. This not only indicated 100% decontamination of VX, it was also indicative of the absence of methyl ester of its toxic degradation product EA-2192.
 | ||
| Fig. 3 GC-EI-MS spectrum of methyl-2-(diisopropylamino)ethanesulfonate (MDIES) decontaminated product of VX. Eluting at 12.82 min from the GC column. | ||
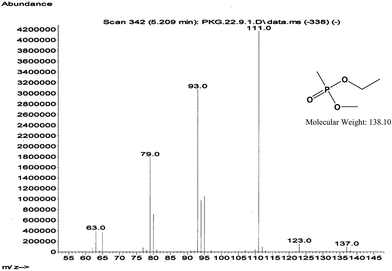 | ||
| Fig. 4 GC-EI-MS spectrum of ethyl methyl methylphosphonate (EMMP) decontaminated product of VX. Eluting at 5.21 min from the GC column. | ||
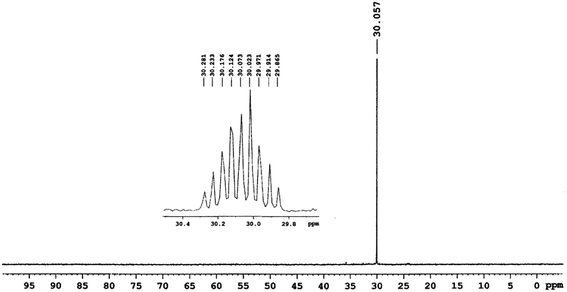
The 31P{1H}, 31P NMR experiments were carried out on VX and the second fraction of the reaction mixture indicated absence of VX (at 57.12 ppm, Fig. 5) and exclusive formation of O-ethyl methylphosphonate (EMPA, 30.06 ppm, Fig. 6 and 7) as the organophosphorus degradation product. The presence of EMPA and absence of degradation product EA-2192 was also confirmed by 1H-31P HSQC experiments on the reaction mixture (Fig. 7).
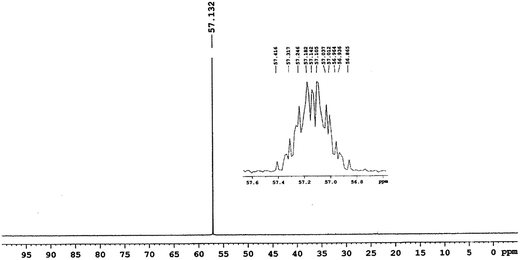 | ||
| Fig. 5 The purity of VX was ascertained from 31P{1H}-NMR (inset 31P-NMR) spectrum of VX recorded prior to reaction. | ||
Similar sample preparation procedure was used to study the decontamination products of OSDEMP by GC-MS. The hexane extract did not show the presence of OSDEMP. When the aqueous fraction was analyzed after methylation, ethyl methyl methylphosphonate (EMMP) was found to be present. These observations were also corroborated by the results obtained from 31P, 31P{1H} and 1H-31P HSQC NMR experiments.
On the basis of the identified major degradation products valeric acid (VA), ethyl methylphosphonic acid (EMPA), 2-(diisopropylamino)ethanesulfonate (DIES) and valeramide (VAmide) and reported literature;31,68 plausible mechanism of the reaction is proposed below (Fig. 8). The reaction is initiated by attack of the electrophilic Cl+ on the lone pair of electrons of bivalent sulfur of VX.31 This leads to the formation of sulfonium cation intermediate (b) and subsequently the nucleophilic attack of water on phosphorus atom of (b) leads to cleavage of the P–S bond of VX gives protonated ethyl methylphosphonate (EMPA) (c) and 2-(N,N-diisopropylamino)ethanesulfenyl chloride (d). Removal of proton from intermediate (c) gives ethyl methylphosphonate (EMPA). Furthermore in the step (iii) hydrolysis S–Cl bond and subsequent removal of HCl forms intermediate (d) which further hydrolysis to form (e). In the step (iv) again the lone pair of electrons present on sulfur atom of (e) attacks on positive chlorine to form a specie (f). This happens due to the presence of positive charge and vacant d-orbitals on the sulfur atom in (f) and lone pair of oxygen moiety of water molecule attacks on this sulfur and leads to the formation of unstable dicationic specie (g). This specie gets converted into intermediate (h). Elimination of HCl from intermediate (h) in the step (vii) produces sulfinic acid derivative (i). Furthermore repetition of steps iv–vii leads to the formation of sulfonic acid product 2-(N,N-diisopropylamino)ethanesulfonate (j).
4 Conclusion
In conclusion, the study reveals that NCV works as an effective decontaminating agent against OSDEMP and VX to form non-toxic products at room temperature. This reagent has advantage over earlier reported reagent in terms of ease of synthesis, cost, effectiveness, decontamination efficiency and stability.Author contributions
PKG was responsible for conception of the study, synthesis of NCV, reaction monitoring by GC/GC-MS and drafting of manuscript. AM was responsible for sample preparation, acquisition of NMR experiments, interpretation of experimental data, drafting and editing the manuscript. GR synthesized VX for the study.References
- C. Fest and K. J. Schmidt, The chemistry of organophosphorus pesticides, Springer Science & Business Media, 2012 Search PubMed.
- J. E. Franz, M. K. Mao and J. A. Sikorski, Glyphosate: a unique global herbicide, American Chemical Society, 1997 Search PubMed.
- H. R. Hudson, Phosphorus, Sulfur Silicon Relat. Elem., 1999, 144, 441–444 CrossRef.
- H. Martin, Insecticide and fungicide handbook for crop protection, 1965 Search PubMed.
- S. E. Kunz and D. H. Kemp, Rev. Inform. Sci. Tech., 1994, 13, 1249–1286 CAS.
- P. Moy, J. Vinyl Addit. Technol., 2004, 10, 187–192 CrossRef CAS.
- D. S. Flett, J. Organomet. Chem., 2005, 690, 2426–2438 CrossRef CAS.
- G. Broze, Handbook of Detergents: Properties, CRC Press, 1999 Search PubMed.
- K. A. Booman and R. I. Sedlak, J. - Water Pollut. Control Fed., 1986, 1092–1100 CAS.
- B. M. Liederer and R. T. Borchardt, J. Pharm. Sci., 2006, 95, 1177–1195 CrossRef CAS PubMed.
- D. H. Moreton, US Pat 2684336, 1954.
- R. M. Black and J. M. Harrison, in The chemistry of organophosphorus compounds, John Wiley & Sons, Ltd, 1996, pp. 781–840, DOI:10.1002/0470034351.ch10.
- H. John, F. Balszuweit, K. Kehe, F. Worek and H. Thiermann, in Handbook of toxicology of chemical warfare agents, ed. R. C. Gupta, Academic Press, San Diego, 2009, pp. 755–790, DOI:10.1016/b978-012374484-5.00050-x.
- L. Szinicz, Toxicology, 2005, 214, 167–181 CrossRef CAS PubMed.
- F. Bolz Jr, K. J. Dudonis and D. P. Schulz, The counterterrorism handbook: tactics, procedures, and techniques, CRC Press, 2011 Search PubMed.
- C. Macilwain, Nature, 1993, 363, 3 CAS.
- T. Okumura, T. Hisaoka, A. Yamada, T. Naito, H. Isonuma, S. Okumura, K. Miura, M. Sakurada, H. Maekawa, S. Ishimatsu, N. Takasu and K. Suzuki, Toxicol. Appl. Pharmacol., 2005, 207, 471–476 CrossRef CAS PubMed.
- J. A. Romano, B. J. Lukey and H. Salem, Chemical warfare agents: chemistry, pharmacology, toxicology, and therapeutics, CRC Press, 2007 Search PubMed.
- H. M. Hartmann, Regul. Toxicol. Pharmacol., 2002, 35, 347–356 CrossRef CAS PubMed.
- A. Watson, D. Opresko, R. Young and V. Hauschild, J. Toxicol. Environ. Health, Part B, 2006, 9, 173–263 CAS.
- R. C. Gupta, G. T. Patterson and W.-D. Dettbarn, Toxicol. Sci., 1991, 16, 449–458 CrossRef CAS.
- T. Okumura, N. Takasu, S. Ishimatsu, S. Miyanoki, A. Mitsuhashi, K. Kumada, K. Tanaka and S. Hinohara, Ann. Emerg. Med., 1996, 28, 129–135 CrossRef CAS PubMed.
- K. MacPhee-Quigley, P. Taylor and S. Taylor, J. Biol. Chem., 1985, 260, 12185–12189 CAS.
- J. Bajgar, in Advances in Clinical Chemistry, Elsevier, 2004, vol. 38, pp. 151–216 Search PubMed.
- J. Newmark, in Clinical Neurotoxicology, ed. M. R. Dobbs and W. B. Saunders, Philadelphia, 2009, pp. 646–659, DOI:10.1016/b978-032305260-3.50062-9.
- Convention on the Prohibition of the Development, Stockpiling and Use of Chemical Weapons and their Destruction, Technical Secretariat of the Organization for Prohibition of Chemical Weapons, The Hague, The Netherlands, 1997, http://www.opcw.org Search PubMed.
- M. A. S. Khan, M. K. Kesharwani, T. Bandyopadhyay and B. Ganguly, J. Mol. Graphics Modell., 2009, 28, 177–182 CrossRef CAS PubMed.
- K. A. Daniel, L. A. Kopff and E. V. Patterson, J. Phys. Org. Chem., 2008, 21, 321–328 CrossRef CAS.
- E. Gershonov, I. Columbus and Y. Zafrani, J. Org. Chem., 2009, 74, 329–338 CrossRef CAS PubMed.
- B. B. Dhar, D. R. Edwards and R. S. Brown, Inorg. Chem., 2011, 50, 3071–3077 CrossRef CAS PubMed.
- Y. C. Yang, J. A. Baker and J. R. Ward, Chem. Rev., 1992, 92, 1729–1743 CrossRef CAS.
- Y.-C. Yang, Acc. Chem. Res., 1998, 32, 109–115 CrossRef CAS.
- S. L. Hoenig, Compendium of chemical warfare agents, Springer, New York, 2007 Search PubMed.
- S. S. Talmage, A. P. Watson, V. Hauschild, N. B. Munro and J. King, Curr. Org. Chem., 2007, 11, 285–298 CrossRef CAS.
- A. F. Kingery and H. E. Allen, Toxicol. Environ. Chem., 1995, 47, 155–184 CrossRef CAS.
- A. H. Love, A. L. Vance, J. G. Reynolds and M. L. Davisson, Chemosphere, 2004, 57, 1257–1264 CrossRef CAS PubMed.
- C. P. Holstege, M. Kirk and F. R. Sidell, Crit. Care Clin., 1997, 13, 923–942 CrossRef CAS PubMed.
- C. H. Dalton, I. J. Hattersley, S. J. Rutter and R. P. Chilcott, Toxicol. In Vitro, 2006, 20, 1532–1536 CrossRef CAS PubMed.
- M. J. van der Schans, B. J. Lander, H. V. D. Wiel, J. P. Langenberg and H. P. Benschop, Toxicol. Appl. Pharmacol., 2003, 191, 48–62 CrossRef CAS PubMed.
- Y. C. Yang, L. L. Szafraniec, W. T. Beaudry and D. K. Rohrbaugh, J. Am. Chem. Soc., 1990, 112, 6621–6627 CrossRef CAS.
- A. N. Bigley, C. Xu, T. J. Henderson, S. P. Harvey and F. M. Raushel, J. Am. Chem. Soc., 2013, 135, 10426–10432 CrossRef CAS PubMed.
- P. V. Iyer and L. Ananthanarayan, Process Biochem., 2008, 43, 1019–1032 CrossRef CAS.
- P. A. Fields, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2001, 129, 417–431 CrossRef CAS.
- G. W. Wagner, L. R. Procell, D. C. Sorrick, G. E. Lawson, C. M. Wells, C. M. Reynolds, D. B. Ringelberg, K. L. Foley, G. J. Lumetta and D. L. Blanchard, Ind. Eng. Chem. Res., 2010, 49, 3099–3105 CrossRef CAS.
- E. Raber and R. McGuire, J. Hazard. Mater., 2002, 93, 339–352 CrossRef CAS PubMed.
- G. K. Prasad, P. Ramacharyulu and B. Singh, J. Sci. Ind. Res., 2011, 70, 91–104 CAS.
- G. Sun, S. Worley and R. Broughton Jr, Military Textiles, Woodhead Publishing, 2008, p. 281 Search PubMed.
- A. Zenerino, T. Boutard, C. Bignon, S. Amigoni, D. Josse, T. Devers and F. Guittard, Toxicol. Rep., 2015, 2, 1007–1013 CrossRef.
- A. Komano, T. Hirakawa, K. Sato, S. Kishi, C. K. Nishimoto, N. Mera, M. Kugishima, T. Sano, N. Negishi, H. Ichinose, Y. Seto and K. Takeuchi, Appl. Catal., B, 2013, 134–135, 19–25 CrossRef CAS.
- H. Morales-Rojas and R. A. Moss, Chem. Rev., 2002, 102, 2497–2522 CrossRef CAS PubMed.
- B. Salter, J. Owens, R. Hayn, R. McDonald and E. Shannon, J. Mater. Sci., 2009, 44, 2069–2078 CrossRef CAS.
- G. W. Wagner, Ind. Eng. Chem. Res., 2011, 50, 12285–12287 CrossRef CAS.
- P. K. Gutch and A. Mazumder, Ind. Eng. Chem. Res., 2012, 51, 5830–5837 CrossRef CAS.
- R. Singh, P. K. Gutch and A. Mazumder, Ind. Eng. Chem. Res., 2013, 52, 4689–4694 CrossRef CAS.
- R. Singh, P. K. Gutch, J. Acharya and S. Prabha, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 1504–1509 Search PubMed.
- P. K. Gutch, R. Singh and M. V. S. Suryanarayana, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 1677 Search PubMed.
- A. Mazumder and D. K. Dubey, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2013, DOI:10.1016/b978-0-12-409547-2.05891-1.
- A. Mazumder, P. Garg, D. Pardasani, A. Kumar, A. K. Purohit and D. K. Dubey, Anal. Methods, 2011, 3, 1574–1581 RSC.
- A. Mazumder, H. K. Gupta, P. Garg, R. Jain and D. K. Dubey, J. Chromatogr. A, 2009, 1216, 5228–5234 CrossRef CAS PubMed.
- A. Mazumder, H. K. Gupta, R. K. Srivastava and D. Dubey, Def. Sci. J., 2010, 60, 502–506 CrossRef CAS.
- A. Mazumder, A. Kumar and D. K. Dubey, J. Chromatogr. A, 2013, 1284, 88–99 CrossRef CAS PubMed.
- A. Mazumder, A. Kumar and D. K. Dubey, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 95–101 CAS.
- A. Mazumder, A. Kumar, A. K. Purohit and D. K. Dubey, J. Chromatogr. A, 2010, 1217, 2887–2894 CrossRef CAS PubMed.
- A. Mazumder, P. K. Gutch and D. K. Dubey, J. Chromatogr. A, 2015, 1393, 26–36 CrossRef CAS PubMed.
- A. Mazumder, A. Kumar, A. K. Purohit and D. K. Dubey, Anal. Bioanal. Chem., 2012, 402, 1643–1652 CrossRef CAS PubMed.
- H. Koskela, U. Hakala and P. Vanninen, Anal. Chem., 2010, 82, 5331–5340 CrossRef CAS PubMed.
- J. Mendham, Vogels textbook of quantitative chemical analysis, Pearson Education, India, 2006 Search PubMed.
- D. K. Dubey, R. C. Malhotra, R. Vaidyanathaswamy and R. Vijayaraghavan, J. Org. Chem., 1999, 64, 8031–8033 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |